Published online Mar 24, 2022. doi: 10.5306/wjco.v13.i3.168
Peer-review started: February 23, 2021
First decision: July 29, 2021
Revised: September 7, 2021
Accepted: February 19, 2022
Article in press: February 19, 2022
Published online: March 24, 2022
Processing time: 393 Days and 19.9 Hours
Adult stem cells are necessary for self-renewal tissues and regeneration after damage. Especially in the intestine, which self-renews every few days, they play a key role in tissue homeostasis. Therefore, complex regulatory mechanisms are needed to prevent hyperproliferation, which can lead in the worst case to carcinogenesis or under-activation of stem cells, which can result in dysfunctional epithelial. One main regulatory signaling pathway is the Wnt/β-catenin signaling pathway. It is a highly conserved pathway, with β-catenin, a transcription factor, as target protein. Translocation of β-catenin from cytoplasm to nucleus activates the transcription of numerous genes involved in regulating stem cell pluripo-tency, proliferation, cell differentiation and regulation of cell death. This review presents a brief overview of the Wnt/β-catenin signaling pathway, the regulatory mechanism of this pathway and its role in intestinal homeostasis. Additionally, this review highlights the molecular mechanisms and the histomorphological features of Wnt hyperactivation. Furthermore, the central role of the Wnt signaling pathway in intestinal carcinogenesis as well as its clinical relevance in colorectal carcinoma are discussed.
Core Tip: Wnt signaling pathway is a key regulator of intestinal stem cells. Mutations in this pathway are frequently found in adenomas and carcinomas of the colorectum. Therefore, it represents a potential target for anticancer therapy. This review sums up the physiological role and the regulatory mechanism of Wnt signaling in the human intestine, and moreover, discusses the central role of the Wnt signaling pathway in intestinal carcinogenesis, the morphological features associated with Wnt hyperactivation and clinical relevance of Wnt in the colorectal carcinoma.
- Citation: Swoboda J, Mittelsdorf P, Chen Y, Weiskirchen R, Stallhofer J, Schüle S, Gassler N. Intestinal Wnt in the transition from physiology to oncology. World J Clin Oncol 2022; 13(3): 168-185
- URL: https://www.wjgnet.com/2218-4333/full/v13/i3/168.htm
- DOI: https://dx.doi.org/10.5306/wjco.v13.i3.168
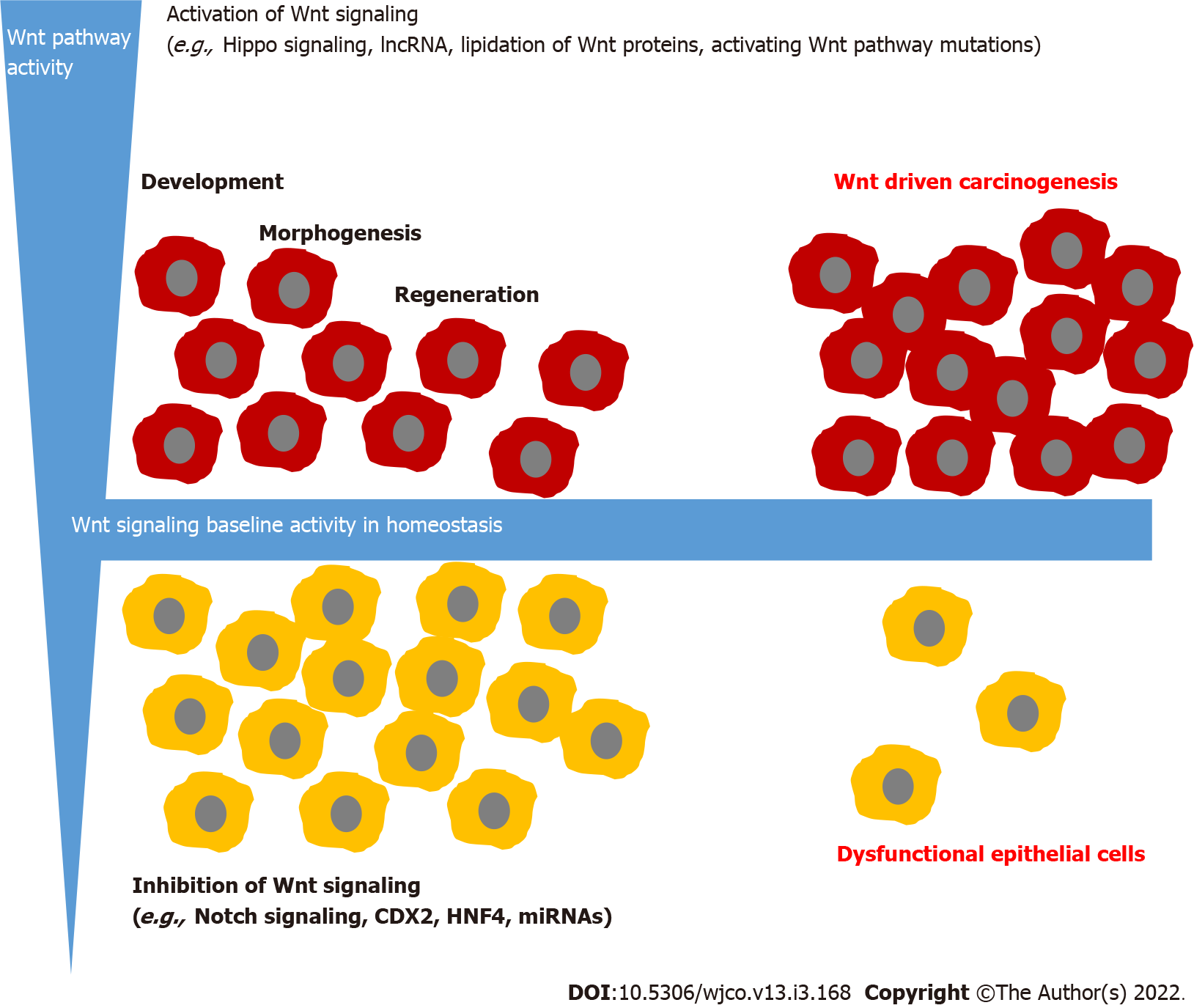
The gastrointestinal epithelia are tissues that self-renew every few days. Therefore, pluripotent stem cells are needed, which have the potential to develop into different epithelial cells. These highly complex mechanisms need complex fine-tuning. An overactivation of pluripotent stem cells could lead to hyperproliferation and in the worst case to cancer development. Conversely, under-activation could lead to insufficient development of the epithelia with dysfunction of the epithelia. One main regulatory signaling responsible for intestinal epithelial development is Wnt signaling.
Since 1976 it has been known that the Wingless (WNT) gene in Drosophila not only influences development, but also provokes abnormalities of the mesothorax[1]. In recent decades, other genes of the Wnt family have been found and the signaling pathways around Wnt in humans have also become more and more clear. Today 19 WNT genes in humans are known and the Wnt pathway is known to play a critical role in embryonic development and tissue homeostasis[2]. An imbalance in Wnt signaling can lead to several diseases including carcinogenesis, neurodegenerative, metabolic and cardiovascular diseases[3]. In addition to the canonical Wnt/β-catenin pathway, which is the main focus of this review, there is also the noncanonical pathway and the noncanonical Wnt/calcium pathway[4].
This work focuses on the regulation and the role of the canonical Wnt/β-catenin signaling pathway in physiological epithelial differentiation and the molecular activities of Wnt contributing to autonomous hyperproliferation and injured cell death as hallmarks of carcinogenesis.
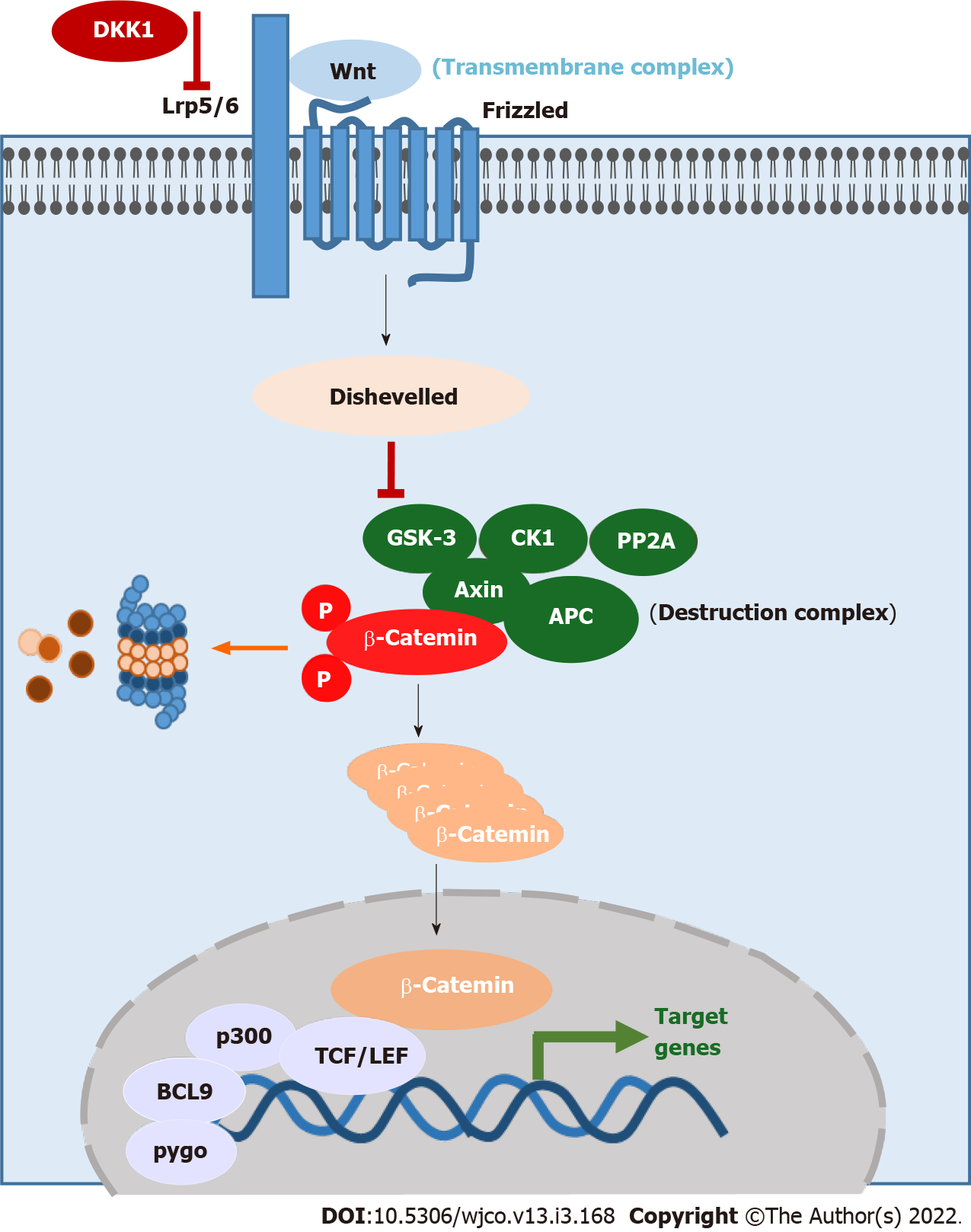
The most common Wnt pathway and evolutionarily conserved pathway is the canonical Wnt/β-catenin signaling (Figure 1). It consists of the transmembrane complex (Lrp5/6 and Frizzled), a destruction complex [Axin, Adenomatous polyposis coli (APC), glycogen synthase kinase-3 (GSK3), casein kinase 1 (CK1), protein phosphatase 2A (PP2A)] and β-catenin[5-7]. In the absence of the Wnt ligand, β-catenin is phosphorylated by the kinases CK1 and GSK3[8]. The phosphorylation leads to the ubiquitination and degradation of β-catenin. If Wnt binds to the transmembrane complex, the protein Disheveled is activated and turns down the destruction complex, resulting in accumulation of β-catenin in the cytoplasm[9,10]. Then, β-catenin is translocated into the nucleus and acts there as a transcription factor together with P300, B-cell CLL/lymphoma 9, pygo and T-cell factor/lymphoid enhancer-binding factor (TCF/LEF) as cofactors[11-13]. Moreover, there are inhibitors of this pathway like Dickkopf 1 (Dkk1), which binds to Lrp5 and inhibits the binding of Wnt at the transmembrane complex[14,15].
The role of Wnt/β-catenin signaling in the development of the gastrointestinal tract becomes clear when we look at the main genes which are regulated by the Wnt signaling pathway. Nuclear β-catenin activates genes which code for proteins involved in important pathways as well as processes including embryogenesis, proliferation, cell differentiation and the regulation of cell death (Table 1)[16-18].
| Gene | Function of the protein | Ref. |
| ATOH1 | Transcription factor, secretory cell line differentiation | [137,138] |
| AXIN2 | Part of destruction complex Wnt signaling | [139] |
| BCL2 | Antiapoptotic | [140] |
| BIRC5 | Apoptosis inhibitor | [141] |
| BMP4 | Possible Wnt inhibitor | [142] |
| CCND1 | Cell proliferation | [143] |
| CDKN2A | Cell cycle inhibitor | [144] |
| CDX1 | Transcription factor, intestinal cell differentiation | [145] |
| CDX2 | Transcription factor, intestinal cell differentiation | [146] |
| DKK1/4 | Inhibitor of Wnt signaling | [147,148] |
| EPHB2/3 | Migration and proliferation in intestine epithelial | [149] |
| HD5/6 | Defensine, microbial defense | [150] |
| HEF1 | Supports activation of oncogenic signaling pathways | [151] |
| HES1 | Regulation of Notch signaling | [152] |
| JAG1 | Ligand of Notch signaling | [153] |
| JUN | Cell cycle progression, apoptosis inhibitor | [154,155] |
| LGR5 | Part of Wnt signaling | [156] |
| MDR1 | Plasma membrane protein involved in the drug resistance | [123,124] |
| MET | Differentiation of intestinal epithelium | [157] |
| MYC | Protooncogene | [158] |
| MYCBP | Control of transcriptional activity of c-MYC | [159] |
| NOTCH2 | Notch receptor | [160] |
| SGK1 | Inhibits pro-apoptotic transcription factors | [161] |
| SOX9 | Paneth cell differentiation | [32,162] |
| YAP | Transcription factor (Hippo signaling) activates genes involved in cell proliferation, suppresses apoptotic genes | [163] |
In the intestinal tract, the canonical Wnt is an essential and fundamental molecular cascade to establish and constitute the mucosal barrier. However, in the different segments of the intestinal tract, the Wnt shows different cellular and molecular players as well as facets that are characteristic for each compartment. Wnt signaling is required in all parts for stem cell renewal, while Wnt overactivation in the stomach can lead to intestinal shift. Mutations in the Wnt ligands affect all parts of the intestine[19,20]. These points are addressed further in the following paragraphs.
The stomach can be divided, based on its local glands, into two main parts: The corpus/fundus and the antrum. The corpus and fundus contain oxyntic glands with chief cells, parietal cells and endocrine cells, while the antrum glands mainly contain mucous and endocrine cells[21]. Wnt/β-catenin signaling was required for the development of the embryonic fundus and in the β-catenin-deficient epithelium, parietal cells were absent[22]. In the antrum glands, Lgr5+ and Axin2+ stem cells were found[23]. Both proteins are regulated throughout Wnt signaling. Wnts are necessary for the maintenance of Lgr5+ cells and are necessary for the zymogenic cell line from Lgr5+ cells[24]. Moreover, they suppress the differentiation along the pit cell lineage. The Wnt ligands in the stomach will be secreted by pericyte-like stromal cells[25]. These cells are conserved and exist in the colon as well as in the stomach. Besides, activation of Wnt signaling in the stomach can lead to an intestinal fate in the stomach. Therefore, the mesenchymal transcription factor Barx1 represses the Wnt signaling and inhibits an intestinal shift of the stomach epithelium[26].
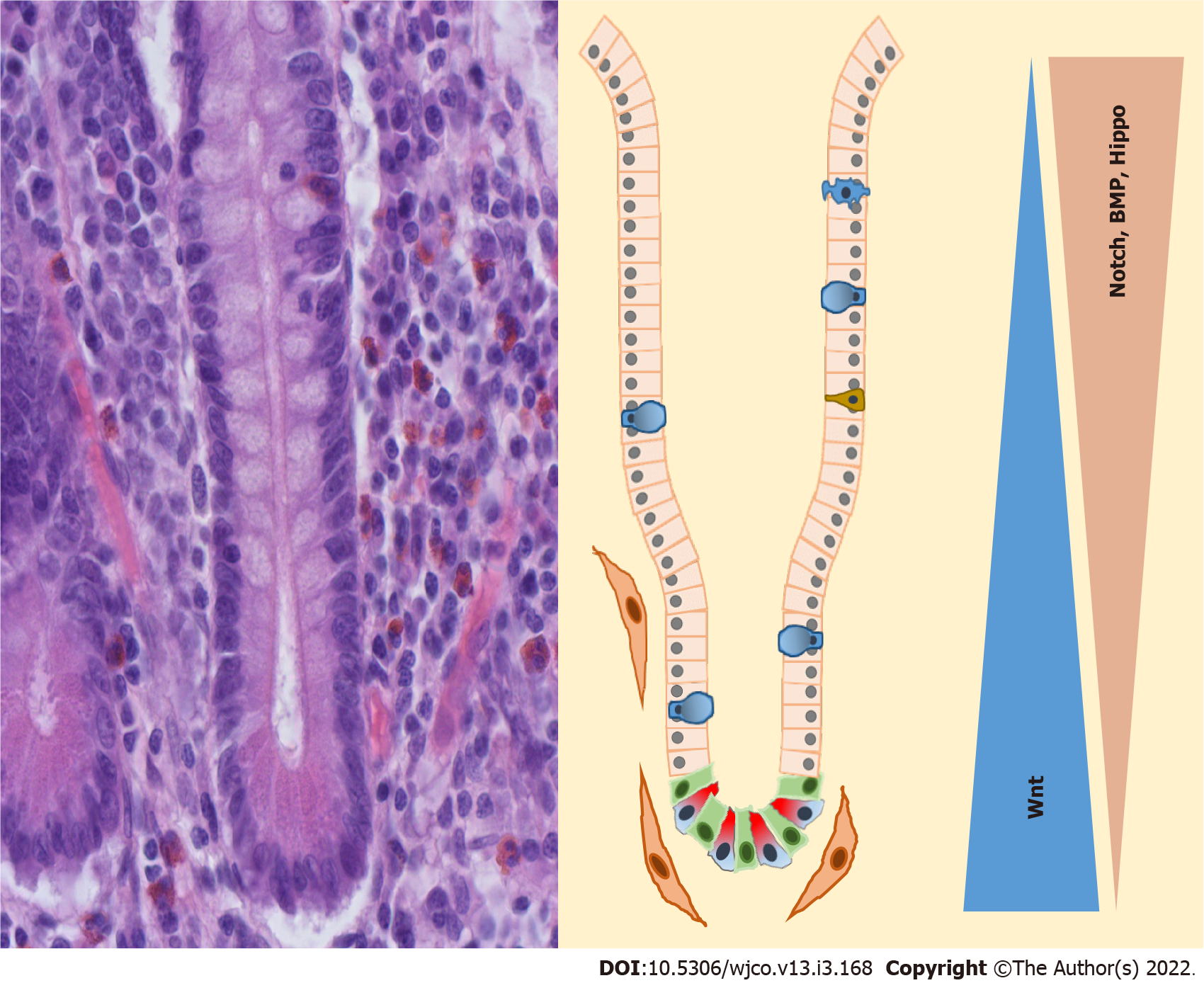
The small intestine consists of finger-like villi with an absorptive function and crypts of Lieberkühn (Figure 2). In the crypts, two different populations of intestinal stem cells (ISC) are located[27]. At the bottom of the crypts are columnar ISCs which express Lgr5, have a high division rate and are preferred for the renewal of the intestinal epithelia[28]. These cells can be activated throughout Wnt. On the other hand, there are quiescent ISCs that have a slow division rate, are less vulnerable to radiation and Wnt signaling is not activated. These cells are located above the Paneth cells and are also called +4 cells[29]. The role of these cells has not been fully investigated yet. But in the absence of columnar ISCs, quiescent ISCs can be activated and assume the tasks of columnar ISCs[30]. The localization of the subpopulation of ISC in the crypt is controlled by the surrounding mesenchymal cells through bone morphogenetic protein (BMP) signaling[27]. The regulation of the ISC occurs through Wnt3A which is secreted by Paneth cells[31].
Paneth cells are located in the base of the crypt of the small intestine next to Lgr5+ cells. Their differentiation is induced by SOX9, a transcriptional target and a critical regulator of Wnt signaling[32]. In contrast to other differentiated intestine cells, they do not migrate upwards to the top of the villus tip and their lifetime is, at 30 d, much longer[33]. Their main role is to synthesize and secrete defensins, anti-microbial peptides and trophic factors. Nevertheless, they seem to have an impact on crypt homeostasis.
Above the Paneth cells and stem cells is the transit-amplifying zone. The progenitor cells of the differentiated enterocytes are settled here, which can divide themselves two to five times[34,35]. All differentiated cells with the exception of Paneth cells migrate from the crypts upwards to the villi. The main parts of differentiated cells are enterocytes, which make up 80%-90% and have an absorptive function. In addition to them, there are tuft cells, goblet cells, enteroendocrine cells and microfold cells that are also termed M cells[35,36].
That Wnt signaling is essential for intestinal development has been already shown in the work of Pinto et al[37]. Overexpression of the Wnt inhibitor Dkk1 leads to a loss of crypts and reduced epithelial proliferation[37]. Furthermore, inhibition of Dkk leads to a reduced rate of fission of crypts in postnatal growth[38]. A negative autoregulatory feedback loop of Wnt signaling prevents a hyperactivation of Wnt signaling[28,39].
The colon has, in contrast to the small intestine, crypts, but no villi. The so-called colonocytes are functionally equivalent to the enterocytes[35]. Like the small intestine, the colon epithelia renew themselves through crypt-based columnar ISCs[35]. The work of Davies et al[40] revealed that Wnt activity is lower in the colon than in the small intestine. This may be influenced by the fact that instead of Paneth cells the colon epithelia have deep secretory cells with similar functions to Paneth cells, but in contrast to Paneth cells, they do not secrete Wnt ligands[35,41]. Furthermore, in vitro studies show that the reaction of Wnt-signaling activation also differs between the left and the right colon[42]. In embryonic development, a Wnt3A gradient plays an important role in hindgut extension and colon formation[43]. Like the small intestine, the colon epithelia include goblet cells, tuft cells and enteroendocrine cells[35].
As mentioned above, the Wnt signaling pathway is a highly conserved pathway and essential for intestinal homeostasis. To preserve this homeostasis, precise fine-tuning is absolutely necessary. The regulation of Wnt ligands occurs on different pathway levels. The mechanisms involved in this regulation are explained below and summed up in Figure 3.
Notch signaling is one of the most important signaling pathways in terms of adjacent cellular commun-ication and regulation of gastrointestinal stem cells[44]. It plays a crucial role in determining whether a cell develops into a secretory or an absorptive cell[44]. Deletion of NOTCH1 and NOTCH2 leads to hyperplasia of secretory cells[45]. It is not surprising that Wnt and Notch signaling act closely together and regulate each other[46,47]. The amount of Notch correlates here inversely with the amount of β-catenin[48,49]. On the other hand, Disheveled, which is part of the Wnt signaling, inhibits Notch signaling[50,51]. As Notch signaling requires cell-cell contact, Paneth cells are important for controlling the Notch signaling of small ISC[52]. In conclusion, Notch signaling determines cell fate to absorptive cell lines, while Wnt signaling drives cells to secretory cell lines[35,53].
Caudal-related homeobox transcription factor 2 (CDX2) is essential for human development. In the gastrointestinal tract, it determines gastric and intestinal development[54]. In adult mice, the absence of CDX2 leads to a cessation of intestinal differentiation[54]. In various works it has been shown that CDX2 activates Axin 2, which is part of the destruction complex in Wnt/β-catenin signaling[55,56]. Yu et al[56] showed in their work that CDX2 upregulates not only Axin 2 but also GSK-3β, which is also part of the destruction complex. The absence of CDX2, which in colorectal cancer is directly correlated with a higher tumor grade, leads to an activation of Wnt signaling[57].
BMPs belong to the transforming growth factor-β (TGF-β) family. They are produced by mesenchymal cells especially at the tip of the villus and generate a contrary gradient with Wnt through the crypt-villus axis[58]. At the crypt base, BMP signaling is repressed by BMP inhibitors like gremlin and chordin-like 1 secreted by smooth muscle cells or myofibroblasts[59]. BMP represses ISC proliferation, while the influence of BMP on Wnt signaling is the subject of controversial debate. The work of He et al[60] postulates that BMP inhibits Wnt signaling, while the work of Qi et al[61] describes a direct suppression of Lgr5+ cells through BMP without changes in the Wnt target genes.
Hippo signaling is a highly conserved pathway and important for intestinal homeostasis and regeneration. Inactivation of Hippo signaling leads to an activation of the transcription factor Yes-associated protein 1 (YAP1), which has the highest activity at the bottom of the crypts[62]. YAP1 is an oncogene that is a facultative regulator of stem cell homeostasis and an essential regulator for the regeneration of the intestinal epithelial after injury[62]. Hippo and Wnt signaling are closely linked to each other[63]. YAP1 increases the transcriptional activity of β-catenin, while active Hippo signaling leads to the formation of the destruction complex of Wnt signaling[64,65].
Hepatocyte nuclear factor 4 (HNF4) is a transcription factor family that mainly regulates metabolism in cells. Especially fatty acids have a high impact on ISC homeostasis[66]. Chen et al[67] show in in vitro studies that HNF4α and HNF4γ activate genes involved in fatty acid oxidation and that HNF4 is necessary for stem cell renewal in the intestine. Studies about the interaction of HNF4 and Wnt are rare, few studies indicate that HNF4 may regulate Wnt signaling. The study by Yao et al[68] demonstrated that HNF4α is downregulated in human colon carcinoma and showed in in vitro experiments that
Wnt ligands need posttranslational modifications before they can activate Wnt signaling. In the endoplasmic reticulum, Wnt ligands were glycosylated and lipidated[70]. These modifications are essential for intracellular transport, secretion of Wnt ligands and signaling[71,72].
Wnt signaling could also be inhibited by posttranslational palmitoylation. Acyl-CoA synthetase 5 (ACSL5), a mitochondrial enzyme, activates long-chain fatty acids, while binding a thioester. ACSL5-dependent palmitoylation of Wnt2β leads to an accumulation of Wnt2β in the mitochondrion and a decrease in Wnt signaling activity[73].
Furthermore, the degradation of Wnt components by the proteasome can be regulated via ubiquitination through ligases. For example a phosphor switch in the E3 ubiquitin ligase RNF43 leads to a lack of degradation of Frizzled and therefore to Wnt activation[74]. The ligase RNF43 itself is inhibited by receptor Lgr4[75]. Park et al[76] summed up the different regulation possibilities of Wnt signaling throughout ubiquitination and deubiquitination. The ubiquitination is done by E3 Ligases while deubiquitination is done by deubiquitinating enzymes. In Wnt signaling, every protein component is targeted by ubiquitination or deubiquitination[76]. Therefore, it is an important regulator of Wnt signaling.
Long non-coding RNAs are over 200 nt long non-coding RNA molecules. As reviewed in Zarkou et al[77], they can act as a Wnt enhancer by transcriptional activation of genes coding for Wnt proteins or by interaction with transcription factors regulating Wnt signaling.
MicroRNAs (miRNAs) are small 18-25 nt long non-coding RNA molecules and can bind on their target messenger-RNA (mRNA) and suppress translation. Rahmani et al[78] summed up about 17 miRNAs that target mRNAs encoding for proteins of Wnt signaling. Here, they can act as an activator of Wnt signaling by suppressing translation of mRNA encoding for the destruction complex or as a suppressor of Wnt signaling, by inhibiting translation of mRNAs encoding for transmembrane complex or β-catenin. Kim et al[79] examined the crosstalk between stress-driven ribosome dysfunction and Wnt signaling. A proteinkinase R-activating ribosomal insult leads to changes in the Wnt and connective tissue growth factor crosstalk, which leads to progression in cancer stemness.
Despite the above-described pathways, growing evidence demonstrates that other pathways including the mitogen-activated protein kinase (MAPK) pathway, TGF-β signaling, and phosphoinositide 3-kinase/protein kinase B (PI3K/AKT) pathways involved in cell proliferation and survival have an influence on Wnt signaling[80]. It is reported that MAPK signaling regulates Wnt activity on stemness phenotypes in colorectal carcinoma cells[80,81]. Moreover, it has been found that Wnt and TGF-β pathways interact with each other to regulate genes participating in epithelial to mesenchymal transition (EMT)[82]. Hu et al[83] depict that epidermal growth factor receptor mediated PI3K/AKT activation enhances Wnt signaling activity through promoting β-catenin translocation, leading to increased tumor cell invasiveness.
In spite of these regulatory mechanisms, Wnt hyperactivation is not always avoidable. In this context, controlled activation must be distinguished from autonomous activation. Controlled activation is triggered by a stimulus outside the cell and determined through the presence of the stimulus, while autonomous activation is mainly triggered through modifications of proteins involved in the pathway and independently of the regulatory mechanism. The detailed mechanisms which lead to hyper-activation of Wnt signaling and the histomorphological correlation will be discussed hereafter.
As mentioned above, Wnt signaling is a complex regulated signaling pathway and many possibilities lead to hyperactivation of Wnt signaling in the intestine. Especially Wnt activation, while the loss of APC gene is well-studied in vitro and in vivo. In Drosophila, APC loss induced intestinal tumorigenesis[84]. A germline mutation in the APC gene with a loss-of-function mutation leads to familial adenomatous polyposis, representing a hereditary disease characterized by hundreds of colorectal adenomas[85]. But hyperactivation is not always accompanied by pathological tissue growth. In intestinal epithelial after injury, Wnt is also hyperactivated and enables regeneration[86]. Nevertheless, there is a fine line between Wnt activation for tissue regeneration and tissue hyperplasia. Ahmed et al[87] show in mice that Wnt and Notch signaling balance transmissible murine colonic hyperplasia and colitis induced by citrobacter rodentium. In the chronically inflamed intestine such as bowel disease, Wnt signaling is activated[88]. These patients had an increased risk of developing dysplasia and colorectal carcinoma[89]. Abnormal β-catenin expression was more closely linked to E-cadherin alterations in inflammatory bowel disease-related cancers than in sporadic cancers suggesting that specific alterations in this pathway may differ in these two cancer groups[90].
As long as Wnt signaling is controlled by other pathways, hyperproliferation of epithelial is stoppable. Problematic is uncontrolled Wnt activation, which leads to a permanent-growth stimulus. This could be caused by loss-of-function mutations in the genes encoding for the destruction complex. As mentioned above, familial adenomatous polyposis is a good example of this. But growth stimulation alone is not sufficient for carcinoma development. Fearon and Vogelstein generate the model of the adenoma-carcinoma-sequence[91]. They postulate that stepwise genetic alterations in oncogenes and tumor suppressor genes lead to hyperproliferative epithelial, low-grade and high-grade adenoma to carcinoma development. Besides APC mutations, which are hypothesized as a key event in adenoma development, gain-of-function mutations in KRAS and loss of functions in P16-INK4, TP53 and Smad4 are described in the model of multiple step carcinogenesis[92]. It is assumed that this model applies to 80% of colorectal carcinoma[93]. Nonetheless, not only APC mutations but also mutations in KRAS influence Wnt/β-catenin signaling[84]. In cell culture, KRAS stabilizes β-catenin through inhibition of GSK-3β, while others postulate that KRAS mutations activate Wnt signaling through DNA demethy
In the stomach, bile acid reflux leads to an epigenetic downregulation of Dkk1, an inhibitor of Wnt signaling[96]. The bile acid-induced downregulation of Dkk1 is correlated with gastric intestinal metaplasia and might be triggered by Wnt activation. Other studies have demonstrated high expression of Dkk1 in gastric carcinomas[97].
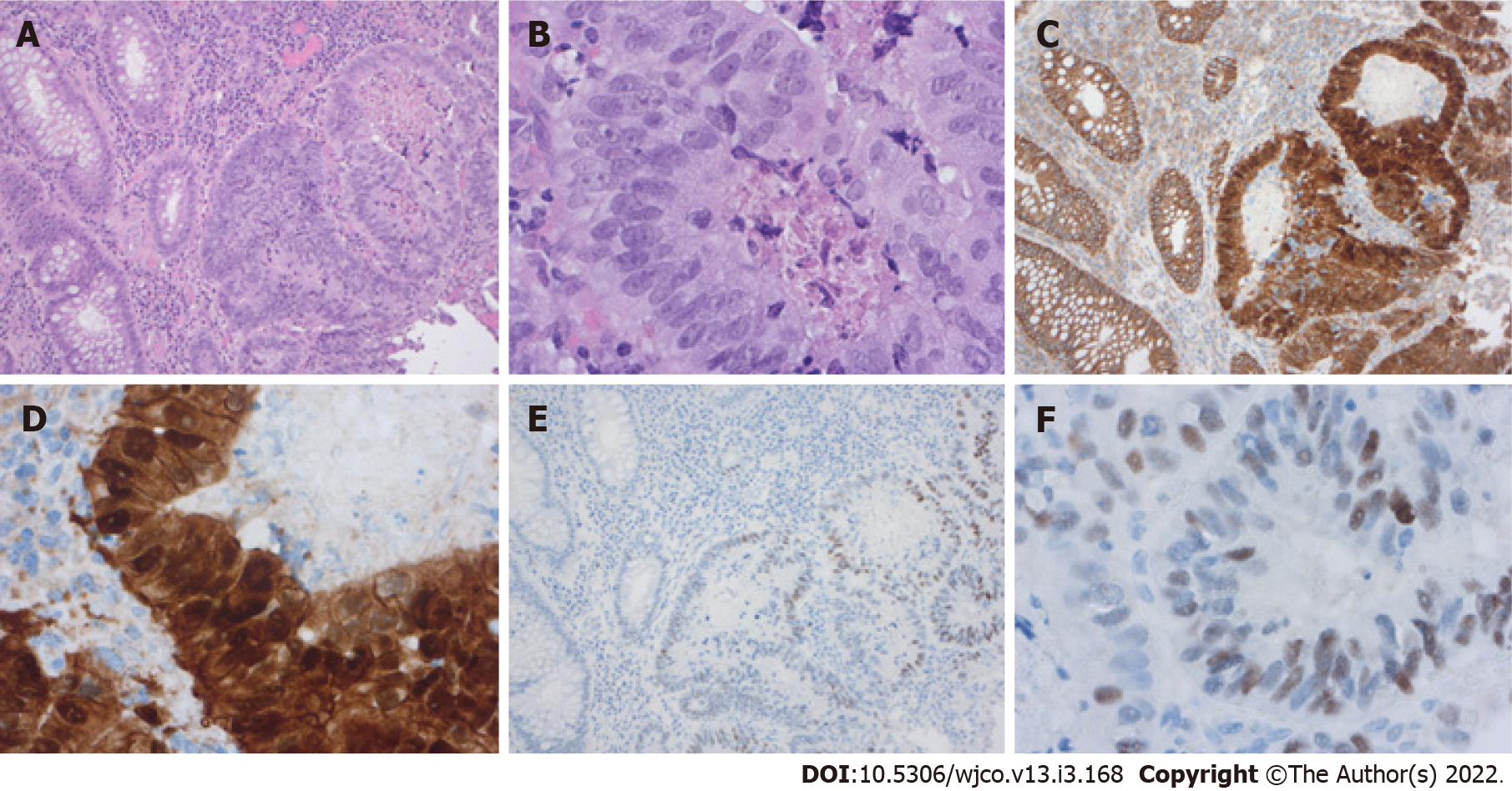
The genotypic changes in colorectal adenomas lead to phenotypic changes (Figure 4). Adenoma with the classical adenoma-carcinoma-sequence often present macroscopically or endoscopically as polypoid lesions, while tumors with CpG island hypermethylation and BRAF mutations often present as flat mucosal lesions[92]. APC mutations are more often in adenomas with villous or tubulovillous formation, which are reminiscent of small intestinal villi, but APC mutation is also found in tubular adenomas which had elongated crypts[98]. Furthermore, Paneth cell metaplasia is also a common finding in conventional adenoma, following the adenoma-carcinoma-sequence. Joo et al[99] examined colonic epithelial neoplasms for Paneth cell metaplasia and Paneth cells were found in 38.5% of the conventional adenoma. This Paneth cell metaplasia was always associated with positive nuclear β-catenin staining[99]. The adenoma cells also show, depending on their grading, enlarged, hyper
As in the intestine, APC downregulation occurs in gastric adenomas[101]. In the stomach, the downregulation of APC is mostly caused by hypermethylation of the APC promoter and might be triggered by Helicobacter pylori infection[102]. Koushyar et al[103] summed up the parts of Wnt signaling which are deregulated in gastric cancer. In gastric cancer organoids, Wnt inactivation leads to a shift from morphological poorly carcinoma not other specified to signet-ring cell carcinoma[104].
In studies, Wnt signaling was upregulated in more than 80% of the examined gastric cancers and may mark Lgr5 stem cells[105]. The detailed mechanism which leads to Wnt activation is similar to colorectal cancer and is reviewed in detail by Chiurillo[106]. Mao et al[107] examined that Wnt1 overexpression accelerated the growth of gastric cancer. Wnt/β-catenin signaling inhibitors suppress gastric tumor growth in a mice model[108].
Chen et al[109] showed cells of the Paneth cell lineage are present in intestinal adenomas. They secrete Wnt 3 and a deletion of Paneth cells leads to reduced growth of adenomas in the small intestine in APCmin mice. The authors concluded that Wnt3 is required for early tumorigenesis in the small bowel.
In recent decades, the role of genetic aberration as a prognostic value has moved increasingly to the fore. It is therefore evident that APC mutations, which occur in the majority of microsatellite stable colorectal cancers, are examined to determine whether they had a prognostic value of colorectal cancer. Jorissen et al[110] analyzed over seven hundred patients with sporadic colorectal cancer and found that wild-type APC correlates with poor prognosis (5-year survival) in microsatellite stable proximal colon cancer. On the other hand, some studies indicate that nuclear β-catenin promotes metastasis of colon cancer, which usually display poor prognosis, by EMT[111,112].
As mentioned above, mutations that activate Wnt/β-catenin signaling are common genetic events in colorectal cancer and usually occur in an early state of carcinogenesis. Therefore, Wnt inactivation is a possible target for preventing tumor progression and as a potential treatment of colorectal cancer. 5-aminosalicylic acid (5-ASA) is a well-established treatment against inflammatory bowel disease, especially in ulcerative colitis. Therefore, it has not only anti-inflammatory but also anti-proliferative effects[113]. Several cohort studies and case-control studies have demonstrated that 5-ASA treatment is associated with a reduced colorectal cancer risk in patients with ulcerative colitis[114-116]. Therefore, guidelines recommend 5-ASA treatment for ulcerative colitis patients also under the aspect of cancer prevention. The anti-proliferative effect is forced by PP2A-dependent accumulation of nuclear β-catenin[117]. Munding et al[118] examined the role of the chemopreventive effects of 5-ASA in vivo. After three years, there were no significant differences regarding the progression of adenomas between the patients treated with 5-ASA and the placebo group. But in the group treated with 5-ASA, a significant decrease in nuclear β-catenin expression was found[118]. Further studies with a longer treatment time were necessary because the development of carcinoma through the adenoma-carcinoma sequence takes about ten to fifteen years[119]. Serafino et al[120] examined in their study the β-catenin expression and the expression of the β-catenin regulated proteins c-Myc and Cyclin D1 in bowel disease and found elevated expression levels of these proteins especially in low-grade and high-grade dysplasia. These results emphasize the potential benefit of Wnt signaling inactivation as a predictive cancer therapy.
As reviewed by Zhu et al[121], Wnt activation has an impact on the resistance to chemotherapy in colorectal adenocarcinoma. Hu et al[122] determined that Wnt activation through exosomal Wnt secretion of fibroblasts leads to an increase in chemoresistance of cancer stem cells. Zhang et al[123] also identified the tumor microenvironment as a crucial factor in Wnt-induced chemoresistance. The increased chemoresistance in Wnt upregulated cancers is not only caused by enhancing the expression of antiapoptotic proteins, but also by enhancing the expression of multidrug resistance proteins[123,124]. Zhong et al[125] summarized different studies where chemoresistance is associated with Wnt activation in conventional radiochemotherapy, but also in targeted and immunotherapy. Wnt signaling seems to have a big impact on the response to cancer therapy. Hence, the development of a personalized therapy targeting components of the Wnt signaling pathway in treatment of cancer is required.
Application of Wnt inhibitors might be a possible therapeutic strategy to inactivate the Wnt pathway in cancer, for example obviation of binding of Wnt to Frizzled, stabilization of Dkk or destruction complex, inhibition of the transmembrane complex or Disheveled, application of β-catenin antagonist and antagonist of β-catenin cofactors, etc. Different drugs targeting Wnt pathway are currently in clinical trials, as reviewed in detail in Caspi et al[126]. Kleeman et al[127] postulate that there may be a difference in the therapeutic approach in ligand-dependent and ligand-independent tumors. Therefore, the localization of the mutation should be taken into account in the choice of Wnt signaling-targeting therapy. Ligand-dependent tumors should be targeted to the ligands or the transmembrane complex. In ligand-independent tumors, such as APC mutated tumors, targeting transmembrane complex is useless. A therapeutic option in these tumors is increased degradation of β-catenin. This is achieved by a stabilization of the destruction complex or directly by an increase of β-catenin degradation. One way to stabilize the destruction complex is an increased polymerization of conductin/axin2[128]. In vitro it represses the growth of colorectal cancer cells[128]. An opportunity to strengthen the degradation of β-catenin is via the proteasome through binding of molecules, which induces proteolysis. Kessler et al[129] examined potential binding sites of β-catenin proteolysis targeting chimeras (PROTACs). The first PROTACs are tested in mice and showed, in APCmin/+ mice, prevention and regression of colorectal cancer[130]. The E3 Ligase, TRIM58 enhances β-catenin degradation in gastric cancer and is a potential therapeutic target[131]. A different approach would be oncolytic viruses. In vitro and in a mice model, the adenovirus CD55-Smad4 represses tumor proliferation in metastasis by, inter alia, suppression of Wnt signaling[132]. Adenoviruses that inhibit tumor growth by repressing the Wnt pathway have also been developed for other carcinomas such as hepatocellular carcinoma[133]. Another possible therapeutic approach in Wnt-activated tumors would be the inhibition of the ribosome biogenesis. Raveux et al[134] show that ribosome biogenesis dysfunction alleviates Wnt-driven tumor initiation and reduces cancer cell proliferation. In a study, kinase inhibitors in gastric cancer were screened for Wnt pathway inhibition and 34 kinases inhibit Wnt signaling more than 50%[135]. Potential targets to inhibit Wnt/β-catenin signaling are summarized in Table 2.
| Target | Effect | Ref. |
| Ligand-dependent Wnt signaling activation | ||
| Wnt ligands | Wnt inhibitors | [164] |
| Posttranslational modification | [165] | |
| Dkk1 | Stabilization, increase of Dkk1 | [128,166] |
| Transmembrane complex | Inhibition of Lgr5/6 | [167] |
| Inhibition of Frizzled | [168,169] | |
| Dishevelled | Inhibition | [170] |
| Ligand independent Wnt signaling activation | ||
| Destruction complex | Stabilization of the destruction complex | [171,172] |
| β-catenin | Increase of degradation | [130,131] |
| Inhibition of translocation to the nucleolus | [173] | |
| β-catenin cofactors | [174] | |
| Ribosome biogenesis | [134] | |
| Oncolytic viruses | [132,133] | |
However, it must be noted that there could be a YAP/TAZ-dependent transcriptional reprogram-ming which leads to a lineage reversion and a Wnt-independent tumor growth, which can lead to failure of Wnt signaling inhibitors[136].
Development of therapeutic approaches by targeting Wnt signaling main players is challenging though it brings new hope for the management of colorectal cancer in the future.
The Wnt/β-catenin signaling pathway is a highly regulated pathway and essential for intestinal homeostasis. Disruption of this homeostasis with Wnt signaling hyperactivation can lead to tumor development and indeed Wnt activation is common in human colorectal cancer. The prognostic value of Wnt activation in colorectal cancer has not been fully elucidated yet. Furthermore, components of the Wnt signaling pathway have been brought into focus as possible targets in anti-cancer therapy and as possible adjuvant treatment for chemoresistant cancers.
Provenance and peer review: Invited article; Externally peer reviewed.
Peer-review model: Single blind
Specialty type: Oncology
Country/Territory of origin: Germany
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): B
Grade C (Good): C
Grade D (Fair): D
Grade E (Poor): 0
P-Reviewer: Feng R, Wang YG S-Editor: Wang JJ L-Editor: A P-Editor: Wang JJ
| 1. | Sharma RP, Chopra VL. Effect of the Wingless (wg1) mutation on wing and haltere development in Drosophila melanogaster. Dev Biol. 1976;48:461-465. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 233] [Cited by in RCA: 236] [Article Influence: 4.8] [Reference Citation Analysis (0)] |
| 2. | Nusse R. Wnt signaling in disease and in development. Cell Res. 2005;15:28-32. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 739] [Cited by in RCA: 757] [Article Influence: 37.9] [Reference Citation Analysis (0)] |
| 3. | Ng LF, Kaur P, Bunnag N, Suresh J, Sung ICH, Tan QH, Gruber J, Tolwinski NS. WNT Signaling in Disease. Cells. 2019;8. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 100] [Cited by in RCA: 173] [Article Influence: 28.8] [Reference Citation Analysis (0)] |
| 4. | Duchartre Y, Kim YM, Kahn M. The Wnt signaling pathway in cancer. Crit Rev Oncol Hematol. 2016;99:141-149. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 255] [Cited by in RCA: 410] [Article Influence: 41.0] [Reference Citation Analysis (0)] |
| 5. | Parker TW, Neufeld KL. APC controls Wnt-induced β-catenin destruction complex recruitment in human colonocytes. Sci Rep. 2020;10:2957. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 29] [Cited by in RCA: 67] [Article Influence: 13.4] [Reference Citation Analysis (0)] |
| 6. | Kimelman D, Xu W. beta-catenin destruction complex: insights and questions from a structural perspective. Oncogene. 2006;25:7482-7491. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 451] [Cited by in RCA: 503] [Article Influence: 27.9] [Reference Citation Analysis (0)] |
| 7. | MacDonald BT, He X. Frizzled and LRP5/6 receptors for Wnt/β-catenin signaling. Cold Spring Harb Perspect Biol. 2012;4. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 340] [Cited by in RCA: 472] [Article Influence: 36.3] [Reference Citation Analysis (0)] |
| 8. | Cruciat CM. Casein kinase 1 and Wnt/β-catenin signaling. Curr Opin Cell Biol. 2014;31:46-55. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 82] [Cited by in RCA: 99] [Article Influence: 9.0] [Reference Citation Analysis (0)] |
| 9. | Klingensmith J, Nusse R, Perrimon N. The Drosophila segment polarity gene dishevelled encodes a novel protein required for response to the wingless signal. Genes Dev. 1994;8:118-130. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 295] [Cited by in RCA: 312] [Article Influence: 10.1] [Reference Citation Analysis (0)] |
| 10. | Schaefer KN, Pronobis MI, Williams CE, Zhang S, Bauer L, Goldfarb D, Yan F, Major MB, Peifer M. Wnt regulation: exploring Axin-Disheveled interactions and defining mechanisms by which the SCF E3 ubiquitin ligase is recruited to the destruction complex. Mol Biol Cell. 2020;31:992-1014. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 13] [Cited by in RCA: 17] [Article Influence: 3.4] [Reference Citation Analysis (0)] |
| 11. | Cantù C, Felker A, Zimmerli D, Prummel KD, Cabello EM, Chiavacci E, Méndez-Acevedo KM, Kirchgeorg L, Burger S, Ripoll J, Valenta T, Hausmann G, Vilain N, Aguet M, Burger A, Panáková D, Basler K, Mosimann C. Mutations in Bcl9 and Pygo genes cause congenital heart defects by tissue-specific perturbation of Wnt/β-catenin signaling. Genes Dev. 2018;32:1443-1458. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 42] [Cited by in RCA: 33] [Article Influence: 4.7] [Reference Citation Analysis (0)] |
| 12. | Rieger ME, Zhou B, Solomon N, Sunohara M, Li C, Nguyen C, Liu Y, Pan JH, Minoo P, Crandall ED, Brody SL, Kahn M, Borok Z. p300/β-Catenin Interactions Regulate Adult Progenitor Cell Differentiation Downstream of WNT5a/Protein Kinase C (PKC). J Biol Chem. 2016;291:6569-6582. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 58] [Cited by in RCA: 72] [Article Influence: 8.0] [Reference Citation Analysis (0)] |
| 13. | Doumpas N, Lampart F, Robinson MD, Lentini A, Nestor CE, Cantù C, Basler K. TCF/LEF dependent and independent transcriptional regulation of Wnt/β-catenin target genes. EMBO J. 2019;38. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 99] [Cited by in RCA: 149] [Article Influence: 24.8] [Reference Citation Analysis (0)] |
| 14. | Bafico A, Liu G, Yaniv A, Gazit A, Aaronson SA. Novel mechanism of Wnt signalling inhibition mediated by Dickkopf-1 interaction with LRP6/Arrow. Nat Cell Biol. 2001;3:683-686. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 618] [Cited by in RCA: 628] [Article Influence: 26.2] [Reference Citation Analysis (0)] |
| 15. | Semënov MV, Tamai K, Brott BK, Kühl M, Sokol S, He X. Head inducer Dickkopf-1 is a ligand for Wnt coreceptor LRP6. Curr Biol. 2001;11:951-961. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 555] [Cited by in RCA: 571] [Article Influence: 23.8] [Reference Citation Analysis (0)] |
| 16. | Herbst A, Jurinovic V, Krebs S, Thieme SE, Blum H, Göke B, Kolligs FT. Comprehensive analysis of β-catenin target genes in colorectal carcinoma cell lines with deregulated Wnt/β-catenin signaling. BMC Genomics. 2014;15:74. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 153] [Cited by in RCA: 182] [Article Influence: 16.5] [Reference Citation Analysis (0)] |
| 17. | Alexandre C, Baena-Lopez A, Vincent JP. Patterning and growth control by membrane-tethered Wingless. Nature. 2014;505:180-185. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 266] [Cited by in RCA: 227] [Article Influence: 20.6] [Reference Citation Analysis (0)] |
| 18. | Chin AM, Tsai YH, Finkbeiner SR, Nagy MS, Walker EM, Ethen NJ, Williams BO, Battle MA, Spence JR. A Dynamic WNT/β-CATENIN Signaling Environment Leads to WNT-Independent and WNT-Dependent Proliferation of Embryonic Intestinal Progenitor Cells. Stem Cell Reports. 2016;7:826-839. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 23] [Cited by in RCA: 25] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 19. | O'Connell AE, Zhou F, Shah MS, Murphy Q, Rickner H, Kelsen J, Boyle J, Doyle JJ, Gangwani B, Thiagarajah JR, Kamin DS, Goldsmith JD, Richmond C, Breault DT, Agrawal PB. Neonatal-Onset Chronic Diarrhea Caused by Homozygous Nonsense WNT2B Mutations. Am J Hum Genet. 2018;103:131-137. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 18] [Cited by in RCA: 22] [Article Influence: 3.1] [Reference Citation Analysis (0)] |
| 20. | Zhang YJ, Jimenez L, Azova S, Kremen J, Chan YM, Elhusseiny AM, Saeed H, Goldsmith J, Al-Ibraheemi A, O'Connell AE, Kovbasnjuk O, Rodan L, Agrawal PB, Thiagarajah JR. Novel variants in the stem cell niche factor WNT2B define the disease phenotype as a congenital enteropathy with ocular dysgenesis. Eur J Hum Genet. 2021;29:998-1007. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 14] [Cited by in RCA: 18] [Article Influence: 4.5] [Reference Citation Analysis (0)] |
| 21. | Kim TH, Shivdasani RA. Stomach development, stem cells and disease. Development. 2016;143:554-565. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 98] [Cited by in RCA: 109] [Article Influence: 12.1] [Reference Citation Analysis (0)] |
| 22. | McCracken KW, Aihara E, Martin B, Crawford CM, Broda T, Treguier J, Zhang X, Shannon JM, Montrose MH, Wells JM. Wnt/β-catenin promotes gastric fundus specification in mice and humans. Nature. 2017;541:182-187. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 176] [Cited by in RCA: 167] [Article Influence: 20.9] [Reference Citation Analysis (0)] |
| 23. | Fischer AS, Sigal M. The Role of Wnt and R-spondin in the Stomach During Health and Disease. Biomedicines. 2019;7. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 23] [Cited by in RCA: 22] [Article Influence: 3.7] [Reference Citation Analysis (0)] |
| 24. | Sayols S, Klassek J, Werner C, Möckel S, Ritz S, Mendez-Lago M, Soshnikova N. Signalling codes for the maintenance and lineage commitment of embryonic gastric epithelial progenitors. Development. 2020;147. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 6] [Cited by in RCA: 11] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 25. | Kim JE, Fei L, Yin WC, Coquenlorge S, Rao-Bhatia A, Zhang X, Shi SSW, Lee JH, Hahn NA, Rizvi W, Kim KH, Sung HK, Hui CC, Guo G, Kim TH. Single cell and genetic analyses reveal conserved populations and signaling mechanisms of gastrointestinal stromal niches. Nat Commun. 2020;11:334. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 33] [Cited by in RCA: 76] [Article Influence: 15.2] [Reference Citation Analysis (0)] |
| 26. | Kim BM, Buchner G, Miletich I, Sharpe PT, Shivdasani RA. The stomach mesenchymal transcription factor Barx1 specifies gastric epithelial identity through inhibition of transient Wnt signaling. Dev Cell. 2005;8:611-622. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 159] [Cited by in RCA: 157] [Article Influence: 7.9] [Reference Citation Analysis (0)] |
| 27. | Li L, Clevers H. Coexistence of quiescent and active adult stem cells in mammals. Science. 2010;327:542-545. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1015] [Cited by in RCA: 954] [Article Influence: 63.6] [Reference Citation Analysis (0)] |
| 28. | Mah AT, Yan KS, Kuo CJ. Wnt pathway regulation of intestinal stem cells. J Physiol. 2016;594:4837-4847. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 72] [Cited by in RCA: 107] [Article Influence: 11.9] [Reference Citation Analysis (0)] |
| 29. | Potten CS, Booth C, Pritchard DM. The intestinal epithelial stem cell: the mucosal governor. Int J Exp Pathol. 1997;78:219-243. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 370] [Cited by in RCA: 352] [Article Influence: 12.6] [Reference Citation Analysis (0)] |
| 30. | Tian H, Biehs B, Warming S, Leong KG, Rangell L, Klein OD, de Sauvage FJ. A reserve stem cell population in small intestine renders Lgr5-positive cells dispensable. Nature. 2011;478:255-259. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 836] [Cited by in RCA: 935] [Article Influence: 66.8] [Reference Citation Analysis (0)] |
| 31. | Farin HF, Van Es JH, Clevers H. Redundant sources of Wnt regulate intestinal stem cells and promote formation of Paneth cells. Gastroenterology. 2012;143:1518-1529.e7. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 464] [Cited by in RCA: 509] [Article Influence: 39.2] [Reference Citation Analysis (0)] |
| 32. | Bastide P, Darido C, Pannequin J, Kist R, Robine S, Marty-Double C, Bibeau F, Scherer G, Joubert D, Hollande F, Blache P, Jay P. Sox9 regulates cell proliferation and is required for Paneth cell differentiation in the intestinal epithelium. J Cell Biol. 2007;178:635-648. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 353] [Cited by in RCA: 395] [Article Influence: 21.9] [Reference Citation Analysis (0)] |
| 33. | Clevers HC, Bevins CL. Paneth cells: maestros of the small intestinal crypts. Annu Rev Physiol. 2013;75:289-311. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 427] [Cited by in RCA: 490] [Article Influence: 40.8] [Reference Citation Analysis (0)] |
| 34. | Umar S. Intestinal stem cells. Curr Gastroenterol Rep. 2010;12:340-348. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 174] [Cited by in RCA: 213] [Article Influence: 14.2] [Reference Citation Analysis (0)] |
| 35. | Beumer J, Clevers H. Cell fate specification and differentiation in the adult mammalian intestine. Nat Rev Mol Cell Biol. 2021;22:39-53. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 120] [Cited by in RCA: 398] [Article Influence: 79.6] [Reference Citation Analysis (0)] |
| 36. | Clevers H. The intestinal crypt, a prototype stem cell compartment. Cell. 2013;154:274-284. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 755] [Cited by in RCA: 894] [Article Influence: 74.5] [Reference Citation Analysis (0)] |
| 37. | Pinto D, Gregorieff A, Begthel H, Clevers H. Canonical Wnt signals are essential for homeostasis of the intestinal epithelium. Genes Dev. 2003;17:1709-1713. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 758] [Cited by in RCA: 793] [Article Influence: 36.0] [Reference Citation Analysis (0)] |
| 38. | Dudhwala ZM, Hammond PD, Howarth GS, Cummins AG. Intestinal stem cells promote crypt fission during postnatal growth of the small intestine. BMJ Open Gastroenterol. 2020;7. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 14] [Cited by in RCA: 14] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 39. | van der Flier LG, Clevers H. Stem cells, self-renewal, and differentiation in the intestinal epithelium. Annu Rev Physiol. 2009;71:241-260. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1156] [Cited by in RCA: 1327] [Article Influence: 82.9] [Reference Citation Analysis (0)] |
| 40. | Davies PS, Dismuke AD, Powell AE, Carroll KH, Wong MH. Wnt-reporter expression pattern in the mouse intestine during homeostasis. BMC Gastroenterol. 2008;8:57. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 48] [Cited by in RCA: 49] [Article Influence: 2.9] [Reference Citation Analysis (0)] |
| 41. | Humphries A, Wright NA. Colonic crypt organization and tumorigenesis. Nat Rev Cancer. 2008;8:415-424. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 244] [Cited by in RCA: 241] [Article Influence: 14.2] [Reference Citation Analysis (0)] |
| 42. | Adam RS, van Neerven SM, Pleguezuelos-Manzano C, Simmini S, Léveillé N, de Groot NE, Holding AN, Markowetz F, Vermeulen L. Intestinal region-specific Wnt signalling profiles reveal interrelation between cell identity and oncogenic pathway activity in cancer development. Cancer Cell Int. 2020;20:578. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 8] [Cited by in RCA: 10] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 43. | Garriock RJ, Chalamalasetty RB, Zhu J, Kennedy MW, Kumar A, Mackem S, Yamaguchi TP. A dorsal-ventral gradient of Wnt3a/β-catenin signals controls mouse hindgut extension and colon formation. Development. 2020;147. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 10] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 44. | Demitrack ES, Samuelson LC. Notch regulation of gastrointestinal stem cells. J Physiol. 2016;594:4791-4803. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 80] [Cited by in RCA: 102] [Article Influence: 11.3] [Reference Citation Analysis (0)] |
| 45. | Carulli AJ, Keeley TM, Demitrack ES, Chung J, Maillard I, Samuelson LC. Notch receptor regulation of intestinal stem cell homeostasis and crypt regeneration. Dev Biol. 2015;402:98-108. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 94] [Cited by in RCA: 104] [Article Influence: 10.4] [Reference Citation Analysis (0)] |
| 46. | Hayward P, Kalmar T, Arias AM. Wnt/Notch signalling and information processing during development. Development. 2008;135:411-424. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 214] [Cited by in RCA: 232] [Article Influence: 13.6] [Reference Citation Analysis (0)] |
| 47. | Hurlbut GD, Kankel MW, Lake RJ, Artavanis-Tsakonas S. Crossing paths with Notch in the hyper-network. Curr Opin Cell Biol. 2007;19:166-175. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 170] [Cited by in RCA: 184] [Article Influence: 10.2] [Reference Citation Analysis (0)] |
| 48. | Acosta H, López SL, Revinski DR, Carrasco AE. Notch destabilises maternal beta-catenin and restricts dorsal-anterior development in Xenopus. Development. 2011;138:2567-2579. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 26] [Cited by in RCA: 24] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 49. | Kwon C, Cheng P, King IN, Andersen P, Shenje L, Nigam V, Srivastava D. Notch post-translationally regulates β-catenin protein in stem and progenitor cells. Nat Cell Biol. 2011;13:1244-1251. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 212] [Cited by in RCA: 235] [Article Influence: 16.8] [Reference Citation Analysis (0)] |
| 50. | Collu GM, Hidalgo-Sastre A, Acar A, Bayston L, Gildea C, Leverentz MK, Mills CG, Owens TW, Meurette O, Dorey K, Brennan K. Dishevelled limits Notch signalling through inhibition of CSL. Development. 2012;139:4405-4415. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 43] [Cited by in RCA: 52] [Article Influence: 4.3] [Reference Citation Analysis (0)] |
| 51. | Axelrod JD, Matsuno K, Artavanis-Tsakonas S, Perrimon N. Interaction between Wingless and Notch signaling pathways mediated by dishevelled. Science. 1996;271:1826-1832. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 338] [Cited by in RCA: 335] [Article Influence: 11.6] [Reference Citation Analysis (0)] |
| 52. | Mei X, Gu M, Li M. Plasticity of Paneth cells and their ability to regulate intestinal stem cells. Stem Cell Res Ther. 2020;11:349. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 15] [Cited by in RCA: 42] [Article Influence: 8.4] [Reference Citation Analysis (0)] |
| 53. | Kaemmerer E, Jeon MK, Berndt A, Liedtke C, Gassler N. Targeting Wnt Signaling via Notch in Intestinal Carcinogenesis. Cancers (Basel). 2019;11. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 32] [Cited by in RCA: 26] [Article Influence: 4.3] [Reference Citation Analysis (0)] |
| 54. | Stringer EJ, Duluc I, Saandi T, Davidson I, Bialecka M, Sato T, Barker N, Clevers H, Pritchard CA, Winton DJ, Wright NA, Freund JN, Deschamps J, Beck F. Cdx2 determines the fate of postnatal intestinal endoderm. Development. 2012;139:465-474. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 81] [Cited by in RCA: 79] [Article Influence: 6.1] [Reference Citation Analysis (0)] |
| 55. | Boyd M, Hansen M, Jensen TG, Perearnau A, Olsen AK, Bram LL, Bak M, Tommerup N, Olsen J, Troelsen JT. Genome-wide analysis of CDX2 binding in intestinal epithelial cells (Caco-2). J Biol Chem. 2010;285:25115-25125. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 59] [Cited by in RCA: 62] [Article Influence: 4.1] [Reference Citation Analysis (0)] |
| 56. | Yu J, Liu D, Sun X, Yang K, Yao J, Cheng C, Wang C, Zheng J. CDX2 inhibits the proliferation and tumor formation of colon cancer cells by suppressing Wnt/β-catenin signaling via transactivation of GSK-3β and Axin2 expression. Cell Death Dis. 2019;10:26. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 57] [Cited by in RCA: 131] [Article Influence: 21.8] [Reference Citation Analysis (0)] |
| 57. | Dalerba P, Sahoo D, Paik S, Guo X, Yothers G, Song N, Wilcox-Fogel N, Forgó E, Rajendran PS, Miranda SP, Hisamori S, Hutchison J, Kalisky T, Qian D, Wolmark N, Fisher GA, van de Rijn M, Clarke MF. CDX2 as a Prognostic Biomarker in Stage II and Stage III Colon Cancer. N Engl J Med. 2016;374:211-222. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 314] [Cited by in RCA: 358] [Article Influence: 39.8] [Reference Citation Analysis (0)] |
| 58. | McCarthy N, Manieri E, Storm EE, Saadatpour A, Luoma AM, Kapoor VN, Madha S, Gaynor LT, Cox C, Keerthivasan S, Wucherpfennig K, Yuan GC, de Sauvage FJ, Turley SJ, Shivdasani RA. Distinct Mesenchymal Cell Populations Generate the Essential Intestinal BMP Signaling Gradient. Cell Stem Cell. 2020;26:391-402.e5. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 114] [Cited by in RCA: 240] [Article Influence: 48.0] [Reference Citation Analysis (0)] |
| 59. | Kosinski C, Li VS, Chan AS, Zhang J, Ho C, Tsui WY, Chan TL, Mifflin RC, Powell DW, Yuen ST, Leung SY, Chen X. Gene expression patterns of human colon tops and basal crypts and BMP antagonists as intestinal stem cell niche factors. Proc Natl Acad Sci U S A. 2007;104:15418-15423. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 411] [Cited by in RCA: 460] [Article Influence: 25.6] [Reference Citation Analysis (0)] |
| 60. | He XC, Zhang J, Tong WG, Tawfik O, Ross J, Scoville DH, Tian Q, Zeng X, He X, Wiedemann LM, Mishina Y, Li L. BMP signaling inhibits intestinal stem cell self-renewal through suppression of Wnt-beta-catenin signaling. Nat Genet. 2004;36:1117-1121. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 805] [Cited by in RCA: 815] [Article Influence: 38.8] [Reference Citation Analysis (0)] |
| 61. | Qi Z, Li Y, Zhao B, Xu C, Liu Y, Li H, Zhang B, Wang X, Yang X, Xie W, Li B, Han JJ, Chen YG. BMP restricts stemness of intestinal Lgr5+ stem cells by directly suppressing their signature genes. Nat Commun. 2017;8:13824. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 147] [Cited by in RCA: 226] [Article Influence: 28.3] [Reference Citation Analysis (0)] |
| 62. | Hong AW, Meng Z, Guan KL. The Hippo pathway in intestinal regeneration and disease. Nat Rev Gastroenterol Hepatol. 2016;13:324-337. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 152] [Cited by in RCA: 232] [Article Influence: 25.8] [Reference Citation Analysis (0)] |
| 63. | Li N, Lu N, Xie C. The Hippo and Wnt signalling pathways: crosstalk during neoplastic progression in gastrointestinal tissue. FEBS J. 2019;286:3745-3756. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 37] [Cited by in RCA: 64] [Article Influence: 10.7] [Reference Citation Analysis (0)] |
| 64. | Kriz V, Korinek V. Wnt, RSPO and Hippo Signalling in the Intestine and Intestinal Stem Cells. Genes (Basel). 2018;9. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 29] [Cited by in RCA: 51] [Article Influence: 7.3] [Reference Citation Analysis (0)] |
| 65. | Ward D, Montes Olivas S, Fletcher A, Homer M, Marucci L. Cross-talk between Hippo and Wnt signalling pathways in intestinal crypts: Insights from an agent-based model. Comput Struct Biotechnol J. 2020;18:230-240. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 7] [Cited by in RCA: 7] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 66. | Mihaylova MM, Cheng CW, Cao AQ, Tripathi S, Mana MD, Bauer-Rowe KE, Abu-Remaileh M, Clavain L, Erdemir A, Lewis CA, Freinkman E, Dickey AS, La Spada AR, Huang Y, Bell GW, Deshpande V, Carmeliet P, Katajisto P, Sabatini DM, Yilmaz ÖH. Fasting Activates Fatty Acid Oxidation to Enhance Intestinal Stem Cell Function during Homeostasis and Aging. Cell Stem Cell. 2018;22:769-778.e4. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 174] [Cited by in RCA: 309] [Article Influence: 51.5] [Reference Citation Analysis (0)] |
| 67. | Chen L, Vasoya RP, Toke NH, Parthasarathy A, Luo S, Chiles E, Flores J, Gao N, Bonder EM, Su X, Verzi MP. HNF4 Regulates Fatty Acid Oxidation and Is Required for Renewal of Intestinal Stem Cells in Mice. Gastroenterology. 2020;158:985-999.e9. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 73] [Cited by in RCA: 152] [Article Influence: 30.4] [Reference Citation Analysis (0)] |
| 68. | Yao HS, Wang J, Zhang XP, Wang LZ, Wang Y, Li XX, Jin KZ, Hu ZQ, Wang WJ. Hepatocyte nuclear factor 4α suppresses the aggravation of colon carcinoma. Mol Carcinog. 2016;55:458-472. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 20] [Cited by in RCA: 29] [Article Influence: 2.9] [Reference Citation Analysis (0)] |
| 69. | Wu N, Zhang YL, Wang HT, Li DW, Dai HJ, Zhang QQ, Zhang J, Ma Y, Xia Q, Bian JM, Hang HL. Overexpression of hepatocyte nuclear factor 4α in human mesenchymal stem cells suppresses hepatocellular carcinoma development through Wnt/β-catenin signaling pathway downregulation. Cancer Biol Ther. 2016;17:558-565. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 32] [Cited by in RCA: 42] [Article Influence: 5.3] [Reference Citation Analysis (0)] |
| 70. | Kaemmerer E, Gassler N. Wnt Lipidation and Modifiers in Intestinal Carcinogenesis and Cancer. Cancers (Basel). 2016;8. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 25] [Cited by in RCA: 21] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 71. | Takada R, Satomi Y, Kurata T, Ueno N, Norioka S, Kondoh H, Takao T, Takada S. Monounsaturated fatty acid modification of Wnt protein: its role in Wnt secretion. Dev Cell. 2006;11:791-801. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 548] [Cited by in RCA: 595] [Article Influence: 33.1] [Reference Citation Analysis (0)] |
| 72. | Kurayoshi M, Yamamoto H, Izumi S, Kikuchi A. Post-translational palmitoylation and glycosylation of Wnt-5a are necessary for its signalling. Biochem J. 2007;402:515-523. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 167] [Cited by in RCA: 171] [Article Influence: 9.5] [Reference Citation Analysis (0)] |
| 73. | Klaus C, Schneider U, Hedberg C, Schütz AK, Bernhagen J, Waldmann H, Gassler N, Kaemmerer E. Modulating effects of acyl-CoA synthetase 5-derived mitochondrial Wnt2B palmitoylation on intestinal Wnt activity. World J Gastroenterol. 2014;20:14855-14864. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 26] [Cited by in RCA: 23] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 74. | Tsukiyama T, Zou J, Kim J, Ogamino S, Shino Y, Masuda T, Merenda A, Matsumoto M, Fujioka Y, Hirose T, Terai S, Takahashi H, Ishitani T, Nakayama KI, Ohba Y, Koo BK, Hatakeyama S. A phospho-switch controls RNF43-mediated degradation of Wnt receptors to suppress tumorigenesis. Nat Commun. 2020;11:4586. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 23] [Cited by in RCA: 43] [Article Influence: 8.6] [Reference Citation Analysis (0)] |
| 75. | Park S, Wu L, Tu J, Yu W, Toh Y, Carmon KS, Liu QJ. Unlike LGR4, LGR5 potentiates Wnt-β-catenin signaling without sequestering E3 Ligases. Sci Signal. 2020;13. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 11] [Cited by in RCA: 28] [Article Influence: 5.6] [Reference Citation Analysis (0)] |
| 76. | Park HB, Kim JW, Baek KH. Regulation of Wnt Signaling through Ubiquitination and Deubiquitination in Cancers. Int J Mol Sci. 2020;21. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 34] [Cited by in RCA: 94] [Article Influence: 18.8] [Reference Citation Analysis (0)] |
| 77. | Zarkou V, Galaras A, Giakountis A, Hatzis P. Crosstalk mechanisms between the WNT signaling pathway and long non-coding RNAs. Noncoding RNA Res. 2018;3:42-53. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 37] [Cited by in RCA: 42] [Article Influence: 6.0] [Reference Citation Analysis (0)] |
| 78. | Rahmani F, Avan A, Hashemy SI, Hassanian SM. Role of Wnt/β-catenin signaling regulatory microRNAs in the pathogenesis of colorectal cancer. J Cell Physiol. 2018;233:811-817. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 56] [Cited by in RCA: 76] [Article Influence: 9.5] [Reference Citation Analysis (0)] |
| 79. | Kim KH, Lee SJ, Kim J, Moon Y. Dynamic Malignant Wave of Ribosome-Insulted Gut Niche via the Wnt-CTGF/CCN2 Circuit. iScience. 2020;23:101076. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 4] [Cited by in RCA: 9] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 80. | Wei G, Gao N, Chen J, Fan L, Zeng Z, Gao G, Li L, Fang G, Hu K, Pang X, Fan HY, Clevers H, Liu M, Zhang X, Li D. Erk and MAPK signaling is essential for intestinal development through Wnt pathway modulation. Development. 2020;147. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 10] [Cited by in RCA: 19] [Article Influence: 3.8] [Reference Citation Analysis (0)] |
| 81. | Horst D, Chen J, Morikawa T, Ogino S, Kirchner T, Shivdasani RA. Differential WNT activity in colorectal cancer confers limited tumorigenic potential and is regulated by MAPK signaling. Cancer Res. 2012;72:1547-1556. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 93] [Cited by in RCA: 99] [Article Influence: 7.6] [Reference Citation Analysis (0)] |
| 82. | Cheruku HR, Mohamedali A, Cantor DI, Tan SH, Nice EC, Baker MS. Transforming growth factor-β, MAPK and Wnt signaling interactions in colorectal cancer. EuPA Open Proteomics. 2015;8:104-115. [RCA] [DOI] [Full Text] [Cited by in Crossref: 26] [Cited by in RCA: 31] [Article Influence: 3.1] [Reference Citation Analysis (0)] |
| 83. | Hu T, Li C. Convergence between Wnt-β-catenin and EGFR signaling in cancer. Mol Cancer. 2010;9:236. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 177] [Cited by in RCA: 223] [Article Influence: 14.9] [Reference Citation Analysis (0)] |
| 84. | Wang C, Zhao R, Huang P, Yang F, Quan Z, Xu N, Xi R. APC loss-induced intestinal tumorigenesis in Drosophila: Roles of Ras in Wnt signaling activation and tumor progression. Dev Biol. 2013;378:122-140. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 40] [Cited by in RCA: 39] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 85. | Half E, Bercovich D, Rozen P. Familial adenomatous polyposis. Orphanet J Rare Dis. 2009;4:22. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 308] [Cited by in RCA: 376] [Article Influence: 23.5] [Reference Citation Analysis (0)] |
| 86. | Cordero JB, Sansom OJ. Wnt signalling and its role in stem cell-driven intestinal regeneration and hyperplasia. Acta Physiol (Oxf). 2012;204:137-143. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 41] [Cited by in RCA: 43] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 87. | Ahmed I, Chandrakesan P, Tawfik O, Xia L, Anant S, Umar S. Critical roles of Notch and Wnt/β-catenin pathways in the regulation of hyperplasia and/or colitis in response to bacterial infection. Infect Immun. 2012;80:3107-3121. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 46] [Cited by in RCA: 51] [Article Influence: 3.9] [Reference Citation Analysis (0)] |
| 88. | Moparthi L, Koch S. Wnt signaling in intestinal inflammation. Differentiation. 2019;108:24-32. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 66] [Cited by in RCA: 119] [Article Influence: 19.8] [Reference Citation Analysis (0)] |
| 89. | Mark-Christensen A, Laurberg S, Haboubi N. Dysplasia in Inflammatory Bowel Disease: Historical Review, Critical Histopathological Analysis, and Clinical Implications. Inflamm Bowel Dis. 2018;24:1895-1903. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 15] [Cited by in RCA: 12] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 90. | Aust DE, Terdiman JP, Willenbucher RF, Chew K, Ferrell L, Florendo C, Molinaro-Clark A, Baretton GB, Löhrs U, Waldman FM. Altered distribution of beta-catenin, and its binding proteins E-cadherin and APC, in ulcerative colitis-related colorectal cancers. Mod Pathol. 2001;14:29-39. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 56] [Cited by in RCA: 58] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 91. | Fearon ER, Vogelstein B. A genetic model for colorectal tumorigenesis. Cell. 1990;61:759-767. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 8087] [Cited by in RCA: 8006] [Article Influence: 228.7] [Reference Citation Analysis (1)] |
| 92. | Bosman F, Yan P. Molecular pathology of colorectal cancer. Pol J Pathol. 2014;65:257-266. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 24] [Cited by in RCA: 29] [Article Influence: 2.9] [Reference Citation Analysis (0)] |
| 93. | Li J, Mizukami Y, Zhang X, Jo WS, Chung DC. Oncogenic K-ras stimulates Wnt signaling in colon cancer through inhibition of GSK-3beta. Gastroenterology. 2005;128:1907-1918. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 75] [Cited by in RCA: 76] [Article Influence: 3.8] [Reference Citation Analysis (0)] |
| 94. | Wong CC, Xu J, Bian X, Wu JL, Kang W, Qian Y, Li W, Chen H, Gou H, Liu D, Yat Luk ST, Zhou Q, Ji F, Chan LS, Shirasawa S, Sung JJ, Yu J. In Colorectal Cancer Cells With Mutant KRAS, SLC25A22-Mediated Glutaminolysis Reduces DNA Demethylation to Increase WNT Signaling, Stemness, and Drug Resistance. Gastroenterology. 2020;159:2163-2180.e6. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 50] [Cited by in RCA: 115] [Article Influence: 23.0] [Reference Citation Analysis (0)] |
| 95. | Schrock AB, Devoe CE, McWilliams R, Sun J, Aparicio T, Stephens PJ, Ross JS, Wilson R, Miller VA, Ali SM, Overman MJ. Genomic Profiling of Small-Bowel Adenocarcinoma. JAMA Oncol. 2017;3:1546-1553. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 139] [Cited by in RCA: 165] [Article Influence: 20.6] [Reference Citation Analysis (0)] |
| 96. | Lu W, Ni Z, Tong M, Jiang S, Zhang J, Feng C, Han C, Yuan T, Wang N, Zhao J, Sun N, Liu C, Jia Q, Wu Q, Ning H, Shi Y. DKK1 is epigenetically downregulated by promoter methylation and inhibits bile acid-induced gastric intestinal metaplasia. Biochem Biophys Res Commun. 2020;523:780-786. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 6] [Cited by in RCA: 6] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 97. | Guan E, Tian F, Liu Z. A novel risk score model for stomach adenocarcinoma based on the expression levels of 10 genes. Oncol Lett. 2020;19:1351-1367. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1] [Cited by in RCA: 3] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 98. | De Benedetti L, Sciallero S, Gismondi V, James R, Bafico A, Biticchi R, Masetti E, Bonelli L, Heouaine A, Picasso M. Association of APC gene mutations and histological characteristics of colorectal adenomas. Cancer Res. 1994;54:3553-3556. [PubMed] |
| 99. | Joo M, Shahsafaei A, Odze RD. Paneth cell differentiation in colonic epithelial neoplasms: evidence for the role of the Apc/beta-catenin/Tcf pathway. Hum Pathol. 2009;40:872-880. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 45] [Cited by in RCA: 49] [Article Influence: 3.1] [Reference Citation Analysis (0)] |
| 100. | Bosman FT, Carneiro F, Hruban RH, Theise ND. WHO classification of tumours of the digestive system. WHO Classification of Tumours. 2010;3. |
| 101. | Wang ZK, Liu J, Liu C, Wang FY, Chen CY, Zhang XH. Hypermethylation of adenomatous polyposis coli gene promoter is associated with novel Wnt signaling pathway in gastric adenomas. J Gastroenterol Hepatol. 2012;27:1629-1634. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 23] [Cited by in RCA: 20] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 102. | Wang Z, Ye Y, Liu D, Yang X, Wang F. Hypermethylation of multiple Wnt antagonist genes in gastric neoplasia: Is H pylori infection blasting fuse? Medicine (Baltimore). 2018;97:e13734. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 8] [Cited by in RCA: 8] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 103. | Koushyar S, Powell AG, Vincan E, Phesse TJ. Targeting Wnt Signaling for the Treatment of Gastric Cancer. Int J Mol Sci. 2020;21. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 41] [Cited by in RCA: 54] [Article Influence: 10.8] [Reference Citation Analysis (0)] |
| 104. | Togasaki K, Sugimoto S, Ohta Y, Nanki K, Matano M, Takahashi S, Fujii M, Kanai T, Sato T. Wnt Signaling Shapes the Histologic Variation in Diffuse Gastric Cancer. Gastroenterology. 2021;160:823-830. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 23] [Cited by in RCA: 46] [Article Influence: 11.5] [Reference Citation Analysis (0)] |
| 105. | Tan SH, Swathi Y, Tan S, Goh J, Seishima R, Murakami K, Oshima M, Tsuji T, Phuah P, Tan LT, Wong E, Fatehullah A, Sheng T, Ho SWT, Grabsch HI, Srivastava S, Teh M, Denil SLIJ, Mustafah S, Tan P, Shabbir A, So J, Yeoh KG, Barker N. AQP5 enriches for stem cells and cancer origins in the distal stomach. Nature. 2020;578:437-443. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 47] [Cited by in RCA: 92] [Article Influence: 18.4] [Reference Citation Analysis (0)] |
| 106. | Chiurillo MA. Role of the Wnt/β-catenin pathway in gastric cancer: An in-depth literature review. World J Exp Med. 2015;5:84-102. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 207] [Cited by in RCA: 248] [Article Influence: 24.8] [Reference Citation Analysis (0)] |
| 107. | Mao J, Fan S, Ma W, Fan P, Wang B, Zhang J, Wang H, Tang B, Zhang Q, Yu X, Wang L, Song B, Li L. Roles of Wnt/β-catenin signaling in the gastric cancer stem cells proliferation and salinomycin treatment. Cell Death Dis. 2014;5:e1039. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 189] [Cited by in RCA: 215] [Article Influence: 19.5] [Reference Citation Analysis (0)] |
| 108. | Yu Z, Jiang X, Qin L, Deng H, Wang J, Ren W, Li H, Zhao L, Liu H, Yan H, Shi W, Wang Q, Luo C, Long B, Zhou H, Sun H, Jiao Z. A novel UBE2T inhibitor suppresses Wnt/β-catenin signaling hyperactivation and gastric cancer progression by blocking RACK1 ubiquitination. Oncogene. 2021;40:1027-1042. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 49] [Cited by in RCA: 76] [Article Influence: 19.0] [Reference Citation Analysis (0)] |
| 109. | Chen Q, Suzuki K, Sifuentes-Dominguez L, Miyata N, Song J, Lopez A, Starokadomskyy P, Gopal P, Dozmorov I, Tan S, Ge B, Burstein E. Paneth cell-derived growth factors support tumorigenesis in the small intestine. Life Sci Alliance. 2021;4. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 3] [Cited by in RCA: 8] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 110. | Jorissen RN, Christie M, Mouradov D, Sakthianandeswaren A, Li S, Love C, Xu ZZ, Molloy PL, Jones IT, McLaughlin S, Ward RL, Hawkins NJ, Ruszkiewicz AR, Moore J, Burgess AW, Busam D, Zhao Q, Strausberg RL, Lipton L, Desai J, Gibbs P, Sieber OM. Wild-type APC predicts poor prognosis in microsatellite-stable proximal colon cancer. Br J Cancer. 2015;113:979-988. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 29] [Cited by in RCA: 34] [Article Influence: 3.4] [Reference Citation Analysis (0)] |
| 111. | Yue B, Liu C, Sun H, Liu M, Song C, Cui R, Qiu S, Zhong M. A Positive Feed-Forward Loop between LncRNA-CYTOR and Wnt/β-Catenin Signaling Promotes Metastasis of Colon Cancer. Mol Ther. 2018;26:1287-1298. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 91] [Cited by in RCA: 159] [Article Influence: 22.7] [Reference Citation Analysis (0)] |
| 112. | Ormanns S, Neumann J, Horst D, Kirchner T, Jung A. WNT signaling and distant metastasis in colon cancer through transcriptional activity of nuclear β-Catenin depend on active PI3K signaling. Oncotarget. 2014;5:2999-3011. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 45] [Cited by in RCA: 49] [Article Influence: 4.9] [Reference Citation Analysis (0)] |
| 113. | Reinacher-Schick A, Seidensticker F, Petrasch S, Reiser M, Philippou S, Theegarten D, Freitag G, Schmiegel W. Mesalazine changes apoptosis and proliferation in normal mucosa of patients with sporadic polyps of the large bowel. Endoscopy. 2000;32:245-254. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 69] [Cited by in RCA: 61] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 114. | Velayos FS, Loftus EV Jr, Jess T, Harmsen WS, Bida J, Zinsmeister AR, Tremaine WJ, Sandborn WJ. Predictive and protective factors associated with colorectal cancer in ulcerative colitis: A case-control study. Gastroenterology. 2006;130:1941-1949. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 288] [Cited by in RCA: 254] [Article Influence: 13.4] [Reference Citation Analysis (0)] |
| 115. | Velayos FS, Terdiman JP, Walsh JM. Effect of 5-aminosalicylate use on colorectal cancer and dysplasia risk: a systematic review and metaanalysis of observational studies. Am J Gastroenterol. 2005;100:1345-1353. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 426] [Cited by in RCA: 379] [Article Influence: 19.0] [Reference Citation Analysis (0)] |
| 116. | Magro F, Gionchetti P, Eliakim R, Ardizzone S, Armuzzi A, Barreiro-de Acosta M, Burisch J, Gecse KB, Hart AL, Hindryckx P, Langner C, Limdi JK, Pellino G, Zagórowicz E, Raine T, Harbord M, Rieder F; European Crohn’s and Colitis Organisation [ECCO]. Third European Evidence-based Consensus on Diagnosis and Management of Ulcerative Colitis. Part 1: Definitions, Diagnosis, Extra-intestinal Manifestations, Pregnancy, Cancer Surveillance, Surgery, and Ileo-anal Pouch Disorders. J Crohns Colitis. 2017;11:649-670. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1446] [Cited by in RCA: 1298] [Article Influence: 162.3] [Reference Citation Analysis (0)] |
| 117. | Bos CL, Diks SH, Hardwick JC, Walburg KV, Peppelenbosch MP, Richel DJ. Protein phosphatase 2A is required for mesalazine-dependent inhibition of Wnt/beta-catenin pathway activity. Carcinogenesis. 2006;27:2371-2382. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 60] [Cited by in RCA: 62] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 118. | Munding J, Ziebarth W, Pox CP, Ladigan S, Reiser M, Hüppe D, Brand L, Schmiegel W, Tannapfel A, Reinacher-Schick AC. The influence of 5-aminosalicylic acid on the progression of colorectal adenomas via the β-catenin signaling pathway. Carcinogenesis. 2012;33:637-643. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 29] [Cited by in RCA: 37] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 119. | Day DW, Morson BC. The adenoma-carcinoma sequence. Major Probl Pathol. 1978;10:58-71. [PubMed] |
| 120. | Serafino A, Moroni N, Zonfrillo M, Andreola F, Mercuri L, Nicotera G, Nunziata J, Ricci R, Antinori A, Rasi G, Pierimarchi P. WNT-pathway components as predictive markers useful for diagnosis, prevention and therapy in inflammatory bowel disease and sporadic colorectal cancer. Oncotarget. 2014;5:978-992. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 56] [Cited by in RCA: 54] [Article Influence: 4.9] [Reference Citation Analysis (0)] |
| 121. | Zhu GX, Gao D, Shao ZZ, Chen L, Ding WJ, Yu QF. Wnt/βcatenin signaling: Causes and treatment targets of drug resistance in colorectal cancer (Review). Mol Med Rep. 2021;23. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 11] [Cited by in RCA: 36] [Article Influence: 7.2] [Reference Citation Analysis (0)] |
| 122. | Hu YB, Yan C, Mu L, Mi YL, Zhao H, Hu H, Li XL, Tao DD, Wu YQ, Gong JP, Qin JC. Exosomal Wnt-induced dedifferentiation of colorectal cancer cells contributes to chemotherapy resistance. Oncogene. 2019;38:1951-1965. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 122] [Cited by in RCA: 157] [Article Influence: 26.2] [Reference Citation Analysis (0)] |
| 123. | Zhang ZM, Wu JF, Luo QC, Liu QF, Wu QW, Ye GD, She HQ, Li BA. Pygo2 activates MDR1 expression and mediates chemoresistance in breast cancer via the Wnt/β-catenin pathway. Oncogene. 2016;35:4787-4797. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 51] [Cited by in RCA: 75] [Article Influence: 8.3] [Reference Citation Analysis (0)] |
| 124. | Cao F, Yin LX. miR-122 enhances sensitivity of hepatocellular carcinoma to oxaliplatin via inhibiting MDR1 by targeting Wnt/β-catenin pathway. Exp Mol Pathol. 2019;106:34-43. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 28] [Cited by in RCA: 51] [Article Influence: 7.3] [Reference Citation Analysis (0)] |
| 125. | Zhong Z, Virshup DM. Wnt Signaling and Drug Resistance in Cancer. Mol Pharmacol. 2020;97:72-89. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 173] [Cited by in RCA: 166] [Article Influence: 33.2] [Reference Citation Analysis (0)] |
| 126. | Caspi M, Wittenstein A, Kazelnik M, Shor-Nareznoy Y, Rosin-Arbesfeld R. Therapeutic targeting of the oncogenic Wnt signaling pathway for treating colorectal cancer and other colonic disorders. Adv Drug Deliv Rev. 2021;169:118-136. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 36] [Cited by in RCA: 60] [Article Influence: 15.0] [Reference Citation Analysis (0)] |
| 127. | Kleeman SO, Koelzer VH, Jones HJ, Vazquez EG, Davis H, East JE, Arnold R, Koppens MA, Blake A, Domingo E, Cunningham C, Beggs AD, Pestinger V, Loughrey MB, Wang LM, Lannagan TR, Woods SL, Worthley D; Consortium SC; Tomlinson I, Dunne PD, Maughan T, Leedham SJ. Exploiting differential Wnt target gene expression to generate a molecular biomarker for colorectal cancer stratification. Gut. 2020;69:1092-1103. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 50] [Cited by in RCA: 55] [Article Influence: 11.0] [Reference Citation Analysis (0)] |
| 128. | Bernkopf DB, Brückner M, Hadjihannas MV, Behrens J. An aggregon in conductin/axin2 regulates Wnt/β-catenin signaling and holds potential for cancer therapy. Nat Commun. 2019;10:4251. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 11] [Cited by in RCA: 32] [Article Influence: 5.3] [Reference Citation Analysis (0)] |
| 129. | Kessler D, Mayer M, Zahn SK, Zeeb M, Wöhrle S, Bergner A, Bruchhaus J, Ciftci T, Dahmann G, Dettling M, Döbel S, Fuchs JE, Geist L, Hela W, Kofink C, Kousek R, Moser F, Puchner T, Rumpel K, Scharnweber M, Werni P, Wolkerstorfer B, Breitsprecher D, Baaske P, Pearson M, McConnell DB, Böttcher J. Getting a Grip on the Undrugged: Targeting β-Catenin with Fragment-Based Methods. ChemMedChem. 2021;16:1420-1424. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 14] [Cited by in RCA: 18] [Article Influence: 4.5] [Reference Citation Analysis (0)] |
| 130. | Liao H, Li X, Zhao L, Wang Y, Wang X, Wu Y, Zhou X, Fu W, Liu L, Hu HG, Chen YG. A PROTAC peptide induces durable β-catenin degradation and suppresses Wnt-dependent intestinal cancer. Cell Discov. 2020;6:35. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 53] [Cited by in RCA: 105] [Article Influence: 21.0] [Reference Citation Analysis (0)] |
| 131. | Liu X, Long Z, Cai H, Yu S, Wu J. TRIM58 suppresses the tumor growth in gastric cancer by inactivation of β-catenin signaling via ubiquitination. Cancer Biol Ther. 2020;21:203-212. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 20] [Cited by in RCA: 23] [Article Influence: 3.8] [Reference Citation Analysis (0)] |
| 132. | Xiao B, Zhang L, Liu H, Fang H, Wang C, Huang B, Liu X, Zhou X, Wang Y. Oncolytic Adenovirus CD55-Smad4 Suppresses Cell Proliferation, Metastasis, and Tumor Stemness in Colorectal Cancer by Regulating Wnt/β-Catenin Signaling Pathway. Biomedicines. 2020;8. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 22] [Cited by in RCA: 18] [Article Influence: 3.6] [Reference Citation Analysis (0)] |
| 133. | Zhang J, Lai W, Li Q, Yu Y, Jin J, Guo W, Zhou X, Liu X, Wang Y. A novel oncolytic adenovirus targeting Wnt signaling effectively inhibits cancer-stem like cell growth via metastasis, apoptosis and autophagy in HCC models. Biochem Biophys Res Commun. 2017;491:469-477. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 33] [Cited by in RCA: 53] [Article Influence: 6.6] [Reference Citation Analysis (0)] |
| 134. | Raveux A, Stedman A, Coqueran S, Vandormael-Pournin S, Owens N, Romagnolo B, Cohen-Tannoudji M. Compensation between Wnt-driven tumorigenesis and cellular responses to ribosome biogenesis inhibition in the murine intestinal epithelium. Cell Death Differ. 2020;27:2872-2887. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4] [Cited by in RCA: 5] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 135. | Bhaskar Rao D, Devanandan HJ, Ganesan K. Identification of kinases and kinase inhibitors for the differential targeting of Wnt/β-catenin signaling in gastric cancer subtypes. Drug Dev Res. 2021;82:1182-1192. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 1] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 136. | Han T, Goswami S, Hu Y, Tang F, Zafra MP, Murphy C, Cao Z, Poirier JT, Khurana E, Elemento O, Hechtman JF, Ganesh K, Yaeger R, Dow LE. Lineage Reversion Drives WNT Independence in Intestinal Cancer. Cancer Discov. 2020;10:1590-1609. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 54] [Cited by in RCA: 59] [Article Influence: 11.8] [Reference Citation Analysis (0)] |
| 137. | Shi F, Cheng YF, Wang XL, Edge AS. Beta-catenin up-regulates Atoh1 expression in neural progenitor cells by interaction with an Atoh1 3' enhancer. J Biol Chem. 2010;285:392-400. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 86] [Cited by in RCA: 100] [Article Influence: 6.3] [Reference Citation Analysis (0)] |
| 138. | Castillo-Azofeifa D, Fazio EN, Nattiv R, Good HJ, Wald T, Pest MA, de Sauvage FJ, Klein OD, Asfaha S. Atoh1+ secretory progenitors possess renewal capacity independent of Lgr5+ cells during colonic regeneration. EMBO J. 2019;38. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 41] [Cited by in RCA: 60] [Article Influence: 10.0] [Reference Citation Analysis (0)] |
| 139. | Jho EH, Zhang T, Domon C, Joo CK, Freund JN, Costantini F. Wnt/beta-catenin/Tcf signaling induces the transcription of Axin2, a negative regulator of the signaling pathway. Mol Cell Biol. 2002;22:1172-1183. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1301] [Cited by in RCA: 1404] [Article Influence: 61.0] [Reference Citation Analysis (0)] |
| 140. | Lapham A, Adams JE, Paterson A, Lee M, Brimmell M, Packham G. The Bcl-w promoter is activated by beta-catenin/TCF4 in human colorectal carcinoma cells. Gene. 2009;432:112-117. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 22] [Cited by in RCA: 24] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 141. | Kim PJ, Plescia J, Clevers H, Fearon ER, Altieri DC. Survivin and molecular pathogenesis of colorectal cancer. Lancet. 2003;362:205-209. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 247] [Cited by in RCA: 259] [Article Influence: 11.8] [Reference Citation Analysis (0)] |
| 142. | Kim JS, Crooks H, Dracheva T, Nishanian TG, Singh B, Jen J, Waldman T. Oncogenic beta-catenin is required for bone morphogenetic protein 4 expression in human cancer cells. Cancer Res. 2002;62:2744-2748. [PubMed] |
| 143. | Tetsu O, McCormick F. Beta-catenin regulates expression of cyclin D1 in colon carcinoma cells. Nature. 1999;398:422-426. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2796] [Cited by in RCA: 2853] [Article Influence: 109.7] [Reference Citation Analysis (0)] |
| 144. | Wassermann S, Scheel SK, Hiendlmeyer E, Palmqvist R, Horst D, Hlubek F, Haynl A, Kriegl L, Reu S, Merkel S, Brabletz T, Kirchner T, Jung A. p16INK4a is a beta-catenin target gene and indicates low survival in human colorectal tumors. Gastroenterology. 2009;136:196-205.e2. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 36] [Cited by in RCA: 35] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 145. | Lickert H, Domon C, Huls G, Wehrle C, Duluc I, Clevers H, Meyer BI, Freund JN, Kemler R. Wnt/(beta)-catenin signaling regulates the expression of the homeobox gene Cdx1 in embryonic intestine. Development. 2000;127:3805-3813. [PubMed] |
| 146. | Zhao T, Gan Q, Stokes A, Lassiter RN, Wang Y, Chan J, Han JX, Pleasure DE, Epstein JA, Zhou CJ. β-catenin regulates Pax3 and Cdx2 for caudal neural tube closure and elongation. Development. 2014;141:148-157. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 53] [Cited by in RCA: 63] [Article Influence: 5.3] [Reference Citation Analysis (0)] |
| 147. | Niida A, Hiroko T, Kasai M, Furukawa Y, Nakamura Y, Suzuki Y, Sugano S, Akiyama T. DKK1, a negative regulator of Wnt signaling, is a target of the beta-catenin/TCF pathway. Oncogene. 2004;23:8520-8526. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 408] [Cited by in RCA: 448] [Article Influence: 21.3] [Reference Citation Analysis (0)] |
| 148. | Pendás-Franco N, García JM, Peña C, Valle N, Pálmer HG, Heinäniemi M, Carlberg C, Jiménez B, Bonilla F, Muñoz A, González-Sancho JM. DICKKOPF-4 is induced by TCF/beta-catenin and upregulated in human colon cancer, promotes tumour cell invasion and angiogenesis and is repressed by 1alpha,25-dihydroxyvitamin D3. Oncogene. 2008;27:4467-4477. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 128] [Cited by in RCA: 131] [Article Influence: 7.7] [Reference Citation Analysis (0)] |
| 149. | Batlle E, Henderson JT, Beghtel H, van den Born MM, Sancho E, Huls G, Meeldijk J, Robertson J, van de Wetering M, Pawson T, Clevers H. Beta-catenin and TCF mediate cell positioning in the intestinal epithelium by controlling the expression of EphB/ephrinB. Cell. 2002;111:251-263. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 857] [Cited by in RCA: 860] [Article Influence: 37.4] [Reference Citation Analysis (0)] |
| 150. | Beisner J, Teltschik Z, Ostaff MJ, Tiemessen MM, Staal FJ, Wang G, Gersemann M, Perminow G, Vatn MH, Schwab M, Stange EF, Wehkamp J. TCF-1-mediated Wnt signaling regulates Paneth cell innate immune defense effectors HD-5 and -6: implications for Crohn's disease. Am J Physiol Gastrointest Liver Physiol. 2014;307:G487-G498. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 28] [Cited by in RCA: 36] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 151. | Li Y, Bavarva JH, Wang Z, Guo J, Qian C, Thibodeau SN, Golemis EA, Liu W. HEF1, a novel target of Wnt signaling, promotes colonic cell migration and cancer progression. Oncogene. 2011;30:2633-2643. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 75] [Cited by in RCA: 73] [Article Influence: 5.2] [Reference Citation Analysis (0)] |
| 152. | Kay SK, Harrington HA, Shepherd S, Brennan K, Dale T, Osborne JM, Gavaghan DJ, Byrne HM. The role of the Hes1 crosstalk hub in Notch-Wnt interactions of the intestinal crypt. PLoS Comput Biol. 2017;13:e1005400. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 41] [Cited by in RCA: 37] [Article Influence: 4.6] [Reference Citation Analysis (0)] |
| 153. | Rodilla V, Villanueva A, Obrador-Hevia A, Robert-Moreno A, Fernández-Majada V, Grilli A, López-Bigas N, Bellora N, Albà MM, Torres F, Duñach M, Sanjuan X, Gonzalez S, Gridley T, Capella G, Bigas A, Espinosa L. Jagged1 is the pathological link between Wnt and Notch pathways in colorectal cancer. Proc Natl Acad Sci U S A. 2009;106:6315-6320. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 279] [Cited by in RCA: 306] [Article Influence: 19.1] [Reference Citation Analysis (0)] |
| 154. | Mann B, Gelos M, Siedow A, Hanski ML, Gratchev A, Ilyas M, Bodmer WF, Moyer MP, Riecken EO, Buhr HJ, Hanski C. Target genes of beta-catenin-T cell-factor/Lymphoid-enhancer-factor signaling in human colorectal carcinomas. Proc Natl Acad Sci U S A. 1999;96:1603-1608. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 608] [Cited by in RCA: 612] [Article Influence: 23.5] [Reference Citation Analysis (0)] |
| 155. | Wisdom R, Johnson RS, Moore C. c-Jun regulates cell cycle progression and apoptosis by distinct mechanisms. EMBO J. 1999;18:188-197. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 494] [Cited by in RCA: 527] [Article Influence: 20.3] [Reference Citation Analysis (0)] |
| 156. | Barker N, van Es JH, Kuipers J, Kujala P, van den Born M, Cozijnsen M, Haegebarth A, Korving J, Begthel H, Peters PJ, Clevers H. Identification of stem cells in small intestine and colon by marker gene Lgr5. Nature. 2007;449:1003-1007. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3854] [Cited by in RCA: 4336] [Article Influence: 240.9] [Reference Citation Analysis (0)] |
| 157. | Boon EM, van der Neut R, van de Wetering M, Clevers H, Pals ST. Wnt signaling regulates expression of the receptor tyrosine kinase met in colorectal cancer. Cancer Res. 2002;62:5126-5128. [PubMed] |
| 158. | He TC, Sparks AB, Rago C, Hermeking H, Zawel L, da Costa LT, Morin PJ, Vogelstein B, Kinzler KW. Identification of c-MYC as a target of the APC pathway. Science. 1998;281:1509-1512. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3533] [Cited by in RCA: 3604] [Article Influence: 133.5] [Reference Citation Analysis (0)] |
| 159. | Jung HC, Kim K. Identification of MYCBP as a beta-catenin/LEF-1 target using DNA microarray analysis. Life Sci. 2005;77:1249-1262. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 26] [Cited by in RCA: 31] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 160. | Ungerbäck J, Elander N, Grünberg J, Sigvardsson M, Söderkvist P. The Notch-2 gene is regulated by Wnt signaling in cultured colorectal cancer cells. PLoS One. 2011;6:e17957. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 44] [Cited by in RCA: 52] [Article Influence: 3.7] [Reference Citation Analysis (0)] |
| 161. | Dehner M, Hadjihannas M, Weiske J, Huber O, Behrens J. Wnt signaling inhibits Forkhead box O3a-induced transcription and apoptosis through up-regulation of serum- and glucocorticoid-inducible kinase 1. J Biol Chem. 2008;283:19201-19210. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 85] [Cited by in RCA: 96] [Article Influence: 5.6] [Reference Citation Analysis (0)] |
| 162. | Blache P, van de Wetering M, Duluc I, Domon C, Berta P, Freund JN, Clevers H, Jay P. SOX9 is an intestine crypt transcription factor, is regulated by the Wnt pathway, and represses the CDX2 and MUC2 genes. J Cell Biol. 2004;166:37-47. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 354] [Cited by in RCA: 398] [Article Influence: 19.0] [Reference Citation Analysis (0)] |
| 163. | Konsavage WM Jr, Kyler SL, Rennoll SA, Jin G, Yochum GS. Wnt/β-catenin signaling regulates Yes-associated protein (YAP) gene expression in colorectal carcinoma cells. J Biol Chem. 2012;287:11730-11739. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 196] [Cited by in RCA: 237] [Article Influence: 18.2] [Reference Citation Analysis (0)] |
| 164. | Wei W, Chua MS, Grepper S, So SK. Blockade of Wnt-1 signaling leads to anti-tumor effects in hepatocellular carcinoma cells. Mol Cancer. 2009;8:76. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 69] [Cited by in RCA: 83] [Article Influence: 5.2] [Reference Citation Analysis (0)] |
| 165. | Shah K, Panchal S, Patel B. Porcupine inhibitors: Novel and emerging anti-cancer therapeutics targeting the Wnt signaling pathway. Pharmacol Res. 2021;167:105532. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 17] [Cited by in RCA: 74] [Article Influence: 18.5] [Reference Citation Analysis (0)] |
| 166. | Dunbar K, Valanciute A, Lima ACS, Vinuela PF, Jamieson T, Rajasekaran V, Blackmur J, Ochocka-Fox AM, Guazzelli A, Cammareri P, Arends MJ, Sansom OJ, Myant KB, Farrington SM, Dunlop MG, Din FVN. Aspirin Rescues Wnt-Driven Stem-like Phenotype in Human Intestinal Organoids and Increases the Wnt Antagonist Dickkopf-1. Cell Mol Gastroenterol Hepatol. 2021;11:465-489. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 19] [Cited by in RCA: 21] [Article Influence: 4.2] [Reference Citation Analysis (0)] |
| 167. | Novellasdemunt L, Kucharska A, Jamieson C, Prange-Barczynska M, Baulies A, Antas P, van der Vaart J, Gehart H, Maurice MM, Li VS. NEDD4 and NEDD4L regulate Wnt signalling and intestinal stem cell priming by degrading LGR5 receptor. EMBO J. 2020;39:e102771. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 62] [Cited by in RCA: 74] [Article Influence: 14.8] [Reference Citation Analysis (0)] |
| 168. | Nile AH, de Sousa E Melo F, Mukund S, Piskol R, Hansen S, Zhou L, Zhang Y, Fu Y, Gogol EB, Kömüves LG, Modrusan Z, Angers S, Franke Y, Koth C, Fairbrother WJ, Wang W, de Sauvage FJ, Hannoush RN. A selective peptide inhibitor of Frizzled 7 receptors disrupts intestinal stem cells. Nat Chem Biol. 2018;14:582-590. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 32] [Cited by in RCA: 59] [Article Influence: 8.4] [Reference Citation Analysis (0)] |
| 169. | Gurney A, Axelrod F, Bond CJ, Cain J, Chartier C, Donigan L, Fischer M, Chaudhari A, Ji M, Kapoun AM, Lam A, Lazetic S, Ma S, Mitra S, Park IK, Pickell K, Sato A, Satyal S, Stroud M, Tran H, Yen WC, Lewicki J, Hoey T. Wnt pathway inhibition via the targeting of Frizzled receptors results in decreased growth and tumorigenicity of human tumors. Proc Natl Acad Sci U S A. 2012;109:11717-11722. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 378] [Cited by in RCA: 483] [Article Influence: 37.2] [Reference Citation Analysis (0)] |
| 170. | Grandy D, Shan J, Zhang X, Rao S, Akunuru S, Li H, Zhang Y, Alpatov I, Zhang XA, Lang RA, Shi DL, Zheng JJ. Discovery and characterization of a small molecule inhibitor of the PDZ domain of dishevelled. J Biol Chem. 2009;284:16256-16263. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 163] [Cited by in RCA: 155] [Article Influence: 9.7] [Reference Citation Analysis (0)] |
| 171. | Alula KM, Delgado-Deida Y, Jackson DN, Venuprasad K, Theiss AL. Nuclear partitioning of Prohibitin 1 inhibits Wnt/β-catenin-dependent intestinal tumorigenesis. Oncogene. 2021;40:369-383. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 3] [Cited by in RCA: 11] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 172. | Mizutani A, Yashiroda Y, Muramatsu Y, Yoshida H, Chikada T, Tsumura T, Okue M, Shirai F, Fukami T, Yoshida M, Seimiya H. RK-287107, a potent and specific tankyrase inhibitor, blocks colorectal cancer cell growth in a preclinical model. Cancer Sci. 2018;109:4003-4014. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 44] [Cited by in RCA: 63] [Article Influence: 9.0] [Reference Citation Analysis (0)] |
| 173. | Tian M, Wang X, Sun J, Lin W, Chen L, Liu S, Wu X, Shi L, Xu P, Cai X. IRF3 prevents colorectal tumorigenesis via inhibiting the nuclear translocation of β-catenin. Nat Commun. 2020;11:5762. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 68] [Reference Citation Analysis (0)] |
| 174. | Yan M, Li G, An J. Discovery of small molecule inhibitors of the Wnt/β-catenin signaling pathway by targeting β-catenin/Tcf4 interactions. Exp Biol Med (Maywood). 2017;242:1185-1197. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 51] [Cited by in RCA: 69] [Article Influence: 8.6] [Reference Citation Analysis (0)] |