Published online Aug 24, 2022. doi: 10.5306/wjco.v13.i8.702
Peer-review started: February 28, 2022
First decision: May 31, 2022
Revised: June 24, 2022
Accepted: July 26, 2022
Article in press: July 26, 2022
Published online: August 24, 2022
Processing time: 176 Days and 0.2 Hours
Delays in sentinel lymph node (SLN) biopsy may affect the positivity of non-SLNs. For these reasons, effort is being directed at obtaining reliable information regarding SLN positivity prior to surgical excision. However, the existing tools, e.g., dermoscopy, do not recognize statistically significant predictive criteria for SLN positivity in melanomas.
To investigate the possible association of computer-assisted objectively obtained color, color texture, sharpness and geometry variables with SLN positivity.
We retrospectively reviewed and analyzed the computerized medical records of all patients diagnosed with cutaneous melanoma in a tertiary hospital in Germany during a 3-year period. The study included patients with histologically confirmed melanomas with Breslow > 0.75 mm who underwent lesion excision and SLN biopsy during the study period and who had clinical images shot with a digital camera and a handheld ruler aligned beside the lesion.
Ninety-nine patients with an equal number of lesions met the inclusion criteria and were included in the analysis. Overall mean (± standard deviation) age was 66 (15) years. The study group consisted of 20 patients with tumor-positive SLN (SLN+) biopsy, who were compared to 79 patients with tumor-negative SLN biopsy specimen (control group). The two groups differed significantly in terms of age (61 years vs 68 years) and histological subtype, with the SLN+ patients being younger and presenting more often with nodular or secondary nodular tumors (P < 0.05). The study group patients showed significantly higher eccentricity (i.e. distance between color and geometrical midpoint) as well as higher sharpness (i.e. these lesions were more discrete from the surrounding normal skin, P < 0.05). Regarding color variables, SLN+ patients demonstrated higher range in all four color intensities (gray, red, green, blue) and significantly higher skewness in three color intensities (gray, red, blue), P < 0.05. Color texture variables, i.e. lacunarity, were comparable in both groups.
SLN+ patients demonstrated significantly higher eccentricity, higher sharpness, higher range in all four color intensities (gray, red, green, blue) and significantly higher skewness in three color intensities (gray, red, blue). Further prospective studies are needed to better understand the effectiveness of clinical image processing in SLN+ melanoma patients.
Core Tip: Computer-aided image analysis can facilitate prediction of sentinel lymph-node positivity. Several color, sharpness and geometry parameters can predict positive lymph node occurrence, while color texture cannot determine sentinel lymph node positivity.
- Citation: Papadakis M, Paschos A, Papazoglou AS, Manios A, Zirngibl H, Manios G, Koumaki D. Computer-aided clinical image analysis as a predictor of sentinel lymph node positivity in cutaneous melanoma. World J Clin Oncol 2022; 13(8): 702-711
- URL: https://www.wjgnet.com/2218-4333/full/v13/i8/702.htm
- DOI: https://dx.doi.org/10.5306/wjco.v13.i8.702
Cutaneous melanoma is a highly aggressive tumor that often spreads to local lymph nodes. Sentinel lymph node biopsy (SLNB) is commonly performed to identify nodal metastases because sentinel lymph node (SLN) status is a strong prognostic factor for survival in melanoma patients, especially in those without evidence of clinically positive lymph nodes[1]. In some subgroups, e.g., in patients with thick melanomas (i.e. Breslow thickness > 4 mm) and patients with melanomas of the scalp, SLN status is considered the most important prognostic survival factor[2,3].
According to the existing guidelines, the decision for SLNB is based on the thickness of the primary tumor. SLNB is indicated for all primary tumors thicker than 1 mm and tumors thicker than 0.75 mm in the presence of ulceration or high mitotic rate (> 1 mm²). SLNB is of crucial importance in disease management because positive SLNB should be followed by lymph node dissection for regional disease control and staging purposes. Delays in SLNB may affect the positivity of non-SLNs[4]. For these reasons, an effort is being directed at obtaining reliable information regarding SLN positivity before surgical excision.
Dermoscopy is a non-invasive technique that facilitates early melanoma detection by revealing skin features invisible to the naked eye. However, dermoscopy does not recognize statistically significant predictive criteria for SLN positivity in melanomas because specific melanoma criteria strongly associated with a higher Breslow thickness, such as gray-blue areas or an atypical vascular pattern, do not seem to associate with SLN positivity[5].
Computer-aided clinical image analysis is also used to improve diagnostic accuracy for skin melanoma. We have shown that geometrical and color parameters objectively extracted by computer-aided clinical image processing may correlate with tumor thickness in patients with cutaneous melanoma[6]. However, to the best of our knowledge, there is no study investigating the possible association of computer-assisted objectively obtained color, color texture, sharpness and geometry variables with SLN positivity. The aim of this study was to investigate whether such an association exists.
We retrospectively reviewed and analyzed the computerized medical records of all patients diagnosed with cutaneous melanoma in a tertiary hospital in Germany during a 3-year period. The study included patients with histologically confirmed melanomas with Breslow > 0.75 mm who underwent lesion excision and SLN biopsy during the study period and who had clinical images shot with a digital camera and a handheld ruler aligned beside the lesion. Patients with melanomas with Breslow < 0.75 mm and in situ melanomas as well as patients without digital images were excluded from the study. Patients referred to our center after primary excision to undergo SLN biopsy were also excluded from the study.
The study group consisted of patients with a positive SLN biopsy who were compared to patients with a negative SLN biopsy (control group). Clinical features studied included age, sex, tumor location and diagnosis date. Histopathologic features included tumor subtype [superficial spreading (de novo and nevus-associated) and nodular, including secondary nodular], Breslow thickness, Clark level, presence of ulceration, nevus pre-existence and SLN status (positive or negative). The study was approved by the institutional review board of the University of Witten-Herdecke.
All lesions were photographed at admission with the same commercial digital camera at a resolution of 1600 × 1200 pixels and with a handheld ruler aligned beside the lesion to allow for correct image scaling. All photos were obtained from the same educated nurse to minimize inconsistencies in methodology and were uploaded to a local server. The lesions were then excised under local anesthesia, and the diagnosis was histologically confirmed.
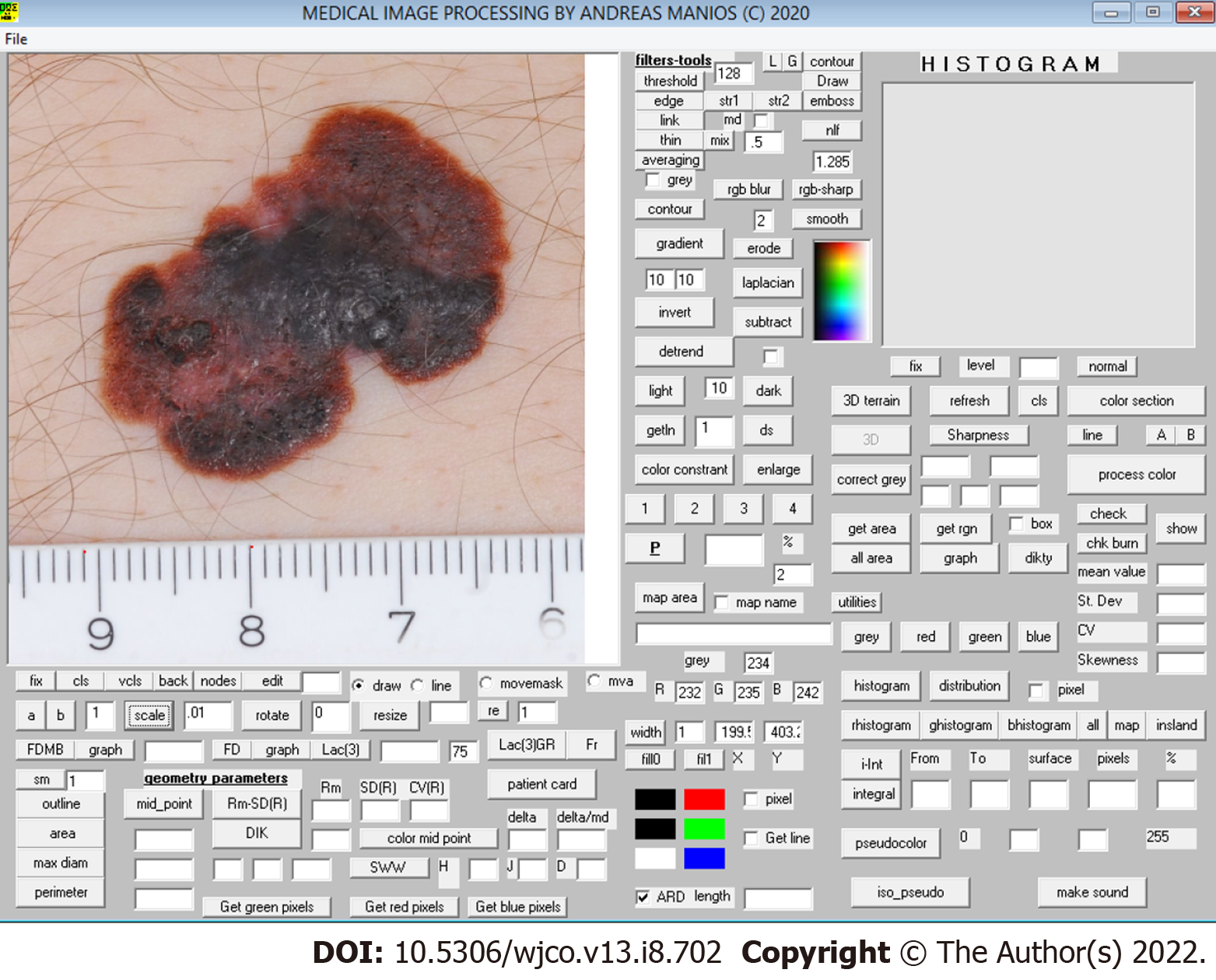
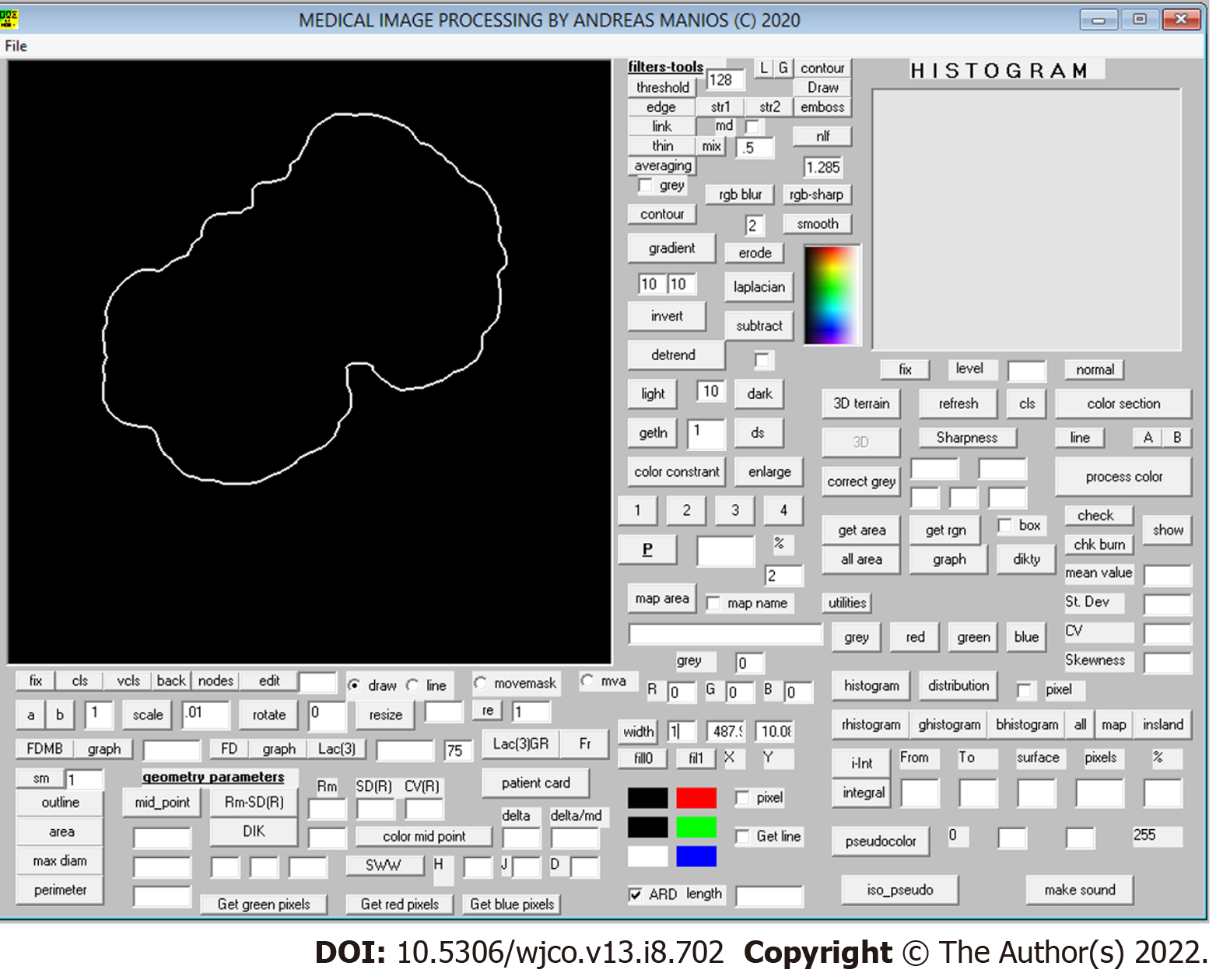
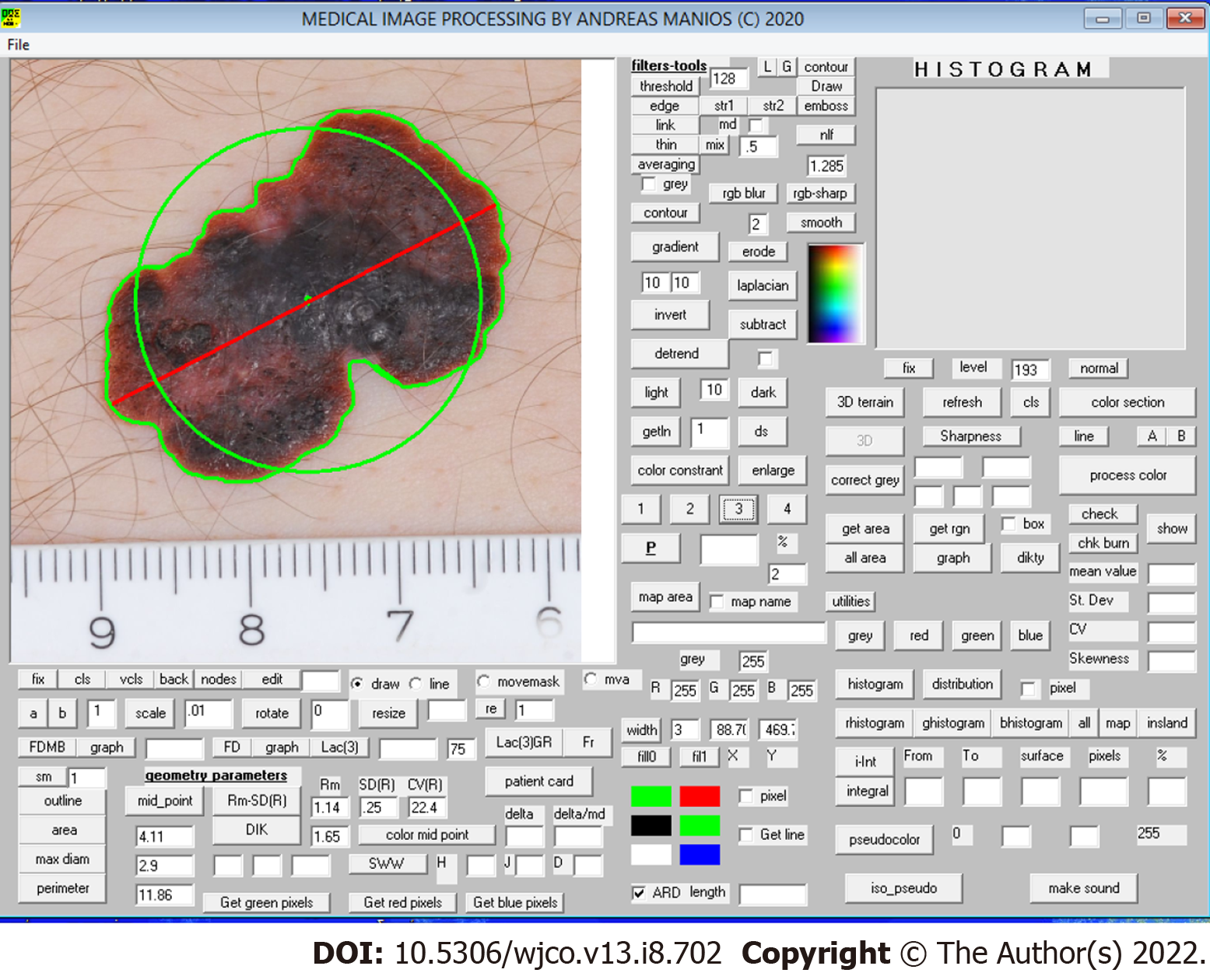
The color images obtained underwent digital processing with an almost fully automated noncommercial software developed by one of the authors for study purposes. The software applies several kinds of algorithms to allow image segmentation and geometry, color and color texture analysis (Figure 1). The only manual involvement was the selection of the lesion border with the mouse cursor when the algorithm failed to do so (i.e. in very small lesions) (Figure 2). Such cases were independently analyzed twice by two of the authors (MP and GM) to avoid intraobserver errors (Figure 3). In cases of discrepancies, the mean scores were accepted and further analyzed. The 34 variables studied are classified as follows: (1) Geometrical variables (i.e. area, maximum diameter, perimeter, circularity, eccentricity and mean radius); (2) Color variables [i.e. range, standard deviation, coefficient of variation and skewness for all four color intensities (gray, red, green, blue)]; (3) Sharpness variables; and (4) Color texture variables (i.e. lacunarity)[7]. All variables are thoroughly described in Table 1.
| Classification | Parameter | Explanation |
| Geometry variables | Area | Lesion surface area, measured in cm2 |
| Maximum diameter | The longest line that joins two points on the border of the lesion, measured in cm | |
| Perimeter | Total boundary length of the region of interest (i.e. lesion), measured in cm | |
| Circularity | Ratio of the perimeter of the lesion divided by the perimeter of a circle with the same midpoint and same area as the lesion | |
| Mean radius (Rm) | Mean value of the lesion’s radii | |
| Standard deviation of Rm | Standard deviation of the mean radius | |
| Coefficient of variation of Rm | Expresses the standard deviation as a percentage of the mean | |
| Eccentricity | Distance between color and geometric midpoint within the lesion | |
| Eccentricity ratio | Distance between midpoint and color midpoint expressed as a fraction of the maximum diameter | |
| Sharpness variables | SD of gray intensity | Intensity of gray on the border of the lesion |
| Coefficient of variation of SD of gray intensity | SD of gray intensity in grayscale image. The higher the value, the more discrete the lesion is from the surrounding normal skin (Manousaki et al[7], 2016) | |
| Color texture variables | Grayscale lacunarity of lesion (Lac gray) | It is estimated in grayscale image and assesses image texture heterogeneity or incomplete space filling within the lesion |
| Color variables | Range of gray, red, green, blue | Range of values of gray, red, green, blue intensity |
| Mean gray, red, green, blue | Mean value of gray, red, green, blue intensity within the lesion | |
| SD of gray, red, green, blue | Standard deviation of gray, red, green, blue intensity within the lesion | |
| Coefficient of variation of gray, red, green, blue | Expresses the standard deviation of gray, red, green, blue intensity values as mean percentage | |
| Skewness from Gaussian curve (gray, red, green, blue) | Deviation of each color’s histogram from the normal distribution curve |
Normal distribution was determined using histogram plots, box plots and the Shapiro-Wilk test. Continuous data are presented in mean-standard deviation form. Categorical variables were compared using the two-tailed Fisher’s exact test and continuous variables using the two-tailed Student’s t test. A P value of less than 0.05 was considered statistically significant. Univariate and multivariate analysis using logistic regression were employed to identify potential independent determinants of a positive SLN result. Data analyses were performed using SPSS 23.
Ninety-nine patients with an equal number of lesions met the inclusion criteria and were included in the analysis. Of these, 52 (52%) were males and the rest (48%) females. Histogram plots, box plots and the Shapiro-Wilk test demonstrated an almost normal distribution appearance for all continuous variables. The overall mean (standard deviation) age was 66 (15) years. The youngest patient was 14-years-old and the oldest 92-years-old. The study group consisted of 20 patients with tumor-positive SLN (SLN+) biopsy who were compared to 79 patients with tumor-negative SLN biopsy specimens (control group).
The two groups differed significantly in terms of age (61 years vs 68 years) and histological subtype; the SLN+ patients were younger and presented more often with nodular or secondary nodular tumors (P < 0.05).
The study group patients also showed significantly higher eccentricity (i.e. the distance between color and geometrical midpoint) and higher sharpness (i.e. these lesions were more discrete from the surrounding normal skin, P < 0.05). Regarding color variables, SLN+ patients demonstrated a higher range in all four color intensities (gray, red, green, blue) and significantly higher skewness in three color intensities (gray, red, blue), P < 0.05. Color texture variables (i.e. lacunarity) were similar in both groups. Comparative data are summarized in Table 2.
| Variable | Study group, SLNB+; n = 20 | Control group, SLNB-; n = 79 | P value | |
| Demographics | ||||
| Sex | ||||
| Male | 12 (60%) | 40 (50%) | 0.62 | |
| Female | 8 (40%) | 39 (50%) | ||
| Age (yr) | 61 (13) | 68 (15) | 0.05 | |
| Tumor thickness (mm) | 2.6 (2.7) | 2.2 (3.0) | 0.64 | |
| Subtype | Superficial spread | 3 (15%) | 44 (56%) | 0.04 |
| Nodular | 10 (50%) | 25 (32%) | ||
| Secondary nodular | 7 (35%) | 10 (12%) | ||
| Geometric variables | ||||
| Area (cm2) | 3.4 (2.9) | 2.8 (4.7) | 0.49 | |
| MaxD (cm) | 2.4 (1.2) | 2 (1.1) | 0.22 | |
| Perimeter (cm) | 6.6 (3.0) | 5.7 (3.3) | 0.25 | |
| Circularity (ratio) | 1.1 (0.1) | 1.1 (0.1) | 0.44 | |
| Rm (cm) | 0.98 (0.5) | 0.84 (0.5) | 0.21 | |
| SDRm | 0.14 (0.1) | 0.12 (0.1) | 0.48 | |
| CVRm | 13 (7.1) | 13 (5.5) | 0.88 | |
| Delta (cm) | 0.04 (0.03) | 0.03 (0.03) | 0.04 | |
| Delta ratio | 1.8 (0.9) | 1.6 (1.3) | 0.42 | |
| Sharpness variables | ||||
| Sharpness | 31 (9) | 26 (8) | 0.02 | |
| CV sharpness | 22 (6) | 18 (7) | 0.02 | |
| Color texture variables | ||||
| Lac gray | 2 (0.28) | 1.97 (0.30) | 0.65 | |
| Color variables | ||||
| Mean gray | 104 (21) | 113 (24) | 0.12 | |
| SD gray | 32 (6) | 30 (7) | 0.12 | |
| CV gray | 8 (2) | 10 (1) | 0.07 | |
| Range gray | 201 (21) | 187 (32) | 0.03 | |
| Skewness gray | 0.52 (0.5) | 0.23 (0.6) | 0.03 | |
| Mean red | 144 (26) | 157 (32) | 0.06 | |
| SD red | 37 (9) | 33 (11) | 0.14 | |
| CV red | 27 (10) | 23 (11) | 0.14 | |
| Range red | 205 (23) | 190 (37) | 0.04 | |
| Skewness red | 0.03 (0.43) | -0.04 (0.83) | 0.003 | |
| Mean green | 89 (23) | 96 (23) | 0.24 | |
| SD green | 33 (7) | 31 (6) | 0.19 | |
| CV green | 39 (12) | 35 (13) | 0.13 | |
| Range green | 209 (24) | 195 (33) | 0.04 | |
| Skewness green | 0.64 (0.46) | 0.41 (0.60) | 0.08 | |
| Mean blue | 87 (22) | 94 (25) | 0.22 | |
| SD blue | 33 (7) | 31 (6) | 0.24 | |
| CV blue | 40 (10) | 35 (13) | 0.14 | |
| Range blue | 218 (23) | 201 (34) | 0.02 | |
| Skewness blue | 0.67 (0.47) | 0.42 (0.56) | 0.05 | |
Multivariate analysis of univariately significant variables (P < 0.05) revealed that younger age and higher eccentricity were independently associated with a higher probability of positive lymph node occurrence [for age: adjusted odds ratio (aOR) = 0.95, 95% confidence interval (CI): 0.91 to 0.99 and for eccentricity: aOR = 1.45, 95%CI: 1.12 to 1.89]. Nevus, nodular and secondary nodular histotypes were also significantly linked with higher odds of positive lymph node presence when compared to the superficial spreading histotype (for nevus: aOR = 14.19, 95%CI: 1.15 to 174.76, for nodular: aOR = 10.71, 95%CI: 1.48 to 77.48 and for secondary nodular: aOR = 18.21, 95%CI: 2.19 to 151.22). The proposed multivariate model can predict the presence of SLN+ with an accuracy of 85% and is summarized in Table 3.
| Variable | Coefficient (β) | Standard Error | Wald χ2 | P value | Odds ratio | 95%CI |
| Age | -0.05 | 0.20 | 7.60 | 0.006 | 0.95 | 0.91 to 0.99 |
| Subtype, nevus-associated | 2.65 | 1.28 | 4.28 | 0.038 | 14.19 | 1.15 to 174.76 |
| Subtype, nodular | 2.37 | 1.01 | 5.51 | 0.019 | 10.71 | 1.48 to 77.48 |
| Subtype, secondary nodular | 2.90 | 1.08 | 7.22 | 0.007 | 18.21 | 2.19 to 151.22 |
| Eccentricity | 0.38 | 0.13 | 7.84 | 0.005 | 1.46 | 1.12 to 1.89 |
Computer-aided image analysis is a noninvasive method and as such an established tool in the physicians’ armamentarium to obtain reliable information regarding malignancy before surgical excision. SLN status is a strong prognostic factor for survival in melanoma patients (the tumor thickness threshold for SLNB being 1 mm) in the absence of risk factors. We herein investigated the possible association of computer-assisted objectively obtained color, texture and geometric variables with SLN positivity.
SLN+ patients have a higher range in all four color intensities (gray, red, green, blue) and significantly higher skewness in three color intensities (gray, red, blue). Blue and black pigmentation is associated with the presence of nodular melanoma, which in our study accounted for 50% of the SLN+ tumors[8]. Malignant epidermal structures, (e.g., atypical pigment network, radial streaks and pseudopods) are rarely seen in SLN+ melanomas, while they are observed in one-fourth of SLN negative lesions[5].
Despite the significant differences in all color intensities, we found that lacunarity (a measure of the variation of the color intensity) cannot predict SLN status in melanoma patients although it is a proven promising parameter in the automated differentiation of melanoma from non-melanoma[9]. We also found the lesions of SLN+ patients have significantly higher eccentricity, which is an index of uneven lesion coloration. Eccentricity represents a special case of asymmetry, and as shown before eccentric lesions may be thicker[6]. Dermoscopically, only the presence of ulceration and blotch correlate with positive SLNB[10].
Regarding histological type, González-Álvarez et al[10] found nodular melanomas to be the most associated with SLN positivity, reporting an OR of 3.98. This is consistent with our findings, where half of the SLN+ tumors were nodular, and the OR was 14.4. Moreover, we found that secondary nodular tumors are much more often associated with SLN+, with the OR exceeding 25. This may reflect more aggressive tumor growth because angiolymphatic invasion is observed in the majority of nodular melanomas with SLN+[5]. According to our findings, a multivariate model consisting of age, histological type and eccentricity can predict the presence of SLN+ with an accuracy of up to 85%.
A recent study described a deep learning-based digital biomarker to predict SLN+ from digitized hematoxylin and eosin slides of primary melanoma tumors. Artificial neural networks predicted SLN status with an accuracy of 55%-62%[11]. This relatively low accuracy is attributed to morphological changes of the tumor cells or tumor architecture. Moreover, the histopathological workup may have caused tumor cells to be missed in the lymph nodes. Neural networks failed to detect features other than thickness and age that predict SLN+. We found that higher values of eccentricity, sharpness, blue, gray, green range, red skewness and red mean could also predict positive lymph node occurrence.
Our study is limited regarding its retrospective nature and small sample size. First, it is a single-center study of German individuals, and therefore the results cannot be easily generalized. Second, only melanoma patients with available clinical images made before the diagnosis was established were included. Therefore, there is a high risk of bias due to no consecutive cases being included. Selection bias may have led to suspect cases being more frequently photographed. Moreover, we excluded all patients with melanomas with Breslow thickness < 0.75 mm. However, the specimens were analyzed by different pathologists during the study period, so the interpretation bias of tumor thickness may have led to eligible cases being excluded.
In conclusion, computer-aided image analysis can facilitate the prediction of SLN+. SLN+ patients demonstrated significantly higher eccentricity, higher sharpness and higher range in all four color intensities (gray, red, green, blue) as well as significantly higher skewness in three color intensities (gray, red, blue). Further prospective studies are needed to better understand the effectiveness of clinical image processing in SLN+ melanoma patients.
Computer-aided clinical image analysis is used to improve diagnostic accuracy for skin melanoma.
To the best of our knowledge, there is no study investigating the possible association of computer-assisted objectively obtained color, color texture, sharpness and geometry variables with sentinel lymph node positivity (SLN+).
To investigate a possible association of computer-assisted objectively obtained color, color texture, sharpness and geometry variables with SLN+.
The study included patients with histologically confirmed melanomas with Breslow > 0.75 mm who underwent lesion excision and SLN biopsy during the 3-year study period and who had clinical images shot with a digital camera and a handheld ruler aligned beside the lesion. All the color images obtained underwent digital processing with an almost fully automated noncommercial software developed by one of the authors for study purposes.
Ninety-nine patients with an equal number of lesions met the inclusion criteria and were included in the analysis. The study group consisted of 20 patients with SLN+ biopsy who were compared to 79 patients with tumor-negative SLN biopsy specimen (control group). The study group patients showed significantly higher eccentricity (i.e. distance between color and geometrical midpoint) as well as higher sharpness (i.e. these lesions were more discrete from the surrounding normal skin, P < 0.05). Regarding color variables, SLN+ patients demonstrated higher range in all four color intensities (gray, red, green, blue) and significantly higher skewness in three color intensities (gray, red, blue), P < 0.05. Color texture variables, i.e. lacunarity, were comparable in both groups.
Computer-aided image analysis can facilitate the prediction of SLN+. SLN+ patients demonstrated significantly higher eccentricity, higher sharpness and higher range in all four color intensities (gray, red, green, blue) as well as significantly higher skewness in three color intensities (gray, red, blue).
Further prospective studies are needed to better understand the effectiveness of clinical image processing in SLN+ melanoma patients.
Provenance and peer review: Invited article; Externally peer reviewed.
Peer-review model: Single blind
Specialty type: Oncology
Country/Territory of origin: Germany
Peer-review report’s scientific quality classification
Grade A (Excellent): A
Grade B (Very good): B
Grade C (Good): C
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Mangla A, United States; Wang P, China S-Editor: Wang LL L-Editor: Filipodia P-Editor: Wang LL
| 1. | Narang J, Hue JJ, Bingmer K, Hardacre JM, Winter JM, Ocuin LM, Ammori JB, Mangla A, Bordeaux J, Rothermel LD. Sentinel lymph node biopsy guideline concordance in melanoma: Analysis of the National Cancer Database. J Surg Oncol. 2021;124:669-678. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3] [Cited by in RCA: 16] [Article Influence: 4.0] [Reference Citation Analysis (0)] |
| 2. | Gutzmer R, Satzger I, Thoms KM, Völker B, Mitteldorf C, Kapp A, Bertsch HP, Kretschmer L. Sentinel lymph node status is the most important prognostic factor for thick (> or = 4 mm) melanomas. J Dtsch Dermatol Ges. 2008;6:198-203. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 37] [Cited by in RCA: 42] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 3. | Cappello ZJ, Augenstein AC, Potts KL, McMasters KM, Bumpous JM. Sentinel lymph node status is the most important prognostic factor in patients with melanoma of the scalp. Laryngoscope. 2013;123:1411-1415. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 19] [Cited by in RCA: 21] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 4. | Richtig G, Richtig E, Neiss AN, Quehenberger F, Gmainer DG, Kamolz LP, Lumenta DB. Does the time interval between sentinel lymph node biopsy and completion lymph node dissection affect outcome in malignant melanoma? Int J Surg. 2020;75:160-164. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3] [Cited by in RCA: 5] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 5. | Pagnanelli G, Bono R, Pizzichetta MA, Talamini R, Ascierto PA, Testori A, Stanganelli I; Italian Melanoma Intergroup (IMI). Clinical and dermoscopic criteria related to melanoma sentinel lymph node positivity. Anticancer Res. 2007;27:2939-2944. [PubMed] |
| 6. | Papadakis M, Paschos A, Manios A, Lehmann P, Manios G, Zirngibl H. Computer-aided clinical image analysis for non-invasive assessment of tumor thickness in cutaneous melanoma. BMC Res Notes. 2021;14:232. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 7] [Cited by in RCA: 3] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 7. | Manousaki AG, Manios AG, Tsompanaki EI, Panayiotides JG, Tsiftsis DD, Kostaki AK, Tosca AD. A simple digital image processing system to aid in melanoma diagnosis in an everyday melanocytic skin lesion unit: a preliminary report. Int J Dermatol. 2006;45:402-410. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 46] [Cited by in RCA: 28] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 8. | Argenziano G, Fabbrocini G, Carli P, De Giorgi V, Delfino M. Clinical and dermatoscopic criteria for the preoperative evaluation of cutaneous melanoma thickness. J Am Acad Dermatol. 1999;40:61-68. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 79] [Cited by in RCA: 72] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 9. | Gilmore S, Hofmann-Wellenhof R, Muir J, Soyer HP. Lacunarity analysis: a promising method for the automated assessment of melanocytic naevi and melanoma. PLoS One. 2009;4:e7449. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 37] [Cited by in RCA: 28] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 10. | González-Álvarez T, Carrera C, Bennassar A, Vilalta A, Rull R, Alos L, Palou J, Vidal-Sicart S, Malvehy J, Puig S. Dermoscopy structures as predictors of sentinel lymph node positivity in cutaneous melanoma. Br J Dermatol. 2015;172:1269-1277. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 14] [Cited by in RCA: 17] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 11. | Brinker TJ, Kiehl L, Schmitt M, Jutzi TB, Krieghoff-Henning EI, Krahl D, Kutzner H, Gholam P, Haferkamp S, Klode J, Schadendorf D, Hekler A, Fröhling S, Kather JN, Haggenmüller S, von Kalle C, Heppt M, Hilke F, Ghoreschi K, Tiemann M, Wehkamp U, Hauschild A, Weichenthal M, Utikal JS. Deep learning approach to predict sentinel lymph node status directly from routine histology of primary melanoma tumours. Eur J Cancer. 2021;154:227-234. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 9] [Cited by in RCA: 30] [Article Influence: 7.5] [Reference Citation Analysis (0)] |