Published online Aug 24, 2022. doi: 10.5306/wjco.v13.i8.712
Peer-review started: April 7, 2021
First decision: June 28, 2021
Revised: August 8, 2021
Accepted: July 18, 2022
Article in press: July 18, 2022
Published online: August 24, 2022
Processing time: 502 Days and 17.5 Hours
There are concerns that tamoxifen is less effective in Asian women because of the high prevalence of impaired function cytochrome P450 2D6 (CYP2D6) polymor-phisms.
To evaluate how knowledge of CYP2D6 genotype impacted the choice of hormonal agent and how CYP2D6 genotype and agent were associated with clinical outcomes.
Eighty-two women were recruited. Seventy-eight completed CYP2D6 genotyping and were categorized into poor, intermediate (IM) and extensive or ultra metabolizer phenotypes. Women with poor metabolizer and IM phenotypes were recommended aromatase inhibitors as the preferred agent.
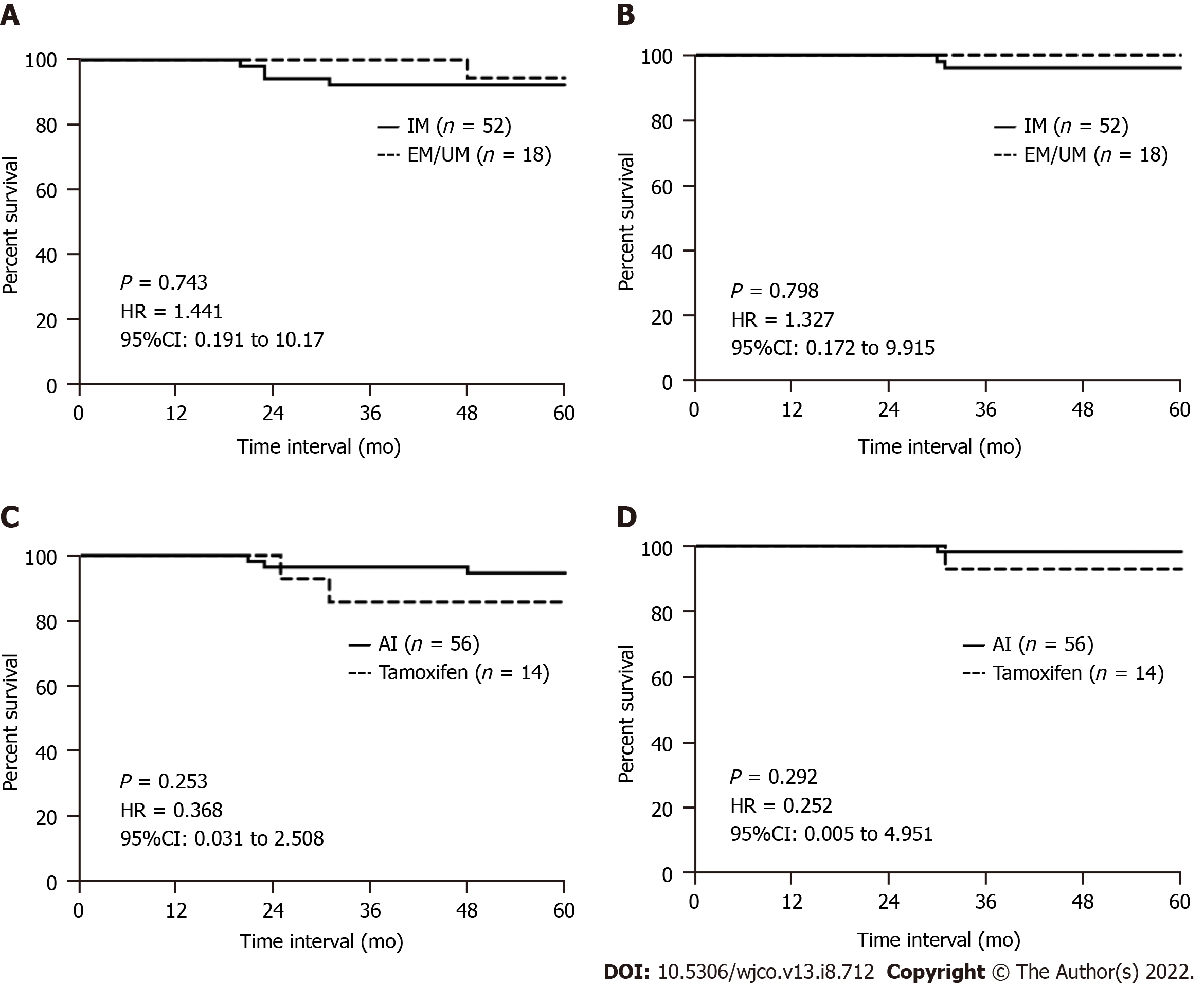
More than 70% of the women had an IM phenotype, 32% an extensive or ultra metabolizer phenotype, and 0% had a poor metabolizer phenotype. Regardless of genotype, more women opted for aromatase inhibitors. Overall, 80% of women completed 5 years of hormonal therapy. Five women developed recurrence, 3 contralateral breast cancer, 5 died, and 1 was diagnosed with a second primary cancer. Five-year recurrence-free and overall survival were slightly better in women with the extensive or ultra metabolizer phenotype compared to those with the IM phenotype, though not statistically significant [P = 0.743, hazard ratio (HR): 1.441, 95% confidence interval (CI): 0.191 to 10.17 and P = 0.798, HR: 1.327, 95%CI: 0.172 to 9.915, respectively]. Women receiving aromatase inhibitors also appeared to have a better, but also nonsignificant, 5-year recurrence-free and overall survival (P = 0.253, HR: 0.368, 95%CI: 0.031 to 0.258 and P = 0.292, HR: 0.252, 95%CI: 0.005 to 4.951, respectively).
The IM phenotype was highly prevalent but was not associated with clinical outcome.
Core Tip: We studied the role of cytochrome P450 2D6 (CYP2D6) polymorphisms in guiding the selection of hormonal agents in women with hormone-responsive breast cancer. The CYP2D6 intermediate metabolizer phenotype was highly prevalent in our women, while the poor metabolizer phenotype was rare. We did not observe any significant association between the CYP2D6 phenotypes and recurrence-free or overall survival in our study, although it could be because most women opted for aromatase inhibitors regardless of CYP2D6 phenotype. There was a non-significant trend towards better survival associated with aromatase inhibitor use over tamoxifen.
- Citation: Tan EY, Bharwani L, Chia YH, Soong RCT, Lee SSY, Chen JJC, Chan PMY. Impact of cytochrome P450 2D6 polymorphisms on decision-making and clinical outcomes in adjuvant hormonal therapy for breast cancer. World J Clin Oncol 2022; 13(8): 712-724
- URL: https://www.wjgnet.com/2218-4333/full/v13/i8/712.htm
- DOI: https://dx.doi.org/10.5306/wjco.v13.i8.712
In many centers, aromatase inhibitors (AIs) are now the first-line adjuvant hormonal therapy agents recommended for hormone-responsive breast cancer. While there are several reports of superior efficacy with AIs[1-3], some have questioned whether this is seen only in women with impaired tamoxifen metabolism[4,5]. Tamoxifen undergoes extensive first pass oxidative metabolism by the cytochrome P450 2D6 (CYP2D6) enzyme into the metabolically active derivative endoxifen (4-hydroxy-N-desmethyl-tamoxifen)[6,7]. The effect of impaired tamoxifen metabolism has particular significance among certain patient groups including Asians, where only 50% have functional CYP2D6 alleles. The reduced function allele CYP2D6*10 is highly prevalent among Asians and results in a 60% reduction in CYP2D6 enzyme activity[8-14]. The lower levels of the active metabolite endoxifen could imply that a large number of Asian women may have sub-therapeutic levels of tamoxifen and consequently suboptimal risk reduction. In contrast to tamoxifen, AIs act by inhibiting the aromatase enzymatic conversion of androgens to estradiol and is not affected by CYP2D6 metabolism.
At the time when this study was initiated, cost and the treatment duration were significant factors contributing to patient cost of the hormonal agent. Previously, AIs cost almost 25 times more than tamoxifen, and AIs were recommended for 5 years, whereas tamoxifen began to be recommended for 10 years. These factors are less relevant today. The cost of AIs are now relatively similar to tamoxifen with generic preparations of AIs now available, and extended AI therapy is also more often recommended. In spite of this, tamoxifen remains an important hormonal agent, particularly in premenopausal women, where the use of an AI will require concomitant use of gonadotropin-releasing hormone inhibitors. This is seldom done unless the risk of recurrence risk is high. Tamoxifen also remains a valuable alternative in women who cannot tolerate the musculoskeletal side effects of AIs or who develop osteoporosis from accelerated bone loss.
In this study, we evaluated the frequency of CYP2D6 polymorphisms and examined how the knowledge of the CYP2D6 phenotype impacted patient’s choice of hormonal agent. We also evaluated the association with clinical outcome through endpoints such as disease recurrence, mortality, contralateral breast cancer and 5-year recurrence-free and overall survival. We also evaluated the adverse effects reported by patients and the compliance to each agent, including the frequency of a switch to an alternative agent or premature discontinuation of hormonal therapy.
The study was designed as a single-arm prospective study and recruited 82 women with breast cancer. The study was granted Ethics Committee approval (2011/00017). Women included into the study were: (1) Post-menopausal; (2) Histologically confirmed with invasive breast carcinoma, Stage I to III; (3) Proven to have estrogen receptor (ER) and/or progesterone receptor (PR)-positive tumors (tumors with cells with at least 1% of cells staining positive for ER or PR were considered positive); (4) Had completed curative breast cancer surgery; (5) Had been recommended adjuvant hormonal therapy by the multidisciplinary tumor board; and (6) Were capable of providing informed consent. Exclusion criteria were: (1) Ductal carcinoma in situ; (2) Microinvasive disease; (3) Metastatic disease at presentation (including those found with metastatic disease on staging scans done after surgery); (4) Prior personal history of breast cancer or other primary cancers; and (5) Specific contraindications to tamoxifen and AIs, such as previous deep venous thrombosis, pulmonary embolism, cerebrovascular accident and severe osteoporosis.
Blood was sampled from patients who satisfied both criteria for CYP2D6 genotyping. The Qiagen DNA extraction kit (Qiagen, Hilden, Germany) was used to extract genomic DNA from blood collected; DNA concentration, purity and integrity were verified for all samples. Three poor metabolizer (PM) alleles (*4, *5, *6) and three intermediate metabolizer (IM) alleles (*9, *10 and *41) were identified using the pyrosequencing method, as previously described[15]. These six alleles were selected based on the reported prevalence among women of Chinese and Malay ethnicity, who make up the majority of the study cohort[8-14]. Primers were designed using the pyrosequencing software (http://techsupport.pyrosequencing.com); primer sequences are listed in Table 1.
| Position/change | Forward primer (5’→3’) | Reverse primer (5’→3’) | |
| *4 | 1846G>A | AGA GGC GCT TCT CCG TGT CC | AAA TCC TGC TCT TCC GAG GC |
| *5 | Gene deletion | CTC CAG CCT CCA CCA GTC CAG | CAG GCA TGA GCT AAG GCA CCC AGA C |
| *6 | 1707delT | CGC AAC TTG GGC CTG GGC AAG AAG TCG CTG GAC TAG | CTC GGG AGC TCG CCC TGC AGA GAC TC |
| *9 | 2613AGA>del | GGT CAG TGG TAA GGA CAG GCA GGC CC | CAC CCT TGC CCC CCA CCG TGG CAG CCA CTC TAA GCT |
| *10 | 100C>T | GAT GCA CCG GCG CCA ACG CTG GGC TGC ACG GTA C | CAA ACC TGC TTC CCC TTC TCA GCC |
| *41 | 2988G>A | CGT GAG CCC ATC TGG GAA A | CTG ACA CTC CTT CTT GCC TCC TA |
The PM phenotype was defined by being homozygous or compound heterozygous for two PM alleles. The IM phenotype was defined by being homozygous for two IM alleles or heterozygous for a PM allele and an IM allele. All other combinations are considered either extensive metabolizer (EM) or ultra metabolizer (UM) phenotypes. A PM or IM allele in combination with an EM allele results in an EM phenotype (tamoxifen metabolism being comparable). Patients were classified according to the following combinations: (1) PM: *4/*4, *4/*5, *4/*6*, *5/*5, *5/*6, *6/*6; (2) IM: *9/*9, *9/*10, *9/*41, *10/*10, *10/*41, *41/*41, *9/*4, *9/*5, *9/*6, *10/*4, *10/*5, *10/*6, *41/*4, *41/*5, *41/*6, heterozygotes for *4, *5, *6, *9, *10, *41; (3) EM/UM: all other combinations.
Results of the CYP2D6 genotyping were made known to the patient during the discussion for hormonal therapy. Patients identified to have PM or IM phenotypes were recommended anastrozole at a dose of 1 mg daily or letrozole at a dose of 2.5 mg daily, but they were still permitted to opt for tamoxifen. Those with an EM/UM phenotype were given a choice between an AI or tamoxifen (20 mg daily). All agents were recommended for a duration of 5 years, which was the standard practice at the time of the study. The benefit and potential treatment-related side effects of either agent was discussed with the patient, and the women themselves made the final decision regarding the choice of hormonal agent. The women were assessed every 6 mo with clinical history and physical examination during regular surveillance visits, and any side effects or disease progression were documented. Women receiving AIs also received calcium and vitamin D supplements, and bone mineral density was monitored every year. Women receiving tamoxifen were referred to the gynecology clinic for surveillance that included a yearly ultrasound of the pelvis to evaluate endometrial thickness specially; this was in addition to the routine 3-yearly PAP smear screenings. While this study was originally designed to follow up for the 5-year duration of hormonal treatment, we continued to collect data from the women who remained on extended therapy and on follow-up with the breast clinic in view of the increasing use of hormonal agents beyond 5 years as the study progressed. All 70 women included in the final analyses remained on follow-up until study completion. Median follow-up was 86 mo (30.57 to 99.50 mo), and median overall survival rate was 75.95 mo (30.57 to 94.10 mo).
The associations between CYP2D6 phenotype and the specific hormonal agent received and with standard clinicopathological parameters and outcomes were evaluated with univariate analyses (χ2 test, Fisher’s test, one-way analysis of variance, χ2 test for trend for ordinal data) and were performed using GraphPad Prism version 6 (GraphPad Software, San Diego, CA, United States). Recurrence-free survival was defined as the time interval from surgery to the development of either locoregional or distant recurrence. Overall survival was defined as the time interval from surgery to death, whether from breast cancer specific mortality or from any other causes. Contralateral cancer was defined as the occurrence of a metachronous cancer in the contralateral breast more than 6 mo following the diagnosis of the first cancer. Kaplan Meier survival curves were performed to compare survival rates, and the log rank test was used to compare between the two arms. A two-tailed test was used for all analyses, and a value of P < 0.05 was considered statistically significant.
Over a 3-year period from 2011 to 2013, a total of 82 women were recruited into the study. Eleven women later opted to withdraw for personal reasons, and one woman was withdrawn after she developed metastatic disease while on adjuvant chemotherapy treatment (prior to the start of hormonal treatment). CYP2D6 genotyping was completed in 78 women, and study endpoints were evaluated in the 70 women who remained in the study and completed at least 5 years of follow-up (apart from 3 women who died). Median patient age was 61 years (ranging from 47 years to 86 years), and the majority (70 of 82, 85.4%) were of Chinese ethnicity. More than half (62 of 82, 75.6%) of the women had at least one pre-existing co-morbidity; hypertension was the most common. All the women had undergone curative surgery, with 64.6% having had a mastectomy. Disease was staged as Stage I in 34 of 82 women (41.5%), Stage II in 27 (32.9%) and Stage III in 21 (25.6%); 40.2% of women had node-positive disease. Invasive ductal carcinoma not otherwise specified was the most common histological type (78%). Median invasive tumor size was 2 cm (ranging from 0.1 to 8.0 cm), and median tumor grade was 2.
All but 2 women had ER-positive tumors; they had ER-negative/PR-positive tumors. Median ER staining intensity was strong, and the median proportion of tumor cells staining positive for ER was 90.7%. Details of tumor ER expression is as follows: less than 10% of tumor cells stained positive in 4 of 80 women (4.9%), 11% to 49% of cells stained positive in 8 women (8.9%), 50% to 89% of cells stained positive in 17 women (24.3%) and more than 90% of cells stained positive in 41 women (58.7%). Tumors were positive for PR in 61 women (87.1%) and human epidermal growth factor receptor-2-positive in 31 (44.3%) women.
The majority of women (73.1%) were classified as having an IM phenotype, 25 women (32.1%) as having an EM/UM phenotype, and none were classified as having a PM phenotype (Table 2). The *10 allele was highly prevalent and was found in 52 (66.7%) women. Another 2 (2.5%) women were found with the *41 allele. The *4 and *5 PM alleles were found in 5 (6.4%) women, and in all instances occurred together with a *10 allele. Of the 8 women who withdrew from the study and who were not included in endpoint analyses, 5 were of the IM phenotype and 3 were of the EM/UM phenotype. Women with IM and EM/UM phenotypes shared similar characteristics (Table 3). Specifically, the IM phenotype did not correlate with tumor receptor status nor with the intensity of ER staining or proportion of tumor cells staining positive for ER.
| Phenotype | CYP2D6 genotype | Number of women, n = 78 |
| IM | *10/*10 | 25 |
| *10/41 | 2 | |
| *10/*4 | 2 | |
| *10/*5 | 3 | |
| *10/EM/UM | 20 | |
| *5/EM/UM | 5 | |
| EM/UM | EM/UM | 21 |
| IM phenotype, n = 52 | EM/UM phenotype, n = 18 | P value | |
| Median age in yr | 62.5 (51-86) | 61 (50-84) | 0.574 |
| Disease stage | 0.595 | ||
| I | 24 | 6 | |
| II | 17 | 8 | |
| III | 11 | 4 | |
| Ethnicity | 0.148 | ||
| Chinese | 47 | 13 | |
| Malay | 3 | 3 | |
| Indian | 1 | 2 | |
| Others | 1 | 0 | |
| Comorbidities | 0.728 | ||
| Yes | 43 | 14 | |
| No | 9 | 4 | |
| Tumor histology | 0.518 | ||
| IDC | 40 | 14 | |
| ILC | 7 | 1 | |
| Others | 5 | 3 | |
| Tumor grade | 0.247 | ||
| 1 | 16 | 3 | |
| 2 | 25 | 10 | |
| 3 | 11 | 5 | |
| Median tumor size in mm | 16.5 (1.2 to 70.0) | 20.0 (3.0 to 45.0) | 0.334 |
| Lymphovascular invasion | 0.527 | ||
| Present | 16 | 7 | |
| Absent | 36 | 11 | |
| ER intensity | 0.528 | ||
| Low | 2 | 0 | |
| Moderate | 11 | 2 | |
| High | 38 | 15 | |
| Negative | 1 | 1 | |
| Proportion of tumor cells staining ER-positive | 0.267 | ||
| 1% to 10% | 4 | 0 | |
| 11% to 49% | 6 | 1 | |
| 50% to 89% | 14 | 3 | |
| More than 90% | 27 | 14 | |
| PR intensity | 0.631 | ||
| Low | 5 | 1 | |
| Moderate | 9 | 5 | |
| High | 31 | 11 | |
| Negative | 7 | 1 | |
| Proportion of tumor cells staining PR-positive | 0.785 | ||
| 1% to 10% | 11 | 3 | |
| 11% to 49% | 8 | 2 | |
| 50% to 89% | 18 | 7 | |
| More than 90% | 11 | 6 | |
| HER2 status | 0.495 | ||
| Positive | 11 | 2 | |
| Negative | 41 | 16 | |
| Clinical subtypes | 0.692 | ||
| ER+/HER2- | 41 | 14 | |
| ER+/HER2+ | 10 | 2 | |
| ER-/HER2+ | 1 | 0 |
Following a discussion with their attending clinician, more women opted for an AI over tamoxifen regardless of CYP2D6 phenotype. More than 80% of the women (43 of 52, 82.7%) with an IM phenotype opted for an AI, and 72.2% of women with an EM/UM phenotype (13 of 18) also opted for an AI. Only 14 women (9 with an IM phenotype and 5 with an EM/UM phenotype) opted for tamoxifen. There were no significant differences between the group who opted for an AI compared to those who chose tamoxifen (Table 4). The difference in median ages between the two groups (P = 0.008) was not likely clinically significant. Similar numbers of women on AI and tamoxifen choose mastectomy, but more women opting for AI had received other modes of systemic treatment (chemotherapy with or without trastuzumab) (P = 0.045).
| Aromatase inhibitors, n = 56 | Tamoxifen, n = 14 | P value | |
| CYP2D6 phenotype | 0.339 | ||
| IM | 43 | 9 | |
| EM/UM | 13 | 5 | |
| Median age in yr | 62 (50 to 80) | 63 (52 to 86) | 0.008 |
| Disease stage | 0.795 | ||
| I | 23 | 7 | |
| II | 21 | 4 | |
| III | 12 | 3 | |
| Ethnicity | 0.126 | ||
| Chinese | 50 | 10 | |
| Malay | 3 | 2 | |
| Indian | 3 | 1 | |
| Others | 0 | 1 | |
| Comorbidities | 1.000 | ||
| Yes | 45 | 12 | |
| No | 11 | 2 | |
| Tumor histology | 0.918 | ||
| IDC | 45 | 11 | |
| ILC | 6 | 2 | |
| Others | 5 | 1 | |
| Tumor grade | 0.686 | ||
| 1 | 15 | 4 | |
| 2 | 27 | 8 | |
| 3 | 14 | 2 | |
| Median tumor size in mm | 16.5 (1.2 to 70.0) | 19.0 (1.6 to 53.0) | 0.747 |
| Lymphovascular invasion | 0.526 | ||
| Yes | 17 | 6 | |
| No | 39 | 8 | |
| ER intensity | 0.509 | ||
| Low intensity | 1 | 1 | |
| Moderate intensity | 10 | 4 | |
| High intensity | 44 | 9 | |
| Negative | 1 | 0 | |
| Proportion of tumor cells staining ER-positive | 0.386 | ||
| 1%-10% | 3 | 1 | |
| 11% to 49% | 4 | 3 | |
| 50% to 89% | 15 | 2 | |
| More than 90% | 33 | 8 | |
| PR status | 1.000 | ||
| Positive | 49 | 13 | |
| Negative | 7 | 1 | |
| PR intensity | 0.161 | ||
| Low intensity | 3 | 3 | |
| Moderate intensity | 10 | 4 | |
| High intensity | 36 | 6 | |
| Negative | 7 | 1 | |
| Proportion of tumor cells staining PR-positive | 0.318 | ||
| 1%-10% | 11 | 3 | |
| 11% to 49% | 6 | 4 | |
| 50% to 89% | 21 | 4 | |
| More than 90% | 15 | 2 | |
| HER2 status | 0.700 | ||
| Positive | 10 | 3 | |
| Negative | 46 | 10 | |
| Tumor subtypes | 0.754 | ||
| ER+/HER2- | 46 | 10 | |
| ER+/HER2+ | 9 | 3 | |
| ER-/HER2+ | 1 | 0 | |
| Type of surgery | 0.756 | ||
| Mastectomy | 35 | 10 | |
| Wide local excision | 21 | 4 | |
| Treatments received | 0.045 | ||
| Systemic therapy1 and hormonal therapy | 12 | 2 | |
| Systemic therapy1, radiation and hormonal therapy | 18 | 0 | |
| Radiation and hormonal therapy | 12 | 5 | |
| Hormonal therapy alone | 14 | 7 | |
| Disease recurrence2 | 0.260 | ||
| Yes | 3 | 2 | |
| No | 53 | 12 | |
| Mortality2 | 0.344 | ||
| Yes | 1 | 1 | |
| No | 55 | 13 | |
| Contralateral breast cancer2 | 0.551 | ||
| Yes | 2 | 1 | |
| No | 54 | 13 |
Overall, 56 of the 70 women (80%) completed 5 years of hormonal therapy; comprising 46 of the 56 women (82.1%) who opted for an AI and 10 of the 14 women (71.4%) who opted for tamoxifen. Nineteen women who started on an AI and three of those who started on tamoxifen continued with extended therapy after 5 years of treatment. Thirty of the 56 women (53.5%) completed 5 years of the initial AI agent they were started on. Side effects were reported in 19 women: severe myalgia and arthralgia in 8 women, skin rashes in 4 women and osteoporosis in 7 women. Two women with intolerable musculoskeletal side effects opted to discontinue hormonal therapy. Of the other 12 women with musculoskeletal side effects or rashes, 7 switched to another AI, and 3 switched to tamoxifen. Four of the seven women who developed osteoporosis switched to tamoxifen, while the remaining three remained on AIs but started on bisphosphonates. All the women who switched to another AI agent eventually completed 5 years of treatment, although 2 switched to a third AI agent and 3 switched to tamoxifen before completion. Of the 7 women who switched to tamoxifen, 6 completed 5 years of treatment with tamoxifen, while the remaining patient switched back to another 2 different AI agents before completing the 5 years of treatment. Of the 14 women who started on tamoxifen, 2 women (14.3%) switched to an AI after developing skin rashes; though one of them later discontinued the AI after developing musculoskeletal side effects. The hormonal agent was discontinued in 8 patients upon the development of new events. Another 3 women chose to discontinue hormonal therapy but had not reported any side effects.
Over the follow-up period of 96 mo, 3 women developed contralateral breast cancer, 5 women developed recurrences, 5 women died, and 1 woman was diagnosed with a nasopharyngeal carcinoma. Contralateral breast cancer was diagnosed after a median interval of 48.8 mo (ranging from 47.83 to 59.27 mo). All 3 occurred in women of the IM phenotype, of which 2 women had received AI and 1 received tamoxifen. Recurrence was systemic in all 5 women and had occurred after a median interval of 24.93 mo (ranging from 23.07 to 48.10 mo). Four of these women later died, although one death was attributed to a non-breast cancer-related cause. The last mortality occurred in a woman who had remained disease-free up to the time of death from a non-breast cancer-related cause. Disease recurrence did not show a clear association with the hormonal agent received (P = 0.260) (Table 4). Recurrence developed in 3 women who had received an AI (2 of the IM phenotype and 1 of the EM/UM phenotype) and in 2 women who had received tamoxifen (both of the IM phenotype). One of the women developed both locoregional and systemic recurrence.
Five-year recurrence-free and overall survival appeared slightly better in women with an EM/UM phenotype compared to those with an IM phenotype but was not statistically significant [P = 0.743, hazard ratio (HR): 1.441, 95% confidence interval (CI): 0.191 to 10.17 and P = 0.798, HR: 1.327, 95%CI: 0.172 to 9.915, respectively) (Figure 1A and B). When stratified by the hormonal agent received, women who had received an AI appeared to have better 5-year recurrence-free and overall survival compared to those who had received tamoxifen, but again these were not statistically significant (P = 0.253, HR: 0.368, 95%CI: 0.031 to 0.258 and P = 0.292, HR: 0.252, 95%CI: 0.005 to 4.951, respectively) (Figure 1C and D).
AIs are now widely used as first-line hormonal agent in many clinical units. With the introduction of generic letrozole, AIs have become more affordable for our local women. The common practice here is to start women on AIs unless they have contraindications or develop intolerable side effects. Tamoxifen is now first-line only in premenopausal women or in those with contraindications to AIs. Otherwise, it is often the second-line agent that women are switched to should they develop intolerable side effects from tamoxifen. The move towards adopting AIs as first-line came following reports of superior efficacy and because AIs were previously recommended for 5 years, whereas tamoxifen was recommended for 10 years. However, it has also been suggested that AIs are superior to tamoxifen primarily in women with nonfunctional or reduced function CYP2D6 polymorphisms[4,5], specifically in those with a PM or IM phenotype where impaired CYP2D6 enzyme metabolism results in lower serum concentrations of active metabolite endoxifen responsible for tamoxifen efficacy[16,17]. Observations that serum endoxifen levels correlated with the frequency and severity of adverse effects raised the possibility that clinical outcomes could likewise be adversely affected by impaired CYP2D6 metabolism[18,19].
More than 70% of Caucasians have functional CYP2D6 alleles, yet only 50% of Asians have functional CYP2D6 alleles[8-10]. It has been variously reported that 40% to 70% of Asians carry reduced function alleles, particularly the CYP2D6*10 allele[11-14]. The high prevalence of CYP2D6*10 was confirmed in our study, where it was present in two-thirds of the women included. The *41 allele was present in 2 women. The CYP2D6*10 allele results in an approximately 60% reduction in CYP2D6 enzyme activity, and Asians have been reported to metabolize tamoxifen and other CYP2D6-mediated drugs more slowly than Caucasians[20-23]. Nonfunctional alleles were uncommon, and all 5 women (6.4%) in our study with *4 and *5 PM alleles were heterozygotes. This is relatively similar to the prevalence reported in other studies[9-11,13,14,22,23]. We did not find any women with the CYP2D6*9 allele, which we had included as it was observed in 3% of Malays in Malaysia[9].
Five women (7.1%) in our study developed recurrence during the study, including 4 women who were of the IM phenotype; 2 of whom received an AI. We did observe a trend towards better survival in women with the EM/UM phenotype. This was in agreement with the findings of a prospective study window-of-opportunity study conducted at our unit where women received up to 2 wk of tamoxifen prior to surgery. Women with at least one wild-type CYP2D6 allele demonstrated a significantly greater Ki-67 response, suggesting that tamoxifen produced a greater inhibitory effect in those with functioning CYP2D6 polymorphisms[24]. The lack of statistical significance was likely because of the small sample size and perhaps because there were no women with the PM phenotype in our study. Women with a PM phenotype were reportedly at a 7% higher risk of recurrence, which appeared to be incremental over time[5]. Like others, we did not observe CYP2D6 polymorphisms to correlate with tumor size, grade or nodal status, which meant that survival differences were not likely a result of unfavorable tumor factors[25].
Mathematical modelling showed that survival outcomes in women without nonfunctional alleles were not different whether they received AIs or tamoxifen[5], and reduced function CYP2D6 polymorphisms were only associated with clinical outcomes in those treated with tamoxifen[25,26]. We observed that women who received AIs had a slightly better but nonsignificant survival and that these women were more likely to have received systemic treatments (chemotherapy with or without trastuzumab), perhaps indicating a clinician bias towards AIs in those deemed to have more ‘high-risk’ disease. However, our study numbers are small and too few women received tamoxifen for a meaningful analysis. A larger cohort would be needed to stratify the effect of the hormonal agent used by disease stage and other systemic treatments. Overall, we observed little difference in contralateral breast cancer rates and disease recurrence between women with IM and EM/UM phenotypes, which was perhaps due to many women in our study, including 72% of those with an EM/UM phenotype, opting for an AI over tamoxifen.
Despite the differences in cost at the time of the study, many women opted for the more costly AI. This was so even in those with an EM/UM phenotype, in whom there were suggestions that tamoxifen efficacy was comparable to AI. However, only about half of the women in our study completed 5 years of the AI agent they were initially started on. In 60% of cases, the discontinuation was initiated by the women themselves because of intolerable myalgia, arthralgia and skin rashes [10 (53%) switched to another AI, and 5 had further problems with the second AI and eventually switched to tamoxifen or a third AI]. Four women who started on an AI were switched to tamoxifen by their clinician because of osteoporosis from accelerated bone loss. On the other hand, the majority of women who started on tamoxifen appeared to tolerate it well, and only 2 were switched to an AI after developing skin rashes. Overall, more women who started on tamoxifen completed 5 years of treatment compared to those who started on an AI. Excluding the 8 women who progressed on hormonal therapy, 90% of the women completed 5 years of hormonal therapy.
Like others, we did not find CYP2D6 polymorphisms to correlate with any clinicopathological factors, implying that genotyping would be the only means of ascertaining the phenotype. The prevalence of the IM phenotype in our local women may mean that CYP2D6 genotyping at the offset may be of little benefit since AIs are now the initial hormonal agent of choice. On the other hand, given that a significant number of women do develop AI-related side effects and in those whom a switch is being considered, CYP2D6 genotyping could help clinicians decide whether to switch to another AI or to tamoxifen. Those with an EM/UM phenotype can be switched to tamoxifen since outcomes are probably comparable with those on AIs[5]. Furthermore, tamoxifen-related side effects appear to be less common. An unpublished review of women on follow-up at our unit did not find a higher incidence of endometrial cancer among those treated with tamoxifen. Those with an IM phenotype should consider switching to another AI agent, based on reports of impaired function variants being associated with higher risks of recurrence. This would have particular significance in the setting of ER-positive disease where late recurrences are more common and since the majority of women survive for many more years after breast cancer treatment.
The prevalence of the IM phenotype was high in our study, with more than two-thirds of the women having the CYP2D6*10 allele. We did not observe the IM phenotype to be associated with any clinicopathological parameter and did not observe any correlation with clinical outcome. The hormonal agent used was not associated with a difference in outcome. Compliance was good, and most women completed 5 years of hormonal therapy, although more women who started on an AI required a switch to another hormonal agent because of side effects.
The authors are grateful to Ms Sim E-Jan for contributions to the generation and presentation of Figure 1.
There are concerns that tamoxifen is less effective in Asian women because of the high prevalence of impaired function cytochrome P450 2D6 (CYP2D6) polymorphisms.
Tamoxifen is still the first-line agent for premenopausal women and for those with intolerable AI-related side effects. It is therefore necessary to verify the effectiveness of tamoxifen in view of the high prevalence of reduced function CYP2D6 polymorphisms in Asians.
We evaluated the frequency of CYP2D6 polymorphisms and its association with clinical outcome. We also evaluated treatment-related side effects in order to better determine the risk:benefit ratio.
We designed a single-arm prospective study to evaluate how knowledge of CYP2D6 genotype impacted the choice of hormonal agent and how CYP2D6 genotype and agent were associated with clinical outcomes.
More than 70% of the women in our study had an intermediate metabolizer phenotype. Regardless of genotype, more women opted for aromatase inhibitors. Women with the extensive or ultra metabolizer phenotype had slightly better but nonsignificant 5-year recurrence-free and overall survival compared to women with the intermediate metabolizer phenotype. Women on AIs appeared to have better but also nonsignificant 5-year recurrence-free and overall survival.
The intermediate metabolizer phenotype was highly prevalent in our local women but was not associated with clinical outcome.
Data on the effect of CYP2D6 polymorphisms on tamoxifen efficacy remains conflicting. More studies in Asian women would help to clarify this association.
Provenance and peer review: Invited article; Externally peer reviewed.
Peer-review model: Single blind
Specialty type: Oncology
Country/Territory of origin: Singapore
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): 0
Grade C (Good): C
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Kamalabadi-Farahani M, Iran S-Editor: Gong ZM L-Editor: Filipodia P-Editor: Gong ZM
| 1. | Arimidex, Tamoxifen; Alone or in Combination (ATAC) Trialists' Group. , Forbes JF, Cuzick J, Buzdar A, Howell A, Tobias JS, Baum M. Effect of anastrozole and tamoxifen as adjuvant treatment for early-stage breast cancer: 100-month analysis of the ATAC trial. Lancet Oncol. 2008;9:45-53. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 777] [Cited by in RCA: 744] [Article Influence: 43.8] [Reference Citation Analysis (0)] |
| 2. | Breast International Group (BIG) 1-98 Collaborative Group; Thürlimann B, Keshaviah A, Coates AS, Mouridsen H, Mauriac L, Forbes JF, Paridaens R, Castiglione-Gertsch M, Gelber RD, Rabaglio M, Smith I, Wardley A, Price KN, Goldhirsch A. A comparison of letrozole and tamoxifen in postmenopausal women with early breast cancer. N Engl J Med. 2005;353:2747-2757. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1180] [Cited by in RCA: 1161] [Article Influence: 58.1] [Reference Citation Analysis (0)] |
| 3. | Gnant M, Mlineritsch B, Schippinger W, Luschin-Ebengreuth G, Pöstlberger S, Menzel C, Jakesz R, Seifert M, Hubalek M, Bjelic-Radisic V, Samonigg H, Tausch C, Eidtmann H, Steger G, Kwasny W, Dubsky P, Fridrik M, Fitzal F, Stierer M, Rücklinger E, Greil R; ABCSG-12 Trial Investigators, Marth C. Endocrine therapy plus zoledronic acid in premenopausal breast cancer. N Engl J Med. 2009;360:679-691. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 809] [Cited by in RCA: 787] [Article Influence: 49.2] [Reference Citation Analysis (0)] |
| 4. | Schroth W, Goetz MP, Hamann U, Fasching PA, Schmidt M, Winter S, Fritz P, Simon W, Suman VJ, Ames MM, Safgren SL, Kuffel MJ, Ulmer HU, Boländer J, Strick R, Beckmann MW, Koelbl H, Weinshilboum RM, Ingle JN, Eichelbaum M, Schwab M, Brauch H. Association between CYP2D6 polymorphisms and outcomes among women with early stage breast cancer treated with tamoxifen. JAMA. 2009;302:1429-1436. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 418] [Cited by in RCA: 394] [Article Influence: 24.6] [Reference Citation Analysis (0)] |
| 5. | Punglia RS, Burstein HJ, Winer EP, Weeks JC. Pharmacogenomic variation of CYP2D6 and the choice of optimal adjuvant endocrine therapy for postmenopausal breast cancer: a modeling analysis. J Natl Cancer Inst. 2008;100:642-648. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 78] [Cited by in RCA: 74] [Article Influence: 4.4] [Reference Citation Analysis (0)] |
| 6. | Jordan VC. Metabolites of tamoxifen in animals and man: identification, pharmacology, and significance. Breast Cancer Res Treat. 1982;2:123-138. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 107] [Cited by in RCA: 97] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 7. | Stearns V, Johnson MD, Rae JM, Morocho A, Novielli A, Bhargava P, Hayes DF, Desta Z, Flockhart DA. Active tamoxifen metabolite plasma concentrations after coadministration of tamoxifen and the selective serotonin reuptake inhibitor paroxetine. J Natl Cancer Inst. 2003;95:1758-1764. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 626] [Cited by in RCA: 618] [Article Influence: 29.4] [Reference Citation Analysis (0)] |
| 8. | Bradford LD. CYP2D6 allele frequency in European Caucasians, Asians, Africans and their descendants. Pharmacogenomics. 2002;3:229-243. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 593] [Cited by in RCA: 543] [Article Influence: 23.6] [Reference Citation Analysis (0)] |
| 9. | Teh LK, Ismail R, Yusoff R, Hussein A, Isa MN, Rahman AR. Heterogeneity of the CYP2D6 gene among Malays in Malaysia. J Clin Pharm Ther. 2001;26:205-211. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 34] [Cited by in RCA: 37] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 10. | Kubota T, Yamaura Y, Ohkawa N, Hara H, Chiba K. Frequencies of CYP2D6 mutant alleles in a normal Japanese population and metabolic activity of dextromethorphan O-demethylation in different CYP2D6 genotypes. Br J Clin Pharmacol. 2000;50:31-34. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 112] [Cited by in RCA: 124] [Article Influence: 5.0] [Reference Citation Analysis (0)] |
| 11. | Dahl ML, Yue QY, Roh HK, Johansson I, Säwe J, Sjöqvist F, Bertilsson L. Genetic analysis of the CYP2D locus in relation to debrisoquine hydroxylation capacity in Korean, Japanese and Chinese subjects. Pharmacogenetics. 1995;5:159-164. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 70] [Cited by in RCA: 68] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 12. | Wang SL, Huang JD, Lai MD, Liu BH, Lai ML. Molecular basis of genetic variation in debrisoquin hydroxylation in Chinese subjects: polymorphism in RFLP and DNA sequence of CYP2D6. Clin Pharmacol Ther. 1993;53:410-418. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 110] [Cited by in RCA: 109] [Article Influence: 3.4] [Reference Citation Analysis (0)] |
| 13. | Tateishi T, Chida M, Ariyoshi N, Mizorogi Y, Kamataki T, Kobayashi S. Analysis of the CYP2D6 gene in relation to dextromethorphan O-demethylation capacity in a Japanese population. Clin Pharmacol Ther. 1999;65:570-575. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 98] [Cited by in RCA: 97] [Article Influence: 3.7] [Reference Citation Analysis (0)] |
| 14. | Nishida Y, Fukuda T, Yamamoto I, Azuma J. CYP2D6 genotypes in a Japanese population: low frequencies of CYP2D6 gene duplication but high frequency of CYP2D6*10. Pharmacogenetics. 2000;10:567-570. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 101] [Cited by in RCA: 101] [Article Influence: 4.0] [Reference Citation Analysis (0)] |
| 15. | Tan YH, Liu Y, Eu KW, Ang PW, Li WQ, Salto-Tellez M, Iacopetta B, Soong R. Detection of BRAF V600E mutation by pyrosequencing. Pathology. 2008;40:295-298. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 68] [Cited by in RCA: 71] [Article Influence: 4.2] [Reference Citation Analysis (0)] |
| 16. | Osborne CK. Tamoxifen in the treatment of breast cancer. N Engl J Med. 1998;339:1609-1618. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 910] [Cited by in RCA: 933] [Article Influence: 34.6] [Reference Citation Analysis (0)] |
| 17. | Borges S, Desta Z, Li L, Skaar TC, Ward BA, Nguyen A, Jin Y, Storniolo AM, Nikoloff DM, Wu L, Hillman G, Hayes DF, Stearns V, Flockhart DA. Quantitative effect of CYP2D6 genotype and inhibitors on tamoxifen metabolism: implication for optimization of breast cancer treatment. Clin Pharmacol Ther. 2006;80:61-74. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 363] [Cited by in RCA: 359] [Article Influence: 18.9] [Reference Citation Analysis (0)] |
| 18. | Fisher B, Dignam J, Bryant J, DeCillis A, Wickerham DL, Wolmark N, Costantino J, Redmond C, Fisher ER, Bowman DM, Deschênes L, Dimitrov NV, Margolese RG, Robidoux A, Shibata H, Terz J, Paterson AH, Feldman MI, Farrar W, Evans J, Lickley HL. Five versus more than five years of tamoxifen therapy for breast cancer patients with negative lymph nodes and estrogen receptor-positive tumors. J Natl Cancer Inst. 1996;88:1529-1542. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 661] [Cited by in RCA: 600] [Article Influence: 20.7] [Reference Citation Analysis (0)] |
| 19. | Goetz MP, Rae JM, Suman VJ, Safgren SL, Ames MM, Visscher DW, Reynolds C, Couch FJ, Lingle WL, Flockhart DA, Desta Z, Perez EA, Ingle JN. Pharmacogenetics of tamoxifen biotransformation is associated with clinical outcomes of efficacy and hot flashes. J Clin Oncol. 2005;23:9312-9318. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 604] [Cited by in RCA: 570] [Article Influence: 30.0] [Reference Citation Analysis (0)] |
| 20. | Bertilsson L, Lou YQ, Du YL, Liu Y, Kuang TY, Liao XM, Wang KY, Reviriego J, Iselius L, Sjöqvist F. Pronounced differences between native Chinese and Swedish populations in the polymorphic hydroxylations of debrisoquin and S-mephenytoin. Clin Pharmacol Ther. 1992;51:388-397. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 287] [Cited by in RCA: 279] [Article Influence: 8.5] [Reference Citation Analysis (0)] |
| 21. | Kalow W. Interethnic variation of drug metabolism. Trends Pharmacol Sci. 1991;12:102-107. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 77] [Cited by in RCA: 69] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 22. | Johansson I, Yue QY, Dahl ML, Heim M, Säwe J, Bertilsson L, Meyer UA, Sjöqvist F, Ingelman-Sundberg M. Genetic analysis of the interethnic difference between Chinese and Caucasians in the polymorphic metabolism of debrisoquine and codeine. Eur J Clin Pharmacol. 1991;40:553-556. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5] [Cited by in RCA: 13] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 23. | Johansson I, Oscarson M, Yue QY, Bertilsson L, Sjöqvist F, Ingelman-Sundberg M. Genetic analysis of the Chinese cytochrome P4502D locus: characterization of variant CYP2D6 genes present in subjects with diminished capacity for debrisoquine hydroxylation. Mol Pharmacol. 1994;46:452-459. [PubMed] |
| 24. | Zembutsu H, Nakamura S, Akashi-Tanaka S, Kuwayama T, Watanabe C, Takamaru T, Takei H, Ishikawa T, Miyahara K, Matsumoto H, Hasegawa Y, Kutomi G, Shima H, Satomi F, Okazaki M, Zaha H, Onomura M, Matsukata A, Sagara Y, Baba S, Yamada A, Shimada K, Shimizu D, Tsugawa K, Shimo A, Tan EY, Hartman M, Chan CW, Lee SC, Nakamura Y. Significant Effect of Polymorphisms in CYP2D6 on Response to Tamoxifen Therapy for Breast Cancer: A Prospective Multicenter Study. Clin Cancer Res. 2017;23:2019-2026. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 24] [Cited by in RCA: 24] [Article Influence: 2.7] [Reference Citation Analysis (0)] |
| 25. | Schroth W, Antoniadou L, Fritz P, Schwab M, Muerdter T, Zanger UM, Simon W, Eichelbaum M, Brauch H. Breast cancer treatment outcome with adjuvant tamoxifen relative to patient CYP2D6 and CYP2C19 genotypes. J Clin Oncol. 2007;25:5187-5193. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 334] [Cited by in RCA: 321] [Article Influence: 17.8] [Reference Citation Analysis (0)] |
| 26. | Xu Y, Sun Y, Yao L, Shi L, Wu Y, Ouyang T, Li J, Wang T, Fan Z, Fan T, Lin B, He L, Li P, Xie Y. Association between CYP2D6 *10 genotype and survival of breast cancer patients receiving tamoxifen treatment. Ann Oncol. 2008;19:1423-1429. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 139] [Cited by in RCA: 135] [Article Influence: 7.9] [Reference Citation Analysis (0)] |