Published online Apr 24, 2021. doi: 10.5306/wjco.v12.i4.272
Peer-review started: December 22, 2020
First decision: January 11, 2021
Revised: January 30, 2021
Accepted: March 7, 2021
Article in press: March 7, 2021
Published online: April 24, 2021
Processing time: 119 Days and 4.9 Hours
The management of metastatic progressive radioiodine-resistant differentiated thyroid cancer remains challenging for clinicians. The availability of tyrosine kinase inhibitors (TKIs), sorafenib and lenvatinib, within the last decade has expanded treatment options; however, these lead to significant adverse effects, which may curtail their use.
We report the case of a 47-year-old female with Hurthle cell thyroid cancer who underwent total thyroidectomy followed by radioiodine ablation. During follow-up, she developed noniodine-avid renal and pulmonary metastases. With respect to her pre-existing diabetes, hypertension, and polycystic kidney disease, the tumor board decided against performing renal metastasectomy because of the risk of future renal decline requiring dialysis. Metastases were treated using sorafenib, which provided stability followed by progression within a year. We switched to lenvatinib, which led to disease regression. However, the patient experienced severe adverse effects, including cardiomyopathy, bicytopenia, renal impairment, and the rarely reported nephrotic syndrome. Renal metastasis is a rare manifes-tation of Hurthle cell thyroid cancer with only two reported cases in literature. We report the experience of our first case of renal metastasis and its treatment with TKIs. This case serves as a reminder of the adverse drug reactions associated with TKI use.
We advocate close monitoring of patients’ hematological and renal profiles as well as their cardiac status using an echocardiogram.
Core Tip: The present case study provides a unique learning and case management experience. Our patient had widespread metastasis from a histopathologically low-risk thyroid cancer coupled with two decades of survival despite noniodine-avid metastasis, treatment of renal metastasis with tyrosine kinase inhibitors, and the development of multisystem adverse events, including rare nephrotic syndrome.
- Citation: Butt MI, Khalid Bakhsh AM, Nadri QJ. Lenvatinib-induced multiorgan adverse events in Hurthle cell thyroid cancer: A case report. World J Clin Oncol 2021; 12(4): 272-281
- URL: https://www.wjgnet.com/2218-4333/full/v12/i4/272.htm
- DOI: https://dx.doi.org/10.5306/wjco.v12.i4.272
The worldwide annual incidence of thyroid cancer is increasing, and it is the most common cancer that involves the endocrine organs[1]. This increase is thanks to the improved availability and sophistication of imaging technologies, such as high-resolution ultrasonography of the neck. This has led to early detection of small tumors. According to the Surveillance, Epidemiology, and End Results database, the mortality rate of thyroid cancer is exceptionally low at 0.5 per 100000 individuals per year. Moreover, the 5-year survival rate of thyroid cancer is 98.3% because of early detection as well as a predominantly localized disease[2]. In Saudi Arabia, the incidence of thyroid cancer subtypes show a pattern similar to the rest of the world, with papillary thyroid cancer at 85%, follicular thyroid cancer at 5.7%, and medullary thyroid cancer at 3.1%. However, the incidence of thyroid cancer among Saudi citizens in 2015 was 8.5% of all newly diagnosed cancers[3]. This contrasts with a much lower annual rate of 2.9% in the United States[2]. The exact reason for this is unknown. However, possi-bilities include iodine deficiency in the region, exposure to depleted uranium in the previous Gulf war, and increasing prevalence of obesity in the area.
The prognosis for patients with radioactive iodine (RAI)-avid metastatic disease remains good, with a 56% 10-year survival in avid metastasis vs 10% 10-year survival in noniodine-avid metastasis[4]. Over time, nearly two-thirds of these distant metastases progress to RAI-nonavid disease, similar to our case. Such cases present a treatment challenge. Within the last decade, the US Food and Drug Administration (FDA) approved tyrosine kinase inhibitors (TKIs), sorafenib and lenvatinib, for treating RAI-resistant progressive metastatic differentiated thyroid cancers (DTC). Patients treated with multi-targeted TKIs have shown progression-free survival in the DECISION[5] and SELECT[6] phase 3 clinical trials, albeit the overall survival benefit was not reached in these trials. However, these treatment options have undesirable side effects, which were experienced by > 95% of patients in both trials. We describe a unique case who presented with a confluence of adverse events (AEs) associated with the use of lenvatinib, including nephrotic syndrome, renal impairment, cardiomyopathy, and bicytopenia.
A 47-year-old female patient presented to our clinic in January 2001 with a swelling on the left side of her neck.
The patient noticed that the swelling gradually increased over the preceding six months without any pain or pressure symptoms.
She had a history of hypertension (HTN) and type 2 diabetes (T2DM) that were being treated with oral agents.
Physical examination revealed a non-tender swelling on the left side of her neck. It moved during the swallowing, was firm in consistency, and had no tethering of overlying skin. In addition, no palpable cervical lymph nodes were detected.
Her complete blood count parameters as well as renal and thyroid function tests were normal.
An ultrasound scan of the neck revealed a large solid hypoechoic lesion with ill-defined margins measuring a maximum of 2.8 cm in the left lobe of the thyroid gland.
Fine needle aspiration cytology showed follicular cells (many of which demonstrated a Hurthle cell change), raising the suspicion of Hurthle cell neoplasm.
She underwent total thyroidectomy in January 2001, and the procedure was uneventful. Histopathology showed Hurthle cell thyroid cancer (HCTC) with maximum tumor size of 2.5 cm. There was one focus of capsular and two foci of small vessel invasion, and the tumor extended to the perithyroidal soft tissue. One focus of micropapillary thyroid cancer, measuring 0.5 cm, was present in the left lobe without any invasion.
She underwent diagnostic iodine 123 whole-body scan (WBS), which showed a tracer uptake of 1% in the thyroid bed. There was no uptake outside the neck. The stimulated thyroglobulin (TG) level upon thyroxine withdrawal was 723 mcg/L (reference 0-55 mcg/L) with thyrotropin stimulating hormone level of 59 mU/L (reference 0.35-5.5 mU/L) and free thyroid hormone (FT4) level of < 2 pmol/L (reference 11-23 pmol/L). Anti-TG antibodies were absent. She was administered a dose of 147 millicuries (mCi) radioiodine (RAI) ablation. Her post-therapy scans four days later did not show any new lesions.
In 2002, she had a recurrence in the neck, which was treated with bilateral neck dissection. Fourteen dissected lymph nodes were free of metastases. However, the pre-tracheal tissue deposit showed HCTC with prominent vascular invasion. This was followed by 60 Grays (Gy) of external beam radiotherapy to the neck delivered in two phases.
She continued to have an exceedingly high TG level, for which we performed diagnostic iodine 123 WBS that did not show any persistent or recurrent disease. In addition, 18F-fluorodeoxyglucose (FDG) positron emission tomography (PET) scan in 2003 showed no evidence of metastatic disease. In 2004, she underwent computed tomography (CT) scan of the chest, abdomen, and pelvis. CT revealed a solitary nodule (7 mm in size) in the middle lobe of the right lung, which remained stable on follow-up monitoring. Moreover, CT showed hepatic and bilateral renal cysts with radiological appearance consistent with polycystic kidney disease (PCKD). We administered another dose of 200 mCi RAI as empirical therapy in 2006 because of persistently elevated TG, although diagnostic iodine 123 WBS was negative. Her post-therapy scan did not show any radiotracer uptake.
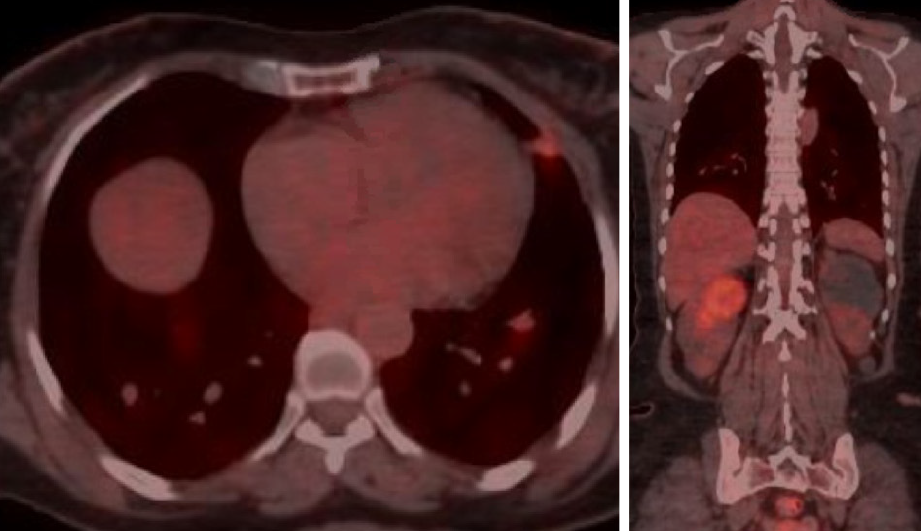
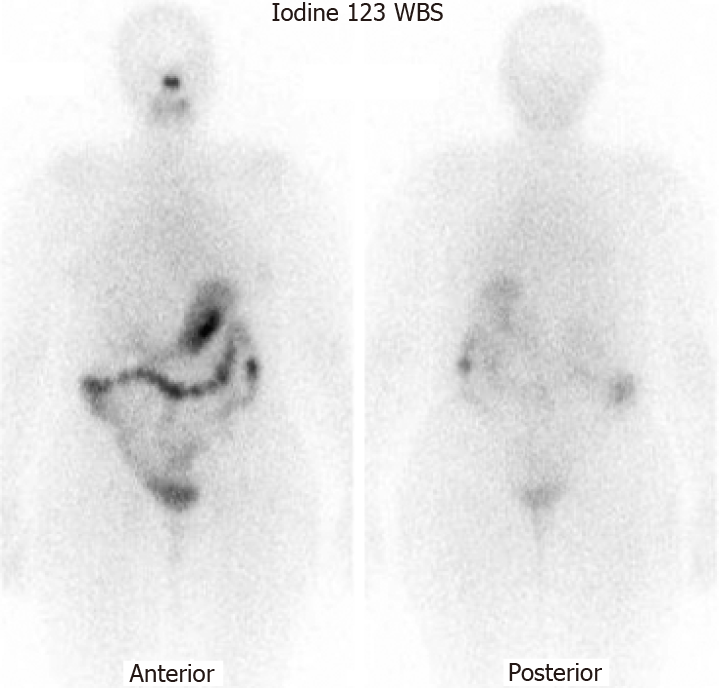
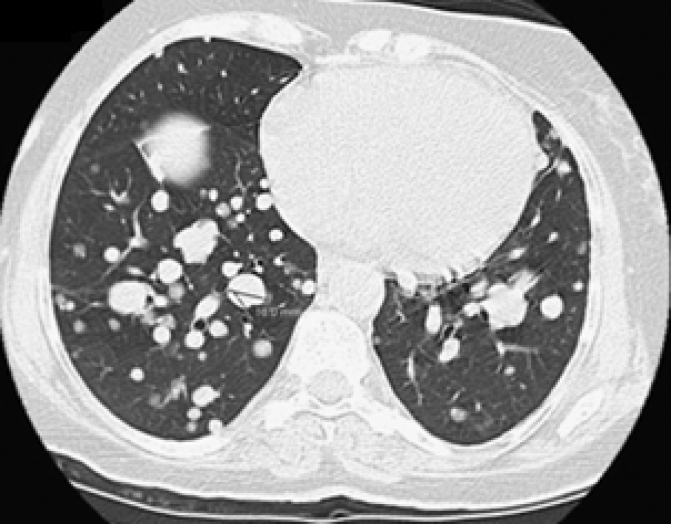
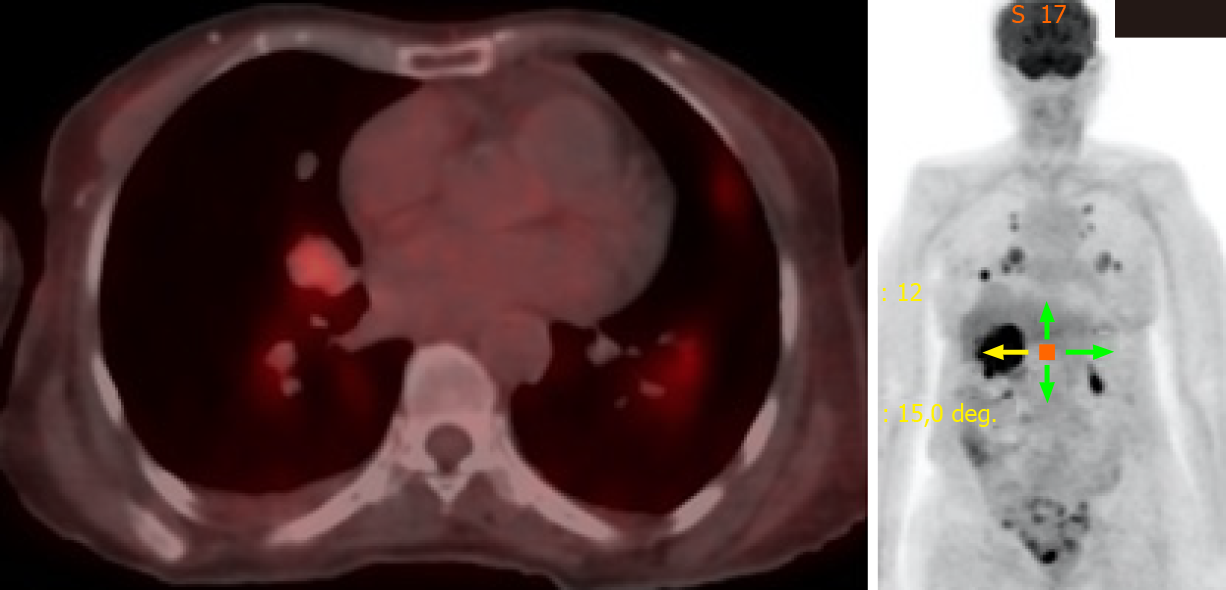
She was continuously monitored, and in 2011, FDG PET scan showed FDG-avid right renal mass measuring 4 cm in size and multiple micronodules in both the lung parenchyma (Figure 1). Iodine 123 WBS did not show any iodine-avid metastases (Figure 2). CT-guided biopsy of the renal mass revealed a renal oncocytoma. Given the benign nature of this renal tumor, she was monitored by urologists with no intervention. Lung nodules continued to increase in number and size, with some increasing to > 1.5-2.0 cm in size. Given this progression, in 2015, we suggested sorafenib. She reported that she felt healthy and elected not to pursue this option and was lost to follow-up. She revisited the clinic toward the end of 2017 when CT of the trunk showed significant progression of the pulmonary metastasis with some nodules > 2.5 cm in size (Figure 3). The renal mass also progressed in size, reaching 6 cm with evidence of invasion to the right renal vein. This etiology was inconsistent with a benign tumor; therefore, we reviewed the original biopsy from the renal mass. Immunohistochemistry was positive for thyroid transcription factor 1, paired box 8, and TG, which confirmed metastases from HCTC to the kidney.
The urology tumor board decided against performing a right nephrectomy because it would have left the patient with a solitary kidney with large cysts. Because of existing T2DM and HTN, there was a significant risk of future renal decline requiring renal replacement therapy; hence, no intervention was offered.
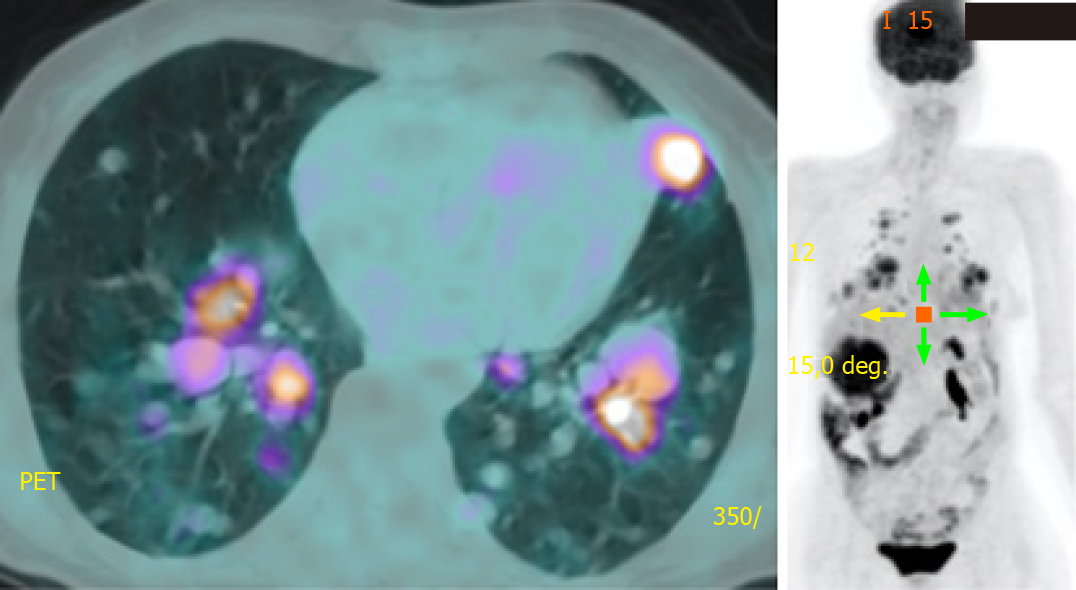
She was prescribed sorafenib 400 mg twice a day in March 2018 and she tolerated it well with a transient mouth ulcer. The dose was briefly reduced to 200 mg twice a day until the ulcer subsided. Surveillance radiological imaging at 3 mo and 6 mo showed disease stability without a reduction in TG levels. At 12 mo, PET showed progression in the number and size of pulmonary metastases (Figure 4). In August 2019, we discontinued sorafenib because of disease progression and initiated lenvatinib 24 mg daily, which has been shown to improve progression-free survival even in patients who have previously progressed with another TKI[6]. She tolerated the medication well with occasional diarrhea. Radiological monitoring at 3 mo and 6 mo showed stability with some reduction in the size of pulmonary metastases. TG levels declined in tandem with radiological stability (Figures 5 and 6A).
The patient experienced asymptomatic bicytopenia with a decline in red blood cells and platelets 10 wk after initiating lenvatinib. Hematological markers, including Coombs test, haptoglobin, reticulocyte count, lactate dehydrogenase, and peripheral blood morphology, did not indicate hemolysis. We managed the condition as an AE of lenvatinib and reduced the dose to 14 mg, which led to an improvement in indices (Figure 6B and C).
Further monitoring showed increasing proteinuria and a new impairment in renal function; hence, treatment was discontinued for two weeks and restarted with a 10 mg daily dose in March 2020. In May 2020, she presented with a 3-d history of diarrhea and acute kidney injury. She was administered intravenous fluid replacement and symptomatic care during hospitalization. Her proteinuria progressed to the nephrotic range, reaching 6 g per 24 h, coupled with a decline in estimated glomerular filtration rate (eGFR) to < 30 mL/min per 1.73 m2 (reference eGFR > 90 mL/min per 1.73 m2). At this stage, we discontinued lenvatinib. Five months later, we noticed a trend toward improvement with proteinuria reducing to 2.4 g per 24 h, plasma albumin rising to normal levels of 32 g/dL (reference 28-46 g/L) , and serum creatinine decreasing from 258 μmol/L to 160 μmol/L (reference 64-115 μmol/L) (Figure 6D and E). We have maintained regular telephone and clinic follow-up, and the patient continues to show improvement in the hematological and renal parameters, although the lung metastasis has shown a slight increase in size. She desires to restart TKI once her metabolic derangements normalize.
Our patient had a long history of T2DM, HTN, and PCKD, all of which can cause varying degrees of compromise to renal function as well as lead to proteinuria. However, she had normal baseline serum urea, creatinine, and eGFR levels. She had microalbuminuria before the initiation of lenvatinib. Renal indices worsened upon the initiation of lenvatinib but improved upon discontinuation, thus providing a clue toward a causal relationship of lenvatinib with respect to renal parameters in our patient.
Our patient had multiple large bilateral renal cysts. Moreover, the right kidney had a large metastatic tumor deposit from the thyroid cancer. In this context, performing a renal biopsy to determine the cause of nephrotic syndrome risked further compromise to an already impaired renal function. It was therefore deemed unsafe and not performed.
Renal impairment is a well-recognized side effect associated with TKI use. Both sorafenib and lenvatinib are FDA-approved TKIs for use in patients with progressive metastatic RAI-resistant thyroid cancer. The DECISION trial did not report on sorafenib-associated renal adverse effects[5]. However, the incidence of sorafenib-associated proteinuria of any grade was 11.6%, whereas the incidence of grade ≥ 3 was 0.9% in a recent meta-analysis[7].
In the SELECT trial involving lenvatinib, nearly one-third of all patients experienced some degree of proteinuria and 10% developed grade ≥ 3 proteinuria[6]. Some degree of renal impairment developed in 14% patients, while 1.9% developed grade ≥ 3 renal impairment. In a subgroup analysis of the Japanese population in the SELECT trial, the incidence of renal adverse effects was higher with any grade proteinuria of 63% and grade 3 proteinuria of 20%, even after adjusting the dose with weight[8].
The real-world experience of using lenvatinib and sorafenib in the Japanese population showed a much higher incidence of proteinuria (all grades) of 60.8% and 27.8%, respectively[9]. The mean change in eGFR was a decrease of 6.75% with lenvatinib vs an increase of 5.9% with sorafenib[9]. The median duration of treatment was 14.9 mo with lenvatinib and 4.6 mo with sorafenib in this retrospective study, which accounts for a higher incidence of proteinuria with lenvatinib. The authors reported five patients who developed nephrotic range proteinuria based on semiquantitative analysis using urine dipstick measurement, unlike our patient who had 24-h urine collection for estimating proteinuria.
An Italian real-life observational study that included 94 patients with DTC treated with lenvatinib reported reduced AE rates with grade ≥ 3 HTN of 4.7% and proteinuria of 1.7%[10]. These results are better than those observed in the SELECT trial[6]. The likely explanation for these superior results could be increased awareness, monitoring, and treatment of HTN and proteinuria by clinicians or gradual increase in the dose. A higher rate of AE observed in Japanese studies[8,9] and a lower rate of AE observed due to lenvatinib in the Italian study[10] suggest that different populations are distinctively predisposed toward AE risks with the same drug. Therefore, it is prudent to conduct an observational study in the Middle Eastern community to quantify the risk to our population.
A recent meta-analysis reported 18.7% of all grade and 2.4% of high-grade proteinuria with a nearly three-fold higher risk using anti-vascular endothelial growth factor (VEGF) TKI therapy[7]. The risk remained the same regardless of the renal or nonrenal site of the underlying cancer. Moreover, the risk varied depending on the use of different TKIs. However, the meta-analysis did not include any studies with lenvatinib. By contrast, Boursiquot et al[11] studied the use of anti-VEGF TKI therapy in patients with renal cell carcinoma. They found that it did not negatively impact the renal function of patients in the long term, even when used in patients with a pre-existing renal impairment or new development of HTN. They did not assess proteinuria pretreatment; however, only 7% of the patients had clinically relevant proteinuria during treatment.
Understanding the structure and normal function of glomeruli provides insight into the mechanism of TKI-induced renal injury. The fenestrated endothelium, the glomerular basement membrane, and the podocytes in the Bowman capsule form a physical barrier against the filtration of macromolecules in the glomeruli. The podocytes produce nephrin, podocin, and VEGF proteins that are essential for the development, maintenance, and function of the vascular endothelium within the glomeruli[12,13]. Anti-VEGF TKI therapies suppress the production of these proteins, which impair the normal morphology and function of glomeruli, causing glomerular injury that leads to renal impairment and proteinuria.
Treatment with anti-VEGF TKI therapies can lead to a new development or worsening of established HTN. Our patient had pre-existing HTN, and we optimized her medications as per requirement. There is some evidence that glomerular injury precedes the new development of HTN; therefore, HTN cannot be the sole trigger for proteinuria[12]. Moreover, some patients remain normotensive, yet they develop proteinuria. By contrast, some patients develop HTN without any accompanying proteinuria. This observation suggests that other factors may contribute to HTN development. Horowitz et al[14] demonstrated that anti-VEGF therapies could block the VEGF-mediated vasodilation via the activation of nitric oxide (NO) synthase within the endothelial cells. Therefore, HTN may be observed in patients on anti-VEGF TKI therapy. A more recent study evaluated lenvatinib-treated patients with DTC, all of whom developed new HTN. They reported a drop in serum NO levels coupled with an increase in VEGF levels 6 d after treatment, suggesting that HTN may be caused by a reduction in NO levels at the level of vascular endothelium due to anti-VEGF therapy[15]. HTN development is considered as a surrogate marker of the therapeutic effect of anti-VEGF therapy, and those who develop new or worsening HTN show a trend toward survival benefit[11].
These targeted TKIs are well known to cause cardiovascular toxicity[16]. This study also carried out a meta-analysis, which included only sorafenib among the FDA-approved therapies for thyroid cancer, showed that it increased the risk of myocardial infarction, HTN, and left ventricular systolic dysfunction[16]. Moreover, both the DECISION[5] and SELECT trials[6] reported cardiac dysfunction presenting as the prolongation of corrected QT as well as impairment of cardiac function. Our patient had a normal echocardiogram during sorafenib treatment. However, she developed mild to moderate left ventricular systolic and diastolic dysfunction and ejection fraction reduction to 40%-45% during lenvatinib treatment. The 12-lead electro-cardiograph did not show any abnormality.
Jensen et al[17] demonstrated that sorafenib-treated mice experienced deleterious effects in the myocardial metabolic pathways leading to systolic cardiac dysfunction, as shown on their echocardiograms after just two weeks of exposure. They noted a significant reduction in the levels of various metabolites that are involved in the taurine and hypotaurine metabolism pathways in the myocardium. Taurine, an amino acid, is available in abundance within the myocardium that functions in reducing injury caused due to oxidants[18]. Moreover, Ramila et al[19] found that taurine-deficient hearts had reduced sarcoplasmic reticulum calcium ATPase activity that affected the calcium sensitivity of myofibril proteins. They concluded that this could be responsible for deleterious effects on myocardial contractility.
Hematological derangements are not uncommon with TKI use. Our patient developed transient anemia and thrombocytopenia. Thrombocytopenia was the most common grade ≥ 3 AE observed in 25% of the patients in the meta-analysis of lenvatinib trials[20]. This observation was in sharp contrast with the 1.5% rate of grade ≥ 3 thrombocytopenia observed in the SELECT trial[6]. These variations could be due to different populations, inclusion/exclusion criteria, durations of exposure, pre-existing comorbidities, and concomitant use of other drugs that could predispose patients to these AEs.
VEGF receptor is present on most hematopoietic cells and peripheral cells, including the platelets and monocytes. The inhibitory effect of these anti-VEGF TKIs on hematopoiesis and myelopoiesis probably causes cytopenia. In addition, these multikinases block the platelet-derived growth factor receptor present on the hematopoietic stem cells and megakaryocytes. This hampers the function of platelet-derived growth factor, which promotes the production of platelets by direct effects on the bone marrow[21]. There is some evidence that thrombocytopenia could be caused by drug-induced immune-mediated response, as shown by the presence of antiplatelet antibodies during TKI treatment[22].
Kidneys are an exceedingly rare metastatic site from HCTC with only two reported cases in literature[23,24]. Both patients had iodine-avid metastasis; one patient underwent nephrectomy and RAI ablation cleared metastases in the other patient. Neither of these cases required treatment with TKIs. Our patient’s cancer was noniodine avid and surgically unresectable, and she had PCKD. She required TKIs but experienced significant multiorgan AEs, all of which added more knowledge to the existing information regarding the treatment of such patients. Another unusual aspect of our patient was a progressive and metastatic tumor with a relatively low-risk pathology that otherwise carried a low risk of recurrence and metastasis[25].
In conclusion, the present case was unique and provided a unique learning and case management experience. The patient had widespread metastases despite a low-risk pathology, prolonged overall survival approaching two decades despite noniodine-avid distant metastases, and a confluence of multiorgan AEs with the use of lenvatinib.
We thank Al-Hindi HN, Alsuhaibani H, Almsaadi S, and the Department of Endocrinology for providing invaluable support required for patient care.
Manuscript source: Unsolicited manuscript
Corresponding Author's Membership in Professional Societies: Fellow of Royal College of Physicians Glasgow UK, No. 89279; and Fellow of American Association of Clinical Endocrinology, No. 12768.
Specialty type: Oncology
Country/Territory of origin: Saudi Arabia
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): B, B
Grade C (Good): 0
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Zhang J S-Editor: Gao CC L-Editor: A P-Editor: Yuan YY
| 1. | Pellegriti G, Frasca F, Regalbuto C, Squatrito S, Vigneri R. Worldwide increasing incidence of thyroid cancer: update on epidemiology and risk factors. J Cancer Epidemiol. 2013;2013:965212. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 633] [Cited by in RCA: 837] [Article Influence: 69.8] [Reference Citation Analysis (0)] |
| 2. | National Cancer Institute. Surveillance, Epidemiology, and End Results Program. [cited November 28, 2020]. Available from: https://seer.cancer.gov/. |
| 3. | Saudi Health Council. Cancer incidence report Saudi Arabia, 2016. [cited July 25, 2020]. Available from: https://nhic.gov.sa/en/eServices/Documents/2016.pdf. |
| 4. | Durante C, Haddy N, Baudin E, Leboulleux S, Hartl D, Travagli JP, Caillou B, Ricard M, Lumbroso JD, De Vathaire F, Schlumberger M. Long-term outcome of 444 patients with distant metastases from papillary and follicular thyroid carcinoma: benefits and limits of radioiodine therapy. J Clin Endocrinol Metab. 2006;91:2892-2899. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1047] [Cited by in RCA: 1113] [Article Influence: 58.6] [Reference Citation Analysis (0)] |
| 5. | Brose MS, Nutting CM, Jarzab B, Elisei R, Siena S, Bastholt L, de la Fouchardiere C, Pacini F, Paschke R, Shong YK, Sherman SI, Smit JW, Chung J, Kappeler C, Peña C, Molnár I, Schlumberger MJ; DECISION investigators. Sorafenib in radioactive iodine-refractory, locally advanced or metastatic differentiated thyroid cancer: a randomised, double-blind, phase 3 trial. Lancet. 2014;384:319-328. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1260] [Cited by in RCA: 1125] [Article Influence: 102.3] [Reference Citation Analysis (1)] |
| 6. | Schlumberger M, Tahara M, Wirth LJ, Robinson B, Brose MS, Elisei R, Habra MA, Newbold K, Shah MH, Hoff AO, Gianoukakis AG, Kiyota N, Taylor MH, Kim SB, Krzyzanowska MK, Dutcus CE, de las Heras B, Zhu J, Sherman SI. Lenvatinib versus placebo in radioiodine-refractory thyroid cancer. N Engl J Med. 2015;372:621-630. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1207] [Cited by in RCA: 1348] [Article Influence: 134.8] [Reference Citation Analysis (0)] |
| 7. | Zhang ZF, Wang T, Liu LH, Guo HQ. Risks of proteinuria associated with vascular endothelial growth factor receptor tyrosine kinase inhibitors in cancer patients: a systematic review and meta-analysis. PLoS One. 2014;9:e90135. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 61] [Cited by in RCA: 62] [Article Influence: 5.6] [Reference Citation Analysis (0)] |
| 8. | Kiyota N, Schlumberger M, Muro K, Ando Y, Takahashi S, Kawai Y, Wirth L, Robinson B, Sherman S, Suzuki T, Fujino K, Gupta A, Hayato S, Tahara M. Subgroup analysis of Japanese patients in a phase 3 study of lenvatinib in radioiodine-refractory differentiated thyroid cancer. Cancer Sci. 2015;106:1714-1721. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 86] [Cited by in RCA: 98] [Article Influence: 9.8] [Reference Citation Analysis (0)] |
| 9. | Iwasaki H, Yamazaki H, Takasaki H, Suganuma N, Sakai R, Nakayama H, Toda S, Masudo K. Renal dysfunction in patients with radioactive iodine-refractory thyroid cancer treated with tyrosine kinase inhibitors: A retrospective study. Medicine (Baltimore). 2019;98:e17588. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 8] [Cited by in RCA: 12] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 10. | Locati LD, Piovesan A, Durante C, Bregni M, Castagna MG, Zovato S, Giusti M, Ibrahim T, Puxeddu E, Fedele G, Pellegriti G, Rinaldi G, Giuffrida D, Verderame F, Bertolini F, Bergamini C, Nervo A, Grani G, Rizzati S, Morelli S, Puliafito I, Elisei R. Real-world efficacy and safety of lenvatinib: data from a compassionate use in the treatment of radioactive iodine-refractory differentiated thyroid cancer patients in Italy. Eur J Cancer. 2019;118:35-40. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 53] [Cited by in RCA: 66] [Article Influence: 11.0] [Reference Citation Analysis (0)] |
| 11. | Boursiquot BC, Zabor EC, Glezerman IG, Jaimes EA. Hypertension and VEGF (Vascular Endothelial Growth Factor) Receptor Tyrosine Kinase Inhibition: Effects on Renal Function. Hypertension. 2017;. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 20] [Cited by in RCA: 24] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 12. | Eremina V, Jefferson JA, Kowalewska J, Hochster H, Haas M, Weisstuch J, Richardson C, Kopp JB, Kabir MG, Backx PH, Gerber HP, Ferrara N, Barisoni L, Alpers CE, Quaggin SE. VEGF inhibition and renal thrombotic microangiopathy. N Engl J Med. 2008;358:1129-1136. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1264] [Cited by in RCA: 1131] [Article Influence: 66.5] [Reference Citation Analysis (0)] |
| 13. | Tryggvason K, Patrakka J, Wartiovaara J. Hereditary proteinuria syndromes and mechanisms of proteinuria. N Engl J Med. 2006;354:1387-1401. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 406] [Cited by in RCA: 383] [Article Influence: 20.2] [Reference Citation Analysis (0)] |
| 14. | Horowitz JR, Rivard A, van der Zee R, Hariawala M, Sheriff DD, Esakof DD, Chaudhry GM, Symes JF, Isner JM. Vascular endothelial growth factor/vascular permeability factor produces nitric oxide-dependent hypotension. Evidence for a maintenance role in quiescent adult endothelium. Arterioscler Thromb Vasc Biol. 1997;17:2793-2800. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 199] [Cited by in RCA: 201] [Article Influence: 7.2] [Reference Citation Analysis (0)] |
| 15. | Sueta D, Suyama K, Sueta A, Tabata N, Yamashita T, Tomiguchi M, Takeshita T, Yamamoto-Ibusuki M, Yamamoto E, Izumiya Y, Kaikita K, Yamamoto Y, Hokimoto S, Iwase H, Tsujita K. Lenvatinib, an oral multi-kinases inhibitor, -associated hypertension: Potential role of vascular endothelial dysfunction. Atherosclerosis. 2017;260:116-120. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 25] [Cited by in RCA: 38] [Article Influence: 4.8] [Reference Citation Analysis (0)] |
| 16. | Escalante CP, Chang YC, Liao K, Rouleau T, Halm J, Bossi P, Bhadriraju S, Brito-Dellan N, Sahai S, Yusuf SW, Zalpour A, Elting LS; Epidemiology Section of the Mucositis Study Group of the Multinational Association of Supportive Care in Cancer; 2013. Meta-analysis of cardiovascular toxicity risks in cancer patients on selected targeted agents. Support Care Cancer. 2016;24:4057-4074. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 17] [Cited by in RCA: 21] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 17. | Jensen BC, Parry TL, Huang W, Beak JY, Ilaiwy A, Bain JR, Newgard CB, Muehlbauer MJ, Patterson C, Johnson GL, Willis MS. Effects of the kinase inhibitor sorafenib on heart, muscle, liver and plasma metabolism in vivo using non-targeted metabolomics analysis. Br J Pharmacol. 2017;174:4797-4811. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 23] [Cited by in RCA: 29] [Article Influence: 3.6] [Reference Citation Analysis (0)] |
| 18. | Schuller-Levis GB, Park E. Taurine: new implications for an old amino acid. FEMS Microbiol Lett. 2003;226:195-202. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 292] [Cited by in RCA: 288] [Article Influence: 13.1] [Reference Citation Analysis (0)] |
| 19. | Ramila KC, Jong CJ, Pastukh V, Ito T, Azuma J, Schaffer SW. Role of protein phosphorylation in excitation-contraction coupling in taurine deficient hearts. Am J Physiol Heart Circ Physiol. 2015;308:H232-H239. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 30] [Cited by in RCA: 41] [Article Influence: 3.7] [Reference Citation Analysis (0)] |
| 20. | Zhu C, Ma X, Hu Y, Guo L, Chen B, Shen K, Xiao Y. Safety and efficacy profile of lenvatinib in cancer therapy: a systematic review and meta-analysis. Oncotarget. 2016;7:44545-44557. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 70] [Cited by in RCA: 66] [Article Influence: 7.3] [Reference Citation Analysis (0)] |
| 21. | Ye JY, Chan GC, Qiao L, Lian Q, Meng FY, Luo XQ, Khachigian LM, Ma M, Deng R, Chen JL, Chong BH, Yang M. Platelet-derived growth factor enhances platelet recovery in a murine model of radiation-induced thrombocytopenia and reduces apoptosis in megakaryocytes via its receptors and the PI3-k/Akt pathway. Haematologica. 2010;95:1745-1753. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 31] [Cited by in RCA: 35] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 22. | Barak AF, Bonstein L, Lauterbach R, Naparstek E, Tavor S. Tyrosine kinase inhibitors induced immune thrombocytopenia in chronic myeloid leukemia? Hematol Rep. 2011;3:e29. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 8] [Cited by in RCA: 8] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 23. | Madani A, Jozaghi Y, Tabah R, How J, Mitmaker E. Rare metastases of well-differentiated thyroid cancers: a systematic review. Ann Surg Oncol. 2015;22:460-466. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 30] [Cited by in RCA: 48] [Article Influence: 4.4] [Reference Citation Analysis (0)] |
| 24. | Claimon A, Suh M, Cheon GJ, Lee DS, Kim EE, Chung JK. Bilateral Renal Metastasis of Hürthle Cell Thyroid Cancer with Discordant Uptake Between I-131 Sodium Iodide and F-18 FDG. Nucl Med Mol Imaging. 2017;51:256-260. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4] [Cited by in RCA: 5] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 25. | Ghossein RA, Hiltzik DH, Carlson DL, Patel S, Shaha A, Shah JP, Tuttle RM, Singh B. Prognostic factors of recurrence in encapsulated Hurthle cell carcinoma of the thyroid gland: a clinicopathologic study of 50 cases. Cancer. 2006;106:1669-1676. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 93] [Cited by in RCA: 84] [Article Influence: 4.4] [Reference Citation Analysis (0)] |