Published online Oct 24, 2022. doi: 10.5306/wjco.v13.i10.802
Peer-review started: July 7, 2022
First decision: August 22, 2022
Revised: August 26, 2022
Accepted: September 9, 2022
Article in press: September 9, 2022
Published online: October 24, 2022
Processing time: 104 Days and 21.4 Hours
Malignant ovarian germ cell tumors (MOGCT) are rare and frequently occur in women of young and reproductive age and the oncologic and reproductive outcomes after fertility-sparing surgery (FSS) for this disease are still limited.
To evaluate the oncology and reproductive outcomes of MOGCT patients who underwent FSS.
All MOGCT patients who underwent FSS defined as the operation with a preserved uterus and at least one side of the ovary at our institute between January 2005 and December 2020 were retrospectively reviewed.
Sixty-two patients were recruited for this study. The median age was 22 years old and over 77% were nulliparous. The three most common histology findings were immature teratoma (32.2%), dysgerminoma (24.2%), and yolk sac tumor (24.2%). The distribution of stage was as follows; Stage I, 74.8%; stage II, 9.7%; stage III, 11.3%; and stage IV, 4.8%. Forty-three (67.7%) patients received adjuvant chemotherapy. With a median follow-up time of 96.3 mo, the 10-year progression-free survival and overall survival were 82.4% and 91%, respectively. For repro
The oncology and reproductive outcomes of MOGCT treated by FSS are excellent. Many patients show a long survival time with normal menstruation. However, the obstetric outcome is not quite satisfactory.
Core Tip: The oncology and reproductive outcomes of malignant ovarian germ cell tumors treated by fertility-sparing surgery were satisfying. Even though most patients developed normal menstruation, nearly 1/3 were successfully pregnant and delivered.
- Citation: Rungoutok M, Suprasert P. Oncology and reproductive outcomes over 16 years of malignant ovarian germ cell tumors treated by fertility sparing surgery. World J Clin Oncol 2022; 13(10): 802-812
- URL: https://www.wjgnet.com/2218-4333/full/v13/i10/802.htm
- DOI: https://dx.doi.org/10.5306/wjco.v13.i10.802
Malignant ovarian germ cell tumors (MOGCT) account for about 5% of all ovarian cancer cases and approximately 70% develop in young women[1]. With the introduction of chemotherapy consisting of bleomycin, etoposide, and cisplatin (BEP) for MOGCT treatment after surgery, the outcome of this malignancy is excellent even in the advanced stage[2]. The cure rate of MOGCT in the early stage and the advanced stage was 100% and 75%, respectively[3]. Therefore, in patients who were of young and reproductive age, the role of fertility-sparing surgery (FSS) defined as cytoreductive surgery with preservation of the contralateral adnexa and uterus is the standard treatment for these patients[4]. We previously reported a 10-year overall survival (OS) rate as high as 86.2% but did not focus on the patients who underwent FSS[5]. Therefore, with the limited data on oncology and reproductive outcomes of FSS especially in Southeast Asia, this study was conducted to identify these outcomes of MOGCT patients who were treated by FSS.
After the protocol was approved by the local ethics committees, the medical records of the MOGCT patients who underwent FSS defined as surgical cytoreduction with preservation of the uterus and unilateral adnexa at Chiang Mai University Hospital from January 2005 through December 2020 were reviewed. The patients who developed other histologic types arising in germ cell tumors were excluded. The basic clinical data, histology, staging, type of surgery, chemotherapy regimen, and outcomes were identified. All pathology specimens were examined by gynecologic pathologists in our institute. The decision of treatment depended on the preference of the physicians.
After complete treatment, the surveillance schedule was set every 3 mo in the first year, every 4 mo in the second year, every 6 mo in the third to fifth year, and annually thereafter. At that time, all of the patients were examined for a blood test for MOGCT and were examined by gynecologic oncologists. Pelvic ultrasonography was done at each visit for unmarried patients. Other imaging modalities such as CT were utilized when clinically indicated or with a rising of tumor markers.
Progression-free survival (PFS) was defined as the time between the month of the primary surgery and the month of tumor progression or recurrence detection or last contact, whereas OS was defined as the similar starting time of PFS to the month of patient death or last contact. The death data was also sought from the Thai Civil registration system via the National identification card number. Both PFS and OS were estimated by the Kaplan-Meier method using the SPSS for Windows program (Version 22; IBM Corporation, Armonk, New York, United States). Descriptive data of all studied patients are presented as the mean with range and discrete data are reported as numbers and percentages.
The reproductive outcome after FSS was identified by collecting the data on the menstrual status during and after treatment, the number of pregnancies and childbirth before and after treatment, the present marital status, the childbearing desire, the method of pregnancy, gestational age at delivery, birth weight of the baby, and obstetrical complications from the medical records and direct contact with the patients by phone for more information.
Among 98 MOGCT, 62 patients underwent FSS in the studied period. The clinical data are presented in Table 1. The median age of these patients was 22 with a range of 4-34 years old. Over 77% of them were nulliparous and the three most common presentations were pelvic mass, abdominal pain, and abdominal distension. Approximately 54.8% of the tumors were located on the right side and 41.9% on the left side.
| n (%) | |
| Median age (range; yr) | 22.0 (4-34) |
| Parity before surgery | |
| 0 | 48 (77.4) |
| 1 | 8 (12.9) |
| 2 | 6 (9.7) |
| Presentation | |
| Pelvic mass | 24 (38.7) |
| Abdominal pain | 18 (29.0) |
| Abdominal distension | 15 (24.2) |
| Others1 | 5 (8.1) |
| Tumor side | |
| Right | 34 (54.8) |
| Left | 26 (41.9) |
| Bilateral | 2 (3.2) |
| Detail of FSS | |
| Unilateral SO | 49 (79.0) |
| Unilateral cystectomy | 4 (6.5) |
| Unilateral SO & cystectomy | 9 (14.5) |
| Frozen section | 26 (41.9) |
| Cytology | |
| Not done | 23 (37.1) |
| Negative | 29 (46.8) |
| Positive | 10 (16.1) |
| Omentectomy | |
| Not done | 18 (29.0) |
| Negative | 40 (64.5) |
| positive | 4 (6.5) |
| Lymphadenectomy | |
| Not done | 32 (51.6) |
| Negative | 26 (41.9) |
| Positive | 4 (6.5) |
| Appendectomy | 32 (51.6) |
| Surgical outcome | |
| No residual | 47 (75.8) |
| Optimal | 5 (8.1) |
| Suboptimal (residual tumor > 1 cm) | 10 (16.1) |
| Histology | |
| Dysgerminoma | 17 (27.4) |
| Immature teratoma | 20 (32.3) |
| Yolk sac tumor | 15 (24.2) |
| Mixed type | 5 (8.1) |
| Others2 | 5 (8.1) |
| Stage | |
| I | 46 (74.2) |
| II | 6 (9.7) |
| III | 7 (11.3) |
| IV | 3 (4.8) |
| Adjuvant chemotherapy | |
| None | 19 (30.6) |
| BEP | 42 (67.7) |
| EMACO | 1 (1.6) |
| Cycles of chemotherapy | |
| 1-3 | 6 |
| 4-6 | 33 |
| > 6 | 4 |
| Long-term side effect | |
| None | 46 (74.2) |
| Numbness | 3 (4.8) |
| Lung fibrosis | 2 (3.2) |
| High-frequency hearing loss | 1 (1.6) |
| Tinnitus | 1 (1.6) |
| Progression of disease | 4 (9.5) |
| Death | 5 (8.1) |
| Alive | 55 (88.7) |
| Missing data | 2 (3.2) |
The details of FSS are as follows: Unilateral salpingo-oophorectomy (SO) in 49 cases, unilateral ovarian cystectomy in four, and unilateral SO with contralateral ovarian cystectomy in the rest. A frozen section was done in 26 cases. About staging procedures, peritoneal cytology was done in 39 cases with ten cases revealing positive malignancy cells, while omentectomy was done in 44 cases and lymphadenectomy was performed in 30, with four cases each having positive results. Half of the studied patients underwent an appendectomy. Regarding the surgical outcomes, 75.8% had complete resections.
The three leading histology types were immature teratoma (32.3%), dysgerminoma (24.2%), and yolk sac tumor (24.2%). The majority of the patients were in stage I (74.2%) and about 4.8% were in stage IV. Nearly 70% of the patients were given adjuvant chemotherapy. All except one was BEP regimen. Only one case was given etoposide + methotrexate + actinomycin D + cyclophosphamide + vincristine (EMACO). This case was diagnosed with stage IV choriocarcinoma. About one-third of the patients received four to six cycles of chemotherapy. Concerning the long-term side effects of chemotherapy, numbness occurred in three cases, lung fibrosis occurred in two, and hearing problems in two. Five patients died; two died from neutropenic sepsis and the rest from the progression of the disease.
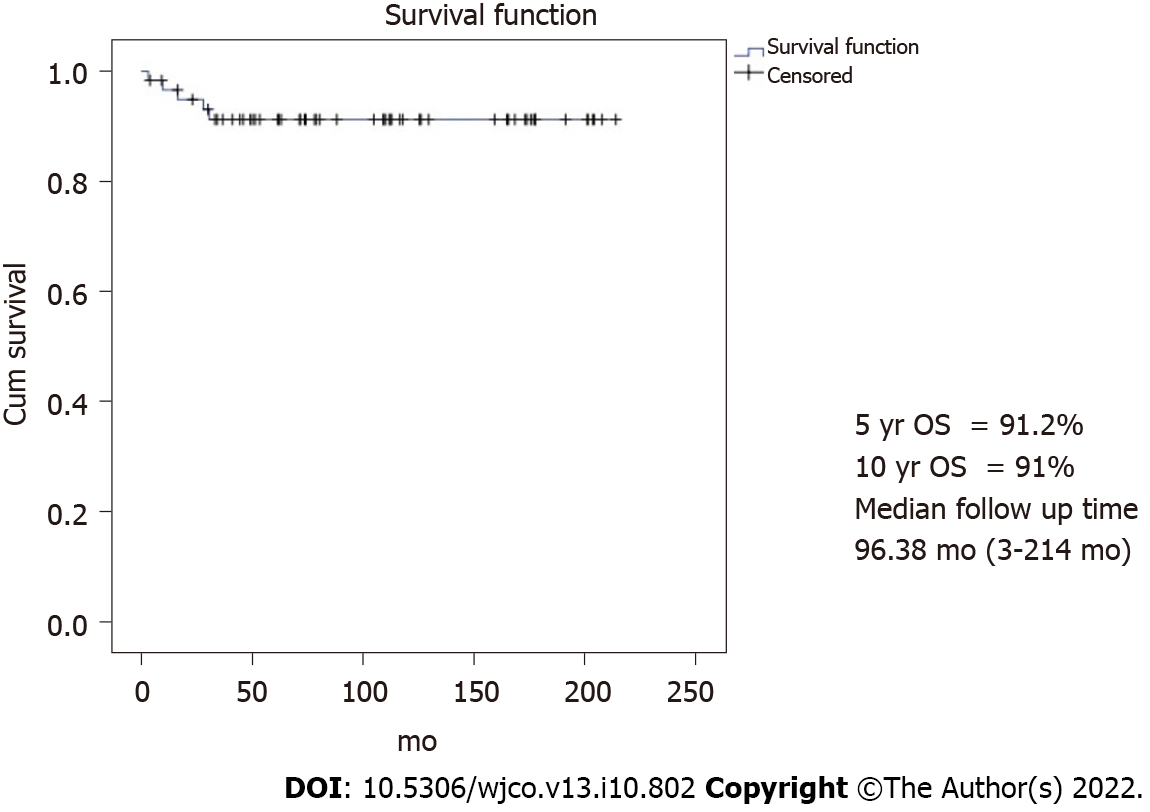
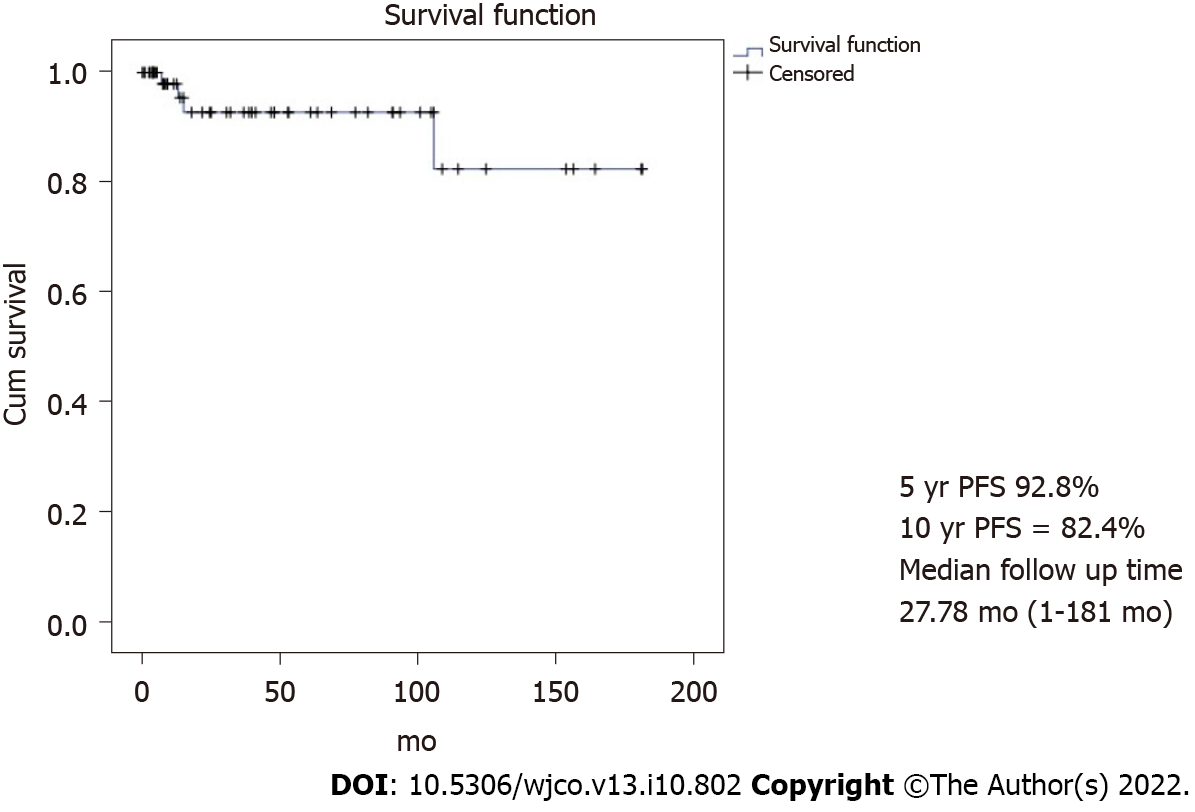
With a median follow-up time of 96.3 mo, the 10-year PFS and OS were 82.4% and 91% as shown in Figures 1 and 2, respectively. However, 62.9% did not continue regular follow-ups.
Four cases developed progression after primary FSS. The details of these patients are summarized in Table 2. One of them lived while the others died from the disease progression during treatment. The surviving case was a 17-year-old with stage IC1 grade 2 immature teratoma. The primary surgery was right SO and appendectomy with pelvic recurrence developing 1 mo after the operation. She underwent tumor debulking and received six cycles of the BEP regimen. She is still alive without disease with an overall survival of 109 mo. The other three cases had stage IV disease. The histology was yolk sac tumor in two cases with choriocarcinoma in the remainder. All of them underwent FSS and received multiple chemotherapy regimens with unfavorable outcomes and died of disease after primary surgery at 16, 28, and 30 mo. One case developed lung fibrosis after being administered two cycles of the BEP regimen.
| SN | Year | Age | Stage | Histology | Chemotherapy | Cycle | Site | Primary surgery | Note |
| 50 | 2013 | 17 | IC1 | Immature teratoma grade 2 | None | - | Right | Right SO and appendectomy | PFS 3 mos →pelvic recurrence → debulking tumor and BEP × 6 cycles → alive without disease DFS 103 mos, overall survival 109 mo |
| 52 | 2013 | 15 | IV | Yolk sac tumor | BEP | 8 | Right | Right SO and left cystectomy and omentectomy | Progression after BEP × 8: Liver & lung metastasis → TIP x 2 → PT × 1 → ifosfamide × 1 → progression → death (overall survival 16 mo) |
| 74 | 2010 | 18 | IV | Choriocarcinoma | EMACO | 6 | Left | Left SO and omentectomy and appendectomy and PAN sampling | EMACO × 6 → progression (PFS 7 mos) → cisplatin and ifosfamide × 5 → paclitaxel × 1 → Act D and 5 FU × 1 → VAC × 1 → TAH and right SO (19/4/2011) → EMA/EP × 9 → TP/TE × 1 → BEP × 2 → palliative treatment → death 5/7/2012 overall survival 28 mo |
| 110 | 2005 | 16 | IV | Yolk sac tumor | BEP × 2 → EP × 11 | 12 | Right | Right SO and appendectomy | Progression after EP × 11 → ifosfamide × 1 → EMA × 1 → single paclitaxel × 1 → palliative RT 25/1/2006 → VAC × 1 → Death 4/7/2007,OS 30 mo (lung fibrosis after BEP × 2) |
Regarding four patients who underwent only a cystectomy, the pathology was immature teratoma in two cases (stage IA grade 2 and stage IC grade 1), papillary thyroid cancer arising from mature teratoma (1), and carcinoid tumor (1). Only one case of stage IA grade 2 immature teratoma received four cycles of BEP regimen while the other received only an operation. All of them are still alive at present with an overall survival of 44-173 mo.
Of 62 patients, 43 received adjuvant chemotherapy with BEP in 41 cases and EMACO in the rest. The menstrual history of these patients is summarized in Table 3. Forty-two percent of the patients had menstruation while receiving chemotherapy, while 39.5% resumed menstruation after complete treatment with a median resumption time of 4 mo. One case was five years old at the treatment time with menarche at age 12 (seven years later).
Eight patients were without menstruation after chemotherapy. The one case without menarche at presentation was 12 years old. She was diagnosed with stage I mixed MOGCT and received six cycles of BEP regimen after undergoing right SO at 5 years of age. She was followed regularly with no evidence of recurrence. The remaining seven patients developed premature menopause. One case was diagnosed as having a stage IIA endodermal sinus tumor at 29 years old. She received six cycles of BEP regimen after undergoing right SO and omentectomy on January 1, 2017. One year after that, she developed a left ovarian tumor measuring 10 cm × 15 cm and received a hysterectomy with left SO. The final pathology revealed grade 1 endometrioid CA. The patient was given six cycles of carboplatin with a disease-free survival of 61 mo and received estradiol valerate 2 mg as hormonal therapy. The other two patients underwent FSS and received three and six cycles of BEP, respectively. Both cases did not resume menstruation after completing treatment. One case received hormonal therapy. However, both cases were followed for only 1 year after FSS. Four cases died, two from neutropenic sepsis, and two from disease progression after multiple chemotherapy regimens. The details of these patients are summarized in Table 4.
| SN | Year | Age | Stage | Histology | Chemotherapy | Cycle | Site | Primary surgery | Note |
| 25 | 2017 | 29 | IIA | Yolk sac tumor | BEP | 6 | Right | Right SO and omentectomy January 6, 2017 | 16/1/18 abdominal pain and pelvic mass size 10 cm × 15 cm, solid and cystic, movable AFP 2.2 → TAH and left SO 19/1/18 → endometrioid CA IA → carboplatin × 6 → complete response → DFS 61 mo, HRT |
| 43 | 2014 | 16 | III | Dysgerminoma | BEP | 4 | Bilateral | Left SO and omentectomy | Partial response during BEP, overall survival of 3 mo, death from sepsis (neutropenia) |
| 52 | 2013 | 15 | IV | Yolk sac tumor | BEP | 8 | Right | Right salpingo-oophorectomy with left ovarian cystectomy | PFS 15 mo → TIP × 2 cycles → PT × 1 → ifosfamide × 1 cycle → death OS 16 mo |
| 87 | 2008 | 28 | I | Dysgerminoma | BEP | 3 | Left | Left SO | HRT icycloprogynova lost to follow up since 2009, unknown status |
| 108 | 2005 | 15 | III | Yolk sac tumor | BEP | 6 | Right | Right SO | Febrile neutropenia → sepsis → death 2005 OS 9 mo |
| 110 | 2005 | 16 | IV | Yolk sac tumor | BEP × 2 → EP × 11 | 12 | Right | Progression after EP × 11 → ifosfamide × 1 → EMA × 1 → single paclitaxel × 1 → palliative RT January 25, 2006 → VAC × 1 → Death July 4, 2007, OS 30 mo (lung fibrosis after BEP × 2) | |
| 114 | 2005 | 23 | II | Dysgerminoma | BEP | 6 | Right | Right SO | Alive, loss after 12 mo since start treatment, no HRT |
Regarding 19 patients who underwent only FSS without adjuvant chemotherapy, one was lost to follow-up since surgery while the remaining 18 had no problem with menstruation. One case was diagnosed with stage I immature teratoma and received left SO with omentectomy and appendectomy at 4 years old. At 15 years old, her menarche occurred.
For pregnancy outcomes, the data was available in 30 patients and revealed that 14 cases attempted to become pregnant and four of them (28.6%) succeeded in delivering a term baby after 1 year for two cases and 6 years for one case. One patient was known to give one term birth due to unavailable contact details. Three cases underwent unilateral SO and the rest received a unilateral ovarian cystectomy. The histology of these four cases was grade 1 carcinoid tumor neuroendocrine tumor (1), dysgerminoma (2), and grade 1 immature teratoma (1). Moreover, one case developed a spontaneous abortion 2 years after treatment and was never pregnant again. She was diagnosed with a steroid cell tumor. None of the patients who attempted to conceive actively tried to become pregnant by going to an infertility clinic. The details of these patients are shown in Table 5.
| SN | Year | Age Dx | Age Preg | Stage | Histology | Site | Chemotherapy | Cycle | Parity1 | Primary surgery | Pregnancy outcome |
| 19 | 2018 | 30 | 31 | IA | Carcinoid tumor neuroendocrine tumor grade 1 arising in mature cystic teratoma | Left | None | - | 1001 | Laparoscopic left ovarian cystectomy June 1, 2018 | 1 Term pregnancy, GA 39 wk, NL August 1, 2019, BW 3030 gm |
| 53 | 2013 | 21 | 27 | IC | Dysgerminoma | Right | BEP | 4 | - | Right SO and omentectomy and appendectomy June 15, 2013 | 1 Term pregnancy, C/S GA 38 wk April 23, 2019, BW 2780 gm, ompholocele 9 cm × 8 cm, and atrial septal defect → surgical correction |
| 84 | 2008 | 19 | 21 | IA | Steroid cell tumor | Right | None | - | - | Right SO and omentectomy and PNS and PAS December 18, 2008 | 1 spontaneous abortion 31/1/2010 |
| 92 | 2005 | 34 | 35 | IA | Immature teratoma grade1 | Left | None | - | 1001 | Left SO | 1 Term pregnancy, GA 38 wk, NL 10/12/2006, BW 2700 gm |
| 94 | 2007 | 24 | 26 | IA | Dysgerminoma | Left | None | - | 1001 | Left SO and omentectomy October 9, 2007 | 1 Term pregnancy with no available data of birth date, GA, and BW |
The outcome of 62 MOGOT patients who were treated by FSS in the present study was excellent with the 10-year PFS and OS being 82.4% and 91%, respectively. These results were close to the previous reports. Zamani et al[6] studied 79 MOGCT over 15 years and showed the 10-year OS as 94.4%. This study recruited only stages I-III while our study recruited all stages including three progressed cases of stage IV. Another study from Korea[1] studied 171 MOGCT patients who underwent FSS for 23 years (1992-2015). They reported the 5-year PFS and OS as 86% and 97%, respectively. About 14.6% developed recurrent disease and the death rate of disease was 2.9%. This recurrence rate was higher than our study which showed the progression of the disease at only 1.6%. However, due to over 2/3 of our patients without regular follow-up, the actual number of recurrence patients might be missed. However, the death rate of this disease in our study was 4.8%, which is near the Korean report. In addition, Beiner et al[7] reviewed eight retrospective studies comparing FSS with the conventional operation for MOGCT patients and found that both types of surgery were not significant for recurrence.
Regarding ovarian cystectomy in MOGCT, although this operation was not the standard of FSS, Tamauchi et al[8] showed an excellent outcome in eight patients who were diagnosed with early-stage immature teratoma treated by ovarian cystectomy. Five patients received chemotherapy. With a median follow-up time of 4.7 years, all patients were still free of disease. The authors suggested that cystectomy followed by adjuvant chemotherapy showed impressive outcomes for early-stage MOGCT, especially in immature teratoma. For our study, four cases underwent ovarian cystectomy with one case of stage IA grade 2 immature teratoma and received adjuvant chemotherapy. All of them were still alive at a duration time of 44-173 mo after surgery.
The 70.8% of patients who had no menstruation during treatment by FSS and chemotherapy in this study resumed menstruation with a median time of 4 mo. The true premature ovarian failure from chemotherapy occurred in only two (3.2%) cases. Both underwent unilateral SO with three and six cycles of the BEP regimen. Turkmen et al[9] used the Tokai Ovarian Tumor Study Group database on ovarian cancer patients and selected 110 MOGCT patients who received FSS with a median follow-up period of 10.4 years for the study. In this Japanese report, 63.9% of the patients received the BEP regimen and about 30.6% received the cisplatin + vincristine + bleomycin regimen. They revealed premature menopause which was close to our study of 2.9%.
Regarding the obstetric outcome, our study reported that the rate of term pregnancy was 28.6%. This result was different from that of a Japanese study[9]. The authors revealed that 45 patients attempted to become pregnant with 40 patients succeeding in deliveries with total pregnancies as term deliveries in 54 (83.1%) cases, preterm delivery in two (3.2%), and abortion in 12 (18.5%). Seven cases received fertility treatment. A publication from Iran reported that 19 of 26 (73%) MOGCT patients who underwent FSS were successful in delivery without infertility treatment[6]. In addition, Mikuš et al[10] reported that the pregnancy rate in 20 German patients with MOGCT who desired to become pregnant of their series was 50%. The pregnancy rate from previous studies was higher than that of our study, which showed the successful pregnancy rate was only 28.6%. The difference might be from the current trend of Thai culture to have fewer children, the missing data from the patients unable to be contacted, and those non-actively who tried to conceive in our patients.
The strength of our study was the real-world series of patients with MOGCT treated by FSS in a single institute to show the oncology and reproductive outcomes. However, with the limitation of the retrospective study, about 2/3 of the patients were not followed for a long time. Therefore, some data were missed.
In conclusion, the oncology and reproductive outcomes of MOGCT treated by FSS were good. Many patients showed a long survival time with normal menstruation. However, the obstetric outcome in patients who attempted to conceive was not quite as high.
Malignant ovarian germ cell tumors (MOGCT) are rare and frequently occur in women of young and reproductive age. Fertility-sparing surgery (FSS) is the main treatment for these patients. However, oncologic and reproductive outcomes after FSS for this disease are still limited.
Due to the limited data on oncology and reproductive outcomes of FSS especially in Southeast Asia, this study was conducted to identify these outcomes of MOGCT patients who were treated by FSS.
To evaluate the oncology and reproductive outcomes of MOGCT who underwent FSS.
All MOGCT patients who underwent FSS defined as the operation with a preserved uterus and at least one side of the ovary at our institute between January 2005 and December 2020 were retrospectively reviewed.
Sixty-two patients were reviewed in this study. The median age was 22 years old and over 77% were nulliparous. The three most common histology findings were immature teratoma (32.2%), dysgerminoma (24.2%), and yolk sac tumor (24.2%). The distribution of stage was as follows: Stage I, 74.8%; stage II, 9.7%; stage III, 11.3%; stage IV, 4.8%. About 2/3 of the patients received adjuvant chemotherapy. With a median follow-up time of 96.3 mo, the 10-year progression-free survival and overall survival were 82.4% and 91%, respectively. For reproductive outcomes, of 43 patients who received adjuvant chemotherapy, 18 (41.9%) had normal menstruation and 17 (39.5%) resumed menstruation with a median time of 4 mo. Of about 14 patients who desired to conceive, four were pregnant and delivered good outcomes. Only one case was aborted. Therefore, the successful pregnancy rate was 28.6%.
The oncology and reproductive outcomes of MOGCT treated by FSS were excellent. Many patients showed a long survival time with normal menstruation. However, the obstetric outcome was not quite high.
The strength of our study was the real-world series of patients with MOGCT treated by FSS in a single institute to show the oncology and reproductive outcomes. However, with the limitation of the retrospective study, about 2/3 of the patients were not followed for a long time. Therefore, some data were missed. A good plan follow-up is needed in the future.
Provenance and peer review: Unsolicited article; Externally peer reviewed.
Peer-review model: Single blind
Specialty type: Obstetrics and gynecology
Country/Territory of origin: Thailand
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): B
Grade C (Good): C
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Jian X, China; Yang YZ, China S-Editor: Liu JH L-Editor: Wang TQ P-Editor: Liu JH
| 1. | Park JY, Kim DY, Suh DS, Kim JH, Kim YM, Kim YT, Nam JH. Analysis of outcomes and prognostic factors after fertility-sparing surgery in malignant ovarian germ cell tumors. Gynecol Oncol. 2017;145:513-518. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 38] [Cited by in RCA: 45] [Article Influence: 5.6] [Reference Citation Analysis (0)] |
| 2. | Williams S, Blessing JA, Liao SY, Ball H, Hanjani P. Adjuvant therapy of ovarian germ cell tumors with cisplatin, etoposide, and bleomycin: a trial of the Gynecologic Oncology Group. J Clin Oncol. 1994;12:701-706. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 254] [Cited by in RCA: 192] [Article Influence: 6.2] [Reference Citation Analysis (0)] |
| 3. | Di Tucci C, Casorelli A, Morrocchi E, Palaia I, Muzii L, Panici PB. Fertility management for malignant ovarian germ cell tumors patients. Crit Rev Oncol Hematol. 2017;120:34-42. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 11] [Cited by in RCA: 18] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 4. | Brown J, Friedlander M, Backes FJ, Harter P, O'Connor DM, de la Motte Rouge T, Lorusso D, Maenpaa J, Kim JW, Tenney ME, Seckl MJ. Gynecologic Cancer Intergroup (GCIG) consensus review for ovarian germ cell tumors. Int J Gynecol Cancer. 2014;24:S48-S54. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 110] [Cited by in RCA: 86] [Article Influence: 7.8] [Reference Citation Analysis (0)] |
| 5. | Neeyalavira V, Suprasert P. Outcomes of malignant ovarian germ-cell tumors treated in Chiang Mai University Hospital over a nine year period. Asian Pac J Cancer Prev. 2014;15:4909-4913. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 7] [Cited by in RCA: 8] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 6. | Zamani N, Rezaei Poor M, Ghasemian Dizajmehr S, Alizadeh S, Modares Gilani M. Fertility sparing surgery in malignant ovarian Germ cell tumor (MOGCT): 15 years experiences. BMC Womens Health. 2021;21:282. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 9] [Cited by in RCA: 1] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 7. | Beiner ME, Gotlieb WH, Korach Y, Shrim A, Stockheim D, Segal Y, Fridman E, Ben-Baruch G. Cystectomy for immature teratoma of the ovary. Gynecol Oncol. 2004;93:381-384. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 12] [Cited by in RCA: 22] [Article Influence: 5.5] [Reference Citation Analysis (0)] |
| 8. | Tamauchi S, Kajiyama H, Yoshihara M, Ikeda Y, Yoshikawa N, Nishino K, Utsumi F, Niimi K, Suzuki S, Kikkawa F. Reproductive outcomes of 105 malignant ovarian germ cell tumor survivors: a multicenter study. Am J Obstet Gynecol. 2018;219:385.e1-385.e7. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 21] [Cited by in RCA: 32] [Article Influence: 4.6] [Reference Citation Analysis (0)] |
| 9. | Turkmen O, Karalok A, Basaran D, Kimyon GC, Tasci T, Ureyen I, Tulunay G, Turan T. Fertility-Sparing Surgery Should Be the Standard Treatment in Patients with Malignant Ovarian Germ Cell Tumors. J Adolesc Young Adult Oncol. 2017;6:270-276. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 16] [Cited by in RCA: 21] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 10. | Mikuš M, Benco N, Matak L, Planinić P, Ćorić M, Lovrić H, Radošević V, Puževski T, Bajt M, Vujić G. Fertility-sparing surgery for patients with malignant ovarian germ cell tumors: 10 years of clinical experience from a tertiary referral center. Arch Gynecol Obstet. 2020;301:1227-1233. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5] [Cited by in RCA: 11] [Article Influence: 2.2] [Reference Citation Analysis (0)] |