Published online Feb 24, 2022. doi: 10.5306/wjco.v13.i2.101
Peer-review started: February 27, 2021
First decision: May 7, 2021
Revised: June 7, 2021
Accepted: January 24, 2022
Article in press: January 24, 2022
Published online: February 24, 2022
Processing time: 360 Days and 10.2 Hours
Stereotactic ablative body radiotherapy (SABR) is an effective technique comparable to surgery in terms of local control and efficacy in early stages of non-small cell lung cancer (NSCLC) and pulmonary metastasis. Several fractionation schemes have proven to be safe and effective, including the single fraction (SF) scheme. SF is an option cost-effectiveness, more convenience and comfortable for the patient and flexible in terms of its management combined with systemic treatments. The outbreak of the severe acute respiratory syndrome coronavirus 2 pandemic has driven this not new but underutilized paradigm, recommending this option to minimize patients’ visits to hospital. SF SABR already has a long experience, strong evidence and sufficient maturity to reliably evaluate outcomes in peripheral primary NSCLC and there are promising outcomes in pulmonary metastases, making it a valid treatment option; although its use in central locations, synchronous and recurrencies tumors requires more prospective safety and efficacy studies. The SABR radiobiology study, together with the combination with systemic therapies, (targeted therapies and immunotherapy) is a direction of research in both advanced disease and early stages whose future includes SF.
Core Tip: There is strong evidence (two phase II prospective studies) to support using single-fraction stereotactic ablative body radiotherapy schemes in early stage peripheral non-small cell lung cancer. In pulmonary oligometastatic disease, there are promising outcomes and publication of one randomized prospective phase II study is pending. The association of this scheme with new systemic therapies looks promising for the future.
- Citation: Fernández C, Navarro-Martin A, Bobo A, Cabrera-Rodriguez J, Calvo P, Chicas-Sett R, Luna J, Rodríguez de Dios N, Couñago F. Single-fraction stereotactic ablative body radiation therapy for primary and metastasic lung tumor: A new paradigm? World J Clin Oncol 2022; 13(2): 101-115
- URL: https://www.wjgnet.com/2218-4333/full/v13/i2/101.htm
- DOI: https://dx.doi.org/10.5306/wjco.v13.i2.101
Stereotactic ablative body radiotherapy (SABR) is an important development in the early stages of non-small cell lung cancer (NSCLC). An effective, non-invasive and well-tolerated treatment which, by delivering high and precise doses over several sessions, improves the survival outcomes of these medically inoperable patients compared with conventional fractionation schemes[1,2]. It achieves a high level of local control in stages I and IIA, and a similar survival to surgery[3] in both primary tumors and lung metastases[4].
In spite of an increasingly widespread use of SABR over the past two decades, no consensus has been reached about the most suitable fractionation schemes, as several have proven to be safe and effective[5-7], including a single fraction scheme (SF)[8].
SF SABR was first utilized in intracranial stereotactic radiosurgery (SRS)[9,10] and showed promising efficacy that was comparable to surgery. Pioneering extracranial developments of SF included the treatment of thoracic malignancies[11], although in clinical practice or research it has been adopted much less than fractionated SABR[12].
The outbreak of the severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) pandemic in 2019 has resulted in an urgent need to reduce the number of patients’ face-to-face visits. There is, therefore, renewed interest in SF as a viable treatment option for primary and metastatic lung cancer[13,14], as it delivers a radical treatment dose in just one hospital visit. In fact, oncological guidelines[15-17] now recommend using SF in these patients.
Moreover, recent studies reporting the use of SABR in carefully-selected patients with sustained metachronic extracranial oligometastases[18-22], will increase the number of indications for this approach, and SF could also be an attractive option in this setting.
Other possible applications for SF could include synchronous or oligorecurrent tumors, after a first treatment.
However, SF differs from other treatments in two important ways. One concerns the radiobiological principles and the other refers to a possible different immunogenicity than with multifraction SABR.
The clinical efficacy of SABR is greater than would be expected by the linear-quadratic model. This is because, in addition to the directly ablative effect of SABR, it also has indirect effects that induce vascular endothelial lesion and immune activation[23,24].
Apart from reoxygenation, perhaps, the other radiobiological principles of the 4Rs are not applicable to SABR. Tumoral hypoxia can persist after vascular lesions caused by SABR[25]. Moreover, it is unlikely that switching from 1 to 5 fractions will permit the initially hypoxic tumor cells to become sufficiently reoxygenated, and could explain the small number of local failures observed in fractionated SABR[8]. To date, no clinical study has measured the effect of SABR on tumoral hypoxia in patients with NSCLC, to determine whether the efficacy of SABR depends on reoxygenation[25].
In the present review, we examine current evidence for the safety and efficacy of SF, its benefits and limitations to use. We also examine possible future directions for new systemic treatments and immunotherapies.
In 2005[26,27], the first published experiences began to appear using single fraction radiation therapy in lung, in which doses of 30-34G achieved local response rates at one-year of 93%, with a G3 toxicity of 2%.
The first prospective studies in dose-escalation (Table 1) were published by Stanford University[28] in 2003. Later, the authors presented the outcomes for different doses[29]. This was a Phase I trial in dose-escalation with a study design with 4 doses of SF increasing from 15 Gy to 30 Gy, by increments of 5 Gy. The primary end-point was to identify the maximum tolerated dose (MTD) three months after dose delivery by SF. A total of 32 inoperable patients were recruited, of whom 20 had NSCLC and 12 were metastatic with lesions smaller than 5 cm. After 5-6 mo, the patients with central tumors and with a PTV > 50 cc presented pneumonitis G2-3. On the other hand, delivery of 25 Gy to patients with prior radiotherapy (RT) produced a significant increase in toxicity effects. Therefore, an addendum was applied for the 30 Gy dose to exclude the population with PTV > 50 cc who had received prior RT. The three G5 toxicities reported all corresponded to centrally located tumors, in patients with prior chemotherapy, one before SABR and two as adjuvant therapy to SABR, and two patients had a PTV > 50 cc. Local control (LC) at one-year was 91% for those delivered a dose higher than 20 Gy and 54% for doses lower than 20 Gy. Local control was significantly less in metastatic lesions than in primary tumors. The authors conclude that SF SABR of 25 Gy is well-tolerated in patients with prior thoracic radiotherapy with a PTV < 50 cc. However, central lesions and the population receiving prior chemotherapy, before or after SABR, could be at greater risk.
| Ref. | Design | Arms | n | Toxicity rates > GIII | LC | PFS | OS | FU3 | SABR technique/prescription |
| Le et al[29], 2006 | Phase I, n = 32 | 15 Gy (1fr) | 9 | 0 | 54 | NSCLC1: 67% | NSCLC1: 85% | 18 | Cyberknife |
| Gold fiducials | |||||||||
| Breathold or Synchrony (Accuray) respiratory tracking system/Isodose coverage: 95% of PTV | |||||||||
| 20 Gy (1fr) | 1 | 0 | |||||||
| 25 Gy (1fr) | 20 | 1p (GIII)3p (GV) | 91 | ||||||
| Metastatic1: 25% | Metastatic1: 56% | ||||||||
| 30 Gy (1fr) | 2 | 0 | |||||||
| Videtic et al[30], 2015 | Phase II, n = 84 | 48 Gy (4fr) | 45 | 6 (13.3%) | 92.7%1 | 71.1%2 | 77.7%2 | 30.2 | Abdominal compression, gating with the respiratory cycle, tumor tracking, and active breath-holding techniques were allowed. Image guidance was required/prescription isodose surface ≥ 60% and < 90% of the maximun dose. |
| RTOG 0915 | |||||||||
| 34 Gy (1fr) | 39 | 4 (10.3%) | 97%1 | 56.4%2 | 62.3%2 | ||||
| Singh et al[31], 2019 | Phase II, n = 98 | 60 Gy (3fr) | 49 | 6 (15%) | 97.1%1 | 50%2 | 62%2 | 53.8 | Body Fix (Elekta) immobilizer. Real-Time Position Management by Varían Medical System or abdominal compression. 3D-CRT was preferred. Image guidance was required/tumor coverage and normal tissue dose constraints followed RTOG 0915 |
| 30 Gy (1fr) | 49 | 8 (17%) | 94.9%2 | 65%2 | 73%2 |
After this experience in dose-escalation, two prospective studies were published. The first was the Radiation Therapy Oncology Group (RTOG) 0915 study, published by Videtic et al[30] in 2015. This is a phase II study that analyzed 84 patients with a median follow up of 30.2 mo. Patients had T1-T2 N0 peripheral NSCLC and were randomized into one of two arms: SF 34 Gy (Arm A) vs 48 Gy delivered in 4F (Arm B). The aim of the study design was to identify the schedule that produced the least G3 adverse events in one year. The results showed a Grade 3 toxicity of 10.3% in Arm A and of 13.3% in Arm B. One G toxicity was recorded in each Arm. This was not related to SABR in Arm A, whereas it was related to treatment in Arm B. Local control at one-year was 97% in Arm A and 92.7% in Arm B with a tendency towards increased overall survival (OS), although this was not statistically significant with the 34 Gy dose.
The study conducted by Singh et al[31] at the Roswell Park Cancer Institute and published in 2019 was a phase II study that recruited 98 patients with T1/T2 primary peripheral lung cancer, randomized to receive a SF of 30 Gy (Arm 1) vs 60Gy delivered in 3 fractions, not correcting for heterogeneity (Arm 2). The primary endpoint of the study was to determine the incidence of toxicity of G3 or higher, with secondary endpoints of LC, survival and quality-of-life. With a mean follow-up of 53.8 mo, no significant differences were found between the two arms in G3 toxicity, LC at two years, which was 94.9% in Arm 1 and 97.1% in Arm 2, or in OS or progression free survival (PFS). A statistically significant improvement was only observed in social functioning in the 30Gy arm.
The results show that SF presents a comparable toxicity profile to multi-fraction radiotherapy without differences in LC. It would, therefore, seem pertinent to propose a phase III trial[8] that compares SF with Timmerman’s classical fractionation schedules of 54 Gy in 3 fractions[32]. Possible limitations to this phase III study would be the problem of obtaining a sufficient sample size, and a possible excess toxicity to the ribs in extreme peripheral lesions.
Because of the SARS-CoV-2 pandemic, the use of hypofractionated schedules are being considered and, specifically, for NSCLC the use of a SF of SABR with a 30 Gy or 34 Gy dose, as both these doses have been reported to produce similar outcomes[33].
Central tumors are defined in the clinical trial protocol of the cooperative group of the RTOG 0236 as those located less than 2cm away from the proximal bronchial tree (PBT)[34]. The initial experience in centrally located tumors treated with SABR but following protocols for non-central targets, showed a high toxicity, including deaths from complications[35,36]. Ultra-central tumors[37], in which the margin is reduced to within 1 cm of the boundary of the PBT, and the lesion touches or immediately invades one of the organs at risk, such as the mediastinum, trachea, bronchus or esophagus, are at greater risk of treatment-related death with SABR, associated with necrosis of the respiratory tract. However, for small (T1, T2) ultra-central lesions, SABR has been used quite safely.
There is growing interest in the treatment of central and ultra-centrally located targets by SABR, and in identifying the optimum dosing schedule that is both effective and safe to administer[38].
The Phase I/II RTOG 0813 study[39] evaluated the maximum tolerated dose, efficacy and safety in 120 patients with NSCLC cT1-2N0 of central location. The maximum tolerated dose was 12 Gy/fraction, and was associated with 7.2% of dose-limiting toxicity and high control rates. Local control rates at two years for the 71 evaluable patients in cohorts of 11.5 and 12.0 Gy/fraction were 89% and 88%per cent, respectively. Analysis of ultra-central vs central locations is still pending.
The fractionation schedules most used in centrally-located tumors are: 45-50 Gy delivered in 4-5 fractions following recommendations of the American Society for Radiation Oncology[6] or 60 Gy in 8 fractions according to the UK SBRT consortium[40].
Ongoing radiation studies in ultra-central tumors are attempting to elucidate the ideal dosing schedule. These include the Canadian trial SUNSET[41] and the EORTC LungTech study[42], which use the 60 Gy scheme delivered in 8 fractions.
SF SABR schedules of 30-34 Gy in phase II trials have been validated in peripheral tumors[30,31] but not in central targets. The Phase I dose-escalation study of Standford[29], which did include centrally-located tumors, reported a greater toxicity in these patients, as mentioned in the previous section. The remaining studies focusing on the use of SF on central targets are retrospective.
One retrospective study published by the Roswell Park Cancer Institute analyzed 42 patients with central tumors, and compared treatment outcomes in 11 patients delivered a single fraction of 26-30 Gy vs 31 patients treated with 52.6-60 Gy in 5 fractions. They found no significant differences in OS, PFS or in local, lymph node, or distant failure at 18 mo[43]. In spite of the higher rate of grade ≥ 3 toxicity (P = 0.28) in the cohort of patients treated with the single fraction, in the univariant analysis dose had no significant effect on risk of toxicity ≥ 3. Local control at one year was 100% in patients treated with SF and 96% in the multiple fraction group.
A retrospective review by Siva et al[44] that included 65 patients with 1-3 pulmonary metastases compared SF (26 Gy to peripheral lesions and 18 Gy to central lesions) vs delivery of multiple fractions (48 Gy in 4 fractions and 50 Gy in 5 fractions). With a mean follow-up of 25 mo they found no significant differences in OS, local or distant progression, or toxicity. There were no cases of grade ≥ 3 toxicity.
To conclude, the treatment of centrally-located pulmonary tumors with SABR is still controversial owing to greater toxicity risks associated with central compared with peripheral locations. Evidence from published studies for SF in central or ultra-central tumors shows a higher toxicity risk, as doses exceed tolerance doses for central structures. Therefore, until prospective studies can establish SF as an alternative to multifraction SABR in this location, it cannot be recommended.
There is little solid evidence about the use of SABR, and even less for SF, in synchronous lung tumors. This setting is particularly complex as there is often no anatomical pathology thus complicating therapeutic planning.
A study at Stanford[45] describes the results of a dosing strategy for SABR adapted to tumor volume, in primary and metastatic pulmonary tumors. In one of the groups, patients with a tumoral volume < 12 mL received a SF of 18-30 Gy. This group studied 48 patients with a total of 62 tumors, so an important proportion of patients treated with SF SABR had more than one tumor (between 2 and 4).
With a median follow up of 13 mo, patients with one or more small tumors treated by SF with a BED < 100 Gy, had a high rate of local control and a low toxicity, equivalent to rates recorded in patients treated with multiple fractions of BED >100 Gy.
In 2014, Kumar et al[46], of the Cleveland Clinic published the data of their updated series of 445 patients with early stage NSCLC treated with SABR, including 26 patients (5.8%) with synchronous pulmonary tumors confirmed by biopsy and/or PET-CT. Both the group of synchronous and of single pulmonary tumors included patients who had received SF (30 Gy in both groups and 34 Gy in the group of single tumors). At one-year of follow-up, there were no differences in survival or progression between the groups.
In a retrospective analysis by Tekatli et al[47] on SABR in both primary and metastatic synchronous pulmonary tumors, out of a total of 84 patients and 188 pulmonary lesions treated, only 7 Lesions (3.7%) were delivered a single session of 34 Gy by multicentric VMAT to simultaneously treat lesions some distance apart. A toxicity ≥ G3 was only recorded in 2% of the patients.
Another setting for which few studies have been published is rescue therapy by SABR, either in cases of tumoral recurrence, or persistence after a first oncological treatment. Most of the published studies of SABR used in tumoral recurrence deliver from 3 to 8 fractions[48-50]. A retrospective series published by Pennathur et al[51] analyzed 100 patients receiving SABR treatment for tumoral recurrence using the following regimens: surgery ± radio/chemotherapy, chemo-or radiochemotherapy, RT or radiofrequency. Of these, 31% were given 20 Gy as SF SABR, whereas the remaining patients were given 45 to 60 Gy in 3-5 fractions. With an important median follow up of 51 mo, the OS estimated for the whole sample at 1, 2 and 5 years was 74%, 49% and 31%, respectively. Although these data are from a retrospective study, they are the best data published to date in the setting of SABR for oligorecurrent or persistent lung cancer after a first treatment. No severe toxicity was reported.
Ultimately, there are few published experiences of SABR delivered in a single session for synchronous tumors, or for tumoral recurrence. Further studies are required to establish the viability of this treatment modality in these settings.
The lung is the second most frequent location of metastases[52]. Although metastectomy is the standard treatment[4], not all patients are candidates for pulmonary resection.
The efficacy and safety of SF SABR in primary or metastatic pulmonary tumors has been known since 2000[11].
Studies and data on SF SABR for the treatment of pulmonary metastases are summarized in Table 2. For series that also include patients treated with fractionated SABR, only data referring to the single fraction treatment are included. Nakagawa et al[11] also include pleural and costal metastases. Hara et al[26], Fritz et al[53], Le et al[29] and Wulf et al[54] combine patients with primary and those with metastatic pulmonary tumors, although only two studies assess local control and distinguish between that achieved in primary vs metastatic tumors. The phase I trial at Standford of Le et al[29] , also mentioned previously, found a lower LC rate at one-year in metastases of 58%, compared to 78% with primary tumors, whereas the prospective series by Fritz et al[53], found no significant differences in LC ( 80% 5 years) or OS (mean survival between 20 and 26 mo) in metastases vs primary tumors.
| Ref. | Study design | Total lesions (n)/LM (n) | Mean, Dose Gy (range)/Location | SABR technique/prescription | Mean GTV (cc) (range) failing this, cm | FU (mo), median | LC | Toxicity ≥ GIII | Comments |
| Nakagawa et al[11] | P | 22/12 | 22.8 (18-25)1/NR | Rotational or StaticTherapy 3D-CRT. Abdominal compression/PTV enclosing isodose. | 4.8 (0.8-13) | 10 | 100%1 | 0 | Non actuarial LC |
| Hara et al[26] | P | 59/48 | 30(20-34)/Periph | Static 3D-CRT. Gating/Minimal dose to GTV | 5 (1-19) | 12(mean) | 1-yr 93% | 1 GIII | LC 52% < 30 Gy |
| LC 83% ≥ 30 Gy | |||||||||
| P = 0.068 | |||||||||
| 2-yr 78% | |||||||||
| Wulf et al[54] | R | 92/31 | 26/Central | Static 3D-CRT. Abdominal compression/65-80%-isodose enclosing PTV | NR | 14 | 100% | NR | SF data are shown |
| Fritz et al[53] | P | 64/31 | 30/Periph | Static 3D-CRT. Abdominal compression/Isocenter, 90% isodose enclosing GTV, 80% isodose enclosing PTV | Median: 6 (2.8-55.8)1 | 221 | 5-yr 80%1 | 0 | No difference LC and OS LM vs primary lung cancer |
| Le et al [29] | Phase I | 32/11 | 22.34 (15-30)/Periph | Cyberknife. Gold fiducials.Breathold or Synchrony (Accuray) respiratory tracking system / Isodose coverage: 95% of PTV | Median: 17.1 (2-103) | 18 | 1-yr 91% (≥ 20 Gy) | 1 GIII (pn) | LC primary vs LM: 78% vs 58% |
| And OS (85% vs 56%) | |||||||||
| 1-yr 54% (< 20 Gy) | 3 GV (central) | ||||||||
| Higher toxicity in central tumors | |||||||||
| Hof et al [63] | P | 0/71 | 24.35 (12-30)/NR | Static 3D-CRT. Abdominal compression/Isocenter: 80% isodose enclosing PTV | 10 (1-53) | 14 | 1-yr 88.6% | 3 GIII (pn) | LC 3 yr 78% 26-30 Gy |
| 2-yr 73.7% | |||||||||
| 3-yr 63.1% | |||||||||
| Gandhidasan et al [56] | R | 186/95 | 18/Central26 or 28/Periph | Static 3D-CRT or IMRT/80% isodose enclosing PTV | NR | 22 | 2yr 84% | 0 | |
| Osti et al [57] | P | 0/103 | 23Gy/Central30 Gy/Periph | Static 3D-CRT. 4DCT. 80% isodose enclosing PTV | NR | 15 | Central vs peripheral:1-yr 79.4% vs 94.7% | 2 GIII (pn) | Prognostic factors for LC: sex and histology |
| Global: 1-yr 89.1%, 2-yr 82.1% | |||||||||
| Filippi et al[58] | R | 0/90 | 26Gy/Periph | Static 3D-CRT or IMRT or VMAT. Abdominal compression/80% isodose enclosing PTV | < 5 cm | 24 | 1-yr 93.4% | 8 GII-IIIlate radiological toxicity | They suggest not to use a SF in lesions close to the chest wall |
| 2-yr 88.1% | |||||||||
| 6 GII-IIIchest wall toxicity | |||||||||
| Siva et al [44] | R | 0/41 | 18/Central26/Periph | Static 3D-CRT or IMRT or VMAT. /70-80% isodose enclosing PTV | < 5 cm | 25 | 2-yr 93% | 0 | LC, OS and toxicity rates between SF and multi-fraction SABR |
| Osti et al [59] | R | 0/166 | 30/Periph | Static 3D-CRT. 4DCT/95% isodose enclosing PTV | 3.46 (0.03-47.48) | 38 | 3-yr 80.1% | 6 GIII (pn) | Lesions ≤ 15 mm from mediastinum were not included in the study |
| 11 GIIIlung fibrosis | |||||||||
| 5-yr 79.2% | |||||||||
| 1 GV at 15 mm PBT | |||||||||
| Sharma et al [61] | R | 32 | 30/Periph | Cyberknife. Radiopaque markers Tumor traking.70-90% isodose enclosing PTV | < 3 cm | 22 | 2-yr 68% | No details for SF | BED10 < 100, delivery of pre-SBRT chemo. and synchronous metastasis: independently < LC |
| 3-yr 63% | |||||||||
| 4-yr 59% | |||||||||
| Sogono et al [60] | R | 167 (95% peripher) | 16-18/Central26-28/Periph | Static 3D-CRT or IMRT or VMAT. 4DCT/99% isodose enclosing PTV | NR | 37 | 1-yr 96% | NR | Several locations |
| 2-yr 92% | |||||||||
| 5-yr 92% | |||||||||
| Siva et al[55] | Phase II | 133 | 28/NR | Static 3D-CRT or IMRT or VMAT. Abdominalo compression/70-80% isodose enclosing PTV | 2.2 cm (mean) | 12 | 1-yr 93% | 2 GIII | Preliminary results (TROG 13.01 SAFRON II) |
| 1-3 metastases non-central targets < 5 cm |
Treatment with SF SABR has been used for pulmonary metastases for over 20 years, in over 1000 cases. In seven publications the lesions included for analysis exceed 90[54-60].
In these series, the mean dose delivered to peripheral lesions was 27.03 Gy (range: 12-30 Gy), and for centrally-located tumors was 18.75 Gy (range: 16-23 Gy). The lowest doses are found in publications of the first exploratory studies of SABR and dose-escalation. In more recent publications (from 2010 onwards), the most frequently used dosing interval in peripheral tumors is from 26 to 30 Gy. Recently, central tumors have also been included in SF SABR protocols[44,54,56,57,60].
With an estimated mean follow up of 22 mo, the mean local control at one- and two-years is 87.1% and 84.2%, respectively; only Osti et al[59] and Sogono et al[60] provide data for LC at 5 years, of 79% and 92%, respectively.
Sharma et al[61] obtained particularly poor results (LC at 2 years of 68%, at 3 years of 63% and at 4 years of 59%). They attribute this to the dose calculation algorithm used[62], and suggest that the real dose delivered was lower than the theoretical dose. Although Filippi et al[58] advise against using SF schemes in tumors close to the chest wall , Sogono et al[60] and Siva et al[44] report no toxicities when dosing limits are of organ at risk are respected.
As shown in Table 2, grade 3 toxicity is very rare in all the cited studies. A total of 4 deaths were reported, all in patients with centrally-located tumors treated with non-adapted regimens. Three of these were reported in the phase I dose-escalation trial at Stanford[29] mentioned in previous sections, and one of them located at 15mm PBT in the retrospective series of Osti et al[59].
As mentioned in the section on centrally-located tumors, in the retrospectrive review of Siva et al[44], with appropriate dose constraints, no significant differences are observed in LC or toxicity compared with peripheral pulmonary tumors in retrospective series.
The phase II multicentric prospective study TROG 13.01 SAFRON II of Siva et al[55] is currently addressing the equivalence of a SF SABR schedule of 28 Gy and a schedule of 48 Gy delivered in four fractions for peripheral pulmonary oligometastases smaller than 5 cm. The prespecified primary evaluation criterion relating to safety was satisfied. Preliminary results point to an equivalence of both schedules for LC, OS and DFS (disease free survival), although more time is required to verify these, and other secondary endpoints such as quality-of-life and cost-effectiveness.
SF SABR schemes are an attractive option in terms of more convenience for the patient, reduced costs (direct and indirect) and greater flexibility for combinations with systemic treatments[58]. These features have become even more critical during the pandemic, to minimize patients´ visits to hospital, and hospital stay.
However, some constraints have prevented the widespread implementation of this technique such as: a fear of severe toxicity (especially in early studies and central tumors[29]), the scarcity of long-term studies compared with fractionated SABR (a scheme the specialist is already familiar with), and the possibility of errors in geographical positioning (which can be fatal in SF-SABR), requiring a high quality SABR control and appropriate technical capability[8,17] , among others (Table 3).
| Benefits | Constraints |
| Low medium-long term toxicity | Fear of severe toxicity in initial studies |
| Prospective efficacy and toxicity data | Insufficient long-term data |
| Convenience for patient, fewer hospital visits (indirect costs), shorter treatment times | |
| Less occupation of accelerators | |
| Reduced positioning errors between fractions | Greater risk of positioning errors |
| Peripheral tumors | Central tumors |
| Reduction in direct costs | |
| Less interference with systemic therapies | Cases of Neumonitis recall with some systemic therapies |
| Convenience for COVID-19 pandemic |
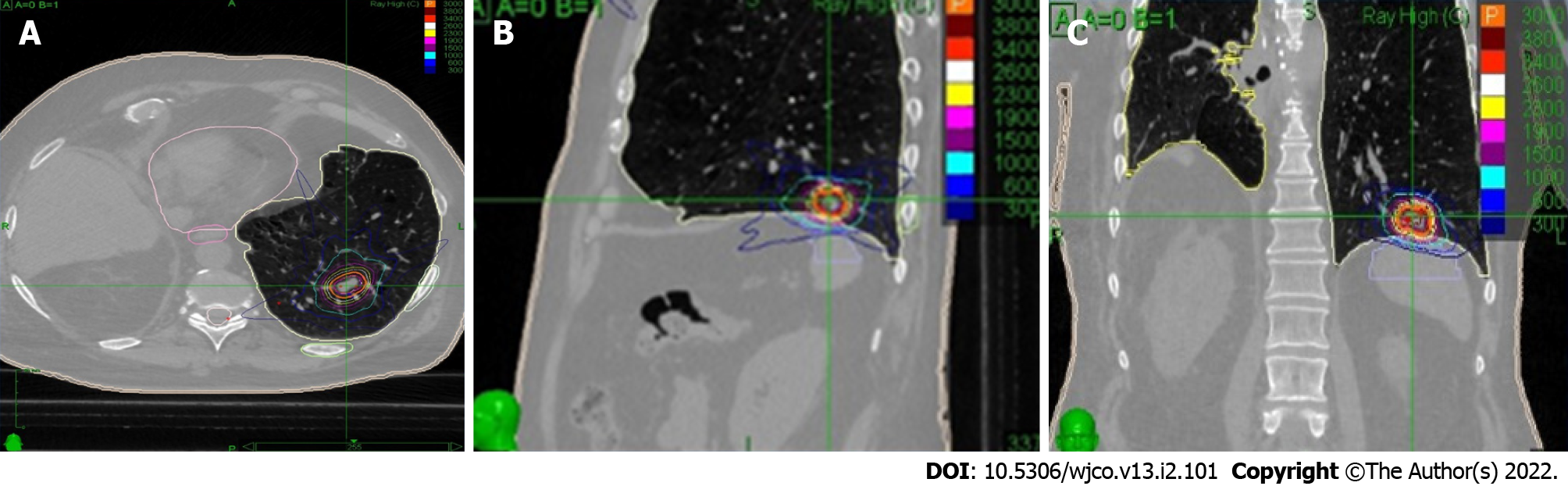
As in fractionated SABR, motion management methods should be used in SF, at planning and/or treatment; depending on available technology and previous experience (Figure 1). The treatment and prescription techniques of the studies mentioned in this article are shown in (Tables 1 and 2).
Regarding the benefits of this therapy, there is now strong evidence (two phase II prospective studies), and of sufficient maturity to reliably evaluate outcomes (5 years), to support using SF SABR schemes in early stage peripheral NSCLC[30,31].
In pulmonary oligometastatic disease, prospective and retrospective studies (Table 2) describe promising outcomes for LC, acceptable toxicity and emphasize benefits for patient adherence, more convenience and less associated costs. Publication of the randomized prospective phase II study, which is also studying cost-effectiveness, is pending[55,63].
Published data on SF-SABR in centrally-located and large-volumed primary tumors (> 50 cc), are controversial, and its use in these settings is not recommended [29,35].
There is some uncertainty about the combined use of some systemic therapies (especially with gemcitabine) due to a possible rise in cases of recall pneumonitis, more associated with higher doses per fraction[29].
Radiobiologically, single fraction schemes use a similar biologically effective dose (BED) to fractionated schemes (BED > 100 Gy). Therefore, theoretically both should have the same effect on the tumor (> 90%). The BED for healthy tissues suggests a possible rise in toxicity, although this has not been demonstrated in prospective studies (Table 4)[30,31,64]. Moreover, toxicity could be mitigated by increasing the precision of the irradiation, with a high dose gradient between the tumor and healthy tissues, adequate inmolibilization to minimize motion and account for intra-fraction movement[17].
| Early tumor effects α/β = 10 | Late tumor effects α/β = 3 | |
| 28 Gy in 1 fraction | 106 Gy | 289 Gy |
| 48 Gy in 4 fractions | 105 Gy | 240 Gy |
The use of single fraction schedules has been found to be 40% less costly than 3 fraction schedules, according to Medicare 2009 rates, approximately 9000$ vs 150000$), although the reimbursement per fraction scheme has been a barrier in countries that implement it (US)[65]. The combination of greater patient comfort, with fewer hospital visits and shorter duration of treatments (one day vs one week), less burden on accelerators, and guarantees of acceptable toxicity and effectiveness[8,66], have all contributed to making SF SABR a valid treatment option to consider.
The SARS-CoV-2 has posed a major challenge for the practice of radiation oncology, especially in lung cancer patients that represents one of the greatest risk groups[67].
The first results of the Thoracic Cancers International COVID-19 Collaboration (TERAVOLT), the first registry created to establish the effects of SARS-CoV-2 infection in patients with thoracic cancers, report a higher mortality in this group of patients and less access to intensive care units, although 74% of cases corresponded to Stage I patients[68].
During the pandemic, we have had to evaluate alternative dose fractionation schemes and RT techniques, with two main goals: (1) To reduce the number of hospital visits and limit exposure to SARS-CoV-2 of patients receiving RT of curative intent for lung cancer; and (2) To make room, in the radiotherapeutic oncology services, to treat operable lung cancer patients who cannot receive surgery during the pandemic.
Several guidelines have been published (Table 5) in order to provide an objective and transparent framework with which to classify patients according to the stage of the pandemic and the healthcare resources available.
| ESTRO-ASTRO | UK | GOECP/SEOR |
| 45-54 Gy in 3 fx, 48 Gy in 4 fx; Maximum hypofractionation supported, 30-34 Gy in 1 fx (90% support if choosing hypofractionation) | Safe zone: 34 Gy in 1 fx | Safe zone: 30-34 Gy, 1 fx (first option); 54 Gy in 3 fx |
| Tumours within 2.5 cm of the Chest Wall: 48-54 Gy in 3 fx | ||
| Peripheral lesions: 48 Gy in 4 fx (first option) | ||
| Moderately central: 50 Gy in 5 fx | ||
| Central tumour: 50-60 Gy in 5 fx, 60 Gy in 8 fx | ||
| Ultra-central: 45-50 Gy in 4-5 fx, 60 Gy in 8 fx | ||
| Central/ultra-central early stage tumours not suitable for stereotactic ablative radiotherapy: 50-60 Gy in 15 fx |
There is unanimous agreement about recommending SABR in operable patients with early stage NSCLC or oligometastatic lesions, owing to closed operating theatres or delayed surgical interventions[15-17].
Some teams have adopted an approach called SABR-BRIDGE (Stereotactic ABlative Radiotherapy Before Resection to avoId Delay for early-stage lunG cancer or oligomEts) in which SABR is used as a bridge to provide a radical treatment based on a combination of immediate SABR followed by programmed surgery 3 to 6 mo later[69].
Of the different SABR schemes available, the guidelines support administration of a SF treatment to reduce the number of visits during the pandemic. The preferred option is SF of 30-34 Gy for tumors ≤ 2 cm and > 1 cm distant from the chest wall that are outside the no-fly zone. However, the timing and the ability to implement changes in doses/fractionation schedules will depend upon the healthcare resources and technology available (for example, daily CBCT, 4 DCT etc.), and previous experience in SABR is preferable if SF dosing schemes are to be implemented.
New systemic treatments have changed the paradigm for lung cancer, benefitting both OS and DFS. Immune checkpoint inhibitors (ICI) are a standard procedure of locally-advanced and metastatic NSCLC[24]. The use of targeted therapies in carriers of actionable mutations (EGFR, ALK, ROS1, BRAF, TRK, RET and MET) has also changed the course of advanced disease[70]. Unfortunately, platinum-based chemotherapy doublets produce low response rates[71]. Moreover, new treatments can present primary and/or secondary resistances that limit their efficacy in most patients. The exclusive use of ICI in monotherapy produces a clinical benefit in fewer than 30% of patients, and 20% of those receiving targeted therapies develop acquired resistance during the first year[24,72].
Several studies have shown that systemic treatment is effective at controlling the microscopic disease, but largely ineffective macroscopically[20]. This has led to studies exploring combinations of systemic treatments with local therapies such as SABR. The clinical benefit of this combination has been demonstrated in two randomized phase II studies in oligometastatic patients with NSCLC[21,22]. In both of these, the patients received induction ChT and were then randomized to receive SABR or surgery vs systemic treatment exclusively. Similar outcomes were obtained, and PFS was three times greater in the group receiving local consolidation therapy.
Randomized retrospective and prospective studies on the combined use of SABR and targeted therapies in stage IV NSCLC have also reported a clinical benefit for LC, PFS and OS[73,74]. This was confirmed in the Phase III randomized study (SINDAS), which showed an increase in PFS and OS[75].
The combination of ICI and SABR (I-SABR) is attracting even more interest. This is because SABR can induce an effective immunogenic death that can reactivate the antitumoral immune response[24]. A systematic review of stage IV NSCLC found that I-SABR increased the objective response rate (ORR) in 40% and also the PFS[76]. The PEMBRO-RT study (phase II randomized) used SABR (24 Gy in 3 fractions) prior to starting pembrolizumab, and reported an ORR, PFS and OS of 36%, 6.6 mo and 15.9 mo in favor of the combination[77]. Bauml et al[78] (phase II study of one arm) evaluated the combination of local ablative therapies with pembrolizumab and found a PFS of 19 mo and an OS of 77.5% at two years. A pooled analysis of the PEMBRO-RT and MDACC trials reported better outcomes for I-SABR with a median PFS and OS of 9 mo and 19.2 mo, respectively[79].
In the light of these promising results for I-SABR in advanced disease, current research is focusing on its benefits in early stages. One example is the PACIFIC-004 study, a multicentric phase III trial that combines SABR with durvalumab in stage I-II[80].
Although current evidence tends to favor hypofractionation, there is still controversy regarding the optimum fractionation schedule. Some ongoing studies are attempting to evaluate the role of single fraction SABR combined with immunotherapy. One example is the NCT03217071 trial that uses induction SABR at 12 Gy associated with pembrolizumab in stages I-IIIA. In advanced disease, the NCT02639026 trial evaluates the 17Gy scheme associated with durvalumab + tremelimumab[81].
Carbon ion radiotherapy (CIRT) has also proven effective in NSCLC. The use of CIRT in single fraction has achieved LC rates of 95% at 5 years with doses higher than 48 GyE[82]. Several possible synergistic mechanisms have been proposed for combinations with immunotherapy, but this research is still ongoing[83].
SF SABR is a valid treatment option in patients with lung cancer owing to an increased convenience of this approach, its lower costs and greater flexibility for combining with systemic therapy. During the SARS-CoV-2 pandemic, there has been renewed interest in hypofractionated and ultra-short schedules including SF, which has transformed the paradigm of radiation oncology.
Results reported in the literature reveal comparable local control, PFS and OS, late onset toxicity and quality-of-life for both SF SABR and multifraction SABR in primary NSCLC and there are promising outcomes in lung metastases.
However, there are some settings in which SF could entail too high a toxicity risk such as: patients who have received prior RT, when PTV > 50 cc, or in peripheral locations where noncompliance of SF with dosing limits for healthy tissues could endanger structures such as the chest wall. Moreover, the use of this scheme in centrally-located tumors with SABR is still controversial owing to toxicity risks and the current evidence so should be used in a clinical trial scenario.
The radiobiology of SF and combinations of this technique with immunotherapy are still under investigation, and studies focusing on high dose ablative regimens will continue.
Combining SABR with systemic treatments is safe and effective. Preclinical trials have reported an immune effect for SABR in a SF, and this is also easier to deliver between one systemic treatment and the next. However, the clinical application of SF with immunotherapy to trigger synergistic effects is still being investigated.
Provenance and peer review: Invited article; Externally peer reviewed.
Peer-review model: Single blind
Specialty type: Oncology
Country/Territory of origin: Spain
Peer-review report’s scientific quality classification
Grade A (Excellent): A
Grade B (Very good): B
Grade C (Good): 0
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Agolli L, Pisani P S-Editor: Gong ZM L-Editor: A P-Editor: Gong ZM
| 1. | Ball D, Mai GT, Vinod S, Babington S, Ruben J, Kron T, Chesson B, Herschtal A, Vanevski M, Rezo A, Elder C, Skala M, Wirth A, Wheeler G, Lim A, Shaw M, Schofield P, Irving L, Solomon B; TROG 09. 02 CHISEL investigators. Stereotactic ablative radiotherapy versus standard radiotherapy in stage 1 non-small-cell lung cancer (TROG 09.02 CHISEL): a phase 3, open-label, randomised controlled trial. Lancet Oncol. 2019;20:494-503. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 240] [Cited by in RCA: 403] [Article Influence: 67.2] [Reference Citation Analysis (0)] |
| 2. | Nyman J, Hallqvist A, Lund JÅ, Brustugun OT, Bergman B, Bergström P, Friesland S, Lewensohn R, Holmberg E, Lax I. SPACE - A randomized study of SBRT vs conventional fractionated radiotherapy in medically inoperable stage I NSCLC. Radiother Oncol. 2016;121:1-8. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 187] [Cited by in RCA: 246] [Article Influence: 27.3] [Reference Citation Analysis (0)] |
| 3. | Soldà F, Lodge M, Ashley S, Whitington A, Goldstraw P, Brada M. Stereotactic radiotherapy (SABR) for the treatment of primary non-small cell lung cancer; systematic review and comparison with a surgical cohort. Radiother Oncol. 2013;109:1-7. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 127] [Cited by in RCA: 140] [Article Influence: 11.7] [Reference Citation Analysis (0)] |
| 4. | Londero F, Grossi W, Morelli A, Parise O, Masullo G, Tetta C, Livi U, Maessen JG, Gelsomino S. Surgery versus stereotactic radiotherapy for treatment of pulmonary metastases. A systematic review of literature. Future Sci OA. 2020;6:FSO471. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 14] [Cited by in RCA: 28] [Article Influence: 5.6] [Reference Citation Analysis (0)] |
| 5. | Timmerman RD, Hu C, Michalski JM, Bradley JC, Galvin J, Johnstone DW, Choy H. Long-term Results of Stereotactic Body Radiation Therapy in Medically Inoperable Stage I Non-Small Cell Lung Cancer. JAMA Oncol. 2018;4:1287-1288. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 92] [Cited by in RCA: 183] [Article Influence: 30.5] [Reference Citation Analysis (0)] |
| 6. | Videtic GMM, Donington J, Giuliani M, Heinzerling J, Karas TZ, Kelsey CR, Lally BE, Latzka K, Lo SS, Moghanaki D, Movsas B, Rimner A, Roach M, Rodrigues G, Shirvani SM, Simone CB 2nd, Timmerman R, Daly ME. Stereotactic body radiation therapy for early-stage non-small cell lung cancer: Executive Summary of an ASTRO Evidence-Based Guideline. Pract Radiat Oncol. 2017;7:295-301. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 220] [Cited by in RCA: 330] [Article Influence: 41.3] [Reference Citation Analysis (0)] |
| 7. | Guckenberger M, Andratschke N, Dieckmann K, Hoogeman MS, Hoyer M, Hurkmans C, Tanadini-Lang S, Lartigau E, Méndez Romero A, Senan S, Verellen D. ESTRO ACROP consensus guideline on implementation and practice of stereotactic body radiotherapy for peripherally located early stage non-small cell lung cancer. Radiother Oncol. 2017;124:11-17. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 161] [Cited by in RCA: 246] [Article Influence: 30.8] [Reference Citation Analysis (0)] |
| 8. | Siva S, Ball DL. Single Fraction SBRT for Early Stage Lung Cancer-Less is More? Int J Radiat Oncol Biol Phys. 2019;103:1085-1087. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 6] [Cited by in RCA: 11] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 9. | LEKSELL L. The stereotaxic method and radiosurgery of the brain. Acta Chir Scand. 1951;102:316-319. [PubMed] |
| 10. | Koga T, Shin M, Saito N. Role of γ knife radiosurgery in neurosurgery: past and future perspectives. Neurol Med Chir (Tokyo). 2010;50:737-748. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 12] [Cited by in RCA: 14] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 11. | Nakagawa K, Aoki Y, Tago M, Terahara A, Ohtomo K. Megavoltage CT-assisted stereotactic radiosurgery for thoracic tumors: original research in the treatment of thoracic neoplasms. Int J Radiat Oncol Biol Phys. 2000;48:449-457. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 104] [Cited by in RCA: 81] [Article Influence: 3.2] [Reference Citation Analysis (0)] |
| 12. | Daly ME, Perks JR, Chen AM. Patterns-of-care for thoracic stereotactic body radiotherapy among practicing radiation oncologists in the United States. J Thorac Oncol. 2013;8:202-207. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 50] [Cited by in RCA: 53] [Article Influence: 4.4] [Reference Citation Analysis (0)] |
| 13. | Salama JK, Giuliani ME, Robinson CG, Daly ME. Single-fraction SBRT for Early Stage NSCLC-A Viable Option in "These Uncertain Times"? Int J Radiat Oncol Biol Phys. 2021;109:1-4. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 7] [Cited by in RCA: 5] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 14. | Ng SSW, Ning MS, Lee P, McMahon RA, Siva S, Chuong MD. Single-Fraction Stereotactic Body Radiation Therapy: A Paradigm During the Coronavirus Disease 2019 (COVID-19) Pandemic and Beyond? Adv Radiat Oncol. 2020;5:761-773. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 24] [Cited by in RCA: 31] [Article Influence: 6.2] [Reference Citation Analysis (0)] |
| 15. | Guckenberger M, Belka C, Bezjak A, Bradley J, Daly ME, DeRuysscher D, Dziadziuszko R, Faivre-Finn C, Flentje M, Gore E, Higgins KA, Iyengar P, Kavanagh BD, Kumar S, Le Pechoux C, Lievens Y, Lindberg K, McDonald F, Ramella S, Rengan R, Ricardi U, Rimner A, Rodrigues GB, Schild SE, Senan S, Simone CB 2nd, Slotman BJ, Stuschke M, Videtic G, Widder J, Yom SS, Palma D. Practice Recommendations for Lung Cancer Radiotherapy During the COVID-19 Pandemic: An ESTRO-ASTRO Consensus Statement. Int J Radiat Oncol Biol Phys. 2020;107:631-640. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 24] [Cited by in RCA: 40] [Article Influence: 8.0] [Reference Citation Analysis (0)] |
| 16. | Faivre-Finn C, Fenwick JD, Franks KN, Harrow S, Hatton MQF, Hiley C, McAleese JJ, McDonald F, O'Hare J, Peedell C, Pope T, Powell C, Rulach R, Toy E. Reduced Fractionation in Lung Cancer Patients Treated with Curative-intent Radiotherapy during the COVID-19 Pandemic. Clin Oncol (R Coll Radiol). 2020;32:481-489. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 45] [Cited by in RCA: 43] [Article Influence: 8.6] [Reference Citation Analysis (0)] |
| 17. | Couñago F, Navarro-Martin A, Luna J, Rodríguez de Dios N, Rodríguez A, Casas F, García R, Gómez-Caamaño A, Contreras J, Serrano J. GOECP/SEOR clinical recommendations for lung cancer radiotherapy during the COVID-19 pandemic. World J Clin Oncol. 2020;11:510-527. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 16] [Cited by in RCA: 14] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 18. | Chalkidou A, Macmillan T, Grzeda MT, Peacock J, Summers J, Eddy S, Coker B, Patrick H, Powell H, Berry L, Webster G, Ostler P, Dickinson PD, Hatton MQ, Henry A, Keevil S, Hawkins MA, Slevin N, van As N. Stereotactic ablative body radiotherapy in patients with oligometastatic cancers: a prospective, registry-based, single-arm, observational, evaluation study. Lancet Oncol. 2021;22:98-106. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 76] [Cited by in RCA: 175] [Article Influence: 43.8] [Reference Citation Analysis (0)] |
| 19. | Lehrer EJ, Singh R, Wang M, Chinchilli VM, Trifiletti DM, Ost P, Siva S, Meng MB, Tchelebi L, Zaorsky NG. Safety and Survival Rates Associated With Ablative Stereotactic Radiotherapy for Patients With Oligometastatic Cancer: A Systematic Review and Meta-analysis. JAMA Oncol. 2021;7:92-106. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 52] [Cited by in RCA: 131] [Article Influence: 32.8] [Reference Citation Analysis (0)] |
| 20. | Palma DA, Olson R, Harrow S, Gaede S, Louie AV, Haasbeek C, Mulroy L, Lock M, Rodrigues GB, Yaremko BP, Schellenberg D, Ahmad B, Griffioen G, Senthi S, Swaminath A, Kopek N, Liu M, Moore K, Currie S, Bauman GS, Warner A, Senan S. Stereotactic ablative radiotherapy versus standard of care palliative treatment in patients with oligometastatic cancers (SABR-COMET): a randomised, phase 2, open-label trial. Lancet. 2019;393:2051-2058. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1019] [Cited by in RCA: 1316] [Article Influence: 219.3] [Reference Citation Analysis (0)] |
| 21. | Gomez DR, Blumenschein GR Jr, Lee JJ, Hernandez M, Ye R, Camidge DR, Doebele RC, Skoulidis F, Gaspar LE, Gibbons DL, Karam JA, Kavanagh BD, Tang C, Komaki R, Louie AV, Palma DA, Tsao AS, Sepesi B, William WN, Zhang J, Shi Q, Wang XS, Swisher SG, Heymach JV. Local consolidative therapy versus maintenance therapy or observation for patients with oligometastatic non-small-cell lung cancer without progression after first-line systemic therapy: a multicentre, randomised, controlled, phase 2 study. Lancet Oncol. 2016;17:1672-1682. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 747] [Cited by in RCA: 836] [Article Influence: 92.9] [Reference Citation Analysis (0)] |
| 22. | Iyengar P, Wardak Z, Gerber DE, Tumati V, Ahn C, Hughes RS, Dowell JE, Cheedella N, Nedzi L, Westover KD, Pulipparacharuvil S, Choy H, Timmerman RD. Consolidative Radiotherapy for Limited Metastatic Non-Small-Cell Lung Cancer: A Phase 2 Randomized Clinical Trial. JAMA Oncol. 2018;4:e173501. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 454] [Cited by in RCA: 781] [Article Influence: 111.6] [Reference Citation Analysis (0)] |
| 23. | Qiu B, Aili A, Xue L, Jiang P, Wang J. Advances in Radiobiology of Stereotactic Ablative Radiotherapy. Front Oncol. 2020;10:1165. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 13] [Cited by in RCA: 41] [Article Influence: 8.2] [Reference Citation Analysis (0)] |
| 24. | Chicas-Sett R, Zafra-Martin J, Morales-Orue I, Castilla-Martinez J, Berenguer-Frances MA, Gonzalez-Rodriguez E, Rodriguez-Abreu D, Couñago F. Immunoradiotherapy as An Effective Therapeutic Strategy in Lung Cancer: From Palliative Care to Curative Intent. Cancers (Basel). 2020;12. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 27] [Cited by in RCA: 24] [Article Influence: 4.8] [Reference Citation Analysis (0)] |
| 25. | Kelada OJ, Decker RH, Nath SK, Johung KL, Zheng MQ, Huang Y, Gallezot JD, Liu C, Carson RE, Oelfke U, Carlson DJ. High Single Doses of Radiation May Induce Elevated Levels of Hypoxia in Early-Stage Non-Small Cell Lung Cancer Tumors. Int J Radiat Oncol Biol Phys. 2018;102:174-183. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 32] [Cited by in RCA: 31] [Article Influence: 4.4] [Reference Citation Analysis (0)] |
| 26. | Hara R, Itami J, Kondo T, Aruga T, Uno T, Sasano N, Ohnishi K, Kiyozuka M, Fuse M, Ito M, Naoi K, Kohno Y. Clinical outcomes of single-fraction stereotactic radiation therapy of lung tumors. Cancer. 2006;106:1347-1352. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 84] [Cited by in RCA: 75] [Article Influence: 3.9] [Reference Citation Analysis (0)] |
| 27. | Singh R, Ansinelli H, Sharma D, Jenkins J, Davis J, Vargo JA, Sharma S. Clinical Outcomes Following Stereotactic Body Radiation Therapy (SBRT) for Stage I Medically Inoperable Small Cell Lung Carcinoma: A Multi-Institutional Analysis From the RSSearch Patient Registry. Am J Clin Oncol. 2019;42:602-606. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 7] [Cited by in RCA: 7] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 28. | Whyte RI, Crownover R, Murphy MJ, Martin DP, Rice TW, DeCamp MM Jr, Rodebaugh R, Weinhous MS, Le QT. Stereotactic radiosurgery for lung tumors: preliminary report of a phase I trial. Ann Thorac Surg. 2003;75:1097-1101. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 259] [Cited by in RCA: 216] [Article Influence: 9.8] [Reference Citation Analysis (0)] |
| 29. | Le QT, Loo BW, Ho A, Cotrutz C, Koong AC, Wakelee H, Kee ST, Constantinescu D, Whyte RI, Donington J. Results of a phase I dose-escalation study using single-fraction stereotactic radiotherapy for lung tumors. J Thorac Oncol. 2006;1:802-809. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 57] [Cited by in RCA: 53] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 30. | Videtic GM, Hu C, Singh AK, Chang JY, Parker W, Olivier KR, Schild SE, Komaki R, Urbanic JJ, Timmerman RD, Choy H. A Randomized Phase 2 Study Comparing 2 Stereotactic Body Radiation Therapy Schedules for Medically Inoperable Patients With Stage I Peripheral Non-Small Cell Lung Cancer: NRG Oncology RTOG 0915 (NCCTG N0927). Int J Radiat Oncol Biol Phys. 2015;93:757-764. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 302] [Cited by in RCA: 293] [Article Influence: 29.3] [Reference Citation Analysis (0)] |
| 31. | Singh AK, Gomez-Suescun JA, Stephans KL, Bogart JA, Hermann GM, Tian L, Groman A, Videtic GM. One Versus Three Fractions of Stereotactic Body Radiation Therapy for Peripheral Stage I to II Non-Small Cell Lung Cancer: A Randomized, Multi-Institution, Phase 2 Trial. Int J Radiat Oncol Biol Phys. 2019;105:752-759. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 90] [Cited by in RCA: 85] [Article Influence: 14.2] [Reference Citation Analysis (0)] |
| 32. | Timmerman R, Paulus R, Galvin J, Michalski J, Straube W, Bradley J, Fakiris A, Bezjak A, Videtic G, Johnstone D, Fowler J, Gore E, Choy H. Stereotactic body radiation therapy for inoperable early stage lung cancer. JAMA. 2010;303:1070-1076. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 2018] [Cited by in RCA: 1955] [Article Influence: 130.3] [Reference Citation Analysis (0)] |
| 33. | Videtic GM, Stephans KL, Woody NM, Reddy CA, Zhuang T, Magnelli A, Djemil T. 30 Gy or 34 Gy? Int J Radiat Oncol Biol Phys. 2014;90:203-208. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 51] [Cited by in RCA: 60] [Article Influence: 5.5] [Reference Citation Analysis (0)] |
| 34. | Xiao Y, Papiez L, Paulus R, Timmerman R, Straube WL, Bosch WR, Michalski J, Galvin JM. Dosimetric evaluation of heterogeneity corrections for RTOG 0236: stereotactic body radiotherapy of inoperable stage I-II non-small-cell lung cancer. Int J Radiat Oncol Biol Phys. 2009;73:1235-1242. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 140] [Cited by in RCA: 131] [Article Influence: 8.2] [Reference Citation Analysis (0)] |
| 35. | Timmerman R, McGarry R, Yiannoutsos C, Papiez L, Tudor K, DeLuca J, Ewing M, Abdulrahman R, DesRosiers C, Williams M, Fletcher J. Excessive toxicity when treating central tumors in a phase II study of stereotactic body radiation therapy for medically inoperable early-stage lung cancer. J Clin Oncol. 2006;24:4833-4839. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1074] [Cited by in RCA: 1134] [Article Influence: 59.7] [Reference Citation Analysis (0)] |
| 36. | Lagerwaard FJ, Haasbeek CJ, Smit EF, Slotman BJ, Senan S. Outcomes of risk-adapted fractionated stereotactic radiotherapy for stage I non-small-cell lung cancer. Int J Radiat Oncol Biol Phys. 2008;70:685-692. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 415] [Cited by in RCA: 421] [Article Influence: 23.4] [Reference Citation Analysis (0)] |
| 37. | Palma DA. An Ultracentral Lung Tumor. Int J Radiat Oncol Biol Phys. 2017;97:651. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5] [Cited by in RCA: 6] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 38. | Owen D, Sio TT. Stereotactic body radiotherapy (SBRT) for central and ultracentral node-negative lung tumors. J Thorac Dis. 2020;12:7024-7031. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 3] [Cited by in RCA: 10] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 39. | Bezjak A, Paulus R, Gaspar LE, Timmerman RD, Straube WL, Ryan WF, Garces YI, Pu AT, Singh AK, Videtic GM, McGarry RC, Iyengar P, Pantarotto JR, Urbanic JJ, Sun AY, Daly ME, Grills IS, Sperduto P, Normolle DP, Bradley JD, Choy H. Safety and Efficacy of a Five-Fraction Stereotactic Body Radiotherapy Schedule for Centrally Located Non-Small-Cell Lung Cancer: NRG Oncology/RTOG 0813 Trial. J Clin Oncol. 2019;37:1316-1325. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 403] [Cited by in RCA: 388] [Article Influence: 64.7] [Reference Citation Analysis (0)] |
| 40. | The Faculty of Clinical Oncology of The Royal College of Radiologists. UK SABR consortium guidelines v6.1. 2019. Available from: https://www.sabr.org.uk/wp-content/uploads/2019/03/SABRconsortium-guidelines-2019-v6.1.0.pdf. |
| 41. | Giuliani M, Mathew AS, Bahig H, Bratman SV, Filion E, Glick D, Louie AV, Raman S, Swaminath A, Warner A, Yau V, Palma D. SUNSET: Stereotactic Radiation for Ultracentral Non-Small-Cell Lung Cancer-A Safety and Efficacy Trial. Clin Lung Cancer. 2018;19:e529-e532. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 44] [Cited by in RCA: 80] [Article Influence: 11.4] [Reference Citation Analysis (0)] |
| 42. | Adebahr S, Collette S, Shash E, Lambrecht M, Le Pechoux C, Faivre-Finn C, De Ruysscher D, Peulen H, Belderbos J, Dziadziuszko R, Fink C, Guckenberger M, Hurkmans C, Nestle U. LungTech, an EORTC Phase II trial of stereotactic body radiotherapy for centrally located lung tumours: a clinical perspective. Br J Radiol. 2015;88:20150036. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 76] [Cited by in RCA: 78] [Article Influence: 7.8] [Reference Citation Analysis (0)] |
| 43. | Ma SJ, Syed YA, Rivers CI, Gomez Suescun JA, Singh AK. Comparison of single- and five-fraction schedules of stereotactic body radiation therapy for central lung tumours: a single institution experience. J Radiother Pract. 2017;16:148-154. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 16] [Cited by in RCA: 13] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 44. | Siva S, Kirby K, Caine H, Pham D, Kron T, Te Marvelde L, Whalley D, Stevens MJ, Foroudi F, MacManus M, Ball D, Eade T. Comparison of Single-fraction and Multi-fraction Stereotactic Radiotherapy for Patients with 18F-fluorodeoxyglucose Positron Emission Tomography-staged Pulmonary Oligometastases. Clin Oncol (R Coll Radiol). 2015;27:353-361. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 27] [Cited by in RCA: 30] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 45. | Trakul N, Chang CN, Harris J, Chapman C, Rao A, Shen J, Quinlan-Davidson S, Filion EJ, Wakelee HA, Colevas AD, Whyte RI, Dieterich S, Maxim PG, Hristov D, Tran P, Le QT, Loo BW Jr, Diehn M. Tumor volume-adapted dosing in stereotactic ablative radiotherapy of lung tumors. Int J Radiat Oncol Biol Phys. 2012;84:231-237. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 57] [Cited by in RCA: 59] [Article Influence: 4.5] [Reference Citation Analysis (0)] |
| 46. | Kumar AMS, Woody NM, Djemil T, Videtic GMM, Stephans KL. Synchronous non small cell lung cancer nodules treated with stereotactic body radiation therapy (SBRT). J Radiosurg SBRT. 2014;3:81-88. [PubMed] |
| 47. | Tekatli H, Tetar SU, Nguyen TK, Warner A, Verbakel WF, Palma DA, Dahele M, Gaede S, Haasbeek C, Spoelstra FO, de Haan PF, Slotman BJ, Senan S. Optimizing SABR delivery for synchronous multiple lung tumors using volumetric-modulated arc therapy. Acta Oncol. 2017;56:548-554. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 10] [Cited by in RCA: 15] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 48. | Kennedy WR, Gabani P, Nikitas J, Robinson CG, Bradley JD, Roach MC. Repeat stereotactic body radiation therapy (SBRT) for salvage of isolated local recurrence after definitive lung SBRT. Radiother Oncol. 2020;142:230-235. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 27] [Cited by in RCA: 32] [Article Influence: 6.4] [Reference Citation Analysis (0)] |
| 49. | Ogawa Y, Shibamoto Y, Hashizume C, Kondo T, Iwata H, Tomita N, Ogino H. Repeat stereotactic body radiotherapy (SBRT) for local recurrence of non-small cell lung cancer and lung metastasis after first SBRT. Radiat Oncol. 2018;13:136. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 22] [Cited by in RCA: 40] [Article Influence: 5.7] [Reference Citation Analysis (0)] |
| 50. | Ricco A, Barlow S, Feng J, Jacob J, Lozano A, Hanlon A, Arrigo S, Obayomi-Davies O, Lamond J, Yang J, Lanciano R. Repeat Thoracic Stereotactic Body Radiation Therapy (SBRT) for Nonsmall Cell Lung Cancer: Long-Term Outcomes, Toxicity, and Dosimetric Considerations. Adv Radiat Oncol. 2020;5:984-993. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 5] [Cited by in RCA: 5] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 51. | Pennathur A, Luketich JD, Heron DE, Schuchert MJ, Bianco V, Clump D, Burton S, Abbas G, Gooding WE, Ozhasoglu C, Landreneau RJ, Christie NA. Stereotactic Radiosurgery/Stereotactic Body Radiotherapy for Recurrent Lung Neoplasm: An Analysis of Outcomes in 100 Patients. Ann Thorac Surg. 2015;100:2019-2024. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 12] [Cited by in RCA: 12] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 52. | Ripley RT, Rusch VW. Lung Metastases. In: Abeloff’s Clinical Oncology: Fifth Edition. Elsevier Inc.; 2013: 764-777. |
| 53. | Fritz P, Kraus HJ, Mühlnickel W, Hammer U, Dölken W, Engel-Riedel W, Chemaissani A, Stoelben E. Stereotactic, single-dose irradiation of stage I non-small cell lung cancer and lung metastases. Radiat Oncol. 2006;1:30. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 85] [Cited by in RCA: 78] [Article Influence: 4.1] [Reference Citation Analysis (0)] |
| 54. | Wulf J, Baier K, Mueller G, Flentje MP. Dose-response in stereotactic irradiation of lung tumors. Radiother Oncol. 2005;77:83-87. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 196] [Cited by in RCA: 182] [Article Influence: 9.1] [Reference Citation Analysis (0)] |
| 55. | Siva S, Bressel M, Kron T, Mai T, Le HV, Montgomery R, Hardcastle N, Rezo A, Gill S, Higgs BG, Pryor DI, De Abreu Lourenco R, Awad R, Chesson B, Eade TN, Skala M, Sasso G, Wong W, Vinod S, Ball D. Stereotactic Ablative Fractionated Radiotherapy vs Radiosurgery for Oligometastatic Neoplasia to the Lung: A Randomized Phase II Trial. Int J Radiat Oncol Biol Phys. 2020;108 Suppl:S3-S4. [RCA] [DOI] [Full Text] [Cited by in Crossref: 4] [Cited by in RCA: 2] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 56. | Gandhidasan S, Ball D, Kron T, Bressel M, Shaw M, Chu J, Chander S, Wheeler G, Plumridge N, Chesson B, David S, Siva S. Single Fraction Stereotactic Ablative Body Radiotherapy for Oligometastasis: Outcomes from 132 Consecutive Patients. Clin Oncol (R Coll Radiol). 2018;30:178-184. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 14] [Cited by in RCA: 14] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 57. | Osti MF, Carnevale A, Valeriani M, De Sanctis V, Minniti G, Cortesi E, Martelli M, Maurizi Enrici R. Clinical outcomes of single dose stereotactic radiotherapy for lung metastases. Clin Lung Cancer. 2013;14:699-703. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 31] [Cited by in RCA: 40] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 58. | Filippi AR, Badellino S, Guarneri A, Levis M, Botticella A, Mantovani C, Ragona R, Racca P, Buffoni L, Novello S, Ricardi U. Outcomes of single fraction stereotactic ablative radiotherapy for lung metastases. Technol Cancer Res Treat. 2014;13:37-45. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 34] [Cited by in RCA: 38] [Article Influence: 3.5] [Reference Citation Analysis (0)] |
| 59. | Osti MF, Agolli L, Valeriani M, Reverberi C, Bracci S, Marinelli L, De Sanctis V, Cortesi E, Martelli M, De Dominicis C, Minniti G, Nicosia L. 30 Gy single dose stereotactic body radiation therapy (SBRT): Report on outcome in a large series of patients with lung oligometastatic disease. Lung Cancer. 2018;122:165-170. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 21] [Cited by in RCA: 36] [Article Influence: 5.1] [Reference Citation Analysis (0)] |
| 60. | Sogono P, Bressel M, David S, Shaw M, Chander S, Chu J, Plumridge N, Byrne K, Hardcastle N, Kron T, Wheeler G, Hanna GG, MacManus M, Ball D, Siva S. Safety, Efficacy, and Patterns of Failure After Single-Fraction Stereotactic Body Radiation Therapy (SBRT) for Oligometastases. Int J Radiat Oncol Biol Phys. 2021;109:756-763. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 8] [Cited by in RCA: 25] [Article Influence: 5.0] [Reference Citation Analysis (0)] |
| 61. | Sharma A, Duijm M, Oomen-de Hoop E, Aerts JG, Verhoef C, Hoogeman M, Nuyttens JJ. Factors affecting local control of pulmonary oligometastases treated with stereotactic body radiotherapy. Acta Oncol. 2018;57:1031-1037. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 16] [Cited by in RCA: 28] [Article Influence: 4.0] [Reference Citation Analysis (0)] |
| 62. | Nuyttens JJ, van der Voort van Zyp NC, Verhoef C, Maat A, van Klaveren RJ, van der Holt B, Aerts J, Hoogeman M. Stereotactic body radiation therapy for oligometastases to the lung: a phase 2 study. Int J Radiat Oncol Biol Phys. 2015;91:337-343. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 55] [Cited by in RCA: 58] [Article Influence: 5.8] [Reference Citation Analysis (0)] |
| 63. | Hof H, Hoess A, Oetzel D, Debus J, Herfarth K. Stereotactic single-dose radiotherapy of lung metastases. Strahlenther Onkol. 2007;183:673-678. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 75] [Cited by in RCA: 64] [Article Influence: 3.6] [Reference Citation Analysis (0)] |
| 64. | Siva S, Kron T, Bressel M, Haas M, Mai T, Vinod S, Sasso G, Wong W, Le H, Eade T, Hardcastle N, Chesson B, Pham D, Høyer M, Montgomery R, Ball D. A randomised phase II trial of Stereotactic Ablative Fractionated radiotherapy versus Radiosurgery for Oligometastatic Neoplasia to the lung (TROG 13.01 SAFRON II). BMC Cancer. 2016;16:183. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 25] [Cited by in RCA: 32] [Article Influence: 3.6] [Reference Citation Analysis (0)] |
| 65. | Sher DJ, Wee JO, Punglia RS. Cost-effectiveness analysis of stereotactic body radiotherapy and radiofrequency ablation for medically inoperable, early-stage non-small cell lung cancer. Int J Radiat Oncol Biol Phys. 2011;81:e767-e774. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 62] [Cited by in RCA: 71] [Article Influence: 5.1] [Reference Citation Analysis (0)] |
| 66. | Nicosia L, Reverberi C, Agolli L, Marinelli L, De Sanctis V, Valeriani M, Osti MF. Long term results of single high dose Stereotactic Body Radiotherapy in the treatment of primary lung tumors. Sci Rep. 2019;9:15498. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 9] [Cited by in RCA: 13] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 67. | Luo J, Rizvi H, Preeshagul IR, Egger JV, Hoyos D, Bandlamudi C, McCarthy CG, Falcon CJ, Schoenfeld AJ, Arbour KC, Chaft JE, Daly RM, Drilon A, Eng J, Iqbal A, Lai WV, Li BT, Lito P, Namakydoust A, Ng K, Offin M, Paik PK, Riely GJ, Rudin CM, Yu HA, Zauderer MG, Donoghue MTA, Łuksza M, Greenbaum BD, Kris MG, Hellmann MD. COVID-19 in patients with lung cancer. Ann Oncol. 2020;31:1386-1396. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 196] [Cited by in RCA: 181] [Article Influence: 36.2] [Reference Citation Analysis (0)] |
| 68. | Garassino MC, Whisenant JG, Huang LC, Trama A, Torri V, Agustoni F, Baena J, Banna G, Berardi R, Bettini AC, Bria E, Brighenti M, Cadranel J, De Toma A, Chini C, Cortellini A, Felip E, Finocchiaro G, Garrido P, Genova C, Giusti R, Gregorc V, Grossi F, Grosso F, Intagliata S, La Verde N, Liu SV, Mazieres J, Mercadante E, Michielin O, Minuti G, Moro-Sibilot D, Pasello G, Passaro A, Scotti V, Solli P, Stroppa E, Tiseo M, Viscardi G, Voltolini L, Wu YL, Zai S, Pancaldi V, Dingemans AM, Van Meerbeeck J, Barlesi F, Wakelee H, Peters S, Horn L; TERAVOLT investigators. COVID-19 in patients with thoracic malignancies (TERAVOLT): first results of an international, registry-based, cohort study. Lancet Oncol. 2020;21:914-922. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 484] [Cited by in RCA: 470] [Article Influence: 94.0] [Reference Citation Analysis (0)] |
| 69. | Kidane B, Spicer J, Kim JO, Fiset PO, Abdulkarim B, Malthaner R, Palma D. SABR-BRIDGE: Stereotactic ABlative Radiotherapy Before Resection to AvoId Delay for Early-Stage LunG Cancer or OligomEts During the COVID-19 Pandemic. Front Oncol. 2020;10:580189. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 14] [Cited by in RCA: 19] [Article Influence: 3.8] [Reference Citation Analysis (0)] |
| 70. | Yang SR, Schultheis AM, Yu H, Mandelker D, Ladanyi M, Büttner R. Precision medicine in non-small cell lung cancer: Current applications and future directions. Semin Cancer Biol. 2020;. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 24] [Cited by in RCA: 140] [Article Influence: 28.0] [Reference Citation Analysis (0)] |
| 71. | NSCLC Meta-Analyses Collaborative Group. Chemotherapy in addition to supportive care improves survival in advanced non-small-cell lung cancer: a systematic review and meta-analysis of individual patient data from 16 randomized controlled trials. J Clin Oncol. 2008;26:4617-4625. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 457] [Cited by in RCA: 444] [Article Influence: 26.1] [Reference Citation Analysis (0)] |
| 72. | Helou J, Thibault I, Poon I, Chiang A, Jain S, Soliman H, Erler D, Yeung L, Cheung P. Stereotactic Ablative Radiation Therapy for Pulmonary Metastases: Histology, Dose, and Indication Matter. Int J Radiat Oncol Biol Phys. 2017;98:419-427. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 40] [Cited by in RCA: 56] [Article Influence: 7.0] [Reference Citation Analysis (0)] |
| 73. | Petrelli F, Ghidini A, Cabiddu M, Tomasello G, De Stefani A, Bruschieri L, Vitali E, Ghilardi M, Borgonovo K, Barni S, Trevisan F. Addition of radiotherapy to the primary tumour in oligometastatic NSCLC: A systematic review and meta-analysis. Lung Cancer. 2018;126:194-200. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 21] [Cited by in RCA: 35] [Article Influence: 5.0] [Reference Citation Analysis (0)] |
| 74. | Couñago F, Luna J, Guerrero LL, Vaquero B, Guillén-Sacoto MC, González-Merino T, Taboada B, Díaz V, Rubio-Viqueira B, Díaz-Gavela AA, Marcos FJ, Del Cerro E. Management of oligometastatic non-small cell lung cancer patients: Current controversies and future directions. World J Clin Oncol. 2019;10:318-339. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 39] [Cited by in RCA: 35] [Article Influence: 5.8] [Reference Citation Analysis (0)] |
| 75. | Wang X, Zeng M. First-line tyrosine kinase inhibitor with or without aggressive upfront local radiation therapy in patients with EGFRm oligometastatic non-small cell lung cancer: Interim results of a randomized phase III, open-label clinical trial (SINDAS)(NCT02893332). J Clin Oncol. 2020;38:9508. [RCA] [DOI] [Full Text] [Cited by in Crossref: 25] [Cited by in RCA: 26] [Article Influence: 5.2] [Reference Citation Analysis (0)] |
| 76. | Chicas-Sett R, Morales-Orue I, Castilla-Martinez J, Zafra-Martin J, Kannemann A, Blanco J, Lloret M, Lara PC. Stereotactic Ablative Radiotherapy Combined with Immune Checkpoint Inhibitors Reboots the Immune Response Assisted by Immunotherapy in Metastatic Lung Cancer: A Systematic Review. Int J Mol Sci. 2019;20. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 40] [Cited by in RCA: 64] [Article Influence: 10.7] [Reference Citation Analysis (0)] |
| 77. | Theelen WSME, Peulen HMU, Lalezari F, van der Noort V, de Vries JF, Aerts JGJV, Dumoulin DW, Bahce I, Niemeijer AN, de Langen AJ, Monkhorst K, Baas P. Effect of Pembrolizumab After Stereotactic Body Radiotherapy vs Pembrolizumab Alone on Tumor Response in Patients With Advanced Non-Small Cell Lung Cancer: Results of the PEMBRO-RT Phase 2 Randomized Clinical Trial. JAMA Oncol. 2019;5:1276-1282. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 362] [Cited by in RCA: 695] [Article Influence: 115.8] [Reference Citation Analysis (0)] |
| 78. | Bauml JM, Mick R, Ciunci C, Aggarwal C, Davis C, Evans T, Deshpande C, Miller L, Patel P, Alley E, Knepley C, Mutale F, Cohen RB, Langer CJ. Pembrolizumab After Completion of Locally Ablative Therapy for Oligometastatic Non-Small Cell Lung Cancer: A Phase 2 Trial. JAMA Oncol. 2019;5:1283-1290. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 128] [Cited by in RCA: 230] [Article Influence: 38.3] [Reference Citation Analysis (0)] |
| 79. | Theelen WSME, Chen D, Verma V, Hobbs BP, Peulen HMU, Aerts JGJV, Bahce I, Niemeijer ALN, Chang JY, de Groot PM, Nguyen QN, Comeaux NI, Simon GR, Skoulidis F, Lin SH, He K, Patel R, Heymach J, Baas P, Welsh JW. Pembrolizumab with or without radiotherapy for metastatic non-small-cell lung cancer: a pooled analysis of two randomised trials. Lancet Respir Med. 2021;9:467-475. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 98] [Cited by in RCA: 345] [Article Influence: 69.0] [Reference Citation Analysis (0)] |
| 80. | Lubas MJ, Kumar SS. The Combined Use of SBRT and Immunotherapy-a Literature Review. Curr Oncol Rep. 2020;22:117. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 9] [Cited by in RCA: 10] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 81. | Yang H, Jin T, Li M, Xue J, Lu B. Synergistic effect of immunotherapy and radiotherapy in non-small cell lung cancer current clinical trials and prospective challenges. Precis Clin Med. 2019;2:57-70. [RCA] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 25] [Cited by in RCA: 25] [Article Influence: 4.2] [Reference Citation Analysis (0)] |
| 82. | Yamamoto N, Miyamoto T, Nakajima M, Karube M, Hayashi K, Tsuji H, Tsujii H, Kamada T, Fujisawa T. A Dose Escalation Clinical Trial of Single-Fraction Carbon Ion Radiotherapy for Peripheral Stage I Non-Small Cell Lung Cancer. J Thorac Oncol. 2017;12:673-680. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 36] [Cited by in RCA: 48] [Article Influence: 5.3] [Reference Citation Analysis (0)] |
| 83. | Helm A, Ebner DK, Tinganelli W, Simoniello P, Bisio A, Marchesano V, Durante M, Yamada S, Shimokawa T. Combining Heavy-Ion Therapy with Immunotherapy: An Update on Recent Developments. Int J Part Ther. 2018;5:84-93. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 19] [Cited by in RCA: 19] [Article Influence: 2.7] [Reference Citation Analysis (0)] |