Published online Jun 24, 2022. doi: 10.5306/wjco.v13.i6.540
Peer-review started: January 12, 2022
First decision: March 24, 2022
Revised: April 6, 2022
Accepted: May 12, 2022
Article in press: May 12, 2022
Published online: June 24, 2022
Processing time: 161 Days and 6 Hours
Epstein-Barr virus associated smooth muscle tumor (EBV-SMT) is a rare oncolo
Our case series includes six cases of EBV-SMTs across different age groups, with different treatment modalities, adding to the limited existing literature on this rare tumor. The median latency time between immunosuppression and disease diagnosis is four years. EBV-SMTs present with variable degrees of aggressiveness and seem to have worse clinical outcomes in patients with tumor multiplicity and worse immunocompetency.
It is imperative to continue building on this knowledge and keeping EBV-SMTs on the differential in immunocompromised individuals.
Core Tip: Epstein-Barr virus associated smooth muscle tumor (EBV-SMT) is a rare oncological entity. Only a handful of case series have shed light on the presence of EBV-SMT in individuals, most of whom are immunocompromised. EBV-SMT should not be confused with post-transplant lymphoproliferative disorder. Histopathology should help guide the diagnosis.
- Citation: Khan AA, Estfan BN, Yalamanchali A, Niang D, Savage EC, Fulmer CG, Gosnell HL, Modaresi Esfeh J. Epstein-Barr virus-associated smooth muscle tumors in immunocompromised patients: Six case reports. World J Clin Oncol 2022; 13(6): 540-552
- URL: https://www.wjgnet.com/2218-4333/full/v13/i6/540.htm
- DOI: https://dx.doi.org/10.5306/wjco.v13.i6.540
Epstein-Barr virus associated smooth muscle tumor (EBV-SMT), first reported in 1970[1], is a rare oncological entity. Though EBV is present in 50%-89% of children and > 90% of adults worldwide, the virus often remains dormant until an individual becomes immunocompromised[2,3]. EBV is better known for other malignancies including nasopharyngeal carcinomas and lymphomas but in a minority of cases, it can trigger the proliferation of smooth muscle cells, resulting in mesenchymal tumors termed EBV-SMT.
There are three different types of EBV-SMTs identified to date: (1) Post-transplant associated smooth muscle tumors (PT-SMT); (2) Human immunodeficiency virus (HIV) associated smooth muscle tumors (HIV-SMT); and (3) Congenital immunodeficiency associated smooth muscle tumors (CI-SMT) such as in GATA2 and CARMIL2 deficiency[4]. EBV-SMTs can be encountered at any age, though it is more common in children[5,6]. EBV-SMTs can arise in any organ system, however, they are most common in the liver, followed by the lungs, central nervous system (CNS), adrenal glands, and gastrointestinal tract[7,8]. Unlike primary leiomyosarcomas where the histological grade is associated with disease severity, EBV-SMTs behave with variable severity, independent of their histological grade[9]. Clinical presentation is thus non-specific and depends on the location, size, and degree of organ involvement. EBV-SMTs can manifest either synchronously or metachronously in multiple organ systems and have a low propensity to metastasize[7,8].
PT-SMT can be confused with EBV associated post-transplant lymphoproliferative disorder (EBV-PTLD) as they are both caused by the same virus and occur in immunocompromised patients. Clinical presentation and radiographic findings cannot be used to tell the two entities apart. Instead, histopathology, immunohistochemistry, and EBV-encoded small RNA in situ hybridization (EBER ISH) are used to aid the diagnosis. Given its rarity, it is difficult to quantify the incidence of EBV-SMTs, however, it is estimated each subtype above may impact < 1%-5% of individuals. EBV-PTLD, on the other hand, is more prevalent than EBV-SMT and has an incidence ranging from 1%-20%[10] with mortality rates around 50%-90%[11,12].
Given how rare these tumors are, there is no standard treatment for EBV-SMTs which are instead treated on a case-by-case basis. Individuals with HIV-SMT are kept on appropriate antiretroviral treatment, and those with PT-SMT are treated with a reduction of immunosuppression. Surgical removal of tumors is considered when tumors impinge on the involved organ. Chemotherapy and radiation have also been utilized but without any obvious benefits on the disease course[6]. Allogeneic hematopoietic stem cell transplantation has also been used to treat CI-SMT with good outcomes[13,14].
Given their rarity, much of what we know about EBV-SMTs is through case reports and case series. However, as the frequency of organ transplantation grows with reliance on immunosuppression to prevent graft loss, it is pertinent to continue gathering information on EBV-SMTs. Many questions remain regarding incidence, prevalence, latency period, survival rates, and appropriate treatment strategies, amongst other crucial facts. Here, we describe six cases of EBV-SMT at a quaternary academic referral center.
Case 1: Fever, thrush, diarrhea.
Case 2: Weight loss, headaches, and myalgias.
Case 3: Severe headaches and altered mental status.
Case 4: New-onset hematuria.
Case 5: Shortness of breath.
Case 6: Neck, back, and shoulder pain.
Case 1: A five-year-old female with a history of idiopathic dilated cardiomyopathy status post orthotopic heart transplant (OHT) at eight months old, on tacrolimus, and previous EBV viremia at age 22 months presented with her third infection-related hospitalization in the previous six months.
Case 2: A 20-year-old male with a history of Idiopathic dilated cardiomyopathy status post OHT at age 17 on tacrolimus and mycophenolate mofetil (MMF) presented to the hospital with weight loss, headaches, and myalgias.
Case 3: A 16-year-old female with a history of dilated cardiomyopathy status post OHT at 12-year-old, on MMF and tacrolimus presented to the hospital with severe headaches and altered mental status.
Case 4: A 61-year-old male with a pre-transplant negative cytomegalovirus (CMV) and EBV serology underwent orthotopic heart and liver transplant from a CMV and EBV positive donor (CMV Donor+/Recipient-, EBV Donor+/Recipient-) at the age of 58 years for cirrhosis secondary to Hereditary Familial Amyloidosis (Thr60A1a mutation) was admitted for new-onset hematuria and acute kidney injury.
Case 5: A 63-year-old male with a history of a living-related donor kidney transplant at age 55 for end-stage renal disease of unknown etiology was hospitalized for community-acquired pneumonia.
Case 6: A 45-year-old female with a long-standing history of HIV (CD 4 count unknown) on anti-retroviral therapy presented to the primary care clinic with neck, back, and shoulder pain with associated proximal and distal left upper extremity numbness and paresthesias.
Case 1, 2: Idiopathic dilated cardiomyopathy status post OHT.
Case 3: History of dilated cardiomyopathy status post OHT at 12-year-old.
Case 4: Status post Orthotopic heart and liver transplant from a CMV and EBV positive donor (CMV Donor+/Recipient-, EBV Donor+/Recipient-) for cirrhosis secondary to Hereditary Familial Amyloidosis (Thr60A1a mutation).
Case 5: History of a living-related donor kidney transplant for end-stage renal disease of unknown etiology.
Case 6: Long-standing history of HIV (CD 4 count unknown) on anti-retroviral therapy with dolutegravir, abacavir, and lamivudine
Cases 1-6: No relevant family history.
Physical examination findings are not applicable.
Laboratory findings are summarized in Table 1 for cases 1-6.
| Cases | Sex | Type of transplant/IS | IS | Age at transplant (yr) | Age at diagnosis (yr) | EBV titer at diagnosis (copies per mL) | Tumor location | Histopathology findings | Treatment | Outcome (years post EBV-SMT) | Follow up |
| 1 | F | OHT | Tacrolimus | 8 mo | 6 | 286000 | Colon | Smooth muscle spindle cell proliferation with strongly positive in-situ EBER hybridization and focal desmin+ | Tacrolimus reduced | Alive (age 16) | None |
| 2 | M | OHT | Tacrolimus, MMF | 17 | 20 | 161476 | Thymus, lung, liver, mesenteric, and retroperitoneal lymph nodes, ascending colon, and proximal left femoral bone marrow | The spindled cells were positive with antibodies to smooth muscle antigen and desmin. Tumor cells were focally positive with antibodies to caldesmon. The spindle cells were negative with antibodies to S100 protein and cytokeratin AE1/3. In situ hybridization revealed that up to 80% of tumor cell nuclei were positive for the presence of EBER | Supportive | Death (age 20) | NA |
| 3 | F | OHT | Tacrolimus; Sirolimus | 12 | 16 | 11848 | Brain, lung, kidney | Hepatic parenchyma focally replaced by spindle cell proliferation. The spindle cells are arranged in a haphazard pattern and contain nuclei with minimal nuclear pleomorphism and abundant eosinophilic cytoplasm. (1) The tumor cells are strongly positive for h-caldesmon and smooth muscle actin; (2) Desmin+; (3) EBER positive by CISH; (4) CD20+, MUM-1+. CD3-, CD10-. EBER ISH+ (CNS); and (5) H-caldesmon+, smooth muscle actin+, desmin+ (focal). EBER ISH+ (liver) | Intrathecal rituximab, methotrexate, systemic chemotherapy, whole brain radiation, and T-cell therapy | Alive (age 22); course c/b seizures | Yearly brain and liver MRI |
| 4 | M | OHT/OLT | Tacrolimus | 58 | 61 | 25458 | Kidney, liver, lung | (1) Monotonous proliferative of relatively uniform spindle cells arranged in intersecting fascicles with pale eosinophilic cytoplasm and elongated, blunt-ended nuclei with dark vesicular chromatin.; (2) Mitotic figures are not readily identified and there is no significant cytologic atypia, pleomorphism, or necrosis; (3) The tumor cells are strongly immunoreactive for SMA and negative for CD117, DOG-1, desmin, S100, SOX10, CD34, and STAT6; and (4) EBER positive by CISH | L lateral segmentectomy w superficial wedge resection;Tacrolimus reduced, stopped, and eventually started given concern for ACR | Death (age 61) | NA |
| 5 | M | LRD kidney | Prednisone, MMF, Sirolimus | 55 | 63 | 4765 | Liver | (1) Fascicles of malignant appearing spindle cells diffusely positive for desmin and H-caldesmon; and (2) EBER positive by CISH | MMF. Sirolimus dosing decreased | Alive (age 73) | Repeat MRI with stable/decreased size hepatic lesions; no new lesions noticed |
| 6 | F | HIV | Abacavir-Lamivudine-Dolutegravir for HIV | NA | 45 | NA | Thoracic spine | (1) Spindle cell neoplasm staining positive for smooth muscle actin; (2) Negative for desmin; and (3) EBV positive by CISH | s/p T8-T10 Laminectomy; no change in HAART | Alive (age 55); total hip arthroplasty for bl avascular necrosis | None |
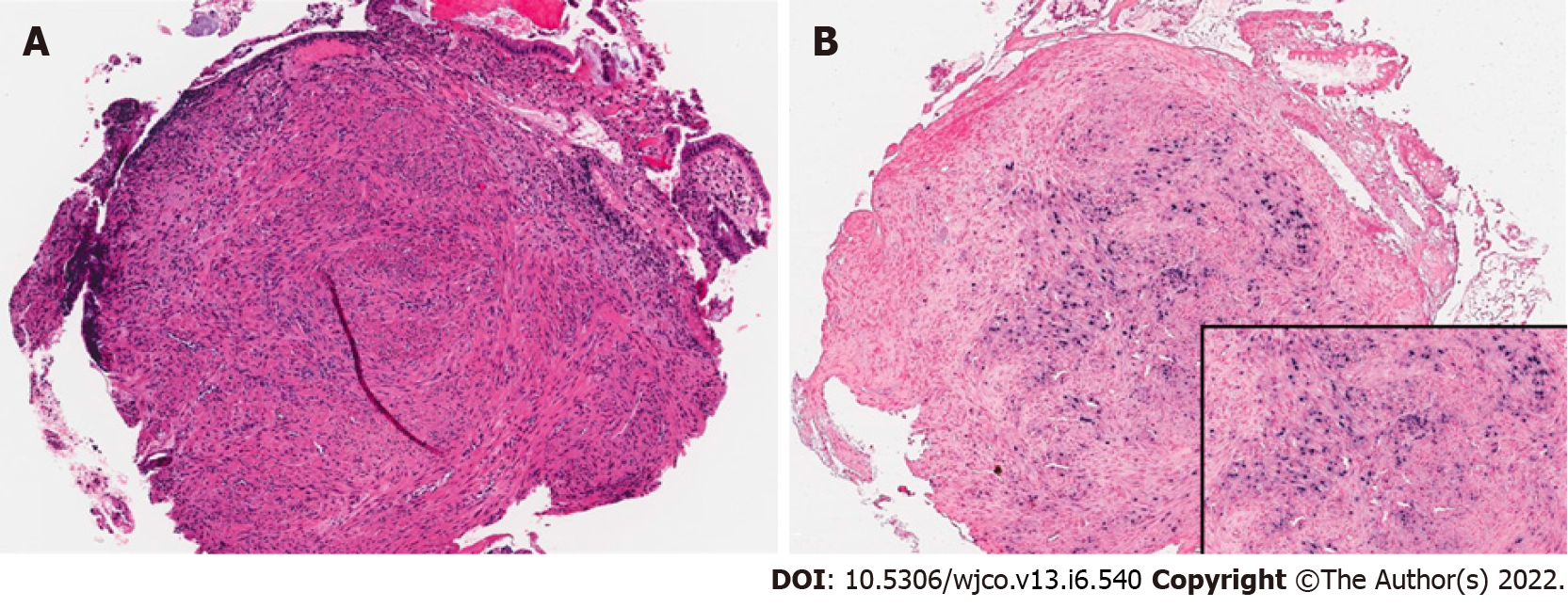
Case 1: Unrevealing total body computed tomography (CT) scan. EGD and colonoscopy with 6-10 mm polypoid lesions with central ulceration in the sigmoid colon and rectum. Biopsy of lesions confirmed EBV-SMT (Figures 1 and 2).
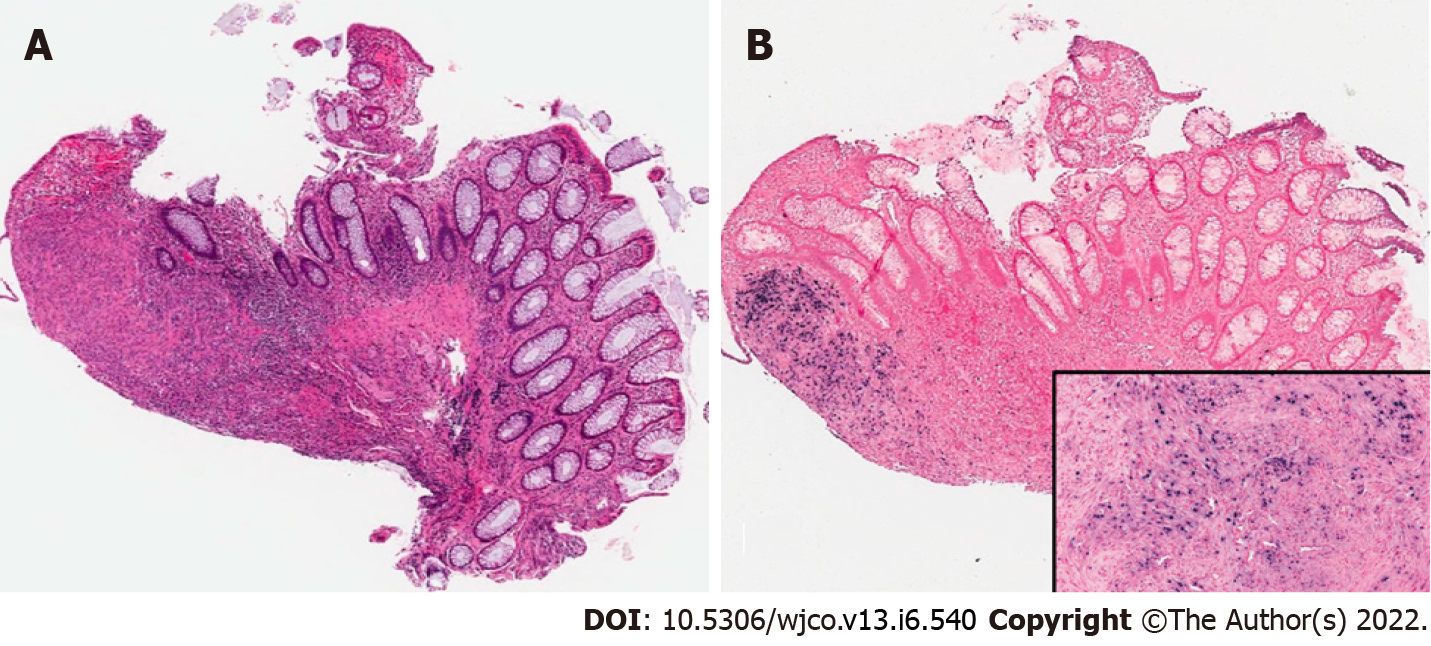
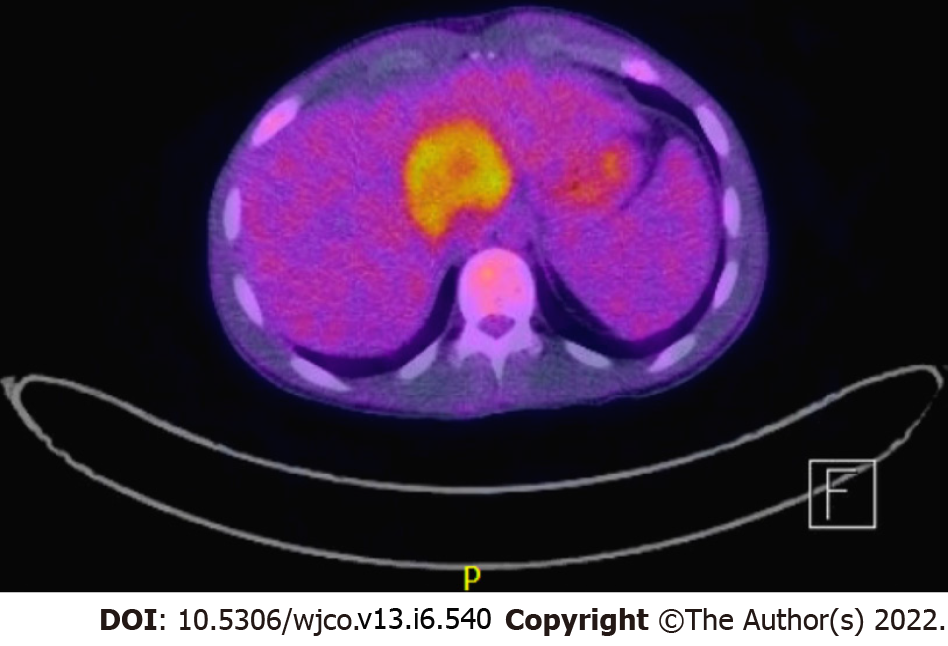
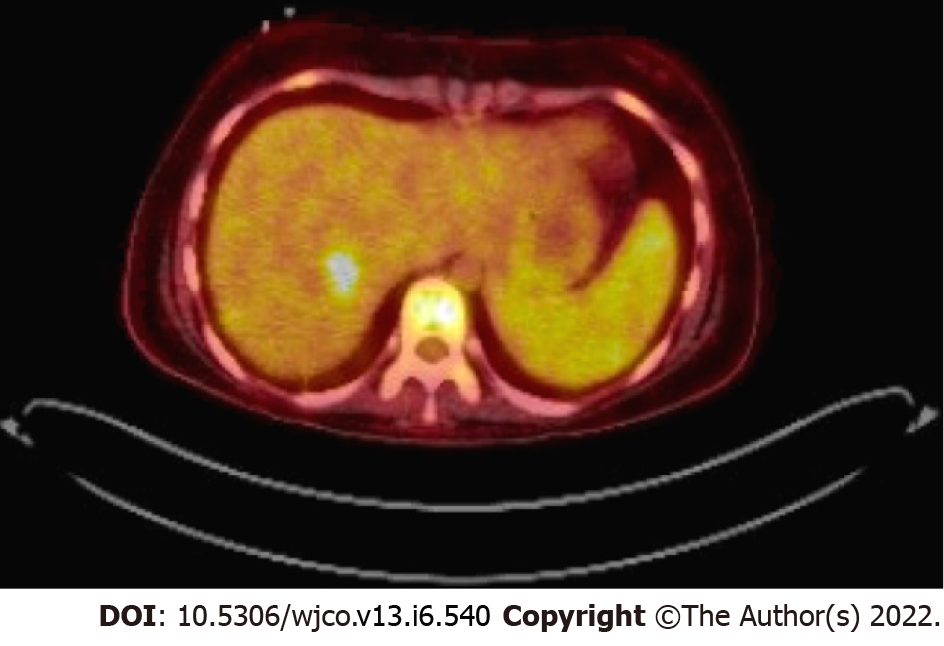
Case 2: Neck magnetic resonance imaging (MRI) revealed a mass in the right Meckel’s cave. Liver biopsy confirmed EBV-SMT (Figure 3). PET scan showed multiple fluorodeoxyglucose avid foci (Figure 4).
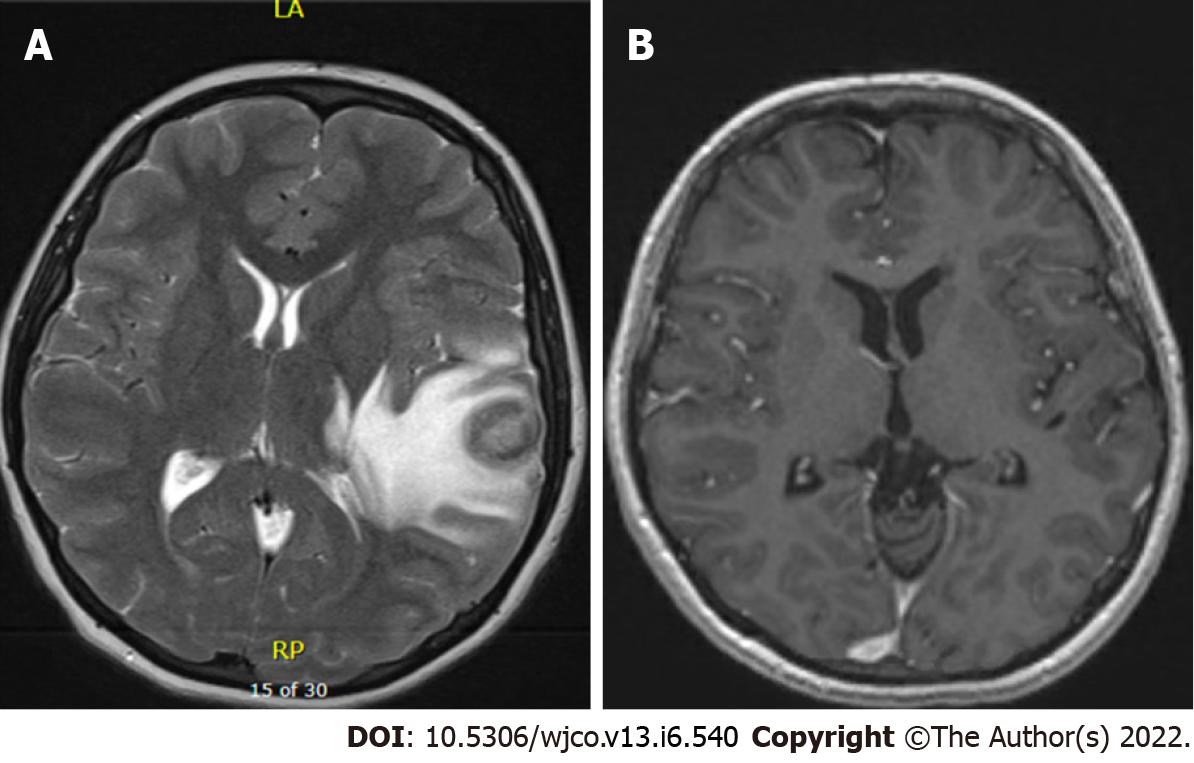
Case 3: MRI brain showed a left temporal ring-enhancing lesion (Figure 5). Whole body PET scan showed right hepatic mass (Figure 6).
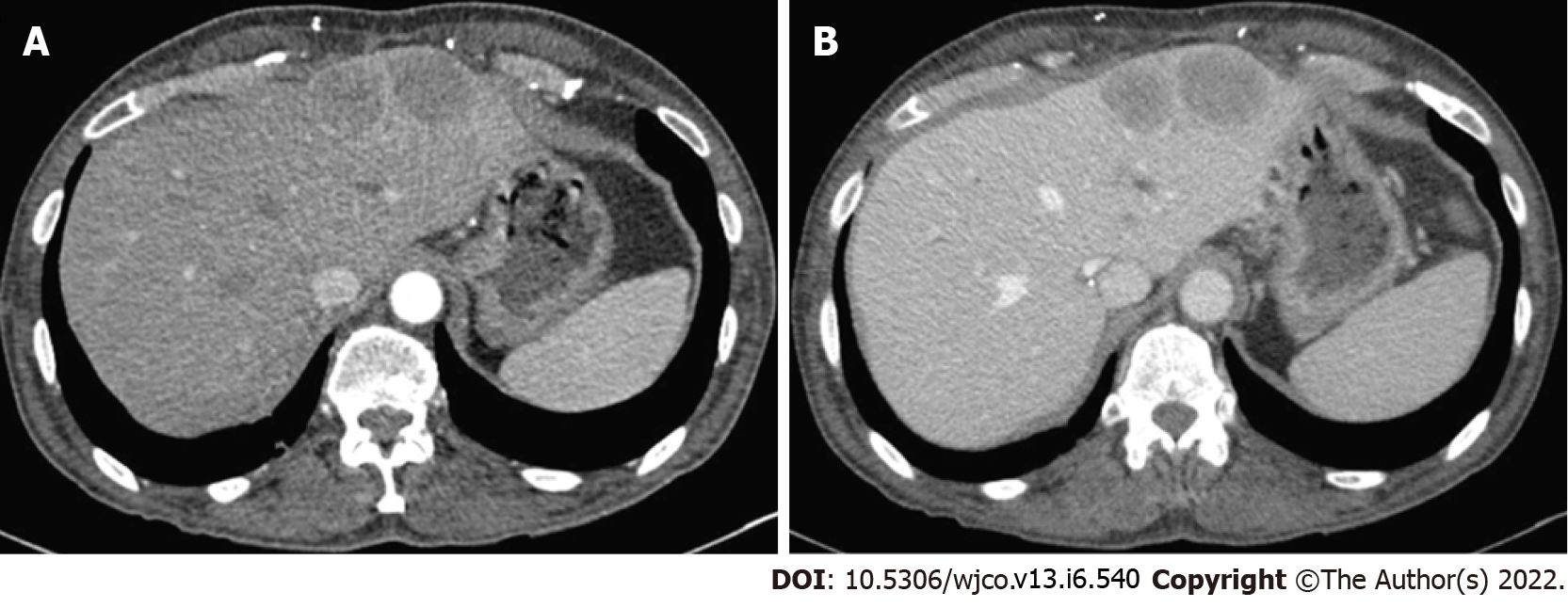
Case 4: CT scan of the liver revealed multiple indeterminate liver masses (Figure 7).
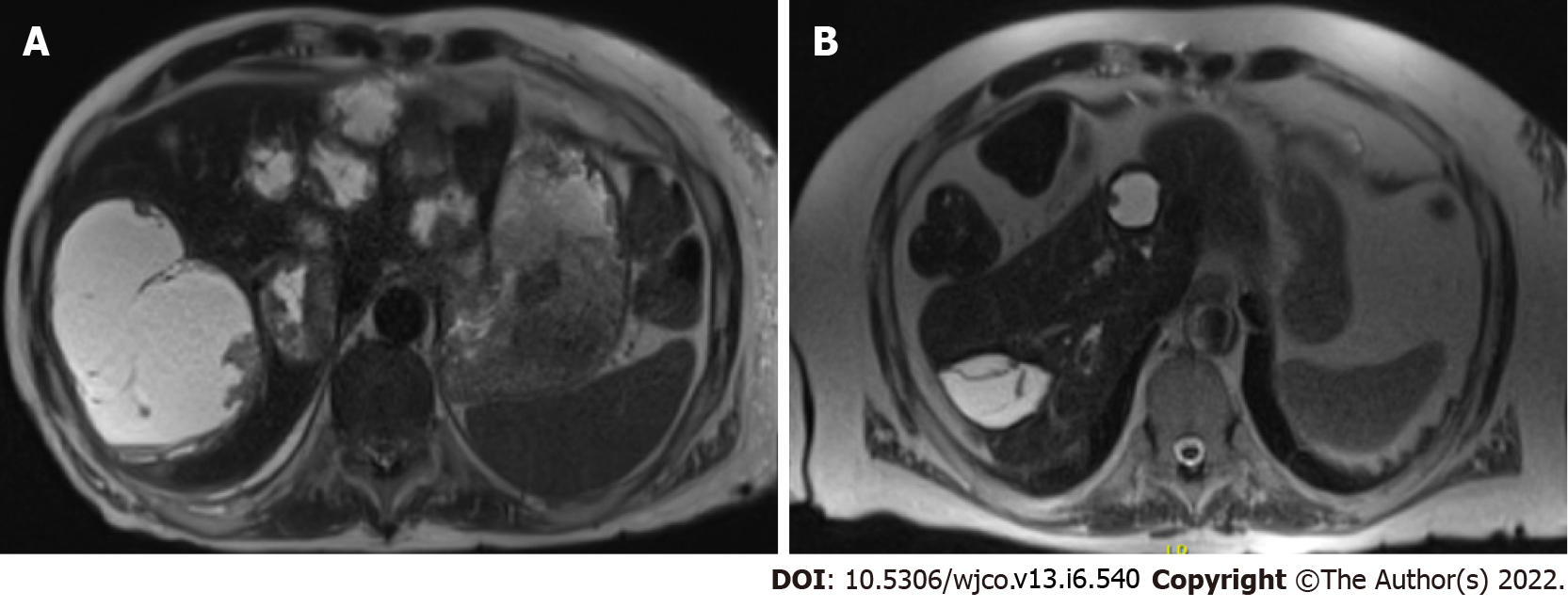
Case 5: MRI imaging of the abdomen showed over 20 cystic hepatic masses, with the largest measuring 12.5 cm x 9.2 cm, resolved on subsequent imaging (Figure 8).
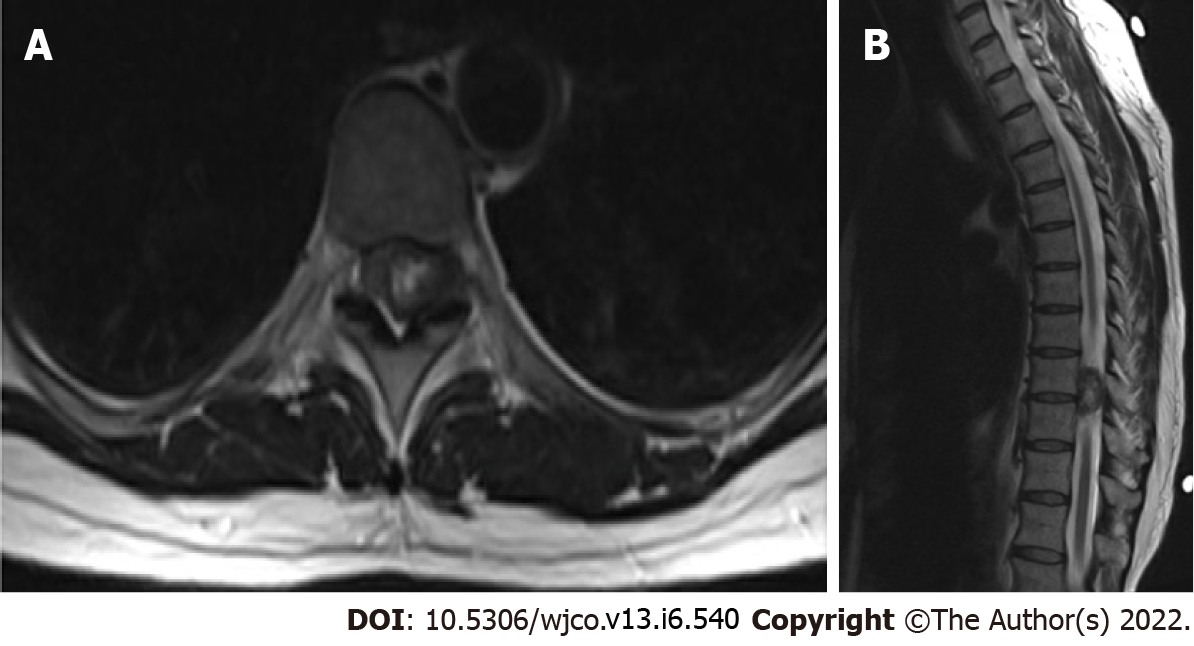
Case 6: Cervical and thoracic spine MRI which showed a 1.9 cm x 1.2 cm x 2.4 cm intradural heterogeneous mass centered within the left T9-T10 neuroforamina, with abutment of the left lateral spinal cord resulting in rightward cord displacement as well as moderate canal stenosis (Figure 9).
Case 1: A five-year-old female with a history of idiopathic dilated cardiomyopathy status post orthotopic heart transplant (OHT) at eight months old, on tacrolimus, and previous EBV viremia at age 22 mo presented with her third infection-related hospitalization in the previous six months. Presenting symptoms included fever, thrush, diarrhea, and neutropenia with an absolute neutrophil count of 260 cells per µL. She was placed on broad-spectrum antibiotics and antifungals but continued to have diarrhea, failure to thrive, as well as new oral ulcers. Due to concern for PTLD, she underwent total body CT which was unrevealing. She also underwent esophagogastroduodenoscopy and colonoscopy. In addition to the presence of colonic candidiasis, colonoscopy revealed 6-10 mm polypoid lesions with central ulceration in the sigmoid colon and rectum with biopsies consistent with an EBV-driven smooth muscle tumor (Figures 1 and 2). The patient was EBV negative before transplant with repeat EBV titers high on admission (Table 1). She was treated for colonic candidiasis as well as a pseudallescheria boydii complex infection of her lungs with symptomatic improvement. She did not undergo any EBV-SMT-directed treatment or surveillance and remains well at age 16 years.
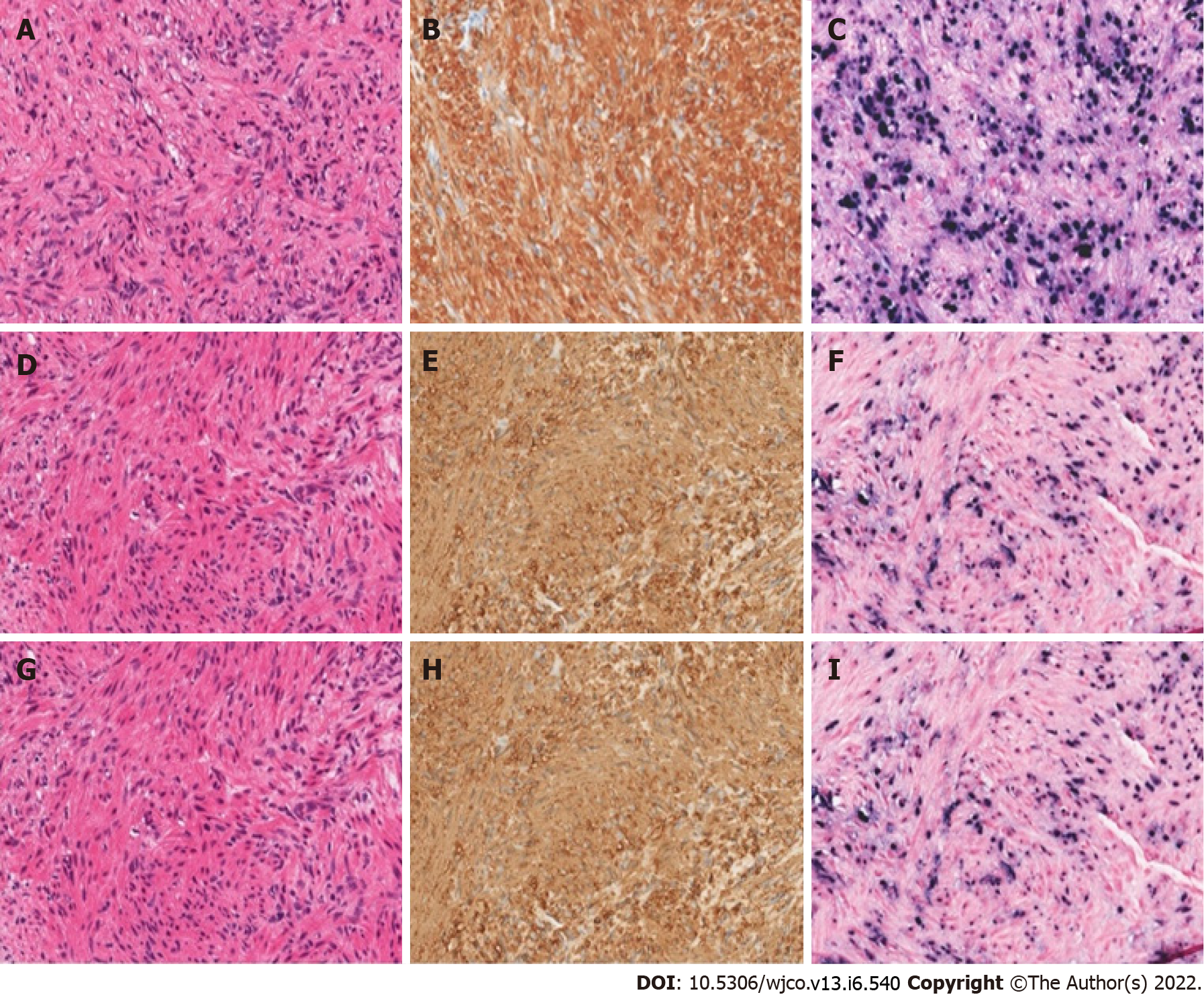
Case 2: A 20-year-old male with a history of idiopathic dilated cardiomyopathy status post OHT at age 17 on tacrolimus and mycophenolate mofetil (MMF), Crohn’s disease, alopecia, juvenile idiopathic arthritis, and common variable immune deficiency (CVID) presented to the hospital with weight loss, headaches, and myalgias. The patient had abruptly stopped his immunosuppression agents a month before admission. He was treated with antibiotics for Group A streptococcus infection. The patient was previously EBV seronegative before transplant but had elevated titers on admission (Table 1). A whole-body positron emission tomography (PET) scan showed multiple fluorodeoxyglucose (FDG) avid foci suspicious for widespread PTLD in the thymus, lung, liver, mesenteric lymph nodes, retroperitoneal lymph nodes, ascending colon, and proximal left femoral bone marrow (Figure 4). A neck magnetic resonance image (MRI) revealed a mass in the right Meckel’s cave. The patient’s tacrolimus was deceased due to suspicion for PTLD. Biopsies taken during colonoscopy and bone marrow biopsies were negative for PTLD. A liver biopsy was performed and showed a proliferation of bland spindle cells with pale eosinophilic cytoplasm and ovoid nuclei with smooth contours and pale chromatin (Figure 3A). Immunohistochemical stain for smooth muscle actin showed strong, diffuse reactivity in the neoplastic cells (Figure 3B). EBER highlighted the presence of viral genetic material, confirming the diagnosis of PT-SMT (Figure 3C). During this admission, he became febrile prompting a chest X-ray which revealed left upper lobe haziness, concerning for pneumonia. He rapidly deteriorated, developing acute renal failure requiring dialysis and respiratory distress with encephalopathy requiring intubation. He then sustained a fatal cardiac arrest. An autopsy revealed severe acute cellular rejection, negative for antibody-mediated rejection, and confirmed multisite PT-SMT.
Case 3: A 16-year-old female with a history of dilated cardiomyopathy status post OHT at 12-year-old, on MMF and tacrolimus presented to the hospital with severe headaches and altered mental status. MRI brain showed a left temporal ring-enhancing lesion (Figure 5). The patient was previously EBV seronegative before transplant but had elevated titers on admission (Table 1). She underwent a brain biopsy which showed EBV-PTLD, with morphology consistent with diffuse large B-cell lymphoma. Her immunosuppression therapy was reduced. She underwent a CT abdomen which showed a 2 cm low-attenuating hypervascular lesion in the right hepatic dome and another 0.6 cm lesion in the right anterior inferior hepatic lobe, redemonstrated on a PET scan (Figure 6). Liver biopsy showed a proliferation of spindle cells with eosinophilic cytoplasm, mild to moderate nuclear pleomorphism, and focal tumor necrosis (Figure 3D). No severe nuclear pleomorphism or mitotic activity was identified. The neoplastic cells were strongly immunoreactive for smooth muscle actin, and showed diffuse nuclear reactivity for EBER, confirming the diagnosis of PT-SMT (Figure 3E and F). A subsequent CT scan of the abdomen and pelvis showed two nonspecific small low density lesions in the left kidney, three months after the initial diagnosis of PTLD. For her CNS-PTLD, the patient had a ventriculoperitoneal shunt placed and underwent six cycles of intrathecal rituximab with methotrexate, systemic chemotherapy, whole-brain radiation, and T-cell therapy with subsequent decrease and ultimate resolution in the CNS lesion on subsequent imaging (Figure 5). Her cerebrospinal fluid studies were negative for infection. She started having generalized tonic-clonic seizures in the post-treatment setting and was treated with anti-epileptics. She remains well at the age of 22 years, with yearly liver MRI showing stable hepatic lesions (Figure 6).
Case 4: A 61-year-old male with a pre-transplant negative CMV and EBV serology underwent orthotopic heart and liver transplant from a CMV and EBV positive donor (CMV Donor+/Recipient-, EBV Donor+/Recipient-) at the age of 58 years for cirrhosis secondary to Hereditary Familial Amyloidosis (Thr60A1a mutation). He was on tacrolimus for immunosuppression and post-transplant developed asymptomatic EBV and CMV viremia as well as stage 3 chronic kidney disease secondary to calcineurin inhibitor nephrotoxicity. He was admitted for new-onset hematuria and acute kidney injury. A kidney ultrasound noted a 2 cm hypoechoic lesion in the right inferior liver lobe. A CT scan of the liver revealed multiple indeterminate liver masses (Figure 7A and B). Biopsy of one lesion showed a monotonous proliferation of relatively uniform spindle cells arranged in intersecting fascicles with pale eosinophilic cytoplasm and elongated, blunt-ended nuclei with darkly staining vesicular chromatin (Figure 3G). There was again, no significant cytologic atypia, nuclear pleomorphism, or tumor necrosis. The tumor cells were strongly immunoreactive for smooth muscle actin and showed diffuse nuclear reactivity for EBER (Figure 3H and I). These morphologic and immunohistochemical findings were consistent with a diagnosis of PT-SMT. He subsequently underwent a left lateral liver segmentectomy with additional superficial hepatic wedge resections in segments 4A, 4B, and 8. Pathology showed positive margins. Immunosuppression was initially lowered and eventually discontinued. He was later found to have elevated liver enzymes on follow-up testing and a liver biopsy confirmed severe acute cellular rejection (ACR). He was treated with steroids and restarted on sirolimus and tacrolimus with resolution of ACR as proven on subsequent biopsy and improvement of liver enzymes on laboratory testing. He also completed a course of valganciclovir for CMV viremia. However, he developed worsening neutropenia with bone marrow suppression, low-grade fevers, altered mental status requiring intubation, and anuria requiring dialysis. He was empirically started on broad-spectrum antibiotics. Infectious workup demonstrated streptococcus Gordonii bacteremia. Despite optimal treatment and supportive measures, his condition deteriorated rapidly and ended up having multi-organ failure, followed by a fatal cardiac arrest.
Case 5: A 63-year-old male with a history of a living-related donor kidney transplant at age 55 for end-stage renal disease of unknown etiology, was maintained on prednisone, MMF, and sirolimus. The patient was hospitalized for community-acquired pneumonia complicated by a parapneumonic effusion necessitating antibiotics and decortication. On CT and subsequent MRI imaging of the abdomen, he was incidentally found to have over 20 cystic hepatic masses, with the largest measuring 12.5 cm x 9.2 cm (Figure 8A). Fine needle aspiration confirmed PT-SMT. EBV DNA quantification was at 4,765 copies per milliliter with no prior pre-transplant levels. He had no evidence of distant or intra-cranial disease on imaging. MMF was stopped and sirolimus was decreased from 1.5 mg to 1 mg daily initially, and down to 0.5 mg one year later. He was also treated with a course of valganciclovir at the time of diagnosis. Annual hepatic MRIs demonstrated initial size reduction followed by stable disease without any new lesions, with the most recent imaging performed at age 72 (Figure 8B). He continues to do well with intact kidney function, despite decreasing his immunosuppression.
Case 6: A 45-year-old female with a long-standing history of HIV (CD 4 count unknown) on anti-retroviral therapy with dolutegravir, abacavir, and lamivudine presented to the primary care clinic with neck, back, and shoulder pain with associated proximal and distal left upper extremity numbness and paresthesia. With worsening symptoms after conservative therapy, she underwent cervical and thoracic spine MRI which showed a 1.9 cm x 1.2 cm x 2.4 cm intradural heterogeneous mass centered within the left T9-T10 neuroforamina, with abutment of the left lateral spinal cord resulting in rightward cord displacement as well as moderate canal stenosis (Figure 9). She underwent T8-T10 Laminectomy with mass excision, with pathology consistent with EBV-SMT. Subsequent PET revealed no FDG avid lesions. Serum EBV viral levels were not obtained and prior seronegative status was also unknown. She did not undergo any further treatment or surveillance and has not had any complications of her disease, now at age 55.
EBV-SMT in all six cases.
Immunosuppression reduction.
Supportive treatment.
Intrathecal rituximab, methotrexate, systemic chemotherapy, whole brain radiation, and T-cell therapy.
Left lateral segmentectomy with superficial wedge resection; reduction in immunosuppression, and eventually started given concern for ACR.
Reduction in immunosuppression.
T8-T10 Laminectomy; no change in HAART regimen.
Cases 2 and 4 died. Cases 1, 3, 5, 6 alive and well with no recurrence in EBV-SMT.
Here we report six cases of EBV-SMT, two of which were in pediatric patients with ages ranging from 6-61. There was an equal number of males and females in the cohort. Five out of six cases were PT-SMT while the sixth patient had HIV-SMT. One patient had CVID in addition to being a transplant recipient. Two patients died at the time of diagnosis, though neither death was attributed directly to the SMTs. Three patients in the group had single organ involvement while the rest had multiple organs involved. Two patients with CNS involvement (one with EBV-PTLD and another with HIV-SMT) underwent surgical removal of the tumor without recurrence. Interestingly, one patient in this cohort had both biopsy-proven PTLD and EBV-SMT.
A systemic review by Chen et al[15] on EBV-SMT with CNS invasion found that HIV-SMTs have a predilection for the central nervous system. This was seen in our patient with HIV-SMT whose EBV-SMT was found in the thoracic spine. In contrast, patients with PT-SMT have a propensity for extra-CNS involvement, primarily lung and liver as confirmed in a study by Jonigk et al[7]. The pathophysiology is unclear but it has been proposed these organ systems are hypervascular and may attract the proliferation of smooth muscle tumors. This is also seen in other lymphoproliferative disorders such as PTLD and non-Hodgkin lymphomas in patients with Acquired Immunodeficiency Syndrome (AIDS). Interestingly, Chen et al[15] notice a concomitant lung and liver involvement in patients with PT-SMT. This was true for two of our patients who had both lung and liver SMTs; one patient with solitary lung involvement. This raises the question of whether a liver lesion increases the chance of getting a lung lesion and not vice versa. Regardless, concomitant lung and liver lesions should be kept in mind during workup.
The latency period between either HIV infection or immunosuppression initiation and the occurrence of EBV-SMT is variable. In PT-SMT patients, previous studies have found an average latency period of three years in children compared to four years in adults[7,15]. The latency period in our patients ranged from three to eight years. For HIV-SMT patients, it was more difficult to determine the timeline between HIV infection and diagnosis of AIDS. However, latency time could be as high as 8.5 years[16,17]. For our patient with HIV, we were unable to determine this latency period. In contrast, PTLD can develop at any point after transplant, up to 10 years later, whereby a majority of cases occur within the first year post transplantation[13,14].
Additionally, multiple cases of synchronous or metachronous EBV-SMTs were seen in our patients, consistent with prior publications[15,18,19]. Jonigk et al[7] showed that patients with multiple organs involvement had worse overall survival than those with single organ involvement, while individuals with intracranial disease had the worst outcomes. However, in the study by Chen et al[15], presence of CNS SMTs, tumor multiplicity, or pre-existing medical conditions did not impact the survival rate. In our case series, one-third of our patients died and had tumor multiplicity in addition to a multitude of chronic medical conditions. These patients did not die directly due to EBV-SMTs but died of the disease while battling other complications. Age differences did not impact survival, raising the question whether age has any role in prognosis. However, it does supplement the theory that the degree of immunocompetency may determine the degree of disease aggressiveness and subsequent survival rates.
EBV-SMTs have been generally thought of as slow-growing tumors with a 1-year overall survival rate of 50%-76% for patients with HIV-SMT and PT-SMT patients and 0% for CI-SMT[8,15]. 5-year survival rate is estimated at 60% for patients with CNS involvement[15]. Patients with HIV-SMTs have been known to have higher survival but with a shorter follow-up period. A separate analysis by Jonigk et al[7] suggested that PT-SMT and CI-SMT have better outcomes than HIV-SMT. Four out of six patients in our group continue to live with stable disease or no evidence of disease. It will be prudent to continue follow-up for all EBV-SMTs to help us study the disease course and prognosis over time.
Previous studies have found that pre-transplant EBV seronegativity is a risk factor for EBV-PTLD[20]. However, the role of this factor is not known for EBV-SMT, though it is postulated that pre-transplant seronegativity and post-transplant primary EBV infection could be considered a risk factor for PT-SMT[21]. In our study, EBV status was not known for HIV patient, however, the other transplant patients were seronegative at the time of transplant and became highly seropositive at the time of diagnosis (table 1). It would be interesting to see if any absolute levels of EBV titers have any bearing on the severity of disease course and outcomes.
There is no standardized treatment for EBV-SMT, given its rarity. In the study by Jonigk et al[7], patients who underwent surgical resection had similar outcomes to those who underwent reduced immunosuppression alone without any surgery, suggesting that either may be an appropriate strategy. Most patients with CNS involvement undergo surgical resection to alleviate parenchymal tumor compression[15]. No statistically significant difference was seen in outcomes between PT-SMT and HIV-SMT in these patients. Surgical resection to alleviate parenchymal tumor compression in individuals with CNS involvement is a reasonable strategy.
EBV-SMTs are a rare oncological entity found in immunocompromised patients with either primary immunodeficiency as in Congenital Immunodeficiency or secondary immunodeficiency as seen in patients with HIV or post-transplant patients on long term immunosuppression. As the number of patients who undergo organ transplantation increases with time, the incidence of EBV-SMT may also increase. It is imperative to keep EBV-SMT on the differential in immunosuppressed individuals who develop tumors. Questions regarding the best treatment modality remain as patients are treated on a case-by-case basis.
We owe this publication to the brave patients who harbor Epstein-Barr virus associated smooth muscle tumors and have graciously allowed us to help inform the world about their rare disease.
Provenance and peer review: Unsolicited article; Externally peer reviewed.
Peer-review model: Single blind
Specialty type: Gastroenterology and hepatology
Country/Territory of origin: United States
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): B
Grade C (Good): C
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Ishikawa E, Japan; Liu D, China S-Editor: Ma YJ L-Editor: A P-Editor: Ma YJ
| 1. | Pritzker KP, Huang SN, Marshall KG. Malignant tumours following immunosuppressive therapy. Can Med Assoc J. 1970;103:1362-1365. [PubMed] |
| 2. | Al Hamed R, Bazarbachi AH, Mohty M. Epstein-Barr virus-related post-transplant lymphoproliferative disease (EBV-PTLD) in the setting of allogeneic stem cell transplantation: a comprehensive review from pathogenesis to forthcoming treatment modalities. Bone Marrow Transplant. 2020;55:25-39. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 45] [Cited by in RCA: 89] [Article Influence: 14.8] [Reference Citation Analysis (0)] |
| 3. | Young SM, Amrith S, Ting E, Wu B, Nga ME, Sundar G. EBV Smooth Muscle Tumour. In: Ocular Adnexal Lesions. Springer, 2019: 257-261. [DOI] [Full Text] |
| 4. | Vij M, Sivasankaran M, Jayaraman D, Sankaranarayanan S, Kumar V, Munirathnam D, Scott J. CARMIL2 Immunodeficiency with Epstein Barr Virus Associated Smooth Muscle Tumor (EBV-SMT). Report of a Case with Comprehensive Review of Literature. Fetal Pediatr Pathol. 2021;1-12. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 8] [Cited by in RCA: 5] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 5. | Lee ES, Locker J, Nalesnik M, Reyes J, Jaffe R, Alashari M, Nour B, Tzakis A, Dickman PS. The association of Epstein-Barr virus with smooth-muscle tumors occurring after organ transplantation. N Engl J Med. 1995;332:19-25. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 355] [Cited by in RCA: 317] [Article Influence: 10.6] [Reference Citation Analysis (0)] |
| 6. | Hussein K, Maecker-Kolhoff B, Donnerstag F, Laenger F, Kreipe H, Jonigk D. Epstein-Barr virus-associated smooth muscle tumours after transplantation, infection with human immunodeficiency virus and congenital immunodeficiency syndromes. Pathobiology. 2013;80:297-301. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 17] [Cited by in RCA: 18] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 7. | Jonigk D, Laenger F, Maegel L, Izykowski N, Rische J, Tiede C, Klein C, Maecker-Kolhoff B, Kreipe H, Hussein K. Molecular and clinicopathological analysis of Epstein-Barr virus-associated posttransplant smooth muscle tumors. Am J Transplant. 2012;12:1908-1917. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 68] [Cited by in RCA: 72] [Article Influence: 5.5] [Reference Citation Analysis (0)] |
| 8. | Hussein K, Rath B, Ludewig B, Kreipe H, Jonigk D. Clinico-pathological characteristics of different types of immunodeficiency-associated smooth muscle tumours. Eur J Cancer. 2014;50:2417-2424. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 49] [Cited by in RCA: 72] [Article Influence: 6.5] [Reference Citation Analysis (0)] |
| 9. | Deyrup AT, Lee VK, Hill CE, Cheuk W, Toh HC, Kesavan S, Chan EW, Weiss SW. Epstein-Barr virus-associated smooth muscle tumors are distinctive mesenchymal tumors reflecting multiple infection events: a clinicopathologic and molecular analysis of 29 tumors from 19 patients. Am J Surg Pathol. 2006;30:75-82. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 186] [Cited by in RCA: 170] [Article Influence: 8.9] [Reference Citation Analysis (0)] |
| 10. | Petrara MR, Giunco S, Serraino D, Dolcetti R, De Rossi A. Post-transplant lymphoproliferative disorders: from epidemiology to pathogenesis-driven treatment. Cancer Lett. 2015;369:37-44. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 92] [Cited by in RCA: 97] [Article Influence: 9.7] [Reference Citation Analysis (0)] |
| 11. | Styczynski J, Gil L, Tridello G, Ljungman P, Donnelly JP, van der Velden W, Omar H, Martino R, Halkes C, Faraci M, Theunissen K, Kalwak K, Hubacek P, Sica S, Nozzoli C, Fagioli F, Matthes S, Diaz MA, Migliavacca M, Balduzzi A, Tomaszewska A, Camara Rde L, van Biezen A, Hoek J, Iacobelli S, Einsele H, Cesaro S; Infectious Diseases Working Party of the European Group for Blood and Marrow Transplantation. Response to rituximab-based therapy and risk factor analysis in Epstein Barr Virus-related lymphoproliferative disorder after hematopoietic stem cell transplant in children and adults: a study from the Infectious Diseases Working Party of the European Group for Blood and Marrow Transplantation. Clin Infect Dis. 2013;57:794-802. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 143] [Cited by in RCA: 169] [Article Influence: 14.1] [Reference Citation Analysis (0)] |
| 12. | Nakanishi C, Kawagishi N, Sekiguchi S, Akamatsu Y, Sato K, Miyagi S, Takeda I, Fukushima D, Kobayashi Y, Ishida K, Niizuma H, Tsuchiya S, Wada M, Nio M, Satomi S. Post-transplantation lymphoproliferative disorder in living-donor liver transplantation: a single-center experience. Surg Today. 2012;42:741-751. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 6] [Cited by in RCA: 5] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 13. | Parta M, Cuellar-Rodriguez J, Freeman AF, Gea-Banacloche J, Holland SM, Hickstein DD. Resolution of Multifocal Epstein-Barr Virus-Related Smooth Muscle Tumor in a Patient with GATA2 Deficiency Following Hematopoietic Stem Cell Transplantation. J Clin Immunol. 2017;37:61-66. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 17] [Cited by in RCA: 16] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 14. | Cavazzana M, Touzot F, Moshous D, Neven B, Blanche S, Fischer A. Stem cell transplantation for primary immunodeficiencies: the European experience. Curr Opin Allergy Clin Immunol. 2014;14:516-520. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 11] [Cited by in RCA: 11] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 15. | Lau KW, Hsu YW, Lin YT, Chen KT. Role of surgery in treating epstein-barr virus-associated smooth muscle tumor (EBV-SMT) with central nervous system invasion: A systemic review from 1997 to 2019. Cancer Med. 2021;10:1473-1484. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 7] [Cited by in RCA: 4] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 16. | Gupta S, Havens PL, Southern JF, Firat SY, Jogal SS. Epstein-Barr virus-associated intracranial leiomyosarcoma in an HIV-positive adolescent. J Pediatr Hematol Oncol. 2010;32:e144-e147. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 28] [Cited by in RCA: 26] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 17. | Suankratay C, Shuangshoti S, Mutirangura A, Prasanthai V, Lerdlum S, Pintong J, Wilde H. Epstein-Barr virus infection-associated smooth-muscle tumors in patients with AIDS. Clin Infect Dis. 2005;40:1521-1528. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 71] [Cited by in RCA: 71] [Article Influence: 3.6] [Reference Citation Analysis (0)] |
| 18. | Dekate J, Chetty R. Epstein-Barr Virus-Associated Smooth Muscle Tumor. Arch Pathol Lab Med. 2016;140:718-722. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 36] [Cited by in RCA: 52] [Article Influence: 6.5] [Reference Citation Analysis (0)] |
| 19. | Jossen J, Chu J, Hotchkiss H, Wistinghausen B, Iyer K, Magid M, Kamath A, Roayaie S, Arnon R. Epstein-Barr virus-associated smooth muscle tumors in children following solid organ transplantation: a review. Pediatr Transplant. 2015;19:235-243. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 33] [Cited by in RCA: 31] [Article Influence: 3.1] [Reference Citation Analysis (0)] |
| 20. | Evens AM, Roy R, Sterrenberg D, Moll MZ, Chadburn A, Gordon LI. Post-transplantation lymphoproliferative disorders: diagnosis, prognosis, and current approaches to therapy. Curr Oncol Rep. 2010;12:383-394. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 106] [Cited by in RCA: 95] [Article Influence: 6.8] [Reference Citation Analysis (0)] |
| 21. | Stubbins RJ, Alami Laroussi N, Peters AC, Urschel S, Dicke F, Lai RL, Zhu J, Mabilangan C, Preiksaitis JK. Epstein-Barr virus associated smooth muscle tumors in solid organ transplant recipients: Incidence over 31 years at a single institution and review of the literature. Transpl Infect Dis. 2019;21:e13010. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 14] [Cited by in RCA: 23] [Article Influence: 3.3] [Reference Citation Analysis (0)] |