Published online Jun 24, 2021. doi: 10.5306/wjco.v12.i6.482
Peer-review started: February 9, 2021
First decision: March 31, 2021
Revised: April 6, 2021
Accepted: June 2, 2021
Article in press: June 2, 2021
Published online: June 24, 2021
Processing time: 131 Days and 10.1 Hours
Personalized nutrition and protective diets and lifestyles represent a key cancer research priority. The association between consumption of specific dietary components and colorectal cancer (CRC) incidence has been evaluated by a number of population-based studies, which have identified certain food items as having protective potential, though the findings have been inconsistent. Herein we present a systematic review and meta-analysis on the potential protective role of five common phytochemically rich dietary components (nuts, cruciferous vegetables, citrus fruits, garlic and tomatoes) in reducing CRC risk.
To investigate the independent impact of increased intake of specific dietary constituents on CRC risk in the general population.
Medline and Embase were systematically searched, from time of database inception to January 31, 2020, for observational studies reporting CRC incidence relative to intake of one or more of nuts, cruciferous vegetables, citrus fruits, garlic and/or tomatoes in the general population. Data were extracted by two independent reviewers and analyzed in accordance with the Meta-analysis of Observational Studies in Epidemiology (MOOSE) and Preferred Reporting Items for Systematic Review and Meta-analysis (PRISMA) reporting guidelines and according to predefined inclusion/exclusion criteria. Effect sizes of studies were pooled using a random-effects model.
Forty-six studies were identified. CRC risk was significantly reduced in patients with higher vs lower consumption of cruciferous vegetables [odds ratio (OR) = 0.90; 95% confidence interval (CI): 0.85-0.95; P < 0.005], citrus fruits (OR = 0.90; 95%CI: 0.84-0.96; P < 0.005), garlic (OR = 0.83; 95%CI: 0.76-0.91; P < 0.005) and tomatoes (OR = 0.89; 95%CI: 0.84-0.95; P < 0.005). Subgroup analysis showed that this association sustained when looking at case-control studies alone, for all of these four food items, but no significant difference was found in analysis of cohort studies alone. Nut consumption exhibited a similar trend, but overall results were not significant (OR = 0.72; 95%CI: 0.50-1.03; P < 0.07; I2 = 90.70%). Putative anticarcinogenic mechanisms are proposed using gene-set enrichment analysis of gene/protein perturbations caused by active compounds within each food item.
Increased cruciferous vegetable, garlic, citrus fruit and tomato consumption are all inversely associated with CRC risk. These findings highlight the potential for developing precision nutrition strategies for CRC prevention.
Core Tip: Personalized nutrition and protective diets and lifestyles represent a key cancer research priority. Herein we present a systematic review and meta-analysis on the potential protective role of five common phytochemically rich dietary components (nuts, cruciferous vegetables, citrus fruits, garlic and tomatoes) in reducing colorectal cancer (CRC) risk. Results show that increased dietary consumption of cruciferous vegetables, citrus fruits, garlic and tomatoes is associated with reduced CRC risk. Active components of these may interact with certain genes to exert this anticarcinogenic effect, highlighting the potential for developing precision nutrition strategies for CRC prevention.
- Citation: Borgas P, Gonzalez G, Veselkov K, Mirnezami R. Phytochemically rich dietary components and the risk of colorectal cancer: A systematic review and meta-analysis of observational studies. World J Clin Oncol 2021; 12(6): 482-499
- URL: https://www.wjgnet.com/2218-4333/full/v12/i6/482.htm
- DOI: https://dx.doi.org/10.5306/wjco.v12.i6.482
Colorectal cancer (CRC) imposes a major public health burden and is the third most commonly diagnosed cancer worldwide[1]. Incidence varies globally between ethnicities and geographical locations[2] with an increase noted amongst migrants moving from low- to high-risk countries[3]. Likewise, socioeconomic disparities correlate with CRC incidence with higher rates occurring in more deprived regions[4,5]. Large-scale epidemiological studies have served to highlight the impact of potentially modifiable factors such as diet, obesity, physical activity and smoking on CRC incidence[6,7].
Diet is an essential component of health and is known to play a key role in the development of a vast array of diseases, including cancers of the digestive system[3]. CRC is strongly associated with inflammation and oxidative stress, both of which are believed to be highly influenced by diet[8-10]. For many decades, considerable research efforts have been directed towards the identification of food groups which increase CRC risk, and broadly these have concluded that diets rich in red- and processed-meat, alcohol, saturated fat, refined grains and sugars, increase the risk of developing CRC[11-15]. Likewise, it is believed that a diet rich in fiber, fruits and vegetables can exert a protective effect[12-14].
The Third Expert Report from the World Cancer Research Fund (2018) has emphasized the importance of protective dietary and lifestyle pattern in creating a metabolic state which is more favorable in preventing cancer as a whole through genetic and epigenetic alterations in carcinogenesis, rather than focusing on specific nutrients or food components alone[14]. However, in order to build a holistic protective lifestyle, it remains important to understand which specific foods have the potential to reduce the risk of certain cancers, so as to establish the determinants of external and endogenous challenges contributing to or preventing cancer carcinogenesis. Additionally, in light of the recently published National Institute of Health (NIH) strategic plan, and the call for research into ‘precision nutrition’ as a potential “holistic approach to developing comprehensive and dynamic nutritional recommendations relevant to both individual and population health”[16], it is vital to explore ways in which specific foods have the potential to beneficially modify cancer incidence and outcomes.
Observational studies have investigated the relationship between CRC incidence and the consumption of certain food items. This study investigated nuts, cruciferous vegetables, citrus fruits, garlic and tomatoes, because these remain amongst those foods with most epidemiological data available. Additionally, Veselkov et al[17] have previously uncovered compounds with anticancer properties within these foods by analyzing the genomic effects of the said compounds which may help explain the underlying cause for any protective associations found through observational studies. Thus, food items were included for analysis in circumstances where a substantial number of studies provided comparative outcome data based on consumption levels and fulfilled all inclusion and no exclusion criteria, as outlined in the methods. Although previous meta-analyses have sought to evaluate the impact of some of these food items[18-23], results have been contradictory and inconclusive. Furthermore, to date there has been no comprehensive meta-analysis, including more recent multinational cohort studies, which unifies all five of these food items, assessing all available and recent evidence. To meet this need, we conducted a systematic review of the literature with pooled analysis of data from all eligible observational studies exploring the effect of increased consumption of nuts, cruciferous vegetables, citrus fruits, garlic and tomatoes on CRC risk in the general population. Furthermore, putative anticarcinogenic mechanisms are highlighted through gene-set enrichment analysis (GSEA).
This systematic review and meta-analysis was conducted according to Meta-analysis of Observational Studies in Epidemiology guidelines (MOOSE)[24] and Preferred Reporting Items for Systematic Review and Meta-analyses (PRISMA)[25] guidelines. Two reviewers (Borgas P and Mirnezami R) independently conducted a systematic literature search of Medline and Embase, from time of database inception to January 31, 2020. A detailed summary of the search terms used is provided in the Supplementary material, Appendix 1. There were no restrictions to language or study population. Duplicates between databases were removed. Food items were included for analysis in circumstances where a substantial amount of studies provided comparative outcome data based on consumption levels and fulfilled all inclusion and no exclusion criteria as outlined below.
Inclusion criteria comprised: (1) Observational studies (i.e., case-control or cohort); (2) The exposure variable being at least one of the following five dietary components; nuts (including peanuts and peanut butter), cruciferous vegetables (including studies reporting data on individual types of cruciferous vegetables as well as grouped data for several types of Cruciferae), citrus fruits, garlic, tomatoes; (3) Colorectal adenocarcinoma incidence being the outcome (studies investigating benign adenomas or polyps were excluded); (4) Provision of adjusted odds ratios (ORs) or relative risks (RRs) with 95% confidence intervals (CIs); (5) Participants aged ≥ 18 without prevalent cancers at baseline; and (6) Newcastle-Ottawa scale (NOS) > 5 indicating sufficient study quality[26]. A sensitivity analysis was performed to assess the robustness of results with respect to the NOS selection criterion.
Further exclusion criteria comprised: (1) Grouping of the above listed food items with other foods (e.g., nuts often with legumes, cruciferous vegetables often with other vegetables); (2) Where multiple publications from the same study population were found, only the study with the largest case cohort and/or most relevant/recent information was included for analysis; and (3) Non-human research.
Additional criteria such as number of cases, method of outcome assessment and, for case-control studies, method cases and controls selected for, were considered and selected for as part of each studies’ individual quality assessment using NOS.
Two authors (Borgas P and Mirnezami R) screened the titles of all identified articles independently, to exclude those that were not relevant to the question at hand, did not meet all inclusion criteria or met at least one exclusion criteria. Abstracts and full texts of potentially relevant remaining articles were then screened for eligibility according to the same principles. Full text articles which met all criteria outlined above were retrieved. Discrepancies were resolved by discussion between the two reviewers (Borgas P and Mirnezami R). Reference lists of all identified articles, and other related review articles, systematic reviews and meta-analyses were hand-searched for additional articles
Two authors (Borgas P and Mirnezami R) independently extracted the following information: name of first author, year of publication, study design, characteristics of study population (age range or mean age, sex, country, specific details on patient demographic), dietary exposure, dietary assessment instrument used, outcomes (including cancer site), comparison, OR or RR (95%CI), adjusted variables, NOS. For case-control studies the following additional information was extracted: number of cases and number of controls. For cohort studies the following additional information was extracted: number of participants at baseline, number of CRC cases, person years, length of follow-up (Supplementary Tables 1 and 2). These variables were judged to be most relevant to the outcome studied. Where multiple estimates for the association of the same outcome were used, the one with the highest number of adjusted variables was extracted.
The NOS was used to grade the quality and assess confounding of each included study by two independent investigators (Borgas P and Mirnezami R)[26]. Studies scoring less than 6 were excluded in analysis. Disagreements were resolved by discussion.
Meta-analyses were conducted in Stata software, version 16.1 (StataCorp LLC). A random-effects model was employed, and the inverse variance method with restricted maximum likelihood was applied to assess the association of intake of each dietary component with the risk of CRC. Between-study heterogeneity was assessed using I2, in order to define the percentage of variability in effect size estimates which were the result of differences between studies. An I2> 50% suggests high heterogeneity. Subgroup analyses were performed, stratifying by study design to account for the increased risk of biases related to the retrospective assessment of diet in case-control studies compared to cohort studies.
For all studies, we selected the effect- sizes computed by comparing the highest vs lowest dietary exposure category and adjusted for confounding variables. For studies reporting gender-specific results only, we included effect size for male and female participants separately and these are therefore displayed as separate studies in the accompanying forest plots, despite originating from the same publication. For studies presenting gender-specific as well as merged male and female data, we included the merged data only. We followed the same procedure for studies reporting data on all CRC types vs site-specific CRC. Given that the absolute risk of CRC is low in human, the ORs should approximate the RRs; therefore, we reported all results as ORs for simplicity. Supplementary Table 3 demonstrates the number of studies reporting sub-type specific data based on sex and/or site of cancer for each food item, confirming a relative lack in sufficient numbers of studies reporting such specified data to be able to perform separate analyses for sex and CRC subsite.
We evaluated the presence of bias in the form of small-study effects using contour-enhanced funnel plots and Egger regression asymmetry testing. For funnel plots, we used contour lines representing 1%, 5% and 10% significance. We assessed publication bias by visually inspecting the funnel plots, with publication bias being suspected when smaller studies were absent from the non-significant regions. Egger’s test was used to obtain a statistical measure of funnel plot asymmetry with the null hypothesis being that the funnel plot is symmetric (i.e., there is no evidence of small-study effects). In this case, a P value > 0.05 implies no evidence of small-study effects.
We investigated potential anticarcinogenic mechanisms of food items by building a profile of genomic perturbations caused by each food item and identifying biological pathways affected by these perturbations. We built food profiles of genomic perturbations by averaging genomic perturbations caused by predicted anticarcinogenic compounds within each food as previously proposed by Veselkov et al[17]. Pathway analytics were performed using Gene Set Enrichment Analysis v4.0.3, and PANTHER[27]. An extended description of this methodology is provided in Supplementary material, Appendix 2.
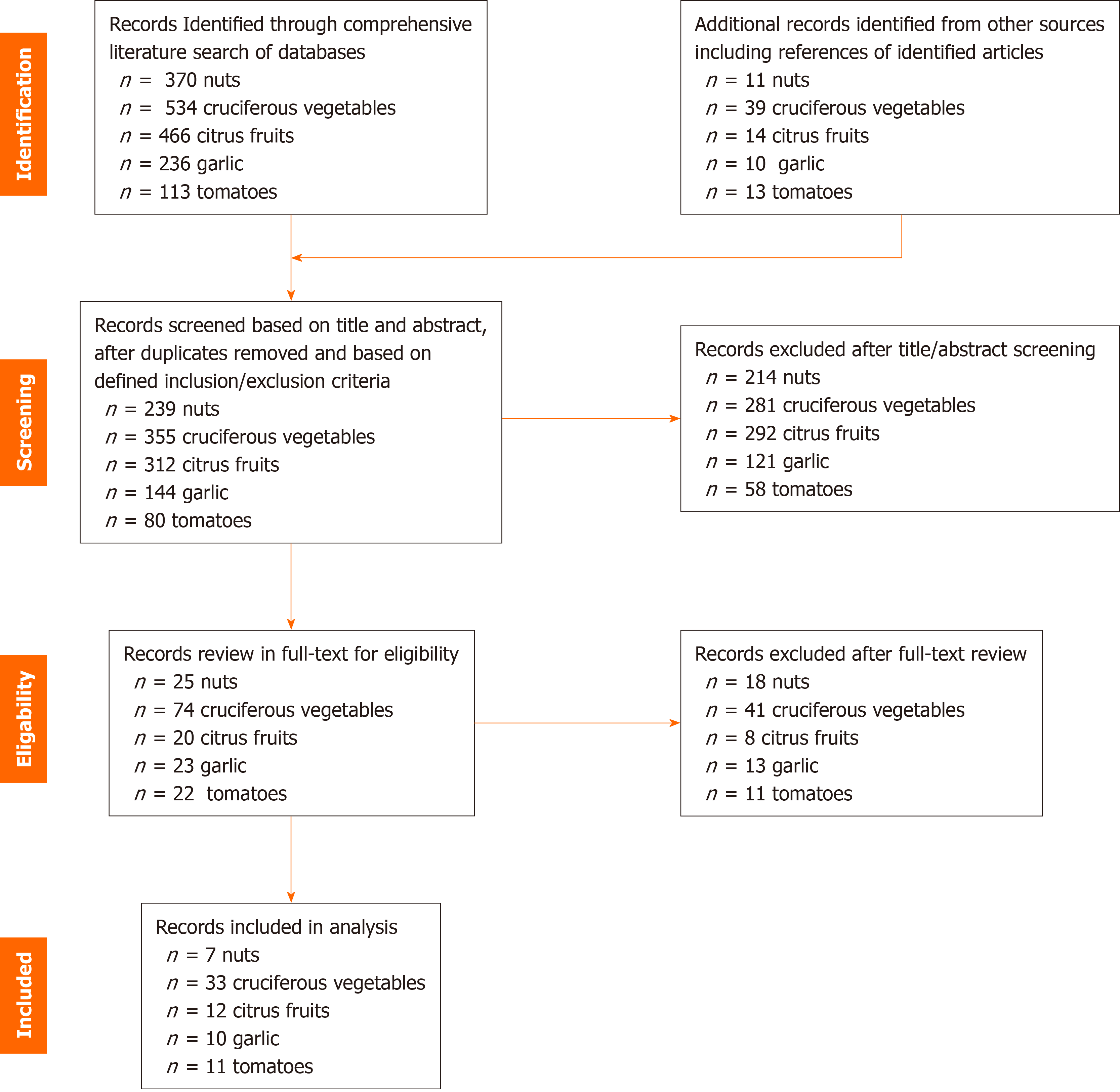
Our initial search strategy identified 534, 236, 466, 113 and 370 records on cruciferous vegetables, citrus fruits, garlic, tomatoes and nuts, respectively. Based on our predefined inclusion/exclusion criteria 33, 12, 10, 11 and 7 records, respectively, have been included in this systematic review and meta-analysis. Figure 1 summarizes the study selection process for each dietary component.
For each of the 5 food items, there was no significant discrepancy between pooled ORs of analyses including all studies, before exclusion based on NOS, compared to analyses after excluding studies with NOS ≤ 5 (Supplementary Table 4). This confirms robustness of the NOS ≤ 5 exclusion criteria used in study selection. All other parameters used in study selection were not arbitrary but rather based on the research question at hand, and thus do not require sensitivity analysis.
Pooled ORs for each of the five food items are presented in Table 1. Overall analysis of case-control and cohort studies combined showed that higher dietary intake of cruciferous vegetables, citrus fruits, garlic and tomatoes were all significantly associated with reduced incidence of CRC. Cohort studies on nut consumption showed the same trend.
| Dietary component | Number of studies (number of publications)1 | Pooled OR (95%CI) | P value | Heterogeneity [I2 (%)] | Egger P value | |
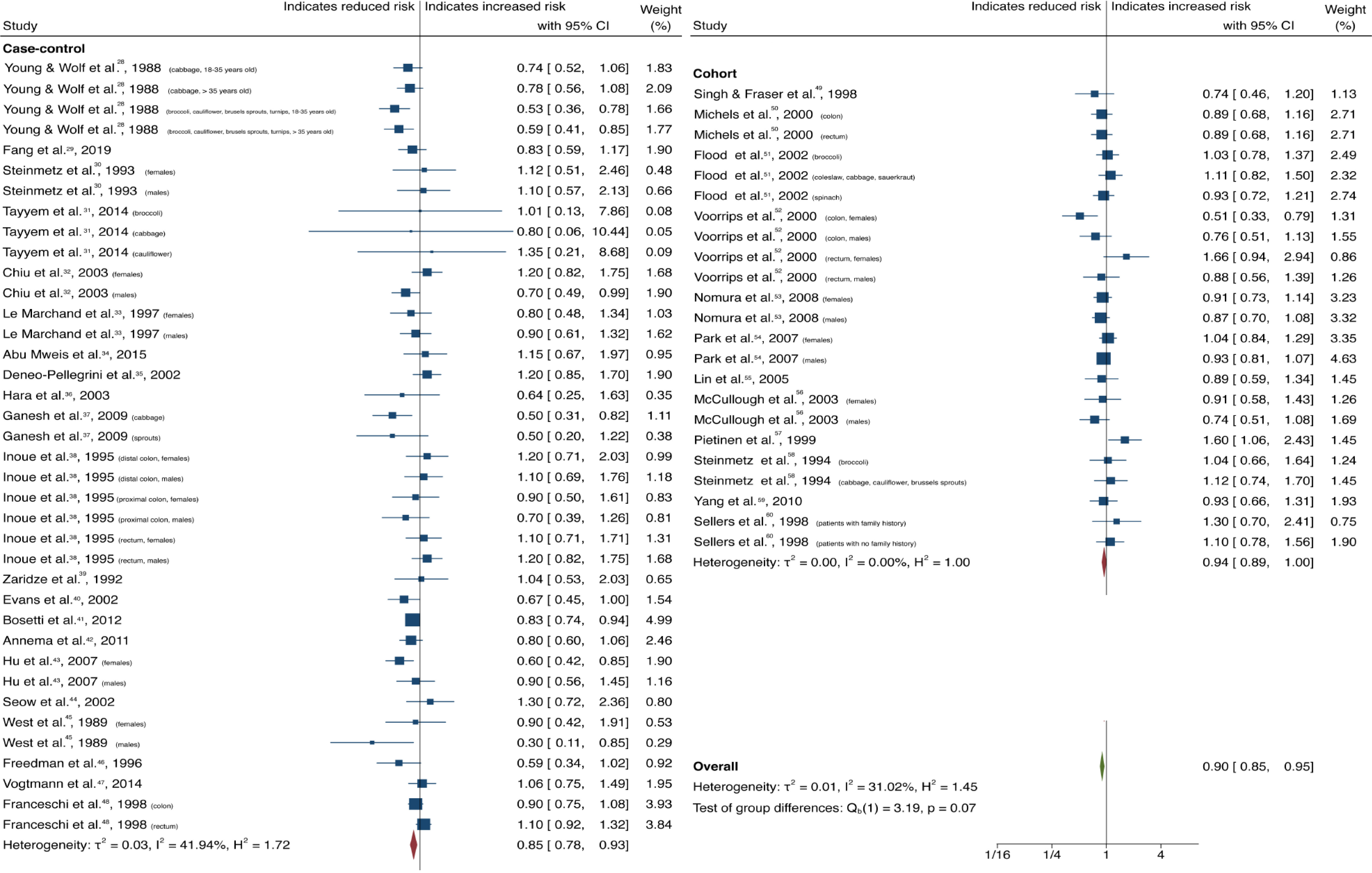
| Cruciferous vegetables | All | 61 (33) | 0.90 (0.85-0.95) | 0.00 | 31.02 | 0.58 |
| Case-control | 38 (21) | 0.85 (0.78-0.93) | 0.00 | 41.94 | ||
| Cohort | 23 (12) | 0.94 (0.89-1.00) | 0.07 | 0.00 | ||
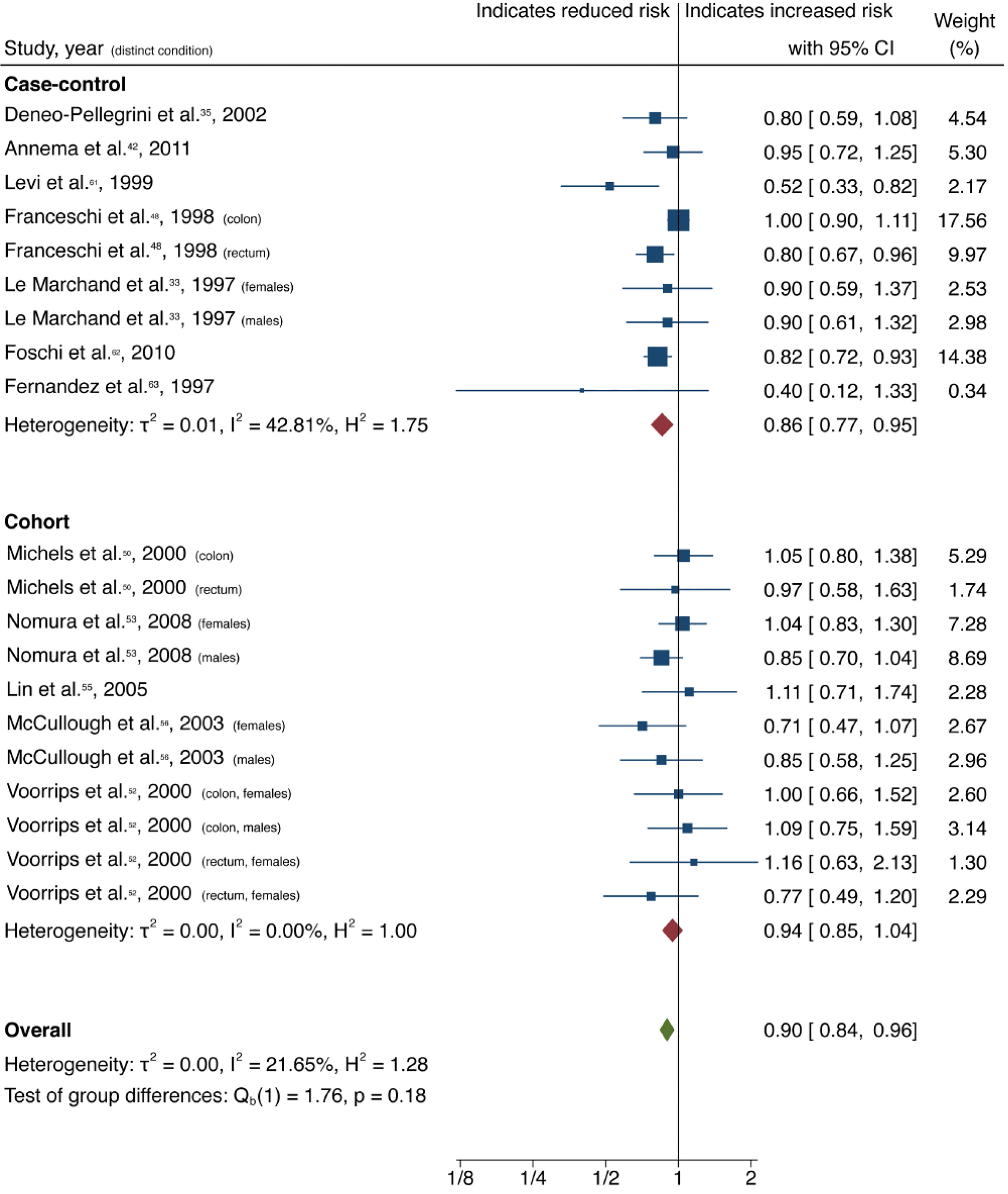
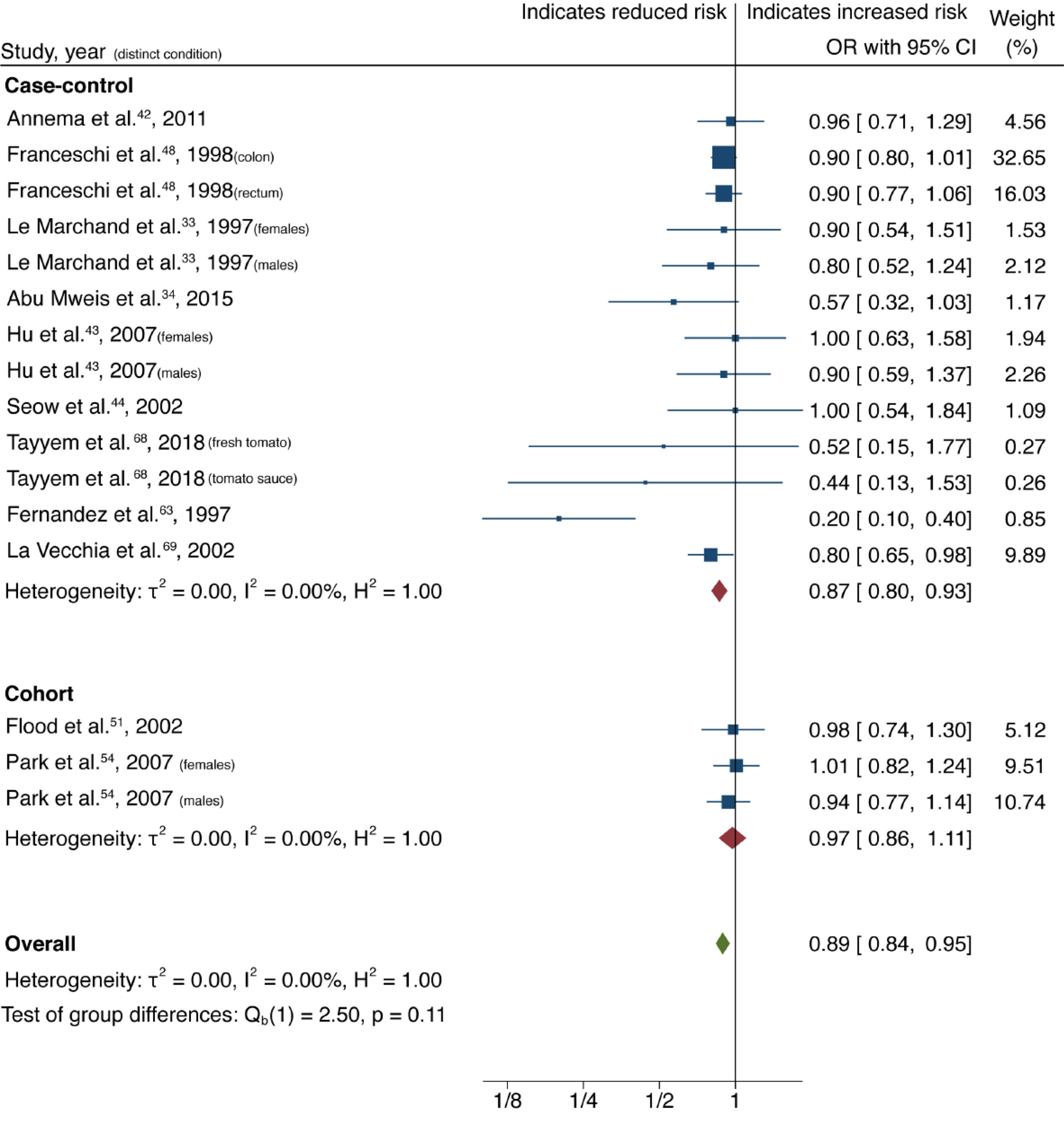
| Citrus fruits | All | 20 (12) | 0.90 (0.84-0.96) | 0.00 | 21.65 | 0.46 |
| Case-control | 9 (7) | 0.94 (0.85-1.04) | 0.00 | 42.81 | ||
| Cohort | 11 (5) | 0.86 (0.77-0.95) | 0.26 | 0.00 | ||
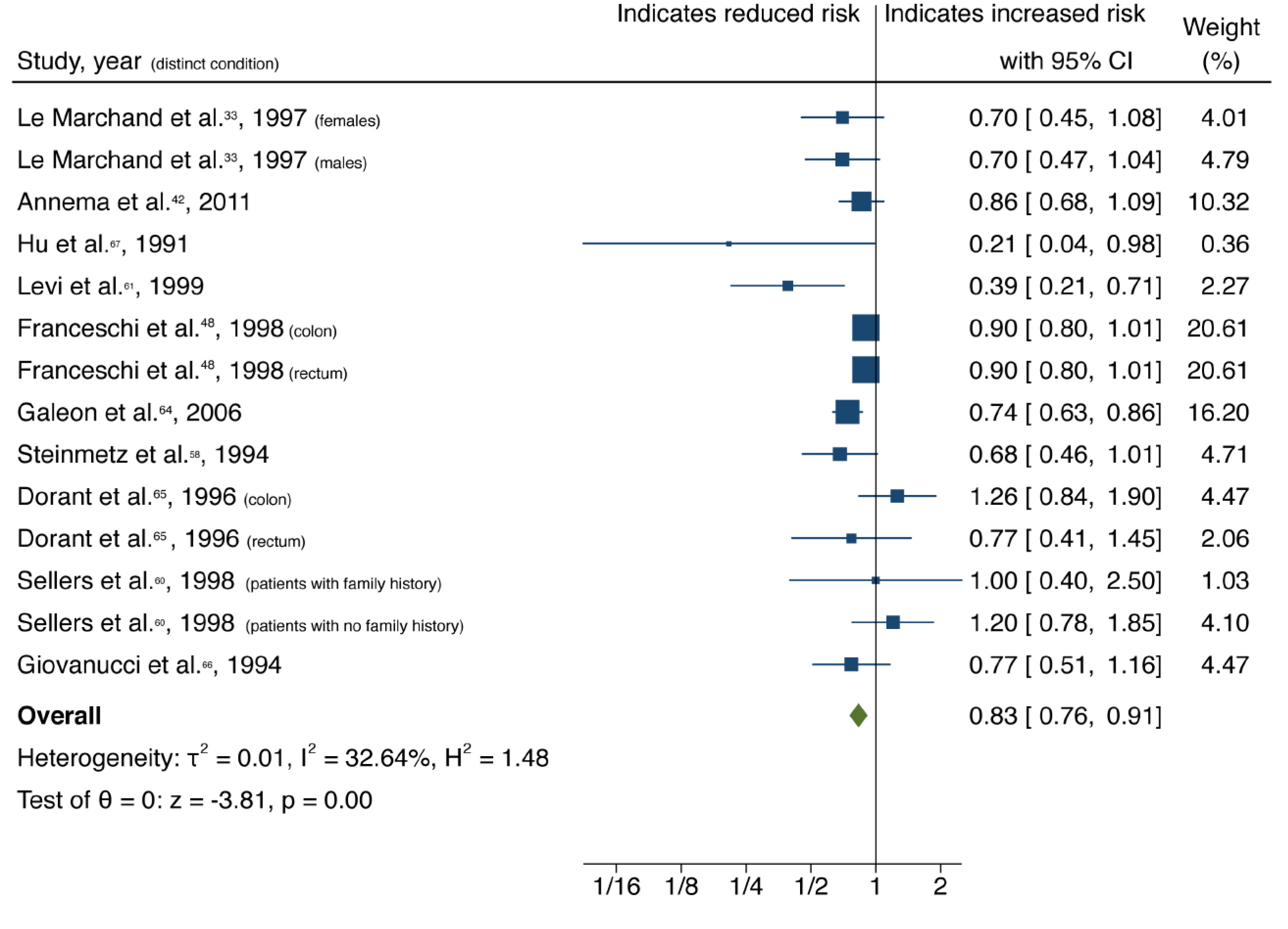
| Garlic | All | 14 (10) | 0.83 (0.76-0.91) | 0.00 | 32.64 | 0.12 |
| Case-control | 8 (6) | 0.92 (0.72-1.18) | 0.00 | 47.80 | ||
| Cohort | 6 (4) | 0.81 (0.72-0.90) | 0.51 | 35.38 | ||
| Tomatoes | All | 16 (11) | 0.89 (0.84-0.95) | 0.00 | 0.00 | 0.02 |
| Case-control | 13 (9) | 0.97 (0.84-0.95) | 0.00 | 0.00 | ||
| Cohort | 3 (2) | 0.87 (0.8-0.93) | 0.68 | 0.00 | ||
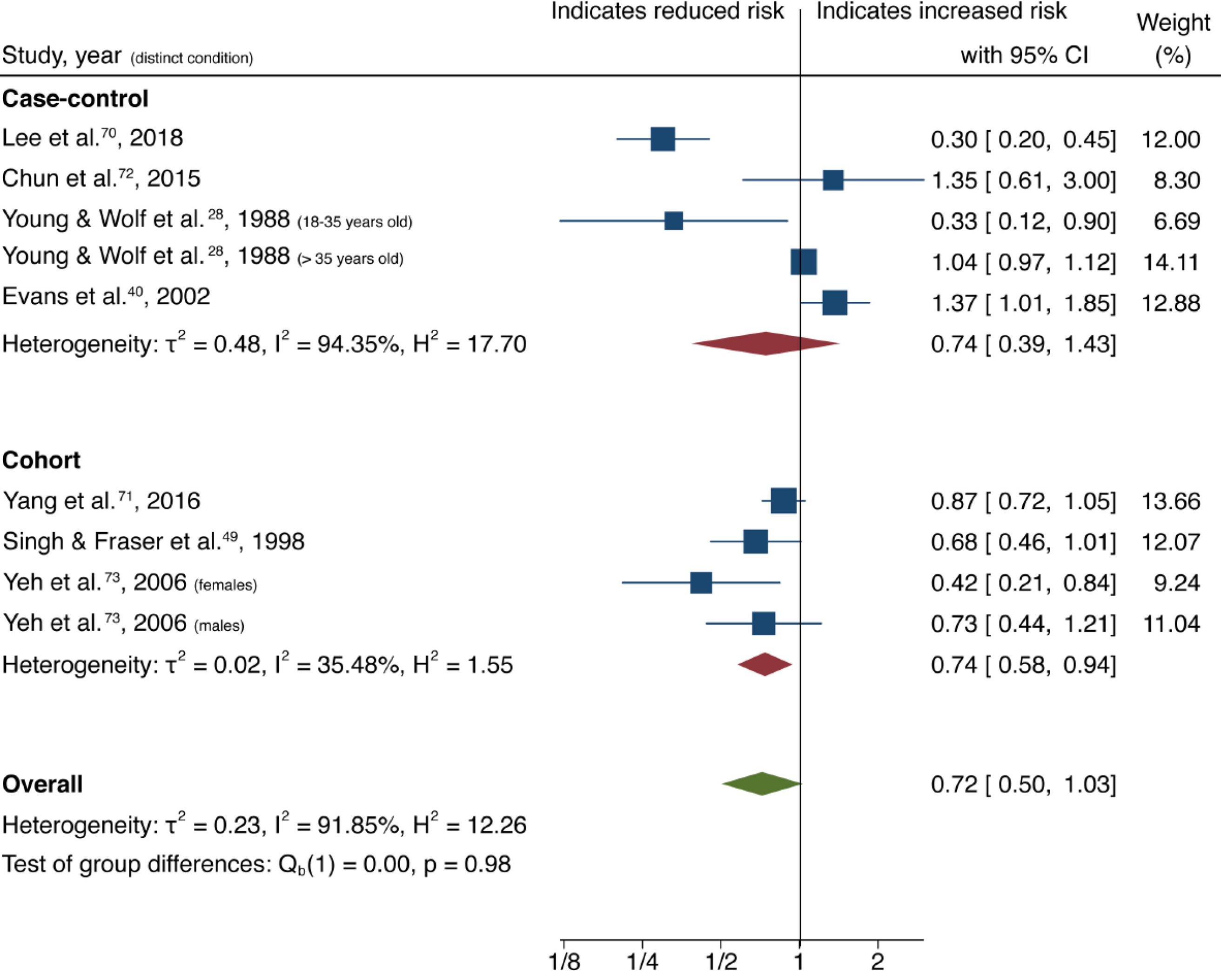
| Nuts | All | 9 (7) | 0.72 (0.50-1.03) | 0.07 | 91.85 | 0.22 |
| Case-control | 5 (4) | 0.74 (0.39-1.43) | 0.38 | 94.35 | ||
| Cohort | 4 (3) | 0.74 (0.58-0.94) | 0.01 | 35.48 | ||
Cruciferous vegetable intake was associated with a 10% reduction in CRC risk, with low heterogeneity and no evidence of small-study effect (61 studies, 33 publications[28-60]; OR = 0.90; 95%CI: 0.85-0.95; P = 0.00; I2= 31.02%, Egger P = 0.57) (Figure 2). Dietary intake of citrus fruits was also associated with a 10% risk reduction in CRC incidence, with low heterogeneity and no evidence of small-study effect (20 studies, 12 publications[33,35,42,48,50,52,53,55,56,61-63]; OR = 0.90; 95%CI: 0.84-0.96; P = 0.00; I2= 21.65%, Egger P = 0.46) (Figure 3). Higher garlic intake was associated with a 17% reduction in risk of CRC with low heterogeneity and no evidence of small-study effect (14 studies, 10 publications[33,42,48,58,60,61,64-67]; OR = 0.83; 95%CI: 0.76-0.91; P = 0.00; I2= 32.64%, Egger P = 0.11) (Figure 4). Higher dietary tomato intake was associated with an 11% reduction in CRC risk, with low heterogeneity (16 studies, 11 publications[33,34,42-44,48,51,54,63,68,69]; OR = 0.89; 95%CI: 0.84-0.95; P = 0.00; I2= 0%, Egger P = 0.02) (Figure 5). However, the P value acquired with Egger’s regression indicates that we could not discard the presence of small-study effects.
The association of increased dietary intake of all four food items with reduced CRC risk, is maintained in subgroup analysis of case-control studies alone, however cohort studies alone show an association which does not reach significance for all four aforementioned food items (Table 1).
Overall analysis of both case-control and cohort studies investigating higher dietary nut consumption demonstrated no statistically significant association with reduced risk of CRC, and significant heterogeneity was observed between studies (9 studies, 7 publications[28,40,49,70-73]; OR = 0.72; 95%CI: 0.50-1.03; P = 0.07; I2= 91.85%, Egger P = 0.21) (Figure 6). However, subgroup analysis of cohort and case-control studies separately showed a significant association between nut consumption and reduced CRC risk for cohort studies with low heterogeneity (4 studies, 3 publications[49,71,73]; OR = 0.74; 95%CI: 0.58-0.94; P = 0.01; I2= 35.48%). Case-control studies showed no significant association with high heterogeneity (5 studies, 4 publications[28,40,70,72]; OR 0.74; 95%CI: 0.39-1.43; P = 0.38; I2= 94.35%).
Funnel plots of citrus fruits and cruciferous vegetables show a clear symmetrical distribution of studies, with both large and small-sized studies in the significant and non-significant regions. Symmetrical distribution is not evident in funnel plots generated for garlic, nuts and tomato. The P values for Egger’s test can be found in Table 1. As expected, tests of symmetry for cruciferous vegetable and citrus fruit studies demonstrated robust P values (0.58 and 0.46, respectively); garlic and nuts studies had more modest but still non-significant P values (0.12 and 0.22 respectively); the P value for tomato studies was 0.02. Funnel plots of all food items can be found in Supplementary Figures 1-5. These analyses suggest that there is no evidence of small study effects (publication bias) in studies evaluating cruciferous vegetables, citrus fruits, garlic, and nuts; whereas this possibility cannot be discarded for studies exploring tomato intake in relation to CRC incidence.
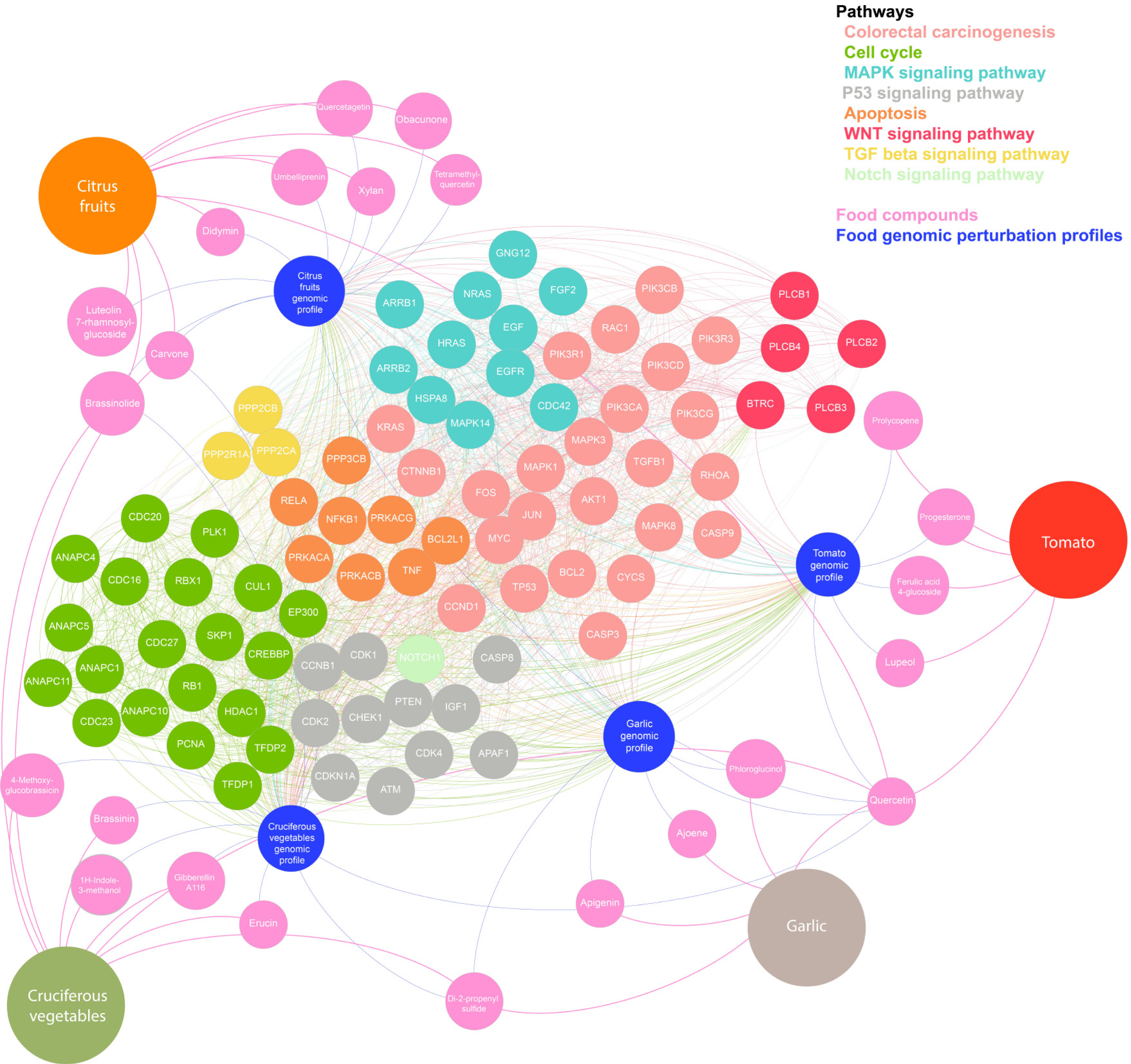
A list of the genes and proteins most affected by active compounds within each food item (Supplementary Tables 5 and 6) was subjected to pathway analytics using gene-set enrichment analysis (see Methods and Supplementary material, Appendix 2). The most affected genes and pathways are presented in Figure 7. The node colour repre
Dietary chemoprevention is emerging as a potentially cost effective and innovative way to prevent and treat cancer and has been highlighted as a key research priority by the NIH. The importance of this in implementing a holistic protective lifestyle is also highlighted in the Third Expert Report from the WCRF (World Cancer Research Fund)[14,16]. The findings of this meta-analysis indicate that higher dietary intake of the phytochemically rich cruciferous vegetables, citrus fruits, garlic and tomatoes is associated with a significant reduction in the risk of CRC, suggesting that promoting increased consumption of these foods should be considered as part of a holistic CRC protective diet. Meta-analysis of cohort studies investigating increased nut consumption showed the same significant association. Compared to existing systematic reviews and meta-analyses which have only explored the effect of single dietary components on CRC or have largely focused on dietary components associated with increased CRC risk[18-23], this is the first study to investigate the protective potential of these five highly investigated dietary components on CRC incidence. Additionally, to the authors’ knowledge, data on increased dietary intake of tomatoes and citrus fruits and CRC risk have not previously been subjected to meta-analytical evaluation.
Combined analysis of case-control and cohort studies showed significant results overall, however sub-group analyses revealed that the results are significant for case-control but not cohort studies. Although the Third Expert Report published by the WCRF has highlighted the importance of focusing evidence on cohort studies, there are a number of factors which make the evidence from cohort studies more difficult to interpret and subject to bias. Case-control studies remain a stronger source of evidence for diseases with long latency periods including CRC for which the incidence increases with age in both men and women. Thus, evidence from cohort studies is stronger if patients are closer to this age group and with longer follow-up period. However, many of the studies included patients as young as approximately 30 or 45 years old[49,50,53,55,59,66,71] and the average years of follow up of all cohort studies in this analysis was 9.4 years with certain studies having follow-up periods as short as 3-6 years[49,54,58,65,66]. Only 3 out of a total of 16 identified cohort studies followed patients up for 10 or more years[50,71,73]. This could lead to significant amounts of bias which is likely to be a major contributing factor as to why the association observed was not significant when analyzing cohort studies alone. For this reason, emphasis in this meta-analysis was not set solely on cohort studies alone but case-control studies were also viewed as highly informative and valuable in analysis despite the potential for biases related to retrospective assessment of diet in case-control studies. Hence, and in view of both types of epidemiological studies having the potential for different types of bias, it was thought to be most accurate to place most emphasis on the overall results in combination rather than focusing on the results of the sub-type analysis alone.
Despite an overall protective correlation having been observed for these foods, variability between outcomes of individual studies is evident, which may be attributable to a number of factors. Firstly, there is much variation in highest vs lower intake comparisons between studies with some not providing numerical data on intake servings, making a continuous dose-response analysis not feasible. Additionally, differences in geographical location may also explain variability in results between individual studies as cultural differences in dietary and lifestyle patterns as a whole may influence any protective effects observed. Although only studies which provided adjusted OR and RR data were included in analysis, there was a degree of variance in stratified analysis between studies and the extent to which confounders and other lifestyle factors were adjusted for between studies was inconsistent.
Significant divergences of individual studies from the mean may be attributable to study design and quality. For example, Levi et al[61], investigating citrus fruits and garlic, included much younger patients (from 27 years) compared to the majority of remaining studies which had an approximate age range between 50-80 years. Others, like Fernandez et al[63], investigating citrus fruits and tomatoes, did not adjust for as many significant variables like smoking, alcohol, total energy intake, education, consumption of other foods, as most other studies. These factors may have contributed to increased variability demonstrated in wide CIs and could explain the reason for the risk association to appear much stronger in these studies compared to the overall result. Conversely, some studies included a relatively low number of participants[31,36,39,44,63,72], participants selected from a defined population group attributing to selection bias[57], did not report details of their study design leading to low NOS[44] or studied specific individual components of the overall food items investigated here
Although the exact mechanisms for the associations reported here remain unclear, all four dietary components are rich in compounds believed to exert anticarcinogenic effect. To investigate these putative mechanisms further, we compiled a list of ‘anti
Garlic contains five anticarcinogenic compounds, belonging to organosulfur (ajoene, Di-2-propenyl sulphide), flavonoid (apigenin, quercetin) and phenol (phloroglucinol) categories. Several of the top 20 genes perturbed by these compounds take part in pathways related to inflammation, apoptosis, and angiogenesis. Furthermore, organosulfur and flavonoid compounds are thought to have chemoprotective properties[74,75] through a number of mechanisms including reduction of aberrant crypt formation[76], antioxidant effects, regulation of cell cycle arrest, induction of apoptosis, and activation of metabolizing enzymes which detoxify carcinogens and inhibit angiogenesis[77,78].
In addition to flavonoids (quercetin), tomatoes contain anticarcinogenic compounds including prenol lipids (prolycopene, lupeol), progesterone and carbohydrate (ferulic acid 4-glucoside). Citrus fruits are also rich in flavonoids (luteolin 7-rhamnosylglucoside, quercetin, tetramethylquercetin, didymin, quercetagetin) and prenol lipids (carvone, obacunon, umbelliprenin) and additionally contain steroids (brassinolide) and carbohydrates (xyan). The genes targeted by these anticarcinogenic compounds were also predominantly found to be involved in apoptosis, angiogenesis and inflammation. GSEA revealed interaction with MAPK and p53 signalling pathways, which are key drivers of CRC development and progression[79,80].
Cruciferous vegetables contain nine anticarcinogenic compounds including flavonoids (quercetin), and prenol lipids (carvone, gibberellin A116) but also isothiocyanates (erucin), indoles (1H-Indole-3-methanol, brassinin), allyl sulphur compounds (di-2-propenyl sulfide), organooxygen compounds (4-methoxyglucobrassicin) and steroids (brassinolide). Glucosinolates are precursors of isothiocyanates and indole-3-carbinol[81,82], which inhibit carcinogen-activating enzyme and angiogenesis, and detoxify carcinogens, inducing apoptosis and preventing cell cycle progression[44,83]. Animal studies have shown that cruciferous vegetables activate phase I (cytochrome P450) and II (glutathionin S-transferase) cell-cycle enzymes, and that this joint induction leads to a favourable metabolic profile, aiding in the elimination of chemical carcinogens by interacting with isothocyanate metabolism[44,59,81-83].
Nuts contain macro- and micro-nutrients including fibre, vitamins (BE, folate, niacin), minerals (zinc, potassium, calcium, magnesium) polyphenols, folate, phytoestrogens, phytochemicals (flavonoids, carotenoid and phytosteroils) and other bioactive substances. Additional anticarcinogenic compounds, as confirmed through our analysis, also include unsaturated fatty acids (dihydroxystearic acid), flavonoids (procyanidin B3 procyanidin B2, quercetin), garlic acid, naphthalene (plumbagin) prenol lipids (betulinic acid) and hydorcycoumarins (aesculetin), all of which harbour anti-inflammatory properties[84-86]. They display anticarcinogenic effect by reducing DNA damage and regulating immunological activity and inflammatory responses[84,87]. In vitro studies have demonstrated that fermented nuts exhibit chemopreventative effects by decreasing tumour-promoting deoxycholic acid, increasing short-chain fatty acids and protecting against oxidative stress[88]. Our results showed a significant association between higher dietary nut intake, and reduced CRC risk, supporting the findings of a previous meta-analysis[18]. However significant heterogeneity was observed meaning that a conclusion cannot reliably be reached based on currently available data.
In addition to these postulated mechanisms specific to each food items’ associated bioactive compounds, pathway enrichment analysis of genomic perturbation profiles of all food items showed an over-representation of several pathways known to be linked with CRC pathogenesis including the MAPK, EGFR, PI3K/AKT, TGF-b and Wnt signalling pathways[89]. This implies that the active compounds present in the five food items studied, interact with genes and pathways involved in the development of CRC. This in turn suggests that the protective influence of these dietary components, as demonstrated by this meta-analysis, may be directly attributable to novel nutrigenomic interactions.
The findings presented herein support the vision recently outlined in the NIH strategic plan, which highlights an urgent need to accelerate nutrition research over the next decade and demonstrate the potentially important role that precision nutrition can play in the prevention and treatment of CRC. These findings should form the foundation on which to design randomized clinical trials for the evaluation of food-based approaches in modifying CRC risk. In the future, we envisage that those at risk of CRC could have personalized ‘food passports’ developed by care providers, specifically composed of food plans to build dietary patterns designed to reduce their risk of cancer. Future research should focus on understanding the underlying mechanisms behind the observed effects and exploring the potential interactions between individual genes/pathways and active compounds of the investigated foods in more detail. Furthermore, the nature of the observed findings should be explored using linear and continuous exposure analyses to identify thresholds and plateaus in the protective effect and investigate dose-response effects of consumption of the foods evaluated. Lastly, the influence of food cultivation, packaging, storage and preparation methods should be explored, all of which may influence the associations observed.
This meta-analysis demonstrates that an increased consumption of cruciferous vegetables, citrus fruits, garlic and tomatoes is associated with a reduction in the incidence of CRC. These findings support the rapidly expanding initiative for exploring the role of nutrition in the prevention and treatment of cancer and could pave the way for personalized CRC protective diets in the future.
Personalized nutrition and protective diets and lifestyles represent a key cancer research priority according to The Third Expert Report from the World Cancer Research Fund (2018) and the recently published National Institute of Health strategic plan. The association between consumption of specific dietary components and colorectal cancer (CRC) incidence has been evaluated by a number of large-scale, population-based, epidemiological studies, which have identified certain food items as having protective potential, though the findings have been inconsistent.
Although previous meta-analyses have sought to evaluate the impact of some of these food items, results have been contradictory and inconclusive. To date there has been no comprehensive meta-analysis, including more recent multinational cohort studies, which unifies all five of these food items, assessing all available and recent evidence. Furthermore, anticancer properties could be explained by genomic effects of certain compounds contained in these phytochemically rich foods. This may help explain the underlying cause for any protective associations. Thus, we present a systematic review and meta-analysis on the potential protective role of five common phytochemically rich dietary components (nuts, cruciferous vegetables, citrus fruits, garlic and tomatoes) in reducing CRC risk. Putative anticarcinogenic mechanisms are highlighted through gene-set enrichment analysis.
To investigate the independent impact of increased intake of five dietary constituents (nuts, cruciferous vegetables, citrus fruits, garlic and tomatoes) on CRC risk in the general population.
Medline and Embase were systematically searched, from time of database inception to January 31, 2020, for observational studies reporting CRC incidence relative to intake of one or more of nuts, cruciferous vegetables, citrus fruits, garlic and/or tomatoes in the general population. Data were extracted by two independent reviewers and analyzed in accordance with the Meta-analysis of Observational Studies in Epidemiology (MOOSE) and Preferred Reporting Items for Systematic Review and Meta-analysis (PRISMA) reporting guidelines and according to predefined inclusion/exclusion criteria. Effect sizes of studies were pooled using a random-effects model. Using gene set enrichment analysis, a profile of genomic perturbations caused by each food item was built and biological pathways affected by these perturbations were identified.
This systematic review and meta-analysis of 46 observational studies found that increased dietary consumption of cruciferous vegetables, citrus fruits, garlic and tomatoes is associated with a significant reduction in the risk of developing CRC. Consumption of nuts exhibited a similar trend, but results were not significant. The underlying mechanisms of the observed effects remain unclear, although putative anticarcinogenic mechanisms are proposed using the results of gene-set enrichment analysis of gene-protein perturbations caused by active compounds within each food item.
Increased cruciferous vegetable, garlic, citrus fruit and tomato consumption are all inversely associated with CRC risk. These findings highlight the potential for precision nutrition strategies and development of personalized food maps in CRC prevention for both the general population and at-risk individuals.
The findings should stimulate future research to focus on understanding the nature of the observed effects, by exploring the epigenetic interactions of active compounds within the investigated foods. Additionally, linear and continuous exposure analyses should be explored to identify consumption thresholds for the observed effects. Results should then encourage the design of randomized controlled trials to assess food-based approached in modifying CRC risk.
Manuscript source: Invited manuscript
Specialty type: Oncology
Country/Territory of origin: United Kingdom
Peer-review report’s scientific quality classification
Grade A (Excellent): A
Grade B (Very good): 0
Grade C (Good): 0
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Swai JD S-Editor: Gao CC L-Editor: A P-Editor: Xing YX
| 1. | Bray F, Ferlay J, Soerjomataram I, Siegel RL, Torre LA, Jemal A. Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin. 2018;68:394-424. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 53206] [Cited by in RCA: 55814] [Article Influence: 7973.4] [Reference Citation Analysis (132)] |
| 2. | Global Burden of Disease Cancer Collaboration, Fitzmaurice C, Allen C, Barber RM, Barregard L, Bhutta ZA, Brenner H, Dicker DJ, Chimed-Orchir O, Dandona R, Dandona L, Fleming T, Forouzanfar MH, Hancock J, Hay RJ, Hunter-Merrill R, Huynh C, Hosgood HD, Johnson CO, Jonas JB, Khubchandani J, Kumar GA, Kutz M, Lan Q, Larson HJ, Liang X, Lim SS, Lopez AD, MacIntyre MF, Marczak L, Marquez N, Mokdad AH, Pinho C, Pourmalek F, Salomon JA, Sanabria JR, Sandar L, Sartorius B, Schwartz SM, Shackelford KA, Shibuya K, Stanaway J, Steiner C, Sun J, Takahashi K, Vollset SE, Vos T, Wagner JA, Wang H, Westerman R, Zeeb H, Zoeckler L, Abd-Allah F, Ahmed MB, Alabed S, Alam NK, Aldhahri SF, Alem G, Alemayohu MA, Ali R, Al-Raddadi R, Amare A, Amoako Y, Artaman A, Asayesh H, Atnafu N, Awasthi A, Saleem HB, Barac A, Bedi N, Bensenor I, Berhane A, Bernabé E, Betsu B, Binagwaho A, Boneya D, Campos-Nonato I, Castañeda-Orjuela C, Catalá-López F, Chiang P, Chibueze C, Chitheer A, Choi JY, Cowie B, Damtew S, das Neves J, Dey S, Dharmaratne S, Dhillon P, Ding E, Driscoll T, Ekwueme D, Endries AY, Farvid M, Farzadfar F, Fernandes J, Fischer F, G/Hiwot TT, Gebru A, Gopalani S, Hailu A, Horino M, Horita N, Husseini A, Huybrechts I, Inoue M, Islami F, Jakovljevic M, James S, Javanbakht M, Jee SH, Kasaeian A, Kedir MS, Khader YS, Khang YH, Kim D, Leigh J, Linn S, Lunevicius R, El Razek HMA, Malekzadeh R, Malta DC, Marcenes W, Markos D, Melaku YA, Meles KG, Mendoza W, Mengiste DT, Meretoja TJ, Miller TR, Mohammad KA, Mohammadi A, Mohammed S, Moradi-Lakeh M, Nagel G, Nand D, Le Nguyen Q, Nolte S, Ogbo FA, Oladimeji KE, Oren E, Pa M, Park EK, Pereira DM, Plass D, Qorbani M, Radfar A, Rafay A, Rahman M, Rana SM, Søreide K, Satpathy M, Sawhney M, Sepanlou SG, Shaikh MA, She J, Shiue I, Shore HR, Shrime MG, So S, Soneji S, Stathopoulou V, Stroumpoulis K, Sufiyan MB, Sykes BL, Tabarés-Seisdedos R, Tadese F, Tedla BA, Tessema GA, Thakur JS, Tran BX, Ukwaja KN, Uzochukwu BSC, Vlassov VV, Weiderpass E, Wubshet Terefe M, Yebyo HG, Yimam HH, Yonemoto N, Younis MZ, Yu C, Zaidi Z, Zaki MES, Zenebe ZM, Murray CJL, Naghavi M. Global, Regional, and National Cancer Incidence, Mortality, Years of Life Lost, Years Lived With Disability, and Disability-Adjusted Life-years for 32 Cancer Groups, 1990 to 2015: A Systematic Analysis for the Global Burden of Disease Study. JAMA Oncol. 2017;3:524-548. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2838] [Cited by in RCA: 2960] [Article Influence: 370.0] [Reference Citation Analysis (0)] |
| 3. | Marmot M, Atinmo T, Byers T, Chen J, Hirohata T, Jackson A, James WPT, Kolonel LN, Kumanyika S, Leitzmann C, Mann J, Hilary JP, Reddy KS, Riboli E, Rivera JA, Schatzkin A, Seidell JC, Shuker DE, Uauy R, Willett WC, Zeisel SH, Beaglehole R. Food, Nutrition, Physical Activity, and the Prevention of Cancer: A Global Perspective. Washington, DC: World Cancer Research Fund/American institute for Cancer Research, 2007. |
| 4. | Doubeni CA, Laiyemo AO, Major JM, Schootman M, Lian M, Park Y, Graubard BI, Hollenbeck AR, Sinha R. Socioeconomic status and the risk of colorectal cancer: an analysis of more than a half million adults in the National Institutes of Health-AARP Diet and Health Study. Cancer. 2012;118:3636-3644. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 143] [Cited by in RCA: 194] [Article Influence: 14.9] [Reference Citation Analysis (0)] |
| 5. | Arnold M, Sierra MS, Laversanne M, Soerjomataram I, Jemal A, Bray F. Global patterns and trends in colorectal cancer incidence and mortality. Gut. 2017;66:683-691. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3058] [Cited by in RCA: 3302] [Article Influence: 412.8] [Reference Citation Analysis (3)] |
| 6. | Willett WC. Diet and cancer: an evolving picture. JAMA. 2005;293:233-234. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 91] [Cited by in RCA: 97] [Article Influence: 4.9] [Reference Citation Analysis (0)] |
| 7. | Doubeni CA, Major JM, Laiyemo AO, Schootman M, Zauber AG, Hollenbeck AR, Sinha R, Allison J. Contribution of behavioral risk factors and obesity to socioeconomic differences in colorectal cancer incidence. J Natl Cancer Inst. 2012;104:1353-1362. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 125] [Cited by in RCA: 167] [Article Influence: 12.8] [Reference Citation Analysis (0)] |
| 8. | Reuter S, Gupta SC, Chaturvedi MM, Aggarwal BB. Oxidative stress, inflammation, and cancer: how are they linked? Free Radic Biol Med. 2010;49:1603-1616. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 4137] [Cited by in RCA: 3700] [Article Influence: 246.7] [Reference Citation Analysis (0)] |
| 9. | Terzić J, Grivennikov S, Karin E, Karin M. Inflammation and colon cancer. Gastroenterology. 2010;138:2101-2114.e5. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1333] [Cited by in RCA: 1508] [Article Influence: 100.5] [Reference Citation Analysis (0)] |
| 10. | Klampfer L. Cytokines, inflammation and colon cancer. Curr Cancer Drug Targets. 2011;11:451-464. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 191] [Cited by in RCA: 223] [Article Influence: 15.9] [Reference Citation Analysis (0)] |
| 11. | Tárraga López PJ, Albero JS, Rodríguez-Montes JA. Primary and secondary prevention of colorectal cancer. Clin Med Insights Gastroenterol. 2014;7:33-46. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 41] [Cited by in RCA: 81] [Article Influence: 7.4] [Reference Citation Analysis (0)] |
| 12. | Aune D, Chan DS, Lau R, Vieira R, Greenwood DC, Kampman E, Norat T. Dietary fibre, whole grains, and risk of colorectal cancer: systematic review and dose-response meta-analysis of prospective studies. BMJ. 2011;343:d6617. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 830] [Cited by in RCA: 721] [Article Influence: 51.5] [Reference Citation Analysis (0)] |
| 13. | Vieira AR, Abar L, Chan DSM, Vingeliene S, Polemiti E, Stevens C, Greenwood D, Norat T. Foods and beverages and colorectal cancer risk: a systematic review and meta-analysis of cohort studies, an update of the evidence of the WCRF-AICR Continuous Update Project. Ann Oncol. 2017;28:1788-1802. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 193] [Cited by in RCA: 288] [Article Influence: 41.1] [Reference Citation Analysis (0)] |
| 14. | World Cancer Research Fund International. Diet, Nutrition, Physical Activity and Cancer: a Global Perspective - The Third Expert Report. Third Expert Report ed. London, UK: World Cancer Research Fund International, 2018. |
| 15. | IARC Monographs on the Evaluation of Carcinogeni Risks To Humans. Red Meat And Processed Meat. Lyon, France: International Agency for Research on Cancer, 2018. |
| 16. | Collins FS, Rodgers GP. 2020-2030 Strategic Plan for NIH Nutrition Research. In: Force TNRT. National Institute of Health, 2020. |
| 17. | Veselkov K, Gonzalez G, Aljifri S, Galea D, Mirnezami R, Youssef J, Bronstein M, Laponogov I. HyperFoods: Machine intelligent mapping of cancer-beating molecules in foods. Sci Rep. 2019;9:9237. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 25] [Cited by in RCA: 36] [Article Influence: 6.0] [Reference Citation Analysis (0)] |
| 18. | Wu L, Wang Z, Zhu J, Murad AL, Prokop LJ, Murad MH. Nut consumption and risk of cancer and type 2 diabetes: a systematic review and meta-analysis. Nutr Rev. 2015;73:409-425. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 73] [Cited by in RCA: 83] [Article Influence: 9.2] [Reference Citation Analysis (0)] |
| 19. | Schwingshackl L, Schwedhelm C, Hoffmann G, Knüppel S, Laure Preterre A, Iqbal K, Bechthold A, De Henauw S, Michels N, Devleesschauwer B, Boeing H, Schlesinger S. Food groups and risk of colorectal cancer. Int J Cancer. 2018;142:1748-1758. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 130] [Cited by in RCA: 209] [Article Influence: 26.1] [Reference Citation Analysis (1)] |
| 20. | Verhoeven DT, Goldbohm RA, van Poppel G, Verhagen H, van den Brandt PA. Epidemiological studies on brassica vegetables and cancer risk. Cancer Epidemiol Biomarkers Prev. 1996;5:733-748. [PubMed] |
| 21. | Wu QJ, Yang Y, Vogtmann E, Wang J, Han LH, Li HL, Xiang YB. Cruciferous vegetables intake and the risk of colorectal cancer: a meta-analysis of observational studies. Ann Oncol. 2013;24:1079-1087. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 154] [Cited by in RCA: 164] [Article Influence: 12.6] [Reference Citation Analysis (0)] |
| 22. | Fleischauer AT, Poole C, Arab L. Garlic consumption and cancer prevention: meta-analyses of colorectal and stomach cancers. Am J Clin Nutr. 2000;72:1047-1052. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 154] [Cited by in RCA: 129] [Article Influence: 5.2] [Reference Citation Analysis (0)] |
| 23. | Chiavarini M, Minelli L, Fabiani R. Garlic consumption and colorectal cancer risk in man: a systematic review and meta-analysis. Public Health Nutr. 2016;19:308-317. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 29] [Cited by in RCA: 24] [Article Influence: 2.7] [Reference Citation Analysis (0)] |
| 24. | Stroup DF, Berlin JA, Morton SC, Olkin I, Williamson GD, Rennie D, Moher D, Becker BJ, Sipe TA, Thacker SB. Meta-analysis of observational studies in epidemiology: a proposal for reporting. Meta-analysis Of Observational Studies in Epidemiology (MOOSE) group. JAMA. 2000;283:2008-2012. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 14425] [Cited by in RCA: 16793] [Article Influence: 671.7] [Reference Citation Analysis (0)] |
| 25. | Moher D, Liberati A, Tetzlaff J, Altman DG; PRISMA Group. Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. BMJ. 2009;339:b2535. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 18665] [Cited by in RCA: 17528] [Article Influence: 1095.5] [Reference Citation Analysis (1)] |
| 26. | Wells G, Shea B, O'Connell D, Peterson J, Welch V, Losos M, Tugwell P. The Newcastle-Ottawa Scale (NOS) for assessing the quality of nonrandomised studies in meta-analyses. Ottawa Hosp Res Instit. 2011;. |
| 27. | Thomas PD, Campbell MJ, Kejariwal A, Mi H, Karlak B, Daverman R, Diemer K, Muruganujan A, Narechania A. PANTHER: a library of protein families and subfamilies indexed by function. Genome Res. 2003;13:2129-2141. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2307] [Cited by in RCA: 2302] [Article Influence: 104.6] [Reference Citation Analysis (0)] |
| 28. | Young TB, Wolf DA. Case-control study of proximal and distal colon cancer and diet in Wisconsin. Int J Cancer. 1988;42:167-175. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 167] [Cited by in RCA: 147] [Article Influence: 4.0] [Reference Citation Analysis (0)] |
| 29. | Fang W, Qu X, Shi J, Li H, Guo X, Wu X, Liu Y, Li Z. Cruciferous vegetables and colorectal cancer risk: a hospital-based matched case-control study in Northeast China. Eur J Clin Nutr. 2019;73:450-457. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4] [Cited by in RCA: 4] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 30. | Steinmetz KA, Potter JD. Food-group consumption and colon cancer in the Adelaide Case-Control Study. I. Vegetables and fruit. Int J Cancer. 1993;53:711-719. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 87] [Cited by in RCA: 80] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 31. | Tayyem RF, Shehadah I, Abu-Mweis SS, Bawadi HA, Bani-Hani KE, Al-Jaberi T, Al-Nusairr M, Heath DD. Fruit and vegetable intake among Jordanians: results from a case-control study of colorectal cancer. Cancer Control. 2014;21:350-360. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 14] [Cited by in RCA: 15] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 32. | Chiu BC, Ji BT, Dai Q, Gridley G, McLaughlin JK, Gao YT, Fraumeni JF Jr, Chow WH. Dietary factors and risk of colon cancer in Shanghai, China. Cancer Epidemiol Biomarkers Prev. 2003;12:201-208. [PubMed] |
| 33. | Le Marchand L, Hankin JH, Wilkens LR, Kolonel LN, Englyst HN, Lyu LC. Dietary fiber and colorectal cancer risk. Epidemiology. 1997;8:658-665. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 26] [Cited by in RCA: 37] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 34. | Abu Mweis SS, Tayyem RF, Shehadah I, Bawadi HA, Agraib LM, Bani-Hani KE, Al-Jaberi T, Al-Nusairr M. Food groups and the risk of colorectal cancer: results from a Jordanian case-control study. Eur J Cancer Prev. 2015;24:313-320. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 9] [Cited by in RCA: 17] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 35. | Deneo-Pellegrini H, Boffetta P, De Stefani E, Ronco A, Brennan P, Mendilaharsu M. Plant foods and differences between colon and rectal cancers. Eur J Cancer Prev. 2002;11:369-375. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 30] [Cited by in RCA: 31] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 36. | Hara M, Hanaoka T, Kobayashi M, Otani T, Adachi HY, Montani A, Natsukawa S, Shaura K, Koizumi Y, Kasuga Y, Matsuzawa T, Ikekawa T, Sasaki S, Tsugane S. Cruciferous vegetables, mushrooms, and gastrointestinal cancer risks in a multicenter, hospital-based case-control study in Japan. Nutr Cancer. 2003;46:138-147. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 72] [Cited by in RCA: 68] [Article Influence: 3.2] [Reference Citation Analysis (0)] |
| 37. | Ganesh B, Talole SD, Dikshit R. A case-control study on diet and colorectal cancer from Mumbai, India. Cancer Epidemiol. 2009;33:189-193. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 16] [Cited by in RCA: 29] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 38. | Inoue M, Tajima K, Hirose K, Hamajima N, Takezaki T, Hirai T, Kato T, Ohno Y. Subsite-specific risk factors for colorectal cancer: a hospital-based case-control study in Japan. Cancer Causes Control. 1995;6:14-22. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 78] [Cited by in RCA: 79] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 39. | Zaridze D, Filipchenko V, Kustov V, Serdyuk V, Duffy S. Diet and colorectal cancer: results of two case-control studies in Russia. Eur J Cancer. 1992;29A:112-115. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 43] [Cited by in RCA: 41] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 40. | Evans RC, Fear S, Ashby D, Hackett A, Williams E, Van Der Vliet M, Dunstan FD, Rhodes JM. Diet and colorectal cancer: an investigation of the lectin/galactose hypothesis. Gastroenterology. 2002;122:1784-1792. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 40] [Cited by in RCA: 46] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 41. | Bosetti C, Filomeno M, Riso P, Polesel J, Levi F, Talamini R, Montella M, Negri E, Franceschi S, La Vecchia C. Cruciferous vegetables and cancer risk in a network of case-control studies. Ann Oncol. 2012;23:2198-2203. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 84] [Cited by in RCA: 71] [Article Influence: 5.5] [Reference Citation Analysis (0)] |
| 42. | Annema N, Heyworth JS, McNaughton SA, Iacopetta B, Fritschi L. Fruit and vegetable consumption and the risk of proximal colon, distal colon, and rectal cancers in a case-control study in Western Australia. J Am Diet Assoc. 2011;111:1479-1490. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 44] [Cited by in RCA: 47] [Article Influence: 3.4] [Reference Citation Analysis (0)] |
| 43. | Hu J, Mery L, Desmeules M, Macleod M; Canadian Cancer Registries Epidemiology Research Group. Diet and vitamin or mineral supplementation and risk of rectal cancer in Canada. Acta Oncol. 2007;46:342-354. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 11] [Cited by in RCA: 8] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 44. | Seow A, Quah SR, Nyam D, Straughan PT, Chua T, Aw TC. Food groups and the risk of colorectal carcinoma in an Asian population. Cancer. 2002;95:2390-2396. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 41] [Cited by in RCA: 43] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 45. | West DW, Slattery ML, Robison LM, Schuman KL, Ford MH, Mahoney AW, Lyon JL, Sorensen AW. Dietary intake and colon cancer: sex- and anatomic site-specific associations. Am J Epidemiol. 1989;130:883-894. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 135] [Cited by in RCA: 129] [Article Influence: 3.6] [Reference Citation Analysis (0)] |
| 46. | Freedman AN, Michalek AM, Marshall JR, Mettlin CJ, Petrelli NJ, Black JD, Zhang ZF, Satchidanand S, Asirwatham JE. Familial and nutritional risk factors for p53 overexpression in colorectal cancer. Cancer Epidemiol Biomarkers Prev. 1996;5:285-291. [PubMed] |
| 47. | Vogtmann E, Xiang YB, Li HL, Cai Q, Wu QJ, Xie L, Li GL, Yang G, Waterbor JW, Levitan EB, Zhang B, Zheng W, Shu XO. Cruciferous vegetables, glutathione S-transferase polymorphisms, and the risk of colorectal cancer among Chinese men. Ann Epidemiol. 2014;24:44-49. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 16] [Cited by in RCA: 13] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 48. | Franceschi S, Parpinel M, La Vecchia C, Favero A, Talamini R, Negri E. Role of different types of vegetables and fruit in the prevention of cancer of the colon, rectum, and breast. Epidemiology. 1998;9:338-341. [PubMed] |
| 49. | Singh PN, Fraser GE. Dietary risk factors for colon cancer in a low-risk population. Am J Epidemiol. 1998;148:761-774. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 203] [Cited by in RCA: 195] [Article Influence: 7.2] [Reference Citation Analysis (0)] |
| 50. | Michels KB, Edward Giovannucci, Joshipura KJ, Rosner BA, Stampfer MJ, Fuchs CS, Colditz GA, Speizer FE, Willett WC. Prospective study of fruit and vegetable consumption and incidence of colon and rectal cancers. J Natl Cancer Inst. 2000;92:1740-1752. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 305] [Cited by in RCA: 260] [Article Influence: 10.4] [Reference Citation Analysis (0)] |
| 51. | Flood A, Velie EM, Chaterjee N, Subar AF, Thompson FE, Lacey JV Jr, Schairer C, Troisi R, Schatzkin A. Fruit and vegetable intakes and the risk of colorectal cancer in the Breast Cancer Detection Demonstration Project follow-up cohort. Am J Clin Nutr. 2002;75:936-943. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 96] [Cited by in RCA: 92] [Article Influence: 4.0] [Reference Citation Analysis (0)] |
| 52. | Voorrips LE, Goldbohm RA, van Poppel G, Sturmans F, Hermus RJ, van den Brandt PA. Vegetable and fruit consumption and risks of colon and rectal cancer in a prospective cohort study: The Netherlands Cohort Study on Diet and Cancer. Am J Epidemiol. 2000;152:1081-1092. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 216] [Cited by in RCA: 197] [Article Influence: 7.9] [Reference Citation Analysis (0)] |
| 53. | Nomura AM, Wilkens LR, Murphy SP, Hankin JH, Henderson BE, Pike MC, Kolonel LN. Association of vegetable, fruit, and grain intakes with colorectal cancer: the Multiethnic Cohort Study. Am J Clin Nutr. 2008;88:730-737. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 55] [Cited by in RCA: 52] [Article Influence: 3.1] [Reference Citation Analysis (0)] |
| 54. | Park Y, Subar AF, Kipnis V, Thompson FE, Mouw T, Hollenbeck A, Leitzmann MF, Schatzkin A. Fruit and vegetable intakes and risk of colorectal cancer in the NIH-AARP diet and health study. Am J Epidemiol. 2007;166:170-180. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 59] [Cited by in RCA: 54] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 55. | Lin J, Zhang SM, Cook NR, Rexrode KM, Liu S, Manson JE, Lee IM, Buring JE. Dietary intakes of fruit, vegetables, and fiber, and risk of colorectal cancer in a prospective cohort of women (United States). Cancer Causes Control. 2005;16:225-233. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 85] [Cited by in RCA: 80] [Article Influence: 4.0] [Reference Citation Analysis (0)] |
| 56. | McCullough ML, Robertson AS, Chao A, Jacobs EJ, Stampfer MJ, Jacobs DR, Diver WR, Calle EE, Thun MJ. A prospective study of whole grains, fruits, vegetables and colon cancer risk. Cancer Causes Control. 2003;14:959-970. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 120] [Cited by in RCA: 98] [Article Influence: 4.7] [Reference Citation Analysis (0)] |
| 57. | Pietinen P, Malila N, Virtanen M, Hartman TJ, Tangrea JA, Albanes D, Virtamo J. Diet and risk of colorectal cancer in a cohort of Finnish men. Cancer Causes Control. 1999;10:387-396. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 240] [Cited by in RCA: 224] [Article Influence: 8.6] [Reference Citation Analysis (0)] |
| 58. | Steinmetz KA, Kushi LH, Bostick RM, Folsom AR, Potter JD. Vegetables, fruit, and colon cancer in the Iowa Women's Health Study. Am J Epidemiol. 1994;139:1-15. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 309] [Cited by in RCA: 255] [Article Influence: 8.2] [Reference Citation Analysis (0)] |
| 59. | Yang G, Gao YT, Shu XO, Cai Q, Li GL, Li HL, Ji BT, Rothman N, Dyba M, Xiang YB, Chung FL, Chow WH, Zheng W. Isothiocyanate exposure, glutathione S-transferase polymorphisms, and colorectal cancer risk. Am J Clin Nutr. 2010;91:704-711. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 47] [Cited by in RCA: 47] [Article Influence: 3.1] [Reference Citation Analysis (0)] |
| 60. | Sellers TA, Bazyk AE, Bostick RM, Kushi LH, Olson JE, Anderson KE, Lazovich D, Folsom AR. Diet and risk of colon cancer in a large prospective study of older women: an analysis stratified on family history (Iowa, United States). Cancer Causes Control. 1998;9:357-367. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 91] [Cited by in RCA: 90] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 61. | Levi F, Pasche C, La Vecchia C, Lucchini F, Franceschi S. Food groups and colorectal cancer risk. Br J Cancer. 1999;79:1283-1287. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 154] [Cited by in RCA: 138] [Article Influence: 5.3] [Reference Citation Analysis (0)] |
| 62. | Foschi R, Pelucchi C, Dal Maso L, Rossi M, Levi F, Talamini R, Bosetti C, Negri E, Serraino D, Giacosa A, Franceschi S, La Vecchia C. Citrus fruit and cancer risk in a network of case-control studies. Cancer Causes Control. 2010;21:237-242. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 35] [Cited by in RCA: 30] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 63. | Fernandez E, La Vecchia C, D'Avanzo B, Negri E, Franceschi S. Risk factors for colorectal cancer in subjects with family history of the disease. Br J Cancer. 1997;75:1381-1384. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 20] [Cited by in RCA: 18] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 64. | Galeone C, Pelucchi C, Levi F, Negri E, Franceschi S, Talamini R, Giacosa A, La Vecchia C. Onion and garlic use and human cancer. Am J Clin Nutr. 2006;84:1027-1032. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 155] [Cited by in RCA: 129] [Article Influence: 6.8] [Reference Citation Analysis (0)] |
| 65. | Dorant E, van den Brandt PA, Goldbohm RA. A prospective cohort study on the relationship between onion and leek consumption, garlic supplement use and the risk of colorectal carcinoma in The Netherlands. Carcinogenesis. 1996;17:477-484. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 74] [Cited by in RCA: 61] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 66. | Giovannucci E, Rimm EB, Stampfer MJ, Colditz GA, Ascherio A, Willett WC. Intake of fat, meat, and fiber in relation to risk of colon cancer in men. Cancer Res. 1994;54:2390-2397. [PubMed] |
| 67. | Hu JF, Liu YY, Yu YK, Zhao TZ, Liu SD, Wang QQ. Diet and cancer of the colon and rectum: a case-control study in China. Int J Epidemiol. 1991;20:362-367. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 111] [Cited by in RCA: 93] [Article Influence: 2.7] [Reference Citation Analysis (0)] |
| 68. | Tayyem RF, Bawadi HA, Shehadah I, Bani-Hani KE, Takruri H, Al-Jaberi T, Heath DD. Fast Foods, Sweets and Beverage Consumption and Risk of Colorectal Cancer: A Case-Control Study in Jordan. Asian Pac J Cancer Prev. 2018;19:261-269. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 10] [Reference Citation Analysis (0)] |
| 69. | La Vecchia C. Tomatoes, lycopene intake, and digestive tract and female hormone-related neoplasms. Exp Biol Med (Maywood). 2002;227:860-863. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 47] [Cited by in RCA: 40] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 70. | Lee J, Shin A, Oh JH, Kim J. The relationship between nut intake and risk of colorectal cancer: a case control study. Nutr J. 2018;17:37. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 28] [Cited by in RCA: 21] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 71. | Yang M, Hu FB, Giovannucci EL, Stampfer MJ, Willett WC, Fuchs CS, Wu K, Bao Y. Nut consumption and risk of colorectal cancer in women. Eur J Clin Nutr. 2016;70:333-337. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 24] [Cited by in RCA: 24] [Article Influence: 2.7] [Reference Citation Analysis (0)] |
| 72. | Chun YJ, Sohn SK, Song HK, Lee SM, Youn YH, Lee S, Park H. Associations of colorectal cancer incidence with nutrient and food group intakes in korean adults: a case-control study. Clin Nutr Res. 2015;4:110-123. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 16] [Cited by in RCA: 27] [Article Influence: 2.7] [Reference Citation Analysis (0)] |
| 73. | Yeh CC, You SL, Chen CJ, Sung FC. Peanut consumption and reduced risk of colorectal cancer in women: a prospective study in Taiwan. World J Gastroenterol. 2006;12:222-227. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 49] [Cited by in RCA: 47] [Article Influence: 2.5] [Reference Citation Analysis (1)] |
| 74. | Iciek M, Kwiecień I, Włodek L. Biological properties of garlic and garlic-derived organosulfur compounds. Environ Mol Mutagen. 2009;50:247-265. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 292] [Cited by in RCA: 267] [Article Influence: 16.7] [Reference Citation Analysis (0)] |
| 75. | El-Bayoumy K, Sinha R, Pinto JT, Rivlin RS. Cancer chemoprevention by garlic and garlic-containing sulfur and selenium compounds. J Nutr. 2006;136:864S-869S. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 97] [Cited by in RCA: 85] [Article Influence: 4.5] [Reference Citation Analysis (0)] |
| 76. | Ross SA, Finley JW, Milner JA. Allyl sulfur compounds from garlic modulate aberrant crypt formation. J Nutr. 2006;136:852S-854S. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 25] [Cited by in RCA: 19] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 77. | Powolny AA, Singh SV. Multitargeted prevention and therapy of cancer by diallyl trisulfide and related Allium vegetable-derived organosulfur compounds. Cancer Lett. 2008;269:305-314. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 212] [Cited by in RCA: 178] [Article Influence: 10.5] [Reference Citation Analysis (0)] |
| 78. | Bayan L, Koulivand PH, Gorji A. Garlic: a review of potential therapeutic effects. Avicenna J Phytomed. 2014;4:1-14. [PubMed] |
| 79. | Tanaka T, Kohno H, Tsukio Y, Honjo S, Tanino M, Miyake M, Wada K. Citrus limonoids obacunone and limonin inhibit azoxymethane-induced colon carcinogenesis in rats. Biofactors. 2000;13:213-218. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 47] [Cited by in RCA: 44] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 80. | Manthey JA, Guthrie N. Antiproliferative activities of citrus flavonoids against six human cancer cell lines. J Agric Food Chem. 2002;50:5837-5843. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 259] [Cited by in RCA: 247] [Article Influence: 10.7] [Reference Citation Analysis (0)] |
| 81. | Hecht SS. Inhibition of carcinogenesis by isothiocyanates. Drug Metab Rev. 2000;32:395-411. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 341] [Cited by in RCA: 302] [Article Influence: 12.1] [Reference Citation Analysis (0)] |
| 82. | Conaway CC, Yang YM, Chung FL. Isothiocyanates as cancer chemopreventive agents: their biological activities and metabolism in rodents and humans. Curr Drug Metab. 2002;3:233-255. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 314] [Cited by in RCA: 285] [Article Influence: 12.4] [Reference Citation Analysis (0)] |
| 83. | Zhang Y, Yao S, Li J. Vegetable-derived isothiocyanates: anti-proliferative activity and mechanism of action. Proc Nutr Soc. 2006;65:68-75. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 77] [Cited by in RCA: 69] [Article Influence: 3.6] [Reference Citation Analysis (0)] |
| 84. | González CA, Salas-Salvadó J. The potential of nuts in the prevention of cancer. Br J Nutr. 2006;96 Suppl 2:S87-S94. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 97] [Cited by in RCA: 82] [Article Influence: 4.3] [Reference Citation Analysis (0)] |
| 85. | Bolling BW, Chen CY, McKay DL, Blumberg JB. Tree nut phytochemicals: composition, antioxidant capacity, bioactivity, impact factors. A systematic review of almonds, Brazils, cashews, hazelnuts, macadamias, pecans, pine nuts, pistachios and walnuts. Nutr Res Rev. 2011;24:244-275. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 336] [Cited by in RCA: 255] [Article Influence: 18.2] [Reference Citation Analysis (0)] |
| 86. | Alasalvar C, Bolling BW. Review of nut phytochemicals, fat-soluble bioactives, antioxidant components and health effects. Br J Nutr. 2015;113 Suppl 2:S68-S78. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 222] [Cited by in RCA: 232] [Article Influence: 23.2] [Reference Citation Analysis (0)] |
| 87. | Kris-Etherton PM, Hecker KD, Bonanome A, Coval SM, Binkoski AE, Hilpert KF, Griel AE, Etherton TD. Bioactive compounds in foods: their role in the prevention of cardiovascular disease and cancer. Am J Med. 2002;113 Suppl 9B:71S-88S. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1508] [Cited by in RCA: 1204] [Article Influence: 52.3] [Reference Citation Analysis (0)] |
| 88. | Lux S, Scharlau D, Schlörmann W, Birringer M, Glei M. In vitro fermented nuts exhibit chemopreventive effects in HT29 colon cancer cells. Br J Nutr. 2012;108:1177-1186. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 23] [Cited by in RCA: 24] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 89. | Koveitypour Z, Panahi F, Vakilian M, Peymani M, Seyed Forootan F, Nasr Esfahani MH, Ghaedi K. Signaling pathways involved in colorectal cancer progression. Cell Biosci. 2019;9:97. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 122] [Cited by in RCA: 255] [Article Influence: 42.5] [Reference Citation Analysis (0)] |
| 90. | Cowen L, Ideker T, Raphael BJ, Sharan R. Network propagation: a universal amplifier of genetic associations. Nat Rev Genet. 2017;18:551-562. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 369] [Cited by in RCA: 395] [Article Influence: 49.4] [Reference Citation Analysis (0)] |