Published online Apr 24, 2021. doi: 10.5306/wjco.v12.i4.238
Peer-review started: January 14, 2021
First decision: February 15, 2021
Revised: March 14, 2021
Accepted: April 5, 2021
Article in press: April 5, 2021
Published online: April 24, 2021
Processing time: 96 Days and 12.5 Hours
Recent studies in non-colorectal malignancy have associated T resident memory (TRM) cells with improved patient survival. It is unknown if TRM plays a role in colorectal cancer (CRC).
To examine the potential role of TRM cells in providing immunogenicity in CRC stratified by microsatellite instability (MSI) and BRAF status.
Patients with known MSI and BRAF mutation status were eligible for inclusion in this study. CRC tumour sections stained with haematoxylin and eosin were microscopically reviewed and the images scanned prior to assessment for location of invading edge and core of tumour. Sequential sections were prepared for quantitative multiplex immunohistochemistry (IHC) staining. Opal Multiplex IHC staining was performed with appropriate positive and negative controls and imaged using a standard fluorescent microscope fitted with a spectral scanning camera (Mantra) in conjunction with Mantra snap software. Images were unmixed and annotated in inForm 2.2.0. Statistical analysis was performed using Graphpad Prism Version 7 and Stata Version 15.
Seventy-two patients with known MSI and BRAF status were included in the study. All patients were assessed for MSI by IHC and high resolution capillary electrophoresis testing and 44 of these patients successfully underwent quantitative multiplex IHC staining. Overall, there was a statistically significant increase in CD8+ TRM cells in the MSI (BRAF mutant and wild type) group over the microsatellite stable (MSS) group. There was a statistically significant difference in CD8+ TRM between high level MSI (MSI-H):BRAF mutant [22.57, 95% confidence interval (CI): 14.31-30.84] vs MSS [8.031 (95%CI: 4.698-11.36)], P = 0.0076 andMSI-H:BRAF wild type [16.18 (95%CI: 10.44-21.93)] vs MSS [8.031 (95%CI: 4.698-11.36)], P = 0.0279. There was no statistically significant difference in CD8 T cells (both CD8+CD103- and CD8+CD103+TRM) between MSI-H: BRAF mutant and wild type CRC.
This study has shown that CD8+ TRM are found in greater abundance in MSI-H CRC, both BRAF mutant and MSI-H:BRAF wild type, when compared with their MSS counterpart. CD8+ TRM may play a role in the immunogenicity in MSI-H CRC (BRAF mutant and BRAF wild type). Further studies should focus on the potential immunogenic qualities of TRM cells and investigate potential immunotherapeutic approaches to improve treatment and survival associated with CRC.
Core Tip: Prior to this study, whether T resident memory (TRM) cells exist in colorectal cancer (CRC) was poorly understood. This study has identified and characterize TRM cells within human CRC. CD8+ TRM cells are found in greater abundance in both high microsatellite instability (MSI-H) BRAF mutant and MSI-H:BRAF wild type CRC when compared with their microsatellite stable counterpart. CD8+ TRM may play a role in the immunogenicity in both BRAF mutant and BRAF wild type MSI-H CRC. Further studies should focus on the potential immunogenic qualities of TRM cells and investigate potential immunotherapeutic approaches to improve treatment and survival associated with CRC based on TRM.
- Citation: Toh JWT, Ferguson AL, Spring KJ, Mahajan H, Palendira U. Cytotoxic CD8+ T cells and tissue resident memory cells in colorectal cancer based on microsatellite instability and BRAF status. World J Clin Oncol 2021; 12(4): 238-248
- URL: https://www.wjgnet.com/2218-4333/full/v12/i4/238.htm
- DOI: https://dx.doi.org/10.5306/wjco.v12.i4.238
Colorectal cancer (CRC) may be divided into microsatellite stable (MSS) and high microsatellite unstable (MSI-H) CRC. Approximately 15% of CRC are MSI-H and are more likely to be immunogenic with abundant tumour infiltrating lymphocytes (TILs)[1-3]. On the other hand, MSS CRC are less likely to be immunogenic, with sparse/limited TILs. Abundance of TILs has been associated improved survival[4-14] and is widely believed to provide MSI-H CRC with a favourable prognosis[15,16] despite the higher grade features also associated with MSI-H CRC, such as larger diameter, poorly differentiated, mucinous tumours.
However, while it is widely known that MSI-H CRC is associated with TILs, there is significant debate as to the composition of TILs within CRC and which component of TILs is most important. Various reports have suggested that within TILs, CD8+, CD4+, CD3+, FOXP3+ and CD45RO+ regulatory cells have important roles in immunosurveillance. There has been no consensus in recent studies. Nosho et al[17] reported on the benefits of CD45RO+ cells, Guidoboni et al[18] on cytotoxic CD3+ and CD8+ cells, Salama et al[19] reported that FOXP3+ cells were associated with favourable prognosis respectively. Potentially, the interaction of all of these cells may provide benefit as these cells have different profiles and roles within an intricate orchestrated immune response. It has been believed for decades that T cells are recruited in response to cancer, and constantly recirculate in the blood and lymphatic system. The benefit of these TILs in CRC has been reported and has been subject to recent reviews[20,21].
More recently a new lineage of T cells has been discovered which is believed to provide immunity to solid tumours[22-24]. Tissue resident memory (TRM) cells, particularly CD8+ TRM, have been identified in non-colorectal malignancies and its presence has already been shown to be associated with better survival outcomes in lung cancer[25], ovarian cancer[26], breast cancer[27-29] and melanoma[24,30], particularly CD8+ TRM[24-27,31-36]. The difference between CD8+ TRM and conventional cytotoxic CD8+ T cells is that TRM cells, by definition, do not recirculate—rather they remain in peripheral tissue. This is because TRM cells are not just a continuous circulation of T cells, but cells committed to their tissue of residence including tumour tissue, thereby providing immediate and long-term protection against invaders. TRM cells are believed to produce interferon-gamma, granzyme B and perforin[30].
TRM cells at epithelial surfaces develop from precursor cells through a process that relies on transcription factors and cytokines[37] and can be identified by the expression of CD103 (and CD69). Both CD103 and CD69 contribute to the retention of these cells within epithelia and prevent these cells from exiting their resident tissue. CD103 is the alpha chain of integrin, which binds to E-Cadherin which is an epithelial adhesion molecule. Furthermore, TRM cells express checkpoint receptors such as programmed death 1 (PD-1), CTLA4 and Tim-3, potential targets for current immunotherapy strategies. If present in CRC, TRM may represent an avenue for improving survival in CRC.
In this study, we have chosen CD103 status to identify TRM cells. CD39, CD69 and CD103 have been associated with TRM[36,37], with CD103 reported to strongly correlate with a favourable prognosis in cancer patients[38]. While there are studies which have suggested the importance of CD4+ TRM cells[39], the majority of studies have associated improved survival with CD8+ TRM cells[24-26,31-36] and for this reason the main outcome measure chosen for this study was the abundance of CD8+ TRM in the CRC tumour microenvironment. Secondary outcomes included assessing the presence of CD8+ TRM and cytotoxic CD8+ non-TRM cells. Results of the above outcome measures were pooled by microsatellite instability (MSI) and BRAF status.
MSI status was chosen because it is a biomarker widely considered to influence immunogenicity. BRAF status was also chosen because in CRC it has been shown that presence of BRAFV600E mutation nearly always excludes Lynch syndrome and absence of BRAF mutation (BRAF wild type) may be associated with Lynch syndrome (although 30%-40% of MSI-H:BRAF wild type may be sporadic)[40]. Thus this study examined the potential role of TRM cells in providing immunogenicity in CRC stratified by MSI and BRAF status, with CRC divided into three groups: MSS, MSI-H:BRAF mutant (representing sporadic MSI-H, usually associated with epigenetic silencing of MLH-1) and MSI-H:BRAF wild type (approximately 60%-70% associated with Lynch syndrome)[40].
Seventy-two patients with known MSI and BRAF status confirmed by immunohistochemistry (IHC) and high resolution capillary electrophoresis were eligible for inclusion in this study. These patients also had detailed clinical, operative, pathological, adjuvant treatment and follow-up data from a cancer database which was analyzed retrospectively. Institutional board approval was obtained, and patients had provided written consent for the use of their information for research (CH62/62011-136 HREC/11/CRGH206).
Histopathology slides prepared with haematoxylin and eosin were reviewed using an Olympus BX53 microscope to find a representative slide which shows the tumour invading edge and core. These slides were then scanned electronically and the scanned images were reexamined by a pathologist to mark out the invading edge and core. From these blocks, representative slides were recut using a microtome in preparation for quantitative multiplex IHC staining.
Of the 72 original patient tumour specimens prepared on representative slides, 44 formalin fixed paraffin embedded (FFPE) tumour sections were successfully stained with quantitative multiplex IHC staining. All IHC staining was performed on 4-µm-thick sections and stained using an Opal Multiplex IHC assay kit (PerkinElmer, Waltham, MA, United States) and optimised in-house. Briefly, FFPE tissue sections were deparaffinized and rehydrated prior to antigen retrieval by boiling in basic (10 mmol/L Tris base, 1 mmol/L EDTA, 0.05% Tween 20, pH 9.0) buffer. The sections were then incubated with 3% hydrogen peroxide for 20 min at room temperature before washing and blocking with Perkin Elmer Antibody diluent/Block buffer. Sections were then incubated with a single unconjugated primary antibody for 35 min, washed and then incubated with Opal Polymer HRP (Akoya Biosciences, United States) for 10 min. After washing, sections were incubated with Opal fluorochromes at a 1:100 dilution made up in tyramide signal amplification reagent (PerkinElmer, Waltham, MA, United States) for 10 min. The antigen retrieval step was repeated, and tissue was stained for subsequent antibodies as described above. Finally, sections were stained with DAPI (cell signalling technologies) for 5 min and then mounted using Prolong Diamond (Life Technologies). Slides were stored in the dark until imaging was complete. The following antibodies were used; CD8 (ab4055 clone; Abcam), CD103 [EPR4116(2) clone; Abcam] PD1 (NAT105 clone, Cell Marque). The Mantra imaging platform (PerkinElmer) was used for imaging in combination with Mantra snap software for data acquisition. For all quantitative analysis up to 10 randomly selected regions of interest per section were analyzed. InForm advanced image analysis software (PerkinElmer, MA, United States) (PerkinElmer) was used to process and analyze images, with each tissue manually segmented into tissue regions.
Statistical analysis was performed using Graphpad Prism Version 7 and Stata Version 15.
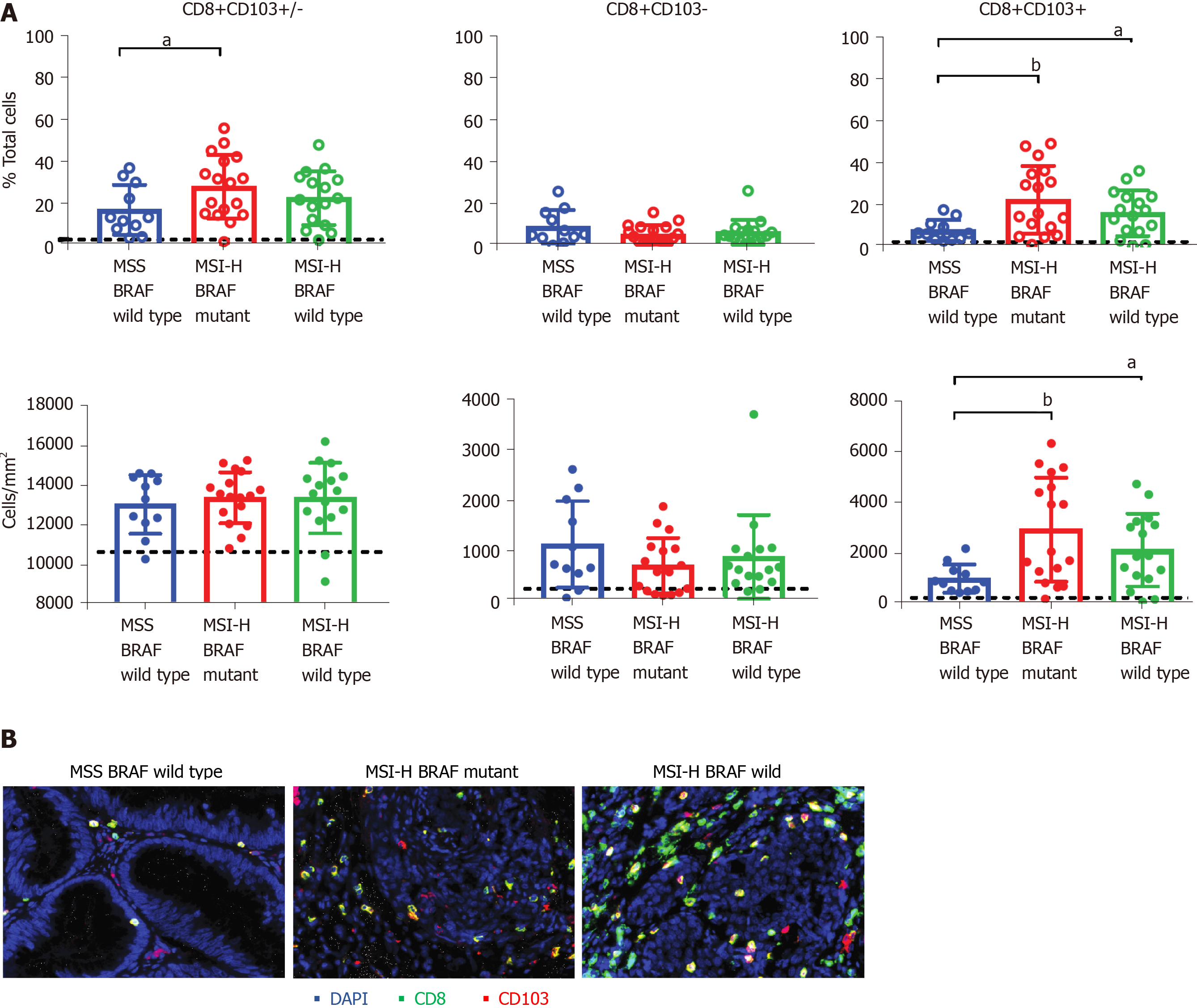
Seventy-two patients with known MSI and BRAF status met the eligibility criteria for this study, and of these 44 patient tumour specimens were successfully stained by quantitative multiplex IHC staining (Figure 1). The percentage and densities of total CD8+ T cells, TRM T cells, and non-TRM CD8+ T cells were calculated for MSS, MSI-H:BRAF mutant and MSI-H:BRAF wild type subgroups. An unpaired student t test was used to compare the mean % TRM/non-TRM cells/total cells between the three groups for each outcome measure. Where there were significant outliers, the median was used instead of mean, and a Mann Whitney U test was performed to compare the median between the three groups. This showed that compared to healthy control all three groups had a statistically greater abundance of CD8+ T cells (Figure 1).
There was a significant difference in the proportion of CD8+ T cells between MSI-H:BRAF mutant and MSS subgroups with 17.24% [95% confidence interval (CI): 9.334-25.15] of nucleated cells in MSS compared to 28.14% (95%CI: 20.38-35.89) in MSI-H:BRAF mutant and 22.82 (95%CI: 16.02-29.62) in MSI-H:BRAF wild type subgroups. On Mann Whitney U analysis, there was a statistically significant difference between MSI-H:BRAF mutant and MSS subgroups (P = 0.0417). Although there was a greater percentage of CD8+ T cells in the MSI-H:BRAF wild type subgroup when compared to MSS, this did not reach statistical significance [22.82 (95%CI: 16.02-29.62) vs 17% (95%CI: 9.334-25.15), P = 0.2841].
Importantly, there were significant differences in the proportions of CD8+ TRM cells between the three subgroups of CRC. Compared to MSS, the MSI-H:BRAF mutant subgroup had a significantly higher proportion of CD8+ TRM cells (Figure 1) with 22.5% (95%CI: 14.31-30.84) in MSI-H:BRAF mutant subgroup and 8.0% in MSS subgroup (95%CI: 4.698-11.36, P = 0.0076). Similarly, the MSI-H:BRAF wild type subgroup had significantly higher CD8+ TRM (16.18%, 95%CI: 10.44-21.93) when compared to MSS (8%, 95%CI: 4.698-11.36 and P = 0.0279). The difference in abundance of TRM between MSI-H: BRAF mutant and MSI-H:BRAF wild type subgroups was not significant (Figure 1). There was no statistically significant difference in non-TRM CD8+ cells between the three subgroups.
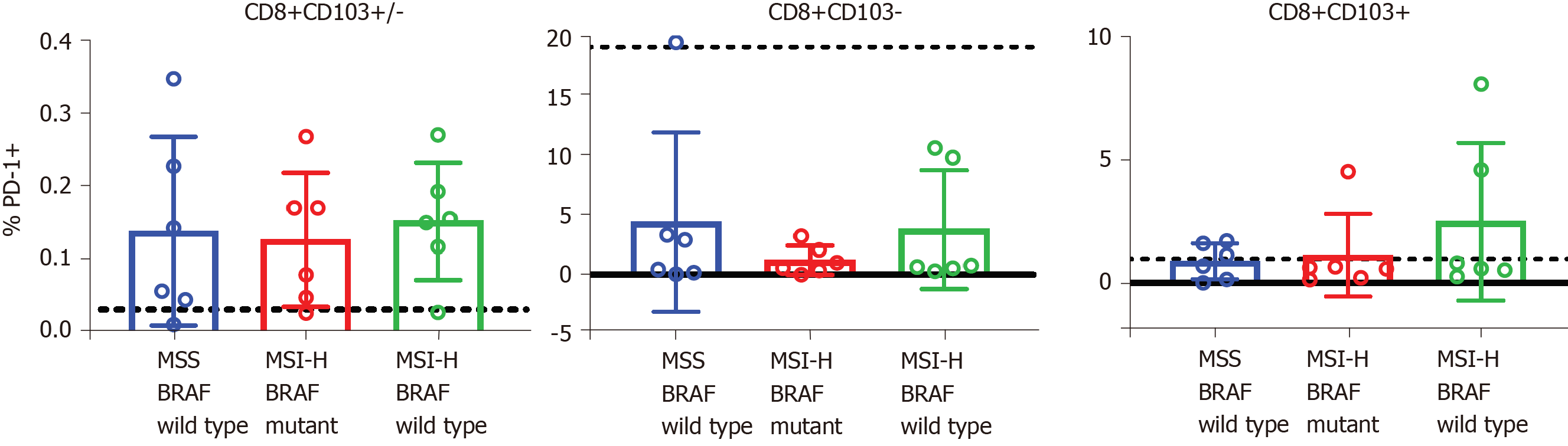
We next sought to determine whether the expression of checkpoint receptor, PD-1 was impacted by MSI status or BRAF mutation. We enumerated the proportion of T cells expressing PD-1 in all three subgroups. PD-1 expression was present in both TRM T cells and non-TRM T cell populations, although the proportion of non-TRM T cells expressing PD-1 was slightly higher than that of the TRM T cell population (Figure 2). While there was a trend to increased PD-1+ expression in the TRM T cell population in MSI-H:BRAF wild type compared to MSI-H:BRAF mutant and MSS subgroups, there was, however, no statistically significant differences in the proportion of T cells expressing PD-1 between the three groups.
Characteristics of TILS within CRC have been poorly defined to date, and there is a paucity of information on TRM T cells in CRC. In humans, within the circulation, two types of memory T cells exist — central memory (TCM) and effector memory (TEM). For decades, it was thought that T lymphocyte populations in peripheral tissues including TILs were largely maintained by the continuous circulation of these memory T cells as well as other circulatory cells. Recent studies have challenged this concept and provided evidence for the existence of another subset of CD8+ T cells — tissue-TRM T cells. These cells are permanently resident (i.e., do not recirculate) in both lymphoid and non-lymphoid tissues and represent a distinct subset to circulatory TEM or TCM.
Prior to this study the existence of TRM cells in CRC was poorly understood and the aim of this study was to identify and characterize TRM cells within human CRC. If present, TRM cells may be important in the protection against recurrence, dissemination and metastasis. A favourable prognosis associated with the presence of TRM cells has already been reported in studies of high-grade serous ovarian cancer patients, non-small cell lung carcinoma patients and breast cancer[41]. In lung cancer, although only a small proportion of CD8+ T cells were reported to be TRM, the majority of TRM cells were tumour-reactive. Studies in melanoma have also shown an association between TRM and favourable prognosis[30,42].
This present study showed that TRM cells exist in MSS, MSI-H:BRAF mutant and MSI-H:BRAF wild type CRC. However, they are in greater abundance in MSI-H than MSS CRC. This is supported by the findings of a recent study by de Vries et al[21] on 35 CRC tissues which demonstrated that CD103+ TRM cells were most abundant in MSI-H CRC. In that study 13 MSI-H CRC were included and BRAF status was not considered. Our study had 42 MSI-H CRC patients which were all subject to immunoscoring, of which 33 went on to have multiplex IHC staining to look for presence of TRM cells and of these 17 were BRAF mutant and 16 were BRAF wild type. Of the 30 MSS CRC patients, 11 went on to have multiplex IHC staining to look for presence of TRM cells. Our study showed that both MSI-H:BRAF mutant and BRAF wild type subgroups had significantly greater abundance of TRM cells when compared to the MSS subgroup. There was no statistically significant difference between MSI-H:BRAF mutant and BRAF wild type subgroups.
There was a trend to increased PD-1+ in the TRM T cell population in MSI-H:BRAF wild type compared to MSI-H:BRAF mutant and MSS subgroups, but this was not statistically significant. This is an important finding as in advanced CRC this T cell subpopulation amongst other PD-1+ immune cells may be exhausted by PD-1/PD-1 ligand binding, a mechanism of escaping immunosurveillance by tumours. Reactivation of exhausted PD-1+ TRM cells may provide therapeutic opportunities that may be exploited in MSI-H:BRAF wild type CRC. PD-1 expression has been reported to be more prominent in cells with a TRM phenotype[25,43], and in the case of CRC it may be their numbers that impacts the outcome.
The potential ways which CD8+ TRM cells can be harnessed to improve the success of immunotherapeutic targets has already been the focus of several general reviews[38,44-46] as well as in breast cancer[31] and melanoma[47,48], with Edwards et al[42] reporting that CD8+ TRM cells being prognostic in metastatic melanoma and potentially initiating response to immunotherapy with anti-PD-1. Therefore, the role of TRM cells could have important implications for checkpoint-inhibition therapy in CRC[49].
The mechanisms by which TRM cells confer immunity remains unclear. Recent studies by Park et al[24] have demonstrated that depletion of TRM cells in melanoma triggered tumour outgrowth of mice with occult melanoma, demonstrating that TRM cells have an important role in cancer surveillance and equilibrium and may keep cancer cells dormant[50]. This effect may be long term as TRM cell populations are able to persist in barrier tissues for a prolonged period of time[51]. Menares et al[52] suggest that the mechanism by which TRM cells provide immunity is via cross-talk with dendritic cells to trigger the spread of cytotoxic CD8+ T cells in response to tumour.
The use of a single marker (CD103) to define TRM cells is a limitation. However, many studies have consistently shown that CD103+CD8+ T cells have the hallmark of TRM T cells[24,53]. Further, the study sample size was small (n = 72) and we were only able to successfully perform quantitative multiplex IHC staining and imaging on 44 samples. Nonetheless, this is to date the largest study on TRM cells in CRC.
This study has shown that in CRC a significant fraction of TILs is made up of TRM cells and are found in greater abundance in MSI-H:BRAF mutant and MSI-H:BRAF wild type compared with MSS CRC tumours. Acknowledging that there is a complex interaction of factors at play in influencing the prognosis in CRC, the abundance of TRM cells may contribute to the favourable prognosis observed in MSI-H CRC when compared to MSS[15,16]. This is because MSS CRC has significantly less TRM cells when compared to MSI-H CRC. The abundance of TRM cells in MSI-H CRC may also explain the reduced likelihood of metastases in patients with MSI-H CRC[54] and also the difference in response to immunotherapy between MSI-H CRC and MSS CRC[20]. It is important to understand that while TRM cells can provide immunity in solid organ tumours, the immunity conferred is by no means absolute and cancers still arise and continue to disseminate despite the presence of TRM cells. Further studies should focus on the potential immunogenic qualities of TRM cells and investigate potential immunotherapeutic approaches to improve treatment and survival associated with CRC.
The presence of resident memory T (TRM) cells has already been reported in studies of high-grade serous ovarian cancer patients, non-small cell lung carcinoma patients and breast cancer, but there has been limited evidence on TRM cells in the scientific literature in colorectal cancer (CRC). This is a landmark study on TRM cells in CRC.
With TRM cells showing promise in several solid organ tumour studies, the premise of this study was to evaluate if TRM cells are present in CRC and thus potentially be a target for immunotherapy/novel target therapy in the future.
The objective of this study was to examine the potential role of TRM cells in providing immunogenicity in CRC stratified by microsatellite instability (MSI) and BRAF status.
Formalin fixed paraffin embedded tumour sections were successfully stained using quantitative multiplex IHC staining. All IHC staining was performed using an Opal Multiplex immunohistochemistry (IHC) assay kit (PerkinElmer, Waltham, MA, United States) and optimised in-house.
This study has shown that CD8+ TRM are found in greater abundance in both high level MSI (MSI-H):BRAF mutant and MSI-H:BRAF wild type CRC when compared with their microsatellite stable (MSS) counterpart. CD8+ TRM may play a role in the immunogenicity in both BRAF mutant and BRAF wild type MSI-H CRC. The abundance of TRM cells may contribute to the favourable prognosis observed in MSI-H CRC when compared to MSS CRC.
TRM cells are found in greater abundance and contributes to the immunogenicity of MSI-H CRCs.
TRM cells may be a target for immunotherapy/novel target therapy in MSI-H CRCs, and future research should focus on the potential value of TRM cells, as well as therapeutic agents that may stimulate TRM activity or adoptive cell transfer aimed at harvesting and utilizing the patients' own immune cells such as specially altered T-cells to precisely and specifically target cancer cells.
The authors thank Chapuis P who maintains the Colorectal Cancer Resection Database and advises on entry of clinical and follow up data as to occurrence, date and cause of death. The authors thank Cancer Council NSW (RG18-08) and Bio-Connect from the University of Sydney.
Manuscript source: Invited manuscript
Corresponding Author's Membership in Professional Societies: Royal Australasian College of Surgeons; and Colorectal Surgery Society of Australia and New Zealand.
Specialty type: Oncology
Country/Territory of origin: Australia
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): 0
Grade C (Good): C
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Taira K S-Editor: Gao CC L-Editor: A P-Editor: Wang LL
| 1. | Le DT, Uram JN, Wang H, Bartlett BR, Kemberling H, Eyring AD, Skora AD, Luber BS, Azad NS, Laheru D, Biedrzycki B, Donehower RC, Zaheer A, Fisher GA, Crocenzi TS, Lee JJ, Duffy SM, Goldberg RM, de la Chapelle A, Koshiji M, Bhaijee F, Huebner T, Hruban RH, Wood LD, Cuka N, Pardoll DM, Papadopoulos N, Kinzler KW, Zhou S, Cornish TC, Taube JM, Anders RA, Eshleman JR, Vogelstein B, Diaz LA Jr. PD-1 Blockade in Tumors with Mismatch-Repair Deficiency. N Engl J Med. 2015;372:2509-2520. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 6096] [Cited by in RCA: 7233] [Article Influence: 723.3] [Reference Citation Analysis (0)] |
| 2. | Gatalica Z, Snyder C, Maney T, Ghazalpour A, Holterman DA, Xiao N, Overberg P, Rose I, Basu GD, Vranic S, Lynch HT, Von Hoff DD, Hamid O. Programmed cell death 1 (PD-1) and its ligand (PD-L1) in common cancers and their correlation with molecular cancer type. Cancer Epidemiol Biomarkers Prev. 2014;23:2965-2970. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 324] [Cited by in RCA: 405] [Article Influence: 36.8] [Reference Citation Analysis (0)] |
| 3. | Topalian SL, Hodi FS, Brahmer JR, Gettinger SN, Smith DC, McDermott DF, Powderly JD, Carvajal RD, Sosman JA, Atkins MB, Leming PD, Spigel DR, Antonia SJ, Horn L, Drake CG, Pardoll DM, Chen L, Sharfman WH, Anders RA, Taube JM, McMiller TL, Xu H, Korman AJ, Jure-Kunkel M, Agrawal S, McDonald D, Kollia GD, Gupta A, Wigginton JM, Sznol M. Safety, activity, and immune correlates of anti-PD-1 antibody in cancer. N Engl J Med. 2012;366:2443-2454. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 8900] [Cited by in RCA: 9899] [Article Influence: 761.5] [Reference Citation Analysis (0)] |
| 4. | Pagès F, Berger A, Camus M, Sanchez-Cabo F, Costes A, Molidor R, Mlecnik B, Kirilovsky A, Nilsson M, Damotte D, Meatchi T, Bruneval P, Cugnenc PH, Trajanoski Z, Fridman WH, Galon J. Effector memory T cells, early metastasis, and survival in colorectal cancer. N Engl J Med. 2005;353:2654-2666. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1553] [Cited by in RCA: 1630] [Article Influence: 81.5] [Reference Citation Analysis (0)] |
| 5. | Mlecnik B, Tosolini M, Kirilovsky A, Berger A, Bindea G, Meatchi T, Bruneval P, Trajanoski Z, Fridman WH, Pagès F, Galon J. Histopathologic-based prognostic factors of colorectal cancers are associated with the state of the local immune reaction. J Clin Oncol. 2011;29:610-618. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 689] [Cited by in RCA: 793] [Article Influence: 56.6] [Reference Citation Analysis (0)] |
| 6. | Ling A, Edin S, Wikberg ML, Öberg Å, Palmqvist R. The intratumoural subsite and relation of CD8(+) and FOXP3(+) T lymphocytes in colorectal cancer provide important prognostic clues. Br J Cancer. 2014;110:2551-2559. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 72] [Cited by in RCA: 94] [Article Influence: 8.5] [Reference Citation Analysis (0)] |
| 7. | Michael-Robinson JM, Biemer-Hüttmann A, Purdie DM, Walsh MD, Simms LA, Biden KG, Young JP, Leggett BA, Jass JR, Radford-Smith GL. Tumour infiltrating lymphocytes and apoptosis are independent features in colorectal cancer stratified according to microsatellite instability status. Gut. 2001;48:360-366. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 140] [Cited by in RCA: 156] [Article Influence: 6.5] [Reference Citation Analysis (0)] |
| 8. | Svennevig JL, Lunde OC, Holter J, Bjørgsvik D. Lymphoid infiltration and prognosis in colorectal carcinoma. Br J Cancer. 1984;49:375-377. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 134] [Cited by in RCA: 149] [Article Influence: 3.6] [Reference Citation Analysis (0)] |
| 9. | Jass JR. Lymphocytic infiltration and survival in rectal cancer. J Clin Pathol. 1986;39:585-589. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 259] [Cited by in RCA: 271] [Article Influence: 6.9] [Reference Citation Analysis (0)] |
| 10. | Shunyakov L, Ryan CK, Sahasrabudhe DM, Khorana AA. The influence of host response on colorectal cancer prognosis. Clin Colorectal Cancer. 2004;4:38-45. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 33] [Cited by in RCA: 34] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 11. | Dahlin AM, Henriksson ML, Van Guelpen B, Stenling R, Oberg A, Rutegård J, Palmqvist R. Colorectal cancer prognosis depends on T-cell infiltration and molecular characteristics of the tumor. Mod Pathol. 2011;24:671-682. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 158] [Cited by in RCA: 182] [Article Influence: 13.0] [Reference Citation Analysis (0)] |
| 12. | Chiba T, Ohtani H, Mizoi T, Naito Y, Sato E, Nagura H, Ohuchi A, Ohuchi K, Shiiba K, Kurokawa Y, Satomi S. Intraepithelial CD8+ T-cell-count becomes a prognostic factor after a longer follow-up period in human colorectal carcinoma: possible association with suppression of micrometastasis. Br J Cancer. 2004;91:1711-1717. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 139] [Cited by in RCA: 160] [Article Influence: 7.6] [Reference Citation Analysis (0)] |
| 13. | Zlobec I, Lugli A, Baker K, Roth S, Minoo P, Hayashi S, Terracciano L, Jass JR. Role of APAF-1, E-cadherin and peritumoral lymphocytic infiltration in tumour budding in colorectal cancer. J Pathol. 2007;212:260-268. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 95] [Cited by in RCA: 107] [Article Influence: 5.9] [Reference Citation Analysis (0)] |
| 14. | Prall F, Dührkop T, Weirich V, Ostwald C, Lenz P, Nizze H, Barten M. Prognostic role of CD8+ tumor-infiltrating lymphocytes in stage III colorectal cancer with and without microsatellite instability. Hum Pathol. 2004;35:808-816. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 174] [Cited by in RCA: 186] [Article Influence: 8.9] [Reference Citation Analysis (0)] |
| 15. | Popat S, Hubner R, Houlston RS. Systematic review of microsatellite instability and colorectal cancer prognosis. J Clin Oncol. 2005;23:609-618. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1267] [Cited by in RCA: 1347] [Article Influence: 67.4] [Reference Citation Analysis (1)] |
| 16. | Guastadisegni C, Colafranceschi M, Ottini L, Dogliotti E. Microsatellite instability as a marker of prognosis and response to therapy: a meta-analysis of colorectal cancer survival data. Eur J Cancer. 2010;46:2788-2798. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 264] [Cited by in RCA: 305] [Article Influence: 20.3] [Reference Citation Analysis (0)] |
| 17. | Nosho K, Baba Y, Tanaka N, Shima K, Hayashi M, Meyerhardt JA, Giovannucci E, Dranoff G, Fuchs CS, Ogino S. Tumour-infiltrating T-cell subsets, molecular changes in colorectal cancer, and prognosis: cohort study and literature review. J Pathol. 2010;222:350-366. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 408] [Cited by in RCA: 397] [Article Influence: 26.5] [Reference Citation Analysis (0)] |
| 18. | Guidoboni M, Gafà R, Viel A, Doglioni C, Russo A, Santini A, Del Tin L, Macrì E, Lanza G, Boiocchi M, Dolcetti R. Microsatellite instability and high content of activated cytotoxic lymphocytes identify colon cancer patients with a favorable prognosis. Am J Pathol. 2001;159:297-304. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 224] [Cited by in RCA: 249] [Article Influence: 10.4] [Reference Citation Analysis (0)] |
| 19. | Salama P, Phillips M, Grieu F, Morris M, Zeps N, Joseph D, Platell C, Iacopetta B. Tumor-infiltrating FOXP3+ T regulatory cells show strong prognostic significance in colorectal cancer. J Clin Oncol. 2009;27:186-192. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 713] [Cited by in RCA: 801] [Article Influence: 47.1] [Reference Citation Analysis (0)] |
| 20. | Toh JWT, de Souza P, Lim SH, Singh P, Chua W, Ng W, Spring KJ. The Potential Value of Immunotherapy in Colorectal Cancers: Review of the Evidence for Programmed Death-1 Inhibitor Therapy. Clin Colorectal Cancer. 2016;15:285-291. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 54] [Cited by in RCA: 68] [Article Influence: 7.6] [Reference Citation Analysis (0)] |
| 21. | de Vries NL, van Unen V, Ijsselsteijn ME, Abdelaal T, van der Breggen R, Farina Sarasqueta A, Mahfouz A, Peeters KCMJ, Höllt T, Lelieveldt BPF, Koning F, de Miranda NFCC. High-dimensional cytometric analysis of colorectal cancer reveals novel mediators of antitumour immunity. Gut. 2020;69:691-703. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 71] [Cited by in RCA: 88] [Article Influence: 17.6] [Reference Citation Analysis (0)] |
| 22. | Dadi S, Li MO. Tissue-resident lymphocytes: sentinel of the transformed tissue. J Immunother Cancer. 2017;5:41. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 7] [Cited by in RCA: 7] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 23. | Dadi S, Chhangawala S, Whitlock BM, Franklin RA, Luo CT, Oh SA, Toure A, Pritykin Y, Huse M, Leslie CS, Li MO. Cancer Immunosurveillance by Tissue-Resident Innate Lymphoid Cells and Innate-like T Cells. Cell. 2016;164:365-377. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 213] [Cited by in RCA: 281] [Article Influence: 31.2] [Reference Citation Analysis (0)] |
| 24. | Park SL, Buzzai A, Rautela J, Hor JL, Hochheiser K, Effern M, McBain N, Wagner T, Edwards J, McConville R, Wilmott JS, Scolyer RA, Tüting T, Palendira U, Gyorki D, Mueller SN, Huntington ND, Bedoui S, Hölzel M, Mackay LK, Waithman J, Gebhardt T. Tissue-resident memory CD8+ T cells promote melanoma-immune equilibrium in skin. Nature. 2019;565:366-371. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 184] [Cited by in RCA: 286] [Article Influence: 40.9] [Reference Citation Analysis (0)] |
| 25. | Djenidi F, Adam J, Goubar A, Durgeau A, Meurice G, de Montpréville V, Validire P, Besse B, Mami-Chouaib F. CD8+CD103+ tumor-infiltrating lymphocytes are tumor-specific tissue-resident memory T cells and a prognostic factor for survival in lung cancer patients. J Immunol. 2015;194:3475-3486. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 344] [Cited by in RCA: 479] [Article Influence: 47.9] [Reference Citation Analysis (0)] |
| 26. | Webb JR, Milne K, Watson P, Deleeuw RJ, Nelson BH. Tumor-infiltrating lymphocytes expressing the tissue resident memory marker CD103 are associated with increased survival in high-grade serous ovarian cancer. Clin Cancer Res. 2014;20:434-444. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 260] [Cited by in RCA: 354] [Article Influence: 29.5] [Reference Citation Analysis (0)] |
| 27. | Egelston CA, Avalos C, Tu TY, Rosario A, Wang R, Solomon S, Srinivasan G, Nelson MS, Huang Y, Lim MH, Simons DL, He TF, Yim JH, Kruper L, Mortimer J, Yost S, Guo W, Ruel C, Frankel PH, Yuan Y, Lee PP. Resident memory CD8+ T cells within cancer islands mediate survival in breast cancer patients. JCI Insight. 2019;4. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 53] [Cited by in RCA: 86] [Article Influence: 14.3] [Reference Citation Analysis (0)] |
| 28. | Schalck A, Bernatchez C, Navin N. Resident Breast T Cells: The Troops Are Already There. Trends Mol Med. 2018;24:821-822. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 1] [Article Influence: 0.1] [Reference Citation Analysis (0)] |
| 29. | Savas P, Virassamy B, Ye C, Salim A, Mintoff CP, Caramia F, Salgado R, Byrne DJ, Teo ZL, Dushyanthen S, Byrne A, Wein L, Luen SJ, Poliness C, Nightingale SS, Skandarajah AS, Gyorki DE, Thornton CM, Beavis PA, Fox SB; Kathleen Cuningham Foundation Consortium for Research into Familial Breast Cancer (kConFab); Darcy PK, Speed TP, Mackay LK, Neeson PJ, Loi S. Single-cell profiling of breast cancer T cells reveals a tissue-resident memory subset associated with improved prognosis. Nat Med. 2018;24:986-993. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 461] [Cited by in RCA: 723] [Article Influence: 103.3] [Reference Citation Analysis (0)] |
| 30. | Khalil S, Bardawil T, Kurban M, Abbas O. Tissue-resident memory T cells in the skin. Inflamm Res. 2020;69:245-254. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 20] [Cited by in RCA: 42] [Article Influence: 8.4] [Reference Citation Analysis (0)] |
| 31. | Byrne A, Savas P, Sant S, Li R, Virassamy B, Luen SJ, Beavis PA, Mackay LK, Neeson PJ, Loi S. Tissue-resident memory T cells in breast cancer control and immunotherapy responses. Nat Rev Clin Oncol. 2020;17:341-348. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 80] [Cited by in RCA: 201] [Article Influence: 40.2] [Reference Citation Analysis (0)] |
| 32. | Yang R, Cheng S, Luo N, Gao R, Yu K, Kang B, Wang L, Zhang Q, Fang Q, Zhang L, Li C, He A, Hu X, Peng J, Ren X, Zhang Z. Distinct epigenetic features of tumor-reactive CD8+ T cells in colorectal cancer patients revealed by genome-wide DNA methylation analysis. Genome Biol. 2019;21:2. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 62] [Cited by in RCA: 93] [Article Influence: 15.5] [Reference Citation Analysis (0)] |
| 33. | Hochheiser K, Aw Yeang HX, Wagner T, Tutuka C, Behren A, Waithman J, Angel C, Neeson PJ, Gebhardt T, Gyorki DE. Accumulation of CD103+ CD8+ T cells in a cutaneous melanoma micrometastasis. Clin Transl Immunology. 2019;8:e1100. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 5] [Cited by in RCA: 8] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 34. | Wu X, Wu P, Shen Y, Jiang X, Xu F. CD8+ Resident Memory T Cells and Viral Infection. Front Immunol. 2018;9:2093. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 31] [Cited by in RCA: 47] [Article Influence: 6.7] [Reference Citation Analysis (0)] |
| 35. | Molodtsov A, Turk MJ. Tissue Resident CD8 Memory T Cell Responses in Cancer and Autoimmunity. Front Immunol. 2018;9:2810. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 60] [Cited by in RCA: 85] [Article Influence: 12.1] [Reference Citation Analysis (0)] |
| 36. | Corgnac S, Boutet M, Kfoury M, Naltet C, Mami-Chouaib F. The Emerging Role of CD8+ Tissue Resident Memory T (TRM) Cells in Antitumor Immunity: A Unique Functional Contribution of the CD103 Integrin. Front Immunol. 2018;9:1904. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 70] [Cited by in RCA: 112] [Article Influence: 16.0] [Reference Citation Analysis (0)] |
| 37. | Duhen T, Duhen R, Montler R, Moses J, Moudgil T, de Miranda NF, Goodall CP, Blair TC, Fox BA, McDermott JE, Chang SC, Grunkemeier G, Leidner R, Bell RB, Weinberg AD. Co-expression of CD39 and CD103 identifies tumor-reactive CD8 T cells in human solid tumors. Nat Commun. 2018;9:2724. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 379] [Cited by in RCA: 641] [Article Influence: 91.6] [Reference Citation Analysis (0)] |
| 38. | Dumauthioz N, Labiano S, Romero P. Tumor Resident Memory T Cells: New Players in Immune Surveillance and Therapy. Front Immunol. 2018;9:2076. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 48] [Cited by in RCA: 77] [Article Influence: 11.0] [Reference Citation Analysis (0)] |
| 39. | Nguyen QP, Deng TZ, Witherden DA, Goldrath AW. Origins of CD4+ circulating and tissue-resident memory T-cells. Immunology. 2019;157:3-12. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 90] [Cited by in RCA: 105] [Article Influence: 17.5] [Reference Citation Analysis (0)] |
| 40. | Newton K, Jorgensen NM, Wallace AJ, Buchanan DD, Lalloo F, McMahon RF, Hill J, Evans DG. Tumour MLH1 promoter region methylation testing is an effective prescreen for Lynch Syndrome (HNPCC). J Med Genet. 2014;51:789-796. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 54] [Cited by in RCA: 53] [Article Influence: 4.8] [Reference Citation Analysis (0)] |
| 41. | Guo L, Cao C, Goswami S, Huang X, Ma L, Guo Y, Yang B, Li T, Chi Y, Zhang X, Wu J. Tumoral PD-1hiCD8+ T cells are partially exhausted and predict favorable outcome in triple-negative breast cancer. Clin Sci (Lond). 2020;134:711-726. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 13] [Cited by in RCA: 27] [Article Influence: 5.4] [Reference Citation Analysis (0)] |
| 42. | Edwards J, Wilmott JS, Madore J, Gide TN, Quek C, Tasker A, Ferguson A, Chen J, Hewavisenti R, Hersey P, Gebhardt T, Weninger W, Britton WJ, Saw RPM, Thompson JF, Menzies AM, Long GV, Scolyer RA, Palendira U. CD103+ Tumor-Resident CD8+ T Cells Are Associated with Improved Survival in Immunotherapy-Naïve Melanoma Patients and Expand Significantly During Anti-PD-1 Treatment. Clin Cancer Res. 2018;24:3036-3045. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 197] [Cited by in RCA: 316] [Article Influence: 45.1] [Reference Citation Analysis (0)] |
| 43. | Boddupalli CS, Bar N, Kadaveru K, Krauthammer M, Pornputtapong N, Mai Z, Ariyan S, Narayan D, Kluger H, Deng Y, Verma R, Das R, Bacchiocchi A, Halaban R, Sznol M, Dhodapkar MV, Dhodapkar KM. Interlesional diversity of T cell receptors in melanoma with immune checkpoints enriched in tissue-resident memory T cells. JCI Insight. 2016;1:e88955. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 89] [Cited by in RCA: 119] [Article Influence: 13.2] [Reference Citation Analysis (0)] |
| 44. | Steinbach K, Vincenti I, Merkler D. Resident-Memory T Cells in Tissue-Restricted Immune Responses: For Better or Worse? Front Immunol. 2018;9:2827. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 48] [Cited by in RCA: 69] [Article Influence: 9.9] [Reference Citation Analysis (0)] |
| 45. | Smazynski J, Webb JR. Resident Memory-Like Tumor-Infiltrating Lymphocytes (TILRM): Latest Players in the Immuno-Oncology Repertoire. Front Immunol. 2018;9:1741. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 33] [Cited by in RCA: 34] [Article Influence: 4.9] [Reference Citation Analysis (0)] |
| 46. | Blanc C, Hans S, Tran T, Granier C, Saldman A, Anson M, Oudard S, Tartour E. Targeting Resident Memory T Cells for Cancer Immunotherapy. Front Immunol. 2018;9:1722. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 40] [Cited by in RCA: 52] [Article Influence: 7.4] [Reference Citation Analysis (0)] |
| 47. | Willemsen M, Linkutė R, Luiten RM, Matos TR. Skin-resident memory T cells as a potential new therapeutic target in vitiligo and melanoma. Pigment Cell Melanoma Res. 2019;32:612-622. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 20] [Cited by in RCA: 31] [Article Influence: 5.2] [Reference Citation Analysis (0)] |
| 48. | Park SL, Mackay LK, Waithman J, Gebhardt T. Tissue-resident memory T cells orchestrate tumour-immune equilibrium. Cell Stress. 2019;3:162-164. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 6] [Cited by in RCA: 8] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 49. | Amsen D, van Gisbergen KPJM, Hombrink P, van Lier RAW. Tissue-resident memory T cells at the center of immunity to solid tumors. Nat Immunol. 2018;19:538-546. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 156] [Cited by in RCA: 220] [Article Influence: 31.4] [Reference Citation Analysis (0)] |
| 50. | Gabriel SS, Kallies A. Tissue-resident memory T cells keep cancer dormant. Cell Res. 2019;29:341-342. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3] [Cited by in RCA: 5] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 51. | Takamura S. Niches for the Long-Term Maintenance of Tissue-Resident Memory T Cells. Front Immunol. 2018;9:1214. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 67] [Cited by in RCA: 102] [Article Influence: 14.6] [Reference Citation Analysis (0)] |
| 52. | Menares E, Gálvez-Cancino F, Cáceres-Morgado P, Ghorani E, López E, Díaz X, Saavedra-Almarza J, Figueroa DA, Roa E, Quezada SA, Lladser A. Tissue-resident memory CD8+ T cells amplify anti-tumor immunity by triggering antigen spreading through dendritic cells. Nat Commun. 2019;10:4401. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 153] [Cited by in RCA: 168] [Article Influence: 28.0] [Reference Citation Analysis (0)] |
| 53. | Woon HG, Braun A, Li J, Smith C, Edwards J, Sierro F, Feng CG, Khanna R, Elliot M, Bell A, Hislop AD, Tangye SG, Rickinson AB, Gebhardt T, Britton WJ, Palendira U. Compartmentalization of Total and Virus-Specific Tissue-Resident Memory CD8+ T Cells in Human Lymphoid Organs. PLoS Pathog. 2016;12:e1005799. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 69] [Cited by in RCA: 76] [Article Influence: 8.4] [Reference Citation Analysis (0)] |
| 54. | Malesci A, Laghi L, Bianchi P, Delconte G, Randolph A, Torri V, Carnaghi C, Doci R, Rosati R, Montorsi M, Roncalli M, Gennari L, Santoro A. Reduced likelihood of metastases in patients with microsatellite-unstable colorectal cancer. Clin Cancer Res. 2007;13:3831-3839. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 172] [Cited by in RCA: 192] [Article Influence: 10.7] [Reference Citation Analysis (0)] |