Published online Sep 24, 2021. doi: 10.5306/wjco.v12.i9.823
Peer-review started: April 29, 2021
First decision: June 16, 2021
Revised: June 29, 2021
Accepted: August 10, 2021
Article in press: August 10, 2021
Published online: September 24, 2021
Processing time: 140 Days and 18.2 Hours
Primary pancreatic lymphoma (PPL) is a rare neoplasm. Being able to distinguish it from other pancreatic malignancies such as pancreatic ductal adenocarcinoma (PDAC) is important for appropriate management. Unlike PDAC, PPL is highly sensitive to chemotherapy and usually does not require surgery. Therefore, being able to identify PPL preoperatively will not only direct physicians towards the correct avenue of treatment, it will also avoid unnecessary surgical intervention.
To evaluate the typical and atypical multi-phasic computed tomography (CT) imaging features of PPL.
A retrospective review was conducted of the clinical, radiological, and patho
Twenty-nine cases of PPL were diagnosed between January 2000 and December 2020 (mean age 66 years; 13 males/16 females). All twenty-nine subjects were symptomatic but only 4 of the 29 subjects (14%) had B symptoms. Obstructive jaundice occurred in 24% of subjects. Elevated lactate dehydrogenase was seen in 81% of cases, whereas elevated cancer antigen 19-9 levels were present in only 10% of cases for which levels were recorded. The vast majority (90%) of tumors involved the pancreatic head and uncinate process. Mean tumor size was 7.8 cm (range, 4.0-13.8 cm). PPL presented homogenous hypoenhancement on CT in 72% of cases. Small volume peripancreatic lymphadenopathy was seen in 28% of subjects. Tumors demonstrated encasement of superior mesenteric vessels in 69% of cases but vascular stenosis or occlusion only manifested in 5 out of the twenty-nine individuals (17%). Mild pancreatic duct dilatation was also infrequent and seen in only 17% of cases, whereas common bile duct (CBD) dilation was seen in 41% of subjects. Necrosis occurred in 10% of cases. Size did not impact the pre
PPL is an uncommon diagnosis best made preoperatively to avoid unnecessary surgery and ensure adequate treatment. In addition to the typical CT findings of PPL, such as homogeneous hypoenhancement, absence of vascular stenosis and occlusion despite encasement, and peripancreatic lymphadenopathy, this study highlighted many less typical findings, including small volume necrosis and pancreatic and bile duct dilation.
Core Tip: Primary pancreatic lymphoma (PPL) is often misdiagnosed as pancreatic adenocarcinoma. This two-center retrospective study of twenty-nine cases emphasized computed tomography (CT) imaging features useful for distinguishing PPL from its mimics. Distinct CT features of PPL, including a large homogenous hypovascular mass, absence of ductal dilation or atrophy, encasement of mesenteric vessels without invasion, and the presence of small volume peripancreatic adenopathy, should alert the clinicians to the potential diagnosis of this rare neoplasm. This study also demonstrated atypical findings such as necrosis and mild pancreatic or biliary ductal dilation should not rule out the diagnosis of PPL.
- Citation: Segaran N, Sandrasegaran K, Devine C, Wang MX, Shah C, Ganeshan D. Features of primary pancreatic lymphoma: A bi-institutional review with an emphasis on typical and atypical imaging features. World J Clin Oncol 2021; 12(9): 823-832
- URL: https://www.wjgnet.com/2218-4333/full/v12/i9/823.htm
- DOI: https://dx.doi.org/10.5306/wjco.v12.i9.823
Primary pancreatic lymphoma (PPL) is an extremely rare malignancy, constituting less than 0.5% of pancreatic neoplasms and approximately 1% of all extranodal lym
Identifying unique preoperative characteristics of PPL so physicians may discri
For this retrospective Health Insurance Portability and Accountability Act-compliant study, clinical, radiology, and pathology databases at the University of Texas MD Anderson Cancer Center, Houston, TX and Mayo Clinic, Phoenix, AZ were reviewed. This review searched the period between January 2000 and December 2020 for reports containing the strings “pancreas lymphoma” or “pancreatic lymphoma”. Institutional review board permission was obtained for retrospective assessment of imaging, clinical, and pathologic data with waiver of informed consent from both the insti
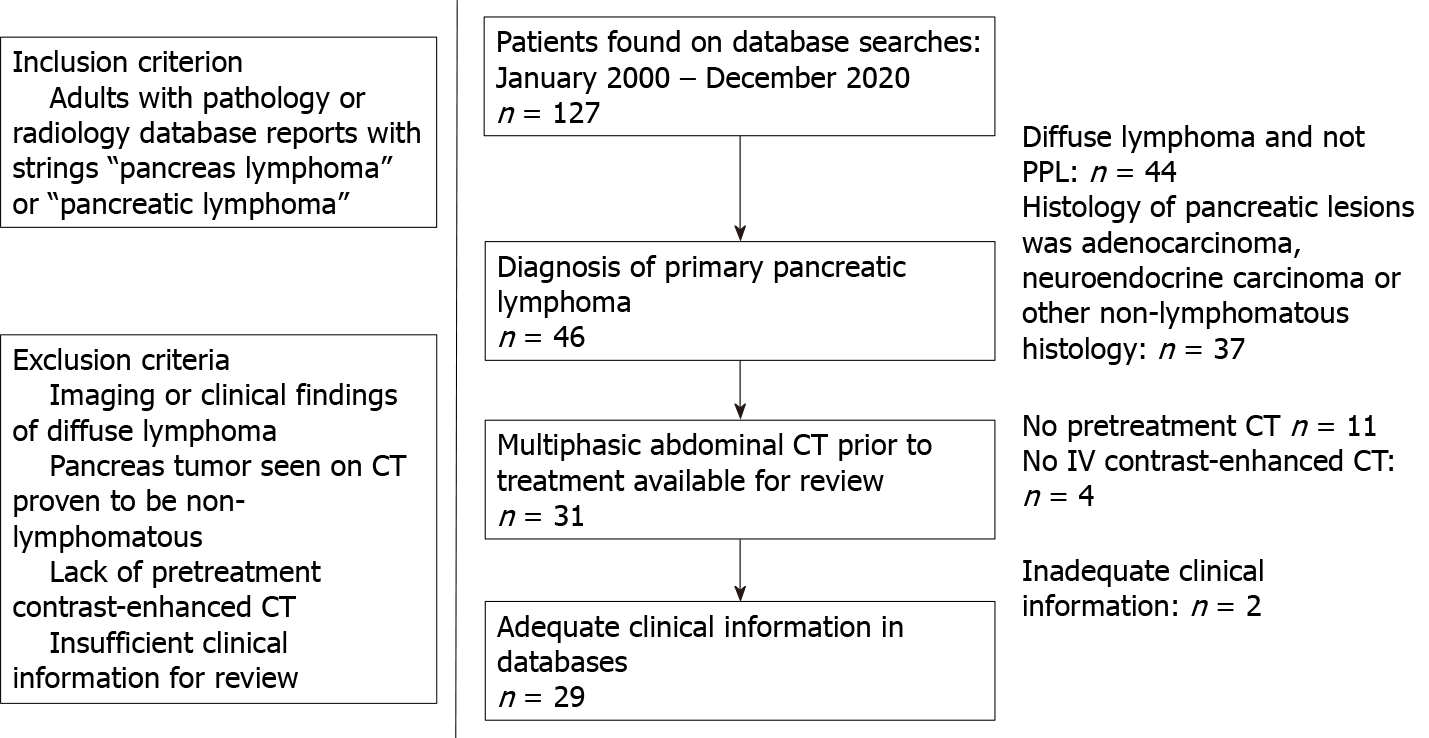
The derivation of a cohort of 29 subjects is shown in Figure 1. All subjects had biopsy-proven PPL with a mass involving the pancreas. There were 13 males and 16 females with a mean age of 66 years (range, 55-88 years).
Multislice CT scans of the abdomen were obtained in the pre-contrast, arterial, venous, and delayed phases. CT findings such as the size, site, morphology and imaging characteristics of PPL including the presence/absence of nodal, vascular and ductal involvement in these subjects were recorded by two reviewers (DG, KS) with 12 and 18 years of post-fellowship experience in Abdominal Imaging.
Descriptive statistics were compiled. Differences in sizes of tumors showing atypical findings (i.e., pancreatic and bile duct dilation, mesenteric root infiltration, and tumor necrosis) and those without these findings was performed using the Mann-Whitney test. Frequency of these findings in tumors larger than 10 cm and less than 10 cm was performed with the Chi-Square test. MedCalc 19.3 (MedCalc Software Ltd, Ostend, Belgium) was used for statistical analysis.
The clinical presentation of the 29 biopsy-confirmed PPL cases used for imaging and clinical feature evaluation are summarized in Table 1. The most common presenting finding were vague abdominal pain (83%) and nausea and vomiting (31%). Weight loss was present in 21% of cases. Classic “B” symptom (i.e. fever, chills, night sweats, weight loss) were only seen in 14% of subjects. Similarly, 14% of cases demonstrated a palpable abdominal mass. Obstructive jaundice appeared in seven out of the twenty-nine subjects (24%).
| Characteristics | n (%) |
| Abdominal pain | 24 (83) |
| Nausea and vomiting | 9 (31) |
| Weight loss | 6 (21) |
| Classic “B” symptoms | 4 (14) |
| Palpable mass | 4 (14) |
| Obstructive jaundice | 7 (24) |
All twenty-nine subjects underwent chemotherapy; the most common treatment was R-CHOP (given in 72% of cases). R-CVP was administered to 2 subjects (7%). In addition, 5 subjects (17%) underwent radiation therapy.
Lactate dehydrogenase (LDH) levels were elevated in 17 of the 21 cases for which LDH levels were recorded (81%). Elevated amylase was seen in four out of 11 subjects with recorded values (36%), all of whom presented with bile duct dilation (n = 3) or con
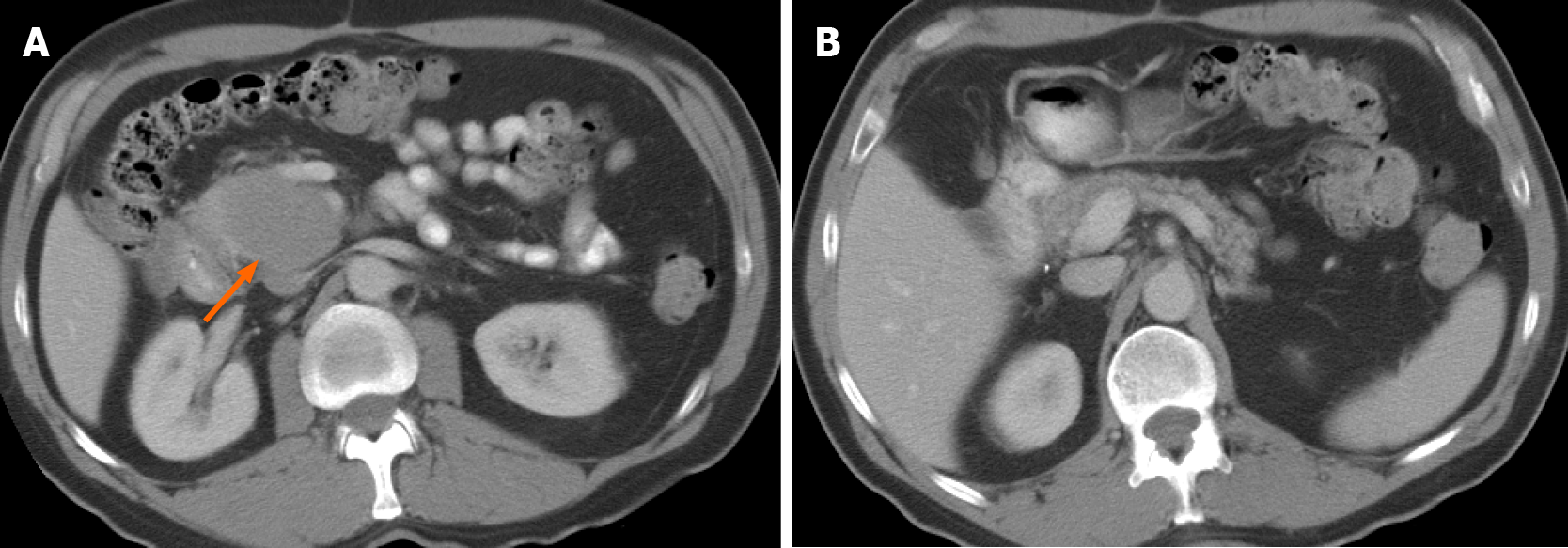
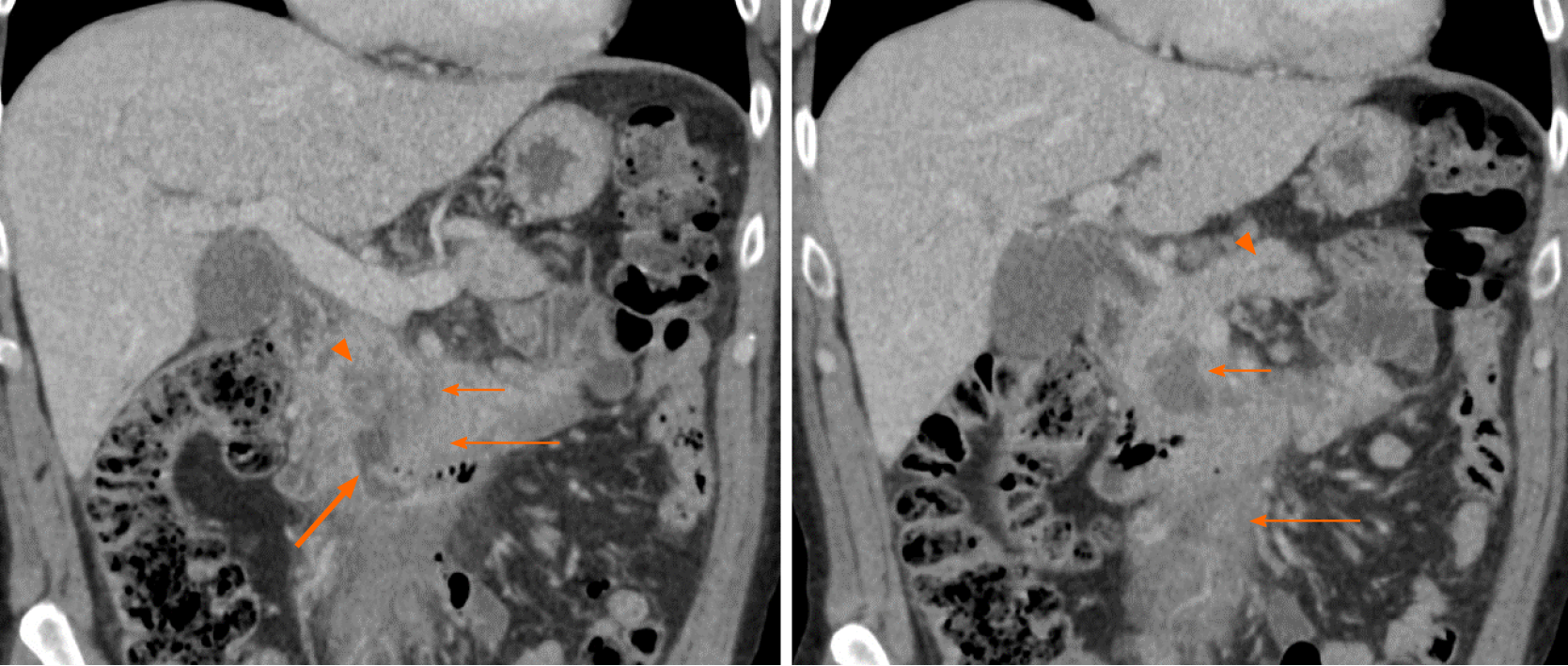
The prevalence of imaging findings are given in Table 2. The majority of tumors involved to the head and uncinate process (90%). Only four tumors (14%) occurred in the tail. Mean tumor size was 7.8 cm (range, 4.0-13.8 cm). Typical manifestation included a large (> 5 cm) infiltrative (52%) or focal, well-defined (48%) pancreatic mass, with homogenous hypoenhancement on post-contrast CT (72%) (Figure 2). In 41% of cases there was extension to the duodenum. Mass effect on the adjacent superior mesenteric vessels was seen in sixteen out of the twenty-nine subjects (55%). In addition, the majority of cases (69%) demonstrated encasement of superior me
| Characteristics | n (%) |
| Location | |
| Head | 23 (80) |
| Body | 3 (10) |
| Tail | 4 (14) |
| Uncinate process | 16 (55) |
| Mean size (cm) | 7.8 |
| Infiltrative mass | 15 (52) |
| Focal mass | 14 (48) |
| Homogeneous hypoenhancement | 21 (72) |
| Tumor necrosis | 3 (10) |
| Extension to nuodenum | 12 (41) |
| Mass effect on SMV | 16 (55) |
| Encasement of SMA/SMV | 20 (69) |
| Vascular stenosis/occlusion | 5 (17) |
| Pancreatic ductal dilation | 5 (17) |
| Common bile duct dilation | 12 (41) |
| Pancreatic atrophy | 0 (0) |
| Infiltration of mesenteric root | 4 (14) |
| Peripancreatic lymphadenopathy | 8 (28) |
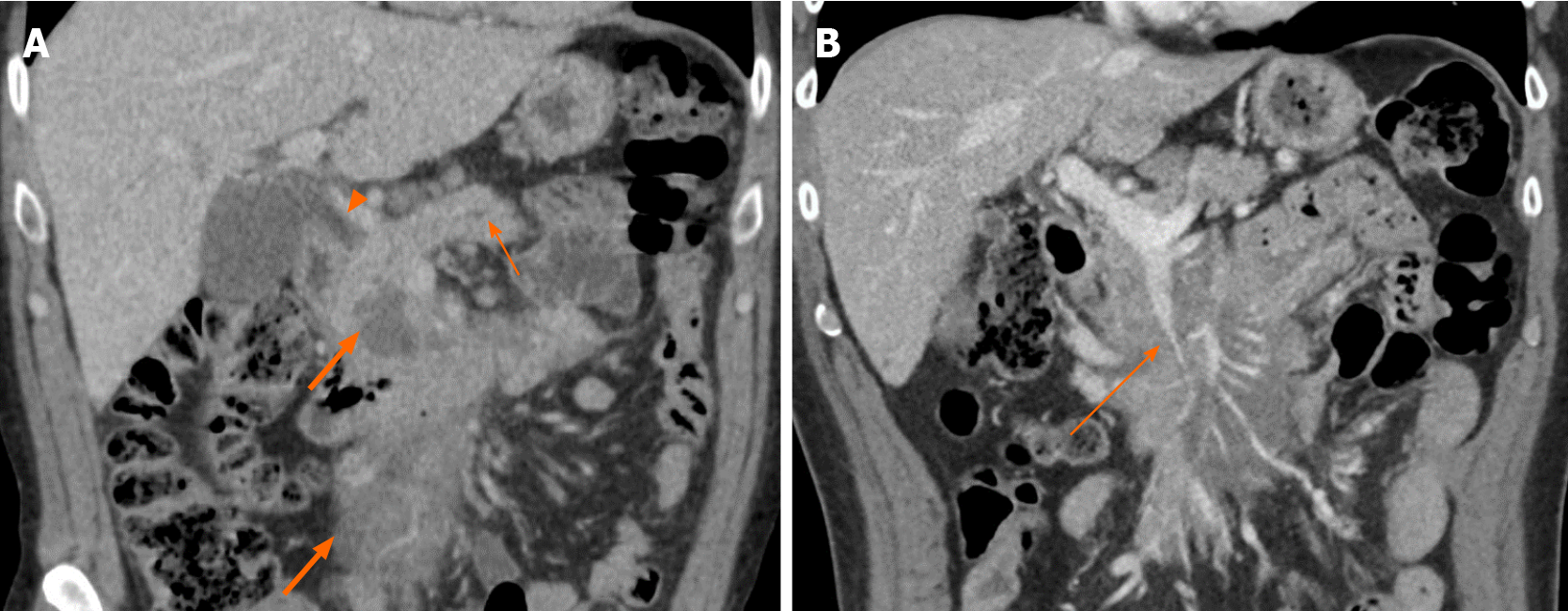
Furthermore, pancreatic ductal dilation was absent in 83% of cases; the remaining five subjects demonstrated mild dilation with duct caliber of less than 5 mm (Figures 3 and 5). There was no difference in size of those without and with pancreatic duct dilation (median sizes of 6.9 vs 6.8 cm, P = 0.525) or in the frequency of pancreatic duct dilation between tumors greater than 10 cm and less than 10 cm (P = 0.366). Common bile duct (CBD) dilation was seen in 41% of cases (Figure 3). Tumor size did not differ between those without and with CBD dilation (median sizes of 5.4 vs 7.1 cm, P = 0.294). There was no difference in frequency of CBD dilation between tumors greater than 10 cm and less than 10 cm (P = 0.823)
No cases presented signs of pancreatic atrophy. Peripancreatic lymphadenopathy manifested in eight subjects (28%), five of which included involvement below the renal vein. These nodes all had a short-axis dimension of less than 1.5 cm. Mesenteric root infiltration was seen in 14% of cases (Figures 3 and 5). There was no significant difference in tumor size of those without and with mesenteric root infiltration (median sizes of 6.8 vs 12.8 cm, P = 0.097), although there was an overall trend for larger tumors to infiltrate the mesentery.
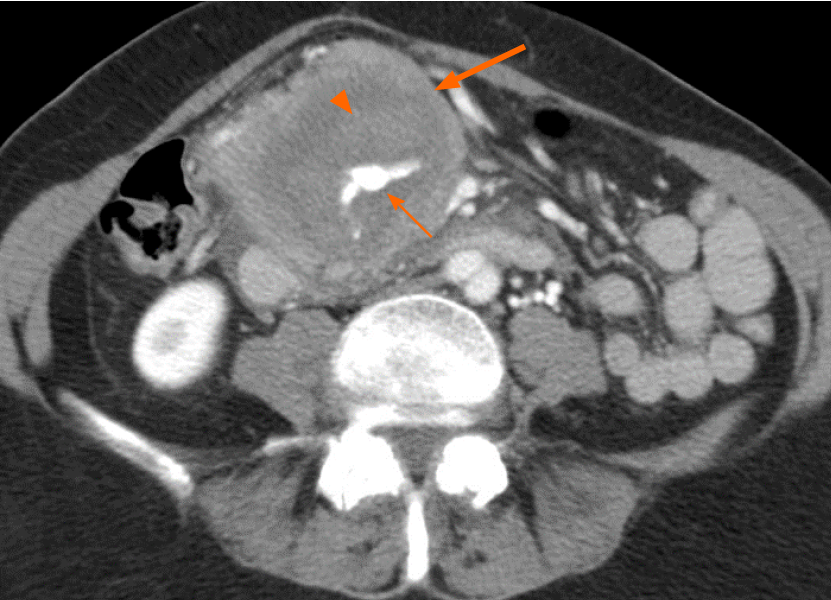
Small foci of low density within tumor less than 10% of volume, and likely to be necrosis, was seen in three cases (10%). These subjects either had acute pancreatitis (n = 2) or a duodenal fistula (n = 1) (Figure 5). There was no significant difference in size of those without and with necrosis (median sizes of 7.0 vs 6.2 cm, P = 0.543) or in frequency of necrosis between tumors greater than 10 cm and less than 10 cm (P = 0.950).
In agreement with prior literature, subjects in this study presented with nonspecific abdominal symptoms including vague epigastric pain, nausea and vomiting, weight loss, obstructive jaundice, and a palpable mass, making PPL difficult to distinguish from other pancreatic pathologies based on clinical presentation alone[4,6]. Out of these presentations, the most prevalent in PPL are abdominal pain, nausea and vomiting, and weight loss. The decreased prevalence of obstructive jaundice among PPL cases in comparison to individuals with PDAC could aid differentiating between the two; nevertheless, obstructive jaundice is still a relatively common potential presentation of PPL[7-10]. Classic “B” symptoms such as fever, chills, night sweats, and weight loss associated with systemic non-Hodgkin lymphoma are rare in PPL, and only occurred in 4 cases from this study[4].
Laboratory values may play a more decisive role in the diagnosis of the PPL. Elevated tumor marker CA 19-9 has been reported in 80% of PDAC cases but is a rare finding of PPL[4]. In this study, CA 19-9 was within normal limits for the vast majority of subjects. PPL cases with biliary dilation may be associated with mild elevation of CA 19-9[11]. In keeping with this study, elevated amylase has been observed in PPL by other studies and numerous case reports, often due to associated acute pancreatitis or biliary obstruction[8-10,12,13]. Other useful biomarkers include LDH which is often elevated in non-Hodgkin lymphomas, including PPL, but is less likely to be elevated in other pancreatic neoplasms such as PDAC[10,11].
Despite the potential use of laboratory values to suggest PPL over its mimics, the overall nonspecificity of clinical and laboratory features of PPL emphasizes the importance of imaging. CT is the most common imaging study performed for diag
Lymphadenopathy localized to the peripancreatic region also suggests PPL[16]. In particular, the presence of lymphadenopathy below the level of renal hilum may be useful in excluding the diagnosis of PDAC[9,17,18]. The majority of cases in this study demonstrating lymphadenopathy presented nodal involvement below the renal hilum. In addition, all nodal involvement in this study was less than 1.5 cm in short-axis diameter, which may be useful for differentiating PPL from diffuse lymphoma secon
Perhaps the most important findings which indicate PPL rather than PDAC are the absence of pancreatic ductal dilation and pancreatic atrophy; only 17% of subjects in this study showed mild pancreatic ductal dilation (< 5 mm) and no subjects presented pancreatic atrophy (Figures 3 and 5)[14]. This is a useful differentiating feature as even small pancreatic adenocarcinoma located in the head of pancreas tends to result in moderate or severe upstream pancreatic ductal dilatation as well as pancreatic atrophy[4,6,20]. Nevertheless, mild pancreatic duct dilation may still be seen in PPL and should not be used to exclude this diagnosis.
In addition to cases of occasional mild pancreatic ductal dilation, this study has demonstrated multiple atypical features of PPL that have not been widely reported. Interestingly, CBD dilation was seen in a substantial proportion (41%) of subjects. While not widely reported in studies on PPL, Boninsegna et al[21] found similar results to this study, finding CBD dilation in six out of the fourteen subjects (43%) included in their series.
Ten percent of cases in this cohort showed partial necrosis of the tumor. This finding is contrary to the existing consensus that the presence of necrosis effectively excludes a diagnosis of PPL[5,7,11,17]. While most PPL do not present necrosis, this study demonstrates that complications such as concomitant acute pancreatitis or intratumoral fluid collection from a duodenal fistula may lead to this atypical finding (Figure 5).
To our knowledge, infiltration of the mesenteric root by PPL has not been reported by previous studies. This finding was demonstrated in 14% of cases in this study (Figures 3 and 5). As expected, the mesenteric vessels were not thrombosed or occluded; however, in one case stenosis of the SMV was seen (Figure 3). There was active extravasation of blood into the tumor in one subject, likely as a result of tumor erosion of the vessels wall (Figure 4).
There are limitations of this study, including its retrospective design. In addition, PPL is a rare entity, and the combined databases of two major institutions only revealed 29 cases of biopsy-proven PPL with pretreatment multiphase CT. Never
PPL is a rare tumor, which may be misdiagnosed as PDAC, but differentiation between the two entities is critical in order to avoid unnecessary surgery and asso
Primary pancreatic lymphoma (PPL) is a rare neoplasm. The ability to differentiate PPL from other pancreatic malignancies including pancreatic ductal adenocarcinoma (PDAC) is important for appropriate management. However, the nonspecific characteristics currently associated with PPL and a lack of information regarding PPL’s distinctive imaging features makes diagnosis difficult.
Identifying typical and atypical features of PPL on computed tomography (CT), as well as other diagnostic features that may differentiate PPL from its mimics, may enable definitive diagnosis. The discovery of features which distinguish PPL from PDAC early-on is critical to avoid unnecessary surgery.
This study aims to evaluate the typical and atypical CT imaging appearances of PPL. In addition, it distinguishes various clinical and laboratory markers which may be useful to identify PPL. An emphasis was placed on differentiating PPL from PDAC, which can be difficult to do using the current characteristics associated with PPL.
Radiology, clinical, and pathology databases from two institutions were searched for reports between January 2000 and December 2020 containing the strings “pancreas lymphoma” or “pancreatic lymphoma”. The exclusion criteria were: (1) Lymphoma with mediastinal or pelvic adenopathy, bone marrow or hepatosplenic involvement which were considered to be systemic lymphoma and not PPL; (2) Pancreatic tumors suspected to be lymphoma on CT and later biopsy-proven to be another diagnosis on histological examination; (3) Subjects without a pretreatment multiphasic CT exa
All twenty-nine subjects were symptomatic, but only 14% demonstrated B symptoms and 24% demonstrated obstructive jaundice. Lactate dehydrogenase (LDH) levels were elevated in 17 of the 21 cases for which LDH levels were recorded (81%), however cancer antigen 19-9 (CA 19-9) levels were within normal limits for 18 out of the 20 cases for which values were recorded (90%). Pancreatic ductal dilation was absent in 83% of cases and no patients presented pancreatic atrophy. Atypical features of PPL included pancreatic bile duct dilation (17%), common bile duct (CBD) dilation (41%), necrosis (10%), and infiltration of the mesenteric root (14%). Size did not impact the prevalence of pancreatic and CBD dilation, necrosis, or mesenteric root infiltration (P = 0.525, P = 0.294, P = 0.543, and P = 0.097, respectively).
The decreased prevalence of obstructive jaundice, elevated CA 19-9 levels, pancreatic ductal dilation, and pancreatic atrophy, as well as the increased elevation of LDH levels, encasement of the small mesenteric artery and/or vein without invasion or stenosis, and lymphadenopathy limited to the peripancreatic region, may be useful for distinguish PPL from its mimics, such as PDAC. However, in addition to the occa
Prospective studies with larger cohorts must be conducted to support the findings of this paper and the potential use of its highlighted imaging and clinical features for definitive diagnosis of PPL. In addition, there is a need for direct comparison of the frequency of these features in PPL vs PDAC, to determine how useful they are in differentiating the two entities.
Manuscript source: Invited manuscript
Specialty type: Oncology
Country/Territory of origin: United States
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): 0
Grade C (Good): C
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Caronna R S-Editor: Fan JR L-Editor: A P-Editor: Yuan YY
| 1. | Battula N, Srinivasan P, Prachalias A, Rela M, Heaton N. Primary pancreatic lymphoma: diagnostic and therapeutic dilemma. Pancreas. 2006;33:192-194. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 46] [Cited by in RCA: 46] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 2. | Behrns KE, Sarr MG, Strickler JG. Pancreatic lymphoma: is it a surgical disease? Pancreas. 1994;9:662-667. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 102] [Cited by in RCA: 89] [Article Influence: 2.9] [Reference Citation Analysis (0)] |
| 3. | Boni L, Benevento A, Dionigi G, Cabrini L, Dionigi R. Primary pancreatic lymphoma. Surg Endosc. 2002;16:1107-1108. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 25] [Cited by in RCA: 23] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 4. | Manning MA, Paal EE, Srivastava A, Mortele KJ. Nonepithelial Neoplasms of the Pancreas, Part 2: Malignant Tumors and Tumors of Uncertain Malignant Potential From the Radiologic Pathology Archives. Radiographics. 2018;38:1047-1072. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 15] [Cited by in RCA: 20] [Article Influence: 2.9] [Reference Citation Analysis (0)] |
| 5. | Dunphy L, Abbas SH, Al Shoek I, Al-Salti W. Primary Pancreatic lymphoma: a rare clinical entity. BMJ Case Rep. 2020;13. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 3] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 6. | Low G, Panu A, Millo N, Leen E. Multimodality imaging of neoplastic and nonneoplastic solid lesions of the pancreas. Radiographics. 2011;31:993-1015. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 150] [Cited by in RCA: 137] [Article Influence: 10.5] [Reference Citation Analysis (0)] |
| 7. | Grossberg AJ, Chu LC, Deig CR, Fishman EK, Hwang WL, Maitra A, Marks DL, Mehta A, Nabavizadeh N, Simeone DM, Weekes CD, Thomas CR Jr. Multidisciplinary standards of care and recent progress in pancreatic ductal adenocarcinoma. CA Cancer J Clin. 2020;70:375-403. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 142] [Cited by in RCA: 319] [Article Influence: 63.8] [Reference Citation Analysis (0)] |
| 8. | Shnitser A, Halegoua-DeMarzio D, Loren DE. Primary Pancreatic Lymphoma Presenting as Acute Pancreatitis. Gastroenterol Hepatol (N Y). 2016;12:456-458. [PubMed] |
| 9. | Merkle EM, Bender GN, Brambs HJ. Imaging findings in pancreatic lymphoma: differential aspects. AJR Am J Roentgenol. 2000;174:671-675. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 157] [Cited by in RCA: 143] [Article Influence: 5.7] [Reference Citation Analysis (0)] |
| 10. | Facchinelli D, Sina S, Boninsegna E, Borin A, Tisi MC, Piazza F, Scapinello G, Maiolo E, Hohaus S, Zamò A, Merli M, Stefani PM, Mellone F, Basso M, Sartori R, Rusconi C, Parisi A, Manfrin E, Krampera M, Ruggeri M, Visco C, Tecchio C. Primary pancreatic lymphoma: Clinical presentation, diagnosis, treatment, and outcome. Eur J Haematol. 2020;105:468-475. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 19] [Cited by in RCA: 23] [Article Influence: 4.6] [Reference Citation Analysis (0)] |
| 11. | Rad N, Khafaf A, Mohammad Alizadeh AH. Primary pancreatic lymphoma: what we need to know. J Gastrointest Oncol. 2017;8:749-757. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 12] [Cited by in RCA: 24] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 12. | Abedi SH, Ahmadzadeh A, Nikmanesh A, Mohammad Alizadeh AH. The role of endoscopic ultrasound in primary pancreatic lymphoma presented with acute pancreatitis: a case report. JOP. 2014;15:493-496. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 3] [Reference Citation Analysis (0)] |
| 13. | Li Z, Zhang S, Vasdani N, Castillo E. Clues for diagnosing primary pancreatic lymphoma. Case Rep Gastroenterol. 2012;6:438-445. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 10] [Cited by in RCA: 11] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 14. | Anand D, Lall C, Bhosale P, Ganeshan D, Qayyum A. Current update on primary pancreatic lymphoma. Abdom Radiol (NY). 2016;41:347-355. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 37] [Cited by in RCA: 35] [Article Influence: 3.9] [Reference Citation Analysis (0)] |
| 15. | Gandhi NS, Feldman MK, Le O, Morris-Stiff G. Imaging mimics of pancreatic ductal adenocarcinoma. Abdom Radiol (NY). 2018;43:273-284. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 22] [Cited by in RCA: 31] [Article Influence: 4.4] [Reference Citation Analysis (0)] |
| 16. | Alzerwi NAN. Primary Pancreatic Lymphoma Masquerading as Carcinoma. Case Rep Oncol Med. 2020;2020:5160545. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1] [Cited by in RCA: 7] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 17. | Wallace D, Dang N, Dhawan M, Kulkarni A. Diagnosis of a patient with primary pancreatic lymphoma. Gastroenterol Hepatol (N Y). 2012;8:850-852. [PubMed] |
| 19. | Sandrasegaran K, Tomasian A, Elsayes KM, Nageswaran H, Shaaban A, Shanbhogue A, Menias CO. Hematologic malignancies of the pancreas. Abdom Imaging. 2015;40:411-423. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 10] [Cited by in RCA: 9] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 20. | Elbanna KY, Jang HJ, Kim TK. Imaging diagnosis and staging of pancreatic ductal adenocarcinoma: a comprehensive review. Insights Imaging. 2020;11:58. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 65] [Cited by in RCA: 82] [Article Influence: 16.4] [Reference Citation Analysis (0)] |
| 21. | Boninsegna E, Zamboni GA, Facchinelli D, Triantopoulou C, Gourtsoyianni S, Ambrosetti MC, Veneri D, Ambrosetti A, Pozzi Mucelli R. CT imaging of primary pancreatic lymphoma: experience from three referral centres for pancreatic diseases. Insights Imaging. 2018;9:17-24. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 15] [Cited by in RCA: 23] [Article Influence: 3.3] [Reference Citation Analysis (0)] |