Published online Apr 24, 2022. doi: 10.5306/wjco.v13.i4.237
Peer-review started: March 21, 2021
First decision: July 27, 2021
Revised: August 27, 2021
Accepted: April 9, 2022
Article in press: April 9, 2022
Published online: April 24, 2022
Processing time: 396 Days and 21.2 Hours
Non-small cell lung cancer (NSCLC) is a heterogeneous disease accounting for approximately 85% of all lung cancers. Only 17% of patients are diagnosed at an early stage. Treatment is multidisciplinary and radiotherapy plays a key role in all stages of the disease. More than 50% of patients with NSCLC are treated with radiotherapy (curative-intent or palliative). Technological advances-including highly conformal radiotherapy techniques, new immobilization and respiratory control systems, and precision image verification systems-allow clinicians to individualize treatment to maximize tumor control while minimizing treatment-related toxicity. Novel therapeutic regimens such as moderate hypofractionation and advanced techniques such as stereotactic body radiotherapy (SBRT) have reduced the number of radiotherapy sessions. The integration of SBRT into routine clinical practice has radically altered treatment of early-stage disease. SBRT also plays an increasingly important role in oligometastatic disease. The aim of the present guidelines is to review the role of radiotherapy in the treatment of localized, locally-advanced, and metastatic NSCLC. We review the main radiotherapy techniques and clarify the role of radiotherapy in routine clinical practice. These guidelines are based on the best available evidence. The level and grade of evidence supporting each recommendation is provided.
Core Tip: Radiotherapy is a critical component of multi-modality treatment of non-small-cell lung cancer (NSCLC). This guideline provides recommendations on the use of radiation therapy to treat patients with different stages of NSCLC. Our goal is to promote medical knowledge among physicians and improve health-care quality on these patients. These guidelines are based on the best available evidence. The level and grade of evidence supporting each recommendation is provided.
- Citation: Rodríguez De Dios N, Navarro-Martin A, Cigarral C, Chicas-Sett R, García R, Garcia V, Gonzalez JA, Gonzalo S, Murcia-Mejía M, Robaina R, Sotoca A, Vallejo C, Valtueña G, Couñago F. GOECP/SEOR radiotheraphy guidelines for non-small-cell lung cancer. World J Clin Oncol 2022; 13(4): 237-266
- URL: https://www.wjgnet.com/2218-4333/full/v13/i4/237.htm
- DOI: https://dx.doi.org/10.5306/wjco.v13.i4.237
Lung cancer is the leading cause of cancer-related mortality in both men and women. In 2018, there were more than 1.7 million cancer-related deaths worldwide. In that same year, more than 2 million people were newly-diagnosed with lung cancer. At diagnosis, approximately 57% of lung cancers are metastatic, 22% present lymph node involvement, and only 17% of cases are diagnosed at early stages[1]. Various environmental and lifestyle factors have been associated with the development of lung cancer. The main risk factor is tobacco use, accounting for 85%-90% of cases[2]. Non-small cell cancer (NSCLC) comprises more than 85% of all lung cancer diagnoses. Despite important treatment advances in recent years, 5-year overall survival (OS) rates remain low, ranging from to 0%-10% in stage IVA-IVB disease to as high as 68% in early stage[3,4].
Advances in treatment and diagnosis include minimally-invasive diagnostic/therapeutic techniques such as endobronchial ultrasound (EBUS) and video-assisted thoracic surgery[5]. In addition, determination of the histological subtypes has become standard practice to assess eligibility-based on tumor histology and molecular status-for systemic therapy[6,7].
Radiotherapy (RT) is one of the three pillars of the multidisciplinary treatment of lung cancer. In recent years, technological advances have greatly improved this treatment modality. It is estimated that more than half of all cancer patients will require curative or palliative-intent RT at some point in the course of the disease[8]. A series of important advances-including simulation with four-dimensional computed tomography (4D-CT), three-dimensional conformal RT (3D-CRT), intensity-modulated RT (IMRT), volumetric modulated arc therapy (VMAT), cone beam CT (CBCT) image verification systems, and control of respiratory movement-have made it possible to maximize tumor control while minimizing toxicity to adjacent healthy organs and tissues[9]. As a result, the radiation dose can be precisely delivered to the target and adapted to the patient’s individual characteristics [anatomy and tumor location, TNM stage, comorbidities, and general performance status (PS)].
In the present guidelines, we review the clinical indications for RT in NSCLC according to disease stage, with a discussion of fractionation schedules, treatment volumes, and organs at risk (OAR). We also discuss the management of the main clinical scenarios seen in routine practice, establishing the grades of recommendation for each treatment according to the strength of evidence.
These guidelines are based on the most relevant studies published in peer reviewed journals. A comprehensive review of the clinical literature in the following databases was performed: MEDLINE (Pubmed), EMBASE (Ovid), Web of science (Web of Knowledge). Article selection was undertaken by the expert authors. The Infectious Diseases Society of America grading system[10] was used to assign levels of evidence and grades of recommendation (Table 1). Statements without grading were considered justified standard clinical practice by the authors.
| Level of evidence | |
| I | Evidence from at least one large randomised controlled trial of good methodological quality (low potential for bias) or meta-analyses of well-conducted randomised trials without heterogeneity |
| II | Small randomised trials or large randomised trials with a suspicion of bias (lower methodological quality) or meta-analyses of such trials or of trials with demonstrated heterogeneity |
| III | Prospective cohort studies |
| IV | Retrospective cohort studies or case-control studies |
| V | Studies without control groups; case reports; expert opinions |
| Grades of recommendation | |
| A | Strong evidence for efficacy with a substantial clinical benefit, strongly recommended |
| B | Strong or moderate evidence for efficacy but with a limited clinical benefit, generally recommended |
| C | Insufficient evidence for efficacy or benefit does not outweigh the risk or the disadvantages (adverse events, costs, etc.), optional |
| D | Moderate evidence against efficacy or for adverse outcomes, generally not recommended |
| E | Strong evidence against efficacy or for adverse outcome, never recommended |
The clinical manifestations of lung cancer are frequently nonspecific. If NSCLC is suspected, the patient should be referred to the pulmonologist and/or the rapid diagnosis unit and be evaluated by a multidisciplinary team (II, C)[11,12]. The evaluation begins with CT[13,14] and positron emission tomography (PET), which are essential for diagnosis, staging, and treatment planning (I, A). Brain magnetic resonance imaging (MRI) is also essential[15]. All nodes > 1.5 cm on the CT scan should be biopsied, even if the PET scan is negative (I, C). A positive PET scan should be further evaluated, regardless of lesion size[16,17], through EBUS or digestive endoscopic ultrasonography[18-20] (I, A). In uncertain cases, conventional mediastinoscopy or video-assisted mediastinoscopy and video-assisted thoracoscopy are surgical alternatives to obtain samples for subsequent analysis[21,22]. Peripheral lesions can be evaluated by CT-guided transthoracic fine-needle aspiration biopsy[14,23]. Pathologic confirmation is required in patients with a single metastatic lesion and uptake on PET[24].
Pathologic diagnosis of NSCLC should be based on the criteria established in the World Health Organization classification system[6]. It is important to differentiate between the histological subtype: Squamous cell carcinoma, adenocarcinoma (the most common), large cell carcinoma, and neuroendocrine tumors (I, B). The International Association for the Study of Lung Cancer (IASLC) has developed a classification system for adenocarcinoma with prognostic implications[25]. Immunohistochemical studies and determination of molecular alterations such as epidermal growth factor receptor (EGFR), Kirsten rat sarcoma, and anaplastic lymphoma kinase (ALK) mutations should be performed, as these alterations can predict sensitivity to certain drugs and/or targeted therapies[26] (I, B). Classification of NSCLC or not otherwise specified histology should be avoided. Staging is based on the IASLC TNM classification system (8th edition), which is used to classify patients according to disease stage to determine the prognosis and appropriate treatment[27].
Indications: Stereotactic body radiation therapy (SBRT), also known as stereotactic ablative body radiation, consists of delivery of high dose radiation to a very specific target volume, with a high dose gradient in all directions[28]. The indication for this technique is based on the patient’s surgical risk category: Inoperable, high-risk, or standard-risk[29]. As follows: (1) Inoperable: Approximately 25% of patients with early-stage NSCLC (ES-NSCLC) are inoperable due to age or comorbidities[30]. In this population, prospective studies of SBRT have reported local control (LC) rates of 90% at 5 years[31] and 91.9% at 7 years[32] and, with a ≥ grade (G)3 toxicity rate under 10%. The well-designed phase II TROG 09.02 CHISEL trial[33] compared SBRT to conventional 3D-RT. SBRT was superior to conventional 3D-RT in terms of LC [hazard ratio (HR) = 0.32, 95% confidence interval (CI): 0.13-0.77, P = 0.0077] with no increase in treatment-related adverse events (AEs). SBRT is therefore the treatment of choice in inoperable patients (III, A); (2) Operable NSCLC: Only one prospective study (a pooled analysis of the ROSEL and STARS trials) has (indirectly) compared SBRT to 3D-CRT in operable patients[34,36]. The findings of that study, published in 2015, were criticised for the underpowered statistical analyses and the poor surgical quality in the two trials[35]. Several non-comparative prospective studies of SBRT have been conducted, most notably the phase II RTOG 0618 trial[36]. In that trial, 33 operable patients received SBRT, with a 4-year LC rate of 96% and ≥ G3 toxicity of only 8%. The findings of retrospective series comparing SBRT to surgery through matched pair analysis are inconclusive[37,38]. However, a recent meta-analysis[39] suggested that surgery may provide better outcomes on various survival parameters, including OS, cancer-specific survival, and disease-free survival (DFS). Prospective phase III trials are needed to confirm these findings. Currently, four prospective trials are underway to compare surgery to SBRT. Of these trials, the only non-randomised study is the Canadian RAXSIA trial (NCT03431415). The POSTILV trial (NCT01753414) is comparing SBRT to surgery in operable patients while the STABLE-MATES trial (NCT02468024) is comparing sublobar resection to SBRT. Although the VALUE trial (NCT02984761) was activated in 2016, they are still recruiting patients as of the last update (December 2020). Therefore, at present, there is no evidence to support SBRT vs surgery in operable patients, unless the patient refuses surgery (III, C); and (3) High-risk patients or patients > 75 years of age. The American Society of Clinical Oncology and the American Society of Radiation Oncology (ASTRO) recommend offering SBRT as an alternative treatment in high-risk patients[40,41] (III, A).
Fractionation: In order to select the appropriate fractionation schedule in SBRT, it is essential to carefully weigh the risks and benefits. LC is poor when the biological equivalent dose (BED) is < 100 Gy[42]. Consequently, the dose should be determined according to the location of the target lesion and, therefore, to the tolerance of adjacent organs. Tumor are classified as central, peripheral, or “safe” (> 2 cm from mediastinal structures and > 1 cm from the chest wall) depending on their location within the thoracic cavity.
Central tumors: Central tumor fractionations as defined by the IASLC[42]: The most important prospective phase I/II trial for central tumors was the RTOG 0813 trial[43], a dose escalation study comparing 50 Gy to 60 Gy, both administered in 5 daily fractions (fx), with an escalation schedule of 0.5 Gy per fraction/arm. The maximum tolerated dose was 12.0 Gy/fx, with a ≥ G3 toxicity rate of 7.2%. Two-year LC rates in patients who received the lowest dose fraction (10 Gy/fx) was 87.5% vs 87.9% in the 12 Gy/fx regimen, with 2-year progression free survival (PFS) rates of 50% vs 54.5%, respectively.
The dose-escalated SUNSET[44] trial (dose level 1:60 Gy/7 fr-dose level 3 60 Gy/5 fr) and the Hilus[45] trial (56 Gy/8 fr to 65%-70% isodose line) were both performed to assess high-dose SBRT in central/ultracentral tumors. The recently published, Hilus trial showed that this fraccionation regimen in tumors located ≤ 1 cm from the main bronchus and trachea had a high risk of G3 to G5 toxicity (33.8%) with 10 patients experiencing G5 toxicity. These regimens contrast with the more conservative Dutch regimen (60 Gy/8 fx)[46], which obtained a 3-year LC rate of 92.6% and ≥ G3 toxicity of 7.9%.
Based on the available evidence, the optimal fractionation in central tumors appears to be 50 to 60 Gy delivered in five fractions. The dose per fraction should be adjusted to OAR tolerances, and can range from 10-12 Gy/fraction with a total dose of 50-60 Gy administered in 5 daily fractions or 8 fx of 7.5 Gy each to a total of 60 Gy.
Lesions adjacent to the chest wall: In patients with tumors located adjacent to or in contact with the chest wall, European guidelines[47] recommend a total dose of 48 Gy in four fractions. Prospective studies[48] have shown that this fractionation schedule yields 3-year LC rates ranging from 85.4% to 87.3%, with a ≥ G3 toxicity rate (rib fracture) of 3%. Other fractionation schedules have been proposed in this location. For example, Haasbeek et al[46] proposed 60 Gy in five fractions, with a 3-year LC rate of 89.3% and a late ≥ G3 toxicity rate (chest wall pain) of 2.1%[46]. Nyman et al[49] proposed 45 Gy in three fractions, which achieved a LC rate of 80% with late toxicity (rib fracture) in 4%.
Tumors located in the “safe” zone: Lesions located in the “safe” zone can be considered non-central tumors located > 2 cm from the chest wall. Evidence from two prospective phase II trials - Singh et al[50] and RTOG 0915[31]-support extreme hypofractionation (single 30-34 Gy fraction). Singh et al[50] found that a single 30 Gy fraction yielded a 2-year LC rate of 94.9%, with G3 toxicity in 17%, and no ≥ G4 toxicity. In RTOG 0915, which evaluated a single 34 Gy dose, the one-year LC rate was 97.0%, with ≥ G3 toxicity rate of 10.3%. Timmerman et al[51] conducted a prospective phase II trial to evaluate SBRT in inoperable ES-NSCLC, the findings of that trial supported the classical Timmerman fractionation scheme, with a 3-year LC rate in peripheral tumors ranging from 90.6%-94% and ≥ G3 AEs ranging from 10% to 16.3% (Table 2).
| Localization | Dose | Ref. | Evidence level |
| Central tumour | 50/5 fx-60/5 fx | Bezjak et al[43], 2019 | II, B |
| 60 Gy/8 fx | Haasbeek et al[46], 2011 | ||
| Chest wall | 48 Gy/4 fx | Guckenberger et al[47], 2017 | II, B |
| 60 Gy/5 fx | Nagata et al[48], 2015 | ||
| 45 Gy/3 fx | Nyman et al[49], 2006 | ||
| Safe zone | 30 Gy/1 fx | Singh et al[50], 2019 | II, B |
| 34 Gy/1 fx | Videtic et al[31], 2019 | ||
| 54 Gy/3 fx | Timmerman et al[36], 2018 |
Radical chemoradiotherapy: Concomitant vs sequential: At diagnosis, approximately 35% of patients with NSCLC present locally-advanced disease, for which the standard treatment is CRT. The recommended RT dose is 60-66 Gy (I, A). Increasing the radiation dose in combination with chemotherapy (ChT) does not improve outcomes but does increase toxicity rates[52].
In patients with good PS, the recommended treatment is concomitant chemoradiotherapy, which has been shown to improve OS vs sequential chemoradiotherapy by 5.7% at 3 years and 4.5% at 5 years, with a mean survival time of 22-25 mo and 5-year OS of 20%[53], probably due to better locoregional control (2.9% at 3 years and 2.2% at 5 years) (I, A). However, concomitant chemoradiotherapy also has a higher incidence of acute non-hematological toxicity[54], mainly G3-G4 esophagitis (range: 4%-18%), but no effect on acute pulmonary toxicity[55]. To date, no differences in treatment outcomes have been observed for the following variables: Type or ChT scheme, age, sex, PS, histology, or disease stage. Neither induction nor consolidation ChT are indicated, although data from the phase III PACIFIC trial showed that consolidation therapy with durvalumab improves both PFS and OS in patients with programmed death ligand 1 > 1% who do not progress after concomitant chemoradiotherapy[56].
Neoadjuvant chemoradiotherapy: Several studies, including the SAKK Lung Cancer Project Group trial[57] and the Lung Intergroup Trial 0139[58], have evaluated the role of neoadjuvant chemoradiotherapy, finding this approach improves PFS in patients who receive trimodal treatment, but without any benefit for OS. This lack of benefit in the surgical arm may be due to higher early mortality rates, especially in patients undergoing right pneumonectomy. A subanalysis found a significant improvement in survival in patients treated with induction chemoradiotherapy followed by lobectomy vs those who received concomitant chemoradiotherapy [58].
Induction chemoradiotherapy has been shown to achieve a greater reduction in nodal downstaging than ChT alone, but with no benefit in OS[53] except for potentially resectable superior sulcus tumors, for which the treatment of choice is concomitant chemoradiotherapy (45-54 Gy, 1.8-2 Gy/d) (III, A). However, it is important to plan radical dose RT in case surgery is ultimately not performed[59].
Adjuvant RT: Adjuvant RT is indicated when complete resection (R0) has not been achieved and salvage surgery is not feasible (I, A). In these cases, sequential chemoradiotherapy (ChT followed by RT) should be offered, although with a less aggressive ChT scheme. Concomitant chemoradiotherapy should be limited to patients with macroscopic residual disease (V, C).
The role of adjuvant RT has long been controversial, especially after a meta-analysis published in 1998 showed higher mortality rates after postoperative RT (PORT) in patients with N0-N1 disease[60]. However, the increased mortality was probably due to the excessive toxicity associated with older radiation therapy techniques. By contrast, no deleterious effects of adjuvant RT have been observed in N2 disease. A recent meta-analysis concluded that adjuvant RT is associated with better OS and PFS rates in these patients[61].
The role of PORT was evaluated in two recent phase III trials. In the phase III Lung-ART trial (definitive results still pending publication) patients with N2 involvement were randomized to receive PORT (54 Gy) or observation after complete tumor resection. The initial results showed that PORT did not improve DFS or OS, although fewer thoracic relapses were observed in the group treated with PORT (25% vs 46.1%). The lack of improvement in survival outcomes (DFS and OS) could be due to higher rates of cardiopulmonary toxicity ≥ G3 (10.8% vs 4.9%). In this regard, it should be noted that 89% of the patients were treated with 3D-CRT and only 11% with IMRT[62].
The second recently-published phase III trial was a single-center study involving 394 patients with stage IIIA-pN2 disease randomized to receive PORT (50 Gy) or observation after complete resection and four cycles of adjuvant chemotherapy. Most of the patients (89.3%) were treated with IMRT (10.7% received 3D-CRT). In the intention-to-treat analysis, PORT did not improve DFS, although it did improve DFS in the per-protocol analysis. The results of a pre-planned exploratory analysis in which patients were stratified according to the number of resected nodes ( 20 vs > 20) and involved nodes [(1-3) vs ≥ 4] revealed a significant improvement in DFS (HR = 0.75; 95%CI: 0.58-0.98; P = 0.04). PORT had no impact on OS. Toxicity rates were lower than observed in the Lung-ART trial, probably due to the high proportion of patients treated with IMRT, stricter dose limits to the OARs, and the exclusion of the contralateral mediastinal nodes from the treatment volume[63].
Despite the significant improvement in LC achieved in both trials, this did not lead to better survival outcomes. For this reason, PORT is not currently recommended as part of standard treatment in patients with N2 involvement and R0 resection. However, stage IIIA-N2 patients are a heterogeneous group and some of these patients could benefit from PORT, as suggested by data from previous studies as well as the recently published findings of the Lung-ART trial (ESMO 2021), which show that a mediastinal nodal involvement ratio (nodes involved/nodes evaluated) ≥ 25% is a prognostic factor for DFS, suggesting that the extent of nodal involvement could help to select patients who may benefit from PORT. Nonetheless, more studies are needed to better determine, through a comprehensive analysis of clinical and molecular characteristics, the patients most likely to benefit from PORT. Based on the postoperative pathologic findings, the recommended PORT doses are as follows: (1) R0: 50-54 Gy, 1.8-2 Gy/fx; (2) Involved margins or microscopic disease: 54-60 Gy; and (3) Macroscopic residual disease: ≥ 60 Gy[59,64].
Altered fractionation schemes: Various dose-intensification strategies have been explored, including accelerated hyperfractionation and other hypofractionated schemes.
Accelerated fractionation and hyperfractionation: Three phase III trials compared different hyperfractionated schemes to conventional RT, demonstrating that hyperfractionated RT yields positive results when administered alone or after induction ChT (I, A). Those trials include the Continuous Hyperfractionated Accelerated Radiotherapy Trial (CHART)[65,66], HART[67], and Continuous Hyperfractionated Accelerated Radiotherapy Weekend Less (CHARTWEL)[68]. The findings of these trials were recently confirmed in a large retrospective series[69].
A meta-analysis evaluated the results of nine trials (2000 patients) - including the CHART, HART, and CHARTWEL trials - comparing conventional RT to various hyperfractionated and accelerated RT schemes. All of the altered fractionation schemes improved OS, although without any significant between-group differences in PFS. The administration or not of ChT did not impact OS. The modified fractionation schemes, particularly very accelerated RT, increased the risk of acute severe esophagitis in Table 3[70].
| Ref. | Study type | Number of patients | Radiotherapy | Chemotherapy | Results | Toxicity |
| [65,66] | Phase III RCT | n = 563: Stage I (29%), II (7%), IIIA (38%), IIIB (23%). Similar in both arms | [cRT: 60 Gy, 2 Gy/d (6 wk). INP 44 Gy + boost 16 Gy tumour and involved nodes] vs (CHART: 54 Gy, 1.5 Gy/3 times/d, 6 h apart, on 12 consecutive days). INP 37.5 Gy in 25 fx + boost 16.5 Gy in 11 Gy to tumour and involved nodes | No | Absolute 2-yr survival improvement of 9%: 20% cRT vs 29% CHART. 21% relative risk reduction for PL. Major improvement in squamous cell disease: 13% 2-yr survival: 20% cRT vs 33% CHART. 25% relative risk reduction of PL | Clinical pneumonitis 19% cRT and 10% CHART |
| [67] | Phase III RCT | n = 141: Stage III A-B unresectable. ECOG 0-1 | [cRT: 64 Gy, 2 Gy/d (6 ½ wk)] vs [HART: 57.6 Gy, 1.5 Gy 2 times/d (2.5 wk)] | Induction: Carboplatin AUC 6 + paclitaxel 225 mg/m2 2 cycles prior to RT | 2-yr OS: 44% HART vs 24% cRT; 3 yr: 34% vs 14%. Non-significant trend towards better survival with HART. Feasible treatment. Trial close early due to slow recruitment | Esophagitis ≥ G3: 23% HART vs 15% cRT. Pneumonitis ≥ G3: 0 HART vs 10% cRT |
| [68] | Phase III RCT | n = 406: Stage I 10%, II 5%, IIIA 38%, IIIB 46%. Similar in both arms | (CHARTWEL: 60 Gy, 1.5 Gy 2 times/d in 2.5 wk) vs (cRT: 66 Gy, 2 Gy/d, 6.5 wk) | Neoadjuvant 27%. Similar in both arms | Better LC in CHARTWEL. No difference between arms in OS at 2, 3, 5 yr. Better LC CHARTWEL trend in advanced stages and after neoadjuvant ChT | Greater acute dysphagia CHARTWEL. Greater radiological pneumonitis CHARTWEL, no differences in clinical pneumonitis |
| [69] | Retrospective | n = 849, 9 United Kingdom centres. Stage I 33%, II 13%, IIIA 24%, IIIB 24%, IV 1% | CHART: 54 Gy, 1.5 Gy/3 times/d, 6 h apart, in 12 d | Induction: 27% patients, 82% stage III (96% platinum doublets: Cisplatin or carboplatin with vinorelbine, gemcitabine or paclitaxel) | OS 2 and 3 yr: 47% and 32%. OS 3 yr: 38% stage I and 27% stage III. Tendency to better survival in stage III after ChT | Esophagitis, pneumonitis ≥ G3 5% |
Moderate hypofractionation: Some patients - due to advanced age, the presence of comorbidities, and/or travel-related difficulties - are poor candidates for conventional (60-66 Gy, 30-36 daily fractions) or hyperfractionated RT. In recent months, due to the coronavirus disease 2019 (COVID-19) pandemic and the consequent need to reduce the number of hospital visits, the use of moderately hypofractionated RT has become more common in patients eligible for radical RT.
The available evidence suggests that dose escalation with standard fractionation techniques (achieved by extending treatment duration) does not improve outcomes[52]. However, radiobiological models show that each 1% increase in the radiation dose improves LC by 1% to 2%[71]. A systematic review of clinical data from dose escalation studies[72] found a BED10 dose-response relationship for NSCLC. That review evaluated studies that applied various fractionation schemes, including standard fractionation, hyperfractionation, and hypofractionation. Although the best results were obtained with hypofractionated RT, the differences were not significant.
Phase I dose escalation trials of hypofractionated RT have evaluated various regimens[73-75]. Prospective and retrospective series[76-80] have found that accelerated RT is both feasible and well-tolerated when administered alone or concurrently/sequentially with ChT, a finding that was also confirmed in the interim analysis of a phase III trial (Iyengar et al[81]) comparing accelerated hypofractionated RT to conventional RT.
The phase III EORTC 08972-22973 trial[82] and the randomised phase II SOCCAR trial[83] compared concurrent to sequential CRT in patients receiving hypofractionated RT. Based on the excellent results obtained with concomitant CRT in the SOCCAR trial[83], this scheme is now widely used in routine practice in the United Kingdom. Iqbal et al[84] showed that modifying the ChT dose, incorporating advanced imaging techniques such as PET-CT for staging, and the use of IMRT and VMAT improved survival outcomes at 2-years (58%), with acceptable rates of acute toxicity (Table 4).
| Ref. | Type of study | Number of patients | Radiotherapy | Chemotherapy | Results | Toxicity |
| [76] | Prospective | 30, stage III-IVA. ECOG ≥ 2 | 60 Gy (20 fx 3 Gy); (BED10 79.4 Gy) | Sequential (80% patients) | LR 37%. OS 2-yr 38.1%. LR 37%. Distant relapse 57% | Acute esophagitis G3 7%. Acute pneumonitis G3 3%. No chronic toxicity |
| [77] | Prospective | 83 (32 stage III) | 66 Gy (24 fx 2.75 Gy); (BED10 84 Gy) | Sequential 90.6% stage III (platinum + vinorelbine) | OS 2 yr 37.5%. SCE 2 yr 41.5% | No toxicity ≥ G3 |
| [78] | Retrospective | 300, stage III, inoperable, MEG | 3 arms: 45 Gy (15 fx 3 Gy); 60-63 Gy (6 wk); > 63 Gy (6 wk) | No significant differences in LC, distant control, or OS. > DFS in 60-63 Gy | Lower in hypofractionated arm | |
| [79] | Retrospective | 609 (9 centres). Stage IA (18%), IB (30.7%), II (14.8%), IIIA (16.4%), IIIB (19.2%). Unresectable or inoperable | 55 Gy (20 fx 2.75 Gy) | ChT 28% (83% stage III). Platinum doublets. Most neoadjuvant | OS at 2, 3 and 5 yr: 50%, 36% and 20%. 2 yr OS: stage IA, 72%, stage Ib 51%, stage IIIA 40%. Adenocarcinoma better median survival (31 m) vs squamous (20.4 m). No difference in OS between ChT vs no ChT. Stage III, trend towards better OS with ChT | No toxicity ≥ G3. Pneumonitis G1-2, 15% |
| [80,82] | Retrospective | 31, stage I (15), II (15), IIIA (57), IIIB (43). Medically inoperable or unresectable | 3 arms: 66 Gy (24 fx 2.75 Gy) + daily cisplatin (6 mg/m2); same sequential RT after 2 cycles cisplatin/gemcitabine; RT alone 66 Gy (24 fx 2.75 Gy) or 60 Gy (20 fx 3 Gy) | Concurrent: Cisplatin daily (6 mg/m2). Sequential: (2 cycles cisplatin/gemcitabine) prior to RT | LR 36%, DM 46%. Better RT + ChT than RT alone. 5 yr OS: Concurrent CRT, 23%. No significant difference between concurrent and sequential CRT. LR 36%, DM 46% | Severe late toxicity greater in CRT (27% concurrent, 23% sequential) than in RT alone (8%) |
| [81] | Phase III RCT1 | 60, stage II/III (11.6%/88.3%). ECOG ≥ 2. Not candidates for ChT/RT | cRT 60-66 Gy/30-33 fx vs accelerated hypofx 60 Gy/15 fx 4 Gy | Non-concurrent ChT. Possible neoadjuvant or adjuvant | OS and PFS without significant differences between cRT and hypofx | No G4 toxicity. G3 toxicity: 35% cRT and 18.75% hypofx |
| [82] | Phase III RCT | 158, stage I (3% sequential, 1% concurrent), II (4% sequential, 5% concurrent), IIIA (45% sequential, 30% concurrent), IIIB (47% sequential, 64% concurrent). Inoperable ECOG 0-1 | 66 Gy (24 fx 2.75 Gy) | Concurrent: Daily cisplatin (6 mg/m2) + RT 66 Gy (24 fx 2.75 Gy) vs sequential: 2 cycles gemcitabine 1250 mg/m2 days 1, 8 and cisplatin (75 mg/m2 day 2, prior to RT 66 Gy (24 fx 2.75 Gy) | No significant differences between the 2 groups in DM, OS, PFS. OS 2 and 3 yr: 39%-34% concurrent and 34%-22% sequential. Both schemes well tolerated. Due to early closure, no conclusions drawn | Acute esophagitis G3/4 more common in concurrent (14% vs 5%). Late esophagitis G3 = 4% in both arms. Pneumonitis G3/4 = 18% concurrent and 14% sequential |
| [83] | Phase II RCT | 130, stage III inoperable. ECOG 0-1 | 55 Gy (20 fx 2.75 Gy) | Concurrent: Cisplatin 20 mg/m2 days 1-4 and 16-19 and vinorelbine 15 mg/m2 days 1, 6, 15 and 20 RT and 1 or 2 post ChT cycles (CDDP) 80 mg/m2 day 1 and vinorelbine 25 mg/m2 days 1 and 8). Sequential: Cisplatin 80 mg/m2 day 1 and vinorelbine 25 mg/m2 days 1 and 8, x 3-4 cycles before RT | No significant differences. OS 1 yr: 70% concurrent vs 83% sequential and 2 yr: 50% concurrent vs 46% sequential. PFS 1 yr: 74% concurrent vs 85% sequential; 2 yr: 47% concurrent vs 45% sequential. Both safe and effective treatments. Non-significant trend towards better survival with concurrent RT/ChT | Similar esophagitis ≥ G3 in both arms (8.8% concurrent and 8.5% sequential. Pneumonitis ≥ G3: 3.1% concurrent vs 5.2% sequential. No grade 4/5 esophagitis. G3 neutropenia lower in concurrent (37%) vs sequential (55%) |
| [84] | Retrospective | 100, stages IIIA-B 95%, II 5%. ECOG 0/1 | 55 Gy (20 fx 2.75 Gy) | Concurrent: Cisplatin 20 mg/m2 days 1-4 and 16-19 RT and vinorelbine 15 mg/m2 days 1, 6, 15, 20 and 2 cycles post RT/ChT | OS 2 yr 58%. PFS 2 yr 49% | Esophagitis G3/4 14%. Pneumonitis G3/4 4% |
A systematic review evaluated 33 studies (1902 patients) involving radical-intent hypofractionated RT for the treatment of stage III NSCLC. The number of fractions in those studies ranged from 15 to 35, with dose fractions ranging from 2.3 Gy to 3.5 Gy, and total doses from 45.0 to 85.5 Gy. Nearly half of those studies (15/33) included concurrent ChT with radiation schemes ranging from 52.5 to 75 Gy at 2.24-3.5 Gy/dose in 15-30 fx. The other studies included neoadjuvant, adjuvant, or no ChT, at RT doses ranging from 45-85.5 Gy (2.25-3.42 Gy/fx, 15-35 fx). There was a linear relationship between BED10 and OS: Every 1 Gy increase in BED10 yielded an absolute survival benefit of 0.36% to 0.70%. Compared to non-concurrent schemes, concurrent CRT was associated with better OS, albeit with higher - but still acceptable - rates of esophageal toxicity[85].
A single-centre study evaluated 563 patients; 43% received CHART and 57% hypofractionated RT (55 Gy in 20 fx of 2.75 Gy). Both treatment regimens yielded comparable results in terms of survival and treatment-related AEs[86]. Based on their findings, the authors concluded that moderately hypofractionated RT with concurrent ChT is safe when delivered with modern RT techniques and may improve treatment outcomes. However, these findings need to be confirmed in phase III trials.
The ongoing COVID-19 pandemic has led to an increase in the use of hypofractionated RT. To address the challenges presented by the pandemic, a group in the United Kingdom[87] and the ESTRO-ASTRO[88] have both published recommendations for hypofractionated schemes during this period. The United Kingdom group recommends 55 Gy in 20 fx of 2.75 Gy with concurrent ChT in patients with good PS. In patients unable to tolerate concurrent CRT, those guidelines recommend either sequential CRT or RT alone. If ChT is not administered, then hypofractionated RT schemes (e.g., 50-58 Gy in 15 fx) can be considered[87]. The ESTRO-ASTRO practice guidelines, developed through a modified Delphi consensus process, proposed recommendations for two different scenarios: (1) Early pandemic phase, focused on risk mitigation; and (2) A later phase (severe pandemic scenario) in which RT resources may be limited. In the first scenario, there was strong support (97% of the expert panel) for hypofractionated RT (60 Gy in 15 fx, 60 Gy in 20 fx, 60-66 Gy in 24-30 fx, or 55 Gy in 20 fx) if treatment was limited to RT alone. For sequential CRT, there was also strong support (97%) for the same fractionation and dose schemes, although with a clear preference for the 55 Gy (20 fx) or 60-66 Gy (24-30 fx at 2.2-2.75 Gy/d) schemes (II, A). There was no consensus to support concomitant hypofractionated CRT. An alternative would be 55-60 Gy in 20 fx[88] (II, B).
RT in oligometastatic patients: Approximately two-thirds (60%-70%) of patients with NSCLC are diagnosed with stage IV disease. Of these, 20% - or more if PET-CT imaging is used for staging - are oligometastatic at diagnosis[89]. Oligometastasis may present in one of two ways: (1) “De novo” oligometastasis: Patient with 3-5 lesions at diagnosis (synchronous) or after 3-6 mo of treatment of the primary tumor (metachronous); and (2) Induced oligometastatic: Polymetastatic patient with metastatic disease in 3-5 locations after systemic therapy.
This recently described concept of oligometastatic disease[90,91] can be further subdivided as follows: (1) Oligopersistence: Persistent disease that is stable on imaging studies, with < 5 lesions after systemic treatment; (2) Oligoprogression: Progression (new lesions or growth of known lesions) in 3 to 5 sites after systemic treatment; and (3) Oligorecurrence: Recurrent disease in 3-5 sites in patients not receiving active systemic therapy.
In these patients, a prior with disseminated disease, the use of local treatments has been shown to improve OS[92] (II, B). In this regard, three prospective[93-95] studies involving patients with oligometastasis at diagnosis have been published (Table 5). Those trials demonstrated that the patients most likely to benefit from local treatments are those whose disease remains stable or responds to systemic therapy, which is why the National Comprehensive Cancer Network guidelines for oligoprogression recommend mutation-directed therapies (EGFR, ALK). However, it is important to keep in mind that patients in the experimental arms of those trials did not receive immunotherapy, an approach that has altered the treatment paradigm in metastatic disease. In this regard, several studies are currently evaluating radioimmunotherapy, which combines local RT with immunotherapy[96].
| Ref. | Type | Design | Palliative treatment | Histology | Presentation | No. of metastases/location | RT type | Follow-up (mo) | PFS (mo) | MFS (mo) | OS (mo) |
| Gomez et al[93], 2019 | Phase II RCT. Multicentre | Induct. ChT: (RT + MT) vs MT | 49 | NSCLC (No EGFR, ALK) | Synchronous. Metachronous | ≤ 3 (1%:65%)/lung, CNS, bone, liver SSRR, nodes | SABR/SBRT (MTX) hypofra. RT (primary) | 38.8 | 14.2 (SABR/SBRT + MT) vs 4.4 (MT) | 11.9 (SABR/SBRT + MT) vs 5.7 | 41 (SABR/SBRT + MT) vs 17 |
| Iyengar et al[95], 2018 | Phase II RCT. Multicentre | Induct. ChT: (SBRT + mChT) vs mChT | 29 | NSCLC (No GFR, ALK) | Synchronous | ≤ 5 (1%:21%, 2%-3%:76%)/lung, lymph, bone, SSRR | SABR/SBRT (MTX) hypofra. RT (primary) | 9.61 | 9.7 (SABR/SBRT + MT) vs 3.5 (MT) | NR | NR (SABR/SBRT + MT) vs 17 |
| Palma et al[94], 2020 | Phase II RCT. Multicentre | (ChT + PT) vs (ChT + SABR/SBRT) | 99 | Lung (18/99) | Synchronous. Metachronous | ≤ 5 (1%-3%:93%)/lung, bone, CNS, liver, SSRR | SABR/SBRT | 51 | 11.6 (SABR/SBRT + MT) vs 5.4 (TP-MT) | NR | 50 (SABR/SBRT + MT) vs 22 |
Multiple studies have sought to identify the characteristics of the “true” oligometastatic patient and those with the best prognosis based on predictors identified in retrospective series (Table 6), as well as other predictive variables currently under investigation[97,98]. These patients are candidates for radical RT, with the dose adjusted for the lesion location and size. The most common metastatic sites in patients with stage IV NSCLC are the brain, lungs, liver, bone, and adrenal glands.
| Factor | Comments |
| Gender | Female > male |
| Histology | Adenocarcinoma > squamous cell carcinoma |
| Presentation | Metachronous > synchronous |
| Karnofsky index - ECOG | 80% < - ≤ 100% |
| Number of lesions | 1 > 2-3 > 4-10 |
| Size | < 3 cm |
| Location | Lung, bone > adrenal glands, lymph nodes > liver, brain |
RT in metastatic patients: In metastatic disease, the main objective of RT is symptom relief and better quality of life (QoL). Prior to RT, it is important to assess the patient’s functional status, social and family situation, and systemic treatment. Thanks for the important advances in targeted therapies and immunotherapy in recent years, survival in this subgroup has substantially improved[99]. The specific symptoms will depend on the tumor location; symptom relief is the main indication for RT in this setting.
Based on currently available data[100], symptom control appears to be similar regardless of the specific palliative RT scheme (I, A). Short course RT is associated with a higher risk of reirradiation, which is why it is recommended only in patients with poor PS or short life expectancy[101,102] (II, A). Higher doses (20-30 Gy in 5-10 fx) have been shown to improve OS by 5% in selected patients[103], which is why this RT scheme is recommended for thoracic lesions (II, B). Another option is endobronchial brachytherapy, which until recently was reserved for the treatment of airway obstruction in previously-irradiated patients. However, a systematic review published in 2012 comparing endobronchial brachytherapy + external beam RT (EBRT) to EBRT alone reported better symptom control in the EBRT group[104] (II, B).
The optimal management of brain metastases is increasingly controversial. In patients ineligible for stereotactic radiosurgery (SRS) and patients with multiple diffuse brain metastases, the treatment of choice is whole-brain RT (WBRT). However, the findings of the QUARTZ trial, a randomised phase III trial comparing WBRT to supportive treatment in patients unsuitable for SRS, which found no benefit for WBRT in terms of OS or QoL, called this indication into question[105] (I, A).
In patients with asymptomatic brain metastases who have not yet started systemic therapy - and could potentially benefit from targeted therapy due to the presence of oncogenic driver mutations (e.g., ALK mutation) - the start of RT can be considered given the intra- and extra-cranial effects of RT[106] (III, B).
At present, there are no clear recommendations on how to best combine RT and immunotherapy. However, two phase II studies (one randomised)[107,108] found that combined treatment was safe and provided adequate symptom control without negatively affecting QoL (III, B).
Systematic errors (inaccurate contouring of the target volume, OARs, and/or margins) reduce the likelihood of LC while increasing treatment-related toxicity. In 2018, the ESTRO published consensus guidelines for target volume definition in the treatment (radical and PORT) of locally-advanced NSCLC, with four grades of recommendation[109].
According to those guidelines, contrast-enhanced CT should be used for treatment planning. If possible, a recent PET-CT scan in the treatment position is recommended[110]. Respiratory motion should be quantified by 4D-CT, particularly in lower lobe tumours or treatments involving SBRT (IV, A). Treatment volumes[111]: (1) Gross tumour volume (GTV): The primary tumor GTV (GTV-P) and lymph nodes (GTV-N) should be delineated separately. It is important to select the correct window on CT (lung window: W = 1600, l = 600 for lesions surrounded by the lung; mediastinum window: W = 400, l = 20, for lymph nodes and tumors invading the mediastinum/chest wall) (III, A); (2) GTV-P: Areas of atelectasis should be excluded[112], which is why PET-CT imaging is particular valuable. If neoadjuvant ChT is administered, the initial volume based on the current CT scan should be used for contouring (III, B); and (3) GTV-N[113-115]: Lymph nodes that are positive on biopsy or pathologic by PET-CT or CT (≥ 1 cm) should be included. Nodes that are highly suspicious on PET-CT imaging but with negative findings on EBUS should be included due to the risk of false negatives (III, A).
If neoadjuvant ChT has been performed, include the lymph nodes or nodal stations involved prior to ChT, regardless of the response. Contouring atlases should be used for nodal station delineation[116-118]. Clinical target volume (CTV): The CTV includes the GTV plus adjacent subclinical disease. It is generally not contoured in SBRT (III, B). CTV-P[119]: For the CTV-P, the GTV should be expanded by 5-8 mm and manually edited to account for surrounding anatomy. CTV-N: The CTV-N can be created in two ways: Either by including the involved nodal station with a margin ≥ 5 mm around the GTV-N[109] or through geometric expansion of the GTV-N (5-8 mm), adapted to anatomical barriers. Elective or prophylactic nodal radiation is not recommended since it does not improve locoregional control but does increase toxicity. PORT[120]: The following areas should be irradiated: Involved lymph nodes, bronchial stump, ipsilateral hilum, and lymph node stations 4 and 7. In left lung cancers, levels 5 and 6 should also be irradiated.
Internal target volume: The internal target volume (ITV) takes into account the internal motion of the tumor. Various systems are available to estimate this motion, which can be limited to reduce the ITV, or monitored with 4D-CT or target lesion tracking[121,122]. One of the most widely used and recommended systems is 4D-CT. The CTV-GTV is contoured in each respiratory phase, or directly in the maximum intensity projection reconstruction. If this is not possible, a slow acquisition CT, or CT on inspiration, expiration and free breathing can be acquired, contouring the CTV-GTV at each point (III, B).
Planning target volume: This is generated by expanding the ITV to account for geometric uncertainties. The planning target volume (PTV) will vary according to the RT centre since differences between centres (e.g., the immobilization system, the method used to compensate for respiratory motion, the specific image-guided technique, etc.) can affect the PTV (III, A).
In many cases, the radiation dose is limited by OARs in the chest cavity. Accurate contouring of these organs is essential, especially for extreme hypofractionated schemes. In 2003, Collier et al[123] described the intra- and inter-observer uncertainty in manual contouring of thoracic OARs, thus making it possible to determine the dosimetric impact of these uncertainties. In the last decade, several different contouring atlases have been published to assist in contouring tissues in this anatomic region[124,125].
Lung (lung window settings): Although each lung should be contoured separately, the dosimetric evaluation should be based on the sum of doses to both lungs, excluding the main bronchial tree, the trachea, areas of atelectasis, and the primary GTV (IV, A).
Esophagus (mediastinal window): All layers (mucosa, submucosa and muscular) from the cricoid cartilage to the gastroesophageal junction should be included (IV, A). Oral contrast can be used to ensure correct visualization. For SBRT, contouring of the esophagus should start ≥ 10 cm above the upper limit of the PTV to ≥ 10 cm below the lower limit.
Heart (mediastinal window): There are various approaches to contouring this organ, although the most common approach is to contour the entire heart, including the pericardium and cardiac base, from the lower limit of the pulmonary artery below the aortic arch to the cardiac apex at the level of the diaphragm (IV, A). The pulmonary artery, aorta, and superior vena cava should be excluded. In some cases, other subvolumes, such as the coronary arteries (IV, C), can be included[125].
Spinal cord (mediastinal window): Generally, for EBRT, the spinal canal is delineated on the planning CT, corresponding to the planning risk volume (PRV) for the spinal cord (IV, B). For SBRT, the GTV should be contoured if it is located close to the spinal cord; MRI images are useful in these cases. Next, a PRV of the area of interest should be created.
Brachial plexus[126]: Tumors located at the lung apex should be contoured to avoid neurotoxicity (IV, B). A contrast-enhanced CT (or fusion MRI/CT) should be performed to ensure contouring accuracy. The brachial plexus is located between the anterior and middle scalene muscles. There are 5 roots (C5-T1), as follows: (1) Upper limit: The exit point between C4-C5; (2) Lower limit: Subclavian artery and vein; (3) Internal limit: The neural foramina extending from the lateral aspect of the spinal canal to the small space between the two scalene muscles; and (4) Outer limit: The space between the two scalene muscles. For tumors located in the right lung base, the liver should also be contoured (IV, C).
SBRT OARs[127]: Chest wall[128] (mediastinal window): The involved hemithorax should be contoured from the sternal border to the vertebral body, including the ribs and intercostal muscles, excluding other muscles and skin (IV, B). In peripheral tumors, the ribs closest to the tumor should be contoured separately in a bone window setting (IV, C). Trachea (mediastinal window): Include the mucosa, submucosa, and tracheal rings from the lower edge of the cricoid to the upper limit of the proximal bronchial tree (2 cm above the carina). This can also be delineated starting 10 cm above the PTV extension or 5 cm above the carina (whichever is more superior). The lower border is the upper limit of the proximal bronchial tree (IV, B). Proximal bronchial tree (mediastinal window for the trachea and carina and lung window for the bronchi). This includes the area 2 cm distal from the trachea, right and left (R/L) main bronchi, upper lobe (R/L), intermediate bronchus, middle lobe bronchus, lingula, and lower lobe (R/L) (IV, B). Aorta and great vessels[129] (mediastinal window): The aorta and superior vena cava should be included. The vascular wall and all muscle layers must be included (IV, B), and contoured starting ≥ 10 cm above the upper limit of the PTV continuing to at least 10 cm below the lower limit. Skin (mediastinal window): This is a hollow organ. Automatically contour the body and subtract 5 mm (IV, B).
Normofractionated radiation therapy: The Quantitative Analysis of Normal Tissue Effects in the Clinic (QUANTEC) study was published in 2010[130]. The aim of this study was to review the available data on the effects of radiation on normal tissue. QUANTEC updated and further refined the tolerance doses for normal tissues described by Emami et al[131] in 1991. QUANTEC provides normal tissue complication probability (NTCP) models, with summary tables of specific results for each organ. However, as the authors indicate, these limitations are not intended to replace comprehensive data provided by organ-specific reviews, and they apply primarily to adult patients. The NTCP according to organ and dose is summarised in Table 7.
| Organ | Volume | Endpoint | Dose (Gy), dose/volume | Rate, % | Ref. |
| Spinal cord | Partial | Myelopathy | Dmax 50, Dmax 60, Dmax 69 | 0.2%, 6%, 50% | |
| Lung | Whole organ, both lungs | Pneumonitis | V20 ≤ 30%, MD = 7, MD = 13, MD = 20, MD = 24, MD = 27 | < 20%, 5%, 10%, 20%, 30%, 40% | Palma et al[133], 2013 Marks et al[130], 2010 |
| Esophagus | Whole organ | ≥ Grade 3 acute esophagitis, ≥ grade 2 acute esophagitis | MD < 34, V60 ≤ 17%, V35 < 50%, V50 < 40%, V70 < 20% | 5%-20%, < 30%, < 30%, < 30% | Al-Halabi et al[135], 2015 |
| Heart | Pericardium. Whole organ | Pericarditis. Cardiac mortality long term | MD < 26, V30 < 46%, V25 < 10%, V50 ≤ 25% | < 15%, < 15%, < 1% | Speirs et al[136], 2017 |
| Brachial plexus | Whole organ | Brachial plexopathy | MD > 69 Gy. Dosis maximum 75 Gy to 2 cc of the brachial plexus | Amini et al[137], 2012 |
The specific limits are as follows: (1) Lung: With conventional fractionation (2 Gy/fx), the recommended V20 limit for both lungs is ≤ 30%-35% and MD ≤ 20-23 Gy to minimize the risk of symptomatic pneumonitis to < 20%[132]. However, several different factors must be considered, included the patient’s age and any concurrent systemic treatments. A meta-analysis of data from 836 patients treated with concurrent CRT (60 Gy; cisplatin-etoposide in 38%, carboplatin-paclitaxel in 26%, other schemes in 36%)[133] found that two variables - the lung volume receiving ≥ 20 Gy (V20) and carboplatin/paclitaxel ChT - were predictors of pneumonitis. The highest risk was observed in patients > 65 years receiving carboplatin/paclitaxel-based chemotherapy. The probability of fatal pneumonitis was greater if the daily dose was > 2 Gy and the tumor was located in the lower lobe. Although the latest results presented at ESMO 2020 have called into question the role of PORT in the absence of a definitive analysis, in patients with involved margins PORT is still indicated. A recent study published by a group from the Memorial Sloan Kettering Cancer Center[134] compared dosimetric parameters in 285 patients with NSCLC treated with PORT between 2004 and 2017. The incidence of pneumonitis ≥ G2 was 12.6%. The following factors were associated with pneumonitis: Lung and heart dose, age, and carboplatin-based ChT. These data suggest that elderly patients may be more susceptible to lower lung doses. To limit the risk of pneumonitis ≥ G2 to less than 5% in patients receiving PORT, the authors recommended the following limits: (1) Lung V5 ≤ 65% in patients < 65 years of age and V5 ≤ 36% in patients ≥ age 65. After pneumonectomy, the recommended limits are lung V5 < 60%, V20 < 4%-10%, and median lung dose < 8 Gy[132]; (2) Esophagus: In a study published in 2015, Al-Halabi et al[135] evaluated 20 patients who underwent CRT for tumours located < 1 cm from the esophagus. The median radiation dose was 70.2 Gy (range: 63-72.15 Gy). Due to measures taken to protect the contralateral esophagus, there were no cases of esophagitis ≥ G3. The proposed dose contraints to the contralateral esophagus were: V45 < 2.5 cc and V55 < 0.5 cc. IMRT and VMAT allow for dose reduction to the esophagus, thus reducing the incidence of esophagitis; (3) Heart: A subanalysis of the RTOG 0617 dose escalation trial[136] evaluated the association between heart dosimetric parameters and OS. Heart V50 < 25% vs ≥ 25% was associated with a significant improvement in OS at both one and two years: 70.2% vs 46.8% and 45.9% vs 26.7% (P < 0.0001), respectively. The median heart V50 was significantly higher (20.8% vs 13.9%, P < 0.0001) in patients with ≥ G1 cardiac toxicity; and (4) Plexus: An analysis of 90 patients with apical lung cancer treated with CRT found an association between brachial plexopathy and the mean dose to the brachial plexus > 69 Gy (60% of doses > 69 Gy vs 13% ≤ 69 Gy) and maximum dose > 75 Gy at 2 cc of the brachial plexus (43% vs 13%)[137].
Hypofractionated radiation therapy: Several different total and fractional dose schedules have been used for moderate hypofractionation, including concurrent CRT with various ChT schemes and sequential RT after ChT, or EBRT alone. The dose constraints were not reported in all studies. Table 8 summarises the recommended dose constraints for the most common moderately hypofractionated schemes published by Faivre-Finn et al[87].
| Organ | Concurrent RT/ChT (55 Gy/20 fx) | Sequential RT/ChT (55 Gy/20 fx) | RT (50-58 Gy/15 fx) | RT (50-60 Gy/15 fx)[138] |
| Spinal cord | MD 44 Gy (0.1 cc) | Dmax ≤ 36 | MD 42 Gy (0.1 cc) | MD < 38 Gy |
| Esophagus1 | MD < 55 Gy (1 cc) | V42 < 32% | MD < 52 Gy (1 cc) | MD < 50 Gy (1 cc), V45 < 10 cc |
| Lungs-GTV | V20 < 35%, MD < 18 Gy | V20 < 25%-30%, MD ≤ 15 Gy | V19 < 35%, MD < 16 Gy | V20 < 30%, V5 < 60%, MD < 20 Gy |
| Heart | V30 < 36% | V33 < 25% | D100% < 33 Gy, D67% < 40 Gy, D33% < 52 Gy | MD 63 Gy, V57 < 10 cc |
| Great vessels | NA | NA | MD 58 Gy | MD 63 Gy, V57 < 10 cc |
| Trachea, carina and main bronchus | NA | NA | MD 58 Gy | MD 63 Gy, V57 < 10cc |
| Rib | MD < 63 Gy | NA | V30 < 30 cc | MD 63 Gy; V30 < 30cc |
SBRT: Several reviews have described the constarints to OARs in SBRT base on the studies shown in Table 9[19,31,36,43,139-145].
| Organ | Single fraction (30-34 Gy) | Three fractions (54-60 Gy) | Four fractions (48 Gy) | Five fractions (50-60 Gy) | Eight fractions (60 Gy) | Ref. | |||||
| Optimal | Mandatory | Optimal | Mandatory | Optimal | Mandatory | Optimal | Mandatory | Optimal | Mandatory | ||
| Brachial plexus | 14 Gy < 3 cc | 17.5 Gy ≤ 0.035 cc | 20.4 Gy < 3cc | 24 Gy ≤ 0.035 cc | 27 Gy < 3 cc | 30.5 Gy ≤ 0.035 cc | Benedict et al[139], Grimm et al[140] | ||||
| 14.4 Gy < 3cc | 17.5 Gy Dmax | 22.5 Gy < 3 cc | 24 Gy | 30 Gy < 3 cc | 32 Gy | Bezjak et al[43] | |||||
| 23.6 Gy < 3 cc, 30 Gy < 10 cc, 35 Gy < 1 cc | 27.2 Gy Dmax, 40 Gy Dmax | Videtic et al[31], Chang et al[146] | |||||||||
| 24 Gy ≤ 0.5 cc | 26 Gy ≤ 0.5 cc | 27 Gy ≤ 0.5 cc | 29 Gy ≤ 0.5 cc | 27 Gy ≤ 0.5 cc | 38 Gy ≤ 0.5 cc | Hanna et al[141] | |||||
| Spinal cord | 10 Gy < 0.35 cc, 7 Gy < 1.2 cc | 14 Gy ≤ 0.035 cc | 14 Gy < 0.35 cc, 12.3 Gy < 1.2 cc | 18 Gy ≤ 0.035 cc | 23 Gy < 0.35 cc, 14.5 Gy < 1.2 cc | 30 Gy ≤ 0.035 cc | Benedict et al[139] | ||||
| 7 Gy < 1.2 cc | 7 Gy < 1.2 cc | 18 Gy < 0.25 cc, 11.1 Gy < 1.2 cc | 18 Gy | 20.8 Gy < 0.35 cc, 13.6 Gy < 1.2 cc | 26 Gy Dmax | 22.5 Gy < 0.25 cc, 13.5 Gy < 1.2 cc, 13.5 Gy < 0.5 cc | Bezjak et al[43], Videtic et al[31], Timmerman et al[36] | ||||
| 18 Gy < 0.1 cc | 21.9 Gy < 0.1 cc | 23 Gy < 0.1 cc | 30 Gy < 0.1 cc | 25 Gy < 0.1 cc | 32 Gy < 0.1 cc | Hanna et al[141] | |||||
| Esophagus | 11.9 Gy < 5 cc, 14.5 Gy < 5 cc | 15.4 Gy Dmax | 17.7 Gy < 5 cc | 25.2 Gy | 19.5 Gy < 5 cc | 35 Gy | Videtic et al[31] | ||||
| 21 Gy < 5 cc | 27 Gy | 18.8 Gy < 5 cc, 30 Gy < 10 cc, 35 Gy < 1 cc | 30 Gy Dmax, 50 Gy Dmax | 27.5 Gy < 5 cc | 35 Gy, 52.5 Gy | Timmerman et al[36], Bezjak et al[43], Chang et al[146] | |||||
| 25.2 Gy < 0.5 cc | 32 Gy < 0.5 cc | 34 Gy < 0.5 cc | 40 Gy < 0.5 cc | Hanna et al[141] | |||||||
| Heart | 16 Gy < 15 cc, 16 Gy < 15 cc | 22 Gy Dmax, 22 Gy Dmax | 24 Gy < 15 cc, 24 Gy < 15 cc | 30 Gy Dmax, 30 Gy Dmax | 28 Gy < 15 cc, 35 Gy < 10 cc, 40 Gy < 1 cc | 34 Gy Dmax, 50 Gy Dmax | 32 Gy < 15 cc, 32 Gy < 15 cc | 38 Gy Dmax, 38 Gy Dmax, 52.5 Gy Dmax | Benedict et al[139], Timmerman et al[36], Bezjak et al[43], Chang et al[146] | ||
| 24 Gy < 0.5 cc | 26 Gy < 0.5 cc | 27 Gy < 0.5 cc | 29 Gy < 0.5 cc | 50 Gy < 0.5 cc | 60 Gy | Hanna et al[141] | |||||
| Great Vessels | 31 Gy < 10 cc | 37 Gy Dmax | 39 Gy < 10 cc | 45 Gy Dmax | 47 Gy < 10 cc | 53 Gy Dmax | Benedict et al[139] | ||||
| 31 Gy < 10 cc | 37 Gy < 0.035 cc | 39 Gy < 10 cc | 45 Gy Dmax | 43 Gy < 10 cc, 35 Gy < 10 cc, 40 Gy < 1 cc | 49 Gy Dmax | 47 Gy < 10 cc | 52.5 Gy Dmax | Bezjak et al[43], Videtic et al[31], Chang et al[146] | |||
| 45 Gy < 0.5 cc | 53 Gy < 5 cc | Hanna et al[141] | |||||||||
| Trachea and bronchus | 10.5 Gy < 4 cc | 20.2 Gy Dmax | 15 Gy < 4 cc | 30 Gy Dmax | 16.5 Gy < 4 cc | 40 Gy Dmax | Benedict et al[139] | ||||
| 8.8 Gy < 4 cc, 10.5 Gy < 4 cc | 22 Gy Dmax, 20.2 Gy < 0.035 cc | 21 Gy < 5 cc | 30 Gy Dmax | 30 Gy < 10 cc, 35 Gy < 1 cc, 15.6 Gy < 4 cc | 50 Gy Dmax, 34.8 Gy Dmax | Bezjak et al[43], Videtic et al[31], Timmerman et al[36], Chang et al[146] | |||||
| 30 Gy < 0.5 cc | 32 Gy < 0.5 cc | 32 Gy < 0.5 cc | 35 Gy < 0.5 cc | 32 Gy < 0.5 cc | 44 Gy < 0.5 cc | Hanna et al[141] | |||||
| Skin | 23 Gy < 10 cc, 14.4 Gy < 10 cc | 26 Gy Dmax, 16 Gy Dmax | 30 Gy < 10 cc, 22.5 Gy < 10 cc | 33 Gy Dmax, 24 Gy Dmax | 35 Gy < 10 cc, 40 Gy < 1 cc, 33.2 Gy < 10 cc | 36 Gy Dmax | 36.5 Gy < 10 cc, 30 Gy < 10 cc | 39.5 Gy Dmax, 32 Gy Dmax | Benedict et al[139], Chang et al[146], Videtic et al[31] | ||
| Chest wall | 22 Gy < 1 cc | 30 Gy Dmax | 28.8 Gy < 1 cc, 30 Gy < 30 cc | 36.9 Gy Dmax | 35 Gy < 1 cc | 43 Gy Dmax | Benedict et al[139] | ||||
| 22 Gy < 1 cc | 30 Gy Dmax | 30 Gy < 30 cc, 50 Gy < 2.3 cc | 35 Gy < 10 cc, 32 Gy < 1 cc | 40 Gy Dmax | 30 Gy < 30 cc, 50 Gy < 2.3 cc, 60 Gy < 1.4 cc | Videtic et al[31], Kong et al[145], Liao et al[147] | |||||
| 37 Gy < 0.5 cc, 30 Gy < 30 cc | 39 Gy < 0.5 cc, 32 Gy < 30 cc | 39 Gy < 0.5 cc, 35 Gy < 30 cc | Hanna et al[141], Dunlap et al[142], Ma et al[143] | ||||||||
| 40 Gy < 5 cc, 60 Gy < 0.5 cc | V30 < 30 cc, V30 < 70 cc | Herth et al[19] | |||||||||
| Normal lungs | Minimal critical volume under threshold. 1500 cc, 1000 cc | Threshold dose: 7 Gy, 7.4 Gy | Threshold dose: 11.6 Gy, 12.4 Gy | Threshold dose: 12.5 Gy, 13.5 Gy | Benedict et al[139] | ||||||
| Minimal critical volume under threshold. 1500 cc, 1000 cc, 1500 cc, 1000 cc | 7 Gy, 7.4 Gy | 20 Gy < 10%, 20 Gy < 15% | 10.5 Gy, 11.4 Gy | 11.6 Gy, 12.4 Gy, 20 Gy < 20%, 30 Gy < 10% | 12.5 Gy, 13.5 Gy, 20 Gy < 20%, 30 Gy < 10% | Bezjak et al[43], Videtic et al[31], Chang et al[146] | |||||
| V20 < 10%, V12.5 < 15% | V20 < 10%, V12.5 < 15% | V20 < 10%, V12.5 < 15% | Hanna et al[141] | ||||||||
| Treatment on lesion: V20 < 10%; treatment 2-3 lesions: V20 < 12.5% (optimal); V20 < 15% (acceptable); V20 < 20% (selected cases) 3-8 fractions on alternating days. If the lesions are not included in the treatment field, alternate the treatment days for the different lesions | Hanna et al[141] | ||||||||||
| In 3-5 fraction Dmean ≤ 8 Gy and V20 ≤ 10%-15% | Kong et al[145] | ||||||||||
Technological advances in recent years have led to significant changes in the radiotherapeutic treatment of NSCLC, which has progressed from 3D-CRT to IMRT and VMAT, together with advances in image-guided RT (IGRT) and the introduction of proton RT.
Based on data from non-randomised studies, these more sophisticated techniques reduce toxicity to OARs and improve tumor control, thereby leader to better survival outcomes when compared to 3D-CRT[147,148]. The phase III RTOG 0617 trial comparing IMRT to 3D-CRT in advanced stage disease showed that IMRT reduced lung doses (V20), leading to lower rates of severe (≥ G3) pneumonitis and lower heart doses, which is a predictor of survival[149,150]. VMAT offers many of the same advantages as IMRT, including a reduction in the number of treatment sessions, similar lung doses and PTV coverage, but with lower heart doses; as a result, VMAT is becoming more common in the treatment of NSCLC[151].
Intrathoracic motion of lung tumor and healthy tissues is a major challenge that can significantly influence treatment delivery. Breathing control techniques can help reduce PTV margins and allow for more precise treatment delivery based on the unique motion of a given tumor, thus providing better tumor control and lower doses to OARs. During planning, several techniques can be used to quantify tumor motion, including “slow” CT, inspiration-expiration CT, or 4D-CT, as well as techniques to control movement, such as abdominal compression, deep-inspiration breath hold, and breath synchronization techniques such as “gating” in which CT acquisition and treatment are performed in specific phases of the respiratory cycle, and “real-time” tumor tracking-used mainly in SBRT[152]. A useful resource for the implementation of respiratory control is the AAPM Task Group 76 report, which can be used to develop institutional guidelines based on the technical resources available at each centre[153].
The incorporation of CBCT has improved IGRT. CBCT allows for more accurate positioning and reduces inter- and intrafraction errors, thus resulting in smaller PTV margins and lower OAR doses. In addition, CBCT can measure changes in location, morphology, and physiology, thus permitting changes in the initial treatment plan[154-156]. This capacity to adjust the treatment plan, known as adaptive RT, permits administration of higher radiation doses to the tumor with lower doses to the OARs[155,157,158]. Data from small studies suggest that adaptive RT improves LC[159]. This technique is currently being evaluated in the phase II RTOG 1106 trial (NCT01507428) comparing standard concomitant CRT (60 Gy) to adaptive RT based on PET-CT imaging.
Data from both retrospective and prospective studies suggest that proton radiation therapy (PRT) may be superior to photon RT in the treatment of NSCLC[160-162]. However, only one randomised study has compared SBRT to PRT in stage I disease and that trial was closed early[163]. In patients with stage III disease, prospective and retrospective studies have shown acceptable locoregional control with PRT combined with ChT[164]. PRT has the potential to reduce toxicity to OARs such as the lung, heart, and esophagus, especially in unresectable central tumors[165-167]. However, to date, only one randomised phase II trial has compared IMRT to PRT, finding no significant advantages for PRT, nor any significant differences between these modalities in terms of pneumonitis or LC[168]. Consequently, the theoretical advantages of PRT need to be validated in randomised trials, such as RTOG 1308, which is currently recruiting patients[169].
Approximately 20%-40% of patients with early stage or locally-advanced NSCLC develop locoregional progression or metachronous disease at 2 years[170]. Most of these recurrences or second primaries are unresectable, which explains the growing interest in reirradiation. Due to technological advances in radiation therapy delivery - IMRT, SBRT, proton therapy, and IGRT - it is now possible to consider reirradiating certain tumors. However, there is no consensus on the optimal approach to RT for local recurrences in previously-irradiated patients[171].
The two most common techniques in the radical dose reirradiation setting are IMRT and SBRT. To select the technique that provides the best local disease control with acceptable toxicity, it is important to consider the following parameters: Type of prior RT, anatomic location of the recurrence, and whether the lesion is located in or outside of the original RT field. Several factors - good PS, lung function, small PTV, and a BED dose > 100 Gy - are predictive of better LC and survival. Consequently, these factors should be considered when determining suitability for reirradiation.
SBRT is the technique of choice for peripheral recurrences located far from the mediastinum[172] because SBRT-related toxicity can be severe when the tumor is located near the bronchial tree and/or esophagus. Vyfhuis et al[173] reported a 92% LC rate in patients treated with 50 Gy in four fractions (SBRT) while Kilburn et al[174] reported a 2-year LC rate of 67% for recurrences located within the prior treatment field, with an acceptable toxicity profile (G2 = 30%, only case of G3 toxicity). The findings of the MD Anderson studies[175] show that IMRT is the most appropriate technique for reirradiation in central tumors, as high doses are required to achieve better LC. IMRT also reduces the dose to healthy tissues, thus limiting toxicity.
Particle therapy (protons/carbon ions) is another option to consider for re-irradiation, mainly to reduce toxicity to OARs, as the physical characteristics of these particles reduces the integral dose (low-dose bath of photons at the beam exit point). However, these patients have a high rate of metastases. Some studys reported a significant decrease in OAR toxicity in patients reirradiated with PRT[176,177].
Proton therapy is increasingly being used as a primary treatment for NSCLC and may also have an important role in the reirradiation setting, mainly due to the lack of exit doses. Although carbon ion radiation therapy (CIRT) appears to be superior to proton therapy, due to greater linear energy transfer and relative biological effectiveness, its use is currently very limited[178].
The ROCOCO dosimetric comparison study[179] showed that PRT reduced the integral dose and doses to OARs, even with dose escalation. Chao et al[180] found that patients treated with PRT had a high rate of toxicity, with 39% of patients developing ≥ G3 toxicity. In that study, the one-year OS and DFS rates were 59% and 58%, respectively. However, given the toxicity findings, the authors recom-mended careful selection of patients.
Several studies are currently evaluating reirradiation in NSCLC. Some of these trials have completed patient recruitment and results are pending. One trial (NCT01808677) is evaluating reirradiation with IMRT or PRT; the main endpoint is severe toxicity (≥ G3) and survival is a secondary endpoint.
Reirradiation with CIRT has shown moderate efficacy and acceptable toxicity, suggesting that this modality could be an effective treatment option in selected patients[181]; however, large multicentre trials are required to confirm these findings.
To conclude this section, the best candidates for reirradiation have the following characteristics: Good PS, small volume recurrences, non-central locations, and the capacity to tolerate high dose radiation (SBRT, IMRT, or particle therapy)[175,182].
Management of the overall treatment time (OTT) is especially important in NSCLC. Depending on the fractionation scheme, the effects of prolonging the OTT may vary, and different strategies can be employed to minimize these deleterious effects. In normofractionated schemes, extending the OTT will negatively impact locoregional control and OS[183-186]. One report suggested that OS rates may decrease by up to 1.8% for each day of treatment prolongation[187]. In hyperfractionated regimens, interruptions that increased the OTT by ≥ 5 d in high dose schemes (≥ 69.6 Gy) negatively impact OS, especially in patients with good prognostic factors, such as Karnofsky Performance Status 90%-100%, weight loss < 5%, and ≤ N2[185].
In the year 2000, the Royal College of Radiologists in the United Kingdom published recommendations for the management of unscheduled treatment interruptions, which were updated in 2019[188]. These recommendations divide the treatment type into three categories: Radical (categories 1 and 2) and palliative (category 3) treatment, as follows: (1) Category 1: Patients whose tumors have a high repopulation rate (e.g., squamous cell tumors) who are being treated with radical curative-intent RT. The United Kingdom recommendations include both NSCLC and SCLC in this group. Treatment prolongation in these patients should be no more than two days beyond the prescribed time in 95% of patients; (2) Category 2: Patients with slow growing cancers (mainly adenocarcinomas) receiving radical-intent RT. This group includes breast, transitional bladder carcinoma, and prostate cancer; and (3) Category 3: Patients undergoing palliative-intent RT. OTT prolongation is less critical in these cases. However, it is advisable to compensate for prolonged (> 7 d) interruptions.
Some authors have suggested that modern RT techniques such as IMRT reduce the incidence of treatment interruptions[189]. Nevertheless, the general principle is to ensure that interruptions are kept as short as possible and to anticipate interruptions whenever possible.
In general, treatment delays can be classified into two main groups: Planned and unplanned interruptions. Two types of measures - universal and specific - can be applied to address these scenarios. Universal measures are useful in both groups, while specific measures will depend on whether the interruption is programmed or not.
There are two main types of universal compensation measures, as follows: (1) Compensation on weekends and holidays; and (2) The use of compatible linear accelerators, which allow for treatment delivery on either machine. Although this is a “planned” measure, it also allows for compensation in the event of unexpected equipment malfunction.
Specific measures can be classified according to whether the interruption was planned or unplanned, as follows: (1) Unplanned: Option 1: Administer two sessions on the same day, 6 h apart, to compensate for the delay. Option 2: Compensate for the dose in the remaining fraction based on the BED, taking into account the a/b for healthy tissue or tumor according to the following formula[190].
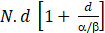
Where N is the number of fractions, d is the dose per fraction, and α/β is the repair coefficient between lethal and sub-lethal damage. If we take into account the accelerated repopulation time, assuming a tumor α/β ratio of 10, the formula would be as follows.
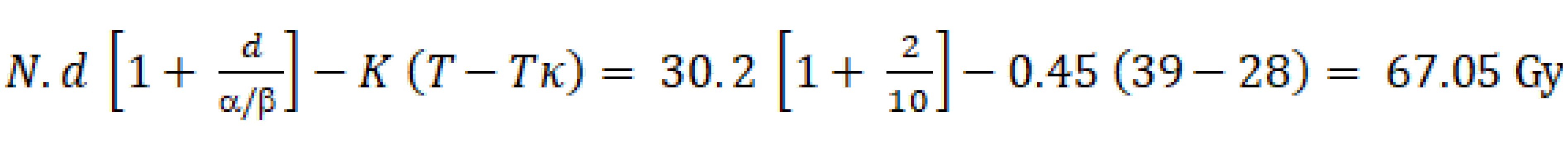
Where: + K (esti-mated loss of biological efficacy in Gy per day of delay that would need to be added to compensate)[183]: (1) Stages T1-3, N0-1: 0.27 Gy/d; stages T1-3 N2-3 or T4: 0.75 Gy/d; all stages: Mean 0.45 Gy/d. + T: Total treatment time. In the example, the T is 39 d and treatments assumed to start on a Monday. + Tκ (time from the start of RT at which accelerated repopulation begins) reported: 3-4 wk[187]: 28 d. Therefore, to calculate the dose per remaining fraction, we need to consider the remaining BED needed to reach 67.05 Gy, and the remaining fractions not to exceed two days of treatment extension. Using this equation, we calculate the d (dose per fraction); and (2) Planned: Option 1: Compensate on a holiday. Option 2: Perform the dose calculation per fraction to compensate for the missed treatment days using the formulas described above, provided that the dose is ≤ 3.5 Gy/fx and the OAR dose tolerance is within the stipulated limits, after adjusting for the relevant biological calculation.
Recommendations: Prioritise patients with squamous cell tumors. Use IMRT whenever possible, especially in locoregionally-advanced cases. Conventional fractionation: Keep delays to a minimum. Compensate if the OTT is > 45-50 d and/or the interruption is ≥ 4-5 d. Adjuvant RT: Although there are no published data in this scenario, as a precautionary measure, avoid delays ≥ 5-10 d, especially in patients without signs of poor prognosis or squamous cell tumors. In hyperfractionated schemes, compensation strategies are more complex, which is why treatment on holidays is preferred. However, if the treatment delay is ≥ 10 d, full compensation is not recommended due to the risk of excess toxicity[188]. The number of indications for moderately hypofractionated RT and SBRT has increased substantially during the COVID-19 pandemic. Specific guidelines for these cases have been published[191].
Approximately 40% of patients with lung cancer will develop a distant recurrence from 3 to 5 years after treatment completion. At 3-years, approximately 30% of patients will develop a locoregional recurrence (potentially-curable)[55]. After SBRT, approximately 12% of patients develop locoregional recurrence at 4 years[36].
The risk for development of a second primary lung cancer after treatment ranges from 1% to 6% per patient per year and this risk does not decrease over time. The mean interval from the first to the second primary tumor ranges from 59 to 62 mo[192]. Early management of these relapses, whether curative or palliative intent, is associated with better survival and QoL, which underscores the importance of close follow-up[193,194]. For the assessment of treatment-related toxicity and recurrence, we recommend the following follow-up measures.
Most recurrences occur more than 6 mo after treatment. Based on recommendations from the ESTRO[47], the United Kingdom SBRT consensus statement[195], and updates on high-risk CT features[196] , the following follow-up procedures are recommended.
First year post-treatment: The first clinical follow-up visit (complete medical history and physical examination) should take place within 4-6 wk of treatment completion. The first CT scan should be performed at least 3 mo after treatment. Clinical evaluation, including contrast-enhanced CT, should be performed every 3 mo for at least one year.
Second to third year after treatment: After the first year, follow-up should be performed every 3-6 mo for three years. CT images performed every 3 mo should be compared to previous CTs.
Third to fifth year after treatment: CT imaging should be performed every 6 mo from year three to year five. Low-dose CT should be performed annually from that time if risk factors are present. If the CT scan reveals risk factors[197,198], then a PET scan (III, B) should be ordered. If salvage therapy is feasible, then a biopsy should be performed to confirm the PET findings (III, B). Lung function testing should be performed annually.
Based on recommendations from ESMO[59], the Italian Association of Medical Oncology[199], and SEPAR[15], we recommend the following: (1) Unsalvageable patients. Perform clinical evaluations (complete medical history, physical examination, and blood tests) every 6 mo for two years. A chest CT should be performed at months 12 and 24, with annual follow-up thereafter (III, B); and (2) Salvageable patients. First three years: CT IV contrast every 3-6 mo (III, B). Years four and five: Follow-up every 6 mo; thereafter, annual low-dose CT without contrast. If pathologic findings are detected on CT, perform PET-CT and brain MRI. Obtain histopathologic confirmation of PET findings in accordance with the therapeutic option (III, B). Maintain follow-up for at least 5 years.
General recommendations: The treating physician should actively participate in follow-up (I, C). In patients unlikely to benefit from salvage therapy, the frequency of follow-up should be adapted to the patient’s individual needs (V, B). Follow-up with PET-CT or abdominal ultrasound is not recommended (I, C). Smoking cessation[200] (III, A). Behavioral therapy combined with pharmacological intervention (I, A). Influenza and pneumococcal vaccination should be offered if not contraindicated.
Summary of recommendations is provided in Table 10.
| Diagnosis | Level of evidence, grade of recommendation |
| If lung cancer is suspected, refer patient to a rapid diagnostic service for evaluation by a multidisciplinary team | II, C |
| PET-CT is recommended for initial staging in patients with stage I-III disease who are candidates for radical treatment | I, A |
| EBUS/EUS is recommended for clinical staging in patients with enlarged lymph nodes without distant metastases, with or without PET uptake | I, C |
| EBUS/EUS is recommended for stating in patients with positive PET-CT scans and normal-sized lymph nodes without distant metastases | I, A |
| Histological confirmation of the mediastinum by EBUS/EUS is recommended in central tumours, tumours > 3 cm, and N1 cases | I, C |
| Histological confirmation is required in cases with a single metastatic lesion and positive PET-CT | II, A |
| Brain MRI is recommended in candidates for curative-intent treatment | II, A |
| VAMS should be performed when EBUS/EUS findings are not evaluable | I, B |
| Differentiation between adenocarcinomas and squamous cell carcinomas is recommended even for small biopsies or cytology | I, B |
| EGFR mutations and ALK rearrangements should be assessed in patients with stage IV, non-squamous cell carcinomas. This determination should be performed in all cases (regardless of smoking status) and in all non-smokers independently of tumour histology | I, B |
| Early stage NSCLC - SBRT | |
| Inoperable | II, A |
| Operable | III, C |
| High surgical risk | III, A |
| Locally-advanced disease | |
| Concomitant radiotherapy: This is the treatment of choice for unresectable stage IIIA/IIIB with ECOG 0-1 and weight loss < 5% in 3 mo | I, A |
| 60-66 Gy in 30-33 daily fractions of 2 Gy/fx and 2-4 ChT cycles | I, A |
| Platinum-based ChT | I, A |
| Treatment should be completed in < 7 wk | III, B |
| Sequential radiotherapy | |
| If concomitant treatment is not possible, the alternative is sequential CRT | I, A |
| Treatment should be completed in a short period of time | I, A |
| Neoadjuvant radiotherapy | |
| Assessment by a multidisciplinary team is recommended | IV, C |
| In potentially-resectable upper sulcus tumours, the recommended approach is neoadjuvant CRT followed by surgery | III, A |
| This approach can be considered in potentially-resectable T3/T4 tumours, but only in well-selected cases at experienced centres | III, B |
| Surgery must be performed within 4 wk after completion of RT | III, B |
| Adjuvant radiotherapy | |
| Not recommended in early stage disease with complete resection (R0) | I, A |
| It should be considered if resection is incomplete or margins are involved (R1) | IV, B |
| Not recommended as standard in R0 cases with N2 involvement | I, A |
| In N2 disease, adjuvant RT could be considered based on risk factors for local recurrence | IV, C |
| If adjuvant ChT and RT are both administered, the recommended sequence is ChT followed by RT | V, C |
| Altered fractionation schemes | |
| Accelerated hyperfractionation schemes provide better disease control than conventional RT | I, A |
| Recommended fractionation schemes for RT administered alone or sequentially after ChT: 55 Gy (20 fx, 2.75 Gy), 60 Gy (20 fx, 3 Gy), 60 Gy (15 fx, 4 Gy), 45-50 Gy (15 fx, 3-3.33 Gy) | II, A |
| If RT administered concurrently with ChT in patients with good performance status: 55 Gy (20 fx 2.75 Gy) | II, B |
| General considerations: There is no evidence to support prophylactic WBRT in stage III disease | II, A |
Radiotherapy is a critical component of multi-modality treatment of NSCLC. This GOECP/SEOR guidelines, can help physicians to improve medical knowledge and find better ways to treat their NSCLC patients. Following the level of evidence this guidelines are summarized in Table 10.
Provenance and peer review: Invited article; Externally peer reviewed.
Peer-review model: Single blind
Corresponding Author's Membership in Professional Societies: Oncologic Group for the Study of lung Cancer-Spanish Radiation Oncology Group.
Specialty type: Oncology
Country/Territory of origin: Spain
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): 0
Grade C (Good): C
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Kawabata H, Japan S-Editor: Wang JJ L-Editor: A P-Editor: Wang JJ
| 1. | National Cancer Institute. Cancer stat facts: Lung and bronchus cancer. [cited 15 January 2021]. Available from: https://seer.cancer.gov/statfacts/html/Lungb.html. |
| 2. | Haugen A. [Lung cancer]. Tidsskr Nor Laegeforen. 1998;118:2362-2365. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 342] [Cited by in RCA: 536] [Article Influence: 89.3] [Reference Citation Analysis (0)] |
| 3. | Goldstraw P, Chansky K, Crowley J, Rami-Porta R, Asamura H, Eberhardt WE, Nicholson AG, Groome P, Mitchell A, Bolejack V; International Association for the Study of Lung Cancer Staging and Prognostic Factors Committee, Advisory Boards, and Participating Institutions; International Association for the Study of Lung Cancer Staging and Prognostic Factors Committee Advisory Boards and Participating Institutions. The IASLC Lung Cancer Staging Project: Proposals for Revision of the TNM Stage Groupings in the Forthcoming (Eighth) Edition of the TNM Classification for Lung Cancer. J Thorac Oncol. 2016;11:39-51. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3190] [Cited by in RCA: 3101] [Article Influence: 344.6] [Reference Citation Analysis (0)] |
| 4. | Duma N, Santana-Davila R, Molina JR. Non-Small Cell Lung Cancer: Epidemiology, Screening, Diagnosis, and Treatment. Mayo Clin Proc. 2019;94:1623-1640. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 747] [Cited by in RCA: 1388] [Article Influence: 231.3] [Reference Citation Analysis (0)] |
| 5. | Rami-Porta R, Call S, Dooms C, Obiols C, Sánchez M, Travis WD, Vollmer I. Lung cancer staging: a concise update. Eur Respir J. 2018;51. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 39] [Cited by in RCA: 86] [Article Influence: 12.3] [Reference Citation Analysis (0)] |
| 6. | Herbst RS, Morgensztern D, Boshoff C. The biology and management of non-small cell lung cancer. Nature. 2018;553:446-454. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1607] [Cited by in RCA: 3087] [Article Influence: 441.0] [Reference Citation Analysis (0)] |
| 7. | Travis WD, Brambilla E, Nicholson AG, Yatabe Y, Austin JHM, Beasley MB, Chirieac LR, Dacic S, Duhig E, Flieder DB, Geisinger K, Hirsch FR, Ishikawa Y, Kerr KM, Noguchi M, Pelosi G, Powell CA, Tsao MS, Wistuba I; WHO Panel. The 2015 World Health Organization Classification of Lung Tumors: Impact of Genetic, Clinical and Radiologic Advances Since the 2004 Classification. J Thorac Oncol. 2015;10:1243-1260. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2160] [Cited by in RCA: 3112] [Article Influence: 345.8] [Reference Citation Analysis (0)] |
| 8. | Kong FM, Zhao J, Wang J, Faivre-Finn C. Radiation dose effect in locally advanced non-small cell lung cancer. J Thorac Dis. 2014;6:336-347. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 31] [Reference Citation Analysis (0)] |
| 9. | Brown S, Banfill K, Aznar MC, Whitehurst P, Faivre Finn C. The evolving role of radiotherapy in non-small cell lung cancer. Br J Radiol. 2019;92:20190524. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 63] [Cited by in RCA: 97] [Article Influence: 16.2] [Reference Citation Analysis (0)] |
| 10. | Dykewicz CA; Centers for Disease Control and Prevention (U. S.); Infectious Diseases Society of America; American Society of Blood and Marrow Transplantation. Summary of the Guidelines for Preventing Opportunistic Infections among Hematopoietic Stem Cell Transplant Recipients. Clin Infect Dis. 2001;33:139-144. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 414] [Cited by in RCA: 466] [Article Influence: 19.4] [Reference Citation Analysis (0)] |
| 11. | Ost DE, Jim Yeung SC, Tanoue LT, Gould MK. Clinical and organizational factors in the initial evaluation of patients with lung cancer: Diagnosis and management of lung cancer, 3rd ed: American College of Chest Physicians evidence-based clinical practice guidelines. Chest. 2013;143:e121S-e141S. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 117] [Cited by in RCA: 122] [Article Influence: 10.2] [Reference Citation Analysis (0)] |
| 12. | Buccheri G, Ferrigno D. Lung cancer: clinical presentation and specialist referral time. Eur Respir J. 2004;24:898-904. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 75] [Cited by in RCA: 84] [Article Influence: 4.2] [Reference Citation Analysis (0)] |
| 13. | de Hoop B, De Boo DW, Gietema HA, van Hoorn F, Mearadji B, Schijf L, van Ginneken B, Prokop M, Schaefer-Prokop C. Computer-aided detection of lung cancer on chest radiographs: effect on observer performance. Radiology. 2010;257:532-540. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 53] [Cited by in RCA: 56] [Article Influence: 3.7] [Reference Citation Analysis (0)] |
| 14. | Gould MK, Donington J, Lynch WR, Mazzone PJ, Midthun DE, Naidich DP, Wiener RS. Evaluation of individuals with pulmonary nodules: when is it lung cancer? Chest. 2013;143:e93S-e120S. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 950] [Cited by in RCA: 985] [Article Influence: 82.1] [Reference Citation Analysis (0)] |
| 15. | Sánchez de Cos J, Hernández Hernández J, Jiménez López MF, Padrones Sánchez S, Rosell Gratacós A, Rami Porta R. Normativa SEPAR sobre estadificación del cáncer de pulmón. Arch Bronconeumol. 2011;47:454-465. [RCA] [DOI] [Full Text] [Cited by in Crossref: 45] [Cited by in RCA: 35] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 16. | Silvestri GA, Gonzalez AV, Jantz MA, Margolis ML, Gould MK, Tanoue LT, Harris LJ, Detterbeck FC. Methods for staging non-small cell lung cancer: Diagnosis and management of lung cancer, 3rd ed: American College of Chest Physicians evidence-based clinical practice guidelines. Chest. 2013;143:e211S-e250S. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 825] [Cited by in RCA: 1015] [Article Influence: 84.6] [Reference Citation Analysis (0)] |
| 17. | De Leyn P, Dooms C, Kuzdzal J, Lardinois D, Passlick B, Rami-Porta R, Turna A, Van Schil P, Venuta F, Waller D, Weder W, Zielinski M. Revised ESTS guidelines for preoperative mediastinal lymph node staging for non-small-cell lung cancer. Eur J Cardiothorac Surg. 2014;45:787-798. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 391] [Cited by in RCA: 561] [Article Influence: 51.0] [Reference Citation Analysis (0)] |
| 18. | Czarnecka K, Yasufuku K. Interventional pulmonology: focus on pulmonary diagnostics. Respirology. 2013;18:47-60. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 16] [Cited by in RCA: 22] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 19. | Herth F, Becker HD, Ernst A. Conventional vs endobronchial ultrasound-guided transbronchial needle aspiration: a randomized trial. Chest. 2004;125:322-325. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 259] [Cited by in RCA: 251] [Article Influence: 12.0] [Reference Citation Analysis (0)] |
| 20. | Zhang R, Ying K, Shi L, Zhang L, Zhou L. Combined endobronchial and endoscopic ultrasound-guided fine needle aspiration for mediastinal lymph node staging of lung cancer: a meta-analysis. Eur J Cancer. 2013;49:1860-1867. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 86] [Cited by in RCA: 91] [Article Influence: 7.6] [Reference Citation Analysis (0)] |
| 21. | Adebibe M, Jarral OA, Shipolini AR, McCormack DJ. Does video-assisted mediastinoscopy have a better lymph node yield and safety profile than conventional mediastinoscopy? Interact Cardiovasc Thorac Surg. 2012;14:316-319. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 6] [Cited by in RCA: 4] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 22. | Sebastián-Quetglás F, Molins L, Baldó X, Buitrago J, Vidal G; Spanish Video-assisted Thoracic Surgery Study Group. Clinical value of video-assisted thoracoscopy for preoperative staging of non-small cell lung cancer. A prospective study of 105 patients. Lung Cancer. 2003;42:297-301. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 31] [Cited by in RCA: 24] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 23. | Baldwin DR, Eaton T, Kolbe J, Christmas T, Milne D, Mercer J, Steele E, Garrett J, Wilsher ML, Wells AU. Management of solitary pulmonary nodules: how do thoracic computed tomography and guided fine needle biopsy influence clinical decisions? Thorax. 2002;57:817-822. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 33] [Cited by in RCA: 26] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 24. | De Wever W, Vankan Y, Stroobants S, Verschakelen J. Detection of extrapulmonary lesions with integrated PET/CT in the staging of lung cancer. Eur Respir J. 2007;29:995-1002. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 42] [Cited by in RCA: 41] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 25. | Travis WD, Brambilla E, Noguchi M, Nicholson AG, Geisinger KR, Yatabe Y, Beer DG, Powell CA, Riely GJ, Van Schil PE, Garg K, Austin JH, Asamura H, Rusch VW, Hirsch FR, Scagliotti G, Mitsudomi T, Huber RM, Ishikawa Y, Jett J, Sanchez-Cespedes M, Sculier JP, Takahashi T, Tsuboi M, Vansteenkiste J, Wistuba I, Yang PC, Aberle D, Brambilla C, Flieder D, Franklin W, Gazdar A, Gould M, Hasleton P, Henderson D, Johnson B, Johnson D, Kerr K, Kuriyama K, Lee JS, Miller VA, Petersen I, Roggli V, Rosell R, Saijo N, Thunnissen E, Tsao M, Yankelewitz D. International association for the study of lung cancer/american thoracic society/european respiratory society international multidisciplinary classification of lung adenocarcinoma. J Thorac Oncol. 2011;6:244-285. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 3651] [Cited by in RCA: 3621] [Article Influence: 258.6] [Reference Citation Analysis (0)] |
| 26. | Nicholson AG, Gonzalez D, Shah P, Pynegar MJ, Deshmukh M, Rice A, Popat S. Refining the diagnosis and EGFR status of non-small cell lung carcinoma in biopsy and cytologic material, using a panel of mucin staining, TTF-1, cytokeratin 5/6, and P63, and EGFR mutation analysis. J Thorac Oncol. 2010;5:436-441. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 157] [Cited by in RCA: 152] [Article Influence: 10.1] [Reference Citation Analysis (0)] |
| 27. | Carter BW, Lichtenberger JP 3rd, Benveniste MK, de Groot PM, Wu CC, Erasmus JJ, Truong MT. Revisions to the TNM Staging of Lung Cancer: Rationale, Significance, and Clinical Application. Radiographics. 2018;38:374-391. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 35] [Cited by in RCA: 54] [Article Influence: 7.7] [Reference Citation Analysis (0)] |
| 28. | Timmerman RD, Herman J, Cho LC. Emergence of stereotactic body radiation therapy and its impact on current and future clinical practice. J Clin Oncol. 2014;32:2847-2854. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 152] [Cited by in RCA: 182] [Article Influence: 16.5] [Reference Citation Analysis (0)] |
| 29. | Brunelli A, Kim AW, Berger KI, Addrizzo-Harris DJ. Physiologic evaluation of the patient with lung cancer being considered for resectional surgery: Diagnosis and management of lung cancer, 3rd ed: American College of Chest Physicians evidence-based clinical practice guidelines. Chest. 2013;143:e166S-e190S. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 489] [Cited by in RCA: 542] [Article Influence: 45.2] [Reference Citation Analysis (0)] |
| 30. | Schwartz RM, Alpert N, Rosenzweig K, Flores R, Taioli E. Changes in quality of life after surgery or radiotherapy in early-stage lung cancer. J Thorac Dis. 2019;11:154-161. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 11] [Cited by in RCA: 21] [Article Influence: 3.5] [Reference Citation Analysis (0)] |
| 31. | Videtic GM, Paulus R, Singh AK, Chang JY, Parker W, Olivier KR, Timmerman RD, Komaki RR, Urbanic JJ, Stephans KL, Yom SS, Robinson CG, Belani CP, Iyengar P, Ajlouni MI, Gopaul DD, Gomez Suescun JB, McGarry RC, Choy H, Bradley JD. Long-term Follow-up on NRG Oncology RTOG 0915 (NCCTG N0927): A Randomized Phase 2 Study Comparing 2 Stereotactic Body Radiation Therapy Schedules for Medically Inoperable Patients With Stage I Peripheral Non-Small Cell Lung Cancer. Int J Radiat Oncol Biol Phys. 2019;103:1077-1084. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 214] [Cited by in RCA: 210] [Article Influence: 35.0] [Reference Citation Analysis (0)] |
| 32. | Sun B, Brooks ED, Komaki RU, Liao Z, Jeter MD, McAleer MF, Allen PK, Balter PA, Welsh JD, O'Reilly MS, Gomez D, Hahn SM, Roth JA, Mehran RJ, Heymach JV, Chang JY. 7-year follow-up after stereotactic ablative radiotherapy for patients with stage I non-small cell lung cancer: Results of a phase 2 clinical trial. Cancer. 2017;123:3031-3039. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 78] [Cited by in RCA: 125] [Article Influence: 15.6] [Reference Citation Analysis (0)] |
| 33. | Ball D, Mai GT, Vinod S, Babington S, Ruben J, Kron T, Chesson B, Herschtal A, Vanevski M, Rezo A, Elder C, Skala M, Wirth A, Wheeler G, Lim A, Shaw M, Schofield P, Irving L, Solomon B; TROG 09. 02 CHISEL investigators. Stereotactic ablative radiotherapy vs standard radiotherapy in stage 1 non-small-cell lung cancer (TROG 09.02 CHISEL): a phase 3, open-label, randomised controlled trial. Lancet Oncol. 2019;20:494-503. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 240] [Cited by in RCA: 403] [Article Influence: 67.2] [Reference Citation Analysis (0)] |
| 34. | Chang JY, Bezjak A, Mornex F; IASLC Advanced Radiation Technology Committee. Stereotactic ablative radiotherapy for centrally located early stage non-small-cell lung cancer: what we have learned. J Thorac Oncol. 2015;10:577-585. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 91] [Cited by in RCA: 123] [Article Influence: 12.3] [Reference Citation Analysis (0)] |
| 35. | Meyers BF, Puri V, Broderick SR, Samson P, Keogan K, Crabtree TD. Lobectomy vs stereotactic body radiotherapy for stage I non-small cell lung cancer: Post hoc analysis dressed up as level-1 evidence? J Thorac Cardiovasc Surg. 2015;150:468-471. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 25] [Cited by in RCA: 31] [Article Influence: 3.1] [Reference Citation Analysis (0)] |
| 36. | Timmerman RD, Paulus R, Pass HI, Gore EM, Edelman MJ, Galvin J, Straube WL, Nedzi LA, McGarry RC, Robinson CG, Schiff PB, Chang G, Loo BW Jr, Bradley JD, Choy H. Stereotactic Body Radiation Therapy for Operable Early-Stage Lung Cancer: Findings From the NRG Oncology RTOG 0618 Trial. JAMA Oncol. 2018;4:1263-1266. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 158] [Cited by in RCA: 271] [Article Influence: 45.2] [Reference Citation Analysis (0)] |
| 37. | Zhang B, Zhu F, Ma X, Tian Y, Cao D, Luo S, Xuan Y, Liu L, Wei Y. Matched-pair comparisons of stereotactic body radiotherapy (SBRT) vs surgery for the treatment of early stage non-small cell lung cancer: a systematic review and meta-analysis. Radiother Oncol. 2014;112:250-255. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 75] [Cited by in RCA: 71] [Article Influence: 6.5] [Reference Citation Analysis (0)] |
| 38. | Crabtree TD, Puri V, Robinson C, Bradley J, Broderick S, Patterson GA, Liu J, Musick JF, Bell JM, Yang M, Meyers BF. Analysis of first recurrence and survival in patients with stage I non-small cell lung cancer treated with surgical resection or stereotactic radiation therapy. J Thorac Cardiovasc Surg. 2014;147:1183-1191; discussion 1191. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 58] [Cited by in RCA: 78] [Article Influence: 7.1] [Reference Citation Analysis (0)] |
| 39. | Cao C, Wang D, Chung C, Tian D, Rimner A, Huang J, Jones DR. A systematic review and meta-analysis of stereotactic body radiation therapy vs surgery for patients with non-small cell lung cancer. J Thorac Cardiovasc Surg. 2019;157:362-373.e8. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 50] [Cited by in RCA: 60] [Article Influence: 10.0] [Reference Citation Analysis (0)] |
| 40. | Schneider BJ, Daly ME, Kennedy EB, Antonoff MB, Broderick S, Feldman J, Jolly S, Meyers B, Rocco G, Rusthoven C, Slotman BJ, Sterman DH, Stiles BM. Stereotactic Body Radiotherapy for Early-Stage Non-Small-Cell Lung Cancer: American Society of Clinical Oncology Endorsement of the American Society for Radiation Oncology Evidence-Based Guideline. J Clin Oncol. 2018;36:710-719. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 80] [Cited by in RCA: 135] [Article Influence: 19.3] [Reference Citation Analysis (0)] |
| 41. | Videtic GMM, Donington J, Giuliani M, Heinzerling J, Karas TZ, Kelsey CR, Lally BE, Latzka K, Lo SS, Moghanaki D, Movsas B, Rimner A, Roach M, Rodrigues G, Shirvani SM, Simone CB 2nd, Timmerman R, Daly ME. Stereotactic body radiation therapy for early-stage non-small cell lung cancer: Executive Summary of an ASTRO Evidence-Based Guideline. Pract Radiat Oncol. 2017;7:295-301. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 220] [Cited by in RCA: 330] [Article Influence: 41.3] [Reference Citation Analysis (0)] |
| 42. | Onishi H, Araki T, Shirato H, Nagata Y, Hiraoka M, Gomi K, Yamashita T, Niibe Y, Karasawa K, Hayakawa K, Takai Y, Kimura T, Hirokawa Y, Takeda A, Ouchi A, Hareyama M, Kokubo M, Hara R, Itami J, Yamada K. Stereotactic hypofractionated high-dose irradiation for stage I nonsmall cell lung carcinoma: clinical outcomes in 245 subjects in a Japanese multiinstitutional study. Cancer. 2004;101:1623-1631. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 687] [Cited by in RCA: 663] [Article Influence: 31.6] [Reference Citation Analysis (0)] |
| 43. | Bezjak A, Paulus R, Gaspar LE, Timmerman RD, Straube WL, Ryan WF, Garces YI, Pu AT, Singh AK, Videtic GM, McGarry RC, Iyengar P, Pantarotto JR, Urbanic JJ, Sun AY, Daly ME, Grills IS, Sperduto P, Normolle DP, Bradley JD, Choy H. Safety and Efficacy of a Five-Fraction Stereotactic Body Radiotherapy Schedule for Centrally Located Non-Small-Cell Lung Cancer: NRG Oncology/RTOG 0813 Trial. J Clin Oncol. 2019;37:1316-1325. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 403] [Cited by in RCA: 388] [Article Influence: 64.7] [Reference Citation Analysis (0)] |
| 44. | Giuliani M, Mathew AS, Bahig H, Bratman SV, Filion E, Glick D, Louie AV, Raman S, Swaminath A, Warner A, Yau V, Palma D. SUNSET: Stereotactic Radiation for Ultracentral Non-Small-Cell Lung Cancer-A Safety and Efficacy Trial. Clin Lung Cancer. 2018;19:e529-e532. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 44] [Cited by in RCA: 80] [Article Influence: 11.4] [Reference Citation Analysis (0)] |
| 45. | Lindberg K, Bergström PP, Brustugun OT, Engelholm S, Grozman V, Høyer M, Karlsson K, Khalil AA, Kristiansen C, Lax I, Löden B, Nyman J, Persson GF, Rogg L, Wersäll PJ, Lewensohn R. The Nordic HILUS-Trial - First Report of a Phase II Trial of SBRT of Centrally Located Lung Tumors. J Thorac Oncol. 2017;12:S340. [RCA] [DOI] [Full Text] [Cited by in Crossref: 36] [Cited by in RCA: 36] [Article Influence: 4.5] [Reference Citation Analysis (0)] |
| 46. | Haasbeek CJ, Lagerwaard FJ, Slotman BJ, Senan S. Outcomes of stereotactic ablative radiotherapy for centrally located early-stage lung cancer. J Thorac Oncol. 2011;6:2036-2043. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 175] [Cited by in RCA: 197] [Article Influence: 15.2] [Reference Citation Analysis (0)] |
| 47. | Guckenberger M, Andratschke N, Dieckmann K, Hoogeman MS, Hoyer M, Hurkmans C, Tanadini-Lang S, Lartigau E, Méndez Romero A, Senan S, Verellen D. ESTRO ACROP consensus guideline on implementation and practice of stereotactic body radiotherapy for peripherally located early stage non-small cell lung cancer. Radiother Oncol. 2017;124:11-17. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 161] [Cited by in RCA: 246] [Article Influence: 30.8] [Reference Citation Analysis (0)] |
| 48. | Nagata Y, Hiraoka M, Shibata T, Onishi H, Kokubo M, Karasawa K, Shioyama Y, Onimaru R, Kozuka T, Kunieda E, Saito T, Nakagawa K, Hareyama M, Takai Y, Hayakawa K, Mitsuhashi N, Ishikura S. Prospective Trial of Stereotactic Body Radiation Therapy for Both Operable and Inoperable T1N0M0 Non-Small Cell Lung Cancer: Japan Clinical Oncology Group Study JCOG0403. Int J Radiat Oncol Biol Phys. 2015;93:989-996. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 241] [Cited by in RCA: 307] [Article Influence: 30.7] [Reference Citation Analysis (0)] |
| 49. | Nyman J, Johansson KA, Hultén U. Stereotactic hypofractionated radiotherapy for stage I non-small cell lung cancer--mature results for medically inoperable patients. Lung Cancer. 2006;51:97-103. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 208] [Cited by in RCA: 188] [Article Influence: 9.9] [Reference Citation Analysis (1)] |
| 50. | Singh R, Ansinelli H, Sharma D, Jenkins J, Davis J, Vargo JA, Sharma S. Clinical Outcomes Following Stereotactic Body Radiation Therapy (SBRT) for Stage I Medically Inoperable Small Cell Lung Carcinoma: A Multi-Institutional Analysis From the RSSearch Patient Registry. Am J Clin Oncol. 2019;42:602-606. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 7] [Cited by in RCA: 7] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 51. | Timmerman R, Paulus R, Galvin J, Michalski J, Straube W, Bradley J, Fakiris A, Bezjak A, Videtic G, Johnstone D, Fowler J, Gore E, Choy H. Stereotactic body radiation therapy for inoperable early stage lung cancer. JAMA. 2010;303:1070-1076. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 2018] [Cited by in RCA: 1955] [Article Influence: 130.3] [Reference Citation Analysis (0)] |
| 52. | Bradley JD, Paulus R, Komaki R, Masters G, Blumenschein G, Schild S, Bogart J, Hu C, Forster K, Magliocco A, Kavadi V, Garces YI, Narayan S, Iyengar P, Robinson C, Wynn RB, Koprowski C, Meng J, Beitler J, Gaur R, Curran W Jr, Choy H. Standard-dose vs high-dose conformal radiotherapy with concurrent and consolidation carboplatin plus paclitaxel with or without cetuximab for patients with stage IIIA or IIIB non-small-cell lung cancer (RTOG 0617): a randomised, two-by-two factorial phase 3 study. Lancet Oncol. 2015;16:187-199. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1197] [Cited by in RCA: 1525] [Article Influence: 152.5] [Reference Citation Analysis (0)] |
| 53. | Majem M, Hernández-Hernández J, Hernando-Trancho F, Rodríguez de Dios N, Sotoca A, Trujillo-Reyes JC, Vollmer I, Delgado-Bolton R, Provencio M. Multidisciplinary consensus statement on the clinical management of patients with stage III non-small cell lung cancer. Clin Transl Oncol. 2020;22:21-36. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 20] [Cited by in RCA: 32] [Article Influence: 5.3] [Reference Citation Analysis (0)] |
| 54. | Curran WJ Jr, Paulus R, Langer CJ, Komaki R, Lee JS, Hauser S, Movsas B, Wasserman T, Rosenthal SA, Gore E, Machtay M, Sause W, Cox JD. Sequential vs. concurrent chemoradiation for stage III non-small cell lung cancer: randomized phase III trial RTOG 9410. J Natl Cancer Inst. 2011;103:1452-1460. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 760] [Cited by in RCA: 917] [Article Influence: 65.5] [Reference Citation Analysis (1)] |
| 55. | Aupérin A, Le Péchoux C, Rolland E, Curran WJ, Furuse K, Fournel P, Belderbos J, Clamon G, Ulutin HC, Paulus R, Yamanaka T, Bozonnat MC, Uitterhoeve A, Wang X, Stewart L, Arriagada R, Burdett S, Pignon JP. Meta-analysis of concomitant vs sequential radiochemotherapy in locally advanced non-small-cell lung cancer. J Clin Oncol. 2010;28:2181-2190. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1439] [Cited by in RCA: 1368] [Article Influence: 91.2] [Reference Citation Analysis (0)] |
| 56. | Paz-Ares L, Spira A, Raben D, Planchard D, Cho BC, Özgüroğlu M, Daniel D, Villegas A, Vicente D, Hui R, Murakami S, Spigel D, Senan S, Langer CJ, Perez BA, Boothman AM, Broadhurst H, Wadsworth C, Dennis PA, Antonia SJ, Faivre-Finn C. Outcomes with durvalumab by tumour PD-L1 expression in unresectable, stage III non-small-cell lung cancer in the PACIFIC trial. Ann Oncol. 2020;31:798-806. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 68] [Cited by in RCA: 163] [Article Influence: 32.6] [Reference Citation Analysis (0)] |
| 57. | Pless M, Stupp R, Ris HB, Stahel RA, Weder W, Thierstein S, Gerard MA, Xyrafas A, Früh M, Cathomas R, Zippelius A, Roth A, Bijelovic M, Ochsenbein A, Meier UR, Mamot C, Rauch D, Gautschi O, Betticher DC, Mirimanoff RO, Peters S; SAKK Lung Cancer Project Group. Induction chemoradiation in stage IIIA/N2 non-small-cell lung cancer: a phase 3 randomised trial. Lancet. 2015;386:1049-1056. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 227] [Cited by in RCA: 277] [Article Influence: 27.7] [Reference Citation Analysis (0)] |
| 58. | Albain KS, Swann RS, Rusch VW, Turrisi AT 3rd, Shepherd FA, Smith C, Chen Y, Livingston RB, Feins RH, Gandara DR, Fry WA, Darling G, Johnson DH, Green MR, Miller RC, Ley J, Sause WT, Cox JD. Radiotherapy plus chemotherapy with or without surgical resection for stage III non-small-cell lung cancer: a phase III randomised controlled trial. Lancet. 2009;374:379-386. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1135] [Cited by in RCA: 1077] [Article Influence: 67.3] [Reference Citation Analysis (0)] |
| 59. | Postmus PE, Kerr KM, Oudkerk M, Senan S, Waller DA, Vansteenkiste J, Escriu C, Peters S; ESMO Guidelines Committee. Early and locally advanced non-small-cell lung cancer (NSCLC): ESMO Clinical Practice Guidelines for diagnosis, treatment and follow-up. Ann Oncol. 2017;28:iv1-iv21. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1256] [Cited by in RCA: 1343] [Article Influence: 167.9] [Reference Citation Analysis (0)] |
| 60. | Postoperative radiotherapy in non-small-cell lung cancer: systematic review and meta-analysis of individual patient data from nine randomised controlled trials. PORT Meta-analysis Trialists Group. Lancet. 1998;352:257-263. [PubMed] |
| 61. | Harling L, Jayakumar S, Ashrafian H, Bille A, Toufektzian L, Smith D. Mediastinal Radiotherapy after Adjuvant Chemotherapy for Resected Non-Small Cell Lung Cancer with N2 Lymphadenopathy: A Novel Meta-Analysis. JTCVS Open. 2021;5:121-130. [RCA] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1] [Cited by in RCA: 1] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 62. | Le Pechoux C, Pourel N, Barlesi F, Faivre-Finn C, Lerouge D, Zalcman G, Antoni D, Lamezec B, Nestle U, Boisselier P, Thillays F, Paumier A, Dansin E, Peignaux K, Madelaine J, Pichon E, Larrouy A, Riesterer O, Lavole A, Bardet A. LBA3_PR An international randomized trial, comparing post-operative conformal radiotherapy (PORT) to no PORT, in patients with completely resected non-small cell lung cancer (NSCLC) and mediastinal N2 involvement: Primary end-point analysis of LungART (IFCT-0503, UK NCRI, SAKK) NCT00410683. Ann Oncol. 2020;31:S1178. [RCA] [DOI] [Full Text] [Cited by in Crossref: 63] [Cited by in RCA: 67] [Article Influence: 13.4] [Reference Citation Analysis (0)] |
| 63. | Hui Z, Men Y, Hu C, Kang J, Sun X, Bi N, Zhou Z, Liang J, Lv J, Feng Q, Xiao Z, Chen D, Wang Y, Li J, Wang J, Gao S, Wang L, He J. Effect of Postoperative Radiotherapy for Patients With pIIIA-N2 Non-Small Cell Lung Cancer After Complete Resection and Adjuvant Chemotherapy: The Phase 3 PORT-C Randomized Clinical Trial. JAMA Oncol. 2021;7:1178-1185. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 40] [Cited by in RCA: 150] [Article Influence: 37.5] [Reference Citation Analysis (0)] |
| 64. | Rodrigues G, Choy H, Bradley J, Rosenzweig KE, Bogart J, Curran WJ Jr, Gore E, Langer C, Louie AV, Lutz S, Machtay M, Puri V, Werner-Wasik M, Videtic GMM. Adjuvant radiation therapy in locally advanced non-small cell lung cancer: Executive summary of an American Society for Radiation Oncology (ASTRO) evidence-based clinical practice guideline. Pract Radiat Oncol. 2015;5:149-155. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 32] [Cited by in RCA: 40] [Article Influence: 4.4] [Reference Citation Analysis (1)] |
| 65. | Saunders M, Dische S, Barrett A, Harvey A, Gibson D, Parmar M. Continuous hyperfractionated accelerated radiotherapy (CHART) vs conventional radiotherapy in non-small-cell lung cancer: a randomised multicentre trial. CHART Steering Committee. Lancet. 1997;350:161-165. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 452] [Cited by in RCA: 405] [Article Influence: 14.5] [Reference Citation Analysis (0)] |
| 66. | Saunders M, Dische S, Barrett A, Harvey A, Griffiths G, Palmar M. Continuous, hyperfractionated, accelerated radiotherapy (CHART) vs conventional radiotherapy in non-small cell lung cancer: mature data from the randomised multicentre trial. CHART Steering committee. Radiother Oncol. 1999;52:137-148. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 385] [Cited by in RCA: 350] [Article Influence: 13.5] [Reference Citation Analysis (0)] |
| 67. | Belani CP, Wang W, Johnson DH, Wagner H, Schiller J, Veeder M, Mehta M; Eastern Cooperative Oncology Group. Phase III study of the Eastern Cooperative Oncology Group (ECOG 2597): induction chemotherapy followed by either standard thoracic radiotherapy or hyperfractionated accelerated radiotherapy for patients with unresectable stage IIIA and B non-small-cell lung cancer. J Clin Oncol. 2005;23:3760-3767. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 126] [Cited by in RCA: 118] [Article Influence: 5.9] [Reference Citation Analysis (0)] |
| 68. | Baumann M, Herrmann T, Koch R, Matthiessen W, Appold S, Wahlers B, Kepka L, Marschke G, Feltl D, Fietkau R, Budach V, Dunst J, Dziadziuszko R, Krause M, Zips D; CHARTWEL-Bronchus studygroup. Final results of the randomized phase III CHARTWEL-trial (ARO 97-1) comparing hyperfractionated-accelerated vs conventionally fractionated radiotherapy in non-small cell lung cancer (NSCLC). Radiother Oncol. 2011;100:76-85. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 110] [Cited by in RCA: 117] [Article Influence: 8.4] [Reference Citation Analysis (0)] |
| 69. | Sanganalmath P, Lester JE, Bradshaw AG, Das T, Esler C, Roy AEF, Toy E, Lester JF, Button M, Wilson P, Comins C, Atherton P, Pickles R, Foweraker K, Walker GA, Keni M, Hatton MQ. Continuous Hyperfractionated Accelerated Radiotherapy (CHART) for Non-small Cell Lung Cancer (NSCLC): 7 Years' Experience From Nine UK Centres. Clin Oncol (R Coll Radiol). 2018;30:144-150. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 6] [Cited by in RCA: 7] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 70. | Mauguen A, Le Péchoux C, Saunders MI, Schild SE, Turrisi AT, Baumann M, Sause WT, Ball D, Belani CP, Bonner JA, Zajusz A, Dahlberg SE, Nankivell M, Mandrekar SJ, Paulus R, Behrendt K, Koch R, Bishop JF, Dische S, Arriagada R, De Ruysscher D, Pignon JP. Hyperfractionated or accelerated radiotherapy in lung cancer: an individual patient data meta-analysis. J Clin Oncol. 2012;30:2788-2797. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 173] [Cited by in RCA: 190] [Article Influence: 14.6] [Reference Citation Analysis (0)] |
| 71. | Fenwick JD, Nahum AE, Malik ZI, Eswar CV, Hatton MQ, Laurence VM, Lester JF, Landau DB. Escalation and intensification of radiotherapy for stage III non-small cell lung cancer: opportunities for treatment improvement. Clin Oncol (R Coll Radiol). 2009;21:343-360. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 46] [Cited by in RCA: 44] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 72. | Partridge M, Ramos M, Sardaro A, Brada M. Dose escalation for non-small cell lung cancer: analysis and modelling of published literature. Radiother Oncol. 2011;99:6-11. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 100] [Cited by in RCA: 95] [Article Influence: 6.8] [Reference Citation Analysis (0)] |
| 73. | Cannon DM, Mehta MP, Adkison JB, Khuntia D, Traynor AM, Tomé WA, Chappell RJ, Tolakanahalli R, Mohindra P, Bentzen SM, Cannon GM. Dose-limiting toxicity after hypofractionated dose-escalated radiotherapy in non-small-cell lung cancer. J Clin Oncol. 2013;31:4343-4348. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 108] [Cited by in RCA: 126] [Article Influence: 10.5] [Reference Citation Analysis (0)] |
| 74. | Urbanic JJ, Wang X, Bogart JA, Stinchcombe TE, Hodgson L, Schild SE, Bazhenova L, Hahn O, Salgia R, Vokes EE. Phase 1 Study of Accelerated Hypofractionated Radiation Therapy With Concurrent Chemotherapy for Stage III Non-Small Cell Lung Cancer: CALGB 31102 (Alliance). Int J Radiat Oncol Biol Phys. 2018;101:177-185. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 20] [Cited by in RCA: 36] [Article Influence: 5.1] [Reference Citation Analysis (0)] |
| 75. | Westover KD, Loo BW Jr, Gerber DE, Iyengar P, Choy H, Diehn M, Hughes R, Schiller J, Dowell J, Wardak Z, Sher D, Christie A, Xie XJ, Corona I, Sharma A, Wadsworth ME, Timmerman R. Precision Hypofractionated Radiation Therapy in Poor Performing Patients With Non-Small Cell Lung Cancer: Phase 1 Dose Escalation Trial. Int J Radiat Oncol Biol Phys. 2015;93:72-81. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 43] [Cited by in RCA: 54] [Article Influence: 5.4] [Reference Citation Analysis (0)] |
| 76. | Osti MF, Agolli L, Valeriani M, Falco T, Bracci S, De Sanctis V, Enrici RM. Image guided hypofractionated 3-dimensional radiation therapy in patients with inoperable advanced stage non-small cell lung cancer. Int J Radiat Oncol Biol Phys. 2013;85:e157-e163. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 35] [Cited by in RCA: 39] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 77. | de Dios NR, Sanz X, Foro P, Membrive I, Reig A, Ortiz A, Jiménez R, Algara M. Accelerated hypofractionated radiation therapy (AHRT) for non-small-cell lung cancer: can we leave standard fractionation? Clin Transl Oncol. 2017;19:440-447. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 8] [Cited by in RCA: 8] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 78. | Amini A, Lin SH, Wei C, Allen P, Cox JD, Komaki R. Accelerated hypofractionated radiation therapy compared to conventionally fractionated radiation therapy for the treatment of inoperable non-small cell lung cancer. Radiat Oncol. 2012;7:33. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 36] [Cited by in RCA: 43] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 79. | Din OS, Harden SV, Hudson E, Mohammed N, Pemberton LS, Lester JF, Biswas D, Magee L, Tufail A, Carruthers R, Sheikh G, Gilligan D, Hatton MQ. Accelerated hypo-fractionated radiotherapy for non small cell lung cancer: results from 4 UK centres. Radiother Oncol. 2013;109:8-12. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 52] [Cited by in RCA: 56] [Article Influence: 4.7] [Reference Citation Analysis (0)] |
| 80. | Uitterhoeve AL, Koolen MG, van Os RM, Koedooder K, van de Kar M, Pieters BR, Koning CC. Accelerated high-dose radiotherapy alone or combined with either concomitant or sequential chemotherapy; treatments of choice in patients with Non-Small Cell Lung Cancer. Radiat Oncol. 2007;2:27. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 22] [Cited by in RCA: 24] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 81. | Iyengar P, Westover KD, Court LE, Patel MK, Shivnani AT, Saunders MW, Li Y, Chang JY, Gao A, Ahn C, Choy H, Timmerman RD. A Phase III Randomized Study of Image Guided Conventional (60 Gy/30 fx) Versus Accelerated, Hypofractionated (60 Gy/15 fx) Radiation for Poor Performance Status Stage II and III NSCLC Patients—An Interim Analysis. Int J Radiat Oncol. 2016;96:E451. [RCA] [DOI] [Full Text] [Cited by in Crossref: 17] [Cited by in RCA: 14] [Article Influence: 1.6] [Reference Citation Analysis (3)] |
| 82. | Belderbos J, Uitterhoeve L, van Zandwijk N, Belderbos H, Rodrigus P, van de Vaart P, Price A, van Walree N, Legrand C, Dussenne S, Bartelink H, Giaccone G, Koning C; EORTC LCG and RT Group. Randomised trial of sequential vs concurrent chemo-radiotherapy in patients with inoperable non-small cell lung cancer (EORTC 08972-22973). Eur J Cancer. 2007;43:114-121. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 135] [Cited by in RCA: 140] [Article Influence: 7.4] [Reference Citation Analysis (0)] |
| 83. | Maguire J, Khan I, McMenemin R, O'Rourke N, McNee S, Kelly V, Peedell C, Snee M. SOCCAR: A randomised phase II trial comparing sequential vs concurrent chemotherapy and radical hypofractionated radiotherapy in patients with inoperable stage III Non-Small Cell Lung Cancer and good performance status. Eur J Cancer. 2014;50:2939-2949. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 82] [Cited by in RCA: 98] [Article Influence: 8.9] [Reference Citation Analysis (0)] |
| 84. | Iqbal MS, Vashisht G, McMenemin R, Atherton P, McDonald F, Simmons T, Bradshaw A, Kovarik J, Turnbull H, Dodd L, Mulvenna P, Greystoke A. Hypofractionated Concomitant Chemoradiation in Inoperable Locally Advanced Non-small Cell Lung Cancer: A Report on 100 Patients and a Systematic Review. Clin Oncol (R Coll Radiol). 2019;31:e1-e10. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 15] [Cited by in RCA: 19] [Article Influence: 2.7] [Reference Citation Analysis (0)] |
| 85. | Kaster TS, Yaremko B, Palma DA, Rodrigues GB. Radical-intent hypofractionated radiotherapy for locally advanced non-small-cell lung cancer: a systematic review of the literature. Clin Lung Cancer. 2015;16:71-79. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 41] [Cited by in RCA: 60] [Article Influence: 5.5] [Reference Citation Analysis (0)] |
| 86. | Robinson SD, Tahir BA, Absalom KAR, Lankathilake A, Das T, Lee C, Fisher PM, Bates E, Hatton MQF. Radical accelerated radiotherapy for non-small cell lung cancer (NSCLC): A 5-year retrospective review of two dose fractionation schedules. Radiother Oncol. 2020;143:37-43. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 8] [Cited by in RCA: 19] [Article Influence: 3.2] [Reference Citation Analysis (0)] |
| 87. | Faivre-Finn C, Fenwick JD, Franks KN, Harrow S, Hatton MQF, Hiley C, McAleese JJ, McDonald F, O'Hare J, Peedell C, Pope T, Powell C, Rulach R, Toy E. Reduced Fractionation in Lung Cancer Patients Treated with Curative-intent Radiotherapy during the COVID-19 Pandemic. Clin Oncol (R Coll Radiol). 2020;32:481-489. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 45] [Cited by in RCA: 43] [Article Influence: 8.6] [Reference Citation Analysis (0)] |
| 88. | Guckenberger M, Belka C, Bezjak A, Bradley J, Daly ME, DeRuysscher D, Dziadziuszko R, Faivre-Finn C, Flentje M, Gore E, Higgins KA, Iyengar P, Kavanagh BD, Kumar S, Le Pechoux C, Lievens Y, Lindberg K, McDonald F, Ramella S, Rengan R, Ricardi U, Rimner A, Rodrigues GB, Schild SE, Senan S, Simone CB 2nd, Slotman BJ, Stuschke M, Videtic G, Widder J, Yom SS, Palma D. Practice Recommendations for Lung Cancer Radiotherapy During the COVID-19 Pandemic: An ESTRO-ASTRO Consensus Statement. Int J Radiat Oncol Biol Phys. 2020;107:631-640. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 24] [Cited by in RCA: 40] [Article Influence: 8.0] [Reference Citation Analysis (0)] |
| 89. | Nieder C, Tollåli T, Reigstad A, Pawinski A, Haukland E, Dalhaug A. Oligometastatic non-small cell lung cancer: a significant entity outside of specialized cancer centers? Med Princ Pract. 2014;23:526-531. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 18] [Cited by in RCA: 20] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 90. | Guckenberger M, Lievens Y, Bouma AB, Collette L, Dekker A, deSouza NM, Dingemans AC, Fournier B, Hurkmans C, Lecouvet FE, Meattini I, Méndez Romero A, Ricardi U, Russell NS, Schanne DH, Scorsetti M, Tombal B, Verellen D, Verfaillie C, Ost P. Characterisation and classification of oligometastatic disease: a European Society for Radiotherapy and Oncology and European Organisation for Research and Treatment of Cancer consensus recommendation. Lancet Oncol. 2020;21:e18-e28. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 532] [Cited by in RCA: 673] [Article Influence: 134.6] [Reference Citation Analysis (0)] |
| 91. | Lievens Y, Guckenberger M, Gomez D, Hoyer M, Iyengar P, Kindts I, Méndez Romero A, Nevens D, Palma D, Park C, Ricardi U, Scorsetti M, Yu J, Woodward WA. Defining oligometastatic disease from a radiation oncology perspective: An ESTRO-ASTRO consensus document. Radiother Oncol. 2020;148:157-166. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 162] [Cited by in RCA: 422] [Article Influence: 84.4] [Reference Citation Analysis (0)] |
| 92. | Corbin KS, Hellman S, Weichselbaum RR. Extracranial oligometastases: a subset of metastases curable with stereotactic radiotherapy. J Clin Oncol. 2013;31:1384-1390. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 141] [Cited by in RCA: 142] [Article Influence: 11.8] [Reference Citation Analysis (0)] |
| 93. | Gomez DR, Tang C, Zhang J, Blumenschein GR Jr, Hernandez M, Lee JJ, Ye R, Palma DA, Louie AV, Camidge DR, Doebele RC, Skoulidis F, Gaspar LE, Welsh JW, Gibbons DL, Karam JA, Kavanagh BD, Tsao AS, Sepesi B, Swisher SG, Heymach JV. Local Consolidative Therapy Vs. Maintenance Therapy or Observation for Patients With Oligometastatic Non-Small-Cell Lung Cancer: Long-Term Results of a Multi-Institutional, Phase II, Randomized Study. J Clin Oncol. 2019;37:1558-1565. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 837] [Cited by in RCA: 922] [Article Influence: 153.7] [Reference Citation Analysis (0)] |
| 94. | Palma DA, Olson R, Harrow S, Gaede S, Louie AV, Haasbeek C, Mulroy L, Lock M, Rodrigues GB, Yaremko BP, Schellenberg D, Ahmad B, Senthi S, Swaminath A, Kopek N, Liu M, Moore K, Currie S, Schlijper R, Bauman GS, Laba J, Qu XM, Warner A, Senan S. Stereotactic Ablative Radiotherapy for the Comprehensive Treatment of Oligometastatic Cancers: Long-Term Results of the SABR-COMET Phase II Randomized Trial. J Clin Oncol. 2020;38:2830-2838.. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 309] [Cited by in RCA: 832] [Article Influence: 166.4] [Reference Citation Analysis (0)] |
| 95. | Iyengar P, Wardak Z, Gerber DE, Tumati V, Ahn C, Hughes RS, Dowell JE, Cheedella N, Nedzi L, Westover KD, Pulipparacharuvil S, Choy H, Timmerman RD. Consolidative Radiotherapy for Limited Metastatic Non-Small-Cell Lung Cancer: A Phase 2 Randomized Clinical Trial. JAMA Oncol. 2018;4:e173501. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 454] [Cited by in RCA: 781] [Article Influence: 111.6] [Reference Citation Analysis (0)] |
| 96. | Leaman Alcibar O, Candini D, López-Campos F, Albert Antequera M, Morillo Macías V, Conde AJ, Rodríguez Pérez A, Hervás Morón A, Contreras Martínez J, Ferrer Albiach C, Navarro Aguilar S, Rodríguez-Ruiz ME. Time for radioimmunotherapy: an overview to bring improvements in clinical practice. Clin Transl Oncol. 2019;21:992-1004. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 6] [Cited by in RCA: 12] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 97. | Wong AC, Watson SP, Pitroda SP, Son CH, Das LC, Stack ME, Uppal A, Oshima G, Khodarev NN, Salama JK, Weichselbaum RR, Chmura SJ. Clinical and molecular markers of long-term survival after oligometastasis-directed stereotactic body radiotherapy (SBRT). Cancer. 2016;122:2242-2250. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 83] [Cited by in RCA: 102] [Article Influence: 11.3] [Reference Citation Analysis (0)] |
| 98. | Greco C, Zelefsky MJ, Lovelock M, Fuks Z, Hunt M, Rosenzweig K, Zatcky J, Kim B, Yamada Y. Predictors of local control after single-dose stereotactic image-guided intensity-modulated radiotherapy for extracranial metastases. Int J Radiat Oncol Biol Phys. 2011;79:1151-1157. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 83] [Cited by in RCA: 87] [Article Influence: 5.8] [Reference Citation Analysis (0)] |
| 99. | Chicas-Sett R, Zafra-Martin J, Morales-Orue I, Castilla-Martinez J, Berenguer-Frances MA, Gonzalez-Rodriguez E, Rodriguez-Abreu D, Couñago F. Immunoradiotherapy as An Effective Therapeutic Strategy in Lung Cancer: From Palliative Care to Curative Intent. Cancers (Basel). 2020;12. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 27] [Cited by in RCA: 24] [Article Influence: 4.8] [Reference Citation Analysis (0)] |
| 100. | Stevens R, Macbeth F, Toy E, Coles B, Lester JF. Palliative radiotherapy regimens for patients with thoracic symptoms from non-small cell lung cancer. Cochrane Database Syst Rev. 2015;1:CD002143. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 13] [Cited by in RCA: 32] [Article Influence: 3.2] [Reference Citation Analysis (0)] |
| 101. | A Medical Research Council (MRC) randomised trial of palliative radiotherapy with two fractions or a single fraction in patients with inoperable non-small-cell lung cancer (NSCLC) and poor performance status. Medical Research Council Lung Cancer Working Party. Br J Cancer. 1992;65:934-941. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 204] [Cited by in RCA: 189] [Article Influence: 5.7] [Reference Citation Analysis (0)] |
| 102. | Lutz S, Berk L, Chang E, Chow E, Hahn C, Hoskin P, Howell D, Konski A, Kachnic L, Lo S, Sahgal A, Silverman L, von Gunten C, Mendel E, Vassil A, Bruner DW, Hartsell W; American Society for Radiation Oncology (ASTRO). Palliative radiotherapy for bone metastases: an ASTRO evidence-based guideline. Int J Radiat Oncol Biol Phys. 2011;79:965-976. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 597] [Cited by in RCA: 564] [Article Influence: 40.3] [Reference Citation Analysis (0)] |
| 103. | Kramer GW, Wanders SL, Noordijk EM, Vonk EJ, van Houwelingen HC, van den Hout WB, Geskus RB, Scholten M, Leer JW. Results of the Dutch National study of the palliative effect of irradiation using two different treatment schemes for non-small-cell lung cancer. J Clin Oncol. 2005;23:2962-2970. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 86] [Cited by in RCA: 79] [Article Influence: 4.0] [Reference Citation Analysis (0)] |
| 104. | Reveiz L, Rueda JR, Cardona AF. Palliative endobronchial brachytherapy for non-small cell lung cancer. Cochrane Database Syst Rev. 2012;12:CD004284. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 15] [Cited by in RCA: 23] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 105. | Mulvenna P, Nankivell M, Barton R, Faivre-Finn C, Wilson P, McColl E, Moore B, Brisbane I, Ardron D, Holt T, Morgan S, Lee C, Waite K, Bayman N, Pugh C, Sydes B, Stephens R, Parmar MK, Langley RE. Dexamethasone and supportive care with or without whole brain radiotherapy in treating patients with non-small cell lung cancer with brain metastases unsuitable for resection or stereotactic radiotherapy (QUARTZ): results from a phase 3, non-inferiority, randomised trial. Lancet. 2016;388:2004-2014. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 471] [Cited by in RCA: 471] [Article Influence: 52.3] [Reference Citation Analysis (0)] |
| 106. | Robinet G, Thomas P, Breton JL, Léna H, Gouva S, Dabouis G, Bennouna J, Souquet PJ, Balmes P, Thiberville L, Fournel P, Quoix E, Riou R, Rebattu P, Pérol M, Paillotin D, Mornex F. Results of a phase III study of early versus delayed whole brain radiotherapy with concurrent cisplatin and vinorelbine combination in inoperable brain metastasis of non-small-cell lung cancer: Groupe Français de Pneumo-Cancérologie (GFPC) Protocol 95-1. Ann Oncol. 2001;12:59-67. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 207] [Cited by in RCA: 177] [Article Influence: 7.4] [Reference Citation Analysis (0)] |
| 107. | Theelen WSME, Peulen HMU, Lalezari F, van der Noort V, de Vries JF, Aerts JGJV, Dumoulin DW, Bahce I, Niemeijer AN, de Langen AJ, Monkhorst K, Baas P. Effect of Pembrolizumab After Stereotactic Body Radiotherapy vs Pembrolizumab Alone on Tumor Response in Patients With Advanced Non-Small Cell Lung Cancer: Results of the PEMBRO-RT Phase 2 Randomized Clinical Trial. JAMA Oncol. 2019;5:1276-1282. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 362] [Cited by in RCA: 694] [Article Influence: 115.7] [Reference Citation Analysis (0)] |
| 108. | Bauml JM, Mick R, Ciunci C, Aggarwal C, Davis C, Evans T, Deshpande C, Miller L, Patel P, Alley E, Knepley C, Mutale F, Cohen RB, Langer CJ. Pembrolizumab After Completion of Locally Ablative Therapy for Oligometastatic Non-Small Cell Lung Cancer: A Phase 2 Trial. JAMA Oncol. 2019;5:1283-1290. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 128] [Cited by in RCA: 230] [Article Influence: 38.3] [Reference Citation Analysis (0)] |
| 109. | Nestle U, De Ruysscher D, Ricardi U, Geets X, Belderbos J, Pöttgen C, Dziadiuszko R, Peeters S, Lievens Y, Hurkmans C, Slotman B, Ramella S, Faivre-Finn C, McDonald F, Manapov F, Putora PM, LePéchoux C, Van Houtte P. ESTRO ACROP guidelines for target volume definition in the treatment of locally advanced non-small cell lung cancer. Radiother Oncol. 2018;127:1-5. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 122] [Cited by in RCA: 139] [Article Influence: 19.9] [Reference Citation Analysis (1)] |
| 110. | Nestle U, Schimek-Jasch T, Kremp S, Schaefer-Schuler A, Mix M, Küsters A, Tosch M, Hehr T, Eschmann SM, Bultel YP, Hass P, Fleckenstein J, Thieme A, Stockinger M, Dieckmann K, Miederer M, Holl G, Rischke HC, Gkika E, Adebahr S, König J, Grosu AL; PET-Plan study group. Imaging-based target volume reduction in chemoradiotherapy for locally advanced non-small-cell lung cancer (PET-Plan): a multicentre, open-label, randomised, controlled trial. Lancet Oncol. 2020;21:581-592. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 63] [Cited by in RCA: 124] [Article Influence: 24.8] [Reference Citation Analysis (0)] |
| 111. | Prescribing, Recording, and Reporting Photon-Beam Intensity-Modulated Radiation Therapy (IMRT): Contents. J ICRU. 2010;10:1-3. [RCA] [DOI] [Full Text] [Cited by in Crossref: 182] [Cited by in RCA: 186] [Article Influence: 12.4] [Reference Citation Analysis (0)] |
| 112. | Konert T, Vogel W, MacManus MP, Nestle U, Belderbos J, Grégoire V, Thorwarth D, Fidarova E, Paez D, Chiti A, Hanna GG. PET/CT imaging for target volume delineation in curative intent radiotherapy of non-small cell lung cancer: IAEA consensus report 2014. Radiother Oncol. 2015;116:27-34. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 96] [Cited by in RCA: 101] [Article Influence: 10.1] [Reference Citation Analysis (0)] |
| 113. | Schaefer A, Vermandel M, Baillet C, Dewalle-Vignion AS, Modzelewski R, Vera P, Massoptier L, Parcq C, Gibon D, Fechter T, Nemer U, Gardin I, Nestle U. Impact of consensus contours from multiple PET segmentation methods on the accuracy of functional volume delineation. Eur J Nucl Med Mol Imaging. 2016;43:911-924. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 29] [Cited by in RCA: 25] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 114. | Peeters ST, Dooms C, Van Baardwijk A, Dingemans AM, Martinussen H, Vansteenkiste J, Decaluwé H, De Leyn P, Yserbyt J, Nackaerts K, De Wever W, Deroose CM, De Ruysscher D. Selective mediastinal node irradiation in non-small cell lung cancer in the IMRT/VMAT era: How to use E(B)US-NA information in addition to PET-CT for delineation? Radiother Oncol. 2016;120:273-278. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 23] [Cited by in RCA: 29] [Article Influence: 3.2] [Reference Citation Analysis (0)] |
| 115. | Nestle U, Rischke HC, Eschmann SM, Holl G, Tosch M, Miederer M, Plotkin M, Essler M, Puskas C, Schimek-Jasch T, Duncker-Rohr V, Rühl F, Leifert A, Mix M, Grosu AL, König J, Vach W. Improved inter-observer agreement of an expert review panel in an oncology treatment trial--Insights from a structured interventional process. Eur J Cancer. 2015;51:2525-2533. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 17] [Cited by in RCA: 23] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 116. | Lynch R, Pitson G, Ball D, Claude L, Sarrut D. Computed tomographic atlas for the new international lymph node map for lung cancer: A radiation oncologist perspective. Pract Radiat Oncol. 2013;3:54-66. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 25] [Cited by in RCA: 20] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 117. | Rusch VW, Asamura H, Watanabe H, Giroux DJ, Rami-Porta R, Goldstraw P; Members of IASLC Staging Committee. The IASLC lung cancer staging project: a proposal for a new international lymph node map in the forthcoming seventh edition of the TNM classification for lung cancer. J Thorac Oncol. 2009;4:568-577. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 736] [Cited by in RCA: 812] [Article Influence: 50.8] [Reference Citation Analysis (1)] |
| 118. | Itazawa T, Tamaki Y, Komiyama T, Nishimura Y, Nakayama Y, Ito H, Ohde Y, Kusumoto M, Sakai S, Suzuki K, Watanabe H, Asamura H. The Japan Lung Cancer Society-Japanese Society for Radiation Oncology consensus-based computed tomographic atlas for defining regional lymph node stations in radiotherapy for lung cancer. J Radiat Res. 2017;58:86-105. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 10] [Cited by in RCA: 8] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 119. | Giraud P, Antoine M, Larrouy A, Milleron B, Callard P, De Rycke Y, Carette MF, Rosenwald JC, Cosset JM, Housset M, Touboul E. Evaluation of microscopic tumor extension in non-small-cell lung cancer for three-dimensional conformal radiotherapy planning. Int J Radiat Oncol Biol Phys. 2000;48:1015-1024. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 341] [Cited by in RCA: 316] [Article Influence: 12.6] [Reference Citation Analysis (0)] |
| 120. | Le Péchoux C. Role of postoperative radiotherapy in resected non-small cell lung cancer: a reassessment based on new data. Oncologist. 2011;16:672-681. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 90] [Cited by in RCA: 100] [Article Influence: 7.1] [Reference Citation Analysis (0)] |
| 121. | Janssens G, Jacques L, Orban de Xivry J, Geets X, Macq B. Diffeomorphic registration of images with variable contrast enhancement. Int J Biomed Imaging. 2011;2011:891585. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 59] [Cited by in RCA: 60] [Article Influence: 4.0] [Reference Citation Analysis (0)] |
| 122. | van Herk M, Remeijer P, Rasch C, Lebesque JV. The probability of correct target dosage: dose-population histograms for deriving treatment margins in radiotherapy. Int J Radiat Oncol Biol Phys. 2000;47:1121-1135. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1101] [Cited by in RCA: 1147] [Article Influence: 45.9] [Reference Citation Analysis (0)] |
| 123. | Collier DC, Burnett SS, Amin M, Bilton S, Brooks C, Ryan A, Roniger D, Tran D, Starkschall G. Assessment of consistency in contouring of normal-tissue anatomic structures. J Appl Clin Med Phys. 2003;4:17-24. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 32] [Cited by in RCA: 41] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 124. | Kong FM, Ritter T, Quint DJ, Senan S, Gaspar LE, Komaki RU, Hurkmans CW, Timmerman R, Bezjak A, Bradley JD, Movsas B, Marsh L, Okunieff P, Choy H, Curran WJ Jr. Consideration of dose limits for organs at risk of thoracic radiotherapy: atlas for lung, proximal bronchial tree, esophagus, spinal cord, ribs, and brachial plexus. Int J Radiat Oncol Biol Phys. 2011;81:1442-1457. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 207] [Cited by in RCA: 282] [Article Influence: 18.8] [Reference Citation Analysis (0)] |
| 125. | Feng M, Moran JM, Koelling T, Chughtai A, Chan JL, Freedman L, Hayman JA, Jagsi R, Jolly S, Larouere J, Soriano J, Marsh R, Pierce LJ. Development and validation of a heart atlas to study cardiac exposure to radiation following treatment for breast cancer. Int J Radiat Oncol Biol Phys. 2011;79:10-18. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 583] [Cited by in RCA: 556] [Article Influence: 39.7] [Reference Citation Analysis (0)] |
| 126. | Hall WH, Guiou M, Lee NY, Dublin A, Narayan S, Vijayakumar S, Purdy JA, Chen AM. Development and validation of a standardized method for contouring the brachial plexus: preliminary dosimetric analysis among patients treated with IMRT for head-and-neck cancer. Int J Radiat Oncol Biol Phys. 2008;72:1362-1367. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 105] [Cited by in RCA: 104] [Article Influence: 6.1] [Reference Citation Analysis (0)] |
| 127. | De Rose F, Franceschini D, Reggiori G, Stravato A, Navarria P, Ascolese AM, Tomatis S, Mancosu P, Scorsetti M. Organs at risk in lung SBRT. Phys Med. 2017;44:131-138. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 20] [Cited by in RCA: 23] [Article Influence: 2.9] [Reference Citation Analysis (0)] |
| 128. | Kimsey F, McKay J, Gefter J, Milano MT, Moiseenko V, Grimm J, Berg R. Dose-Response Model for Chest Wall Tolerance of Stereotactic Body Radiation Therapy. Semin Radiat Oncol. 2016;26:129-134. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 14] [Cited by in RCA: 19] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 129. | Xue J, Kubicek G, Patel A, Goldsmith B, Asbell SO, LaCouture TA. Validity of Current Stereotactic Body Radiation Therapy Dose Constraints for Aorta and Major Vessels. Semin Radiat Oncol. 2016;26:135-139. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 19] [Cited by in RCA: 23] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 130. | Marks LB, Yorke ED, Jackson A, Ten Haken RK, Constine LS, Eisbruch A, Bentzen SM, Nam J, Deasy JO. Use of normal tissue complication probability models in the clinic. Int J Radiat Oncol Biol Phys. 2010;76:S10-S19. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1352] [Cited by in RCA: 1172] [Article Influence: 78.1] [Reference Citation Analysis (1)] |
| 131. | Emami B, Lyman J, Brown A, Coia L, Goitein M, Munzenrider JE, Shank B, Solin LJ, Wesson M. Tolerance of normal tissue to therapeutic irradiation. Int J Radiat Oncol Biol Phys. 1991;21:109-122. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3227] [Cited by in RCA: 3074] [Article Influence: 90.4] [Reference Citation Analysis (0)] |
| 132. | Marks LB, Bentzen SM, Deasy JO, Kong FM, Bradley JD, Vogelius IS, El Naqa I, Hubbs JL, Lebesque JV, Timmerman RD, Martel MK, Jackson A. Radiation dose-volume effects in the lung. Int J Radiat Oncol Biol Phys. 2010;76:S70-S76. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 843] [Cited by in RCA: 793] [Article Influence: 52.9] [Reference Citation Analysis (0)] |
| 133. | Palma DA, Senan S, Tsujino K, Barriger RB, Rengan R, Moreno M, Bradley JD, Kim TH, Ramella S, Marks LB, De Petris L, Stitt L, Rodrigues G. Predicting radiation pneumonitis after chemoradiation therapy for lung cancer: an international individual patient data meta-analysis. Int J Radiat Oncol Biol Phys. 2013;85:444-450. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 510] [Cited by in RCA: 499] [Article Influence: 41.6] [Reference Citation Analysis (0)] |
| 134. | Shepherd AF, Iocolano M, Leeman J, Imber BS, Wild AT, Offin M, Chaft JE, Huang J, Rimner A, Wu AJ, Gelblum DY, Shaverdian N, Simone CB 2nd, Gomez DR, Yorke ED, Jackson A. Clinical and Dosimetric Predictors of Radiation Pneumonitis in Patients With Non-Small Cell Lung Cancer Undergoing Postoperative Radiation Therapy. Pract Radiat Oncol. 2021;11:e52-e62. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 8] [Cited by in RCA: 14] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 135. | Al-Halabi H, Paetzold P, Sharp GC, Olsen C, Willers H. A Contralateral Esophagus-Sparing Technique to Limit Severe Esophagitis Associated With Concurrent High-Dose Radiation and Chemotherapy in Patients With Thoracic Malignancies. Int J Radiat Oncol Biol Phys. 2015;92:803-810. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 20] [Cited by in RCA: 22] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 136. | Speirs CK, DeWees TA, Rehman S, Molotievschi A, Velez MA, Mullen D, Fergus S, Trovo M, Bradley JD, Robinson CG. Heart Dose Is an Independent Dosimetric Predictor of Overall Survival in Locally Advanced Non-Small Cell Lung Cancer. J Thorac Oncol. 2017;12:293-301. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 150] [Cited by in RCA: 211] [Article Influence: 23.4] [Reference Citation Analysis (0)] |
| 137. | Amini A, Yang J, Williamson R, McBurney ML, Erasmus J Jr, Allen PK, Karhade M, Komaki R, Liao Z, Gomez D, Cox J, Dong L, Welsh J. Dose constraints to prevent radiation-induced brachial plexopathy in patients treated for lung cancer. Int J Radiat Oncol Biol Phys. 2012;82:e391-e398. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 47] [Cited by in RCA: 51] [Article Influence: 3.9] [Reference Citation Analysis (0)] |
| 138. | Zeng KL, Poon I, Ung YC, Zhang L, Cheung P. Accelerated Hypofractionated Radiation Therapy for Centrally Located Lung Tumors Not Suitable for Stereotactic Body Radiation Therapy (SBRT) or Concurrent Chemoradiotherapy (CRT). Int J Radiat Oncol Biol Phys. 2018;102:e719-e720. [RCA] [DOI] [Full Text] [Cited by in Crossref: 6] [Cited by in RCA: 3] [Article Influence: 0.4] [Reference Citation Analysis (1)] |
| 139. | Benedict SH, Yenice KM, Followill D, Galvin JM, Hinson W, Kavanagh B, Keall P, Lovelock M, Meeks S, Papiez L, Purdie T, Sadagopan R, Schell MC, Salter B, Schlesinger DJ, Shiu AS, Solberg T, Song DY, Stieber V, Timmerman R, Tomé WA, Verellen D, Wang L, Yin FF. Stereotactic body radiation therapy: the report of AAPM Task Group 101. Med Phys. 2010;37:4078-4101. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1283] [Cited by in RCA: 1497] [Article Influence: 99.8] [Reference Citation Analysis (0)] |
| 140. | Grimm J, LaCouture T, Croce R, Yeo I, Zhu Y, Xue J. Dose tolerance limits and dose volume histogram evaluation for stereotactic body radiotherapy. J Appl Clin Med Phys. 2011;12:3368. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 128] [Cited by in RCA: 132] [Article Influence: 9.4] [Reference Citation Analysis (0)] |
| 141. | Hanna GG, Murray L, Patel R, Jain S, Aitken KL, Franks KN, van As N, Tree A, Hatfield P, Harrow S, McDonald F, Ahmed M, Saran FH, Webster GJ, Khoo V, Landau D, Eaton DJ, Hawkins MA. UK Consensus on Normal Tissue Dose Constraints for Stereotactic Radiotherapy. Clin Oncol (R Coll Radiol). 2018;30:5-14. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 178] [Cited by in RCA: 191] [Article Influence: 27.3] [Reference Citation Analysis (0)] |
| 142. | Dunlap NE, Cai J, Biedermann GB, Yang W, Benedict SH, Sheng K, Schefter TE, Kavanagh BD, Larner JM. Chest wall volume receiving >30 Gy predicts risk of severe pain and/or rib fracture after lung stereotactic body radiotherapy. Int J Radiat Oncol Biol Phys. 2010;76:796-801. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 201] [Cited by in RCA: 195] [Article Influence: 12.2] [Reference Citation Analysis (0)] |
| 143. | Ma JT, Liu Y, Sun L, Milano MT, Zhang SL, Huang LT, Jing W, Zhao JZ, Han CB, Kong FS. Chest Wall Toxicity After Stereotactic Body Radiation Therapy: A Pooled Analysis of 57 Studies. Int J Radiat Oncol Biol Phys. 2019;103:843-850. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 19] [Cited by in RCA: 26] [Article Influence: 3.7] [Reference Citation Analysis (0)] |
| 144. | Milano MT, Mihai A, Kang J, Singh DP, Verma V, Qiu H, Chen Y, Kong FS. Stereotactic body radiotherapy in patients with multiple lung tumors: a focus on lung dosimetric constraints. Expert Rev Anticancer Ther. 2019;19:959-969. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5] [Cited by in RCA: 7] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 145. | Kong FS, Moiseenko V, Zhao J, Milano MT, Li L, Rimner A, Das S, Li XA, Miften M, Liao Z, Martel M, Bentzen SM, Jackson A, Grimm J, Marks LB, Yorke E. Organs at Risk Considerations for Thoracic Stereotactic Body Radiation Therapy: What Is Safe for Lung Parenchyma? Int J Radiat Oncol Biol Phys. 2021;110:172-187. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 25] [Cited by in RCA: 56] [Article Influence: 8.0] [Reference Citation Analysis (0)] |
| 146. | Chang JY, Senan S, Paul MA, Mehran RJ, Louie AV, Balter P, Groen HJ, McRae SE, Widder J, Feng L, van den Borne BE, Munsell MF, Hurkmans C, Berry DA, van Werkhoven E, Kresl JJ, Dingemans AM, Dawood O, Haasbeek CJ, Carpenter LS, De Jaeger K, Komaki R, Slotman BJ, Smit EF, Roth JA. Stereotactic ablative radiotherapy vs lobectomy for operable stage I non-small-cell lung cancer: a pooled analysis of two randomised trials. Lancet Oncol. 2015;16:630-637. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1013] [Cited by in RCA: 1108] [Article Influence: 110.8] [Reference Citation Analysis (0)] |
| 147. | Liao ZX, Komaki RR, Thames HD Jr, Liu HH, Tucker SL, Mohan R, Martel MK, Wei X, Yang K, Kim ES, Blumenschein G, Hong WK, Cox JD. Influence of technologic advances on outcomes in patients with unresectable, locally advanced non-small-cell lung cancer receiving concomitant chemoradiotherapy. Int J Radiat Oncol Biol Phys. 2010;76:775-781. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 204] [Cited by in RCA: 212] [Article Influence: 13.3] [Reference Citation Analysis (0)] |
| 148. | Li Y, Wang J, Tan L, Hui B, Ma X, Yan Y, Xue C, Shi X, Drokow EK, Ren J. Dosimetric comparison between IMRT and VMAT in irradiation for peripheral and central lung cancer. Oncol Lett. 2018;15:3735-3745. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 9] [Cited by in RCA: 10] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 149. | Chun SG, Hu C, Choy H, Komaki RU, Timmerman RD, Schild SE, Bogart JA, Dobelbower MC, Bosch W, Galvin JM, Kavadi VS, Narayan S, Iyengar P, Robinson CG, Wynn RB, Raben A, Augspurger ME, MacRae RM, Paulus R, Bradley JD. Impact of Intensity-Modulated Radiation Therapy Technique for Locally Advanced Non-Small-Cell Lung Cancer: A Secondary Analysis of the NRG Oncology RTOG 0617 Randomized Clinical Trial. J Clin Oncol. 2017;35:56-62. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 383] [Cited by in RCA: 541] [Article Influence: 60.1] [Reference Citation Analysis (0)] |
| 150. | Eaton BR, Pugh SL, Bradley JD, Masters G, Kavadi VS, Narayan S, Nedzi L, Robinson C, Wynn RB, Koprowski C, Johnson DW, Meng J, Curran WJ Jr. Institutional Enrollment and Survival Among NSCLC Patients Receiving Chemoradiation: NRG Oncology Radiation Therapy Oncology Group (RTOG) 0617. J Natl Cancer Inst. 2016;108. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 66] [Cited by in RCA: 82] [Article Influence: 9.1] [Reference Citation Analysis (0)] |
| 151. | Chio JH. Comparison of IMRT and VMAT Plan for Advanced Stage Non-Small Cell Lung Cancer Treatment. Ann Behav Sci. 2018;. [RCA] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 2] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 152. | Cole AJ, Hanna GG, Jain S, O'Sullivan JM. Motion management for radical radiotherapy in non-small cell lung cancer. Clin Oncol (R Coll Radiol). 2014;26:67-80. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 49] [Cited by in RCA: 54] [Article Influence: 4.9] [Reference Citation Analysis (0)] |
| 153. | Keall PJ, Mageras GS, Balter JM, Emery RS, Forster KM, Jiang SB, Kapatoes JM, Low DA, Murphy MJ, Murray BR, Ramsey CR, Van Herk MB, Vedam SS, Wong JW, Yorke E. The management of respiratory motion in radiation oncology report of AAPM Task Group 76. Med Phys. 2006;33:3874-3900. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1537] [Cited by in RCA: 1611] [Article Influence: 84.8] [Reference Citation Analysis (0)] |
| 154. | Yeung AR, Li J, Shi W, Newlin H, Morris CG, Samant S, Saito A, Chvetsov A, Liu C, Palta J, Olivier K. Optimal image-guidance scenario with cone-beam computed tomography in conventionally fractionated radiotherapy for lung tumors. Am J Clin Oncol. 2010;33:276-280. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 9] [Cited by in RCA: 11] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 155. | Jaffray DA, Langen KM, Mageras G, Dawson LA, Yan D, EdD RA, Mundt AJ, Fraass B. Safety considerations for IGRT: Executive summary. Pract Radiat Oncol. 2013;3:167-170. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 47] [Cited by in RCA: 47] [Article Influence: 3.9] [Reference Citation Analysis (0)] |
| 156. | Molitoris JK, Diwanji T, Snider JW 3rd, Mossahebi S, Samanta S, Badiyan SN, Simone CB 2nd, Mohindra P. Advances in the use of motion management and image guidance in radiation therapy treatment for lung cancer. J Thorac Dis. 2018;10:S2437-S2450. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 37] [Cited by in RCA: 45] [Article Influence: 6.4] [Reference Citation Analysis (0)] |
| 157. | Matsuo Y. A promising result of locoregional tumor control with biologically adaptive radiotherapy in patients with locally advanced non-small cell lung cancer. Transl Lung Cancer Res. 2018;7:S111-S113. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 2] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 158. | Kavanaugh J, Hugo G, Robinson CG, Roach MC. Anatomical Adaptation-Early Clinical Evidence of Benefit and Future Needs in Lung Cancer. Semin Radiat Oncol. 2019;29:274-283. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 10] [Cited by in RCA: 16] [Article Influence: 2.7] [Reference Citation Analysis (0)] |
| 159. | Tvilum M, Khalil AA, Møller DS, Hoffmann L, Knap MM. Clinical outcome of image-guided adaptive radiotherapy in the treatment of lung cancer patients. Acta Oncol. 2015;54:1430-1437. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 33] [Cited by in RCA: 34] [Article Influence: 3.4] [Reference Citation Analysis (0)] |
| 160. | Grutters JP, Kessels AG, Pijls-Johannesma M, De Ruysscher D, Joore MA, Lambin P. Comparison of the effectiveness of radiotherapy with photons, protons and carbon-ions for non-small cell lung cancer: a meta-analysis. Radiother Oncol. 2010;95:32-40. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 234] [Cited by in RCA: 248] [Article Influence: 16.5] [Reference Citation Analysis (0)] |
| 161. | Brooks ED, Ning MS, Verma V, Zhu XR, Chang JY. Proton therapy for non-small cell lung cancer: the road ahead. Transl Lung Cancer Res. 2019;8:S202-S212. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 10] [Cited by in RCA: 14] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 162. | Vyfhuis MAL, Onyeuku N, Diwanji T, Mossahebi S, Amin NP, Badiyan SN, Mohindra P, Simone CB 2nd. Advances in proton therapy in lung cancer. Ther Adv Respir Dis. 2018;12:1753466618783878. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 31] [Cited by in RCA: 42] [Article Influence: 6.0] [Reference Citation Analysis (0)] |
| 163. | Nantavithya C, Gomez DR, Wei X, Komaki R, Liao Z, Lin SH, Jeter M, Nguyen QN, Li H, Zhang X, Poenisch F, Zhu XR, Balter PA, Feng L, Choi NC, Mohan R, Chang JY. Phase 2 Study of Stereotactic Body Radiation Therapy and Stereotactic Body Proton Therapy for High-Risk, Medically Inoperable, Early-Stage Non-Small Cell Lung Cancer. Int J Radiat Oncol Biol Phys. 2018;101:558-563. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 36] [Cited by in RCA: 55] [Article Influence: 7.9] [Reference Citation Analysis (0)] |
| 164. | Chang JY, Komaki R, Lu C, Wen HY, Allen PK, Tsao A, Gillin M, Mohan R, Cox JD. Phase 2 study of high-dose proton therapy with concurrent chemotherapy for unresectable stage III nonsmall cell lung cancer. Cancer. 2011;117:4707-4713. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 138] [Cited by in RCA: 130] [Article Influence: 9.3] [Reference Citation Analysis (0)] |
| 165. | Hoppe BS, Henderson R, Pham D, Cury JD, Bajwa A, Morris CG, D'Agostino H Jr, Flampouri S, Huh S, Li Z, McCook B, Nichols RC Jr. A Phase 2 Trial of Concurrent Chemotherapy and Proton Therapy for Stage III Non-Small Cell Lung Cancer: Results and Reflections Following Early Closure of a Single-Institution Study. Int J Radiat Oncol Biol Phys. 2016;95:517-522. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 39] [Cited by in RCA: 44] [Article Influence: 4.4] [Reference Citation Analysis (0)] |
| 166. | Sejpal S, Komaki R, Tsao A, Chang JY, Liao Z, Wei X, Allen PK, Lu C, Gillin M, Cox JD. Early findings on toxicity of proton beam therapy with concurrent chemotherapy for nonsmall cell lung cancer. Cancer. 2011;117:3004-3013. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 87] [Cited by in RCA: 91] [Article Influence: 6.5] [Reference Citation Analysis (0)] |
| 167. | Nguyen QN, Ly NB, Komaki R, Levy LB, Gomez DR, Chang JY, Allen PK, Mehran RJ, Lu C, Gillin M, Liao Z, Cox JD. Long-term outcomes after proton therapy, with concurrent chemotherapy, for stage II-III inoperable non-small cell lung cancer. Radiother Oncol. 2015;115:367-372. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 67] [Cited by in RCA: 76] [Article Influence: 7.6] [Reference Citation Analysis (0)] |
| 168. | Liao Z, Lee JJ, Komaki R, Gomez DR, O'Reilly MS, Fossella FV, Blumenschein GR Jr, Heymach JV, Vaporciyan AA, Swisher SG, Allen PK, Choi NC, DeLaney TF, Hahn SM, Cox JD, Lu CS, Mohan R. Bayesian Adaptive Randomization Trial of Passive Scattering Proton Therapy and Intensity-Modulated Photon Radiotherapy for Locally Advanced Non-Small-Cell Lung Cancer. J Clin Oncol. 2018;36:1813-1822. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 161] [Cited by in RCA: 236] [Article Influence: 33.7] [Reference Citation Analysis (0)] |
| 169. | Giaddui T, Chen W, Yu J, Lin L, Simone CB 2nd, Yuan L, Gong YU, Wu QJ, Mohan R, Zhang X, Bluett JB, Gillin M, Moore K, O'Meara E, Presley J, Bradley JD, Liao Z, Galvin J, Xiao Y. Establishing the feasibility of the dosimetric compliance criteria of RTOG 1308: phase III randomized trial comparing overall survival after photon vs proton radiochemotherapy for inoperable stage II-IIIB NSCLC. Radiat Oncol. 2016;11:66. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 48] [Cited by in RCA: 88] [Article Influence: 9.8] [Reference Citation Analysis (0)] |
| 170. | De Ruysscher D, Faivre-Finn C, Le Pechoux C, Peeters S, Belderbos J. High-dose re-irradiation following radical radiotherapy for non-small-cell lung cancer. Lancet Oncol. 2014;15:e620-e624. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 58] [Cited by in RCA: 73] [Article Influence: 6.6] [Reference Citation Analysis (0)] |
| 171. | Fischer-Valuck BW, Robinson CG, Simone CB 2nd, Gomez DR, Bradley JD. Challenges in Re-Irradiation in the Thorax: Managing Patients with Locally Recurrent Non-Small Cell Lung Cancer. Semin Radiat Oncol. 2020;30:223-231. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4] [Cited by in RCA: 9] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 172. | Sumodhee S, Bondiau PY, Poudenx M, Cohen C, Naghavi AO, Padovani B, Maneval D, Gal J, Leysalle A, Ghalloussi H, Otto J, Doyen J. Long term efficacy and toxicity after stereotactic ablative reirradiation in locally relapsed stage III non-small cell lung cancer. BMC Cancer. 2019;19:305. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 13] [Cited by in RCA: 13] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 173. | Vyfhuis MAL, Rice S, Remick J, Mossahebi S, Badiyan S, Mohindra P, Simone CB 2nd. Reirradiation for locoregionally recurrent non-small cell lung cancer. J Thorac Dis. 2018;10:S2522-S2536. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 19] [Cited by in RCA: 21] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 174. | Kilburn JM, Kuremsky JG, Blackstock AW, Munley MT, Kearns WT, Hinson WH, Lovato JF, Miller AA, Petty WJ, Urbanic JJ. Thoracic re-irradiation using stereotactic body radiotherapy (SBRT) techniques as first or second course of treatment. Radiother Oncol. 2014;110:505-510. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 54] [Cited by in RCA: 65] [Article Influence: 5.9] [Reference Citation Analysis (0)] |
| 175. | McAvoy S, Ciura K, Wei C, Rineer J, Liao Z, Chang JY, Palmer MB, Cox JD, Komaki R, Gomez DR. Definitive reirradiation for locoregionally recurrent non-small cell lung cancer with proton beam therapy or intensity modulated radiation therapy: predictors of high-grade toxicity and survival outcomes. Int J Radiat Oncol Biol Phys. 2014;90:819-827. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 53] [Cited by in RCA: 68] [Article Influence: 6.2] [Reference Citation Analysis (0)] |
| 176. | McAvoy SA, Ciura KT, Rineer JM, Allen PK, Liao Z, Chang JY, Palmer MB, Cox JD, Komaki R, Gomez DR. Feasibility of proton beam therapy for reirradiation of locoregionally recurrent non-small cell lung cancer. Radiother Oncol. 2013;109:38-44. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 56] [Cited by in RCA: 59] [Article Influence: 4.9] [Reference Citation Analysis (0)] |
| 177. | Carsten N, Langendijk J. Re-Irradiation: New Frontiers. Cham: Springer, 2017. |
| 178. | Giaj-Levra N, Borghetti P, Bruni A, Ciammella P, Cuccia F, Fozza A, Franceschini D, Scotti V, Vagge S, Alongi F. Current radiotherapy techniques in NSCLC: challenges and potential solutions. Expert Rev Anticancer Ther. 2020;20:387-402. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 35] [Cited by in RCA: 33] [Article Influence: 6.6] [Reference Citation Analysis (0)] |
| 179. | Roelofs E, Engelsman M, Rasch C, Persoon L, Qamhiyeh S, de Ruysscher D, Verhaegen F, Pijls-Johannesma M, Lambin P; ROCOCO Consortium. Results of a multicentric in silico clinical trial (ROCOCO): comparing radiotherapy with photons and protons for non-small cell lung cancer. J Thorac Oncol. 2012;7:165-176. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 73] [Cited by in RCA: 77] [Article Influence: 5.9] [Reference Citation Analysis (0)] |
| 180. | Chao HH, Berman AT, Simone CB 2nd, Ciunci C, Gabriel P, Lin H, Both S, Langer C, Lelionis K, Rengan R, Hahn SM, Prabhu K, Fagundes M, Hartsell W, Mick R, Plastaras JP. Multi-Institutional Prospective Study of Reirradiation with Proton Beam Radiotherapy for Locoregionally Recurrent Non-Small Cell Lung Cancer. J Thorac Oncol. 2017;12:281-292. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 55] [Cited by in RCA: 83] [Article Influence: 10.4] [Reference Citation Analysis (0)] |
| 181. | Hayashi K, Yamamoto N, Karube M, Nakajima M, Tsuji H, Ogawa K, Kamada T. Feasibility of carbon-ion radiotherapy for re-irradiation of locoregionally recurrent, metastatic, or secondary lung tumors. Cancer Sci. 2018;109:1562-1569. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 13] [Cited by in RCA: 19] [Article Influence: 2.7] [Reference Citation Analysis (0)] |
| 182. | Berman AT, James SS, Rengan R. Proton Beam Therapy for Non-Small Cell Lung Cancer: Current Clinical Evidence and Future Directions. Cancers (Basel). 2015;7:1178-1190. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 33] [Cited by in RCA: 36] [Article Influence: 3.6] [Reference Citation Analysis (0)] |
| 183. | Koukourakis M, Hlouverakis G, Kosma L, Skarlatos J, Damilakis J, Giatromanolaki A, Yannakakis D. The impact of overall treatment time on the results of radiotherapy for nonsmall cell lung carcinoma. Int J Radiat Oncol Biol Phys. 1996;34:315-322. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 71] [Cited by in RCA: 71] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 184. | Topkan E, Ozdemir Y, Kucuk A, Besen AA, Mertsoylu H, Sezer A, Selek U. Significance of overall concurrent chemoradiotherapy duration on survival outcomes of stage IIIB/C non-small-cell lung carcinoma patients: Analysis of 956 patients. PLoS One. 2019;14:e0218627. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 10] [Cited by in RCA: 10] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 185. | Cox JD, Pajak TF, Asbell S, Russell AH, Pederson J, Byhardt RW, Emami B, Roach M 3rd. Interruptions of high-dose radiation therapy decrease long-term survival of favorable patients with unresectable non-small cell carcinoma of the lung: analysis of 1244 cases from 3 Radiation Therapy Oncology Group (RTOG) trials. Int J Radiat Oncol Biol Phys. 1993;27:493-498. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 176] [Cited by in RCA: 163] [Article Influence: 5.1] [Reference Citation Analysis (0)] |
| 186. | Chen M, Jiang GL, Fu XL, Wang LJ, Qian H, Chen GY, Zhao S, Liu TF. The impact of overall treatment time on outcomes in radiation therapy for non-small cell lung cancer. Lung Cancer. 2000;28:11-19. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 50] [Cited by in RCA: 51] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 187. | Fowler JF, Chappell R. Non-small cell lung tumors repopulate rapidly during radiation therapy. Int J Radiat Oncol Biol Phys. 2000;46:516-517. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 95] [Cited by in RCA: 98] [Article Influence: 3.9] [Reference Citation Analysis (0)] |
| 188. | The Royal College of Radiologists. The timely delivery of radical radiotherapy: guidelines for the management of unscheduled treatment interruptions Fourth edition. London: The Royal College of Radiologists, 2019. |
| 189. | Koshy M, Malik R, Spiotto M, Mahmood U, Rusthoven CG, Sher DJ. Association between intensity modulated radiotherapy and survival in patients with stage III non-small cell lung cancer treated with chemoradiotherapy. Lung Cancer. 2017;108:222-227. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 15] [Cited by in RCA: 19] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 190. | Joiner MC, Van der Kogel A. Basic Clinical Radiobiology Fourth Edition. Boca Raton: CRC Press, 2009. |
| 191. | Banfill K, Croxford W, Fornacon-Wood I, Wicks K, Ahmad S, Britten A, Carson C, Dorey N, Hatton M, Hiley C, Thippu Jayaprakash K, Jegannathen A, Koh P, Panakis N, Peedell C, Pope A, Powell C, Stilwell C, Thomas B, Toy E, Wood V, Yahya S, Zhou SY, Price G, Faivre-Finn C. Changes in the Management of Patients having Radical Radiotherapy for Lung Cancer during the First Wave of the COVID-19 Pandemic in the UK. Clin Oncol (R Coll Radiol). 2022;34:19-27. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 7] [Cited by in RCA: 10] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 192. | Thakur MK, Ruterbusch JJ, Schwartz AG, Gadgeel SM, Beebe-Dimmer JL, Wozniak AJ. Risk of Second Lung Cancer in Patients with Previously Treated Lung Cancer: Analysis of Surveillance, Epidemiology, and End Results (SEER) Data. J Thorac Oncol. 2018;13:46-53. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 51] [Cited by in RCA: 84] [Article Influence: 10.5] [Reference Citation Analysis (0)] |
| 193. | Bakitas M, Lyons KD, Hegel MT, Balan S, Brokaw FC, Seville J, Hull JG, Li Z, Tosteson TD, Byock IR, Ahles TA. Effects of a palliative care intervention on clinical outcomes in patients with advanced cancer: the Project ENABLE II randomized controlled trial. JAMA. 2009;302:741-749. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1256] [Cited by in RCA: 1323] [Article Influence: 82.7] [Reference Citation Analysis (0)] |
| 194. | Chandrasekar D, Tribett E, Ramchandran K. Integrated Palliative Care and Oncologic Care in Non-Small-Cell Lung Cancer. Curr Treat Options Oncol. 2016;17:23. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 14] [Cited by in RCA: 11] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 195. | SABR UK Consortium. Stereotactic Ablative Body Radiation Therapy (SABR): A Resource. London: The Royal College of Radiologists, 2019. |
| 196. | Kim TH, Woo S, Halpenny DF, Kim YJ, Yoon SH, Suh CH. Can high-risk CT features suggest local recurrence after stereotactic body radiation therapy for lung cancer? Eur J Radiol. 2020;127:108978. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3] [Cited by in RCA: 4] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 197. | Huang K, Senthi S, Palma DA, Spoelstra FO, Warner A, Slotman BJ, Senan S. High-risk CT features for detection of local recurrence after stereotactic ablative radiotherapy for lung cancer. Radiother Oncol. 2013;109:51-57. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 91] [Cited by in RCA: 106] [Article Influence: 8.8] [Reference Citation Analysis (0)] |
| 198. | Peulen H, Mantel F, Guckenberger M, Belderbos J, Werner-Wasik M, Hope A, Giuliani M, Grills I, Sonke JJ. Validation of High-Risk Computed Tomography Features for Detection of Local Recurrence After Stereotactic Body Radiation Therapy for Early-Stage Non-Small Cell Lung Cancer. Int J Radiat Oncol Biol Phys. 2016;96:134-141. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 26] [Cited by in RCA: 32] [Article Influence: 3.6] [Reference Citation Analysis (0)] |
| 199. | Passiglia F, Bertolaccini L, Del Re M, Facchinetti F, Ferrara R, Franchina T, Malapelle U, Menis J, Passaro A, Pilotto S, Ramella S, Rossi G, Trisolini R, Novello S. Diagnosis and treatment of early and locally advanced non-small-cell lung cancer: The 2019 AIOM (Italian Association of Medical Oncology) clinical practice guidelines. Crit Rev Oncol Hematol. 2020;148:102862. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 22] [Cited by in RCA: 29] [Article Influence: 5.8] [Reference Citation Analysis (0)] |
| 200. | De Ruysscher D, Faivre-Finn C, Nackaerts K, Jordan K, Arends J, Douillard JY, Ricardi U, Peters S. Recommendation for supportive care in patients receiving concurrent chemotherapy and radiotherapy for lung cancer. Ann Oncol. 2020;31:41-49. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 30] [Cited by in RCA: 63] [Article Influence: 12.6] [Reference Citation Analysis (0)] |








