Published online Dec 24, 2021. doi: 10.5306/wjco.v12.i12.1101
Peer-review started: April 28, 2021
First decision: June 16, 2021
Revised: June 20, 2021
Accepted: November 26, 2021
Article in press: November 26, 2021
Published online: December 24, 2021
Processing time: 241 Days and 2.5 Hours
The liver has remarkable regenerative potential, with the capacity to regenerate after 75% hepatectomy in humans and up to 90% hepatectomy in some rodent models, enabling it to meet the challenge of diverse injury types, including physical trauma, infection, inflammatory processes, direct toxicity, and immunological insults. Current understanding of liver regeneration is based largely on animal research, historically in large animals, and more recently in rodents and zebrafish, which provide powerful genetic manipulation experimental tools. Whilst immensely valuable, these models have limitations in extrapolation to the human situation. In vitro models have evolved from 2-dimensional culture to complex 3 dimensional organoids, but also have shortcomings in replicating the complex hepatic micro-anatomical and physiological milieu. The process of liver regeneration is only partially understood and characterized by layers of complexity. Liver regeneration is triggered and controlled by a multitude of mitogens acting in autocrine, paracrine, and endocrine ways, with much redundancy and cross-talk between biochemical pathways. The regenerative response is variable, involving both hypertrophy and true proliferative hyperplasia, which is itself variable, including both cellular phenotypic fidelity and cellular trans-differentiation, according to the type of injury. Complex interactions occur between parenchymal and non-parenchymal cells, and regeneration is affected by the status of the liver parenchyma, with differences between healthy and diseased liver. Finally, the process of termination of liver regeneration is even less well understood than its triggers. The complexity of liver regeneration biology combined with limited understanding has restricted specific clinical interventions to enhance liver regeneration. Moreover, manipulating the fundamental biochemical pathways involved would require cautious assessment, for fear of unintended consequences. Nevertheless, current knowledge provides guiding principles for strategies to optimise liver regeneration potential.
Core Tip: The liver has remarkable regenerative potential, allowing recovery from 90% hepatectomy in some rodent models. Current understanding of liver regeneration comes from in vitro and animal models. Liver regeneration is controlled by mitogens acting in autocrine, paracrine, and endocrine ways. Complex cross talk occurs between parenchymal and non-parenchymal cells. Regeneration involves hypertrophy and hyperplasia, with both cellular phenotypic fidelity and transdifferentiation, which come into play according to the nature and magnitude of the injury, and the presence of underlying liver disease. Current knowledge provides guiding principles for strategies to optimise liver regeneration potential in the treatment of liver tumours.
- Citation: Hadjittofi C, Feretis M, Martin J, Harper S, Huguet E. Liver regeneration biology: Implications for liver tumour therapies. World J Clin Oncol 2021; 12(12): 1101-1156
- URL: https://www.wjgnet.com/2218-4333/full/v12/i12/1101.htm
- DOI: https://dx.doi.org/10.5306/wjco.v12.i12.1101
The process of liver regeneration is highly complex, and incompletely understood. Moreover, the components of this complexity are multiple. Firstly, liver regeneration may be triggered by a wide range of diverse injury types, occurring in isolation or combination, and including physical trauma, infection, inflammatory processes, direct toxicity, and immunological insults. Commensurate with the range of injuries, the biochemical mechanisms which trigger liver regeneration in the first place are also diverse, but only partly identified. Second, the response to injury is not only dependent on the type of injury, but also its magnitude. For example, liver growth after 30% partial hepatectomy in the rat model is predominantly by hepatocyte hypertrophy (liver growth by hepatocyte volume increase), in contrast to the hyperplasia (liver growth by hepatocyte proliferation) seen after 70% hepatectomy. The mechanism underlying this observation is poorly understood. Third, even in the context of liver regeneration by proliferation, different pathways are activated depending on the magnitude of the injury and the status of the background liver. Thus, when the default pathway of phenotypic fidelity (hepatocytes dividing to produce more hepatocytes, cholangiocytes dividing to produce more cholangiocytes, and so on) fails, alternative pathways are recruited whereby intrahepatic bipotential cells transdifferentiate to hepatocytes or cholangiocytes to meet the deficit. Fourth, the triggers and drivers to liver regeneration are an expanding multitude of cytokines, hormones, and growth factors (collectively referred to as hepatic mitogens), from hepatic and extra-hepatic sources, acting either synchronously or metachronously, each subject to complicated and ill-defined control mechanisms and feedback loops. The mitogen maelstrom is characterized by much redundancy (ablation of particular mitogens is compensated by others) and overlapping ‘biochemical promiscuity’ (with mitogens impacting on more than one receptor, or intracellular signalling pathway). This degree of overlap and redundancy is understandably a highly valuable evolutionary adaptation to meet the diverse insults the liver is exposed to. Whilst many mitogens have been identified and characterized, the complexity of the interactions make it extremely difficult to assign quantitative relative contributions or importance. Fifth, the complexity of interactions in mitogenic stimuli is further enhanced by the interplay between parenchymal cells (hepatocytes and cholangiocytes) and non-parenchymal cells [Kupffer cells (KC), hepatic stellate cells (HSC), liver sinusoidal endothelial cells (LSEC)], with the latter group, though present in much smaller numbers, playing critical roles. Sixth, the regenerative response is significantly affected not only by the nature of the injury and its magnitude, but also by the health of the underlying liver. Thus, liver regeneration in the face of established steatosis, steatohepatitis, fibrosis, cirrhosis, or biliary outflow obstruction is much altered to that in healthy liver tissue. Seventh, although the processes driving liver regeneration are only partially understood, those controlling the stop signals, once the liver has grown sufficiently, are even less well defined. Lastly, although many in vitro and animal models are available for the study of liver regeneration, all have their limitations, and their results cannot necessarily be extrapolated to the human situation where information is most limited.
This review provides an overview of liver regeneration biology, and the implications of our current understanding for the treatment of liver tumours. We discuss the subject in separate sections listed below. It is emphasized that the presentation of the subject in this way, though designed to orientate the reader, is somewhat artificial in the context of a biological process characterized by multiple synchronous and overlapping events. There is therefore a degree of overlap between sections, with references made to key events in one section subsequently expanded upon in later ones.
Section 1 describes the models of liver regeneration and provides an account of the in vitro, animal, and human models that provide our current knowledge of liver regeneration.
Section 2 describes the very early events post liver injury (provided by the hepatectomy model) and provides an account of the known early triggers to liver regeneration.
Section 3 provides an account of the multiple hepatic mitogens which contribute to initiating and maintaining liver regeneration.
Section 4 describes the contribution of non-parenchymal cells to liver regeneration.
Section 5 describes the ‘alternative pathways’ of liver regeneration, in which stem cell trans differentiation is recruited as a mechanism to deal with situations when phenotypic fidelity fails.
Section 6 describes the influence of underlying liver disease to liver regeneration.
Section 7 describes current knowledge of the mechanisms underlying ceasing of liver regeneration.
Section 8 considers how our current knowledge of liver regeneration affects therapy for liver tumours currently and in terms of future developments.
Although the clinician’s perspective may aim to use understanding of liver reg
Early research and the flow theory: The history and evolution of animal models used for the study of liver regeneration is described in detail within the excellent review by Mortensen et al[1]. The very first liver regeneration research is attributed to Nicolas Eck, a 29-year-old Russian military surgeon, in his investigation of portocaval fistula in dogs[2].
From this early period and into the early 1900s, the prevailing view, referred to as ‘the flow theory’ hypothesized that liver homeostasis and regeneration could be maintained provided that the liver sinusoids were supplied with mechanical flow of blood, irrespective of its source.
The theory was seemingly supported by experiments showing liver regeneration in dogs after 70% hepatectomy who had undergone total portocaval transposition (thus delivering exclusively systemic venous blood to the sinusoids)[3], and by similar experiments showing liver regeneration in dogs after 42% hepatectomy who had undergone portocaval shunt and arterialization of the hepatic portal stump[4] (thus delivering exclusively arterial blood to the sinusoids). With hindsight, the inte
The humoral theory: The concept that constituents of portal blood were essential to liver homeostasis and regeneration only gradually gained acceptance, despite early evidence form Hahn who described liver failure in dogs undergoing portocaval shunts[5]. In the 1920s, Rous and Larimore reported that unilateral portal ligation produced ipsilateral atrophy with contralateral hypertrophy in a rabbit model[6]. From the 1960s onwards many more investigators pursued the idea of portal flow as critical in liver regeneration, in experiments including those of Marchioro et al[7], who carried out canine split portocaval transposition in which one portal branch is supplied with venous blood from the inferior vena cava and the second portal branch receives portal blood, showing atrophy and hypertrophy of the respective parts of liver parenchyma. Furthermore it was demonstrated that adjusting flow and oxygenation alone did not, in a dog model, compensate for the absence of portal blood[8].
Characterising portal blood constituents: With the recognition of the importance of portal blood came an impetus to define the source and nature of vital portal blood constituents. Thus splanchnic portal flow separation experiments were carried out separating portal flow of distal stomach, duodenum, pancreas and spleen from that of small intestine, with the overall finding that the grafts supplied with small intestinal portal flow atrophied, in contrast to those supplied with portal blood from the upper intestinal tract[9,10].
Thereafter, searches for candidate hepato-trophic factors were carried out by infusing individual growth factors and hormones in portal deprived parenchyma to see if rescue could be achieved. In this way, it was demonstrated that insulin infusion into one portal branch of liver after portocaval shunt could partially rescued atrophy of the liver[11], though insulin was unable to prevent liver atrophy following complete splanchnic evisceration[12].
This portocaval shunt rescue model of experimentation allowed the identification of other factors which promoted liver regeneration including thyroxine (T 3), insulin-like growth factor II, transforming growth factor alpha (TGFα) and hepatocyte growth factor (HGF)[13]. Although portal in origin, the systemic blood dissemination of the factors involved in liver regeneration were shown in canine experiments with auto-transplantation of small liver grafts to the jejunal mesentery, then randomising animals to sham surgery or 70% hepatectomy. In contrast to sham surgery, autografts in hepatectomised animals did not atrophy, indicating a growth stimulus via the systemic circulation[14].
Similar results were obtained in parabiosis experiments. Thus, using rats with surgically conjoined systemic circulations, partial hepatectomy in one rat, resulted in liver hypertrophy in the non-hepatectomised rat[15,16].
Thus, the early experiments establishing the underlying principles of liver regeneration were performed using predominantly large animal models. In the more recent era, small animal models have preferentially been used because, as well as providing similar physiology and anatomy to the large animal models, they presented advantages in terms of cost, animal husbandry, rapidity of experimentation, and, in the mouse in particular, greater opportunity for genetic modification as an investigative tool. The sections below follow on to describe the rat and mouse models, with the subsequent evolution to the zebrafish model.
It should be emphasised that drawing conclusions from these different models presents additional complexity per se, in that the observations of one species model may not necessarily be extrapolated to the others. Moreover, even within one species, different liver injury types may present differing characteristics. For example, in the mouse model, epidermal growth factor receptor (EGFR) blockade markedly inhibits liver regeneration after paracetamol injury[17], but only delays it after partial hepatectomy (PH)[18].
Rat model: The rat model has gained favour over larger animal models (i.e., dogs, rabbits, baboons and pigs) due to advantages in terms of ethics, costs, and practicalities such as husbandry, handling, and shorter experimental times[19] although their size renders surgery more intricate.
As early as 1931, Higgins & Anderson described a standardized technique for partial hepatectomy in rats, which resulted in liver regeneration[20]. Two decades later, Bucher et al[15] reported on parabiotic experiments, whereby rats that underwent partial hepatectomy were joined to partner rats with intact livers by way of an abdominal wall anastomosis. The authors found that mitosis increased both within the operated and the intact livers, thus concluding that liver regeneration is influenced by factors in the systemic circulation. In a contemporaneous report of parabiotic rats, Wenneker & Sussman[16] found that liver weight and number of hepatic cells increased both in hepatectomized and “normal” rats, thus reaching the same conclusion. Moolten & Bucher[21] investigated this further by establishing carotid-to-jugular cross-circulation from partial hepatectomy to normal rats, and demonstrating that DNA synthesis increased in the normal livers, dependent on the extent of hepatectomy in the parabiotic partner. Since these early experiments, a variety of surgical and hepatotoxic rat models have been developed for the study of regeneration in acute and chronic liver disease.
The rat liver consists of four main lobes: middle (38% liver mass), left lateral (30%), right (22%), and caudate (8%)[19]. In descriptions where the paracaval portion is considered separate from the caudate, this amounts to 2% of liver mass. These lobes, and their subdivisions, are analogous to the human liver segments described by Couinaud[22]. Specifically, the caudate lobe (which consists of the Spiegel lobe and paracaval portion) corresponds to the human segments (Sg) I and IX, the left lobe to Sg II, the left component of the middle lobe to Sg III, the right component to Sg IV, V, and VIII, and the right lobe to Sg VI and VII[23].
The classical surgical model involves a 70% (2/3) hepatectomy, as described by Higgins & Anderson[20], and remains the most common surgical model for liver regeneration. Impressively, rat liver can completely regenerate within 8 d of 70% hepatectomy[24]. Variations to this model can result in 5%-97% partial hepatectomies, depending on the combination of liver lobes resected[19]. Impressively, 90% hepatectomy in rats is survivable[25]. Furthermore, survival can (perhaps counterintuitively) be enhanced by suppressing the abrupt early regenerative response of the remnant liver via the mitogenactivated protein kinase pathway, thus rendering regeneration linear in the acute phase[26] or by selective bowel decontamination with gentamicin[27]. These phenomena point towards a substantial regenerative reserve in rats, which unfortunately is not found in humans and which limits extrapolation from rodent models to humans. Bile duct ligation (BDL) is another commonly used surgical model, which involves dividing the common bile duct between ligatures, thus providing a model for the study of cholestatic disease[28]. Yet another surgical model is portal branch ligation, after which ipsilateral atrophy and contralateral hyperplasia is observed in rats[29] analogous to human clinical scenarios such as portal vein embolisation (PVE) or associating liver partition and portal vein ligation for staged hepatectomy (ALPPS).
Hepatotoxic models have been extensively studied in both rats and mice, shown in Table 1, with the aim of replicating acute or chronic liver disease. Their mechanisms are also described below in the context of mouse models. The hepatotoxic approach has been used to demonstrate the protective effects of flavonoids[30], thiamine[31] protocatechuic acid[32], Lactococcus lactis in probiotic preparation[33] and 5-methoxytryptophan[34], to mention a few examples. An alternative approach to hepatotoxicity is the manipulation of the cell cycle. Specifically, 2-acetaminofluorene (AAF) has been shown to inhibit hepatocyte proliferation, whilst inducing the proliferation and transdifferentiation of oval cells (hepatic progenitor cells) to hepatocytes after partial hepatectomy[35,36], thus shedding light on alternative liver regenerative pathways.
| Toxin | Mechanism | Necrosis pattern |
| Acetaminophen (paracetamol)[19,36,37] | Free radical enhancement and Kupffer cell activation | Pericentral |
| Carbon tetrachloride[19,30,37] | Free radical enhancement and Kupffer cell activation | Pericentral |
| Concanavalin A[37] | T-cell activation; cytokine release; ICAM-1 & VCAM-1 upregulation. | Centrilobular |
| D-Galactosamine[19,37] | Uridine metabolite deficiency | Random |
| Ethanol[19,31] | Increases production of reactive oxygen species and infiltration of inflammatory cells | None |
| Lipopolysaccharide[37] | Kupffer cell activation | Centrilobular |
| Thioacetamide[19,37,38] | Increases production of toxic metabolites and reactive oxygen species | Pericentral |
Mouse model: Although much knowledge on liver regeneration has been generated from partial hepatectomy rat models, the mouse model provides an attractive alternative due to lower costs (mice generally require fewer expensive reagents and less expensive housing)[39] relative ease of handling, and immense experimental potential afforded by genetically altered (transgenic and knockout) mice[19].
Mouse models of liver regeneration have been described in various contexts, including: partial hepatectomy[40], portal branch occlusion[41], bile duct ligation[42], chemical, pharmacological or immune-mediated injury[43-47], and chronic conditions such as non-alcoholic fatty liver disease[48] and liver cancer[49].
The lobar anatomy of the mouse and rat liver is broadly similar, and the inferior vena cava is intrahepatic in both species[39]. A significant distinction is the absence of a gallbladder in the rat[19]. In the mouse, the normal liver consists of seven lobes with the following mass proportions: left posterior (37%), left anterior (12%), right anterior (22%), right posterior (14%), right middle (8%), and two omental lobes (7%)[50]. The classical surgical model in rodents is the partial hepatectomy, which most commonly results in removal of 70% of the liver mass (also referred to as a “2/3 hepatectomy”)[19]. Portal branch occlusion can be performed radiologically in humans and in large animals but requires an open surgical approach in rodents[19]. BDL has also been developed as a model of cholestasis[42], with relevance to the study of malignant biliary obstruction. Although the total BDL rat model has existed for decades, mice have been used more recently in the partial BDL model, whereby (rather than transecting the bile duct between ligatures) a 7-0 needle is ligated onto the duct. When the needle is removed, a reproducibly narrow bile duct lumen is left, which results in less liver necrosis[42] and may more closely resemble chronic cholestasis. These surgical models are of particular interest with regards to single or staged hepatectomies with or without portal vein occlusion in human patients with liver tumours, where physiological reserve, oncological and technical resectability, as well as liver tumour burden and status of background parenchyma will determine the most optimal approach. However, recapitulating human liver procedures in mouse models is limited by the fact that mice are relatively very small, and (as in humans) there is significant anatomical variability in their hepatic vascular and biliary systems[39]. Furthermore, rodents can typically survive with much smaller liver remnants than humans, and the kinetics of liver regeneration vary between species. Nevertheless, surgical techniques in mice are well established and are characterized by reproducibility and minimal operative mortality[39].
The most frequently used hepatotoxins used to induce liver injury in mouse models are carbon tetrachloride (CCl4), d-galactosamine, paracetamol (acetaminophen), ethanol[51] and thioacetamide[19]. CCl4 can induce acute and chronic liver injury through its action on cytochrome P450, leading to the production of free peroxide radicals which cause lipid peroxidation of hepatocyte[19]. The disadvantage of the CCl4 model is the inflammatory and immune response caused during hepatocyte injury, which may confound models of liver regeneration[19]. D-galactosamine is thought to induce liver injury via intracellular deficiency in uridine metabolites and can additionally induce hepatocyte apoptosis when combined with lipopolysaccharide[19]. Paracetamol is metabolized by cytochrome P450 and in overdose leads to toxic levels of N-acetyl-benzoquinone imine, free radical formation and centrilobular apoptosis/necrosis[19]. The kinetics of liver regeneration after CCl4, D-Galactosamine and paracetamol-induced injury are similar[19]. Ethanol induces liver injury via mitochondrial dysfunction, oxidative stress, inflammatory cell infiltration and translocation of intestinal bacteria which can then enter the portal and systemic circulation[19]. Finally, thioacetamide leads to oxidative stress via its conversion to thioacetamide disulfoxide which increases the production of reactive oxygen species[19].
In addition to the hepatotoxic models mentioned above, several dietary models are used in mice to model liver disease. These include the 1,4dihydro2,4,6trimethyl-pyridine-3,5-dicarboxylate (DDC) diet, which leads to biliary injury and regeneration[52], the modified choline-deficient ethionine diet, which leads to hepatocellular injury, steatosis and spread of ductular cells from the portal tract[53]. More recently, a mouse model with rapid progression from normal liver to non-alcoholic steatohepatitis (NASH), fibrosis, cirrhosis, and hepatocellular carcinoma (HCC) within 24 wk has been described by Tsuchida et al[49]. This was achieved by feeding C57BL/6J mice a western diet (high-fat, high-fructose and high- cholesterol) and administering weekly intraperitoneal doses of CCl4.
The development of transgenic and knockout mouse models has enabled closer scrutiny of pathophysiological mechanisms with regards to liver regeneration after surgery or chemical/diet-induced injury, also highlighting the importance of the innate and adaptive immune system in liver regeneration[54].
The opportunities offered by these models and their relevance to the treatment of liver tumours in humans will be elaborated in the sections to follow. Table 2, whilst non-exhaustive, gives an impression of the breadth and potential of transgenic and KO mouse models in the study of liver regeneration.
| Yr | First author | Gene product | Study title | Ref. |
| 1994 | Webber | TGF-α | “Overexpression of transforming growth factor-alpha causes liver enlargement and increased hepatocyte proliferation in transgenic mice” | [55] |
| 1996 | Cressman | IL-6 | “Liver failure and defective hepatocyte regeneration in interleukin-6-deficient mice” | [56] |
| 1997 | Yamada | TNF | “Initiation of liver growth by tumor necrosis factor: deficient liver regeneration in mice lacking type I tumor necrosis factor receptor” | [57] |
| 1998 | Greenbaum | C/EBP-β | “CCAAT enhancer-binding protein beta is required for normal hepatocyte proliferation in mice after partial hepatectomy | [58] |
| 1998 | Rai | iNOS | “Impaired liver regeneration in inducible nitric oxide synthase-deficient mice” | [59] |
| 1998 | Roselli | uPA | “Liver regeneration is transiently impaired in urokinase-deficient mice” | [60] |
| 1998 | Yamada | TNFR-1TNFR-2 | “Analysis of liver regeneration in mice lacking type 1 or type 2 tumor necrosis factor receptor: requirement for type 1 but not type 2 receptor” | [61] |
| 2002 | Anderson | PPAR-α | “Delayed liver regeneration in peroxisome proliferator-activated receptor-alpha-null mice” | [62] |
| 2003 | Leu | IGFBP-1 | “Impaired hepatocyte DNA synthetic response posthepatectomy in insulin-like growth factor binding protein 1-deficient mice with defects in C/EBP beta and mitogen-activated protein kinase/extracellular signal-regulated kinase regulation” | [63] |
| 2003 | Strey | C3a/C5a | “The proinflammatory mediators C3a and C5a are essential for liver regeneration” | [64] |
| 2004 | Borowiak | Met | “Met provides essential signals for liver regeneration” | [65] |
| 2004 | Mohammed | TIMP3 | “Abnormal TNF activity in Timp3(–/–) mice leads to chronic hepatic inflammation and failure of liver regeneration | [66] |
| 2004 | Nakamura | OSM | “Hepatocyte proliferation and tissue remodeling is impaired after liver injury in oncostatin M receptor knockout mice” | [67] |
| 2004 | Oe | TGF-β | “Intact signaling by transforming growth factor beta is not required for termination of liver regeneration in mice” | [68] |
| 2005 | Duffield | DTR | “Selective depletion of macrophages reveals distinct, opposing roles during liver injury and repair” | [69] |
| 2005 | Mitchell | HB-EGF | “Heparin-binding epidermal growth factor-like growth factor links hepatocyte priming with cell cycle progression during liver regeneration” | [70] |
| 2005 | Oliver | MT | “Impaired hepatic regeneration in metallothionein-I/II knockout mice” | [71] |
| 2005 | Seki | MyD88 | “Contribution of Toll-like receptor/myeloid differentiation factor 88 signaling to murine liver regeneration” | [72] |
| 2006 | Fernández | Caveolin-1 | “Caveolin-1 is essential for liver regeneration” | [73] |
| 2006 | Olle | MMP9 | “Matrix metalloproteinase-9 is an important factor in hepatic regeneration after partial hepatectomy in mice” | [74] |
| 2007 | Mayoral | Caveolin-1 | “Dispensability and dynamics of caveolin-1 during liver regeneration and in isolated hepatic Cells” | [75] |
| 2009 | Tumanov | Rag1LT | “T cell-derived lymphotoxin regulates liver regeneration” | [54] |
| 2010 | Erhardt | CCR5, CXCR3 | “Tolerance induction in response to liver inflammation” | [47] |
| 2010 | Liu | GPC3 | “Suppression of liver regeneration and hepatocyte proliferation in hepatocyte-targeted glypican 3 transgenic mice” | [76] |
| 2012 | Borude | FXR | “Hepatocyte-Specific Deletion of Farnesoid X Receptor Delays But Does Not Inhibit Liver Regeneration After Partial Hepatectomy in Mice” | [77] |
| 2013 | Bhave | GPC3 | “Regulation of Liver Growth by Glypican 3, CD81, Hedgehog, and Hhex” | [78] |
| 2014 | Kong | FGF15 | “Fibroblast growth factor 15 deficiency impairs liver regeneration in mice” | [79] |
| 2014 | Yang | Lrp5/6 | “β-catenin signaling in murine liver zonation and regeneration: a Wnt-Wnt situation!” | [80] |
| 2015 | Lu | Mdm2 | “Hepatic progenitor cells of biliary origin with liver repopulation capacity” | [81] |
| 2016 | Swiderska-Syn | Cre recombinase | “Hedgehog regulates yes-associated protein 1 in regenerating mouse liver” | [82] |
| 2018 | Tsagianni | MET | “Combined Systemic Disruption of MET and Epidermal Growth Factor Receptor Signaling Causes Liver Failure in Normal Mice” | [83] |
| 2019 | Asrud | Epac | “Mice depleted for Exchange Proteins Directly Activated by cAMP (Epac) exhibit irregular liver regeneration in response to partial hepatectomy” | [84] |
| 2019 | Fortier | p38α MAPK | “Hepatospecific ablation of p38α MAPK governs liver regeneration through modulation of inflammatory response to CCl 4-induced acute injury” | [85] |
| 2019 | Modares | IL-6R | “IL-6 Trans-signaling Controls Liver Regeneration After Partial Hepatectomy” | [86] |
| 2019 | Zhou | Rictor | “Mammalian Target of Rapamycin Complex 2 Signaling Is Required for Liver Regeneration in a Cholestatic Liver Injury Murine Model” | [87] |
| 2020 | Laschinger | CGRP-RAMP1 | “The CGRP receptor component RAMP1 links sensory innervation with YAP activity in the regenerating liver” | [88] |
| 2020 | Seguin | Mfrn1, Mfrn2 | “The mitochondrial metal transporters mitoferrin1 and mitoferrin2 are required for liver regeneration and cell proliferation in mice” | [89] |
| 2020 | Xue | GPC3 | “Phosphorylated Ezrin (Thr567) Regulates Hippo Pathway and Yes-Associated Protein (Yap) in Liver” | [90] |
Zebrafish model: Following their discovery in the Ganges River in the late 19th century, zebrafish (Danio rerio) were initially used by embryologists to investigate developmental biology[91]. Their relative low cost, rapid development from one-cell embryo to free-swimming larva 5 d post-fertilisation, optical transparency enabling direct observation using light and fluorescent microscopy, and relative genetic conservation compared to the human genome with approximately 70% of human genes having a zebrafish orthologue[92] has led to their role within medical research expanding considerably. In the realm of liver biology, applications include the study of high throughput drug discovery and hepatotoxicity screening, forward genetic screening, heritable and developmental liver diseases, the molecular and cellular factors that contribute to human liver disease, liver cancer biology and liver regeneration[91,93-95]. The research opportunities and disadvantages presented by zebrafish are summarized in Table 3.
| Advantages | Disadvantages |
| Vertebrate body plan | Partial genome duplication in teleosts |
| Ease of husbandry | Differences in microanatomy and liver architecture |
| Inexpensive to maintain | Less conserved physiology than mammalian models |
| Large numbers of embryos produced rapidly | Less conserved morphogenesis than mammals |
| External development | Less developed cell culture technology |
| Optical clarity during development | Poorly developed embryonic stem cell technology |
| Zebrafish liver not required for foetal haematopoiesis | |
| Amenable to forward and reverse genetics | |
| Molecular conservation of development | |
| Amenable to high-throughput screening: (1) Phenotype assessment; and (2) Drug/chemical screening |
Cell types are highly conserved between zebrafish and mammalian livers, with the exception of hepatic immune cells (Kupffer cells), which have not been identified in zebrafish. Whilst zebrafish provide immensely useful models, this difference highlights the caution needed in the extrapolation of results between species. As discussed below, Kupffer cells play an important part in cytokine priming of hepatocytes, implying that a different priming mechanism operates in zebrafish, or that this role is played by different cell type. Cellular morphology and physiology are also largely conserved with zebrafish livers demonstrating similar functions to mammalian livers including secretion of bile, glycogen and lipid storage, insulin responsiveness, ammonia metabolism and the production and secretion of proteins including complement, clotting factors and a protein resembling albumin. The morphological composition of the zebrafish, however, is distinct to the mammalian liver with the liver arranged into 3 Lobes that lack a pedicle that separates the lobes in the mammalian liver. Moreover, the portal architecture of mammalian livers is not observed. In fish, the hepatocytes are arranged into tubules with bile ductules running between two rows of hepatocytes[91].
Liver regeneration in mammalian livers involves a compensatory regeneration with hepatocyte proliferation and hypertrophy. In contrast, zebrafish demonstrate true epimorphic regeneration in response to partial hepatectomy with regrowth of the resected lobe, again highlighting significant inter-species differences. Genome-wide gene expression studies have demonstrated that liver regeneration is the result of a coordinated expression of thousands of genes, and whilst several pathways have been identified as important in liver regeneration in both mammals and zebrafish including WNT, fibroblast growth factor receptor (FGFR) and bone morphogenic protein receptor, in isolation they are insufficient to drive the complex process of liver regeneration. The mechanisms underlying the difference between mammalian liver regeneration and zebrafish, and epimorphic regeneration are still to be elucidated[96].
The observation of interspecies variability, the ethical guiding principles of the 3R principles (replacement, refinement, and reduction of animal testing), and the opportunity of better-defined experimental conditions have motivated the development in vitro models to study liver biology. Thus, two- and three-dimensional (2D and 3D) in vitro models have increased our understanding of the mechanisms of liver injury, hepatotoxicity, and mechanisms of liver regeneration[97].
2D culture models: 2D in vitro liver models, have traditionally used immortalised cell lines such as the HepG2 and the HepaRG cell lines derived from human progenitor cells[98], or mechanically and enzymatically dissociated primary cells[99] expanded on plastic surfaces, or supported by extracellular matrix (ECM) scaffolding[100]. Though presenting advantages in terms of ease of tissue culture, such systems have limitations; for example, cells lines have fundamentally different gene expression profiles to primary hepatocytes, owing to their immortalised nature[101]. Primary hepatocytes have some benefits in this regard, but are difficult to source (in the human case), exhibit donor variability[102], and rapidly lose their differentiation and function (such as morphology and toxicant related genes expression) in plastic culture[100,103,104].
The presence of ECM partially addresses these shortcomings. Culturing primary hepatocytes between two layers of collagen, termed sandwich-cultured hepatocytes (SCH)[105], results in extended viability, retained cellular polarity with correct localization of basolateral and canalicular transporters[106] as well as formation of functional bile networks[107]. However, despite their promising properties compared to monolayer cultures, sandwich cultures have their own disadvantages including the barrier to introduced materials created by the collagen layers, and low levels of expression of cell-to-cell adhesion proteins that are critical for cell function and differentiation[108]. As such the role of sandwich culture in the experimental process is often limited to short term studies.
3D culture models: Significant progress has been made using 3D in vitro hepatic models with benefits in terms of maturity of hepatocytes, long term viability, and more precise representations of the microenvironment of the in vivo liver[109]. In vitro liver modelling studies with human cells have allowed investigation of liver development, liver disease modelling, liver regeneration, and therapeutic tran
Human-induced pluripotent stem cells (hIPSCs) offer an effectively unlimited source of genetically diverse cell lines that can be generated from both healthy and diseased livers. Furthermore, these cells are amenable to genetic modification using the CRISPR technology in order to facilitate disease modelling[111]. hIPSCs have further expanded the opportunity for 3D in vitro culture systems by the development of hepatic organoids from hIPSCs[112]. Thus, reports describe the design of organoids involving multiple cell types by co-differentiating hepatocytes and cholangiocytes[113], or hepatocytes with other supporting cell types including stellate-like and Kupffer-like cells[114]. However, an important limitation is the relative immaturity of hepatocyte-like cells generated from hIPSCs. This is demonstrated by continued alpha-fetoprotein (αFP), low albumin expression, and distinctive CYP expression and function[115]. The problem of functional maturity has been partially addressed by modifying culture conditions, including the medium composition (e.g., inclusion of specific growth factors, hormones)[115]. More recent approaches to circumvent the disadvantages of hIPSCs have involved the use of primary cells to form organoids. Thus, Huch et al[116] generated human liver organoids from primary ductal Epidermal Cell Adhesion Molecule positive cells grown in a defined human liver media allowing culture with stable function for over 6 mo, and Justin et al[117] describe the formation of biliary organoids from primary cholangiocytes.
In spite of these advances, 3D ex vivo cultures do carry their own drawback including difficulties in controlling cellular distribution, innervation, and vascularization-with the latter aspect of particular importance given the central role of the liver sinusoids to hepatic function.
Repopulation of decellularised livers: Repopulation of decellularised liver scaffolds with cells has offered a further refinement to the in vitro investigation of liver regeneration as well as potential therapeutic opportunities[118].
Earlier approaches to generating functional livers include hepatocyte trans
More recently, techniques in whole liver decellularization and repopulation have moved the field further, although significant challenges remain. In general terms, the process involves removal of the liver’s cellular and immunogenic components, thus creating a scaffold which retains the ultrastructure and properties of the ECM[126]. This is usually followed by static cultivation of cells (e.g., hepatocytes) and their subsequent infusion into the scaffold.
In one of the first such studies, Uygun et al[127] demonstrated that ischaemic rat livers can be decellularised whilst preserving structural and basement-membrane-based components of the ECM, as well as the microvasculature. The investigators achieved decellularisation by portal vein perfusion using sodium dodecylsulfate (an anionic detergent), and repopulation with primary rat hepatocytes via the same route. Recellularised grafts were implanted in rats for 8 h, and after explantation underwent ex vivo blood perfusion for 24 h, demonstrating ongoing hepatocyte metabolic activity. Others have demonstrated that implanting repopulated ECM liver scaffolds into rats which had undergone extended hepatectomy improved liver function and extended their mean lifespan from 16 to 72 h[128]. In the last 10 years, a variety of animal models, decellularisation techniques, repopulation routes and cell sources have been described, with promising outcomes in terms of vascular repopulation[118,129,130], hepatocyte survival[131] as well as formation of biliary duct-like structures and activation of liver detoxification enzymes[132]. One of the commonest sources of liver scaffolds is the rat[118,127,132-137] repopulated with rat hepatocytes (although cholangiocytes[136] and lineages from pluripotent stem cells, mesenchymal cells, and fibroblasts have also been described[137] usually via the portal vein. With regards to human tissue, Verstegen et al[138] demonstrated that decellularised human livers can be repopulated with human umbilical vein endothelial cells, leading to re-endothelialisation of the vascular tree. Table 4 presents further examples of the different approaches to liver decellularisation-repopulation developed thus far.
| First author | Yr | Liver scaffold source | Cell source & type | Repopulation route | Outcomes | Ref. |
| Uygun | 2010 | Rat | Rat hepatocytes | Portal vein | Recellularised liver grafts implanted in rats, perfused in vivo for 8 h, explanted and assessed after 24 h, demonstrating hepatocyte survival, albumin secretion, urea synthesis and cytochrome P450 expression. | Uygun 2010[127] |
| Zhou | 2011 | Mouse | Human foetal hepatocytes | Portal vein | Recellularised liver matrix implanted in mice, achieving hepatocyte survival after 6 wk, with albumin secretion and cytochrome P450 expression. | Zhou 2011[131] |
| Ko | 2014 | Pig | Murine endothelial cells, after scaffold conjugation with rat anti-mouse CD31 antibodies | Portal veinHepatic arteryInferior vena cava | Recellularised liver grafts implanted in pigs, demonstrating good blood flow and patency throughout vascular network over 24 h after transplantation. | Ko 2015[130] |
| Navarro-Tableros | 2015 | Rat | Human liver stem-like cells | Portal vein | Loss of embryonic markers, expression of albumin, lactate dehydrogenase and cytochrome P450 subtypes. Production of urea and nitrogen. | Navarro-Tableros 2015[133] |
| Ogiso | 2016 | Rat | Mouse hepatocytes | Biliary tree; Portal vein | (1) > 80% of cells seeded via biliary tree entered the parenchyma; (2) Approximate 20% of cells seeded via portal vein entered the parenchyma; and (3) Increased gene expression of foetal hepatocyte albumin, glucose 6-phosphatase, transferrin, cytokeratin 19, and gamma-glutamyl transpeptidase, activation of liver detoxification enzymes, formation of biliary duct-like structures. | Ogiso 2016[132] [PMID 27767181] |
| Verstegen | 2017 | Human | Human umbilical vein endothelial cells. | - | Re-endothelialisation of vascular tree, demonstrated by luminal vimentin and von Willebrand Factor/F8 staining. | Verstegen 2017[138] |
| Butter | 2018 | Rat | Rat hepatocytes | Hepatic artery and portal vein | In vitro demonstration of hepatocyte spread to all liver lobes, with proliferation, and production of aminotransferases, lactate dehydrogenase and albumin. | Butter 2018[134] |
| Chen | 2018 | Rat | Rat hepatocytes | Portal vein | None (description of materials and methods). | Chen 2018[135] |
| Chen | 2019 | Rat | Rat cholangiocytes Rat hepatocytes | Common bile duct; Portal vein | In vitro viability and function demonstrated by albumin and urea secretion, and gene expression of functional proteins. | Chen 2019[136] |
| Harper | 2020 | Rat | Rat bone marrow cells | Portal vein | Stem cells engrafted in portal, sinusoidal and hepatic vein compartments, achieving expression of endothelial cell surface markers for up to 30 d. | Harper 2020[118] |
| Takeishi | 2020 | Rat | Human hepatocytes, biliary epithelial cells, and vascular endothelial cells derived from pluripotent stem cells, mesenchymal cells, and fibroblasts. | Biliary tree; Portal vein; Central veins | Auxiliary grafts implanted in rats, achieving in vivo functionality for 4 d. | Takeishi 2020[137] |
The main challenges in producing a viable whole organ from liver decellularisation-repopulation techniques include heterogeneity of cell engraftment, thrombosis (partly related to incomplete or suboptimally functional endothelium as well as microvascular injury[121,130]), the re-creation of an intact and functional biliary tree, as well as attaining the specific distribution of liver cell types seen in the native healthy organ. Mesenchymal and pluripotent stem cells for repopulation are currently considered attractive research avenues[121] as they may lead to more clinically applicable models.
The study of human liver regeneration is limited to observational data in the context of clinical pathology and applied therapies, and thus contrasts to the directed experimental approaches possible in animal models. Moreover, access to human liver tissue during the regenerative process is not possible as liver biopsy can only be justified by clinical need, given the risks of the procedure including a measurable mortality[139]. The available observational data comes from a combination of clinical findings, serum biomarker measurements, and imaging. Clinical observations and blood bio markers are subject to difficult interpretation because of the confounding effects of the heterogeneity of the study population, diverse pathologies, and varied clinical scenarios even within a defined patient group. Although there are reports of serum biomarkers such as αFP and micro RNAs correlating with liver regeneration, their clinical applicability remains to be established. Combining clinical and serological measurements, scores such as the Acute Liver Failure Study Group index has allowed the identification of patients likely to require liver transplant[140-142].
In this context, the relatively non-invasive nature of modern imaging techniques has provided the main means of assessing liver growth and function, as markers of regeneration. Although liver function correlates well with liver volume in uncompromised livers, this relationship is less clear in patients with pre-existing parenchymal liver[143,144]. Estimation of remnant liver function instead of remnant liver volume is a better predictor of clinical outcome after liver resection in patients with decreased liver function[145]. In order to avoid PHLF, clinicians must ensure that the future remnant liver (FRL) will be sufficient to sustain life. Traditionally, this functionality assessment is made by pre-operatively measuring the volume of the FRL as a surrogate measure of functionality[144]. Volumetry, however, assumes liver parenchymal homogeneity and normal underlying liver function, which are not always present in patients undergoing extensive hepatic resections. This lack of homogeneity in hepatic function can cause a discrepancy between FRL volume (FRL-V%) and FRL function (FRL-F)[146] which is especially important in patients who present with pre-existing liver disease or who have previously received chemotherapy that resulted in steatotic or microvascular liver changes[146]. As such, FRL-V% cut-off values may not accurately predict the quality of the FRL in some patients, with implication on the development of PHLF and associated mortality. The radiological modalities most used to predict the FLR are outlined below.
Standard liver volumes can be calculated from the patient’s body surface area or mass using the formulas originally proposed by Vauthey et al[144]. However, these formulas are limited by subject demographics (healthy individuals) and by their modest correlation to liver sizes calculated by more advanced forms of volumetry[147]. CT volumetry of the liver was first performed on cadavers by Heymsfield et al[148] in 1979 and was shown to be accurate within 5% of water displacement volumetry. CT is more commonly used due to its greater accessibility, higher spatial resolution, and short acquisition time. MRI, conversely, offers multiple contrast mechanisms and the ability to assess vascular and biliary anatomy in addition to parenchymal pathology. Additionally, MRI also minimises the risk of contrast induced nephrotoxicity and eliminates concerns of radiation exposure[149]. Liver segmentation has emerged as the preferred technique CT volumetry can be used to calculate the volume of the FLR and is widely used to exclude patients from liver resection or to select patients who will benefit from a procedure to increase the volume of the future remnant, such as PVE[150]. However, the outcomes of previous reports correlating the findings of CT volumetric analysis of the future remnant with post-resectional outcome, have not been consistent and the role alternative imaging modalities has been examined[150,151].
Hepatobiliary scintigraphy using 99mTc-iminodiacetic acid analogues, such as 99mTc-mebrofenin, can be used to measure segmental liver function. 99mTc-mebrofenin is excreted into the bile by adenosine triphosphate–dependent export pumps the multidrug-resistance-associated proteins 1 and 2 without undergoing biotransformation during transit through the hepatocytes[146,152,153] . Previous reports in the literature have shown that 99mTc-mebrofenin hepatobiliary scintigraphy (HBS) can provide clinicians with information on FRL-F instead of volumetric information alone[153]. HBS provides visual and quantitative information of global and regional liver function as well as excretory function (intrahepatic and extrahepatic bile transport). 99mTc-mebrofenin is intravenously injected and consequently excreted in bile by the hepatocytes without undergoing biotransformation. As such, the clearance measurement of Technetium-99m mebrofenin using scintigraphy can quantify hepatic function[146,153]. FRL-F assessment using HBS has been proven to be superior to volumetry in the prediction of PHLF and PHLF(M), making HBS the imaging modality of choice prior to proceeding with major hepatectomy. Reports in the literature have illustrated that an HBS cut-off value of 2.7%/min/m2 can outperform volumetry cut-off values in the prediction and prevention of PHLF and PHLF(M) by identifying high-risk patients with borderline predicted remnant liver function, and consequent selection for pre-operative PVE or other hypertrophic strategies (e.g., ALPPS[154,155]. Certain hepatobiliary units have already implemented HBS in favor of CT volumetry before hepatic resection based on emerging evidence in the literature.
The PH model in rodents has allowed the examination of immediate events which occur within minutes of liver resection and provides an insight into the mechanisms that trigger the process of liver regeneration. These early events relate to vascular portal flow, tissue hypoxia, haemostatic mechanisms, and changes in extracellular matrix integrity.
Following PH, the increased portal blood flow through the remnant liver exerts a heightened shear stress on the LSECs[156]. Shear stress on LSEC induces numerous physiological changes[157] including microscopically visible ones such as increased sinusoidal diameter and changes to LSEC fenestrae and sieve plates[158,159]. Shear stress also induces biochemical responses including the release of vascular endothelial cell growth factor (VEGF) from LSEC[160], the secretion of VEGF and HGF from hepatic stellate cells[161], and the LSEC production of nitric oxide (NO) by Nitric Oxide synthase (NOS), which increases hepatocyte sensitivity to HGF[162,163]. The physiological importance of NO is suggested by the finding that inhibition of NOS severely impairs liver regeneration in mice after PH[59].
Shear stress also induces the hepatocyte priming cytokine interleukin 6 (IL6) in LSEC[164], as well as expression in of liver regeneration associated WNT, VEGF, and epithelial cell adhesion molecules in hepatic progenitor cells[165].
Another consequence of increased portal flow through the remnant liver is increased exposure to lipopolysaccharide (LPS), which is derived from gut bacteria, and which translocates from the gut into portal blood. PH increases the concentration of LPS in the remnant liver not only because of diversion of more portal blood to the remnant liver, but also because the rise in portal pressure increases intestinal permeability, allowing greater LPS translocation[166,167]. In the sinusoids, LPS binds Toll like receptors (TLR) on Kupffer cells, resulting in the secretion of the hepatocyte priming cytokines IL6 and tumour necrosis factor alpha TNFα[168], in a signalling pathway that is dependent on myeloid differentiation factor 88[169].
The increased expression of liver regeneration promoting biochemicals is not confined to the liver. Following PH in the rat, increased expression of VEGF, HGF, and hypoxia inducible factor (HIF) is also observed portal vein drained tissues such as the spleen and small intestine, whereupon portal VEGF concentrations exceed those of the systemic circulation. The mechanism stimulating this extrahepatic expression of growth factors from portal drained tissues is unclear but may also be related to portal pressure changes[170].
Following PH, increased portal flow brings about a reflex arterial vasoconstriction (the arterial buffer response), which can result in hypoxia in the remnant liver, given the low partial pressure of oxygen (pO2) in portal venous blood[171].
An important outcome of hypoxia is the induction of HIF, which in turn leads to the activation of multiple genes involved in tissue adaptations to hypoxia ranging from glycolytic metabolism to angiogenesis[172].
In the liver, PH leads to increased expression of HIF and subsequently VEGF[173]. In elegant experiments, Dirscherl et al[174] show that the hypoxic environment triggers hepatic stellate cell expression of HIF, resulting in increased expression of VEGF, which then elicits a range of responses in LSEC including proliferation and an
The injury to liver tissue in PH results in the activation of mechanisms for haemostasis carried out by platelets and the coagulation cascade. The role of platelets is not confined to haemostasis, but also includes functions relating to liver regeneration[177], and studies in animals and humans suggest impaired liver regeneration in individuals with low platelet counts[178,179].
Following PH, platelets migrate to the space of Disse, where they release liver regeneration promoting biochemical including serotonin, VEGF, and HGF from secreted cytoplasmic granules[180]. In addition to growth factor containing vesicles, platelets contain cytoplasmic RNA, which can be transferred to nearby hepatocytes, resulting in gene expression, and promoting hepatocyte proliferation[181]. Finally, platelets may stimulate liver regeneration by activation of immune cells which also have an important role in cellular cross-talk[180].
In addition to the role of platelets, the coagulation cascade both individually and in combination with ‘damage associated molecular patterns’ (DAMPs) (including mitochondrial DNA and peptides)[182], activates elements of the complement cascade[183]. These include C3a and C5a, which have a role in stimulating pathways involved in the priming of hepatocytes[184], enabling them to respond to growth factors, as discussed in “Priming of hepatocytes”.
Other elements of the coagulation cascade may also play key roles in liver regeneration. Thus, Groeneveld et al[185] report that intrahepatic deposition of fibrinogen after PH is a key driver to platelet accumulation in the liver. Fibrinogen depletion was associated with impaired liver regeneration in a mouse model, and in humans undergoing liver resection, low intrahepatic fibrinogen and low post op serum fibrinogen levels were associated with poor liver function an increased mortality.
Urokinase-type plasminogen activator (uPA) activity increases within one minute of PH in rats[186], resulting in the activation of plasminogen to plasmin, which then activates key metalloproteinases (MMP) such as MMP-9[187,188], which remodel the hepatic ECM, where HGF is present in its inactive form. uPA also activates HGF to its active form[189], releasing it locally in the liver parenchyma and also into the circulation in significant quantities[190]. uPA knockout mice show impaired liver regeneration[60]. As well as HGF, the ECM contains other inactive forms of growth factors including HB-EGF and fibroblast growth factor (FGF)[191]. Moreover, the importance of matrix alteration in the initiation goes beyond the release of growth factor stores in the ECM in that hepatocyte response to key growth factors is ineffective in the presence of intact ECM, and that ECM changes are required for growth factor driven hepatocyte proliferation[192,193].
Thus, the rapid action of uPA following PH provides a mechanism to kick start the liver regenerative process by liberating ECM stored growth factors, until such time as other mechanisms begin to contribute to maintaining the liver regenerative process.
Liver regeneration is characterized by hypertrophy and rapid proliferation allowing return to the starting volume of liver even if recovering from a 25% remnant in humans, or a 10% remnant in some rodent models. The proliferation of hepatocytes is controlled by a maelstrom of growth factors with different but overlapping effects. Within this complexity exists a hierarchy of functions, whereby hepatocytes first require to be primed (“Priming of hepatocytes”), after which they become responsive to a range of mitogens referred to as complete (“Complete mitogens”) auxiliary (“Auxiliary mitogens”), and complex (“Complex mitogens”). “Intracellular signalling pathways” summarizes the intracellular pathways which transmit the effect of the growth factors described in sections “Priming of hepatocytes” - “Complex mitogens”.
Hepatocyte transition from G0 to G1: Although proliferation of hepatocytes is stimulated by a wide range of biochemicals in response to injury, most hepatocytes in uninjured liver do not proliferate[194], although there is some heterogeneity in this regard as discussed in “Hepatocyte response heterogeneity after PH” entitled ‘hepatocyte regenerative heterogeneity’. The stimulus to proliferation from the multitude of mitogens requires hepatocytes to be ‘primed’, a complex phenomenon characterized by the induction of > 100 genes[195], which then enables the hepatocytes to respond to these mitogenic stimuli.
Although cell cycle biology is outside the scope of this review, a brief summary of key events is useful to frame the subsequent sections relating to the priming effects of cytokines and proliferative stimulus of mitogens on hepatocytes.
The cell cycle is divided into 2 main phases: mitosis (the actual process of cell division) and interphase (the phase preparing the cell for mitosis). Interphase is further divided into 3 stages, which, in order, consist of the G1 phase (during which the cell synthesises protein and organelles), S phase (during which DNA is replicated) and G2 phase (during which the machinery for mitosis is assembled). Although some cells undergo this cycle continuously, others exit the cycle and enter a stationary phase G0. In order for a cell in G0 to replicate, it first needs to be ‘primed’ by molecular signals to return to G1, whereupon a different set of signals will determine the speed of replication and how long it continues.
Hepatocytes provide an example of this situation, and are, in the absence of injury, almost entirely in G0[194]. Their proliferation therefore requires priming factors to return them to G1. The priming function is carried out by cytokines TNFα and IL6.
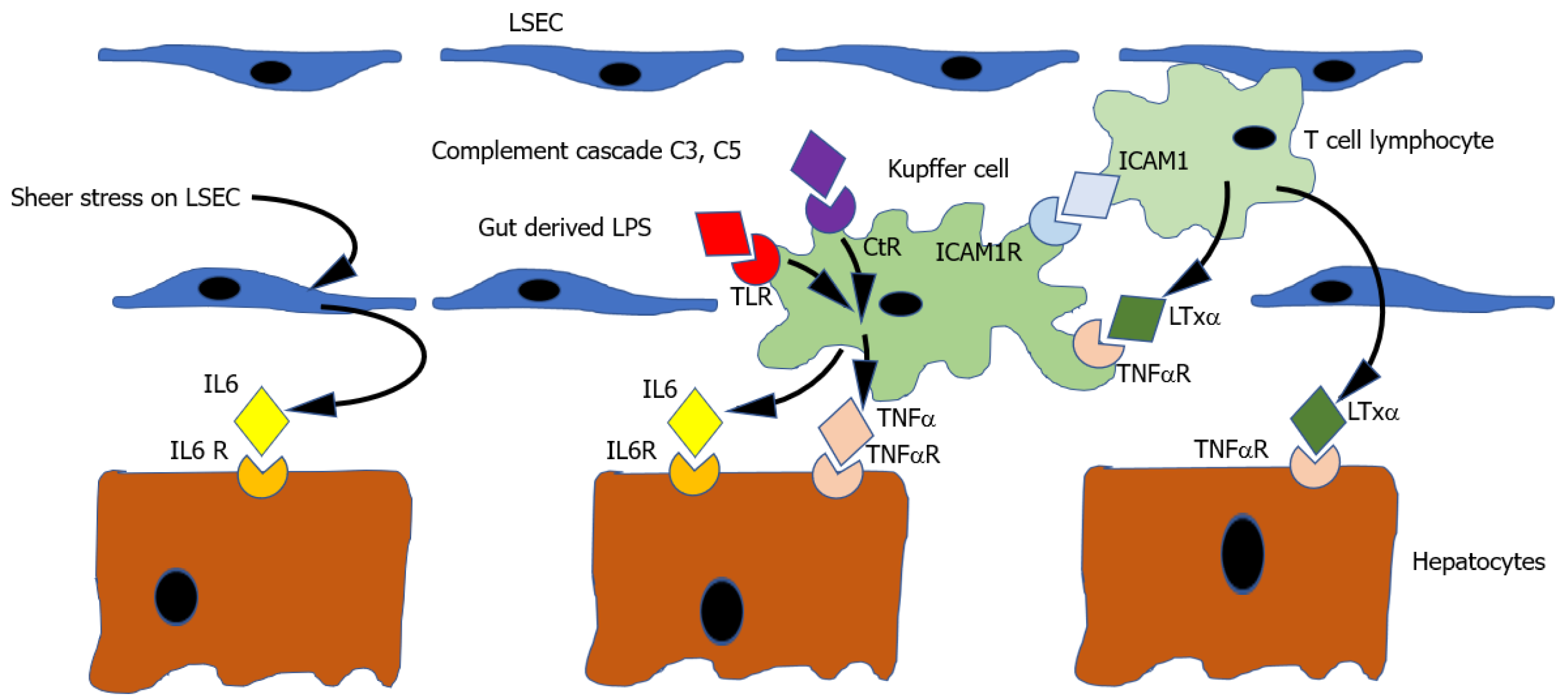
Thus the current working model[184] (illustrated in Figure 1) suggests that the cytokine priming mechanism starts with the activation of nuclear factor-kappa B (NFκB) in Kupffer cells. NFκB activation may be triggered by several stimuli including (1) Binding of TNFα to its receptor; (2) Binding of complement components C3a & C5a to their receptor; or (3) Binding of lipo-polysaccharide to the TLR receptor. Activation of NFκB results in increased expression of both TNFα and IL6. TNFα may stimulate its own further expression in the Kupfer cell in an autocrine manner. IL6 binds IL6R on hepatocytes, producing activation of signal transducer and activator of transcription 3 (STAT3), which results in the transcription of multiple other genes which push hepatocyte from G0 to G1, thus priming the cell to be responsive to circulating growth factors.
Crucially, in vivo, infusion of the powerful complete mitogens EGF and HGF produces only modest hepatocyte proliferation, whereas marked hepatocyte proliferation is observed if EGF and HGF infusion is preceded by the priming effect of a single TNFα injection[196].
Consistent with this model the following events are observed in the minutes after PH: (1) TNFα and IL6 mRNA and protein increase immediately[197,198]; and (2) Activation of the transcription factors NFκB and STAT3[199,200]. Moreover, DNA replication in hepatocytes is blocked by TNFα antibodies[201], TNF receptor (TNFR)[57] and IL6[56] knockout mice show impaired liver regeneration, and liver regeneration in TNFR knockout mice is rescued with IL6 infusion[57].
Of note, highlighting the necessary caution needed before extrapolating between animal models, TNFα levels after PH differ between rats and mice, with higher levels in rats. Also, the model exemplifies the recurring theme of redundancy in the system with the TNFα knockout mice showing normal liver regeneration because of the ability of other ligands to bind the TNFR[202]. Similarly, the activation of STAT3 may be achieved by other cytokines than IL6, such as Stem Cell Factor[203] and Oncostatin[203].
Triggers to cytokine priming: The initial triggers to expression of the priming cytokines TNFα and IL6 after PH are doubtless numerous and not all identified, but at least 5 stimuli have been demonstrated.
Firstly, PH results in an immediate increase in portal venous pressure which causes a sheer stress on liver sinusoidal endothelial cells[156]. This physical stimulus has many consequences[159] which are discussed in more detail in section 2 on early events post hepatectomy, but which include the induction of IL6 expression in LSECs[164], thereby contributing to the priming of hepatocytes .
Secondly, another trigger to cytokine expression after PH is binding of LPS derived from gut bacteria and translocated to portal blood, to the TLR, and producing expression of IL6 and TNFα. The increase in portal pressure resulting from PH increases gut permeability and may therefore result in exposure of the remnant liver to higher concentrations of LPS[204]. Supporting the physiological relevance of this hypothesis, is the observation that rodents with germ free guts have impaired liver regeneration[205]. The effects of LPS on liver regeneration may not be limited to induction of the priming molecules IL6 and TNFα, but also producing an increase in secretion of hepatic mitogens including insulin, epidermal growth factor, and triiodothyronine[204] (see “Complete mitogens” and “Auxiliary mitogens” on complete and auxiliary mitogens).
Thirdly, it is also known that binding of complement cascade components C3 and C5 to the complement receptors on Kupfer cells also triggers an NFκB dependant increase in both IL6 and TNF. Thus, complement activation resulting from physical injury to liver in PH may also contribute to the initiation of cytokine priming of hepatocytes. The significance of this mechanism is suggested by the finding that following PH, C3-5 knockout mice show diminished activation of NFκB and STAT3, decreased expression of TNFα and IL6 impaired liver regeneration[64].
Fourthly, it is observed that mice lacking the receptor intercellular cell adhesion molecule 1 (ICAM1) show diminished TNFα and IL6 expression and impaired liver regeneration after PH. It is thought that leucocytes, attracted to a liver injury site may mediate triggering of ICAM 1 on Kupfer cells, thus providing another stimulus to initiating the cytokine cascade[206].
Fifth, it is known that the TNFR may be activated not only by TNFα, but also by the protein lymphotoxin alpha (LTxα). This is markedly upregulated in intra-hepatic T lymphocytes after PH[207] and may thus allow T cells to contribute to initiation of the cytokine cascade by activation of TNFR on Kupffer cells. Consistent with this, mice lacking both TNFα and LTα show impaired liver regeneration[208]. Moreover, LTxα may act directly on hepatocytes.
Thus, having been primed by the initial injury triggered cytokine cascade, hepatocytes return to the G1 phase of the cell cycle where they are susceptible to stimulation by mitogens including growth factors, hormones and other biochemicals to accelerate the rate of proliferation.
The concept of mitogen hierarchy: A multitude of different hepatocyte mitogens have been identified which originate from a variety of different tissues, different cell types within a given tissue, acting via different receptors, or sometimes overlapping in their receptor binding, and producing a variety of different effects on the target hepatocyte. This complexity exemplifies a key feature of liver regeneration biology, which is the existence of high levels of redundancy, presumably an evolutionary outcome enabling the liver to cope meet the wide range of physical, biochemical and infectious injuries it may encounter.
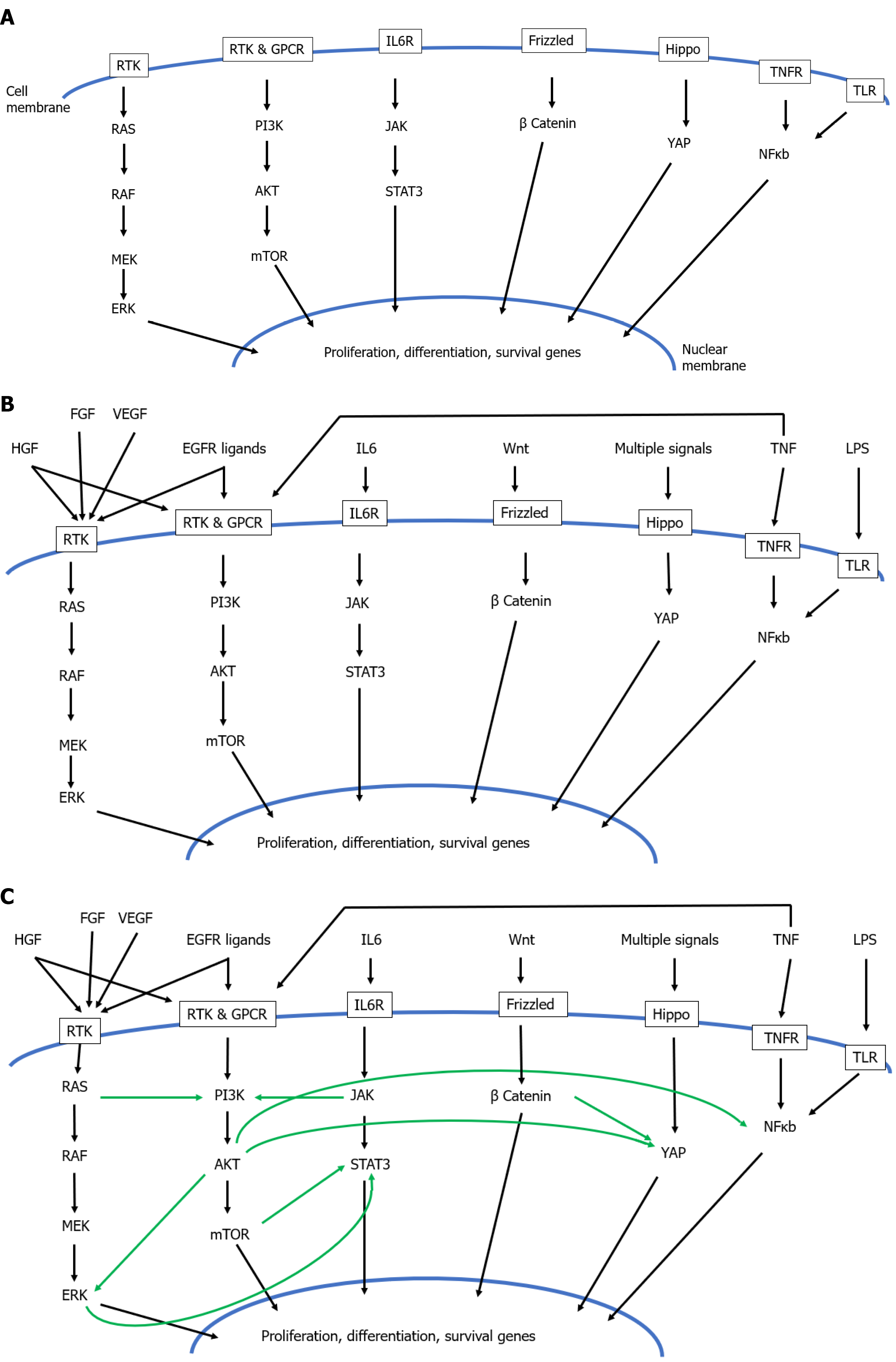
Amongst this complexity however, as arisen the concept of a hierarchy amongst hepatic mitogens, classifying them as ‘complete mitogens’, ‘auxiliary mitogens’, and ‘complex mitogens’[209]: (1) Complete mitogens cause proliferation of hepatocyte cultures in serum- free media, and, when injected into whole animals, cause liver enlargement and hepatocyte DNA synthesis. Moreover, ablation of both the MET and EGFR pathways leads to complete inhibition of liver regeneration. The complete mitogens are (a) Hepatocyte growth factor which binds to its receptor MET; and (b) Ligands of the EGFR: EGF, transforming growth factor-α (TGFα), heparin- binding EGF- like growth factor and amphiregulin; (2) Auxiliary mitogens do not cause hepatocyte proliferation in culture in serum free media, do not cause hepatocyte DNA synthesis and liver enlargement when injected in vivo, and ablation of their signalling pathways delays but does not abolish liver regeneration. The auxiliary mitogens are noradrenaline and the α1- adrenergic receptor, VEGF and its receptors (VEGFR1 and VEGFR2), bile acids, serotonin, insulin, and growth hormone; and (3) Complex mitogens are the third category and are much less well defined than complete or auxiliary mitogens, with pathways involving multiple overlapping extracellular signals, disruption of which delays but does not abolish liver regeneration. The complex mitogens are the proteins involved in the Wnt, β-catenin, Hippo and Yap pathways.
Hepatocyte growth factor: HGF was the first complete hepatic mitogen, identified in 1984 with the human homolog cloned in 1989[210]. Thus, HGF produces hepatocyte proliferation in serum free media in vitro, and liver enlargement when infused in vivo. HGF mediates its effect on hepatocytes by binding to its receptor MET, a receptor tyrosine kinase with wide ranging roles in diverse areas of cell biology including not only cell survival and proliferation[211], but also metabolism[212], growth and development[213]. MET signalling is dependent on the transcription factor CCAAT/enhancer-binding protein beta C/EBP beta[214], and Inhibition of MET signalling results in blocking of mitosis and increased expression of apoptosis genes after PH[215].
After PH, HGF is mobilised in a biphasic manner, first with the activation and recruitment of ECM bound inactive HGF in the immediate minutes after PH, and then secondly by secretion of newly expressed HGF in a second wave.
Thus, whilst HGF is bound in inactive form in the ECM in resting liver[216], ECM remodelling[187] resulting from PH results in activation of HGF with binding to hepatocytes and released into the circulation[217], which peaks 30-60 min after PH.
Thereafter, peaking at 24 h post PH[218], a second wave of HGF is observed, newly synthesized by LSEC and stellate cells in the liver, but also from extra-hepatic cells and tissues including platelets[219], lung[220], kidney, spleen[221], thyroid, brain, and salivary glands[221]. In spite of these multiple sites of HGF production, experiments using genetically altered mice showed that inhibiting HGF production specifically in LSECs resulted in impaired liver regeneration, suggesting that extra-hepatic HGF production cannot compensate for depletion of hepatic HGF production[222]. The factors that stimulate HGF expression in the second wave after its release from ECM include noradrenergic signals[223], insulin like growth factors[224].
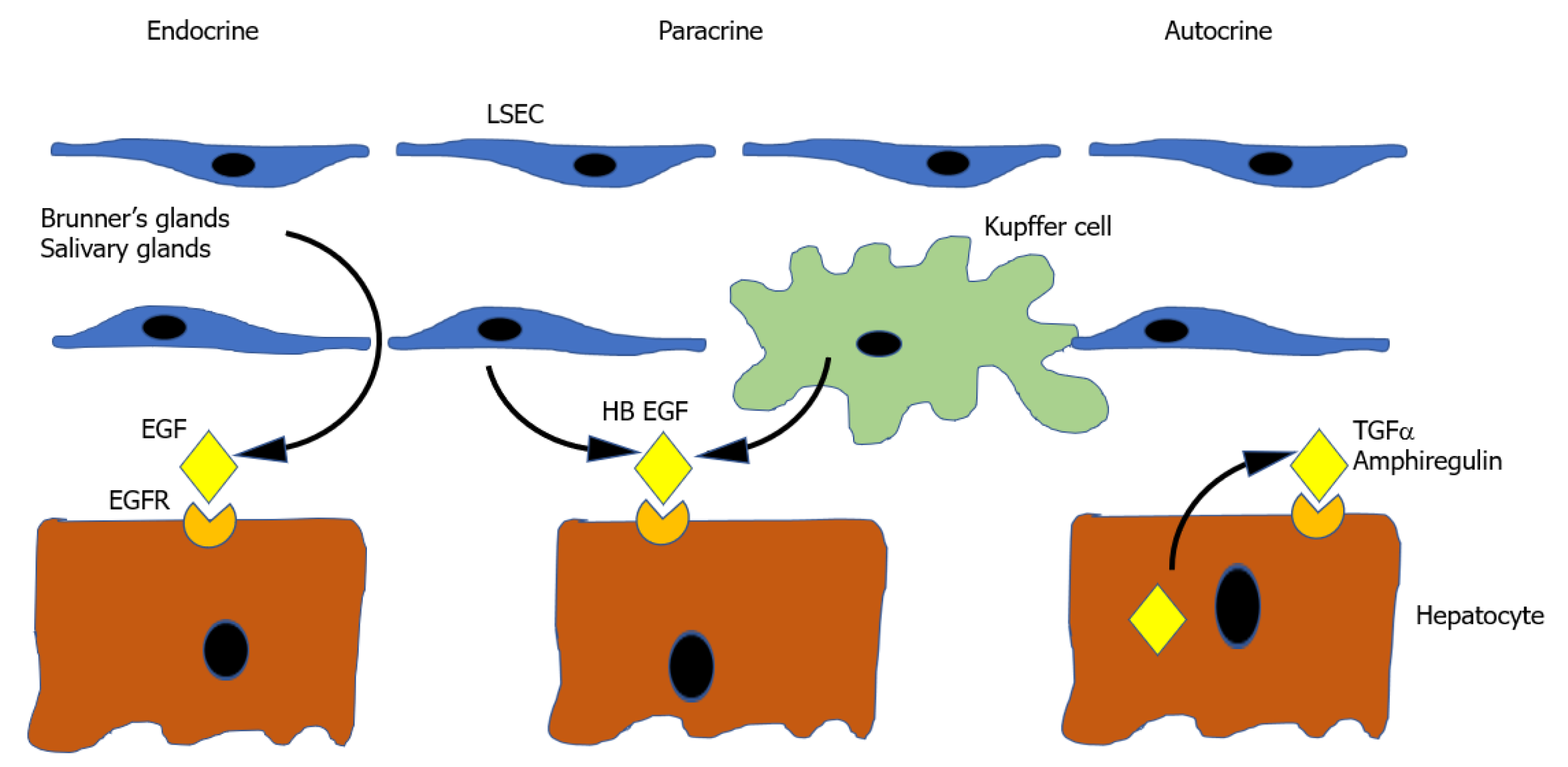
Epidermal growth factor: Ligands of the EGFR make up the other known complete mitogens. EGF is one member of a family of 7 Ligands which bind a group of 4 receptors (EGFR/ErbB1, HER2/ErbB2, HER3/ErbB3 and HER4/ErbB4)[225]. Of the 7 known ligands, the ones that relate to liver regeneration are epidermal EGF, transforming growth factor-alpha (TGFα), Amphiregulin (AR), and Heparin bound EGF-like growth factor (HB-EGF), with their role illustrated in Figure 2.
EGF is a complete mitogen and produces hepatocyte proliferation in vitro and in vivo when infused[226]. It is produced in many tissues[227], but the most relevant sites of production are the Brunner glands of the duodenum which provide a constant supply of EGF to the liver via the portal vein[228]. EGF production is increased by nora
TGFα is produced by hepatocytes themselves during liver regeneration[231] and therefore functions as a mitogen in an autocrine or paracrine way[232]. TGFα knockout mice have no liver regeneration deficiency however, presumably as a result of the considerable redundancy in the EGFR signalling pathway[233].
Amphiregulin, like TGFα, is produced by hepatocytes. Its expression is in part regulated by inflammatory mediators providing a mechanism for its upregulation following PH. Its significance is suggested by the observation that AR knockout mice have impaired liver regeneration[234,235].
HB EGF is produced Kupffer cells and sinusoidal endothelial cells[236]. Its expression seems to be in part determined by the magnitude of liver resection, as it is increased in 2/3 PH but not 1/3 PH. Its physiological significance is emphasised by the fact that HBEGF transgenic mice[237] and HB EGF knockout mice[70] have accelerated and delayed liver regeneration, respectively.
In the midst of these multiple ligand binding events, EGFR activation peaks at 60 minutes post PH[238], and ablation of EGFR by antisense RNA impairs liver regeneration[239].
Bile acids: Primary bile acids are synthesized in the liver by a multistep oxidative metabolism of cholesterol and secreted in bile. In the intestine, bile acids emulsify fats thus facilitating their digestion. Bile acids are metabolized by gut bacteria to produce secondary bile acids, and although some are lost through faecal excretion, a significant proportion are reabsorbed in the gut and recycled in the liver, in the entero-hepatic circulation[240].
Above a certain concentration, bile acids are toxic to liver and may induce apoptosis and necrosis, such that bile salt synthesis is tightly regulated by means of negative feedback loops involving bile acid receptors in the ileum[241]. At non-toxic concentrations however, bile acids play an important part in regulation of liver regeneration. Both the negative feedback controls and liver regenerative roles are mediated by bile acid receptors which comprise the extracellular TGR5 receptor (TGR5) on Kupffer cells[242], and intracellular Farnesoid X receptor (FXR) within hepatocytes[243].
Bile acids were first investigated as candidate factors for controlling liver regeneration in part because of their exclusively hepatic synthesis, offering the potential for a feedback loop hepatostatic mechanism. Thus, dietary bile acid supplementation was found to produce hepatomegaly in mice with non-injured livers, and increase liver regeneration after PH, in an effect that was dependent on the FXR. Conversely, bile acid sequestering agents resulted in impaired liver regeneration[243]. Furthermore, genetically engineered bile salt deficient mice also show impaired liver regeneration after PH[244], and rats having undergone PH with biliary fistula also show impaired liver regeneration, which can be rescued by intestinal delivery of bile acids[245].
After PH, serum bile acid concentration increases in blood within minutes, peaks at 24 h, and diminishes again by 48 h. The mechanism of this serum bile acid increase is not fully understood, but may involve neurological pathways activated by PH related changes in portal pressure[246], consistent with the observation that bile acid increase is also seen after portal vein embolization[247].
The binding of bile acids to the FXR stimulates activation of transcription factor Forkhead box M1b (FoxM1b), an injury-induced transcription factor that promotes cell cycle progression[248]. In addition, bile acids also contribute to liver regeneration by binding extra-hepatic FXR situated in the ileum, resulting in expression of fibroblast growth factor (Fgf15/FGF19). Fgf15/FGF19, which then binds it receptor FGFR4[249] on hepatocytes, stimulating cell cycle progression[241]. In comparison to the FXR receptor, the role of bile acid binding to the TGR5 receptor on Kupffer cells is less well understood, but is clearly important as TGR5 knockout mice show impaired liver regeneration after PH, as well as severe hepatic necrosis[250].
Thus, bile acids have an important role in the control of liver regeneration and may contribute to the post liver injury hepatostat.
Noradrenaline: Noradrenaline secretion increases following PH[251] and is produced by the adrenal medulla, sympathetic neurons, as well as by hepatic stellate cells.
Noradrenalin not only stimulates the production of EGF (from Brunner’s glands) and HGF from fibroblasts, but also augments their mitogenic effect[252], and activates the proliferation associated STAT3 pathway[253], whilst reducing the mito-inhibitory effects of TGFβ[254]. Thus, α1 receptor blockade, and also hepatic sympathectomy significantly delays liver regeneration after PH[251]. Noradrenaline may also stimulate liver regeneration by activating WNT and β-catenin pathways via β-adrenergic receptors[255].
Serotonin: Serotonin is a neurotransmitter stored by platelets and which has a role in the control of inflammation. Mice with absent platelets or lacking tryptophan hydroxylase 1 (a key enzyme in serotonin synthesis) show significantly delayed liver regeneration after PH[256], which is rescued by serotonin infusion. Moreover, serotonin agonist produces LSEC fenestration changes, and a VEGF dependent increase in hepatocyte proliferation[257]. Serotonin may also act via the Hippo proliferative pathway[258]. Although serotonin deficient mice show significantly impaired liver regeneration, serotonin exemplifies the need for caution in assuming that the results of one animal model may be extrapolated to others, as it is noted that rats lacking the serotonin transporter which are unable to store serotonin in platelets do not show any liver regeneration impairment after PH[259].
Insulin: Insulin is produced by the beta cells of the pancreas, and was one of the earliest identified hepatic mitogens, having been found to prevent liver atrophy when infused directly into the liver via the portal stump in dogs having undergone portocaval shunt[11,13]. Although insulin is not a complete mitogen in that it does not induce hepatocyte proliferation in vitro, its presence is essential for hepatocyte survival in culture[260] and is essential for the effects of complete mitogens EGF and HGF in vitro[261].
The paradox that insulin is not a complete mitogen in vitro, but able to prevent liver atrophy after portal diversion is not fully understood but may be partly explained by interactions of the insulin receptor with EGFR and MET, thus triggering those proliferative pathways[212].
Growth hormone and insulin-like growth factor: Growth hormone is synthesized in the pituitary gland and has widespread growth-related roles in many tissues[262]. The effects of GH can be mediated directly via the GH receptor, or indirectly by insulin-like growth factor (IGF), which is synthesized by hepatocytes in response to GH and secreted into the circulation bound to IGF binding protein (IGFBP). Whilst hepatocytes do not have IGF receptors[263], Kupffer cells and stellate cells do[264], allowing a possible paracrine role for IGF in the liver. GH may also act directly on hepatocytes by upregulating the EGFR[265] and also stimulating activity of the EGFR in cross-talk with the GHR[266]. Consistent with this, in the rat model, exogenous dietary or infused GH enhances liver regeneration after PH, and mice lacking IGFBP show impaired liver regeneration after PH[63].
In terms of the physiological relative importance of these pathways, Pennisi et al[267] showed that GH lacking mice showed the greatest impairment to liver regeneration, with less marked liver regeneration impairment seen in IGF and IGFBP lacking mice, suggesting that whilst both direct and indirect GH actions impact on liver regeneration, the direct effect of GH is more significant that IGF mediated effect.
Thyroid hormone: The thyroid hormones Triiodothyronine (T3) and thyroxine (T4) are produced in the follicular cells of the thyroid gland, and have extensive roles in carbohydrate, protein, and lipid metabolism, regulation of metabolic rate, oxygen consumption, thermal regulation, muscle function, and roles in tissue growth and development[268].
In terms of liver regeneration, thyroid hormones have been shown to act as incomplete mitogens with impaired liver regeneration seen in thyroid receptor knockout mice[269], and conversely accelerated liver regeneration in T3 treated rats after PH[270].
In terms of molecular mechanisms of action in promoting liver regeneration, the thyroid hormones do not act via the NFκB or STAT3 pathways which are typically activated by the complete mitogens. Rather, thyroid hormones mediate hepatocyte proliferation by a number of pathways including (1) Increase in expression of transcription factors of the E2F family, which accelerates the transition of hepatocytes from G1 to S phase[271]; (2) Increased expression of cell cycle promotion genes Cyclins A, D1, and E, and diminished expression of their inhibitors[272]; (3) Decreased levels of p53 and p73 (tumour suppressor proteins involved in growth arrest and apoptosis)[269]; and (4) Activation of the Wnt/b-catenin signalling pathway[273].
VEGF: The VEGF family of growth factors comprises a group of at least 6 isoforms (VEGF A, B, C, D, E, F), which bind to the 3 different receptors (VEGFR 1, 2, 3), with roles in cell proliferation, migration, metabolism, vasodilation, blood vessel formation and remodelling[274]. Though not directly mitogenic on hepatocytes directly, VEGF plays a central role in liver regeneration in several ways, including the orchestration of proliferation of LSECs, and inducing the LSEC population to produce key hepatocyte mitogens including HGF[175, 275].
In a rat 70% PH model, Bockhorn et al[276] showed that blocking VEGF signalling with anti VEGF antibodies almost completely suppressed hepatic proliferation in the first 24 h after surgery, and conversely that exogenous VEGF promoted hepatocyte proliferation, suggesting a physiologically relevant role for VEGF in the early stages of liver regeneration.
WNT/β-catenin signalling pathway: The WNT family of genes are named after the gene responsible for the Wingless-type phenotype in Drosophila melanogaster, and int-1 (a target for insertional activation of mouse mammary tumour virus, and a secretory glycolipoprotein-encoding gene which is regarded as the prototype for several mammalian genes)[277]. The resulting glycolipoproteins participate in several fundamental signalling events, which influence cell proliferation and tissue homeostasis[278].
β-catenin is a protein encoded by the CTNNB1 and is a subunit of the cadherin protein complex which acts as an intracellular signal transducer in the context of WNT signalling, but which also interacts with a variety of transcription factors such as T-cell factor and hypoxia-inducible factor 1α[279]. β-catenin plays an important role in human embryogenesis, including liver development[280]. It is widely expressed in the adult liver and is always active in the pericentral region. Usually bound to a multiprotein degradation complex, it can be activated by several pathways, including WNT.
The WNT/β-catenin pathway has been shown to be active during liver regeneration, contributing towards mass and functional recovery from a the very early stages of liver injury[281]. β-catenin is in fact detected in rat hepatocyte nuclei within 5 minutes of partial hepatectomy[282]. Upon WNT binding to its receptor (Frizzled), β-catenin translocates to the nucleus, where it promotes the expression of key genes, such as high-level controllers of transcription like c-myc and cell cycle regulating genes like cyclin D1[279].
In the normal liver, β-catenin regulates the expression of genes in pericentral hepatocytes and influences hepatic lobular zonation[279] and is involved in cell-cell adhesion[280]. Additionally, it implicated in a variety of diseased liver states, although the exact mechanisms remain incompletely understood. Specifically, β-catenin appears to be involved in the development of NASH, partly by binding to TCF4 and HNF4α, thus regulating hepatic gluconeogenesis and lipogenesis. In hepatic fibrosis, the literature is currently conflicting regarding the role of WNT/β-catenin signalling. Nevertheless, evidence is accumulating to show that this signalling pathway is activating during hepatic stellate cell activation and fibrosis, and that WNT blockade is associated with an antifibrotic effect[279].
This pathway has also been identified in hepatic neoplasia. In focal nodular hyperplasia (FNH), glutathione synthetase (the expression of which is regulated by WNT/β-catenin signalling) stains FNH, which may be of diagnostic value[279]. Other relevant neoplastic processes include hepatocellular adenoma both with and without the presence of CTNNB1 mutations, HCC where mutations may lead to autonomous WNT-mediated activation of β-catenin, and hepatoblastoma, where 90% of tumours are associated with CTNNB1 mutations[279].
Hedgehog signalling pathway: Emerging evidence in the literature has shown the importance of the activation of the Hedgehog (Hh) pathway in the context of liver regeneration. Hh is a protein produced as a 45-kDa precursor that undergoes proteolytic processing in the endoplasmic reticulum[283]. The Hh pathway is a highly complex signalling cascade, which may be summarised in four fundamental components: (1) The ligand Hedgehog; (2) The receptor Patched (Patch); (3) The signal transducer Smoothened (Smo); and (4) The effector transcription factor, Gli. Components of the Hh pathway concentrate in the Primary cilia and a complex Primary cilium trafficking system regulates the interaction of Hh pathway components to enhance, or block, the Hh-initiated signal[284-286].
Previous work in adult rodents has demonstrated that Hh ligand expression increases transiently but significantly following partial hepatectomy[287]. Furthermore, inhibiting Hh pathway induction with a direct pharmacologic antagonist of Smo was found to decrease both recovery of liver volume and overall survival[288]. Evidence in the literature suggests that, mice subjected to portal vein ligation (a procedure commonly done in humans to allow the remnant liver to enlarge prior to hepatectomy) with simultaneous administration of systemic Hh, performed as well as mice submitted to ALPPS, supporting the evidence that Hh signaling plays a major role in promoting liver regeneration[289]. Further evidence suggests that the extracellular matrix of the healthy adult liver, the proteoglycan glypican-3 binds normally to Hh to prevent Hh from binding to Patch in order to constrain activation of the Hh pathway[78].
The evidence above highlights the role of the Hh pathway in post-hepatectomy liver regeneration. Further translational studies are required in order to explore the role of administering a recombinant form of Hh in the pre-operative setting in patients undergoing major hepatectomy who are potentially at risk of liver insufficiency.
Hippo Yap signalling pathway: The Hippo signalling pathway was originally identified in Drosophila melanogaster and its components have mammalian homologs[290]. The Hippo signalling pathway exerts a controlling influence on organ size by regulatory effects on cell proliferation, apoptosis, and stem cell self-renewal[291].
The pathway consists of a series of protein kinases, activation of which results in the phosphorylation of yes-associated protein (YAP), thus preventing its translocation to the nucleus. In the nucleus YAP interacts with a family of transcription enhancer factors. This family of nuclear proteins are involved in the modulation and regulation of multiple genes involved in promoting cell proliferation[292-294].
Multiple signals may activate Hippo signalling, including mechanical stimuli and cell attachment. Thus, in situations of high cell density, activation of Hippo signalling leads to the inhibition of YAP nuclear translocation, and thereby a break on cell proliferation[295]. Conversely, decreased Hippo pathway signalling allows YAP mediated pro-proliferation signals and is associated with an increase in organ size through excessive proliferation and inhibition of apoptosis[292]. Consistent with this, and suggesting its physiological importance, mice with liver specific YAP deletions show significant impairment in liver regeneration[296].
However, the Hippo-YAP signalling pathway is the final step of multiple opposing signalling pathways that contribute to liver regeneration and repair and the conflicting nature of these signals makes the study and understanding of the role of this pathway challenging[209].
The complexity inherent in the multitude of extracellular molecules implicated in the control of liver regeneration is reflected by a similarly complex array of intracellular signalling pathways which transmit the effect of ligand-receptor binding to the nucleus to activate effector genes.
In the same way that there is much redundancy in growth factor function extracellularly, there is also much overlap in the intracellular pathways, probably reflecting an evolutionary mechanism to safeguard against failure of any one individual pathway.
The complexity of each pathway, the activation of different pathways by diverse ligands, and the intracellular cross-talk between pathways makes it difficult to assign quantitative importance to any one pathway. Nevertheless, the section describes the main recognized intracellular pathways relating to liver regeneration. A full account of this area is beyond the scope of this review, but the summary below is intended to give a general overview and an impression of the ramifying complexity of the processes involved.
Figure 3A shows the pathways separately in summary form. Figure 3B shows the overlap in ligand binding, and Figure 3C provides an impression of the cross-talk between the various pathways.
Ras/Raf/MEK/ERK pathway: The Ras/Raf/MEK/ERK Pathway is triggered by binding of ligands to receptor tyrosine kinase receptors, which triggers autophosphorylation of tyrosine residues on the intracellular aspect of the receptor, resulting in the sequential activation of downstream components, ultimately controlling the expression of multiple growth controlling genes including high level ‘master genes’ such as c-myc, c-fos, and c-jun[297]. The first molecule, RAS, once activated, can activate multiple different signalling intracellular pathways including not only the Raf/MEK/ERK pathway, but also the MEKK/SEK/JNK pathway, and pathways involving NFκB[298,299].
In liver regeneration, the growth factor which activate this pathway include HGF, the EGFR ligand family members, fibroblast growth factor, and VEGF[299].
Phosphatidylinositol 3’-kinase/AKT kinase (also known as protein kinase B)/ mammalian target of rapamycin (PI3K/Akt/mTOR) pathway: The PI3K/ Akt/mTOR is a ubiquitous pathway is involved in the regulation of fundamental physiological processes including transcription, apoptosis, cell cycle progression, and translation[299]. The pathway is activated by binding of ligands to receptor tyrosine kinases or G-protein-coupled receptors[300], ultimately promoting cell growth, proliferation, survival [301], and malignancy when dysregulated[302]. In the context of liver regeneration, the main growth factors activating the PI3K/Akt/mTOR pathway include TNF-α, IL-6, HGF, EGF, and transforming growth factor (TGF)-α[303] (Figure 3B and C).
Janus Kinase pathway: Activation of the Janus Kinase (JAK) kinase pathway promotes cell proliferation, differentiation, migration, growth. Like RTKs, JAKs activate by autophosphorylation. STAT3 is the key downstream messenger which translocates to the nucleus and functions as a high level transcription factor in liver regeneration with resultant induction of gene expression including cytokines[304]. In the context of liver regeneration, this pathway is primarily activated by IL-6 and its receptor, IL-6R.
Interestingly, following PH, a circulating (rather than membrane bound) form of the IL6R cleaved by matrix metalloproteases appears to have a key role in initiating liver regeneration[86].
NF-κB pathway: In priming of hepatocytes, Kupffer cells are induced to secrete TNF-α and IL-6 after stimulation by a variety of stimuli (see section on hepatocyte priming) including complement factors and LPS from the gut. This action of Kupffer cells is mediated by the NF-κB signaling pathway. Once activated, NF-κB migrates to the nucleus, where it promotes the further expression of TNF, IL-6, and VEGF[305].
WNT/β-Catenin pathway: The Wnt/β-catenin pathway regulates processes including cell proliferation, and tissue morphology[282]. Upon WNT binding to its receptor (Frizzled), β-catenin translocates to the nucleus, where it promotes the expression of key genes, such as high-level controllers of transcription like c-myc and cell cycle regulating genes like cyclin D1[279].
Hippo pathway: The Hippo signalling pathway is involved in cell proliferation, apoptosis, and stem cell self-renewal[291], and may have a key role in the ending of hepatocyte proliferation after regeneration[295]. YAP is a key downstream effector of the Hippo pathway, which translocates to the nucleus, once activated to promotes expression of target genes. The pathway is activated by numerous factors including organ size, cell attachment, mechanical stress, hormones, growth factors[295], as well as vascular shear stress[306].
Cross-talk between pathways: Mirroring the redundancy and overlap in extracellular growth factors and receptor binding, there exists a complex crosstalk between pathways. Just a few examples of the known positive feedback interactions are shown in Figure 3C, which, albeit incomplete and without including negative feedback interactions, provides an impression of the intricacy of intracellular interactions between pathways[297]. Thus, Ras interacts with phosphatidylinositol 3’-kinase (PI3K)[307]. The NF-κB pathway cross-talks with the PI3K/Akt/mTOR, pathways[308]. β-catenin interacts with the Hippo signalling pathways[195]. YAP cross-talks with PI3K/Akt pathway[309].
The proliferation of hepatocytes in liver regeneration is in critical ways dependant on the role of non-parenchymal cells (Kupffer cells, stellate cells, and liver sinusoidal endothelial cells). Conversely, proliferating hepatocytes provide many growth factors that elicit non parenchymal cell proliferation, thus suggesting an interdependence allowing proportionate expansion of each cell type to produce liver tissue containing all key constituents. Thus, proliferating hepatocytes produceVEGF and angiopoietins 1 and 2 (LSECs mitogen), TGFα (LSECs and stellate cell mitogen), fibroblast growth factor 1 (FGF1) and FGF2 (HSC and LSEC mitogen), and granulocyte–macrophage colonystimulating factor (GMCSF) (Kupffer cells mitogen)[191,310,311]. In this section we examine in more detail the part played by non-parenchymal cells in liver regeneration.
LSECs have a key role in the immediate events that trigger the onset of hepatocyte proliferation after PH. Thus, shear stress resulting from portal pressure changes after PH induces the expression of the hepatocyte priming IL6[164].
Once liver regeneration is initiated, hepatocytes form clusters which are initially avascular. The production of VEGF, and angiopoietin 1 & 2 stimulates endothelial cells to migrate into the avascular structures, proliferate, differentiate to a liver sinusoidal phenotype[312]. LSECs also produce VEGF[313], as well as the potent mitogen HGF, and NO[160,162] which increases hepatocyte sensitivity to HGF[163].
In the rat hepatectomy model, liver sinusoidal cell repopulation is not only achieved by resident LSECs, but also by recruitment of LSEC progenitors in the bone marrow which, under the influence of VEGF, undergo proliferation, migration into the bloodstream, and engraftment in the liver, where they contribute a significant proportion of the total HGF production.
Of note, the importance of bone marrow derived LSECs in this model is emphasised by the fact that bone marrow ablation by irradiation abolishes liver regeneration, which can be rescued by exogenous infusion of LSECs[314,315]. Thus, LSECs play a key role, not only in allowing the vasculature of the liver to keep pace with regenerating hepatocytes, but also in providing the very growth factors (such as HGF) that allow hepatocyte to proliferate.
Stellate cells are situated in the Space of Disse between LSECs and hepatocytes [316], and though representing only approximately 8% of cells in the liver, have multiple long cytoplasmic projections[317] which contact hepatocytes, LSECs, and Kupffer cells, allowing a central role in intercellular signalling in part by production of growth factors such as HGF and VEGF. Stellate cells also exhibit cellular contraction, permitting the control of sinusoidal blood flow[318], and have a key role in the regulation of ECM, both in its production and degradation[319].
After hepatic injury, HSC become activated myofibroblast-like cells. In the midst of liver regeneration, the initiation, perpetuation and resolution of HSC activation only adds further complexity, in processes which are poorly understood[320], but which ultimately result in the laying down of ECM to provide the vital framework on which regeneration may proceed[321].
The triggering of HSC activation is multifactorial, but includes the secretion by hepatocytes of growth factors such as FGF1 & 2, and PDGF, the latter being a potent mitogen and chemo-attractant for HSCs[322], and highlighting an example of interdependent complex paracrine stimulation (with HSCs producing the hepatocyte mitogen HGF and hepatocytes producing the HSC mitogen PDGF).
Following activation, HSC production of HGF increases, in a mechanism dependant on the neurotrophin receptor P75NTR[323], and its downstream mediator Rho[324]. Activated HSCs also produce Noradrenaline, which enhances HGF production by mesenchymal cells[223] and production of EGF from Brunner’s glands[229]. HSCs interact directly with LSECs to stabilise and remodel sinusoids[325], via combined actions of PDGF, TGF-β1, FGF, VEGF, and angiopoietin. HSC are the principal cell source of ECM constituent production, and of ECM remodelling control by expression of matrix metalloproteases, thus providing the scaffold in which liver cells can regenerate[326].
The importance of HSC activation in liver regeneration is suggested by the observation that following ablation of HCS activation, liver regeneration is markedly impaired in both the mouse acetaminophen[327] and rat acetyl amino fluorene[328] models of liver injury with much reduced proliferation of hepatocytes and oval cells respectively, and with rescue of liver regeneration by infusion of medium conditioned by HSC[329].
Amongst the different resident intrahepatic macrophages, Kupffer cells are the predominant type, and originate from erythromyeloid progenitors in the foetal liver[330]. In the homeostatic situation, Kupffer cells have wide-ranging roles in (1) Clearance of cellular debris in blood[331]; (2) Maintenance of iron homeostasis via phagocytosis of red blood cells[332]; (3) Regulation of cholesterol homeostasis[333]; (4) Antimicrobial defence[334]; and (5) Promotion of immunological tolerance[335].
Kupffer cells have a limited half-life (of approximately 12 d in mice)[336]. Their maintenance is achieved by self-replenishment in the healthy liver[337], but is to an extent dependant on extra-hepatic progenitors in the case of liver injury[338].
There is evidence that replenishment of Kupffer cells following injury may be achieved by engraftment and differentiation of monocyte derived macrophages into a Kupffer cell phenotype, in a manner controlled by HCS and LSEC, highlighting once again the complex cross-talk between the non-parenchymal cell types[339]. The monocyte derived macrophage precursors may originate from within the liver, but also from the peritoneum[340] and spleen[341].
Kupffer cells are strategically placed with access to the sinusoidal lumen and space of Disse, and carry multiple surface receptors to injury related molecules (the Pattern Recognition Receptors), suggesting a key role for Kupffer cells as sensors of hepatic injury[342].
Thus, Kupffer cells are able to detect, and become activated by: (1) DAMPs, e.g., mitochondrial DNA (mtDNA), and ATP, from damaged hepatocytes; (2) Pathogen-associated molecular patterns (PAMPs)[343]; (3) Hypoxic liver environment[344]; and (4) Extracellular vesicles secreted from various cells containing proinflammatory stimuli[345].
The activation of Kupffer cells by any of the above stimuli results in the secretion a wide range of bioactive molecules including chemokines such as C chemokine 2 which attract inflammatory and immune response cells to the injury site[346], and proinflammatory cytokines such as IL6 and TNFα which prime hepatocytes out of G0 phase an also activate HSCs[347].
Thus, Kupffer cells have a central role in the initiation and orchestration of liver regeneration, and their importance is suggested by the observation that depletion of macrophages[206] or inhibition of monocyte[348] recruitment results in impaired liver regeneration following PH.
The liver regenerative response varies not only according to the nature of the injury, but also its magnitude and the status of the underlying liver parenchyma. These different contexts dictate how regenerating cells behave, but also the recruitment of different types of cells to accomplish the task. Current knowledge on the mechanisms of liver regeneration is largely derived from experimental models involving 2/3 PH in rodents[195], where “standard” liver regeneration occurs. This involves the proliferation of hepatocytes and cholangiocytes from homotypic precursors[209] and is addressed in the first subsection below. In the second subsection, the “alternative” liver regeneration pathways, which involve liver progenitor cells (LPCs) and transdifferentiation, will be examined. As seen in other aspects of liver regeneration, the mechanisms outlined below are subject to ongoing scientific scrutiny and are currently incompletely understood.
Liver regeneration after PH is achieved in different ways according to the magnitude of the liver resection. Thus regeneration after 1/3 PH is achieved principally by hypertrophy, with few cell divisions[349]. In contrast, during liver regeneration after resections larger than 1/3PH, although hypertrophy precedes hyperplasia, hyperplasia occurs increasingly as well, such that hypertrophy and hyperplasia contribute equally to liver regeneration in 70% PH. Moreover, during the hyperplastic response, although the majority of hepatocytes entered S phase of the cell cycle, not all undergo actual cell division, and the known significant number of polyploid hepatocytes[350] are shown to undergo division to produce mononuclear hepatocytes.
Thus, hepatocyte behaviour during liver regeneration is not uniform. Hepatocytes within a liver lobule are not equivalent and show functional heterogeneity. The liver lobule may be separated into 3 zones: Zone 1 (periportal) hepatocytes are in the vicinity of the portal triad, zone 3 (perivenular) hepatocytes are situated near the central vein, and zone 2 (pericentral) hepatocytes reside between zones 1 and 2. Metabolically speaking, zone 1 hepatocytes carry out gluconeogenesis and b-oxidation, in contrast to glycolysis, lipogenesis, and detoxification performed by zone 3 hepatocytes[195].
Moreover, there is some evidence that there is heterogeneity in baseline hepatocyte turnover during homeostasis, with a population of cells in zone 3 replenishing the lobule population, albeit slowly[351].
Hepatocyte proliferation heterogeneity is also apparent in the context of the regenerative response. In the context of true proliferative response after PH, lineage experiments have identified a population of hepatocytes in the periportal region zone 1 which appear to have greater proliferative potential. These are referred to as ‘hybrid hepatocytes ‘in that in addition to hepatocyte markers, they express progenitor cell genes and biliary transcription factors[352]. There appears to be further heterogeneity in proliferative response in that hepatocytes in proximity to LSEC proliferate faster than ones which are more distant[175].
The alternative liver regenerative pathways are characterized by deviation from the phenotypic fidelity in which hepatocytes or cholangiocytes proliferate to produce more of the same cell type. In this “alternative” context, liver epithelial cells (i.e., hepatocytes and cholangiocytes) can operate as facultative stem cells for one another in conditions where regeneration of one or other is impaired[209,353], presumably as a rescue mechanism. This mechanism appears beneficial from an evolutionary standpoint, is plausible from a developmental biology perspective given that both hepatocytes and cholangiocytes are derived from hepatoblasts[354], and has been demonstrated in previous studies[81,355-357]. Nevertheless, there is controversy in the field regarding the in vivo capability, conditions, and extent to which liver epithelial cells can transdifferentiate and achieve regeneration.
The term “LPCs” is seen in the literature, yet such cells have not been identified on microscopy or tissue dissociation of liver lobules in the resting state and are thought to possibly arise from the transdifferentiation of hepatocytes and cholangiocytes[353] as mentioned above. A central event in this process is the “ductular reaction”, which occurs when hepatocyte proliferation is suppressed, thus leading to the expansion of progenitor cells[209] and which can be observed both in acute and chronic liver disease models, typically after extensive hepatocyte injury.
In conditions such as fulminant hepatic failure, some liver epithelial cells demonstrate an overlapping set of biomarkers (e.g., cholangiocytes may express “hepatocytic” biomarkers such as HNF4, albumin, and HEPPAR3)[353]. The extensive necrosis and apoptosis characteristic of fulminant hepatic failure is thought to pivot liver regeneration towards LPCs, as reflected in elevated α-fetoprotein levels[358] and as demonstrated by histological findings of “regenerative clusters”, which consist of atypical ductules lined by cells exhibiting a combination of cholangiocyte and hepatocyte biomarkers[209].
In the context of PH, once a certain resection threshold is exceeded (e.g., > 80%), adequate liver regeneration cannot be achieved by the relatively small number of remaining hepatocytes, and the alternative pathway is thus activated. In this process, biliary epithelial cells (cholangiocytes) de-differentiate into progenitor cells and then re-differentiate into hepatocytes in order to repopulate the liver[195]. As described in the rat models subsection above, Evarts et al[359] demonstrated that administration of 2-AAF to rats which had undergone PH was associated with differentiation of oval cells (a putative hepatic progenitor cell) to hepatocytes. However, this model did not allow for genomic-based cell lineage tagging[209].
More recently, Lu et al[81] induced widespread hepatocyte injury in mice through Mdm 2 deletion, which results in p53 upregulation with p53-induced hepatocyte death and senescence. The authors found that widespread hepatocyte injury was associated with a ductular reaction, whereby hepatocyte progenitor cell populations expanded and where bromodeoxyuridine-positive hepatocyte progenitor cells were often closely associated with bromodeoxyuridine-positive hepatocytes, thus suggesting that (in this context) hepatocytes arise from progenitor cells[81]. In a zebrafish model, Choi et al[355] found that after severe hepatocyte depletion, biliary epithelial cells de-differentiated into hepatoblast-like cells and then differentiated into highly proliferative hepatocytes, thus leading to liver regeneration.
Although the above studies focused on transdifferentiation from cholangiocytes to hepatocytes, the inverse has also been demonstrated in a murine model of Alagille syndrome (a human genetic condition associated with biliary underdevelopment). In this study, Schaub et al[360] found that hepatocytes converted to mature cholangiocytes that were effective in supporting biliary drainage and remained so after cholestasis resolved, in a TGFβ signalling mediated process. This persistent phenotypic change is distinct from the reversible conversion of human or murine hepatocytes to progenitor cells seen in other studies[361] and which may more accurately be described as “metaplasia” rather than “transdifferentiation”. In their chimeric liver rat model, Michalopoulos et al[362] injected dipeptidyl peptidase IV (DPPIV)-positive rat hepatocytes into DPPIV-negative rats which then underwent partial hepatectomy and bile duct ligation, with or without additional biliary injury by methylene diamiline (DAPM) administration. On animal sacrifice after 30 d, the authors found that ductules exhibited the DPPIV marker, and that this was enhanced 36-fold in rats with additional DAPM-mediated biliary toxicity.
Evidence in support of liver epithelial cell transdifferentiation for regeneration is accumulating, yet several areas of controversy remain to be resolved. In addition to the findings described above, self-renewing facultative stem cells have been located in peribiliary glands and liver progenitor cells of bipotential differentiation capacity have been located in association with the canals of Hering. However, their role in liver regeneration has been disputed[195]. Also, it remains unclear whether all mature hepatocytes are capable of dedifferentiation to progenitor cells, or whether this is only possible in a subset of cells[195]. Finally, various signalling pathways (e.g., YAP, Rho kinase, TGF-β, glycogen synthase kinase 3) have been implicated in hepatocyte dedifferentiation in animal models[195], yet their role with respect to human hepatocytes remains to be clarified.
The processes and mechanisms of liver regeneration are not only influenced by the magnitude and type of injury, but also by the status of the underlying liver parenchyma prior to injury. Although the PH model has contributed much information relating to events relating to regeneration of normal liver, significant differences come to light when the underlying liver is diseased. This section describes the ways in which liver regeneration is altered in the instances of age-related liver impairment, acute liver injury, hepatic steatosis, fibrosis, and cirrhosis.
Although the adult liver retains regenerative capacity throughout life, this is reduced in old age[363], through several suggested mechanisms. FoxM1B is a transcription factor expressed during embryonal development and also in liver regeneration[364]. Its expression is diminished in aged mice, whose liver regeneration can be rescued through its transgenic overexpression[365]. Age-related liver regeneration impairment may also be mediated by changes in the expression and function of cell cycle affecting genes such as CCAAT/enhancer-binding protein (C/EBP)α, which is an inhibitor of Cyclin D[366]. Budding uninhibited by benzimidazole-related 1 (BubR1) is involved in the control of mitosis and is found to be diminished in old age. Genetically manipulated mice expressing low levels of BubR1 show impaired liver regeneration[367].
In addition to these mechanisms, Conboy et al[368] and Liu et al[369] have demonstrated in parabiotic experiments that the blood or plasma of young mice partially rescues the liver regenerative compromise seen in old mice, suggesting the presence of currently unidentified circulating factors, in work reminiscent of the early experimental approaches used to demonstrate the existence of portal mitogens.
Severe acute liver injury may result from a variety of insults including viral infection (e.g., Hepatitis A, B, C), poisoning (e.g., paracetamol) or auto-immune disease. Though different in nature, these diverse insults nevertheless have the common feature of causing significant necrosis and apoptosis, in the midst of which regeneration must happen.
Specific models provide some mechanistic information. For example, the mouse model of paracetamol injury suggests that beyond a threshold of injury, regeneration fails, and that this is associated with failure of β catenin activation, consistent with the correlation of β-catenin activation and regeneration seen in patients[370].
In the setting of widespread necrosis, the contribution of non-parenchymal cells may be particularly important. Macrophages are essential in clearing toxic cellular debris[371], and thus mice deficient in CSF1 which promotes the maturation of macrophages have impaired liver regeneration[372], and CSF1 serum levels correlate with recovery from paracetamol liver injury[373].
Hepatic steatosis is known to be detrimental to liver regeneration not only in experimental models[374], but also in the clinical setting[375]. The mechanism is not fully understood but may include cell cycle machinery defects in steatosis[376]. Down regulation of the EGFR pathway may also contribute, and EGFR overexpression has been shown to rescue liver regeneration in a mouse model of PH in steatosis[377]. Steatosis may also compromise liver regeneration by inhibition of NFκB[378]. Finally, failure to activate growth arrest and DNA damage-inducible protein GADD34 in fatty liver may partly contribute to impaired liver regeneration, which can be rescued by transgenic overexpression of growth arrest and DNA damage-inducible protein GADD34 in a mouse experimental model[379].
In the setting of overwhelming injury where the capacity of hepatocytes to proliferate is overwhelmed, oval cell transdifferentiation provides a mechanism to assist cellular repopulation. Such injury is, however, also associated with activation of stellate cells to myofibroblasts, which secrete ECM[380]. Excessive ECM secretion is nonetheless harmful because it inhibits the ductular reaction[381] and hepatocyte proliferation[382]. Moreover, excessive fibrosis impairs portal flow, leading to arterialisation of the liver, and senescence of hepatocytes and cholangiocytes[383]. The distortion of the macro and micro-anatomy of the liver results in major compromise to liver function leaving affected patients in an extremely precarious state characterized by rapid and severe decompensation, which can be triggered by relatively minor physiologically stresses.
In the rodent PH model, liver regeneration proceeds until the liver have returned to its pre-PH weight, approximately 10 d later, at which point regeneration ceases. The mechanisms resulting in termination of liver regeneration have received less attention than those driving it, but are equally important, not only from the perspective of the study of liver regeneration biology but also in terms of the light they shed on other pathologies including liver malignancy. Nevertheless, several regeneration termination pathways have been identified, relating to TGFβ, the activins, the ECM, and glypican-3 (GPC3).
TGFβ is a multifunction cytokine with wide ranging roles in growth and development. It exists in 3 isoforms resulting from differential protein cleaving and binds to 3 different TGFβ receptors. Binding to the TGFβR results in autophosphorylation, and activation of SMAD, which translocates to the nucleus, delivering an inhibitory signal to cell proliferation[384]. Although TGFβ does inhibits hepatocyte proliferation in vitro[385], other experimental results in vivo cast some doubt on its role in termination of liver regeneration in that liver specific TGFβR knockout mice terminate liver regeneration appropriately[68]. However, this result does not necessarily rule out TGFβ as a significant factor in liver regeneration termination: given the redundancy seen in the processes that drive regeneration, it seems likely that similar redundancy exists in its termination, such that ablation of one mechanism may readily be rescued by other pathways.
The activins are a family of proteins which are similar in structure to the TGFβ family, which also transduce signals via receptors that activate SMAD, and convey a growth inhibitory effect. Activins are upregulated during the liver regeneration[386], and blocking their action pharmacologically results in excessive regeneration and hepatomegaly following PH in rats[387].
The ECM is thought to convey a growth controlling influence on liver cells in a mechanism whereby integrin proteins in intact ECM bind hepatocyte cell membrane Integrin linked kinase (ILK) receptors, which deliver a growth inhibitory signal[388]. Consistent with this, the growth response of hepatocytes to mitogens in vitro is much reduced when grown in the presence of ECM in comparison to plastic[389]. Moreover, ILK knockout mice show not only hepatomegaly in the native state[390], but also an exaggerated regeneration after PH[391]. Thus, it may be that the activation of matrix metalloproteases that occurs early after liver injury[188] results in degradation of the controlling influence of integrins, and that this is gradually recovered during regeneration as new ECM is laid down.
GPC3 is a heparan sulphate proteoglycan found on the cell surface of many tissues which conveys a growth inhibitory effect[392]. It is not detectable in quiescent liver but is expressed coinciding with the end of regeneration after PH in rats[393]. Moreover, loss of function mutations of GPC3 results in organ overgrowth[394], and transgenic over-expression of GPC3 delays liver regeneration after PH[393].
C/EBP is one member of a family of transcription factors with a role in producing cell cycle arrest. Although complete ablation of C/EBP is fatal in mice[395], altering the function of the protein by mutation results in partial loss of function with mice exhibiting excessive regeneration and hepatomegaly after PH and CCl4 injury[396], thus suggesting that the native protein has a role in the control of liver regeneration.
The cyclins are a group of proteins which impact on the progression of the cell through the cell cycle. Amongst these cyclins E1 and E2 influence the advancement of the cell from the G1 phase (during which the cell synthesizes protein and organelles), to S phase (during which DNA is replicated)[397]. Cyclin E1 and E2 have opposing roles, with Cyclin E1 promoting entry into S phase[398], and Cyclin E2 halting it[399]. Thus, mice with ablated Cyclin E2 show increased DNA synthesis and hepatomegaly after PH suggesting a role for Cyclin E2[398,400].
Of note the hepatomegaly seen after CyclinE2 ablation is not due to cell division, but hypertrophy, providing a possible mechanism to explain to the observation that liver growth after 30% PH is hypertrophic rather than the hyperplasia seen in 70% PH.
The Hippo/Yap signalling pathway is conserved in a wide range of organisms and associated with growth suppression[401]. YAP is key downstream effector of Hippo and its activation when dephosphorylated leads to massive liver overgrowth[402], suggesting a possible regulatory role in control of liver regeneration.
Micro RNAs are short RNA molecules which bind to messenger RNA and thus affect expression of the gene product by interfering with translation of mRNA to protein[403]. Several micro RNAs have been identified which target the mRNAs of key regeneration promoting proteins, and thus may play a part in controlling liver regeneration. Thus miR-23b targets the growth inhibiting SMAD protein such that the observed downregulation of miR-23b following PH may provide a mechanism for slowing liver regeneration[404]. miR-34a targets several mRNAs including that which codes for the HGF receptor MET, again providing a potential mechanism for limiting hepatocyte proliferation[405].
The complexity of liver regeneration biology, combined with our currently limited understanding has to date much restricted specific clinical interventions to enhance liver regeneration. Moreover, the processes involve such fundamental biochemical pathways that attempts to manipulate these would require very careful assessment, for fear of unintended consequences in the liver and other organ systems. Nevertheless, current knowledge allows clinicians to anticipate what scenarios or treatments may compromise liver regeneration and provides guiding principles which may allow planning treatment strategies to optimise liver regeneration potential.
Liver tumours, be they primary or metastatic, may be treated by chemotherapy in systemic or locoregional delivery methods, or by surgical intervention with resection or local ablation techniques. Radiotherapy, although used to an extent, has a much lesser role and evidence base. In this section, we discuss how the biology of liver regeneration affects treatment choices and delivery, not only in terms of chemotherapy and surgery, but also in relation to wider organ system physiology that relates to liver regeneration.
The principal scenario in which chemotherapy impacts on liver regeneration is the use of neoadjuvant chemotherapy prior to a planned liver resection, or in the instance of downsizing an initially unresectable lesion, most usually in the context of colorectal liver metastases[406]. Chemotherapy affects not only individual cell types within liver parenchyma, but also key extra-hepatic tissues pertinent to the liver regenerative process such as the bone marrow, and in some cases affects key liver regenerative pathways.
The main toxicities inflicted on the liver by chemotherapy are steatosis, steatohepatitis, and sinusoidal obstruction syndrome (SOS). Steatosis is the excessive deposition of fat within hepatocytes. This may trigger an inflammatory reaction leading to steatohepatitis, and in turn to fibrosis and cirrhosis. Sinusoidal obstruction syndrome is a separate entity with direct toxicity to LSEC leading to occlusive phenomena in the sinusoids[407].
In the context of treatment of (colorectal liver metastases) CRLM, 5FU is associated with steatosis[408], probably as a result of impaired oxidation of fatty acids[409]. 5FU also triggers the activation of pro-inflammatory genes, which may contribute to the evolution of steatohepatitis[410]. Chemotherapy associated Steatohepatitis (CASH) is also particularly associated with regimens containing irinotecan[411] which also inhibits fatty acid oxidation, but elicits steatohepatitis by activation of ERK[412], which if inhibited, leads to a reduction in steatohepatitis. SOS is associated with oxaliplatin regimens. Although not fully understood, a contributing mechanism is the increased expression of matrix metalloproteases, resulting in lifting of LSEC from the basement membrane and allowing infiltration of red blood cells into the space of Disse, thus causing an occlusive phenomenon in the lumen of the sinusoid, and an inflammatory reaction with stellate cell activation and perisinusoidal fibrosis[413].
In addition to these directly hepatotoxic effects, the above agents are myelosuppressive, and may thus compromise the bone marrow constituents that play important roles in liver regeneration, including (1) LSEC progenitors which are important in repopulating LSEC after PH and a key source of the important mitogen HGF[314,315]; (2) Macrophage progenitors which have a key role in clearing cell debris in preparation for regeneration[206]; and (3) Megakaryocytes which replenish platelets, with their important role in delivering liver regenerative signals[178,179].
5FU, oxaliplatin, and irinotecan are frequently used in combination with biological agents targeting specific proliferative pathways within tumours, which overlap with biochemical pathways promoting liver regeneration. Thus, the anti-EGFR antibody cetuximab blocks binding of EGFR ligands including the key mitogen EGF, acting via the RAS-RAF-MEK pathway. Another example is the anti-angiogenic antibody bevacizumab which delivers antitumor effects by blocking VEGF-binding to its receptor, but thereby also interfering with LSEC repopulation in the liver[406]. Thus, the commonly used chemotherapeutic agents and antibodies used in the treatment of CRLM have a multitude of liver regeneration compromising properties. The challenge for clinicians is to find the optimal balance between the oncological benefits and liver regenerative toxicity.
Clinical trials provide some guidance in this regard. Thus 5FU, oxaliplatin and irinotecan-based regimens have been shown to produce CRLM response rates allowing increased potential for curative resection[414,415], with additional benefits attributed to the use of anti EGFR[416] and anti VEGF antibodies[417]. Interestingly, whilst the use of EGFR antibody regimens is established as a means to downsize tumours to increase operability, their perioperative use in primarily resectable CRLM is detrimental[418].
In terms of duration of chemotherapy, the theoretical ideal is the delivery of the oncological hit to the tumour whilst minimising liver toxicity. In this regard Karoui et al[419] found that patient receiving fewer than 6 chemotherapy cycles experienced significantly fewer post liver resection complications than those who had received more than 6 cycles (19% vs 54% complication rate) although there was no impact on mortality rates. Similarly, Nguyen et al [420]showed a greater incidence of post-operative liver failure in patients undergoing more than 10 cycles of chemotherapy. Moreover, the general practice of leaving an interval of 4-6 wk between chemotherapy and surgery is intended to allow reversible inflammatory changes and bone marrow to recover[421].
The prospect of specific interventions to minimise chemotherapy related injury is the subject of research but has not yet reached widespread clinical application. Nevertheless, there have been reports that bevacizumab may reduce SOS in oxaliplatin regimens[422], and S-adenosylmethionine (SAMe) may have a protective effect in chemotherapy-induced liver injury[423], with SAMe infusion associated with lower serum concentrations of aspartate transaminase and alanine transaminase during chemotherapy treatment[424].
In the context of a healthy underlying liver parenchyma, up to 75% of the liver may be resected. However, this is the very limit and liver surgeons are often faced with the problem of some degree of parenchymal pathology or dysfunction, which may present a higher requirement in terms of the remnant liver volume. As discussed in section 1.4, pure volume assessments are gradually being complemented by liver functional assessments, principally by hepatobiliary scintigraphy[146,152].
In instances where future remnant liver volume/function is deemed insufficient, PVE has become an established technique to produce atrophy of the tumour bearing liver parenchyma, and compensatory growth of the future remnant liver, allowing a safer hepatectomy[425]. In the context of resections for CRLM, this technique increases resection rates by 10%-20%[426].
In the instance where PVE fails to produce sufficient hypertrophy, perhaps caused by the development of collateral intrahepatic portal vessels between the embolized and non-embolized parts of the liver, further growth of the future remnant liver may be achieved by parenchymal section to interrupt the collateral vessels.
This approach, as a salvage manoeuvre after PVE is a modification of the originally described ALPPS technique[427] that combined single stage portal vein embolization and parenchymal transection. Whilst undoubtedly producing significant additional growth, the technique remains debated owing to questions regarding the functionality of the rapidly expanded liver, and high post-operative mortality in some series[428].
The continuum of steatosis, steatohepatitis, fibrosis, and cirrhosis presents a major challenge to liver resection. In terms of steatosis, the observed experimental and clinical compromise to liver regeneration as a result of steatosis translates to a diminished tolerance to liver resection[429]. Thus, studies examining the outcome of hepatic resection in patients with steatotic livers suggest more marked abnormalities in postoperative liver dysfunction, more morbidity and increased complication rates[143,430], with steatosis identified as an independent predictor of complications[431]. Increased mortality is identified in some studies[430], and although this has not been a universal finding, the presumption is that this relates to careful patient selection.
The tolerance of the liver to resection progressively decreases with more severe underlying liver disease. Thus, cirrhosis is associated with increased mortality rates for all abdominal surgery including[432] liver resection[433].
Interventions to mitigate the risk of liver surgery in the context of underlying liver disease are limited, and practice has focused on patient selection to avoid prohibitively hazardous resections. In the context of steatosis, the response of the steatotic liver to ischaemic insult[434] has motivated research in the concept of ischaemic preconditioning, whereby a short period of ischaemia prior to liver resection is applied with a view to improving subsequent perfusion[435]. Although benefits have been shown in rodent models and in the clinical setting[436], results have not been universal[437], and the practice not widely accepted.
One of the key biochemical triggers which initiates liver regeneration is LPS from the gut, which translocates into portal blood following liver resection in part as a result of the rise in portal pressure[204] (see “Priming of hepatocytes”). The evidence supporting the importance of LPS is that germ free mice show impaired liver regeneration, which can be rescued by exogenous LPS administration[205].
Though the presence of LPS in the blood is important, there appears to be a delicate balance as excessive translocation of LPS into portal blood is detrimental to liver regeneration. Thus, in the rat model after 90% PH, gut mucosal permeability is disrupted with loss of tight junctions, resulting in high levels of portal blood LPS, severe inflammatory changes in the liver with necrosis, associated with high mortality. By decontaminating the gut with gentamycin, gut permeability, portal blood LPS, and liver necrosis is much improved, and associated with a significant improvement in survival from 24% to 56%[27].
No clinical trials have been carried out to test the potential benefit of this animal experimental result in humans, where its applicability could be investigated in patients undergoing major liver resection. Additional considerations would come into play, including the risk of clostridium difficile sepsis associated with alteration of bowel flora. Another potential approach, also uninvestigated, could be the use of bowel preparation similar to that used prior to colorectal surgery.
The data from early experimental result suggested that liver regeneration was significantly dependent on portal blood growth factors derived from the upper gastrointestinal tract (stomach, duodenum, pancreas) as portal flow separation experiments comparing isolated portal flow of distal stomach, duodenum, pancreas and spleen to portal flow of small intestine showed that the grafts supplied with intestinal portal flow atrophied, in contrast to those supplied with portal blood from the upper gastrointestinal tract[9,10].
On this basis, there might be a similar risk in humans in cases where liver resection is combined with a significant resection of the upper gastro-intestinal tract, such as Whipples resection (resection of distal stomach, duodenum and head of pancreas) or total pancreatectomy (resection of distal stomach, duodenum, pancreas, and spleen). However, existing case series describing such multivisceral resections do not report problems relating to liver regeneration[438-440].
Thus it may be that the situation in the original animal experiments cannot be extrapolated to humans owing to different physiology, although interpretation of the human literature has to be taken with the caveats of selective reporting (bias against reporting poor outcomes with liver failure), case selection of surgical candidates (likely good performance status individuals for such major resections), and likely small liver resections which might not manifest with post-operative liver failure.
Bile salts are important auxiliary mitogens, with rodent models showing impaired liver regeneration with bile acid sequestering agents[243], bile-salt-deficient transgenic mice[244], and in rats with external biliary fistula[245].
This observation in rodent models is mirrored in clinical practice, with a randomised trial comparing patients undergoing major liver resection with and without cystic duct biliary drainage showing significantly lower bile salt concentrations in the drained group as well as lesser liver regeneration assessed by volumetric CT[441]. Moreover, in the context of portal vein embolisation, increased systemic bile salt levels predicted hypertrophy of the non-embolised lobe[442].
Thus, the presence of bile in the intestine following liver resection appears to be important and would argue against the use of external biliary drains after liver resection, or in circumstances requiring such drains, to consider enteric bile recycling via nasogastric or nasoenteric tube.
The rise in portal venous pressure following liver resection appears to contribute to providing important triggers to liver regeneration in the form of induction of cytokines, hepatic mitogens and angiogenic growth factors, as described in “Vascular events”section. However, an excessive increase in portal pressure is thought to be detrimental in eliciting a reduced arterial inflow as a result of the arterial buffer response, and a subsequent hypoxia[171], which is hyptothesised to contribute to post liver resection liver failure[443], in combination with direct mechanical injury occurs to the liver sinusoids[444].
Thus, a number of investigators have examined a variety of interventions to decrease portal venous pressure by surgical (splenectomy, splenic artery ligation, porto-systemic shunt), interventional radiological (pre-operative splenic artery ligation), and pharmacological means (non-selective beta-blockers, terlipressin), with successful reductions in portal pressure and improvement in small-for-size syndrome[444].
Intrahepatic hypoxia is one of the stimuli which may contribute to the early triggers of liver regeneration[175], via a number of mechanisms including the hypoxia induced secretion of complex regeneration promoting molecules from stem cells[176]. Given the critical necessity of maintaining normoxia in other tissues, manipulating pO2 for hepatic regeneration benefits seems an unlikely strategy, however, pharmacological manipulation of HIF has been used to treat renal anaemia[445], and in vitro studies suggest that such agents could produce angiogenesis in the liver, as well as a hepatocyte cytoprotective effect[174].
The finding that surgical denervation of the liver or pharmacological alpha adrenergic blockade significantly impairs liver regeneration, is consistent with known mod
Hepatic sympathectomy is effectively carried out in humans in the context of hilar cholangiocarcinoma resections (when the entire hepatic hilum is skeletonized) and in liver transplantation, when the implanted graft is totally denervated, however there is no obvious clinical evidence that this has a detrimental effect on liver regeneration.
The absence of reported clinical compromise to liver regeneration following sympathectomy in humans may reflect differences in autonomic supply[447], compensatory effects from adrenal catecholamine secretion, or perhaps relatively rapid re-innervation, as seen in some animal models[448]. Finally, the effect of catecholamine stimulation is complicated by the fact that inhibition of alpha adrenergic signals may have a beneficial effect in minimising hepatic stellate cell activation and therefore reduce detrimental excessive fibrosis[446].
Platelets clearly have an important role in promoting liver regeneration with animals and humans studies showing impaired liver regeneration in individuals thrombocytopenia[178,179], and the association between thrombocytopenia and post-operative mortality after major liver resection[449]. Moreover, fibrinogen deposition in the liver appears key in driving platelet accumulation in the liver, with post hepatectomy hypofibrinogenaemia being associated with liver dysfunction and mortality. Moreover, studies in rodents suggest platelets are the key source of the auxiliary hepatic mitogen serotonin in liver regeneration, and that serotonin infusion rescues impaired liver regeneration observed in thrombocytopenic mice[256].
Correction of platelet count and fibrinogen levels in patients post hepatectomy has not been investigated in humans, but could potentially have beneficial effects, although thromboembolic risks would have to be taken into account. Serotonin infusion has also not been investigated in humans in the context of liver regeneration, but has been carried out in other clinical contexts to stimulate prolactin[450], and as a desired effect in amitriptyline treatment of depression[451].
In the setting of liver regeneration with significant hepatic necrosis, the role of non-parenchymal cells may be particularly important. Macrophages clear toxic cellular debris, and mice deficient in macrophage colony stimulating factor 1 (CSF1) which promotes the maturation of macrophages have impaired liver regeneration[372]. Moreover, CSF1 correlates with recovery from paracetamol liver injury in patients with liver failure[373]. In addition to clearing metabolic debris, macrophages may in part act by stimulating the hepatic ductular reaction[452] and limiting fibrosis[453].
Although macrophage colony-stimulating factor-1 has not been used in humans in the context of liver failure and regeneration, it does have a multitude of other clinical applications[454].
Similarly, in rodent models at least, the bone marrow is a significant source of LSEC progenitor cells, which emigrate from the marrow into the bloodstream from which they engraft into regenerating liver, where they produce a significant quantities of the complete hepatic mitogen HGF, in a VEGF driven process[314,315]. Specific LSEC progenitor stimulation therefore offers a theoretical therapeutic opportunity but has not been investigated.
The characterization of the multiple cytokines, hormones and growth factors involved in liver regeneration has motivated investigation of means to modulate the hepatic proliferative response. Such approaches have involved the infusion of specific mitogens, or the use growth enhancing progenitor cells or their secretome and are to date at experimental animal model stage.
These wide-ranging experimental approaches are beyond the scope of this review, but a few examples provide an insight into potential avenues for the future. In a murine model of 85% PH, Cataldegirmen et al[455] investigated the potential therapeutic opportunity of blocking the Receptor for advanced glycation end-products (RAGE), which is upregulated in massive hepatectomy and associated with cell stress when binding its ligands. Blockage of RAGE pharmacologically or by transgenic means resulted in significant improvement in survival post massive PH[455]. In a mouse model of partial biliary ligation, Mangieri et al[456] report improved liver regeneration produced by infusion of the complete mitogen HGF. Similarly, but targeting the other complete mitogen pathway of the EGFR ligands, Zimmers et al[377] demonstrated improved liver regeneration after plasmid delivery of EGF receptor.
In addition to the focus on individual hepatic mitogens, investigation has also been carried out in the infusion of whole cells[457], ranging from primary human hepatocytes[458], pluripotent stem cell derived hepatocyte-like cells[459], and mesenchymal stem cells[460]. Cell based therapies have met several obstacles including sourcing, immuno-compatibility, and potential malignant transformation, thus motivating research into potential for using the secretome of stem cells, thus obviating the difficulties presented by the whole cell therapies[176,461-463].
Liver regeneration is highly complex, and current understanding is based largely on animal and in vitro models. The likelihood that not all hepatic mitogens have been identified, the multitude of known ones, the complexity and incomplete understanding of their associated biochemical pathways, the equally complex and poorly understood cross talk between cell types, and our even poorer understanding of the factors that cease liver regeneration all suggest that a comprehensive working understanding of the process is improbable in the foreseeable future. Consequently, specific interventions to influence liver regeneration in the clinical setting are commensurately limited, though allow clinicians to at least optimise conditions for liver regeneration to occur. The implications of this in relation to the treatment of liver tumours are most notably applicable in the context of liver resection for malignancy, where assessment and optimisation of remnant liver function not only increases the proportion of patients eligible for treatment, but also improves patient safety. The increasingly sophisticated in vitro organoid models, and potential opportunities presented by repopulation of decellularised scaffolds may allow the creation of constructs that allow not only deeper understanding, but also novel therapeutic options.
Provenance and peer review: Invited article; Externally peer reviewed.
Peer-review model: Single blind
Specialty type: Oncology
Country/Territory of origin: United Kingdom
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): B
Grade C (Good): 0
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Zharikov YO S-Editor: Wu YXJ L-Editor: A P-Editor: Wu YXJ
| 1. | Mortensen KE, Revhaug A. Liver regeneration in surgical animal models - a historical perspective and clinical implications. Eur Surg Res. 2011;46:1-18. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 31] [Cited by in RCA: 28] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 2. | Eck NVK. Concerning ligation of the vena porta (in Russian). Voen Med Zh 1877; 130 (English translation: Child CG: Eck’s fistula. Surg Gynecol Obstet. 1953;96: 375-376. |
| 3. | Child CG 3rd, Barr D, Holswade GR, Harrison CS. Liver regeneration following portacaval transposition in dogs. Ann Surg. 1953;138:600-608. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 106] [Cited by in RCA: 120] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 4. | Mccredie JA, Doggart JR, Welbourn RB. Total arterialization of the liver. Br J Surg. 1957;45:83-100. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 22] [Cited by in RCA: 23] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 5. | Hahn M, Massen O, Nencki M, Pavlov J. Die Eck’sche Fistel zwischen der unteren Hohlvene und der Pfortader und Folgen für den Organismus. Arch Exp Pathol Pharmakol. 1893;189:161-210. [RCA] [DOI] [Full Text] [Cited by in Crossref: 190] [Cited by in RCA: 137] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 6. | Rous P, Larimore LD. Relation of the portal blood to liver maintenance: A demonstration of liver atrophy conditional on compensation. J Exp Med. 1920;31:609-632. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 248] [Cited by in RCA: 226] [Article Influence: 15.1] [Reference Citation Analysis (0)] |
| 7. | Marchioro TL, Porter KA, Brown BI, Faris TD, Herrmann TJ, Sudweeks A, Starzl TE. The specific influence of nonhepatic splanchnic venous blood flow on the liver. Surg Forum. 1965;16:280-282. [PubMed] |
| 8. | Marchioro TL, Porter KA, Brown BI, Otte JB, Starzl TE. The effect of partial portacaval transposition on the canine liver. Surgery. 1967;61:723-732. [PubMed] |
| 9. | Marchioro TL, Porter KA, Dickinson TC, Faris TD, Starzi TE. Physiologic requirements for auxiliary liver homotransplantation. Surg Gynecol Obstet. 1965;121:17-31. [PubMed] |
| 10. | Starzl TE, Francavilla A, Halgrimson CG, Francavilla FR, Porter KA, Brown TH, Putnam CW. The origin, hormonal nature, and action of hepatotrophic substances in portal venous blood. Surg Gynecol Obstet. 1973;137:179-199. [PubMed] |
| 11. | Duguay LR, Orloff MJ. Role of the pancreas in regulation of liver regeneration in dogs. Surg Forum. 1977;28:387-390. [PubMed] |
| 12. | Starzl TE, Francavilla A, Porter KA, Benichou J. The effect upon the liver of evisceration with or without hormone replacement. Surg Gynecol Obstet. 1978;146:524-532. [PubMed] |
| 13. | Francavilla A, Starzl TE, Porter K, Foglieni CS, Michalopoulos GK, Carrieri G, Trejo J, Azzarone A, Barone M, Zeng QH. Screening for candidate hepatic growth factors by selective portal infusion after canine Eck's fistula. Hepatology. 1991;14:665-670. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 12] [Reference Citation Analysis (0)] |
| 14. | Sigel B, Dunn MR, Butterfield J. Effect of partial hepatectomy and ECK fistula on autotransplanted liver tissue. Evidence for a humoral mechanism in liver regeneration. Surg Forum. 1963;14:72-74. [PubMed] |
| 15. | Bucher NL, Scott JF, Aub JC. Regeneration of the liver in parabiotic rats. Cancer Res. 1951;11:457-465. [PubMed] |
| 16. | Wenneker AS, Sussman N. Regeneration of liver tissue following partial hepatectomy in parabiotic rats. Proc Soc Exp Biol Med. 1951;76:683-686. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 34] [Cited by in RCA: 38] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 17. | Bhushan B, Chavan H, Borude P, Xie Y, Du K, McGill MR, Lebofsky M, Jaeschke H, Krishnamurthy P, Apte U. Dual Role of Epidermal Growth Factor Receptor in Liver Injury and Regeneration after Acetaminophen Overdose in Mice. Toxicol Sci. 2017;155:363-378. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 39] [Cited by in RCA: 56] [Article Influence: 6.2] [Reference Citation Analysis (0)] |
| 18. | Bhushan B, Stoops JW, Mars WM, Orr A, Bowen WC, Paranjpe S, Michalopoulos GK. TCPOBOP-Induced Hepatomegaly and Hepatocyte Proliferation are Attenuated by Combined Disruption of MET and EGFR Signaling. Hepatology. 2019;69:1702-1718. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 42] [Cited by in RCA: 40] [Article Influence: 6.7] [Reference Citation Analysis (0)] |
| 19. | Wei W, Dirsch O, Mclean AL, Zafarnia S, Schwier M, Dahmen U. Rodent models and imaging techniques to study liver regeneration. Eur Surg Res. 2015;54:97-113. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 17] [Cited by in RCA: 18] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 20. | Higgins G, M, Anderson RM. Experimental pathology of the liver – Restoration of the liver of the white rat following partial surgical removal. Arch Pathol. 1931;12:186-202. |
| 21. | Moolten FL, Bucher NL. Regeneration of rat liver: transfer of humoral agent by cross circulation. Science. 1967;158:272-274. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 198] [Cited by in RCA: 170] [Article Influence: 2.9] [Reference Citation Analysis (0)] |
| 22. | Couinaud C. [Liver lobes and segments: notes on the anatomical architecture and surgery of the liver]. Presse Med. 1954;62:709-712. [PubMed] |
| 23. | Kogure K, Ishizaki M, Nemoto M, Kuwano H, Makuuchi M. A comparative study of the anatomy of rat and human livers. J Hepatobiliary Pancreat Surg. 1999;6:171-175. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 67] [Cited by in RCA: 61] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 24. | Andersen KJ, Knudsen AR, Kannerup AS, Sasanuma H, Nyengaard JR, Hamilton-Dutoit S, Erlandsen EJ, Jørgensen B, Mortensen FV. The natural history of liver regeneration in rats: description of an animal model for liver regeneration studies. Int J Surg. 2013;11:903-908. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 23] [Cited by in RCA: 31] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 25. | Madrahimov N, Dirsch O, Broelsch C, Dahmen U. Marginal hepatectomy in the rat: from anatomy to surgery. Ann Surg. 2006;244:89-98. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 103] [Cited by in RCA: 117] [Article Influence: 6.2] [Reference Citation Analysis (0)] |
| 26. | Ninomiya M, Shirabe K, Terashi T, Ijichi H, Yonemura Y, Harada N, Soejima Y, Taketomi A, Shimada M, Maehara Y. Deceleration of regenerative response improves the outcome of rat with massive hepatectomy. Am J Transplant. 2010;10:1580-1587. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 53] [Cited by in RCA: 60] [Article Influence: 4.0] [Reference Citation Analysis (0)] |
| 27. | Ren W, Wang X, Zhang A, Li C, Chen G, Ge X, Pan K, Dong JH. Selective bowel decontamination improves the survival of 90% hepatectomy in rats. J Surg Res. 2015;195:454-464. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 8] [Cited by in RCA: 10] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 28. | Mariotti V, Strazzabosco M, Fabris L, Calvisi DF. Animal models of biliary injury and altered bile acid metabolism. Biochim Biophys Acta Mol Basis Dis. 2018;1864:1254-1261. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 76] [Cited by in RCA: 128] [Article Influence: 16.0] [Reference Citation Analysis (0)] |
| 29. | Rozga J, Jeppsson B, Bengmark S. Portal branch ligation in the rat. Reevaluation of a model. Am J Pathol. 1986;125:300-308. [PubMed] |
| 30. | Khan RA, Khan MR, Sahreen S. CCl4-induced hepatotoxicity: protective effect of rutin on p53, CYP2E1 and the antioxidative status in rat. BMC Complement Altern Med. 2012;12:178. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 119] [Cited by in RCA: 130] [Article Influence: 10.0] [Reference Citation Analysis (0)] |
| 31. | Vidhya A, Renjugopal V, Indira M. Impact of thiamine supplementation in the reversal of ethanol induced toxicity in rats. Indian J Physiol Pharmacol. 2013;57:406-417. [PubMed] |
| 32. | Adeyanju AA, Asejeje FO, Molehin OR, Owoeye O, Olatoye EO, Ekpo EN. Protective role of protocatechuic acid in carbon tetrachloride-induced oxidative stress via modulation of proinflammatory cytokines levels in brain and liver of Wistar rats. J Basic Clin Physiol Pharmacol. 2021;. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3] [Cited by in RCA: 6] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 33. | Delgado-Venegas CS, Martínez-Hernández SL, Cervantes-García D, Montes de Oca-Luna R, de Jesús Loera-Arias M, Mata-Martínez MG, Ventura-Juárez J, Muñoz-Ortega MH. Modulating effects of the probiotic Lactococcus lactis on the hepatic fibrotic process induced by CCl4 in Wistar rats. Exp Ther Med. 2021;21:339. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 2] [Cited by in RCA: 8] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 34. | Tong M, Zheng Q, Liu M, Chen L, Lin YH, Tang SG, Zhu YM. 5-methoxytryptophan alleviates liver fibrosis by modulating FOXO3a/miR-21/ATG5 signaling pathway mediated autophagy. Cell Cycle. 2021;20:676-688. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4] [Cited by in RCA: 16] [Article Influence: 4.0] [Reference Citation Analysis (0)] |
| 35. | Evarts RP, Nagy P, Marsden E, Thorgeirsson SS. A precursor-product relationship exists between oval cells and hepatocytes in rat liver. Carcinogenesis. 1987;8:1737-1740. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 318] [Cited by in RCA: 284] [Article Influence: 7.5] [Reference Citation Analysis (0)] |
| 36. | Trautwein C, Will M, Kubicka S, Rakemann T, Flemming P, Manns MP. 2-acetaminofluorene blocks cell cycle progression after hepatectomy by p21 induction and lack of cyclin E expression. Oncogene. 1999;18:6443-6453. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 28] [Cited by in RCA: 27] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 37. | Rahman TM, Hodgson HJ. Animal models of acute hepatic failure. Int J Exp Pathol. 2000;81:145-157. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 135] [Cited by in RCA: 131] [Article Influence: 5.2] [Reference Citation Analysis (0)] |
| 38. | Peeling J, Shoemaker L, Gauthier T, Benarroch A, Sutherland GR, Minuk GY. Cerebral metabolic and histological effects of thioacetamide-induced liver failure. Am J Physiol. 1993;265:G572-G578. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 9] [Cited by in RCA: 19] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 39. | Martins PN, Theruvath TP, Neuhaus P. Rodent models of partial hepatectomies. Liver Int. 2008;28:3-11. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 104] [Cited by in RCA: 119] [Article Influence: 7.0] [Reference Citation Analysis (0)] |
| 40. | Sakamoto T, Ezure T, Lunz J, Murase N, Tsuji H, Fung JJ, Demetris AJ. Concanavalin A simultaneously primes liver hematopoietic and epithelial progenitor cells for parallel expansion during liver regeneration after partial hepatectomy in mice. Hepatology. 2000;32:256-267. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 24] [Cited by in RCA: 24] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 41. | Kollmar O, Corsten M, Scheuer C, Vollmar B, Schilling MK, Menger MD. Tumour growth following portal branch ligation in an experimental model of liver metastases. Br J Surg. 2010;97:917-926. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 11] [Cited by in RCA: 12] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 42. | Heinrich S, Georgiev P, Weber A, Vergopoulos A, Graf R, Clavien PA. Partial bile duct ligation in mice: a novel model of acute cholestasis. Surgery. 2011;149:445-451. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 40] [Cited by in RCA: 46] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 43. | Bruccoleri A, Gallucci R, Germolec DR, Blackshear P, Simeonova P, Thurman RG, Luster MI. Induction of early-immediate genes by tumor necrosis factor alpha contribute to liver repair following chemical-induced hepatotoxicity. Hepatology. 1997;25:133-141. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 114] [Cited by in RCA: 114] [Article Influence: 4.1] [Reference Citation Analysis (0)] |
| 44. | Koniaris LG, Zimmers-Koniaris T, Hsiao EC, Chavin K, Sitzmann JV, Farber JM. Cytokine-responsive gene-2/IFN-inducible protein-10 expression in multiple models of liver and bile duct injury suggests a role in tissue regeneration. J Immunol. 2001;167:399-406. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 70] [Cited by in RCA: 66] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 45. | Gardner CR, Laskin JD, Dambach DM, Sacco M, Durham SK, Bruno MK, Cohen SD, Gordon MK, Gerecke DR, Zhou P, Laskin DL. Reduced hepatotoxicity of acetaminophen in mice lacking inducible nitric oxide synthase: potential role of tumor necrosis factor-alpha and interleukin-10. Toxicol Appl Pharmacol. 2002;184:27-36. [PubMed] |
| 46. | Diehl AM. Effect of ethanol on tumor necrosis factor signaling during liver regeneration. Clin Biochem. 1999;32:571-578. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 27] [Cited by in RCA: 31] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 47. | Erhardt A, Tiegs G. Tolerance induction in response to liver inflammation. Dig Dis. 2010;28:86-92. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 21] [Cited by in RCA: 25] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 48. | Imajo K, Yoneda M, Kessoku T, Ogawa Y, Maeda S, Sumida Y, Hyogo H, Eguchi Y, Wada K, Nakajima A. Rodent models of nonalcoholic fatty liver disease/nonalcoholic steatohepatitis. Int J Mol Sci. 2013;14:21833-21857. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 64] [Cited by in RCA: 76] [Article Influence: 6.3] [Reference Citation Analysis (0)] |
| 49. | Tsuchida T, Lee YA, Fujiwara N, Ybanez M, Allen B, Martins S, Fiel MI, Goossens N, Chou HI, Hoshida Y, Friedman SL. A simple diet- and chemical-induced murine NASH model with rapid progression of steatohepatitis, fibrosis and liver cancer. J Hepatol. 2018;69:385-395. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 331] [Cited by in RCA: 393] [Article Influence: 56.1] [Reference Citation Analysis (0)] |
| 50. | Nikfarjam M, Malcontenti-Wilson C, Fanartzis M, Daruwalla J, Christophi C. A model of partial hepatectomy in mice. J Invest Surg. 2004;17:291-294. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 31] [Cited by in RCA: 31] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 51. | Palmes D, Spiegel HU. Animal models of liver regeneration. Biomaterials. 2004;25:1601-1611. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 103] [Cited by in RCA: 103] [Article Influence: 4.9] [Reference Citation Analysis (0)] |
| 52. | Preisegger KH, Factor VM, Fuchsbichler A, Stumptner C, Denk H, Thorgeirsson SS. Atypical ductular proliferation and its inhibition by transforming growth factor beta1 in the 3,5-diethoxycarbonyl-1,4-dihydrocollidine mouse model for chronic alcoholic liver disease. Lab Invest. 1999;79:103-109. [PubMed] |
| 53. | Akhurst B, Croager EJ, Farley-Roche CA, Ong JK, Dumble ML, Knight B, Yeoh GC. A modified choline-deficient, ethionine-supplemented diet protocol effectively induces oval cells in mouse liver. Hepatology. 2001;34:519-522. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 172] [Cited by in RCA: 161] [Article Influence: 6.7] [Reference Citation Analysis (0)] |
| 54. | Tumanov AV, Koroleva EP, Christiansen PA, Khan MA, Ruddy MJ, Burnette B, Papa S, Franzoso G, Nedospasov SA, Fu YX, Anders RA. T cell-derived lymphotoxin regulates liver regeneration. Gastroenterology. 2009;136:694-704.e4. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 62] [Cited by in RCA: 62] [Article Influence: 3.9] [Reference Citation Analysis (0)] |
| 55. | Webber EM, Wu JC, Wang L, Merlino G, Fausto N. Overexpression of transforming growth factor-alpha causes liver enlargement and increased hepatocyte proliferation in transgenic mice. Am J Pathol. 1994;145:398-408. [PubMed] |
| 56. | Cressman DE, Greenbaum LE, DeAngelis RA, Ciliberto G, Furth EE, Poli V, Taub R. Liver failure and defective hepatocyte regeneration in interleukin-6-deficient mice. Science. 1996;274:1379-1383. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1181] [Cited by in RCA: 1213] [Article Influence: 41.8] [Reference Citation Analysis (0)] |
| 57. | Yamada Y, Kirillova I, Peschon JJ, Fausto N. Initiation of liver growth by tumor necrosis factor: deficient liver regeneration in mice lacking type I tumor necrosis factor receptor. Proc Natl Acad Sci USA. 1997;94:1441-1446. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 740] [Cited by in RCA: 755] [Article Influence: 27.0] [Reference Citation Analysis (0)] |
| 58. | Greenbaum LE, Li W, Cressman DE, Peng Y, Ciliberto G, Poli V, Taub R. CCAAT enhancer- binding protein beta is required for normal hepatocyte proliferation in mice after partial hepatectomy. J Clin Invest. 1998;102:996-1007. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 219] [Cited by in RCA: 235] [Article Influence: 8.7] [Reference Citation Analysis (0)] |
| 59. | Rai RM, Lee FY, Rosen A, Yang SQ, Lin HZ, Koteish A, Liew FY, Zaragoza C, Lowenstein C, Diehl AM. Impaired liver regeneration in inducible nitric oxide synthasedeficient mice. Proc Natl Acad Sci USA. 1998;95:13829-13834. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 191] [Cited by in RCA: 192] [Article Influence: 7.1] [Reference Citation Analysis (0)] |
| 60. | Roselli HT, Su M, Washington K, Kerins DM, Vaughan DE, Russell WE. Liver regeneration is transiently impaired in urokinase-deficient mice. Am J Physiol. 1998;275:G1472-G1479. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 13] [Cited by in RCA: 23] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 61. | Yamada Y, Webber EM, Kirillova I, Peschon JJ, Fausto N. Analysis of liver regeneration in mice lacking type 1 or type 2 tumor necrosis factor receptor: requirement for type 1 but not type 2 receptor. Hepatology. 1998;28:959-970. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 212] [Cited by in RCA: 203] [Article Influence: 7.5] [Reference Citation Analysis (0)] |
| 62. | Anderson SP, Yoon L, Richard EB, Dunn CS, Cattley RC, Corton JC. Delayed liver regeneration in peroxisome proliferator-activated receptor-alpha-null mice. Hepatology. 2002;36:544-554. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 112] [Cited by in RCA: 111] [Article Influence: 4.8] [Reference Citation Analysis (0)] |
| 63. | Leu JI, Crissey MA, Craig LE, Taub R. Impaired hepatocyte DNA synthetic response posthepatectomy in insulin-like growth factor binding protein 1-deficient mice with defects in C/EBP beta and mitogen-activated protein kinase/extracellular signal-regulated kinase regulation. Mol Cell Biol. 2003;23:1251-1259. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 79] [Cited by in RCA: 84] [Article Influence: 3.8] [Reference Citation Analysis (0)] |
| 64. | Strey CW, Markiewski M, Mastellos D, Tudoran R, Spruce LA, Greenbaum LE, Lambris JD. The proinflammatory mediators C3a and C5a are essential for liver regeneration. J Exp Med. 2003;198:913-923. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 314] [Cited by in RCA: 327] [Article Influence: 14.9] [Reference Citation Analysis (0)] |
| 65. | Borowiak M, Garratt AN, Wüstefeld T, Strehle M, Trautwein C, Birchmeier C. Met provides essential signals for liver regeneration. Proc Natl Acad Sci USA. 2004;101:10608-10613. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 371] [Cited by in RCA: 399] [Article Influence: 19.0] [Reference Citation Analysis (0)] |
| 66. | Mohammed FF, Smookler DS, Taylor SE, Fingleton B, Kassiri Z, Sanchez OH, English JL, Matrisian LM, Au B, Yeh WC, Khokha R. Abnormal TNF activity in Timp3-/- mice leads to chronic hepatic inflammation and failure of liver regeneration. Nat Genet. 2004;36:969-977. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 220] [Cited by in RCA: 226] [Article Influence: 10.8] [Reference Citation Analysis (0)] |
| 67. | Nakamura K, Nonaka H, Saito H, Tanaka M, Miyajima A. Hepatocyte proliferation and tissue remodeling is impaired after liver injury in oncostatin M receptor knockout mice. Hepatology. 2004;39:635-644. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 117] [Cited by in RCA: 122] [Article Influence: 5.8] [Reference Citation Analysis (0)] |
| 68. | Oe S, Lemmer ER, Conner EA, Factor VM, Levéen P, Larsson J, Karlsson S, Thorgeirsson SS. Intact signaling by transforming growth factor beta is not required for termination of liver regeneration in mice. Hepatology. 2004;40:1098-1105. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 91] [Cited by in RCA: 97] [Article Influence: 4.6] [Reference Citation Analysis (0)] |
| 69. | Duffield JS, Forbes SJ, Constandinou CM, Clay S, Partolina M, Vuthoori S, Wu S, Lang R, Iredale JP. Selective depletion of macrophages reveals distinct, opposing roles during liver injury and repair. J Clin Invest. 2005;115:56-65. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 38] [Cited by in RCA: 729] [Article Influence: 36.5] [Reference Citation Analysis (0)] |
| 70. | Mitchell C, Nivison M, Jackson LF, Fox R, Lee DC, Campbell JS, Fausto N. Heparin-binding epidermal growth factor-like growth factor links hepatocyte priming with cell cycle progression during liver regeneration. J Biol Chem. 2005;280:2562-2568. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 113] [Cited by in RCA: 128] [Article Influence: 6.1] [Reference Citation Analysis (0)] |
| 71. | Oliver JR, Mara TW, Cherian MG. Impaired hepatic regeneration in metallothionein-I/II knockout mice after partial hepatectomy. Exp Biol Med (Maywood). 2005;230:61-67. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 21] [Cited by in RCA: 21] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 72. | Seki E, Tsutsui H, Iimuro Y, Naka T, Son G, Akira S, Kishimoto T, Nakanishi K, Fujimoto J. Contribution of Toll-like receptor/myeloid differentiation factor 88 signaling to murine liver regeneration. Hepatology. 2005;41:443-450. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 133] [Cited by in RCA: 128] [Article Influence: 6.4] [Reference Citation Analysis (0)] |
| 73. | Fernández MA, Albor C, Ingelmo-Torres M, Nixon SJ, Ferguson C, Kurzchalia T, Tebar F, Enrich C, Parton RG, Pol A. Caveolin-1 is essential for liver regeneration. Science. 2006;313:1628-1632. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 196] [Cited by in RCA: 208] [Article Influence: 10.9] [Reference Citation Analysis (0)] |
| 74. | Olle EW, Ren X, McClintock SD, Warner RL, Deogracias MP, Johnson KJ, Colletti LM. Matrix metalloproteinase-9 is an important factor in hepatic regeneration after partial hepatectomy in mice. Hepatology. 2006;44:540-549. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 66] [Cited by in RCA: 66] [Article Influence: 3.5] [Reference Citation Analysis (0)] |
| 75. | Mayoral R, Fernández-Martínez A, Roy R, Boscá L, Martín-Sanz P. Dispensability and dynamics of caveolin-1 during liver regeneration and in isolated hepatic cells. Hepatology. 2007;46:813-822. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 40] [Cited by in RCA: 39] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 76. | Liu B, Bell AW, Paranjpe S, Bowen WC, Khillan JS, Luo JH, Mars WM, Michalopoulos GK. Suppression of liver regeneration and hepatocyte proliferation in hepatocyte-targeted glypican 3 transgenic mice. Hepatology. 2010;52:1060-1067. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 64] [Cited by in RCA: 63] [Article Influence: 4.2] [Reference Citation Analysis (0)] |
| 77. | Borude P, Edwards G, Walesky C, Li F, Ma X, Kong B, Guo GL, Apte U. Hepatocyte-specific deletion of farnesoid X receptor delays but does not inhibit liver regeneration after partial hepatectomy in mice. Hepatology. 2012;56:2344-2352. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 78] [Cited by in RCA: 75] [Article Influence: 5.8] [Reference Citation Analysis (0)] |
| 78. | Bhave VS, Mars W, Donthamsetty S, Zhang X, Tan L, Luo J, Bowen WC, Michalopoulos GK. Regulation of liver growth by glypican 3, CD81, hedgehog, and Hhex. Am J Pathol. 2013;183:153-159. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 32] [Cited by in RCA: 30] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 79. | Kong B, Huang J, Zhu Y, Li G, Williams J, Shen S, Aleksunes LM, Richardson JR, Apte U, Rudnick DA, Guo GL. Fibroblast growth factor 15 deficiency impairs liver regeneration in mice. Am J Physiol Gastrointest Liver Physiol. 2014;306:G893-G902. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 77] [Cited by in RCA: 82] [Article Influence: 7.5] [Reference Citation Analysis (0)] |
| 80. | Yang J, Mowry LE, Nejak-Bowen KN, Okabe H, Diegel CR, Lang RA, Williams BO, Monga SP. β-catenin signaling in murine liver zonation and regeneration: a Wnt-Wnt situation! Hepatology. 2014;60:964-976. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 163] [Cited by in RCA: 187] [Article Influence: 17.0] [Reference Citation Analysis (0)] |
| 81. | Lu WY, Bird TG, Boulter L, Tsuchiya A, Cole AM, Hay T, Guest RV, Wojtacha D, Man TY, Mackinnon A, Ridgway RA, Kendall T, Williams MJ, Jamieson T, Raven A, Hay DC, Iredale JP, Clarke AR, Sansom OJ, Forbes SJ. Hepatic progenitor cells of biliary origin with liver repopulation capacity. Nat Cell Biol. 2015;17:971-983. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 356] [Cited by in RCA: 372] [Article Influence: 37.2] [Reference Citation Analysis (0)] |
| 82. | Swiderska-Syn M, Xie G, Michelotti GA, Jewell ML, Premont RT, Syn WK, Diehl AM. Hedgehog regulates yes-associated protein 1 in regenerating mouse liver. Hepatology. 2016;64:232-244. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 77] [Cited by in RCA: 99] [Article Influence: 11.0] [Reference Citation Analysis (0)] |
| 83. | Tsagianni A, Mars WM, Bhushan B, Bowen WC, Orr A, Stoops J, Paranjpe S, Tseng GC, Liu S, Michalopoulos GK. Combined Systemic Disruption of MET and Epidermal Growth Factor Receptor Signaling Causes Liver Failure in Normal Mice. Am J Pathol. 2018;188:2223-2235. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 22] [Cited by in RCA: 22] [Article Influence: 3.1] [Reference Citation Analysis (0)] |
| 84. | Sivertsen Åsrud K, Pedersen L, Aesoy R, Muwonge H, Aasebø E, Nitschke Pettersen IK, Herfindal L, Dobie R, Jenkins S, Berge RK, Henderson NC, Selheim F, Døskeland SO, Bakke M. Mice depleted for Exchange Proteins Directly Activated by cAMP (Epac) exhibit irregular liver regeneration in response to partial hepatectomy. Sci Rep. 2019;9:13789. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 7] [Cited by in RCA: 6] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 85. | Fortier M, Cadoux M, Boussetta N, Pham S, Donné R, Couty JP, Desdouets C, Celton-Morizur S. Hepatospecific ablation of p38α MAPK governs liver regeneration through modulation of inflammatory response to CCl4-induced acute injury. Sci Rep. 2019;9:14614. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 5] [Cited by in RCA: 8] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 86. | Fazel Modares N, Polz R, Haghighi F, Lamertz L, Behnke K, Zhuang Y, Kordes C, Häussinger D, Sorg UR, Pfeffer K, Floss DM, Moll JM, Piekorz RP, Ahmadian MR, Lang PA, Scheller J. IL-6 Trans-signaling Controls Liver Regeneration After Partial Hepatectomy. Hepatology. 2019;70:2075-2091. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 53] [Cited by in RCA: 85] [Article Influence: 14.2] [Reference Citation Analysis (0)] |
| 87. | Zhou Y, Xu M, Liu P, Liang B, Qian M, Wang H, Song X, Nyshadham P, Che L, Calvisi DF, Li F, Lin S, Chen X. Mammalian Target of Rapamycin Complex 2 Signaling Is Required for Liver Regeneration in a Cholestatic Liver Injury Murine Model. Am J Pathol. 2020;190:1414-1426. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5] [Cited by in RCA: 9] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 88. | Laschinger M, Wang Y, Holzmann G, Wang B, Stöß C, Lu M, Brugger M, Schneider A, Knolle P, Wohlleber D, Schulze S, Steiger K, Tsujikawa K, Altmayr F, Friess H, Hartmann D, Hüser N, Holzmann B. The CGRP receptor component RAMP1 Links sensory innervation with YAP activity in the regenerating liver. FASEB J. 2020;34:8125-8138. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5] [Cited by in RCA: 20] [Article Influence: 4.0] [Reference Citation Analysis (0)] |
| 89. | Seguin A, Jia X, Earl AM, Li L, Wallace J, Qiu A, Bradley T, Shrestha R, Troadec MB, Hockin M, Titen S, Warner DE, Dowdle PT, Wohlfahrt ME, Hillas E, Firpo MA, Phillips JD, Kaplan J, Paw BH, Barasch J, Ward DM. The mitochondrial metal transporters mitoferrin1 and mitoferrin2 are required for liver regeneration and cell proliferation in mice. J Biol Chem. 2020;295:11002-11020. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 14] [Cited by in RCA: 43] [Article Influence: 8.6] [Reference Citation Analysis (0)] |
| 90. | Xue Y, Bhushan B, Mars WM, Bowen W, Tao J, Orr A, Stoops J, Yu Y, Luo J, Duncan AW, Michalopoulos GK. Phosphorylated Ezrin (Thr567) Regulates Hippo Pathway and Yes-Associated Protein (Yap) in Liver. Am J Pathol. 2020;190:1427-1437. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 17] [Cited by in RCA: 16] [Article Influence: 3.2] [Reference Citation Analysis (0)] |
| 91. | Goessling W, Sadler KC. Zebrafish: an important tool for liver disease research. Gastroenterology. 2015;149:1361-1377. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 211] [Cited by in RCA: 222] [Article Influence: 22.2] [Reference Citation Analysis (0)] |
| 92. | Howe K, Clark MD, Torroja CF, Torrance J, Berthelot C, Muffato M, Collins JE, Humphray S, McLaren K, Matthews L, McLaren S, Sealy I, Caccamo M, Churcher C, Scott C, Barrett JC, Koch R, Rauch GJ, White S, Chow W, Kilian B, Quintais LT, Guerra-Assunção JA, Zhou Y, Gu Y, Yen J, Vogel JH, Eyre T, Redmond S, Banerjee R, Chi J, Fu B, Langley E, Maguire SF, Laird GK, Lloyd D, Kenyon E, Donaldson S, Sehra H, Almeida-King J, Loveland J, Trevanion S, Jones M, Quail M, Willey D, Hunt A, Burton J, Sims S, McLay K, Plumb B, Davis J, Clee C, Oliver K, Clark R, Riddle C, Elliot D, Threadgold G, Harden G, Ware D, Begum S, Mortimore B, Kerry G, Heath P, Phillimore B, Tracey A, Corby N, Dunn M, Johnson C, Wood J, Clark S, Pelan S, Griffiths G, Smith M, Glithero R, Howden P, Barker N, Lloyd C, Stevens C, Harley J, Holt K, Panagiotidis G, Lovell J, Beasley H, Henderson C, Gordon D, Auger K, Wright D, Collins J, Raisen C, Dyer L, Leung K, Robertson L, Ambridge K, Leongamornlert D, McGuire S, Gilderthorp R, Griffiths C, Manthravadi D, Nichol S, Barker G, Whitehead S, Kay M, Brown J, Murnane C, Gray E, Humphries M, Sycamore N, Barker D, Saunders D, Wallis J, Babbage A, Hammond S, Mashreghi-Mohammadi M, Barr L, Martin S, Wray P, Ellington A, Matthews N, Ellwood M, Woodmansey R, Clark G, Cooper J, Tromans A, Grafham D, Skuce C, Pandian R, Andrews R, Harrison E, Kimberley A, Garnett J, Fosker N, Hall R, Garner P, Kelly D, Bird C, Palmer S, Gehring I, Berger A, Dooley CM, Ersan-Ürün Z, Eser C, Geiger H, Geisler M, Karotki L, Kirn A, Konantz J, Konantz M, Oberländer M, Rudolph-Geiger S, Teucke M, Lanz C, Raddatz G, Osoegawa K, Zhu B, Rapp A, Widaa S, Langford C, Yang F, Schuster SC, Carter NP, Harrow J, Ning Z, Herrero J, Searle SM, Enright A, Geisler R, Plasterk RH, Lee C, Westerfield M, de Jong PJ, Zon LI, Postlethwait JH, Nüsslein-Volhard C, Hubbard TJ, Roest Crollius H, Rogers J, Stemple DL. The zebrafish reference genome sequence and its relationship to the human genome. Nature. 2013;496:498-503. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2735] [Cited by in RCA: 3412] [Article Influence: 284.3] [Reference Citation Analysis (0)] |
| 93. | Wilkins BJ, Pack M. Zebrafish models of human liver development and disease. Compr Physiol. 2013;3:1213-1230. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 40] [Cited by in RCA: 67] [Article Influence: 5.6] [Reference Citation Analysis (0)] |
| 94. | Wang S, Miller SR, Ober EA, Sadler KC. Making It New Again: Insight Into Liver Development, Regeneration, and Disease From Zebrafish Research. Curr Top Dev Biol. 2017;124:161-195. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 46] [Cited by in RCA: 49] [Article Influence: 6.1] [Reference Citation Analysis (0)] |
| 95. | Ko S, Russell JO, Tian J, Gao C, Kobayashi M, Feng R, Yuan X, Shao C, Ding H, Poddar M, Singh S, Locker J, Weng HL, Monga SP, Shin D. Hdac1 Regulates Differentiation of Bipotent Liver Progenitor Cells During Regeneration via Sox9b and Cdk8. Gastroenterology. 2019;156:187-202.e14. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 72] [Cited by in RCA: 64] [Article Influence: 10.7] [Reference Citation Analysis (0)] |
| 96. | Chu J, Sadler KC. New school in liver development: lessons from zebrafish. Hepatology. 2009;50:1656-1663. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 200] [Cited by in RCA: 169] [Article Influence: 10.6] [Reference Citation Analysis (0)] |
| 97. | Godoy P, Hewitt NJ, Albrecht U, Andersen ME, Ansari N, Bhattacharya S, Bode JG, Bolleyn J, Borner C, Böttger J, Braeuning A, Budinsky RA, Burkhardt B, Cameron NR, Camussi G, Cho CS, Choi YJ, Craig Rowlands J, Dahmen U, Damm G, Dirsch O, Donato MT, Dong J, Dooley S, Drasdo D, Eakins R, Ferreira KS, Fonsato V, Fraczek J, Gebhardt R, Gibson A, Glanemann M, Goldring CE, Gómez-Lechón MJ, Groothuis GM, Gustavsson L, Guyot C, Hallifax D, Hammad S, Hayward A, Häussinger D, Hellerbrand C, Hewitt P, Hoehme S, Holzhütter HG, Houston JB, Hrach J, Ito K, Jaeschke H, Keitel V, Kelm JM, Kevin Park B, Kordes C, Kullak-Ublick GA, LeCluyse EL, Lu P, Luebke-Wheeler J, Lutz A, Maltman DJ, Matz-Soja M, McMullen P, Merfort I, Messner S, Meyer C, Mwinyi J, Naisbitt DJ, Nussler AK, Olinga P, Pampaloni F, Pi J, Pluta L, Przyborski SA, Ramachandran A, Rogiers V, Rowe C, Schelcher C, Schmich K, Schwarz M, Singh B, Stelzer EH, Stieger B, Stöber R, Sugiyama Y, Tetta C, Thasler WE, Vanhaecke T, Vinken M, Weiss TS, Widera A, Woods CG, Xu JJ, Yarborough KM, Hengstler JG. Recent advances in 2D and 3D in vitro systems using primary hepatocytes, alternative hepatocyte sources and non-parenchymal liver cells and their use in investigating mechanisms of hepatotoxicity, cell signaling and ADME. Arch Toxicol. 2013;87:1315-1530. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1026] [Cited by in RCA: 975] [Article Influence: 81.3] [Reference Citation Analysis (0)] |
| 98. | Ramboer E, Vanhaecke T, Rogiers V, Vinken M. Immortalized Human Hepatic Cell Lines for In Vitro Testing and Research Purposes. Methods Mol Biol. 2015;1250:53-76. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 40] [Cited by in RCA: 42] [Article Influence: 4.2] [Reference Citation Analysis (0)] |
| 99. | Michalopoulos G, Cianciulli HD, Novotny AR, Kligerman AD, Strom SC, Jirtle RL. Liver regeneration studies with rat hepatocytes in primary culture. Cancer Res. 1982;42:4673-4682. [PubMed] |
| 100. | Jiang J, Wolters JE, van Breda SG, Kleinjans JC, de Kok TM. Development of novel tools for the in vitro investigation of drug-induced liver injury. Expert Opin Drug Metab Toxicol. 2015;11:1523-1537. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 41] [Cited by in RCA: 35] [Article Influence: 3.5] [Reference Citation Analysis (0)] |
| 101. | Hart SN, Li Y, Nakamoto K, Subileau EA, Steen D, Zhong XB. A comparison of whole genome gene expression profiles of HepaRG cells and HepG2 cells to primary human hepatocytes and human liver tissues. Drug Metab Dispos. 2010;38:988-994. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 229] [Cited by in RCA: 220] [Article Influence: 14.7] [Reference Citation Analysis (0)] |
| 102. | Gómez-Lechón MJ, Tolosa L, Conde I, Donato MT. Competency of different cell models to predict human hepatotoxic drugs. Expert Opin Drug Metab Toxicol. 2014;10:1553-1568. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 126] [Cited by in RCA: 152] [Article Influence: 13.8] [Reference Citation Analysis (0)] |
| 103. | Smith CM, Nolan CK, Edwards MA, Hatfield JB, Stewart TW, Ferguson SS, Lecluyse EL, Sahi J. A comprehensive evaluation of metabolic activity and intrinsic clearance in suspensions and monolayer cultures of cryopreserved primary human hepatocytes. J Pharm Sci. 2012;101:3989-4002. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 61] [Cited by in RCA: 74] [Article Influence: 5.7] [Reference Citation Analysis (0)] |
| 104. | Tuschl G, Lauer B, Mueller SO. Primary hepatocytes as a model to analyze species-specific toxicity and drug metabolism. Expert Opin Drug Metab Toxicol. 2008;4:855-870. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 46] [Cited by in RCA: 42] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 105. | Bell CC, Dankers ACA, Lauschke VM, Sison-Young R, Jenkins R, Rowe C, Goldring CE, Park K, Regan SL, Walker T, Schofield C, Baze A, Foster AJ, Williams DP, van de Ven AWM, Jacobs F, Houdt JV, Lähteenmäki T, Snoeys J, Juhila S, Richert L, Ingelman-Sundberg M. Comparison of Hepatic 2D Sandwich Cultures and 3D Spheroids for Long-term Toxicity Applications: A Multicenter Study. Toxicol Sci. 2018;162:655-666. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 211] [Cited by in RCA: 224] [Article Influence: 32.0] [Reference Citation Analysis (0)] |
| 106. | Liu X, Brouwer KL, Gan LS, Brouwer KR, Stieger B, Meier PJ, Audus KL, LeCluyse EL. Partial maintenance of taurocholate uptake by adult rat hepatocytes cultured in a collagen sandwich configuration. Pharm Res. 1998;15:1533-1539. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 65] [Cited by in RCA: 64] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 107. | LeCluyse EL, Audus KL, Hochman JH. Formation of extensive canalicular networks by rat hepatocytes cultured in collagen-sandwich configuration. Am J Physiol. 1994;266:C1764-C1774. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 211] [Cited by in RCA: 187] [Article Influence: 6.0] [Reference Citation Analysis (0)] |
| 108. | Du Y, Han R, Wen F, Ng San San S, Xia L, Wohland T, Leo HL, Yu H. Synthetic sandwich culture of 3D hepatocyte monolayer. Biomaterials. 2008;29:290-301. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 60] [Cited by in RCA: 52] [Article Influence: 2.9] [Reference Citation Analysis (0)] |
| 109. | Underhill GH, Khetani SR. Emerging trends in modeling human liver disease in vitro. APL Bioeng. 2019;3:040902. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 18] [Cited by in RCA: 20] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 110. | Edmondson R, Broglie JJ, Adcock AF, Yang L. Three-dimensional cell culture systems and their applications in drug discovery and cell-based biosensors. Assay Drug Dev Technol. 2014;12:207-218. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1372] [Cited by in RCA: 1630] [Article Influence: 163.0] [Reference Citation Analysis (1)] |
| 111. | Hsia GSP, Esposito J, da Rocha LA, Ramos SLG, Okamoto OK. Clinical Application of Human Induced Pluripotent Stem Cell-Derived Organoids as an Alternative to Organ Transplantation. Stem Cells Int. 2021;2021:6632160. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 7] [Cited by in RCA: 18] [Article Influence: 4.5] [Reference Citation Analysis (0)] |
| 112. | Ramli MNB, Lim YS, Koe CT, Demircioglu D, Tng W, Gonzales KAU, Tan CP, Szczerbinska I, Liang H, Soe EL, Lu Z, Ariyachet C, Yu KM, Koh SH, Yaw LP, Jumat NHB, Lim JSY, Wright G, Shabbir A, Dan YY, Ng HH, Chan YS. Human Pluripotent Stem Cell-Derived Organoids as Models of Liver Disease. Gastroenterology. 2020;159:1471-1486.e12. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 83] [Cited by in RCA: 165] [Article Influence: 33.0] [Reference Citation Analysis (1)] |
| 113. | Wang B, Jakus AE, Baptista PM, Soker S, Soto-Gutierrez A, Abecassis MM, Shah RN, Wertheim JA. Functional Maturation of Induced Pluripotent Stem Cell Hepatocytes in Extracellular Matrix-A Comparative Analysis of Bioartificial Liver Microenvironments. Stem Cells Transl Med. 2016;5:1257-1267. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 81] [Cited by in RCA: 90] [Article Influence: 10.0] [Reference Citation Analysis (0)] |
| 114. | Ouchi R, Togo S, Kimura M, Shinozawa T, Koido M, Koike H, Thompson W, Karns RA, Mayhew CN, McGrath PS, McCauley HA, Zhang RR, Lewis K, Hakozaki S, Ferguson A, Saiki N, Yoneyama Y, Takeuchi I, Mabuchi Y, Akazawa C, Yoshikawa HY, Wells JM, Takebe T. Modeling Steatohepatitis in Humans with Pluripotent Stem Cell-Derived Organoids. Cell Metab. 2019;30:374-384.e6. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 210] [Cited by in RCA: 355] [Article Influence: 59.2] [Reference Citation Analysis (0)] |
| 115. | Olgasi C, Cucci A, Follenzi A. iPSC-Derived Liver Organoids: A Journey from Drug Screening, to Disease Modeling, Arriving to Regenerative Medicine. Int J Mol Sci. 2020;21. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 21] [Cited by in RCA: 61] [Article Influence: 12.2] [Reference Citation Analysis (0)] |
| 116. | Huch M, Gehart H, van Boxtel R, Hamer K, Blokzijl F, Verstegen MM, Ellis E, van Wenum M, Fuchs SA, de Ligt J, van de Wetering M, Sasaki N, Boers SJ, Kemperman H, de Jonge J, Ijzermans JN, Nieuwenhuis EE, Hoekstra R, Strom S, Vries RR, van der Laan LJ, Cuppen E, Clevers H. Long-term culture of genome-stable bipotent stem cells from adult human liver. Cell. 2015;160:299-312. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1153] [Cited by in RCA: 1133] [Article Influence: 113.3] [Reference Citation Analysis (0)] |
| 117. | Justin AW, Saeb-Parsy K, Markaki AE, Vallier L, Sampaziotis F. Advances in the generation of bioengineered bile ducts. Biochim Biophys Acta Mol Basis Dis. 2018;1864:1532-1538. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 13] [Cited by in RCA: 17] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 118. | Harper S, Hoff M, Skepper J, Davies S, Huguet E. Portal venous repopulation of decellularised rat liver scaffolds with syngeneic bone marrow stem cells. J Tissue Eng Regen Med. 2020;14:1502-1512. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 4] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 119. | Mito M, Kusano M, Kawaura Y. Hepatocyte transplantation in man. Transplant Proc. 1992;24:3052-3053. [PubMed] |
| 120. | Davis MW, Vacanti JP. Toward development of an implantable tissue engineered liver. Biomaterials. 1996;17:365-372. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 99] [Cited by in RCA: 78] [Article Influence: 2.7] [Reference Citation Analysis (0)] |
| 121. | Meng F, Assiri A, Dhar D, Broering D. Whole liver engineering: A promising approach to develop functional liver surrogates. Liver Int. 2017;37:1759-1772. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 14] [Cited by in RCA: 13] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 122. | Li J, Pan J, Zhang L, Guo X, Yu Y. Culture of primary rat hepatocytes within porous chitosan scaffolds. J Biomed Mater Res A. 2003;67:938-943. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 64] [Cited by in RCA: 45] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 123. | Harada K, Mitaka T, Miyamoto S, Sugimoto S, Ikeda S, Takeda H, Mochizuki Y, Hirata K. Rapid formation of hepatic organoid in collagen sponge by rat small hepatocytes and hepatic nonparenchymal cells. J Hepatol. 2003;39:716-723. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 31] [Cited by in RCA: 32] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 124. | Liu Tsang V, Chen AA, Cho LM, Jadin KD, Sah RL, DeLong S, West JL, Bhatia SN. Fabrication of 3D hepatic tissues by additive photopatterning of cellular hydrogels. FASEB J. 2007;21:790-801. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 369] [Cited by in RCA: 295] [Article Influence: 15.5] [Reference Citation Analysis (0)] |
| 125. | Douglas DN, Kneteman NM. Mice with Chimeric Human Livers and Their Applications. Methods Mol Biol. 2019;1911:459-479. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4] [Cited by in RCA: 4] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 126. | Hillebrandt KH, Everwien H, Haep N, Keshi E, Pratschke J, Sauer IM. Strategies based on organ decellularization and recellularization. Transpl Int. 2019;32:571-585. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 26] [Cited by in RCA: 57] [Article Influence: 11.4] [Reference Citation Analysis (0)] |
| 127. | Uygun BE, Soto-Gutierrez A, Yagi H, Izamis ML, Guzzardi MA, Shulman C, Milwid J, Kobayashi N, Tilles A, Berthiaume F, Hertl M, Nahmias Y, Yarmush ML, Uygun K. Organ reengineering through development of a transplantable recellularized liver graft using decellularized liver matrix. Nat Med. 2010;16:814-820. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1162] [Cited by in RCA: 999] [Article Influence: 66.6] [Reference Citation Analysis (0)] |
| 128. | Bao J, Shi Y, Sun H, Yin X, Yang R, Li L, Chen X, Bu H. Construction of a portal implantable functional tissue-engineered liver using perfusion-decellularized matrix and hepatocytes in rats. Cell Transplant. 2011;20:753-766. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 117] [Cited by in RCA: 104] [Article Influence: 6.9] [Reference Citation Analysis (0)] |
| 129. | Huppert SS, Campbell KM. Emerging advancements in liver regeneration and organogenesis as tools for liver replacement. Curr Opin Organ Transplant. 2016;21:581-587. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 13] [Cited by in RCA: 14] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 130. | Ko IK, Peng L, Peloso A, Smith CJ, Dhal A, Deegan DB, Zimmerman C, Clouse C, Zhao W, Shupe TD, Soker S, Yoo JJ, Atala A. Bioengineered transplantable porcine livers with re-endothelialized vasculature. Biomaterials. 2015;40:72-79. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 104] [Cited by in RCA: 111] [Article Influence: 11.1] [Reference Citation Analysis (0)] |
| 131. | Zhou P, Lessa N, Estrada DC, Severson EB, Lingala S, Zern MA, Nolta JA, Wu J. Decellularized liver matrix as a carrier for the transplantation of human fetal and primary hepatocytes in mice. Liver Transpl. 2011;17:418-427. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 92] [Cited by in RCA: 74] [Article Influence: 5.3] [Reference Citation Analysis (0)] |
| 132. | Ogiso S, Yasuchika K, Fukumitsu K, Ishii T, Kojima H, Miyauchi Y, Yamaoka R, Komori J, Katayama H, Kawai T, Yoshitoshi EY, Kita S, Yasuda K, Uemoto S. Efficient recellularisation of decellularised whole-liver grafts using biliary tree and foetal hepatocytes. Sci Rep. 2016;6:35887. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 36] [Cited by in RCA: 45] [Article Influence: 5.0] [Reference Citation Analysis (0)] |
| 133. | Navarro-Tableros V, Herrera Sanchez MB, Figliolini F, Romagnoli R, Tetta C, Camussi G. Recellularization of rat liver scaffolds by human liver stem cells. Tissue Eng Part A. 2015;21:1929-1939. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 41] [Cited by in RCA: 52] [Article Influence: 5.2] [Reference Citation Analysis (0)] |
| 134. | Butter A, Aliyev K, Hillebrandt KH, Raschzok N, Kluge M, Seiffert N, Tang P, Napierala H, Muhamma AI, Reutzel-Selke A, Andreou A, Pratschke J, Sauer IM, Struecker B. Evolution of graft morphology and function after recellularization of decellularized rat livers. J Tissue Eng Regen Med. 2018;12:e807-e816. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 14] [Cited by in RCA: 12] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 135. | Chen Y, Geerts S, Jaramillo M, Uygun BE. Preparation of Decellularized Liver Scaffolds and Recellularized Liver Grafts. Methods Mol Biol. 2018;1577:255-270. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 9] [Cited by in RCA: 14] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 136. | Chen Y, Devalliere J, Bulutoglu B, Yarmush ML, Uygun BE. Repopulation of intrahepatic bile ducts in engineered rat liver grafts. Technology (Singap World Sci). 2019;7:46-55. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 12] [Cited by in RCA: 11] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 137. | Takeishi K, Collin de l'Hortet A, Wang Y, Handa K, Guzman-Lepe J, Matsubara K, Morita K, Jang S, Haep N, Florentino RM, Yuan F, Fukumitsu K, Tobita K, Sun W, Franks J, Delgado ER, Shapiro EM, Fraunhoffer NA, Duncan AW, Yagi H, Mashimo T, Fox IJ, Soto-Gutierrez A. Assembly and Function of a Bioengineered Human Liver for Transplantation Generated Solely from Induced Pluripotent Stem Cells. Cell Rep. 2020;31:107711. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 47] [Cited by in RCA: 87] [Article Influence: 21.8] [Reference Citation Analysis (0)] |
| 138. | Verstegen MMA, Willemse J, van den Hoek S, Kremers GJ, Luider TM, van Huizen NA, Willemssen FEJA, Metselaar HJ, IJzermans JNM, van der Laan LJW, de Jonge J. Decellularization of Whole Human Liver Grafts Using Controlled Perfusion for Transplantable Organ Bioscaffolds. Stem Cells Dev. 2017;26:1304-1315. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 49] [Cited by in RCA: 67] [Article Influence: 8.4] [Reference Citation Analysis (0)] |
| 139. | Neuberger J, Patel J, Caldwell H, Davies S, Hebditch V, Hollywood C, Hubscher S, Karkhanis S, Lester W, Roslund N, West R, Wyatt JI, Heydtmann M. Guidelines on the use of liver biopsy in clinical practice from the British Society of Gastroenterology, the Royal College of Radiologists and the Royal College of Pathology. Gut. 2020;69:1382-1403. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 257] [Cited by in RCA: 229] [Article Influence: 45.8] [Reference Citation Analysis (0)] |
| 140. | Schiødt FV, Ostapowicz G, Murray N, Satyanarana R, Zaman A, Munoz S, Lee WM. Alpha-fetoprotein and prognosis in acute liver failure. Liver Transpl. 2006;12:1776-1781. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 86] [Cited by in RCA: 93] [Article Influence: 4.9] [Reference Citation Analysis (0)] |
| 141. | John K, Hadem J, Krech T, Wahl K, Manns MP, Dooley S, Batkai S, Thum T, Schulze-Osthoff K, Bantel H. MicroRNAs play a role in spontaneous recovery from acute liver failure. Hepatology. 2014;60:1346-1355. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 63] [Cited by in RCA: 78] [Article Influence: 7.1] [Reference Citation Analysis (0)] |
| 142. | Rutherford A, King LY, Hynan LS, Vedvyas C, Lin W, Lee WM, Chung RT; ALF Study Group. Development of an accurate index for predicting outcomes of patients with acute liver failure. Gastroenterology. 2012;143:1237-1243. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 96] [Cited by in RCA: 101] [Article Influence: 7.8] [Reference Citation Analysis (0)] |
| 143. | Belghiti J, Hiramatsu K, Benoist S, Massault P, Sauvanet A, Farges O. Seven hundred forty-seven hepatectomies in the 1990s: an update to evaluate the actual risk of liver resection. J Am Coll Surg. 2000;191:38-46. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 800] [Cited by in RCA: 798] [Article Influence: 31.9] [Reference Citation Analysis (0)] |
| 144. | Vauthey JN, Chaoui A, Do KA, Bilimoria MM, Fenstermacher MJ, Charnsangavej C, Hicks M, Alsfasser G, Lauwers G, Hawkins IF, Caridi J. Standardized measurement of the future liver remnant prior to extended liver resection: methodology and clinical associations. Surgery. 2000;127:512-519. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 494] [Cited by in RCA: 489] [Article Influence: 19.6] [Reference Citation Analysis (0)] |
| 145. | Schindl MJ, Redhead DN, Fearon KC, Garden OJ, Wigmore SJ; Edinburgh Liver Surgery and Transplantation Experimental Research Group (eLISTER). The value of residual liver volume as a predictor of hepatic dysfunction and infection after major liver resection. Gut. 2005;54:289-296. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 430] [Cited by in RCA: 416] [Article Influence: 20.8] [Reference Citation Analysis (0)] |
| 146. | Bennink RJ, Dinant S, Erdogan D, Heijnen BH, Straatsburg IH, Van Vliet AK, Van Gulik TM. Preoperative assessment of postoperative remnant liver function using hepatobiliary scintigraphy. J Nuclear Med. 2004;45:965-971. |
| 147. | Martel G, Cieslak KP, Huang R, van Lienden KP, Wiggers JK, Belblidia A, Dagenais M, Lapointe R, van Gulik TM, Vandenbroucke-Menu F. Comparison of techniques for volumetric analysis of the future liver remnant: implications for major hepatic resections. HPB (Oxford). 2015;17:1051-1057. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 23] [Cited by in RCA: 36] [Article Influence: 3.6] [Reference Citation Analysis (0)] |
| 148. | Heymsfield SB, Fulenwider T, Nordlinger B, Barlow R, Sones P, Kutner M. Accurate measurement of liver, kidney, and spleen volume and mass by computerized axial tomography. Ann Intern Med. 1979;90:185-187. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 337] [Cited by in RCA: 313] [Article Influence: 6.8] [Reference Citation Analysis (0)] |
| 149. | Fulcher AS, Szucs RA, Bassignani MJ, Marcos A. Right lobe living donor liver transplantation: preoperative evaluation of the donor with MR imaging. AJR Am J Roentgenol. 2001;176:1483-1491. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 102] [Cited by in RCA: 94] [Article Influence: 3.9] [Reference Citation Analysis (0)] |
| 150. | Gotra A, Sivakumaran L, Chartrand G, Vu KN, Vandenbroucke-Menu F, Kauffmann C, Kadoury S, Gallix B, de Guise JA, Tang A. Liver segmentation: indications, techniques and future directions. Insights Imaging. 2017;8:377-392. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 96] [Cited by in RCA: 106] [Article Influence: 13.3] [Reference Citation Analysis (0)] |
| 151. | Shoup M, Gonen M, D'Angelica M, Jarnagin WR, DeMatteo RP, Schwartz LH, Tuorto S, Blumgart LH, Fong Y. Volumetric analysis predicts hepatic dysfunction in patients undergoing major liver resection. J Gastrointest Surg. 2003;7:325-330. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 350] [Cited by in RCA: 339] [Article Influence: 15.4] [Reference Citation Analysis (0)] |
| 152. | Dinant S, de Graaf W, Verwer BJ, Bennink RJ, van Lienden KP, Gouma DJ, van Vliet AK, van Gulik TM. Risk assessment of posthepatectomy liver failure using hepatobiliary scintigraphy and CT volumetry. J Nucl Med. 2007;48:685-692. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 115] [Cited by in RCA: 136] [Article Influence: 7.6] [Reference Citation Analysis (0)] |
| 153. | Erdogan D, Heijnen BH, Bennink RJ, Kok M, Dinant S, Straatsburg IH, Gouma DJ, van Gulik TM. Preoperative assessment of liver function: a comparison of 99mTc-Mebrofenin scintigraphy with indocyanine green clearance test. Liver Int. 2004;24:117-123. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 82] [Cited by in RCA: 88] [Article Influence: 4.2] [Reference Citation Analysis (0)] |
| 154. | Olthof PB, Coelen RJS, Bennink RJ, Heger M, Lam MF, Besselink MG, Busch OR, van Lienden KP, van Gulik TM. 99mTc-mebrofenin hepatobiliary scintigraphy predicts liver failure following major liver resection for perihilar cholangiocarcinoma. HPB (Oxford). 2017;19:850-858. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 46] [Cited by in RCA: 69] [Article Influence: 8.6] [Reference Citation Analysis (0)] |
| 155. | de Graaf W, van Lienden KP, Dinant S, Roelofs JJ, Busch OR, Gouma DJ, Bennink RJ, van Gulik TM. Assessment of future remnant liver function using hepatobiliary scintigraphy in patients undergoing major liver resection. J Gastrointest Surg. 2010;14:369-378. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 167] [Cited by in RCA: 204] [Article Influence: 13.6] [Reference Citation Analysis (0)] |
| 156. | Sato Y, Koyama S, Tsukada K, Hatakeyama K. Acute portal hypertension reflecting shear stress as a trigger of liver regeneration following partial hepatectomy. Surg Today. 1997;27:518-526. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 100] [Cited by in RCA: 107] [Article Influence: 3.8] [Reference Citation Analysis (0)] |
| 157. | Song Z, Gupta K, Ng IC, Xing J, Yang YA, Yu H. Mechanosensing in liver regeneration. Semin Cell Dev Biol. 2017;71:153-167. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 32] [Cited by in RCA: 41] [Article Influence: 5.1] [Reference Citation Analysis (0)] |
| 158. | Sato Y, Tsukada K, Hatakeyama K. Role of shear stress and immune responses in liver regeneration after a partial hepatectomy. Surg Today. 1999;29:1-9. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 74] [Cited by in RCA: 83] [Article Influence: 3.2] [Reference Citation Analysis (0)] |
| 159. | Morsiani E, Aleotti A, Ricci D. Haemodynamic and ultrastructural observations on the rat liver after two-thirds partial hepatectomy. J Anat. 1998;192:507-515. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 31] [Cited by in RCA: 35] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 160. | Carnovale CE, Ronco MT. Role of nitric oxide in liver regeneration. Ann Hepatol. 2012;11:636-647. [PubMed] |
| 161. | Fausto N, Campbell JS, Riehle KJ. Liver regeneration. J Hepatol. 2012;57:692-694. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 137] [Cited by in RCA: 148] [Article Influence: 11.4] [Reference Citation Analysis (0)] |
| 162. | Schoen JM, Wang HH, Minuk GY, Lautt WW. Shear stress-induced nitric oxide release triggers the liver regeneration cascade. Nitric Oxide. 2001;5:453-464. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 116] [Cited by in RCA: 129] [Article Influence: 5.6] [Reference Citation Analysis (0)] |
| 163. | Poisson J, Lemoinne S, Boulanger C, Durand F, Moreau R, Valla D, Rautou PE. Liver sinusoidal endothelial cells: Physiology and role in liver diseases. J Hepatol. 2017;66:212-227. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 475] [Cited by in RCA: 706] [Article Influence: 88.3] [Reference Citation Analysis (0)] |
| 164. | Kawai M, Naruse K, Komatsu S, Kobayashi S, Nagino M, Nimura Y, Sokabe M. Mechanical stress-dependent secretion of interleukin 6 by endothelial cells after portal vein embolization: clinical and experimental studies. J Hepatol. 2002;37:240-246. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 44] [Cited by in RCA: 53] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 165. | Nishii K, Brodin E, Renshaw T, Weesner R, Moran E, Soker S, Sparks JL. Shear stress upregulates regeneration-related immediate early genes in liver progenitors in 3D ECM-like microenvironments. J Cell Physiol. 2018;233:4272-4281. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 14] [Cited by in RCA: 15] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 166. | Abu Rmilah A, Zhou W, Nelson E, Lin L, Amiot B, Nyberg SL. Understanding the marvels behind liver regeneration. Wiley Interdiscip Rev Dev Biol. 2019;8:e340. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 46] [Cited by in RCA: 81] [Article Influence: 13.5] [Reference Citation Analysis (0)] |
| 167. | Arab JP, Martin-Mateos RM, Shah VH. Gut-liver axis, cirrhosis and portal hypertension: the chicken and the egg. Hepatol Int. 2018;12:24-33. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 91] [Cited by in RCA: 155] [Article Influence: 19.4] [Reference Citation Analysis (0)] |
| 168. | Ozaki M. Cellular and molecular mechanisms of liver regeneration: Proliferation, growth, death and protection of hepatocytes. Semin Cell Dev Biol. 2020;100:62-73. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 24] [Cited by in RCA: 56] [Article Influence: 9.3] [Reference Citation Analysis (0)] |
| 169. | Campbell JS, Riehle KJ, Brooling JT, Bauer RL, Mitchell C, Fausto N. Proinflammatory cytokine production in liver regeneration is Myd88-dependent, but independent of Cd14, Tlr2, and Tlr4. J Immunol. 2006;176:2522-2528. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 82] [Cited by in RCA: 80] [Article Influence: 4.2] [Reference Citation Analysis (0)] |
| 170. | Yamamoto C, Yagi S, Hori T, Iida T, Taniguchi K, Isaji S, Uemoto S. Significance of portal venous VEGF during liver regeneration after hepatectomy. J Surg Res. 2010;159:e37-e43. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 25] [Cited by in RCA: 26] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 171. | Eipel C, Abshagen K, Vollmar B. Regulation of hepatic blood flow: the hepatic arterial buffer response revisited. World J Gastroenterol. 2010;16:6046-6057. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 339] [Cited by in RCA: 368] [Article Influence: 24.5] [Reference Citation Analysis (2)] |
| 172. | Schödel J, Oikonomopoulos S, Ragoussis J, Pugh CW, Ratcliffe PJ, Mole DR. High-resolution genome-wide mapping of HIF-binding sites by ChIP-seq. Blood. 2011;117:e207-e217. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 612] [Cited by in RCA: 562] [Article Influence: 40.1] [Reference Citation Analysis (0)] |
| 173. | Maeno H, Ono T, Dhar DK, Sato T, Yamanoi A, Nagasue N. Expression of hypoxia inducible factor-1alpha during liver regeneration induced by partial hepatectomy in rats. Liver Int. 2005;25:1002-1009. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 47] [Cited by in RCA: 50] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 174. | Dirscherl K, Schläpfer M, Roth Z'graggen B, Wenger RH, Booy C, Flury-Frei R, Fatzer R, Aloman C, Bartosch B, Parent R, Kurtcuoglu V, de Zélicourt D, Spahn DR, Beck Schimmer B, Schadde E. Hypoxia sensing by hepatic stellate cells leads to VEGF-dependent angiogenesis and may contribute to accelerated liver regeneration. Sci Rep. 2020;10:4392. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 19] [Cited by in RCA: 29] [Article Influence: 5.8] [Reference Citation Analysis (0)] |
| 175. | Ding BS, Nolan DJ, Butler JM, James D, Babazadeh AO, Rosenwaks Z, Mittal V, Kobayashi H, Shido K, Lyden D, Sato TN, Rabbany SY, Rafii S. Inductive angiocrine signals from sinusoidal endothelium are required for liver regeneration. Nature. 2010;468:310-315. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 700] [Cited by in RCA: 641] [Article Influence: 42.7] [Reference Citation Analysis (0)] |
| 176. | Lee SC, Jeong HJ, Lee SK, Kim SJ. Hypoxic Conditioned Medium From Human Adipose-Derived Stem Cells Promotes Mouse Liver Regeneration Through JAK/STAT3 Signaling. Stem Cells Transl Med. 2016;5:816-825. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 53] [Cited by in RCA: 73] [Article Influence: 8.1] [Reference Citation Analysis (0)] |
| 177. | Starlinger P, Luyendyk JP, Groeneveld DJ. Hemostasis and Liver Regeneration. Semin Thromb Hemost. 2020;46:735-742. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4] [Cited by in RCA: 11] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 178. | Matsuo R, Nakano Y, Ohkohchi N. Platelet administration via the portal vein promotes liver regeneration in rats after 70% hepatectomy. Ann Surg. 2011;253:759-763. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 84] [Cited by in RCA: 97] [Article Influence: 6.9] [Reference Citation Analysis (0)] |
| 179. | Han S, Park HW, Song JH, Gwak MS, Lee WJ, Kim G, Lee SK, Ko JS. Association Between Intraoperative Platelet Transfusion and Early Graft Regeneration in Living Donor Liver Transplantation. Ann Surg. 2016;264:1065-1072. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 41] [Cited by in RCA: 54] [Article Influence: 6.8] [Reference Citation Analysis (0)] |
| 180. | Lisman T, Porte RJ. Mechanisms of platelet-mediated liver regeneration. Blood. 2016;128:625-629. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 37] [Cited by in RCA: 52] [Article Influence: 5.8] [Reference Citation Analysis (0)] |
| 181. | Kirschbaum M, Karimian G, Adelmeijer J, Giepmans BN, Porte RJ, Lisman T. Horizontal RNA transfer mediates platelet-induced hepatocyte proliferation. Blood. 2015;126:798-806. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 54] [Cited by in RCA: 70] [Article Influence: 7.0] [Reference Citation Analysis (0)] |
| 182. | Zhang Q, Raoof M, Chen Y, Sumi Y, Sursal T, Junger W, Brohi K, Itagaki K, Hauser CJ. Circulating mitochondrial DAMPs cause inflammatory responses to injury. Nature. 2010;464:104-107. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 2990] [Cited by in RCA: 2800] [Article Influence: 186.7] [Reference Citation Analysis (0)] |
| 183. | Satyam A, Graef ER, Lapchak PH, Tsokos MG, Dalle Lucca JJ, Tsokos GC. Complement and coagulation cascades in trauma. Acute Med Surg. 2019;6:329-335. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 17] [Cited by in RCA: 40] [Article Influence: 6.7] [Reference Citation Analysis (0)] |
| 184. | Fausto N, Campbell JS, Riehle KJ. Liver regeneration. Hepatology. 2006;43:S45-S53. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1126] [Cited by in RCA: 1204] [Article Influence: 63.4] [Reference Citation Analysis (0)] |
| 185. | Groeneveld D, Pereyra D, Veldhuis Z, Adelmeijer J, Ottens P, Kopec AK, Starlinger P, Lisman T, Luyendyk JP. Intrahepatic fibrin(ogen) deposition drives liver regeneration after partial hepatectomy in mice and humans. Blood. 2019;133:1245-1256. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 27] [Cited by in RCA: 46] [Article Influence: 7.7] [Reference Citation Analysis (0)] |
| 186. | Mars WM, Liu ML, Kitson RP, Goldfarb RH, Gabauer MK, Michalopoulos GK. Immediate early detection of urokinase receptor after partial hepatectomy and its implications for initiation of liver regeneration. Hepatology. 1995;21:1695-1701. [PubMed] |
| 187. | Kim TH, Mars WM, Stolz DB, Petersen BE, Michalopoulos GK. Extracellular matrix remodeling at the early stages of liver regeneration in the rat. Hepatology. 1997;26:896-904. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 157] [Cited by in RCA: 140] [Article Influence: 5.0] [Reference Citation Analysis (0)] |
| 188. | Kim TH, Mars WM, Stolz DB, Michalopoulos GK. Expression and activation of pro-MMP-2 and pro-MMP-9 during rat liver regeneration. Hepatology. 2000;31:75-82. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 130] [Cited by in RCA: 121] [Article Influence: 4.8] [Reference Citation Analysis (0)] |
| 189. | Mars WM, Kim TH, Stolz DB, Liu ML, Michalopoulos GK. Presence of urokinase in serum-free primary rat hepatocyte cultures and its role in activating hepatocyte growth factor. Cancer Res. 1996;56:2837-2843. [PubMed] |
| 190. | Lindroos PM, Zarnegar R, Michalopoulos GK. Hepatocyte growth factor (hepatopoietin A) rapidly increases in plasma before DNA synthesis and liver regeneration stimulated by partial hepatectomy and carbon tetrachloride administration. Hepatology. 1991;13:743-750. [PubMed] |
| 191. | Michalopoulos GK. Principles of liver regeneration and growth homeostasis. Compr Physiol. 2013;3:485-513. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 191] [Cited by in RCA: 174] [Article Influence: 14.5] [Reference Citation Analysis (0)] |
| 192. | Roos F, Ryan AM, Chamow SM, Bennett GL, Schwall RH. Induction of liver growth in normal mice by infusion of hepatocyte growth factor/scatter factor. Am J Physiol. 1995;268:G380-G386. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 6] [Cited by in RCA: 13] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 193. | Liu ML, Mars WM, Zarnegar R, Michalopoulos GK. Collagenase pretreatment and the mitogenic effects of hepatocyte growth factor and transforming growth factor-alpha in adult rat liver. Hepatology. 1994;19:1521-1527. [PubMed] |
| 194. | Michalopoulos GK. Hepatostat: Liver regeneration and normal liver tissue maintenance. Hepatology. 2017;65:1384-1392. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 353] [Cited by in RCA: 318] [Article Influence: 39.8] [Reference Citation Analysis (0)] |
| 195. | Gilgenkrantz H, Collin de l'Hortet A. Understanding Liver Regeneration: From Mechanisms to Regenerative Medicine. Am J Pathol. 2018;188:1316-1327. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 79] [Cited by in RCA: 113] [Article Influence: 16.1] [Reference Citation Analysis (0)] |
| 196. | Webber EM, Bruix J, Pierce RH, Fausto N. Tumor necrosis factor primes hepatocytes for DNA replication in the rat. Hepatology. 1998;28:1226-1234. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 210] [Cited by in RCA: 214] [Article Influence: 7.9] [Reference Citation Analysis (0)] |
| 197. | Trautwein C, Rakemann T, Niehof M, Rose-John S, Manns MP. Acute-phase response factor, increased binding, and target gene transcription during liver regeneration. Gastroenterology. 1996;110:1854-1862. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 99] [Cited by in RCA: 106] [Article Influence: 3.7] [Reference Citation Analysis (0)] |
| 198. | Iwai M, Cui TX, Kitamura H, Saito M, Shimazu T. Increased secretion of tumour necrosis factor and interleukin 6 from isolated, perfused liver of rats after partial hepatectomy. Cytokine. 2001;13:60-64. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 52] [Cited by in RCA: 57] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 199. | FitzGerald MJ, Webber EM, Donovan JR, Fausto N. Rapid DNA binding by nuclear factor kappa B in hepatocytes at the start of liver regeneration. Cell Growth Differ. 1995;6:417-427. [PubMed] |
| 200. | Cressman DE, Diamond RH, Taub R. Rapid activation of the Stat3 transcription complex in liver regeneration. Hepatology. 1995;21:1443-1449. [PubMed] |
| 201. | Akerman P, Cote P, Yang SQ, McClain C, Nelson S, Bagby GJ, Diehl AM. Antibodies to tumor necrosis factor-alpha inhibit liver regeneration after partial hepatectomy. Am J Physiol. 1992;263:G579-G585. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 41] [Cited by in RCA: 106] [Article Influence: 3.2] [Reference Citation Analysis (0)] |
| 202. | Hayashi H, Nagaki M, Imose M, Osawa Y, Kimura K, Takai S, Imao M, Naiki T, Kato T, Moriwaki H. Normal liver regeneration and liver cell apoptosis after partial hepatectomy in tumor necrosis factor-alpha-deficient mice. Liver Int. 2005;25:162-170. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 34] [Cited by in RCA: 35] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 203. | Ren X, Hogaboam C, Carpenter A, Colletti L. Stem cell factor restores hepatocyte proliferation in IL-6 knockout mice following 70% hepatectomy. J Clin Invest. 2003;112:1407-1418. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 62] [Cited by in RCA: 60] [Article Influence: 2.7] [Reference Citation Analysis (0)] |
| 204. | Cornell RP. Gut-derived endotoxin elicits hepatotrophic factor secretion for liver regeneration. Am J Physiol. 1985;249:R551-R562. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 13] [Cited by in RCA: 26] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 205. | Cornell RP, Liljequist BL, Bartizal KF. Depressed liver regeneration after partial hepatectomy of germ-free, athymic and lipopolysaccharide-resistant mice. Hepatology. 1990;11:916-922. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 133] [Cited by in RCA: 130] [Article Influence: 3.7] [Reference Citation Analysis (0)] |
| 206. | Selzner N, Selzner M, Odermatt B, Tian Y, Van Rooijen N, Clavien PA. ICAM-1 triggers liver regeneration through leukocyte recruitment and Kupffer cell-dependent release of TNF-alpha/IL-6 in mice. Gastroenterology. 2003;124:692-700. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 137] [Cited by in RCA: 154] [Article Influence: 7.0] [Reference Citation Analysis (0)] |
| 207. | Anders RA, Subudhi SK, Wang J, Pfeffer K, Fu YX. Contribution of the lymphotoxin beta receptor to liver regeneration. J Immunol. 2005;175:1295-1300. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 54] [Cited by in RCA: 61] [Article Influence: 3.1] [Reference Citation Analysis (0)] |
| 208. | Knight B, Yeoh GC. TNF/LTalpha double knockout mice display abnormal inflammatory and regenerative responses to acute and chronic liver injury. Cell Tissue Res. 2005;319:61-70. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 36] [Cited by in RCA: 36] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 209. | Michalopoulos GK, Bhushan B. Liver regeneration: biological and pathological mechanisms and implications. Nat Rev Gastroenterol Hepatol. 2021;18:40-55. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 647] [Cited by in RCA: 577] [Article Influence: 144.3] [Reference Citation Analysis (1)] |
| 210. | Nakamura T, Nishizawa T, Hagiya M, Seki T, Shimonishi M, Sugimura A, Tashiro K, Shimizu S. Molecular cloning and expression of human hepatocyte growth factor. Nature. 1989;342:440-443. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1628] [Cited by in RCA: 1626] [Article Influence: 45.2] [Reference Citation Analysis (0)] |
| 211. | Wang X, DeFrances MC, Dai Y, Pediaditakis P, Johnson C, Bell A, Michalopoulos GK, Zarnegar R. A mechanism of cell survival: sequestration of Fas by the HGF receptor Met. Mol Cell. 2002;9:411-421. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 180] [Cited by in RCA: 176] [Article Influence: 7.7] [Reference Citation Analysis (0)] |
| 212. | Fafalios A, Ma J, Tan X, Stoops J, Luo J, Defrances MC, Zarnegar R. A hepatocyte growth factor receptor (Met)-insulin receptor hybrid governs hepatic glucose metabolism. Nat Med. 2011;17:1577-1584. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 75] [Cited by in RCA: 84] [Article Influence: 6.0] [Reference Citation Analysis (0)] |
| 213. | Schmidt C, Bladt F, Goedecke S, Brinkmann V, Zschiesche W, Sharpe M, Gherardi E, Birchmeier C. Scatter factor/hepatocyte growth factor is essential for liver development. Nature. 1995;373:699-702. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1076] [Cited by in RCA: 1033] [Article Influence: 34.4] [Reference Citation Analysis (0)] |
| 214. | Wang B, Gao C, Ponder KP. C/EBPbeta contributes to hepatocyte growth factor-induced replication of rodent hepatocytes. J Hepatol. 2005;43:294-302. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 9] [Cited by in RCA: 13] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 215. | Paranjpe S, Bowen WC, Bell AW, Nejak-Bowen K, Luo JH, Michalopoulos GK. Cell cycle effects resulting from inhibition of hepatocyte growth factor and its receptor c-Met in regenerating rat livers by RNA interference. Hepatology. 2007;45:1471-1477. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 81] [Cited by in RCA: 81] [Article Influence: 4.5] [Reference Citation Analysis (0)] |
| 216. | Schuppan D, Schmid M, Somasundaram R, Ackermann R, Ruehl M, Nakamura T, Riecken EO. Collagens in the liver extracellular matrix bind hepatocyte growth factor. Gastroenterology. 1998;114:139-152. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 115] [Cited by in RCA: 106] [Article Influence: 3.9] [Reference Citation Analysis (0)] |
| 217. | Pediaditakis P, Lopez-Talavera JC, Petersen B, Monga SP, Michalopoulos GK. The processing and utilization of hepatocyte growth factor/scatter factor following partial hepatectomy in the rat. Hepatology. 2001;34:688-693. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 106] [Cited by in RCA: 99] [Article Influence: 4.1] [Reference Citation Analysis (0)] |
| 218. | Zarnegar R, DeFrances MC, Kost DP, Lindroos P, Michalopoulos GK. Expression of hepatocyte growth factor mRNA in regenerating rat liver after partial hepatectomy. Biochem Biophys Res Commun. 1991;177:559-565. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 171] [Cited by in RCA: 166] [Article Influence: 4.9] [Reference Citation Analysis (0)] |
| 219. | Nakamura T, Teramoto H, Ichihara A. Purification and characterization of a growth factor from rat platelets for mature parenchymal hepatocytes in primary cultures. Proc Natl Acad Sci USA. 1986;83:6489-6493. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 420] [Cited by in RCA: 424] [Article Influence: 10.9] [Reference Citation Analysis (0)] |
| 220. | Yanagita K, Nagaike M, Ishibashi H, Niho Y, Matsumoto K, Nakamura T. Lung may have an endocrine function producing hepatocyte growth factor in response to injury of distal organs. Biochem Biophys Res Commun. 1992;182:802-809. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 143] [Cited by in RCA: 145] [Article Influence: 4.4] [Reference Citation Analysis (0)] |
| 221. | Kono S, Nagaike M, Matsumoto K, Nakamura T. Marked induction of hepatocyte growth factor mRNA in intact kidney and spleen in response to injury of distant organs. Biochem Biophys Res Commun. 1992;186:991-998. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 93] [Cited by in RCA: 99] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 222. | Zhang XJ, Olsavszky V, Yin Y, Wang B, Engleitner T, Öllinger R, Schledzewski K, Koch PS, Rad R, Schmid RM, Friess H, Goerdt S, Hüser N, Géraud C, von Figura G, Hartmann D. Angiocrine Hepatocyte Growth Factor Signaling Controls Physiological Organ and Body Size and Dynamic Hepatocyte Proliferation to Prevent Liver Damage during Regeneration. Am J Pathol. 2020;190:358-371. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 9] [Cited by in RCA: 27] [Article Influence: 4.5] [Reference Citation Analysis (0)] |
| 223. | Broten J, Michalopoulos G, Petersen B, Cruise J. Adrenergic stimulation of hepatocyte growth factor expression. Biochem Biophys Res Commun. 1999;262:76-79. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 46] [Cited by in RCA: 47] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 224. | Skrtic S, Wallenius V, Ekberg S, Brenzel A, Gressner AM, Jansson JO. Insulin-like growth factors stimulate expression of hepatocyte growth factor but not transforming growth factor beta1 in cultured hepatic stellate cells. Endocrinology. 1997;138:4683-4689. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 28] [Cited by in RCA: 34] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 225. | Burgess AW. EGFR family: structure physiology signalling and therapeutic targets. Growth Factors. 2008;26:263-274. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 180] [Cited by in RCA: 213] [Article Influence: 12.5] [Reference Citation Analysis (0)] |
| 226. | McGowan JA, Strain AJ, Bucher NL. DNA synthesis in primary cultures of adult rat hepatocytes in a defined medium: effects of epidermal growth factor, insulin, glucagon, and cyclic-AMP. J Cell Physiol. 1981;108:353-363. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 259] [Cited by in RCA: 273] [Article Influence: 6.2] [Reference Citation Analysis (0)] |
| 227. | Bucher NL, Patel U, Cohen S. Hormonal factors concerned with liver regeneration. Ciba Found Symp. 1977;95-107. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 11] [Cited by in RCA: 20] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 228. | St Hilaire RJ, Jones AL. Epidermal growth factor: its biologic and metabolic effects with emphasis on the hepatocyte. Hepatology. 1982;2:601-613. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 45] [Cited by in RCA: 48] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 229. | Olsen PS, Poulsen SS, Kirkegaard P. Adrenergic effects on secretion of epidermal growth factor from Brunner's glands. Gut. 1985;26:920-927. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 58] [Cited by in RCA: 55] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 230. | Jones DE Jr, Tran-Patterson R, Cui DM, Davin D, Estell KP, Miller DM. Epidermal growth factor secreted from the salivary gland is necessary for liver regeneration. Am J Physiol. 1995;268:G872-G878. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 7] [Cited by in RCA: 20] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 231. | Webber EM, FitzGerald MJ, Brown PI, Bartlett MH, Fausto N. Transforming growth factor-alpha expression during liver regeneration after partial hepatectomy and toxic injury, and potential interactions between transforming growth factor-alpha and hepatocyte growth factor. Hepatology. 1993;18:1422-1431. [PubMed] |
| 232. | Mead JE, Fausto N. Transforming growth factor alpha may be a physiological regulator of liver regeneration by means of an autocrine mechanism. Proc Natl Acad Sci USA. 1989;86:1558-1562. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 347] [Cited by in RCA: 349] [Article Influence: 9.7] [Reference Citation Analysis (0)] |
| 233. | Russell WE, Kaufmann WK, Sitaric S, Luetteke NC, Lee DC. Liver regeneration and hepatocarcinogenesis in transforming growth factor-alpha-targeted mice. Mol Carcinog. 1996;15:183-189. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 2] [Reference Citation Analysis (0)] |
| 234. | Berasain C, García-Trevijano ER, Castillo J, Erroba E, Lee DC, Prieto J, Avila MA. Amphiregulin: an early trigger of liver regeneration in mice. Gastroenterology. 2005;128:424-432. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 140] [Cited by in RCA: 158] [Article Influence: 7.9] [Reference Citation Analysis (0)] |
| 235. | Michalopoulos GK, Khan Z. Liver regeneration, growth factors, and amphiregulin. Gastroenterology. 2005;128:503-506. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 85] [Cited by in RCA: 90] [Article Influence: 4.5] [Reference Citation Analysis (0)] |
| 236. | Kiso S, Kawata S, Tamura S, Higashiyama S, Ito N, Tsushima H, Taniguchi N, Matsuzawa Y. Role of heparin-binding epidermal growth factor-like growth factor as a hepatotrophic factor in rat liver regeneration after partial hepatectomy. Hepatology. 1995;22:1584-1590. [PubMed] |
| 237. | Kiso S, Kawata S, Tamura S, Inui Y, Yoshida Y, Sawai Y, Umeki S, Ito N, Yamada A, Miyagawa J, Higashiyama S, Iwawaki T, Saito M, Taniguchi N, Matsuzawa Y, Kohno K. Liver regeneration in heparin-binding EGF-like growth factor transgenic mice after partial hepatectomy. Gastroenterology. 2003;124:701-707. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 63] [Cited by in RCA: 68] [Article Influence: 3.1] [Reference Citation Analysis (0)] |
| 238. | Stolz DB, Mars WM, Petersen BE, Kim TH, Michalopoulos GK. Growth factor signal transduction immediately after two-thirds partial hepatectomy in the rat. Cancer Res. 1999;59:3954-3960. [PubMed] |
| 239. | Paranjpe S, Bowen WC, Tseng GC, Luo JH, Orr A, Michalopoulos GK. RNA interference against hepatic epidermal growth factor receptor has suppressive effects on liver regeneration in rats. Am J Pathol. 2010;176:2669-2681. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 61] [Cited by in RCA: 56] [Article Influence: 3.7] [Reference Citation Analysis (1)] |
| 240. | Russell DW. The enzymes, regulation, and genetics of bile acid synthesis. Annu Rev Biochem. 2003;72:137-174. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1372] [Cited by in RCA: 1488] [Article Influence: 67.6] [Reference Citation Analysis (0)] |
| 241. | Schaap FG, Trauner M, Jansen PL. Bile acid receptors as targets for drug development. Nat Rev Gastroenterol Hepatol. 2014;11:55-67. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 451] [Cited by in RCA: 518] [Article Influence: 47.1] [Reference Citation Analysis (0)] |
| 242. | Keitel V, Kubitz R, Häussinger D. Endocrine and paracrine role of bile acids. World J Gastroenterol. 2008;14:5620-5629. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 88] [Cited by in RCA: 94] [Article Influence: 5.5] [Reference Citation Analysis (0)] |
| 243. | Evans RM, Mangelsdorf DJ. Nuclear Receptors, RXR, and the Big Bang. Cell. 2014;157:255-266. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 743] [Cited by in RCA: 895] [Article Influence: 81.4] [Reference Citation Analysis (0)] |
| 244. | Meng Z, Liu N, Fu X, Wang X, Wang YD, Chen WD, Zhang L, Forman BM, Huang W. Insufficient bile acid signaling impairs liver repair in CYP27(-/-) mice. J Hepatol. 2011;55:885-895. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 39] [Cited by in RCA: 36] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 245. | Naugler WE. Bile acid flux is necessary for normal liver regeneration. PLoS One. 2014;9:e97426. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 44] [Cited by in RCA: 51] [Article Influence: 4.6] [Reference Citation Analysis (0)] |
| 246. | Doignon I, Julien B, Serrière-Lanneau V, Garcin I, Alonso G, Nicou A, Monnet F, Gigou M, Humbert L, Rainteau D, Azoulay D, Castaing D, Gillon MC, Samuel D, Duclos-Vallée JC, Tordjmann T. Immediate neuroendocrine signaling after partial hepatectomy through acute portal hyperpressure and cholestasis. J Hepatol. 2011;54:481-488. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 57] [Cited by in RCA: 60] [Article Influence: 4.3] [Reference Citation Analysis (0)] |
| 247. | Hoekstra LT, Rietkerk M, van Lienden KP, van den Esschert JW, Schaap FG, van Gulik TM. Bile salts predict liver regeneration in rabbit model of portal vein embolization. J Surg Res. 2012;178:773-778. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 14] [Cited by in RCA: 15] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 248. | Chen WD, Wang YD, Zhang L, Shiah S, Wang M, Yang F, Yu D, Forman BM, Huang W. Farnesoid X receptor alleviates age-related proliferation defects in regenerating mouse livers by activating forkhead box m1b transcription. Hepatology. 2010;51:953-962. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 39] [Cited by in RCA: 61] [Article Influence: 4.1] [Reference Citation Analysis (0)] |
| 249. | Baier P, Wolf-Vorbeck G, Hempel S, Hopt UT, von Dobschuetz E. Effect of liver regeneration after partial hepatectomy and ischemia-reperfusion on expression of growth factor receptors. World J Gastroenterol. 2006;12:3835-3840. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 5] [Cited by in RCA: 5] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 250. | Jourdainne V, Péan N, Doignon I, Humbert L, Rainteau D, Tordjmann T. The Bile Acid Receptor TGR5 and Liver Regeneration. Dig Dis. 2015;33:319-326. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 22] [Cited by in RCA: 25] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 251. | Cruise JL, Knechtle SJ, Bollinger RR, Kuhn C, Michalopoulos G. Alpha 1-adrenergic effects and liver regeneration. Hepatology. 1987;7:1189-1194. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 152] [Cited by in RCA: 141] [Article Influence: 3.7] [Reference Citation Analysis (0)] |
| 252. | Cruise JL, Houck KA, Michalopoulos GK. Induction of DNA synthesis in cultured rat hepatocytes through stimulation of alpha 1 adrenoreceptor by norepinephrine. Science. 1985;227:749-751. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 191] [Cited by in RCA: 185] [Article Influence: 4.6] [Reference Citation Analysis (0)] |
| 253. | Han C, Bowen WC, Michalopoulos GK, Wu T. Alpha-1 adrenergic receptor transactivates signal transducer and activator of transcription-3 (Stat3) through activation of Src and epidermal growth factor receptor (EGFR) in hepatocytes. J Cell Physiol. 2008;216:486-497. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 31] [Cited by in RCA: 31] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 254. | Houck KA, Cruise JL, Michalopoulos G. Norepinephrine modulates the growth-inhibitory effect of transforming growth factor-beta in primary rat hepatocyte cultures. J Cell Physiol. 1988;135:551-555. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 64] [Cited by in RCA: 64] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 255. | Soeda J, Mouralidarane A, Ray S, Novelli M, Thomas S, Roskams T, Diehl AM, Oben JA. The β-adrenoceptor agonist isoproterenol rescues acetaminophen-injured livers through increasing progenitor numbers by Wnt in mice. Hepatology. 2014;60:1023-1034. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 23] [Cited by in RCA: 32] [Article Influence: 2.9] [Reference Citation Analysis (0)] |
| 256. | Lesurtel M, Graf R, Aleil B, Walther DJ, Tian Y, Jochum W, Gachet C, Bader M, Clavien PA. Platelet-derived serotonin mediates liver regeneration. Science. 2006;312:104-107. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 572] [Cited by in RCA: 594] [Article Influence: 31.3] [Reference Citation Analysis (0)] |
| 257. | Furrer K, Rickenbacher A, Tian Y, Jochum W, Bittermann AG, Käch A, Humar B, Graf R, Moritz W, Clavien PA. Serotonin reverts age-related capillarization and failure of regeneration in the liver through a VEGF-dependent pathway. Proc Natl Acad Sci USA. 2011;108:2945-2950. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 65] [Cited by in RCA: 79] [Article Influence: 5.6] [Reference Citation Analysis (0)] |
| 258. | Fang Y, Liu C, Shu B, Zhai M, Deng C, He C, Luo M, Han T, Zheng W, Zhang J, Liu S. Axis of serotonin -pERK-YAP in liver regeneration. Life Sci. 2018;209:490-497. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 15] [Cited by in RCA: 13] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 259. | Matondo RB, Punt C, Homberg J, Toussaint MJ, Kisjes R, Korporaal SJ, Akkerman JW, Cuppen E, de Bruin A. Deletion of the serotonin transporter in rats disturbs serotonin homeostasis without impairing liver regeneration. Am J Physiol Gastrointest Liver Physiol. 2009;296:G963-G968. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 36] [Cited by in RCA: 33] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 260. | Michalopoulos G, Pitot HC. Primary culture of parenchymal liver cells on collagen membranes. Morphological and biochemical observations. Exp Cell Res. 1975;94:70-78. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 627] [Cited by in RCA: 603] [Article Influence: 12.1] [Reference Citation Analysis (0)] |
| 261. | Block GD, Locker J, Bowen WC, Petersen BE, Katyal S, Strom SC, Riley T, Howard TA, Michalopoulos GK. Population expansion, clonal growth, and specific differentiation patterns in primary cultures of hepatocytes induced by HGF/SF, EGF and TGF alpha in a chemically defined (HGM) medium. J Cell Biol. 1996;132:1133-1149. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 402] [Cited by in RCA: 366] [Article Influence: 12.6] [Reference Citation Analysis (0)] |
| 262. | Lu M, Flanagan JU, Langley RJ, Hay MP, Perry JK. Targeting growth hormone function: strategies and therapeutic applications. Signal Transduct Target Ther. 2019;4:3. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 46] [Cited by in RCA: 88] [Article Influence: 14.7] [Reference Citation Analysis (0)] |
| 263. | McElduff A, Poronnik P, Baxter RC, Williams P. A comparison of the insulin and insulin-like growth factor I receptors from rat brain and liver. Endocrinology. 1988;122:1933-1939. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 35] [Cited by in RCA: 32] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 264. | Zindy F, Lamas E, Schmidt S, Kirn A, Brechot C. Expression of insulin-like growth factor II (IGF-II) and IGF-II, IGF-I and insulin receptors mRNAs in isolated non-parenchymal rat liver cells. J Hepatol. 1992;14:30-34. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 26] [Cited by in RCA: 26] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 265. | Thompson BJ, Shang CA, Waters MJ. Identification of genes induced by growth hormone in rat liver using cDNA arrays. Endocrinology. 2000;141:4321-4324. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 40] [Cited by in RCA: 39] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 266. | Yamauchi T, Ueki K, Tobe K, Tamemoto H, Sekine N, Wada M, Honjo M, Takahashi M, Takahashi T, Hirai H, Tushima T, Akanuma Y, Fujita T, Komuro I, Yazaki Y, Kadowaki T. Tyrosine phosphorylation of the EGF receptor by the kinase Jak2 is induced by growth hormone. Nature. 1997;390:91-96. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 210] [Cited by in RCA: 212] [Article Influence: 7.6] [Reference Citation Analysis (0)] |
| 267. | Pennisi PA, Kopchick JJ, Thorgeirsson S, LeRoith D, Yakar S. Role of growth hormone (GH) in liver regeneration. Endocrinology. 2004;145:4748-4755. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 55] [Cited by in RCA: 60] [Article Influence: 2.9] [Reference Citation Analysis (0)] |
| 268. | Tanase DM, Gosav EM, Neculae E, Costea CF, Ciocoiu M, Hurjui LL, Tarniceriu CC, Floria M. Hypothyroidism-Induced Nonalcoholic Fatty Liver Disease (HIN): Mechanisms and Emerging Therapeutic Options. Int J Mol Sci. 2020;21. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 12] [Cited by in RCA: 42] [Article Influence: 8.4] [Reference Citation Analysis (0)] |
| 269. | López-Fontal R, Zeini M, Través PG, Gómez-Ferrería M, Aranda A, Sáez GT, Cerdá C, Martín-Sanz P, Hortelano S, Boscá L. Mice lacking thyroid hormone receptor Beta show enhanced apoptosis and delayed liver commitment for proliferation after partial hepatectomy. PLoS One. 2010;5:e8710. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 36] [Cited by in RCA: 35] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 270. | Bockhorn M, Frilling A, Benko T, Best J, Sheu SY, Trippler M, Schlaak JF, Broelsch CE. Tri-iodothyronine as a stimulator of liver regeneration after partial and subtotal hepatectomy. Eur Surg Res. 2007;39:58-63. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 24] [Cited by in RCA: 27] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 271. | Pibiri M, Ledda-Columbano GM, Cossu C, Simbula G, Menegazzi M, Shinozuka H, Columbano A. Cyclin D1 is an early target in hepatocyte proliferation induced by thyroid hormone (T3). FASEB J. 2001;15:1006-1013. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 69] [Cited by in RCA: 87] [Article Influence: 3.6] [Reference Citation Analysis (0)] |
| 272. | Alisi A, Demori I, Spagnuolo S, Pierantozzi E, Fugassa E, Leoni S. Thyroid status affects rat liver regeneration after partial hepatectomy by regulating cell cycle and apoptosis. Cell Physiol Biochem. 2005;15:69-76. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 31] [Cited by in RCA: 37] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 273. | Alvarado TF, Puliga E, Preziosi M, Poddar M, Singh S, Columbano A, Nejak-Bowen K, Monga SP. Thyroid Hormone Receptor β Agonist Induces β-Catenin-Dependent Hepatocyte Proliferation in Mice: Implications in Hepatic Regeneration. Gene Expr. 2016;17:19-34. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 35] [Cited by in RCA: 37] [Article Influence: 4.1] [Reference Citation Analysis (0)] |
| 274. | Shaik F, Cuthbert GA, Homer-Vanniasinkam S, Muench SP, Ponnambalam S, Harrison MA. Structural Basis for Vascular Endothelial Growth Factor Receptor Activation and Implications for Disease Therapy. Biomolecules. 2020;10. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 24] [Cited by in RCA: 63] [Article Influence: 12.6] [Reference Citation Analysis (0)] |
| 275. | Jia C. Advances in the regulation of liver regeneration. Expert Rev Gastroenterol Hepatol. 2011;5:105-121. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 56] [Cited by in RCA: 65] [Article Influence: 4.6] [Reference Citation Analysis (0)] |
| 276. | Bockhorn M, Goralski M, Prokofiev D, Dammann P, Grünewald P, Trippler M, Biglarnia A, Kamler M, Niehues EM, Frilling A, Broelsch CE, Schlaak JF. VEGF is important for early liver regeneration after partial hepatectomy. J Surg Res. 2007;138:291-299. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 80] [Cited by in RCA: 92] [Article Influence: 5.1] [Reference Citation Analysis (0)] |
| 277. | Nusse R, Brown A, Papkoff J, Scambler P, Shackleford G, McMahon A, Moon R, Varmus H. A new nomenclature for int-1 and related genes: the Wnt gene family. Cell. 1991;64:231. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 211] [Cited by in RCA: 225] [Article Influence: 6.6] [Reference Citation Analysis (0)] |
| 278. | MacDonald BT, Tamai K, He X. Wnt/beta-catenin signaling: components, mechanisms, and diseases. Dev Cell. 2009;17:9-26. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 4613] [Cited by in RCA: 4507] [Article Influence: 281.7] [Reference Citation Analysis (0)] |
| 279. | Monga SP. β-Catenin Signaling and Roles in Liver Homeostasis, Injury, and Tumorigenesis. Gastroenterology. 2015;148:1294-1310. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 363] [Cited by in RCA: 398] [Article Influence: 39.8] [Reference Citation Analysis (0)] |
| 280. | Perugorria MJ, Olaizola P, Labiano I, Esparza-Baquer A, Marzioni M, Marin JJG, Bujanda L, Banales JM. Wnt-β-catenin signalling in liver development, health and disease. Nat Rev Gastroenterol Hepatol. 2019;16:121-136. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 428] [Cited by in RCA: 401] [Article Influence: 66.8] [Reference Citation Analysis (0)] |
| 281. | Hu S, Monga SP. Wnt/-Catenin Signaling and Liver Regeneration: Circuit, Biology, and Opportunities. Gene Expr. 2021;20:189-199. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 11] [Cited by in RCA: 24] [Article Influence: 6.0] [Reference Citation Analysis (0)] |
| 282. | Monga SP, Pediaditakis P, Mule K, Stolz DB, Michalopoulos GK. Changes in WNT/beta-catenin pathway during regulated growth in rat liver regeneration. Hepatology. 2001;33:1098-1109. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 233] [Cited by in RCA: 219] [Article Influence: 9.1] [Reference Citation Analysis (0)] |
| 283. | Chen Y, Sasai N, Ma G, Yue T, Jia J, Briscoe J, Jiang J. Sonic Hedgehog dependent phosphorylation by CK1α and GRK2 is required for ciliary accumulation and activation of smoothened. PLoS Biol. 2011;9:e1001083. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 192] [Cited by in RCA: 177] [Article Influence: 12.6] [Reference Citation Analysis (0)] |
| 284. | Roy S. Cilia and Hedgehog: when and how was their marriage solemnized? Differentiation. 2012;83:S43-S48. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 24] [Cited by in RCA: 24] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 285. | Ramsbottom SA, Pownall ME. Regulation of Hedgehog Signalling Inside and Outside the Cell. J Dev Biol. 2016;4:23. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 49] [Cited by in RCA: 53] [Article Influence: 5.9] [Reference Citation Analysis (0)] |
| 286. | Merchant JL, Saqui-Salces M. Inhibition of Hedgehog signaling in the gastrointestinal tract: targeting the cancer microenvironment. Cancer Treat Rev. 2014;40:12-21. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 29] [Cited by in RCA: 28] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 287. | Ochoa B, Syn WK, Delgado I, Karaca GF, Jung Y, Wang J, Zubiaga AM, Fresnedo O, Omenetti A, Zdanowicz M, Choi SS, Diehl AM. Hedgehog signaling is critical for normal liver regeneration after partial hepatectomy in mice. Hepatology. 2010;51:1712-1723. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 149] [Cited by in RCA: 144] [Article Influence: 9.6] [Reference Citation Analysis (0)] |
| 288. | Swiderska-Syn M, Syn WK, Xie G, Krüger L, Machado MV, Karaca G, Michelotti GA, Choi SS, Premont RT, Diehl AM. Myofibroblastic cells function as progenitors to regenerate murine livers after partial hepatectomy. Gut. 2014;63:1333-1344. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 87] [Cited by in RCA: 93] [Article Influence: 8.5] [Reference Citation Analysis (0)] |
| 289. | Langiewicz M, Schlegel A, Saponara E, Linecker M, Borger P, Graf R, Humar B, Clavien PA. Hedgehog pathway mediates early acceleration of liver regeneration induced by a novel two-staged hepatectomy in mice. J Hepatol. 2017;66:560-570. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 27] [Cited by in RCA: 39] [Article Influence: 4.9] [Reference Citation Analysis (0)] |
| 290. | Patel SH, Camargo FD, Yimlamai D. Hippo Signaling in the Liver Regulates Organ Size, Cell Fate, and Carcinogenesis. Gastroenterology. 2017;152:533-545. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 209] [Cited by in RCA: 230] [Article Influence: 28.8] [Reference Citation Analysis (0)] |
| 291. | Zheng Y, Pan D. The Hippo Signaling Pathway in Development and Disease. Dev Cell. 2019;50:264-282. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 441] [Cited by in RCA: 626] [Article Influence: 104.3] [Reference Citation Analysis (0)] |
| 292. | Grijalva JL, Huizenga M, Mueller K, Rodriguez S, Brazzo J, Camargo F, Sadri-Vakili G, Vakili K. Dynamic alterations in Hippo signaling pathway and YAP activation during liver regeneration. Am J Physiol Gastrointest Liver Physiol. 2014;307:G196-G204. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 101] [Cited by in RCA: 114] [Article Influence: 10.4] [Reference Citation Analysis (0)] |
| 293. | Chen L, Loh PG, Song H. Structural and functional insights into the TEAD-YAP complex in the Hippo signaling pathway. Protein Cell. 2010;1:1073-1083. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 39] [Cited by in RCA: 48] [Article Influence: 3.4] [Reference Citation Analysis (0)] |
| 294. | Miesfeld JB, Link BA. Establishment of transgenic lines to monitor and manipulate Yap/Taz-Tead activity in zebrafish reveals both evolutionarily conserved and divergent functions of the Hippo pathway. Mech Dev. 2014;133:177-188. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 46] [Cited by in RCA: 42] [Article Influence: 3.8] [Reference Citation Analysis (0)] |
| 295. | Meng Z, Moroishi T, Guan KL. Mechanisms of Hippo pathway regulation. Genes Dev. 2016;30:1-17. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 890] [Cited by in RCA: 1252] [Article Influence: 139.1] [Reference Citation Analysis (0)] |
| 296. | Oh SH, Swiderska-Syn M, Jewell ML, Premont RT, Diehl AM. Liver regeneration requires Yap1-TGFβ-dependent epithelial-mesenchymal transition in hepatocytes. J Hepatol. 2018;69:359-367. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 73] [Cited by in RCA: 118] [Article Influence: 16.9] [Reference Citation Analysis (0)] |
| 297. | Yagi S, Hirata M, Miyachi Y, Uemoto S. Liver Regeneration after Hepatectomy and Partial Liver Transplantation. Int J Mol Sci. 2020;21. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 27] [Cited by in RCA: 122] [Article Influence: 24.4] [Reference Citation Analysis (0)] |
| 298. | Wellbrock C, Karasarides M, Marais R. The RAF proteins take centre stage. Nat Rev Mol Cell Biol. 2004;5:875-885. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 869] [Cited by in RCA: 914] [Article Influence: 43.5] [Reference Citation Analysis (0)] |
| 299. | Asati V, Mahapatra DK, Bharti SK. PI3K/Akt/mTOR and Ras/Raf/MEK/ERK signaling pathways inhibitors as anticancer agents: Structural and pharmacological perspectives. Eur J Med Chem. 2016;109:314-341. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 316] [Cited by in RCA: 437] [Article Influence: 48.6] [Reference Citation Analysis (0)] |
| 300. | Ersahin T, Tuncbag N, Cetin-Atalay R. The PI3K/AKT/mTOR interactive pathway. Mol Biosyst. 2015;11:1946-1954. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 226] [Cited by in RCA: 245] [Article Influence: 24.5] [Reference Citation Analysis (0)] |
| 301. | Wullschleger S, Loewith R, Hall MN. TOR signaling in growth and metabolism. Cell. 2006;124:471-484. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4260] [Cited by in RCA: 4433] [Article Influence: 233.3] [Reference Citation Analysis (0)] |
| 302. | Fresno Vara JA, Casado E, de Castro J, Cejas P, Belda-Iniesta C, González-Barón M. PI3K/Akt signalling pathway and cancer. Cancer Treat Rev. 2004;30:193-204. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1407] [Cited by in RCA: 1787] [Article Influence: 85.1] [Reference Citation Analysis (0)] |
| 303. | Jackson LN, Larson SD, Silva SR, Rychahou PG, Chen LA, Qiu S, Rajaraman S, Evers BM. PI3K/Akt activation is critical for early hepatic regeneration after partial hepatectomy. Am J Physiol Gastrointest Liver Physiol. 2008;294:G1401-G1410. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 84] [Cited by in RCA: 79] [Article Influence: 4.6] [Reference Citation Analysis (0)] |
| 304. | Gao B, Wang H, Lafdil F, Feng D. STAT proteins - key regulators of anti-viral responses, inflammation, and tumorigenesis in the liver. J Hepatol. 2012;57:430-441. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 116] [Cited by in RCA: 146] [Article Influence: 11.2] [Reference Citation Analysis (0)] |
| 305. | Solt LA, May MJ. The IkappaB kinase complex: master regulator of NF-kappaB signaling. Immunol Res. 2008;42:3-18. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 231] [Cited by in RCA: 225] [Article Influence: 13.2] [Reference Citation Analysis (0)] |
| 306. | Moya IM, Halder G. Hippo-YAP/TAZ signalling in organ regeneration and regenerative medicine. Nat Rev Mol Cell Biol. 2019;20:211-226. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 334] [Cited by in RCA: 665] [Article Influence: 110.8] [Reference Citation Analysis (0)] |
| 307. | Simanshu DK, Nissley DV, McCormick F. RAS Proteins and Their Regulators in Human Disease. Cell. 2017;170:17-33. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1234] [Cited by in RCA: 1328] [Article Influence: 166.0] [Reference Citation Analysis (0)] |
| 308. | Taniguchi K, Karin M. NF-κB, inflammation, immunity and cancer: coming of age. Nat Rev Immunol. 2018;18:309-324. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1132] [Cited by in RCA: 1920] [Article Influence: 274.3] [Reference Citation Analysis (0)] |
| 309. | Yimlamai D, Fowl BH, Camargo FD. Emerging evidence on the role of the Hippo/YAP pathway in liver physiology and cancer. J Hepatol. 2015;63:1491-1501. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 137] [Cited by in RCA: 148] [Article Influence: 14.8] [Reference Citation Analysis (0)] |
| 310. | Michalopoulos GK, DeFrances MC. Liver regeneration. Science. 1997;276:60-66. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2649] [Cited by in RCA: 2467] [Article Influence: 88.1] [Reference Citation Analysis (0)] |
| 311. | Michalopoulos GK. Liver regeneration. J Cell Physiol. 2007;213:286-300. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1246] [Cited by in RCA: 1158] [Article Influence: 64.3] [Reference Citation Analysis (0)] |
| 312. | Ross MA, Sander CM, Kleeb TB, Watkins SC, Stolz DB. Spatiotemporal expression of angiogenesis growth factor receptors during the revascularization of regenerating rat liver. Hepatology. 2001;34:1135-1148. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 119] [Cited by in RCA: 128] [Article Influence: 5.3] [Reference Citation Analysis (0)] |
| 313. | LeCouter J, Moritz DR, Li B, Phillips GL, Liang XH, Gerber HP, Hillan KJ, Ferrara N. Angiogenesis-independent endothelial protection of liver: role of VEGFR-1. Science. 2003;299:890-893. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 512] [Cited by in RCA: 514] [Article Influence: 23.4] [Reference Citation Analysis (0)] |
| 314. | Wang L, Wang X, Wang L, Chiu JD, van de Ven G, Gaarde WA, Deleve LD. Hepatic vascular endothelial growth factor regulates recruitment of rat liver sinusoidal endothelial cell progenitor cells. Gastroenterology. 2012;143:1555-1563.e2. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 68] [Cited by in RCA: 63] [Article Influence: 4.8] [Reference Citation Analysis (0)] |
| 315. | Wang L, Wang X, Xie G, Wang L, Hill CK, DeLeve LD. Liver sinusoidal endothelial cell progenitor cells promote liver regeneration in rats. J Clin Invest. 2012;122:1567-1573. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 132] [Cited by in RCA: 144] [Article Influence: 11.1] [Reference Citation Analysis (0)] |
| 316. | Yin C, Evason KJ, Asahina K, Stainier DY. Hepatic stellate cells in liver development, regeneration, and cancer. J Clin Invest. 2013;123:1902-1910. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 439] [Cited by in RCA: 571] [Article Influence: 47.6] [Reference Citation Analysis (0)] |
| 317. | Kandilis AN, Koskinas J, Tiniakos DG, Nikiteas N, Perrea DN. Liver regeneration: focus on cell types and topographic differences. Eur Surg Res. 2010;44:1-12. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 14] [Cited by in RCA: 18] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 318. | Reynaert H, Urbain D, Geerts A. Regulation of sinusoidal perfusion in portal hypertension. Anat Rec (Hoboken). 2008;291:693-698. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 32] [Cited by in RCA: 41] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 319. | Hellerbrand C. Hepatic stellate cells--the pericytes in the liver. Pflugers Arch. 2013;465:775-778. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 87] [Cited by in RCA: 114] [Article Influence: 9.5] [Reference Citation Analysis (0)] |
| 320. | Tsuchida T, Friedman SL. Mechanisms of hepatic stellate cell activation. Nat Rev Gastroenterol Hepatol. 2017;14:397-411. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1221] [Cited by in RCA: 1988] [Article Influence: 248.5] [Reference Citation Analysis (0)] |
| 321. | Bansal MB. Hepatic stellate cells: fibrogenic, regenerative or both? Hepatol Int. 2016;10:902-908. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 26] [Cited by in RCA: 32] [Article Influence: 3.6] [Reference Citation Analysis (0)] |
| 322. | Friedman SL. Liver fibrosis -- from bench to bedside. J Hepatol. 2003;38 Suppl 1:S38-S53. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1199] [Cited by in RCA: 1292] [Article Influence: 58.7] [Reference Citation Analysis (0)] |
| 323. | Passino MA, Adams RA, Sikorski SL, Akassoglou K. Regulation of hepatic stellate cell differentiation by the neurotrophin receptor p75NTR. Science. 2007;315:1853-1856. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 143] [Cited by in RCA: 144] [Article Influence: 8.0] [Reference Citation Analysis (0)] |
| 324. | Kato M, Iwamoto H, Higashi N, Sugimoto R, Uchimura K, Tada S, Sakai H, Nakamuta M, Nawata H. Role of Rho small GTP binding protein in the regulation of actin cytoskeleton in hepatic stellate cells. J Hepatol. 1999;31:91-99. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 36] [Cited by in RCA: 37] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 325. | Fernández M, Semela D, Bruix J, Colle I, Pinzani M, Bosch J. Angiogenesis in liver disease. J Hepatol. 2009;50:604-620. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 417] [Cited by in RCA: 445] [Article Influence: 27.8] [Reference Citation Analysis (0)] |
| 326. | Schachtrup C, Le Moan N, Passino MA, Akassoglou K. Hepatic stellate cells and astrocytes: Stars of scar formation and tissue repair. Cell Cycle. 2011;10:1764-1771. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 28] [Cited by in RCA: 40] [Article Influence: 2.9] [Reference Citation Analysis (0)] |
| 327. | Shen K, Chang W, Gao X, Wang H, Niu W, Song L, Qin X. Depletion of activated hepatic stellate cell correlates with severe liver damage and abnormal liver regeneration in acetaminophen-induced liver injury. Acta Biochim Biophys Sin (Shanghai). 2011;43:307-315. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 33] [Cited by in RCA: 40] [Article Influence: 2.9] [Reference Citation Analysis (0)] |
| 328. | Pintilie DG, Shupe TD, Oh SH, Salganik SV, Darwiche H, Petersen BE. Hepatic stellate cells' involvement in progenitor-mediated liver regeneration. Lab Invest. 2010;90:1199-1208. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 63] [Cited by in RCA: 56] [Article Influence: 3.7] [Reference Citation Analysis (0)] |
| 329. | Chang W, Song L, Chang X, Ji M, Wang H, Qin X, Niu W. Early activated hepatic stellate cell-derived paracrine molecules modulate acute liver injury and regeneration. Lab Invest. 2017;97:318-328. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 23] [Cited by in RCA: 27] [Article Influence: 3.4] [Reference Citation Analysis (0)] |
| 330. | Gomez Perdiguero E, Klapproth K, Schulz C, Busch K, Azzoni E, Crozet L, Garner H, Trouillet C, de Bruijn MF, Geissmann F, Rodewald HR. Tissue-resident macrophages originate from yolk-sac-derived erythro-myeloid progenitors. Nature. 2015;518:547-551. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1340] [Cited by in RCA: 1727] [Article Influence: 157.0] [Reference Citation Analysis (0)] |
| 331. | Willekens FL, Werre JM, Kruijt JK, Roerdinkholder-Stoelwinder B, Groenen-Döpp YA, van den Bos AG, Bosman GJ, van Berkel TJ. Liver Kupffer cells rapidly remove red blood cell-derived vesicles from the circulation by scavenger receptors. Blood. 2005;105:2141-2145. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 176] [Cited by in RCA: 208] [Article Influence: 10.4] [Reference Citation Analysis (0)] |
| 332. | Theurl I, Hilgendorf I, Nairz M, Tymoszuk P, Haschka D, Asshoff M, He S, Gerhardt LM, Holderried TA, Seifert M, Sopper S, Fenn AM, Anzai A, Rattik S, McAlpine C, Theurl M, Wieghofer P, Iwamoto Y, Weber GF, Harder NK, Chousterman BG, Arvedson TL, McKee M, Wang F, Lutz OM, Rezoagli E, Babitt JL, Berra L, Prinz M, Nahrendorf M, Weiss G, Weissleder R, Lin HY, Swirski FK. On-demand erythrocyte disposal and iron recycling requires transient macrophages in the liver. Nat Med. 2016;22:945-951. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 301] [Cited by in RCA: 314] [Article Influence: 34.9] [Reference Citation Analysis (0)] |
| 333. | Wang Y, van der Tuin S, Tjeerdema N, van Dam AD, Rensen SS, Hendrikx T, Berbée JF, Atanasovska B, Fu J, Hoekstra M, Bekkering S, Riksen NP, Buurman WA, Greve JW, Hofker MH, Shiri-Sverdlov R, Meijer OC, Smit JW, Havekes LM, van Dijk KW, Rensen PC. Plasma cholesteryl ester transfer protein is predominantly derived from Kupffer cells. Hepatology. 2015;62:1710-1722. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 49] [Cited by in RCA: 65] [Article Influence: 6.5] [Reference Citation Analysis (0)] |
| 334. | Helmy KY, Katschke KJ Jr, Gorgani NN, Kljavin NM, Elliott JM, Diehl L, Scales SJ, Ghilardi N, van Lookeren Campagne M. CRIg: a macrophage complement receptor required for phagocytosis of circulating pathogens. Cell. 2006;124:915-927. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 397] [Cited by in RCA: 430] [Article Influence: 22.6] [Reference Citation Analysis (0)] |
| 335. | You Q, Cheng L, Kedl RM, Ju C. Mechanism of T cell tolerance induction by murine hepatic Kupffer cells. Hepatology. 2008;48:978-990. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 271] [Cited by in RCA: 257] [Article Influence: 15.1] [Reference Citation Analysis (0)] |
| 336. | Wacker HH, Radzun HJ, Parwaresch MR. Kinetics of Kupffer cells as shown by parabiosis and combined autoradiographic/immunohistochemical analysis. Virchows Arch B Cell Pathol Incl Mol Pathol. 1986;51:71-78. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 31] [Cited by in RCA: 30] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 337. | Sieweke MH, Allen JE. Beyond stem cells: self-renewal of differentiated macrophages. Science. 2013;342:1242974. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 336] [Cited by in RCA: 381] [Article Influence: 31.8] [Reference Citation Analysis (0)] |
| 338. | Yona S, Kim KW, Wolf Y, Mildner A, Varol D, Breker M, Strauss-Ayali D, Viukov S, Guilliams M, Misharin A, Hume DA, Perlman H, Malissen B, Zelzer E, Jung S. Fate mapping reveals origins and dynamics of monocytes and tissue macrophages under homeostasis. Immunity. 2013;38:79-91. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2014] [Cited by in RCA: 2424] [Article Influence: 202.0] [Reference Citation Analysis (0)] |
| 339. | Bonnardel J, T'Jonck W, Gaublomme D, Browaeys R, Scott CL, Martens L, Vanneste B, De Prijck S, Nedospasov SA, Kremer A, Van Hamme E, Borghgraef P, Toussaint W, De Bleser P, Mannaerts I, Beschin A, van Grunsven LA, Lambrecht BN, Taghon T, Lippens S, Elewaut D, Saeys Y, Guilliams M. Stellate Cells, Hepatocytes, and Endothelial Cells Imprint the Kupffer Cell Identity on Monocytes Colonizing the Liver Macrophage Niche. Immunity. 2019;51:638-654.e9. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 336] [Cited by in RCA: 417] [Article Influence: 69.5] [Reference Citation Analysis (0)] |
| 340. | Wang J, Kubes P. A Reservoir of Mature Cavity Macrophages that Can Rapidly Invade Visceral Organs to Affect Tissue Repair. Cell. 2016;165:668-678. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 318] [Cited by in RCA: 410] [Article Influence: 45.6] [Reference Citation Analysis (0)] |
| 341. | Swirski FK, Nahrendorf M, Etzrodt M, Wildgruber M, Cortez-Retamozo V, Panizzi P, Figueiredo JL, Kohler RH, Chudnovskiy A, Waterman P, Aikawa E, Mempel TR, Libby P, Weissleder R, Pittet MJ. Identification of splenic reservoir monocytes and their deployment to inflammatory sites. Science. 2009;325:612-616. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1790] [Cited by in RCA: 1731] [Article Influence: 108.2] [Reference Citation Analysis (0)] |
| 342. | Guillot A, Tacke F. Liver Macrophages: Old Dogmas and New Insights. Hepatol Commun. 2019;3:730-743. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 156] [Cited by in RCA: 256] [Article Influence: 42.7] [Reference Citation Analysis (0)] |
| 343. | Shim YR, Jeong WI. Recent advances of sterile inflammation and inter-organ cross-talk in alcoholic liver disease. Exp Mol Med. 2020;52:772-780. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 18] [Cited by in RCA: 48] [Article Influence: 9.6] [Reference Citation Analysis (0)] |
| 344. | Koh MY, Gagea M, Sargis T, Lemos R Jr, Grandjean G, Charbono A, Bekiaris V, Sedy J, Kiriakova G, Liu X, Roberts LR, Ware C, Powis G. A new HIF-1α/RANTES-driven pathway to hepatocellular carcinoma mediated by germline haploinsufficiency of SART1/HAF in mice. Hepatology. 2016;63:1576-1591. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 34] [Cited by in RCA: 36] [Article Influence: 4.0] [Reference Citation Analysis (0)] |
| 345. | Lee JH, Shim YR, Seo W, Kim MH, Choi WM, Kim HH, Kim YE, Yang K, Ryu T, Jeong JM, Choi HG, Eun HS, Kim SH, Mun H, Yoon JH, Jeong WI. Mitochondrial Double-Stranded RNA in Exosome Promotes Interleukin-17 Production Through Toll-Like Receptor 3 in Alcohol-associated Liver Injury. Hepatology. 2020;72:609-625. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 62] [Cited by in RCA: 99] [Article Influence: 19.8] [Reference Citation Analysis (0)] |
| 346. | Marra F, Tacke F. Roles for chemokines in liver disease. Gastroenterology. 2014;147:577-594.e1. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 507] [Cited by in RCA: 628] [Article Influence: 57.1] [Reference Citation Analysis (0)] |
| 347. | Wen Y, Lambrecht J, Ju C, Tacke F. Hepatic macrophages in liver homeostasis and diseases-diversity, plasticity and therapeutic opportunities. Cell Mol Immunol. 2021;18:45-56. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 116] [Cited by in RCA: 433] [Article Influence: 86.6] [Reference Citation Analysis (0)] |
| 348. | Wyler SL, D'Ingillo SL, Lamb CL, Mitchell KA. Monocyte chemoattractant protein-1 is not required for liver regeneration after partial hepatectomy. J Inflamm (Lond). 2016;13:28. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 10] [Cited by in RCA: 12] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 349. | Miyaoka Y, Ebato K, Kato H, Arakawa S, Shimizu S, Miyajima A. Hypertrophy and unconventional cell division of hepatocytes underlie liver regeneration. Curr Biol. 2012;22:1166-1175. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 286] [Cited by in RCA: 344] [Article Influence: 26.5] [Reference Citation Analysis (0)] |
| 350. | Donne R, Saroul-Aïnama M, Cordier P, Celton-Morizur S, Desdouets C. Polyploidy in liver development, homeostasis and disease. Nat Rev Gastroenterol Hepatol. 2020;17:391-405. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 77] [Cited by in RCA: 115] [Article Influence: 23.0] [Reference Citation Analysis (0)] |
| 351. | Wang B, Zhao L, Fish M, Logan CY, Nusse R. Self-renewing diploid Axin2(+) cells fuel homeostatic renewal of the liver. Nature. 2015;524:180-185. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 600] [Cited by in RCA: 540] [Article Influence: 54.0] [Reference Citation Analysis (0)] |
| 352. | Font-Burgada J, Shalapour S, Ramaswamy S, Hsueh B, Rossell D, Umemura A, Taniguchi K, Nakagawa H, Valasek MA, Ye L, Kopp JL, Sander M, Carter H, Deisseroth K, Verma IM, Karin M. Hybrid Periportal Hepatocytes Regenerate the Injured Liver without Giving Rise to Cancer. Cell. 2015;162:766-779. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 320] [Cited by in RCA: 380] [Article Influence: 42.2] [Reference Citation Analysis (0)] |
| 353. | Michalopoulos GK, Khan Z. Liver Stem Cells: Experimental Findings and Implications for Human Liver Disease. Gastroenterology. 2015;149:876-882. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 88] [Cited by in RCA: 86] [Article Influence: 8.6] [Reference Citation Analysis (0)] |
| 354. | Zong Y, Stanger BZ. Molecular mechanisms of liver and bile duct development. Wiley Interdiscip Rev Dev Biol. 2012;1:643-655. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 43] [Cited by in RCA: 51] [Article Influence: 3.9] [Reference Citation Analysis (0)] |
| 355. | Choi TY, Ninov N, Stainier DY, Shin D. Extensive conversion of hepatic biliary epithelial cells to hepatocytes after near total loss of hepatocytes in zebrafish. Gastroenterology. 2014;146:776-788. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 152] [Cited by in RCA: 189] [Article Influence: 17.2] [Reference Citation Analysis (0)] |
| 356. | Rodrigo-Torres D, Affò S, Coll M, Morales-Ibanez O, Millán C, Blaya D, Alvarez-Guaita A, Rentero C, Lozano JJ, Maestro MA, Solar M, Arroyo V, Caballería J, van Grunsven LA, Enrich C, Ginès P, Bataller R, Sancho-Bru P. The biliary epithelium gives rise to liver progenitor cells. Hepatology. 2014;60:1367-1377. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 136] [Cited by in RCA: 155] [Article Influence: 14.1] [Reference Citation Analysis (0)] |
| 357. | Verfaillie CM. Biliary cells to the rescue of Prometheus. Gastroenterology. 2014;146:611-614. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4] [Cited by in RCA: 4] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 358. | Kiseleva YV, Antonyan SZ, Zharikova TS, Tupikin KA, Kalinin DV, Zharikov YO. Molecular pathways of liver regeneration: A comprehensive review. World J Hepatol. 2021;13:270-290. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 37] [Cited by in RCA: 38] [Article Influence: 9.5] [Reference Citation Analysis (3)] |
| 359. | Evarts RP, Hu Z, Omori N, Omori M, Marsden ER, Thorgeirsson SS. Precursor-product relationship between oval cells and hepatocytes: comparison between tritiated thymidine and bromodeoxyuridine as tracers. Carcinogenesis. 1996;17:2143-2151. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 59] [Cited by in RCA: 56] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 360. | Schaub JR, Huppert KA, Kurial SNT, Hsu BY, Cast AE, Donnelly B, Karns RA, Chen F, Rezvani M, Luu HY, Mattis AN, Rougemont AL, Rosenthal P, Huppert SS, Willenbring H. De novo formation of the biliary system by TGFβ-mediated hepatocyte transdifferentiation. Nature. 2018;557:247-251. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 151] [Cited by in RCA: 221] [Article Influence: 31.6] [Reference Citation Analysis (0)] |
| 361. | Tarlow BD, Pelz C, Naugler WE, Wakefield L, Wilson EM, Finegold MJ, Grompe M. Bipotential adult liver progenitors are derived from chronically injured mature hepatocytes. Cell Stem Cell. 2014;15:605-618. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 351] [Cited by in RCA: 417] [Article Influence: 37.9] [Reference Citation Analysis (0)] |
| 362. | Michalopoulos GK, Barua L, Bowen WC. Transdifferentiation of rat hepatocytes into biliary cells after bile duct ligation and toxic biliary injury. Hepatology. 2005;41:535-544. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 269] [Cited by in RCA: 260] [Article Influence: 13.0] [Reference Citation Analysis (0)] |
| 363. | Pibiri M. Liver regeneration in aged mice: new insights. Aging (Albany NY). 2018;10:1801-1824. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 24] [Cited by in RCA: 40] [Article Influence: 6.7] [Reference Citation Analysis (0)] |
| 364. | Le Lay J, Kaestner KH. The Fox genes in the liver: from organogenesis to functional integration. Physiol Rev. 2010;90:1-22. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 72] [Cited by in RCA: 68] [Article Influence: 4.5] [Reference Citation Analysis (0)] |
| 365. | Wang X, Quail E, Hung NJ, Tan Y, Ye H, Costa RH. Increased levels of forkhead box M1B transcription factor in transgenic mouse hepatocytes prevent age-related proliferation defects in regenerating liver. Proc Natl Acad Sci USA. 2001;98:11468-11473. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 169] [Cited by in RCA: 168] [Article Influence: 7.0] [Reference Citation Analysis (0)] |
| 366. | Iakova P, Awad SS, Timchenko NA. Aging reduces proliferative capacities of liver by switching pathways of C/EBPalpha growth arrest. Cell. 2003;113:495-506. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 203] [Cited by in RCA: 203] [Article Influence: 9.2] [Reference Citation Analysis (0)] |
| 367. | Ikawa-Yoshida A, Matsumoto T, Okano S, Aoyagi Y, Matsubara Y, Furuyama T, Nakatsu Y, Tsuzuki T, Onimaru M, Ohkusa T, Nomura M, Maehara Y. BubR1 Insufficiency Impairs Liver Regeneration in Aged Mice after Hepatectomy through Intercalated Disc Abnormality. Sci Rep. 2016;6:32399. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 8] [Cited by in RCA: 9] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 368. | Conboy IM, Conboy MJ, Wagers AJ, Girma ER, Weissman IL, Rando TA. Rejuvenation of aged progenitor cells by exposure to a young systemic environment. Nature. 2005;433:760-764. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1544] [Cited by in RCA: 1639] [Article Influence: 82.0] [Reference Citation Analysis (0)] |
| 369. | Liu A, Guo E, Yang J, Yang Y, Liu S, Jiang X, Hu Q, Dirsch O, Dahmen U, Zhang C, Gewirtz DA, Fang H. Young plasma reverses age-dependent alterations in hepatic function through the restoration of autophagy. Aging Cell. 2018;17. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 33] [Cited by in RCA: 56] [Article Influence: 8.0] [Reference Citation Analysis (0)] |
| 370. | Apte U, Singh S, Zeng G, Cieply B, Virji MA, Wu T, Monga SP. Beta-catenin activation promotes liver regeneration after acetaminophen-induced injury. Am J Pathol. 2009;175:1056-1065. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 119] [Cited by in RCA: 134] [Article Influence: 8.4] [Reference Citation Analysis (0)] |
| 371. | Holt MP, Cheng L, Ju C. Identification and characterization of infiltrating macrophages in acetaminophen-induced liver injury. J Leukoc Biol. 2008;84:1410-1421. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 288] [Cited by in RCA: 337] [Article Influence: 19.8] [Reference Citation Analysis (0)] |
| 372. | Amemiya H, Kono H, Fujii H. Liver regeneration is impaired in macrophage colony stimulating factor deficient mice after partial hepatectomy: the role of M-CSF-induced macrophages. J Surg Res. 2011;165:59-67. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 29] [Cited by in RCA: 38] [Article Influence: 2.7] [Reference Citation Analysis (0)] |
| 373. | Stutchfield BM, Antoine DJ, Mackinnon AC, Gow DJ, Bain CC, Hawley CA, Hughes MJ, Francis B, Wojtacha D, Man TY, Dear JW, Devey LR, Mowat AM, Pollard JW, Park BK, Jenkins SJ, Simpson KJ, Hume DA, Wigmore SJ, Forbes SJ. CSF1 Restores Innate Immunity After Liver Injury in Mice and Serum Levels Indicate Outcomes of Patients With Acute Liver Failure. Gastroenterology. 2015;149:1896-1909.e14. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 125] [Cited by in RCA: 158] [Article Influence: 15.8] [Reference Citation Analysis (0)] |
| 374. | Veteläinen R, van Vliet AK, van Gulik TM. Severe steatosis increases hepatocellular injury and impairs liver regeneration in a rat model of partial hepatectomy. Ann Surg. 2007;245:44-50. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 112] [Cited by in RCA: 123] [Article Influence: 6.8] [Reference Citation Analysis (0)] |
| 375. | Truant S, Bouras AF, Petrovai G, Buob D, Ernst O, Boleslawski E, Hebbar M, Pruvot FR. Volumetric gain of the liver after major hepatectomy in obese patients: a case-matched study in 84 patients. Ann Surg. 2013;258:696-702; discussion 702. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 28] [Cited by in RCA: 34] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 376. | Selzner M, Clavien PA. Failure of regeneration of the steatotic rat liver: disruption at two different levels in the regeneration pathway. Hepatology. 2000;31:35-42. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 188] [Cited by in RCA: 191] [Article Influence: 7.6] [Reference Citation Analysis (0)] |
| 377. | Zimmers TA, Jin X, Zhang Z, Jiang Y, Koniaris LG. Epidermal growth factor receptor restoration rescues the fatty liver regeneration in mice. Am J Physiol Endocrinol Metab. 2017;313:E440-E449. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 13] [Cited by in RCA: 18] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 378. | DeAngelis RA, Markiewski MM, Taub R, Lambris JD. A high-fat diet impairs liver regeneration in C57BL/6 mice through overexpression of the NF-kappaB inhibitor, IkappaBalpha. Hepatology. 2005;42:1148-1157. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 106] [Cited by in RCA: 124] [Article Influence: 6.2] [Reference Citation Analysis (0)] |
| 379. | Inaba Y, Furutani T, Kimura K, Watanabe H, Haga S, Kido Y, Matsumoto M, Yamamoto Y, Harada K, Kaneko S, Oyadomari S, Ozaki M, Kasuga M, Inoue H. Growth arrest and DNA damage-inducible 34 regulates liver regeneration in hepatic steatosis in mice. Hepatology. 2015;61:1343-1356. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 33] [Cited by in RCA: 36] [Article Influence: 3.6] [Reference Citation Analysis (0)] |
| 380. | Henderson NC, Forbes SJ. Hepatic fibrogenesis: from within and outwith. Toxicology. 2008;254:130-135. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 45] [Cited by in RCA: 48] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 381. | Kallis YN, Robson AJ, Fallowfield JA, Thomas HC, Alison MR, Wright NA, Goldin RD, Iredale JP, Forbes SJ. Remodelling of extracellular matrix is a requirement for the hepatic progenitor cell response. Gut. 2011;60:525-533. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 86] [Cited by in RCA: 78] [Article Influence: 5.6] [Reference Citation Analysis (0)] |
| 382. | Issa R, Zhou X, Trim N, Millward-Sadler H, Krane S, Benyon C, Iredale J. Mutation in collagen-1 that confers resistance to the action of collagenase results in failure of recovery from CCl4-induced liver fibrosis, persistence of activated hepatic stellate cells, and diminished hepatocyte regeneration. FASEB J. 2003;17:47-49. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 159] [Cited by in RCA: 151] [Article Influence: 6.9] [Reference Citation Analysis (0)] |
| 383. | Marshall A, Rushbrook S, Davies SE, Morris LS, Scott IS, Vowler SL, Coleman N, Alexander G. Relation between hepatocyte G1 arrest, impaired hepatic regeneration, and fibrosis in chronic hepatitis C virus infection. Gastroenterology. 2005;128:33-42. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 143] [Cited by in RCA: 148] [Article Influence: 7.4] [Reference Citation Analysis (0)] |
| 384. | Bierie B, Moses HL. Transforming growth factor beta (TGF-beta) and inflammation in cancer. Cytokine Growth Factor Rev. 2010;21:49-59. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 250] [Cited by in RCA: 270] [Article Influence: 16.9] [Reference Citation Analysis (0)] |
| 385. | Nakamura T, Tomita Y, Hirai R, Yamaoka K, Kaji K, Ichihara A. Inhibitory effect of transforming growth factor-beta on DNA synthesis of adult rat hepatocytes in primary culture. Biochem Biophys Res Commun. 1985;133:1042-1050. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 233] [Cited by in RCA: 226] [Article Influence: 5.7] [Reference Citation Analysis (0)] |
| 386. | Yasuda H, Mine T, Shibata H, Eto Y, Hasegawa Y, Takeuchi T, Asano S, Kojima I. Activin A: an autocrine inhibitor of initiation of DNA synthesis in rat hepatocytes. J Clin Invest. 1993;92:1491-1496. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 141] [Cited by in RCA: 148] [Article Influence: 4.6] [Reference Citation Analysis (0)] |
| 387. | Kogure K, Omata W, Kanzaki M, Zhang YQ, Yasuda H, Mine T, Kojima I. A single intraportal administration of follistatin accelerates liver regeneration in partially hepatectomized rats. Gastroenterology. 1995;108:1136-1142. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 68] [Cited by in RCA: 73] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 388. | Gkretsi V, Bowen WC, Yang Y, Wu C, Michalopoulos GK. Integrin-linked kinase is involved in matrix-induced hepatocyte differentiation. Biochem Biophys Res Commun. 2007;353:638-643. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 29] [Cited by in RCA: 29] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 389. | Rana B, Mischoulon D, Xie Y, Bucher NL, Farmer SR. Cell-extracellular matrix interactions can regulate the switch between growth and differentiation in rat hepatocytes: reciprocal expression of C/EBP alpha and immediate-early growth response transcription factors. Mol Cell Biol. 1994;14:5858-5869. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 160] [Cited by in RCA: 150] [Article Influence: 4.8] [Reference Citation Analysis (0)] |
| 390. | Gkretsi V, Apte U, Mars WM, Bowen WC, Luo JH, Yang Y, Yu YP, Orr A, St-Arnaud R, Dedhar S, Kaestner KH, Wu C, Michalopoulos GK. Liver-specific ablation of integrin-linked kinase in mice results in abnormal histology, enhanced cell proliferation, and hepatomegaly. Hepatology. 2008;48:1932-1941. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 75] [Cited by in RCA: 66] [Article Influence: 3.9] [Reference Citation Analysis (0)] |
| 391. | Apte U, Gkretsi V, Bowen WC, Mars WM, Luo JH, Donthamsetty S, Orr A, Monga SP, Wu C, Michalopoulos GK. Enhanced liver regeneration following changes induced by hepatocyte-specific genetic ablation of integrin-linked kinase. Hepatology. 2009;50:844-851. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 134] [Cited by in RCA: 126] [Article Influence: 7.9] [Reference Citation Analysis (0)] |
| 392. | Song HH, Filmus J. The role of glypicans in mammalian development. Biochim Biophys Acta. 2002;1573:241-246. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 111] [Cited by in RCA: 109] [Article Influence: 4.7] [Reference Citation Analysis (0)] |
| 393. | Liu B, Paranjpe S, Bowen WC, Bell AW, Luo JH, Yu YP, Mars WM, Michalopoulos GK. Investigation of the role of glypican 3 in liver regeneration and hepatocyte proliferation. Am J Pathol. 2009;175:717-724. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 48] [Cited by in RCA: 49] [Article Influence: 3.1] [Reference Citation Analysis (0)] |
| 394. | Cano-Gauci DF, Song HH, Yang H, McKerlie C, Choo B, Shi W, Pullano R, Piscione TD, Grisaru S, Soon S, Sedlackova L, Tanswell AK, Mak TW, Yeger H, Lockwood GA, Rosenblum ND, Filmus J. Glypican-3-deficient mice exhibit developmental overgrowth and some of the abnormalities typical of Simpson-Golabi-Behmel syndrome. J Cell Biol. 1999;146:255-264. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 151] [Cited by in RCA: 198] [Article Influence: 7.6] [Reference Citation Analysis (0)] |
| 395. | Wang ND, Finegold MJ, Bradley A, Ou CN, Abdelsayed SV, Wilde MD, Taylor LR, Wilson DR, Darlington GJ. Impaired energy homeostasis in C/EBP alpha knockout mice. Science. 1995;269:1108-1112. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 734] [Cited by in RCA: 740] [Article Influence: 24.7] [Reference Citation Analysis (0)] |
| 396. | Jin J, Hong IH, Lewis K, Iakova P, Breaux M, Jiang Y, Sullivan E, Jawanmardi N, Timchenko L, Timchenko NA. Cooperation of C/EBP family proteins and chromatin remodeling proteins is essential for termination of liver regeneration. Hepatology. 2015;61:315-325. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 46] [Cited by in RCA: 44] [Article Influence: 4.4] [Reference Citation Analysis (0)] |
| 397. | Geng Y, Yu Q, Sicinska E, Das M, Schneider JE, Bhattacharya S, Rideout WM, Bronson RT, Gardner H, Sicinski P. Cyclin E ablation in the mouse. Cell. 2003;114:431-443. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 495] [Cited by in RCA: 497] [Article Influence: 22.6] [Reference Citation Analysis (0)] |
| 398. | Hu W, Nevzorova YA, Haas U, Moro N, Sicinski P, Geng Y, Barbacid M, Trautwein C, Liedtke C. Concurrent deletion of cyclin E1 and cyclin-dependent kinase 2 in hepatocytes inhibits DNA replication and liver regeneration in mice. Hepatology. 2014;59:651-660. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 34] [Cited by in RCA: 41] [Article Influence: 3.7] [Reference Citation Analysis (0)] |
| 399. | Lauper N, Beck AR, Cariou S, Richman L, Hofmann K, Reith W, Slingerland JM, Amati B. Cyclin E2: a novel CDK2 partner in the late G1 and S phases of the mammalian cell cycle. Oncogene. 1998;17:2637-2643. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 108] [Cited by in RCA: 112] [Article Influence: 4.1] [Reference Citation Analysis (0)] |
| 400. | Nevzorova YA, Tschaharganeh D, Gassler N, Geng Y, Weiskirchen R, Sicinski P, Trautwein C, Liedtke C. Aberrant cell cycle progression and endoreplication in regenerating livers of mice that lack a single E-type cyclin. Gastroenterology. 2009;137:691-703, 703.e1. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 53] [Cited by in RCA: 52] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 401. | Dong J, Feldmann G, Huang J, Wu S, Zhang N, Comerford SA, Gayyed MF, Anders RA, Maitra A, Pan D. Elucidation of a universal size-control mechanism in Drosophila and mammals. Cell. 2007;130:1120-1133. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 2037] [Cited by in RCA: 1931] [Article Influence: 107.3] [Reference Citation Analysis (0)] |
| 402. | Avruch J, Zhou D, Fitamant J, Bardeesy N. Mst1/2 signalling to Yap: gatekeeper for liver size and tumour development. Br J Cancer. 2011;104:24-32. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 83] [Cited by in RCA: 92] [Article Influence: 6.1] [Reference Citation Analysis (0)] |
| 403. | Bartel DP. MicroRNAs: target recognition and regulatory functions. Cell. 2009;136:215-233. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 14460] [Cited by in RCA: 16076] [Article Influence: 1004.8] [Reference Citation Analysis (2)] |
| 404. | Yuan B, Dong R, Shi D, Zhou Y, Zhao Y, Miao M, Jiao B. Down-regulation of miR-23b may contribute to activation of the TGF-β1/Smad3 signalling pathway during the termination stage of liver regeneration. FEBS Lett. 2011;585:927-934. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 67] [Cited by in RCA: 74] [Article Influence: 5.3] [Reference Citation Analysis (0)] |
| 405. | Chen H, Sun Y, Dong R, Yang S, Pan C, Xiang D, Miao M, Jiao B. Mir-34a is upregulated during liver regeneration in rats and is associated with the suppression of hepatocyte proliferation. PLoS One. 2011;6:e20238. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 59] [Cited by in RCA: 62] [Article Influence: 4.4] [Reference Citation Analysis (0)] |
| 406. | Martin J, Petrillo A, Smyth EC, Shaida N, Khwaja S, Cheow HK, Duckworth A, Heister P, Praseedom R, Jah A, Balakrishnan A, Harper S, Liau S, Kosmoliaptsis V, Huguet E. Colorectal liver metastases: Current management and future perspectives. World J Clin Oncol. 2020;11:761-808. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 111] [Cited by in RCA: 147] [Article Influence: 29.4] [Reference Citation Analysis (6)] |
| 407. | Gangi A, Lu SC. Chemotherapy-associated liver injury in colorectal cancer. Therap Adv Gastroenterol. 2020;13:1756284820924194. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 13] [Cited by in RCA: 33] [Article Influence: 6.6] [Reference Citation Analysis (0)] |
| 408. | Pawlik TM, Olino K, Gleisner AL, Torbenson M, Schulick R, Choti MA. Preoperative chemotherapy for colorectal liver metastases: impact on hepatic histology and postoperative outcome. J Gastrointest Surg. 2007;11:860-868. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 186] [Cited by in RCA: 179] [Article Influence: 9.9] [Reference Citation Analysis (0)] |
| 409. | Zeng J, Deng S, Wang Y, Li P, Tang L, Pang Y. Specific Inhibition of Acyl-CoA Oxidase-1 by an Acetylenic Acid Improves Hepatic Lipid and Reactive Oxygen Species (ROS) Metabolism in Rats Fed a High Fat Diet. J Biol Chem. 2017;292:3800-3809. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 55] [Cited by in RCA: 86] [Article Influence: 10.8] [Reference Citation Analysis (0)] |
| 410. | Sommer J, Mahli A, Freese K, Schiergens TS, Kuecuekoktay FS, Teufel A, Thasler WE, Müller M, Bosserhoff AK, Hellerbrand C. Analysis of molecular mechanisms of 5-fluorouracil-induced steatosis and inflammation in vitro and in mice. Oncotarget. 2017;8:13059-13072. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 20] [Cited by in RCA: 37] [Article Influence: 4.6] [Reference Citation Analysis (0)] |
| 411. | Vauthey JN, Pawlik TM, Ribero D, Wu TT, Zorzi D, Hoff PM, Xiong HQ, Eng C, Lauwers GY, Mino-Kenudson M, Risio M, Muratore A, Capussotti L, Curley SA, Abdalla EK. Chemotherapy regimen predicts steatohepatitis and an increase in 90-day mortality after surgery for hepatic colorectal metastases. J Clin Oncol. 2006;24:2065-2072. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 970] [Cited by in RCA: 953] [Article Influence: 50.2] [Reference Citation Analysis (0)] |
| 412. | Mahli A, Saugspier M, Koch A, Sommer J, Dietrich P, Lee S, Thasler R, Schulze-Luehrmann J, Luehrmann A, Thasler WE, Müller M, Bosserhoff A, Hellerbrand C. ERK activation and autophagy impairment are central mediators of irinotecan-induced steatohepatitis. Gut. 2018;67:746-756. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 11] [Cited by in RCA: 22] [Article Influence: 3.1] [Reference Citation Analysis (0)] |
| 413. | Aloia T, Sebagh M, Plasse M, Karam V, Lévi F, Giacchetti S, Azoulay D, Bismuth H, Castaing D, Adam R. Liver histology and surgical outcomes after preoperative chemotherapy with fluorouracil plus oxaliplatin in colorectal cancer liver metastases. J Clin Oncol. 2006;24:4983-4990. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 395] [Cited by in RCA: 392] [Article Influence: 20.6] [Reference Citation Analysis (0)] |
| 414. | de Gramont A, Figer A, Seymour M, Homerin M, Hmissi A, Cassidy J, Boni C, Cortes-Funes H, Cervantes A, Freyer G, Papamichael D, Le Bail N, Louvet C, Hendler D, de Braud F, Wilson C, Morvan F, Bonetti A. Leucovorin and fluorouracil with or without oxaliplatin as first-line treatment in advanced colorectal cancer. J Clin Oncol. 2000;18:2938-2947. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2950] [Cited by in RCA: 2825] [Article Influence: 113.0] [Reference Citation Analysis (1)] |
| 415. | Douillard JY, Cunningham D, Roth AD, Navarro M, James RD, Karasek P, Jandik P, Iveson T, Carmichael J, Alakl M, Gruia G, Awad L, Rougier P. Irinotecan combined with fluorouracil compared with fluorouracil alone as first-line treatment for metastatic colorectal cancer: a multicentre randomised trial. Lancet. 2000;355:1041-1047. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2407] [Cited by in RCA: 2381] [Article Influence: 95.2] [Reference Citation Analysis (1)] |
| 416. | Petrelli F, Barni S; Anti-EGFR agents for liver metastases. Resectability and outcome with anti-EGFR agents in patients with KRAS wild-type colorectal liver-limited metastases: a meta-analysis. Int J Colorectal Dis. 2012;27:997-1004. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 49] [Cited by in RCA: 50] [Article Influence: 3.8] [Reference Citation Analysis (1)] |
| 417. | Gruenberger T, Bridgewater J, Chau I, García Alfonso P, Rivoire M, Mudan S, Lasserre S, Hermann F, Waterkamp D, Adam R. Bevacizumab plus mFOLFOX-6 or FOLFOXIRI in patients with initially unresectable liver metastases from colorectal cancer: the OLIVIA multinational randomised phase II trial. Ann Oncol. 2015;26:702-708. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 196] [Cited by in RCA: 250] [Article Influence: 22.7] [Reference Citation Analysis (0)] |
| 418. | Bridgewater JA, Pugh SA, Maishman T, Eminton Z, Mellor J, Whitehead A, Stanton L, Radford M, Corkhill A, Griffiths GO, Falk S, Valle JW, O'Reilly D, Siriwardena AK, Hornbuckle J, Rees M, Iveson TJ, Hickish T, Garden OJ, Cunningham D, Maughan TS, Primrose JN; New EPOC investigators. Systemic chemotherapy with or without cetuximab in patients with resectable colorectal liver metastasis (New EPOC): long-term results of a multicentre, randomised, controlled, phase 3 trial. Lancet Oncol. 2020;21:398-411. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 138] [Cited by in RCA: 160] [Article Influence: 32.0] [Reference Citation Analysis (0)] |
| 419. | Karoui M, Penna C, Amin-Hashem M, Mitry E, Benoist S, Franc B, Rougier P, Nordlinger B. Influence of preoperative chemotherapy on the risk of major hepatectomy for colorectal liver metastases. Ann Surg. 2006;243:1-7. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 539] [Cited by in RCA: 519] [Article Influence: 27.3] [Reference Citation Analysis (0)] |
| 420. | Nguyen-Khac E, Lobry C, Chatelain D, Fuks D, Joly JP, Brevet M, Tramier B, Mouly C, Hautefeuille V, Chauffert B, Regimbeau JM. A Reappraisal of Chemotherapy-Induced Liver Injury in Colorectal Liver Metastases before the Era of Antiangiogenics. Int J Hepatol. 2013;2013:314868. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 15] [Cited by in RCA: 13] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 421. | Vigano L, De Rosa G, Toso C, Andres A, Ferrero A, Roth A, Sperti E, Majno P, Rubbia-Brandt L. Reversibility of chemotherapy-related liver injury. J Hepatol. 2017;67:84-91. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 70] [Cited by in RCA: 66] [Article Influence: 8.3] [Reference Citation Analysis (0)] |
| 422. | Ribero D, Wang H, Donadon M, Zorzi D, Thomas MB, Eng C, Chang DZ, Curley SA, Abdalla EK, Ellis LM, Vauthey JN. Bevacizumab improves pathologic response and protects against hepatic injury in patients treated with oxaliplatin-based chemotherapy for colorectal liver metastases. Cancer. 2007;110:2761-2767. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 297] [Cited by in RCA: 280] [Article Influence: 15.6] [Reference Citation Analysis (0)] |
| 423. | Santini D, Vincenzi B, Massacesi C, Picardi A, Gentilucci UV, Esposito V, Liuzzi G, La Cesa A, Rocci L, Marcucci F, Montesarchio V, Groeger AM, Bonsignori M, Tonini G. S-adenosylmethionine (AdoMet) supplementation for treatment of chemotherapy-induced liver injury. Anticancer Res. 2003;23:5173-5179. [PubMed] |
| 424. | Vincenzi B, Daniele S, Frezza AM, Berti P, Vespasiani U, Picardi A, Tonini G. The role of S-adenosylmethionine in preventing oxaliplatin-induced liver toxicity: a retrospective analysis in metastatic colorectal cancer patients treated with bevacizumab plus oxaliplatin-based regimen. Support Care Cancer. 2012;20:135-139. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 23] [Cited by in RCA: 17] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 425. | Makuuchi M, Thai BL, Takayasu K, Takayama T, Kosuge T, Gunvén P, Yamazaki S, Hasegawa H, Ozaki H. Preoperative portal embolization to increase safety of major hepatectomy for hilar bile duct carcinoma: a preliminary report. Surgery. 1990;107:521-527. |
| 426. | van Lienden KP, van den Esschert JW, de Graaf W, Bipat S, Lameris JS, van Gulik TM, van Delden OM. Portal vein embolization before liver resection: a systematic review. Cardiovasc Intervent Radiol. 2013;36:25-34. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 345] [Cited by in RCA: 333] [Article Influence: 27.8] [Reference Citation Analysis (0)] |
| 427. | Schnitzbauer AA, Lang SA, Goessmann H, Nadalin S, Baumgart J, Farkas SA, Fichtner-Feigl S, Lorf T, Goralcyk A, Hörbelt R, Kroemer A, Loss M, Rümmele P, Scherer MN, Padberg W, Königsrainer A, Lang H, Obed A, Schlitt HJ. Right portal vein ligation combined with in situ splitting induces rapid left lateral liver lobe hypertrophy enabling 2-staged extended right hepatic resection in small-for-size settings. Ann Surg. 2012;255:405-414. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 837] [Cited by in RCA: 932] [Article Influence: 71.7] [Reference Citation Analysis (0)] |
| 428. | Wigmore SJ. ALPPS: The argument against. Eur J Surg Oncol. 2017;43:249-251. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5] [Cited by in RCA: 6] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 429. | Veteläinen R, van Vliet A, Gouma DJ, van Gulik TM. Steatosis as a risk factor in liver surgery. Ann Surg. 2007;245:20-30. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 215] [Cited by in RCA: 226] [Article Influence: 12.6] [Reference Citation Analysis (0)] |
| 430. | Behrns KE, Tsiotos GG, DeSouza NF, Krishna MK, Ludwig J, Nagorney DM. Hepatic steatosis as a potential risk factor for major hepatic resection. J Gastrointest Surg. 1998;2:292-298. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 306] [Cited by in RCA: 306] [Article Influence: 11.3] [Reference Citation Analysis (0)] |
| 431. | Kooby DA, Fong Y, Suriawinata A, Gonen M, Allen PJ, Klimstra DS, DeMatteo RP, D'Angelica M, Blumgart LH, Jarnagin WR. Impact of steatosis on perioperative outcome following hepatic resection. J Gastrointest Surg. 2003;7:1034-1044. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 341] [Cited by in RCA: 317] [Article Influence: 14.4] [Reference Citation Analysis (0)] |
| 432. | Bhangui P, Laurent A, Amathieu R, Azoulay D. Assessment of risk for non-hepatic surgery in cirrhotic patients. J Hepatol. 2012;57:874-884. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 89] [Cited by in RCA: 80] [Article Influence: 6.2] [Reference Citation Analysis (0)] |
| 433. | Berzigotti A, Reig M, Abraldes JG, Bosch J, Bruix J. Portal hypertension and the outcome of surgery for hepatocellular carcinoma in compensated cirrhosis: a systematic review and meta-analysis. Hepatology. 2015;61:526-536. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 219] [Cited by in RCA: 285] [Article Influence: 28.5] [Reference Citation Analysis (0)] |
| 434. | Selzner M, Rüdiger HA, Sindram D, Madden J, Clavien PA. Mechanisms of ischemic injury are different in the steatotic and normal rat liver. Hepatology. 2000;32:1280-1288. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 218] [Cited by in RCA: 213] [Article Influence: 8.5] [Reference Citation Analysis (0)] |
| 435. | Serafín A, Roselló-Catafau J, Prats N, Xaus C, Gelpí E, Peralta C. Ischemic preconditioning increases the tolerance of Fatty liver to hepatic ischemia-reperfusion injury in the rat. Am J Pathol. 2002;161:587-601. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 163] [Cited by in RCA: 168] [Article Influence: 7.3] [Reference Citation Analysis (0)] |
| 436. | Clavien PA, Harvey PR, Strasberg SM. Preservation and reperfusion injuries in liver allografts. An overview and synthesis of current studies. Transplantation. 1992;53:957-978. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 639] [Cited by in RCA: 614] [Article Influence: 18.6] [Reference Citation Analysis (0)] |
| 437. | Clavien PA, Selzner M, Rüdiger HA, Graf R, Kadry Z, Rousson V, Jochum W. A prospective randomized study in 100 consecutive patients undergoing major liver resection with vs without ischemic preconditioning. Ann Surg. 2003;238:843-50; discussion 851. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 352] [Cited by in RCA: 370] [Article Influence: 16.8] [Reference Citation Analysis (0)] |
| 438. | Hemming AW, Magliocca JF, Fujita S, Kayler LK, Hochwald S, Zendejas I, Kim RD. Combined resection of the liver and pancreas for malignancy. J Am Coll Surg. 2010;210:808-814, 814. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 41] [Cited by in RCA: 41] [Article Influence: 2.7] [Reference Citation Analysis (0)] |
| 439. | Klein F, Puhl G, Guckelberger O, Pelzer U, Pullankavumkal JR, Guel S, Neuhaus P, Bahra M. The impact of simultaneous liver resection for occult liver metastases of pancreatic adenocarcinoma. Gastroenterol Res Pract. 2012;2012:939350. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 39] [Cited by in RCA: 57] [Article Influence: 4.4] [Reference Citation Analysis (0)] |
| 440. | Singh A, Singh T, Chaudhary A. Synchronous resection of solitary liver metastases with pancreaticoduodenectomy. JOP. 2010;11:434-438. [PubMed] |
| 441. | Otao R, Beppu T, Isiko T, Mima K, Okabe H, Hayashi H, Masuda T, Chikamoto A, Takamori H, Baba H. External biliary drainage and liver regeneration after major hepatectomy. Br J Surg. 2012;99:1569-1574. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 42] [Cited by in RCA: 56] [Article Influence: 4.3] [Reference Citation Analysis (0)] |
| 442. | Hayashi H, Beppu T, Sugita H, Horino K, Komori H, Masuda T, Okabe H, Takamori H, Baba H. Increase in the serum bile acid level predicts the effective hypertrophy of the nonembolized hepatic lobe after right portal vein embolization. World J Surg. 2009;33:1933-1940. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 14] [Cited by in RCA: 15] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 443. | Rahbari NN, Garden OJ, Padbury R, Brooke-Smith M, Crawford M, Adam R, Koch M, Makuuchi M, Dematteo RP, Christophi C, Banting S, Usatoff V, Nagino M, Maddern G, Hugh TJ, Vauthey JN, Greig P, Rees M, Yokoyama Y, Fan ST, Nimura Y, Figueras J, Capussotti L, Büchler MW, Weitz J. Posthepatectomy liver failure: a definition and grading by the International Study Group of Liver Surgery (ISGLS). Surgery. 2011;149:713-724. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1224] [Cited by in RCA: 1724] [Article Influence: 123.1] [Reference Citation Analysis (0)] |
| 444. | Riddiough GE, Christophi C, Jones RM, Muralidharan V, Perini MV. A systematic review of small for size syndrome after major hepatectomy and liver transplantation. HPB (Oxford). 2020;22:487-496. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 37] [Cited by in RCA: 33] [Article Influence: 6.6] [Reference Citation Analysis (0)] |
| 445. | Fishbane S, El-Shahawy MA, Pecoits-Filho R, Van BP, Houser MT, Frison L, Little DJ, Guzman NJ, Pergola PE. Roxadustat for Treating Anemia in Patients with CKD Not on Dialysis: Results from a Randomized Phase 3 Study. J Am Soc Nephrol. 2021;32:737-755. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 59] [Cited by in RCA: 116] [Article Influence: 29.0] [Reference Citation Analysis (0)] |
| 446. | Roskams T, Cassiman D, De Vos R, Libbrecht L. Neuroregulation of the neuroendocrine compartment of the liver. Anat Rec A Discov Mol Cell Evol Biol. 2004;280:910-923. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 63] [Cited by in RCA: 64] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 447. | Kiba T. The role of the autonomic nervous system in liver regeneration and apoptosis--recent developments. Digestion. 2002;66:79-88. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 38] [Cited by in RCA: 45] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 448. | Takahashi T, Kakita A, Sakamoto I, Takahashi Y, Hayashi K, Tadokoro F, Yamashina S. Immunohistochemical and electron microscopic study of extrinsic hepatic reinnervation following orthotopic liver transplantation in rats. Liver. 2001;21:300-308. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 14] [Cited by in RCA: 15] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 449. | Golriz M, Ghamarnejad O, Khajeh E, Sabagh M, Mieth M, Hoffmann K, Ulrich A, Hackert T, Weiss KH, Schirmacher P, Büchler MW, Mehrabi A. Preoperative Thrombocytopenia May Predict Poor Surgical Outcome after Extended Hepatectomy. Can J Gastroenterol Hepatol. 2018;2018:1275720. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 10] [Cited by in RCA: 14] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 450. | MacIndoe JH, Turkington RW. Stimulation of human prolactin secretion by intravenous infusion of L-tryptophan. J Clin Invest. 1973;52:1972-1978. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 138] [Cited by in RCA: 114] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 451. | Banki CM, Vojnik M. Effect of intravenous infusion of amitriptyline on total blood serotonin content. Eur J Clin Pharmacol. 1978;13:259-262. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5] [Cited by in RCA: 5] [Article Influence: 0.1] [Reference Citation Analysis (0)] |
| 452. | Bird TG, Lu WY, Boulter L, Gordon-Keylock S, Ridgway RA, Williams MJ, Taube J, Thomas JA, Wojtacha D, Gambardella A, Sansom OJ, Iredale JP, Forbes SJ. Bone marrow injection stimulates hepatic ductular reactions in the absence of injury via macrophage-mediated TWEAK signaling. Proc Natl Acad Sci USA. 2013;110:6542-6547. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 131] [Cited by in RCA: 138] [Article Influence: 11.5] [Reference Citation Analysis (0)] |
| 453. | Thomas JA, Pope C, Wojtacha D, Robson AJ, Gordon-Walker TT, Hartland S, Ramachandran P, Van Deemter M, Hume DA, Iredale JP, Forbes SJ. Macrophage therapy for murine liver fibrosis recruits host effector cells improving fibrosis, regeneration, and function. Hepatology. 2011;53:2003-2015. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 297] [Cited by in RCA: 281] [Article Influence: 20.1] [Reference Citation Analysis (0)] |
| 454. | Hume DA, MacDonald KP. Therapeutic applications of macrophage colony-stimulating factor-1 (CSF-1) and antagonists of CSF-1 receptor (CSF-1R) signaling. Blood. 2012;119:1810-1820. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 433] [Cited by in RCA: 567] [Article Influence: 40.5] [Reference Citation Analysis (0)] |
| 455. | Cataldegirmen G, Zeng S, Feirt N, Ippagunta N, Dun H, Qu W, Lu Y, Rong LL, Hofmann MA, Kislinger T, Pachydaki SI, Jenkins DG, Weinberg A, Lefkowitch J, Rogiers X, Yan SF, Schmidt AM, Emond JC. RAGE limits regeneration after massive liver injury by coordinated suppression of TNF-alpha and NF-kappaB. J Exp Med. 2005;201:473-484. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 108] [Cited by in RCA: 117] [Article Influence: 5.9] [Reference Citation Analysis (0)] |
| 456. | Mangieri CW, McCartt JC, Strode MA, Lowry JE, Balakrishna PM. Perioperative hepatocyte growth factor (HGF) infusions improve hepatic regeneration following portal branch ligation (PBL) in rodents. Surg Endosc. 2017;31:2789-2797. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 11] [Cited by in RCA: 9] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 457. | Alwahsh SM, Rashidi H, Hay DC. Liver cell therapy: is this the end of the beginning? Cell Mol Life Sci. 2018;75:1307-1324. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 54] [Cited by in RCA: 46] [Article Influence: 6.6] [Reference Citation Analysis (0)] |
| 458. | Muraca M, Gerunda G, Neri D, Vilei MT, Granato A, Feltracco P, Meroni M, Giron G, Burlina AB. Hepatocyte transplantation as a treatment for glycogen storage disease type 1a. Lancet. 2002;359:317-318. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 314] [Cited by in RCA: 271] [Article Influence: 11.8] [Reference Citation Analysis (0)] |
| 459. | Liu H, Kim Y, Sharkis S, Marchionni L, Jang YY. In vivo liver regeneration potential of human induced pluripotent stem cells from diverse origins. Sci Transl Med. 2011;3:82ra39. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 181] [Cited by in RCA: 168] [Article Influence: 12.0] [Reference Citation Analysis (0)] |
| 460. | Liu WH, Song FQ, Ren LN, Guo WQ, Wang T, Feng YX, Tang LJ, Li K. The multiple functional roles of mesenchymal stem cells in participating in treating liver diseases. J Cell Mol Med. 2015;19:511-520. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 100] [Cited by in RCA: 106] [Article Influence: 10.6] [Reference Citation Analysis (0)] |
| 461. | Lee SC, Jeong HJ, Lee SK, Kim SJ. Lipopolysaccharide preconditioning of adipose-derived stem cells improves liver-regenerating activity of the secretome. Stem Cell Res Ther. 2015;6:75. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 69] [Cited by in RCA: 64] [Article Influence: 6.4] [Reference Citation Analysis (0)] |
| 462. | Lee SC, Kim JO, Kim SJ. Secretome from human adipose-derived stem cells protects mouse liver from hepatic ischemia-reperfusion injury. Surgery. 2015;157:934-943. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 26] [Cited by in RCA: 33] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 463. | Lee SK, Lee SC, Kim SJ. A novel cell-free strategy for promoting mouse liver regeneration: utilization of a conditioned medium from adipose-derived stem cells. Hepatol Int. 2015;9:310-320. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 29] [Cited by in RCA: 33] [Article Influence: 3.0] [Reference Citation Analysis (0)] |