Published online Feb 24, 2022. doi: 10.5306/wjco.v13.i2.147
Peer-review started: June 22, 2021
First decision: July 16, 2021
Revised: August 4, 2021
Accepted: January 17, 2022
Article in press: January 17, 2022
Published online: February 24, 2022
Processing time: 245 Days and 11.3 Hours
The targeted therapy cetuximab [directed at the epidermal growth factor receptor (EGFR)] in combination with 5-fluorouracil and platinum-based chemotherapy (the EXTREME regimen) has shown substantial efficacy for patients with recurrent or metastatic squamous cell carcinoma of the head and neck (R/M SCCHN). Thus, this scheme has been established as the preferred first-line option for these patients. However, more recently, a new strategy combining platinum, taxanes, and cetuximab (the TPEx regimen) has demonstrated similar efficacy with a more favorable toxicity profile in clinical trials.
To evaluate the safety and efficacy of the TPEx scheme as first-line therapy in advanced SCCHN in a multicenter cohort study.
This retrospective multicenter cohort study included patients with histologically confirmed recurrent or metastatic SCCHN treated with first-line TPEx at five medical centers in Argentina between January 1, 2017 and April 31, 2020. Chemotherapy consisted of four cycles of docetaxel, cisplatin, and cetuximab followed by cetuximab maintenance therapy. Clinical outcomes and toxicity profiles were collected from medical charts. Treatment response was assessed by the investigator in accordance with Response Evaluation Criteria in Solid Tumors (version 1.1). Adverse events were graded according to the National Cancer Institute Common Terminology Criteria for Adverse Events (version 4.0).
Twenty-four patients were included. The median age at diagnosis was 58 years (range: 36-77 years). The majority of patients (83.3%) received at least four chemotherapy cycles in the initial phase. In the included group, the overall response rate was 62.5%, and 3 patients achieved a complete response (12.5%). The median time to response was 2.4 mo [95% confidence interval (CI): 1.3-3.5]. With a median follow-up of 12.7 mo (95%CI: 8.8-16.6), the median progression-free survival (PFS) was 6.9 mo (95%CI: 6.5-7.3), and the overall survival rate at 12 mo was 82.4%. Patients with documented tumor response showed a better PFS than those with disease stabilization or progression [8.5 mo (95%CI: 5.5-11.5) and 4.5 mo (95%CI: 2.5-6.6), respectively; P = 0.042]. Regarding the safety analysis, two-thirds of patients reported at least one treatment-related adverse event, and 25% presented grade 3 toxicities. Of note, no patient experienced grade 4 adverse events.
TPEx was an adequately tolerated regimen in our population, with low incidence of grade 3-4 adverse events. The median PFS were consistent with those in recent reports of clinical trials evaluating this treatment combination. This regimen may be considered an attractive therapeutic strategy due to its simplified administration, decreased total number of chemotherapy cycles, and treatment tolerability.
Core Tip: We evaluated the safety and efficacy of the combination platinum, taxanes, and cetuximab scheme as a first-line therapy for patients with recurrent or metastatic squamous cell carcinoma of the head and neck in a real-world setting. Among the 24 patients included, the median progression-free survival was 6.9 mo (95% confidence interval: 6.5-7.3), and the overall survival rate at 12 mo was 82.4%, which was consistent with previous clinical trials. Patients with documented tumor response showed statistically better progression-free survival than those with disease stabilization or progression (P = 0.034). The combination platinum, taxanes, and cetuximab regimen was adequately tolerated by most of the analyzed patients, as the incidence of grade 3-4 adverse events was surprisingly lower than expected (25%).
- Citation: Falco A, Leiva M, Blanco A, Cefarelli G, Rodriguez A, Melo J, Cayol F, Rizzo MM, Sola A, Rodríguez Montani H, Chacon M, Enrico D, Waisberg F. First-line cisplatin, docetaxel, and cetuximab for patients with recurrent or metastatic head and neck cancer: A multicenter cohort study. World J Clin Oncol 2022; 13(2): 147-158
- URL: https://www.wjgnet.com/2218-4333/full/v13/i2/147.htm
- DOI: https://dx.doi.org/10.5306/wjco.v13.i2.147
Squamous cell carcinoma of the head and neck (SCCHN) cases represent 5% of all newly diagnosed cancer cases, leading to over 300000 deaths per year[1]. Despite appropriate primary treatments, in approximately 50% to 60% of patients with stage III to IV disease locoregional relapse occurs[2]. Given that a significant proportion of these patients are not suitable for surgery or radiotherapy, systemic treatments and best supportive care are the preferred therapeutic options.
Up to the early 2000s, the median overall survival (OS) of patients with metastatic disease was only 6 mo[3,4]. This poor prognosis encouraged significant research efforts to develop novel drugs in the last 15 years. In this setting, the targeted therapy cetuximab [directed at the epidermal growth factor receptor (EGFR)] has shown substantial efficacy for recurrent or metastatic (R/M) SCCHN treatment in combination with 5-fluorouracil and platinum-based chemotherapy (the EXTREME regimen)[3]. More recently, a new strategy using the immune checkpoint inhibitor pembrolizumab alone or in combination with 5-fluorouracil and platinum has become an appropriate first-line treatment for R/M SCCHN patients[5-7].
Currently, the EXTREME regimen still represents a recommended first-line treatment option in selected scenarios, such as cases with programmed death-ligand 1-negative tumors or when immunotherapy is contraindicated. Notably, this treatment regimen may represent an attractive approach for patients with disease progression after first-line immune checkpoint inhibitors are given as monotherapy[8].
Taxanes have maintained widespread clinical use, particularly in solid tumors since their discovery in the early 1970s, and several clinical trials have shown their antineoplastic activity against SCCHN[9-11]. The addition of fluorouracil to a taxane seeks to take advantage of the potential immunogenic and proapoptotic synergy between cetuximab and docetaxel or paclitaxel[12,13]. Cetuximab-, platinum-, and taxane-based schedules have been associated with promising survival results and cytoreductive properties in clinical studies[14-18]. TPExtreme was the first large, phase 3, randomized trial comparing the TPEx regimen (cetuximab, taxane, and platinum) with the EXTREME scheme in a first-line setting[19]. This trial demonstrated similar efficacy outcomes in 539 R/M HNSCC patients, showing a median OS of 14.5 and 13.4 mo using the TPEx and EXTREME regimens, respectively. Furthermore, the TPEx arm had a more favorable toxicity profile, leading to better compliance of the planned treatment (72% vs 44%) and fewer dose interruptions (10% vs 27%).
Based on these considerations and given the scarce real-world studies including patients treated with this scheme, we retrospectively evaluated the efficacy and safety of the TPEx regimen as first-line therapy in patients with R/M SCCHN.
This retrospective multicenter cohort study included patients seen between January 1, 2017 and April 31, 2020 with a histologically confirmed diagnosis of R/M SCCHN who received TPEx as first-line treatment at five medical centers in Argentina. Chemotherapy consisted of four cycles of docetaxel 75 mg/m² and cisplatin 75 mg/m² every 3 wk and cetuximab (400 mg/m2 on day 1 of cycle 1 and then 250 mg/m2 weekly), with systemic granulocyte colony-stimulating factor support during each cycle. Patients with controlled disease continued with weekly cetuximab 250 mg/m2 or cetuximab 500 mg/m² every 2 wk as maintenance until disease progression or unacceptable toxicity. Demographic and clinicopathological characteristics, including age, Eastern Cooperative Oncology Group performance status, smoking status, alcohol consumption, primary tumor site, and previous treatments, were collected from medical charts and entered into a predefined centralized database. Efficacy and safety information was also retrieved, and treatment strategies, responses, adverse events, and discontinuation were also documented.
Disease progression and treatment response were collected from medical charts. Treatment response was assessed by the investigator using computed tomography or magnetic resonance imaging scans in accordance with Response Evaluation Criteria in Solid Tumors (RECIST version 1.1). Adverse events were graded according to the National Cancer Institute Common Terminology Criteria for Adverse Events (version 4.0). The study was reviewed by our expert biostatistician Santiago Duarte, MD.
Data are summarized as frequencies and percentages for categorical variables and as medians, ranges, and interquartile ranges for continuous variables. The progression-free survival (PFS) and OS of patients treated with TPEx as first-line treatment were calculated from the date of therapy initiation to first documented relapse (PFS) or death due to any cause (OS). Data were censored at the last follow-up if the patient was alive. The duration of response (DOR) was defined as the time from the first complete response (CR) or partial response to progressive disease or death. Survival curves were generated using the Kaplan-Meier method, and differences between groups were calculated using the log-rank test. Relevant prognostic factors were stratified by univariate Cox regression models for PFS. All statistical analyses were performed using SPSS software version 23.0 (SPSS, Inc., Armonk, NY, United States).
In this multicenter retrospective study, 24 patients with R/M SCCHN were included from five Argentinian medical centers. All patients received first-line chemotherapy with TPEx. The median age at diagnosis was 58 years (range: 36-77), males made up 62.5% of the population (n = 15), and the majority of patients had an Eastern Cooperative Oncology Group score of 0-1 (22, 91.7%) (Table 1). A smoking history was reported in 13 patients (54.2%), and approximately one-third of the patients reported alcohol consumption. Of note, only 2 patients (8.3%) had a body mass index < 18.5.
| Characteristics | Number of patients (%) |
| Total | 24 |
| Median age (range), yr | 58 (36-77) |
| Sex | |
| Male | 15 (62.5) |
| Female | 9 (37.5) |
| ECOG at TPEx treatment initiation | |
| 0-1 | 22 (91.7) |
| 2 | 2 (8.3) |
| Smoking history | |
| Never | 6 (25) |
| Current or former | 13 (54.2) |
| NS | 5 (20.8) |
| Alcohol consumption | |
| Occasional or regular | 8 (33.3) |
| None | 7 (29.2) |
| NS | 9 (37.5) |
| Primary tumor site | |
| Larynx | 7 (29.2) |
| Oropharynx | 6 (25) |
| Oral cavity | 5 (20.8) |
| Hypopharynx | 1 (4.2) |
| Other | 5 (20.8) |
| Previous treatment | |
| Concomitant chemoradiotherapy only | 8 (33.3) |
| Surgery only | 5 (20.8) |
| Surgery + concomitant chemoradiotherapy | 5 (20.8) |
| Surgery + radiotherapy | 3 (12.5) |
| Radiotherapy only | 1 (4.2) |
| No | 2 (8.3) |
| Extent of disease at TPEx treatment initiation | |
| Locoregional recurrence only | 14 (58.3) |
| Locoregional recurrence + distant metastasis | 5 (20.8) |
| Metastatic disease | 5 (20.8) |
| Time from initial diagnosis to recurrence(Median, IQR), mo | 16.2 (5.4-37.5) |
| Metastatic or unresectable disease at diagnosis | 11 (45.8) |
Previous treatments included definitive concomitant chemoradiotherapy (33.3%), surgery (20.8%), surgery plus radiotherapy (12.5%), chemoradiotherapy (20.8%), and definitive radiotherapy alone (4.2%). Approximately half of the population had previously received cisplatin (n = 13, 54.2%), and only 2 patients (8.3%) had metastatic disease at diagnosis. The most common reason for treatment discontinuation was disease progression (58.3%), and only 2 patients (8.3%) discontinued treatment prematurely due to unacceptable toxicity. Notably, most patients (83.3%) received at least four chemotherapy cycles during induction therapy.
A total of 3 patients achieved a complete response (12.5%), and in half of the patients, a partial response was documented (Table 2). Remarkably, most of the patients benefited from TPEx therapy since the overall response rate (ORR) and disease control rate (DCR) were 62.5% and 87.5%, respectively. The median time to response was 2.4 mo [95% confidence interval (CI): 1.3-3.5).
| TPEx (n = 24) | |
| Type of response, n (%) | |
| Complete | 3 (12.5) |
| Partial | 12 (50) |
| Stable disease | 6 (25) |
| Progression | 1 (4.2) |
| Nonassessable | 2 (8.3) |
| Objective response rate - % of patients (95%CI)1 | 62.5 |
| Disease-control rate - % of patients (95%CI)2 | 87.5 |
| Time to response – mo3 | |
| Median (95%CI) | 2.4 (1.3-3.5) |
| Duration of response – mo4 | |
| Median (95%CI) | 5.1 (3.0-7.2) |
No statistical differences were observed in terms of ORR or DCR between patients with only locoregional recurrence prior to TEPx initiation and the rest of the included patients [ORR 50% (7/14), DCR 85.7% (12/14) and ORR 80% (8/10), DCR 90% (9/10), respectively; P = 0.21 and P = 1.0].
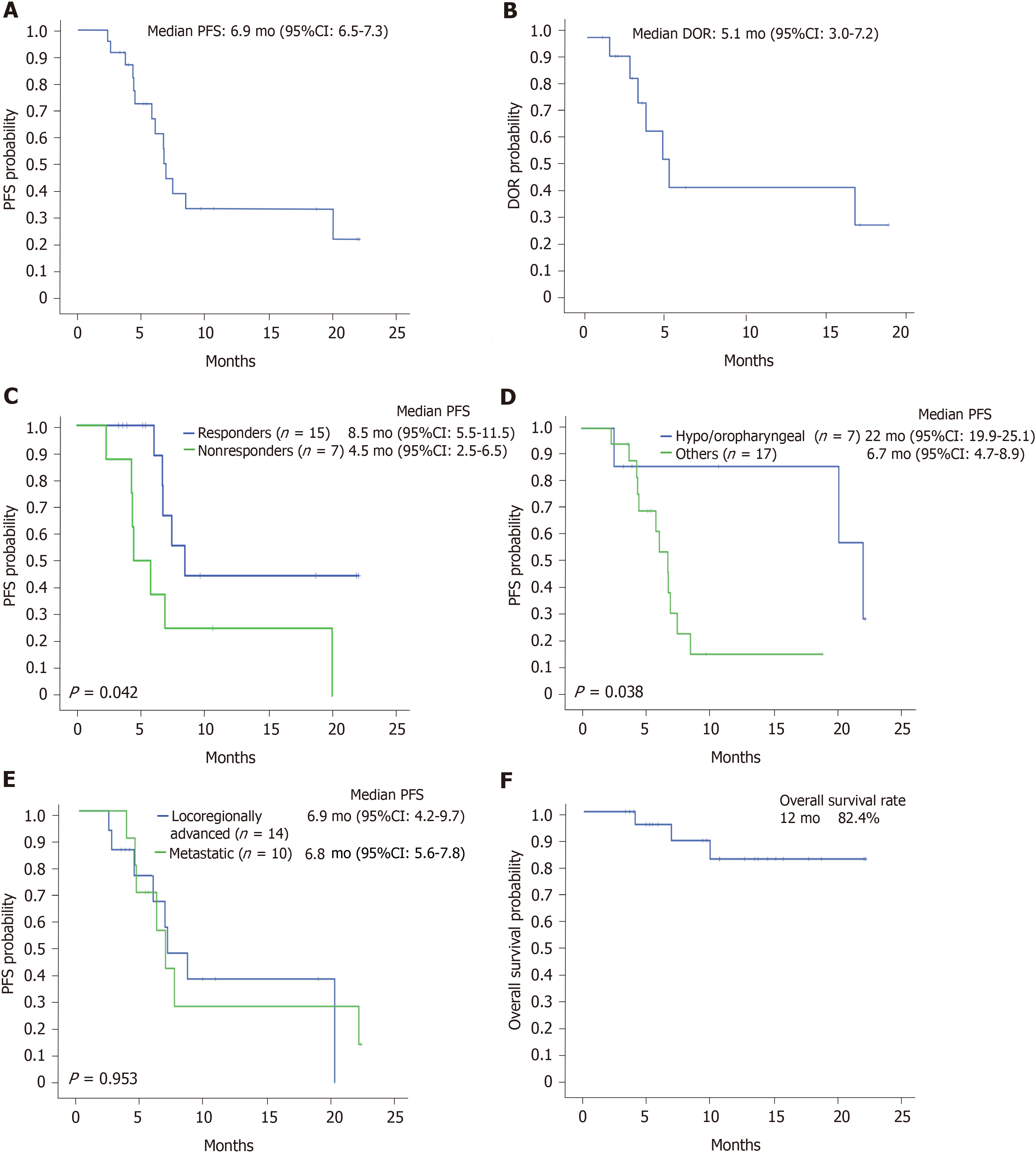
After a median follow-up of 12.7 mo (95%CI: 8.8-16.6), 14 progression events occurred. The median PFS and DOR were 6.9 mo (95%CI: 6.5-7.3) (Figure 1A) and 5.1 mo (95%CI: 3.0-7.2), respectively (Figure 1B). Univariate relevant prognostic factor analyses for first-line TPEx PFS are reported in Table 3. As expected, patients with documented tumor response showed a better PFS than those with disease stabilization or progression [8.5 mo (95%CI: 5.5-11.5) and 4.5 mo (95%CI: 2.5-6.5), respectively; P = 0.042] (Figure 1C). Notably, in 2 out of the 3 patients with documented CR, substantially longer PFS (22.3 and 18.8 mo) and DOR (16.6 and 16.9) were observed. Patients with hypo/oropharyngeal tumors had a better PFS compared to those with other primary sites [22 mo (95%CI: 19.9-25.1) and 6.7 mo (95%CI: 4.7-8.9), respectively; P = 0.038] (Figure 1D). No difference was observed when comparing patients with advanced and metastatic disease (P = 0.953) (Figure 1E). The OS rate at 12 mo was 82.4% (Figure 1F). Remarkably, among the 14 patients who experienced disease progression on TPEx, 13 received second-line treatment with immunotherapy [pembrolizumab (n = 9) and nivolumab (n = 4)].
| Variable | HR (95%CI) | P value | Median PFS (95%CI) |
| ECOG(0 vs 1-2) | 0.91 (0.30-2.80) | 0.87 | 6.9 mo (5.1-8.8) vs 6.8 mo (4.7-8.9) |
| Primary tumor site (Hypo/oropharyngeal vs Others) | 0.15 (0.02-1.17) | 0.04 | 22 mo (19.9-25.1) vs 6.7 mo (4.7-8.9) |
| Response (Responders vs Nonresponders) | 0.34 (0.12–0.97) | 0.04 | 8.5 mo (5.5-11.5) vs 4.5 mo (2.5-6.5) |
| Extent of disease at TPEx initiation (Locoregionally advanced vs Metastatic) | 0.95 (0.33-2.85) | 0.95 | 6.9 mo (4.2-9.7) vs 6.8 mo (5.6-7.8) |
| Relapse-free survival of the primary treatment (≤ 24 vs > 24 mo)1 | 0.37 (0.11-1.21) | 0.09 | 6.1 mo (3.6-8.6) vs 8.5 mo (4.5-12.5) |
| Previous treatment2(Multimodality vs Unimodality) | 0.44 (0.14–1.41) | 0.17 | 7.5 mo (6.3-8.7) vs 6.1 mo (2.7-9.4) |
| Treatment interruption, discontinuation, or dose reduction (Yes vs No) | 1.15 (0.39-3.41) | 0.80 | 6.9 mo (6.4-7.4) vs 6.8 mo (5.0-8.5) |
| Adverse events (Grade 1-2 vs 3-4) | 0.74 (0.23-2.44) | 0.62 | 6.9 mo (5.2-8.6) vs 6.7 mo (0.3-13.9) |
Two-thirds of the patients reported at least one treatment-related adverse event, and 25% reported at least one grade 3 adverse event. Of note, no patient experienced grade 4 toxicity. A summary of the safety profile is listed in Table 4. The most commonly reported hematological adverse events were febrile neutropenia (12.5%), anemia (12.5%), and hyponatremia/hypokalemia (12.5%). Among nonhematological events, acne-like rash was the most frequent (33.3%) related adverse event. Grade 3 nausea-vomiting, asthenia, and renal failure were noted in 4.2% of the patients. Only 1 patient experienced a grade 1 hypersensitivity reaction during taxane infusion.
| Event, n (%) | TPEx (n = 24) | ||
| Any grade | Grade 3 | Grade 4 | |
| Any treatment-related adverse event1 | 18 (75) | 6 (25) | 0 |
| Hematological | |||
| Febrile neutropenia | 3 (12.5) | 3 (12.5) | 0 |
| Anemia | 3 (12.5) | 0 | |
| Hyponatremia and/or hypokalemia | 3 (12.5) | 2 (8.3) | 0 |
| Hypomagnesemia | 2 (8.3) | 1 (4.2) | 0 |
| Thrombocytopenia | 1 (4.2) | 0 | 0 |
| Nonhematological | |||
| Acne-like rash | 8 (33.3) | 0 | 0 |
| Nausea - vomiting | 4 (16.7) | 1 (4.2) | 0 |
| Asthenia | 4 (16.7) | 1 (4.2) | 0 |
| Diarrhea | 2 (8.3) | 0 | 0 |
| Renal failure | 1 (4.2) | 1 (4.2) | 0 |
| Hypersensitivity | 1 (4.2) | 0 | |
| Oral mucositis | 1 (4.2) | 0 | 0 |
| Any serious adverse event2 | - | 5 (20.8) | 0 |
| Treatment-related death | 0 | - | - |
| Event leading to interruption of any treatment component3 | 3 (12.5) | - | - |
| Chemotherapy | 2 (8.3) | - | - |
| Cetuximab | 1 (4.2) | - | - |
| Event leading to discontinuation of any treatment component3 | 2 (8.3) | - | - |
| Chemotherapy | 2 (8.3) | - | - |
| Cetuximab | 0 | ||
| Event leading to dose reduction | 2 (8.3) | - | - |
Overall, serious adverse events were reported in 5 patients (20.8%). Three of the patients developed febrile neutropenia, 1 developed acute renal failure, and the remaining patient was hospitalized due to grade 3 vomiting that required intravenous hydration. All patients continued treatment after the toxicity resolved. The median duration of hospitalization among patients with severe adverse events was 6 d (range: 2-22). Additionally, no fatal events were reported. Globally, TPEx was associated with a low rate of adverse events leading to treatment interruption (12.5%), discontinuation (8.3%), or dose reduction (8.3%).
Despite substantial advances in the last decade, R/M SCCHN remains a significant clinical challenge because of its associated high mortality rate. As such, increasing the tumor response rate is an important goal in these patients given its association with symptom improvement and better quality of life.
Over the past years, the EXTREME regimen has become a preferred first-line strategy for R/M SCCHN patients[3]. While significant improvements in OS, PFS, and ORR were demonstrated in the cetuximab plus platinum–fluorouracil arm of a pivotal phase 3 trial, 82% of the included patients experienced grade 3-4 adverse events, mostly related to 5-fluorouracil continuous infusion. Of note, all these findings were observed in fit patients; hence, treatment decisions in this setting should be analyzed on a case-by-case basis. Clinical comorbidities, performance status, nutritional assessment results, access to infusion pumps, or even availability for patient hospitalization are some of the considerations made in clinical practice before treatment decisions are made.
Given that not all patients can tolerate the EXTREME regimen, alternative treatment protocols have been developed, mostly replacing 5-fluorouracil with taxanes (Table 5). The phase 2 GORTEC study evaluated cisplatin, docetaxel, and cetuximab as a first-line treatment in 54 patients with R/M SCCHN[14]. The median OS, PFS, and ORR were 14 mo, 6.2 mo, and 44.4%, respectively. In this selected population, only 12 patients (22.2%) experienced grade 4 adverse events. In another phase 2 trial, Bossi et al[15] randomized 201 patients with R/M SCCHN to receive first-line cetuximab plus cisplatin with or without paclitaxel. The authors reported a median PFS of 7 mo and an ORR of 51.7% in the cetuximab, cisplatin, and paclitaxel arm. With this regimen, 72.5% and 33% of the included patients presented grade ≥ 3 and 4 adverse events, respectively.
Guigay and collaborators[19] have recently published the results of a phase 2 trial that compared TPEx with EXTREME as first-line treatment for 541 patients. PFS and ORR values were 14.5 vs 13.4 mo, 6.0 vs 6.2 mo, and 57.6% vs 57%, respectively, and there were no significant differences between the two arms. The TPEx regimen was associated with a grade 4 adverse event incidence of 33%, which was significantly lower than the 46% incidence reported with the EXTREME scheme. Furthermore, an exploratory analysis for this trial showed a better quality of life in patients who received TPEx, mainly in terms of global health status, physical functioning, role functioning, and scores of appetite[20].
Remarkably, real-world data in this setting are scarce. Before the GORTEC trial, Even and collaborators[21] presented the results of 30 patients treated with TPEx at Gustave Roussy Institute between 2011 and 2013. In this group of patients, the median PFS and OS were 6.0 and 13.6 mo, respectively. A total of eight grade 3-4 adverse events were documented, including vomiting, mucositis, skin rash, diarrhea, hypersensitivity, and neutropenia. Additionally, Fuchs et al[22] reported similar results in a retrospective single-institution study, including 38 R/M SCCHN patients treated with TPEx at the Medical University of Vienna. In this study, the median OS, PFS, and ORR values were 10.8 mo, 6.3 mo, and 50%, respectively.
To the best of our knowledge, our study presents the first multicenter cohort (including data from South America) of patients treated with the TPEx schema. Notably, the PFS and ORR were consistent with those reported in previous clinical trials. Intriguingly, 2 patients with complete responses had longer PFS, which may support that depth of response could be studied as a prognostic factor in patients with R/M HNSCC.
In our study, the TPEx regimen was adequately tolerated by most of the analyzed patients. The incidence of grade 3-4 adverse events was surprisingly lower than expected (25%), but it should be noted that 5 patients had treatment-related hospitalizations. Fortunately, no fatal toxicities were experienced.
Our experience confirms that the replacement of 5-fluorouracil with docetaxel may be a reasonable treatment strategy for R/M SCCHN patients. TPEx has been incorporated as a standard regimen in our centers, considering that this regimen is associated with a lower duration of treatment infusions and lower total number of cycles and the recent reports of safety and quality of life outcomes. These particular characteristics are essential in low- and middle-income countries with limited access to infusion pumps. Furthermore, the instauration of simplified regimens has become extremely important during the coronavirus disease 2019 pandemic[23].
Our results should be interpreted with caution considering the study limitations. This observational study was conducted in five private care centers, which may have been responsible for the high proportion of patients with access to immunotherapy after disease progression (92.9%). The low number of included patients and the retrospective nature of the study may also hamper the extrapolation of our results to Hispanic and Latino American populations. Additionally, our follow-up was not long enough to analyze adequately OS in our sample. Accordingly, the high response rate and the low incidence of grade 3-4 adverse events and serious toxicity may also be explained by a patient selection bias. Although public and private care centers were invited to register their experience with the TPEX regimen, only private-care physicians reported patients that received this treatment strategy.
Finally, it should also be highlighted that the landscape in R/M SCCHN is evolving. First-line treatment strategies currently include immunotherapy given alone or in combination with chemotherapy[7]. Nevertheless, TPEx represents an adequate alternative for patients with R/M HNSCC without programmed death-ligand 1 expression or as a subsequent treatment after disease progression on immune checkpoint inhibitors given as monotherapy. It should be emphasized that drug combination regimens, such as TPEx, have proven to be associated with a higher ORR, which is particularly beneficial in patients with a high tumor burden.
TPEx was a well-tolerated regimen in our population, showing a lower incidence of grade 3-4 adverse events than previously reported. PFS was comparable to those of recently reported clinical trials using the same treatment scheme. We observed a higher ORR compared to the previous results in phase 2 trials. This regimen may be considered an attractive therapeutic strategy due to its simplified administration, decreased total number of chemotherapy cycles, and treatment tolerability. Overall, quality of life, cost of hospitalization, and adverse event management should be carefully analyzed before deciding the best therapeutic plan for patients with R/M SCCHN.
The targeted therapy cetuximab in combination with 5-fluorouracil and platinum-based chemotherapy (the EXTREME regimen) has shown substantial efficacy for patients with recurrent or metastatic squamous cell carcinoma of the head and neck (R/M SCCHN). However, a new strategy combining platinum, taxanes, and cetuximab (the TPEx regimen) has demonstrated similar efficacy with a more favorable toxicity profile in clinical trials.
There is scarce evidence outside randomized clinical trials including patients treated with TPEx scheme.
To evaluate the safety and efficacy of the TPEx scheme as first-line therapy in advanced SCCHN in a multicenter cohort study.
This retrospective multicenter cohort study included patients with histologically confirmed recurrent or metastatic SCCHN treated with first-line TPEx at five medical centers in Argentina between January 1, 2017, and April 31, 2020. Chemotherapy consisted of four cycles of docetaxel, cisplatin, and cetuximab followed by cetuximab maintenance therapy. Clinical outcomes and toxicity profiles were collected from medical charts. Treatment response was assessed by the investigator in accordance with Response Evaluation Criteria in Solid Tumors (RECIST; version 1.1). Adverse events were graded according to the National Cancer Institute Common Terminology Criteria for Adverse Events (version 4.0).
Among the 24 patients included, the majority of patients (83.3%) received at least four chemotherapy cycles in the initial phase. The overall response rate was 62.5%, and 3 patients achieved a complete response (12.5%). The median time to response was 2.4 mo (95%CI: 1.3-3.5). With a median follow-up of 12.7 mo [95% confidence interval (CI): 8.8-16.6), the median progression-free survival (PFS) was 6.9 mo (95%CI: 6.5-7.3), and the overall survival rate at 12 mo was 82.4%. Patients with documented tumor response showed a better PFS than those with disease stabilization or progression [8.5 mo (95% CI: 5.5-11.5) and 4.5 mo (95%CI: 2.5-6.6), respectively; P = 0.042]. Regarding the safety analysis, two-thirds of patients reported at least one treatment-related adverse event, and 25% presented grade 3 toxicities. Of note, no patient experienced grade 4 adverse events.
TPEx was a well-tolerated regimen in our population, showing a lower incidence of grade 3-4 adverse events than previously reported. PFS was comparable to those of recently reported clinical trials using the same treatment scheme. We observed a higher overall response rate compared to the previous results in phase 2 trials.
This regimen may be considered an attractive therapeutic strategy due to its simplified administration, decreased total number of chemotherapy cycles, and treatment tolerability.
The English editing of this publication was financially supported by Merck KGaA, Darmstadt, German.
Provenance and peer review: Unsolicited article; Externally peer reviewed.
Peer-review model: Single blind
Specialty type: Oncology
Country/Territory of origin: Argentina
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): 0
Grade C (Good): 0
Grade D (Fair): D
Grade E (Poor): 0
P-Reviewer: Budai B S-Editor: Ma YJ L-Editor: Filipodia P-Editor: Ma YJ
| 1. | Mehanna H, Paleri V, West CM, Nutting C. Head and neck cancer--Part 1: Epidemiology, presentation, and prevention. BMJ. 2010;341:c4684. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 160] [Cited by in RCA: 183] [Article Influence: 12.2] [Reference Citation Analysis (0)] |
| 2. | Vokes EE, Weichselbaum RR, Lippman SM, Hong WK. Head and neck cancer. N Engl J Med. 1993;328:184-194. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1231] [Cited by in RCA: 1201] [Article Influence: 37.5] [Reference Citation Analysis (0)] |
| 3. | Vermorken JB, Mesia R, Rivera F, Remenar E, Kawecki A, Rottey S, Erfan J, Zabolotnyy D, Kienzer HR, Cupissol D, Peyrade F, Benasso M, Vynnychenko I, De Raucourt D, Bokemeyer C, Schueler A, Amellal N, Hitt R. Platinum-based chemotherapy plus cetuximab in head and neck cancer. N Engl J Med. 2008;359:1116-1127. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2546] [Cited by in RCA: 2546] [Article Influence: 149.8] [Reference Citation Analysis (0)] |
| 4. | Mendenhall WM, Werning JW, Pfister DG. Cancer of the Head and Neck. In: Devita, Hellman, and Rosenberg’s cancer: principles & practice of oncology. 10th edition. Wolters Kluwer Health. [DOI] [Full Text] |
| 5. | Ferris RL, Blumenschein G Jr, Fayette J, Guigay J, Colevas AD, Licitra L, Harrington K, Kasper S, Vokes EE, Even C, Worden F, Saba NF, Iglesias Docampo LC, Haddad R, Rordorf T, Kiyota N, Tahara M, Monga M, Lynch M, Geese WJ, Kopit J, Shaw JW, Gillison ML. Nivolumab for Recurrent Squamous-Cell Carcinoma of the Head and Neck. N Engl J Med. 2016;375:1856-1867. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3044] [Cited by in RCA: 3697] [Article Influence: 410.8] [Reference Citation Analysis (0)] |
| 6. | Cohen EEW, Soulières D, Le Tourneau C, Dinis J, Licitra L, Ahn MJ, Soria A, Machiels JP, Mach N, Mehra R, Burtness B, Zhang P, Cheng J, Swaby RF, Harrington KJ; KEYNOTE-040 investigators. Pembrolizumab versus methotrexate, docetaxel, or cetuximab for recurrent or metastatic head-and-neck squamous cell carcinoma (KEYNOTE-040): a randomised, open-label, phase 3 study. Lancet. 2019;393:156-167. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 835] [Cited by in RCA: 1180] [Article Influence: 196.7] [Reference Citation Analysis (0)] |
| 7. | Burtness B, Harrington KJ, Greil R, Soulières D, Tahara M, de Castro G Jr, Psyrri A, Basté N, Neupane P, Bratland Å, Fuereder T, Hughes BGM, Mesía R, Ngamphaiboon N, Rordorf T, Wan Ishak WZ, Hong RL, González Mendoza R, Roy A, Zhang Y, Gumuscu B, Cheng JD, Jin F, Rischin D; KEYNOTE-048 Investigators. Pembrolizumab alone or with chemotherapy versus cetuximab with chemotherapy for recurrent or metastatic squamous cell carcinoma of the head and neck (KEYNOTE-048): a randomised, open-label, phase 3 study. Lancet. 2019;394:1915-1928. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2237] [Cited by in RCA: 2079] [Article Influence: 346.5] [Reference Citation Analysis (0)] |
| 8. | Guidi A, Codecà C, Ferrari D. Chemotherapy and immunotherapy for recurrent and metastatic head and neck cancer: a systematic review. Med Oncol. 2018;35:37. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 38] [Cited by in RCA: 58] [Article Influence: 8.3] [Reference Citation Analysis (0)] |
| 9. | Golden EB, Formenti SC, Schiff PB. Taxanes as radiosensitizers. Anticancer Drugs. 2014;25:502-511. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 16] [Cited by in RCA: 26] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 10. | Mekhail TM, Markman M. Paclitaxel in cancer therapy. Expert Opin Pharmacother. 2002;3:755-766. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 251] [Cited by in RCA: 280] [Article Influence: 12.2] [Reference Citation Analysis (0)] |
| 11. | Misiukiewicz K, Gupta V, Bakst R, Posner M. Taxanes in cancer of the head and neck. Anticancer Drugs. 2014;25:561-570. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 12] [Cited by in RCA: 18] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 12. | Morelli MP, Cascone T, Troiani T, De Vita F, Orditura M, Laus G, Eckhardt SG, Pepe S, Tortora G, Ciardiello F. Sequence-dependent antiproliferative effects of cytotoxic drugs and epidermal growth factor receptor inhibitors. Ann Oncol. 2005;16 Suppl 4:iv61-iv68. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 81] [Cited by in RCA: 88] [Article Influence: 5.2] [Reference Citation Analysis (0)] |
| 13. | Rose WC, Wild R. Therapeutic synergy of oral taxane BMS-275183 and cetuximab versus human tumor xenografts. Clin Cancer Res. 2004;10:7413-7417. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 53] [Cited by in RCA: 50] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 14. | Guigay J, Fayette J, Dillies AF, Sire C, Kerger JN, Tennevet I, Machiels JP, Zanetta S, Pointreau Y, Bozec Le Moal L, Henry S, Schilf A, Bourhis J. Cetuximab, docetaxel, and cisplatin as first-line treatment in patients with recurrent or metastatic head and neck squamous cell carcinoma: a multicenter, phase II GORTEC study. Ann Oncol. 2015;26:1941-1947. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 88] [Cited by in RCA: 91] [Article Influence: 9.1] [Reference Citation Analysis (0)] |
| 15. | Bossi P, Miceli R, Locati LD, Ferrari D, Vecchio S, Moretti G, Denaro N, Caponigro F, Airoldi M, Moro C, Vaccher E, Sponghini A, Caldara A, Rinaldi G, Ferrau F, Nolè F, Lo Vullo S, Tettamanzi F, Hollander L, Licitra L. A randomized, phase 2 study of cetuximab plus cisplatin with or without paclitaxel for the first-line treatment of patients with recurrent and/or metastatic squamous cell carcinoma of the head and neck. Ann Oncol. 2017;28:2820-2826. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 56] [Cited by in RCA: 56] [Article Influence: 7.0] [Reference Citation Analysis (0)] |
| 16. | Tahara M, Kiyota N, Yokota T, Hasegawa Y, Muro K, Takahashi S, Onoe T, Homma A, Taguchi J, Suzuki M, Minato K, Yane K, Ueda S, Hara H, Saijo K, Yamanaka T. Phase II trial of combination treatment with paclitaxel, carboplatin and cetuximab (PCE) as first-line treatment in patients with recurrent and/or metastatic squamous cell carcinoma of the head and neck (CSPOR-HN02). Ann Oncol. 2018;29:1004-1009. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 39] [Cited by in RCA: 44] [Article Influence: 6.3] [Reference Citation Analysis (0)] |
| 17. | Tsakonas G, Specht L, Kristensen CA, Moreno MHC, Cange HH, Soderstrom K, Friesland S. Randomized Phase II Study with Cetuximab in Combination with 5-FU and Cisplatin or Carboplatin vs. Cetuximab in Combination with Paclitaxel and Carboplatin for Treatment of Patients with Relapsed or Metastatic Squamous Cell Carcinoma of the Head and Neck (CETMET Trial). Cancers (Basel). 2020;12. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 5] [Cited by in RCA: 14] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 18. | Adkins D, Ley J, Atiq O, Rigden C. Multicenter phase II trial of carbo- or cis-platin, nanoparticle albumin bound (nab)-paclitaxel, and ceTUXimabas first line therapy for recurrent/metastatic HNSCC: “the CACTUX Trial”. Multidisciplinary Head and Neck Cancers Symposium, 2018. [RCA] [DOI] [Full Text] [Cited by in Crossref: 5] [Cited by in RCA: 2] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 19. | Guigay J, Aupérin A, Fayette J, Saada-Bouzid E, Lafond C, Taberna M, Geoffrois L, Martin L, Capitain O, Cupissol D, Castanie H, Vansteene D, Schafhausen P, Johnson A, Even C, Sire C, Duplomb S, Evrard C, Delord JP, Laguerre B, Zanetta S, Chevassus-Clément C, Fraslin A, Louat F, Sinigaglia L, Keilholz U, Bourhis J, Mesia R; GORTEC; AIO; TTCC, and UniCancer Head and Neck groups. Cetuximab, docetaxel, and cisplatin versus platinum, fluorouracil, and cetuximab as first-line treatment in patients with recurrent or metastatic head and neck squamous-cell carcinoma (GORTEC 2014-01 TPExtreme): a multicentre, open-label, randomised, phase 2 trial. Lancet Oncol. 2021;22:463-475. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 32] [Cited by in RCA: 122] [Article Influence: 30.5] [Reference Citation Analysis (0)] |
| 20. | Guigay J, Fayette J, Mesia R, Saada-Bouzid E, Lafond C, Geoffrois L, Martin L, Capitain O, Cupissol D, Castanie H, Johnson AC, Vansteene D, Even C, Sire C, Kapso R, Delhommeau M, Chevassus-Clement C, Keilholz U, Bourhis J, Auperin A. TPExtreme randomized trial: Quality of Life (QoL) and survival according to second-line treatments in patients with recurrent/metastatic head and neck squamous cell carcinoma (R/M HNSCC). J Clin Oncol. 2020;38:6507-6507. [RCA] [DOI] [Full Text] [Cited by in Crossref: 12] [Cited by in RCA: 14] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 21. | Even C, Bobillot B, Mayache-Badis L, Ferrand FR, Lezghed N, Bidault F, Auperin A, Temam S, Janot F, Schilf A, Guigay J. 997P - Results of Tpex (Docetaxel, Cisplatin, Cetuximab) Regimen Use in First Line Patients with Recurrent/Metastatic Squamous Cell Carcinoma of the Head and Neck (R/M Scchn) in a Single Institution. Ann Oncol. 2014;25:iv340-iv356. [RCA] [DOI] [Full Text] [Cited by in Crossref: 3] [Cited by in RCA: 3] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 22. | Fuchs H, Pammer J, Minichsdorfer C, Posch D, Kornek G, Aretin MB, Fuereder T. Modified biweekly cisplatin, docetaxel plus cetuximab (TPEx) as first-line treatment for patients with recurrent/metastatic head and neck cancer. Med Oncol. 2018;35:32. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 10] [Cited by in RCA: 9] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 23. | Waisberg F, Enrico D, Angel M, Chacón M. Cancer Treatment Adaptations in the COVID-19 Era. JCO Oncol Pract. 2020;16:305-307. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 15] [Cited by in RCA: 20] [Article Influence: 4.0] [Reference Citation Analysis (0)] |