Published online May 24, 2022. doi: 10.5306/wjco.v13.i5.388
Peer-review started: August 26, 2021
First decision: October 22, 2021
Revised: November 3, 2021
Accepted: May 5, 2022
Article in press: May 5, 2022
Published online: May 24, 2022
Processing time: 271 Days and 6.1 Hours
The outcomes of patients diagnosed with head and neck squamous cell carcinoma (HNSCC) who are not candidates for local salvage therapy and of those diagnosed with recurrent or metastatic disease are dismal. A relatively new systemic therapy option that emerged in recent years in the treatment of advanced HNSCC is immunotherapy using immune checkpoint inhibitors (ICIs). The safety profile and anti-tumor activity of these agents demonstrated in early phase clinical trials paved the way to the initiation of several promising phase-3 trials in the field.
To evaluate the evidence on the effectiveness of ICIs in HNSCC, based on published phase-3 clinical trials.
We searched PubMed, Cochrane Library, Embase, and Scopus to identify published literature evaluating immunotherapy using ICIs in recurrent or metastatic HNSCC (R/M HNSCC) and locally advanced head and neck squamous cell carcinoma (LAHNSCC). We used a combination of standardized search terms and keywords including head and neck squamous cell carcinoma, recurrent, metastatic, locally advanced, immunotherapy, immune checkpoint inhibitors, monoclonal antibodies, programmed cell death protein-1 (PD-1), programmed death-ligand 1 (PD-L1), cytotoxic T- lymphocyte associated protein-4 (CTLA-4), and phase-3 clinical trial. A sensitive search filter was used to limit our results to randomized controlled trials.
Five phase-3 clinical trials have reported the data on the effectiveness of immunotherapy in HNSCC so far: Four in R/M HNSCC and one in LAHNSCC. In patients with R/M HNSCC, anti-PD-1 agents nivolumab and pembrolizumab demonstrated improved survival benefits in the second-line treatment setting compared to the standard of care (standard single-agent systemic therapy). While the net gain in overall survival (OS) with nivolumab was 2.4 mo [hazard ratio (HR) = 0.69, P = 0.01], that with pembrolizumab was 1.5 mo (HR = 0.80 nominal P = 0.0161). The anti-PD-L1 agent durvalumab with or without the anti-cytotoxic T- lymphocyte associated protein-4 agent tremelimumab did not result in any beneficial outcomes. In the first-line setting, in R/M HNSCC, pembrolizumab plus platinum-based chemotherapy resulted in significant improvement in survival with a net gain in OS of 2.3 mo (HR = 0.77, P = 0.0034) in the overall population and a net gain in OS of 4.2 mo in the PD-L1 positive (combined positive score > 20) population compared to standard of care (EXTREME regime). In patients with PD-L1 positive R/M HNSCC, monotherapy with pembrolizumab also demonstrated statistically significant improvement in survival compared to EXTREME. In LAHNSCC, immunotherapy using avelumab (an anti-PD-L1 agent) along with standard chemoradiation therapy did not result in improved outcomes compared to placebo plus chemoradiation therapy.
Anti-PD-1 agents provide survival benefits in R/M HNSCC in the first and second-line settings, with acceptable toxicity profiles compared to standard therapy. There is no proven efficacy in the curative setting to date.
Core Tip: Immune checkpoint inhibitors have demonstrated better survival outcomes and acceptable toxicity profiles in recurrent/metastatic head and neck squamous cell carcinoma in the first and second-line treatment settings. While anti- programmed cell death protein-1 agents demonstrated efficacy, evidence on the effectiveness of anti-programmed death ligand-1 and anti-cytotoxic T lymphocyte-associated antigen-4 agents is lacking. There is no proven efficacy in the curative setting to date. Gaps in knowledge were found in terms of predictive biomarkers and identification of patients who would benefit from immunotherapy based on biomarker assessment. Several promising trials are currently ongoing to fill this knowledge gap. Novel combination strategies to potentiate and prolong the anti-tumor activity of immune checkpoint inhibitors are also being evaluated currently.
- Citation: Poulose JV, Kainickal CT. Immune checkpoint inhibitors in head and neck squamous cell carcinoma: A systematic review of phase-3 clinical trials. World J Clin Oncol 2022; 13(5): 388-411
- URL: https://www.wjgnet.com/2218-4333/full/v13/i5/388.htm
- DOI: https://dx.doi.org/10.5306/wjco.v13.i5.388
Head and neck squamous cell carcinoma (HNSCC) is one of the major causes of cancer-associated morbidity and mortality globally[1-3]. Treatment approaches for HNSCC vary according to the stage of the disease at presentation. Around 40% of HNSCCs present at an early stage and are treated by a single treatment modality, either radical radiotherapy or surgery. The remaining 60% of cases present as locally advanced disease, and treatment options include chemoradiation or surgery followed by adjuvant therapy. However, within 3 years, over 50% of these patients relapse locally or at distant sites. Salvage approaches for the locally recurrent disease include surgery, surgery followed by re-irradiation, or re-irradiation with or without concurrent chemotherapy[4,5]. For a recurrent disease that is not amenable to salvage approach and for metastatic disease, platinum-based chemotherapy was the only available treatment option until recently. While the median survival of recurrent/metastatic HNSCC (R/M HNSCC) patients receiving platinum-based chemotherapy is 7.4 mo, some patients become refractory to platinum and die within a period of 4 mo[6-12]. Subsequently, the addition of the anti-epidermal growth factor receptor (EGFR) targeted agent cetuximab to platinum-based chemotherapy showed improvement in survival compared to platinum-based chemotherapy alone, as demonstrated in a landmark phase-3 trial in 2008[12-16].
A relatively new systemic therapy option that emerged in recent years in the treatment of advanced HNSCC is immunotherapy using immune checkpoint inhibitors (ICIs). The checkpoint pathways in the tumor microenvironment are responsible for immune escape and T cell exhaustion related to the survival of the cancer cells. ICIs are monoclonal antibodies that can block these pathways by inhibiting the binding of checkpoint proteins on the T cells to similar proteins on the tumor cells. Thus, these agents act by reinvigorating the immune cells and re-establishing the anti-tumor immune responses that promote the elimination of cancer cells. Programmed cell death protein-1 (PD-1) receptors, programmed death-ligand 1 (PD-L1) receptors, and cytotoxic T- lymphocyte associated protein-4 (CTLA-4) are the major established targets for cancer immunotherapy with ICIs, and the therapeutic effects of ICIs result from blockade of these receptors[17-20].
In recent years, many interventional studies have evaluated ICI therapy for the treatment of HNSCC. The objective of this systematic review is to gather the evidence from published phase-3 randomized controlled trials (RCTs) comparing immunotherapy with the standard of care (SOC), among patients with R/M HNSCC or locally advanced HNSCC (LAHNSCC). We aimed to evaluate and synthesize the evidence from the published phase-3 studies investigating immunotherapy in advanced head and neck cancer using checkpoint inhibitors, either alone or in combination with chemotherapy, radiation therapy, or another checkpoint inhibitor.
The study followed the preferred reporting items for systematic reviews and meta-analyses (PRISMA) guidelines[21]. We systematically searched PubMed, SCOPUS, EMBASE, and COCHRANE Library without any language limit. We used a combination of standardized search terms and keywords including head and neck squamous cell carcinoma, recurrent, metastatic, locally advanced, immunotherapy, checkpoint inhibitors, monoclonal antibodies, PD-1, PD-L1, CTLA-4, and phase-3 clinical trial. A sensitive search filter was used to limit our results to RCTs reported from January 2000 till February 2021. The initial search was conducted in February 2021. We also looked for any updates on the selected studies till April 2021. The search syntax is given in the Supplementary file.
Studies were included if they were completed phase-3 RCTs conducted among patients with R/M HNSCC or LAHNSCC, in which the intervention patients received ICI either alone or in combination with chemotherapy, radiation therapy, or with another IO and the control patients received SOC. Anatomical sites of primary tumors were oral cavity, oropharynx, hypopharynx, and larynx in the included studies. Early phase trials and observational studies were excluded. Studies involving patients with nasopharyngeal carcinoma were also excluded.
Titles generated from the initial search results were exported to EndNote. Duplicates were removed, and the remaining titles were scanned for relevance. Abstracts of articles pertaining to potentially eligible studies were independently reviewed by both authors and uncertainties were resolved through discussion. Potentially eligible studies were further evaluated for relevance, trial status (completed/ ongoing/withdrawn), and availability of results.
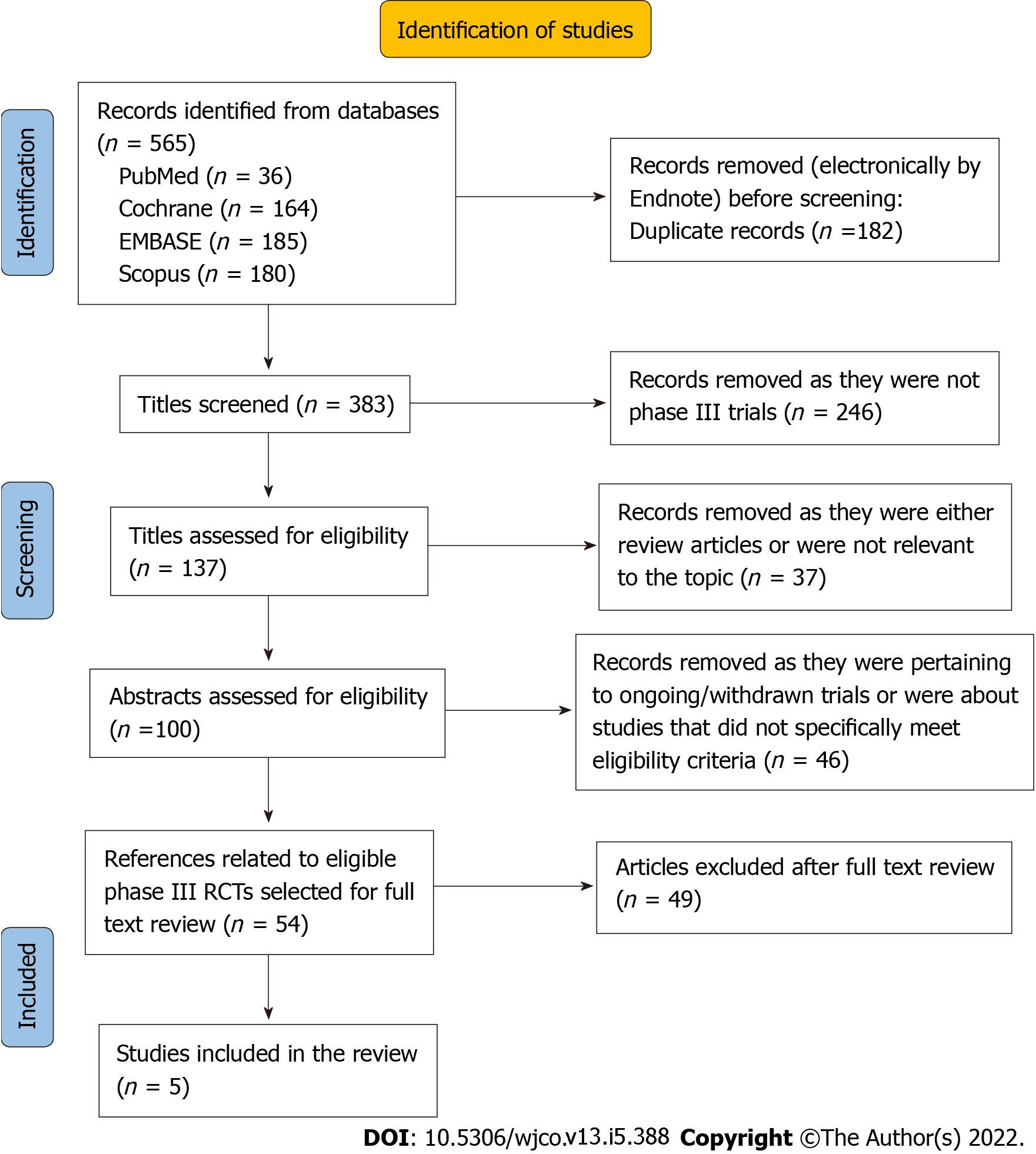
The following descriptive data were extracted from the included studies: Study design, population, details of the intervention, details of treatment received by the control arm, and the primary and secondary endpoints. Information on adverse events and statistical data on the outcomes were also extracted, which included, overall survival (OS), progression-free survival (PFS), overall response rate (ORR), biomarker effect, and patient-reported outcomes. The flow chart of study selection (PRISMA) is given in Figure 1.
The original literature search generated 565 titles altogether, of which 100 titles were eventually selected for abstract review for identification of potentially eligible studies. Others were excluded as they were related to phase-1 or phase-2 studies or not precisely relevant to the topic of the review. Through the abstract review, we identified 56 references (including one conference abstract) pertaining to potentially eligible studies. Through full-text review of these references, we selected five original phase-3 RCTs to be included in the systematic review[22-26]. In four of the trials[22-25], participants were patients with R/M HNSCC, while in one trial, participants were patients diagnosed with LAHNSCC[26,27]. All four studies among patients with R/M HNSCC were open-label RCTs; three of them investigated the effectiveness of ICI as second-line treatment[22-24], while in one study[25], ICI was evaluated as first-line treatment. The study among LAHSCC patients was a double-blinded placebo-controlled RCT[26,27].
ICIs assessed in these studies were nivolumab, pembrolizumab, durvalumab, tremelimumab, and avelumab. While nivolumab and pembrolizumab are anti-PD-1 monoclonal antibodies, durvalumab and avelumab are anti-PD-L1 antibodies. The monoclonal antibody tremelimumab is an anti-CTLA-4 agent[28-31].
We classified the studies into three groups based on the disease status and the treatment setting. The details of these studies in terms of the study population, intervention, comparator, outcomes, and adverse events are given in Table 1.
| Ref. | Design | Population | Intervention (I) | Control (C) | OS | PFS | ORR | QOL measures/symptom burden | Biomarker effect | AE Grade 3 or more |
| Phase-3 clinical trials evaluating ICI as second line therapy in R/M HNSCC | ||||||||||
| Ferris et al[22], 2016 | RCT (2:1), open-label phase-3 trial | Patients with R/M HNSCC not amenable to curative therapy | Nivolumab 3 mg/kg IV Q2W | SOC: Investigator’s, choice of methotrexate 40 mg/m2 IV weekly, docetaxel 30 mg/m2 IV weekly, or cetuximab 400 mg/m2 IV once followed by 250 mg/m2 weekly | Nivolumab: 7.5 mo (95%CI: 5.5-9.1) | Nivolumab: 2.0 mo, 95%CI: 1.9-2.1 | Nivolumab: 13.3%, 95%CI: 9.3-18.3 | Between group differences in favor of Nivolumab group | OS | Nivolumab: 13.1% |
| Checkmate 141 | n = 361 | MoA: PD-1 inhibition | n = 121 | SOC: 5.1 mo, 95%CI: 4.0-6.0; HR 0.69, 95%CI: 0.53-0.91, P = 0.01 | SOC: 2.3 mo, 95%CI: 1.9-3.1; HR 0.89, 95%CI: 0.70-1.13, P = 0.32 | SOC: 5.8%, 95%CI: 2.4-11.6 | Physical functioning: at 9 wk P = 0.01; at 15 wk, P < 0.001 | PD-L1 ≥ 1%: Nivolumab 8.7mo; SOC: 4.6 mo, HR for death 0.55 (95%CI: 0.36-0.83) | Two treatment related deaths | |
| n = 240, median follow up = 5.1 mo (range: 0 to 16.8) | Estimated 1-yr survival rate 36.0% in the nivolumab group vs 16.6% in the control group | Role functioning: at 9 wk, P = 0.003; at 15 wk, P < 0.001 | PD-L1 < 1%: Nivolumab, 5.7 mo; SOC: 5.8 mo, HR for death 0.89 (95%CI: 0.54-1.45) P for int. = 0.17 | SOC: 35.0% | ||||||
| Social functioning: at 9 wk P = 0.002; at 15 wk P < 0.001 | P16 + ve tumors: Nivolumab 9.1 mo; SOC: 4.4 mo, HR for death 0.56 (95%CI: 0.32-0.99) | One treatment related death | ||||||||
| Symptom burden pain: at 9 wk, P < 0.001; at 15 wk, P = 0.02 | P16 -ve tumours: Nivolumab 7.5 mo; SOC: 5.8 mo, HR 0.73 (95%CI: 0.42-1.25), P for Interaction = 0.55 | |||||||||
| Sensory problems: at 9 wk, P = 0.01; at 15 wk, P < 0.001 | ||||||||||
| Social contact problems: at 9 wk, P = 0.26; at 15 wk, P < 0.001 | ||||||||||
| Cohen et al[23], 2019 | RCT (1:1), open-label phase-3 trial | Patients with R/M HNSCC | Pembrolizumab: 200 mg IV Q3W | SOC: methotrexate 40 mg/m2 weekly (in absence of toxicity could increase to 60 | Pembrolizumab: 8.4 mo, 95%CI: 6.4-9.4 | Pembrolizumab: 2.1 mo 95% CI: 2.1-2.3 | Pembrolizumab: 14.6%, 95%CI: 10.4-19.6 | Exploratory HRQOL analysis (published separately) by means of EORTC QOLQ-C30, EORTC QOLQ- H&N35, and EuroQOL-5 dimensions questionnaires | OS | Pembrolizumab: 13%, treatment related death in four patients |
| KEYNOTE 040 | 3-6 mo after multimodal treatment with platinum or progression after platinum-based treatment | MoA: PD-1 inhibition | n = 248, median follow-up 7.1 mo (IQR 3.7-12.4) | SOC: 6.9 mo, 95%CI: 5.9-8.0; HR 0.80, 95%CI: 0.65-0.98, nominal p = 0.0161 | SOC: 2.3 mo, 95%CI: 2.1-2.8; HR 0.96, 95%CI: 0.79-1.16, nominal P = 0.325 | SOC: 10.1%, 95%CI: 6.6-14.5, nominal P = 0.061 | At 15 wk, GHS/QOL scores were stable with pembrolizumab: least square mean (LSM) 0.39; 95%CI: -3.00 to 3.78 | TPS ≥ 50% | SOC: 36.1%, treatment related death in two patients | |
| n = 495 | n = 247, median follow up = 7.5 mo (IQR 3.4-13.3) until data cut-off /8.4 mo (IQR 3.3-14.5) until death | PFS based on modified RECIST 1.1 | At 15 wk, GHS/QOL scores declined with SOC; (LSM -5.86; 95%CI: -9.68 to -2.04) | Pembrolizumab 11.6 mo (95%CI: 8.3-19.5); SOC: 6.6 mo (95%CI: 4.8-9.2), HR 0.53 (95%CI: 0.35-0.81; nominal P = 00014) | ||||||
| Pembrolizumab: 3.5 mo | LSM between-group difference was 6.25 points (95%CI: 1.32-11.18: nominal 2-sided P = 0.01) | TPS < 50% | ||||||||
| SOC: 4.8 mo | Pembrolizumab: 6.5 mo (95% CI 5.6-8.8); SOC: 7.1 mo (95%CI: 5.7-8.1), HR for death 0.93 (95%CI: 0.73-1.17; nominal P = 0.2675), P for int. = 0.015 | |||||||||
| CPS ≥ 1 | ||||||||||
| Pembrolizumab: 8.7 mo (95%CI: 6.9-11.4); SOC: 7.1 mo (95%CI: 5.7-8.3), HR for death = 0.74 (95%CI: 0.58-0.93) nominal P = 0.0049) | ||||||||||
| CPS < 1 | ||||||||||
| Pembrolizumab: 6.3 mo (95% CI 3.9-8.9); SOC: 7 mo (95%CI: 5.1-9.0), HR for death 1.28 (95%CI: 0.8-2.07; P = 08476) P for int.= 0.07 | ||||||||||
| PFS | ||||||||||
| Based on modified RECIST1.1 | ||||||||||
| TPS ≥ 50%: PFS longer with Pembrolizumab than with SOC | ||||||||||
| CPS ≥ 1: PFS almost equal to that in the overall population for both Pembrolizumab and SOC (3.6 mo vs 4.8 mo) | ||||||||||
| CPS < 1, & TPS < 50%: PFS longer with SOC than with Pembrolizumab | ||||||||||
| Ferris et al[24], 2020 | RCT (1:1:1), open-label phase-3 trial | R/M HNSCC not amenable to curative therapy | Arm 1 | SoC | Durvalumab: 7.6 mo 95%CI: 6.1-9.8 | Durvalumab: 2.1 mo, 95%CI: 1.9-3.0 | Durvalumab: 17.9%, 95%CI: 13.3-23.3 | Not assessed | OS | Durvalumab: 10.1%, four treatment related deaths |
| EAGLE | n = 736 | Durvalumab MoA: PD-L1 inhibition 10 mg/kg every 2 wk | Single-agent systemic therapy using one of the following: cetuximab paclitaxel, docetaxel, methotrexate, 5 FU, TS-1, or capecitabine | Durvalumab + Tremelimumab: 6.5 mo, 95%CI: 5.5-8.2 | Durvalumab + Tremelimumab: 2.0 mo, 95%CI: 1.9-2.3 | Durvalumab + Tremelimumab: 18.2%, 95%CI: 13.6-23.6 | TC ≥ 25% | Durvalumab + Tremelimumab, 16.3 %, two treatment related deaths | ||
| n = 240, median follow-up: 7.6 mo | n = 249, median follow-up = 7.8 mo | SoC: 8.3 mo, 95%CI: 7.3-9.2 | SoC: 3.7 mo, 95%CI: 3.1-3.7 | SoC: 17.3%, 95%CI: 12.8-22.5 | Durvalumab: 9.8 mo (95%CI: 4.3-14.1); Durvalumab + Tremelimumab: 4.8 mo (95%CI: 3.3-6.4); SoC: 9 mo (95%CI: 6.8-11.0) | SoC: 24.2%, No treatment related deaths | ||||
| Arm 2 | Durvalumab vs SoC: HR = 0.88, 95%CI: 0.72-1.08, P = 0.20 | Durvalumab vs SoC: HR = 1.02, 95%CI: 0.84-1.25, P = 0.75 | TC < 25% | |||||||
| Durvalumab plus Tremelimumab MoA: CTLA-4 blockade | Durvalumab + Tremelimumab vs SoC.: HR = 1.04, 95%CI: 0.85-1.26, P = 0.76 | Durvalumab + Tremelimumab vs SoC: HR = 1.09, 95%CI: 0.90-1.33, P = 0.54 | Durvalumab: 7.6 mo (95%CI: 6.2-9.5); Durvalumab + Tremelimumab: 7.8 mo (95%CI: 5.9-10.3); SoC: 8 mo (95%CI: 6.7-8.9) | |||||||
| Durvalumab: 20 mg/kg plus Tremelimumab 1 mg/kg every 4 wk-4 times, then Durvalumab: 10 mg /kg every 2 wk | TC ≥ 1%: Both treatment arms vs SoC had no difference in OS | |||||||||
| n = 247, median follow-up: 6.3 mo | TC < 1%: OS was longer for Durvalumab vs SoC; but no difference for Durvalumab + Tremelimumab vs SOC | |||||||||
| Phase-3 clinical trials evaluating ICI as first line therapy in R/M HNSCC | ||||||||||
| Burtness et al[25], 2019 | RCT (1:1:1), open-label phase-3 trial | Patients with R/M HNSCC | Arm 1: Pembrolizumab (MoA: PD-1 inhibition), monotherapy; Pembrolizumab 200 mg once every 3 wk | EXTREME regime: cetuximab 400 mg/m² loading dose, then 250 mg/m², per week plus, carboplatin (AUC 5 mg/m2) or cisplatin (100 mg/m2) and 5-FU (1000 mg/m2 for 4 consecutive days) every 3 wk | Arm 1: Pembrolizumabalone, 11.6 mo, 95%CI: 10.5-13.6 | Arm 1: Pembrolizumab alone, 2.3 mo (95%CI: 2.2-3.3) | Arm 1: Pembrolizumab, 17% | NA | OS | Pembrolizumab alone: 55% (all cause), 17% (TRAE)AE led to death in 8% of pts |
| KEYNOTE 048 | Three arms | n = 301, median follow-up: 11.5 mo | n = 300, median follow-up: 10.7 mo | Arm 2: Pembrolizumab + CT, 13.0 mo, 95%CI: 10.9-14.7 | Control arm: Cetuximab + CT 5.2 mo (95%CI: 4.9-6) | Arm 2: Pembrolizumab + CT, 36% | CPS of ≥ 20: Pembrolizumab alone vs EXTREME: 14.9 mo vs 10.7 mo, HR 0.61; 95%CI: 0.45-0.83, P = 0.0007 | Pembrolizumab + CT: 85% (all cause), 72%(TRAE), AE led to death in 12% of pts | ||
| n = 882 | Arm 2: Pembrolizumab + CT (platinum-FU), Pembrolizumab 200 mg once every 3 wk plus carboplatin (AUC 5 mg/m2) or cisplatin (100 mg/m2) and 5-FU (1000 mg/m2 for 4 consecutive days) every 3 wk | Control arm: Cetuximab + CT, 10.7 mo, 95%CI: 9.3-11.7 | Arm 2: Pembrolizumab + CT, 4.9 mo (95%CI: 4.7-6) | Control arm: Cetuximab + CT, 36% | Pembrolizumab + CT vs EXTREME: 14.7 mo vs 11.0 mo, HR 0.60; 95%CI: 0.45-0.82, P = 0.0004 | Cetuximab + CT: 83% (all cause), 69%(TRAE), AE led to death in 10% of pts | ||||
| n = 281, median follow-up: 13.0 mo | Pembrolizumab alone vs EXTREMEHR 0.85, 95%CI: 0.71-1.03, P = 0.0456 | Control arm: Cetuximab + CT, 5.1 mo (95%CI:4.9-6) | CPS of ≥ 1: Pembrolizumab alone vs EXTREME: 12.3 mo vs 10.3 mo, HR 0.78 [0.64-0.96], P = 0.0086 | |||||||
| Pembrolizumab + CT vs EXTREME, HR 0.77, 95%CI: 0.63-0.93, P = 0.0034 | Pembrolizumab alone vs EXTREME: HR = 1.34; 95%CI: 1.13-1.59 | Pembrolizumab + CT vs EXTREME: 13.6 mo vs 10.4 moHR 0.65; 95%CI: 0.53-0.80, P < 0.0001 | ||||||||
| Pembrolizumab + CT vs EXTREME: HR = 0.92, 95%CI: 0.77-1.10, P = 0.169 | PFS | |||||||||
| CPS of ≥ 20: Pembrolizumab alone vs EXTREME, 3.4 mo vs 5.0 mo, HR 0.99; 95%CI: 0.75-1.29, P = 0.456 | ||||||||||
| Pembrolizumab + CT vs EXTREME: 5.8 mo vs 5.2 mo, HR 0.73; 95%CI: 0.55-0.97, P = 0.0162 | ||||||||||
| CPS of ≥ 1: Pembrolizumab alone vs EXTREME, 3.2 mo vs 5.0 mo, HR 1.16; 95%CI: 0.96-1.39 | ||||||||||
| Pembrolizumab + CT vs EXTREME: 5.0 mo vs 5.0 mo, HR 0.82; 95% CI: 0.67-1.00 | ||||||||||
| Phase-3 clinical trials evaluating ICI for treatment of LAHNSCC | ||||||||||
| Cohen et al[26], 2020 | RCT (1:1) double blind placebo-controlled | Patients with pathologically confirmed previously untreated LA HNSCC who were eligible for definitive CRT with curative intent | Avelumab (PD-L1 inhibitor) 10 mg/kg iv every 2 wk plus CRT with cisplatin 100 mg/m2 every 3 wk plus standard fractionation of 70 Gy in 35 fractions over 7 wk | Placebo plus CRT with cisplatin 100 mg/m2 every 3 wk plus standard fractionation of 70 Gy in 35 fractions over 7 wk | OS: not reached, HR: 1.31, 95%CI: 0.93-1.85; one sided P = 0.94 | PFS: not reached, HR: 1.21, 95%CI: 0.93-1.57; one sided P = 0.92 | Avelumab + CRT: 74%, 95%CI: 69-79; based on modified RECIST 1.1 | NA | PFS | Intervention: 80 %, serious AEs in 36% pts, treatment related death 1%, 7% pts discontinued due to TRAEs |
| Lee et al[31], 2021 | Phase-3 trial | n = 697 | n = 350, median follow-up for PFS = 14.6 mo (IQR 8.5-19.6) for OS =16.7 mo (IQR 12.8-21.2) | n = 347, median follow-up for: PFS = 14.8 mo (11.6-18.8), OS =16.8 mo (IQR 13.1-20.8) | Favors control arm | Favors control arm | Placebo + CRT: 75%; 95%CI: 70-79; based on modified RECIST 1.1 | Avelumab + CRT vs Placebo + CRT, PD-L1 ≥ 25%: HR 0.59 (95%CI: 0.28-1.22); PD-L1 < 25%, HR: 1.37 (95%CI: 1.00-1.88), P for int. = 0.03 | Control: 74%, serious AEs in 32% pts, treatment related death < 1%, 3% pts discontinued due to TRAEs | |
| JAVELIN head and neck 100 trial | OR = 0.95; 95%CI: 0.66-1.35, P = 0.62 | |||||||||
So far, three phase-3 RCTs have compared the effectiveness of ICI against the existing SOC (single-agent systemic therapy with methotrexate, docetaxel, or cetuximab) in the second-line treatment setting[22-24] (Table 1).
Ferris et al[22] conducted a randomized, open-label, phase-3 study (n = 361) among patients with platinum-refractory recurrent HNSCC (recurrence within 6 mo after platinum-based chemotherapy) to investigate the effectiveness of the anti-PD-1 checkpoint inhibitor agent nivolumab. The intervention arm (n = 240) received nivolumab at a dose of 3 mg/kg body weight every 2 wk, while the control patients (n = 121) received SOC in the form of standard single-agent systemic therapy with methotrexate [40 mg/m2 intravenously (IV) weekly], docetaxel (30 mg/m2 IV weekly), or cetuximab (400 mg/m2 IV once followed by 250 mg/m2 weekly). OS was the primary endpoint of the study. Secondary endpoints included PFS, ORR, and biomarker effects on survival, safety, and quality of life assessments. The median duration of follow-up was 5.1 mo (range, 0 to 16.8).
OS: The median OS was 7.5 mo [95% confidence interval (CI): 5.5-9.1] with nivolumab vs 5.1 mo (95%CI: 4.0-6.0) with SOC [hazard ratio (HR) = 0.69; 97.73%CI: 0.53-0.91; P = 0.01]. The estimated 1-year survival rate was 36.0% in the nivolumab group vs 16.6% in the control group.
PFS: PFS was reported as 2 mo (95%CI: 1.9-2.1) with nivolumab vs 2.3 mo (95%CI: 1.9-3.1) with SOC (HR = 0.89; 95%CI: 0.70-1.13; P = 0.32).
ORR: ORR was 13.3% (95%CI: 9.3-18.3) in the intervention arm with nivolumab, whereas it was 5.8% (95%CI: 2.4-11.6) in the control arm (SOC).
Patient-reported outcomes (quality of life): Physical, role, and social functioning (assessed by means of EORTC QOLQ-C30) as well as symptom burden (assessed using EORTC QLQ-H&N35) remained stable or slightly improved with nivolumab, while SOC patients had a decline in QOL. Statistical analysis showed significant between-group differences in physical functioning (P = 0.01 at 9 wk; P < 0.001 at 15 wk), role functioning (P = 0.003 at 9 wk; P < 0.001 at 15 wk), social functioning (P = 0.002 at 9 wk; P < 0.001 at 15 wk), pain (P < 0.001 at 9 wk; P = 0.02 at 15 wk), sensory problems (P = 0.01 at 9 wk; P < 0.001 at 15 wk), and social contact problems (P = 0.26 at 9 wk; P < 0.001 at 15 wk).
Biomarker effect: Biomarker effect on OS was evaluated after stratifying patients based on their PD-L1 expression status (≥ 1% vs < 1%). Among patients with PD-L1 ≥ 1%, median OS was 8.7 mo with nivolumab vs 4.6 mo with SOC (HR = 0.55; 95%CI: 0.36-0.83), whereas in patients with PD-L1 < 1%, median OS was 5.7 mo with nivolumab vs 5.8 mo with SOC (HR for death = 0.89; 95%CI: 0.54-1.45; P for interaction = 0.17). Post-hoc exploratory subgroup analysis based on p16 status was also done in this study. Among patients with p16 positive tumors, the median OS was 9.1 mo with nivolumab vs 4.4 mo with SOC (HR for death 0.56; 95%CI: 0.32-0.99), whereas, among patients with p16 negative tumors, the median OS was 7.5 mo with nivolumab vs 5.8 mo with SOC (HR =0.73; 95%CI: 0.42-1.25; P for interaction = 0.55).
Adverse events: In CheckMate 141, adverse events of grade 3 or more occurred in 13.1% of patients with nivolumab vs 35% with SOC. Two patients in the nivolumab arm and 1 patient in the control arm had treatment-related death. The most common adverse events (of any grade) with nivolumab were fatigue, nausea, decreased appetite, pruritis, and rash. Gastrointestinal side effects (primarily diarrhea) were less in the nivolumab group (6.8%) compared to SOC patients (14.4%), whereas adverse events of skin (rash and pruritus) were more common in the nivolumab group (15.7%) than in the SOC patients (12.6%). Endocrine system-related side effects (hypothyroidism) were also more with nivolumab (7.6%) compared to SOC (0.9%)[22].
In this open-label phase-3 RCT, the investigators tested the efficacy and safety of the immune checkpoint inhibitor pembrolizumab (an anti-PD-1 monoclonal antibody) compared to standard therapy for the treatment of metastatic/recurrent head and neck cancer[23]. This was a multi-center study involving 97 medical centers across 20 countries. There were 247 patients in the intervention arm, while the control arm included 248 patients. Patients with platinum-refractory recurrent or metastatic (or both) HNSCC were included in this study. PD-L1 expression was assessed and categorized according to the tumor proportion score (≥ 50% vs < 50%) as well as the combined positive score (≥ 1 vs < 1) The intervention arm received pembrolizumab 200 mg every 3 wk, while the control arm received investigator’s choice of standard doses of methotrexate (40 mg/m2 IV weekly), docetaxel (75 mg/m2 IV every 3 wk) or cetuximab (250 mg/m2 IV weekly following a loading dose of 400 mg/m2).
OS: Primary outcome of the study was OS. The median OS was 8.4 mo (95%CI: 6.4-9.4) with pembrolizumab vs 6.9 mo (95%CI: 5.9-8.0) with SOC (HR = 0.80; 95%CI: 0.65-0.98; nominal P = 0.0161).
PFS: PFS was 2.1 mo (95%CI: 5.9-8.0) with pembrolizumab vs 2.3 mo (95%CI: 2.1-2.8) with SOC (HR = 0.96; 95%CI: 0.79-1.16; nominal P = 0.325).
ORR: ORR was 14.6% (95%CI: 10.4-19.6) with pembrolizumab vs 10.1% (95%CI: 6.6-14.5) with SOC (nominal P = 0.061).
Patient-reported outcomes: Results (published separately in another article) of an exploratory health-related quality of life analysis showed that at 15 wk, global health status/quality of life (GHS/QOL) scores were stable with pembrolizumab with a least square mean (LSM) of 0.39; 95%CI: -3.00-3.78), while GHS/QOL scores declined with SOC (LSM -5.86; 95%CI: -9.68 to -2.04). LSM between-group difference was 6.25 points (95%CI: 1.32-11.18: nominal 2-sided P = 0.01)[32].
Biomarker effect: Cohen et al[23] found statistically significant interaction between PD-L1 expression [in terms of tumor proportion score (TPS) and combined positive score (CPS)] and treatment effect in KEYNOTE 040. Among patients with TPS ≥ 50%, median OS was 11.6 mo (95%CI: 8.3-19.5) with pembrolizumab vs 6.6 mo (95%CI: 4.8-9.2) with SOC (HR = 0.53;95%CI: 0.35-0.81; nominal P = 00014). Among patients with TPS < 50%, OS was 6.5 mo (95%CI: 5.6-8.8) with pembrolizumab vs 7.1 mo (95%CI: 5.7-8.1) with SOC (HR = 0.93;95%CI: 0.73-1.17; nominal P = 0.2675; P for interaction = 0.015). Similarly, among patients with CPS ≥ 1, median OS was 8.7 mo (95%CI: 6.9-11.4) with pembrolizumab vs 7.1 mo (95%CI: 5.7-8.3) with SOC (HR = 0.74; 95%CI: 0.58-0.93; nominal P = 0.0049). Among patients with CPS < 1, OS was 6.3 mo (95%CI: 3.9-8.9) with pembrolizumab vs 7.0 mo (95%CI: 5.1-9.0) with SOC (HR = 1.28; 95%CI: 0.8-2.07; P = 08476; P for interaction = 0.07). In terms of PFS, based on the modified RECIST1.1, for patients with TPS ≥ 50%, PFS was longer with pembrolizumab than with SOC, whereas for patients with CPS ≥ 1, PFS was slightly lower (3.6 mo) with pembrolizumab compared to SOC (4.8 mo). Among patients with CPS < 1 and those with TPS < 50%, PFS was longer for SOC compared to pembrolizumab[23].
Adverse events: In KEYNOTE 040, adverse events of grade 3 or more occurred in 13% of patients with pembrolizumab vs 36.1% with SOC. Four patients in the pembrolizumab arm and 2 patients in the control arm had treatment-related death. While hypothyroidism was the most common treatment-related adverse event with pembrolizumab (13%), fatigue was the most common adverse event with SOC (18%)[23].
Ferris et al[24] conducted an open-label phase-3 RCT among 736 patients with R/M HNSCC not amenable to curative therapy[24]. In this three-arm study (1:1:1), one of the intervention arms (n = 240, median follow-up 7.6 mo) received the anti PD-L1 agent durvalumab (10mg/kg every 2 wk), and the other intervention arm (n = 247, median follow-up 6.3 mo) received durvalumab (20 mg/kg every 4 wk-4 times followed by 10 mg /kg every 2 wk) plus the anti CTLA-4 agent tremelimumab (1 mg/kg every 4 wk-4 times). The control arm (n = 240 median follow-up 7.8 mo) received investigator’s choice of a standard single-agent [cetuximab, paclitaxel, docetaxel, methotrexate, 5-fluorouracil (5-FU), TS-1, or capecitabine] systemic therapy (SOC) dosed and administered according to local regulations.
OS: Primary outcome of the EAGLE study was OS. The median OS was reported as 7.6 mo (95%CI: 6.1-9.8) with durvalumab vs 8.3 mo (95%CI: 7.3-9.2) with SOC (HR = 0.88; 95%CI: 0.72-1.08, P = 0.20), whereas it was 6.5 mo (95%CI: 5.5-8.2) with durvalumab plus tremelimumab vs 8.3 mo with SOC (HR = 1.04; 95%CI: 0.85-1.26, P = 0.76).
PFS: PFS was 2.1 mo with durvalumab (95%CI: 1.9-3.0) vs 3.7 mo (95%CI: 3.1-3.7) with SOC (HR = 1.02; 95%CI: 0.84-1.25, P = 0.75). PFS with durvalumab plus tremelimumab was 2.0 mo (95%CI: 1.9-2.3) vs 3.7 mo (95%CI: 3.1-3.7) with SOC (HR = 1.09; 95%CI: 0.90-1.33, P = 0.54).
ORR: ORRs were 17.9% (95%CI: 13.3-23.3) with durvalumab monotherapy, 18.2% (95%CI: 13.6-23.6) with durvalumab plus tremelimumab, and 17.3% (95%CI: 12.8-22.5) with SOC.
Patient-reported outcomes: QOL measures were not assessed in the study.
Biomarker effect: In the EAGLE study, investigators measured PD-L1 expression in terms of percentage of tumor cell (TC). Among patients with TC ≥ 25%, the median OS was 9.8 mo (95%CI: 4.3-14.1) with durvalumab and 4.8 mo (95%CI: 3.3-6.4) with durvalumab plus tremelimumab, while SOC patients had an OS of 9.0 mo (95%CI: 6.8-11.0). Among patients with TC < 25%, the median OS with SOC was 8.0 mo (95%CI: 6.7-8.9), whereas it was 7.6 mo (95%CI: 6.2-9.5) with durvalumab and 7.8 mo (95%CI: 5.9-10.3) with durvalumab plus tremelimumab. In patients with TC ≥ 1%, both intervention groups had no difference in OS compared to SOC. In patients with TC < 1%, OS was higher with durvalumab compared to SOC, but no difference in OS was found between the durvalumab plus tremelimumab arm and the SOC arm.
Adverse events: In the EAGLE study, 10.1% of patients in the durvalumab arm, 16.3% patients in the durvalumab plus tremelimumab arm, and 24.2% patients in the control arm developed adverse events of grade 3 or more. Six patients died due to treatment-related issues: 4 with durvalumab, 2 with durvalumab plus tremelimumab, and 0 with SOC. Hypothyroidism was the most common treatment-related adverse event (of any grade) in the durvalumab (11.4%) arm as well as in the durvalumab plus tremelimumab arm (12.2%). Anemia was the most common treatment-related adverse event in the SOC arm (17.5%)[24].
Prior to immunotherapy, the standard first-line treatment option for R/M HNSCC was the EXTREME regime, a combination of cetuximab, platinum (carboplatin or cisplatin), and 5-FU[13]. So far, one phase-3 trial has evaluated immunotherapy against the EXTREME regime in the first-line treatment setting for patients diagnosed with R/M HNSCC.
In this large three-arm RCT (n = 882), one of the intervention arms (n = 301, median follow-up: 11.5 mo) received pembrolizumab as monotherapy (pembrolizumab 200 mg once every 3 wk), while the second intervention arm (n = 281, median follow-up: 13.0 mo) received pembrolizumab (200 mg once every 3 wk) along with platinum-based chemotherapy {carboplatin [area under the curve (AUC) 5 mg/m2] or cisplatin (100 mg/m2) and 5-FU (1000 mg/m2 for 4 consecutive d) every 3 wk}. The control arm (n = 300, median follow-up: 10.7 mo) received the EXTREME regime [cetuximab 400 mg/m² loading dose, then 250 mg/m² per week plus carboplatin (AUC 5 mg/m2) or cisplatin (100 mg/m2) and 5-FU (1000 mg/m2 for 4 consecutive days) every 3 wk][25] (Table 1).
OS: The median OS (primary end point) was 11.6 mo (95%CI: 10.5-13.6) with pembrolizumab monotherapy vs 10.7 mo (95%CI: 9.3-11.7) with EXTREME (HR = 0.85; 95%CI: 0.71-1.03; P = 0.0456). In the pembrolizumab plus chemotherapy arm, median OS was 13.0 mo vs 10.7 mo (95%CI: 9.3-11.7) in the EXTREME arm (HR = 0.77; 95%CI: 0.63-0.93; P = 0.0034).
PFS: PFS was assessed as a primary outcome and was reported as 2.3 mo (95%CI: 2.2-3.3) with pembrolizumab monotherapy vs 5.2 mo (95%CI: 4.9-6.0) with EXTREME (HR = 1.34; 95%CI: 1.13-1.59). In the pembrolizumab plus chemotherapy arm PFS was 4.9 mo (95%CI: 4.7-6.0) vs 5.1 mo (95%CI: 4.9-6) in the EXTREME arm (HR = 0.92; 95%CI: 0.77-1.10; P = 0.169).
ORR: The pembrolizumab monotherapy arm had an ORR of 17% compared to 36% in the EXTREME arm. With pembrolizumab plus chemotherapy, ORR was similar to that with EXTREME (36%).
Biomarker effect: In KEYNOTE 048, PD-L1 expression was measured as CPS. For patients with CPS ≥ 20, median OS with pembrolizumab monotherapy was 14.9 mo vs 10.7 mo with EXTREME (HR = 0.61; 95%CI: 0.45-0.83; P = 0.0007), while median OS with pembrolizumab plus chemotherapy was 14.7 mo vs 11.0 mo with EXTREME (HR = 0.60; 95%CI: 0.45-0.82; P = 0.0004). Similarly, for patients with CPS ≥ 1, median OS with pembrolizumab monotherapy was 12.3 mo vs 10.3 mo with EXTREME (HR = 0.78; 95%CI: 0.64-0.96; P = 0.0086), whereas OS was 13.6 mo in the pembrolizumab plus chemotherapy arm vs 10.4 mo with EXTREME (HR = 0.65; 95%CI: 0.53-0.80; P < 0.0001).
For patients with CPS ≥ 20, median PFS with pembrolizumab monotherapy was 3.4 mo vs 5.0 mo with EXTREME (HR = 0.99; 95%CI: 0.75-1.29; P = 0.456). Median PFS with pembrolizumab plus chemotherapy was 5.8 mo vs 5.2 mo with EXTREME (HR = 0.73; 95%CI: 0.55-0.97; P = 0.0162). Similarly, for patients with CPS ≥ 1, median PFS with pembrolizumab monotherapy was 3.2 mo vs 5.0 mo with EXTREME (HR = 1.16; 95%CI: 0.96-1.39), whereas PFS was 5.0 mo with pembrolizumab plus chemotherapy vs 5.0 mo with EXTREME (HR = 0.82; 95%CI: 0.67-1.00).
Adverse events: In KEYNOTE 048, 55% patients in the pembrolizumab arm, 85% patients in the pembrolizumab plus chemotherapy arm, and 83% patients in the control arm developed grade 3 or more adverse events of any cause. Of these, treatment-related adverse events consisted of 17% in the pembrolizumab alone group, 72% in the pembrolizumab plus chemotherapy group, and 69% in the control group. While adverse events led to death in 8% of patients in the pembrolizumab arm and 12% of patients in the pembrolizumab plus chemotherapy arm, 10% in the control arm also died of adverse events. Major adverse events (of any grade) in the intervention groups were anemia, fatigue, hypothyroidism, and nausea[25].
The current SOC for the treatment of LAHNSCC is concurrent chemoradiation therapy (CRT)[33]. So far, only one phase-3 trial has investigated the usefulness of adding an ICI to concurrent CRT.
The preliminary results of the study were presented in the 2020 European Society for Medical Oncology annual meeting by Cohen et al[26] followed by a recent journal publication[27].
This study (n = 697) was conducted among patients with previously untreated LA HNSCC who were eligible for definitive CRT with curative intent. The intervention arm (n = 350; median follow-up for PFS 14.6 mo, for OS 16.7 mo) received the PD-L1 inhibitor avelumab (10 mg/kg IV every 2 wk) plus CRT, which consisted of cisplatin (100 mg/m2 every 3 wk) concurrently with intensity-modulated radiotherapy (standard fractionation of 70 Gy in 35 fractions over 7 wk). The control arm (n = 347; median follow-up for PFS 14.8 mo, for OS 16.8 mo) received placebo plus CRT (Table 1).
PFS: Median PFS (primary endpoint) was not reached in the intervention group or the control group. Statistical reports showed that hazard ratio (HR= 1.21; 95%CI: 0.93-1.5; one-sided P = 0.92) did not favor the avelumab plus CRT arm.
OS: OS was one of the secondary endpoints in this trial. Median OS was not reached in either study group. Statistical reports showed that the hazard ratio for death (HR = 1.31; 95%CI: 0.93-1.85; one-sided P = 0.937) did not favor the avelumab plus CRT arm.
ORR: Based on modified RECIST 1.1, ORR in the intervention arm was 74% (95%CI: 69-79) and that in the control arm was 75% (95%CI: 70-79) with an OR of 0.95 (95%CI: 0.66-1.35, P = 0.62).
Biomarker: Exploratory subgroup analysis of PFS based on PD-L1 expression showed that patients with PD-L1 ≥ 25% had an HR of 0.59 (95%CI: 0.28-1.22), while patients with PD-L1 < 25% had an HR of 1.37 (95%CI: 1.00-1.88) with avelumab plus CRT compared to placebo plus CRT (P for interaction = 0.03).
Adverse events: Treatment-related adverse events of grade 3 or more occurred in 80% of patients in the avelumab arm and in 74% of patients in the control arm. Serious adverse events occurred in 36% of patients in the intervention arm and in 32% of patients in the control arm. In the intervention arm, 7% of patients discontinued due to treatment-related adverse events vs 3% in the control arm[27].
ICIs have emerged as a novel treatment strategy for HNSCC in recent years. The safety profile and anti-tumor activity of these agents demonstrated in early phase clinical trials paved the way for the initiation of several promising phase-3 trials in the field. Safety profile and clinical activity of pembrolizumab were first reported in KEYNOTE 012, an open-label phase 1b trial among patients with R/M HNSCC[34]. KEYNOTE 055, a phase-2 trial conducted among patients with platinum-resistant R/M HNSCC also reported manageable toxicity and an acceptable safety profile of pembrolizumab[35]. The study demonstrated a clinically meaningful anti-tumor activity of the agent in terms of ORRs and survival. These findings led to the initiation of KEYNOTE 040, the phase-3 trial investigating pembrolizumab for treating patients with platinum-refractory R/M HNSCC, and KEYNOTE 048, the phase-3 trial investigating pembrolizumab as first-line therapy in R/M HNSCC[23,25]. Similarly, two phase-2 trials, the HAWK study (a single-arm study investigating durvalumab monotherapy in R/M HNSCC with > 25% tumor PD-L1 expression) and the CONDOR phase-2 trial (an RCT investigating durvalumab with or without tremelimumab in PD-L1 Low/negative R/M HNSCC) served as the rationale for investigating combination immunotherapy regimens in platinum-refractory R/M HNSCC and to initiate the EAGLE study[24,36,37]. Studies on the effectiveness of nivolumab in other solid tumors supported the initiation of CheckMate 141 trial, the first phase-3 trial of nivolumab among patients with platinum-resistant R/M HNSCC[22,38]. Chemotherapy and radiotherapy, alone or in combination, have demonstrated potential synergetic effects when combined with immunotherapy in early phase studies. This phenomenon and the proven effectiveness of the anti-PD-L1 agent avelumab in other advanced solid tumors paved the way to the JAVELIN head and neck 100 trial, the first phase-3 RCT to investigate the effectiveness of combining ICI with chemoradiation in locally advanced head and neck cancer[27,39,40].
In this systematic review, we included the published phase-3 clinical trials evaluating the effectiveness of ICIs in HNSCC. Five studies met our eligibility criteria. Three studies (CheckMate 141, KEYNOTE 040, and EAGLE study) evaluated ICI as second-line treatment for R/M HSCC, one study (KEYNOTE 048) evaluated ICI as first-line treatment for R/M HSCC, while one phase-3 trial (JAVELIN head and neck 100 trial) evaluated the effectiveness of immunotherapy in LAHNSCC[22-27].
In the second-line treatment setting, nivolumab in CheckMate 141 and pembrolizumab in KEYNOTE 040 demonstrated promising outcomes among patients with platinum-refractory R/M HNSCC[22,23]. In CheckMate 141, the anti-PD-1 agent nivolumab showed a statistically significant 31% reduction in risk of death (HR = 0.69, P = 0.01) and a net gain of 2.4 mo in terms of OS. A 2.3-fold increase in ORR was also reported with nivolumab compared to SOC. A favorable toxicity profile was another finding with nivolumab, with lower rates of treatment-related adverse events of grade 3 or more compared to SOC (13.1% vs 35%). Patient-reported QOL measures remained stable with nivolumab, while a decline in QOL occurred among the control patients. However, the study did not demonstrate any significant PFS benefits with nivolumab (HR = 0.89, 95%CI: 0.70-1.13; P = 0.32). Regarding the impact of biomarkers, survival benefit with nivolumab was found to be irrespective of PD-L1 expression (P for int. = 0.17) in the subgroup analyses based on PD-L1 status, although patients with PD-L1 ≥ 1% had a better magnitude of effect (HR = 0.55) than those with PD-L1 < 1 (HR = 0.89)[22,41,42]. Similarly, based on the post-hoc exploratory subgroup analysis according to p16 status, the investigators concluded that the longer median OS with nivolumab was irrespective of the p16 status (P for interaction = 0.55).
In KEYNOTE 040, the anti-PD-1 agent pembrolizumab demonstrated statistically significant improvement in OS with a 20% reduction in risk of death (HR = 0.80, P = 0.016) compared to SOC in the overall study population[23]. Higher ORR (14.6% vs 10.1%, nominal P = 0.061) and lower rates of adverse events of grade 3 or more (13% vs 36.1%) were also demonstrated with pembrolizumab compared to SOC. At 15 wk, stable GHS/QOL scores were reported with pembrolizumab, while the control patients had a decline in QOL. The study did not, however, demonstrate any PFS benefits with pembrolizumab (HR = 0.96, nominal P = 0.325) compared to SOC. Exploratory subgroup analyses based on PD-L1 expression demonstrated statistically significant interactions between treatment effects and PD-L1 status. For patients with TPS ≥ 50% and CPS > 1, the treatment effects of pembrolizumab vs SOC were found to be higher than in those with TPS < 50% and CPS < 1[23]. For instance, in terms of OS, patients with TPS ≥ 50% had a net gain of 5 mo with a 47% reduction in risk of death with pembrolizumab compared to SOC (HR = 0.53, nominal P = 00014), suggesting PD-L1 expression may be explored as a predictive biomarker while selecting patients for pembrolizumab therapy. Based on the findings of CheckMate 141 and KEYNOTE 040, nivolumab and pembrolizumab were approved as standard second-line treatment options for platinum-resistant R/M HNSCC[22,23,43].
The EAGLE study did not detect any statistically significant improvements in OS with durvalumab (HR = 0.88, P = 0.20) or with durvalumab plus tremelimumab (HR = 1.04, P = 0.76) compared to SOC. Again, there were no significant benefits in terms of PFS with durvalumab or with durvalumab plus tremelimumab, compared to SOC. However, investigators of EAGLE have postulated that control patients in the study had an unexpectedly high OS as the data were confounded by discrepancies in performance status favoring the control arm. Option of using paclitaxel as SOC (paclitaxel was not an option in the other two studies in the second-line setting), and subsequent immunotherapy after discontinuation of SOC treatment by control patients were also mentioned as reasons for this finding[24]. Although the primary objectives were not met, one positive finding was that the rates of adverse events of grade 3 or more were lower with immunotherapy compared to SOC.
In the first-line treatment setting, in KEYNOTE 048, pembrolizumab with platinum-based chemotherapy demonstrated statistically significant improvements in OS (13.0 vs 10.7 mo) with a 23% reduction in risk of death (HR = 0.77, P = 0.0034) compared to cetuximab plus platinum-based chemotherapy (EXTREME) in the total population. Pembrolizumab monotherapy was found to be non-inferior to EXTREME (HR = 0.85; 95%CI: 0.71-1.03; P = 0.0456) in terms of OS (11.6 mo vs 10.7 mo) in the total population. No significant impact on PFS was detected with pembrolizumab alone or pembrolizumab with chemotherapy compared to EXTREME in the overall population. Pembrolizumab alone had a lower ORR (17%) compared to EXTREME (36%), while pembrolizumab plus chemotherapy had an ORR (36%) like that of EXTREME. Interestingly, biomarker (PD-L1) based stratified analysis demonstrated superiority in terms of OS in the CPS ≥ 20 and CPS ≥ subgroups with pembrolizumab alone as well as with pembrolizumab plus chemotherapy compared to EXTREME. For instance, within the CPS ≥ 20 population, pembrolizumab monotherapy compared to EXTREME resulted in a net gain of 4.2 mo in terms of OS (14.9 mo vs 10.7 mo) with a highly significant 39% reduction in risk of death (HR = 0.61, P = 0.0007). In the CPS ≥ subgroup pembrolizumab monotherapy also demonstrated superiority in terms of OS (12.3 mo vs 10.3 mo) compared to EXTREME (HR = 0.78, P = 0.0086), indicating that pembrolizumab monotherapy is a suitable treatment option for PD-L1 positive R/M HNSCC. Similarly, in both subgroups, pembrolizumab with chemotherapy resulted in statistically significant improvements in OS compared to EXTREME. For instance, R/M HNSCC patients with CPS ≥ 20 had a highly significant 40% reduction in risk of death with pembrolizumab plus chemotherapy compared to EXTREME (HR = 0.60, P = 0.0004). Patients with CPS ≥ 1 also had a significant reduction in risk of death with pembrolizumab plus chemotherapy compared to EXTREME (HR = 0.65, P < 0.0001). These findings indicate that tumor PD-L1 expression can be a predictive biomarker for identifying patients who will benefit from pembrolizumab[25,44].
Based on the findings from KEYNOTE 048, pembrolizumab monotherapy was approved as an appropriate SOC for PD-L1 positive R/M HNSCC, and pembrolizumab plus platinum-based chemotherapy became the new SOC for the treatment of R/M HNSCC in the first-line setting[25,43]. In this study, rates of treatment-related adverse events of grade 3 or more were lower with pembrolizumab monotherapy (17%) compared to EXTREME (69%). However, rates of treatment-related adverse events of grade 3 or more were noticeably high (72%) in the combination therapy arm[25]. This finding highlights the importance of weighing up the survival benefits of the pembrolizumab plus chemotherapy regime against its adverse events profile while making treatment decisions for patients with R/M HNSCC.
Regarding immunotherapy in LAHNSCC, there is no definite evidence of benefit according to the primary results of the JAVELIN study[26,27]. The combination of avelumab and CRT did not demonstrate any beneficial outcomes in terms of PFS or OS over placebo plus CRT, and based on the modified RECIST 1.1, there were no ORR benefits (74% vs 75%) either. Moreover, avelumab plus CRT resulted in slightly higher rates of adverse events of grade 3 or more compared to CRT plus placebo (80% vs 74%). As an explanation for the absence of PFS benefits, the investigators postulated that the dysfunction of T cells or changes in the tumor microenvironment after radiotherapy might have reduced the ability of the immune system to eliminate the microscopic disease. A recent phase-2 randomized trial of pembrolizumab with radiation therapy against cetuximab with radiotherapy in LAHNSCC also failed to demonstrate significant treatment benefits, although the combination therapy had a favorable toxicity profile[45]. Similarly, a previous randomized phase-2 trial of nivolumab with stereotactic body radiotherapy compared to nivolumab alone did not result in tumor shrinkage in R/M HNSCC[46]. Interestingly, an exploratory subgroup analysis of patients with high PD-L1 expression in the JAVELIN study indicated a potential PFS benefit with avelumab plus CRT compared to placebo plus CRT. Although definite conclusions cannot be made based on this small subgroup analysis, this is a finding that should be explored further to understand the role of biomarker analysis to select patients for immunotherapy.
In terms of PFS, none of the studies included in this review demonstrated any beneficial outcomes. A recent meta-analysis by Gyawali et al[47] found no correlation between median OS and median PFS in studies evaluating anti-PD-1 agents. Defining PFS based on the traditional RECIST criteria (developed in the pre-immunotherapy era) that do not properly capture the concept of disease progression with immunotherapy was hypothesized as a probable reason for the finding.
While immunotherapy involving anti-PD-1 checkpoint inhibitors resulted in significant improvements in survival, PD-L1 and CTLA-4 blockade did not demonstrate any encouraging outcomes. More studies are needed to build evidence on the role of anti-PD-L1 and CTLA-4 blocking agents in the treatment of advanced HNSCC. Again, in the first-line setting, the evidence on the effectiveness of immunotherapy for R/M HNSCC is based on one single phase-3 trial (KEYNOTE 048), and currently, pembrolizumab is the only ICI approved for treating this group of patients[25]. During our literature search, we identified some of the ongoing phase-3 clinical trials investigating various checkpoint inhibitor agents either alone or as part of combination therapy. Subsequently, we searched the ‘clinical trials.org’ database and identified the major ongoing clinical trials and confirmed the status of those trials.
Studies investigating the combination of two different ICI agents or ICI in combination with another immunomodulatory agent in R/M HNSCC in the first-line treatment setting[48-51]: An ongoing open-label phase-3 trial (KESTREL) is currently evaluating anti-PDL-1 agent durvalumab alone and in combination with the anti-CTLA-4 agent tremelimumab for R/M HNSCC against the EXTREME regime in the first-line treatment setting[48]. Checkmate 651, another ongoing phase-3 study, is currently evaluating the anti-PD-1 agent nivolumab in combination with the CTLA-4 blocking agent ipilimumab for R/M HNSCC against the EXTREME regime in the first-line setting[49]. In a phase-3 trial among R/M HNSCC, patients with a PD-L1 biomarker expression of CPS ≥ 1, the combination of pembrolizumab and lenvatinib, an anti-vascular endothelial growth factor-multiple kinase inhibitor, is being investigated as first-line treatment against pembrolizumab plus placebo[50]. Similarly, ICI in combination with another immunomodulatory agent is being investigated in the ECHO-304/KEYNOTE 669 study[51]. In this phase-3 trial, the combination of pembrolizumab and epacadostat, an indoleamine 2,3-dioxygenase 1, inhibitor agent is being investigated against pembrolizumab monotherapy, and the EXTREME regime, in R/M HNSCC as first-line treatment[51].
Studies investigating ICI plus CRT vs CRT alone in LAHNSCC[52,53]: In KEYNOTE 412, the effectiveness of pembrolizumab given concurrently with CRT and as maintenance therapy is being evaluated against placebo plus standard CRT for the treatment of LAHNSCC[52]. In REACH, the superiority of avelumab in combination with RT-cetuximab compared to cisplatin -RT and/or to RT-cetuximab alone is being evaluated[53].
Studies investigating ICI plus RT vs cetuximab plus RT in platinum ineligible LAHNSCC[54,55]: In HN004, durvalumab plus RT is being compared to cetuximab plus RT in platinum ineligible patients[54]. In a recently completed phase-3 trial with no published results (CheckMate 9TM), cisplatin-ineligible patients received nivolumab plus RT as intervention while control patients received cetuximab plus RT[55].
Studies investigating ICI as neoadjuvant/adjuvant therapy[56-59]: In KEYNOTE 689, pembrolizumab with RT (with or without cisplatin) before and after surgery is compared to RT (with or without cisplatin) given after surgery[56]. Atezolizumab, an anti-PD-L1 agent, is being evaluated as an adjuvant therapy against placebo in the ongoing trial iMvoke010[57]. In IMSTAR-HN, nivolumab alone or in combination with the anti-CTLA-4 agent ipilimumab is evaluated as follow-up after adjuvant therapy against standard follow-up in surgically resectable LAHNSCC[58]. In NIVOPOSTOP, the efficacy of postoperative adjuvant nivolumab along with CRT is compared to post-operative CRT alone[59].
The details of these ongoing phase-3 studies are given in Table 2.
| Study | Status/trial ID | Population | Intervention | Control | No of participants | Target receptor |
| KESTREL[47] | Active, not recruiting/NCT02551159 | R/M HNSCC | Arm 1: Durvalumab | EXTREMEregime | 823 | PDL-1, CTLA-4 |
| Arm 2: Durvalumab with Tremelimumab | ||||||
| Checkmate 651[48] | Active, not recruiting/NCT02741570 | R/M HNSCC | Nivolumab with Ipilimumab | EXTREME regime | 947 | PD-1, CTLA-4 |
| LEAP-10[49] | Active, recruiting/NCT04199104 | R/M HNSCC | Pembrolizumab with Lenvatinib | Pembrolizumabwith placebo | 500 | PD-1, VEGF-multiple kinase |
| ECHO-304/KEYNOTE 669[50] | Active, not recruiting/NCT03358472 | R/M HNSCC | Arm1: Pembrolizumab with Epacadostat | EXTREME | 625 | PD-1,IDO1 |
| Arm 2: Pembrolizumab alone | ||||||
| KEYNOTE 412[51] | Active, not recruiting/NCT03040999 | LAHNSCC | Pembrolizumab with CRT concurrently and as maintenance | Standard CRT plus placebo | 780 | PD-1 |
| REACH[52] | Active, not recruiting/NCT02999087 | LAHNSCC | Avelumab in combination with RT-cetuximab | Cisplatin-RT and/or RT-cetuximab alone | 707 | PD-L1 |
| HN004[53] | Active, recruiting/NCT03258554 | LAHNSCC | Durvalumab plus RT | Cetuximab plus RT | 474 | PD-L1 |
| Platinum in eligible patients | ||||||
| CheckMate 9TM[54] | Completed awaiting results/NCT03349710 | LAHNSCC | Nivolumab plus RT | Cetuximab plus RT | 68 | PD-1 |
| Platinum ineligible cohort | ||||||
| LAHNSCC | Nivolumab pluscisplatin plus RT | Cisplatin plus RT | ||||
| Platinum eligible cohort | ||||||
| KEYNOTE 689[55] | Active, recruiting/NCT03765918 | LAHNSCC | Pembrolizumab with RT (with or without cisplatin) before and after surgery | RT (with or without cisplatin) given after surgery | 704 | PD-1 |
| iMvoke010[56] | Active, recruiting/NCT03452137 | LAHNSCC | Atezolizumab as adjuvant therapy after definitive local therapy | Placebo | 400 | PD-L1 |
| IMSTAR-HN[57] | Active, not recruiting/NCT03700905 | Surgically resectable LAHNSCC | Nivolumab alone or in combination Ipilimumab as follow up after adjuvant therapy | Standard follow-up after adjuvant therapy | 276 | PD-1, CTLA-4 |
| NIVOPOSTOP[58] | Active, recruiting/NCT03576417 | LAHNSCC | Adjuvant Nivolumab with CRT postoperatively | CRT alone post operatively | 680 | PD-1 |
Novel combination strategies to potentiate and prolong the anti-tumor activity of ICI are being evaluated currently. Thus, several early phase clinical trials (phase 1/2) investigating combination strategies of ICIs and other novel immunomodulatory agents are in the pipeline[60,61]. For example, a randomized phase-2 trial to study the safety and tolerability of nivolumab administered alone or in combination with relatlimab (antibody targeting the novel immunomodulatory receptor lymphocyte activation gene-3) or the anti-CTLA-4 agent ipilimumab is currently ongoing among patients with locally advanced surgically resectable HNSCC[62]. Immune biomarker modulation in response to nivolumab given along with Toll-like receptor 8 agonist motolimod is being analyzed in an ongoing phase-1b pre-operative biomarker trial[63]. Combination of pembrolizumab and the vascular endothelial growth factor-multiple kinase inhibitor lenvatinib demonstrated good anti-tumor activity and manageable toxicity among R/M HNSCC patients in a phase-1b/2 trial, and LEAP 010, a phase-3 trial of this combination strategy is currently ongoing[50,64]. The combination of pembrolizumab and the anti-EGFR agent cetuximab had demonstrated encouraging outcomes in the interim analysis of an ongoing multi-arm phase-2 trial[65,66]. A recently completed study among R/M HNSCC patients investigating pembrolizumab in combination with epacadostat has shown clinically meaningful results, and a larger phase-3 trial (ECHO 304/KEYNOTE 669) of this combination strategy is ongoing currently[51,67]. Combination therapy of pembrolizumab with the EGFR-tyrosine kinase inhibitor afatinib, which also included predictive biomarker analysis, had been evaluated recently in a phase-2 clinical trial (the ALPHA study) in R/M HNSC[68]. The study demonstrated augmentation of the anti-tumor activity of pembrolizumab by afatinib, and the results of biomarker analysis suggested that PD-L1 and EGFR amplification could be predictive biomarkers for cancer immunotherapy. EACH, a randomized phase-2 trial among R/M HNSCC is investigating the superiority of avelumab and cetuximab combination compared to avelumab monotherapy[69]. Another recently completed early phase study on the combination of pembrolizumab with the therapeutic vaccine talimogene laherparepvec demonstrated a tolerable safety profile among patients with R/M HNSCC. However, this investigation did not progress into a phase-3 trial as the efficacy of the combination was found to be similar to pembrolizumab monotherapy[70].
Immunotherapy trials among patients with p16-positive head and neck cancer (oropharyngeal squamous cell carcinoma) are also currently underway. In this group of patients, p16 positivity is a known independent predictive biomarker for survival[71]. The efficacy and tolerability of the combination of ipilimumab (anti-CTLA-4) and nivolumab (anti-PD-1) along with RT in locoregionally advanced human papilloma virus-positive oropharyngeal squamous cell carcinoma are being evaluated in an ongoing phase-2 single-arm trial[72]. Another phase-2 randomized study (KEYCHAIN trial) is investigating RT along with concurrent and adjuvant pembrolizumab against concurrent chemoradiation among p16-positive HNSCC[73,74].
Regarding biomarkers, in addition to p-16 positivity and PD-L1 expression, other biomarkers like microsatellite instability (MSI) and tumor mutation burden were also found to be associated with favorable outcomes with ICI therapy in HNSCC[75]. Tardy et al[76] recently reported a case of complete response to anti-PD-L1 therapy in HNSCC in a patient with high tumor MSI (MSI-H) and a negative PD-L1 histochemical status. Similarly, Hanna et al[77] reported that higher tumor mutation burden predicted response to ICI and better treatment outcomes in virus-negative head and neck cancer. Again, some subtypes of tumor-infiltrating lymphocytes (TILs) such as PD-1+TIM-3+CD8+ TILs and PD-1+LAG-3+ CD8+ TILs have also predicted treatment response to ICIs[75,77]. The data on these emerging predictive biomarkers is still not conclusive; therefore, further research is essential. PRECISION 01, an ongoing prospective observational study is currently evaluating biomarker signatures in tissue samples of platinum-refractory HNSCC patients who received nivolumab monotherapy; the findings may contribute to the knowledge on predictive biomarkers for ICIs[78].
In future studies, patient-reported outcomes like QOL should be evaluated meticulously since such outcomes are very crucial for advanced HNSCC patients and their families[79,80]. Cost-effectiveness is another issue to be considered before including ICIs in the routine treatment guidelines for patients from developing countries and resource-poor settings[81,82]. The impact of factors like age, comorbidities, and performance status on outcomes of patients receiving immunotherapy also needs to be determined[83].
There are very few published phase-3 clinical trials evaluating checkpoint inhibitor immunotherapy among patients diagnosed with HNSCC, and the evidence we gathered in this review is based on the five phase-3 RCTs published so far. A previous systematic review on this topic included eight studies, of which two were phase-3 RCTs[84]. Wang et al[85] conducted a systematic review and meta-analysis of nine studies on the effectiveness of checkpoint inhibitors in HNSCC, of which two were phase-3 trials.
To our knowledge, this is the first systematic review conducted on the effectiveness of ICIs in HNSCC incorporating phase-3 trials alone. The evidence we presented based on the five studies in this review will help the practicing clinicians to make informed decisions. We further explored the literature and identified a variety of promising clinical studies that are ongoing currently focusing on combination strategies in enhancing and prolonging the anti-tumor effects of ICIs. We also identified the gaps in knowledge on some important issues such as predictive biomarkers and about the identification of patients who will benefit from immunotherapy based on biomarker assessment[86,87].
ICIs have shown improved survival outcomes with acceptable toxicity profile in R/MHNSCC in the first and second-line treatment settings. The marginal improvement in survival should be weighed against the cost of these therapeutic agents and the QOL of patients. While anti-PD-1 agents demonstrated efficacy, evidence on the effectiveness of anti-PD-L1 and anti-CTLA-4 agents is lacking. There is no proven efficacy in the curative setting to date. The ongoing clinical trials may better define the role of ICI in R/M HNSCC and LAHNSCC in the future.
Head and neck squamous cell carcinoma (HNSCC) is one of the major causes of cancer-associated morbidity and mortality globally, especially in developing countries. Treatment approaches for HNSCC vary according to the stage of the disease at presentation. For recurrent/metastatic HNSCC (R/M HNSCC), platinum-based chemotherapy was the only available treatment option until recently. A relatively new systemic therapy option that emerged in recent years in the treatment of advanced HNSCC is immunotherapy using immune checkpoint inhibitors (ICI).
Advanced HNSCCs are often associated with significant functional limitations, and aggressive treatment may adversely affect the quality of life of these patients who are already suffering from the effect of advanced cancer. The median survival of R/M HNSCC patients receiving platinum-based chemotherapy is 7.4 mo. Some patients become refractory to platinum and die within a period of 4 mo. The safety profile and anti-tumor activity of ICIs demonstrated in early phase clinical trials paved the way to the initiation of several promising phase-3 trials in the field. Therefore, we decided to gather the current evidence on the effectiveness of these agents in advanced head and neck cancer based on the findings from phase-3 clinical trials of ICI published so far. We also wanted to examine the feasibility of incorporating these agents into routine clinical practice in resource-poor settings.
The objective of this systematic review was to gather the evidence from phase-3 randomized controlled trials (RCTs) evaluating the effectiveness of immunotherapy among patients with advanced HNSCC. We aimed to synthesize the evidence from the published phase-3 studies that investigated the efficacy and toxicity profile of ICIs administered either alone or in combination with chemotherapy, radiation therapy, or with another checkpoint inhibitor, in advanced HNSCC.
We conducted this systematic review according to the PRISMA (preferred reporting items for systematic reviews and meta-analyses) guidelines. We searched four major databases including PubMed, Scopus, Embase, and COCHRANE library, without any language limit. A combination of standardized search terms and keywords including head and neck squamous cell carcinoma, recurrent, metastatic, locally advanced, immunotherapy, checkpoint inhibitors, monoclonal antibodies, programmed cell death protein-1 (PD-1), programmed death-ligand 1 (PD-L1), cytotoxic T- lymphocyte associated protein-4 (CTLA-4), and phase-3 clinical trial were used for searching the literature. Studies were included if they were completed phase-3 RCTs conducted among patients with R/M HNSCC or LAHNSCC, in which the intervention patients received ICI either alone or in combination with chemotherapy, radiation therapy, or with another ICI and the control patients received the standard of care treatment (SOC). Anatomical sites of primary tumors were oral cavity, oropharynx, hypopharynx, and larynx in the included studies.
Five phase-3 clinical trials have reported the data on the effectiveness of immunotherapy in HNSCC so far: Four in R/M HNSCC and one in LAHNSCC. In patients with R/M HNSCC, anti-PD-1 agents nivolumab and pembrolizumab demonstrated improvement in overall survival (OS) in the second-line treatment setting compared to the SOC. While the net gain in OS with nivolumab was 2.4 mo, that with pembrolizumab was 1.5 mo. However, the study that investigated the anti-PD-L1 agent durvalumab with or without the anti-CTLA-4 agent tremelimumab in the second-line treatment setting did not demonstrate any beneficial outcomes.
In the first-line setting, pembrolizumab together with platinum-based chemotherapy demonstrated statistically significant improvement in survival with a net gain in OS of 2.3 mo in the overall population and a net gain in OS of 4.2 mo in the population with a combined positive score of > 20 compared to the SOC treatment. Pembrolizumab monotherapy was found to be non-inferior to EXTREME in terms of OS (11.6 mo vs 10.7 mo) in the total population. In patients with PD-L1 positive R/M HNSCC, monotherapy with pembrolizumab also demonstrated statistically significant improvement in survival compared to SOC. In LAHNSCC, immunotherapy using the anti-PD-L1 agent avelumab along with standard chemoradiation therapy did not result in improved outcomes compared to placebo plus chemoradiation therapy.
This systematic review helped us to conclude that anti-PD-1 agents provide survival benefits in R/M HNSCC in the first and second-line settings with manageable toxicity profiles. However, it is important to weigh the marginal survival benefits provided by these therapeutic agents against their cost, especially in resource-poor settings. The review showed that the evidence on the effectiveness of anti-PD-L1 and anti-CTLA-4 agents in advanced head and neck cancer is lacking. To date, there is no evidence on the effectiveness of ICIs in the curative setting either. We believe that the ongoing clinical trials (discussed in the article) will help to define better the role of ICI in R/M HNSCC and LAHNSCC in the future.
Novel combination strategies to potentiate and prolong the anti-tumor activity of ICI are being evaluated currently. Gaps in knowledge exist on some important issues such as predictive biomarkers, and about the identification of patients who will benefit from immunotherapy based on biomarker assessment. Future studies should focus on these issues.
The authors would like to thank Dr. Vijo Poulose for his assistance in searching the databases.
Provenance and peer review: Unsolicited article; Externally peer reviewed.
Peer-review model: Single blind
Corresponding Author's Membership in Professional Societies: Association of Radiation Oncologists of India, No. 1058.
Specialty type: Oncology
Country/Territory of origin: India
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): B, B
Grade C (Good): 0
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Awidi M, United States; Chen YH, China S-Editor: Gong ZM L-Editor: Filipodia P-Editor: Gong ZM
| 1. | Gupta B, Johnson NW, Kumar N. Global Epidemiology of Head and Neck Cancers: A Continuing Challenge. Oncology. 2016;91:13-23. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 248] [Cited by in RCA: 266] [Article Influence: 29.6] [Reference Citation Analysis (0)] |
| 2. | Vigneswaran N, Williams MD. Epidemiologic trends in head and neck cancer and aids in diagnosis. Oral Maxillofac Surg Clin North Am. 2014;26:123-141. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 421] [Cited by in RCA: 579] [Article Influence: 57.9] [Reference Citation Analysis (0)] |
| 3. | Bray F, Ferlay J, Soerjomataram I, Siegel RL, Torre LA, Jemal A. Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin. 2018;68:394-424. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 53206] [Cited by in RCA: 55853] [Article Influence: 7979.0] [Reference Citation Analysis (132)] |
| 4. | Cognetti DM, Weber RS, Lai SY. Head and neck cancer: an evolving treatment paradigm. Cancer. 2008;113:1911-1932. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 199] [Cited by in RCA: 179] [Article Influence: 10.5] [Reference Citation Analysis (0)] |
| 5. | Marur S, Forastiere AA. Head and Neck Squamous Cell Carcinoma: Update on Epidemiology, Diagnosis, and Treatment. Mayo Clin Proc. 2016;91:386-396. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 611] [Cited by in RCA: 809] [Article Influence: 89.9] [Reference Citation Analysis (0)] |
| 6. | Molin Y, Fayette J. Current chemotherapies for recurrent/metastatic head and neck cancer. Anticancer Drugs. 2011;22:621-625. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 31] [Cited by in RCA: 32] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 7. | Colevas AD. Systemic Therapy for Metastatic or Recurrent Squamous Cell Carcinoma of the Head and Neck. J Natl Compr Canc Netw. 2015;13:e37-e48. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 14] [Cited by in RCA: 17] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 8. | Jacob LA, Chaudhuri T, Lakshmaiah KC, Babu KG, Dasappa L, Babu M, Rudresha AH, Lokesh KN, Rajeev LK. Current status of systemic therapy for recurrent and/or metastatic squamous cell carcinoma of the head and neck. Indian J Cancer. 2016;53:471-477. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 6] [Cited by in RCA: 8] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 9. | Price KA, Cohen EE. Current treatment options for metastatic head and neck cancer. Curr Treat Options Oncol. 2012;13:35-46. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 147] [Cited by in RCA: 166] [Article Influence: 12.8] [Reference Citation Analysis (0)] |
| 10. | Lau A, Yang WF, Li KY, Su YX. Systemic Therapy in Recurrent or Metastatic Head and Neck Squamous Cell Carcinoma- A Systematic Review and Meta-Analysis. Crit Rev Oncol Hematol. 2020;153:102984. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 43] [Cited by in RCA: 36] [Article Influence: 7.2] [Reference Citation Analysis (0)] |
| 11. | León X, Hitt R, Constenla M, Rocca A, Stupp R, Kovács AF, Amellal N, Bessa EH, Bourhis J. A retrospective analysis of the outcome of patients with recurrent and/or metastatic squamous cell carcinoma of the head and neck refractory to a platinum-based chemotherapy. Clin Oncol (R Coll Radiol). 2005;17:418-424. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 126] [Cited by in RCA: 131] [Article Influence: 6.6] [Reference Citation Analysis (0)] |
| 12. |
Kainickal CT, Aparna MP, Kumar RR, et al Targeted therapy in recurrent or metastatic head and neck carcinoma.
|
| 13. | Vermorken JB, Mesia R, Rivera F, Remenar E, Kawecki A, Rottey S, Erfan J, Zabolotnyy D, Kienzer HR, Cupissol D, Peyrade F, Benasso M, Vynnychenko I, De Raucourt D, Bokemeyer C, Schueler A, Amellal N, Hitt R. Platinum-based chemotherapy plus cetuximab in head and neck cancer. N Engl J Med. 2008;359:1116-1127. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2546] [Cited by in RCA: 2546] [Article Influence: 149.8] [Reference Citation Analysis (0)] |
| 14. | Sacco AG, Cohen EE. Current Treatment Options for Recurrent or Metastatic Head and Neck Squamous Cell Carcinoma. J Clin Oncol. 2015;33:3305-3313. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 207] [Cited by in RCA: 255] [Article Influence: 25.5] [Reference Citation Analysis (0)] |
| 15. | Blasco MA, Svider PF, Raza SN, Jacobs JR, Folbe AJ, Saraf P, Eloy JA, Baredes S, Fribley AM. Systemic therapy for head and neck squamous cell carcinoma: Historical perspectives and recent breakthroughs. Laryngoscope. 2017;127:2565-2569. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 20] [Cited by in RCA: 21] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 16. | Vermorken JB, Specenier P. Optimal treatment for recurrent/metastatic head and neck cancer. Ann Oncol. 2010;21 Suppl 7:vii252-vii261. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 228] [Cited by in RCA: 241] [Article Influence: 16.1] [Reference Citation Analysis (0)] |
| 17. | Bauml JM, Aggarwal C, Cohen RB. Immunotherapy for head and neck cancer: where are we now and where are we going? Ann Transl Med. 2019;7:S75. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 24] [Cited by in RCA: 36] [Article Influence: 6.0] [Reference Citation Analysis (0)] |
| 18. | Gauduchon T, Reverdy T, Gau M, Karabajakian A, Collet L, Neidhardt EM, Fayette J. Head and neck cancer and immunotherapy: current knowledge and perspective. J Cancer Metastasis Treat. 2019;5:72. [DOI] [Full Text] |
| 19. | Moy JD, Moskovitz JM, Ferris RL. Biological mechanisms of immune escape and implications for immunotherapy in head and neck squamous cell carcinoma. Eur J Cancer. 2017;76:152-166. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 62] [Cited by in RCA: 80] [Article Influence: 10.0] [Reference Citation Analysis (0)] |
| 20. | Yang Y. Cancer immunotherapy: harnessing the immune system to battle cancer. J Clin Invest. 2015;125:3335-3337. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 653] [Cited by in RCA: 994] [Article Influence: 99.4] [Reference Citation Analysis (0)] |
| 21. | Moher D, Shamseer L, Clarke M, Ghersi D, Liberati A, Petticrew M, Shekelle P, Stewart LA; PRISMA-P Group. Preferred reporting items for systematic review and meta-analysis protocols (PRISMA-P) 2015 statement. Syst Rev. 2015;4:1. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 15040] [Cited by in RCA: 15892] [Article Influence: 1589.2] [Reference Citation Analysis (1)] |
| 22. | Ferris RL, Blumenschein G Jr, Fayette J, Guigay J, Colevas AD, Licitra L, Harrington K, Kasper S, Vokes EE, Even C, Worden F, Saba NF, Iglesias Docampo LC, Haddad R, Rordorf T, Kiyota N, Tahara M, Monga M, Lynch M, Geese WJ, Kopit J, Shaw JW, Gillison ML. Nivolumab for Recurrent Squamous-Cell Carcinoma of the Head and Neck. N Engl J Med. 2016;375:1856-1867. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3044] [Cited by in RCA: 3697] [Article Influence: 410.8] [Reference Citation Analysis (0)] |
| 23. | Cohen EEW, Soulières D, Le Tourneau C, Dinis J, Licitra L, Ahn MJ, Soria A, Machiels JP, Mach N, Mehra R, Burtness B, Zhang P, Cheng J, Swaby RF, Harrington KJ; KEYNOTE-040 investigators. Pembrolizumab versus methotrexate, docetaxel, or cetuximab for recurrent or metastatic head-and-neck squamous cell carcinoma (KEYNOTE-040): a randomised, open-label, phase 3 study. Lancet. 2019;393:156-167. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 835] [Cited by in RCA: 1180] [Article Influence: 196.7] [Reference Citation Analysis (0)] |
| 24. | Ferris RL, Haddad R, Even C, Tahara M, Dvorkin M, Ciuleanu TE, Clement PM, Mesia R, Kutukova S, Zholudeva L, Daste A, Caballero-Daroqui J, Keam B, Vynnychenko I, Lafond C, Shetty J, Mann H, Fan J, Wildsmith S, Morsli N, Fayette J, Licitra L. Durvalumab with or without tremelimumab in patients with recurrent or metastatic head and neck squamous cell carcinoma: EAGLE, a randomized, open-label phase III study. Ann Oncol. 2020;31:942-950. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 128] [Cited by in RCA: 274] [Article Influence: 54.8] [Reference Citation Analysis (0)] |
| 25. | Burtness B, Harrington KJ, Greil R, Soulières D, Tahara M, de Castro G Jr, Psyrri A, Basté N, Neupane P, Bratland Å, Fuereder T, Hughes BGM, Mesía R, Ngamphaiboon N, Rordorf T, Wan Ishak WZ, Hong RL, González Mendoza R, Roy A, Zhang Y, Gumuscu B, Cheng JD, Jin F, Rischin D; KEYNOTE-048 Investigators. Pembrolizumab alone or with chemotherapy versus cetuximab with chemotherapy for recurrent or metastatic squamous cell carcinoma of the head and neck (KEYNOTE-048): a randomised, open-label, phase 3 study. Lancet. 2019;394:1915-1928. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2237] [Cited by in RCA: 2079] [Article Influence: 346.5] [Reference Citation Analysis (0)] |
| 26. | Cohen EE, Ferris RL, Psyrri A, Haddad R, Tahara M, Bourhis J, Harrington KJ, Chang PM, Lin J, Razaq M, Teixeira MM, J. Lovey S, J. Chamois13, A. Rueda Dominguez14, C. Hu15, M. Dvorkin16, De Beukelaer17, Pavlov D, Thurm H, Lee N. Primary results of the phase III JAVELIN head & neck 100 trial: avelumab plus chemoradiotherapy (CRT) followed by avelumab maintenance vs CRT in patients with locally advanced squamous cell carcinoma of the head and neck (LA SCCHN). Ann Oncol. 2020;31:S599-S628. [RCA] [DOI] [Full Text] [Cited by in Crossref: 26] [Cited by in RCA: 26] [Article Influence: 5.2] [Reference Citation Analysis (0)] |
| 27. | Lee NY, Ferris RL, Psyrri A, Haddad RI, Tahara M, Bourhis J, Harrington K, Chang PM, Lin JC, Razaq MA, Teixeira MM, Lövey J, Chamois J, Rueda A, Hu C, Dunn LA, Dvorkin MV, De Beukelaer S, Pavlov D, Thurm H, Cohen E. Avelumab plus standard-of-care chemoradiotherapy versus chemoradiotherapy alone in patients with locally advanced squamous cell carcinoma of the head and neck: a randomised, double-blind, placebo-controlled, multicentre, phase 3 trial. Lancet Oncol. 2021;22:450-462. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 270] [Cited by in RCA: 398] [Article Influence: 99.5] [Reference Citation Analysis (0)] |
| 28. | Guo L, Zhang H, Chen B. Nivolumab as Programmed Death-1 (PD-1) Inhibitor for Targeted Immunotherapy in Tumor. J Cancer. 2017;8:410-416. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 114] [Cited by in RCA: 172] [Article Influence: 21.5] [Reference Citation Analysis (0)] |
| 29. | McDermott J, Jimeno A. Pembrolizumab: PD-1 inhibition as a therapeutic strategy in cancer. Drugs Today (Barc). 2015;51:7-20. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 52] [Cited by in RCA: 61] [Article Influence: 6.8] [Reference Citation Analysis (1)] |
| 30. | Wang BC, Li PC, Fan JQ, Lin GH, Liu Q. Durvalumab and tremelimumab combination therapy versus durvalumab or tremelimumab monotherapy for patients with solid tumors: A systematic review and meta-analysis. Medicine (Baltimore). 2020;99:e21273. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 9] [Cited by in RCA: 12] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 31. | Cristina V, Herrera-Gómez RG, Szturz P, Espeli V, Siano M. Immunotherapies and Future Combination Strategies for Head and Neck Squamous Cell Carcinoma. Int J Mol Sci. 2019;20. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 34] [Cited by in RCA: 38] [Article Influence: 6.3] [Reference Citation Analysis (0)] |
| 32. | Harrington KJ, Soulières D, Le Tourneau C, Dinis J, Licitra LF, Ahn MJ, Soria A, Machiels JH, Mach N, Mehra R, Burtness B, Ellison MC, Cheng JD, Chirovsky DR, Swaby RF, Cohen EEW. Quality of Life With Pembrolizumab for Recurrent and/or Metastatic Head and Neck Squamous Cell Carcinoma: KEYNOTE-040. J Natl Cancer Inst. 2021;113:171-181. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 22] [Cited by in RCA: 22] [Article Influence: 5.5] [Reference Citation Analysis (0)] |
| 33. | Lacas B, Carmel A, Landais C, Wong SJ, Licitra L, Tobias JS, Burtness B, Ghi MG, Cohen EEW, Grau C, Wolf G, Hitt R, Corvò R, Budach V, Kumar S, Laskar SG, Mazeron JJ, Zhong LP, Dobrowsky W, Ghadjar P, Fallai C, Zakotnik B, Sharma A, Bensadoun RJ, Ruo Redda MG, Racadot S, Fountzilas G, Brizel D, Rovea P, Argiris A, Nagy ZT, Lee JW, Fortpied C, Harris J, Bourhis J, Aupérin A, Blanchard P, Pignon JP; MACH-NC Collaborative Group. Meta-analysis of chemotherapy in head and neck cancer (MACH-NC): An update on 107 randomized trials and 19,805 patients, on behalf of MACH-NC Group. Radiother Oncol. 2021;156:281-293. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 54] [Cited by in RCA: 207] [Article Influence: 51.8] [Reference Citation Analysis (0)] |
| 34. | Seiwert TY, Burtness B, Mehra R, Weiss J, Berger R, Eder JP, Heath K, McClanahan T, Lunceford J, Gause C, Cheng JD, Chow LQ. Safety and clinical activity of pembrolizumab for treatment of recurrent or metastatic squamous cell carcinoma of the head and neck (KEYNOTE-012): an open-label, multicentre, phase 1b trial. Lancet Oncol. 2016;17:956-965. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1025] [Cited by in RCA: 1317] [Article Influence: 146.3] [Reference Citation Analysis (0)] |
| 35. | Bauml J, Seiwert TY, Pfister DG, Worden F, Liu SV, Gilbert J, Saba NF, Weiss J, Wirth L, Sukari A, Kang H, Gibson MK, Massarelli E, Powell S, Meister A, Shu X, Cheng JD, Haddad R. Pembrolizumab for Platinum- and Cetuximab-Refractory Head and Neck Cancer: Results From a Single-Arm, Phase II Study. J Clin Oncol. 2017;35:1542-1549. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 459] [Cited by in RCA: 514] [Article Influence: 64.3] [Reference Citation Analysis (0)] |
| 36. | Zandberg DP, Algazi AP, Jimeno A, Good JS, Fayette J, Bouganim N, Ready NE, Clement PM, Even C, Jang RW, Wong S, Keilholz U, Gilbert J, Fenton M, Braña I, Henry S, Remenar E, Papai Z, Siu LL, Jarkowski A, Armstrong JM, Asubonteng K, Fan J, Melillo G, Mesía R. Durvalumab for recurrent or metastatic head and neck squamous cell carcinoma: Results from a single-arm, phase II study in patients with ≥25% tumour cell PD-L1 expression who have progressed on platinum-based chemotherapy. Eur J Cancer. 2019;107:142-152. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 130] [Cited by in RCA: 197] [Article Influence: 28.1] [Reference Citation Analysis (0)] |
| 37. | Siu LL, Even C, Mesía R, Remenar E, Daste A, Delord JP, Krauss J, Saba NF, Nabell L, Ready NE, Braña I, Kotecki N, Zandberg DP, Gilbert J, Mehanna H, Bonomi M, Jarkowski A, Melillo G, Armstrong JM, Wildsmith S, Fayette J. Safety and Efficacy of Durvalumab With or Without Tremelimumab in Patients With PD-L1-Low/Negative Recurrent or Metastatic HNSCC: The Phase 2 CONDOR Randomized Clinical Trial. JAMA Oncol. 2019;5:195-203. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 150] [Cited by in RCA: 236] [Article Influence: 39.3] [Reference Citation Analysis (0)] |
| 38. | Brahmer J, Reckamp KL, Baas P, Crinò L, Eberhardt WE, Poddubskaya E, Antonia S, Pluzanski A, Vokes EE, Holgado E, Waterhouse D, Ready N, Gainor J, Arén Frontera O, Havel L, Steins M, Garassino MC, Aerts JG, Domine M, Paz-Ares L, Reck M, Baudelet C, Harbison CT, Lestini B, Spigel DR. Nivolumab versus Docetaxel in Advanced Squamous-Cell Non-Small-Cell Lung Cancer. N Engl J Med. 2015;373:123-135. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5686] [Cited by in RCA: 6742] [Article Influence: 674.2] [Reference Citation Analysis (0)] |
| 39. | Dovedi SJ, Adlard AL, Lipowska-Bhalla G, McKenna C, Jones S, Cheadle EJ, Stratford IJ, Poon E, Morrow M, Stewart R, Jones H, Wilkinson RW, Honeychurch J, Illidge TM. Acquired resistance to fractionated radiotherapy can be overcome by concurrent PD-L1 blockade. Cancer Res. 2014;74:5458-5468. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 914] [Cited by in RCA: 993] [Article Influence: 90.3] [Reference Citation Analysis (0)] |
| 40. | Heery CR, O'Sullivan-Coyne G, Madan RA, Cordes L, Rajan A, Rauckhorst M, Lamping E, Oyelakin I, Marté JL, Lepone LM, Donahue RN, Grenga I, Cuillerot JM, Neuteboom B, Heydebreck AV, Chin K, Schlom J, Gulley JL. Avelumab for metastatic or locally advanced previously treated solid tumours (JAVELIN Solid Tumor): a phase 1a, multicohort, dose-escalation trial. Lancet Oncol. 2017;18:587-598. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 201] [Cited by in RCA: 241] [Article Influence: 30.1] [Reference Citation Analysis (0)] |
| 41. | Ferris RL, Blumenschein GR, Fayette J, Guigay J, Colevas AD, Licitra L, Harrington KJ, Kasper S, Vokes EE, Even C, Worden F, Saba NF, Docampo LCI, Haddad R, Rordorf T, Kiyota N, Tahara M, Lynch M, Jayaprakash V, Li L, Gillison ML. Nivolumab (Nivo) vs investigator's choice (IC) in recurrent or metastatic (R/M) squamous cell carcinoma of the head and neck (SCCHN): 2-yr outcomes in the overall population and PD-L1 subgroups of CheckMate 141. Cancer Res. 2018;78:CT116. [RCA] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 2] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 42. | Ferris RL, Concha-Benavente F, Blumenschein GR, Harrington KJ, Fayette J, Dimitrios Colevas A, Licitra LF, Kasper S, Even C, Gillison ML, Worden F, Saba NF, Haddad RI, Tahara M, Hasegawa Y, Yen CJ, Lynch MJ, Monga M, Geese WJ, Vokes EE. Characterization of potential predictive biomarkers of response to nivolumab in CheckMate-141 in patients with squamous cell carcinoma of the head and neck (SCCHN). J Clin Oncol. 2017;35:5. [RCA] [DOI] [Full Text] [Cited by in Crossref: 7] [Cited by in RCA: 7] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 43. | Cohen EEW, Bell RB, Bifulco CB, Burtness B, Gillison ML, Harrington KJ, Le QT, Lee NY, Leidner R, Lewis RL, Licitra L, Mehanna H, Mell LK, Raben A, Sikora AG, Uppaluri R, Whitworth F, Zandberg DP, Ferris RL. The Society for Immunotherapy of Cancer consensus statement on immunotherapy for the treatment of squamous cell carcinoma of the head and neck (HNSCC). J Immunother Cancer. 2019;7:184. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 350] [Cited by in RCA: 488] [Article Influence: 81.3] [Reference Citation Analysis (0)] |
| 44. | Burtness B, Rischin D, Greil R, Soulieres D, Tahara M, De Castro G, Psyrri A, Basté N, Neupane P, Bratland Å, Fuereder T, Hughes BG, Mesia R, Ngamphaiboon N, Rordorf T, Wan Ishak WZ, Ge J, Swaby R, Gumuscu B, Harrington K. Efficacy of first-line (1L) pembrolizumab by PD-L1 combined positive score <1, 1-19, and ≥20 in recurrent and/or metastatic (R/M) head and neck squamous cell carcinoma (HNSCC): KEYNOTE-048 subgroup analysis. Cancer Res. 2020;80:LB-258. [DOI] [Full Text] |
| 45. | Bourhis J, Sire C, Tao Y, Martin L, Alfonsi M, Prevost JB, Rives M, Lafond C, Tourani JM, Biau J, Geoffrois L, Coutte A, Liem X, Vauleon E, Drouet F, Pechery A, Guigay J, Wanneveich M, Aupérin A, Sun X. LBA38 Pembrolizumab vs cetuximab, concomitant with radiotherapy (RT) in locally advanced head and neck squamous cell carcinoma (LA-HNSCC): Results of the GORTEC 2015-01 "PembroRad" randomized trial. Ann Oncol. 2020;31:S1168. [RCA] [DOI] [Full Text] [Cited by in Crossref: 22] [Cited by in RCA: 28] [Article Influence: 5.6] [Reference Citation Analysis (0)] |
| 46. | McBride S, Sherman E, Tsai CJ, Baxi S, Aghalar J, Eng J, Zhi WI, McFarland D, Michel LS, Young R, Lefkowitz R, Spielsinger D, Zhang Z, Flynn J, Dunn L, Ho A, Riaz N, Pfister D, Lee N. Randomized Phase II Trial of Nivolumab With Stereotactic Body Radiotherapy Versus Nivolumab Alone in Metastatic Head and Neck Squamous Cell Carcinoma. J Clin Oncol. 2021;39:30-37. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 103] [Cited by in RCA: 279] [Article Influence: 55.8] [Reference Citation Analysis (0)] |
| 47. | Gyawali B, Hey SP, Kesselheim AS. A Comparison of Response Patterns for Progression-Free Survival and Overall Survival Following Treatment for Cancer With PD-1 Inhibitors: A Meta-analysis of Correlation and Differences in Effect Sizes. JAMA Netw Open. 2018;1:e180416. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 35] [Cited by in RCA: 50] [Article Influence: 7.1] [Reference Citation Analysis (0)] |
| 48. | Seiwert TY, Weiss J, Baxi SS, Ahn MJ, Fayette J, Gillison ML, Machiels JPH, Takahashi S, Melillo G, Franks A, Emeribe U, Raben D, McDevitt M, Psyrri A. A phase 3, randomized, open-label study of first-line durvalumab (MEDI4736) ± tremelimumab vs standard of care (SoC; EXTREME regimen) in recurrent/metastatic (R/M) SCCHN: KESTREL. J Clin Oncol. 2016;34:TPS6101. [RCA] [DOI] [Full Text] [Cited by in Crossref: 19] [Cited by in RCA: 20] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 49. |
Argiris A, Gillison M, Ferris RL, Harrington K, Sanchez TK, Baudelet C, et al A randomized, open-label, phase 3 study of nivolumab in combination with ipilimumab vs extreme regimen (cetuximab + cisplatin/carboplatin + fluorouracil) as first-line therapy in patients with recurrent or metastatic squamous cell carcinoma of the head and neck-CheckMate 651.
|
| 50. | ClinicalTrials. gov [Internet]. A Study of Pembrolizumab (MK-3475) With or Without Lenvatinib (E7080/MK-7902) as First Line (1L) Intervention in a Programmed Cell Death-ligand 1 (PD-L1) Selected Population With Recurrent or Metastatic Head and Neck Squamous Cell Carcinoma (R/M HNSCC) (LEAP-010) (MK-7902-010). 2021. Available from: https://clinicaltrials.gov/ct2/show/NCT04199104. |
| 51. | Cohen EEW, Rischin D, Pfister DG, Vermorken JB, Zhao Y, Gowda H, Ge JY, Jin F, Harrington KJ. A phase 3, randomized, open-label study of epacadostat plus pembrolizumab, pembrolizumab monotherapy, and the EXTREME regimen as first-line treatment for recurrent/metastatic head and neck squamous cell carcinoma (R/M SCCHN): ECHO-304/KEYNOTE-669. J Clin Oncol. 2018;36:TPS6090. [RCA] [DOI] [Full Text] [Cited by in Crossref: 10] [Cited by in RCA: 10] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 52. | Machiels JP, Tao Y, Burtness B, Tahara M, Licitra L, Rischin D, Waldron J, Simon C, Gregoire V, Harrington K, Alves GV, Figueiredo Lima IP, Pointreau Y, M Hughes BG, Aksoy S, Hetnal M, Ge JY, Brown H, Cheng J, Bidadi B, Siu LL. Pembrolizumab given concomitantly with chemoradiation and as maintenance therapy for locally advanced head and neck squamous cell carcinoma: KEYNOTE-412. Future Oncol. 2020;16:1235-1243. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 35] [Cited by in RCA: 44] [Article Influence: 8.8] [Reference Citation Analysis (0)] |
| 53. | Tao Y, Aupérin A, Sun X, Sire C, Martin L, Coutte A, Lafond C, Miroir J, Liem X, Rolland F, Even C, Nguyen F, Saada E, Maillard A, Colin-Batailhou N, Thariat J, Guigay J, Bourhis J. Avelumab-cetuximab-radiotherapy versus standards of care in locally advanced squamous-cell carcinoma of the head and neck: The safety phase of a randomised phase III trial GORTEC 2017-01 (REACH). Eur J Cancer. 2020;141:21-29. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 24] [Cited by in RCA: 52] [Article Influence: 10.4] [Reference Citation Analysis (0)] |
| 54. | Mell LK, Torres-Saavedra PA, Wong SJ, Chang S, Kish JA, Minn A, Jordan RC, Liu T, Truong MT, Bauman JE, Powell SF, Khomani A, Riaz MK, Raben D, Le QT. Safety of radiotherapy with concurrent and adjuvant MEDI4736 (durvalumab) in patients with locoregionally advanced head and neck cancer with a contraindication to cisplatin: NRG-HN004. J Clin Oncol. 2019;37:6065. [RCA] [DOI] [Full Text] [Cited by in Crossref: 9] [Cited by in RCA: 9] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 55. | Haddad R, Harrington K, Licitra L, Brossart P, Soulières D, Mell L. CheckMate 9TM: Phase 3 study of nivolumab + radiotherapy (RT) vs cetuximab + RT in cisplatin-ineligible patients with intermediate-/high-risk locally advanced squamous cell carcinoma of the head/neck. J ImmunoTher Cancer. 2018;. |
| 56. | Lee NY, Uppaluri R, Westra W, Cohen EE, Haddad RI, Temam S, Tourneau CL, Chernock R, Safina S, Klochikhin A, Meirovitz A, Brana I, Ge JY, Swaby RF, Pinheiro C, Adkins D. KEYNOTE-689: A phase 3 study of neoadjuvant and adjuvant pembrolizumab plus standard of care (SOC) in locally advanced (LA) head and necksquamous cell carcinoma (HNSCC). Cancer Res. 2020;80:CT285. [RCA] [DOI] [Full Text] [Cited by in Crossref: 3] [Cited by in RCA: 7] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 57. | Wong DJ, Fayette J, Guo Y, Kowgier M, Cohen E, Nin RM, Dechaphunkul A, Prabhash K, Geiger J, Bishnoi S, Schafer H, Matheny C, Kabbinavar F, Sandler A, Raben D, Haddad R. IMvoke010: Randomized Phase III study of atezolizumab as adjuvant monotherapy after definitive therapy of squamous cell carcinoma of the head and neck (SCCHN). Cancer Res. 2019;79:CT123. [RCA] [DOI] [Full Text] [Cited by in Crossref: 3] [Cited by in RCA: 5] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 58. | Zech HB, Moeckelmann N, Boettcher A, Muenscher A, Binder M, Vettorazzi E, Bokemeyer C, Schafhausen P, Betz CS, Busch CJ. Phase III study of nivolumab alone or combined with ipilimumab as immunotherapy versus standard of care in resectable head and neck squamous cell carcinoma. Future Oncol. 2020;16:3035-3043. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 11] [Cited by in RCA: 18] [Article Influence: 3.6] [Reference Citation Analysis (0)] |
| 59. | ClinicalTrials. gov [Internet]. A trial evaluating the addition of nivolumab to cisplatin-RT for treatment of cancers of the head and neck (NIVOPOSTOP). 2021 Available from: https://clinicaltrials.gov/ct2/show/NCT03576417. |
| 60. | Dogan V, Rieckmann T, Münscher A, Busch CJ. Current studies of immunotherapy in head and neck cancer. Clin Otolaryngol. 2018;43:13-21. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 38] [Cited by in RCA: 51] [Article Influence: 6.4] [Reference Citation Analysis (0)] |
| 61. | Cramer JD, Burtness B, Ferris RL. Immunotherapy for head and neck cancer: Recent advances and future directions. Oral Oncol. 2019;99:104460. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 177] [Cited by in RCA: 218] [Article Influence: 36.3] [Reference Citation Analysis (0)] |
| 62. | ClinicalTrials. gov [Internet]. Study of Safety and Tolerability of Nivolumab Treatment Alone or in Combination With Relatlimab or Ipilimumab in Head and Neck Cancer. 2021 Available from: https://clinicaltrials.gov/ct2/show/NCT04080804. |
| 63. | ClinicalTrials. gov [Internet]. A Study to Evaluate Immune Biomarker Modulation in Response to VTX-2337 in Combination With an Anti- PD-1 Inhibitor in Head and Neck Cancer. 2021. Available from: https://clinicaltrials.gov/ct2/show/NCT03906526. |
| 64. | Taylor MH, Rasco DW, Brose MS, Vogelzang NJ, Richey SL, Cohn AL, Richards DA, Stepan DE, Dutcus CE, Guo M, Shumaker RC, Schmidt EV, Wirth LJ. A phase 1b/2 trial of lenvatinib plus pembrolizumab in patients with squamous cell carcinoma of the head and neck. J Clin Oncol. 2018;36:6016. [RCA] [DOI] [Full Text] [Cited by in Crossref: 24] [Cited by in RCA: 24] [Article Influence: 3.4] [Reference Citation Analysis (0)] |
| 65. | Sacco AG, Chen R, Ghosh D, Wong DJL, Worden FP, Adkins D, Pittman E, Messer K, Gold KA, Daniels GA, Swiecicki P, Sutton B, Natsuhara A, Cohen EEW. An open label, nonrandomized, multi-arm, phase II trial evaluating pembrolizumab combined with cetuximab in patients with recurrent/metastatic (R/M) head and neck squamous cell carcinoma (HNSCC): Results of cohort 1 interim analysis. J Clin Oncol. 2019;37:6033. [RCA] [DOI] [Full Text] [Cited by in Crossref: 11] [Cited by in RCA: 12] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 66. | Sacco AG, Chen R, Worden FP, Wong DJL, Adkins D, Swiecicki P, Chai-Ho W, Oppelt P, Ghosh D, Bykowski J, Molinolo A, Pittman E, Estrada MV, Gold K, Daniels G, Lippman SM, Natsuhara A, Messer K, Cohen EEW. Pembrolizumab plus cetuximab in patients with recurrent or metastatic head and neck squamous cell carcinoma: an open-label, multi-arm, non-randomised, multicentre, phase 2 trial. Lancet Oncol. 2021;22:883-892. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 42] [Cited by in RCA: 169] [Article Influence: 42.3] [Reference Citation Analysis (0)] |
| 67. | Hamid O, Bauer TM, Spira AI, Olszanski AJ, Patel SP, Wasser JS, Smith DC, Balmanoukian AS, Aggarwal C, Schmidt EV, Zhao Y, Gowda H, Gangadhar TC. Epacadostat plus pembrolizumab in patients with SCCHN: Preliminary phase I/II results from ECHO-202/KEYNOTE-037. J Clin Oncol. 2017;35:6010. [RCA] [DOI] [Full Text] [Cited by in Crossref: 40] [Cited by in RCA: 42] [Article Influence: 5.3] [Reference Citation Analysis (0)] |
| 68. | Kao HF, Liao BC, Huang YL, Huang HC, Chen CN, Chen TC, Hong YJ, Chan CY, Hong RL. Afatinib and pembrolizumab for recurrent or metastatic head and neck squamous cell carcinoma (ALPHA Study): A phase II study with biomarker analysis. J Clin Oncol. 2021;39:6024. [RCA] [DOI] [Full Text] [Cited by in Crossref: 3] [Cited by in RCA: 3] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 69. | Forster MD, Sacco JJ, Kong AH, Wheeler G, Forsyth S, Bhat R, Blair K, Lowe H, Spanswick VJ, Ensell L, Hartley JA, White L. EACH: A randomised phase II study evaluating the safety and anti-tumour activity of the combination of avelumab and cetuximab relative to avelumab monotherapy in recurrent/metastatic head and neck squamous cell cancer. J Clin Oncol. 2019;37:TPS6091. [RCA] [DOI] [Full Text] [Cited by in Crossref: 4] [Cited by in RCA: 4] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 70. | Harrington KJ, Kong A, Mach N, Chesney JA, Fernandez BC, Rischin D, Cohen EEW, Radcliffe HS, Gumuscu B, Cheng J, Snyder W, Siu LL. Talimogene Laherparepvec and Pembrolizumab in Recurrent or Metastatic Squamous Cell Carcinoma of the Head and Neck (MASTERKEY-232): A Multicenter, Phase 1b Study. Clin Cancer Res. 2020;26:5153-5161. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 29] [Cited by in RCA: 71] [Article Influence: 14.2] [Reference Citation Analysis (0)] |
| 71. | Ang KK, Harris J, Wheeler R, Weber R, Rosenthal DI, Nguyen-Tân PF, Westra WH, Chung CH, Jordan RC, Lu C, Kim H, Axelrod R, Silverman CC, Redmond KP, Gillison ML. Human papillomavirus and survival of patients with oropharyngeal cancer. N Engl J Med. 2010;363:24-35. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 5387] [Cited by in RCA: 4979] [Article Influence: 331.9] [Reference Citation Analysis (0)] |
| 72. | ClinicalTrials. gov [Internet]. Ipilimumab, Nivolumab, and Radiation Therapy in TreatingPatients With HPV Positive Advanced Oropharyngeal Squamous Cell Carcinoma. Available from: https://clinicaltrials.gov/ct2/show/NCT03799445. |
| 73. | ClinicalTrials. gov [Internet]. Chemoradiation vs Immunotherapy and Radiation for Head and Neck Cancer. Available from: https://clinicaltrials.gov/ct2/show/NCT03383094. |
| 74. | Sacco AG, Sharabi A, Jing Z, Pittman E, Gold KA, Sumner W. Radiotherapy with Concurrent and Adjuvant Pembrolizumab in Patients with P16-Positive Locoregionally Advanced Head and Neck Cancer: KEYCHAIN Trial Lead-In Results. Int J Radiat Oncol Biol Phys. 2019;105:E363-E364. [RCA] [DOI] [Full Text] [Cited by in Crossref: 7] [Cited by in RCA: 8] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 75. | Hsieh JC, Wang HM, Wu MH, Chang KP, Chang PH, Liao CT, Liau CT. Review of emerging biomarkers in head and neck squamous cell carcinoma in the era of immunotherapy and targeted therapy. Head Neck. 2019;41 Suppl 1:19-45. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 34] [Cited by in RCA: 59] [Article Influence: 11.8] [Reference Citation Analysis (0)] |
| 76. | Tardy MP, Di Mauro I, Ebran N, Refae S, Bozec A, Benezery K, Peyrade F, Guigay J, Sudaka-Bahadoran A, Badoual C, Pedeutour F, Saada-Bouzid E. Microsatellite instability associated with durable complete response to PD-L1 inhibitor in head and neck squamous cell carcinoma. Oral Oncol. 2018;80:104-107. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 15] [Cited by in RCA: 16] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 77. | Hanna GJ, Lizotte P, Cavanaugh M, Kuo FC, Shivdasani P, Frieden A, Chau NG, Schoenfeld JD, Lorch JH, Uppaluri R, MacConaill LE, Haddad RI. Frameshift events predict anti-PD-1/L1 response in head and neck cancer. JCI Insight. 2018;3. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 130] [Cited by in RCA: 208] [Article Influence: 29.7] [Reference Citation Analysis (0)] |
| 78. | ClinicalTrials. gov [Internet]. WGA in Platinum-refractory HNSCC Underwent Nivolumab. 2021 Available from: https://clinicaltrials.gov/ct2/show/NCT03917537. |
| 79. | Gandhi AK, Roy S, Thakar A, Sharma A, Mohanti BK. Symptom Burden and Quality of Life in Advanced Head and Neck Cancer Patients: AIIMS Study of 100 Patients. Indian J Palliat Care. 2014;20:189-193. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 24] [Cited by in RCA: 32] [Article Influence: 2.9] [Reference Citation Analysis (0)] |
| 80. | Rajendra A, Noronha V, Joshi A, Patil VM, Menon N, Prabhash K. Palliative chemotherapy in head and neck cancer: balancing between beneficial and adverse effects. Expert Rev Anticancer Ther. 2020;20:17-29. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 7] [Cited by in RCA: 24] [Article Influence: 4.8] [Reference Citation Analysis (0)] |
| 81. | Liu M, Han S, Zheng B, Cai H, Yang J, Zhuang Q, Li N. Cost-Effectiveness Analysis Of Pembrolizumab In The Treatment Of Advanced Recurrent Metastatic Head And Neck Squamous Cell Carcinoma In China And The United States. Cancer Manag Res. 2019;11:9483-9493. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 8] [Cited by in RCA: 13] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 82. | Haddad R, Cohen EEW, Venkatachalam M, Young K, Singh P, Shaw JW, Korytowsky B, Abraham P, Harrington KJ. Cost-effectiveness analysis of nivolumab for the treatment of squamous cell carcinoma of the head and neck in the United States. J Med Econ. 2020;23:442-447. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 12] [Cited by in RCA: 20] [Article Influence: 4.0] [Reference Citation Analysis (0)] |
| 83. | Sanderson CR, Currow DC. Palliative care meets immunotherapy: what happens as cancer paradigms change? BMJ Support Palliat Care. 2018;8:431-432. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4] [Cited by in RCA: 4] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 84. | Ghanizada M, Jakobsen KK, Grønhøj C, von Buchwald C. The effects of checkpoint inhibition on head and neck squamous cell carcinoma: A systematic review. Oral Oncol. 2019;90:67-73. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 31] [Cited by in RCA: 36] [Article Influence: 6.0] [Reference Citation Analysis (0)] |
| 85. | Wang BC, Cao RB, Li PD, Fu C. The effects and safety of PD-1/PD-L1 inhibitors on head and neck cancer: A systematic review and meta-analysis. Cancer Med. 2019;8:5969-5978. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 16] [Cited by in RCA: 25] [Article Influence: 4.2] [Reference Citation Analysis (0)] |
| 86. | Galot R, Le Tourneau C, Guigay J, Licitra L, Tinhofer I, Kong A, Caballero C, Fortpied C, Bogaerts J, Govaerts AS, Staelens D, Raveloarivahy T, Rodegher L, Laes JF, Saada-Bouzid E, Machiels JP. Personalized biomarker-based treatment strategy for patients with squamous cell carcinoma of the head and neck: EORTC position and approach. Ann Oncol. 2018;29:2313-2327. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 40] [Cited by in RCA: 48] [Article Influence: 8.0] [Reference Citation Analysis (0)] |
| 87. | Wang HC, Yeh TJ, Chan LP, Hsu CM, Cho SF. Exploration of Feasible Immune Biomarkers for Immune Checkpoint Inhibitors in Head and Neck Squamous Cell Carcinoma Treatment in Real World Clinical Practice. Int J Mol Sci. 2020;21. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 10] [Cited by in RCA: 13] [Article Influence: 2.6] [Reference Citation Analysis (0)] |