Published online Nov 24, 2022. doi: 10.5306/wjco.v13.i11.918
Peer-review started: September 19, 2022
First decision: October 13, 2022
Revised: October 13, 2022
Accepted: November 4, 2022
Article in press: November 4, 2022
Published online: November 24, 2022
Processing time: 62 Days and 21.5 Hours
Presence of microvascular invasion (MVI) indicates poorer prognosis post-curative resection of hepatocellular carcinoma (HCC), with an increased chance of tumour recurrence. By present standards, MVI can only be diagnosed post-operatively on histopathology. Texture analysis potentially allows identification of patients who are considered ‘high risk’ through analysis of pre-operative magnetic resonance imaging (MRI) studies. This will allow for better patient selection, improved individualised therapy (such as extended surgical margins or adjuvant therapy) and pre-operative prognostication.
This study aims to evaluate the accuracy of texture analysis on pre-operative MRI in predicting MVI in HCC.
Retrospective review of patients with new cases of HCC who underwent hepatectomy between 2007 and 2015 was performed. Exclusion criteria: No pre-operative MRI, significant movement artefacts, loss-to-follow-up, ruptured HCCs, previous hepatectomy and adjuvant therapy. Fifty patients were divided into MVI (n = 15) and non-MVI (n = 35) groups based on tumour histology. Selected images of the tumour on post-contrast-enhanced T1-weighted MRI were analysed. Both qualitative (performed by radiologists) and quantitative data (performed by software) were obtained. Radiomics texture parameters were extracted based on the largest cross-sectional area of each tumor and analysed using MaZda software. Five separate methods were performed. Methods 1, 2 and 3 exclusively made use of features derived from arterial, portovenous and equilibrium phases respectively. Methods 4 and 5 made use of the comparatively significant features to attain optimal performance.
Method 5 achieved the highest accuracy of 87.8% with sensitivity of 73% and specificity of 94%.
Texture analysis of tumours on pre-operative MRI can predict presence of MVI in HCC with accuracies of up to 87.8% and can potentially impact clinical management.
Core Tip: This study demonstrates the utility of texture analysis on pre-operative magnetic resonance imaging to potentially impact clinical management in patients with surgically resectable hepatocellular carcinoma.
- Citation: Sim JZT, Hui TCH, Chuah TK, Low HM, Tan CH, Shelat VG. Efficacy of texture analysis of pre-operative magnetic resonance imaging in predicting microvascular invasion in hepatocellular carcinoma. World J Clin Oncol 2022; 13(11): 918-928
- URL: https://www.wjgnet.com/2218-4333/full/v13/i11/918.htm
- DOI: https://dx.doi.org/10.5306/wjco.v13.i11.918
Macrovascular invasion and microvascular invasion (MVI) are independent prognostic factors of hepatocellular carcinoma (HCC) recurrence after curative partial hepatectomy or liver transplantation[1-3]. Previous studies have shown MVI to shorten disease-free survival and overall survival post-liver transplantation and liver resection[4]. While presence of macrovascular invasion can often be determined definitively on pre-operative cross-sectional imaging, microvascular invasion in HCC is typically only diagnosed post-operatively on histopathology. Moreover, the lack of consensus definition and grading of MVI, coupled with inter/intra-observer variability, has resulted in great heterogeneity in evaluation of this histopathological feature[5]. Previous studies have shown that the incidence of MVI ranges between 15% and 57.1%[4]. Pre-operative diagnosis of microvascular invasion to identify patients who are considered ‘high risk’ will allow for better patient selection, improved individualised therapy (such as extended surgical margins or adjuvant therapy) and pre-operative prognostication.
Texture analysis (TA) is a branch of computer vision that analyses and objectifies imaging characteristics that may be imperceptible to the human eye. It can be applied to any cross-sectional imaging and has been proven to reflect underlying heterogeneity. This technique may provide quantitative data and insight into tumor biology and thus has the potential to be used to diagnose and prognosticate disease[6-9]. Several groups have attempted to identify MVI pre-operatively using clinical data and imaging scoring systems albeit with variable and sometimes conflicting results[10-12], limiting its translation into clinical practice. Moreover, the detection of MVI by using pre-operative biopsy has proven to be unreliable as it did not correlate well with post-operative pathology[13]. Recent studies have sought to use TA to predict MVI on MRI and have identified certain imaging and textural features (such as tumour entropy) that may be associated with bad tumour behaviour[14,15]. While TA continues to grow as an emerging technology, before it can be considered for widespread clinical implementation, we sought to validate and replicate those findings. In this study, we aim to evaluate the accuracy of texture analysis on pre-operative contrast-enhanced MRI to predict microvascular invasion in HCC.
Institutional review board was obtained and the requirement to obtain written consent was waived. All patients who underwent hepatectomy between January 1, 2007 and December 31, 2015 were considered for study inclusion. Clinical and pathologic parameters were retrospectively reviewed on electronic medical records. Inclusion criteria included histologically proven HCC with pre-operative MRI (done with 1.5T), tumour size ≥ 1 cm, treatment-naïve HCC. Exclusion criteria included loss to follow-up, collision tumours, ruptured HCCs, previous hepatectomy and interval adjuvant therapy, such as transarterial chemoembolization or radiofrequency ablation. For texture analysis evaluation, additional imaging-specific exclusion criteria included 3 T MRI, outdated MRI protocols, and images degraded by motion artefacts.
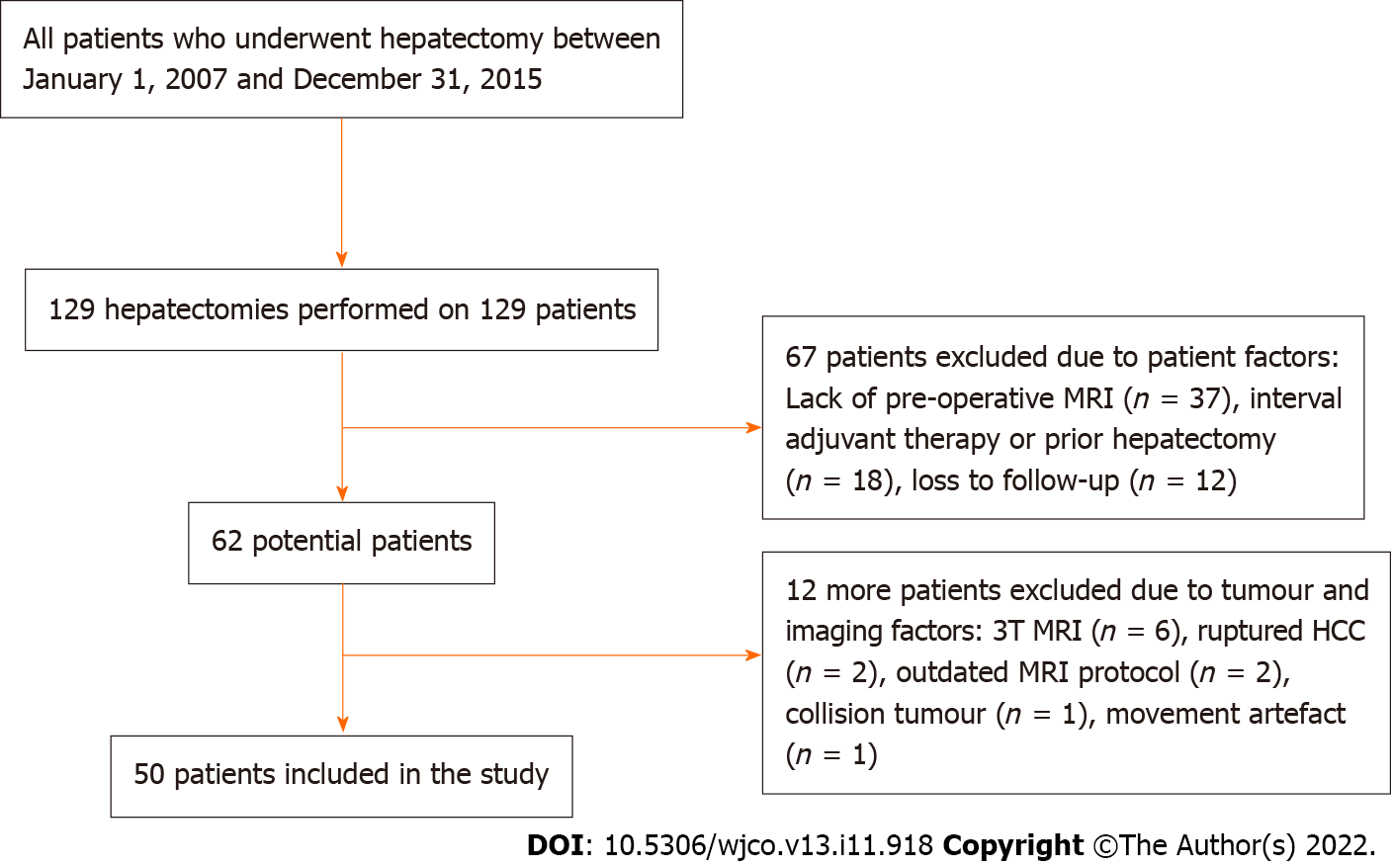
A total of 129 hepatectomies were performed on 129 HCC patients within the study period. 79 patients were excluded from the study for the following reasons: absence of pre-operative MRI (n = 37), interval adjuvant therapy or prior hepatectomy (n = 18), loss to follow-up (n = 12), 3 T MRI (n = 6), ruptured HCC (n = 2), old MRI protocol (n = 2), collision tumour (n = 1), movement artefact (n = 1). A representative flowchart is shown in Figure 1.
The primary outcome measure was the presence of MVI based on histopathological findings. Clinical factors that were potentially associated with MVI were analysed: age, gender, aetiology, hepatitis B surface antigen (HBsAg), serum alpha-fetoprotein, albumin, bilirubin, alanine amino-transferase, aspartate amino-transferase, alkaline phosphatase, ɣ-glutamyltranspeptidase (GGT) and Child-Pugh score.
Magnetic resonance (MR) imaging studies were performed using 1.5 T scanners (Signa HDxt, GE Medical Systems, Milwaukee, WI, United States; Ingenia, Philips, Amsterdam, The Netherlands). The liver imaging protocol included the following sequences: Breath-hold gradient-echo T1-weighted [4.2 ms repetition time (TR)/2 ms echo time (TE), 40 cm field of view, 12o flip angle, 5 mm section thickness], free-breathing spin-echo T2-weighted (10000 ms TR/82.8 ms TE, 40 cm field of view, 90o flip angle, 6 mm section thickness), diffusion-weighted (6000 ms TR / 66.1 ms TE, 40 cm field of view, 90o flip angle, 5 mm section thickness, b = 1000 s/mm2) and T1-weighted contrast-enhanced sequences performed in the arterial (20-second scanning delay), portal venous (70-second scanning delay), and equilibrium phases (180-second scanning delay). Of note, one of the cases had no useable equilibrium phase image. Gadobenate dimeglumine (n = 36; MultiHance, BRACCO Altana Pharma, Constance, Switzerland), gadoterate meglumine (n = 8; Dotarem, Guerbet Roissy, France) and gadoxetic acid (n = 6; Primovist, Bayer Schering Pharma AG, Berlin, Germany) were used as contrast material. All MR examinations utilised 10 cc of the respective contrast agents.
The pre-operative MR images were first independently evaluated on the picture archiving communication system by two radiologists with 13 and five years of experience (Tan CH and Low HM). The reviewers were aware that the patients had HCC but were blinded to the diagnosis of MVI by pathologic examination. The images were evaluated for tumour size (mm), tumour multiplicity, T1 pre-contrast signal intensity, post-contrast enhancement pattern in all phases, T2 hyperintensity, restricted diffusion, visibility of vessels in post-contrast sequences, peri-tumoral features, presence of hypodense halo, definition of border between tumour and liver, tumour margin smoothness and LI-RADS score (see Table 1). The reviewers reviewed the images in consensus and assessed the imaging features subjectively, with a binary “Yes/No” output.
| Total | MVI group | Non-MVI group | P value | |
| Tumors, n (%) | 50 (100) | 15 (30) | 35 (70) | |
| Size (mm), mean (SD) | 56.4 (34.6) | 41.3 (29.1) | 0.107 | |
| Multiplicity, n (%) | 0.087 | |||
| Single | 43 (86) | 15 (100) | 28 (80) | |
| Multifocal | 7 (14) | 0 | 7 (20) | |
| T1 (unenhanced), n (%) | 0.346 | |||
| Hypointense | 46 (92) | 13 (87) | 33 (94) | |
| Isointense | 2 (4) | 1 (7) | 1 (3) | |
| Hyperintense | 2 (4) | 1 (7) | 1 (3) | |
| T1 (arterial phase), n (%) | 0.663 | |||
| Hypointense | 6 (12) | 1 (7) | 5 (14) | |
| Isointense | 6 (12) | 1 (7) | 5 (14) | |
| Hyperintense | 38 (76) | 13 (87) | 25 (71) | |
| T1 (portal venous phase), n (%) | 0.231 | |||
| Hypointense | 42 (84) | 14 (93) | 28 (80) | |
| Isointense | 6 (12) | 0 (0) | 6 (17) | |
| Hyperintense | 2 (4) | 1 (7) | 1 (3) | |
| T1 (equilibrium phase), n (%) | 0.654 | |||
| Hypointense | 45 (90) | 14 (93) | 31 (89) | |
| Isointense | 4 (8) | 1 (7) | 3 (9) | |
| Hyperintense | 0 | 0 (0) | 0 (0) | |
| T1 (HPB phase), n (%) | 0.664 | |||
| Hypointense | 33 (66) | 9 (60) | 24 (69) | |
| Isointense | 1 (2) | 0 | 1 (3) | |
| Not performed | 16 (32) | 6 (40) | 10 (28) | |
| T2 hyperintensity, n (%) | 0.451 | |||
| Present | 42 (84) | 12 (80) | 30 (86) | |
| Absent | 8 (16) | 3 (20) | 5 (14) | |
| Restricted diffusion, n (%) | 0.745 | |||
| Present | 44 (88) | 13 (87) | 31 (89) | |
| Absent | 5 (10) | 2 (13) | 3 (9) | |
| Not performed | 1 (2) | 0 (0) | 1 (3) | |
| Visible vessels (arterial phase), n (%) | 0.001 | |||
| Present | 11 (22) | 8 (53) | 3 (9) | |
| Absent | 39 (78) | 7 (47) | 32 (91) | |
| Visible vessels (portal venous phase), n (%) | 0.043 | |||
| Present | 8 (16) | 5 (33) | 3 (9) | |
| Absent | 42 (84) | 10 (67) | 32 (91) | |
| Peritumoral enhancement, n (%) | 0.248 | |||
| Present | 15 (30) | 6 (40) | 9 (26) | |
| Absent | 35 (70) | 9 (60) | 26 (74) | |
| Hypodense halo, n (%) | 0.524 | |||
| Present | 5 (10) | 1 (7) | 4 (11) | |
| Absent | 45 (90) | 14 (93) | 31 (89) | |
| Border between tumour and liver, n (%) | 0.428 | |||
| Sharp | 34 (68) | 11 (73) | 23 (66) | |
| Ill-defined | 16 (32) | 4 (27) | 12 (34) | |
| Tumor margins, n (%) | 0.477 | |||
| Smooth | 28 (56) | 9 (60) | 19 (54) | |
| Non-smooth | 22 (44) | 6 (40) | 16 (46) | |
| LI-RADS, n (%) | 0.05 | |||
| LR-4 | 10 (20) | 0 (0) | 10 (29) | |
| LR-5 | 27 (54) | 10 (67) | 17 (49) | |
| LR-M | 13 (26) | 5 (33) | 8 (22) |
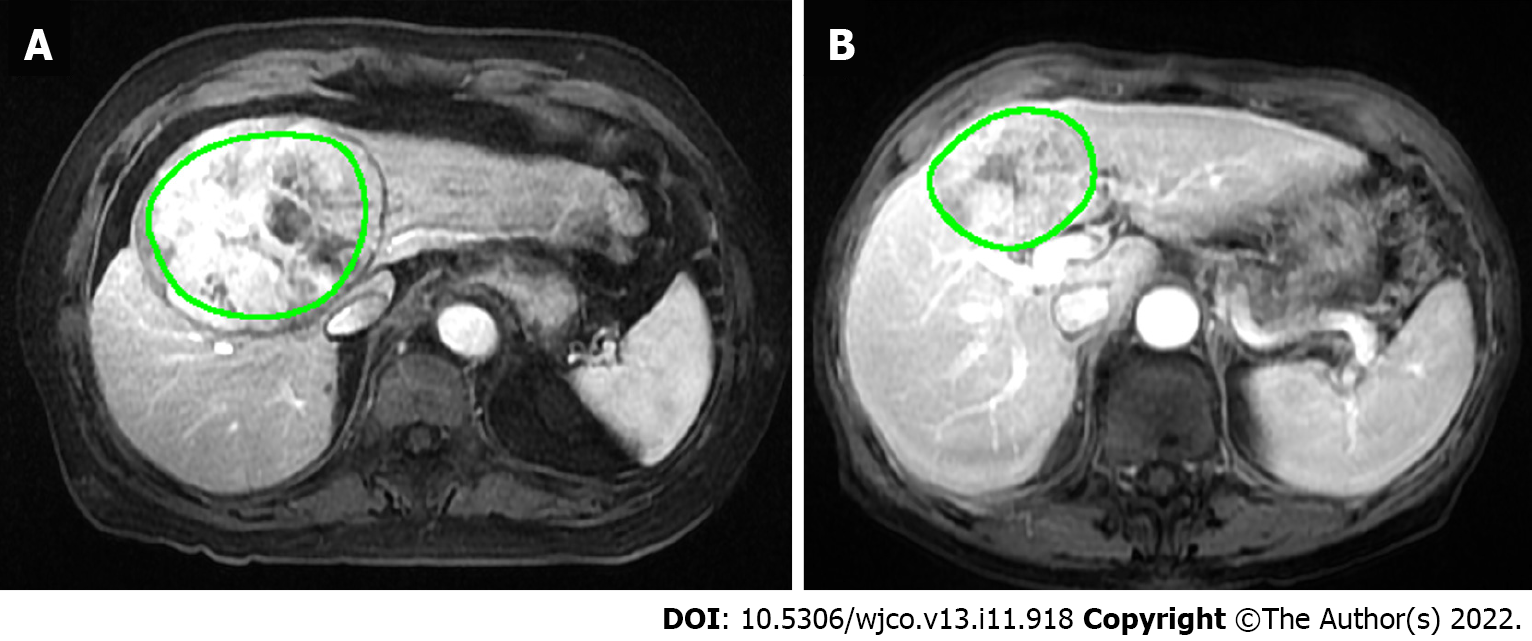
T1W post-contrast sequences in the arterial, portal venous (PV) and equilibrium phases were exported as DICOM (digital imaging and communications in medicine) files. A polygonal region of interest (ROI) was manually drawn on the largest cross-sectional area of the tumour. The segmented images were checked visually by a researcher and vetted by a third radiologist. Discordant findings were verified by one of the two senior radiologists (Tan CH). A typical segmentation result is shown in Figure 2.
Texture analysis was performed using MaZda software (MaZda, Technical University of Lodz)[16]. The MaZda software automatically extracts and analyses 290 texture parameters including area, histogram-based, gradient based, co-occurrence matrix based, autoregressive model, run-length matrix-based and wavelet analysis.
Five distinct methods were evaluated in an attempt to optimise the accuracy of the binary classification (presence or absence of MVI). Support vector machine classifiers developed for each study were tested using either bootstrapping method (1000 runs of five randomly selected test samples at a time, thus obtaining the average accuracy) or leave-one-out method.
Methods 1, 2 and 3 exclusively used features from the arterial, PV, and equilibrium phase respectively. To optimise performance, 14 of the most significant features from methods 1-3 were manually selected and put through mutual information feature selection method for methods 4 and 5. Method 4 employed three features selected purely through mutual information, while study 5 incorporated recursive pruning and subsequent manual feature selection after mutual information. Method 5 eventually employed three features and was then tested with leave-one-out classification method to attain optimal performance.
Statistical analysis was performed using SPSS 20.0. The Mann-Whitney U-test or independent t-test were used for continuous variables, while the Fisher exact test was used for categorical variables.
Fifty patients (43 males, 7 females, and mean age 67 years, range 53-81 years) were included in the present study. All were new cases of histology-proven HCC in treatment-naive patients. The aetiologies include hepatitis B (n = 28%, 56%), hepatitis C (n = 3%, 6%), hepatitis B and C (n = 1%, 2%), de novo (n = 9%, 18%), cryptogenic (n = 5%, 10%), alcoholic cirrhosis (n = 3%, 6%), and non-alcoholic steatotic hepatitis (n = 1%, 2%).
MVI was present in 30% (n = 15) of the resected specimens. Univariate analysis between MVI group and non-MVI group is shown in Table 2. Univariate analysis was also performed for MR imaging features collected for qualitative analysis (Table 1). We found a statistically significant difference of the pre-operative serum ɣ-glutamyltranspeptidase (GGT; P ≤ 0.01) level between the two groups. We also noted a statistically significant difference in the rates of the following imaging features between the two groups: Visible intra-tumoral vessels in arterial phase (P = 0.01) and in PV phase (P = 0.043).
| Variable | Total | MVI | P value | |
| MVI group = 15 | Non-MVI group = 35 | |||
| Age (yr), mean (SD) | 67.0 (7.0) | 68.3 (8.8) | 66.4 (6.1) | 0.491 |
| Gender | 0.348 | |||
| Male, n (%) | 43 (86) | 12 (80) | 31 (89) | |
| Female, n (%) | 7 (14) | 3 (20) | 4 (11) | |
| Aetiology | 0.377 | |||
| Alcohol, n (%) | 3 (6) | 0 (0) | 3 (8) | |
| Hepatitis B, n (%) | 28 (56) | 7 (46) | 21 (60) | |
| Hepatitis C, n (%) | 3 (6) | 1 (7) | 2 (6) | |
| Hepatitis B and C, n (%) | 1 (2) | 1 (7) | 0 (0) | |
| NAon-alcoholic steatohepatitis, n (%) | 1 (2) | 1 (7) | 0 (0) | |
| Cryptogenic cirrhosis, n (%) | 5 (10) | 2 (13) | 3 (8) | |
| De novo, n (%) | 9 (18) | 3 (20) | 6 (17) | |
| HbSAg | 0.676 | |||
| Positive, n (%) | 27 (54) | 7 (46) | 20 (57) | |
| Negative, n (%) | 22 (44) | 8 (54) | 14 (40) | |
| Unknown, n (%) | 1 (2) | 0 (0) | 1 (3) | |
| Pre-operative serology | ||||
| Alpha-fetoprotein (UG/L), mean (SD) | 248.0 (893.2) | 777.7 (1571.4) | 32.9 (105.6) | 0.065 |
| Albumin (g/L), mean (SD) | 36.3 (5.8) | 35.4 (5.6) | 36.7 (5.9) | 0.374 |
| Bilirubin (µmol/L), mean (SD) | 19.5 (12.1) | 18.6 (7.1) | 19.9 (13.8) | 0.797 |
Of the five methods, Method 5 achieved the highest accuracy of 87.8%. Method 5 also achieved a sensitivity of 73.3%, specificity of 94.1% with positive and negative predictive value of 85% and 89% respectively. Methods 1 through 4 achieved accuracies between 70.0% to 85.5%. The details and results of the 5 methods are summarized in Table 3. The three TA parameters in method 5 were Arterial S(4,0) Correlat, Arterial S(4,-4) InvDfMom and PV (3,0)SumAverg. Incidentally, these textural features are found in relatively close proximity along the horizontal and diagonal position of the image (at a distance of 3 and 4 pixels), which could imply particular areas of the tumours contribute more to accuracy than others.
| Method 1 | Method 2 | Method 3 | Method 4 | Method 5 | |
| Feature set used | Arterial phase | Portovenous phase | Equilibrium phase | Selected features from all phases | Selected features put through recursive pruning |
| Classification accuracy (%) | 81.4 | 84.7 | 70 | 85.5 | 87.8 |
The results of our study show that texture analysis of pre-operative MRI can predict the presence of MVI pre-operatively with an accuracy of up to 87.8%. Although the exact relationship between the selected texture parameters and the image appearance is not easily explainable, this study shows that the grey value variation in the horizontal and the diagonal direction show usable differences between the two groups. The study by Ahn et al[14] also showed that application of TA increased diagnostic performance [area under the curve (AUC) improved from 0.7 to 0.83]. Unlike ours, the same study also found sphericity and discrete compactness to be the texture analysis variables that are significantly associated with MVI[14]. Wilson et al[15] achieved an AUC of 0.83 with their final texture analysis model and found tumour entropy to be significantly associated with MVI. Other studies have also identified entropy on post-contrast CT images to be an independent predictor of disease-free survival and overall survival[17,18].
TA is a post-processing technique for quantification of tissue heterogeneity. The method is based on analysis of the grey value distribution and relationship of pixels within any given ROI. The potential applications of texture analysis and radiomics in general are expanding, especially in solid tumour imaging[19-22]. In its present state, this technique is able to achieve good results without the need to obtain additional sequences or interventions. TA can also potentially be incorporated into future predictive models that incorporate clinicoradiological risk factors and radiomic features; early work has shown some promise in predicting MVI[21].
Qualitative MRI features of HCC may be useful in predicting MVI. Kim et al[23] found irregular circumferential peritumoral enhancement to be the only significant variable in MVI-present HCC, while Chandarana et al[24] demonstrated tumor multifocality takes precedence over all other imaging features. The presence of the aforementioned imaging features leads to high specificity for diagnosis of MVI, but with low sensitivity[25,26]. Furthermore, interobserver agreement tends to be only fair to moderate[27].
Most recently, Hong et al[28] conducted a systematic review and meta-analysis on MRI features for predicting MVI and found 7 MRI features to be significantly associated with MVI: Larger tumour size (> 5 cm), rim arterial enhancement, arterial peritumoral enhancement, peritumoral hypointensity on hepatobiliary phase, non-smooth tumour margin, multifocality and hypointensity on T1W imaging.
Our study evaluated most of the aforementioned features, but found that only intra-tumoral vessel visibility on arterial and PV phase was significantly associated with MVI. Interestingly, vessel visibility in post-contrast sequences has not been extensively studied and may be a subject of future study. Further analysis of these two statistically significant features show sensitivity of 53.3% and 33.3% for vessel visibility on arterial and PV phase respectively. Both features demonstrate specificity of 91.4%. Using these as a measure of the qualitative analysis by radiologists, our model outperforms both features, achieving sensitivity of 73.3% and specificity of 94.1%.
The imaging features described in other papers, such as peritumoral arterial phase hyperenhancement, were not found to be significant for diagnosis of MVI; this may be due to small sample size in our current study. Of note, none of the parameters selected for Methods 4 and 5 included features from the equilibrium phase. This is consistent with the observations in current literature, where arterial phase and hepatobiliary phase findings predominate[28].
Current diagnosis of MVI relies on histopathologic examination that is done post-operatively. The presence of MVI indicates more aggressive tumour behaviour and poorer prognosis[1,2]. Furthermore, our study cohort comprises a high proportion of Hepatitis B aetiology (n = 29%; 58%) which has been shown to be associated with development of vascular invasion[29]. The ability to diagnose pre-operative MVI can significantly alter the clinical management for patients in several ways. Firstly, the informed consent process could include equivalent non-invasive options (e.g. Chemoembolisation and/or ablation techniques) which have been shown to produce similar oncologic outcomes, but with the added benefit of lower morbidity[30]. Secondly, the presence of MVI may prompt the use of more aggressive treatment options such as extended surgical margins or additional bridging treatment while awaiting liver transplantation[31-34]. Additionally, adjuvant therapy after surgical resection have also been shown to improve survival in patients with MVI[35,36]. Lastly, these patients could be followed up more closely given the susceptibility for early recurrence.
This study is not without its limitations. Firstly, this is a retrospective single-institution study with a small sample size, limiting our ability to compare it against the diagnostic performance of visual analysis by radiologists. The low sample size may also account for the relative paucity of statistically significant differences among the two study groups in terms of both clinical and radiological characteristics. Despite this, TA was able to achieve relatively high accuracy rates while identifying features that were significantly associated with MVI. Secondly, this study included MR examinations that were done with two types of hepatobiliary contrast agents (gadoterate meglumine, gadoxetate and gadobenate dimeglumine). Utilising a single (hepatocyte-specific) contrast agent would have helped to reduce heterogeneity of the MR images and allowed further analysis of the hepatobiliary phase. Due to small sample size, sub-group analysis of scans performed with each type of contrast agent could not be performed. However, standard imaging parameters were performed, in particular fixed timing of contrast enhanced phases, which reduced technical variability. Lastly, TA was only done across the largest cross-sectional area of the tumour. While analysing the entire volume of the tumour and the peritumoral tissues, would have been ideal, enough information was extracted from just a two-dimensional representation of the HCC to yield relatively accurate results.
In conclusion, texture analysis of HCC performed on pre-operative MR images can accurately predict the presence of MVI with an accuracy of up to 87.8%. It has potential to be incorporated into clinical routine as a reliable tool for making pre-operative treatment decisions. Larger studies should be performed to validate the texture parameters and its value over qualitative visual analysis.
Presence of microvascular invasion (MVI) indicates poorer prognosis post-curative resection of hepatocellular carcinoma (HCC), with an increased chance of tumour recurrence. By present standards, MVI can only be diagnosed post-operatively on histopathology.
Texture analysis potentially allows identification of patients who are considered ‘high risk’ through analysis of pre-operative magnetic resonance imaging (MRI) studies. These findings may or may not be readily apparent to the human eye, thus the need for an analytic software. This will in turn allow for better patient selection, improved individualised therapy (such as extended surgical margins or adjuvant therapy) and pre-operative prognostication.
To evaluate the accuracy of texture analysis on pre-operative MRI in predicting MVI in HCC.
We recruited patients who underwent hepatectomy. Both qualitative (performed by radiologists) and quantitative data (performed by software) were obtained. Radiomics texture parameters were extracted based on the largest cross-sectional area of each tumor and analysed using MaZda software. Final histology of the tumour was used as ground truth.
Texture analysis of tumours on pre-operative MRI can predict presence of MVI in HCC with accuracies of up to 87.8%.
Texture analysis of HCC performed on pre-operative MR images can accurately predict the presence of MVI with an accuracy of up to 87.8%. It has potential to be incorporated into clinical routine as a reliable tool for making pre-operative treatment decisions. Larger studies should be performed to validate the texture parameters and its value over qualitative visual analysis.
This study demonstrates the utility of texture analysis on pre-operative MRI to potentially impact clinical management in patients with surgically resectable hepatocellular carcinoma.
Provenance and peer review: Invited article; Externally peer reviewed.
Peer-review model: Single blind
Specialty type: Radiology, nuclear medicine and medical imaging
Country/Territory of origin: Singapore
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): 0
Grade C (Good): C, C
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Inmutto N, Thailand; Zimmitti G, Italy S-Editor: Liu JH L-Editor: A P-Editor: Liu JH
| 1. | Ikai I, Arii S, Kojiro M, Ichida T, Makuuchi M, Matsuyama Y, Nakanuma Y, Okita K, Omata M, Takayasu K, Yamaoka Y. Reevaluation of prognostic factors for survival after liver resection in patients with hepatocellular carcinoma in a Japanese nationwide survey. Cancer. 2004;101:796-802. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 346] [Cited by in RCA: 353] [Article Influence: 16.8] [Reference Citation Analysis (0)] |
| 2. | Lauwers GY, Terris B, Balis UJ, Batts KP, Regimbeau JM, Chang Y, Graeme-Cook F, Yamabe H, Ikai I, Cleary KR, Fujita S, Flejou JF, Zukerberg LR, Nagorney DM, Belghiti J, Yamaoka Y, Vauthey JN; International Cooperative Study Group on Hepatocellular Carcinoma. Prognostic histologic indicators of curatively resected hepatocellular carcinomas: a multi-institutional analysis of 425 patients with definition of a histologic prognostic index. Am J Surg Pathol. 2002;26:25-34. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 152] [Cited by in RCA: 149] [Article Influence: 6.5] [Reference Citation Analysis (0)] |
| 3. | Lim KC, Chow PK, Allen JC, Chia GS, Lim M, Cheow PC, Chung AY, Ooi LL, Tan SB. Microvascular invasion is a better predictor of tumor recurrence and overall survival following surgical resection for hepatocellular carcinoma compared to the Milan criteria. Ann Surg. 2011;254:108-113. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 284] [Cited by in RCA: 412] [Article Influence: 29.4] [Reference Citation Analysis (0)] |
| 4. | Rodríguez-Perálvarez M, Luong TV, Andreana L, Meyer T, Dhillon AP, Burroughs AK. A systematic review of microvascular invasion in hepatocellular carcinoma: diagnostic and prognostic variability. Ann Surg Oncol. 2013;20:325-339. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 504] [Cited by in RCA: 493] [Article Influence: 41.1] [Reference Citation Analysis (0)] |
| 5. | Wang W, Guo Y, Zhong J, Wang Q, Wang X, Wei H, Li J, Xiu P. The clinical significance of microvascular invasion in the surgical planning and postoperative sequential treatment in hepatocellular carcinoma. Sci Rep. 2021;11:2415. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 30] [Cited by in RCA: 35] [Article Influence: 8.8] [Reference Citation Analysis (0)] |
| 6. | Zhou Y, He L, Huang Y, Chen S, Wu P, Ye W, Liu Z, Liang C. CT-based radiomics signature: a potential biomarker for preoperative prediction of early recurrence in hepatocellular carcinoma. Abdom Radiol (NY). 2017;42:1695-1704. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 128] [Cited by in RCA: 162] [Article Influence: 20.3] [Reference Citation Analysis (0)] |
| 7. | Hui TCH, Chuah TK, Low HM, Tan CH. Predicting early recurrence of hepatocellular carcinoma with texture analysis of preoperative MRI: a radiomics study. Clin Radiol. 2018;73:1056.e11-1056.e16. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 26] [Cited by in RCA: 52] [Article Influence: 7.4] [Reference Citation Analysis (0)] |
| 8. | Liu J, Mao Y, Li Z, Zhang D, Zhang Z, Hao S, Li B. Use of texture analysis based on contrast-enhanced MRI to predict treatment response to chemoradiotherapy in nasopharyngeal carcinoma. J Magn Reson Imaging. 2016;44:445-455. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 62] [Cited by in RCA: 83] [Article Influence: 9.2] [Reference Citation Analysis (0)] |
| 9. | Ahmed A, Gibbs P, Pickles M, Turnbull L. Texture analysis in assessment and prediction of chemotherapy response in breast cancer. J Magn Reson Imaging. 2013;38:89-101. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 147] [Cited by in RCA: 158] [Article Influence: 12.2] [Reference Citation Analysis (0)] |
| 10. | Yamashita Y, Tsuijita E, Takeishi K, Fujiwara M, Kira S, Mori M, Aishima S, Taketomi A, Shirabe K, Ishida T, Maehara Y. Predictors for microinvasion of small hepatocellular carcinoma ≤ 2 cm. Ann Surg Oncol. 2012;19:2027-2034. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 90] [Cited by in RCA: 118] [Article Influence: 8.4] [Reference Citation Analysis (0)] |
| 11. | Cucchetti A, Piscaglia F, Grigioni AD, Ravaioli M, Cescon M, Zanello M, Grazi GL, Golfieri R, Grigioni WF, Pinna AD. Preoperative prediction of hepatocellular carcinoma tumour grade and micro-vascular invasion by means of artificial neural network: a pilot study. J Hepatol. 2010;52:880-888. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 130] [Cited by in RCA: 162] [Article Influence: 10.8] [Reference Citation Analysis (0)] |
| 12. | Lei Z, Li J, Wu D, Xia Y, Wang Q, Si A, Wang K, Wan X, Lau WY, Wu M, Shen F. Nomogram for Preoperative Estimation of Microvascular Invasion Risk in Hepatitis B Virus-Related Hepatocellular Carcinoma Within the Milan Criteria. JAMA Surg. 2016;151:356-363. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 276] [Cited by in RCA: 444] [Article Influence: 49.3] [Reference Citation Analysis (0)] |
| 13. | Pawlik TM, Gleisner AL, Anders RA, Assumpcao L, Maley W, Choti MA. Preoperative assessment of hepatocellular carcinoma tumor grade using needle biopsy: implications for transplant eligibility. Ann Surg. 2007;245:435-442. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 160] [Cited by in RCA: 172] [Article Influence: 9.6] [Reference Citation Analysis (0)] |
| 14. | Ahn SJ, Kim JH, Park SJ, Kim ST, Han JK. Hepatocellular carcinoma: preoperative gadoxetic acid-enhanced MR imaging can predict early recurrence after curative resection using image features and texture analysis. Abdom Radiol (NY). 2019;44:539-548. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 44] [Cited by in RCA: 73] [Article Influence: 12.2] [Reference Citation Analysis (0)] |
| 15. | Wilson GC, Cannella R, Fiorentini G, Shen C, Borhani A, Furlan A, Tsung A. Texture analysis on preoperative contrast-enhanced magnetic resonance imaging identifies microvascular invasion in hepatocellular carcinoma. HPB (Oxford). 2020;22:1622-1630. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 14] [Cited by in RCA: 23] [Article Influence: 4.6] [Reference Citation Analysis (0)] |
| 16. | Strzelecki M, Szczypinski P, Materka A, Klepaczko A. A software tool for automatic classification and segmentation of 2D/3D medical images. Nucl Instruments Methods Phys Res Sect A Accel Spectrometers, Detect Assoc Equip. 2013;702:137-140. [DOI] [Full Text] |
| 17. | Mulé S, Thiefin G, Costentin C, Durot C, Rahmouni A, Luciani A, Hoeffel C. Advanced Hepatocellular Carcinoma: Pretreatment Contrast-enhanced CT Texture Parameters as Predictive Biomarkers of Survival in Patients Treated with Sorafenib. Radiology. 2018;288:445-455. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 59] [Cited by in RCA: 81] [Article Influence: 11.6] [Reference Citation Analysis (0)] |
| 18. | Akai H, Yasaka K, Kunimatsu A, Nojima M, Kokudo T, Kokudo N, Hasegawa K, Abe O, Ohtomo K, Kiryu S. Predicting prognosis of resected hepatocellular carcinoma by radiomics analysis with random survival forest. Diagn Interv Imaging. 2018;99:643-651. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 51] [Cited by in RCA: 73] [Article Influence: 10.4] [Reference Citation Analysis (0)] |
| 19. | Lubner MG, Smith AD, Sandrasegaran K, Sahani DV, Pickhardt PJ. CT Texture Analysis: Definitions, Applications, Biologic Correlates, and Challenges. Radiographics. 2017;37:1483-1503. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 422] [Cited by in RCA: 569] [Article Influence: 71.1] [Reference Citation Analysis (1)] |
| 20. | Wei J, Jiang H, Zeng M, Wang M, Niu M, Gu D, Chong H, Zhang Y, Fu F, Zhou M, Chen J, Lyv F, Wei H, Bashir MR, Song B, Li H, Tian J. Prediction of Microvascular Invasion in Hepatocellular Carcinoma via Deep Learning: A Multi-Center and Prospective Validation Study. Cancers (Basel). 2021;13. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 29] [Cited by in RCA: 39] [Article Influence: 9.8] [Reference Citation Analysis (0)] |
| 21. | Yang L, Gu D, Wei J, Yang C, Rao S, Wang W, Chen C, Ding Y, Tian J, Zeng M. A Radiomics Nomogram for Preoperative Prediction of Microvascular Invasion in Hepatocellular Carcinoma. Liver Cancer. 2019;8:373-386. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 120] [Cited by in RCA: 236] [Article Influence: 39.3] [Reference Citation Analysis (0)] |
| 22. | Feng ST, Jia Y, Liao B, Huang B, Zhou Q, Li X, Wei K, Chen L, Li B, Wang W, Chen S, He X, Wang H, Peng S, Chen ZB, Tang M, Chen Z, Hou Y, Peng Z, Kuang M. Preoperative prediction of microvascular invasion in hepatocellular cancer: a radiomics model using Gd-EOB-DTPA-enhanced MRI. Eur Radiol. 2019;29:4648-4659. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 149] [Cited by in RCA: 146] [Article Influence: 24.3] [Reference Citation Analysis (0)] |
| 23. | Kim H, Park MS, Choi JY, Park YN, Kim MJ, Kim KS, Choi JS, Han KH, Kim E, Kim KW. Can microvessel invasion of hepatocellular carcinoma be predicted by pre-operative MRI? Eur Radiol. 2009;19:1744-1751. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 115] [Cited by in RCA: 148] [Article Influence: 9.3] [Reference Citation Analysis (0)] |
| 24. | Chandarana H, Robinson E, Hajdu CH, Drozhinin L, Babb JS, Taouli B. Microvascular invasion in hepatocellular carcinoma: is it predictable with pretransplant MRI? AJR Am J Roentgenol. 2011;196:1083-1089. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 77] [Cited by in RCA: 88] [Article Influence: 6.3] [Reference Citation Analysis (0)] |
| 25. | Zhang L, Yu X, Wei W, Pan X, Lu L, Xia J, Zheng W, Jia N, Huo L. Prediction of HCC microvascular invasion with gadobenate-enhanced MRI: correlation with pathology. Eur Radiol. 2020;30:5327-5336. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 18] [Cited by in RCA: 44] [Article Influence: 8.8] [Reference Citation Analysis (0)] |
| 26. | Hu HT, Shen SL, Wang Z, Shan QY, Huang XW, Zheng Q, Xie XY, Lu MD, Wang W, Kuang M. Peritumoral tissue on preoperative imaging reveals microvascular invasion in hepatocellular carcinoma: a systematic review and meta-analysis. Abdom Radiol (NY). 2018;43:3324-3330. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 17] [Cited by in RCA: 34] [Article Influence: 4.9] [Reference Citation Analysis (0)] |
| 27. | Min JH, Lee MW, Park HS, Lee DH, Park HJ, Lim S, Choi SY, Lee J, Lee JE, Ha SY, Cha DI, Carriere KC, Ahn JH. Interobserver Variability and Diagnostic Performance of Gadoxetic Acid-enhanced MRI for Predicting Microvascular Invasion in Hepatocellular Carcinoma. Radiology. 2020;297:573-581. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 83] [Cited by in RCA: 69] [Article Influence: 13.8] [Reference Citation Analysis (0)] |
| 28. | Hong SB, Choi SH, Kim SY, Shim JH, Lee SS, Byun JH, Park SH, Kim KW, Kim S, Lee NK. MRI Features for Predicting Microvascular Invasion of Hepatocellular Carcinoma: A Systematic Review and Meta-Analysis. Liver Cancer. 2021;10:94-106. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 30] [Cited by in RCA: 105] [Article Influence: 26.3] [Reference Citation Analysis (0)] |
| 29. | Wei X, Li N, Li S, Shi J, Guo W, Zheng Y, Cheng S. Hepatitis B virus infection and active replication promote the formation of vascular invasion in hepatocellular carcinoma. BMC Cancer. 2017;17:304. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 25] [Cited by in RCA: 40] [Article Influence: 5.0] [Reference Citation Analysis (0)] |
| 30. | Gui CH, Baey S, D'cruz RT, Shelat VG. Trans-arterial chemoembolization + radiofrequency ablation vs surgical resection in hepatocellular carcinoma - A meta-analysis. Eur J Surg Oncol. 2020;46:763-771. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 51] [Cited by in RCA: 59] [Article Influence: 11.8] [Reference Citation Analysis (0)] |
| 31. | Iguchi T, Shirabe K, Aishima S, Wang H, Fujita N, Ninomiya M, Yamashita Y, Ikegami T, Uchiyama H, Yoshizumi T, Oda Y, Maehara Y. New Pathologic Stratification of Microvascular Invasion in Hepatocellular Carcinoma: Predicting Prognosis After Living-donor Liver Transplantation. Transplantation. 2015;99:1236-1242. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 65] [Cited by in RCA: 105] [Article Influence: 10.5] [Reference Citation Analysis (0)] |
| 32. | Erstad DJ, Tanabe KK. Prognostic and Therapeutic Implications of Microvascular Invasion in Hepatocellular Carcinoma. Ann Surg Oncol. 2019;26:1474-1493. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 139] [Cited by in RCA: 286] [Article Influence: 47.7] [Reference Citation Analysis (0)] |
| 33. | Sumie S, Nakashima O, Okuda K, Kuromatsu R, Kawaguchi A, Nakano M, Satani M, Yamada S, Okamura S, Hori M, Kakuma T, Torimura T, Sata M. The significance of classifying microvascular invasion in patients with hepatocellular carcinoma. Ann Surg Oncol. 2014;21:1002-1009. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 106] [Cited by in RCA: 154] [Article Influence: 12.8] [Reference Citation Analysis (0)] |
| 34. | Shi C, Zhao Q, Liao B, Dong Z, Wang C, Yang J, Shen W. Anatomic resection and wide resection margin play an important role in hepatectomy for hepatocellular carcinoma with peritumoural micrometastasis. ANZ J Surg. 2019;89:E482-E486. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 11] [Cited by in RCA: 24] [Article Influence: 4.0] [Reference Citation Analysis (0)] |
| 35. | Zhang XP, Chai ZT, Gao YZ, Chen ZH, Wang K, Shi J, Guo WX, Zhou TF, Ding J, Cong WM, Xie D, Lau WY, Cheng SQ. Postoperative adjuvant sorafenib improves survival outcomes in hepatocellular carcinoma patients with microvascular invasion after R0 Liver resection: a propensity score matching analysis. HPB (Oxford). 2019;21:1687-1696. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 29] [Cited by in RCA: 58] [Article Influence: 9.7] [Reference Citation Analysis (0)] |
| 36. | Chen ZH, Zhang XP, Zhou TF, Wang K, Wang H, Chai ZT, Shi J, Guo WX, Cheng SQ. Adjuvant transarterial chemoembolization improves survival outcomes in hepatocellular carcinoma with microvascular invasion: A systematic review and meta-analysis. Eur J Surg Oncol. 2019;45:2188-2196. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 28] [Cited by in RCA: 56] [Article Influence: 9.3] [Reference Citation Analysis (0)] |