Published online Sep 24, 2021. doi: 10.5306/wjco.v12.i9.746
Peer-review started: May 3, 2021
First decision: June 16, 2021
Revised: June 19, 2021
Accepted: July 30, 2021
Article in press: July 30, 2021
Published online: September 24, 2021
Processing time: 136 Days and 19 Hours
High-dose chemotherapy (HDCT) with autologous hematopoietic stem cell transplantation has been explored and has played an important role in the ma
Core tip: High-dose chemotherapy (HDCT) followed by autologous stem cell trans
- Citation: Porfyriou E, Letsa S, Kosmas C. Hematopoietic stem cell mobilization strategies to support high-dose chemotherapy: A focus on relapsed/refractory germ cell tumors. World J Clin Oncol 2021; 12(9): 746-766
- URL: https://www.wjgnet.com/2218-4333/full/v12/i9/746.htm
- DOI: https://dx.doi.org/10.5306/wjco.v12.i9.746
High-dose chemotherapy (HDCT) followed by autologous hematopoietic stem cell transplantation (ASCT) has been a major breakthrough in oncology. It has broad applicability in patients with metastatic germ cell tumors (GCTs) who experience one or even more relapses after previous chemotherapy, or in those with a poor prognosis on diagnosis (e.g., with extragonadal primary or incomplete response to first-line cisplatin-based chemotherapy)[1,2]. The efficacy of HDCT and ASCT depends largely on successful and adequate hematopoietic stem cell (HSC) mobilization, which ensures faster neutrophil and platelet engraftment and therefore decreased infection risk and hospitalization[2]. Collection of at least 2.0 × 106 CD34+ HSCs has been considered the minimum for a subsequent successful ASCT[3,4]. However, successful mobilization remains a great challenge, as a significant number of patients, somewhere between 5%-30%, are unable to mobilize enough HSCs to support subsequent ASCT. That has been attributed to extensive and prolonged prior exposure to bone marrow-sup
Testicular cancer and GCTs typically subdivided into two main histologic subtypes, seminomas and non-seminomas, are the most common solid tumor in men between 20 and 35 years of age[6,7]. Approximately 50% of testicular cancers are non-seminomas, which are typically more malignant and usually associated with a more aggressive clinical presentation[8]. The cure rates are between 41%-92%[9,10]. About 20%-30% of patients with metastatic disease at initial presentation will eventually require salvage treatment. Second-line therapy options include conventional dose cisplatin-based regimens, or high-dose chemotherapy regimens, currently consisting of carboplatin and etoposide plus ASCT support[10,11].
To date, the main conventional dose chemotherapy (CDCT) salvage regimens include etoposide-ifosfamide-cisplatin, vinblastine-ifosfamide-cisplatin, and paclitaxel (taxol)-ifosfamide-cisplatin (TIP)[12,13]. Randomized data are lacking, and retro
In HDCT, cytotoxic agents are administered at much higher doses than the standard dose applied in CDCT. The observation of a larger therapeutic impact even at minor increases of dosage, proved the dose-response relationship of many chemotherapeutic agents, and thus supported the efficiency of HDCT regimens in eradicating residual drug-resistant tumor cells[14]. Increased doses lead also to more severe side effects, with prolonged myelosuppression being the main reason to delay subsequent cycles, thus leading to failure[15]. To reduce the duration of pancytopenia, and therefore the failure rate, HSCs are harvested from the patient’s peripheral blood by apheresis before the administration of HDCT. After completion of HDCT the harvested stem cells are reinfused to repopulate the bone marrow and ultimately re-establish he
Total-body irradiation (TBI) prior to autologous transplantation was first applied in animals in the 1930’s. The early studies had fatal outcomes because of severe gas
Bone marrow was the first source of HSCs, which were obtained by repeated aspi
Goldman et al[22] was the first to use HSCs collected from the peripheral blood for autologous transplantation after high-dose cytotoxic therapy in patients with CML. Körbling et al[23] followed with a report of autologous transplantation in a patient with CML, and a patient with Burkitt’s lymphoma. Körbling et al[23] reported the collection of peripheral blood stem cells after the use of granulocyte-macrophage colony-stimulating factor (GM-CSF) during leukocyte recovery after myelosuppressive chemotherapy. That was the first example of chemotherapy-induced “mobilization”. Subsequently Kessinger et al[24] used the same mobilization method and documented that performing multiple leukapheresis sessions resulted in a sufficient number of circulating HSCs in the peripheral blood to ensure engraftment after HDCT.
Traditionally, as HSCs reside in the bone marrow at steady-state conditions, collection has been carried out by bone marrow harvesting from the posterior iliac crests and possibly the sternum under general or epidural anesthesia[25]. Bone marrow harves
Since the early 90’s, HSCs mobilized from the bone marrow into the peripheral blood (PB) have been established as the preferred source of HSCs for transplantation because they are easily accessible, and the evidence indicates that they engraft faster after transplantation than HSCs directly harvested from bone marrow (BM). Clinical findings from randomized/comparative trials indicate that patients experience faster neutrophil, platelet, and immune recovery after PB stem cell transplantation; and in allogeneic transplantation, a higher incidence of chronic graft vs host disease and lower probability of relapse[29].
HSCs are multipotent precursors with self-renewal potency that reside predominantly in the bone marrow. A small number of HSCs circulate in the blood (< 0.02%) under steady-state conditions[30]. Several methods have demonstrated effectiveness in increasing the percentage of HSCs in PB and maximize the number collected with the intention of restoring marrow function and reduce the time required for neutrophil and platelet engraftment following HDCT. Initial mobilization strategies include: (1) Administration of hematopoietic CSFs alone; (2) A course of myelosuppressive chemotherapy prior to collection; and (3) Chemotherapy followed by cytokine ad
In turn, the administration of mobilization agents alone not only has the benefit of relatively predictable kinetics of mobilization, but also a reduced need for hospital care compared with chemotherapy because of the minimal side effects of G-CSF[33,34]. The most commonly used myeloid growth factor for peripheral stem cell harvesting is G-CSF. Other alternatives are its pegylated form; pegfilgrastim, and sargramostim; the recombinant human GM-CSF. Several studies now confirm higher successful rates and twice as many progenitor cells in the circulation when a combination of chemotherapy and G-CSF is used. Consequently, that approach is favored by many investigators[35,36].
Having said that, the use of newer agents, such as chemokine receptor antagonists, along with the conventional ways of autografting mentioned above has expanded in recent years, with promising synergistic results. Plerixafor, a bicyclam molecule derivative that reversibly competes with and inhibits stromal-derived factor-1a (SDF-1a; also known as CXCL12) binding to CXCR4, causes an absolute peak of CD34+ cells 6-9 h after administration. Administration is preferable in the evening before aphe
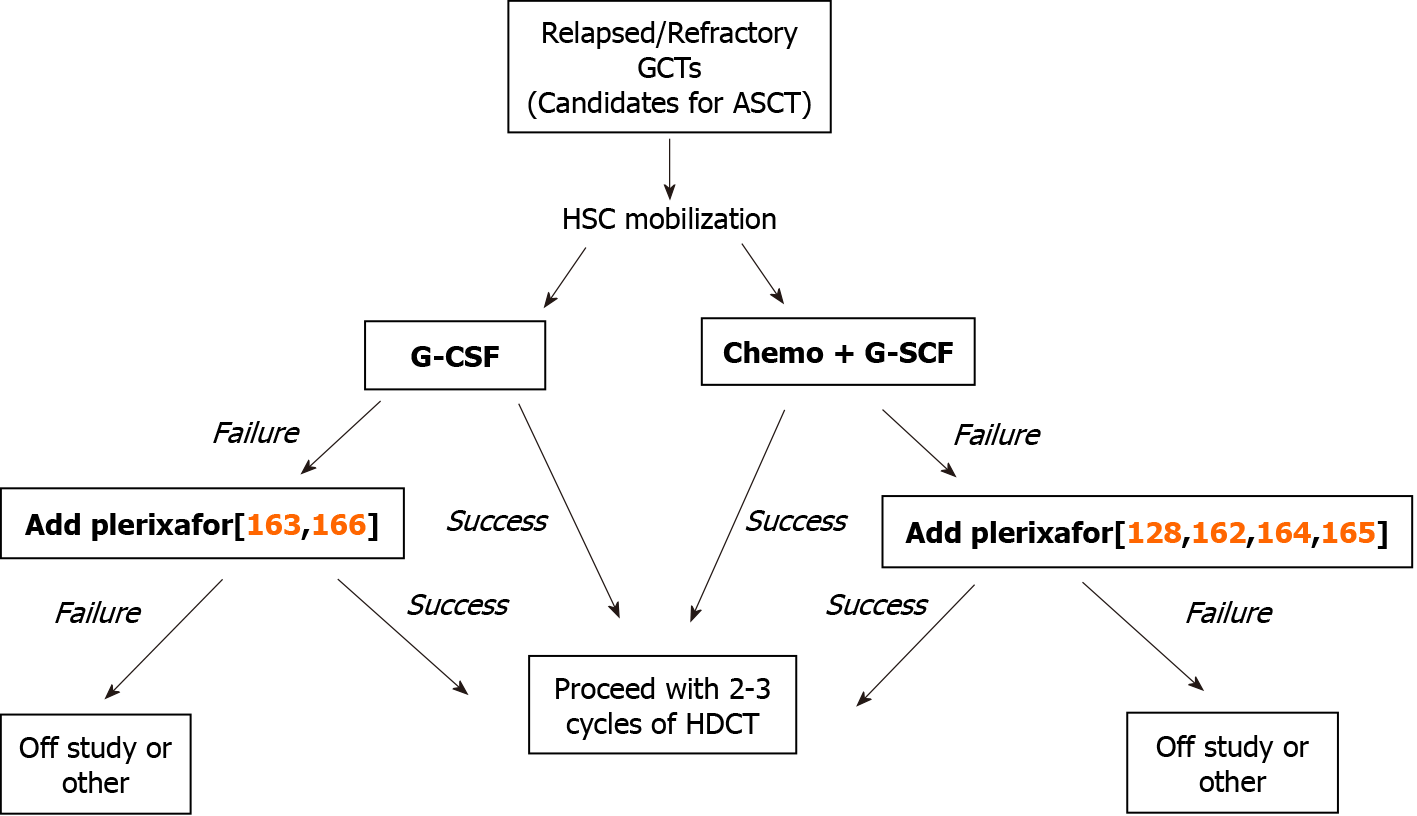
In patients with relapsed/refractory GCTs, we and others attempt HSC mobilization preferably after 1 or 2 salvage chemotherapy cycles with TIP or TI followed by the administration of G-CSF between days 3 and 11 or until the day when sufficient numbers of CD34+ HSCs have been obtained. This approach is accompanied by frequent measurement of circulating PB CD34+/μL counts by flow cytometry, usually starting on day 10-11, in order to decide when to perform the apheresis. A mobi
Schofield was the first to propose the concept of HSCs in 1978[45]. Since then, many have attempted to virtually define this area[46-49], and as a result, we now refer to stem cell niche as the microenvironment where localization and regulation of stem cells takes place. The area is anatomically located near to the endosteum and is composed by two major compartments, the perivascular and the endosteal niches, where cells and molecules dynamically interact[50,51]. The endosteal niche compart
The vascular niche is rich in oxygen, and it is thought that HSCs migrating towards the niche proliferate and regenerate. This compartment is subcategorized into arterial-perivascular, mesenchymal, and sinusoidal endothelial niches. Recent studies showed that the arterial-perivascular niche mostly consists of nestin-bright (nestin+)-smooth muscle perivascular cells[57,58] that express high levels of CXCL12/SDF1 under steady-state conditions and therefore appear to be strongly associated with both proliferation and maintenance of primitive hematopoietic cells in a quiescent state[58,59]. The endothelial sinusoidal niche is composed of endothelial cells that are nestin-dim/leptin receptor-2 (LEPR2) and CXCL12-abudant reticular (CAR) cells with high amounts of CXC-L12, which contribute to regeneration after myelotoxic stress[58]. Several studies showed that as HSCs enter the cell cycle they relocate from areas rich in nestin-bright perivascular cells to those rich in LEPR2+ cells and are mobilized into the circulation[58-60]. In addition to cellular interactions, stem cells are attracted to the bone marrow niche cells through dynamic interactions involving soluble factors (e.g., growth factors, chemokines and cytokines, and adhesion molecules).
One of the most critical chemotactic factors, SDF1a (CXCL12), mainly derived from osteoblasts and endothelial cells, attract HSCs by attaching to their surface chemokine receptor; CXCR4[61]. Other important adhesion molecules are VCAM1 (CD106), which binds to integrin α4β1, very late antigen-4 (VLA-4) on HSCs, and a transmembrane SCF that binds to c-kit (CD117) on HSCs[62,63]. It is well understood that the breaking down of those tethers is necessary for the release of HSCs into the circulation.
Other cells, such as adipocytes, and macrophages have supporting roles in the BM environment. CD169 macrophages secrete oncostatin-M, which leads to increased CXCL12 production by nestin+ and other mesenchymal cells via the MAPK-p38 signaling pathway[64,65]. Depletion of the macrophages results in downregulation of VCAM1, SDF1a, and SCF expression that disrupts the normal niche functions[64,65]. The percentage of adipocytes in the BM, derived from mesenchymal cells, increases with age, leading to a fatty marrow with limited cell proliferation ability[66].
Brief history: In 1966, Ray Bradley and Don Metcalf were the first to identify agents that can stimulate colony formation in hematopoietic cells in semi-solid culture[67]. Later, in 1985 Welte et al[68] purified human G-CSF. Nagata et al[69] in Japan and independently Souza et al[70] from AMGEN in 1986 cloned the G-CSF gene, resulting in the production and clinical application of this cytokine. The first preclinical data to demonstrate mobilization of hematopoietic cells following the administration of G-CSF in mice was in 1986 in a study conducted by Tamura et al[71], where an observation of increasing neutrophil counts approximately 2 h after injection made. The following year, Duhrsen et al[72], confirmed the mobilizing activity of G-CSF in cancer patients, where an increase of mature and progenitor cells into the circulation was observed. The observations were the stimuli for further animal studies to determine whether the progenitor cells could be effective for hematopoietic reconstitution[73].
Mechanism of action: The G-CSF receptor (G-CSFR) is expressed on a range of he
For years there have been trials to establish a universal chemotherapeutic regimen, but without success because of uncontrolled or unknown variables. The optimal che
One of the often administered regimens is an intermediate dose of CY at 2-4.5 g/m2, whereas high doses at 7 g/m2 have been used as well, followed by the administration of G-CSF at a dose of 5-10 μg/kg/d[102]. Others used etoposide in combination with CY and/or cisplatin or added paclitaxel and concluded that the regimens were more effective for stem cell mobilization than CY alone. Moreover, Weaver et al[103] in 1998, used taxanes, either paclitaxel or docetaxel, in combination with CY, followed by G-CSF, and observed more efficient mobilization, almost three times more efficient than CY + G-CSF alone in patients with metastatic breast cancer[103].
The most frequently used regimen in patients with GCTs is paclitaxel at 200 mg/m2 on day 1 plus ifosfamide at 2 g/m2/d on days 1-3 (TI) supported with G-CSF at 10 μg/kg/d, starting on day 4[104,105]. TI was shown by Rick et al[104] more efficient than TI with the addition of cisplatin; i.e. the TIP regimen. An interesting mobilization regimen was used in the TAXIF study, wherein the epirubicin was added to paclitaxel. Despite the different chemotherapy mobilization regimens that have been used, the most commonly applied are TI or TIP, as was shown in a retrospective study by Hamid et al[106] (see also Table 1 for detailed references to the studies).
| Ref. | Number of patients | Successful mobilization | Mobilization regimen |
| Fruehauf et al[149] 1995 (prospective analysis) | 15 | Median BM 31.49 × 106/kg PB 0.46 × 106/kg 100% | Cisplatin 100 mg/m2 etoposide 75 mg/m2 ifosfamide 2 g/m2 + G-CSF |
| Tada et al[150] 1999 (retrospective analysis) | 6 | 2.5 × 108/kg 100% | Cisplatin 200 mg/m2 ifosfamide 4 g/m2 etoposide 100 mg/m2 d1-d3 + G-CSF |
| Rodenhuis et al[151] 1999 (multicenter prospective phase II) | 35 | 10.3 × 106/kg 100% | Cisplatin 200 mg/m2 ifosfamide 4 g/m2 etoposide 100 mg/m2 d1-d3 + G-CSF |
| Lotz et al[152] 2005 TAXIF 2005 (retrospective analysis) | 45 | 9 × 106/kg (for 3 HDCT) 100% | Epirubicin 120 mg/m2 - paclitaxel 200 mg/m2 + G-CSF |
| Argawal et al[102] 2009 (retrospective analysis) | 37 | 3-6 × 106/kg 100% | ifosfamide 2-4.5 g/m2 + G-CSF |
| Feldman et al[153] 2010 (prospective phase I/II) | 107 | > 2 × 106/kg 100% | TI: paclitaxel 200 mg/m2 d1 ifosfamide 2 g/m2 d1-d3 + G-CSF |
| Haugnes et al[154] 2012 (prospective analysis) | 882 | > 2 × 106/kg 100% | BEP-ifosfamide + G-CSF |
| Mohr et al[155] 2012 (retrospective analysis) | 44 | > 4 × 106/kg 100% | PEI (cisplatin, etoposide, ifosfamide) + G-CSF Plerixafor in poor mobilizers |
| Necchi et al[156] 2015 (review) | 42 | > 2 × 106/kg 100% | BEP + G-CSF |
| Moeung et al[157] 2017 (pharmacokinetic phase II study) | 89 | > 9 × 106/kg (for 3 HDCT) (1-2 cycles) 100% | TI: paclitaxel, ifosfamide + G-CSF |
| Hamid et al[106] 2018 (retrospective analysis) | 35 | 10/35 plerixafor + G-CSF 95% | TI: paclitaxel, ifosfamide or TIP |
| Argawal et al[158] 2019 (retrospective analysis) | 321 | 172 allogeneic 95% 149 autologous 73% 77/149 without plerixafor → 64% success 72/149 with plerixafor → 82% success | G-CSF ± Plerixafor |
| Yildiz et al[159] 2020 (retrospective analysis) | 50 | > 2 × 106/kg 100% | TIP + G-CSF |
| Ussowicz et al[160] 2020 (retrospective analysis) | 18 (children) | Median: 4.56 × 106/kg 100% | Cyclophosphamide 4 g/m2 + G-CSF |
| Chevreau et al[161] 2020 (multicenter prospective phase II) | 89 | > 9 × 106/kg (for 3 HDCT) 100% | TI: paclitaxel, ifosfamide + G-CSF |
Higher doses of G-CSF agents have been suggested as a strategy to improve mo
Lenograstim: Lenograstim, a glycosylated form of G-CSF, also widely used for HSC transplantation, was hypothesized to induce increased mobilization compared to conventional G-CSF agents. In fact, it was proposed that its unique structure and glycosylation pattern provided protection against elastase-dependent inactivation, and could thereby lead to prolonged activity and increased mobilization[110,111]. Several studies though did not find any differences on HSC mobilization with collection results and patient outcomes comparable to conventional G-CSF-mobilized patients. Therefore, data on its efficacy remains to date both limited and inconclusive[112-114].
Pegfilgrastim: Pegfilgrastim is a pegylated form of G-CSF with long half-life characteristics because of its significantly reduced renal excretion[115]. It promotes stem cell mobilization with a single dose administration, as opposed to the daily injections of the regular short half-life G-CSF[116,117]. The results of recent studies have been controversial, as a number of them supported a significant increase in peripheral stem cells collected, while others found no difference in terms of stem cell mobilization, when a double dose of 12 mg-compared to the 6mg dose after conventional chemo
Ancestim: Ancestim is a recombinant human SCF that, through its binding to the c-kit receptor on HSCs, modulates their proliferation and adhesion, and has shown promising synergy in HSC mobilization when combined with G-CSF[119,120]. Limited efficacy when administered alone has also been noted[119]. Unfortunately, data avai
GM-CSF: GM-CSF and its synergistic effect when combined with chemotherapy are no longer in use because the superiority of G-CSF in terms of mobilization and safety profile has been proved in a number of studies (e.g., faster neutrophil recovery and fewer transfusions required)[122,123]. GM-CSF is sometimes used in combination with G-CSF in patients who failed an initial mobilization attempt, as a second or even as a third agent[124], despite the fact that several studies reported that the association of the two cytokines was not superior to G-CSF alone[125].
Plerixafor (Mozobil): Briefly, plerixafor was first studied as an agent against HIV[126]. During those clinical trials, neutrophilia was observed that sparked numerous studies[127]. In December 2008, plerixafor was approved by the Federal Drug Ad
Despite the fact that the efficacy of plerixafor as a stem cell mobilization agent in patients with GCTs undergoing HDCT and ASCT has been reported in a number of small patient series and case studies, its use has not yet been approved, because of the lack of prospective studies. Thus, the indications for the use of plerixafor as a mobilization agent in patients with relapsed/refractory GCTs are not yet clear and rely on the opinions of the authors who published the studies (see Table 2 for details).
| Ref. | Number of patients participating | Successful mobilization rates on previously failed chemotherapy + G-SCF driven mobilization (> 2 × 106) | Mobilization techniques |
| Kobold et al[128] 2011 (Retrospective analysis) | 6 | 66.67% (4) | Chemo + G-CSF failed |
| Plerixafor + G-CSF | |||
| Horwitz et al[162] 2012 (Retrospective analysis) | 21 | 76% (17) | Chemo + G-CSF failed |
| Plerixafor + G-CSF | |||
| Worel et al[163] 2012 (Retrospective analysis) | 11 | 91% (10) | Plerixafor + G-CSF |
| Garcia-Escobar et al[164] 2014 (Case series) | 5 | 80% (4) | Chemo + G-CSF failed |
| Plerixafor + G-CSF | |||
| Kosmas et al[165] 2014 (Pilot study) | 14 (3) | 100% (3) | Chemo + G-CSF failed |
| Chemo + Plerixafor + G-CSF | |||
| O’Hara et al[166] 2014 (Retrospective analysis) | 9 (3) | 100% (3) | Plerixafor + G-CSF |
Structure and mechanism of action are as follows. Plerixafor (or AMD3100) is a bicyclam derivative that reversibly competes with and inhibits SDF-1a binding to CXCR4. CXCR4 is expressed on many cell types including white blood cells, epithelial, endothelial cells, and HPCs. It plays a critical role in the homing and trafficking of HPCs, as well as their retention and maintenance in the bone marrow niche. CXCR4 is a member of one of the two major families of chemokines. Chemokines are defined by the number and spacing of cysteine residues at the N-terminal end of the protein. CC cytokines have two cysteine residues that are adjacent; in CXC cytokines they separated by one amino-acid residue[129]. CXCR4 ligand, the chemokine SDF-1a (CXCL12), is produced by bone marrow stromal cells including osteoblasts, en
A possible mechanism for plerixafor-stimulated HSCs mobilization was proposed by Dar et al[132], in which an increase in CXCL12 circulating in the plasma was observed after the administration of plerixafor. At the same time, CXCL12 levels in BM fluids were decreased. The changes correlated with an increase of circulating pro
The pharmacokinetics of plerixafor after subcutaneous injection show a peak plasma concentration within 30-60 min. Up to 58% of plerixafor is bound to plasma proteins, and it is eliminated by the urinary route with a half-life of 4 h. Similar increases in HSC levels are observed after multiple daily injections, suggesting no cumulative drug effect after consecutive injections[37,38]. An interesting fact about the timing of plerixafor injection and the mobilization of CD34+ was reported by Lefrere et al[38]. They found that in good mobilizers, the PB CD34 + count remained high for at least 12 h after G-CSF plus plerixafor administration[38]. In contrast, in poor mo
Future novel approaches: Most novel HSC mobilizing agents are initially tested in MM and NHL patients, and ASCT candidates. Successful application in that setting allows further testing in patients with relapsed/refractory GCTs and other solid tumors where HDCT and autografting are indicated at some point during the disease course. CXCR4 antagonists like plerixafor, emerged as potent agents to rescue “hard-to-mobilize” patients with MM, NHL, GCTs, and some rare solid tumors. Research in that area has expanded with the development of novel CXCR4 inhibitors, such as motixafortide (BL-8040) and BKT140 (4F-benzoyl-TN14003), a 14-residue biostable synthetic peptide that binds CXCR4 with much greater affinity than plerixafor (84 nmol/L vs 4 nmol/L). An interim analysis of the phase 3 GENESIS trial of motixafortide vs placebo, both with G-CSF, for HSC mobilization in MM demonstrated an almost 4.9-fold increased efficacy in obtaining the primary endpoint of a target of 6.0 × 106 CD34+ cells/kg with up to two apheresis sessions and that 5.6-fold more patients achieved that target with one apheresis. Moreover, the motixafortide arm allowed 88.3% of patients to proceed to transplant, as opposed to 10.8% in the placebo arm[133]. Another peptide CXCR4 antagonist, a clinical stage compound balixafortide (POL6326) was evaluated in healthy volunteers and proved to be safe, well tolerated, and induced effective mobilization of HSCs at doses ≥ 1500 µg/kg and was predicted to yield an adequate collection of 4 × 106 CD34+ cells/kg in a single apheresis[134].
Another area of interest in HSC mobilization is the role of the sphingosine-1-phosphate/S1P receptor 1 (S1P/S1P1) axis, and studies in mice demonstrated an additional PB HSC mobilization benefit of S1P1 agonist (SEW2871) treatment in combination with a CXCR4 antagonist, but not human G-CSF[135]. However, that approach still remains experimental, with no apparent clinical testing so far.
Small molecule inhibitors of VLA-4 such as BIO5192 and monoclonal IgG4 anti
Bortezomib (Velcade, PS-341) is a proteasome inhibitor that interferes with the activation of nuclear factor-kappa B (NFκB) by preventing proteasomal degradation of IκBa. VCAM-1 expression is upregulated by the VCAM-1 promoter. The latter is activated by binding to NFκB6. As proteasome inhibitors can indirectly inhibit tran
Hypoxia-inducible factor (HIF) prolyl hydroxylase (PHD) inhibitors, such as FG-4497, synergize with G-CSF and plerixafor to enhance mouse HSC mobilization. Deletion of the Hif1a gene weakens the effect[141]. A potential mechanism of FG-4497 proposed in recent studies includes stabilizing HIF-1a protein and increased VEGF-A secretion by BM macrophages[64,65]. FMS-like tyrosine kinase-3 Ligand (FLT3L) binds the FLT3 (CD135) receptor expressed on HSCs and induces proliferation, differentiation, development, and mobilization. Its efficacy has been shown either as a single agent, or in combination with other molecules mentioned above, such as IL-8 or G-CSF[142]. As chemokine-chemokine receptor axes are involved in retention of HSCs in the BM microenvironment, chemokine receptor agonists have been proposed as thera
A novel mobilization strategy was developed and tested in mice through combined targeting of the chemokine receptor CXCR2 on granulocytes and VLA4 in HSCs. Treatment resulted in rapid and synergistic mobilization along with an enhanced recruitment of long-term repopulating of HSCs. That was achieved when a CXCR2 agonist, a truncated form of GRO-β; (tGRO-β) was administered in conjunction with a VLA4 inhibitor, leading to rapid and potent HSC mobilization, which represents an exciting potential strategy that warrants clinical development[147]. A G-CSF-free mobilization regimen using a tGRO-β compound, MGTA-145, which is a CXCR2 agonist, in combination with plerixafor was developed in the context of in vivo HSC transduction as a gene therapy approach in a mouse model of β-thalassemia[148]. The MGTA-145+plerixafor combination resulted in robust mobilization of HSCs. Im
Despite the fact that GCTs are currently considered as curable tumors, almost 30% of patients presenting with metastatic disease at diagnosis are likely to experience disease progression at some point. The use of HDCT and ASCT has been established as a salvage therapeutic option, but a number of patients fail to mobilize with conventional strategies. Such poor mobilizers endanger the safety of the procedure. Along with conventional mobilization strategies, such as G-CSF and chemo-mobilization, the use of newer mobilizing agents like plerixafor has emerged with promising results for this group of patients.
Algorithms to improve the efficiency of HSC mobilization, for example “just in time” and preemptive, aim to minimize failures, obtain the desired CD34+ HSCs dose for one or more transplants with the least apheresis sessions, and thus reduce overall healthcare costs, are urgently required. As novel HSC mobilizing agents are initially tested in preclinical experimental models and hematologic malignancies, such as NHL and MM, their application in solid tumors, candidates for ASCT, and in particular GCTs, is lagging behind.
Two axes responsible for HSC retention in the BM stroma that have been explored are the CXCR4-CXCL12 (SDF-1) and the VLA4 (α4/β1)-VCAM1 pathways. Novel inhibitors of those interactions have been evaluated, either alone or in combination with G-CSF, or with GRO-β/CXCR2 axis co-stimulation. Nevertheless, as studies in this area are limited, future investigation should concentrate on finding new agents or establishing proper mobilization algorithms to achieve an adequate CD34+ dose required for a successful ASCT.
Manuscript source: Invited manuscript
Specialty type: Oncology
Country/Territory of origin: Greece
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): 0
Grade C (Good): C
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Leardini D S-Editor: Gong ZM L-Editor: Filipodia P-Editor: Yuan YY
| 1. | Copelan EA. Hematopoietic stem-cell transplantation. N Engl J Med. 2006;354:1813-1826. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1657] [Cited by in RCA: 1759] [Article Influence: 92.6] [Reference Citation Analysis (0)] |
| 2. | Bortin MM. A compendium of reported human bone marrow transplants. Transplantation. 1970;9:571-587. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 155] [Cited by in RCA: 113] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 3. | Abrahamsen JF, Stamnesfet S, Liseth K, Hervig T, Bruserud O. Large-volume leukapheresis yields more viable CD34+ cells and colony-forming units than normal-volume leukapheresis, especially in patients who mobilize low numbers of CD34+ cells. Transfusion. 2005;45:248-253. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 35] [Cited by in RCA: 39] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 4. | Bojanic I, Dubravcic K, Batinic D, Cepulic BG, Mazic S, Hren D, Nemet D, Labar B. Large volume leukapheresis: Efficacy and safety of processing patient's total blood volume six times. Transfus Apher Sci. 2011;44:139-147. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 16] [Cited by in RCA: 24] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 5. | Necchi A, Miceli R, Pedrazzoli P, Giannatempo P, Secondino S, Di Nicola M, Farè E, Raggi D, Magni M, Matteucci P, Longoni P, Milanesi M, Paternò E, Ravagnani F, Arienti F, Nicolai N, Salvioni R, Carlo-Stella C, Gianni AM. Predictors of CD34+ cell mobilization and collection in adult men with germ cell tumors: implications for the salvage treatment strategy. Clin Genitourin Cancer. 2014;12:196-202.e1. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 3] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 6. | Greenlee RT, Murray T, Bolden S, Wingo PA. Cancer statistics, 2000. CA Cancer J Clin. 2000;50:7-33. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2959] [Cited by in RCA: 2762] [Article Influence: 110.5] [Reference Citation Analysis (0)] |
| 7. | Parkin DM, Ferlay J, Curado MP, Bray F, Edwards B, Shin HR, Forman D. Fifty years of cancer incidence: CI5 I-IX. Int J Cancer. 2010;127:2918-2927. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 140] [Cited by in RCA: 165] [Article Influence: 11.0] [Reference Citation Analysis (0)] |
| 8. | Horwich A, Shipley J, Huddart R. Testicular germ-cell cancer. Lancet. 2006;367:754-765. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 298] [Cited by in RCA: 273] [Article Influence: 14.4] [Reference Citation Analysis (0)] |
| 9. | Nauman M, Leslie SW. Nonseminomatous Testicular Tumors. In: StatPearls [Internet]. Treasure Island (FL): StatPearls Publishing; 2021. [PubMed] |
| 10. | Vasdev N, Moon A, Thorpe AC. Classification, epidemiology and therapies for testicular germ cell tumours. Int J Dev Biol. 2013;57:133-139. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 36] [Cited by in RCA: 52] [Article Influence: 4.7] [Reference Citation Analysis (0)] |
| 11. | Gilligan T, Lin DW, Aggarwal R, Chism D, Cost N, Derweesh IH, Emamekhoo H, Feldman DR, Geynisman DM, Hancock SL, LaGrange C, Levine EG, Longo T, Lowrance W, McGregor B, Monk P, Picus J, Pierorazio P, Rais-Bahrami S, Saylor P, Sircar K, Smith DC, Tzou K, Vaena D, Vaughn D, Yamoah K, Yamzon J, Johnson-Chilla A, Keller J, Pluchino LA. Testicular Cancer, Version 2.2020, NCCN Clinical Practice Guidelines in Oncology. J Natl Compr Canc Netw. 2019;17:1529-1554. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 73] [Cited by in RCA: 179] [Article Influence: 35.8] [Reference Citation Analysis (0)] |
| 12. | Kondagunta GV, Bacik J, Donadio A, Bajorin D, Marion S, Sheinfeld J, Bosl GJ, Motzer RJ. Combination of paclitaxel, ifosfamide, and cisplatin is an effective second-line therapy for patients with relapsed testicular germ cell tumors. J Clin Oncol. 2005;23:6549-6555. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 280] [Cited by in RCA: 287] [Article Influence: 14.4] [Reference Citation Analysis (0)] |
| 13. | Loehrer PJ Sr, Einhorn LH, Williams SD. VP-16 plus ifosfamide plus cisplatin as salvage therapy in refractory germ cell cancer. J Clin Oncol. 1986;4:528-536. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 123] [Cited by in RCA: 110] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 14. | Porrata LF, Adjei AA. The pharmacologic basis of high dose chemotherapy with haematopoietic stem cell support for solid tumours. Br J Cancer. 2001;85:484-489. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 24] [Cited by in RCA: 27] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 15. | Motzer RJ, Nichols CJ, Margolin KA, Bacik J, Richardson PG, Vogelzang NJ, Bajorin DF, Lara PN Jr, Einhorn L, Mazumdar M, Bosl GJ. Phase III randomized trial of conventional-dose chemotherapy with or without high-dose chemotherapy and autologous hematopoietic stem-cell rescue as first-line treatment for patients with poor-prognosis metastatic germ cell tumors. J Clin Oncol. 2007;25:247-256. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 266] [Cited by in RCA: 225] [Article Influence: 12.5] [Reference Citation Analysis (0)] |
| 16. | Einhorn LH, Williams SD, Chamness A, Brames MJ, Perkins SM, Abonour R. High-dose chemotherapy and stem-cell rescue for metastatic germ-cell tumors. N Engl J Med. 2007;357:340-348. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 360] [Cited by in RCA: 337] [Article Influence: 18.7] [Reference Citation Analysis (0)] |
| 17. | Pico JL, Rosti G, Kramar A, Wandt H, Koza V, Salvioni R, Theodore C, Lelli G, Siegert W, Horwich A, Marangolo M, Linkesch W, Pizzocaro G, Schmoll HJ, Bouzy J, Droz JP, Biron P; Genito-Urinary Group of the French Federation of Cancer Centers (GETUG-FNCLCC), France; European Group for Blood and Marrow Transplantation (EBMT). A randomised trial of high-dose chemotherapy in the salvage treatment of patients failing first-line platinum chemotherapy for advanced germ cell tumours. Ann Oncol. 2005;16:1152-1159. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 201] [Cited by in RCA: 186] [Article Influence: 9.3] [Reference Citation Analysis (0)] |
| 18. | Rodenhuis S, Westermann A, Holtkamp MJ, Nooijen WJ, Baars JW, van der Wall E, Slaper-Cortenbach IC, Schornagel JH. Feasibility of multiple courses of high-dose cyclophosphamide, thiotepa, and carboplatin for breast cancer or germ cell cancer. J Clin Oncol. 1996;14:1473-1483. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 68] [Cited by in RCA: 73] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 19. | Motzer RJ, Mazumdar M, Sheinfeld J, Bajorin DF, Macapinlac HA, Bains M, Reich L, Flombaum C, Mariani T, Tong WP, Bosl GJ. Sequential dose-intensive paclitaxel, ifosfamide, carboplatin, and etoposide salvage therapy for germ cell tumor patients. J Clin Oncol. 2000;18:1173-1180. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 151] [Cited by in RCA: 135] [Article Influence: 5.4] [Reference Citation Analysis (0)] |
| 20. | Thorsby E. A short history of HLA. Tissue Antigens. 2009;74:101-116. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 83] [Cited by in RCA: 94] [Article Influence: 5.9] [Reference Citation Analysis (0)] |
| 21. | Bojanic I, Mazic S, Rajic L, Jakovljevic G, Stepan J, Cepulic BG. Large volume leukapheresis is efficient and safe even in small children up to 15 kg body weight. Blood Transfus. 2017;15:85-92. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 2] [Reference Citation Analysis (0)] |
| 22. | Goldman JM, Johnson SA, Catovsky D, Wareham NJ, Galton DA. Autografting for chronic granulocytic leukemia. N Engl J Med. 1981;305:700. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 15] [Cited by in RCA: 18] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 23. | Körbling M, Dörken B, Ho AD, Pezzutto A, Hunstein W, Fliedner TM. Autologous transplantation of blood-derived hemopoietic stem cells after myeloablative therapy in a patient with Burkitt's lymphoma. Blood. 1986;67:529-532. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 3] [Article Influence: 0.1] [Reference Citation Analysis (0)] |
| 24. | Kessinger A, Armitage JO, Landmark JD, Weisenburger DD. Reconstitution of human hematopoietic function with autologous cryopreserved circulating stem cells. Exp Hematol. 1986;14:192-196. [PubMed] |
| 25. | Thomas ED, Storb R. Technique for human marrow grafting. Blood. 1970;36:507-515. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 309] [Cited by in RCA: 266] [Article Influence: 4.8] [Reference Citation Analysis (0)] |
| 26. | Becker PS, Adair J, Choi G, Lee A, Kiem HP. From bone marrow to mobilized peripheral blood stem cells: The circuitous path to clinical gene therapy for fanconi anemia. Blood. 2018;132 (Supple 1):2208. [RCA] [DOI] [Full Text] [Cited by in RCA: 1] [Reference Citation Analysis (0)] |
| 27. | Körbling M, Anderlini P. Peripheral blood stem cell versus bone marrow allotransplantation: does the source of hematopoietic stem cells matter? Blood. 2001;98:2900-2908. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 263] [Cited by in RCA: 249] [Article Influence: 10.4] [Reference Citation Analysis (0)] |
| 28. | Stroncek DF, Dittmar K, Shawker T, Heatherman A, Leitman SF. Transient spleen enlargement in peripheral blood progenitor cell donors given G-CSF. J Transl Med. 2004;2:25. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 19] [Cited by in RCA: 17] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 29. | Körbling M, Freireich EJ. Twenty-five years of peripheral blood stem cell transplantation. Blood. 2011;117:6411-6416. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 96] [Cited by in RCA: 112] [Article Influence: 8.0] [Reference Citation Analysis (0)] |
| 30. | Sutherland DR, Anderson L, Keeney M, Nayar R, Chin-Yee I. The ISHAGE guidelines for CD34+ cell determination by flow cytometry. International Society of Hematotherapy and Graft Engineering. J Hematother. 1996;5:213-226. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 940] [Cited by in RCA: 965] [Article Influence: 33.3] [Reference Citation Analysis (0)] |
| 31. | Baertsch MA, Schlenzka J, Lisenko K, Krzykalla J, Becker N, Weisel K, Noppeney R, Martin H, Lindemann HW, Haenel M, Nogai A, Scheid C, Salwender H, Fenk R, Graeven U, Reimer P, Schmidt-Hieber M, Goerner M, Schmidt-Wolf IGH, Klein S, Ho AD, Goldschmidt H, Wuchter P. Cyclophosphamide-based stem cell mobilization in relapsed multiple myeloma patients: A subgroup analysis from the phase III trial ReLApsE. Eur J Haematol. 2017;99:42-50. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 12] [Cited by in RCA: 16] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 32. | Nowrousian MR, Waschke S, Bojko P, Welt A, Schuett P, Ebeling P, Flasshove M, Moritz T, Schuette J, Seeber S. Impact of chemotherapy regimen and hematopoietic growth factor on mobilization and collection of peripheral blood stem cells in cancer patients. Ann Oncol. 2003;14 Suppl 1:i29-i36. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 31] [Cited by in RCA: 30] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 33. | Anderlini P, Przepiorka D, Seong D, Miller P, Sundberg J, Lichtiger B, Norfleet F, Chan KW, Champlin R, Körbling M. Clinical toxicity and laboratory effects of granulocyte-colony-stimulating factor (filgrastim) mobilization and blood stem cell apheresis from normal donors, and analysis of charges for the procedures. Transfusion. 1996;36:590-595. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 141] [Cited by in RCA: 130] [Article Influence: 4.5] [Reference Citation Analysis (0)] |
| 34. | Stroncek DF, Clay ME, Petzoldt ML, Smith J, Jaszcz W, Oldham FB, McCullough J. Treatment of normal individuals with granulocyte-colony-stimulating factor: donor experiences and the effects on peripheral blood CD34+ cell counts and on the collection of peripheral blood stem cells. Transfusion. 1996;36:601-610. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 191] [Cited by in RCA: 179] [Article Influence: 6.2] [Reference Citation Analysis (0)] |
| 35. | Kriegsmann K, Schmitt A, Kriegsmann M, Bruckner T, Anyanwu A, Witzens-Harig M, Müller-Tidow C, Klein S, Wuchter P. Orchestration of Chemomobilization and G-CSF Administration for Successful Hematopoietic Stem Cell Collection. Biol Blood Marrow Transplant. 2018;24:1281-1288. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 9] [Cited by in RCA: 14] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 36. | Gertz MA, Kumar SK, Lacy MQ, Dispenzieri A, Hayman SR, Buadi FK, Dingli D, Gastineau DA, Winters JL, Litzow MR. Comparison of high-dose CY and growth factor with growth factor alone for mobilization of stem cells for transplantation in patients with multiple myeloma. Bone Marrow Transplant. 2009;43:619-625. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 102] [Cited by in RCA: 100] [Article Influence: 6.3] [Reference Citation Analysis (0)] |
| 37. | Kessans MR, Gatesman ML, Kockler DR. Plerixafor: a peripheral blood stem cell mobilizer. Pharmacotherapy. 2010;30:485-492. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 31] [Cited by in RCA: 29] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 38. | Lefrère F, Mauge L, Réa D, Ribeil JA, Dal Cortivo L, Brignier AC, Aoun C, Larghéro J, Cavazzana-Calvo M, Micléa JM. A specific time course for mobilization of peripheral blood CD34+ cells after plerixafor injection in very poor mobilizer patients: impact on the timing of the apheresis procedure. Transfusion. 2013;53:564-569. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 29] [Cited by in RCA: 30] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 39. | Baertsch MA, Kriegsmann K, Pavel P, Bruckner T, Hundemer M, Kriegsmann M, Ho AD, Goldschmidt H, Wuchter P. Platelet Count before Peripheral Blood Stem Cell Mobilization Is Associated with the Need for Plerixafor But Not with the Collection Result. Transfus Med Hemother. 2018;45:24-31. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 13] [Cited by in RCA: 12] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 40. | Hsu YM, Cushing MM. Autologous Stem Cell Mobilization and Collection. Hematol Oncol Clin North Am. 2016;30:573-589. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 18] [Cited by in RCA: 19] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 41. | Teng HW, Hsiao LT, Chaou SC, Gau JP, Lee TC, Shih YY, Liu CY, Hong YC, Chen MH, Chang MH, Yang YH, Chen PM. A new model for predicting the timing of leukapheresis on the basis of CD34+ cell and hematopoietic progenitor cell levels. J Clin Apher. 2007;22:195-203. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 3] [Article Influence: 0.2] [Reference Citation Analysis (0)] |
| 42. | Wood WA, Whitley J, Moore D, Sharf A, Irons R, Rao K, Serody J, Coghill J, Gabriel D, Shea T. Chemomobilization with Etoposide is Highly Effective in Patients with Multiple Myeloma and Overcomes the Effects of Age and Prior Therapy. Biol Blood Marrow Transplant. 2011;17:141-146. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 36] [Cited by in RCA: 36] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 43. | Costa LJ, Nista EJ, Buadi FK, Lacy MQ, Dispenzieri A, Kramer CP, Edwards KH, Kang Y, Gertz MA, Stuart RK, Kumar S. Prediction of poor mobilization of autologous CD34+ cells with growth factor in multiple myeloma patients: implications for risk-stratification. Biol Blood Marrow Transplant. 2014;20:222-228. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 30] [Cited by in RCA: 33] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 44. | Giralt S, Costa L, Schriber J, Dipersio J, Maziarz R, McCarty J, Shaughnessy P, Snyder E, Bensinger W, Copelan E, Hosing C, Negrin R, Petersen FB, Rondelli D, Soiffer R, Leather H, Pazzalia A, Devine S. Optimizing autologous stem cell mobilization strategies to improve patient outcomes: consensus guidelines and recommendations. Biol Blood Marrow Transplant. 2014;20:295-308. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 226] [Cited by in RCA: 300] [Article Influence: 25.0] [Reference Citation Analysis (0)] |
| 45. | Schofield R. The relationship between the spleen colony-forming cell and the haemopoietic stem cell. Blood Cells. 1978;4:7-25. [PubMed] |
| 46. | Doetsch F, Caillé I, Lim DA, García-Verdugo JM, Alvarez-Buylla A. Subventricular zone astrocytes are neural stem cells in the adult mammalian brain. Cell. 1999;97:703-716. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2818] [Cited by in RCA: 2975] [Article Influence: 114.4] [Reference Citation Analysis (0)] |
| 47. | Kimble JE, White JG. On the control of germ cell development in Caenorhabditis elegans. Dev Biol. 1981;81:208-219. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 455] [Cited by in RCA: 449] [Article Influence: 10.2] [Reference Citation Analysis (0)] |
| 48. | Nilsson SK, Johnston HM, Coverdale JA. Spatial localization of transplanted hemopoietic stem cells: inferences for the localization of stem cell niches. Blood. 2001;97:2293-2299. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 430] [Cited by in RCA: 411] [Article Influence: 17.1] [Reference Citation Analysis (0)] |
| 49. | Quiñones-Hinojosa A, Sanai N, Soriano-Navarro M, Gonzalez-Perez O, Mirzadeh Z, Gil-Perotin S, Romero-Rodriguez R, Berger MS, Garcia-Verdugo JM, Alvarez-Buylla A. Cellular composition and cytoarchitecture of the adult human subventricular zone: a niche of neural stem cells. J Comp Neurol. 2006;494:415-434. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 398] [Cited by in RCA: 417] [Article Influence: 21.9] [Reference Citation Analysis (0)] |
| 50. | Wilson A, Oser GM, Jaworski M, Blanco-Bose WE, Laurenti E, Adolphe C, Essers MA, Macdonald HR, Trumpp A. Dormant and self-renewing hematopoietic stem cells and their niches. Ann N Y Acad Sci. 2007;1106:64-75. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 176] [Cited by in RCA: 163] [Article Influence: 9.1] [Reference Citation Analysis (0)] |
| 51. | Kopp HG, Avecilla ST, Hooper AT, Rafii S. The bone marrow vascular niche: home of HSC differentiation and mobilization. Physiology (Bethesda). 2005;20:349-356. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 297] [Cited by in RCA: 333] [Article Influence: 16.7] [Reference Citation Analysis (0)] |
| 52. | Zhu J, Garrett R, Jung Y, Zhang Y, Kim N, Wang J, Joe GJ, Hexner E, Choi Y, Taichman RS, Emerson SG. Osteoblasts support B-lymphocyte commitment and differentiation from hematopoietic stem cells. Blood. 2007;109:3706-3712. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 287] [Cited by in RCA: 285] [Article Influence: 15.8] [Reference Citation Analysis (0)] |
| 53. | Zhu CH, Xie T. Clonal expansion of ovarian germline stem cells during niche formation in Drosophila. Development. 2003;130:2579-2588. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 123] [Cited by in RCA: 121] [Article Influence: 5.5] [Reference Citation Analysis (0)] |
| 54. | Eliasson P, Jönsson JI. The hematopoietic stem cell niche: low in oxygen but a nice place to be. J Cell Physiol. 2010;222:17-22. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 322] [Cited by in RCA: 346] [Article Influence: 23.1] [Reference Citation Analysis (0)] |
| 55. | Mohyeldin A, Garzón-Muvdi T, Quiñones-Hinojosa A. Oxygen in stem cell biology: a critical component of the stem cell niche. Cell Stem Cell. 2010;7:150-161. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1249] [Cited by in RCA: 1146] [Article Influence: 76.4] [Reference Citation Analysis (0)] |
| 56. | Zhang CC, Sadek HA. Hypoxia and metabolic properties of hematopoietic stem cells. Antioxid Redox Signal. 2014;20:1891-1901. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 98] [Cited by in RCA: 118] [Article Influence: 10.7] [Reference Citation Analysis (0)] |
| 57. | Panvini FM, Pacini S, Montali M, Barachini S, Mazzoni S, Morganti R, Ciancia EM, Carnicelli V, Petrini M. High NESTIN Expression Marks the Endosteal Capillary Network in Human Bone Marrow. Front Cell Dev Biol. 2020;8:596452. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 9] [Cited by in RCA: 12] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 58. | Boulais PE, Frenette PS. Making sense of hematopoietic stem cell niches. Blood. 2015;125:2621-2629. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 284] [Cited by in RCA: 320] [Article Influence: 32.0] [Reference Citation Analysis (0)] |
| 59. | Kunisaki Y, Bruns I, Scheiermann C, Ahmed J, Pinho S, Zhang D, Mizoguchi T, Wei Q, Lucas D, Ito K, Mar JC, Bergman A, Frenette PS. Arteriolar niches maintain haematopoietic stem cell quiescence. Nature. 2013;502:637-643. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 837] [Cited by in RCA: 941] [Article Influence: 78.4] [Reference Citation Analysis (0)] |
| 60. | Mendelson A, Frenette PS. Hematopoietic stem cell niche maintenance during homeostasis and regeneration. Nat Med. 2014;20:833-846. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 528] [Cited by in RCA: 599] [Article Influence: 54.5] [Reference Citation Analysis (0)] |
| 61. | Bleul CC, Fuhlbrigge RC, Casasnovas JM, Aiuti A, Springer TA. A highly efficacious lymphocyte chemoattractant, stromal cell-derived factor 1 (SDF-1). J Exp Med. 1996;184:1101-1109. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1161] [Cited by in RCA: 1184] [Article Influence: 40.8] [Reference Citation Analysis (0)] |
| 62. | Papayannopoulou T, Craddock C, Nakamoto B, Priestley GV, Wolf NS. The VLA4/VCAM-1 adhesion pathway defines contrasting mechanisms of lodgement of transplanted murine hemopoietic progenitors between bone marrow and spleen. Proc Natl Acad Sci USA. 1995;92:9647-9651. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 409] [Cited by in RCA: 396] [Article Influence: 13.2] [Reference Citation Analysis (0)] |
| 63. | Lennartsson J, Rönnstrand L. Stem cell factor receptor/c-Kit: from basic science to clinical implications. Physiol Rev. 2012;92:1619-1649. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 471] [Cited by in RCA: 600] [Article Influence: 46.2] [Reference Citation Analysis (1)] |
| 64. | Chow A, Lucas D, Hidalgo A, Méndez-Ferrer S, Hashimoto D, Scheiermann C, Battista M, Leboeuf M, Prophete C, van Rooijen N, Tanaka M, Merad M, Frenette PS. Bone marrow CD169+ macrophages promote the retention of hematopoietic stem and progenitor cells in the mesenchymal stem cell niche. J Exp Med. 2011;208:261-271. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 591] [Cited by in RCA: 638] [Article Influence: 45.6] [Reference Citation Analysis (0)] |
| 65. | Winkler IG, Sims NA, Pettit AR, Barbier V, Nowlan B, Helwani F, Poulton IJ, van Rooijen N, Alexander KA, Raggatt LJ, Lévesque JP. Bone marrow macrophages maintain hematopoietic stem cell (HSC) niches and their depletion mobilizes HSCs. Blood. 2010;116:4815-4828. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 584] [Cited by in RCA: 621] [Article Influence: 41.4] [Reference Citation Analysis (0)] |
| 66. | Boroumand P, Klip A. Bone marrow adipose cells - cellular interactions and changes with obesity. J Cell Sci. 2020;133. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 15] [Cited by in RCA: 28] [Article Influence: 5.6] [Reference Citation Analysis (0)] |
| 67. | Bradley TR, Metcalf D. The growth of mouse bone marrow cells in vitro. Aust J Exp Biol Med Sci. 1966;44:287-299. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1381] [Cited by in RCA: 1239] [Article Influence: 21.0] [Reference Citation Analysis (0)] |
| 68. | Welte K, Platzer E, Lu L, Gabrilove JL, Levi E, Mertelsmann R, Moore MA. Purification and biochemical characterization of human pluripotent hematopoietic colony-stimulating factor. Proc Natl Acad Sci USA. 1985;82:1526-1530. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 327] [Cited by in RCA: 312] [Article Influence: 7.8] [Reference Citation Analysis (0)] |
| 69. | Nagata S, Tsuchiya M, Asano S, Kaziro Y, Yamazaki T, Yamamoto O, Hirata Y, Kubota N, Oheda M, Nomura H. Molecular cloning and expression of cDNA for human granulocyte colony-stimulating factor. Nature. 1986;319:415-418. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 549] [Cited by in RCA: 529] [Article Influence: 13.6] [Reference Citation Analysis (0)] |
| 70. | Souza LM, Boone TC, Gabrilove J, Lai PH, Zsebo KM, Murdock DC, Chazin VR, Bruszewski J, Lu H, Chen KK, Barendt J, Platzer E, Moore MAS, Mertelsmann R, Welte K. Recombinant human granulocyte colony-stimulating factor: effects on normal and leukemic myeloid cells. Science. 1986;232:61-65. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 744] [Cited by in RCA: 747] [Article Influence: 19.2] [Reference Citation Analysis (0)] |
| 71. | Tamura M, Hattori K, Nomura H, Oheda M, Kubota N, Imazeki I, Ono M, Ueyama Y, Nagata S, Shirafuji N. Induction of neutrophilic granulocytosis in mice by administration of purified human native granulocyte colony-stimulating factor (G-CSF). Biochem Biophys Res Commun. 1987;142:454-460. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 86] [Cited by in RCA: 82] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 72. | Dührsen U, Villeval JL, Boyd J, Kannourakis G, Morstyn G, Metcalf D. Effects of recombinant human granulocyte colony-stimulating factor on hematopoietic progenitor cells in cancer patients. Blood. 1988;72:2074-2081. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4] [Cited by in RCA: 5] [Article Influence: 0.1] [Reference Citation Analysis (0)] |
| 73. | Molineux G, Pojda Z, Hampson IN, Lord BI, Dexter TM. Transplantation potential of peripheral blood stem cells induced by granulocyte colony-stimulating factor. Blood. 1990;76:2153-2158. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 2] [Article Influence: 0.1] [Reference Citation Analysis (0)] |
| 74. | Touw IP, van de Geijn GJ. Granulocyte colony-stimulating factor and its receptor in normal myeloid cell development, leukemia and related blood cell disorders. Front Biosci. 2007;12:800-815. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 89] [Cited by in RCA: 102] [Article Influence: 5.7] [Reference Citation Analysis (0)] |
| 75. | Christopher MJ, Link DC. Granulocyte colony-stimulating factor induces osteoblast apoptosis and inhibits osteoblast differentiation. J Bone Miner Res. 2008;23:1765-1774. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 95] [Cited by in RCA: 93] [Article Influence: 5.5] [Reference Citation Analysis (0)] |
| 76. | Link DC. Mechanisms of granulocyte colony-stimulating factor-induced hematopoietic progenitor-cell mobilization. Semin Hematol. 2000;37:25-32. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 53] [Cited by in RCA: 51] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 77. | Christopher MJ, Rao M, Liu F, Woloszynek JR, Link DC. Expression of the G-CSF receptor in monocytic cells is sufficient to mediate hematopoietic progenitor mobilization by G-CSF in mice. J Exp Med. 2011;208:251-260. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 248] [Cited by in RCA: 245] [Article Influence: 17.5] [Reference Citation Analysis (0)] |
| 78. | Lévesque JP, Hendy J, Takamatsu Y, Williams B, Winkler IG, Simmons PJ. Mobilization by either cyclophosphamide or granulocyte colony-stimulating factor transforms the bone marrow into a highly proteolytic environment. Exp Hematol. 2002;30:440-449. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 223] [Cited by in RCA: 219] [Article Influence: 9.5] [Reference Citation Analysis (0)] |
| 79. | Christopherson KW, Cooper S, Hangoc G, Broxmeyer HE. CD26 is essential for normal G-CSF-induced progenitor cell mobilization as determined by CD26-/- mice. Exp Hematol. 2003;31:1126-1134. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 36] [Cited by in RCA: 49] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 80. | Lévesque JP, Hendy J, Takamatsu Y, Simmons PJ, Bendall LJ. Disruption of the CXCR4/CXCL12 chemotactic interaction during hematopoietic stem cell mobilization induced by GCSF or cyclophosphamide. J Clin Invest. 2003;111:187-196. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 552] [Cited by in RCA: 566] [Article Influence: 25.7] [Reference Citation Analysis (0)] |
| 81. | Bonig H, Papayannopoulou T. Mobilization of hematopoietic stem/progenitor cells: general principles and molecular mechanisms. Methods Mol Biol. 2012;904:1-14. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 47] [Cited by in RCA: 35] [Article Influence: 2.7] [Reference Citation Analysis (0)] |
| 82. | Bendall LJ, Bradstock KF. G-CSF: From granulopoietic stimulant to bone marrow stem cell mobilizing agent. Cytokine Growth Factor Rev. 2014;25:355-367. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 191] [Cited by in RCA: 238] [Article Influence: 21.6] [Reference Citation Analysis (0)] |
| 83. | Lévesque JP, Takamatsu Y, Nilsson SK, Haylock DN, Simmons PJ. Vascular cell adhesion molecule-1 (CD106) is cleaved by neutrophil proteases in the bone marrow following hematopoietic progenitor cell mobilization by granulocyte colony-stimulating factor. Blood. 2001;98:1289-1297. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 393] [Cited by in RCA: 374] [Article Influence: 15.6] [Reference Citation Analysis (0)] |
| 84. | Petit I, Szyper-Kravitz M, Nagler A, Lahav M, Peled A, Habler L, Ponomaryov T, Taichman RS, Arenzana-Seisdedos F, Fujii N, Sandbank J, Zipori D, Lapidot T. G-CSF induces stem cell mobilization by decreasing bone marrow SDF-1 and up-regulating CXCR4. Nat Immunol. 2002;3:687-694. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1020] [Cited by in RCA: 1006] [Article Influence: 43.7] [Reference Citation Analysis (0)] |
| 85. | Levesque JP, Liu F, Simmons PJ, Betsuyaku T, Senior RM, Pham C, Link DC. Characterization of hematopoietic progenitor mobilization in protease-deficient mice. Blood. 2004;104:65-72. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 185] [Cited by in RCA: 185] [Article Influence: 8.8] [Reference Citation Analysis (0)] |
| 86. | Valenzuela-Fernández A, Planchenault T, Baleux F, Staropoli I, Le-Barillec K, Leduc D, Delaunay T, Lazarini F, Virelizier JL, Chignard M, Pidard D, Arenzana-Seisdedos F. Leukocyte elastase negatively regulates Stromal cell-derived factor-1 (SDF-1)/CXCR4 binding and functions by amino-terminal processing of SDF-1 and CXCR4. J Biol Chem. 2002;277:15677-15689. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 161] [Cited by in RCA: 163] [Article Influence: 7.1] [Reference Citation Analysis (0)] |
| 87. | Kajita M, Itoh Y, Chiba T, Mori H, Okada A, Kinoh H, Seiki M. Membrane-type 1 matrix metalloproteinase cleaves CD44 and promotes cell migration. J Cell Biol. 2001;153:893-904. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 580] [Cited by in RCA: 573] [Article Influence: 23.9] [Reference Citation Analysis (0)] |
| 88. | Semerad CL, Christopher MJ, Liu F, Short B, Simmons PJ, Winkler I, Levesque JP, Chappel J, Ross FP, Link DC. G-CSF potently inhibits osteoblast activity and CXCL12 mRNA expression in the bone marrow. Blood. 2005;106:3020-3027. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 378] [Cited by in RCA: 386] [Article Influence: 19.3] [Reference Citation Analysis (0)] |
| 89. | Lévesque JP, Helwani FM, Winkler IG. The endosteal 'osteoblastic' niche and its role in hematopoietic stem cell homing and mobilization. Leukemia. 2010;24:1979-1992. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 203] [Cited by in RCA: 207] [Article Influence: 13.8] [Reference Citation Analysis (0)] |
| 90. | Katayama Y, Battista M, Kao WM, Hidalgo A, Peired AJ, Thomas SA, Frenette PS. Signals from the sympathetic nervous system regulate hematopoietic stem cell egress from bone marrow. Cell. 2006;124:407-421. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 993] [Cited by in RCA: 1023] [Article Influence: 53.8] [Reference Citation Analysis (0)] |
| 91. | Takamatsu Y, Simmons PJ, Moore RJ, Morris HA, To LB, Lévesque JP. Osteoclast-mediated bone resorption is stimulated during short-term administration of granulocyte colony-stimulating factor but is not responsible for hematopoietic progenitor cell mobilization. Blood. 1998;92:3465-3473. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 6] [Cited by in RCA: 7] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 92. | Golan K, Vagima Y, Ludin A, Itkin T, Cohen-Gur S, Kalinkovich A, Kollet O, Kim C, Schajnovitz A, Ovadya Y, Lapid K, Shivtiel S, Morris AJ, Ratajczak MZ, Lapidot T. S1P promotes murine progenitor cell egress and mobilization via S1P1-mediated ROS signaling and SDF-1 release. Blood. 2012;119:2478-2488. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 150] [Cited by in RCA: 156] [Article Influence: 12.0] [Reference Citation Analysis (0)] |
| 93. | Ratajczak MZ, Lee H, Wysoczynski M, Wan W, Marlicz W, Laughlin MJ, Kucia M, Janowska-Wieczorek A, Ratajczak J. Novel insight into stem cell mobilization-plasma sphingosine-1-phosphate is a major chemoattractant that directs the egress of hematopoietic stem progenitor cells from the bone marrow and its level in peripheral blood increases during mobilization due to activation of complement cascade/membrane attack complex. Leukemia. 2010;24:976-985. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 215] [Cited by in RCA: 211] [Article Influence: 14.1] [Reference Citation Analysis (0)] |
| 94. | Reca R, Cramer D, Yan J, Laughlin MJ, Janowska-Wieczorek A, Ratajczak J, Ratajczak MZ. A novel role of complement in mobilization: immunodeficient mice are poor granulocyte-colony stimulating factor mobilizers because they lack complement-activating immunoglobulins. Stem Cells. 2007;25:3093-3100. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 51] [Cited by in RCA: 52] [Article Influence: 2.9] [Reference Citation Analysis (0)] |
| 95. | Méndez-Ferrer S, Lucas D, Battista M, Frenette PS. Haematopoietic stem cell release is regulated by circadian oscillations. Nature. 2008;452:442-447. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 921] [Cited by in RCA: 988] [Article Influence: 58.1] [Reference Citation Analysis (0)] |
| 96. | Golan K, Kumari A, Kollet O, Khatib-Massalha E, Subramaniam MD, Ferreira ZS, Avemaria F, Rzeszotek S, García-García A, Xie S, Flores-Figueroa E, Gur-Cohen S, Itkin T, Ludin-Tal A, Massalha H, Bernshtein B, Ciechanowicz AK, Brandis A, Mehlman T, Bhattacharya S, Bertagna M, Cheng H, Petrovich-Kopitman E, Janus T, Kaushansky N, Cheng T, Sagi I, Ratajczak MZ, Méndez-Ferrer S, Dick JE, Markus RP, Lapidot T. Daily Onset of Light and Darkness Differentially Controls Hematopoietic Stem Cell Differentiation and Maintenance. Cell Stem Cell. 2018;23:572-585.e7. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 77] [Cited by in RCA: 89] [Article Influence: 12.7] [Reference Citation Analysis (0)] |
| 97. | Albiero M, Poncina N, Tjwa M, Ciciliot S, Menegazzo L, Ceolotto G, Vigili de Kreutzenberg S, Moura R, Giorgio M, Pelicci P, Avogaro A, Fadini GP. Diabetes causes bone marrow autonomic neuropathy and impairs stem cell mobilization via dysregulated p66Shc and Sirt1. Diabetes. 2014;63:1353-1365. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 106] [Cited by in RCA: 121] [Article Influence: 11.0] [Reference Citation Analysis (0)] |
| 98. | Fadini GP, Albiero M, Vigili de Kreutzenberg S, Boscaro E, Cappellari R, Marescotti M, Poncina N, Agostini C, Avogaro A. Diabetes impairs stem cell and proangiogenic cell mobilization in humans. Diabetes Care. 2013;36:943-949. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 129] [Cited by in RCA: 139] [Article Influence: 11.6] [Reference Citation Analysis (0)] |
| 99. | Sung AD, Grima DT, Bernard LM, Brown S, Carrum G, Holmberg L, Horwitz ME, Liesveld JL, Kanda J, McClune B, Shaughnessy P, Tricot GJ, Chao NJ. Outcomes and costs of autologous stem cell mobilization with chemotherapy plus G-CSF vs G-CSF alone. Bone Marrow Transplant. 2013;48:1444-1449. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 26] [Cited by in RCA: 27] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 100. | Lévesque JP, Hendy J, Winkler IG, Takamatsu Y, Simmons PJ. Granulocyte colony-stimulating factor induces the release in the bone marrow of proteases that cleave c-KIT receptor (CD117) from the surface of hematopoietic progenitor cells. Exp Hematol. 2003;31:109-117. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 154] [Cited by in RCA: 147] [Article Influence: 6.7] [Reference Citation Analysis (0)] |
| 101. | Winkler IG, Pettit AR, Raggatt LJ, Jacobsen RN, Forristal CE, Barbier V, Nowlan B, Cisterne A, Bendall LJ, Sims NA, Lévesque JP. Hematopoietic stem cell mobilizing agents G-CSF, cyclophosphamide or AMD3100 have distinct mechanisms of action on bone marrow HSC niches and bone formation. Leukemia. 2012;26:1594-1601. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 116] [Cited by in RCA: 132] [Article Influence: 10.2] [Reference Citation Analysis (0)] |
| 102. | Agarwal R, Dvorak CC, Stockerl-Goldstein KE, Johnston L, Srinivas S. High-dose chemotherapy followed by stem cell rescue for high-risk germ cell tumors: the Stanford experience. Bone Marrow Transplant. 2009;43:547-552. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5] [Cited by in RCA: 7] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 103. | Weaver A, Chang J, Wrigley E, de Wynter E, Woll PJ, Lind M, Jenkins B, Gill C, Wilkinson PM, Pettengell R, Radford JA, Collins CD, Dexter TM, Testa NG, Crowther D. Randomized comparison of progenitor-cell mobilization using chemotherapy, stem-cell factor, and filgrastim or chemotherapy plus filgrastim alone in patients with ovarian cancer. J Clin Oncol. 1998;16:2601-2612. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 34] [Cited by in RCA: 30] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 104. | Rick O, Schwella N, Beyer J, Dubiel M, Krusch A, Hildebrandt M, Schleicher J, Serke S, Siegert W. PBPC mobilization with paclitaxel, ifosfamide, and G-CSF with or without amifostine: results of a prospective randomized trial. Transfusion. 2001;41:196-200. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 3] [Article Influence: 0.1] [Reference Citation Analysis (0)] |
| 105. | Feldman DR, Powles T. Salvage high-dose chemotherapy for germ cell tumors. Urol Oncol. 2015;33:355-362. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 11] [Cited by in RCA: 12] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 106. | Hamid AA, Markt SC, Vicier C, McDermott K, Richardson P, Ho VT, Sweeney CJ. Autologous Stem-Cell Transplantation Outcomes for Relapsed Metastatic Germ-Cell Tumors in the Modern Era. Clin Genitourin Cancer. 2019;17:58-64.e1. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5] [Cited by in RCA: 6] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 107. | André M, Baudoux E, Bron D, Canon JL, D'Hondt V, Fassotte MF, D'Hondt L, Fillet G, Humblet Y, Jerusalem G, Vermeulen P, Symann M, Beguin Y. Phase III randomized study comparing 5 or 10 microg per kg per day of filgrastim for mobilization of peripheral blood progenitor cells with chemotherapy, followed by intensification and autologous transplantation in patients with nonmyeloid malignancies. Transfusion. 2003;43:50-57. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 42] [Cited by in RCA: 43] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 108. | Demirer T, Ayli M, Ozcan M, Gunel N, Haznedar R, Dagli M, Fen T, Genc Y, Dincer S, Arslan O, Gürman G, Demirer S, Ozet G, Uysal A, Konuk N, Ilhan O, Koc H, Akan H. Mobilization of peripheral blood stem cells with chemotherapy and recombinant human granulocyte colony-stimulating factor (rhG-CSF): a randomized evaluation of different doses of rhG-CSF. Br J Haematol. 2002;116:468-474. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 54] [Cited by in RCA: 52] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 109. | Kim S, Kim HJ, Park JS, Lee J, Chi HS, Park CJ, Huh J, Suh C. Prospective randomized comparative observation of single- vs split-dose lenograstim to mobilize peripheral blood progenitor cells following chemotherapy in patients with multiple myeloma or non-Hodgkin's lymphoma. Ann Hematol. 2005;84:742-747. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 25] [Cited by in RCA: 24] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 110. | Oh-eda M, Hasegawa M, Hattori K, Kuboniwa H, Kojima T, Orita T, Tomonou K, Yamazaki T, Ochi N. O-linked sugar chain of human granulocyte colony-stimulating factor protects it against polymerization and denaturation allowing it to retain its biological activity. J Biol Chem. 1990;265:11432-11435. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 124] [Cited by in RCA: 116] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 111. | Pedrazzoli P, Gibelli N, Pavesi L, Preti P, Piolini M, Bertolini F, Robustelli della Cuna G. Effects of glycosylated and non-glycosylated G-CSFs, alone and in combination with other cytokines, on the growth of human progenitor cells. Anticancer Res. 1996;16:1781-1785. [PubMed] |
| 112. | Kopf B, De Giorgi U, Vertogen B, Monti G, Molinari A, Turci D, Dazzi C, Leoni M, Tienghi A, Cariello A, Argnani M, Frassineti L, Scarpi E, Rosti G, Marangolo M. A randomized study comparing filgrastim versus lenograstim versus molgramostim plus chemotherapy for peripheral blood progenitor cell mobilization. Bone Marrow Transplant. 2006;38:407-412. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 33] [Cited by in RCA: 30] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 113. | Bönig H, Silbermann S, Weller S, Kirschke R, Körholz D, Janssen G, Göbel U, Nürnberger W. Glycosylated vs non-glycosylated granulocyte colony-stimulating factor (G-CSF)--results of a prospective randomised monocentre study. Bone Marrow Transplant. 2001;28:259-264. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 37] [Cited by in RCA: 36] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 114. | Sourgens H, Lefrère F. A systematic review of available clinical evidence - filgrastim compared with lenograstim. Int J Clin Pharmacol Ther. 2011;49:510-518. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 20] [Cited by in RCA: 20] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 115. | Molineux G, Kinstler O, Briddell B, Hartley C, McElroy P, Kerzic P, Sutherland W, Stoney G, Kern B, Fletcher FA, Cohen A, Korach E, Ulich T, McNiece I, Lockbaum P, Miller-Messana MA, Gardner S, Hunt T, Schwab G. A new form of Filgrastim with sustained duration in vivo and enhanced ability to mobilize PBPC in both mice and humans. Exp Hematol. 1999;27:1724-1734. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 180] [Cited by in RCA: 173] [Article Influence: 6.7] [Reference Citation Analysis (0)] |
| 116. | Putkonen M, Rauhala A, Pelliniemi TT, Remes K. Single-dose pegfilgrastim is comparable to daily filgrastim in mobilizing peripheral blood stem cells: a case-matched study in patients with lymphoproliferative malignancies. Ann Hematol. 2009;88:673-680. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 27] [Cited by in RCA: 25] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 117. | Bassi S, Rabascio C, Nassi L, Steffanoni S, Babic A, Bertazzoni P, Gigli F, Antoniotti P, Orlando L, Sammassimo S, Quarna J, Negri M, Martinelli G. A single dose of Pegfilgrastim versus daily Filgrastim to evaluate the mobilization and the engraftment of autologous peripheral hematopoietic progenitors in malignant lymphoma patients candidate for high-dose chemotherapy. Transfus Apher Sci. 2010;43:321-326. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 26] [Cited by in RCA: 29] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 118. | Bruns I, Steidl U, Kronenwett R, Fenk R, Graef T, Rohr UP, Neumann F, Fischer J, Scheid C, Hübel K, Haas R, Kobbe G. A single dose of 6 or 12 mg of pegfilgrastim for peripheral blood progenitor cell mobilization results in similar yields of CD34+ progenitors in patients with multiple myeloma. Transfusion. 2006;46:180-185. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 53] [Cited by in RCA: 54] [Article Influence: 2.8] [Reference Citation Analysis (1)] |
| 119. | To LB, Bashford J, Durrant S, MacMillan J, Schwarer AP, Prince HM, Gibson J, Lewis I, Swart B, Marty J, Rawling T, Ashman L, Charles S, Cohen B. Successful mobilization of peripheral blood stem cells after addition of ancestim (stem cell factor) in patients who had failed a prior mobilization with filgrastim (granulocyte colony-stimulating factor) alone or with chemotherapy plus filgrastim. Bone Marrow Transplant. 2003;31:371-378. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 41] [Cited by in RCA: 39] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 120. | Briddell RA, Hartley CA, Smith KA, McNiece IK. Recombinant rat stem cell factor synergizes with recombinant human granulocyte colony-stimulating factor in vivo in mice to mobilize peripheral blood progenitor cells that have enhanced repopulating potential. Blood. 1993;82:1720-1723. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4] [Cited by in RCA: 5] [Article Influence: 0.2] [Reference Citation Analysis (0)] |
| 121. | da Silva MG, Pimentel P, Carvalhais A, Barbosa I, Machado A, Campilho F, Sousa SR, Miranda N, da Costa FL, Campos A, Vaz CP, Antas J, Passos-Coelho JL. Ancestim (recombinant human stem cell factor, SCF) in association with filgrastim does not enhance chemotherapy and/or growth factor-induced peripheral blood progenitor cell (PBPC) mobilization in patients with a prior insufficient PBPC collection. Bone Marrow Transplant. 2004;34:683-691. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 16] [Cited by in RCA: 13] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 122. | Peters WP, Rosner G, Ross M, Vredenburgh J, Meisenberg B, Gilbert C, Kurtzberg J. Comparative effects of granulocyte-macrophage colony-stimulating factor (GM-CSF) and granulocyte colony-stimulating factor (G-CSF) on priming peripheral blood progenitor cells for use with autologous bone marrow after high-dose chemotherapy. Blood. 1993;81:1709-1719. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 1] [Reference Citation Analysis (0)] |
| 123. | Lane TA, Law P, Maruyama M, Young D, Burgess J, Mullen M, Mealiffe M, Terstappen LW, Hardwick A, Moubayed M. Harvesting and enrichment of hematopoietic progenitor cells mobilized into the peripheral blood of normal donors by granulocyte-macrophage colony-stimulating factor (GM-CSF) or G-CSF: potential role in allogeneic marrow transplantation. Blood. 1995;85:275-282. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 217] [Cited by in RCA: 194] [Article Influence: 6.5] [Reference Citation Analysis (0)] |
| 124. | Bot FJ, van Eijk L, Schipper P, Backx B, Löwenberg B. Synergistic effects between GM-CSF and G-CSF or M-CSF on highly enriched human marrow progenitor cells. Leukemia. 1990;4:325-328. [PubMed] |
| 125. | Spitzer G, Adkins D, Mathews M, Velasquez W, Bowers C, Dunphy F, Kronmueller N, Niemeyer R, McIntyre W, Petruska P. Randomized comparison of G-CSF + GM-CSF vs G-CSF alone for mobilization of peripheral blood stem cells: effects on hematopoietic recovery after high-dose chemotherapy. Bone Marrow Transplant. 1997;20:921-930. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 58] [Cited by in RCA: 55] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 126. | De Clercq E. The bicyclam AMD3100 story. Nat Rev Drug Discov. 2003;2:581-587. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 366] [Cited by in RCA: 383] [Article Influence: 17.4] [Reference Citation Analysis (0)] |
| 127. | Flomenberg N, Devine SM, Dipersio JF, Liesveld JL, McCarty JM, Rowley SD, Vesole DH, Badel K, Calandra G. The use of AMD3100 plus G-CSF for autologous hematopoietic progenitor cell mobilization is superior to G-CSF alone. Blood. 2005;106:1867-1874. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 370] [Cited by in RCA: 350] [Article Influence: 17.5] [Reference Citation Analysis (0)] |
| 128. | Kobold S, Isernhagen J, Hübel K, Kilic N, Bogner C, Frickhofen N, Bokemeyer C, Fiedler W. Plerixafor is effective and safe for stem cell mobilization in heavily pretreated germ cell tumor patients. Bone Marrow Transplant. 2011;46:1053-1056. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 23] [Cited by in RCA: 24] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 129. | Hughes CE, Nibbs RJB. A guide to chemokines and their receptors. FEBS J. 2018;285:2944-2971. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 413] [Cited by in RCA: 914] [Article Influence: 130.6] [Reference Citation Analysis (0)] |
| 130. | Fricker SP. Physiology and pharmacology of plerixafor. Transfus Med Hemother. 2013;40:237-245. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 48] [Cited by in RCA: 60] [Article Influence: 5.0] [Reference Citation Analysis (0)] |
| 131. | Lee HM, Wysoczynski M, Liu R, Shin DM, Kucia M, Botto M, Ratajczak J, Ratajczak MZ. Mobilization studies in complement-deficient mice reveal that optimal AMD3100 mobilization of hematopoietic stem cells depends on complement cascade activation by AMD3100-stimulated granulocytes. Leukemia. 2010;24:573-582. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 64] [Cited by in RCA: 63] [Article Influence: 4.2] [Reference Citation Analysis (0)] |
| 132. | Dar A, Schajnovitz A, Lapid K, Kalinkovich A, Itkin T, Ludin A, Kao WM, Battista M, Tesio M, Kollet O, Cohen NN, Margalit R, Buss EC, Baleux F, Oishi S, Fujii N, Larochelle A, Dunbar CE, Broxmeyer HE, Frenette PS, Lapidot T. Rapid mobilization of hematopoietic progenitors by AMD3100 and catecholamines is mediated by CXCR4-dependent SDF-1 release from bone marrow stromal cells. Leukemia. 2011;25:1286-1296. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 174] [Cited by in RCA: 167] [Article Influence: 11.9] [Reference Citation Analysis (0)] |
| 133. | Crees ZD, Stockerl-Goldstein K, Vainstein A, Chen H, DiPersio JF. GENESIS: Phase III trial evaluating BL-8040 + G-CSF to mobilize hematopoietic cells for autologous transplant in myeloma. Future Oncol. 2019;15:3555-3563. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 12] [Cited by in RCA: 20] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 134. | Karpova D, Bräuninger S, Wiercinska E, Krämer A, Stock B, Graff J, Martin H, Wach A, Escot C, Douglas G, Romagnoli B, Chevalier E, Dembowski K, Hooftman L, Bonig H. Mobilization of hematopoietic stem cells with the novel CXCR4 antagonist POL6326 (balixafortide) in healthy volunteers-results of a dose escalation trial. J Transl Med. 2017;15:2. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 37] [Cited by in RCA: 39] [Article Influence: 4.9] [Reference Citation Analysis (0)] |
| 135. | Juarez JG, Harun N, Thien M, Welschinger R, Baraz R, Pena AD, Pitson SM, Rettig M, DiPersio JF, Bradstock KF, Bendall LJ. Sphingosine-1-phosphate facilitates trafficking of hematopoietic stem cells and their mobilization by CXCR4 antagonists in mice. Blood. 2012;119:707-716. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 104] [Cited by in RCA: 111] [Article Influence: 8.5] [Reference Citation Analysis (0)] |
| 136. | Craddock CF, Nakamoto B, Andrews RG, Priestley GV, Papayannopoulou T. Antibodies to VLA4 integrin mobilize long-term repopulating cells and augment cytokine-induced mobilization in primates and mice. Blood. 1997;90:4779-4788. [PubMed] |
| 137. | Bonig H, Watts KL, Chang KH, Kiem HP, Papayannopoulou T. Concurrent blockade of alpha4-integrin and CXCR4 in hematopoietic stem/progenitor cell mobilization. Stem Cells. 2009;27:836-837. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 51] [Cited by in RCA: 46] [Article Influence: 2.9] [Reference Citation Analysis (0)] |
| 138. | Ramirez P, Rettig MP, Uy GL, Deych E, Holt MS, Ritchey JK, DiPersio JF. BIO5192, a small molecule inhibitor of VLA-4, mobilizes hematopoietic stem and progenitor cells. Blood. 2009;114:1340-1343. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 135] [Cited by in RCA: 137] [Article Influence: 8.6] [Reference Citation Analysis (0)] |
| 139. | Rettig MP, Ansstas G, DiPersio JF. Mobilization of hematopoietic stem and progenitor cells using inhibitors of CXCR4 and VLA-4. Leukemia. 2012;26:34-53. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 142] [Cited by in RCA: 150] [Article Influence: 11.5] [Reference Citation Analysis (0)] |
| 140. | Ghobadi A, Fiala MA, Rettig M, Schroeder M, Uy GL, Stockerl-Goldstein K, Westervelt P, Vij R, DiPersio JF. A Phase I Study of the Safety and Feasibility of Bortezomib in Combination With G-CSF for Stem Cell Mobilization in Patients With Multiple Myeloma. Clin Lymphoma Myeloma Leuk. 2019;19:e588-e593. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 2] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 141. | Forristal CE, Nowlan B, Jacobsen RN, Barbier V, Walkinshaw G, Walkley CR, Winkler IG, Levesque JP. HIF-1α is required for hematopoietic stem cell mobilization and 4-prolyl hydroxylase inhibitors enhance mobilization by stabilizing HIF-1α. Leukemia. 2015;29:1366-1378. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 39] [Cited by in RCA: 43] [Article Influence: 4.3] [Reference Citation Analysis (0)] |
| 142. | He S, Chu J, Vasu S, Deng Y, Yuan S, Zhang J, Fan Z, Hofmeister CC, He X, Marsh HC, Devine SM, Yu J. FLT3L and plerixafor combination increases hematopoietic stem cell mobilization and leads to improved transplantation outcome. Biol Blood Marrow Transplant. 2014;20:309-313. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 16] [Cited by in RCA: 16] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 143. | Ratajczak MZ, Kim C. The use of chemokine receptor agonists in stem cell mobilization. Expert Opin Biol Ther. 2012;12:287-297. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 12] [Cited by in RCA: 12] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 144. | Pelus LM, Fukuda S. Peripheral blood stem cell mobilization: the CXCR2 ligand GRObeta rapidly mobilizes hematopoietic stem cells with enhanced engraftment properties. Exp Hematol. 2006;34:1010-1020. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 100] [Cited by in RCA: 102] [Article Influence: 5.4] [Reference Citation Analysis (0)] |
| 145. | Nervi B, Link DC, DiPersio JF. Cytokines and hematopoietic stem cell mobilization. J Cell Biochem. 2006;99:690-705. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 191] [Cited by in RCA: 201] [Article Influence: 11.2] [Reference Citation Analysis (0)] |
| 146. | Ha H, Debnath B, Neamati N. Role of the CXCL8-CXCR1/2 Axis in Cancer and Inflammatory Diseases. Theranostics. 2017;7:1543-1588. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 314] [Cited by in RCA: 565] [Article Influence: 70.6] [Reference Citation Analysis (0)] |
| 147. | Karpova D, Rettig MP, Ritchey J, Cancilla D, Christ S, Gehrs L, Chendamarai E, Evbuomwan MO, Holt M, Zhang J, Abou-Ezzi G, Celik H, Wiercinska E, Yang W, Gao F, Eissenberg LG, Heier RF, Arnett SD, Meyers MJ, Prinsen MJ, Griggs DW, Trumpp A, Ruminski PG, Morrow DM, Bonig HB, Link DC, DiPersio JF. Targeting VLA4 integrin and CXCR2 mobilizes serially repopulating hematopoietic stem cells. J Clin Invest. 2019;129:2745-2759. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 21] [Cited by in RCA: 36] [Article Influence: 6.0] [Reference Citation Analysis (0)] |
| 148. | Li C, Goncalves KA, Raskó T, Pande A, Gil S, Liu Z, Izsvák Z, Papayannopoulou T, Davis JC, Kiem HP, Lieber A. Single-dose MGTA-145/plerixafor leads to efficient mobilization and in vivo transduction of HSCs with thalassemia correction in mice. Blood Adv. 2021;5:1239-1249. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 6] [Cited by in RCA: 15] [Article Influence: 3.8] [Reference Citation Analysis (0)] |
| 149. | Fruehauf S, Haas R, Conradt C, Murea S, Witt B, Möhle R, Hunstein W. Peripheral blood progenitor cell (PBPC) counts during steady-state hematopoiesis allow to estimate the yield of mobilized PBPC after filgrastim (R-metHuG-CSF)-supported cytotoxic chemotherapy. Blood. 1995;85:2619-2626. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 60] [Cited by in RCA: 52] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 150. | Tada T, Takizawa T, Nakazato F, Kobayashi S, Koike K, Oguchi M, Ishii E, Amano Y. Treatment of intracranial nongerminomatous germ-cell tumor by high-dose chemotherapy and autologous stem-cell rescue. J Neurooncol. 1999;44:71-76. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 32] [Cited by in RCA: 27] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 151. | Rodenhuis S, de Wit R, de Mulder PH, Keizer HJ, Sleijfer DT, Lalisang RI, Bakker PJ, Mandjes I, Kooi M, de Vries EG. A multi-center prospective phase II study of high-dose chemotherapy in germ-cell cancer patients relapsing from complete remission. Ann Oncol. 1999;10:1467-1473. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 49] [Cited by in RCA: 50] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 152. | Lotz JP, Bui B, Gomez F, Théodore C, Caty A, Fizazi K, Gravis G, Delva R, Peny J, Viens P, Duclos B, De Revel T, Curé H, Gligorov J, Guillemaut S, Ségura C, Provent S, Droz JP, Culine S, Biron P; Groupe d'Etudes des Tumeurs Uro-Génitales (GETUG). Sequential high-dose chemotherapy protocol for relapsed poor prognosis germ cell tumors combining two mobilization and cytoreductive treatments followed by three high-dose chemotherapy regimens supported by autologous stem cell transplantation. Results of the phase II multicentric TAXIF trial. Ann Oncol. 2005;16:411-418. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 46] [Cited by in RCA: 40] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 153. | Feldman DR, Sheinfeld J, Bajorin DF, Fischer P, Turkula S, Ishill N, Patil S, Bains M, Reich LM, Bosl GJ, Motzer RJ. TI-CE high-dose chemotherapy for patients with previously treated germ cell tumors: results and prognostic factor analysis. J Clin Oncol. 2010;28:1706-1713. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 155] [Cited by in RCA: 181] [Article Influence: 12.1] [Reference Citation Analysis (0)] |
| 154. | Haugnes HS, Laurell A, Stierner U, Bremnes RM, Dahl O, Cavallin-Ståhl E, Cohn-Cedermark G. High-dose chemotherapy with autologous stem cell support in patients with metastatic non-seminomatous testicular cancer - a report from the Swedish Norwegian Testicular Cancer Group (SWENOTECA). Acta Oncol. 2012;51:168-176. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 16] [Cited by in RCA: 16] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 155. | Mohr M, Hartig I, Kessler T, Hamisch C, Kliesch S, Krug U, Spieker T, Semik M, Wiebe K, Pühse G, Hertle L, Liersch R, Müller-Tidow C, Mesters RM, Berdel WE. High-dose chemotherapy with autologous PBSC transplantation for poor prognosis germ cell tumors: a retrospective monocenter analysis of 44 cases. Bone Marrow Transplant. 2012;47:1321-1325. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3] [Cited by in RCA: 3] [Article Influence: 0.2] [Reference Citation Analysis (0)] |
| 156. | Necchi A, Lanza F, Rosti G, Martino M, Farè E, Pedrazzoli P; European Society for Blood and Marrow Transplantation, Solid Tumors Working Party (EBMT-STWP) and the Italian Germ Cell Cancer Group (IGG). High-dose chemotherapy for germ cell tumors: do we have a model? Expert Opin Biol Ther. 2015;15:33-44. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 14] [Cited by in RCA: 14] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 157. | Moeung S, Chevreau C, Broutin S, Guitton J, Lelièvre B, Ciccolini J, Massart C, Fléchon A, Delva R, Gravis G, Lotz JP, Bay JO, Gross-Goupil M, Paci A, Marsili S, Malard L, Chatelut E, Thomas F. Therapeutic Drug Monitoring of Carboplatin in High-Dose Protocol (TI-CE) for Advanced Germ Cell Tumors: Pharmacokinetic Results of a Phase II Multicenter Study. Clin Cancer Res. 2017;23:7171-7179. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 13] [Cited by in RCA: 13] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 158. | Agarwal P, Tejwani N, Pathak A, Kumar D, Agrawal N, Mehta A. Benefits of Pre-harvest Peripheral Blood CD34 Counts Guided Single Dose Therapy with PLERIXAFOR in Autologous Hematopoietic Stem Cell Transplantation: A Retrospective Study at a Tertiary Care Institute in India. Indian J Hematol Blood Transfus. 2019;35:72-76. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 1] [Reference Citation Analysis (0)] |
| 159. | Yildiz F, Durnali A, Eraslan E, Ilhan A, Tufan G, Aslan F, Arslan UY, Alkis N, Demirci U, Altuntas F, Oksuzoglu B. Outcomes of Autologous Stem Cell Transplantation (ASCT) in Relapsed/Refractory Germ Cell Tumors: Single Center Experience from Turkey. Urol J. 2020;17:497-500. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 1] [Reference Citation Analysis (0)] |
| 160. | Ussowicz M, Mielcarek-Siedziuk M, Musiał J, Stachowiak M, Węcławek-Tompol J, Sęga-Pondel D, Frączkiewicz J, Trelińska J, Raciborska A. Melphalan, Etoposide, and Carboplatin Megatherapy with Autologous Stem Cell Transplantation in Children with Relapsing or Therapy-Resistant Extracranial Germ-Cell Tumors-A Retrospective Analysis. Cancers (Basel). 2020;12. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 2] [Cited by in RCA: 2] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 161. | Chevreau C, Massard C, Flechon A, Delva R, Gravis G, Lotz JP, Bay JO, Gross-Goupil M, Fizazi K, Mourey L, Paci A, Guitton J, Thomas F, Lelièvre B, Ciccolini J, Moeung S, Gallois Y, Olivier P, Culine S, Filleron T, Chatelut E. Multicentric phase II trial of TI-CE high-dose chemotherapy with therapeutic drug monitoring of carboplatin in patients with relapsed advanced germ cell tumors. Cancer Med. 2021;10:2250-2258. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 1] [Reference Citation Analysis (0)] |
| 162. | Horwitz ME, Long G, Holman P, Libby E, Calandra GC, Schriber JR. Efficacy and safety of hematopoietic stem cell remobilization with plerixafor+G-CSF in adult patients with germ cell tumors. Bone Marrow Transplant. 2012;47:1283-1286. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 11] [Cited by in RCA: 13] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 163. | Worel N, Apperley JF, Basak GW, Douglas KW, Gabriel IH, Geraldes C, Hübel K, Jaksic O, Koristek Z, Lanza F, Lemoli R, Mikala G, Selleslag D, Duarte RF, Mohty M. European data on stem cell mobilization with plerixafor in patients with nonhematologic diseases: an analysis of the European consortium of stem cell mobilization. Transfusion. 2012;52:2395-2400. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 13] [Cited by in RCA: 14] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 164. | García-Escobar I, Parrilla L, Ortega LM, Castellanos D, Pallarés MA, Cortés-Funés H. Clinical experience with plerixafor as a mobilization regimen for autologous peripheral blood stem cell transplantation in patients with refractory germ cell tumors. Mol Clin Oncol. 2014;2:923-926. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 8] [Cited by in RCA: 9] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 165. | Kosmas C, Athanasopoulos A, Dimitriadis G, Miltiadous C, Zilakos M, Lydakis D, Magiorkinis E, Gekas C, Daladimos T, Mylonakis N, Ziras N. Plerixafor added to G-CSF-supported paclitaxel-ifosfamide-cisplatin salvage chemotherapy enhances mobilization of adequate numbers of hematopoietic stem cells for subsequent autografting in hard-to-mobilize patients with relapsed/refractory germ-cell tumors: a single-center experience. Anticancer Drugs. 2014;25:841-847. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 8] [Cited by in RCA: 9] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 166. | Daphne O'Hara VJ, Karr AH, Srivastava S, Kiel PJ. Experience with plerixafor for hematopoietic cell mobilization in nine patients with germ cell tumors. Pharmacotherapy. 2014;34:85-88. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 6] [Cited by in RCA: 7] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 167. | Saure C, Weigelt C, Schroeder T, Klärner V, Galonska L, Haas R, Kobbe G. Plerixafor enables successful hematopoietic stem cell collection in an extensively pretreated patient with testicular cancer. Acta Haematol. 2010;124:235-238. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 14] [Cited by in RCA: 15] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 168. | Tuffaha H, Abdel-Rahman FA. Successful stem-cell mobilization and transplantation using plerixafor in a patient with a germ cell tumor. Hematol Oncol Stem Cell Ther. 2010;3:203-205. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 8] [Cited by in RCA: 9] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 169. | De Blasio A, Rossi L, Zappone E, Ortu La Barbera E, Salvatori R, Pacilli M, Carbone A, Zaccarelli E, Papa A, Tomao S. Plerixafor and autologous stem cell transplantation: impressive result in a chemoresistant testicular cancer patient treated with high-dose chemotherapy. Anticancer Drugs. 2013;24:653-657. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4] [Cited by in RCA: 6] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 170. | Miltiadous C, Dimitriadis GK, Roditis P, Kosmas C. Plerixafor mobilization of peripheral blood hematopoietic progenitors to support further high-dose chemotherapy cycles in a patient with germ-cell tumor relapsing after previous tandem high-dose chemotherapy and hematopoietic cell transplantation: report of a case. Anticancer Drugs. 2017;28:237-241. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5] [Cited by in RCA: 5] [Article Influence: 0.6] [Reference Citation Analysis (0)] |