Published online Nov 24, 2021. doi: 10.5306/wjco.v12.i11.966
Peer-review started: March 2, 2021
First decision: July 27, 2021
Revised: August 2, 2021
Accepted: November 5, 2021
Article in press: November 5, 2021
Published online: November 24, 2021
Processing time: 265 Days and 23.8 Hours
Different subpopulations of monocytes and dendritic cells (DCs) may have a key impact on the modulation of the immune response in malignancy. In this review, we summarize the monocyte and DCs heterogeneity and their function in the context of modulating the immune response in cancer. Subgroups of monocytes may play opposing roles in cancer, depending on the tumour growth and progression as well as the type of cancer. Monocytes can have pro-tumour and anti-tumour functions and can also differentiate into monocyte-derived DCs (moDCs). MoDCs have a similar antigen presentation ability as classical DCs, including cross-priming, a process by which DCs activate CD8 T-cells by cross-presenting exogenous antigens. DCs play a critical role in generating anti-tumour CD8 T-cell immunity. DCs have plastic characteristics and show distinct phenotypes depending on their mature state and depending on the influence of the tumour microenvironment. MoDCs and other DC subsets have been attracting increased interest owing to their possible beneficial effects in cancer immunotherapy. This review also highlights key strategies deploying specific DC subpopulations in combination with other therapies to enhance the anti-tumour response and summarizes the latest ongoing and completed clinical trials using DCs in lung cancer.
Core Tip: Monocytes and dendritic cells (DCs) as heterogeneous subpopulations may play a key role in the modulation of the immune response in malignant tumours. Monocytes may have a pro- and anti-tumour function and may differentiate into monocyte-derived DC. DCs have the properties of antigen presenting cells. These cells show a different phenotype depending on their maturity and on the influence of the tumour microenvironment. The DCs are of growing interest for their possible beneficial effects in lung cancer immunotherapy. This review highlights specific DC subpopulations in the anti-tumour response and summarizes the latest ongoing DC clinical trials in lung cancer.
- Citation: Kwiecień I, Rutkowska E, Raniszewska A, Rzepecki P, Domagała-Kulawik J. Modulation of the immune response by heterogeneous monocytes and dendritic cells in lung cancer. World J Clin Oncol 2021; 12(11): 966-982
- URL: https://www.wjgnet.com/2218-4333/full/v12/i11/966.htm
- DOI: https://dx.doi.org/10.5306/wjco.v12.i11.966
Lung cancer is responsible for approximately 1.8 million deaths annually worldwide and is now one of the most common cancers and the leading cause of cancer mortality[1,2]. The prognosis for lung cancer remains poor despite advances in treatment strategies including immunotherapy with immune check inhibitors (ICIs)[3,4]. Further investigation of tumour immunology and the different cells subpopulations influencing the anti-tumour immune response could enable the development of novel immunomodulatory strategies such as targeted monoclonal antibodies against specific cell receptors.
The results of research on lung cancer show that cells of the same type can have both pro-cancer and anti-cancer properties. Tumour heterogeneity drives a diverse and plastic spectrum of various subpopulations of non-cancer cells. In this review, we focus on assessing different subpopulations of monocytes and dendritic cells (DCs) that may have a key impact on the modulation of immune response in lung cancer.
The role of macrophages, mainly of tumour associated macrophages (TAM) is well recognized. However, the place of monocytes in the anticancer immune response is not fully understoood. We previously presented the results of investigation of macrophages in the direct lung cancer milieu[5] and preliminary studies on monocytes maturation in lung cancer[6]. Monocytes have both pro-inflammatory and anti-inflammatory properties. A phenotype of monocytes can be divided into classical (pro-inflammatory) and non-classical (anti-inflammatory). Both monocyte subpopulations have been detected among the peripheral blood (PB) mononuclear cells and may differentiate into macrophages. Studies demonstrate that monocytes are capable of both inhibition and stimulation of tumour growth[7]. Previous research on monocytes shows that their function in different cancer microenvironments may vary[8,9].
DCs form another heterogeneous population with the most efficient function of antigen presenting cells (APCs)[10]. They take up antigens and pathogens, generate major histocompatibility complex (MHC) peptide complexes, migrate from antigen acquisition sites to secondary lymphoid organs and finally interact with T lymphocytes. DCs infiltrate a tumour, next they process it and then they present tumour-derived antigens to naïve T-cells. DCs play a critical role in priming anti-tumour T-cell immunity and thereby represent a possible therapeutic target for cancer immunotherapy[11].
Moreover, various cell types and factors within the tumour microenvironment (TME) can act on monocytes and DCs, control their differentiation, and affect their biology, function and longevity. The local TME can also influence the activation and the direction of maturation of monocytes and DCs. Specific local microenvironmental factors may influence the formation of monocytes and DCs with tolerogenic or immunosuppressive activity and conversely, specific subpopulations of these cells can stimulate and inhibit the anti-tumour response.
In this review, we summarize the ongoing investigations on monocyte and DCs heterogeneity and function in the context of modulation of the immune response in lung cancer and highlight key strategies using specific monocyte subpopulations and DCs to improve cancer therapies.
We discuss the heterogeneity of monocytes, their relationship with DCs and the potential of monocyte-derived DCs (moDCs) in the design of vaccines against lung cancer.
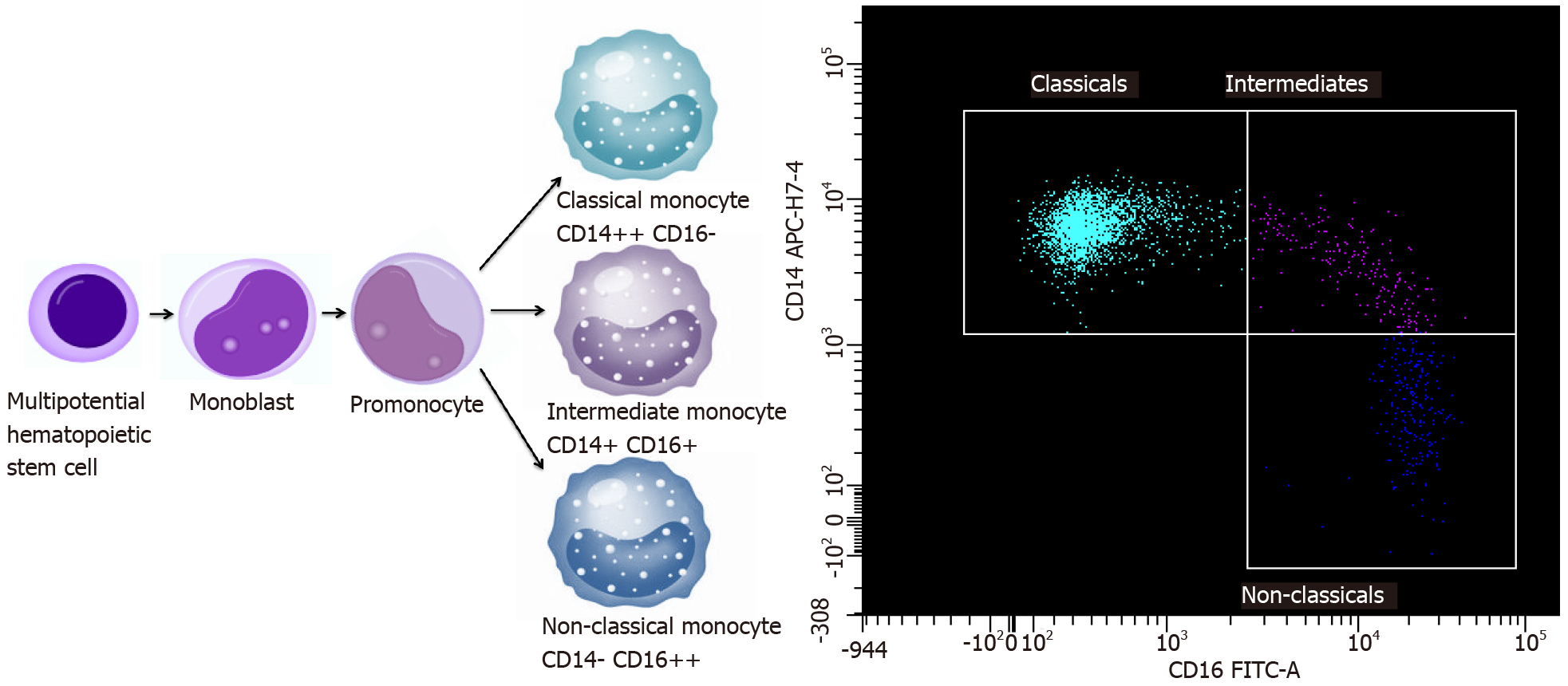
Monocytes are mononuclear immune cells that circulate in PB and direct to tissues at a steady state and at an increased rate during inflammation. Apart from their key role in supporting tissue homeostasis and promoting the immune response to pathogens monocytes take part as regulators of cancer development and progression[12]. As a heterogeneous population, monocytes play opposing roles in inhibiting and stimulating tumour growth and metastases. Monocytes are also precursors of TAM and DCs which are involved in shaping the TME[13]. Monocyte subpopulations perform functions that are involved in both pro- and anti-tumour immunity, including promoting angiogenesis, tumour mediators secretion, phagocytosis, remodelling of the extracellular matrix, influencing lymphocytes, and differentiating into TAM and DCs[14]. Human monocytes express the MHC-II receptor Human Leukocyte Antigen–DR isotype (HLA-DR), integrin αM (CD11b) and CD86. Recent studies demonstrate that monocytes can be divided into three subsets based on the specific surface markers[15,16]. They develop from the lineage-associated bone marrow (BM) precursor, a common monocyte progenitor (cMoP)[17]. cMoPs are monocyte progenitors that express stem cell marker CD117, C-type lectin CLEC12A, CD64 and CD135, a cytokine receptor and an early hematopoietic marker. cMoP may differentiate into classical monocytes and then convert to non-classical monocytes in the blood, with intermediate monocytes at a transition state[18,19].
These cells perform specific functions: Classical (approximately 85%), intermediate (approximately 5%) and non-classical (approximately 10% of the monocyte population), which are characterized by the degree of CD14 and the expression of CD16[20,21]. There are three types of monocytes in PB: Classical monocytes with high the expression of CD14 cell surface receptor and no CD16 expression (CD14++ CD16-), non-classical monocytes with the low/negative level of CD14 expression and the co-expression of CD16 receptor (CD14- CD16++) and intermediate monocytes with the expression of CD14 and the expression of CD16 (CD14+ CD16+)[22,23]. The majority of non-classical monocytes appears to be derived from classical monocytes. However, the current studies show that there may be a limited progenitor lineage capable of differentiating into non-classical monocytes without classical monocyte origin[19,24,25]. After differentiation, classical monocytes exit the BM using C-C chemokine receptor type 2 (CCR2) and next migrate into tissues and lymph nodes by l-selectin (CD62L)[26,27]. Monocyte maturation and a scheme of monocyte subpopulations are presented in Figure 1. Classical monocytes and nonclassical monocytes have a different half-life in the circulation: For classical monocytes it is less than 1 d and for nonclassical monocytes it is 7 d[28]. The mechanisms involved in the recruitment of tissue-specific monocytes remain unclear, possibly they depend on the environmental and tissue availability during both homeostasis and inflammation. However, it is known that classical monocytes are more quickly targeted at the site of inflammation and are able to attract other immune cells by secreting cytokines[26,29]. Non-classical monocytes remain in a state of homeostasis mainly in the vascular system and are likely to be able to exit vessels at a slower rate than classical monocytes during inflammation. They are likely to shift into alternative TAMs and exhibit anti-inflammatory properties[28,30].
Different subgroups of monocytes may play various roles in cancer, depending on the tumour growth and progression, differences in the type of cancer, and depending on the influence of TME[14]. Both classical and non-classical monocytes can have pro-tumour and anti-tumour functions. The protumoural phenotype properties consist of: Differentiation into pro-tumoural TAMs, metastatic cell seeding, the suppression of T-cell function, the recruitment of T regulatory cells (Tregs), the promotion of angiogenesis and contribution to extracellular matrix remodelling (ECM)[31,32]. Classical monocytes exit the vasculature to the primary tumour sites using CC-chemokine ligand 2 (CCL2). They produce carcinogenic mediators and are reprogrammed in the TME to limit their cytotoxicity[32]. Then, they differentiate into TAMs or moDCs in the tumour. TAMs are involved in promoting immunosuppression by inhibiting the activity of CD8 T-cells and in stimulating the formation of Tregs[33]. Moreover, they participate in remodelling of ECM and promote angiogenesis[34]. They have a similar protumoural effect at the metastatic sitesand are capable of promoting the spread of metastases. The number of protumoural signals at the tumour site and metastatic sites leads to the predominance of the anti-tumour response from the host's immune system. On the other hand, monocytes have a number of antitumoural functions such as: Antigen presentation, tumour cytotoxicity, the recruitment of natural killer cells, the inhibition of Tregs, the prevention of metastasis[35].
Long-lived non-classical monocytes are well adapted to the removal of cancer cells and debris. Non-classical monocytes migrate towards the sites of cancer spread where they engulf tumour material and produce cytokines that regulate the anti-tumour immunity[36,37]. This population of monocytes could control metastatic process[37]. The third population is a subset of intermediate monocytes, the function of which is under investigation. However, it is known that the relationship between non-classical and intermediate subsets is close[38,39]. The exact maturation and functional relationship between the individual blood monocyte subpopulations and their tissue distribution profiles have yet to be discovered[40,41]. The results of current research confirm that each subpopulation may play a different role depending on the homeostatic and pathological conditions[16]. Infections, inflammation as well as malignant disease can lead to sudden monopoiesis and the formation of a new subset of monocytes with altered functions[35,42].
Monocytes can differentiate into moDCs. MoDCs have a similar antigen presenting ability as classical DCs, including cross-presentation. Blood monocytes can be a reservoir of DC in response to inflammation[43].
Understanding DCs heterogeneity and their role in modulating the immune response in cancer is critical to the better recognition of cancer's ability to bypass the immune system and, consequently, to the ultimate design of novel therapies aiming at boosting anti-cancer immunity.
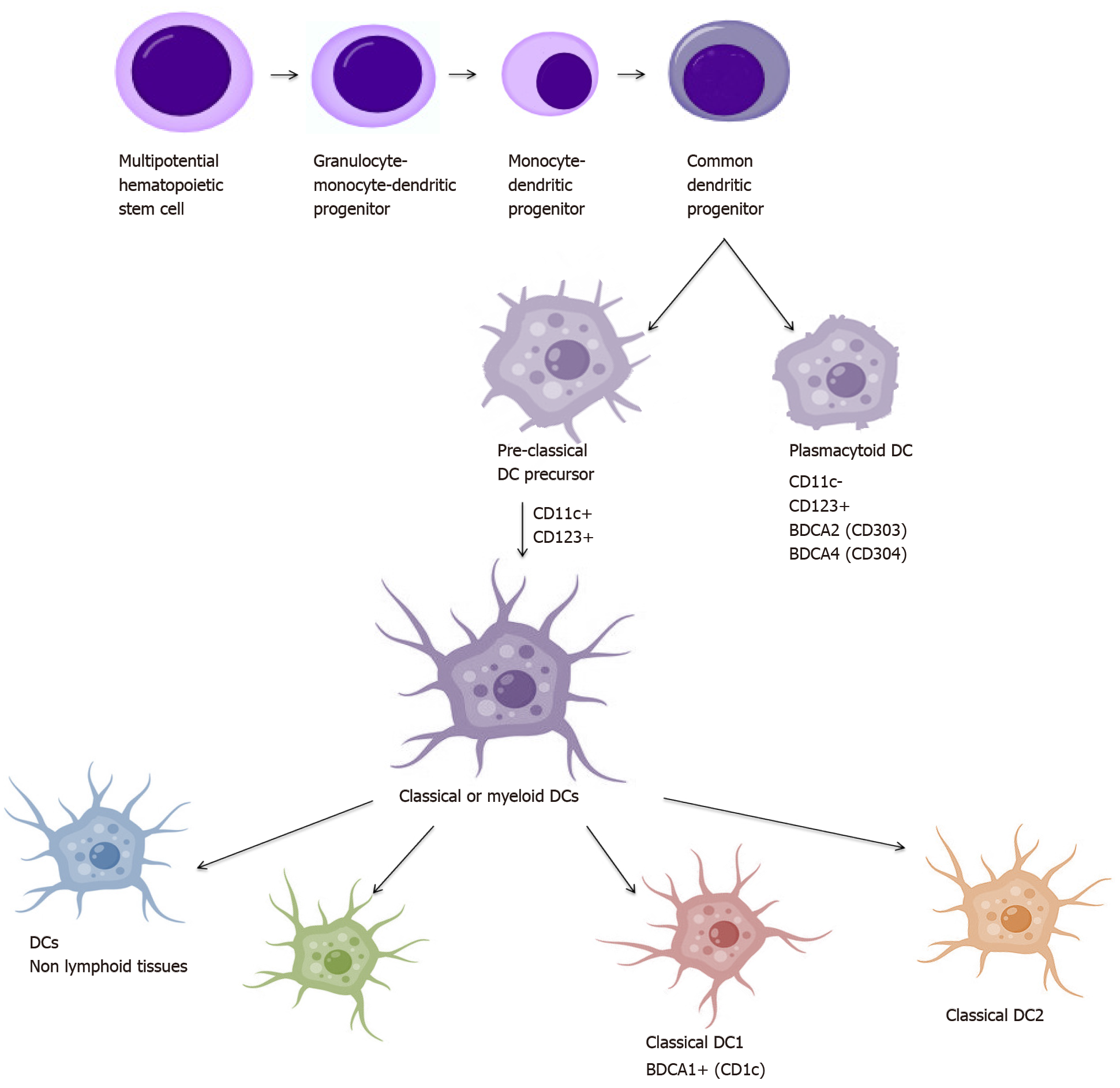
The studies conducted in order to gain understanding of the biology of DCs have resulted in the identification of a large number of their populations. The main criterion of division is the origin, which distinguishes DCs on plasmacytoid origin cells (pDCs): CD123+ CD11c- and myeloid origin cells: CD123+ CD11c+, also called conventional (cDCs)[44,45]. Identification of antigens called blood DC antigens: BDCA-2, BDCA-3 and BDCA-4 and BDCA-1 (CD1c) allowed further discrimination of human blood DCs into two major subsets: cDC1 and cDC2: cDC1 expresses CD1c, while cDC2 (cCD141+) is characterized by the expression of BDCA-3 (CD141) and Clec9A. BDCA-2 (CD303) and BDCA-4 (CD304), together with CD123, characterize pDC. Additionally, cDCs can be divided into resident and migrating cells[46,47]. DCs derive from the CD34+ hematopoietic stem cell that produces BM myeloid precursors (MPs) and lymphoid precursors (LPs). MPs develop into monocytes, macrophages, and DC precursor (MDP) from which they differentiate to monocytes and DC precursors (CDP). CDP are precursors of both cDCs and pDCs[48]. Also, cDCs can differentiate directly from monocytes under the influence of various cytokines[49]. Maturation and DC subpopulations scheme is presented in Figure 2.
PDCs usually complete their differentiation in BM during the development process and as completely differentiated cells circulate into PB. They are mainly located in the vicinity of the endothelium from where they can easily circulate to the lymph nodes to reside in the T-cell zone[50]. They express the CD123 antigen and the low levels of major MHC-II, the wide range of costimulatory molecules without the expression of CD11c. Additionally, the presence of pattern recognition receptors such as Toll-like receptors (TLRs) allows them to recognize pathogen-associated molecular patterns (PAMP) derived from various microbes and secrete a large amount of type I interferon (INF), tumor necrosis factor (TNF)-α and interleukin (IL)-6[45,51]. What is more, TLR-mediated pDC activation promotes efficient antigen presentation and stimulation of T lymphocytes to the immunological response, but in a less effective manner compared to cDCs[51-53]. Nevertheless, pDCs have also been shown to stimulate Tregs for the production of IL-10, suggesting that this subgroup may also play an immunosuppressive role[54].
On the contrary, cDC precursors emerge from the BM transiently transported through the blood and accumulate as cDC pool in tissues[55]. CDCs, also defined as myeloid DCs, expressing CD11c, refer to all DC subsets other than pDC[56]. cCD1c and cCD141 cells belong to the migrating subset of DCs, while epidermal Langerhans cells and interstitial cells are residual cDCs.
CD1c DC subpopulation is the main population of cDCs detected in blood and tissues and lymphatic organs. CD1c cells are identified by major markers CD1c, CD11c, MHC-II (HLA-DR), CD141 and also express other antigens as: CD13, CD33, CD172 and CD45RO. However, they can show a slightly different phenotype, which depends on the place they occur. CD1c+ DCs present in the skin have additional CD1a expression, whereas those present in the gut express CD103[53]. DCs heterogeneity results not only from phenotypic differences but also from the maturity stage. Immature DCs (iDC) are usually found in peripheral tissue, then migrating to the lymph nodes carrying their own antigens, maintaining immune tolerance on their own tissues. They are characterized by increased endocytosis, a decreased expression of MHC and costimulatory molecules and a low ability to produce cytokines[57]. After the antigen is absorbed, the cells begin to mature and change phenotypically and functionally. A peptide complex is formed and an MHC molecule is transported to the cell surface[58]. Maturing DCs migrate to the lymph nodes, increase the expression of MHC-peptide complex, up-regulate the costimulatory molecules and the production of cytokines essential for the T lymphocyte response[55,59]. DCs are considered to be the most important APC, activating T-cells and inducing an immune response. Many factors such as an inflammatory process, an immune response in TME, tissue damage or viruses may promote DCs maturation[60,61].
Many studies show a positive correlation of the number of DCs in the tumour area with a significant extension of patients' survival[62,63]. DCs are able to recognize cancer cells and present neoplastic antigens to effector T lymphocytes. This process depends on the state of maturity and the number of DCs and a local immune status[64].
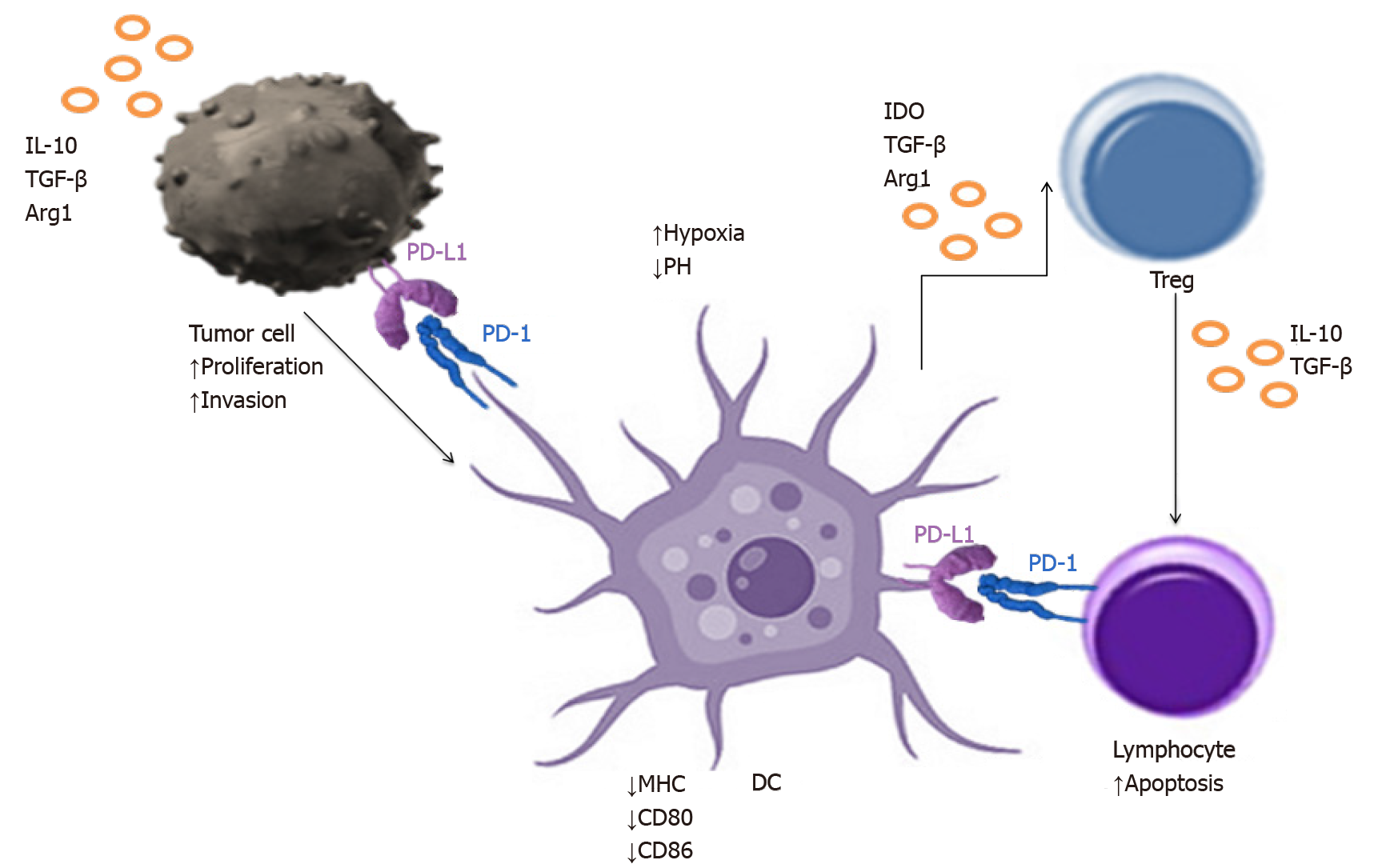
In fact, amount of data confirm that the accumulation of DCs in the tumour area is influenced by the TME, which modulates their maturation and activation. DCs undergo incomplete differentiation and the number of mature cells decreases with the growth of immature cells[65]. Tumour cells secrete suppressor factors such as transforming growth factor β (TGF-β), IL-10 that reduce the expression of cancer antigens and costimulatory molecules on DCs and that convert them into regulatory DCs (DCreg). DCregs occur among the main cells of the immune system responsible for inhibiting of the immune response, which is conducive to the further development and tumour growth[66,67]. DCs in TME can show an increased expression of programmed death ligand (PD-L1), which interacts with PD-1 molecules on the lymphocytes T surface, inducing their apoptosis, causing the immune response to be muted. DCregs also begin to secrete IL-10, thereby stimulating the proliferation of Tregs and their own polarity to DCregs[68]. Therefore, it seems important to investigate the ways to direct DCs for activating the immune system and inducing an anti-tumour response in an attempt to reverse their suppressive effects. It was supported by our studies on the identification of DCs in the aspirates from lymph nodes in lung cancer patients. We found an elevated proportion of DCs in metastatic lymph nodes with a high expression of check- point molecules and the phenotype of DCregs.
To sum up, the aforementioned findings confirm the significant participation of DCs in TME. Considering the high heterogeneity of DCs and their plasticity in anti-tumour activity, it seems reasonable to look for a specific subpopulation of these cells.
Due to the extensive subject matter, we find it valuable to focus on the moDCs population in this review and to discuss DC subpopulation’s role in cancer therapy and a possible therapeutic value associated with these populations in lung cancer.
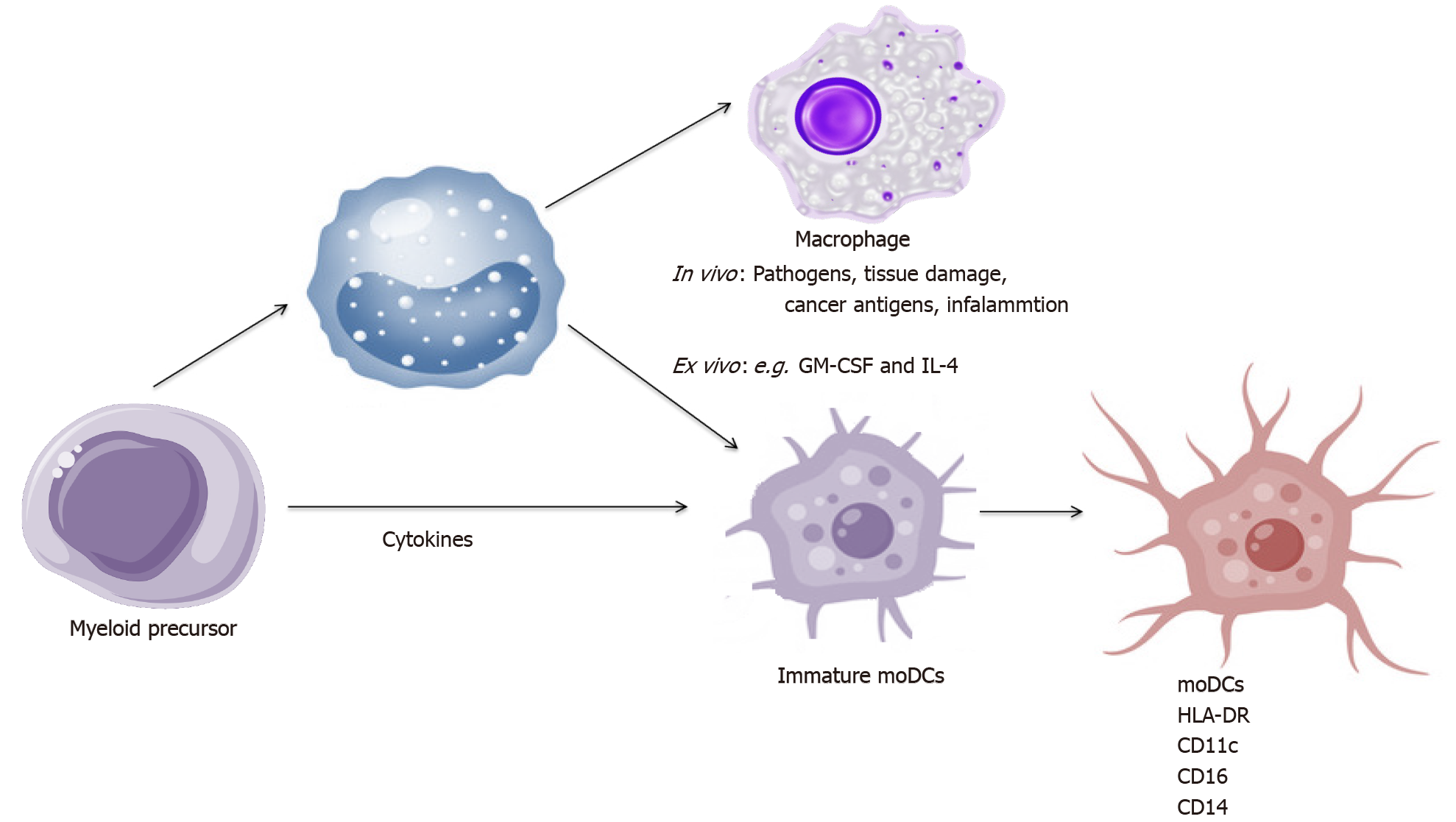
MoDCs arise from monocytes recruited into tissues and become the most abundant DC population during inflammation[69]. In vivo, the maturation of DCs into moDCs is induced by pathogens, tissue damage and cancer antigens. In vitro human moDCs arise from CD14+ monocytes cultured in the presence of granulocyte-macrophage colony-stimulating factor (GM-CSF) and IL-4. This process is triggered in vitro by incubation with pathogen recognition receptor agonists or a pro-inflammatory cytokines cocktail such as: TNF-α, IL-1β, IL-6, IFN-α and prostaglandin E2 (PGE2) or medium conditioned with monocytes with TNF-α and PGE2[70-72]. TLRs or electroporation with coding proteins have recently been used to induce moDCs maturation[73-75].
Human moDCs always express: HLA-DR, CD11c and frequently express CD16, CD14 due to their monocytic origin upon differentiation[76,77]. Maturation scheme of moDCs is presented in Figure 3.
As an APCs, DCs are crucial in the innate and adaptive response of the immune system and play a crucial role in inducing anti-tumour immunity[78]. Mature DCs present exogenous antigens to naive CD4+ T-cells by MHC-II and endogenous peptides to CD8+ T-cells by MHC-I. What is more, they have the ability to cross-present exogenous MHC-I antigens to CD8+ T-cells, which induces a cytotoxic T-cell response against neoplastic cells[79,80]. MoDC cross-presentation plays a key role in the rapid activation of CD8+ memory T-cells residing in the tissues after infection. This process has been found to be active in immunostimulatory anticancer therapies or chemotherapy[81-84].
In order to stimulate T lymphocytes in lymphoid tissues, it requires three signals between DCs and T lymphocytes. Firstly, the antigen is presented by the MHC peptide complex, secondly stimulation by costimulatory molecules from DC to the T-cell occurs. The third one is the secretion of immunostimulating cytokines in the microenvironment[85-87].
Ex vivo produced moDCs are commonly used in clinical trials. Mature antigen loaded moDCs can be easily obtained from PB derived CD14+ monocytes or a hematopoietic stem and progenitor cell CD34+ by treatment with GM-CSF and IL-4[88,89]. Multiple clinical trials have demonstrated the safety and immunogenicity of moDC vaccines. However, clinical responses have been largely disappointing. Admittedly, studies show that ex vivo produced moDCs were able to cross-prime T-cells and produce anti-cancer cytokines such as IL-12[90-92]. MoDCs seemed to be a promising population in anti-cancer therapy. Although, in clinical practice, only in a few groups of treated patients an active anti-cancer response was achieved. This may be due to functional cell deficiencies in conventional vaccines-such as insufficient antigen presentation, impaired migration, and impaired cytokine release, which is insufficient for gaining a strong immunosuppressive TME[88,93,94]. Some studies show that ex vivo stimulation of DCs precursors leads to the production of moDCs that are transcriptionally and phenotypically different from their naturally occurring (primary) cells[92,95]. Ex vivo derived moDCs have a reduced ability to stimulate T-cells compared to natural moDCs isolated from PB and may have a limited ability to migrate to lymph nodes, contributing to reduced vaccine efficacy[56,96-98]. All the aforementioned findings explain the lack of efficace vaccine in lung cancer.
Although, moDCs can be produced in large quantities with minimal side effects from therapy, their effectiveness remains limited in cancer therapy[99,100].
Therefore, other ways of using personalized vaccines with DCs are also being considered. Recent studies show their use in combination with other therapies.
Emerging data suggests that combining DCs vaccination with other cancer treatments could fully unlock the potential of DCs cancer vaccines and improve patient survival. With the advent of combination immunotherapy, personalized DCs vaccination could integrate the current standard of care in the treatment of a wide variety of cancers, among them in lung cancer[88].
The first method is to combine vaccination with chemotherapy to obtain a synergistic effect. In addition to immunosuppressive activity, chemotherapy also strengthens immunity by depleting myeloid derived suppressor cells (MDSCs), Tregs and increases the permeability of cancer cells to cytolytic factors derived from CD8+ T lymphocytes[101,102]. Chemotherapy creates a specific cytokine environment by depleting immune cells, and its combination with DC vaccination and adoptive T-cell transfer has been tested in many trials[103-105]. So far, numerous studies are ongoing in which combination chemotherapy and other methods with DCs vaccines are tested. Chemotherapy with DCs vaccination has been tested with the addition of a Cytochrome c oxidase subunit II inhibitor in patients with melanoma in a phase III trial showing encouraging data. Such a therapy with the addition of autologous T-cells showed longer overall survival compared to chemotherapy alone in two randomized trials in lung cancer[106-108].
Other treatment method is the combination with immunotherapy. For example, preclinical studies showed that this combination decreased MDSCs in the TME, downregulated the PD-1 expression on DCs, and decreased the secretion of immunosuppressive cytokines[109,110]. One clinical trial in advanced renal cell carcinoma patients showed the expansion of CD8+ T-cells and promising survival data[111].
In general, the combination of DCs vaccines with ICIs seems to be promising. A lot of ICIs are currently being tested in clinical trials; many of them, such as: Anti–PD-L1/PD-1 and anti-cytotoxic T-cell antigen 4 (CTLA-4) blocking antibodies have been approved by the Food and Drug Administration[112]. The combination of ICIs with the DCs vaccine seems to have the potential to drive T-cell response into a more specific action[113,114]. In addition, DCs unique ability to cross-present antigens helps to elicit the immune response to more cancer antigens when used in conjunction with ICIs[115]. The anti–CTLA-4 treatment after DCs vaccination may indeed enhance DCs vaccine–induced T-cell responses and there is some evidence that anti–CTLA-4 antibodies might be more effective after DCs vaccination[116,117]. Other studies have shown that DC-based immunotherapy in combination with anti–CTLA-4 antibodies seem to be more effective than the use of these agents alone[118,119]. Anti–PD-1 antibodies are being investigated in combination with DC vaccination, which also opens new avenues of anti-tumour therapy design[120]. The aforementioned studies are conducted in various types of cancer, mainly melanoma, pancreatic cancer, prostate cancer, renal cell carcinoma, and acute myeloid leukemia. Immunotherapy with DCs appears to be capable of eliciting strong tumour-specific responses in combination with other therapies, and is workable and safe[121]. In the recent years, the use of naturally circulating DCs (nDCs) instead of cultured moDCs may have represented the next logical step in anti-cancer therapy and had an impact on long-term clinical benefits[83,122,123].
Lung cancer TME is composed of a large number of phenotypically and functionally different types of cells[124]. A major hallmark of immunosuppression in the TME is the inactivation of cytotoxic CD8+ T-cells, which is achieved through diverse pathways[61,125-130]. Immature DCs produce TGF-β, which expands the population of immunosuppressive Tregs, which in turn inhibit CD8+ T-cells. DCs are recruited into the TME and induced to upregulate PD-1 and PD-L1 in order to directly suppress CD8+ T-cells. Interactions of PD-1 with PD-L1 in the TME blocks responsiveness to danger signals and prevents T-cell activation. T-cells are preferentially drawn to tumour induced DCs as they enter the TME. In addition to the lack of appropriate activating signals, T-cell response is blocked by the engagement of PD-1 by PD-L1 on the DC surface[61]. Tregs are also recruited by the tumour induced DCs to establish a tolerogenic environment[124]. TME's effect on DCs infiltrating the lung cancer tissue is presented in Figure 4. A preclinical study conducted by Lee et al[131]. showed that the administration of DCs transduced with the chemoattractant CCL21 led to the increased infiltration of DCs, CD4+ and CD8+ T-cells in the lung TME, resulting in reduced tumour burden[131]. Given that the efficacy of DC vaccines as a monotherapy is limited by immunosuppressive mechanisms in the TME, these results provide a rationale for combining DCs vaccination with immunotherapy. Combinational approach to the lung cancer treatment in order to increase the effectiveness of DCs therapy is an attractive way to promote and stimulate the anti-cancer immunity. As the molecular basis of an effective DCs therapy inducing T-cell response are still incompletely understood, it has been difficult to identify factors associated with therapeutic success. There is also no consensus how DCs vaccination efficacy should be evaluated. However, various clinical trials have been recently conducted to evaluate the immune response and clinical efficacy of DCs in lung cancer and other tumours (Table 1). Unfortunately, it is unknown whether naturally occurring DCs outperform cultured moDCs as a source for DCs therapy in lung cancer patients, because clinical trials comparing different DCs subsets as a source for DCs therapy have not been performed.
| Status | Major trial | Condition | Study intervention | Official trial code |
| Recruiting | MIDRIXNEO | NSCLC | Dendritic cell immunotherapy | NCT04078269[132] |
| Recruiting | MIDRIX4-LUNG | Metastatic NSCLC | Dendritic cell immunotherapy | NCT04082182[132] |
| Completed | Vaccine Therapy in Treating Patients With Stage I, Stage II, or Stage III Non-small Cell Lung Cancer | Lung cancer | Autologous dendritic cell cancer vaccine | NCT00103116[133,134] |
| Recruiting | Combination Immunotherapy-Ipilimumab-Nivolumab-Dendritic Cell p53 Vac-Patients With SCLC | SCLC; Lung cancer; Relapsed | Combination immunotherapy with Ipilimumab and Nivolumab plus a Dendritic Cell based p53 Vaccine | NCT03406715 |
| Completed | Dendritic Cells in Lung Cancer | NSCLC | Allogeneic Tumour Lysate | NCT00442754 |
| Completed | Chemotherapy Followed By Vaccine Therapy in Treating Patients With Extensive-Stage Small Cell Lung Cancer | Lung cancer | Autologous dendritic cell-adenovirus p53 vaccine combined with Carboplatin and Etoposide | NCT00049218 |
| Completed | Vaccine Therapy in Treating Patients With Stage IIIB, Stage IV, or Recurrent NSCLC | Lung cancer | Autologous dendritic cell-adenovirus CCL21 vaccine | NCT00601094 |
| Completed | CSET 1437 | NSCLC | Dendritic cell-derived exosomes | NCT01159288 |
| Completed | Vaccine Therapy in Treating Patients With Stage IIINSCLC | Lung cancer | Mutant p53 peptide pulsed dendritic cell vaccine combined with adjuvant therapy | NCT00019929 |
| Completed | Vaccine Therapy in Treating Patients With NSCLC | Lung cancer | Autologous tumor cell vaccine therapeutic autologous dendritic cells combined with conventional surgery | NCT00023985 |
| Recruiting | AST-VAC2 Vaccine in Patients With NSCLC | NSCLC in the advanced and adjuvant settings | AST-VAC2 Vaccine | NCT03371485 |
| Recruiting | Luscid | NSCLC | Pembrolizumab with or without intratumoral avelumab/ipilimumab plus CD1c (BDCA-1)+/CD141 (BDCA-3) + myeloid dendritic cells | NCT04571632 |
| Completed | To Immunize Patients With Extensive Stage SCLC Combined With Chemo With or Without ATRA | SCLC | Paclitaxel Ad.p53-DC vaccines. ATRA | NCT00617409 |
| Completed | Denileukin Diftitox Followed by Vaccine Therapy in Treating Patients With Metastatic Cancer | Lung cancer; Breast cancer; Colorectal cancer; Pancreatic cancer | Denileukin diftitox recombinant fowlpox-CEA(6D)/TRICOM vaccine therapeutic autologous dendritic cells | NCT00128622 |
| Completed | Biological Therapy in Treating Patients With Metastatic Cancer | Lung cancer; Breast cancer; Colorectal cancer; Extrahepatic Bile Duct cancer; Gallbladder cancer; Gastric cancer; Head and Neck cancer; Liver cancer; Ovarian cancer; Pancreatic cancer | CEA RNA-pulsed DC cancer vaccine | NCT00004604 |
| Completed | Vaccine Therapy and Biological Therapy in Treating Patients With Advanced Cancer | Lung cancer; Breast cancer; Cervical cancer; Colorectal cancer; Ovarian cancer; Pancreatic cancer | Combining DCs vaccine therapy with interleukin-2 | NCT00019084 |
The heterogeneity of monocytes and DCs has been extensively studied and individual subpopulations of these cells have been well described. As our understanding of monocyte and DCs heterogeneity is growing, their key role as anti-tumour response modulating cells is going to become more useful and targeting the specific subgroups to modulate or stimulate their function is going to become an attractive therapeutic approach. The role of DC in TME is of particular interest in immunological research, but our knowledge is limited, especially in lung cancer. However, the present review emphasizes the role of the DC subpopulation in cancer treatment and a possible therapeutic value associated with these populations in lung cancer. Careful definition of the different subpopulations of DCs and their role in cancer will allow for more accurate targeting of immune cells and a better understanding of their role in modulating the immune response.
Provenance and peer review: Invited article; Externally peer reviewed.
Corresponding Author's Membership in Professional Societies: European Respiratory Society, No. 302693.
Specialty type: Oncology
Country/Territory of origin: Poland
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): 0
Grade C (Good): C
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Yakar M S-Editor: Fan JR L-Editor: A P-Editor: Fan JR
| 1. | Siegel RL, Miller KD, Jemal A. Cancer Statistics, 2017. CA Cancer J Clin. 2017;67:7-30. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 11065] [Cited by in RCA: 12186] [Article Influence: 1523.3] [Reference Citation Analysis (3)] |
| 2. | Ferlay J, Colombet M, Soerjomataram I, Mathers C, Parkin DM, Piñeros M, Znaor A, Bray F. Estimating the global cancer incidence and mortality in 2018: GLOBOCAN sources and methods. Int J Cancer. 2019;144:1941-1953. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3585] [Cited by in RCA: 4896] [Article Influence: 699.4] [Reference Citation Analysis (1)] |
| 3. | Costantini A, Grynovska M, Lucibello F, Moisés J, Pagès F, Tsao MS, Shepherd FA, Bouchaab H, Garassino M, Aerts JGJV, Mazières J, Mondini M, Berghmans T, Meert AP, Cadranel J. Immunotherapy: a new standard of care in thoracic malignancies? Eur Respir J. 2018;51. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 9] [Cited by in RCA: 11] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 4. | Dudnik E, Moskovitz M, Daher S, Shamai S, Hanovich E, Grubstein A, Shochat T, Wollner M, Bar J, Merimsky O, Zer A, Goldstein DA, Hammerman A, Cyjon A, Shechtman Y, Abu-Amna M, Flex D, Roisman LC, Peled N; Israel Lung Cancer Group. Effectiveness and safety of nivolumab in advanced non-small cell lung cancer: The real-life data. Lung Cancer. 2018;126:217-223. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 64] [Cited by in RCA: 84] [Article Influence: 10.5] [Reference Citation Analysis (0)] |
| 5. | Kwiecień I, Polubiec-Kownacka M, Dziedzic D, Wołosz D, Rzepecki P, Domagała-Kulawik J. CD163 and CCR7 as markers for macrophage polarization in lung cancer microenvironment. Cent Eur J Immunol. 2019;44:395-402. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 17] [Cited by in RCA: 48] [Article Influence: 9.6] [Reference Citation Analysis (0)] |
| 6. | Kwiecień I, Rutkowska E, Polubiec-Kownacka M, Raniszewska A, Rzepecki P, Domagała-Kulawik J. Blood Monocyte Subsets with Activation Markers in Relation with Macrophages in Non-Small Cell Lung Cancer. Cancers (Basel). 2020;12. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 9] [Cited by in RCA: 19] [Article Influence: 3.8] [Reference Citation Analysis (0)] |
| 7. | Mytar B, Baj-Krzyworzeka M, Majka M, Stankiewicz D, Zembala M. Human monocytes both enhance and inhibit the growth of human pancreatic cancer in SCID mice. Anticancer Res. 2008;28:187-192. [PubMed] |
| 8. | Vuk-Pavlović S, Bulur PA, Lin Y, Qin R, Szumlanski CL, Zhao X, Dietz AB. Immunosuppressive CD14+HLA-DRlow/- monocytes in prostate cancer. Prostate. 2010;70:443-455. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 207] [Cited by in RCA: 213] [Article Influence: 14.2] [Reference Citation Analysis (0)] |
| 9. | Brooks N, Stojanovska L, Grant P, Apostolopoulos V, McDonald CF, Pouniotis DS. Characterization of blood monocyte phenotype in patients with endometrial cancer. Int J Gynecol Cancer. 2012;22:1500-1508. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5] [Cited by in RCA: 10] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 10. | Collin M, Bigley V. Human dendritic cell subsets: an update. Immunology. 2018;154:3-20. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 809] [Cited by in RCA: 905] [Article Influence: 129.3] [Reference Citation Analysis (0)] |
| 11. | Wylie B, Macri C, Mintern JD, Waithman J. Dendritic Cells and Cancer: From Biology to Therapeutic Intervention. Cancers (Basel). 2019;11. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 90] [Cited by in RCA: 108] [Article Influence: 18.0] [Reference Citation Analysis (0)] |
| 12. | Kapellos TS, Bonaguro L, Gemünd I, Reusch N, Saglam A, Hinkley ER, Schultze JL. Human Monocyte Subsets and Phenotypes in Major Chronic Inflammatory Diseases. Front Immunol. 2019;10:2035. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 281] [Cited by in RCA: 593] [Article Influence: 98.8] [Reference Citation Analysis (0)] |
| 13. | Heim CE, West SC, Ali H, Kielian T. Heterogeneity of Ly6G+ Ly6C+ Myeloid-Derived Suppressor Cell Infiltrates during Staphylococcus aureus Biofilm Infection. Infect Immun. 2018;86. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 17] [Cited by in RCA: 40] [Article Influence: 5.7] [Reference Citation Analysis (0)] |
| 14. | Olingy CE, Dinh HQ, Hedrick CC. Monocyte heterogeneity and functions in cancer. J Leukoc Biol. 2019;106:309-322. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 338] [Cited by in RCA: 388] [Article Influence: 64.7] [Reference Citation Analysis (0)] |
| 15. | Ziegler-Heitbrock L, Ancuta P, Crowe S, Dalod M, Grau V, Hart DN, Leenen PJ, Liu YJ, MacPherson G, Randolph GJ, Scherberich J, Schmitz J, Shortman K, Sozzani S, Strobl H, Zembala M, Austyn JM, Lutz MB. Nomenclature of monocytes and dendritic cells in blood. Blood. 2010;116:e74-e80. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1942] [Cited by in RCA: 1828] [Article Influence: 121.9] [Reference Citation Analysis (0)] |
| 16. | Wong KL, Yeap WH, Tai JJ, Ong SM, Dang TM, Wong SC. The three human monocyte subsets: implications for health and disease. Immunol Res. 2012;53:41-57. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 439] [Cited by in RCA: 518] [Article Influence: 39.8] [Reference Citation Analysis (0)] |
| 17. | Kawamura S, Onai N, Miya F, Sato T, Tsunoda T, Kurabayashi K, Yotsumoto S, Kuroda S, Takenaka K, Akashi K, Ohteki T. Identification of a Human Clonogenic Progenitor with Strict Monocyte Differentiation Potential: A Counterpart of Mouse cMoPs. Immunity. 2017;46:835-848.e4. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 57] [Cited by in RCA: 69] [Article Influence: 8.6] [Reference Citation Analysis (0)] |
| 18. | Sunderkötter C, Nikolic T, Dillon MJ, Van Rooijen N, Stehling M, Drevets DA, Leenen PJ. Subpopulations of mouse blood monocytes differ in maturation stage and inflammatory response. J Immunol. 2004;172:4410-4417. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 824] [Cited by in RCA: 880] [Article Influence: 41.9] [Reference Citation Analysis (0)] |
| 19. | Yona S, Kim KW, Wolf Y, Mildner A, Varol D, Breker M, Strauss-Ayali D, Viukov S, Guilliams M, Misharin A, Hume DA, Perlman H, Malissen B, Zelzer E, Jung S. Fate mapping reveals origins and dynamics of monocytes and tissue macrophages under homeostasis. Immunity. 2013;38:79-91. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2014] [Cited by in RCA: 2426] [Article Influence: 202.2] [Reference Citation Analysis (0)] |
| 20. | Yang J, Zhang L, Yu C, Yang XF, Wang H. Monocyte and macrophage differentiation: circulation inflammatory monocyte as biomarker for inflammatory diseases. Biomark Res. 2014;2:1. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 723] [Cited by in RCA: 756] [Article Influence: 68.7] [Reference Citation Analysis (0)] |
| 21. | Belge KU, Dayyani F, Horelt A, Siedlar M, Frankenberger M, Frankenberger B, Espevik T, Ziegler-Heitbrock L. The proinflammatory CD14+CD16+DR++ monocytes are a major source of TNF. J Immunol. 2002;168:3536-3542. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 613] [Cited by in RCA: 659] [Article Influence: 28.7] [Reference Citation Analysis (0)] |
| 22. | Ziegler-Heitbrock L. The CD14+ CD16+ blood monocytes: their role in infection and inflammation. J Leukoc Biol. 2007;81:584-592. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 690] [Cited by in RCA: 743] [Article Influence: 39.1] [Reference Citation Analysis (0)] |
| 23. | Grage-Griebenow E, Flad HD, Ernst M. Heterogeneity of human peripheral blood monocyte subsets. J Leukoc Biol. 2001;69:11-20. [PubMed] |
| 24. | Mildner A, Schönheit J, Giladi A, David E, Lara-Astiaso D, Lorenzo-Vivas E, Paul F, Chappell-Maor L, Priller J, Leutz A, Amit I, Jung S. Genomic Characterization of Murine Monocytes Reveals C/EBPβ Transcription Factor Dependence of Ly6C- Cells. Immunity. 2017;46:849-862.e7. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 173] [Cited by in RCA: 220] [Article Influence: 27.5] [Reference Citation Analysis (0)] |
| 25. | Satoh T, Nakagawa K, Sugihara F, Kuwahara R, Ashihara M, Yamane F, Minowa Y, Fukushima K, Ebina I, Yoshioka Y, Kumanogoh A, Akira S. Identification of an atypical monocyte and committed progenitor involved in fibrosis. Nature. 2017;541:96-101. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 186] [Cited by in RCA: 238] [Article Influence: 26.4] [Reference Citation Analysis (0)] |
| 26. | Serbina NV, Pamer EG. Monocyte emigration from bone marrow during bacterial infection requires signals mediated by chemokine receptor CCR2. Nat Immunol. 2006;7:311-317. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1146] [Cited by in RCA: 1305] [Article Influence: 68.7] [Reference Citation Analysis (0)] |
| 27. | Jakubzick C, Gautier EL, Gibbings SL, Sojka DK, Schlitzer A, Johnson TE, Ivanov S, Duan Q, Bala S, Condon T, van Rooijen N, Grainger JR, Belkaid Y, Ma'ayan A, Riches DW, Yokoyama WM, Ginhoux F, Henson PM, Randolph GJ. Minimal differentiation of classical monocytes as they survey steady-state tissues and transport antigen to lymph nodes. Immunity. 2013;39:599-610. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 607] [Cited by in RCA: 591] [Article Influence: 49.3] [Reference Citation Analysis (0)] |
| 28. | Auffray C, Fogg D, Garfa M, Elain G, Join-Lambert O, Kayal S, Sarnacki S, Cumano A, Lauvau G, Geissmann F. Monitoring of blood vessels and tissues by a population of monocytes with patrolling behavior. Science. 2007;317:666-670. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1565] [Cited by in RCA: 1469] [Article Influence: 81.6] [Reference Citation Analysis (0)] |
| 29. | Jakubzick CV, Randolph GJ, Henson PM. Monocyte differentiation and antigen-presenting functions. Nat Rev Immunol. 2017;17:349-362. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 427] [Cited by in RCA: 649] [Article Influence: 81.1] [Reference Citation Analysis (0)] |
| 30. | Olingy CE, San Emeterio CL, Ogle ME, Krieger JR, Bruce AC, Pfau DD, Jordan BT, Peirce SM, Botchwey EA. Non-classical monocytes are biased progenitors of wound healing macrophages during soft tissue injury. Sci Rep. 2017;7:447. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 121] [Cited by in RCA: 157] [Article Influence: 19.6] [Reference Citation Analysis (0)] |
| 31. | Mitchem JB, Brennan DJ, Knolhoff BL, Belt BA, Zhu Y, Sanford DE, Belaygorod L, Carpenter D, Collins L, Piwnica-Worms D, Hewitt S, Udupi GM, Gallagher WM, Wegner C, West BL, Wang-Gillam A, Goedegebuure P, Linehan DC, DeNardo DG. Targeting tumor-infiltrating macrophages decreases tumor-initiating cells, relieves immunosuppression, and improves chemotherapeutic responses. Cancer Res. 2013;73:1128-1141. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 639] [Cited by in RCA: 776] [Article Influence: 59.7] [Reference Citation Analysis (0)] |
| 32. | Qian BZ, Li J, Zhang H, Kitamura T, Zhang J, Campion LR, Kaiser EA, Snyder LA, Pollard JW. CCL2 recruits inflammatory monocytes to facilitate breast-tumour metastasis. Nature. 2011;475:222-225. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 2327] [Cited by in RCA: 2227] [Article Influence: 159.1] [Reference Citation Analysis (0)] |
| 33. | Schmall A, Al-Tamari HM, Herold S, Kampschulte M, Weigert A, Wietelmann A, Vipotnik N, Grimminger F, Seeger W, Pullamsetti SS, Savai R. Macrophage and cancer cell cross-talk via CCR2 and CX3CR1 is a fundamental mechanism driving lung cancer. Am J Respir Crit Care Med. 2015;191:437-447. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 121] [Cited by in RCA: 195] [Article Influence: 19.5] [Reference Citation Analysis (0)] |
| 34. | Porrello A, Leslie PL, Harrison EB, Gorentla BK, Kattula S, Ghosh SK, Azam SH, Holtzhausen A, Chao YL, Hayward MC, Waugh TA, Bae S, Godfrey V, Randell SH, Oderup C, Makowski L, Weiss J, Wilkerson MD, Hayes DN, Earp HS, Baldwin AS, Wolberg AS, Pecot CV. Factor XIIIA-expressing inflammatory monocytes promote lung squamous cancer through fibrin cross-linking. Nat Commun. 2018;9:1988. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 41] [Cited by in RCA: 75] [Article Influence: 10.7] [Reference Citation Analysis (0)] |
| 35. | Guilliams M, Mildner A, Yona S. Developmental and Functional Heterogeneity of Monocytes. Immunity. 2018;49:595-613. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 375] [Cited by in RCA: 646] [Article Influence: 92.3] [Reference Citation Analysis (0)] |
| 36. | Kubo H, Mensurado S, Gonçalves-Sousa N, Serre K, Silva-Santos B. Primary Tumors Limit Metastasis Formation through Induction of IL15-Mediated Cross-Talk between Patrolling Monocytes and NK Cells. Cancer Immunol Res. 2017;5:812-820. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 33] [Cited by in RCA: 52] [Article Influence: 6.5] [Reference Citation Analysis (0)] |
| 37. | Hanna RN, Cekic C, Sag D, Tacke R, Thomas GD, Nowyhed H, Herrley E, Rasquinha N, McArdle S, Wu R, Peluso E, Metzger D, Ichinose H, Shaked I, Chodaczek G, Biswas SK, Hedrick CC. Patrolling monocytes control tumor metastasis to the lung. Science. 2015;350:985-990. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 277] [Cited by in RCA: 351] [Article Influence: 35.1] [Reference Citation Analysis (0)] |
| 38. | Ziegler-Heitbrock L, Hofer TP. Toward a refined definition of monocyte subsets. Front Immunol. 2013;4:23. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 208] [Cited by in RCA: 231] [Article Influence: 19.3] [Reference Citation Analysis (0)] |
| 39. | Wong KL, Tai JJ, Wong WC, Han H, Sem X, Yeap WH, Kourilsky P, Wong SC. Gene expression profiling reveals the defining features of the classical, intermediate, and nonclassical human monocyte subsets. Blood. 2011;118:e16-e31. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 636] [Cited by in RCA: 824] [Article Influence: 58.9] [Reference Citation Analysis (0)] |
| 40. | Damasceno D, Teodosio C, van den Bossche WBL, Perez-Andres M, Arriba-Méndez S, Muñoz-Bellvis L, Romero A, Blanco JF, Remesal A, Puig N, Matarraz S, Vicente-Villardón JL, van Dongen JJM, Almeida J, Orfao A; TiMaScan Study Group. Distribution of subsets of blood monocytic cells throughout life. J Allergy Clin Immunol. 2019;144:320-323.e6. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 18] [Cited by in RCA: 31] [Article Influence: 5.2] [Reference Citation Analysis (0)] |
| 41. | Zawada AM, Rogacev KS, Rotter B, Winter P, Marell RR, Fliser D, Heine GH. SuperSAGE evidence for CD14++CD16+ monocytes as a third monocyte subset. Blood. 2011;118:e50-e61. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 350] [Cited by in RCA: 428] [Article Influence: 30.6] [Reference Citation Analysis (0)] |
| 42. | Takizawa H, Boettcher S, Manz MG. Demand-adapted regulation of early hematopoiesis in infection and inflammation. Blood. 2012;119:2991-3002. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 280] [Cited by in RCA: 328] [Article Influence: 25.2] [Reference Citation Analysis (0)] |
| 43. | Macri C, Pang ES, Patton T, O'Keeffe M. Dendritic cell subsets. Semin Cell Dev Biol. 2018;84:11-21. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 111] [Cited by in RCA: 186] [Article Influence: 23.3] [Reference Citation Analysis (0)] |
| 44. | Steinman RM, Idoyaga J. Features of the dendritic cell lineage. Immunol Rev. 2010;234:5-17. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 153] [Cited by in RCA: 161] [Article Influence: 10.7] [Reference Citation Analysis (0)] |
| 45. | Klechevsky E, Liu M, Morita R, Banchereau R, Thompson-Snipes L, Palucka AK, Ueno H, Banchereau J. Understanding human myeloid dendritic cell subsets for the rational design of novel vaccines. Hum Immunol. 2009;70:281-288. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 62] [Cited by in RCA: 60] [Article Influence: 3.8] [Reference Citation Analysis (0)] |
| 46. | Dzionek A, Fuchs A, Schmidt P, Cremer S, Zysk M, Miltenyi S, Buck DW, Schmitz J. BDCA-2, BDCA-3, and BDCA-4: three markers for distinct subsets of dendritic cells in human peripheral blood. J Immunol. 2000;165:6037-6046. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 956] [Cited by in RCA: 964] [Article Influence: 38.6] [Reference Citation Analysis (0)] |
| 47. | Haniffa M, Collin M, Ginhoux F. Ontogeny and functional specialization of dendritic cells in human and mouse. Adv Immunol. 2013;120:1-49. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 127] [Cited by in RCA: 135] [Article Influence: 11.3] [Reference Citation Analysis (0)] |
| 48. | Lee J, Breton G, Oliveira TY, Zhou YJ, Aljoufi A, Puhr S, Cameron MJ, Sékaly RP, Nussenzweig MC, Liu K. Restricted dendritic cell and monocyte progenitors in human cord blood and bone marrow. J Exp Med. 2015;212:385-399. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 190] [Cited by in RCA: 204] [Article Influence: 20.4] [Reference Citation Analysis (0)] |
| 49. | See P, Dutertre CA, Chen J, Günther P, McGovern N, Irac SE, Gunawan M, Beyer M, Händler K, Duan K, Sumatoh HRB, Ruffin N, Jouve M, Gea-Mallorquí E, Hennekam RCM, Lim T, Yip CC, Wen M, Malleret B, Low I, Shadan NB, Fen CFS, Tay A, Lum J, Zolezzi F, Larbi A, Poidinger M, Chan JKY, Chen Q, Rénia L, Haniffa M, Benaroch P, Schlitzer A, Schultze JL, Newell EW, Ginhoux F. Mapping the human DC lineage through the integration of high-dimensional techniques. Science. 2017;356. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 326] [Cited by in RCA: 416] [Article Influence: 52.0] [Reference Citation Analysis (0)] |
| 50. | Cox K, North M, Burke M, Singhal H, Renton S, Aqel N, Islam S, Knight SC. Plasmacytoid dendritic cells (PDC) are the major DC subset innately producing cytokines in human lymph nodes. J Leukoc Biol. 2005;78:1142-1152. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 43] [Cited by in RCA: 44] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 51. | Diebold SS. Activation of dendritic cells by toll-like receptors and C-type lectins. Handb Exp Pharmacol. 2009;3-30. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 50] [Cited by in RCA: 52] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 52. | Reizis B. Regulation of plasmacytoid dendritic cell development. Curr Opin Immunol. 2010;22:206-211. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 92] [Cited by in RCA: 86] [Article Influence: 5.7] [Reference Citation Analysis (0)] |
| 53. | O'Keeffe M, Mok WH, Radford KJ. Human dendritic cell subsets and function in health and disease. Cell Mol Life Sci. 2015;72:4309-4325. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 152] [Cited by in RCA: 142] [Article Influence: 14.2] [Reference Citation Analysis (0)] |
| 54. | Moseman EA, Liang X, Dawson AJ, Panoskaltsis-Mortari A, Krieg AM, Liu YJ, Blazar BR, Chen W. Human plasmacytoid dendritic cells activated by CpG oligodeoxynucleotides induce the generation of CD4+CD25+ regulatory T cells. J Immunol. 2004;173:4433-4442. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 468] [Cited by in RCA: 478] [Article Influence: 22.8] [Reference Citation Analysis (0)] |
| 55. | Merad M, Sathe P, Helft J, Miller J, Mortha A. The dendritic cell lineage: ontogeny and function of dendritic cells and their subsets in the steady state and the inflamed setting. Annu Rev Immunol. 2013;31:563-604. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1510] [Cited by in RCA: 1765] [Article Influence: 147.1] [Reference Citation Analysis (0)] |
| 56. | Osugi Y, Vuckovic S, Hart DN. Myeloid blood CD11c(+) dendritic cells and monocyte-derived dendritic cells differ in their ability to stimulate T lymphocytes. Blood. 2002;100:2858-2866. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 153] [Cited by in RCA: 170] [Article Influence: 7.4] [Reference Citation Analysis (0)] |
| 57. | Hubo M, Trinschek B, Kryczanowsky F, Tuettenberg A, Steinbrink K, Jonuleit H. Costimulatory molecules on immunogenic vs tolerogenic human dendritic cells. Front Immunol. 2013;4:82. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 139] [Cited by in RCA: 168] [Article Influence: 14.0] [Reference Citation Analysis (0)] |
| 58. | Mellman I. Dendritic cells: master regulators of the immune response. Cancer Immunol Res. 2013;1:145-149. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 158] [Cited by in RCA: 218] [Article Influence: 21.8] [Reference Citation Analysis (0)] |
| 59. | Palucka K, Banchereau J. Dendritic-cell-based therapeutic cancer vaccines. Immunity. 2013;39:38-48. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 554] [Cited by in RCA: 642] [Article Influence: 53.5] [Reference Citation Analysis (0)] |
| 60. | Fan Y, Moon JJ. Nanoparticle Drug Delivery Systems Designed to Improve Cancer Vaccines and Immunotherapy. Vaccines (Basel). 2015;3:662-685. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 175] [Cited by in RCA: 203] [Article Influence: 20.3] [Reference Citation Analysis (0)] |
| 61. | Tran Janco JM, Lamichhane P, Karyampudi L, Knutson KL. Tumor-infiltrating dendritic cells in cancer pathogenesis. J Immunol. 2015;194:2985-2991. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 340] [Cited by in RCA: 354] [Article Influence: 35.4] [Reference Citation Analysis (0)] |
| 62. | Lijun Z, Xin Z, Danhua S, Xiaoping L, Jianliu W, Huilan W, Lihui W. Tumor-infiltrating dendritic cells may be used as clinicopathologic prognostic factors in endometrial carcinoma. Int J Gynecol Cancer. 2012;22:836-841. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 31] [Cited by in RCA: 30] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 63. | Dieu-Nosjean MC, Antoine M, Danel C, Heudes D, Wislez M, Poulot V, Rabbe N, Laurans L, Tartour E, de Chaisemartin L, Lebecque S, Fridman WH, Cadranel J. Long-term survival for patients with non-small-cell lung cancer with intratumoral lymphoid structures. J Clin Oncol. 2008;26:4410-4417. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 608] [Cited by in RCA: 748] [Article Influence: 44.0] [Reference Citation Analysis (0)] |
| 64. | Kocián P, Šedivcová M, Drgáč J, Cerná K, Hoch J, Kodet R, Bartůňková J, Špíšek R, Fialová A. Tumor-infiltrating lymphocytes and dendritic cells in human colorectal cancer: their relationship to KRAS mutational status and disease recurrence. Hum Immunol. 2011;72:1022-1028. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 32] [Cited by in RCA: 40] [Article Influence: 2.9] [Reference Citation Analysis (0)] |
| 65. | Veglia F, Gabrilovich DI. Dendritic cells in cancer: the role revisited. Curr Opin Immunol. 2017;45:43-51. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 248] [Cited by in RCA: 337] [Article Influence: 42.1] [Reference Citation Analysis (0)] |
| 66. | Schmidt SV, Nino-Castro AC, Schultze JL. Regulatory dendritic cells: there is more than just immune activation. Front Immunol. 2012;3:274. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 112] [Cited by in RCA: 151] [Article Influence: 11.6] [Reference Citation Analysis (0)] |
| 67. | Shurin MR, Naiditch H, Zhong H, Shurin GV. Regulatory dendritic cells: new targets for cancer immunotherapy. Cancer Biol Ther. 2011;11:988-992. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 40] [Cited by in RCA: 37] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 68. | Ma Y, Shurin GV, Gutkin DW, Shurin MR. Tumor associated regulatory dendritic cells. Semin Cancer Biol. 2012;22:298-306. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 91] [Cited by in RCA: 95] [Article Influence: 7.3] [Reference Citation Analysis (0)] |
| 69. | Tang-Huau TL, Gueguen P, Goudot C, Durand M, Bohec M, Baulande S, Pasquier B, Amigorena S, Segura E. Human in vivo-generated monocyte-derived dendritic cells and macrophages cross-present antigens through a vacuolar pathway. Nat Commun. 2018;9:2570. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 99] [Cited by in RCA: 157] [Article Influence: 22.4] [Reference Citation Analysis (0)] |
| 70. | de Vries IJ, Eggert AA, Scharenborg NM, Vissers JL, Lesterhuis WJ, Boerman OC, Punt CJ, Adema GJ, Figdor CG. Phenotypical and functional characterization of clinical grade dendritic cells. J Immunother. 2002;25:429-438. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 107] [Cited by in RCA: 106] [Article Influence: 4.6] [Reference Citation Analysis (0)] |
| 71. | Jonuleit H, Kühn U, Müller G, Steinbrink K, Paragnik L, Schmitt E, Knop J, Enk AH. Pro-inflammatory cytokines and prostaglandins induce maturation of potent immunostimulatory dendritic cells under fetal calf serum-free conditions. Eur J Immunol. 1997;27:3135-3142. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 892] [Cited by in RCA: 908] [Article Influence: 32.4] [Reference Citation Analysis (0)] |
| 72. | Hervas-Stubbs S, Mancheño U, Riezu-Boj JI, Larraga A, Ochoa MC, Alignani D, Alfaro C, Morales-Kastresana A, Gonzalez I, Larrea E, Pircher H, Le Bon A, Lopez-Picazo JM, Martín-Algarra S, Prieto J, Melero I. CD8 T cell priming in the presence of IFN-α renders CTLs with improved responsiveness to homeostatic cytokines and recall antigens: important traits for adoptive T cell therapy. J Immunol. 2012;189:3299-3310. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 29] [Cited by in RCA: 39] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 73. | Bonehill A, Van Nuffel AM, Corthals J, Tuyaerts S, Heirman C, François V, Colau D, van der Bruggen P, Neyns B, Thielemans K. Single-step antigen loading and activation of dendritic cells by mRNA electroporation for the purpose of therapeutic vaccination in melanoma patients. Clin Cancer Res. 2009;15:3366-3375. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 127] [Cited by in RCA: 127] [Article Influence: 7.9] [Reference Citation Analysis (0)] |
| 74. | Kalinski P, Okada H. Polarized dendritic cells as cancer vaccines: directing effector-type T cells to tumors. Semin Immunol. 2010;22:173-182. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 56] [Cited by in RCA: 56] [Article Influence: 3.7] [Reference Citation Analysis (0)] |
| 75. | Bol KF, Aarntzen EH, Pots JM, Olde Nordkamp MA, van de Rakt MW, Scharenborg NM, de Boer AJ, van Oorschot TG, Croockewit SA, Blokx WA, Oyen WJ, Boerman OC, Mus RD, van Rossum MM, van der Graaf CA, Punt CJ, Adema GJ, Figdor CG, de Vries IJ, Schreibelt G. Prophylactic vaccines are potent activators of monocyte-derived dendritic cells and drive effective anti-tumor responses in melanoma patients at the cost of toxicity. Cancer Immunol Immunother. 2016;65:327-339. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 40] [Cited by in RCA: 53] [Article Influence: 5.9] [Reference Citation Analysis (0)] |
| 76. | Sander J, Schmidt SV, Cirovic B, McGovern N, Papantonopoulou O, Hardt AL, Aschenbrenner AC, Kreer C, Quast T, Xu AM, Schmidleithner LM, Theis H, Thi Huong LD, Sumatoh HRB, Lauterbach MAR, Schulte-Schrepping J, Günther P, Xue J, Baßler K, Ulas T, Klee K, Katzmarski N, Herresthal S, Krebs W, Martin B, Latz E, Händler K, Kraut M, Kolanus W, Beyer M, Falk CS, Wiegmann B, Burgdorf S, Melosh NA, Newell EW, Ginhoux F, Schlitzer A, Schultze JL. Cellular Differentiation of Human Monocytes Is Regulated by Time-Dependent Interleukin-4 Signaling and the Transcriptional Regulator NCOR2. Immunity. 2017;47:1051-1066.e12. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 89] [Cited by in RCA: 125] [Article Influence: 15.6] [Reference Citation Analysis (0)] |
| 77. | Collin M, McGovern N, Haniffa M. Human dendritic cell subsets. Immunology. 2013;140:22-30. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 356] [Cited by in RCA: 355] [Article Influence: 29.6] [Reference Citation Analysis (0)] |
| 78. | Tuyaerts S, Aerts JL, Corthals J, Neyns B, Heirman C, Breckpot K, Thielemans K, Bonehill A. Current approaches in dendritic cell generation and future implications for cancer immunotherapy. Cancer Immunol Immunother. 2007;56:1513-1537. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 121] [Cited by in RCA: 122] [Article Influence: 6.8] [Reference Citation Analysis (0)] |
| 79. | Trombetta ES, Mellman I. Cell biology of antigen processing in vitro and in vivo. Annu Rev Immunol. 2005;23:975-1028. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 843] [Cited by in RCA: 823] [Article Influence: 41.2] [Reference Citation Analysis (0)] |
| 80. | Segura E, Durand M, Amigorena S. Similar antigen cross-presentation capacity and phagocytic functions in all freshly isolated human lymphoid organ-resident dendritic cells. J Exp Med. 2013;210:1035-1047. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 194] [Cited by in RCA: 225] [Article Influence: 18.8] [Reference Citation Analysis (0)] |
| 81. | Kuhn S, Yang J, Ronchese F. Monocyte-Derived Dendritic Cells Are Essential for CD8(+) T Cell Activation and Antitumor Responses After Local Immunotherapy. Front Immunol. 2015;6:584. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 48] [Cited by in RCA: 68] [Article Influence: 6.8] [Reference Citation Analysis (0)] |
| 82. | Segura E. Cross-Presentation Assay for Human Dendritic Cells. Methods Mol Biol. 2016;1423:189-198. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5] [Cited by in RCA: 6] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 83. | Wculek SK, Cueto FJ, Mujal AM, Melero I, Krummel MF, Sancho D. Dendritic cells in cancer immunology and immunotherapy. Nat Rev Immunol. 2020;20:7-24. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 741] [Cited by in RCA: 1705] [Article Influence: 284.2] [Reference Citation Analysis (0)] |
| 84. | Sánchez-Paulete AR, Teijeira A, Cueto FJ, Garasa S, Pérez-Gracia JL, Sánchez-Arráez A, Sancho D, Melero I. Antigen cross-presentation and T-cell cross-priming in cancer immunology and immunotherapy. Ann Oncol. 2017;28:xii74. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 35] [Cited by in RCA: 48] [Article Influence: 6.0] [Reference Citation Analysis (0)] |
| 85. | Steinman RM. Lasker Basic Medical Research Award. Dendritic cells: versatile controllers of the immune system. Nat Med. 2007;13:1155-1159. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 222] [Cited by in RCA: 245] [Article Influence: 13.6] [Reference Citation Analysis (0)] |
| 86. | Ni K, O'Neill HC. The role of dendritic cells in T cell activation. Immunol Cell Biol. 1997;75:223-230. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 95] [Cited by in RCA: 94] [Article Influence: 3.4] [Reference Citation Analysis (0)] |
| 87. | Benvenuti F. The Dendritic Cell Synapse: A Life Dedicated to T Cell Activation. Front Immunol. 2016;7:70. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 49] [Cited by in RCA: 57] [Article Influence: 6.3] [Reference Citation Analysis (0)] |
| 88. | Mastelic-Gavillet B, Balint K, Boudousquie C, Gannon PO, Kandalaft LE. Personalized Dendritic Cell Vaccines-Recent Breakthroughs and Encouraging Clinical Results. Front Immunol. 2019;10:766. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 86] [Cited by in RCA: 145] [Article Influence: 24.2] [Reference Citation Analysis (0)] |
| 89. | Huber A, Dammeijer F, Aerts JGJV, Vroman H. Current State of Dendritic Cell-Based Immunotherapy: Opportunities for in vitro Antigen Loading of Different DC Subsets? Front Immunol. 2018;9:2804. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 87] [Cited by in RCA: 94] [Article Influence: 13.4] [Reference Citation Analysis (0)] |
| 90. | Carreno BM, Becker-Hapak M, Huang A, Chan M, Alyasiry A, Lie WR, Aft RL, Cornelius LA, Trinkaus KM, Linette GP. IL-12p70-producing patient DC vaccine elicits Tc1-polarized immunity. J Clin Invest. 2013;123:3383-3394. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 120] [Cited by in RCA: 138] [Article Influence: 11.5] [Reference Citation Analysis (0)] |
| 91. | Helft J, Böttcher J, Chakravarty P, Zelenay S, Huotari J, Schraml BU, Goubau D, Reis e Sousa C. GM-CSF Mouse Bone Marrow Cultures Comprise a Heterogeneous Population of CD11c(+)MHCII(+) Macrophages and Dendritic Cells. Immunity. 2015;42:1197-1211. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 602] [Cited by in RCA: 643] [Article Influence: 64.3] [Reference Citation Analysis (0)] |
| 92. | Briseño CG, Haldar M, Kretzer NM, Wu X, Theisen DJ, Kc W, Durai V, Grajales-Reyes GE, Iwata A, Bagadia P, Murphy TL, Murphy KM. Distinct Transcriptional Programs Control Cross-Priming in Classical and Monocyte-Derived Dendritic Cells. Cell Rep. 2016;15:2462-2474. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 115] [Cited by in RCA: 167] [Article Influence: 18.6] [Reference Citation Analysis (0)] |
| 93. | Belderbos RA, Aerts JGJV, Vroman H. Enhancing Dendritic Cell Therapy in Solid Tumors with Immunomodulating Conventional Treatment. Mol Ther Oncolytics. 2019;13:67-81. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 28] [Cited by in RCA: 48] [Article Influence: 8.0] [Reference Citation Analysis (0)] |
| 94. | Saxena M, Balan S, Roudko V, Bhardwaj N. Towards superior dendritic-cell vaccines for cancer therapy. Nat Biomed Eng. 2018;2:341-346. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 81] [Cited by in RCA: 95] [Article Influence: 13.6] [Reference Citation Analysis (0)] |
| 95. | Balan S, Ollion V, Colletti N, Chelbi R, Montanana-Sanchis F, Liu H, Vu Manh TP, Sanchez C, Savoret J, Perrot I, Doffin AC, Fossum E, Bechlian D, Chabannon C, Bogen B, Asselin-Paturel C, Shaw M, Soos T, Caux C, Valladeau-Guilemond J, Dalod M. Human XCR1+ dendritic cells derived in vitro from CD34+ progenitors closely resemble blood dendritic cells, including their adjuvant responsiveness, contrary to monocyte-derived dendritic cells. J Immunol. 2014;193:1622-1635. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 89] [Cited by in RCA: 111] [Article Influence: 10.1] [Reference Citation Analysis (0)] |
| 96. | Morse MA, Coleman RE, Akabani G, Niehaus N, Coleman D, Lyerly HK. Migration of human dendritic cells after injection in patients with metastatic malignancies. Cancer Res. 1999;59:56-58. [PubMed] |
| 97. | De Vries IJ, Krooshoop DJ, Scharenborg NM, Lesterhuis WJ, Diepstra JH, Van Muijen GN, Strijk SP, Ruers TJ, Boerman OC, Oyen WJ, Adema GJ, Punt CJ, Figdor CG. Effective migration of antigen-pulsed dendritic cells to lymph nodes in melanoma patients is determined by their maturation state. Cancer Res. 2003;63:12-17. [PubMed] |
| 98. | Shinde P, Fernandes S, Melinkeri S, Kale V, Limaye L. Compromised functionality of monocyte-derived dendritic cells in multiple myeloma patients may limit their use in cancer immunotherapy. Sci Rep. 2018;8:5705. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 44] [Cited by in RCA: 49] [Article Influence: 7.0] [Reference Citation Analysis (0)] |
| 99. | Anguille S, Smits EL, Lion E, van Tendeloo VF, Berneman ZN. Clinical use of dendritic cells for cancer therapy. Lancet Oncol. 2014;15:e257-e267. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 452] [Cited by in RCA: 548] [Article Influence: 49.8] [Reference Citation Analysis (0)] |
| 100. | Garg AD, Coulie PG, Van den Eynde BJ, Agostinis P. Integrating Next-Generation Dendritic Cell Vaccines into the Current Cancer Immunotherapy Landscape. Trends Immunol. 2017;38:577-593. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 211] [Cited by in RCA: 257] [Article Influence: 32.1] [Reference Citation Analysis (0)] |
| 101. | Rosenberg SA, Restifo NP. Adoptive cell transfer as personalized immunotherapy for human cancer. Science. 2015;348:62-68. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1877] [Cited by in RCA: 1842] [Article Influence: 184.2] [Reference Citation Analysis (0)] |
| 102. | Maus MV, June CH. Making Better Chimeric Antigen Receptors for Adoptive T-cell Therapy. Clin Cancer Res. 2016;22:1875-1884. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 184] [Cited by in RCA: 209] [Article Influence: 23.2] [Reference Citation Analysis (0)] |
| 103. | Poschke I, Lövgren T, Adamson L, Nyström M, Andersson E, Hansson J, Tell R, Masucci GV, Kiessling R. A phase I clinical trial combining dendritic cell vaccination with adoptive T cell transfer in patients with stage IV melanoma. Cancer Immunol Immunother. 2014;63:1061-1071. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 60] [Cited by in RCA: 57] [Article Influence: 5.2] [Reference Citation Analysis (0)] |
| 104. | Kandalaft LE, Powell DJ Jr, Chiang CL, Tanyi J, Kim S, Bosch M, Montone K, Mick R, Levine BL, Torigian DA, June CH, Coukos G. Autologous lysate-pulsed dendritic cell vaccination followed by adoptive transfer of vaccine-primed ex vivo co-stimulated T cells in recurrent ovarian cancer. Oncoimmunology. 2013;2:e22664. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 118] [Cited by in RCA: 130] [Article Influence: 11.8] [Reference Citation Analysis (0)] |
| 105. | Chodon T, Comin-Anduix B, Chmielowski B, Koya RC, Wu Z, Auerbach M, Ng C, Avramis E, Seja E, Villanueva A, McCannel TA, Ishiyama A, Czernin J, Radu CG, Wang X, Gjertson DW, Cochran AJ, Cornetta K, Wong DJ, Kaplan-Lefko P, Hamid O, Samlowski W, Cohen PA, Daniels GA, Mukherji B, Yang L, Zack JA, Kohn DB, Heath JR, Glaspy JA, Witte ON, Baltimore D, Economou JS, Ribas A. Adoptive transfer of MART-1 T-cell receptor transgenic lymphocytes and dendritic cell vaccination in patients with metastatic melanoma. Clin Cancer Res. 2014;20:2457-2465. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 170] [Cited by in RCA: 189] [Article Influence: 17.2] [Reference Citation Analysis (0)] |
| 106. | Kimura H, Matsui Y, Ishikawa A, Nakajima T, Yoshino M, Sakairi Y. Randomized controlled phase III trial of adjuvant chemo-immunotherapy with activated killer T cells and dendritic cells in patients with resected primary lung cancer. Cancer Immunol Immunother. 2015;64:51-59. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 45] [Cited by in RCA: 57] [Article Influence: 5.2] [Reference Citation Analysis (0)] |
| 107. | Ellebaek E, Engell-Noerregaard L, Iversen TZ, Froesig TM, Munir S, Hadrup SR, Andersen MH, Svane IM. Metastatic melanoma patients treated with dendritic cell vaccination, Interleukin-2 and metronomic cyclophosphamide: results from a phase II trial. Cancer Immunol Immunother. 2012;61:1791-1804. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 94] [Cited by in RCA: 97] [Article Influence: 7.5] [Reference Citation Analysis (0)] |
| 108. | Zhao M, Li H, Li L, Zhang Y. Effects of a gemcitabine plus platinum regimen combined with a dendritic cell-cytokine induced killer immunotherapy on recurrence and survival rate of non-small cell lung cancer patients. Exp Ther Med. 2014;7:1403-1407. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 31] [Cited by in RCA: 38] [Article Influence: 3.5] [Reference Citation Analysis (0)] |
| 109. | Ko JS, Zea AH, Rini BI, Ireland JL, Elson P, Cohen P, Golshayan A, Rayman PA, Wood L, Garcia J, Dreicer R, Bukowski R, Finke JH. Sunitinib mediates reversal of myeloid-derived suppressor cell accumulation in renal cell carcinoma patients. Clin Cancer Res. 2009;15:2148-2157. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 643] [Cited by in RCA: 683] [Article Influence: 42.7] [Reference Citation Analysis (0)] |
| 110. | Ozao-Choy J, Ma G, Kao J, Wang GX, Meseck M, Sung M, Schwartz M, Divino CM, Pan PY, Chen SH. The novel role of tyrosine kinase inhibitor in the reversal of immune suppression and modulation of tumor microenvironment for immune-based cancer therapies. Cancer Res. 2009;69:2514-2522. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 413] [Cited by in RCA: 427] [Article Influence: 26.7] [Reference Citation Analysis (0)] |
| 111. | Amin A, Dudek AZ, Logan TF, Lance RS, Holzbeierlein JM, Knox JJ, Master VA, Pal SK, Miller WH Jr, Karsh LI, Tcherepanova IY, DeBenedette MA, Williams WL, Plessinger DC, Nicolette CA, Figlin RA. Survival with AGS-003, an autologous dendritic cell-based immunotherapy, in combination with sunitinib in unfavorable risk patients with advanced renal cell carcinoma (RCC): Phase 2 study results. J Immunother Cancer. 2015;3:14. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 131] [Cited by in RCA: 155] [Article Influence: 15.5] [Reference Citation Analysis (0)] |
| 112. | Zarour HM. Reversing T-cell Dysfunction and Exhaustion in Cancer. Clin Cancer Res. 2016;22:1856-1864. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 245] [Cited by in RCA: 331] [Article Influence: 36.8] [Reference Citation Analysis (0)] |
| 113. | Zhao J, Chen Y, Ding ZY, Liu JY. Safety and Efficacy of Therapeutic Cancer Vaccines Alone or in Combination With Immune Checkpoint Inhibitors in Cancer Treatment. Front Pharmacol. 2019;10:1184. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 60] [Cited by in RCA: 57] [Article Influence: 9.5] [Reference Citation Analysis (0)] |
| 114. | Lamberti MJ, Nigro A, Mentucci FM, Rumie Vittar NB, Casolaro V, Dal Col J. Dendritic Cells and Immunogenic Cancer Cell Death: A Combination for Improving Antitumor Immunity. Pharmaceutics. 2020;12. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 36] [Cited by in RCA: 63] [Article Influence: 12.6] [Reference Citation Analysis (0)] |
| 115. | Sánchez-Paulete AR, Cueto FJ, Martínez-López M, Labiano S, Morales-Kastresana A, Rodríguez-Ruiz ME, Jure-Kunkel M, Azpilikueta A, Aznar MA, Quetglas JI, Sancho D, Melero I. Cancer Immunotherapy with Immunomodulatory Anti-CD137 and Anti-PD-1 Monoclonal Antibodies Requires BATF3-Dependent Dendritic Cells. Cancer Discov. 2016;6:71-79. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 275] [Cited by in RCA: 408] [Article Influence: 40.8] [Reference Citation Analysis (0)] |
| 116. | Ribas A, Camacho LH, Lopez-Berestein G, Pavlov D, Bulanhagui CA, Millham R, Comin-Anduix B, Reuben JM, Seja E, Parker CA, Sharma A, Glaspy JA, Gomez-Navarro J. Antitumor activity in melanoma and anti-self responses in a phase I trial with the anti-cytotoxic T lymphocyte-associated antigen 4 monoclonal antibody CP-675,206. J Clin Oncol. 2005;23:8968-8977. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 457] [Cited by in RCA: 417] [Article Influence: 20.9] [Reference Citation Analysis (0)] |
| 117. | Boudewijns S, Koornstra RH, Westdorp H, Schreibelt G, van den Eertwegh AJ, Geukes Foppen MH, Haanen JB, de Vries IJ, Figdor CG, Bol KF, Gerritsen WR. Ipilimumab administered to metastatic melanoma patients who progressed after dendritic cell vaccination. Oncoimmunology. 2016;5:e1201625. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 20] [Cited by in RCA: 21] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 118. | Ribas A, Comin-Anduix B, Chmielowski B, Jalil J, de la Rocha P, McCannel TA, Ochoa MT, Seja E, Villanueva A, Oseguera DK, Straatsma BR, Cochran AJ, Glaspy JA, Hui L, Marincola FM, Wang E, Economou JS, Gomez-Navarro J. Dendritic cell vaccination combined with CTLA4 blockade in patients with metastatic melanoma. Clin Cancer Res. 2009;15:6267-6276. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 161] [Cited by in RCA: 168] [Article Influence: 10.5] [Reference Citation Analysis (0)] |
| 119. | Wilgenhof S, Corthals J, Heirman C, van Baren N, Lucas S, Kvistborg P, Thielemans K, Neyns B. Phase II Study of Autologous Monocyte-Derived mRNA Electroporated Dendritic Cells (TriMixDC-MEL) Plus Ipilimumab in Patients With Pretreated Advanced Melanoma. J Clin Oncol. 2016;34:1330-1338. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 201] [Cited by in RCA: 207] [Article Influence: 23.0] [Reference Citation Analysis (0)] |
| 120. | Schetters STT, Rodriguez E, Kruijssen LJW, Crommentuijn MHW, Boon L, Van den Bossche J, Den Haan JMM, Van Kooyk Y. Monocyte-derived APCs are central to the response of PD1 checkpoint blockade and provide a therapeutic target for combination therapy. J Immunother Cancer. 2020;8. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 14] [Cited by in RCA: 46] [Article Influence: 11.5] [Reference Citation Analysis (0)] |
| 121. | Calmeiro J, Carrascal MA, Tavares AR, Ferreira DA, Gomes C, Falcão A, Cruz MT, Neves BM. Dendritic Cell Vaccines for Cancer Immunotherapy: The Role of Human Conventional Type 1 Dendritic Cells. Pharmaceutics. 2020;12. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 34] [Cited by in RCA: 67] [Article Influence: 13.4] [Reference Citation Analysis (0)] |
| 122. | Bol KF, Tel J, de Vries IJ, Figdor CG. Naturally circulating dendritic cells to vaccinate cancer patients. Oncoimmunology. 2013;2:e23431. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 24] [Cited by in RCA: 25] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 123. | Wojas K, Tabarkiewicz J, Roliński J. Dendritic cells in cancer immunotherapy - a short review. Folia Morphol (Warsz). 2003;62:317-318. [PubMed] |
| 124. | Altorki NK, Markowitz GJ, Gao D, Port JL, Saxena A, Stiles B, McGraw T, Mittal V. The lung microenvironment: an important regulator of tumour growth and metastasis. Nat Rev Cancer. 2019;19:9-31. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 546] [Cited by in RCA: 781] [Article Influence: 130.2] [Reference Citation Analysis (0)] |
| 125. | Domagala-Kulawik J, Osinska I, Hoser G. Mechanisms of immune response regulation in lung cancer. Transl Lung Cancer Res. 2014;3:15-22. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 30] [Reference Citation Analysis (0)] |
| 126. | Osińska I, Stelmaszczyk-Emmel A, Polubiec-Kownacka M, Dziedzic D, Domagała-Kulawik J. CD4+/CD25(high)/FoxP3+/CD127- regulatory T cells in bronchoalveolar lavage fluid of lung cancer patients. Hum Immunol. 2016;77:912-915. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 12] [Cited by in RCA: 17] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 127. | Kwiecien I, Stelmaszczyk-Emmel A, Polubiec-Kownacka M, Dziedzic D, Domagala-Kulawik J. Elevated regulatory T cells, surface and intracellular CTLA-4 expression and interleukin-17 in the lung cancer microenvironment in humans. Cancer Immunol Immunother. 2017;66:161-170. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 25] [Cited by in RCA: 43] [Article Influence: 4.8] [Reference Citation Analysis (0)] |
| 128. | Domagala-Kulawik J, Kwiecien I, Pankowski J, Pasieka-Lis M, Wolosz D, Zielinski M. Elevated Foxp3/CD8 Ratio in Lung Adenocarcinoma Metastatic Lymph Nodes Resected by Transcervical Extended Mediastinal Lymphadenectomy. Biomed Res Int. 2017;2017:5185034. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 5] [Cited by in RCA: 8] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 129. | Galli F, Aguilera JV, Palermo B, Markovic SN, Nisticò P, Signore A. Relevance of immune cell and tumor microenvironment imaging in the new era of immunotherapy. J Exp Clin Cancer Res. 2020;39:89. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 177] [Cited by in RCA: 185] [Article Influence: 37.0] [Reference Citation Analysis (0)] |
| 130. | Stankovic B, Bjørhovde HAK, Skarshaug R, Aamodt H, Frafjord A, Müller E, Hammarström C, Beraki K, Bækkevold ES, Woldbæk PR, Helland Å, Brustugun OT, Øynebråten I, Corthay A. Immune Cell Composition in Human Non-small Cell Lung Cancer. Front Immunol. 2018;9:3101. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 109] [Cited by in RCA: 233] [Article Influence: 38.8] [Reference Citation Analysis (0)] |
| 131. | Lee JM, Lee MH, Garon E, Goldman JW, Salehi-Rad R, Baratelli FE, Schaue D, Wang G, Rosen F, Yanagawa J, Walser TC, Lin Y, Park SJ, Adams S, Marincola FM, Tumeh PC, Abtin F, Suh R, Reckamp KL, Lee G, Wallace WD, Lee S, Zeng G, Elashoff DA, Sharma S, Dubinett SM. Phase I Trial of Intratumoral Injection of CCL21 Gene-Modified Dendritic Cells in Lung Cancer Elicits Tumor-Specific Immune Responses and CD8+ T-cell Infiltration. Clin Cancer Res. 2017;23:4556-4568. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 97] [Cited by in RCA: 175] [Article Influence: 21.9] [Reference Citation Analysis (0)] |
| 132. | Brabants E, Heyns K, De Smet S, Devreker P, Ingels J, De Cabooter N, Debacker V, Dullaers M, VAN Meerbeeck JP, Vandekerckhove B, Vermaelen KY. An accelerated, clinical-grade protocol to generate high yields of type 1-polarizing messenger RNA-loaded dendritic cells for cancer vaccination. Cytotherapy. 2018;20:1164-1181. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 7] [Cited by in RCA: 13] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 133. | Hirschowitz EA, Yannelli JR. Immunotherapy for lung cancer. Proc Am Thorac Soc. 2009;6:224-232. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 28] [Cited by in RCA: 28] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 134. | Hirschowitz EA, Foody T, Hidalgo GE, Yannelli JR. Immunization of NSCLC patients with antigen-pulsed immature autologous dendritic cells. Lung Cancer. 2007;57:365-372. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 56] [Cited by in RCA: 55] [Article Influence: 3.1] [Reference Citation Analysis (0)] |