Published online Apr 24, 2021. doi: 10.5306/wjco.v12.i4.262
Peer-review started: December 3, 2020
First decision: December 24, 2020
Revised: January 7, 2021
Accepted: March 22, 2021
Article in press: March 22, 2021
Published online: April 24, 2021
Processing time: 137 Days and 17.5 Hours
Liver tumors with dual differentiations [combined hepatocellular carcinoma (HCC) and cholangiocarcinoma] are common. However, liver tumors that exhibit hepatocellular, biliary, and neuroendocrine differentiation are exceedingly rare, with only three previous case reports in the literature.
A 65-year-old female with a previous history of hepatitis C and a distant history of low grade, well-differentiated rectal neuroendocrine tumor was found to have two liver lesions in segment 4 and segment 7 on imaging. Serum alpha-fetoprotein and chromogranin A were elevated. Biopsy of the larger lesion in segment 4 revealed a high-grade tumor, with morphologic and immunohistochemical features of a neuroendocrine tumor. Given the previous history of rectal neuroendocrine tumor, imaging investigation, serologic markers, and biopsy findings, metastatic neuroendocrine tumor was considered. Subsequent regional resection of these hepatic lesions revealed the segment 4 lesion to be a HCC with additional biliary and neuroendocrine differentiation and the segment 7 lesion to be a cholangiocarcinoma with neuroendocrine differentiation. Follow-up of the patient revealed disease recurrence in the dome of the liver and metastasis in retro-pancreatic lymph nodes. The patient eventually expired due to complications of chemotherapy.
HCC cases with additional biliary and neuroendocrine differentiation are exceedingly rare, posing a diagnostic challenge for clinicians and pathologists.
Core Tip: Hepatocellular carcinoma (HCC) exhibiting additional biliary and neuroendocrine differentiation are exceedingly rare, with only three previous case reports in the literature. We present a case of HCC with this triple differentiation, which developed in a patient with a history of previously excised rectal neuroendocrine tumor. This case highlights the diagnostic difficulties encountered during the evaluation of these patients, especially in cases where patients have a previous history of neuroendocrine tumors elsewhere in the body. The aggressive nature of these tumors is demonstrated in addition.
- Citation: Dimopoulos YP, Winslow ER, He AR, Ozdemirli M. Hepatocellular carcinoma with biliary and neuroendocrine differentiation: A case report. World J Clin Oncol 2021; 12(4): 262-271
- URL: https://www.wjgnet.com/2218-4333/full/v12/i4/262.htm
- DOI: https://dx.doi.org/10.5306/wjco.v12.i4.262
Hepatocellular carcinoma (HCC) is the most frequent subtype of primary liver cancer (PLC), accounting for 70%-85% of all PLC cases[1]. Traditionally, HCC has been separated from cholangiocarcinoma (CC), with differences in clinical management, treatment, and prognostic stratification of patients[2]. PLCs exhibiting multiple differentiation pathways have been described. For example, PLCs of both hepatocytic and biliary differentiation (combined HCC-CC (cHCC-CC)) are relatively common, with an incidence ranging between 0.4%-14.2%[1,3]. Apart from cHCC-CC, other rare PLCs with combined differentiations exist. Tumors with an HCC and neuroendocrine carcinoma (NEC) differentiation have been reported previously[4]. Additionally, cases of CC of the intra- and extrahepatic biliary tree with neuroendocrine differentiation have been designated as mixed adeno NEC (mANEC)[5,6].
Hepatocellular tumors exhibiting three differentiation pathways are exceedingly rare. The combination of hepatocytic, biliary and neuroendocrine differentiation has been previously described in 3 patients[7,8] (Table 1). We report, herein, an additional case of similar tumor arising in a 65-year-old female.
| Cases of liver tumor with triple differentiation described in the literature | Ref. |
| Chinese male patients (n = 2; average age: 57.5 yr). Both were positive for hepatitis B and exhibited hepatic tumors with triple differentiation. | He et al[8], 2013 |
| An otherwise healthy 19-year-old Caucasian male developed a large hepatic tumor showing triple differentiation. | Beard et al[7], 2017 |
| A 65-year-old female with a previous history of hepatitis C and rectal “carcinoid” developed a hepatic tumor with triple differentiation. | Current case report |
A 65-year-old female presented with vague symptoms of peri-umbilical and epigastric abdominal pain, poor appetite, unintentional weight loss of 10 pounds, and night sweats.
Computed topography (CT) scan performed during the work-up of the patient revealed two liver lesions.
The patient had a history of neuroendocrine tumors in the rectum. Specifically, in a screening colonoscopy performed in 2012, a 1 cm polypoid lesion was seen in the rectum. This was removed endoscopically, with saline injection and hot snare cautery. Review of the histological slides revealed a low-grade, well-differentiated neuroendocrine tumor (“carcinoid”) with no evidence of lymphatic or vascular invasion. Resection margins were not able to be assessed on this excision pathologically. On subsequent colonoscopy in 2017, a more extensive endoscopic mucosal resection was performed in the region of the previously identified carcinoid, with final margins negative on pathologic examination. No recurrence in the region of the rectum or additional neuroendocrine tumors in her colon were seen on subsequent colonoscopies, and no liver lesions were seen on CT imaging.
The patient additionally had a previous medical history of hypothyroidism (treated with levothyroxine) and hypertension (treated with clonidine, amlodipine, and hydrochlorothiazide-triamterene).
Mild diffuse tenderness was found on abdominal examination. No masses or hepatomegaly were detected. The rest of the examination was unremarkable.
Serum chromogranin and alpha-fetoprotein (AFP) were both elevated, at 450 ng/mL (normal range: < 93 ng/mL) and 2198 ng/mL (normal range: 10-20 ng/mL), respectively. The percentage of AFP binding to Lens culinaris agglutinin (AFP-L3%) was also elevated (86.6%). The patient was found to be positive for hepatitis C virus (HCV) by polymerase chain reaction. HCV genotype was determined to be 1a. Laboratory examinations at the time were additionally notable for mildly increased aspartate aminotransferase (72 U/L; normal range: 0-35 U/L) and alanine transaminase (86 U/L; normal range: 19-25 U/L).
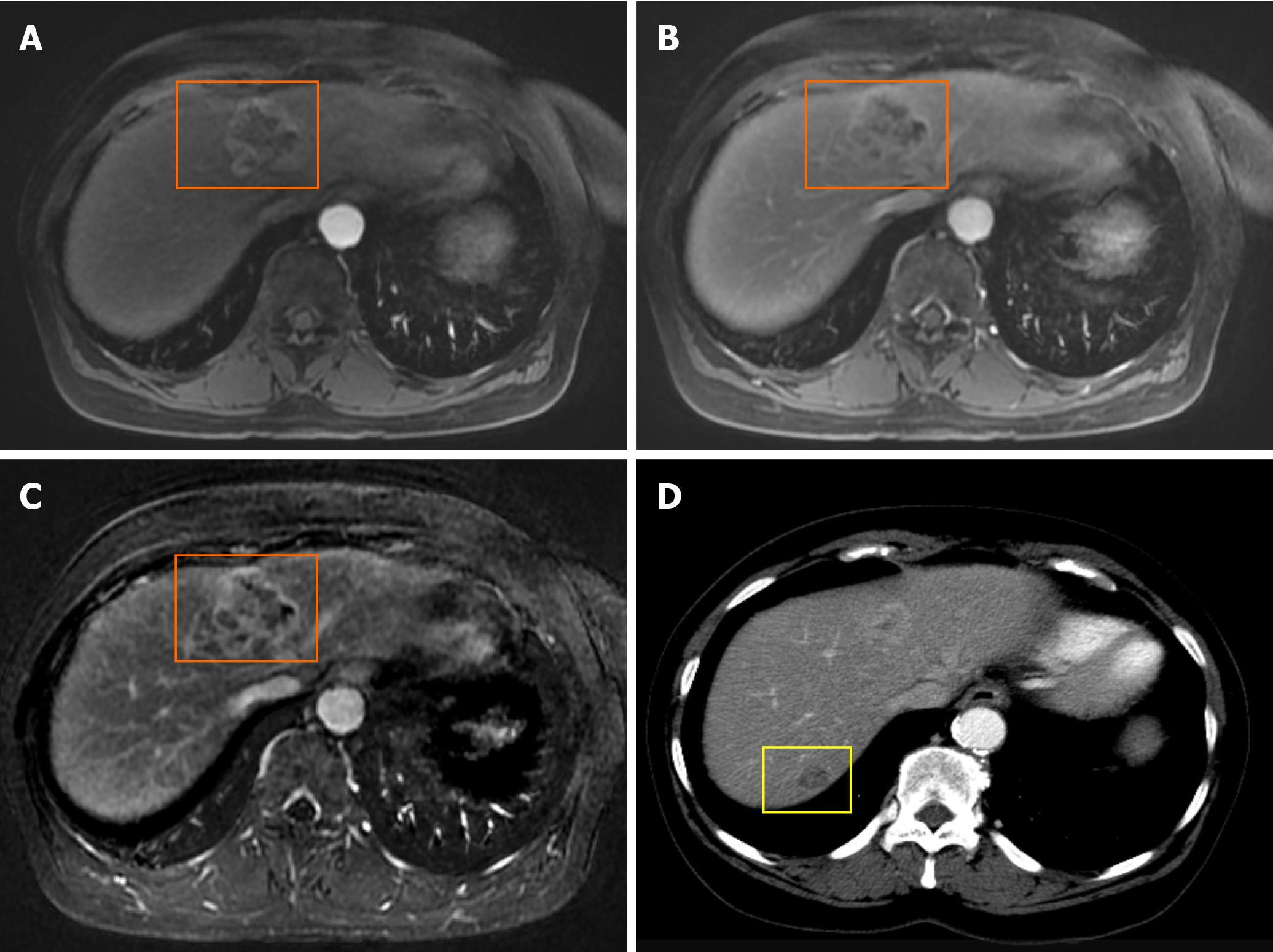
Standard dynamic CT imaging of the abdomen and pelvis with intravenous contrast revealed a total of two hepatic masses: A 2.2 cm × 2.1 cm peripherally enhancing mass in the left lobe (segment 4) with central hypodensity, and a 1.4 cm × 1 cm lesion in the right lobe (segment 7) that was low density (Figure 1). Additionally, prominent portacaval lymph nodes were noted.
To characterize the lesions further, magnetic resonance imaging (MRI) with gadolinium-based contrast was subsequently performed, with multiple pulse sequences, including T1 and T2 weighted and in phase/opposed phase gradient-echo images. Diffusion imaging was also performed. These images showed a 2.7 cm × 2.5 cm × 2.3 cm T2 hyperintense lesion in the left lobe (segment 4) with peripheral rim-like enhancement on the arterial phase and persistent enhancement on the venous phase (Figure 1). The lesion in the right lobe (segment 7) was 1.5 cm × 1.5 cm × 1.1 cm and similarly T2 hyperintense but was too small to evaluate its enhancement characteristics adequately.
Fluorodeoxyglucose (FDG) positron emission tomography (PET) showed FDG avidity in the left lobe lesion (standard uptake value of 6.6) but no avidity in the right lobe lesion. No extrahepatic FDG avidity in the nodal basins or pelvis was seen.
Gallium-68 dotatate PET scan was also performed and showed no tracer uptake in the rectum, liver, or portal lymph nodes.
The left lobe segment 4 lesion of the liver was biopsied under CT guidance, with final pathology findings being consistent with a high-grade neuroendocrine tumor (due to morphology and synaptophysin expression detected by immunohistochemistry). A Ki-67 immunohistochemical investigation revealed 30% of the cells to be positive. Concurrent biopsy of uninvolved hepatic parenchyma revealed absence of cirrhosis or significant fibrosis. A repeat MRI scan performed showed an interval increase in the size of the segment 4 lesion, which measured 4.1 cm × 3.6 cm × 2.3 cm. Chest CT showed no evidence of metastatic disease.
A combination of clinical, imaging, and pathologic findings raised the possibility of metastatic neuroendocrine tumor to the liver at the time, with the main differential diagnosis considered being a primary hepatic tumor (most likely HCC). Given that there was no indication of extra-abdominal spread on imaging studies and the absence of cirrhosis in the hepatic parenchyma, the decision to proceed with segmental resection of the two hepatic lesions with portacaval lymph node sampling was made, as this was felt to be beneficial for either differential diagnoses considered. Furthermore, plans were made to treat the patient’s HCV infection following the surgical intervention.
A staging laparoscopy was performed and revealed no evidence of metastatic disease outside of the liver and confirmed the absence of cirrhosis. Open exploration with intraoperative ultrasound revealed only the two known liver lesions. Anatomic resection of segment 4 and nonanatomic resection of the lesion in segment 7 were performed, as well as a portal lymphadenectomy.
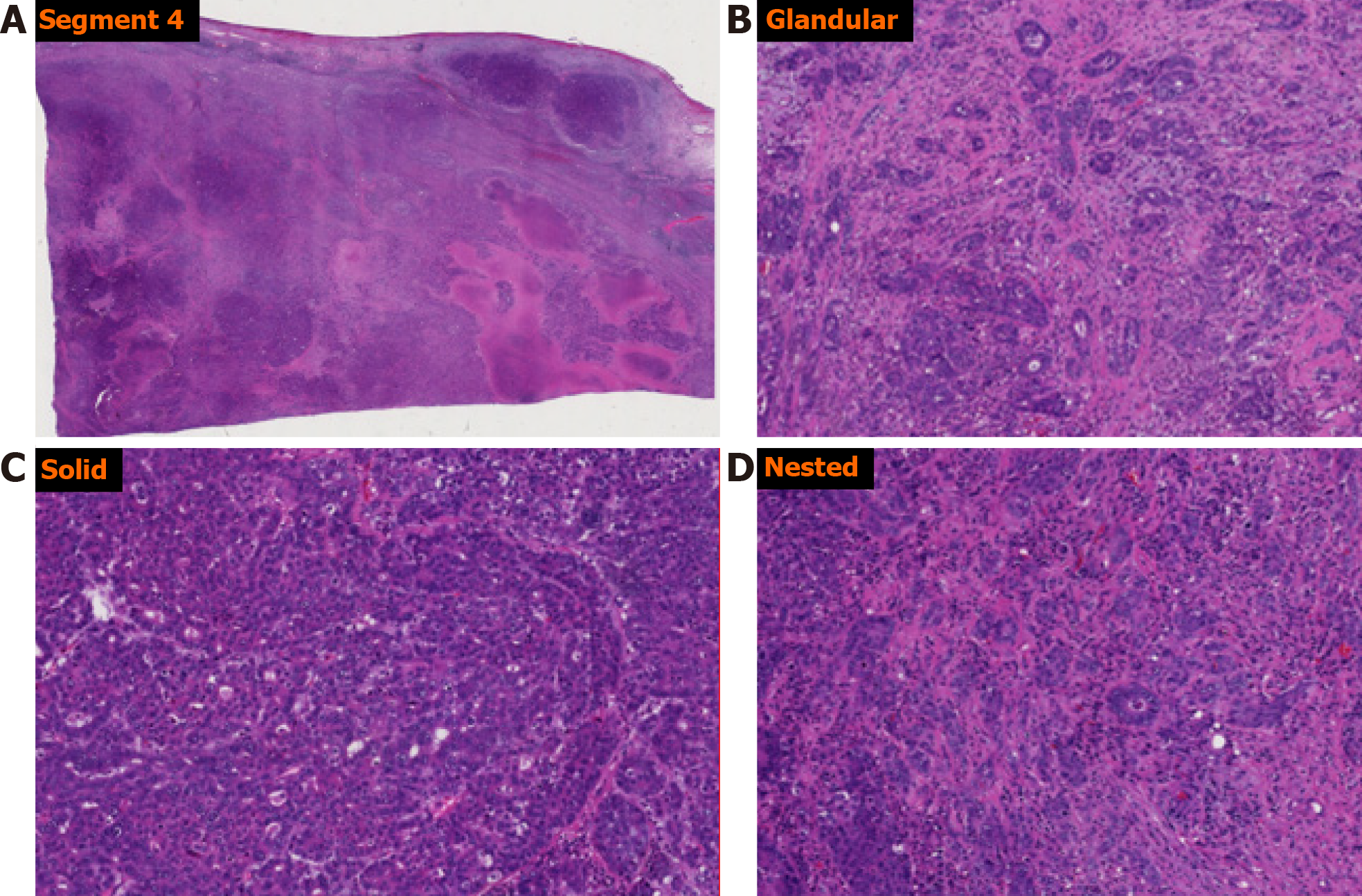
Pathologic examination of the left lobe segment 4 resection specimen revealed a segment of red-brown liver parenchyma measuring 10.2 cm × 5.1 cm × 4.1 cm. On serial sectioning, a tan-white to tan-green mass measuring 4.7 cm × 4.0 cm × 2.3 cm was identified. The surgical resection margin was negative. Microscopic evaluation of this tumor revealed a poorly differentiated malignant tumor with variegated and intermixed histology. Based on morphology, foci resembling HCC, CC, and neuroendocrine tumor were identified (Figure 2). Immunohistochemical analysis showed the malignant cells to be focally positive for AFP, cytokeratin (CK)-19, CK-7, CK-18, CK-20, p53, polyclonal carcinoembryonic antigen, Hep-Par-1, arginase 1, CDX-2, as well as focally positive for synaptophysin and neuron-specific enolase (Figure 3). Ki-67 proliferation index was high, estimated at approximately 60%. The tumor cells were negative for chromogranin, prostatic acid phosphatase, and cancer antigen 19-9 (CA19-9).
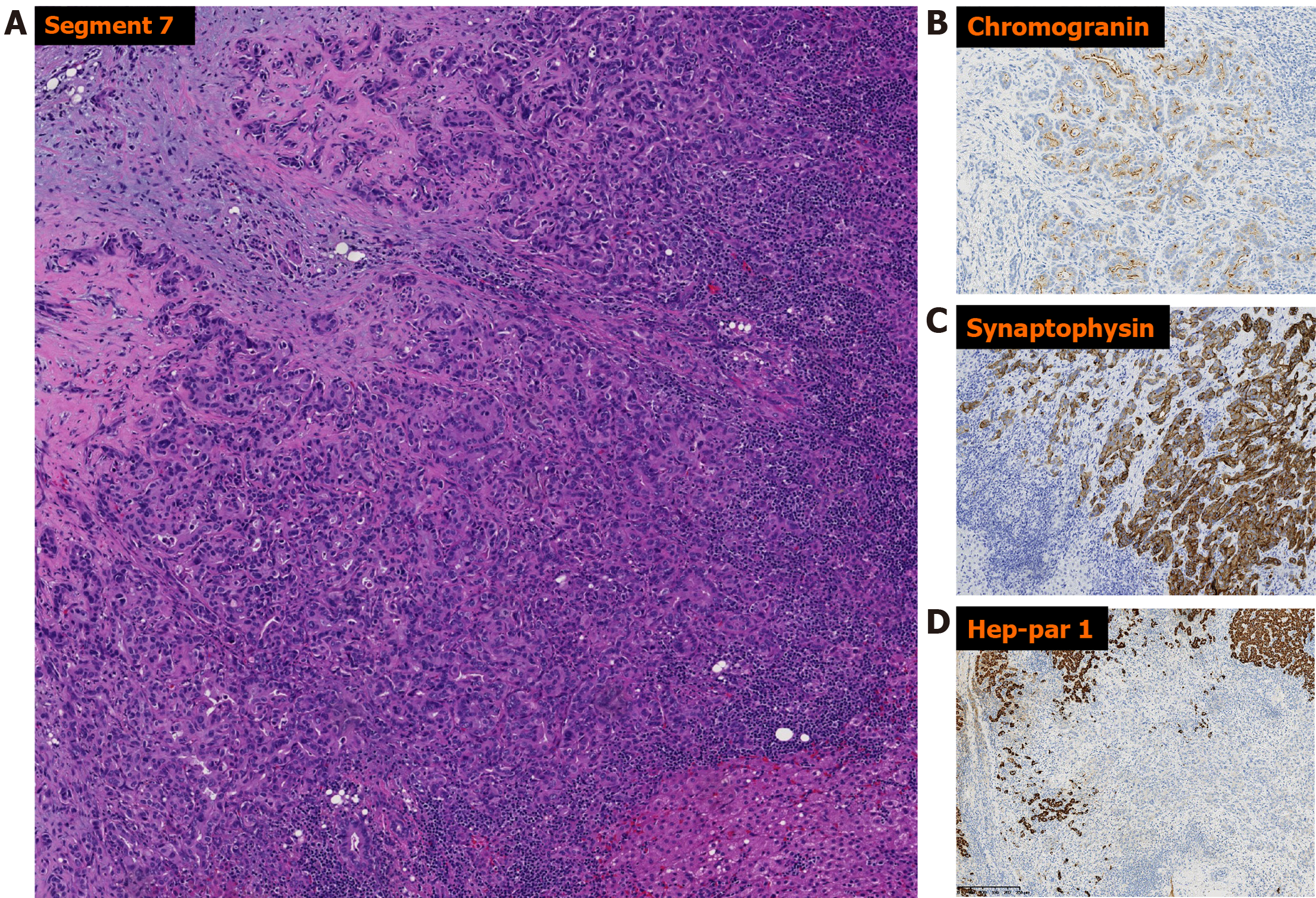
Pathologic examination of the right lobe segment 7 resection specimen revealed a 1.5 cm × 1.5 cm × 1.0 cm segment of hepatic parenchyma, which was serially sectioned to reveal a 1.0 cm × 0.5 cm × 0.5 cm white nodule. The surgical resection margin was negative. Histologic examination of the nodule revealed malignant cells forming glands on a sclerotic background (Figure 4). Immunohistochemical analysis (Figure 4) revealed the malignant cells to be positive for CK-18, CK-19, CK-7, carcinoembryonic antigen, synaptophysin (strong diffuse), chromogranin (scattered and weak), and CDX-2 (scattered and weak). Ki-67 proliferation index was approximately 20%. The cells were negative for AFP, CD56, Hep-Par-1, and arginase 1.
Intra-operative consultation on a portacaval lymph node was initially reported as negative. Final pathologic examination of the resected portal lymph nodes revealed a microscopic focus of metastatic HCC in one of the lymph nodes.
An oncology consultation was pursued and adjuvant chemotherapy was advocated, following additional imaging investigation.
Based on the morphologic and immunophenotypic characteristics, the final diagnosis of HCC with additional biliary and neuroendocrine differentiation was rendered for the left lobe segment 4 mass, with the hepatocellular component being the predominant morphology/immunophenotype observed. The right lobe segment 7 mass was diagnosed as a mANEC. Given the propensity of HCC to metastasize intra-hepatically, the relative size of the two masses (with the left lobe segment 4 mass being larger than the right lobe segment 7 mass), and findings of metastatic HCC to the portacaval lymph node, and the fact that both tumors showed neuroendocrine differentiation, the right lobe segment 7 mass was favored to represent an intra-hepatic metastatic focus of the larger segment 4 mass. The pathologic staging of the patient’s tumors was pT2N1.
Following the resection of the two hepatic tumors, the patient had an uneventful post-surgical recovery and was subsequently discharged home with instructions for post-surgical follow-up.
Immediate postoperative imaging was performed, including an FDG PET/CT, which demonstrated no FDG avid lesions. The patient was followed up by a community oncologist near her home and did not initially receive adjuvant therapy. CT performed 1-mo post-surgery revealed disease recurrence in the hepatic dome. Additionally, an MRI confirmed recurrent disease in the liver dome and new pathologic lymphadenopathy posterior to the pancreatic head. Endoscopic, ultrasound-guided, trans-gastric fine needle aspiration of these enlarged lymph nodes revealed metastatic adenocarcinoma, consistent with metastasis from the CC component of the patient’s tumors.
Formalin fixed, paraffin embedded tissue from the patient’s larger tumor (left lobe, segment 4) was submitted for molecular profiling with next-generation sequencing. This revealed that there was no fibroblast growth factor receptor 2 fusion, IDH1/2 mutation, BRAF mutation, or HER2 amplification. The tumor was found to be microsatellite stable. There was a CDKN2A exon 2 p.H83Y mutation and a TP53 exon 7 p.Y234C mutation. Both pathologic mutations detected would not have any impact on the selection of first-line systemic therapy. Thus, standard first-line palliative systemic therapy with cisplatin and gemcitabine was initiated.
Five months later, while receiving systemic therapy, the patient developed gastrointestinal bleeding (the patient was on anticoagulation for a port-related thrombosis she had developed) and subsequently multiorgan system failure. Imaging obtained on presentation revealed stable disease regarding her relapsed tumor (unchanged size of the retro-pancreatic pathologic lymph node and right hemi-liver recurrence). Unfortunately, the patient passed away secondary to multiorgan failure (see Table 2 for complete timeline of events).
| Dates | Summary from initial and follow-up visits | Diagnostic testing | Intervention/results |
| 2012 and 2017 | Routine colonoscopy | 1 cm polypoid lesion in rectum | Removed endoscopically → well-differentiated neuroendocrine tumor |
| May/June 2019 | Presents with abdominal pain | CT, MRI, FDG/PET → two liver lesions | CT-guided biopsy → poorly differentiated carcinoma with neuroendocrine features |
| September 2019 | Presents for surgical intervention | Presents for surgical intervention | Resection of the 2 liver lesions → Final diagnosis of combined liver tumor |
| October 2019 | Post-surgical follow-up | CT and FDG/PET → New liver lesion in dome | Additional imaging was advocated |
| March 2020 | MRI revealed new portocaval lymphadenopathy | Endoscopic ultrasound-guided FNA of lymph nodes | Lymph nodes positive for metastatic adenocarcinoma |
| May 2020 | Final oncology consultation after imaging | Initiation of cisplatin and gemcitabine chemotherapy | |
| October 2020 | Presents to hospital with altered mental status | Found to be in shock due to a combination of GI bleed/sepsis | Patient expired, despite medical treatment |
Combined PLCs are increasingly being described and prove a diagnostic challenge for clinicians, radiologists, and pathologists. On CT and MRI imaging, our patient’s liver lesions exhibited features more consistent with a metastatic lesion rather than a primary HCC (peripheral rim-like enhancement on the arterial phase and persistent enhancement on the venous phase in the patient’s tumor vs homogenous arterial phase enhancement and washout on the venous phase typically seen in HCC)[9]. It is known however that, based on the predominant histologic component, they may exhibit varying imaging features on CT and MRI[10].
Gallium-68 dotatate PET scan is a widely accepted method for the detection of neuroendocrine tumors and their metastases, with higher reported sensitivity and specificity compared to octreotide scans[11]. Given our patient’s history of a rectal carcinoid, this scan was performed in the context of detecting metastatic disease to the liver, which was suspected clinically. In our patient, the scan failed to reveal uptake in the liver lesions; however, due to the possibility of a false negative result, a metastatic neuroendocrine tumor was not excluded[11].
Serum markers are frequently used in the evaluation of liver tumors, with HCC and CC typically showing an increase in serum AFP and CA19-9, respectively. False positive elevations are well represented in the literature[3,12,13]. Increase in the AFP-L3 percentage has been shown to be relatively specific for HCC[14]. Elevations in AFP have also been reported in the context of metastatic neuroendocrine tumors to the liver[15]. Serum chromogranin has been used as a marker for tumors with neuroendocrine differentiation and for the monitoring of enterochromaffin-like hyperplasia secondary to treatment with acid secretion inhibitors or atrophic gastritis[16]. Our patient exhibited elevations in both serum AFP, AFP-L3 percentage, and chromogranin. Serum CA19-9 was not established pre-operatively, but it was shown to not be elevated post-operatively.
An increased serum AFP and HCV positivity raised the differential diagnosis of a primary HCC. The imaging characteristics and the biopsy results of the lesion in the left liver were not consistent with HCC. Initial CT-guided core biopsy findings of a high-grade tumor with neuroendocrine differentiation highlight the sampling error that has been reported in similar cases of combined PLC and the need to sample multiple areas of the tumor if a combined tumor is suspected[3,10,17]. Our patient had a history of low-grade neuroendocrine neoplasm (carcinoid tumor) that was very different from the high-grade tumors of the liver. Theoretically, metastasis was a possibility given the known rate of discordance in tumoral grade for neuroendocrine tumors between the primary lesion and the liver metastasis[18]. Although the lesion in the right liver was not biopsied, it had similar imaging features by CT and MRI and was thought to be similar in etiology. CC was not strongly considered pre-operatively, given the biopsy finding, imaging features, and tumor markers. Summarizing all the information known at the time, the decision was made for resection of both the liver lesions, which we felt was indicated in either of the main differential diagnoses considered. If truly a metastatic neuroendocrine tumor, these were isolated lesions and amenable to complete resection, which is an established guideline-based recommendation. If the lesions were truly primary lesions (HCC or mixed tumor), resection would be indicated in that case as well, given no distant disease and a liver without fibrosis or cirrhosis.
Histologic examination of the larger (segment 4) tumor in our patient revealed a variegated histology, with regions containing hepatocellular, biliary and neuroendocrine differentiation within the same tumor. By morphology, intermixed components of solid/trabecular architecture with polygonal cells (HCC), glandular architecture (CC), and solid/nested architecture with rounded cells and “salt and pepper” nuclei (neuroendocrine) were seen. Additionally, the triple differentiation was demonstrated immunohistochemically, namely the expression of hepatocellular (HepPar-1, AFP), biliary (CK-7, CK-19) and neuroendocrine (synaptophysin, neuron-specific enolase) markers in the different components of the tumor. This is in contrast with so-called collision tumors, or spatially separate tumor nodules within the same liver, and meets the consensus criteria for a mixed tumor[2,10]. The smaller (segment 7) tumor morphologically resembled a mANEC, with an adenocarcinoma-like architecture and with expression of neuroendocrine markers (synaptophysin). As mentioned previously, given the propensity of HCC for intra-hepatic metastatic spread, the relative size of the two tumors, and the fact that metastasis to the lymph node detected during the surgery had HCC morphology and the fact that both had neuroendocrine differentiation, it was favored that the smaller tumor represented a metastatic focus of the larger tumor. Additionally, morphology and immunophenotype of the tumors excluded metastatic carcinoid tumor, as was suspected initially clinically.
The relative rarity of mixed tumors with three differentiation pathways has made it impossible to reach meaningful conclusions about the risk factors, cell of origin, prognosis, and treatment options. Most reports on these clinical aspects have focused on mHCC-CC or mANEC tumors. It has been postulated that mHCC-CC cases share many of the risk factors with HCC and CC, including cirrhosis and chronic infection with hepatitis B virus or HCV[17]. However, one of the only case reports of a mixed tumor with three differentiation pathways occurred in a young patient with no known identifiable risk factors[7]. Ethnic and environmental influences have been proposed to affect risk[7]. Our patient exhibited chronic infection with HCV without established cirrhosis.
Previous reports have suggested hepatocyte progenitor/stem cells as the likely origin of combined liver tumors[19-21]. As these transformed cells clonally evolve, they may acquire varying and divergent phenotypic and mutational characteristics. This clonal evolution can lead to varying proportions of subtypes in the tumor. The larger tumor in our patient exhibited predominantly hepatocellular differentiation with intermixed regions of biliary and neuroendocrine differentiation, whereas the smaller tumor showed biliary and neuroendocrine differentiation. This is more in keeping with divergent clonal evolution of hepatic stem cells.
As far as prognosis is concerned, mHCC-CCs tend to be considered to have a worse prognosis than HCC but a better prognosis than CC[17]. Previous reports have highlighted the potential importance of the relative contribution of the different components to the final outcome of the patient[17]. The neuroendocrine component is considered to be more aggressive[4,7]. Evidence for the different biological aggressiveness of the different components exists in the form of microdissection experiments, in which different regions of combined tumors were interrogated for molecular alterations[7]. These showed that the neuroendocrine component of the tumor was associated with detectable mutational change and genomic instability and allelic imbalance involving the 5p and 7p chromosomal regions (regions containing tumor suppressor and proto-oncogenes), corresponding to more aggressive behavior[7]. Additionally, it has been noted that the neuroendocrine component usually is involved in metastatic spread in patients with mixed hepatic tumors[4]. Interestingly, in our patient, the micro-metastasis detected during initial surgery in one of the regional lymph nodes had features of HCC rather than NEC, and the subsequent metastatic focus in another lymph node found post-surgery had features of CC, potentially indicating a heterogeneity and different molecular events present in different patients and clonal subpopulations. This is further supported by findings of microsatellite stability on next-generation sequencing performed on our patient’s larger tumor. Granted, microdissection to separate mutational characteristics of the different regions of the tumor was not performed in our patient, and the results most likely are more representative of the predominant component present (i.e. HCC).
Treatment is usually attempted with surgical resection, with liver transplant not proving to be superior to a minor or major hepatectomy[3]. The benefits of lymphadenectomy outside of staging are debatable[3]. Other forms of treatment, such as transarterial chemoembolization, radioembolization, or chemotherapy have not been studied adequately and are used in patients who are poor surgical candidates in the context of palliative treatment[3]. The decision on whether to treat a patient with adjuvant therapy or what type of regime for adjuvant therapy is based on the evaluation of the tumor with worst outcome. In our patient, the features of her segment 4 tumor and positive lymph node weighed more on the decision of adjuvant therapy in comparison to the segment 7 tumor. The segment 4 tumor had mixed CC, HCC, and neuroendocrine features with a Ki-67 proliferation index of 60%, which suggests aggressiveness of the tumor and high risk for cancer recurrence. It is hard to determine which tumor component (CC, HCC, or neuroendocrine) will recur. No randomized clinical trial has supported the benefit of adjuvant therapy for HCC so far. Even though one lymph node was found to have microscopic foci of HCC, no adjuvant therapy for HCC was recommended. Adjuvant therapy with cisplatin and gemcitabine for CC and adjuvant therapy with carboplatin/etoposide for neuroendocrine tumor were initially considered. Our patient developed disease recurrence in the form of a hepatic dome lesion and retro-pancreatic lymphadenopathy with biopsy proven CC, detected on imaging following segmental resection of the two tumors, again in keeping with the more aggressive nature of these tumors previously reported. As such, standard first-line palliative systemic therapy with cisplatin and gemcitabine was favored.
In conclusion, liver tumors with more than two differentiation pathways are exceedingly rare. A heightened index of suspicion needs to be maintained by clinicians, radiologists, and pathologists to reach the correct diagnosis. Most of the clinical aspects, risk factors, and prognostic data currently are extrapolated from cases of mixed tumors with two pathways of differentiation, mainly mHCC-CC and mANEC tumors. More research is needed to reach adequately solid conclusions concerning appropriate management and prognosis.
Manuscript source: Unsolicited manuscript
Specialty type: Oncology
Country/Territory of origin: United States
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): B
Grade C (Good): C
Grade D (Fair): D
Grade E (Poor): 0
P-Reviewer: Ashida R, Di Martino V, Streba LAM S-Editor: Fan JR L-Editor: A P-Editor: Wang LL
| 1. | Rastogi A. Changing role of histopathology in the diagnosis and management of hepatocellular carcinoma. World J Gastroenterol. 2018;24:4000-4013. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 55] [Cited by in RCA: 72] [Article Influence: 10.3] [Reference Citation Analysis (1)] |
| 2. | Brunt E, Aishima S, Clavien PA, Fowler K, Goodman Z, Gores G, Gouw A, Kagen A, Klimstra D, Komuta M, Kondo F, Miksad R, Nakano M, Nakanuma Y, Ng I, Paradis V, Nyun Park Y, Quaglia A, Roncalli M, Roskams T, Sakamoto M, Saxena R, Sempoux C, Sirlin C, Stueck A, Thung S, Tsui WMS, Wang XW, Wee A, Yano H, Yeh M, Zen Y, Zucman-Rossi J, Theise N. cHCC-CCA: Consensus terminology for primary liver carcinomas with both hepatocytic and cholangiocytic differentation. Hepatology. 2018;68:113-126. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 195] [Cited by in RCA: 260] [Article Influence: 37.1] [Reference Citation Analysis (0)] |
| 3. | Stavraka C, Rush H, Ross P. Combined hepatocellular cholangiocarcinoma (cHCC-CC): an update of genetics, molecular biology, and therapeutic interventions. J Hepatocell Carcinoma. 2019;6:11-21. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 59] [Cited by in RCA: 95] [Article Influence: 13.6] [Reference Citation Analysis (0)] |
| 4. | Nishino H, Hatano E, Seo S, Shibuya S, Anazawa T, Iida T, Masui T, Taura K, Haga H, Uemoto S. Histological features of mixed neuroendocrine carcinoma and hepatocellular carcinoma in the liver: a case report and literature review. Clin J Gastroenterol. 2016;9:272-279. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 22] [Cited by in RCA: 27] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 5. | Harada K, Sato Y, Ikeda H, Maylee H, Igarashi S, Okamura A, Masuda S, Nakanuma Y. Clinicopathologic study of mixed adenoneuroendocrine carcinomas of hepatobiliary organs. Virchows Arch. 2012;460:281-289. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 73] [Cited by in RCA: 74] [Article Influence: 5.7] [Reference Citation Analysis (0)] |
| 6. | Zheng SL, Yip VS, Pedica F, Prachalias A, Quaglia A. Intrahepatic bile duct mixed adenoneuroendocrine carcinoma: a case report and review of the literature. Diagn Pathol. 2015;10:204. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 13] [Cited by in RCA: 12] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 7. | Beard RE, Finkelstein SD, Borhani AA, Minervini MI, Marsh JW. A massive hepatic tumor demonstrating hepatocellular, cholangiocarcinoma and neuroendocrine lineages: A case report and review of the literature. Int J Surg Case Rep. 2017;37:26-32. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 5] [Cited by in RCA: 12] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 8. | He C, Yin HF, Liu P, Zhang Y, Zhang JB. [Clinicopathologic features of combined hepatic carcinoma]. Zhonghua Bing Li Xue Za Zhi. 2013;42:824-828. [PubMed] |
| 9. | Ariff B, Lloyd CR, Khan S, Shariff M, Thillainayagam AV, Bansi DS, Khan SA, Taylor-Robinson SD, Lim AK. Imaging of liver cancer. World J Gastroenterol. 2009;15:1289-1300. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 80] [Cited by in RCA: 75] [Article Influence: 4.7] [Reference Citation Analysis (0)] |
| 10. | Gera S, Ettel M, Acosta-Gonzalez G, Xu R. Clinical features, histology, and histogenesis of combined hepatocellular-cholangiocarcinoma. World J Hepatol. 2017;9:300-309. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 62] [Cited by in RCA: 74] [Article Influence: 9.3] [Reference Citation Analysis (0)] |
| 11. | Skoura E, Michopoulou S, Mohmaduvesh M, Panagiotidis E, Al Harbi M, Toumpanakis C, Almukhailed O, Kayani I, Syed R, Navalkissoor S, Ell PJ, Caplin ME, Bomanji J. The Impact of 68Ga-DOTATATE PET/CT Imaging on Management of Patients with Neuroendocrine Tumors: Experience from a National Referral Center in the United Kingdom. J Nucl Med. 2016;57:34-40. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 90] [Cited by in RCA: 110] [Article Influence: 11.0] [Reference Citation Analysis (0)] |
| 12. | Lee H, Yoon JH, Kim H, Yi NJ, Hong SK, Yoon KC, Kim HS, Ahn SW, Choi JY, Choi Y, Lee HW, Yi JY, Lee KB, Lee KW, Suh KS. False Positive Diagnosis of Hepatocellular Carcinoma in Liver Resection Patients. J Korean Med Sci. 2017;32:315-320. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 9] [Cited by in RCA: 10] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 13. | Qin XL, Wang ZR, Shi JS, Lu M, Wang L, He QR. Utility of serum CA19-9 in diagnosis of cholangiocarcinoma: in comparison with CEA. World J Gastroenterol. 2004;10:427-432. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 103] [Cited by in RCA: 127] [Article Influence: 6.0] [Reference Citation Analysis (0)] |
| 14. | Leerapun A, Suravarapu SV, Bida JP, Clark RJ, Sanders EL, Mettler TA, Stadheim LM, Aderca I, Moser CD, Nagorney DM, LaRusso NF, de Groen PC, Menon KV, Lazaridis KN, Gores GJ, Charlton MR, Roberts RO, Therneau TM, Katzmann JA, Roberts LR. The utility of Lens culinaris agglutinin-reactive alpha-fetoprotein in the diagnosis of hepatocellular carcinoma: evaluation in a United States referral population. Clin Gastroenterol Hepatol. 2007;5:394-402; quiz 267. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 96] [Cited by in RCA: 99] [Article Influence: 5.5] [Reference Citation Analysis (0)] |
| 15. | Sobotka LA, Hake T, Kelly C, Mousa L. Metastatic neuroendocrine carcinoma presenting as multifocal liver lesions with elevated alpha-fetoprotein. Clin Case Rep. 2019;7:251-253. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 2] [Cited by in RCA: 2] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 16. | Syversen U, Ramstad H, Gamme K, Qvigstad G, Falkmer S, Waldum HL. Clinical significance of elevated serum chromogranin A levels. Scand J Gastroenterol. 2004;39:969-973. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 35] [Cited by in RCA: 37] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 17. | Wang AQ, Zheng YC, Du J, Zhu CP, Huang HC, Wang SS, Wu LC, Wan XS, Zhang HH, Miao RY, Sang XT, Zhao HT. Combined hepatocellular cholangiocarcinoma: Controversies to be addressed. World J Gastroenterol. 2016;22:4459-4465. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 37] [Cited by in RCA: 42] [Article Influence: 4.7] [Reference Citation Analysis (0)] |
| 18. | Adesoye T, Daleo MA, Loeffler AG, Winslow ER, Weber SM, Cho CS. Discordance of Histologic Grade Between Primary and Metastatic Neuroendocrine Carcinomas. Ann Surg Oncol. 2015;22 Suppl 3:S817-S821. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 12] [Cited by in RCA: 17] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 19. | Choi GH, Ann SY, Lee SI, Kim SB, Song IH. Collision tumor of hepatocellular carcinoma and neuroendocrine carcinoma involving the liver: Case report and review of the literature. World J Gastroenterol. 2016;22:9229-9234. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 25] [Cited by in RCA: 26] [Article Influence: 2.9] [Reference Citation Analysis (0)] |
| 20. | Hu J, Yuan R, Huang C, Shao J, Zou S, Wang K. Double primary hepatic cancer (hepatocellular carcinoma and intrahepatic cholangiocarcinoma) originating from hepatic progenitor cell: a case report and review of the literature. World J Surg Oncol. 2016;14:218. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 4] [Cited by in RCA: 10] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 21. | Ishida M, Seki K, Tatsuzawa A, Katayama K, Hirose K, Azuma T, Imamura Y, Abraham A, Yamaguchi A. Primary hepatic neuroendocrine carcinoma coexisting with hepatocellular carcinoma in hepatitis C liver cirrhosis: report of a case. Surg Today. 2003;33:214-218. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 56] [Cited by in RCA: 49] [Article Influence: 2.2] [Reference Citation Analysis (0)] |