Published online Nov 24, 2023. doi: 10.5306/wjco.v14.i11.445
Peer-review started: September 7, 2023
First decision: September 20, 2023
Revised: September 29, 2023
Accepted: October 16, 2023
Article in press: October 16, 2023
Published online: November 24, 2023
Processing time: 76 Days and 1 Hours
Breast cancer (BC) has become the most common malignancy in women. The incidence and detection rates of BC brain metastasis (BCBM) have increased with the progress of imaging, multidisciplinary treatment techniques and the extension of survival time of BC patients. BM seriously affects the quality of life and sur-vival prognosis of BC patients. Therefore, clinical research on the clinicopathological features and prognostic factors of BCBM is valuable. By analyzing the clinicopathological parameters of BCBM patients, and assessing the risk factors and prognostic indicators, we can perform hierarchical diagnosis and treatment on the high-risk population of BCBM, and achieve clinical benefits of early diagnosis and treatment.
To explore the clinicopathological features and prognostic factors of BCBM, and provide references for diagnosis, treatment and management of BCBM.
The clinicopathological data of 68 BCBM patients admitted to the Air Force Medical Center, Chinese People’s Liberation Army (formerly Air Force General Hospital) from 2000 to 2022 were collected. Another 136 BC patients without BM were matched at a ratio of 1:2 based on the age and site of onset for retrospective analysis. Categorical data were subjected to χ2 test or Fisher’s exact probability test, and the variables with P < 0.05 in the univariate Cox proportional hazards model were incorporated into the multivariate model to identify high-risk factors and independent prognostic factors of BCBM, with a hazard ratio (HR) > 1 suggesting poor prognostic factors. The survival time of patients was estimated by the Kaplan–Meier method, and overall survival was compared between groups by log-rank test.
Multivariate Cox regression analysis showed that patients with stage III/IV tumor at initial diagnosis [HR: 5.58, 95% confidence interval (CI): 1.99–15.68], lung metastasis (HR: 24.18, 95%CI: 6.40–91.43), human epidermal growth factor receptor 2 (HER2)-overexpressing BC and triple-negative BC were more prone to BM. As can be seen from the prognostic data, 52 of the 68 BCBM patients had died by the end of follow-up, and the median time from diagnosis of BC to the occurrence of BM and from the occurrence of BM to death or last follow-up was 33.5 and 14 mo, respectively. It was confirmed by multivariate Cox regression analysis that patients with neurological symptoms (HR: 1.923, 95%CI: 1.005–3.680), with bone metastasis (HR: 2.011, 95%CI: 1.056-3.831), and BM of HER2-overexpressing and triple-negative BC had shorter survival time.
HER2-overexpressing, triple-negative BC, late tumor stage and lung metastasis are risk factors of BM. The presence of neurological symptoms, bone metastasis, and molecular type are influencing prognosis factors of BCBM.
Core Tip: We aimed to identify the high-risk factors of breast cancer brain metastasis (BCBM) and conducted prognostic analyses. Sixty-eight BCBM patients diagnosed and treated in the Air Force Medical Center in 2000–2022 were enrolled. Patients with human epidermal growth factor receptor 2 overexpressing and triple-negative breast cancer were more prone to BM and had shorter survival time. Late tumor stage and lung metastasis were independent risk factors for BM. The presence or absence of neurological symptoms and bone metastasis, and molecular type were independent prognostic factors for BCBM. Early screening of high-risk patients for BM helps improve survival rate.
- Citation: Chen YR, Xu ZX, Jiang LX, Dong ZW, Yu PF, Zhang Z, Gu GL. Analysis of clinicopathological features and prognostic factors of breast cancer brain metastasis. World J Clin Oncol 2023; 14(11): 445-458
- URL: https://www.wjgnet.com/2218-4333/full/v14/i11/445.htm
- DOI: https://dx.doi.org/10.5306/wjco.v14.i11.445
Breast cancer (BC) has become the malignancy with the highest morbidity rate in women[1]. The overall survival (OS) of BC patients has been prolonged with the popularization of universal screening and advances in treatment and management. The proportion of patients with BC brain metastasis (BM) (BCBM) is about 15%[2], which increases with the extension of OS[3]. BM seriously threatens the life expectancy and quality of life of BC patients, and leads to poor prognosis, with a median OS of only 7.4 mo[4]. At present, medical surveillance of the brain is not regarded as a routine follow-up item for BC patients in China and globally. BM has an insidious onset, and most patients are not given targeted diagnosis and treatment of the brain until clinical symptoms emerge, thus losing the best opportunity of diagnosis and treatment and, affecting the survival rate. Therefore, clinical research on the clinicopathological features and prognostic factors of BCBM is required. To identify the clinicopathological features and prognostic factors of BCBM, guide targeted medical monitoring and intervention and raise the survival rate of BCBM patients, 68 BCBM patients were screened from 2238 BC patients admitted to our center from 2000 to 2022, and their clinical data were retrospectively studied.
Inclusion criteria: patients pathologically diagnosed with BC in the Air Force Medical Center (formerly Air Force General Hospital) from 2000 to 2022 were retrospectively collected. Patients with BM (including brain parenchymal metastasis and meningeal metastasis) identified by imaging, cytology or histology were selected.
Exclusion criteria: (1) Patients with concomitant malignancy or with a history of malignancy of other origin; (2) patients with incomplete clinical data; (3) patients diagnosed with other neurological diseases; (4) patients complicated with serious fatal clinical diseases; and (5) male patients with BC.
Enrolled cases and follow-up: 2238 BC patients were admitted to the Air Force Medical Center between 2000 to 2022, of whom, 101 (4.5%) developed BM. After ineligible cases were excluded, 68 patients were enrolled. Another 136 BC patients without BM (control group) were matched at a ratio of 1:2 based on the age and tumor site at initial diagnosis. Follow-up was performed by telephone interview, outpatient re-examination and inpatient examination until April 2023.
Clinicopathological parameters of BC patients were statistically analyzed. Univariate Cox proportional hazards model analysis was performed on age (at diagnosis of BC; menstrual status (at diagnosis of BC); family history of tumors; lymph node stage; primary tumor size; tumor stage at initial diagnosis; estrogen receptor (ER) and progesterone receptor (PR) status; molecular and pathological type; presence or absence of liver, lung and bone metastasis; number of liver, lung and bone metastases; presence or absence of metastasis to other sites; number of metastases to other sites; and whether or not surgical treatment was performed. Covariates with statistical significance were further incorporated into a multivariate Cox proportional hazards model for analysis.
Prognostic indicators: age at diagnosis of BM; time from diagnosis of BC to occurrence of BM; menstrual status at diagnosis of BM; molecular type; presence or absence of liver, lung and bone metastasis; number of liver, lung and bone metastases; presence or absence of metastasis to other sites; number of metastases to other sites; tumor stage at initial diagnosis; lymph node stage; pathological type; presence or absence of symptoms at diagnosis of BM; size and number of BMs; and treatment means for BM (systemic or local therapy).
Parameters of local therapy: surgical resection and radiotherapy [mainly including whole brain radiation therapy (WBRT) and stereotactic radiotherapy (SRT) of brain tumor].
Multidisciplinary treatment (MDT) was defined as systemic therapy combined with radiotherapy or surgery. The presence or absence of symptoms was determined according to whether abnormal vision, ataxia, symptoms of intracranial hypertension (headache, nausea and vomiting, lethargy, etc.), motor dysfunction, and paresthesia occurred in patients diagnosed previously with BC.
Parameters of hormone receptor: hormone receptor and human epidermal growth factor receptor 2 (HER2) status was determined by immunohistochemistry. HER2 positive was defined as HER2(3+), or HER2(2+) was positive in in situ hybridization test, and HER2 negative was defined as HER2(-), HER2(+), or HER2(2+) was negative in in situ hybridization test. The positive threshold of ER and PR in immunohistochemistry was ≥ 1%. Molecular typing of BC was performed according to hormone receptor status and HER2 expression: luminal A type (ER- and/or PR-positive, and HER2-negative); luminal B type (ER- and/or PR-positive, and HER2-positive); HER2-overexpressing type (ER- and PR-negative, and HER2-positive); and triple-negative type (ER-, PR- and HER2-negative). Tumor–node–metastasis (TNM) staging was carried out based on the American Joint Committee on Cancer 8th Edition Staging System.
Numerical data were compared between the two groups by χ2 test or Fisher’s exact probability test. The risk factors associated with BM at initial diagnosis of BC were first subjected to univariate Cox proportional hazards model analysis, and then covariates with P < 0.05 (selected by the backward conditional method) were incorporated into the multivariate Cox proportional hazards model. OS, defined as the time from the initial diagnosis of BM to death from any cause, or last follow-up, was compared between the groups using the log-rank test, and the survival time of patients was estimated by the Kaplan–Meier method. In the prognostic analysis of BCBM patients, univariate Cox proportional hazards model analysis was first performed, and then covariates with P < 0.05 (selected by the forward conditional method) were incorporated into the multivariate Cox proportional hazards model to identify the covariates related to survival outcomes, with a 95% confidence interval (95%CI). P < 0.05 was considered statistically significant. All statistical analyses were conducted with SPSS version 27.0 software (SPSS Inc., Chicago, IL, USA).
Among the 2238 patients with BC, BM was found at the initial diagnosis in two cases (0.089%) and during follow-up in 99 cases (4.42%). In the BCBM group, the median age at diagnosis of BC was 47 (29–69) years; 35 cases (51.5%) were postmenopausal, eight (11.8%) had a family history of malignancy, and most patients (51.5%) had stage III/IV tumors. In terms of molecular type, there were 13 cases (19.1%) of luminal A BC, 22 (32.4%) of luminal B BC, 14 (20.6%) of HER2-overexpressing BC, and 14 (20.6%) of triple-negative BC. Fifty-one cases (75.0%) were pathologically classified as invasive ductal carcinoma. Bone metastasis was the most common (55.9%), followed by lung and liver metastasis. Ten cases (14.7%) developed liver, lung and bone metastasis and BM during the course of disease. Modified radical mastectomy dominated in both groups, and the proportion of patients undergoing neoadjuvant chemotherapy, radiotherapy and HER2-targeted therapy in the BCBM group was higher than that in the non-BCBM group (Table 1).
| Item | Non-BCBM group (n = 136) [n (%)] | BCBM group (n = 68) [n (%)] | P |
| Age at diagnosis of BC (yr) | 0.009 | ||
| ≤ 35 | 9 (6.6) | 12 (17.6) | |
| 35-55 | 79 (58.1) | 43 (63.2) | |
| > 55 | 48 (35.3) | 13 (19.2) | |
| Menstrual status at diagnosis of BC | 0.77 | ||
| Premenopause | 69 (50.7) | 33 (48.5) | |
| Menopause | 67 (49.3) | 35 (51.5) | |
| Family history of cancer | 0.12 | ||
| None | 112 (82.4) | 60 (88.2) | |
| Other malignancies | 16 (11.8) | 8 (11.8) | |
| BC | 8 (5.9) | 0 (0.0) | |
| Lymph node stage | 0.01 | ||
| N0 | 56 (41.2) | 19 (27.9) | |
| N1 | 43 (31.6) | 14 (20.6) | |
| N2 | 25 (18.4) | 17 (25.0) | |
| N3 | 12 (8.8) | 15 (22.1) | |
| Missing | 0 (0.0) | 3 (4.4) | |
| Tumor size | 0.17 | ||
| T1 | 53 (39.0) | 18 (26.5) | |
| T2 | 67 (49.3) | 37 (54.4) | |
| T3 | 13 (9.6) | 11 (16.2) | |
| T4 | 1 (0.7) | 0 (0.0) | |
| Missing | 2 (1.5) | 2 (2.9) | |
| Tumor stage at the initial diagnosis | < 0.001 | ||
| IA | 29 (21.3) | 3 (4.4) | |
| IIA | 39 (28.7) | 19 (27.9) | |
| IIB | 29 (21.3) | 7 (10.3) | |
| IIIA | 22 (16.2) | 17 (25.0) | |
| IIIB | 2 (1.5) | 0 (0.0) | |
| IIIC | 11 (8.1) | 11 (16.2) | |
| IV | 3 (2.2) | 7 (10.3) | |
| Missing | 1 (0.7) | 4 (5.9) | |
| ER | 0.009 | ||
| Negative | 42 (30.9) | 32 (47.1) | |
| Positive | 94 (69.1) | 32 (47.1) | |
| Missing | 0 (0.0) | 4 (5.9) | |
| PR | 0.003 | ||
| Negative | 52 (38.2) | 39 (57.4) | |
| Positive | 84 (61.8) | 25 (36.8) | |
| Missing | 0 (0.0) | 4 (5.9) | |
| Molecular type | < 0.001 | ||
| Luminal A type | 71 (52.2) | 13 (19.1) | |
| Luminal B type | 28 (20.6) | 22 (32.4) | |
| HER2-overexpressing type | 18 (13.2) | 14 (20.6) | |
| Triple-negative type | 19 (14.0) | 14 (20.6) | |
| Missing | 0 (0.0) | 5 (7.4) | |
| Pathological type | 0.42 | ||
| Noninvasive carcinoma | 10 (7.4) | 2 (2.9) | |
| Invasive ductal carcinoma | 95 (69.9) | 51 (75.0) | |
| Invasive lobular carcinoma | 5 (3.7) | 2 (2.9) | |
| Others | 26 (19.1) | 8 (11.8) | |
| Missing | 0 (0.0) | 5 (7.4) | |
| Metastasis | |||
| Bone metastasis | < 0.001 | ||
| Yes | 12 (8.8) | 38 (55.9) | |
| No | 124 (91.2) | 30 (44.1) | |
| Liver metastasis | < 0.001 | ||
| Yes | 7 (5.1) | 20 (29.4) | |
| No | 129 (94.9) | 48 (70.6) | |
| Lung metastasis | < 0.001 | ||
| Yes | 8 (5.9) | 35 (51.5) | |
| No | 128 (94.1) | 33 (48.5) | |
| Number of liver, lung and bone metastases | < 0.001 | ||
| 0 | 123 (90.4) | 21 (30.9) | |
| 1 | 8 (5.9) | 19 (27.9) | |
| 2 | 3 (2.2) | 18 (26.5) | |
| 3 | 2 (1.5) | 10 (14.7) | |
| Number of metastases to other sites | < 0.001 | ||
| 0 | 118 (86.8) | 33 (48.5) | |
| 1 | 15 (11.0) | 24 (35.3) | |
| 2 | 2 (1.5) | 8 (11.8) | |
| 3 | 1 (0.7) | 3 (4.4) | |
| Surgical treatment | < 0.001 | ||
| No | 3 (2.2) | 7 (10.3) | |
| Breast-conserving surgery | 20 (14.7) | 5 (7.4) | |
| Modified radical mastectomy | 106 (77.9) | 43 (63.2) | |
| Others | 7 (5.1) | 13 (19.1) | |
| Radiotherapy | 0.006 | ||
| No | 76 (55.9) | 24 (35.3) | |
| Yes | 60 (44.1) | 44 (64.7) | |
| Chemotherapy | |||
| Neoadjuvant chemotherapy | 0.21 | ||
| No | 119 (87.5) | 55 (80.9) | |
| Yes | 17 (12.5) | 13 (19.1) | |
| Anthracyclines | < 0.001 | ||
| No | 28 (20.6) | 35 (51.5) | |
| Yes | 107 (78.7) | 32 (47.1) | |
| Missing | 1 (0.7) | 1 (1.5) | |
| Taxane | 0.35 | ||
| No | 35 (25.7) | 22 (32.4) | |
| Yes | 101 (74.3) | 46 (67.6) | |
| HER2 targeted therapy | < 0.001 | ||
| No | 118 (86.8) | 36 (52.9) | |
| Yes | 18 (13.2) | 32 (47.1) | |
Sixty-eight BCBM patients were matched at a ratio of 1:2 with 136 BC patients of the same age and tumor site at initial diagnosis. The median time from the diagnosis of BC to the occurrence of BM was 33.5 (0–181) mo in BCBM patients. The risk factors of BCBM are shown in Table 2. In univariate Cox analysis, lymph node stage; tumor stage at the initial diagnosis; ER status; PR status; molecular type; presence or absence of bone metastasis, liver metastasis and lung metastasis; number of bone, liver and lung metastases; number of metastases to other sites; and surgical mode were statistically significant. The above factors were incorporated into multivariate Cox analysis, and it was found that patients with stage III/IV tumor at initial diagnosis [hazard ratio (HR): 5.58, 95%CI: 1.99–15.68], lung metastasis (HR: 24.18, 95%CI: 6.40–91.43), and HER2-overexpressing and triple-negative BC were more prone to BM.
| Item | Univariate Cox | Multivariate Cox | ||
| HR (95%CI) | P | HR (95%CI) | P | |
| Age at diagnosis of BC (yr) | ||||
| ≤ 35 | Reference | |||
| > 35 | 0.008 (0-1.89) | 0.083 | ||
| Menstrual status at diagnosis of BC | ||||
| Premenopause | Reference | |||
| Menopause | 1.29 (0.52-3.20) | 0.59 | ||
| Family history of cancer | ||||
| None | Reference | |||
| Yes | 0.62 (0.26-1.44) | 0.26 | ||
| Lymph node stage | ||||
| N0-N1 | Reference | |||
| N2-N3 | 2.77 (1.40-5.48) | 0.004 | NS | |
| Tumor size | ||||
| T1-T2 | Reference | |||
| T3-T4 | 1.44 (0.65-3.15) | 0.37 | ||
| Tumor stage at the initial diagnosis | ||||
| I-II | Reference | |||
| III-IV | 3.84 (1.89-7.78) | < 0.001 | 5.58 (1.99-15.68) | 0.001 |
| ER | ||||
| Negative | Reference | |||
| Positive | 0.49 (0.27-0.87) | 0.015 | NS | |
| PR | ||||
| Negative | Reference | |||
| Positive | 0.36 (0.18-0.69) | 0.002 | NS | |
| Molecular type | ||||
| Luminal A type | Reference | |||
| Luminal B type | 3.95 (1.71-9.14) | 0.001 | 5.36 (1.61-17.76) | 0.006 |
| HER2-overexpression type | 4.01 (1.61-9.96) | 0.003 | 5.0 (1.30-19.25) | 0.019 |
| Triple-negative type | 4.34 (1.55-12.11) | 0.005 | NS | NS |
| Pathological type | ||||
| Others | Reference | |||
| Invasive ductal carcinoma | 1.83 (0.83-4.05) | 0.14 | ||
| Invasive lobular carcinoma | 1.31 (0.22-7.69) | 0. 77 | ||
| Surgical treatment | ||||
| Others | Reference | |||
| Modified radical mastectomy | 0.48 (0.26-0.90) | 0.021 | NS | |
| Bone metastasis | ||||
| No | Reference | |||
| Yes | 7.19 (3.13-16.52) | < 0.001 | NS | |
| Liver metastasis | ||||
| No | Reference | |||
| Yes | 7.44 (2.77-20.01) | < 0.001 | NS | |
| Lung metastasis | ||||
| No | Reference | |||
| Yes | 15.87 (5.61-44.89) | < 0.001 | 24.18 (6.40-91.43) | < 0.001 |
| Number of liver, lung and bone metastases | ||||
| < 2 | Reference | |||
| ≥ 2 | 17.78 (5.38-58.73) | < 0.001 | NS | |
| Number of metastases to other sites | ||||
| < 2 | Reference | |||
| ≥ 2 | 7.33 (2.05-26.27) | 0.002 | NS | |
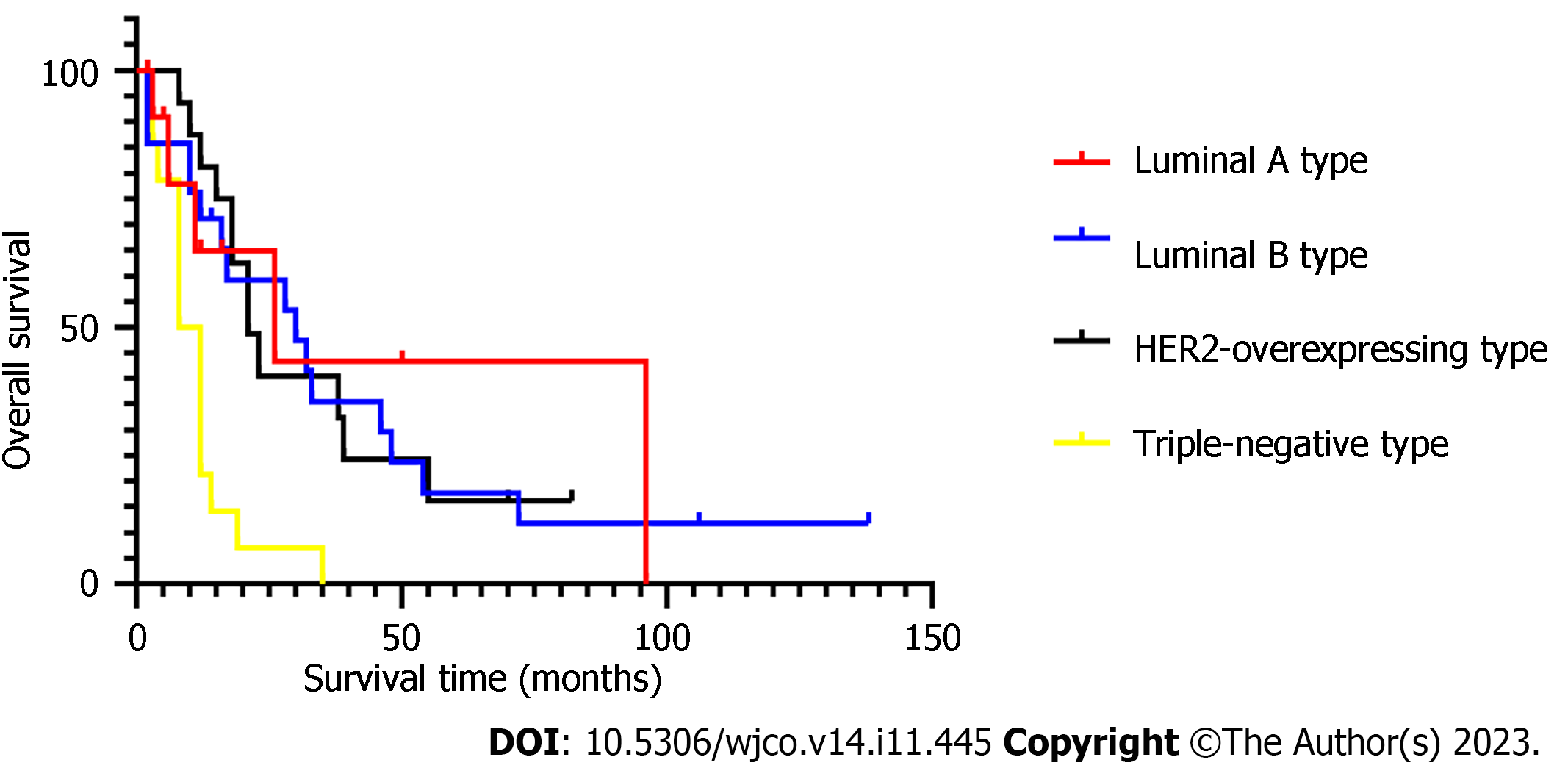
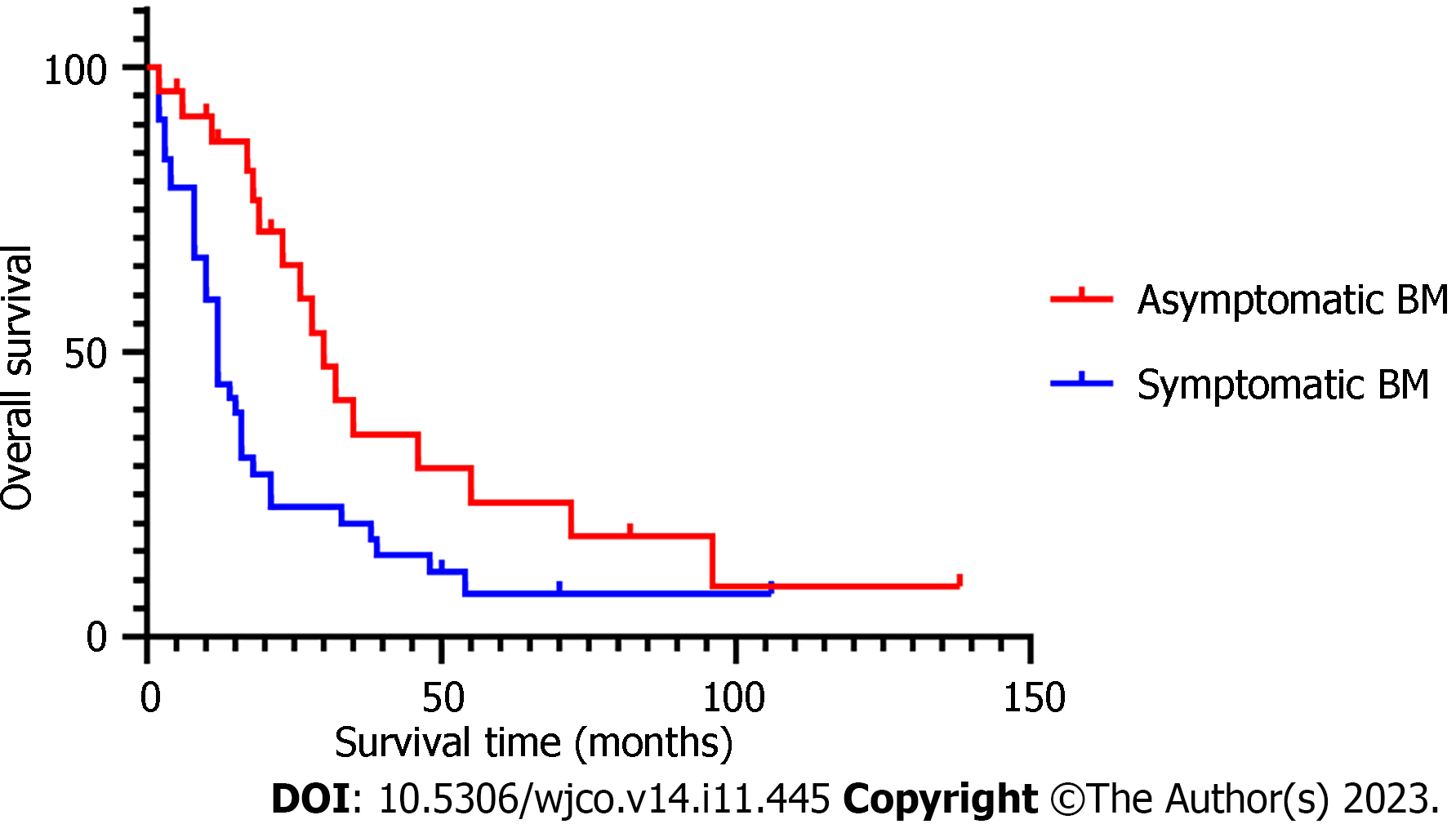
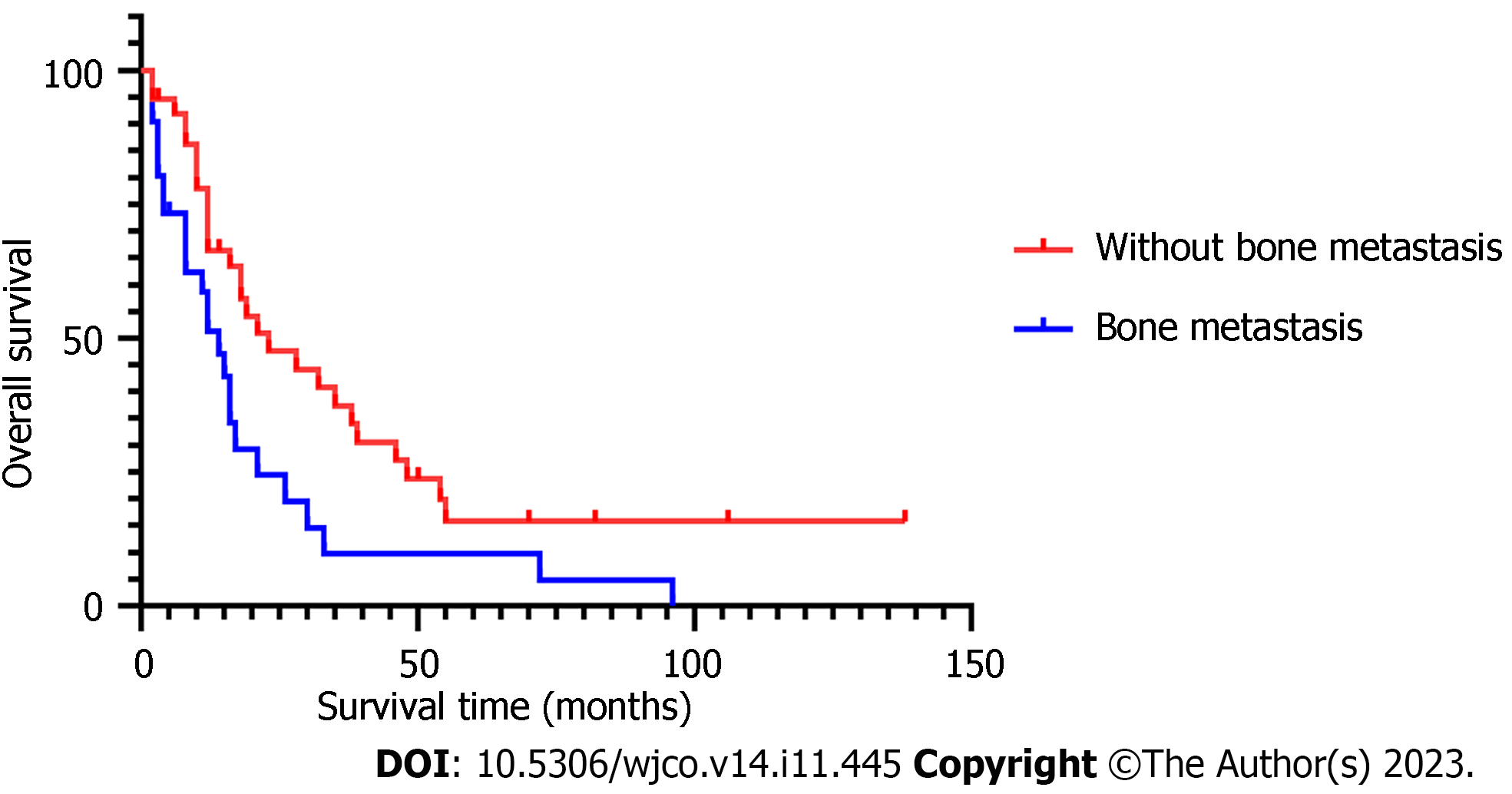
The median age in 68 BCBM patients at diagnosis of BM was 50.5 (30–70) years. Presence or absence of bone metastasis, molecular type, and presence or absence of neurological symptoms at initial diagnosis of BCBM were significant covariates in multivariate Cox analysis. The median time from initial diagnosis of BM to death from any cause or last follow-up was 14 (2–138) mo in the 68 BCBM patients. The survival time of BCBM patients with different molecular types is shown in Figure 1. Of the 68 BCBM patients, 44 (64.7%) were diagnosed with BM due to neurological symptoms; mainly dizziness, which was the initial symptom of 23 (52.3%) patients with BCBM. In addition, typical symptoms of BM included headache (19 cases), nausea and vomiting (10 cases), walking instability (7 cases), blurred vision (5 cases), memory loss (4 cases) and slow response (3 cases), and they often occurred simultaneously. The median survival time was 12 mo among BCBM patients with neurological symptoms, 30 mo among asymptomatic patients (Figure 2), 14 mo among BCBM patients with bone metastasis, and 23 mo among those without bone metastasis (Figure 3). The relevant results are presented in Table 3.
| Item | Univariate Cox | Multivariate Cox | ||
| HR (95%CI) | P | HR (95%CI) | P | |
| Age at diagnosis of BM (yr) | ||||
| ≤ 35 | Reference | |||
| > 35 | 1.033 (0.315-3.385) | 0.957 | ||
| Time from BC to BM (mo) | ||||
| ≤ 24 | Reference | |||
| > 24 | 0.896 (0.482-1.664) | 0.727 | ||
| Menstrual status at diagnosis of BM | ||||
| Premenopause | Reference | |||
| Menopause | 0.685 (0.331-1.416) | 0.307 | ||
| Molecular type | ||||
| Luminal A type | 0.253 (0.087-0.730) | 0.011 | 0.227 (0.074-0.693) | 0.009 |
| Luminal B type | 0.279 (0.128-0.607) | 0.001 | 0.293 (0.129-0.663) | 0.003 |
| HER2-overexpressing type | 0.274 (0.121-0.618) | 0.002 | 0.319 (0.135-0.754) | 0.009 |
| Triple-negative type | Reference | |||
| Bone metastasis | ||||
| No | Reference | |||
| Yes | 1.980 (1.135-3.453) | 0.016 | 2.011 (1.056-3.831) | 0.034 |
| Liver metastasis | ||||
| No | Reference | |||
| Yes | 1.121 (0.626-2.007) | 0.701 | ||
| Lung metastasis | ||||
| No | Reference | |||
| Yes | 1.616 (0.929-2.810) | 0.089 | ||
| Number of liver, lung and bone metastases | ||||
| ≤ 2 | Reference | |||
| > 2 | 1.548 (0.894-2.682) | 0.119 | ||
| Number of metastases to other sites | ||||
| < 2 | Reference | |||
| ≥ 2 | 1.425 (0.711-2.858) | 0.318 | ||
| Tumor stage | ||||
| I-II | Reference | |||
| III-IV | 0.813 (0.466-1.419) | 0.466 | ||
| Lymph node stage | ||||
| N0-N1 | Reference | |||
| N2-N3 | 0.843 (0.486-1.464) | 0.545 | ||
| Pathological type | ||||
| Others | 0.287 (0.073-1.133) | 0.075 | ||
| Invasive lobular carcinoma | Reference | |||
| Invasive ductal carcinoma | 0.208 (0.060-0.725) | 0.014 | NS | |
| Symptoms | ||||
| No | Reference | |||
| Yes | 2.171 (1.191-3.959) | 0.011 | 1.923 (1.005-3.680) | 0.048 |
| Size of BM (cm) | ||||
| ≤ 3 | Reference | |||
| > 3 | 0.803 (0.449-1.439) | 0.462 | ||
| Number of BM | ||||
| ≤ 3 | Reference | |||
| > 3 | 1.248 (0.721-2.160) | 0.428 | ||
| Surgery for BM | ||||
| No | Reference | |||
| Yes | 0.744 (0.348-1.591) | 0.446 | ||
| Treatment after BM | ||||
| Local therapy | Reference | |||
| Medication alone | 1.531 (0.638-3.674) | 0.064 | ||
| Systemic therapy | 0.748 (0.317-1.765) | 0.507 | ||
| Local therapy | ||||
| SRT | 1.350 (0.513-3.549) | 0.543 | ||
| WBRT | 1.600 (0.633-4.043) | 0.320 | ||
| Both | 1.935 (0.680-5.506) | 0.216 | ||
| Neither | Reference | |||
Of the 68 BCBM patients, four (5.9%) underwent no treatment, 27 (39.7%) underwent MDT with local therapy plus systemic medication, 30 (44.1%) were given local therapy only, and seven (10.3%) were given medication only. The median survival time of patients receiving MDT was 21 mo, which was superior to that of patients receiving medication or local therapy alone. Tumor resection was performed in 11 cases and all of them were treated with postoperative radiotherapy. After BM, 16 patients received HER2-targeted therapy, including trastuzumab single targeted therapy for five cases, trastuzumab plus tyrosine kinase inhibitors (TKIs) for two cases, and capecitabine plus TKI for seven cases, and their median survival time was 17, 23 and 54 mo, respectively. The remaining two patients received trastuzumab + pertuzumab dual-targeted therapy, and they were still alive as of the follow-up endpoint (Table 4).
| Item | Patients with BM (n = 68) | Median survival time (mo) |
| Medication | 7 | 12 |
| MDT | 27 | 21 |
| Local therapy alone | 30 | 15 |
| Neither | 4 | |
| Local therapy | n = 57 | |
| SRT alone | 19 | 16 |
| Surgery + SRT | 6 | 19 |
| SRT + WBRT | 10 | 18 |
| Surgery + WBRT | 3 | 30 |
| WBRT | 17 | 12 |
| Surgery + SRT + WBRT | 2 | 21 |
| HER2 targeted therapy | n = 16 | |
| Trastuzumab | 5 | 17 |
| Trastuzumab + pertuzumab | 2 | |
| Trastuzumab + TKI | 2 | 23 |
| Capecitabine + TKI | 7 | 54 |
In this study, the median time from the diagnosis of BC to the occurrence of BCBM was 33.5 (0–181) mo. The risk of BM varied among patients with different molecular subtypes of BC. HER2-overexpressing and triple-negative BC had a high tendency to BM, consistent with previous reports[5]. Patients with advanced stage and lung metastasis were also at high risk of BM. Due to the specificity of the physiological structure of the brain (such as the presence of the blood–brain barrier), there is still a lack of effective intervention means, and BM predicts poor survival outcomes. The results of descriptive statistics, univariate and multivariate Cox proportional hazards model analysis showed that the molecular type, and presence or absence of neurological symptoms and bone metastasis at diagnosis of BM were independent prognosis factors of patients with BM.
In this study, it was found that HER2-overexpressing and triple-negative types were high-risk molecular types of BCBM. Patients with HER2-overexpressing and triple-negative BC accounted for 20.6% of BM patients, in line with research findings that the incidence rate of BM in HER2-overexpressing type and triple-negative BC is 20%–30%[6]. Sixty-nine studies involving 28 countries on risk factors of BCBM concluded that young age, ER-negative, HER2 overexpression, later tumor stage, histological grade, tumor size, and lymph node metastasis are all independent risk factors of BCBM[7]. Univariate analysis of this study showed that lung, liver and bone metastasis, the number of liver, lung and bone metastases, and the number of metastases to other sites were associated with an increased risk of BM in BC patients. In multivariate analysis, only lung metastasis was statistically significant (HR: 24.18, 95%CI: 6.40–91.43). As shown in previous studies, cyclooxygenase 2 and epidermal growth factor receptor ligand can serve as mediators of cancer cells passing through the blood–brain barrier[8], and they are associated with lung cancer infiltration, which may account for the predisposition of patients with lung metastases to BM[9]. Lymph node status and age at diagnosis of BC have been verified to be associated with the risk of BM[10], but no clear association was observed in this study. The later tumor stage often corresponds to later seeking of treatment, greater tumor burden, greater lymph node infiltration, and increased risk of metastasis and recurrence, including BM[11].
Clinically, the treatment regimen is often selected based on the number, location and size of BMs, the patient’s physical condition, extracranial metastasis, and the possible benefits of treatment. SRT is mainly applied to BM patients with < 4 tumor lesions and brain tumor < 3 cm. In this study, the median survival time was 12 mo among patients treated with WBRT alone, 16 mo among patients treated with SRT alone, 18 mo among patients treated with WBRT + SRT, and 18 mo among patients undergoing surgery for brain tumors. Consistent with this study, a study showed that WBRT produces no OS benefit but significant neurocognitive decline[12]. Of the 68 patients, 24 had BMs ≤ 3 cm, and 22 patients (91.7%) underwent SRT. SRT has become the first-line treatment for BC patients with small brain metastases[13].
Despite advances in early diagnosis and effective treatment, distant metastasis remains an important factor threatening the survival of BC patients[14]. In this study, the median survival time of patients with luminal A, luminal B, HER2-overexpressing and triple-negative BC was 26, 30, 21 and 8 mo, respectively. The prognosis of HER2-overexpressing and triple-negative BC patients was poor. It is difficult for most drugs to reach effective blood concentration in the brain due to the presence of the blood–brain barrier. With the progress made in novel targeted drugs in the past decade, breakthroughs have been made in the treatment of HER2-positive BC. Trastuzumab, pertuzumab, antibody-drug conjugate and TKIs (lapatinib, pyrotinib, etc.) have been marketed successively, extending the OS of HER2-positive patients. Studies have revealed that lapatinib + capecitabine can delay the time of WBRT[15]. The PERMEATE study explored the efficacy of lapatinib plus capecitabine in the treatment of patients with HER2-positive BM, and found that the objective response rate of brain can reach 74.6% in patients undergoing the initial neurological radiotherapy[16]. In this study, the median survival time of patients given capecitabine plus TKIs was 54 mo. As of April 2023, the median survival time of HER2-positive BCBM patients enrolled in PERMEATE is up to 31.5 mo[16]. In this study, the patients treated from 2000 to 2022 were enrolled, and trastuzumab was marketed in China since 2002, so some patients did not undergo targeted therapy due to early drug shortage and high treatment cost, which may be one of the reasons for poor prognosis of HER2-overexpressing BC patients. In view of the current effective treatment, HER2-positive patients should be more active in undergoing brain examination and timely treatment.
The results of multivariate Cox regression analysis showed that the HR value of BM patients with neurological symptoms was 1.923 times (95%CI: 1.005–3.680) that of asymptomatic patients (P = 0.048), suggesting that the presence of neurological symptoms at diagnosis is associated with a poor prognosis. The median survival time was 30 mo for asymptomatic patients and 12 mo for symptomatic patients. A study involving long-term survivors of BCBM showed that asymptomatic BCBM patients are more likely to achieve long-term survival of > 15 mo[17]. In this study, asymptomatic patients with BM had a smaller diameter of brain tumor (≤ 3 cm: 95.8% vs 75.0%, P = 0.031), fewer brain metastases (≤ 3: 58.3% vs 43.2%, P = 0.232), and younger age at diagnosis of BC (≤ 35 years: 95.8% vs 75%, P = 0.031) than symptomatic patients, consistent with the characteristics (young age, small diameter of brain tumor, small number of brain tumors, and good physical status without neurological burden) of asymptomatic patients[18]. Due to smaller size and number of brain tumors of asymptomatic patients, a wider range of treatment options is available, and SRT is preferred, which is associated with milder neurological impairment[17]. A prospective study on 1196 asymptomatic patients with BCBM treated with SRT also confirmed that compared with symptomatic patients, asymptomatic BM patients have good neurological status and reduced neurological mortality[18]. A previous study showed that early detection of BM is associated with longer OS as compared to symptomatic BM[19]. Considering the health economic benefits, however, brain screening has not been utilized as routine follow-up for BC patients in China and globally. According to an American study, regular head magnetic resonance imaging screening can save an average of USD 1326 in treatment costs for each BCBM patient. Although there are differences in the medical system between the USA and China, some references are still provided for the formulation of follow-up plans for BC patients in China[20].
The presence of extracranial metastases was identified as an independent prognostic factor in the 2020 version of the Breast Graded Prognostic Assessment. Bone metastasis is the most common mode of metastasis, accounting for 60%–70% of metastatic BC[21]. In this study, 38 (55.9%) patients with BM had bone metastasis. The prognosis of BC bone metastases is better than that of other distant metastases, with a median survival time of 36 mo[22], and the survival rate significantly declines when complicated with metastasis to other sites[23]. In a cohort of 1330 triple-negative BC patients with BM, the median OS was 13 mo (95%CI: 11.5–14.5 mo) for bone metastasis alone and 8 mo (95%CI: 6.3–9.7 mo) for bone metastasis + metastasis to other sites[24]. In this study, the median survival time was 14 mo for patients with BM + bone metastasis and 23 mo for patients without bone metastasis, and the HR value in multivariate analysis was 2.011 times that of patients without bone metastasis (95%CI: 1.056–3.831) (P = 0.034). Common skeletal-related events in patients with bone metastasis include pathological fracture, spinal cord compression, hypercalcemia, and bone pain[21], resulting in limited daily activity and reduced quality of life. In this study, the median age of 68 BCBM patients was 50.5 (30–70) years. The mean menopausal age of Chinese women is 49.5 years, and menopausal women are prone to osteoporosis as well as an increased risk of pathological fracture when complicated with bone metastasis. Fracture-induced immobilization, decrease of physical performance score, and long-term complications related to immobilization (thromboembolism, respiratory tract infection, etc.) are all reasons for the decrease in survival rate. In the case of paralysis caused by spinal cord compression, the survival rate declines further, with a 1-year survival rate of only 17.6%[25].
There were some limitations to this study, such as its small sample size and single-center, retrospective nature, which inevitably introduced selection and recall bias. The clinical data collected from 2000 to 2022 may have had missing follow-up data. Notably, the absence of pathological results and imaging data for some patients diagnosed before 2010 may have influenced the analytical outcomes. Moreover, shifts in clinical guidelines, the introduction of new medications, and advances in the healthcare economy during this period have altered the diagnostic criteria, treatment modalities, and patient management approaches. Other potential confounding factors like the genotype of BC patients were not extensively analyzed due to their low detection rate. The lack of prospective studies introduced uncertainty in pin-pointing the precise onset time for patients with asymptomatic BM. Currently, the efficacy of early screening for BCBM, its potential for early intervention, and whether early detection enhances survival rate, necessitate validation through multicenter prospective studies.
Stage III/IV, lung metastasis, and HER2-overexpressing and triple-negative types are high-risk factors of BCBM, and aggressive monitoring of BM is required. It is recommended that BC patients undergo regular brain examinations since detecting and treating BC before neurological symptoms emerge may produce better outcomes. Patients with central nervous system symptoms, HER2-overexpressing and triple-negative BC, and bone metastasis have poor prognosis.
Breast cancer (BC) brain metastasis (BCBM) is an important influencing factor of the long-term prognosis of BC patients. Triple-negative type is a known risk factor of BCBM, suggesting that patients with different clinicopathological types have differences in survival time.
To explore the influencing factors of the occurrence, development, and prognostic survival of BCBM to provide references for the diagnosis, treatment and management of patients with BM.
To perform more aggressive screening of high-risk patients of BCBM, benefiting patients from early diagnosis and treatment, and producing better outcomes.
Clinicopathological data of 68 BCBM patients admitted to the Air Force Medical Center (formerly Air Force General Hospital) between 2000 and 2022 and another 136 matched BC patients were retrospectively analyzed. The high-risk factors and prognostic factors of BCBM patients were analyzed by univariate and multivariate Cox regression analyses, the survival time of patients was estimated by the Kaplan–Meier method, and the overall survival was compared between two groups by log-rank test.
Stage III/IV, lung metastasis, and human epidermal growth factor receptor 2 (HER2)-overexpressing and triple-negative types were high-risk factors of BCBM. Patients with neurological symptoms, bone metastasis, and HER2-overexpressing and triple-negative BC had poor prognosis, requiring more effective treatment to improve the survival rate of these patients.
The prognosis of BCBM is poor. Active follow-up and screening of the brain should be performed for patients with late stage at initial treatment, lung metastasis, and HER2-overexpressing and triple-negative BC. The median survival time of patients with neurological symptoms, bone metastasis, and HER2-overexpressing and triple-negative BC significantly decreases.
More multicenter large studies on BCBM are required to provide references for the management of high-risk patients, and more effective treatment is needed to raise the survival rate of patients with poor prognosis.
We thank all medical staff and technicians of dialysis centers who agreed to participate in this study.
Provenance and peer review: Unsolicited article; Externally peer reviewed.
Peer-review model: Single blind
Specialty type: Oncology
Country/Territory of origin: China
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): 0
Grade C (Good): C, C
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: King MC, United States; Skarping I, Sweden S-Editor: Lin C L-Editor: Kerr C P-Editor: Chen YX
| 1. | Sung H, Ferlay J, Siegel RL, Laversanne M, Soerjomataram I, Jemal A, Bray F. Global Cancer Statistics 2020: GLOBOCAN Estimates of Incidence and Mortality Worldwide for 36 Cancers in 185 Countries. CA Cancer J Clin. 2021;71:209-249. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 75126] [Cited by in RCA: 64433] [Article Influence: 16108.3] [Reference Citation Analysis (176)] |
| 2. | DeSantis CE, Ma J, Gaudet MM, Newman LA, Miller KD, Goding Sauer A, Jemal A, Siegel RL. Breast cancer statistics, 2019. CA Cancer J Clin. 2019;69:438-451. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1500] [Cited by in RCA: 2024] [Article Influence: 337.3] [Reference Citation Analysis (0)] |
| 3. | Bollig-Fischer A, Michelhaugh S, Ali-Fehmi R, Mittal S. The molecular genomics of metastatic brain tumours. OA Mol Oncol. 2013;1. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 6] [Cited by in RCA: 10] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 4. | Witzel I, Laakmann E, Weide R, Neunhöffer T, Park-Simon TJ, Schmidt M, Fasching PA, Hesse T, Polasik A, Mohrmann S, Würschmidt F, Schem C, Bechtner C, Würstlein R, Fehm T, Möbus V, Burchardi N, Loibl S, Müller V. Treatment and outcomes of patients in the Brain Metastases in Breast Cancer Network Registry. Eur J Cancer. 2018;102:1-9. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 38] [Cited by in RCA: 65] [Article Influence: 9.3] [Reference Citation Analysis (0)] |
| 5. | Kennecke H, Yerushalmi R, Woods R, Cheang MC, Voduc D, Speers CH, Nielsen TO, Gelmon K. Metastatic behavior of breast cancer subtypes. J Clin Oncol. 2010;28:3271-3277. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1283] [Cited by in RCA: 1578] [Article Influence: 105.2] [Reference Citation Analysis (0)] |
| 6. | Kuksis M, Gao Y, Tran W, Hoey C, Kiss A, Komorowski AS, Dhaliwal AJ, Sahgal A, Das S, Chan KK, Jerzak KJ. The incidence of brain metastases among patients with metastatic breast cancer: a systematic review and meta-analysis. Neuro Oncol. 2021;23:894-904. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 93] [Cited by in RCA: 145] [Article Influence: 36.3] [Reference Citation Analysis (0)] |
| 7. | Koniali L, Hadjisavvas A, Constantinidou A, Christodoulou K, Christou Y, Demetriou C, Panayides AS, Pitris C, Pattichis CS, Zamba-Papanicolaou E, Kyriacou K. Risk factors for breast cancer brain metastases: a systematic review. Oncotarget. 2020;11:650-669. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 21] [Cited by in RCA: 51] [Article Influence: 10.2] [Reference Citation Analysis (0)] |
| 8. | Bos PD, Zhang XH, Nadal C, Shu W, Gomis RR, Nguyen DX, Minn AJ, van de Vijver MJ, Gerald WL, Foekens JA, Massagué J. Genes that mediate breast cancer metastasis to the brain. Nature. 2009;459:1005-1009. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1488] [Cited by in RCA: 1406] [Article Influence: 87.9] [Reference Citation Analysis (0)] |
| 9. | Gupta GP, Nguyen DX, Chiang AC, Bos PD, Kim JY, Nadal C, Gomis RR, Manova-Todorova K, Massagué J. Mediators of vascular remodelling co-opted for sequential steps in lung metastasis. Nature. 2007;446:765-770. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 516] [Cited by in RCA: 537] [Article Influence: 29.8] [Reference Citation Analysis (0)] |
| 10. | Aversa C, Rossi V, Geuna E, Martinello R, Milani A, Redana S, Valabrega G, Aglietta M, Montemurro F. Metastatic breast cancer subtypes and central nervous system metastases. Breast. 2014;23:623-628. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 68] [Cited by in RCA: 93] [Article Influence: 8.5] [Reference Citation Analysis (0)] |
| 11. | Azim HA, Abdel-Malek R, Kassem L. Predicting Brain Metastasis in Breast Cancer Patients: Stage Versus Biology. Clin Breast Cancer. 2018;18:e187-e195. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 13] [Cited by in RCA: 24] [Article Influence: 3.4] [Reference Citation Analysis (0)] |
| 12. | Yamamoto M, Serizawa T, Shuto T, Akabane A, Higuchi Y, Kawagishi J, Yamanaka K, Sato Y, Jokura H, Yomo S, Nagano O, Kenai H, Moriki A, Suzuki S, Kida Y, Iwai Y, Hayashi M, Onishi H, Gondo M, Sato M, Akimitsu T, Kubo K, Kikuchi Y, Shibasaki T, Goto T, Takanashi M, Mori Y, Takakura K, Saeki N, Kunieda E, Aoyama H, Momoshima S, Tsuchiya K. Stereotactic radiosurgery for patients with multiple brain metastases (JLGK0901): a multi-institutional prospective observational study. Lancet Oncol. 2014;15:387-395. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 779] [Cited by in RCA: 993] [Article Influence: 90.3] [Reference Citation Analysis (0)] |
| 13. | Churilla TM, Ballman KV, Brown PD, Twohy EL, Jaeckle K, Farace E, Cerhan JH, Anderson SK, Carrero XW, Garces YI, Barker FG 2nd, Deming R, Dixon JG, Burri SH, Chung C, Ménard C, Stieber VW, Pollock BE, Galanis E, Buckner JC, Asher AL. Stereotactic Radiosurgery With or Without Whole-Brain Radiation Therapy for Limited Brain Metastases: A Secondary Analysis of the North Central Cancer Treatment Group N0574 (Alliance) Randomized Controlled Trial. Int J Radiat Oncol Biol Phys. 2017;99:1173-1178. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 67] [Cited by in RCA: 66] [Article Influence: 8.3] [Reference Citation Analysis (0)] |
| 14. | Liang Y, Zhang H, Song X, Yang Q. Metastatic heterogeneity of breast cancer: Molecular mechanism and potential therapeutic targets. Semin Cancer Biol. 2020;60:14-27. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 175] [Cited by in RCA: 565] [Article Influence: 94.2] [Reference Citation Analysis (0)] |
| 15. | Petrelli F, Ghidini M, Lonati V, Tomasello G, Borgonovo K, Ghilardi M, Cabiddu M, Barni S. The efficacy of lapatinib and capecitabine in HER-2 positive breast cancer with brain metastases: A systematic review and pooled analysis. Eur J Cancer. 2017;84:141-148. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 64] [Cited by in RCA: 81] [Article Influence: 10.1] [Reference Citation Analysis (0)] |
| 16. | Yan M, Ouyang Q, Sun T, Niu L, Yang J, Li L, Song Y, Hao C, Chen Z, Orlandi A, Ishii N, Takabe K, Franceschini G, Ricci F, Verschraegen C, Liu Z, Zhang M, Lv H, Liu L, Yang X, Xiao H, Gao Z, Li X, Dong F, Chen X, Qiao J, Zhang G. Pyrotinib plus capecitabine for patients with human epidermal growth factor receptor 2-positive breast cancer and brain metastases (PERMEATE): a multicentre, single-arm, two-cohort, phase 2 trial. Lancet Oncol. 2022;23:353-361. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 66] [Cited by in RCA: 95] [Article Influence: 31.7] [Reference Citation Analysis (0)] |
| 17. | Riecke K, Müller V, Neunhöffer T, Park-Simon TW, Weide R, Polasik A, Schmidt M, Puppe J, Mundhenke C, Lübbe K, Hesse T, Thill M, Wuerstlein R, Denkert C, Decker T, Fehm T, Nekljudova V, Rey J, Loibl S, Laakmann E, Witzel I. Long-term survival of breast cancer patients with brain metastases: subanalysis of the BMBC registry. ESMO Open. 2023;8:101213. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 22] [Reference Citation Analysis (0)] |
| 18. | Gao YK, Kuksis M, Id Said B, Chehade R, Kiss A, Tran W, Sickandar F, Sahgal A, Warner E, Soliman H, Jerzak KJ. Treatment Patterns and Outcomes of Women with Symptomatic and Asymptomatic Breast Cancer Brain Metastases: A Single-Center Retrospective Study. Oncologist. 2021;26:e1951-e1961. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4] [Cited by in RCA: 14] [Article Influence: 3.5] [Reference Citation Analysis (0)] |
| 19. | Laakmann E, Witzel I, Neunhöffer T, Weide R, Schmidt M, Park-Simon TW, Möbus V, Mundhenke C, Polasik A, Lübbe K, Hesse T, Riecke K, Thill M, Fasching PA, Denkert C, Fehm T, Nekljudova V, Rey J, Loibl S, Müller V. Characteristics and Clinical Outcome of Breast Cancer Patients with Asymptomatic Brain Metastases. Cancers (Basel). 2020;12. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 5] [Cited by in RCA: 16] [Article Influence: 3.2] [Reference Citation Analysis (0)] |
| 20. | Lester SC, Taksler GB, Kuremsky JG, Lucas JT Jr, Ayala-Peacock DN, Randolph DM 2nd, Bourland JD, Laxton AW, Tatter SB, Chan MD. Clinical and economic outcomes of patients with brain metastases based on symptoms: an argument for routine brain screening of those treated with upfront radiosurgery. Cancer. 2014;120:433-441. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 58] [Cited by in RCA: 66] [Article Influence: 6.0] [Reference Citation Analysis (0)] |
| 21. | Xiong Z, Deng G, Huang X, Li X, Xie X, Wang J, Shuang Z, Wang X. Bone metastasis pattern in initial metastatic breast cancer: a population-based study. Cancer Manag Res. 2018;10:287-295. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 37] [Cited by in RCA: 86] [Article Influence: 12.3] [Reference Citation Analysis (0)] |
| 22. | Wang R, Zhu Y, Liu X, Liao X, He J, Niu L. The Clinicopathological features and survival outcomes of patients with different metastatic sites in stage IV breast cancer. BMC Cancer. 2019;19:1091. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 111] [Cited by in RCA: 302] [Article Influence: 50.3] [Reference Citation Analysis (0)] |
| 23. | Plunkett TA, Smith P, Rubens RD. Risk of complications from bone metastases in breast cancer. implications for management. Eur J Cancer. 2000;36:476-482. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 120] [Cited by in RCA: 117] [Article Influence: 4.7] [Reference Citation Analysis (0)] |
| 24. | Yao Y, Chu Y, Xu B, Hu Q, Song Q. Risk factors for distant metastasis of patients with primary triple-negative breast cancer. Biosci Rep. 2019;39. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 39] [Cited by in RCA: 70] [Article Influence: 11.7] [Reference Citation Analysis (1)] |
| 25. | Oka H, Kondoh T, Seichi A, Hozumi T, Nakamura K. Incidence and prognostic factors of Japanese breast cancer patients with bone metastasis. J Orthop Sci. 2006;11:13-19. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 16] [Cited by in RCA: 17] [Article Influence: 0.9] [Reference Citation Analysis (0)] |