Published online Aug 24, 2022. doi: 10.5306/wjco.v13.i8.688
- This article has been corrected.
- See: World J Clin Oncol. Jun 24, 2023; 14(6): 227-229
Peer-review started: April 8, 2022
First decision: May 12, 2022
Revised: June 14, 2022
Accepted: July 11, 2022
Article in press: July 11, 2022
Published online: August 24, 2022
Processing time: 136 Days and 17.1 Hours
Cholangiocarcinoma (CC) is a rare tumor that arises from the epithelium of the bile ducts. It is classified according to anatomic location as intrahepatic, perihilar, and distal. Intrahepatic CC (ICC) is rare in patients with cirrhosis due to causes other than primary sclerosing cholangitis. Mixed hepatocellular carcinoma-CC (HCC-CC) is a rare neoplasm that shows histologic findings of both HCC and ICC within the same tumor mass. Due to the difficulties in arriving at the correct diag
To evaluate the outcomes of patients with intrahepatic cholangiocarcinoma or mixed hepatocellular-cholangiocarcinoma on pathological examination after liver transplant.
Propensity score matching was used to analyze tumor recurrence (TR), overall mortality (OM), and recurrence-free survival (RFS) in LT recipients with pathologically confirmed ICC or HCC-CC matched 1:8 to those with HCC. Progression-free survival and overall mortality rates were computed with the Kaplan-Meier method using Cox regression for comparison.
Of 475 HCC LT recipients, 1.7% had the diagnosis of ICC and 1.5% of HCC-CC on pathological examination of the explant. LT recipients with ICC had higher TR (46% vs 11%; P = 0.006), higher OM (63% vs 23%; P = 0.002), and lower RFS (38% vs 89%; P = 0.002) than those with HCC when matched for pretransplant tumor characteristics, as well as higher TR (46% vs 23%; P = 0.083), higher OM (63% vs 35%; P = 0.026), and lower RFS (38% vs 59%; P = 0.037) when matched for posttransplant tumor characteristics. Two pairings were performed to compare the outcomes of LT recipients with HCC-CC vs HCC. There was no significant difference between the outcomes in either pairing.
Patients with ICC had worse outcomes than patients undergoing LT for HCC. The outcomes of patients with HCC-CC did not differ significantly from those of patients with HCC.
Core Tip: This retrospective cohort study analyzes the outcomes of patients undergoing liver trans-plantation (LT) with a presumptive diagnosis of hepatocellular carcinoma (HCC) in which explant analysis identified that they actually had intrahepatic cholangiocarcinoma (ICC) or mixed hepatocellular cholangiocarcinoma (HCC-CC). Propensity score matching was used to analyze tumor recurrence, overall mortality, and recurrence-free survival in LT recipients with pathologically confirmed ICC or HCC-CC matched 1:8 to those with HCC. Patients with ICC have worse outcomes than patients undergoing LT for HCC, even when matched for explant pathology. Outcomes did not differ significantly between patients with HCC-CC and patients with HCC.
- Citation: Brandão ABM, Rodriguez S, Fleck Jr AM, Marroni CA, Wagner MB, Hörbe A, Fernandes MV, Cerski CT, Coral GP. Propensity-matched analysis of patients with intrahepatic cholangiocarcinoma or mixed hepatocellular-cholangiocarcinoma and hepatocellular carcinoma undergoing a liver transplant. World J Clin Oncol 2022; 13(8): 688-701
- URL: https://www.wjgnet.com/2218-4333/full/v13/i8/688.htm
- DOI: https://dx.doi.org/10.5306/wjco.v13.i8.688
Cholangiocarcinoma (CC) is a relatively rare, aggressive tumor that arises from the epithelium of the bile ducts. It is classified according to anatomical location as intrahepatic, perihilar, or distal[1]. CC is the most common tumor of the biliary tree, accounting for approximately 10%-25% of all hepatic malignancies[2]. It is the second most common hepatic malignancy[3].
Intrahepatic CC (ICC) represents 5%-10% of all CCs[1,4,5]. Although rare, its incidence is increasing in many countries[6-9]. In Brazil, ICC-related mortality in persons aged 45-64 years increased by 100% from 2002 to 2012, reaching 0.35 and 0.37 per 100000 person-years for men and women, respectively[9]. The increase is attributed, at least in part, to improved ICC classification, accurate diagnosis, and the negative impact of known risk factors, such as chronic hepatitis C virus (HCV) infection and obesity[10].
Mixed hepatocellular-cholangiocarcinoma (HCC-CC) is a rare neoplasm that histologically resembles both HCC and ICC within the same tumor mass[11]. It has an estimated incidence of 1%-4.7% among hepatic malignancies[12]. HCC-CC and ICC share the same risk factors[13]. The diagnosis of HCC-CC is typically made by pathology after resection or transplant, and a preoperative diagnosis is unlikely[14].
Although imaging findings suggestive of the diagnosis of HCC, ICC, or HCC-CC have been described[15-17], these tumors can be challenging to diagnose because of their rarity. In addition, HCC and ICC can coexist in separate nodules within the same liver or within the same tumor mass. Therefore, due to the difficulties in arriving at the correct diagnosis, patients eventually undergo a liver transplant (LT) with the presumptive imaging diagnosis of HCC when, in fact, they have ICC or HCC-CC[18,19].
The present study aimed to determine the prevalence of ICC or HCC-CC confirmed by explant pathology in patients who underwent LT with the presumptive diagnosis of HCC and to compare recurrence, recurrence-free survival, and overall mortality rates between these patients and LT recipients with HCC.
This retrospective cohort study included patients aged ≥ 18 years with liver cirrhosis and imaging findings suggestive of HCC within the Milan criteria who underwent LT between June 1997 and July 2019 at a transplant referral center/teaching hospital in southern Brazil. Patients were followed up until April 2020 and divided into three groups according to the diagnosis on explant pathology: (1) Patients with HCC; (2) Patients with ICC; and (3) Patients with mixed HCC-CC. Well-established diagnostic criteria were followed, and immunohistochemical analysis was performed if necessary[12,20].
The following variables were analyzed: Age, sex, etiology of liver cirrhosis, Child-Pugh score, pretransplant tumor characteristics, including presence and type of neoadjuvant therapy, highest alpha-fetoprotein (AFP) level, and sum of nodule diameters on imaging; and posttransplant characteristics (explant), including number of nodules and sum of nodule diameters, cases within the Milan criteria or University of California San Francisco (UCSF) criteria, tumor grade/differentiation, presence of total necrosis, and microvascular invasion.
The outcomes analyzed were tumor recurrence, recurrence-free survival, and overall mortality.
In Brazil, patients with liver cirrhosis and imaging findings suggestive of HCC[21,22] can be placed on the LT waiting list upon detection of a lesion ≥ 2 cm and ≤ 5 cm or up to three lesions ≥ 2 cm and ≤ 3 cm.
Patients on the waiting list with an estimated waiting time for LT > 6 mo were treated with transarterial chemoembolization, radiofrequency ablation, or percutaneous ethanol injection.
The statistical methods of this study were reviewed by Mario B. Wagner, MD PhD DLSHTM, Full Professor of Epidemiology and Biostatistics, School of Medicine, Federal University of Rio Grande do Sul, Brazil.
Baseline patient characteristics were described using standard statistical methods. Continuous variables were compared using t-test or Mann-Whitney test when distributional assumptions were in doubt. Categorical variables were compared by the chi-square test or Fisher’s exact test when needed. Propensity score matching (PSM) was used to assess whether tumor recurrence, overall mortality, and recurrence-free survival rates in patients with ICC or HCC-CC differed from those in patients with HCC. Additionally, hazard ratios (HRs) and their confidence intervals (CIs) were calculated. Progression-free survival rate and overall mortality rate were computed with the Kaplan-Meier method using Cox regression for comparison.
Patients with ICC and HCC-CC were matched to those with HCC using PSM based on the nearest neighbor algorithm according to a 1:8 ratio. Considering pretransplant and posttransplant variables, two matching sequences were run for patients with ICC and another two sequences for those with HCC-CC, which resulted in four matching datasets.
The variables considered for the pretransplant matching were highest AFP level, largest nodule diameter or the sum of the largest diameters in the case of multiple lesions, and year of LT. The posttransplant matching was based on variables collected during explant pathology which included tumor grade/differentiation, microvascular invasion, largest nodule diameter or the sum of the largest diameters in the case of multiple lesions, and year of LT.
Simple Cox regression was applied to the four datasets (pretransplant variable-matched sets ICC vs HCC and HCC-CC vs HCC, and posttransplant variable-matched sets ICC vs HCC and HCC-CC vs HCC) to obtain HRs and 95%CIs.
PSM groups were defined using R version 4.0 and the package MatchIT (software package MatchIT in R version 4.0.4; https://www.r-project.org/). Other analyses were conducted with IBM-SPSS version 25. P values < 0.05 were considered statistically significant.
The study followed the guidelines for the publication of observational studies[23]. The Institutional Review Board of Santa Casa de Misericórdia de Porto Alegre approved the study protocol (No. 4.250.889). Informed consent was waived due to the non-interventional design of the study and retrospective nature of data collection. All investigators signed a data use agreement to ensure the ethical and secure use of the data.
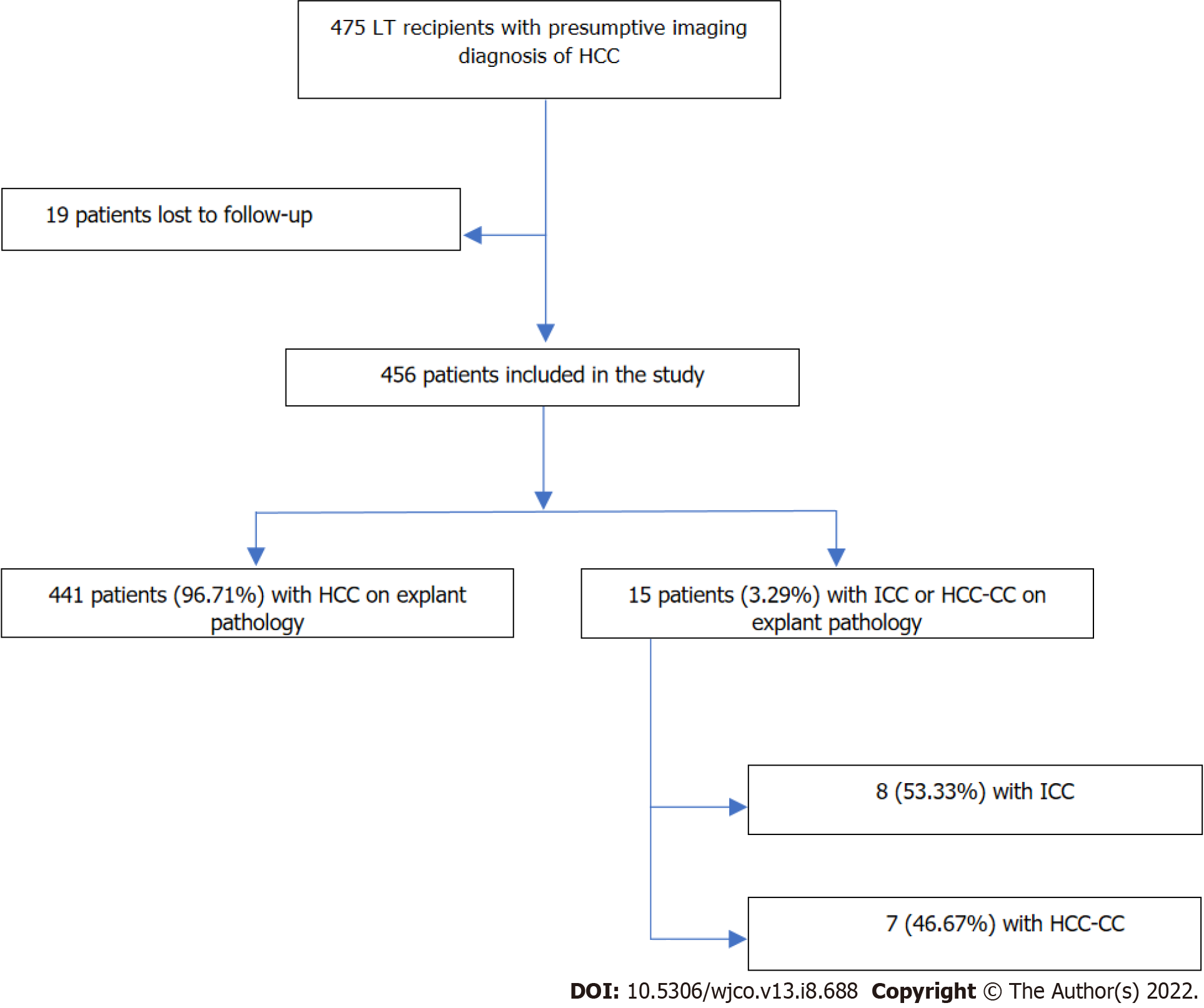
Over a period of 22 years, 475 patients with the presumptive diagnosis of HCC underwent LT at our center. According to a retrospective review of the LT database, 15 of these patients (3.1%) were found to have either ICC (n = 8) or HCC-CC (n = 7) detected in the pathological examination of the explant. The remaining 460 patients had the diagnosis of HCC confirmed by explant pathology (Figure 1). Most ICCs (6/8; 75.0%) were moderately or poorly differentiated and had the largest nodule diameter or the sum of the largest diameters < 5 cm. The patients with HCC-CC (7/7; 100%) were also moderately or poorly differentiated. In most HCC-CC cases (5/7; 71.4%), the largest nodule diameter or the sum of the largest diameters did not exceed 5 cm.
Table 1 shows the comparison of patients with ICC (n = 8) matched 1:8 to those with HCC (n = 64) who underwent LT in the same year and had similar pretransplant tumor characteristics (median highest AFP level and cumulative radiologic tumor diameter). Demographic characteristics and mean age did not differ significantly between the two groups: most patients were men and the most common etiology of liver cirrhosis was HCV infection. The median highest AFP level of patients with ICC was higher than that of patients with HCC, although without statistical significance. Patients with ICC more commonly received bridging therapy for transplant (100% vs 67.2%; P = 0.036), but they were less responsive than patients with HCC (total necrosis: 12.5% vs 58.1%; P = 0.008). Also, according to explant pathology, patients with ICC had less differentiated tumors (grade 2 + 3: 75% vs 56.2%; P = 0.022) and higher rates of microvascular invasion (37.5% vs 9.4%; P = 0.056) (Table 1).
| Variable | Pre-LT factors | Explant factors | |||
| ICC (n = 8) | HCC (n = 64) | P value | HCC (n = 64) | P value | |
| Recipient characteristics | |||||
| Age, mean ± SD | 59.4 ± 7.6 | 61.5 ± 8.0 | 0.489 | 60.3 ± 8.6 | 0.7742 |
| Male, n (%) | 5 (62.5) | 44 (68.8) | 0.704 | 50 (78.1) | 0.3821 |
| Etiology of liver disease, n (%) | 0.201 | 0.7451 | |||
| HCV | 6 (75.0) | 50 (78.1) | 45 (70.3) | ||
| Alcohol | 0 | 9 (14.1) | 10 (15.6) | ||
| HBV | 0 | 1 (1.6) | 3 (4.7) | ||
| NAFLD | 1 (12.5) | 2 (3.1) | 1 (1.6) | ||
| Cryptogenic | 1 (12.5) | 1 (1.6) | 2 (3.1) | ||
| Other | 0 | 1 (1.6) | 3 (4.7) | ||
| CTP class, n (%) | 0.168 | 0.2101 | |||
| A | 7 (87.5) | 43 (67.2) | 40 (63.5) | ||
| B | 0 | 17 (26.6) | 19 (30.2) | ||
| C | 1 (12.5) | 4 (6.3) | 4 (6.3) | ||
| Maximum pretransplant AFP, ng/mL | 28.5 (1.60-801.0) | 10.8 (1.7-1133.0) | 0.324 | 12.5 (1.3-6123.0) | 0.6203 |
| Radiographic tumor characteristics | |||||
| Cumulative tumor diameter, cm, n (%) | 0.072 | 0.8621 | |||
| < 2.1 | 1 (12.5) | 21 (32.8) | 9 (14.1) | ||
| 2.2-5.0 | 4 (50.0) | 37 (57.8) | 39 (60.9) | ||
| > 5.1 | 3 (37.5) | 6 (9.4) | 16 (25.0) | ||
| Neoadjuvant therapy, n (%) | 0.036 | 0.0161 | |||
| None | 0 | 21 (32.8) | 21 (32.8) | ||
| TACE | 8 (100.0) | 32 (50.0) | 29 (45.3) | ||
| Other | 0 | 11 (17.2) | 14 (21.9) | ||
| Pathologic tumor characteristics, n (%) | |||||
| Total necrosis among treated patients, n/total n (%) | 1/8 (12.5) | 25/43 (58.1) | 0.008 | 7/43 (16.3) | 0.7411 |
| Within Milan criteria | 3 (37.5) | 52 (81.3) | 0.015 | 35 (54.7) | 0.4631 |
| Within UCSF criteria | 6 (75.0) | 56 (87.5) | 0.307 | 46 (71.9) | > 0.9991 |
| Median cumulative nodule size | 0.072 | 0.8621 | |||
| < 2.1 | 1 (12.5) | 21 (32.8) | 9 (14.1) | ||
| 2.2-5.0 | 4 (50.0) | 37 (57.8) | 39 (60.9) | ||
| > 5.1 | 3 (37.5) | 6 (9.4) | 16 (25.0) | ||
| Tumor grade, n/total n (%) | 0.225 | 0.2141 | |||
| 1 | 2 (25.0) | 28 (43.8) | 4 (6.3) | ||
| 2 | 4 (50.0) | 31 (48.4) | 40 (62.5) | ||
| 3 | 2 (25.0) | 5 (7.8) | 20 (31.3) | ||
| Microvascular invasion | 3 (37.5) | 6 (9.4) | 0.056 | 20 (31.3) | 0.7411 |
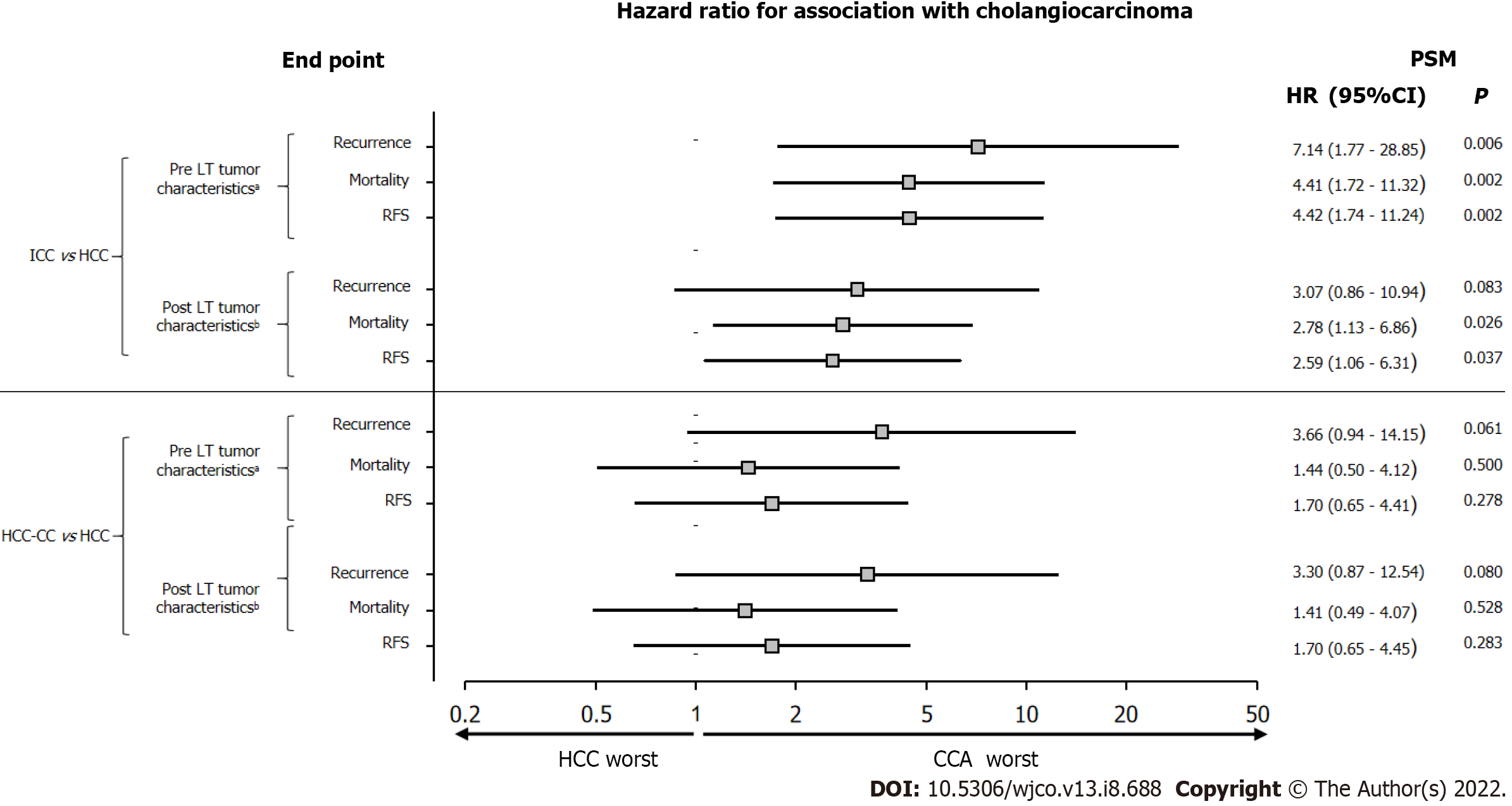
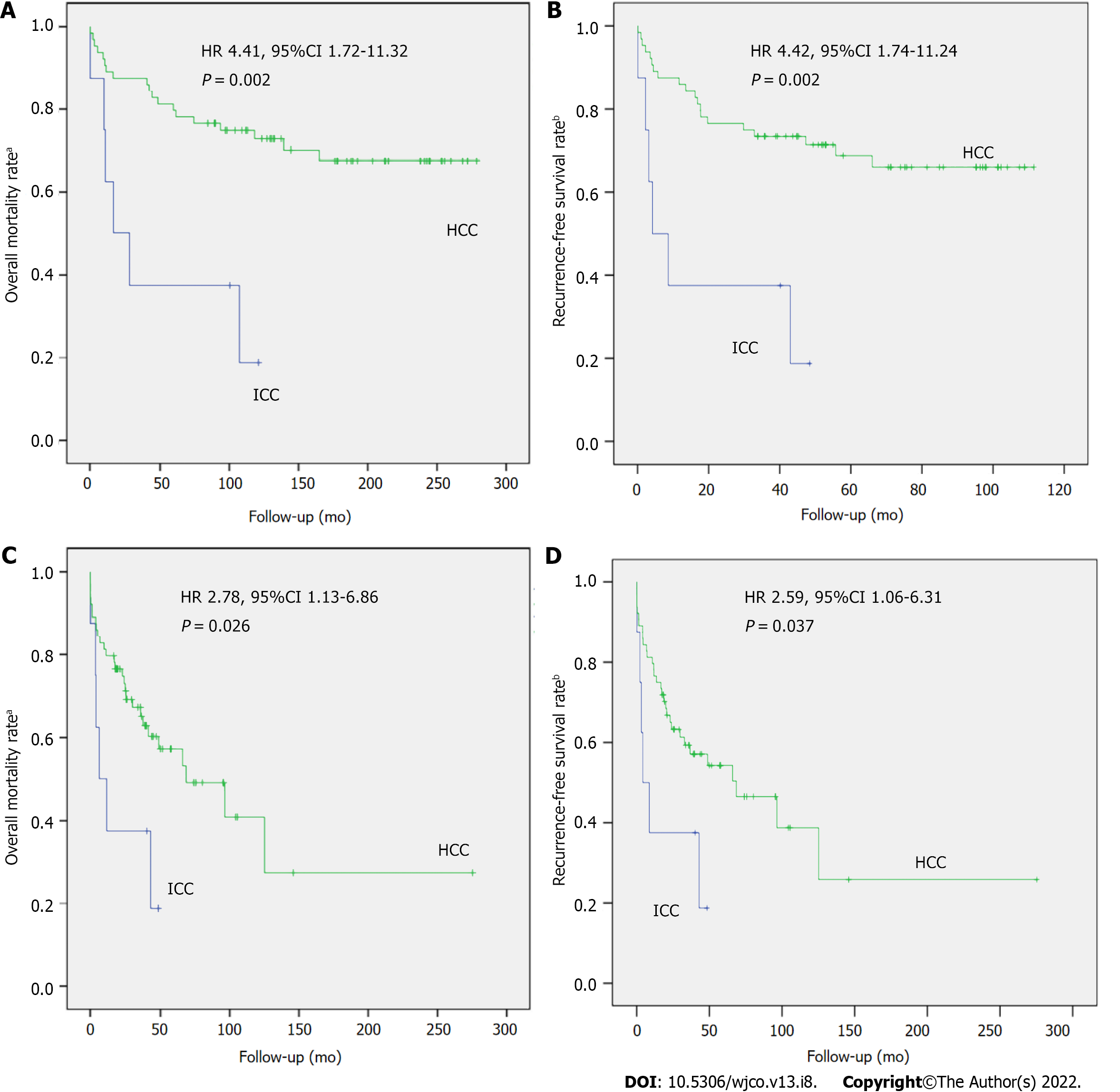
Figure 2 shows the risk of tumor recurrence, overall mortality, and recurrence-free survival. When comparing these risks between patients with ICC and HCC matched for pretransplant tumor characteristics, estimated by the simple Cox regression model, patients with ICC had a higher 3-year risk of recurrence (46% vs 11%; HR 7.14 [95%CI, 1.77-28.85]; P = 0.006) and overall mortality (63% vs 23%; HR 4.41 [95%CI, 1.72-11.32]; P = 0.002) and a lower recurrence-free survival rate (38% vs 77%; HR 4.42 [95%CI, 1.74-11.24]; P = 0.002).
Given the poorer outcomes of LT recipients with ICC and pretransplant tumor characteristics like those of LT recipients with HCC, we sought to assess whether these results would be explained by the potentially more aggressive nature of ICC. To this end, an additional PSM was performed by pairing patients with ICC and HCC with similar explant pathology (median cumulative tumor diameter, nuclear grade/differentiation, and microvascular invasion), but the groups did not differ significantly in these variables (Table 1). Compared with patients with HCC, those with ICC had a higher 3-year cumulative risk of tumor recurrence (46% vs 23%; HR 3.07 [95%CI, 0.86-10.94]; P = 0.083) and overall mortality (63% vs 35%; HR 2.78 [95%CI, 1.13-6.86]; P = 0.026) and a lower recurrence-free survival rate (38% vs 65%; HR 2.59 [95%CI, 1.06-6.31]; P = 0.037) (Figure 2).
Compared with HCC transplant recipients with similar pretransplant characteristics, patients with ICC had significantly higher 1- and 5-year overall mortality (62.5% and 81.2% vs 12.5% and 29.8%; P = 0.002) and lower 1- and 5-year RFS (37.5% and 18.8% vs 87.5% and 70.2%; P = -0.002). Compared with those with similar posttransplant characteristics (explant pathologic features), patients with ICC had significantly higher 1- and 5- year mortality (20.3% and 42.8% vs 12.5% and 29.8%; P = 0.002) and lower 1- and 5-year RFS (79.7% and 57.2% vs 87.5% and 70.2%; P = 0.002) (Figure 3).
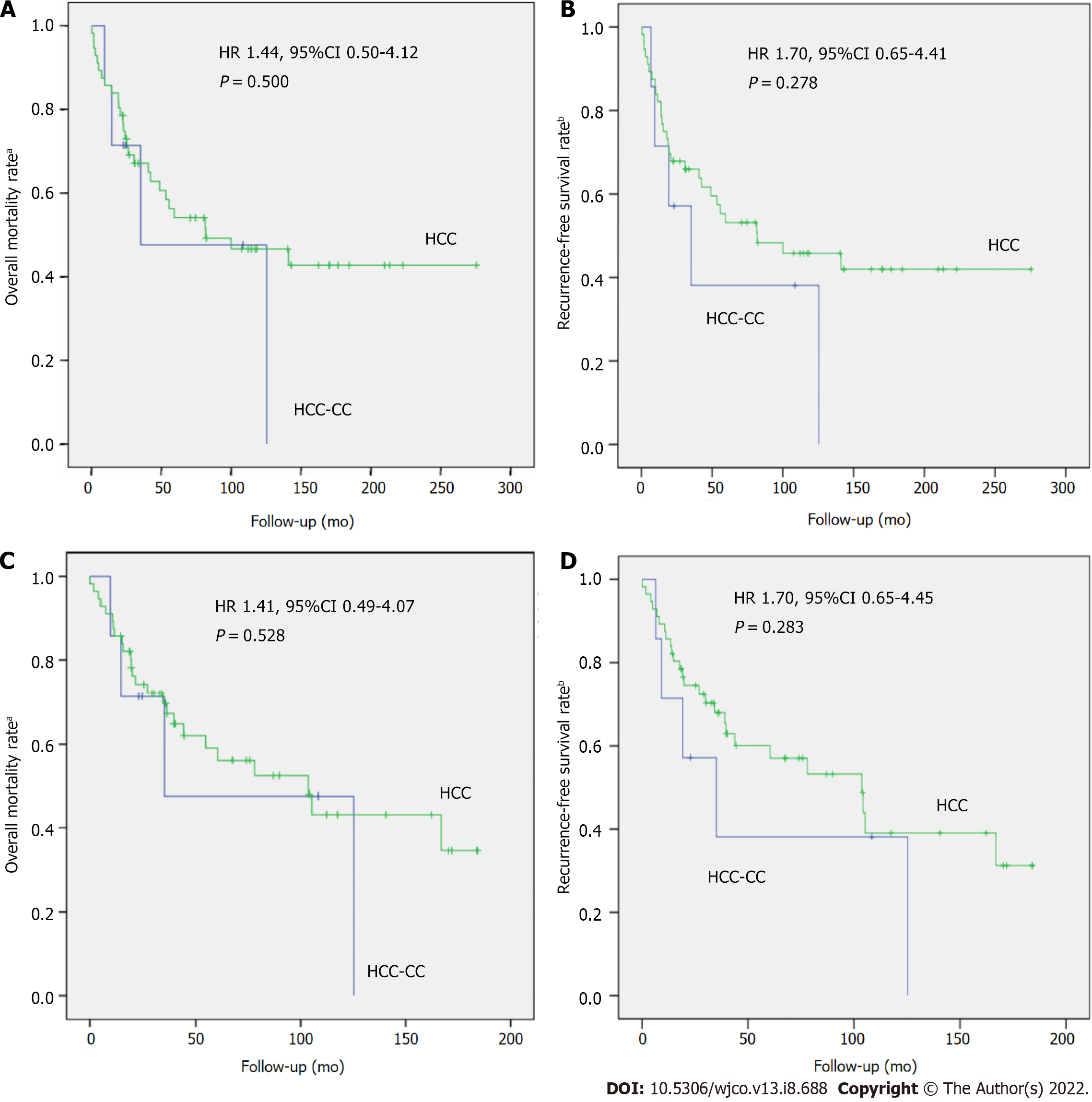
Two pairings were also performed, in a 1:8 ratio, between patients with HCC-CC (n = 7) and HCC (n = 56) who underwent LT in the same year. The first pairing considered similar pretransplant tumor characteristics (imaging findings and highest AFP level), whereas the second pairing considered similar explant pathology. There was no statistically significant difference between the two groups (Table 2). Most patients were men, and HCV infection was the most common etiology of liver cirrhosis. Also, there was no statistically significant difference between recurrence, overall mortality, or recurrence-free survival rates in either pairing (by pretransplant or posttransplant tumor characteristics) (Figure 2).
| Variable | Pre-LT factors | Explant factors | |||
| HCC-CC (n = 7) | HCC (n = 56) | P value | HCC (n = 56) | P value | |
| Recipient characteristics | |||||
| Age, mean ± SD | 58.0 ± 6.9 | 60.3 ± 9.2 | 0.317 | 60.8 ± 7.1 | 0.2892 |
| Male, n (%) | 4 (57.1) | 41 (73.2) | 0.397 | 4 (57.1) | 0.3751 |
| Etiology of liver disease, n (%) | 0.192 | 0.7891 | |||
| HCV | 6 (85.7) | 42 (75.0) | 41 (73.2) | ||
| Alcohol | 0 | 7 (12.5) | 3 (5.4) | ||
| HBV | 0 | 5 (8.9) | 5 (8.9) | ||
| NAFLD | 1 (14.3) | 0 | 3 (5.4) | ||
| Cryptogenic | 0 | 2 (3.6) | 4 (7.1) | ||
| CTP class, n (%) | 0.201 | 0.5561 | |||
| A | 3 (42.9) | 21 (37.5) | 29 (51.8) | ||
| B | 2 (28.6) | 30 (53.6) | 20 (35.7) | ||
| C | 2 (28.6) | 5 (8.9) | 7 (12.5) | ||
| Maximum pretransplant AFP, ng/mL | 35.3(4.3-357.0) | 9.6(1.1-628.0) | 0.150 | 16.5(1.1-6123.0) | 0.6683 |
| Radiographic tumor characteristics | |||||
| Cumulative tumor diameter, cm, n (%) | 0.224 | 0.723t | |||
| < 2.1 | 1 (14.3) | 25 (44.6) | 11 (19.6) | ||
| 2.2-5.0 | 4 (57.1) | 23 (41.1) | 36 (64.3) | ||
| 5.1 | 2 (28.6) | 8 (14.3) | 9 (16.1) | ||
| Neoadjuvant therapy, n (%) | 0.085 | 0.0811 | |||
| None | 0 | 14 (25.0) | 12 (21.4) | ||
| TACE | 3 (42.9) | 22 (39.3) | 28 (50.0) | ||
| Other | 4 (57.1) | 20 (35.7) | 16 (28.6) | ||
| Pathologic tumor characteristics, n (%) | |||||
| Total necrosis among treated patients, n/total n (%) | 3/7 (33.3) | 20/42 (47.6) | 0.8000 | 18/44 (40.1) | 0.2231 |
| Within Milan criteria | 4 (57.1) | 39 (69.6) | 0.669 | 35 (62.5) | > 0.9991 |
| Within UCSF criteria | 6 (85.7) | 49 (87.5) | > 0.999 | 45 (80.4) | > 0.9991 |
| Median cumulative nodule size | 0.224 | 0.7231 | |||
| < 2.1 | 1 (14.3) | 25 (44.6) | 11 (19.6) | ||
| 2.2-5.0 | 4 (57.1) | 23 (41.1) | 36 (64.3) | ||
| > 5.1 | 2 (28.6) | 8 (14.3) | 9 (16.1) | ||
| Tumor grade, n/total n (%) | 0.722 | 0.2331 | |||
| 1 | 0 | 1/36 (2.8) | 0 | ||
| 2 | 4/7 (57.1) | 24/36 (66.7) | 18/55 (32.7) | ||
| 3 | 3/7 (42.9) | 11/36 (30.6) | 37/55 (67.3) | ||
| Microvascular invasion | 0 | 9 (16.1) | 0.580 | 0 | 0 |
Compared with HCC transplant recipients with similar pretransplant characteristics, patients with HCC-CC showed no significant differences in 1- and 5-year overall mortality (14.3% and 52.4% vs 14.3% and 45.9%; P = 0.500) and RFS (85.7% and 47.6% vs 85.7% and 54.1%; P = 0.278). Compared with those with similar posttransplant characteristics, patients with HCC-CC also showed no statistical differences in 1- and 5-year overall mortality (14.3% and 40.9% vs 14.3% and 45;9%; P = 0.528) and 1- and 5-year RFS (85.7% and 59.1% vs 85.7% and 54,1%; P = 0.283) (Figure 4).
The present study described the experience of a Brazilian LT center with the outcomes of LT recipients with ICC or HCC-CC who had a pretransplant radiological diagnosis of HCC. Over a 22-year period, the rate of incorrect diagnosis of ICC or HCC-CC and unintentional LT was 3.1%, similar to that identified in a single-center Spanish study analyzing a 10-year period[24].
In order to assess outcomes of these entities (ICC or HCC-CC) after LT, we compared the outcomes of patients who had ICC or HCC-CC with the outcomes of patients transplanted for HCC. At first, we matched LT recipients with ICC and LT recipients with HCC for pretransplant tumor characteristics. Patients with ICC were more likely to have poorer tumor differentiation and higher microvascular invasion rates on explant pathology. To estimate the risk of recurrence, overall mortality, and recurrence-free survival in both groups, we used PSM followed by simple Cox regression. This comparative, propensity-matched analysis showed a higher risk of poorer outcomes after LT for ICC than HCC when patients were matched for pretransplant tumor characteristics. A previous study reported that worse tumor differentiation and presence of microvascular invasion are risk factors for recurrence in LT recipients with ICC[25]. Therefore, in order to assess the role of the potentially more aggressive nature of ICC, we matched patients with ICC and patients with HCC for explant pathology, which included nuclear differentiation, microvascular invasion, and cumulative tumor diameter, and repeated the same statistical analyses. Again, patients with ICC had worse outcomes (tumor recurrence, overall mortality, and recurrence-free survival) than those with HCC. That is, ICC was associated with worse outcomes even when high-risk factors for tumor recurrence were considered, indicating that ICC is an inherently more aggressive tumor whose risk factors for recurrence differ from those traditionally described for HCC. To our knowledge, this is the first time that posttransplant outcomes of patients with ICC and HCC have been comparatively evaluated by matching patients for explant pathology.
LT has been contraindicated in patients with ICC due to poor results[26-28]. The possibility of successfully transplanting patients with ICC began to change as it became clear that better patient selection was likely to impact posttransplant outcomes. Satisfactory results have been recently reported in LT of cirrhotic patients with grafts showing incidental ICC on explant pathology. Retrospective data from these patients demonstrated suitable 5-year overall and recurrence-free survival in patients with “very early” ICC (≤ 2 cm)[18,25,29]. A Japanese study found that patients with and without cirrhosis who underwent liver resection for ICC ≤ 2 cm reached a 100% 5-year survival rate. The authors identified 2 cm as a good cutoff point when selecting patients for hepatectomy[30]. Recently, French researchers suggested that this ≤ 2 cm limit could be expanded by showing, in a retrospective multicenter study analyzing posttransplant outcomes of cirrhotic patients with incidental ICC detected on the pathological examination of the explant, that patients with clearly differentiated ICC up to 3 cm had similar survival to patients with tumors ≤ 2 cm. In this study, the only independent variable associated with tumor recurrence was its differentiation[31]. Prospective multicenter clinical trials are needed to confirm these results. The 2 cm cutoff point seems safe but limited because preoperative radiological diagnosis of these small tumors is challenging[15,16] and ICC features are still often underestimated during pre-LT diagnostic evaluation. Nevertheless, studies indirectly state that ICC is a more aggressive tumor by suggesting that LT should only be an option for patients with tumors ≤ 2 cm. This differs from the indication for LT in patients with HCC, who can undergo LT with tumors up to 5 cm in diameter, with acceptable recurrence rates[32]. It is important to note that, in our series, all patients with liver cirrhosis had ICCs > 2 cm. In order to expand the indication criteria for LT in patients with liver cirrhosis and unresectable ICC, the effectiveness of pretransplant neoadjuvant chemotherapy is being evaluated[33]. The International Liver Transplantation Society (ILTS) recommends resection as the treatment of choice for patients with ICC. When the procedure is contraindicated, LT may be considered when the tumor is ≤ 2 cm; if the tumor is > 2 cm, LT may be performed under strict clinical protocols and only when the disease remains stable after neoadjuvant therapy[34].
We performed the same comparisons, using pretransplant and posttransplant tumor characteristics, for LT recipients with HCC-CC vs HCC, but no statistically significant differences were observed between the two groups. The statistical analyses (PSM and simple Cox regression) yielded similar risks for tumor recurrence, overall mortality, and recurrence-free survival when patients were matched for pretransplant or posttransplant tumor characteristics. As observed in ICC, patients with HCC-CC also had a worse prognosis than those with HCC, but the differences were smaller than those found for ICC vs HCC; consequently, in most outcomes, the differences did not reach statistical significance. This may suggest that LT recipients with ICC or HCC-CC have worse outcomes than those with HCC, but ICC appears to be more aggressive. Lunsford et al[35] analyzed posttransplant outcomes of 12 patients with HCC-CC vs 36 patients with HCC matched for the pretransplant and posttransplant variables reproduced in the present study. When patients were matched for explant pathology, those with HCC-CC had a slightly higher recurrence rate, without statistical significance, whereas recurrence-free survival and overall survival rates were equivalent to those of LT recipients with HCC[35]. Other authors also consider that a diagnosis of HCC-CC should not be an impediment to LT in well-selected cases[24,36,37]. However, for patients with HCC-CC, the ILTS expert panel believes that this tumor is not an established indication for LT due to the limited worldwide experience, and prognostic factors need to be identified to improve patient selection and to obtain better results with the procedure[30].
Transplant oncology is a new concept encompassing multiple disciplines of transplantation medicine and oncology (transplant oncologists, hepatologists, gastroenterologists, transplant hepatobiliary surgeons, interventional radiologists, and immunologists) designed to push the envelope of the treatment and research of hepatobiliary cancers[38,39]. This field will certainly improve treatments and cure rates for patients with HCC, ICC, or HCC-CC, as well as other cancer types.
This study has limitations that need to be addressed. First, it is a retrospective study conducted at a single center with a limited number of cases. However, given the rarity of these tumors, most studies are retrospective and have also included a small number of patients, which makes it difficult to perform statistical analyses that can identify factors potentially associated with the outcomes[40]. Furthermore, because LT is a current contraindication for patients with ICC or HCC-CC, the diagnosis was made on explant. Finally, the study included patients receiving care over a long period of time. To minimize any bias that may have resulted from advances in research, management, and treatment during the study period, patients were also matched for year of transplant.
In this series, LT for ICC (all excepted one were larger than 2 cm) was associated with worse outcomes compared with LT for HCC, even when patients were matched for explant pathology. However, the outcomes after LT for mixed HCC-CC, despite being worse than those of LT recipients with HCC, did not reach statistical significance. Improvement in the detection of these rare tumors during pretransplant evaluation is essential for the eventual adoption of LT as an effective treatment for these patients.
Cholangiocarcinoma (CC) is a rare tumor that arises from the epithelium of the bile ducts. It is classified according to anatomic location as intrahepatic, perihilar, or distal. Intrahepatic cholangiocarcinoma (ICC) is rare in patients with cirrhosis due to causes other than primary sclerosing cholangitis. Mixed hepatocellular-cholangiocarcinoma (HCC-CC) is a rare neoplasm with histologic findings of both hepatocellular carcinoma (HCC) and ICC within the same tumor mass.
Because of difficulties in reaching the correct diagnosis, patients eventually undergo liver transplantation (LT) with a presumptive diagnosis of HCC on imaging when, in fact, they have ICC or HCC-CC.
To determine the prevalence of ICC or HCC-CC confirmed by explant pathology in patients who underwent LT with the presumptive diagnosis of HCC and to compare tumor recurrence (TR), recurrence-free survival (RFS), and overall mortality (OM) rates between these patients and LT recipients with HCC.
This retrospective cohort study included patients aged ≥ 18 years with liver cirrhosis and imaging findings suggestive of HCC within the Milan criteria who underwent LT between June 1997 and July 2019. Patients were divided into three groups according to the diagnosis on explant pathology: (1) Patients with HCC; (2) Patients with ICC; and (3) Patients with mixed HCC-CC. The analyzed outcomes were TR, RFS, and OM. Propensity score matching was used to assess whether TR, OM, and RFS rates in patients with ICC or HCC-CC differed from those in patients with HCC. Additionally, hazard ratios (HRs) and their confidence intervals were calculated. Progression-free survival and OM rates were computed with the Kaplan-Meier method using Cox regression for comparison.
Over a 22-year period, 475 patients with the presumptive diagnosis of HCC underwent LT, and 15 (3.1%) were found to have either ICC (n = 8) or HCC-CC (n = 7) detected in the pathological examination of the explant. LT recipients with ICC had higher TR (46% vs 11%; P = 0.006), higher OM (63% vs 23%; P = 0.002), and lower RFS (38% vs 89%; P = 0.002) than those with HCC when matched for pretransplant tumor characteristics, as well as higher TR (46% vs 23%; P = 0.083), higher OM (63% vs 35%; P = 0.026), and lower RFS (38% vs 59%; P = 0.037) when matched for posttransplant tumor characteristics. Two pairings were performed to compare the outcomes of LT recipients with HCC-CC vs HCC. There was no significant difference between the outcomes in either pairing.
Patients with ICC had worse outcomes than patients with HCC undergoing LT. Preoperative diagnosis of HCC-CC should not prompt the exclusion of these patients from transplant options.
This study reinforces the need for more accurate criteria: (1) To identify these rare tumors in pretransplant evaluation; and (2) To select patients who may benefit from LT.
To the Liver Transplantation Group at Santa Casa de Misericórdia de Porto Alegre, RS, Brazil (Guido Cantisani Team), and to the Hospital Vozandes Quito-HVQ AS, Quito, Ecuador.
Provenance and peer review: Unsolicited article; Externally peer reviewed.
Peer-review model: Single blind
Specialty type: Oncology
Country/Territory of origin: Brazil
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): B
Grade C (Good): C, C, C, C
Grade D (Fair): D
Grade E (Poor): 0
P-Reviewer: Bellini MI, Italy; Imai Y, Japan; Kao JT, Taiwan; Nakano M, Japan; Park M, South Korea S-Editor: Wang LL L-Editor: A P-Editor: Qi WW
| 1. | Razumilava N, Gores GJ. Cholangiocarcinoma. Lancet. 2014;383:2168-2179. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1072] [Cited by in RCA: 1381] [Article Influence: 125.5] [Reference Citation Analysis (1)] |
| 2. | Tyson GL, El-Serag HB. Risk factors for cholangiocarcinoma. Hepatology. 2011;54:173-184. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 722] [Cited by in RCA: 692] [Article Influence: 49.4] [Reference Citation Analysis (0)] |
| 3. | Rizvi S, Khan SA, Hallemeier CL, Kelley RK, Gores GJ. Cholangiocarcinoma - evolving concepts and therapeutic strategies. Nat Rev Clin Oncol. 2018;15:95-111. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1052] [Cited by in RCA: 1141] [Article Influence: 163.0] [Reference Citation Analysis (0)] |
| 4. | Esnaola NF, Meyer JE, Karachristos A, Maranki JL, Camp ER, Denlinger CS. Evaluation and management of intrahepatic and extrahepatic cholangiocarcinoma. Cancer. 2016;122:1349-1369. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 135] [Cited by in RCA: 208] [Article Influence: 23.1] [Reference Citation Analysis (0)] |
| 5. | Petrick JL, Yang B, Altekruse SF, Van Dyke AL, Koshiol J, Graubard BI, McGlynn KA. Risk factors for intrahepatic and extrahepatic cholangiocarcinoma in the United States: A population-based study in SEER-Medicare. PLoS One. 2017;12:e0186643. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 87] [Cited by in RCA: 144] [Article Influence: 18.0] [Reference Citation Analysis (0)] |
| 6. | Patel T. Increasing incidence and mortality of primary intrahepatic cholangiocarcinoma in the United States. Hepatology. 2001;33:1353-1357. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 773] [Cited by in RCA: 797] [Article Influence: 33.2] [Reference Citation Analysis (0)] |
| 7. | Khan SA, Emadossadaty S, Ladep NG, Thomas HC, Elliott P, Taylor-Robinson SD, Toledano MB. Rising trends in cholangiocarcinoma: is the ICD classification system misleading us? J Hepatol. 2012;56:848-854. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 202] [Cited by in RCA: 223] [Article Influence: 17.2] [Reference Citation Analysis (0)] |
| 8. | Gad MM, Saad AM, Faisaluddin M, Gaman MA, Ruhban IA, Jazieh KA, Al-Husseini MJ, Simons-Linares CR, Sonbol MB, Estfan BN. Epidemiology of Cholangiocarcinoma; United States Incidence and Mortality Trends. Clin Res Hepatol Gastroenterol. 2020;44:885-893. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 70] [Cited by in RCA: 64] [Article Influence: 12.8] [Reference Citation Analysis (0)] |
| 9. | Bertuccio P, Malvezzi M, Carioli G, Hashim D, Boffetta P, El-Serag HB, La Vecchia C, Negri E. Global trends in mortality from intrahepatic and extrahepatic cholangiocarcinoma. J Hepatol. 2019;71:104-114. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 210] [Cited by in RCA: 425] [Article Influence: 70.8] [Reference Citation Analysis (0)] |
| 10. | Gupta A, Dixon E. Epidemiology and risk factors: intrahepatic cholangiocarcinoma. Hepatobiliary Surg Nutr. 2017;6:101-104. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 79] [Cited by in RCA: 133] [Article Influence: 16.6] [Reference Citation Analysis (0)] |
| 11. | Torbenson MS. Morphologic Subtypes of Hepatocellular Carcinoma. Gastroenterol Clin North Am. 2017;46:365-391. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 70] [Cited by in RCA: 89] [Article Influence: 11.1] [Reference Citation Analysis (1)] |
| 12. | Yeh MM. Pathology of combined hepatocellular-cholangiocarcinoma. J Gastroenterol Hepatol. 2010;25:1485-1492. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 90] [Cited by in RCA: 83] [Article Influence: 5.5] [Reference Citation Analysis (0)] |
| 13. | Wang Z, Sheng YY, Dong QZ, Qin LX. Hepatitis B virus and hepatitis C virus play different prognostic roles in intrahepatic cholangiocarcinoma: A meta-analysis. World J Gastroenterol. 2016;22:3038-3051. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 24] [Cited by in RCA: 28] [Article Influence: 3.1] [Reference Citation Analysis (0)] |
| 14. | Bergquist JR, Groeschl RT, Ivanics T, Shubert CR, Habermann EB, Kendrick ML, Farnell MB, Nagorney DM, Truty MJ, Smoot RL. Mixed hepatocellular and cholangiocarcinoma: a rare tumor with a mix of parent phenotypic characteristics. HPB (Oxford). 2016;18:886-892. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 34] [Cited by in RCA: 38] [Article Influence: 4.2] [Reference Citation Analysis (0)] |
| 15. | Rimola J, Forner A, Reig M, Vilana R, de Lope CR, Ayuso C, Bruix J. Cholangiocarcinoma in cirrhosis: absence of contrast washout in delayed phases by magnetic resonance imaging avoids misdiagnosis of hepatocellular carcinoma. Hepatology. 2009;50:791-798. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 216] [Cited by in RCA: 207] [Article Influence: 12.9] [Reference Citation Analysis (0)] |
| 16. | Fowler KJ, Sheybani A, Parker RA 3rd, Doherty S, M Brunt E, Chapman WC, Menias CO. Combined hepatocellular and cholangiocarcinoma (biphenotypic) tumors: imaging features and diagnostic accuracy of contrast-enhanced CT and MRI. AJR Am J Roentgenol. 2013;201:332-339. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 148] [Cited by in RCA: 156] [Article Influence: 13.0] [Reference Citation Analysis (0)] |
| 17. | Jhaveri KS, Hosseini-Nik H. MRI of cholangiocarcinoma. J Magn Reson Imaging. 2015;42:1165-1179. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 52] [Cited by in RCA: 72] [Article Influence: 6.5] [Reference Citation Analysis (0)] |
| 18. | Sapisochin G, de Lope CR, Gastaca M, de Urbina JO, López-Andujar R, Palacios F, Ramos E, Fabregat J, Castroagudín JF, Varo E, Pons JA, Parrilla P, González-Diéguez ML, Rodriguez M, Otero A, Vazquez MA, Zozaya G, Herrero JI, Antolin GS, Perez B, Ciria R, Rufian S, Fundora Y, Ferron JA, Guiberteau A, Blanco G, Varona MA, Barrera MA, Suarez MA, Santoyo J, Bruix J, Charco R. Intrahepatic cholangiocarcinoma or mixed hepatocellular-cholangiocarcinoma in patients undergoing liver transplantation: a Spanish matched cohort multicenter study. Ann Surg. 2014;259:944-952. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 117] [Cited by in RCA: 140] [Article Influence: 12.7] [Reference Citation Analysis (0)] |
| 19. | Chang CC, Chen YJ, Huang TH, Chen CH, Kuo FY, Eng HL, Yong CC, Liu YW, Lin TL, Li WF, Lin YH, Lin CC, Wang CC, Chen CL. Living Donor Liver Transplantation for Combined Hepatocellular Carcinoma and Cholangiocarcinoma: Experience of a Single Center. Ann Transplant. 2017;22:115-120. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 16] [Cited by in RCA: 21] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 20. | Goodman ZD, Ishak KG, Langloss JM, Sesterhenn IA, Rabin L. Combined hepatocellular-cholangiocarcinoma. A histologic and immunohistochemical study. Cancer. 1985;55:124-135. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 12] [Reference Citation Analysis (0)] |
| 21. | Bruix J, Sherman M, Llovet JM, Beaugrand M, Lencioni R, Burroughs AK, Christensen E, Pagliaro L, Colombo M, Rodés J; EASL Panel of Experts on HCC. Clinical management of hepatocellular carcinoma. Conclusions of the Barcelona-2000 EASL conference. European Association for the Study of the Liver. J Hepatol. 2001;35:421-430. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3252] [Cited by in RCA: 3244] [Article Influence: 135.2] [Reference Citation Analysis (0)] |
| 22. | Bruix J, Sherman M; American Association for the Study of Liver Diseases. Management of hepatocellular carcinoma: an update. Hepatology. 2011;53:1020-1022. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 5972] [Cited by in RCA: 6573] [Article Influence: 469.5] [Reference Citation Analysis (1)] |
| 23. | von Elm E, Altman DG, Egger M, Pocock SJ, Gøtzsche PC, Vandenbroucke JP; STROBE Initiative. The Strengthening the Reporting of Observational Studies in Epidemiology (STROBE) statement: guidelines for reporting observational studies. J Clin Epidemiol. 2008;61:344-349. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3667] [Cited by in RCA: 6888] [Article Influence: 626.2] [Reference Citation Analysis (0)] |
| 24. | Sapisochin G, Fidelman N, Roberts JP, Yao FY. Mixed hepatocellular cholangiocarcinoma and intrahepatic cholangiocarcinoma in patients undergoing transplantation for hepatocellular carcinoma. Liver Transpl. 2011;17:934-942. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 125] [Cited by in RCA: 128] [Article Influence: 9.1] [Reference Citation Analysis (0)] |
| 25. | Sapisochin G, Facciuto M, Rubbia-Brandt L, Marti J, Mehta N, Yao FY, Vibert E, Cherqui D, Grant DR, Hernandez-Alejandro R, Dale CH, Cucchetti A, Pinna A, Hwang S, Lee SG, Agopian VG, Busuttil RW, Rizvi S, Heimbach JK, Montenovo M, Reyes J, Cesaretti M, Soubrane O, Reichman T, Seal J, Kim PT, Klintmalm G, Sposito C, Mazzaferro V, Dutkowski P, Clavien PA, Toso C, Majno P, Kneteman N, Saunders C, Bruix J; iCCA International Consortium. Liver transplantation for "very early" intrahepatic cholangiocarcinoma: International retrospective study supporting a prospective assessment. Hepatology. 2016;64:1178-1188. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 197] [Cited by in RCA: 251] [Article Influence: 27.9] [Reference Citation Analysis (0)] |
| 26. | Pinson CW, Moore DE. Liver transplantation is not indicated for cholangiocarcinoma. HPB (Oxford). 2003;5:203-205. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 9] [Cited by in RCA: 8] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 27. | Meyer CG, Penn I, James L. Liver transplantation for cholangiocarcinoma: results in 207 patients. Transplantation. 2000;69:1633-1637. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 368] [Cited by in RCA: 348] [Article Influence: 13.9] [Reference Citation Analysis (0)] |
| 28. | Mahmud N. Selection for Liver Transplantation: Indications and Evaluation. Curr Hepatol Rep. 2020;19:203-212. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 13] [Cited by in RCA: 57] [Article Influence: 11.4] [Reference Citation Analysis (0)] |
| 29. | Sapisochin G, Rodríguez de Lope C, Gastaca M, Ortiz de Urbina J, Suarez MA, Santoyo J, Castroagudín JF, Varo E, López-Andujar R, Palacios F, Sanchez Antolín G, Perez B, Guiberteau A, Blanco G, González-Diéguez ML, Rodriguez M, Varona MA, Barrera MA, Fundora Y, Ferron JA, Ramos E, Fabregat J, Ciria R, Rufian S, Otero A, Vazquez MA, Pons JA, Parrilla P, Zozaya G, Herrero JI, Charco R, Bruix J. "Very early" intrahepatic cholangiocarcinoma in cirrhotic patients: should liver transplantation be reconsidered in these patients? Am J Transplant. 2014;14:660-667. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 113] [Cited by in RCA: 134] [Article Influence: 12.2] [Reference Citation Analysis (0)] |
| 30. | Sakamoto Y, Kokudo N, Matsuyama Y, Sakamoto M, Izumi N, Kadoya M, Kaneko S, Ku Y, Kudo M, Takayama T, Nakashima O; Liver Cancer Study Group of Japan. Proposal of a new staging system for intrahepatic cholangiocarcinoma: Analysis of surgical patients from a nationwide survey of the Liver Cancer Study Group of Japan. Cancer. 2016;122:61-70. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 97] [Cited by in RCA: 130] [Article Influence: 13.0] [Reference Citation Analysis (0)] |
| 31. | De Martin E, Rayar M, Golse N, Dupeux M, Gelli M, Gnemmi V, Allard MA, Cherqui D, Sa Cunha A, Adam R, Coilly A, Antonini TM, Guettier C, Samuel D, Boudjema K, Boleslawski E, Vibert E. Analysis of Liver Resection Versus Liver Transplantation on Outcome of Small Intrahepatic Cholangiocarcinoma and Combined Hepatocellular-Cholangiocarcinoma in the Setting of Cirrhosis. Liver Transpl. 2020;26:785-798. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 40] [Cited by in RCA: 81] [Article Influence: 16.2] [Reference Citation Analysis (0)] |
| 32. | Mazzaferro V, Regalia E, Doci R, Andreola S, Pulvirenti A, Bozzetti F, Montalto F, Ammatuna M, Morabito A, Gennari L. Liver transplantation for the treatment of small hepatocellular carcinomas in patients with cirrhosis. N Engl J Med. 1996;334:693-699. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5110] [Cited by in RCA: 5313] [Article Influence: 183.2] [Reference Citation Analysis (0)] |
| 33. | Lunsford KE, Javle M, Heyne K, Shroff RT, Abdel-Wahab R, Gupta N, Mobley CM, Saharia A, Victor DW, Nguyen DT, Graviss EA, Kaseb AO, McFadden RS, Aloia TA, Conrad C, Li XC, Monsour HP, Gaber AO, Vauthey JN, Ghobrial RM; Methodist–MD Anderson Joint Cholangiocarcinoma Collaborative Committee (MMAJCCC). Liver transplantation for locally advanced intrahepatic cholangiocarcinoma treated with neoadjuvant therapy: a prospective case-series. Lancet Gastroenterol Hepatol. 2018;3:337-348. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 117] [Cited by in RCA: 199] [Article Influence: 28.4] [Reference Citation Analysis (0)] |
| 34. |
Sapisochin G, Javle M, Lenut J, Ohtsuka M, Ghobrial M, Hibi T, et al Liver transplantation for cholangiocarcinoma and mixed hepatocellular carcinoma: working group report from ILTS transplant oncology consensus conference.
|
| 35. | Lunsford KE, Court C, Seok Lee Y, Lu DS, Naini BV, Harlander-Locke MP, Busuttil RW, Agopian VG. Propensity-Matched Analysis of Patients with Mixed Hepatocellular-Cholangiocarcinoma and Hepatocellular Carcinoma Undergoing Liver Transplantation. Liver Transpl. 2018;24:1384-1397. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 28] [Cited by in RCA: 35] [Article Influence: 5.0] [Reference Citation Analysis (0)] |
| 36. | Jaradat D, Bagias G, Lorf T, Tokat Y, Obed A, Oezcelik A. Liver transplantation for combined hepatocellular-cholangiocarcinoma: Outcomes and prognostic factors for mortality. A multicenter analysis. Clin Transplant. 2021;35:e14094. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5] [Cited by in RCA: 6] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 37. | Chen X, Sun S, Lu Y, Shi X, Wang Z, Chen X, Han G, Zhao J, Gao Y, Wang X. Promising role of liver transplantation in patients with combined hepatocellular-cholangiocarcinoma: a propensity score matching analysis. Ann Transl Med. 2022;10:434. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1] [Cited by in RCA: 1] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 38. | Hibi T, Sapisochin G. What is transplant oncology? Surgery. 2019;165:281-285. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 33] [Cited by in RCA: 39] [Article Influence: 5.6] [Reference Citation Analysis (0)] |
| 39. | Abdelrahim M, Esmail A, Abudayyeh A, Murakami N, Saharia A, McMillan R, Victor D, Kodali S, Shetty A, Nolte Fong JV, Moore LW, Heyne K, Gaber AO, Ghobrial RM. Transplant Oncology: An Evolving Field in Cancer Care. Cancers (Basel). 2021;13. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 14] [Cited by in RCA: 42] [Article Influence: 10.5] [Reference Citation Analysis (0)] |
| 40. | Sapisochín G, Fernández de Sevilla E, Echeverri J, Charco R. Liver transplantation for cholangiocarcinoma: Current status and new insights. World J Hepatol. 2015;7:2396-2403. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 34] [Cited by in RCA: 45] [Article Influence: 4.5] [Reference Citation Analysis (0)] |