Published online Jul 24, 2022. doi: 10.5306/wjco.v13.i7.599
Peer-review started: January 11, 2022
First decision: February 15, 2022
Revised: February 27, 2022
Accepted: June 13, 2022
Article in press: June 13, 2022
Published online: July 24, 2022
Processing time: 191 Days and 18.1 Hours
There are currently three coronavirus disease 2019 (COVID-19) vaccines approved by the United States Food and Drug Administration to prevent coronavirus infection. However, robust data are unavailable on the adverse events of the vaccines in patients with solid tumor malignancies undergoing systemic therapies.
To evaluate the safety of COVID-19 vaccines in patients with solid tumors undergoing systemic therapies.
The study included patients with solid tumors treated in an academic tertiary care center who received COVID-19 vaccination between January 1, 2021 and August 15, 2021, while undergoing systemic therapy. Electronic medical records were accessed to collect information on patient characteristics, systemic therapies, type of vaccine received, and adverse effects associated with the vaccine administration. Adverse events (AEs) were graded according to Common Terminology Criteria for Adverse Events, version 5.0.
The analysis included 210 patients; the median age was 70 years, and 51% of patients were female. The most common chemotherapy, immunotherapy, and targeted therapy administered were taxane-based regimens 14.2% (30/210), anti-programmed death 1 (PD-1) agents 22.8% (48/210), and antiangiogenic agents 7.1% (15/210), respectively. The most common cancers were gastrointestinal 43.8% (92/210), thoracic 30.4% (64/210), and genitourinary 17.6% (37/210). Patients received the following vaccines: 2 doses of BNT162b2 by Pfizer 52% (110/210), 2 doses of mRNA-1273 by Moderna 42% (89/210), and 1 dose of JNJ-78436735 by Johnson & Johnson 5% (11/210). At least 1 AE attributable to the vaccine was observed in 37 patients 17.6% (37/210). The total number of AEs attributable to vaccines was 62: Fifty-three grade 1 and nine grade 2. Most adverse events occurred after the second dose 59.7% (37/62). The most frequent grade 1 AEs included fatigue 17% (9/53), fever 15% (8/53), injection site reaction 13.2% (7/53), and chills 9.4% (5/53). The most frequent grade 2 AEs were fatigue 33.3% (3/9) and generalized weakness 22.2% (2/9). Therapy was delayed by 2 wk because of the AEs possibly related to vaccine administration in 3 patients 1.4% (3/210).
The present study demonstrates that the adverse events associated with COVID-19 vaccination are infrequent, mild, and rarely delay treatment in patients with solid tumors receiving systemic therapies.
Core Tip: The current study evaluates the safety and spectrum of adverse events associated with coronavirus disease 2019 (COVID-19) vaccination in solid tumor patients receiving systemic therapy. While COVID-19 vaccination has been shown to be safe and effective in the healthy population, the data confirming the safety of COVID-19 vaccines in cancer patients are sparse. The lack of safety data in cancer patients has caused significant hesitancy to receive COVID-19 vaccination among the patient population with cancer. Our study showed that the administration of COVID-19 vaccines in solid tumor patients receiving systemic therapy is safe and should be encouraged.
- Citation: Cox RE, Parish M, Oxencis C, Mckenna E, Thapa B, Chakrabarti S. Short term safety of coronavirus disease 2019 vaccines in patients with solid tumors receiving systemic therapy. World J Clin Oncol 2022; 13(7): 599-608
- URL: https://www.wjgnet.com/2218-4333/full/v13/i7/599.htm
- DOI: https://dx.doi.org/10.5306/wjco.v13.i7.599
The coronavirus disease 2019 (COVID-19) pandemic, caused by infection with severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2), has profoundly impacted and transformed healthcare systems across the globe. In addition to comprehensive modification in healthcare delivery, patients have encountered immeasurable emotional and socioeconomic hardships[1,2]. SARS-CoV-2 is a novel single-stranded, enveloped RNA virus that primarily spreads via the respiratory route and causes respiratory infection, including pneumonia with or without multiorgan failures[3]. While many patients remain asymptomatic, infection with the SARS-CoV-2 virus has been shown to cause a myriad of symptoms, including severe acute respiratory distress syndrome[1]. Analysis of comprehensive observational data has shown increased mortality, hospitalization, and intensive care admission in cancer patients who received anticancer therapy within 3 mo of infection[4,5]. A study from China reported a 3.5-fold increased risk of respiratory failure requiring mechanical ventilation in cancer patients infected with the SARS-CoV-2 virus[6]. The interplay between COVID-19 infection and cancer is complex, attributable to a wide variety of factors including immunosuppression, co-morbidities, aging, and the biology of the cancer itself[7].
The United States Food and Drug Administration approved three COVID-19 vaccines to prevent coronavirus infection. These include the BNT162b2 from Pfizer, mRNA-1273 from Moderna, and JNJ-78436735 vaccine from Johnson & Johnson. Patients with cancer should be considered a high-priority group for COVID-19 vaccination due to their higher risk of morbidity and death associated with COVID-19 disease[5,6,8-10]. However, the trials reporting efficacy and safety of COVID-19 vaccines were conducted in healthy volunteers, excluding the immunocompromised cancer patients on treatment[11-13]. Although several cancer societies recommend COVID-19 vaccination in patients with cancer, the data confirming the safety of vaccines are sparse[14,15]. This lack of rigorous scientific inquiry into vaccine safety has led to increased apprehension and hesitation to receive vaccination in the patient population with cancer. As the incidence of cancer continues to rise, solid tumor malignancies continue to emerge among the most prevalent diagnoses. Frequently used therapeutic regimens include chemotherapy, immunotherapy, and targeted therapy. We conducted a study to assess the safety and determine the spectrum of adverse events (AEs) associated with COVID-19 vaccination in patients with solid tumors receiving systemic therapy.
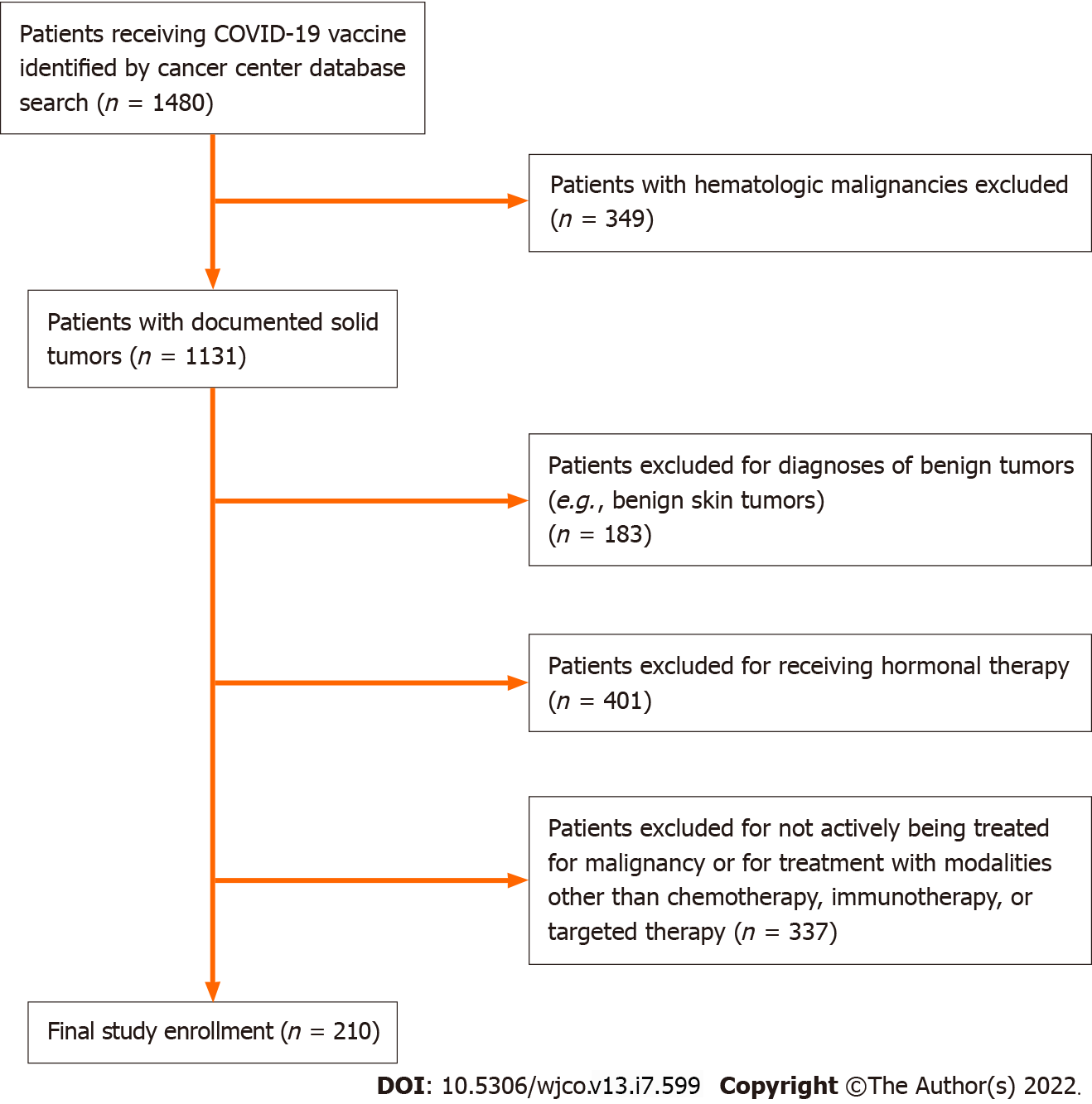
The aim of this study was to determine the real-world incidence and spectrum of AEs in patients with solid tumor malignancies receiving systemic therapy. This was a retrospective study of cancer patients who received COVID-19 vaccination between January 1, 2021 and August 15, 2021 at Froedtert and the Medical College of Wisconsin Cancer Center (Milwaukee, WI, United States of America). Inclusion criteria required that patients be at least 18 years of age at the time of inoculation and have a histologically confirmed solid tumor diagnosis for which they were receiving systemic therapy (chemotherapy, immunotherapy, or targeted therapy). Patients were excluded from the study if they had an active hematologic malignancy, were being treated with hormonal therapy, or had a benign tumor diagnosis that did not require anti-neoplastic treatment. Patients for this study were identified from the cancer center database using a tool available in the electronic medical record software (EPIC SlicerDicer tool). The initial screen identified 1480 cancer patients who received COVID-19 vaccines. Of these, 349 were omitted due to an active hematologic malignancy, and 183 patients were excluded due to diagnoses of benign solid tumors. An additional 401 patients who were receiving hormonal therapies (i.e., leuprolide for prostate cancer or tamoxifen/anastrozole for breast cancer) were excluded. Finally, 337 patients were excluded who were not receiving active treatment for malignancies (e.g., patients on surveillance following completion of their initial treatment) or active malignancies being treated with modalities other than chemotherapy, immunotherapy, or targeted therapies (e.g., radiation therapy). After review, 210 patients were found to meet the study requirements (Figure 1). Electronic medical records for these patients were examined to collect information on patient characteristics, tumor characteristics, details of systemic therapy, type of vaccine received, and any AEs associated with the vaccine administration. Clinic and hospital notes were further analyzed to capture AEs occurring in a period between the first vaccination and day 30 after the second/final vaccination. In the case of the Johnson & Johnson vaccines, patient charts were reviewed for the 30-d period following the single dose of vaccination. AEs were graded in accordance with version 5.0 of the Common Terminology Criteria for Adverse Events[16]. The institutional review board of the Medical College of Wisconsin approved this study protocol.
Between January 1, 2021 and August 15, 2021, 210 patients were included in the study (Table 1). The median age of the cohort was 70 years (range, 23-91), 51% (108/210) of patients were female, and 87.1% (183/210) of the study population was Caucasian. Distribution of vaccine types included BNT162b2 from Pfizer 52.3% (110/210), mRNA-1273 from Moderna 42.3% (89/210), and JNJ-7843 vaccine from J&J 5% (11/210). All patients who received either the Pfizer or Moderna vaccine completed the 2-dose vaccination series. Gastrointestinal cancers were the most frequent diagnoses 43.8% (92/210), followed by thoracic cancers 30.4% (64/210) and genitourinary cancers 17.6% (37/210).
| Patient characteristics | n = 210, % |
| Age at vaccination, median (range), yr | 70 (23-91) |
| Sex | |
| Male | 102 (49) |
| Female | 108 (51) |
| Race | |
| Caucasian | 183 (87) |
| African American | 19 (9) |
| Other | 8 (4) |
| Site of primary tumor | |
| Gastrointestinal | 92 (44) |
| Thoracic | 64 (30) |
| Genitourinary | 37 (18) |
| Other | 17 (8) |
| Type of systemic therapy | |
| Chemotherapy | 117 (56) |
| Immunotherapy | 51 (24) |
| Targeted therapy | 42 (20) |
In the study cohort, 117 patients were receiving systemic chemotherapy at the time of vaccination. The median age of this cohort was 69 years, with a slight female predominance at 53% (62/117). Distribution of vaccine types were BNT162b2 from Pfizer 55.6% (65/117), mRNA-1273 from Moderna 40.1% (47/117), and JNJ-7843 vaccine from J&J 4.2% (5/117). The most common chemotherapeutic regimens included were taxane-based 25.6% (30/117) regimens followed by oxaliplatin-based regimens 22.2% (26/117).
Fifty-one patients were receiving immunotherapy at the time of vaccination. The median age of this cohort was 72 years, with a slight male predominance at 56.9% (29/51). Distribution of vaccine types were BNT162b2 from Pfizer 47% (24/51), mRNA-1273 from Moderna 45.1% (23/51), and JNJ-7843 vaccine from J&J 7.8% (4/51). The most common immunotherapeutic regimens consisted of programmed death 1 (PD-1) blocking agents 94% (48/51).
Forty-two patients were receiving targeted therapy at the time of vaccination. The median age of this cohort was 68 years, with a slight female predominance at 57% (24/42). Distribution of vaccine types were BNT162b2 from Pfizer 50% (21/42), mRNA-1273 from Moderna 45.2% (19/42), and JNJ-7843 vaccine from J&J 4.8% (2/42). The most common targeted therapy treatment administered was Osimertinib 14.2% (6/42).
The total number of AEs attributable to vaccination in the current cohort was 62 (Table 2). At least 1 unique AE was noted in 17.6% of patients (37/210). The number of patients who experienced any grade AEs was 20 in the chemotherapy group, 12 in the immunotherapy group, and 5 in the targeted therapy group. There were 33 AEs related to the Pfizer vaccine, 26 to the Moderna vaccine, and 3 to the J&J vaccine. In total, there were fifty-three grade 1 AEs 85.5% (53/62) and nine grade 2 AEs 14.5% (9/62). Following the first vaccination, there were twenty-one grade 1 and four grade 2 AEs. The most frequent grade 1 AEs were injection site reaction 23.8% (5/21), fatigue 23.8% (5/21), and fever 9.5% (2/21). The four grade 2 AEs noted included fatigue, nausea, chills, and maculopapular rash. Following the second vaccination, there were thirty-two grade 1 and five grade 2 AEs. The most frequent grade 1 AEs were fever 18.8% (6/32), fatigue 12.5% (4/32), chills 12.5% (4/32), and myalgia 12.5% (4/32). The five grade 2 AEs included 2 cases of fatigue, 2 cases of generalized muscle weakness, and 1 case of fever.
| Chemotherapy | Immunotherapy | Targeted therapy | |
| Patient number | 117 | 51 | 42 |
| Median age (yr) | 69 | 72 | 68 |
| Gender (Male/Female) | 55/62 | 29/22 | 18/24 |
| Type of vaccine administered(Moderna/Pfizer/J&J) | 47/65/5 | 23/24/4 | 19/21/2 |
| AEs (Grade 1 + 2), number (%) | 37 (60) | 18 (29) | 7 (11) |
| Therapy delayed because of AEs, # | 1 | 2 | 0 |
Cumulatively, the most frequent grade 1 AEs included fatigue 17% (9/53), fever 15% (8/53), injection site reaction 13.2% (7/53), and chills 9.4% (5/53). The most frequent grade 2 AEs were fatigue 33.3% (3/9) and generalized muscle weakness 22.2% (2/9). Of the grade 2 AEs, 6 were associated with the Pfizer vaccine and 3 with the Moderna vaccine. No grade 2 AEs were noted in the J&J vaccine population. In those who received the Pfizer or Moderna vaccine, the majority of AEs occurred after the second dose of vaccination 59.7% (37/62).
Treatment was delayed in 3 patients 1.4% (3/210) after the second dose of the Moderna vaccine by 2 wk because of AEs possibly related to vaccine administration. None of the patients had displayed any AEs after the first vaccination dose. Two of these 3 patients receiving immunotherapy developed generalized weakness that resolved within 2 wk without any specific treatment. The third patient developed malaise and fatigue, which also resolved spontaneously. No grade 3-5 AEs or anaphylaxis were noted in this patient cohort.
Data on the safety of COVID-19 vaccines in cancer patients undergoing systemic therapies are sparse. The current study aimed to address this unmet need by collecting data on COVID-19 vaccine-associated AEs in real-world cancer patients with solid tumors receiving various systemic therapies. The study revealed that COVID-19 vaccines cause infrequent and minor side effects in this patient population.
The pandemic caused by the novel coronavirus SARS-CoV-2 has significantly impacted cancer care delivery and cancer treatment globally. The COVID-19 pandemic has affected many aspects of cancer care, including delay in cancer diagnosis and treatment, the long-term ramifications of which are yet to be determined[15]. The rapid development of coronavirus vaccines has brought the hope of preventing infection and restoring normalcy. While the initial clinical trials with COVID-19 vaccines demonstrated a high safety profile of the vaccines in the healthy population[11-13], limited safety data have been reported in cancer patients. Consequently, significant hesitancy in adopting widespread vaccination has been observed among patients with active cancer[17-20]. In a cross-sectional, internet-based survey, hesitancy to receive COVID-19 vaccination was reported in 13.4% of patients with cancer[19]. In a study with breast cancer patients, 26% of patients were hesitant to receive vaccination due to their concerns regarding vaccine-related AEs[20]. As patients with cancer are at increased risk of COVID-19 infection-associated complications and mortality[8-10,21,22], data confirming the safety of COVID-19 vaccines in cancer patients are urgently needed. Our study provides important safety information on COVID-19 vaccines in cancer patients undergoing active cancer treatment.
Several studies have investigated the safety of COVID-19 vaccines (summarized in Table 3). Oosting and colleagues have reported a prospective, multicenter study from the Netherlands in which patients with solid tumors received the Moderna vaccine while undergoing treatment with chemotherapy, immunotherapy, or chemoimmunotherapy[23]. In this study, the incidence of grade 3 or worse AEs were reported in 2% of patients treated with immunotherapy, 2% of patients treated with chemotherapy, and 1% of patients treated with chemoimmunotherapy. No vaccine-related death was reported. A similar study from Italy reported that patients with solid tumors undergoing active treatment also demonstrated a low incidence of significant AEs associated with COVID-19 vaccination[24]. In this study, none of the 257 evaluable patients experienced grade 3 or higher AEs. The most frequently reported AE was injection site pain and/or redness occurring in 31.5% and 33.4 % of patients after the first and second vaccinations. The most frequently reported AEs after the first dose were weakness (7%), headache (8%), and muscle pain (2.7%), and after the second dose were weakness (8.9%) and fever (5.8%). A study from Israel also reported a low incidence of AEs in patients with solid tumors receiving immunotherapy with checkpoint inhibitors, with injection site pain being the most frequently reported AE at 21% (28/134)[25]. Several other studies have demonstrated similar results[26-29]. The results of our study, in conjunction with the studies discussed above, indicate that COVID-19 vaccination is safe in solid tumor patients undergoing active treatment. The high mortality rate associated with COVID-19 disease (as high as 40% in certain patient populations)[30] and the safety data available far justify routine COVID-19 vaccination in patients with solid tumors undergoing active treatment. This recommendation is further supported by several oncology societies[14,15] and echoed by the American Society of Clinical Oncology endorsement (https://www.asco.org/covid-resources/vaccines-patients-cancer) which states: At this time, patients undergoing treatment may be offered vaccination against COVID-19 as long as any components of the vaccine are not contraindicated.
| Ref. | Sample size (n) | Cancer type | Systemic therapy | Vaccines administered | Patients with Grade 3 or worse AE, % | Immune-related AEs | Comment |
| Oosting et al[23] | 544 | Solid Tumors | Chemotherapy; Immunotherapy; Chemoimmunotherapy | mRNA-1273 (Moderna) | 10/544 (1.8%) | 4% in both immunotherapy and chemoimmunotherapy group | Total 4 serious AEs were potentially related to the vaccination |
| Cavann et al[24] | 257 | Solid Tumors | Chemotherapy; Immunotherapy; Chemoimmunotherapy; Chemotherapy plus biological therapy; Biologic therapy | PfizerModerna | 0/257 (0%) | NA | Approximately 1/3rd of patients reported mild local reactions (pain, erythema) at the injection site |
| Waissengrin et al[25] | 134 | Solid Tumors | Immune checkpoint inhibitor; Chemoimmunotherapy | BNT162b2 mRNA vaccine (Pfizer) | 0/134 (0%) | Nonattributable to the vaccination | Fatigue (34%), headache (16%), muscle pain (34%) |
| Di Noia et al[26] | 816 | Solid Tumors | Chemotherapy; Immunotherapy; Chemoimmunotherapy; Targeted therapy | Pfizer | 3.3% after the 1st dose, 1.4% after the second dose | NA | AE occurred in 359 (44%) and 301 (38.3%) patients after the first and second dose, respectively |
| Shmueli et al[27] | 129 | Solid Tumors | Chemotherapy; Immunotherapy; Chemoimmunotherapy; Biological Therapy; Hormonal Therapy; Radiotherapy | Pfizer | 0/129 (0%) | NA | AE was reported by 39% of patients after the first dose and 58% of patients after the second dose- all mild to moderate in severity |
| Tamura et al[29] | 120 | Solid Tumor | Chemotherapy; Immunotherapy; Targeted Therapy; Chemoimmunotherapy | Pfizer Moderna | 0/120 (0%); CTCAE was not used | NA | Study limited to patients receiving treatment for lung cancer only. No serious AEs or treatment delay was observed |
| Kian et al[28] | 210 | Solid & Non-Solid Tumors | Chemotherapy; Immunotherapy; Chemoimmunotherapy; Biological Therapy; Hormonal Therapy; Radiotherapy; Radio-hormonal; Chemo-biological | Pfizer | 0.004% after 1st dose, 1.9% after the second dose | NA | AE occurred in 65 (31%) and 65 (31%) patients after the first and second dose, respectively. Injection site pain was the most common AE after both doses |
It is important to reiterate that COVID-19 vaccines in cancer patients treated with immunotherapy did not cause a higher incidence of immune-related AEs, a finding supported by several other studies[23,25]. While 2 patients in our study receiving immune checkpoint inhibitors experienced treatment delay secondary to vaccination-associated AEs, their symptoms resolved quickly with supportive care only. The remaining patients in our immunotherapy cohort demonstrated mild grade 1 AEs with rapid resolution of symptoms.
Although the current study provides valuable information on COVID-19 vaccine safety in a real-world setting, it has several limitations that include the inherent biases associated with a retrospective study design, modest sample size, and reliance on physician documentation for the data related to the AEs.
Our study demonstrates that the COVID-19 vaccines cause infrequent and mild AEs in patients with solid tumors receiving systemic therapies. The study results support routine COVID-19 vaccination in cancer patients receiving active treatment.
In the wake of the coronavirus disease 2019 (COVID-19) pandemic, the United States Food and Drug Administration approved 3 vaccines to prevent coronavirus infection. The rapidity of vaccine approval and the limited scientific inquiry into vaccine-related adverse events notably expanded apprehension towards vaccination in patients with malignancies. Our study reports real-world data on the severity and spectrum of adverse events in solid tumor cancer patients receiving systemic therapy.
The motivation behind this project was to promote awareness regarding the short-term safety of COVID-19 vaccines in cancer patients with solid tumor malignancies. Our results help lessen the societal apprehension and hesitation surrounding the safety of COVID-19 vaccination.
The main objective of this study was to evaluate the short-term safety of COVID-19 vaccines in patients with solid tumors undergoing treatment with systemic therapies. Through rigorous analysis, we were able to document the incidence and spectrum of vaccine-related adverse events in our patient cohort. Our research forms the groundwork for future studies on long-term adverse events secondary to vaccination.
Our study was a retrospective analysis of cancer patients who received COVID-19 vaccination between January 1, 2021 and August 15, 2021. Eligible patients were identified using the EPIC SlicerDicer tool in the Froedtert and the Medical College of Wisconsin Cancer Center database. Once identified, patients were further screened based on study inclusion/exclusion criteria. Electronic medical records for the final patients were examined to collect information on patient characteristics, tumor characteristics, details of systemic therapy, type of vaccine received, and any adverse events associated with the vaccine administration.
Analysis of our 210 patients revealed at least 1 adverse event attributable to vaccination in 17.6% of our study cohort. Of these adverse events, fifty-three were grade 1 and nine were grade 2. Our data further bolsters the sparse scientific literature regarding COVID-19 vaccination in patients with cancer.
The present study demonstrates that the adverse events associated with COVID-19 vaccination are infrequent, mild, and rarely delay treatment in patients with solid tumors receiving systemic therapies. This knowledge further begs the question of whether or not patients receiving systemic therapies are mounting an appropriate response to immunogenic antigens. Further scientific inquiry exploring vaccine efficacy and adverse events in our patient cohort vs a healthy control group could elucidate the role of systemic therapy in vaccine-related adverse events.
Future research will be focused on increasing study enrollment and exploring the long-term adverse events secondary to COVID-19 vaccination.
Provenance and peer review: Invited article; Externally peer reviewed.
Peer-review model: Single blind
Specialty type: Oncology
Country/Territory of origin: United States
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): B
Grade C (Good): C, C, C
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Ata F, Qatar; Mohan S, India; Seid AA, Ethiopia A-Editor: Kołat D, Poland S-Editor: Liu JH L-Editor: A P-Editor: Liu JH
| 1. | Gupta V, Santosh KC, Arora R, Ciano T, Kalid KS, Mohan S. Socioeconomic impact due to COVID-19: An empirical assessment. Inf Process Manag. 2022;59:102810. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 54] [Cited by in RCA: 16] [Article Influence: 5.3] [Reference Citation Analysis (0)] |
| 2. | Gupta V, Jain N, Katariya P, Kumar A, Mohan S, Ahmadian A, Ferrara M. An Emotion Care Model using Multimodal Textual Analysis on COVID-19. Chaos Solitons Fractals. 2021;144:110708. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 62] [Cited by in RCA: 28] [Article Influence: 7.0] [Reference Citation Analysis (0)] |
| 3. | Wiersinga WJ, Rhodes A, Cheng AC, Peacock SJ, Prescott HC. Pathophysiology, Transmission, Diagnosis, and Treatment of Coronavirus Disease 2019 (COVID-19): A Review. JAMA. 2020;324:782-793. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2691] [Cited by in RCA: 3179] [Article Influence: 635.8] [Reference Citation Analysis (0)] |
| 4. | Chavez-MacGregor M, Lei X, Zhao H, Scheet P, Giordano SH. Evaluation of COVID-19 Mortality and Adverse Outcomes in US Patients With or Without Cancer. JAMA Oncol. 2022;8:69-78. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 41] [Cited by in RCA: 154] [Article Influence: 38.5] [Reference Citation Analysis (0)] |
| 5. | Giannakoulis VG, Papoutsi E, Siempos II. Effect of Cancer on Clinical Outcomes of Patients With COVID-19: A Meta-Analysis of Patient Data. JCO Glob Oncol. 2020;6:799-808. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 141] [Cited by in RCA: 161] [Article Influence: 32.2] [Reference Citation Analysis (0)] |
| 6. | Liang W, Guan W, Chen R, Wang W, Li J, Xu K, Li C, Ai Q, Lu W, Liang H, Li S, He J. Cancer patients in SARS-CoV-2 infection: a nationwide analysis in China. Lancet Oncol. 2020;21:335-337. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 3332] [Cited by in RCA: 3125] [Article Influence: 625.0] [Reference Citation Analysis (0)] |
| 7. | Wang L, Sun Y, Yuan Y, Mei Q, Yuan X. Clinical challenges in cancer patients with COVID-19: Aging, immunosuppression, and comorbidities. Aging (Albany NY). 2020;12:24462-24474. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 15] [Cited by in RCA: 15] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 8. | Desai A, Khaki AR, Kuderer NM. Use of Real-World Electronic Health Records to Estimate Risk, Risk Factors, and Disparities for COVID-19 in Patients With Cancer. JAMA Oncol. 2021;7:227-229. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 6] [Cited by in RCA: 4] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 9. | Jee J, Foote MB, Lumish M, Stonestrom AJ, Wills B, Narendra V, Avutu V, Murciano-Goroff YR, Chan JE, Derkach A, Philip J, Belenkaya R, Kerpelev M, Maloy M, Watson A, Fong C, Janjigian Y, Diaz LA Jr, Bolton KL, Pessin MS. Chemotherapy and COVID-19 Outcomes in Patients With Cancer. J Clin Oncol. 2020;38:3538-3546. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 185] [Cited by in RCA: 185] [Article Influence: 37.0] [Reference Citation Analysis (0)] |
| 10. | Wang Q, Berger NA, Xu R. Analyses of Risk, Racial Disparity, and Outcomes Among US Patients With Cancer and COVID-19 Infection. JAMA Oncol. 2021;7:220-227. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 297] [Cited by in RCA: 293] [Article Influence: 73.3] [Reference Citation Analysis (0)] |
| 11. | Baden LR, El Sahly HM, Essink B, Kotloff K, Frey S, Novak R, Diemert D, Spector SA, Rouphael N, Creech CB, McGettigan J, Khetan S, Segall N, Solis J, Brosz A, Fierro C, Schwartz H, Neuzil K, Corey L, Gilbert P, Janes H, Follmann D, Marovich M, Mascola J, Polakowski L, Ledgerwood J, Graham BS, Bennett H, Pajon R, Knightly C, Leav B, Deng W, Zhou H, Han S, Ivarsson M, Miller J, Zaks T; COVE Study Group. Efficacy and Safety of the mRNA-1273 SARS-CoV-2 Vaccine. N Engl J Med. 2021;384:403-416. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 7073] [Cited by in RCA: 7578] [Article Influence: 1894.5] [Reference Citation Analysis (1)] |
| 12. | Polack FP, Thomas SJ, Kitchin N, Absalon J, Gurtman A, Lockhart S, Perez JL, Pérez Marc G, Moreira ED, Zerbini C, Bailey R, Swanson KA, Roychoudhury S, Koury K, Li P, Kalina WV, Cooper D, Frenck RW Jr, Hammitt LL, Türeci Ö, Nell H, Schaefer A, Ünal S, Tresnan DB, Mather S, Dormitzer PR, Şahin U, Jansen KU, Gruber WC; C4591001 Clinical Trial Group. Safety and Efficacy of the BNT162b2 mRNA Covid-19 Vaccine. N Engl J Med. 2020;383:2603-2615. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 10556] [Cited by in RCA: 10717] [Article Influence: 2143.4] [Reference Citation Analysis (1)] |
| 13. | Sadoff J, Gray G, Vandebosch A, Cárdenas V, Shukarev G, Grinsztejn B, Goepfert PA, Truyers C, Fennema H, Spiessens B, Offergeld K, Scheper G, Taylor KL, Robb ML, Treanor J, Barouch DH, Stoddard J, Ryser MF, Marovich MA, Neuzil KM, Corey L, Cauwenberghs N, Tanner T, Hardt K, Ruiz-Guiñazú J, Le Gars M, Schuitemaker H, Van Hoof J, Struyf F, Douoguih M; ENSEMBLE Study Group. Safety and Efficacy of Single-Dose Ad26.COV2.S Vaccine against Covid-19. N Engl J Med. 2021;384:2187-2201. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 2024] [Cited by in RCA: 1817] [Article Influence: 454.3] [Reference Citation Analysis (0)] |
| 14. | Garassino MC, Vyas M, de Vries EGE, Kanesvaran R, Giuliani R, Peters S; European Society for Medical Oncology. The ESMO Call to Action on COVID-19 vaccinations and patients with cancer: Vaccinate. Monitor. Educate. Ann Oncol. 2021;32:579-581. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 51] [Cited by in RCA: 65] [Article Influence: 16.3] [Reference Citation Analysis (0)] |
| 15. | Ribas A, Sengupta R, Locke T, Zaidi SK, Campbell KM, Carethers JM, Jaffee EM, Wherry EJ, Soria JC, D'Souza G; AACR COVID-19 and Cancer Task Force. Priority COVID-19 Vaccination for Patients with Cancer while Vaccine Supply Is Limited. Cancer Discov. 2021;11:233-236. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 157] [Cited by in RCA: 153] [Article Influence: 38.3] [Reference Citation Analysis (0)] |
| 16. | National Cancer Institute. “Cancer Therapy Evaulation Program.” Common Terminology Criteria for Adverse Events (CTCAE). Available from: https://ctep.cancer.gov/protocoldevelopment/adverse_effects.htm. |
| 17. | Barrière J, Gal J, Hoch B, Cassuto O, Leysalle A, Chamorey E, Borchiellini D. Acceptance of SARS-CoV-2 vaccination among French patients with cancer: a cross-sectional survey. Ann Oncol. 2021;32:673-674. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 50] [Cited by in RCA: 72] [Article Influence: 18.0] [Reference Citation Analysis (0)] |
| 18. | Moujaess E, Zeid NB, Samaha R, Sawan J, Kourie H, Labaki C, Chebel R, Chahine G, Karak FE, Nasr F, Ghosn M, Wakim J, Kattan J. Perceptions of the COVID-19 vaccine among patients with cancer: a single-institution survey. Future Oncol. 2021;17:4071-4079. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 29] [Cited by in RCA: 32] [Article Influence: 8.0] [Reference Citation Analysis (0)] |
| 19. | Tsai R, Hervey J, Hoffman K, Wood J, Johnson J, Deighton D, Clermont D, Loew B, Goldberg SL. COVID-19 Vaccine Hesitancy and Acceptance Among Individuals With Cancer, Autoimmune Diseases, or Other Serious Comorbid Conditions: Cross-sectional, Internet-Based Survey. JMIR Public Health Surveill. 2022;8:e29872. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 23] [Cited by in RCA: 112] [Article Influence: 28.0] [Reference Citation Analysis (0)] |
| 20. | Villarreal-Garza C, Vaca-Cartagena BF, Becerril-Gaitan A, Ferrigno AS, Mesa-Chavez F, Platas A. Attitudes and Factors Associated With COVID-19 Vaccine Hesitancy Among Patients With Breast Cancer. JAMA Oncol. 2021;7:1242-1244. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 31] [Cited by in RCA: 57] [Article Influence: 14.3] [Reference Citation Analysis (0)] |
| 21. | Bakouny Z, Hawley JE, Choueiri TK, Peters S, Rini BI, Warner JL, Painter CA. COVID-19 and Cancer: Current Challenges and Perspectives. Cancer Cell. 2020;38:629-646. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 199] [Cited by in RCA: 201] [Article Influence: 40.2] [Reference Citation Analysis (1)] |
| 22. | Yang K, Sheng Y, Huang C, Jin Y, Xiong N, Jiang K, Lu H, Liu J, Yang J, Dong Y, Pan D, Shu C, Li J, Wei J, Huang Y, Peng L, Wu M, Zhang R, Wu B, Li Y, Cai L, Li G, Zhang T, Wu G. Clinical characteristics, outcomes, and risk factors for mortality in patients with cancer and COVID-19 in Hubei, China: a multicentre, retrospective, cohort study. Lancet Oncol. 2020;21:904-913. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 420] [Cited by in RCA: 410] [Article Influence: 82.0] [Reference Citation Analysis (0)] |
| 23. | Oosting SF, van der Veldt AAM, GeurtsvanKessel CH, Fehrmann RSN, van Binnendijk RS, Dingemans AC, Smit EF, Hiltermann TJN, den Hartog G, Jalving M, Westphal TT, Bhattacharya A, van der Heiden M, Rimmelzwaan GF, Kvistborg P, Blank CU, Koopmans MPG, Huckriede ALW, van Els CACM, Rots NY, van Baarle D, Haanen JBAG, de Vries EGE. mRNA-1273 COVID-19 vaccination in patients receiving chemotherapy, immunotherapy, or chemoimmunotherapy for solid tumours: a prospective, multicentre, non-inferiority trial. Lancet Oncol. 2021;22:1681-1691. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 76] [Cited by in RCA: 121] [Article Influence: 30.3] [Reference Citation Analysis (0)] |
| 24. | Cavanna L, Citterio C, Biasini C, Madaro S, Bacchetta N, Lis A, Cremona G, Muroni M, Bernuzzi P, Lo Cascio G, Schiavo R, Mutti M, Tassi M, Mariano M, Trubini S, Bandieramonte G, Maestri R, Mordenti P, Marazzi E, Vallisa D. COVID-19 vaccines in adult cancer patients with solid tumours undergoing active treatment: Seropositivity and safety. A prospective observational study in Italy. Eur J Cancer. 2021;157:441-449. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 40] [Cited by in RCA: 40] [Article Influence: 10.0] [Reference Citation Analysis (0)] |
| 25. | Waissengrin B, Agbarya A, Safadi E, Padova H, Wolf I. Short-term safety of the BNT162b2 mRNA COVID-19 vaccine in patients with cancer treated with immune checkpoint inhibitors. Lancet Oncol. 2021;22:581-583. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 102] [Cited by in RCA: 149] [Article Influence: 37.3] [Reference Citation Analysis (0)] |
| 26. | Di Noia V, Pimpinelli F, Renna D, Barberi V, Maccallini MT, Gariazzo L, Pontone M, Monti A, Campo F, Taraborelli E, Di Santo M, Petrone F, Mandoj C, Ferraresi V, Ferretti G, Carlini P, Di Bella O, Conti L, La Malfa AM, Pellini R, Bracco D, Giannarelli D, Morrone A, Cognetti F. Immunogenicity and Safety of COVID-19 Vaccine BNT162b2 for Patients with Solid Cancer: A Large Cohort Prospective Study from a Single Institution. Clin Cancer Res. 2021;27:6815-6823. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 24] [Cited by in RCA: 43] [Article Influence: 10.8] [Reference Citation Analysis (0)] |
| 27. | Shmueli ES, Itay A, Margalit O, Berger R, Halperin S, Jurkowicz M, Levin EG, Levy I, Olmer L, Regev-Yochay G, Lustig Y, Rahav G. Efficacy and safety of BNT162b2 vaccination in patients with solid cancer receiving anticancer therapy - a single centre prospective study. Eur J Cancer. 2021;157:124-131. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 24] [Cited by in RCA: 38] [Article Influence: 9.5] [Reference Citation Analysis (0)] |
| 28. | Kian W, Zemel M, Kestenbaum EH, Rouvinov K, Alguayn W, Levitas D, Ievko A, Michlin R, Abod MA, Massalha I, Chernomordikov E, Sharb AA, Shalata W, Levison E, Roisman LC, Lavrenkov K, Peled N, Nesher L, Yakobson A. Safety of the BNT162b2 mRNA COVID-19 vaccine in oncologic patients undergoing numerous cancer treatment options: A retrospective single-center study. Medicine (Baltimore). 2022;101:e28561. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 12] [Cited by in RCA: 10] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 29. | Tamura T, Ninomiya K, Kubo T, Kuyama S, Tachibana S, Inoue K, Chikamori K, Kudo K, Ochi N, Harada D, Maeda Y, Kiura K. Short-term safety of an anti-severe acute respiratory syndrome coronavirus 2 messenger RNA vaccine for patients with advanced lung cancer treated with anticancer drugs: A multicenter, prospective, observational study. Thorac Cancer. 2022;13:453-459. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 5] [Cited by in RCA: 4] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 30. | Venkatesulu BP, Chandrasekar VT, Girdhar P, Advani P, Sharma A, Elumalai T, Hsieh CE, Elghazawy HI, Verma V, Krishnan S. A Systematic Review and Meta-Analysis of Cancer Patients Affected by a Novel Coronavirus. JNCI Cancer Spectr. 2021;5:pkaa102. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 51] [Cited by in RCA: 66] [Article Influence: 16.5] [Reference Citation Analysis (0)] |