Published online Sep 24, 2022. doi: 10.5306/wjco.v13.i9.748
Peer-review started: May 25, 2022
First decision: July 13, 2022
Revised: July 25, 2022
Accepted: September 6, 2022
Article in press: September 6, 2022
Published online: September 24, 2022
Processing time: 120 Days and 2.2 Hours
With sentinel node metastasis in breast cancer (BC) patients, axillary lymph node (ALN) dissection is often omitted from cases with breast-conserving surgery. Omission of lymph node dissection reduces the invasiveness of surgery to the patient, but it also obscures the number of metastases to non-sentinel nodes. The possibility of finding ≥ 4 lymph nodes (pN2a/pN3a) preoperatively is important given the ramifications for postoperative treatment.
To search for clinicopathological factors that predicts upstaging from N0 to pN2a/pN3a.
Patients who were sentinel lymph node (SLN)-positive and underwent ALN dissection between September 2007 and August 2018 were selected by retrospective chart review. All patients had BC diagnosed preoperatively as N0 with axillary evaluation by fluorodeoxyglucose (FDG) positron emission tomography/computed tomography and ultrasound (US) examination. When suspicious FDG accumulation was found in ALN, the presence of metastasis was reevaluated by second US. We examined predictors of upstaging from N0 to pN2a/pN3a.
Among 135 patients, we identified 1-3 ALNs (pN1) in 113 patients and ³4 ALNs (pN2a/pN3a) in 22 patients. Multivariate analysis identified the total number of SLN metastasis, the maximal diameter of metastasis in the SLN (SLNDmax), and FDG accumulation of ALN as predictors of upstaging to pN2a/pN3a.
We identified factors involved in upstaging from N0 to pN2a/pN3a. The SLNDmax and number of SLN metastasis are predictors of ≥ 4 ALNs (pN2a/pN3a) and predictors of metastasis to non-sentinel nodes, which have been reported in the past. Attention should be given to axillary accumulations of FDG, even when faint.
Core Tip: This is the first report to include the results of preoperative positron emission tomography/computed tomography (PET/CT) and to examine results related to the upstaging of pN2a/pN3a (more than 4 axillary lymph node metastases) in breast cancer (BC) patients. Specifically, 135 patients who were sentinel lymph node (SLN)-positive and underwent ALN dissection were selected by retrospective chart review, all of whom had BC diagnosed preoperatively as N0 with axillary evaluation by fluorodeoxyglucose (FDG) PET/CT and ultrasound. Our results suggest that the size and number of SLN metastases were still important factors. And, attention should be given to axillary accumulations of FDG, even when faint.
- Citation: Oda G, Nakagawa T, Mori H, Onishi I, Fujioka T, Mori M, Kubota K, Hanazawa R, Hirakawa A, Ishikawa T, Okamoto K, Uetakesszsz H. Factors predicting upstaging from clinical N0 to pN2a/N3a in breast cancer patients. World J Clin Oncol 2022; 13(9): 748-757
- URL: https://www.wjgnet.com/2218-4333/full/v13/i9/748.htm
- DOI: https://dx.doi.org/10.5306/wjco.v13.i9.748
Sentinel lymph node (SLN) biopsy is usually performed in N0 cases. If SLN biopsy yields a positive result, an axillary lymph node (ALN) dissection is performed. However, since the publication of findings from the American College of Surgeons Oncology Group (ACOSOG) Z-0011 trial, many cancer centers have been omitting ALN dissection from breast-conserving surgeries[1]. The problem with omitting axillary dissection is that the number of non-SLN metastases cannot be ascertained, which may lead to over or under-treatment with adjuvant chemotherapy and radiation therapy. For example, patients with pN2a [4-9 ALN metastases (ALNMs)] or pN3a (³10ALNMs) need to be irradiated to not only the residual breast, but also the supraclavicular region after breast-conserving surgery. However, without knowing the number of metastases, no accurate decision on the need for irradiation can be made. Post-mastectomy radiation (PMRT) is also required for pN2a/N3a patients. Some reports have noted that PMRT after reconstruction impairs conformity[2-4]. Further analyses have shown that direct implants and autologous tissue reconstruction have fewer complications from PMRT[5,6]. The ability to predict the necessity of postoperative irradiation before surgery would affect surgical planning, including reconstructive surgery. A variety of factors and nomograms have been reported to allow preoperative prediction of the presence or absence of ALNMs[7-9], but few reports have examined factors predicting the presence of ³4 ALNMs. Preoperative fluorodeoxyglucose (FDG) positron emission tomography (PET)/computed tomography (CT) is reportedly excellent for predicting metastasis preoperatively and may have influenced the prediction of ALNMs in the present study. Although many papers have described predictors of non-SLN metastasis, few have rigorously assessed ALNM preoperatively using PET/CT. In this study, all patients were evaluated preoperatively by PET/CT, and cases with false-negative results on other imaging modalities were excluded. This is the first report to include the results of preoperative PET/CT and to examine results related to the upstaging of pN2a/pN3a.
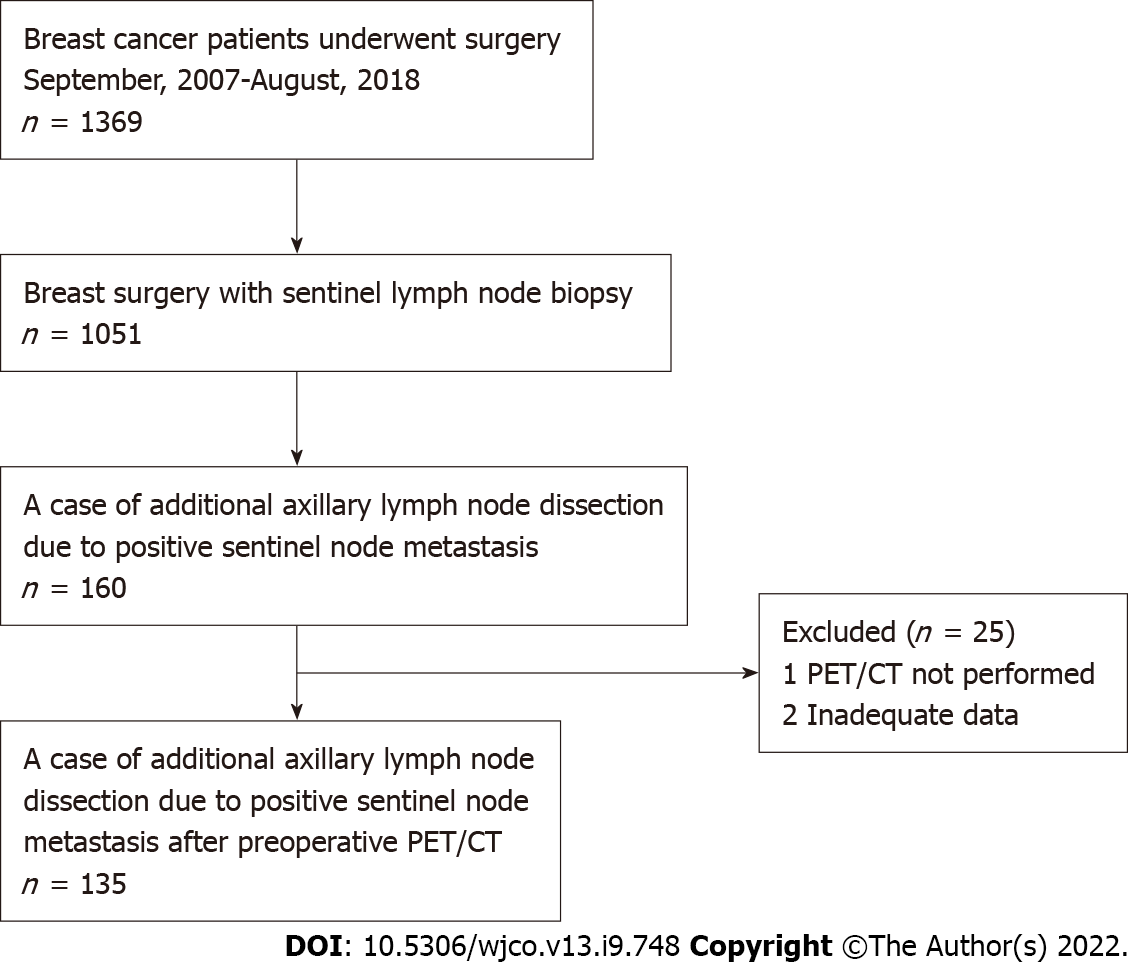
A retrospective chart review was conducted for patients who were SLN-positive and underwent ALND between September 2007 and August 2018. All patients had breast cancer (BC) diagnosed preoperatively as N0 with axillary evaluation by PET/CT and ultrasound (US) examination. This study was conducted with approval from the institutional review board and with the informed consent of each patient. Axillary dissection was performed in all patients with SLN metastasis > 2 mm in diameter. In the case of total mastectomy, axillary dissection was performed for metastases > 0.2 mm in diameter. A flow chart of the eligible/included patients is shown in Figure 1.
A EUB-7500 scanner with a EUP-L54MA 9.75-MHz linear probe (Hitachi Medical Systems, Tokyo, Japan) or Aplio XG scanner with a PLT-805AT 8.0-MHz linear probe (Toshiba Medical Systems, Tochigi, Japan) was used for US examinations. If ultrasound or PET/CT findings were suspicious for metastatic lymph nodes, cytology was performed.
All patients were intravenously administered 18F-FDG (3.7 MBq/kg; 0.1 mCi/kg) after a minimum 4-h fasting period. Next, whole-body images were routinely obtained using a PET/CT system (Aquiduo; Toshiba Medical Systems, Tokyo, Japan). In addition, CT was performed using the following parameters: pitch factor, 0.938; gantry rotation time, 0.5 s; table time, 30 mm/s; auto-exposure control (SD20), 120 kVp; and slice thickness, 2.0 mm. Notably, contrast media were not used for CT examinations. Approximately 60 min after 18F-FDG administration, whole-body PET was performed using the following parameters: emission time per bed, 2 min; bed positions, 7-8; slice thickness, 3.375 mm; and matrix, 128 × 128.
In the present study, 18F-FDG-PET/CT findings at each examination were assessed using a consensus reading by two breast radiologists (T.F. with 14 years of experience in breast imaging; M.M. with 10 years of experience in breast imaging). We performed visual analysis of primary lesions and ALNs without defining a cut-off value. Of note, lesions with an 18F-FDG uptake value higher than that of the background tissue were defined as FDG-positive.
All specimens were analyzed by pathologists from our institution, and specimens were considered estrogen receptor (ER) positive on immunohistochemistry (IHC) for staining rates higher than 10%. For human epidermal growth factor receptor 2 (HER2) values, and IHC result of 3+ was defined as BC with strong, complete membrane staining observed in at least 10% of tumor cells. For HER2 overexpression of 2+, gene amplification with fluorescence in situ hybridization was performed in this study.
Differences in proportions of categorical data were tested using Fisher’s exact probability test. Unless otherwise indicated, significant differences among mean values of numerical data were analyzed using Mann-Whitney test. Relationships between the size of SLN metastases and the number of ALNMs were measured using Spearman rank correlation analysis, which can have a magnitude ranging from 0 to 1, with 0 denoting no correlation at all and 1 denoting complete correlation. Predictors of upstaging to pN2a/pN3a were determined by univariate and multivariate logistic regression analyses. Values of P < 0.05 were regarded as statistically significant. All statistical analyses were performed using EZR (Saitama Medical Center, Jichi Medical University, Saitama, Japan), a graphical user interfaces for R (The R Foundation for Statistical Computing, Vienna, Austria)[10]. More precisely, EZR is a modified version of R Commander designed to add statistical functions frequently used in biostatistics.
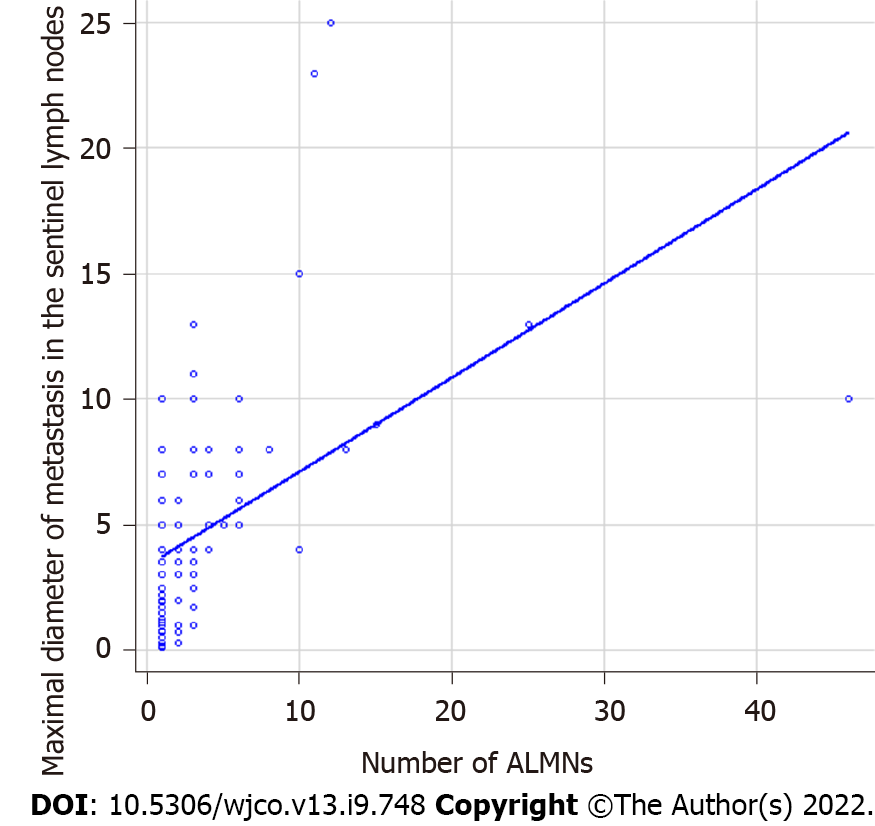
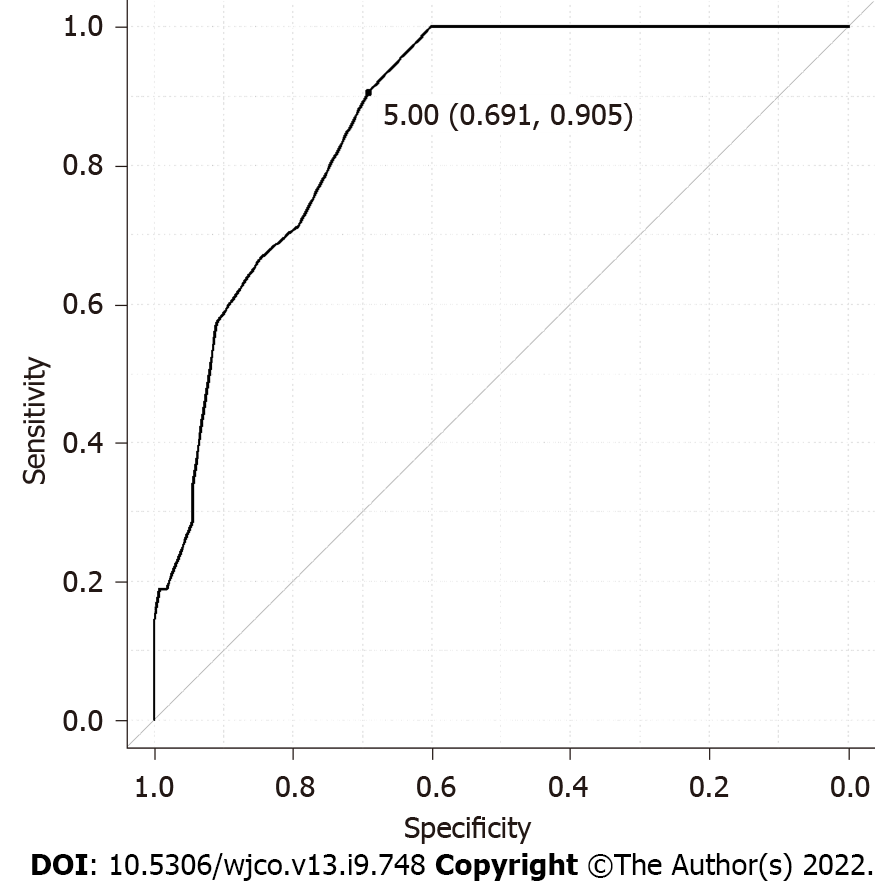
We retrospectively analyzed 135 BC patients who were SLN-positive and had undergone ALN dissection. FDG-PET/CT was performed in all cases preoperatively. The detailed clinicopathological characteristics of subjects are summarized in Table 1. Among these, 113 patients showed 1-3 ALNMs (pN1) and 22 patients had ³4 ALNMs (pN2a/pN3a). FDG accumulation in ALNs was found on PET/CT in 19 cases (14.1%). The mean standardized uptake value (SUV) max was 1.48 (range, 0.8-2.0). Preoperative PET/CT showed accumulation in the ALNs, and second-look US was performed in cases where metastasis could not be ruled out. The mean number of excised SLNs was 1.6 (range, 1-5) and the mean number of SLN metastasis was 1.2 (range, 1-3). The median maximal diameter of metastasis in the SLN (SLNDmax) was 3 mm. The correlation between the SLNDmax and the number of ALNMs is shown in Figure 2. A strong correlation was identified between SLNDmax and the number of ALNMs (P < 0.001). We measured the cut-off value for the SLNDmax from the receiver operating characteristic (ROC) curve and the cut-off value for upstaging from N0 to pN2a/pN3a was set at 5 mm (area under the curve: 0.873; 95%CI: 0.808–0931) (Figure 3). Table 2 shows a comparison between pN1 and pN2a/pN3a.
| Background characteristics (n = 135) | |
| Age (yr) | 56 (35-84) |
| SUVmax of primary tumor | 3.05 (0.8-11.9) |
| pN1 | 113 |
| pN2a or pN3a | 22 |
| The invasion diameter of the primary lesion | |
| ≤ 20 mm | 67 |
| < 50 mm | 55 |
| ≥ 50 mm | 11 |
| NA | 2 |
| Maximal diameter of metastasis in the sentinel lymph nodes (mm) | 3 (0.1-25) |
| The number of SLN metastasis | |
| 1 | 112 |
| 2 | 19 |
| 3 | 4 |
| ER | |
| Positive | 118 |
| Negative | 14 |
| Unknown | 3 |
| HER2 | |
| Positive | 8 |
| Negative | 125 |
| Unknown | 2 |
| Nuclear grade of biopsy specimen | |
| 1, 2 | 103 |
| 3 | 26 |
| Unknown | 6 |
| FDG accumulation of axillary lymph nodes | |
| Yes | 19 |
| No | 116 |
| Total (n = 135) | pN1 | pN2a/pN3a | P value |
| n = 113 | n = 22 | ||
| Age | 57.96 ± 12.14 | 59.64 ± 12.73 | 0.56 |
| The invasion diameter of the primary lesion (mm) | 22.77 ± 14.76 | 39.86 ± 21.73 | < 0.001 |
| Maximal diameter of metastasis in the SLNs | 3.46 ± 2.80 | 9.19 ± 5.66 | < 0.001 |
| The number of SLN metastasis | 0.014 | ||
| 1 | 98 | 14 | |
| 2 and more | 15 | 8 | |
| ER | 0.47 | ||
| Positive | 100 | 18 | |
| Negative | 11 | 3 | |
| Unknown | 2 | 1 | |
| HER2 | 1 | ||
| Positive | 7 | 1 | |
| Negative | 104 | 21 | |
| Unknown | 2 | 0 | |
| Nuclear grade of biopsy specimen | 0.77 | ||
| 1, 2 | 87 | 16 | |
| 3 | 21 | 5 | |
| Unknown | 4 | 1 | |
| SUVmax of the primary tumor | 3.80 ± 2.50 | 4.13 ± 2.77 | 0.61 |
| FDG accumulation of axillary lymph nodes | < 0.001 | ||
| No | 104 | 11 | |
| Yes | 8 | 11 | |
| Unknown | 1 | 0 |
To search for risk factors for upstaging to pN2a/N3a, univariate and multivariate analyses were performed for associations with clinicopathological factors in two groups of pN1 and pN2a/N3a cases. Invasive diameter at the primary site, number of SLN metastasis, FDG accumulation in ALNs, and SLNDmax were associated with upstaging to pN2a/pN3a, but age, ER status, HER 2 status, tumor grade, and SUVmax at the primary site were not (Table 3).
| Factors | Univariate logistic regression analysis to predictive factors of pN2a/pN3a | Multivariate logistic regression analysis to predictive factors of pN2a/pN3a | ||||
| Odds ratio | 95%CI | P value | Odds ratio | 95%CI | P value | |
| Age (yr) (< 50 vs ≥ 50) | 0.863 | 0.333-2.24 | 0.76 | |||
| ER (positive vs negative) | 0.66 | 0.167-2.60 | 0.55 | |||
| HER2 (positive vs negative) | 0.833 | 0.954-7.28 | 0.87 | |||
| Invasive diameter of the primary site (≤ 20 mm vs > 20 mm) | 4.3 | 1.48-12.50 | 0.007 | 3.53 | 0.963-13.0 | 0.057 |
| Nuclear grade (1, 2 vs 3) | 1.29 | 0.426-3.393 | 0.65 | |||
| Total positive SLNs (1 vs 2 and more) | 3.73 | 1.34-10.40 | 0.012 | 3.92 | 1.01-15.3 | 0.048 |
| Maximal diameter of metastasis in the SLNs (< 5 mm vs ≥ 5 mm) | 21.2 | 4.68-96.3 | < 0.001 | 15.6 | 3.08-79.2 | < 0.001 |
| SUVmax of primary tumor (< 3.1 vs ≥ 3.1) | 0.96 | 0.351-2.62 | 0.94 | |||
| FDG accumulation of axillary lymph nodes (yes vs no) | 13 | 4.32-39.2 | < 0.001 | 4.84 | 1.29-18.2 | 0.02 |
Multivariate logistic regression analysis of clinicopathologic factors was used to examine risk factors for upstaging to pN2a/pN3a. The number of SLN metastasis, SLNDmax, and FDG accumulation in ALNs were associated with upstaging (Table 3).
The ACOSOG Z-0011 trial concluded that ALN dissection is not always necessary for women undergoing breast-conserving surgery with 1-2 positive SLNs[1]. However, the overall number of ALNMs represents crucial clinical information. Although avoidance of ALN dissection reduces the degree of surgical invasiveness of a procedure, the number of ALNMs cannot be ascertained. If more than 4 metastatic lymph nodes are present, radiation to the breast or chest wall as well as to the supraclavicular area is necessary[11]. Thus, not knowing the number of ALNMs may lead to over-or under-treatment with radiation therapy postoperatively. The presence or absence of postoperative radiation may also affect the choice of reconstructive technique. Radiation during the insertion of an expander is associated with a greater risk of complications, while radiation after implant placement or to autologous tissue is reported to have fewer complications[2,3]. Pre- and postoperative prediction of the number of ALNMs affects not only the optimal extent of axillary dissection, but also the choice of radiotherapy and, indirectly, reconstruction methods. In the present study, we searched for predictors of upstaging from clinical N0 to pN2a/pN3a using factors identified pre- or intraoperatively, including FDG-PET/CT. This imaging modality is generally considered useful in searching for ALNMs[12]. However, few reports have specified whether preoperative PET/CT was performed when examining factors predicting non-SLN metastasis. As a result of examining various factors for upstaging, we extracted SLNDmax, mild accumulation of FDG in the axilla, and the number of SLN metastasis. The SLNDmax and the number of SLN metastasis have been reported in the past as predictors of metastasis to non-SLNs[13-16]. This is the first study to show that these factors are also important in upstaging from N0 to pN2a/pN3a. Various cut-offs for the SLNDmax have been reported as a predictor of non-SLN metastasis, and we used ROC curves in our search. As a result, the predictor for upstaging from N0 to pN2a/pN3a was set at 5 mm. If the number of SLN metastasis is large (that is, two or more) or the diameter of metastasis is large (more than 5 mm), or if FDG accumulation in the axilla is mild even if no metastases have been confirmed, irradiation to the chest wall and supraclavicular region may need to be considered even in cases where axillary dissection has been omitted. PET/CT is often performed preoperatively for different cancers. On the other hand, findings from this modality are reportedly less significant for low-stage BC, given the low likelihood of distant metastasis. Some reports have suggested that SUV at the primary site may offer a useful predictor of metastasis to non-SLNs[17]. Ueda et al[18] evaluated ALNMs using PET/CT and reported low sensitivity but high specificity. In the present study, we found PET/CT to be useful in predicting multiple ALNMs, but the positive predictive value was not particularly high (57.9%). Many questions remain unanswered, such as the optimal cut-off value for integrated SUV, the influence of the histological type of the primary tumor, and the suitable timing of biopsy.
Further accumulation and analysis of cases are needed. Institutions that aggressively omit axillary dissection should be aware of the dangers of remaining ignorant of the number of ALNMs when multiple risk factors are identified pre- or intraoperatively.
The present study investigated factors predictive of upstaging from clinical N0 to pN2a/pN3a. Factors such as the number of metastases and SLNDmax, which have previously been reported as predictors of metastasis to non-SLNs, were also useful in predicting upstaging to pN2a/pN3a and emphasized the utility of FDG-PET/CT. The only factor that predicts preoperatively, but not intraoperatively, is the result of FDG-PET/CT.
With sentinel node metastasis in breast cancer (BC) patients, axillary lymph node (ALN) dissection is often omitted from cases with breast-conserving surgery. Omission of lymph node dissection reduces the invasiveness of surgery to the patient, but it also obscures the number of metastases to non-sentinel nodes.
The possibility of finding ≥ 4 lymph nodes (pN2a/pN3a) preoperatively is important given the ramifications for postoperative treatment.
The purpose of this study is to search for clinicopathological factors that predict upstaging from N0 to pN2a/N3a.
Patients who were SLN-positive and underwent ALN dissection between September 2007 and August 2018 were selected by retrospective chart review. All patients had BC diagnosed preoperatively as N0 with axillary evaluation by fluorodeoxyglucose (FDG) positron emission tomography/computed tomography and ultrasound (US) examination. When suspicious FDG accumulation was found in ALN, the presence of metastasis was reevaluated by second US. We examined predictors of upstaging from N0 to pN2a/pN3a.
Among 135 patients, we identified 1-3 ALNs (pN1) in 113 patients and ³4 ALNs (pN2a/pN3a) in 22 patients. Multivariate analysis identified the total number of SLN metastasis, the maximal diameter of metastasis in the SLN (SLNDmax), and FDG accumulation of ALN as predictors of upstaging to pN2a/pN3a.
We identified factors involved in upstaging from N0 to pN2a/pN3a. The SLNDmax and number of SLN metastasis are predictors of ≥ 4 ALNs (pN2a/pN3a) and predictors of metastasis to non-sentinel nodes, which have been reported in the past. Attention should be given to axillary accumulations of FDG, even when faint.
It is somewhat possible to predict upstaging to pN2a/pN3a by searching for clinicopathological factors
Provenance and peer review: Unsolicited article; Externally peer reviewed.
Peer-review model: Single blind
Specialty type: Surgery
Country/Territory of origin: Japan
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): B, B
Grade C (Good): C, C, C
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Ding JX, China; Hou L, China; Mao JH, United States S-Editor: Zhang H L-Editor: A P-Editor: Zhang H
| 1. | Giuliano AE, Ballman KV, McCall L, Beitsch PD, Brennan MB, Kelemen PR, Ollila DW, Hansen NM, Whitworth PW, Blumencranz PW, Leitch AM, Saha S, Hunt KK, Morrow M. Effect of Axillary Dissection vs No Axillary Dissection on 10-Year Overall Survival Among Women With Invasive Breast Cancer and Sentinel Node Metastasis: The ACOSOG Z0011 (Alliance) Randomized Clinical Trial. JAMA. 2017;318:918-926. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 806] [Cited by in RCA: 1233] [Article Influence: 154.1] [Reference Citation Analysis (0)] |
| 2. | Reinders FCJ, Young-Afat DA, Batenburg MCT, Bruekers SE, van Amerongen EA, Macaré van Maurik JFM, Braakenburg A, Zonnevylle E, Hoefkens M, Teunis T, Verkooijen HM, van den Bongard HJGD, Maarse W. Higher reconstruction failure and less patient-reported satisfaction after post mastectomy radiotherapy with immediate implant-based breast reconstruction compared to immediate autologous breast reconstruction. Breast Cancer. 2020;27:435-444. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 10] [Cited by in RCA: 35] [Article Influence: 5.8] [Reference Citation Analysis (0)] |
| 3. | Pu Y, Mao TC, Zhang YM, Wang SL, Fan DL. The role of postmastectomy radiation therapy in patients with immediate prosthetic breast reconstruction: A meta-analysis. Medicine (Baltimore). 2018;97:e9548. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 32] [Cited by in RCA: 34] [Article Influence: 4.9] [Reference Citation Analysis (0)] |
| 4. | Magill LJ, Robertson FP, Jell G, Mosahebi A, Keshtgar M. Determining the outcomes of post-mastectomy radiation therapy delivered to the definitive implant in patients undergoing one- and two-stage implant-based breast reconstruction: A systematic review and meta-analysis. J Plast Reconstr Aesthet Surg. 2017;70:1329-1335. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 35] [Cited by in RCA: 48] [Article Influence: 6.0] [Reference Citation Analysis (0)] |
| 5. | Manyam BV, Shah C, Woody NM, Reddy CA, Weller MA, Juloori A, Naik M, Valente S, Grobmyer S, Durand P, Djohan R, Tendulkar RD. Long-Term Outcomes After Autologous or Tissue Expander/Implant-Based Breast Reconstruction and Postmastectomy Radiation for Breast Cancer. Pract Radiat Oncol. 2019;9:e497-e505. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 13] [Cited by in RCA: 24] [Article Influence: 4.0] [Reference Citation Analysis (0)] |
| 6. | Naoum GE, Salama L, Niemierko A, Vieira BL, Belkacemi Y, Colwell AS, Winograd J, Smith B, Ho A, Taghian AG. Single Stage Direct-to-Implant Breast Reconstruction Has Lower Complication Rates Than Tissue Expander and Implant and Comparable Rates to Autologous Reconstruction in Patients Receiving Postmastectomy Radiation. Int J Radiat Oncol Biol Phys. 2020;106:514-524. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 33] [Cited by in RCA: 62] [Article Influence: 12.4] [Reference Citation Analysis (0)] |
| 7. | Pal A, Provenzano E, Duffy SW, Pinder SE, Purushotham AD. A model for predicting non-sentinel lymph node metastatic disease when the sentinel lymph node is positive. Br J Surg. 2008;95:302-309. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 177] [Cited by in RCA: 181] [Article Influence: 10.6] [Reference Citation Analysis (0)] |
| 8. | Gur AS, Unal B, Ozbek U, Ozmen V, Aydogan F, Gokgoz S, Gulluoglu BM, Aksaz E, Ozbas S, Baskan S, Koyuncu A, Soran A; Turkish Federation of Breast Disease Associations Protocol MF08-01 investigators. Validation of breast cancer nomograms for predicting the non-sentinel lymph node metastases after a positive sentinel lymph node biopsy in a multi-center study. Eur J Surg Oncol. 2010;36:30-35. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 59] [Cited by in RCA: 64] [Article Influence: 4.0] [Reference Citation Analysis (0)] |
| 9. | Kohrt HE, Olshen RA, Bermas HR, Goodson WH, Wood DJ, Henry S, Rouse RV, Bailey L, Philben VJ, Dirbas FM, Dunn JJ, Johnson DL, Wapnir IL, Carlson RW, Stockdale FE, Hansen NM, Jeffrey SS; Bay Area SLN Study. New models and online calculator for predicting non-sentinel lymph node status in sentinel lymph node positive breast cancer patients. BMC Cancer. 2008;8:66. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 180] [Cited by in RCA: 193] [Article Influence: 11.4] [Reference Citation Analysis (0)] |
| 10. | Kanda Y. Investigation of the freely available easy-to-use software 'EZR' for medical statistics. Bone Marrow Transplant. 2013;48:452-458. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 9275] [Cited by in RCA: 13321] [Article Influence: 1110.1] [Reference Citation Analysis (0)] |
| 11. | Curigliano G, Burstein HJ, Winer EP, Gnant M, Dubsky P, Loibl S, Colleoni M, Regan MM, Piccart-Gebhart M, Senn HJ, Thürlimann B; St. Gallen International Expert Consensus on the Primary Therapy of Early Breast Cancer 2017, André F, Baselga J, Bergh J, Bonnefoi H, Brucker SY, Cardoso F, Carey L, Ciruelos E, Cuzick J, Denkert C, Di Leo A, Ejlertsen B, Francis P, Galimberti V, Garber J, Gulluoglu B, Goodwin P, Harbeck N, Hayes DF, Huang CS, Huober J, Hussein K, Jassem J, Jiang Z, Karlsson P, Morrow M, Orecchia R, Osborne KC, Pagani O, Partridge AH, Pritchard K, Ro J, Rutgers EJT, Sedlmayer F, Semiglazov V, Shao Z, Smith I, Toi M, Tutt A, Viale G, Watanabe T, Whelan TJ, Xu B. De-escalating and escalating treatments for early-stage breast cancer: the St. Gallen International Expert Consensus Conference on the Primary Therapy of Early Breast Cancer 2017. Ann Oncol. 2017;28:1700-1712. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 682] [Cited by in RCA: 790] [Article Influence: 98.8] [Reference Citation Analysis (0)] |
| 12. | Ulaner GA. PET/CT for Patients With Breast Cancer: Where Is the Clinical Impact? AJR Am J Roentgenol. 2019;213:254-265. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 45] [Cited by in RCA: 46] [Article Influence: 7.7] [Reference Citation Analysis (0)] |
| 13. | Joseph KA, El-Tamer M, Komenaka I, Troxel A, Ditkoff BA, Schnabel F. Predictors of nonsentinel node metastasis in patients with breast cancer after sentinel node metastasis. Arch Surg. 2004;139:648-651. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 40] [Cited by in RCA: 37] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 14. | Degnim AC, Griffith KA, Sabel MS, Hayes DF, Cimmino VM, Diehl KM, Lucas PC, Snyder ML, Chang AE, Newman LA. Clinicopathologic features of metastasis in nonsentinel lymph nodes of breast carcinoma patients. Cancer. 2003;98:2307-2315. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 118] [Cited by in RCA: 117] [Article Influence: 5.3] [Reference Citation Analysis (0)] |
| 15. | Wong SL, Edwards MJ, Chao C, Tuttle TM, Noyes RD, Woo C, Cerrito PB, McMasters KM; University of Louisville Breast Cancer Sentinel Lymph Node Study Group. Predicting the status of the nonsentinel axillary nodes: a multicenter study. Arch Surg. 2001;136:563-568. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 99] [Cited by in RCA: 100] [Article Influence: 4.2] [Reference Citation Analysis (0)] |
| 16. | Boler DE, Uras C, Ince U, Cabioglu N. Factors predicting the non-sentinel lymph node involvement in breast cancer patients with sentinel lymph node metastases. Breast. 2012;21:518-523. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 15] [Cited by in RCA: 20] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 17. | Yamagishi Y, Yamasaki T, Ishida J, Moriya T, Einama T, Koiwai T, Fukumura-Koga M, Kono T, Hayashi K, Ueno H, Yamamoto J, Tsuda H. Utility of 18F-Fluorodeoxyglucose Positron Emission Tomography/Computed Tomography Fusion Imaging for Prediction of Metastasis to Sentinel and Nonsentinel Nodes in Patients with Clinically Node-Negative Breast Cancer. Ann Surg Oncol. 2020;27:2698-2710. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 3] [Cited by in RCA: 3] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 18. | Ueda S, Tsuda H, Asakawa H, Omata J, Fukatsu K, Kondo N, Kondo T, Hama Y, Tamura K, Ishida J, Abe Y, Mochizuki H. Utility of 18F-fluoro-deoxyglucose emission tomography/computed tomography fusion imaging (18F-FDG PET/CT) in combination with ultrasonography for axillary staging in primary breast cancer. BMC Cancer. 2008;8:165. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 95] [Cited by in RCA: 98] [Article Influence: 5.8] [Reference Citation Analysis (0)] |