Published online Jul 24, 2021. doi: 10.5306/wjco.v12.i7.557
Peer-review started: February 25, 2021
First decision: May 4, 2021
Revised: May 10, 2021
Accepted: June 25, 2021
Article in press: June 25, 2021
Published online: July 24, 2021
Processing time: 145 Days and 16.2 Hours
Multimodal treatment is currently the standard of care for locally advanced esophagogastric junction (EGJ) adenocarcinoma due to poor results after surgery alone. Neoadjuvant therapy is intended to shrink the tumor and eliminate potential circulating tumor cells. However, which neoadjuvant treatment is best for patients with EGJ tumors remains controversial. We aimed to compare outcomes of preoperative chemoradiation and perioperative chemotherapy for EGJ adenocarcinomas. For this purpose, we performed a thorough review of the literature describing neoadjuvant treatments for EGJ adenocarcinomas or comparing both therapies. Although some studies have shown better locoregional control and higher rates of complete pathologic response after chemoradiation, data suggest that both types of neoadjuvant therapy have similar survival benefits. As current data are heterogeneous and many studies have included significantly different types of patients in their analysis, future studies with better patient selection are still needed to define which neoadjuvant therapy should be chosen. In addition, targeted therapies and immunotherapy have promising results and should be further explored.
Core Tip: Surgical treatment only has shown poor results in patients with locally advanced esophagogastric junction tumors. Perioperative chemotherapy and neoadjuvant chemoradiation are valid treatment modalities for these patients. This evidence-based review explores the results, advantages, and disadvantages of both approaches. In addition, future directions with potentially effective novel drugs are also discussed.
- Citation: Laxague F, Schlottmann F. Esophagogastric junction adenocarcinoma: Preoperative chemoradiation or perioperative chemotherapy? World J Clin Oncol 2021; 12(7): 557-564
- URL: https://www.wjgnet.com/2218-4333/full/v12/i7/557.htm
- DOI: https://dx.doi.org/10.5306/wjco.v12.i7.557
Esophagogastric junction (EGJ) adenocarcinoma includes tumors originated from the gastric cardia and the distal esophagus, and is the most common pathological type of esophageal cancer in Western countries[1,2]. The prognosis of this entity is unfavo
Surgical resection is the gold standard treatment modality for patients without distant disease. The esophagectomy consists of radical resection of the tumor along with the regional lymph nodes[5]. Nevertheless, the poor results after surgical treatment alone have motivated the adoption of neoadjuvant therapies to improve prognosis. Multiple studies have demonstrated that combined preoperative chemoradiotherapy or perioperative chemotherapy plus surgery provide a greater survival benefit than surgery alone[6-9].
Neoadjuvant therapy is intended to shrink the tumor and eliminate potential circulating tumor cells. However, which neoadjuvant treatment is best for patients with EGJ tumors remains controversial. We aimed to compare outcomes of preoperative chemoradiation and perioperative chemotherapy for EGJ adenocarcinomas. For this purpose, we performed a thorough review of the literature describing neoadjuvant treatments for EGJ adenocarcinomas or comparing both therapies.
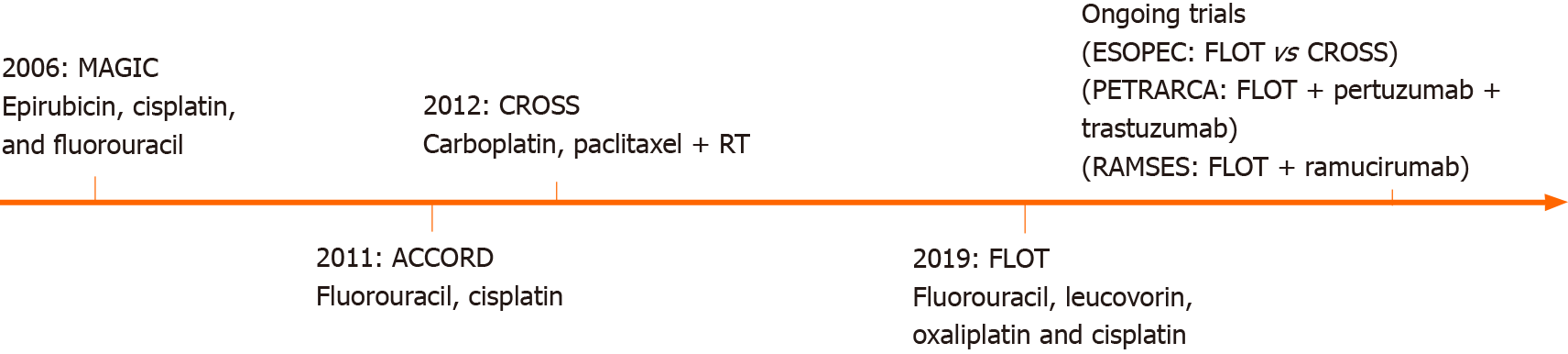
Perioperative chemotherapy for EGJ adenocarcinomas has been explored over time. The first milestone was the British Medical Research Council Adjuvant Gastric Infusional Chemotherapy (MAGIC) trial of 2006, which compared patients with gastric and EGJ adenocarcinomas who underwent 3 cycles of epirubicin, cisplatin, and fluorouracil (ECF) before and after the surgery, vs surgery alone. Results showed a significant improvement in R0 resections and an overall survival benefit in patients receiving perioperative chemotherapy[9].
In 2011, a multicenter phase III trial was conducted (ACCORD-07) including patients with resectable adenocarcinomas of the stomach, EGJ, and distal esophagus. They compared surgery alone vs perioperative chemotherapy with cisplatin and fluorouracil plus surgery. In patients with resectable adenocarcinomas, perioperative chemotherapy significantly improved overall survival, disease-free survival, and curative resection rates[10].
Shortly after, the ChemoRadiotherapy for Oesophageal cancer followed by Surgery Study (CROSS) Group, published the results of a large study that randomized patients with esophageal or EGJ tumors to surgery alone or preoperative chemoradiotherapy followed by surgery. Patients undergoing preoperative chemoradiotherapy with a 5 wk regimen of carboplatin and paclitaxel followed by concurrent radiotherapy, showed a significant improvement in pathological curative resections and overall survival with acceptable adverse events[7,11].
Finally, the German FLOT4 trial in 2019 compared perioperative ECF vs perioperative FLOT (fluorouracil, leucovorin, oxaliplatin, and docetaxel) for gastric and EGJ tumors. This trial was able to demonstrate that patients undergoing FLOT had higher rates of pathological remissions and R0 resections than patients undergoing the MAGIC regimen[12].
Several ongoing trials are currently investigating different neoadjuvant and perioperative therapies in patients with EGJ tumors (Figure 1).
The CROSS trial included 366 patients [275 (75%) adenocarcinomas, 84 (23%) squamous-cell carcinomas, and 7 (2%) large-cell undifferentiated carcinomas]. The vast majority of patients had distal esophageal cancer, with only 22% of EGJ tumors. Patients were randomly assigned to surgery alone (n = 188) or chemoradiotherapy [intravenous carboplatin (AUC 2 mg/mL per min) and intravenous paclitaxel (50 mg/m2 of body-surface area) for 23 d] with concurrent radiotherapy (41.4 Gy, given in 23 fractions of 1.8 Gy on 5 d/wk) followed by surgery (n = 178). The chemoradiotherapy group had significantly higher rates of R0 resections (curative resections) than the surgery alone group (92% vs 69%; P < 0.001). Furthermore, the overall survival was significantly better in the experimental group (49.4 mo vs 24 mo), with a 29% of complete pathological response in the neoadjuvant group. In addition, very few adverse events were reported in the chemoradiotherapy-surgery group (6% leukopenia, 5% anorexia, 3% fatigue, and 2% neutropenia)[7].
Long-term follow-up of the CROSS trial confirmed the benefits of neoadjuvant chemoradiotherapy followed by surgery in patients with EGJ and esophageal cancers. Interestingly, in the subgroup analysis by cancer type, patients with squamous cell carcinomas had a greater overall survival benefit than patients with adenocarcinomas[11].
The addition of radiotherapy to the chemotherapy treatment has shown to improved locoregional control by lymph node downstaging and higher rates of complete pathological response (R0 resections). However, this combination might not be highly effective for reducing the risk of distant metastases[2].
The British MAGIC trial in 2006 introduced the first perioperative chemotherapy regimen for gastric and EGJ tumors, comparing patients who underwent 3 cycles of ECF before and after the surgery, vs patients who underwent surgery alone. The study showed a significant improvement in overall and progression-free survival, as well as higher rates of downsizing of the tumor in the chemotherapy group, with similar complications rates between groups (46% vs 45%)[9]. It is worth mentioning that this trial included only 11% of patients with EGJ adenocarcinomas. In addition, few patients were able to complete the full perioperative treatment (91% completed the 3 preoperative cycles, 66% started the 3 postoperative cycles, and only 76% of these patients completed the three cycles), with only 42% of the patients completing the full 6-cycle regimen. Furthermore, no complete pathological response was observed[9].
The French Actions Concertées dans les cancers COloRectaux et Digestifs (ACCORD)-07 trial in 2011 compared patients receiving 2 or 3 cycles of cisplatin and fluorouracil before and after surgery with patients undergoing surgery alone. The authors observed better overall survival (38% vs 24%), 5 year disease-free survival (34% vs 19%), and higher rates of R0 resections in patients with perioperative chemotherapy[10]. In addition, patients receiving chemotherapy had similar morbidity rates than those undergoing surgery alone. In contrast with the MAGIC-trial, 64% of the patients included in the study had EGJ tumors. However, as well as the MAGIC-trial, one of the main disadvantages was that most patients could not finish the complete regimen due to postoperative morbidity[10].
Based on the results of the MAGIC and ACCORD trials, perioperative chemo
| Study | Year | Number of patients | Included patients | Groups | EGJ tumors | Outcomes |
| MAGIC | 2006 | 503 | Gastric, lower esophagus, and EGJ tumors | ECF + Surgery vs Surgery alone | 11% | Perioperative chemotherapy improves overall survival |
| ACCORD | 2011 | 224 | Gastric, lower esophagus and EGJ tumors | CF + Surgery vs Surgery alone | 64% | Perioperative chemotherapy improves overall survival, disease-free survival and resecability |
| CROSS | 2012 | 366 | Esophageal and EGJ tumors | Chemoradiation + Surgery vs Surgery alone | 22% | Chemoradiotherapy improves overall survival |
| FLOT | 2019 | 716 | Gastric and EGJ tumors | FLOT vs ECF | 56% | FLOT improves overall survival |
To FLOT or to CROSS: that is the question. Regrettably, which is the most effective neoadjuvant therapy for locally advanced EGJ adenocarcinomas remains unclear. In fact, the most important guidelines recommend either perioperative chemotherapy or preoperative chemoradiotherapy for resectable and locally advanced EGJ tumors[13,14].
Unfortunately, scarce studies have compared both therapies. A recent meta-analysis of 13 randomized controlled trials with almost 5000 patients found no significant differences in overall survival between both regimens (FLOT reached a non-significant HR of 0.88 (95%CI: 0.46-1.62) compared to CROSS for overall survival in random-effects models)[15]. Petrelli et al[2] conducted another large systematic review and meta-analysis including 22 studies comparing perioperative chemotherapy and preoperative chemoradiotherapy for GEJ adenocarcinomas, and showed that both therapies had similar overall survival rates. Interestingly, chemoradiotherapy was associated with better locoregional control and higher R0 resection rates but poorer distant metastases control[2].
A propensity score-matched analysis of patients with locally advanced esophageal and EGJ adenocarcinomas compared 40 patients receiving CROSS and 40 receiving FLOT. The study showed that patients undergoing preoperative chemoradiotherapy had higher rates of complete pathological response (97% vs 85%; P = 0.049) and higher rates of negative lymph node metastases (68% vs 40%; P = 0.014) than those receiving perioperative chemotherapy. Nevertheless, despite these benefits associated with the CROSS regimen, no difference in overall survival was found between groups[16].
Recently, a study group conducted a propensity score-matched analysis of 3300 patients (1650 for arm) undergoing preoperative chemoradiation vs perioperative chemotherapy for resectable lower esophageal and EGJ adenocarcinomas. The authors hypothesized that chemoradiation was superior to chemotherapy. They found that although patients undergoing chemoradiation achieved higher rates of complete pathological response (2.7 times), overall survival was similar in both groups[17]. Similarly, a 2-center retrospective analysis, failed to demonstrate a greater benefit between different neoadjuvant therapies for resectable EGJ adenocarcinomas. They analyzed 85 patients (33 received neoadjuvant/perioperative chemotherapy and 52 neoadjuvant chemoradiotherapy). There was a significantly higher pathological complete response after chemoradiotherapy (30% vs 12%; P = 0.01). However, these differences did not translate into a different disease-free or overall survival[18].
At our institution, neoadjuvant chemoradiation is mostly used for patients with distal squamous cell carcinoma (Siewert type I). This strategy is based on the subgroup analysis by cancer type of the CROSS trial, which showed that patients with squamous cell carcinomas had greater overall survival benefit than patients with adenocarcinomas.
In patients with EGJ adenocarcinoma, we try to avoid the morbidity of radiation and we usually offer perioperative chemotherapy based on the multiple trials showing good outcomes with this approach (MAGIC, ACCORD, and FLOT). Currently, we offer FLOT regimen due to the recent results of the FLOT trial. Radiation is usually added in patients with extensive loco-regional involvement (i.e. bulky tumors).
Overall, further studies are needed to clarify which is the best neoadjuvant treatment for EGJ tumors. Each neoadjuvant modality has advantages and disadvantages that should be considered in a case-by-case basis (Table 2).
| Preoperative chemoradiotherapy | Perioperative chemotherapy |
| + Better loco-regional control | + Better systemic control |
| + High rates of complete pathologic response | + No adverse events from radiotherapy |
| - Poorer response in adenocarcinoma (increased radiation sensitivity in squamous cell carcinoma) | - Poorer loco-regional control |
| - Radiation-induced changes in surgical field | - Many patients are not able to complete the postoperative regimen |
As no study could demonstrate greater benefit between chemoradiotherapy or perioperative chemotherapy for resectable EGJ adenocarcinomas, efforts to elucidate the best multimodal treatment are still needed.
The ongoing multicenter randomized controlled phase III ESOPEC-trial compares neoadjuvant CROSS vs FLOT in patients with resectable and potentially curative esophageal adenocarcinoma. The authors hypothesized that the FLOT regimen might improve overall survival and distant metastases disease control. The results of this trial will hopefully help to decide the most suitable neoadjuvant therapy for patients with EGJ adenocarcinoma[4].
Targeted therapies are designed to inhibit specific molecules overexpressed in patients' tumors and are also currently explored for the treatment of esophageal cancer. The human epidermal growth factor receptor 2 (HER2) is involved in diverse cellular functions such as cell growth, differentiation, and survival. Trastuzumab is a monoclonal antibody targeting the extracellular domain of HER2. The trastuzumab for gastric cancer (TOGA) trial evaluated patients with advanced gastroesophageal adenocarcinoma with overexpression of HER2, and found that the addition of trastuzumab to standard chemotherapy was associated with improved overall survival[19]. A recent trial, however, did not show a survival advantage with the addition of trastuzumab to neoadjuvant chemoradiation in patients with HER2 overexpressing esophageal adenocarcinoma[20]. Pertuzumab is another monoclonal antibody targeting HER2. The PETRARCA trial is currently evaluating the outcomes of perioperative trastuzumab and pertuzumab in combination with FLOT vs FLOT alone for patients with HER2-positive resectable esophagogastric adenocarcinoma[21].
The vascular endothelial growth factor (VEGF) and VEGF receptor (VEGFR) regulate angiogenesis and play a key role in tumor growth. Bevacizumab (monoclonal antibody against VEGF-A), ramucirumab (monoclonal antibody against VEGFR-2), and apatinib (molecule inhibitor selective for VEGF-2) are some of the drugs under investigation[22-25].
The advent of immunotherapy has also brought hope for the treatment of esophageal cancer. Immunotherapy utilizes monoclonal antibodies directed against immune checkpoint proteins such as program death 1 (PD-1) receptor, programmed death ligand 1 (PD-L1) or cytotoxic T lymphocyte-associated antigen 4 (CTLA-4). Recent studies have demonstrated survival advantages with monoclonal antibodies targeting PD-1/PD-L1 (e.g., pembrolizumab, nivolumab) in patients with advanced gastric esophageal cancer[26].
Although targeted therapeutics and immunotherapies are indeed promising, further studies are needed to define the safety and efficacy of these drugs.
Although some studies have shown better locoregional control and higher rates of complete pathologic response after chemoradiation as compared to perioperative chemotherapy, current data suggest that both types of neoadjuvant therapy have similar survival benefits. Future studies comparing both treatment modalities and with better patient selection are still needed to define which neoadjuvant therapy should be chosen. Targeted therapeutics and immunotherapies have promising results and might also be part of the treatment armamentarium in the future.
Manuscript source: Invited manuscript
Specialty type: Oncology
Country/Territory of origin: Argentina
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): 0
Grade C (Good): C, C
Grade D (Fair): D
Grade E (Poor): 0
P-Reviewer: Kato K, Luglio G, Tahtabasi M S-Editor: Ma YJ L-Editor: Filipodia P-Editor: Liu JH
| 1. | Rustgi AK, El-Serag HB. Esophageal carcinoma. N Engl J Med. 2014;371:2499-2509. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 817] [Cited by in RCA: 998] [Article Influence: 90.7] [Reference Citation Analysis (0)] |
| 2. | Petrelli F, Ghidini M, Barni S, Sgroi G, Passalacqua R, Tomasello G. Neoadjuvant chemoradiotherapy or chemotherapy for gastroesophageal junction adenocarcinoma: A systematic review and meta-analysis. Gastric Cancer. 2019;22:245-254. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 29] [Cited by in RCA: 45] [Article Influence: 7.5] [Reference Citation Analysis (0)] |
| 3. | Pennathur A, Gibson MK, Jobe BA, Luketich JD. Oesophageal carcinoma. Lancet. 2013;381:400-412. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1956] [Cited by in RCA: 1961] [Article Influence: 163.4] [Reference Citation Analysis (5)] |
| 4. | Hoeppner J, Lordick F, Brunner T, Glatz T, Bronsert P, Röthling N, Schmoor C, Lorenz D, Ell C, Hopt UT, Siewert JR. ESOPEC: prospective randomized controlled multicenter phase III trial comparing perioperative chemotherapy (FLOT protocol) to neoadjuvant chemoradiation (CROSS protocol) in patients with adenocarcinoma of the esophagus (NCT02509286). BMC Cancer. 2016;16:503. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 149] [Cited by in RCA: 249] [Article Influence: 27.7] [Reference Citation Analysis (0)] |
| 5. | Anderegg MCJ, van der Sluis PC, Ruurda JP, Gisbertz SS, Hulshof MCCM, van Vulpen M, Mohammed NH, van Laarhoven HWM, Wiezer MJ, Los M, van Berge Henegouwen MI, van Hillegersberg R. Preoperative Chemoradiotherapy Versus Perioperative Chemotherapy for Patients With Resectable Esophageal or Gastroesophageal Junction Adenocarcinoma. Ann Surg Oncol. 2017;24:2282-2290. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 36] [Cited by in RCA: 35] [Article Influence: 4.4] [Reference Citation Analysis (0)] |
| 6. | Tepper J, Krasna MJ, Niedzwiecki D, Hollis D, Reed CE, Goldberg R, Kiel K, Willett C, Sugarbaker D, Mayer R. Phase III trial of trimodality therapy with cisplatin, fluorouracil, radiotherapy, and surgery compared with surgery alone for esophageal cancer: CALGB 9781. J Clin Oncol. 2008;26:1086-1092. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 941] [Cited by in RCA: 1053] [Article Influence: 61.9] [Reference Citation Analysis (0)] |
| 7. | van Hagen P, Hulshof MC, van Lanschot JJ, Steyerberg EW, van Berge Henegouwen MI, Wijnhoven BP, Richel DJ, Nieuwenhuijzen GA, Hospers GA, Bonenkamp JJ, Cuesta MA, Blaisse RJ, Busch OR, ten Kate FJ, Creemers GJ, Punt CJ, Plukker JT, Verheul HM, Spillenaar Bilgen EJ, van Dekken H, van der Sangen MJ, Rozema T, Biermann K, Beukema JC, Piet AH, van Rij CM, Reinders JG, Tilanus HW, van der Gaast A; CROSS Group. Preoperative chemoradiotherapy for esophageal or junctional cancer. N Engl J Med. 2012;366:2074-2084. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3288] [Cited by in RCA: 4078] [Article Influence: 313.7] [Reference Citation Analysis (0)] |
| 8. | Sjoquist KM, Burmeister BH, Smithers BM, Zalcberg JR, Simes RJ, Barbour A, Gebski V; Australasian Gastro-Intestinal Trials Group. Survival after neoadjuvant chemotherapy or chemoradiotherapy for resectable oesophageal carcinoma: an updated meta-analysis. Lancet Oncol. 2011;12:681-692. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1141] [Cited by in RCA: 1265] [Article Influence: 90.4] [Reference Citation Analysis (0)] |
| 9. | Cunningham D, Allum WH, Stenning SP, Thompson JN, Van de Velde CJ, Nicolson M, Scarffe JH, Lofts FJ, Falk SJ, Iveson TJ, Smith DB, Langley RE, Verma M, Weeden S, Chua YJ, MAGIC Trial Participants. Perioperative chemotherapy vs surgery alone for resectable gastroesophageal cancer. N Engl J Med. 2006;355:11-20. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4899] [Cited by in RCA: 4608] [Article Influence: 242.5] [Reference Citation Analysis (0)] |
| 10. | Ychou M, Boige V, Pignon JP, Conroy T, Bouché O, Lebreton G, Ducourtieux M, Bedenne L, Fabre JM, Saint-Aubert B, Genève J, Lasser P, Rougier P. Perioperative chemotherapy compared with surgery alone for resectable gastroesophageal adenocarcinoma: an FNCLCC and FFCD multicenter phase III trial. J Clin Oncol. 2011;29:1715-1721. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1216] [Cited by in RCA: 1504] [Article Influence: 107.4] [Reference Citation Analysis (0)] |
| 11. | Shapiro J, van Lanschot JJB, Hulshof MCCM, van Hagen P, van Berge Henegouwen MI, Wijnhoven BPL, van Laarhoven HWM, Nieuwenhuijzen GAP, Hospers GAP, Bonenkamp JJ, Cuesta MA, Blaisse RJB, Busch ORC, Ten Kate FJW, Creemers GM, Punt CJA, Plukker JTM, Verheul HMW, Bilgen EJS, van Dekken H, van der Sangen MJC, Rozema T, Biermann K, Beukema JC, Piet AHM, van Rij CM, Reinders JG, Tilanus HW, Steyerberg EW, van der Gaast A; CROSS study group. Neoadjuvant chemoradiotherapy plus surgery vs surgery alone for oesophageal or junctional cancer (CROSS): long-term results of a randomised controlled trial. Lancet Oncol. 2015;16:1090-1098. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1292] [Cited by in RCA: 1824] [Article Influence: 182.4] [Reference Citation Analysis (0)] |
| 12. | Al-Batran SE, Homann N, Pauligk C, Goetze TO, Meiler J, Kasper S, Kopp HG, Mayer F, Haag GM, Luley K, Lindig U, Schmiegel W, Pohl M, Stoehlmacher J, Folprecht G, Probst S, Prasnikar N, Fischbach W, Mahlberg R, Trojan J, Koenigsmann M, Martens UM, Thuss-Patience P, Egger M, Block A, Heinemann V, Illerhaus G, Moehler M, Schenk M, Kullmann F, Behringer DM, Heike M, Pink D, Teschendorf C, Löhr C, Bernhard H, Schuch G, Rethwisch V, von Weikersthal LF, Hartmann JT, Kneba M, Daum S, Schulmann K, Weniger J, Belle S, Gaiser T, Oduncu FS, Güntner M, Hozaeel W, Reichart A, Jäger E, Kraus T, Mönig S, Bechstein WO, Schuler M, Schmalenberg H, Hofheinz RD; FLOT4-AIO Investigators. Perioperative chemotherapy with fluorouracil plus leucovorin, oxaliplatin, and docetaxel vs fluorouracil or capecitabine plus cisplatin and epirubicin for locally advanced, resectable gastric or gastro-oesophageal junction adenocarcinoma (FLOT4): a randomised, phase 2/3 trial. Lancet. 2019;393:1948-1957. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 974] [Cited by in RCA: 1645] [Article Influence: 274.2] [Reference Citation Analysis (0)] |
| 13. | Lordick F, Mariette C, Haustermans K, Obermannová R, Arnold D; ESMO Guidelines Committee. Oesophageal cancer: ESMO Clinical Practice Guidelines for diagnosis, treatment and follow-up. Ann Oncol. 2016;27:v50-v57. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 523] [Cited by in RCA: 665] [Article Influence: 73.9] [Reference Citation Analysis (0)] |
| 14. | National Comprehensive Cancer Network. [cited 19 February 2021] Available from: https://www.nccn.org/professionals/physician_gls/pdf/esophageal.pdf. |
| 15. | van den Ende T, Hulshof MCCM, van Berge Henegouwen MI, van Oijen MGH, van Laarhoven HWM. Gastro-oesophageal junction: to FLOT or to CROSS? Acta Oncol. 2020;59:233-236. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5] [Cited by in RCA: 5] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 16. | Favi F, Bollschweiler E, Berlth F, Plum P, Hescheler DA, Alakus H, Semrau R, Celik E, Mönig SP, Drebber U, Hölscher AH. Neoadjuvant chemotherapy or chemoradiation for patients with advanced adenocarcinoma of the oesophagus? Eur J Surg Oncol. 2017;43:1572-1580. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 18] [Cited by in RCA: 24] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 17. | Zafar SN, Blum M, Chiang YJ, Ajani JA, Estrella JS, Das P, Minsky BD, Hofstetter WL, Mansfield P, Badgwell BD, Ikoma N. Preoperative Chemoradiation Versus Chemotherapy in Gastroesophageal Junction Adenocarcinoma. Ann Thorac Surg. 2020;110:398-405. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 8] [Cited by in RCA: 16] [Article Influence: 3.2] [Reference Citation Analysis (0)] |
| 18. | Wundsam HV, Doleschal B, Prommer R, Venhoda C, Schmitt C, Petzer A, Metz-Gercek S, Rumpold H. Clinical Outcome in Patients with Carcinoma of the Esophagogastric Junction Treated with Neoadjuvant Radiochemotherapy or Perioperative Chemotherapy: A Two-Center Retrospective Analysis. Oncology. 2020;98:706-713. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 2] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 19. | Bang YJ, Van Cutsem E, Feyereislova A, Chung HC, Shen L, Sawaki A, Lordick F, Ohtsu A, Omuro Y, Satoh T, Aprile G, Kulikov E, Hill J, Lehle M, Rüschoff J, Kang YK; ToGA Trial Investigators. Trastuzumab in combination with chemotherapy vs chemotherapy alone for treatment of HER2-positive advanced gastric or gastro-oesophageal junction cancer (ToGA): a phase 3, open-label, randomised controlled trial. Lancet. 2010;376:687-697. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5541] [Cited by in RCA: 5319] [Article Influence: 354.6] [Reference Citation Analysis (3)] |
| 20. | Safran H, Winter KA, Wigle DA, DiPetrillo TA, Haddock MG, Hong TS, Leichman LP, Rajdev L, Resnick MB, Kachnic LA, Seaward SA, Mamon HJ, Diaz Pardo DA, Anderson CM, Shen X, Sharma AK, Katz AW, Salo JC, Leonard KL, Crane CH. Trastuzumab with trimodality treatment for esophageal adenocarcinoma with HER2 overexpression: NRG Oncology/RTOG 1010. J Clin Oncol. 2020;38. [RCA] [DOI] [Full Text] [Cited by in Crossref: 28] [Cited by in RCA: 29] [Article Influence: 5.8] [Reference Citation Analysis (0)] |
| 21. | Hofheinz R, zur Hausen G, Borchert K, Kretzschmar A, Ebert EP, Ettrich TJ. Perioperative trastuzumab and pertuzumab in combination with FLOT vs FLOT alone for HER2 positive resectable esophagogastric adenocarcinoma: Petrarca—A phase II trial of the German AIO. J Clin Oncol. 2017;35 suppl:TPS4133. |
| 22. | Cunningham D, Stenning SP, Smyth EC, Okines AF, Allum WH, Rowley S, Stevenson L, Grabsch HI, Alderson D, Crosby T, Griffin SM, Mansoor W, Coxon FY, Falk SJ, Darby S, Sumpter KA, Blazeby JM, Langley RE. Peri-operative chemotherapy with or without bevacizumab in operable oesophagogastric adenocarcinoma (UK Medical Research Council ST03): primary analysis results of a multicentre, open-label, randomised phase 2-3 trial. Lancet Oncol. 2017;18:357-370. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 203] [Cited by in RCA: 216] [Article Influence: 27.0] [Reference Citation Analysis (0)] |
| 23. | Fuchs CS, Tomasek J, Yong CJ, Dumitru F, Passalacqua R, Goswami C, Safran H, Dos Santos LV, Aprile G, Ferry DR, Melichar B, Tehfe M, Topuzov E, Zalcberg JR, Chau I, Campbell W, Sivanandan C, Pikiel J, Koshiji M, Hsu Y, Liepa AM, Gao L, Schwartz JD, Tabernero J; REGARD Trial Investigators. Ramucirumab monotherapy for previously treated advanced gastric or gastro-oesophageal junction adenocarcinoma (REGARD): an international, randomised, multicentre, placebo-controlled, phase 3 trial. Lancet. 2014;383:31-39. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1541] [Cited by in RCA: 1573] [Article Influence: 143.0] [Reference Citation Analysis (0)] |
| 24. | Wilke H, Muro K, Van Cutsem E, Oh SC, Bodoky G, Shimada Y, Hironaka S, Sugimoto N, Lipatov O, Kim TY, Cunningham D, Rougier P, Komatsu Y, Ajani J, Emig M, Carlesi R, Ferry D, Chandrawansa K, Schwartz JD, Ohtsu A; RAINBOW Study Group. Ramucirumab plus paclitaxel vs placebo plus paclitaxel in patients with previously treated advanced gastric or gastro-oesophageal junction adenocarcinoma (RAINBOW): a double-blind, randomised phase 3 trial. Lancet Oncol. 2014;15:1224-1235. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1613] [Cited by in RCA: 1767] [Article Influence: 160.6] [Reference Citation Analysis (0)] |
| 25. | Li J, Qin S, Xu J, Xiong J, Wu C, Bai Y, Liu W, Tong J, Liu Y, Xu R, Wang Z, Wang Q, Ouyang X, Yang Y, Ba Y, Liang J, Lin X, Luo D, Zheng R, Wang X, Sun G, Wang L, Zheng L, Guo H, Wu J, Xu N, Yang J, Zhang H, Cheng Y, Wang N, Chen L, Fan Z, Sun P, Yu H. Randomized, Double-Blind, Placebo-Controlled Phase III Trial of Apatinib in Patients With Chemotherapy-Refractory Advanced or Metastatic Adenocarcinoma of the Stomach or Gastroesophageal Junction. J Clin Oncol. 2016;34:1448-1454. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 551] [Cited by in RCA: 731] [Article Influence: 81.2] [Reference Citation Analysis (1)] |
| 26. | Chen K, Wang X, Yang L, Chen Z. The Anti-PD-1/PD-L1 Immunotherapy for Gastric Esophageal Cancer: A Systematic Review and Meta-Analysis and Literature Review. Cancer Control. 2021;28:1073274821997430. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 9] [Cited by in RCA: 36] [Article Influence: 9.0] [Reference Citation Analysis (0)] |