Published online Nov 24, 2021. doi: 10.5306/wjco.v12.i11.1037
Peer-review started: February 27, 2021
First decision: May 4, 2021
Revised: May 18, 2021
Accepted: September 3, 2021
Article in press: September 3, 2021
Published online: November 24, 2021
Processing time: 264 Days and 12.3 Hours
Non-clear cell (ncc) metastatic renal-cell carcinoma (RCC) has dismal results with standard systemic therapies and a generally worse prognosis when compared to its clear-cell counterpart. New systemic combination therapies have emerged for metastatic RCC (mRCC), but the pivotal phase III trials excluded patients with nccRCC, which constitute about 30% of metastatic RCC cases.
To provide a piece of real-life evidence on the use of pazopanib in this patient subgroup.
The present study is a multicenter retrospective observational analysis aiming to assess the activity, efficacy, and safety of pazopanib as first-line therapy for advanced nccRCC patients treated in a real-life setting.
Overall, 48 patients were included. At the median follow-up of 40.6 mo, the objective response rate was 27.1%, the disease control rate was 83.3%, and the median progression-free survival and overall survival were 12.3 (95% confidence interval [CI]: 3.6-20.9) and 27.7 (95%CI: 18.2-37.1) mo, respectively. Grade 3 adverse events occurred in 20% of patients, and no grade 4 or 5 toxicities were found.
Pazopanib should be considered as a good first-line option for metastatic RCC with variant histology.
Core Tip: Non-clear cell metastatic renal-cell carcinoma (nccRCC) has dismal results with standard systemic therapies and a poor prognosis. Few therapeutic molecules have been explicitly tested in nccRCC patients. We retrospectively collected 48 advanced nccRCC patients treated with pazopanib in the first-line setting, offering promising findings of quite good response rate (27%), progression-free survival around 12 mo, and overall survival around 28 mo. In light of these results, we suggest that pazopanib can be a good treatment choice in this subgroup of patients, pending the results of ongoing clinical trials with new therapeutic combinations.
- Citation: Buti S, Bersanelli M, Massari F, De Giorgi U, Caffo O, Aurilio G, Basso U, Carteni G, Caserta C, Galli L, Boccardo F, Procopio G, Facchini G, Fornarini G, Berruti A, Fea E, Naglieri E, Petrelli F, Iacovelli R, Porta C, Mosca A. First-line pazopanib in patients with advanced non-clear cell renal carcinoma: An Italian case series. World J Clin Oncol 2021; 12(11): 1037-1046
- URL: https://www.wjgnet.com/2218-4333/full/v12/i11/1037.htm
- DOI: https://dx.doi.org/10.5306/wjco.v12.i11.1037
Non-clear cell renal-cell carcinoma (nccRCC) represents a heterogeneous group of tumors with distinct genomic and metabolic features. Therefore, its clinical behavior can be benign to indolent and even highly malignant with high metastatic potential[1].
Of note, non-clear mRCC has dismal results with standard systemic therapies and a generally worse prognosis when compared to its clear-cell counterpart, as demon
Recent advances have offered the availability of new systemic therapies for me
The previous gold-standard first-line therapies for mRCC, represented by anti-vascular endothelial growth factor receptor tyrosine kinase inhibitors (TKIs) used as a single agent, have been in part investigated in nccRCC patients. In particular, sunitinib was tested in two prospective trials (the ESPN trial and the ASPEN trial) initially planned to show the superiority of the mammalian target of rapamycin inhibitor everolimus over sunitinib as first-line therapy. Both studies, on the contrary, finally supported the use of sunitinib as a primary systemic approach in this population of patients[7,8]. On the other hand, other TKIs have been approved in the first-line setting for all-mRCC histologies, including the alternative with pazopanib, which demonstrated similar efficacy and even better safety profile when compared to sunitinib in randomized trials[9,10]. Nevertheless, little evidence is available about using this drug in nccRCC patients, with a consequent reluctance to prescription in clinical practice, notwithstanding the drug’s good profile, also suitable for frail and elderly patients[11,12].
Given the unmet oncological need for evidence for the systemic approach to nccRCC, the present report of a retrospective multicenter case series aims to provide a piece of real-life evidence on the use of pazopanib in this patient subgroup.
The present study is a retrospective, observational analysis aiming to assess the activity, efficacy, and safety of pazopanib as first-line therapy for advanced nccRCC patients treated in a real-life setting at multiple Italian institutions. The principal inclusion criteria were the diagnosis of nccRCC, including papillary RCC (pRCC), chromophobe RCC (chRCC), RCC with Xp11 translocation, RCC with undefined histology, and mixed-histology RCC with mostly ncc component; receiving the first dose of pazopanib between June 2012 and June 2015; > 18 years old; measurable disease at the computed tomography (CT) scans performed according to clinical practice at the treating centers. Data were collected between February 2017 and February 2018.
The primary objective was to assess the outcome of patients in terms of objective response rate (ORR), disease control rate (DCR), progression-free survival (PFS), OS, and tolerability as co-primary endpoints. PFS was defined as the time between pazopanib initiation and disease progression or death; OS was defined as the time between pazopanib initiation and death or the date of the last follow-up visit for alive patients; DCR as responses plus stable diseases. Objective responses (complete, CR; partial, PR; stable, SD; progressive disease, PD) were evaluated according to Response Evaluation Criteria in Solid Tumors (RECIST 1.1)[13] and assessed every 2-3 mo according to clinical practice.
Treatment-related adverse events (TRAEs) were recorded as clinical practice ac
The characteristics of patients were collected, and their correlation with the outcome was explored.
The study was reviewed and approved by the Local Ethics Committee of Parma (Italy). All study participants provided informed written consent before study enrollment.
Descriptive statistics are used to summarize the data. PFS and OS were estimated using the Kaplan-Meier method with 95% confidence intervals (CI) and compared using the log-rank test. Univariate and multivariate analyses were performed by using Cox proportional hazards models. The comparison between categorical endpoints was performed using the chi-square test. Significance levels were set at a 0.05 value, and all P values were two-sided. SPSS Statistics 24.0 software (IBM Corporation, Armonk, NY, United States) was used to conduct the statistical analyses.
From January 2011 to January 2017, 48 consecutive patients were included in 20 Italian centers. The median follow-up was 40.6 mo (95%CI: 22.3-58.9). The characteristics of patients are reported in Table 1. The median age was 70 (range, 27-86) years, and most patients were male (75.0%). Fifteen patients (31.3%) had distant metastases at disease onset. The majority of patients had pRCC (50.0%) or chRCC (18.8%) as histology. Most patients had previously received nephrectomy (85.4%), and seven (14.6%) had metastasectomy. Thirty-seven patients (77.1%) had an Eastern Cooperative Oncology Group (ECOG) performance status (PS) of 0. All IMDC risk groups were represented in the study population.
| Baseline characteristic | n = 48 |
| Age, median (range) | 70 (27-86) |
| Sex | |
| Males | 36 (75) |
| Females | 12 (25) |
| Histology | |
| Papillary | 24 (50) |
| Chromophobe | 9 (10.8) |
| Xp11 translocation | 1 (2.1) |
| Unclassified | 6 (12.5) |
| Mixed1 | 8 (16.7) |
| Grade (Fuhrman/ISUP) | |
| 1-2 | 6 (12.5) |
| 3 | 15 (31.3) |
| 4 | 4 (8.3) |
| NA | 23 (47.9) |
| Stage at diagnosis | |
| I-III | 33 (68.8) |
| IV | 15 (31.3) |
| Previous nephrectomy | |
| Yes | 41 (85.4) |
| No | 7 (14.6) |
| Metastasectomy | |
| Yes | 7 (14.6) |
| No | 41 (85.4) |
| ECOG PS | |
| 0 | 37 (77.1) |
| 1 | 11 (22.9) |
| IMDC score risk group | |
| Good | 19 (39.6) |
| Intermediate | 25 (52.1) |
| Poor | 2 (4.2) |
| NA | 2 (4.2) |
| Starting dose of pazopanib | |
| 800 mg | 31(64.6) |
| 600 mg | 10 (20.8) |
| 400 mg | 7 (14.6) |
The median duration of treatment was 9.1 (range, 0.6-52.5) mo, and eight patients (16.7%) were still receiving pazopanib at the time of the last follow-up. Twenty-eight patients (58.3%) received a second-line therapy (four received nivolumab and three cabozantinib).
In 17 cases (35.4%), the starting dose of pazopanib was primarily reduced because of the patient conditions (age and/or comorbidity): Ten patients started with 600 mg, and seven patients started with 400 mg. Secondary dose reductions or temporary treatment discontinuations due to TRAEs were required in 19 cases (39.6%), mainly due to grade (G) 3 hepatic toxicity, fatigue, and diarrhea. G3 TRAEs occurred in 20.8% of patients, G1-2 in 81.2%, and no G4 toxicity was observed (Table 2).
| Adverse event | Grade 1/2, n (%) | Grade 3, n (%) |
| Fatigue | 24 (50.0) | |
| Diarrhea | 15 (31.3) | 2 (4.2) |
| Mucositis | 9 (18.8) | |
| Hypertransaminasemia | 7 (14.6) | 4 (8.3) |
| Thrombocytopenia | 6 (12.5) | |
| Anemia | 15 (31.2) | 2 (4.2) |
| Neutropenia | 5 (10.4) | |
| Hypothyroidism | 15 (31.3) | |
| Disgeusia | 5 (10.4) | 1 (2.1) |
| Cutaneous toxicity1 | 8 (16.7) | |
| Nausea/vomiting | 14 (29.2) | |
| Heart failure | 1 (2.1) | |
| Renal failure | 5 (10.4) | 1 (2.1) |
| Other2 | 16 (33.3) |
PR was achieved in 13 patients (27.1%), and no CR was observed. Twenty-seven patients (56.3%) obtained SD; the DCR was 83.3%, whereas six patients (12.5%) had PD as the best response (while 4.2% were not evaluable). Neither ORR nor DCR was significantly correlated with any of the following parameters: Sex, histology, grading, sarcomatoid component, initial pazopanib dose, ECOG PS, and IMDC risk group.
Most cases of primary refractory (PD as best response) were pRCC. Table 3 shows the responses according to the histology.
| Effectiveness outcome | All patients (n = 48) | Papillary RCC (n = 24) | Chromophobe RCC (n = 9) |
| Response rate (RECIST 1.1), n (%) | |||
| Partial responses | 13 (27.1) | 6 (25.0) | 2 (22.2) |
| Complete responses | 0 (0.0) | 0 (0.0) | 0 (0.0) |
| Stable disease | 27 (56.3) | 14 (58.3) | 5 (55.5) |
| Progressions of disease | 6 (12.5) | 3 (12.5) | 1 (11.1) |
| Not evaluable | 2 (4.2) | 1 (4.2) | 1 (11.1) |
| Disease control rate | 40 (83.3) | 20 (83.3) | 7 (77.8) |
| PFS | |||
| Median (mo) (95%CI) | 12.3 (3.6-20.9) | - | - |
| Rate of patients progression free at 6 mo | 67.8% | - | - |
| Rate of patients progression free at 12 mo | 49.0% | - | - |
| OS | |||
| Median (mo) (95%CI) | 27.6 (18.3-37.1) | - | - |
| Rate of patients alive at 12 mo | 82.7% | - | - |
| Rate of patients alive at 24 mo | 62.0% | - | - |
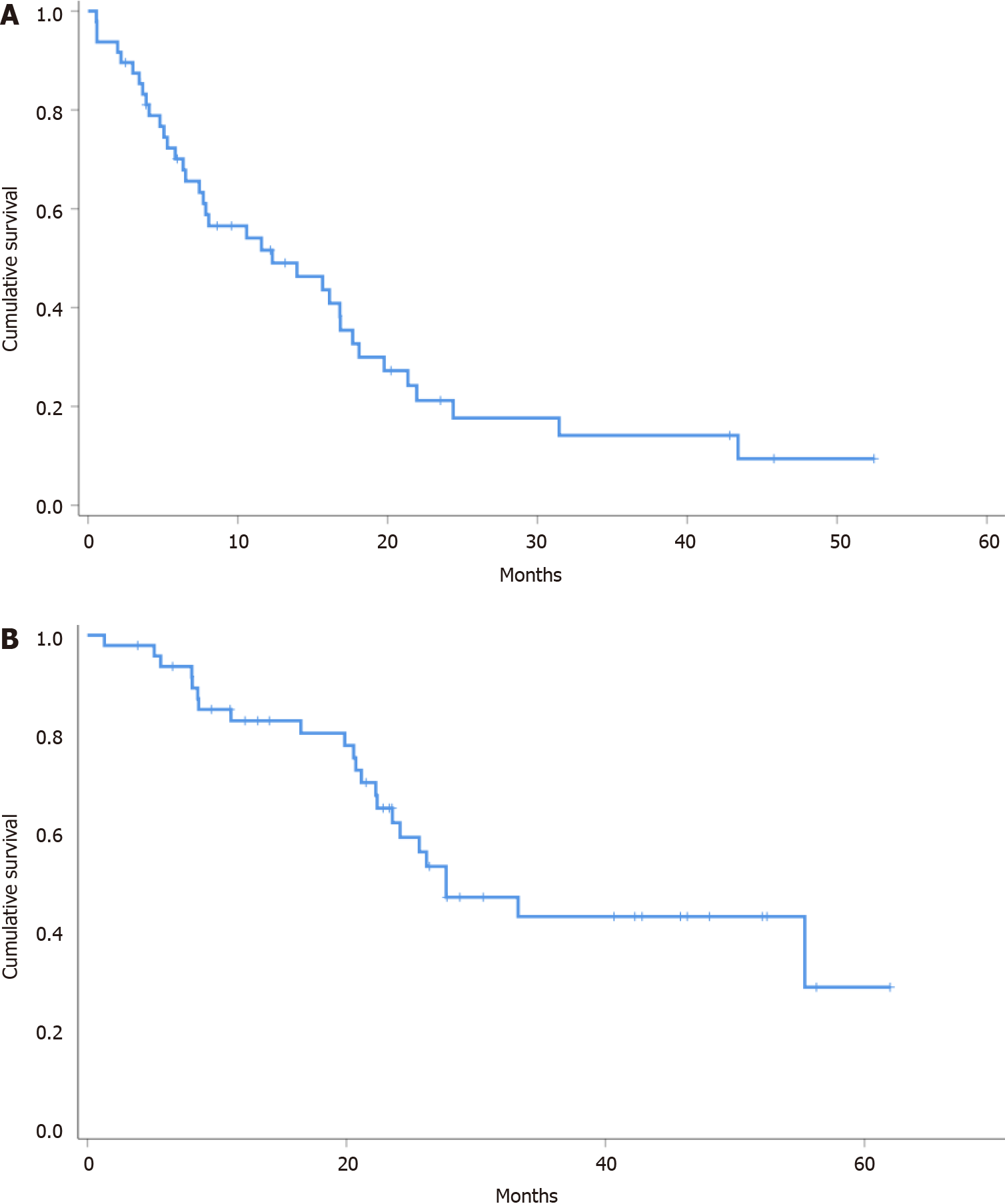
Median PFS and OS were 12.3 (95%CI: 3.6-20.9) and 27.7 (95%CI: 18.2-37.1) mo, respectively (Figure 1, Table 3).
In the univariate analysis, no factors (among sex, previous nephrectomy, histology, grading, metastatic disease at diagnosis, previous metastasectomy, IMDC risk group, ECOG PS, and initial pazopanib dose) were significantly associated with PFS. Con
Notwithstanding the limit of multivariate analysis in such a small sample size, it was performed for OS, and only the absence of metastatic disease at diagnosis (hazard ratio = 8.49, 95%CI: 1.76-40.90; P = 0.008) maintained a positive impact on OS.
The present analysis reported the most extensive case series, to our knowledge, treated with the TKI pazopanib as first-line systemic therapy for advanced nccRCC patients.
The activity and efficacy outcomes are in line with those reported by two prior series from the literature, with an ORR around 27%, a good DCR (over 80%), an mPFS over 1 year, and a good mOS of 27.7 mo. Compared to the other older reports, our study also included patients receiving new-generation drugs as second-line after pazopanib (i.e., nivolumab or cabozantinib), suggesting (in the univariate analysis) this choice as significantly associated with improved survival and providing some evidence for these treatment sequences in nccRCC.
Regarding safety, pazopanib was generally well-tolerated, with G3 TRAEs occur
The limitations of our report are represented by the retrospective nature, the heterogeneous follow-up time (with a wide range, but a good median value), the lack of central independent revision for the histological samples (nccRCC histology based on the original diagnosis), and the limited number of patients receiving new-generation drugs (such as cabozantinib or nivolumab) in other treatment lines. On the other hand, the present analysis corroborates with the largest sample size two previous similar retrospective case series, providing herein clean data for pure first-line setting (as a difference vs Matrana et al[12]), and supporting the use of pazopanib as a single agent for nccRCC patients with advanced disease.
A further single-TKI alternative could be available in the next future as a primary choice for the subgroup of pRCC patients, represented by the third generation TKI cabozantinib. Currently approved as a first-line treatment option for ccRCC patients with poor- or intermediate-risk according to the IMDC model, this drug was investigated in a prospective study as a first- or second-line TKI for advanced pRCC. It demonstrated improved PFS (median 9.0 mo, 95%CI: 6-12) vs the comparator sunitinib (5.6 mo, 95%CI: 3-7; HR = 0.60, 95%CI: 0.37-0.97, P = 0.019) and ORR (23% vs 4% for sunitinib, P = 0.010), at the cost of quite manageable toxicity and a toxic death reported[15].
Beyond TKIs, the advent of immunotherapy has finally landed also in the field of nccRCC, reporting initial findings from prospective monotherapy trials. The single-arm phase II Keynote-427 trial investigated the efficacy and safety of single-agent pembrolizumab, a PD-1 inhibitor, in advanced nccRCC. In this study, 71.5% of patients had confirmed pRCC, 12.7% had chRCC, and 15.8% had unclassified RCC histology. Overall, the ORR was 26.7% (28.8% for papillary, 9.5% for chromophobe, and 30.8% for unclassified RCC); the mPFS was 4.2 mo (95%CI: 2.9-5.6), and the mOS was 28.9 mo. The toxicity was manageable, with 69.7% of patients reporting TRAEs; nevertheless, two deaths were reported as treatment-related[16]. These findings are pretty encou
Another first-line trial (Checkmate-920) recently investigated an immunotherapy combining ipilimumab and nivolumab (anti-CTLA-4 and anti-PD1 respectively) in a nccRCC treatment-naïve population. The first results reported relatively high rates of G3 (92.3%) and G4 (36.5%) toxicities (but without toxic deaths), ORR of 19.6%, and mPFS of 3.7 mo (95%CI: 2.7-4.6)[17]. The SUNNIFORECAST trial, a phase II ran
On the one hand, RCC with sarcomatoid features (irrespective of the primary his
Meanwhile, the single-agent options should include pazopanib, favored by excellent manageability in terms of safety, and supported by high feasibility and excellent cost-effectiveness, especially for elderly patients.
Non-clear cell metastatic renal-cell carcinoma (nccRCC) has dismal results with standard systemic therapies and a generally worse prognosis when compared to its clear-cell counterpart.
We aimed to provide a piece of real-life evidence on the use of pazopanib in this patient subgroup.
To assess the activity, efficacy, and safety of pazopanib as first-line therapy for advanced nccRCC patients treated in a real-life setting.
This was a multicenter retrospective observational analysis.
The objective response rate with pazopanib was 27.1%, the disease control rate was 83.3%, and the median progression-free survival and overall survival were 12.3 (95%CI: 3.6-20.9) mo and 27.7 (95%CI: 18.2-37.1) mo, respectively. Grade 3 adverse events occurred in 20% of patients, and no grade 4 or 5 toxicities were found.
Pazopanib should be considered as a good first-line option for metastatic RCC with variant histology.
Pazopanib warrants further development in metastatic RCC with variant histology.
Provenance and peer review: Invited article; Externally peer reviewed.
Specialty type: Oncology
Country/Territory of origin: Italy
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): B
Grade C (Good): 0
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Watanabe D S-Editor: Gong ZM L-Editor: Wang TQ P-Editor: Yuan YY
| 1. | Signoretti S, Flaifel A, Chen YB, Reuter VE. Renal Cell Carcinoma in the Era of Precision Medicine: From Molecular Pathology to Tissue-Based Biomarkers. J Clin Oncol. 2018;JCO2018792259. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 41] [Cited by in RCA: 55] [Article Influence: 7.9] [Reference Citation Analysis (0)] |
| 2. | Kroeger N, Xie W, Lee JL, Bjarnason GA, Knox JJ, Mackenzie MJ, Wood L, Srinivas S, Vaishamayan UN, Rha SY, Pal SK, Yuasa T, Donskov F, Agarwal N, Kollmannsberger CK, Tan MH, North SA, Rini BI, Choueiri TK, Heng DY. Metastatic non-clear cell renal cell carcinoma treated with targeted therapy agents: characterization of survival outcome and application of the International mRCC Database Consortium criteria. Cancer. 2013;119:2999-3006. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 147] [Cited by in RCA: 188] [Article Influence: 15.7] [Reference Citation Analysis (0)] |
| 3. | Motzer R, Alekseev B, Rha SY, Porta C, Eto M, Powles T, Grünwald V, Hutson TE, Kopyltsov E, Méndez-Vidal MJ, Kozlov V, Alyasova A, Hong SH, Kapoor A, Alonso Gordoa T, Merchan JR, Winquist E, Maroto P, Goh JC, Kim M, Gurney H, Patel V, Peer A, Procopio G, Takagi T, Melichar B, Rolland F, De Giorgi U, Wong S, Bedke J, Schmidinger M, Dutcus CE, Smith AD, Dutta L, Mody K, Perini RF, Xing D, Choueiri TK; CLEAR Trial Investigators. Lenvatinib plus Pembrolizumab or Everolimus for Advanced Renal Cell Carcinoma. N Engl J Med. 2021;384:1289-1300. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 600] [Cited by in RCA: 1145] [Article Influence: 286.3] [Reference Citation Analysis (0)] |
| 4. | Choueiri TK, Powles T, Burotto M, Bourlon MT, Zurawski B, Oyervides Juárez VM, Hsieh JJ, Basso U, Shah AY, Suarez C, Hamzaj A, Barrios CH, Richardet M, Pook D, Tomita Y, Escudier B, Zhang J, Simsek B, Apolo AB, Motzer RJ. Nivolumab + cabozantinib vs. sunitinib in first-line treatment for advanced renal cell carcinoma: First results from the randomized phase III CheckMate 9ER trial. Ann Oncol. 2020;31 (suppl_4):S1142-S1215. [RCA] [DOI] [Full Text] [Cited by in Crossref: 69] [Cited by in RCA: 85] [Article Influence: 17.0] [Reference Citation Analysis (0)] |
| 5. | Rini BI, Plimack ER, Stus V, Gafanov R, Hawkins R, Nosov D, Pouliot F, Alekseev B, Soulières D, Melichar B, Vynnychenko I, Kryzhanivska A, Bondarenko I, Azevedo SJ, Borchiellini D, Szczylik C, Markus M, McDermott RS, Bedke J, Tartas S, Chang YH, Tamada S, Shou Q, Perini RF, Chen M, Atkins MB, Powles T; KEYNOTE-426 Investigators. Pembrolizumab plus Axitinib versus Sunitinib for Advanced Renal-Cell Carcinoma. N Engl J Med. 2019;380:1116-1127. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1797] [Cited by in RCA: 2398] [Article Influence: 399.7] [Reference Citation Analysis (0)] |
| 6. | Motzer RJ, Rini BI, McDermott DF, Arén Frontera O, Hammers HJ, Carducci MA, Salman P, Escudier B, Beuselinck B, Amin A, Porta C, George S, Neiman V, Bracarda S, Tykodi SS, Barthélémy P, Leibowitz-Amit R, Plimack ER, Oosting SF, Redman B, Melichar B, Powles T, Nathan P, Oudard S, Pook D, Choueiri TK, Donskov F, Grimm MO, Gurney H, Heng DYC, Kollmannsberger CK, Harrison MR, Tomita Y, Duran I, Grünwald V, McHenry MB, Mekan S, Tannir NM; CheckMate 214 investigators. Nivolumab plus ipilimumab versus sunitinib in first-line treatment for advanced renal cell carcinoma: extended follow-up of efficacy and safety results from a randomised, controlled, phase 3 trial. Lancet Oncol. 2019;20:1370-1385. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 389] [Cited by in RCA: 615] [Article Influence: 102.5] [Reference Citation Analysis (0)] |
| 7. | Tannir NM, Jonasch E, Albiges L, Altinmakas E, Ng CS, Matin SF, Wang X, Qiao W, Dubauskas Lim Z, Tamboli P, Rao P, Sircar K, Karam JA, McDermott DF, Wood CG, Choueiri TK. Everolimus Versus Sunitinib Prospective Evaluation in Metastatic Non-Clear Cell Renal Cell Carcinoma (ESPN): A Randomized Multicenter Phase 2 Trial. Eur Urol. 2016;69:866-874. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 200] [Cited by in RCA: 278] [Article Influence: 27.8] [Reference Citation Analysis (0)] |
| 8. | Armstrong AJ, Halabi S, Eisen T, Broderick S, Stadler WM, Jones RJ, Garcia JA, Vaishampayan UN, Picus J, Hawkins RE, Hainsworth JD, Kollmannsberger CK, Logan TF, Puzanov I, Pickering LM, Ryan CW, Protheroe A, Lusk CM, Oberg S, George DJ. Everolimus versus sunitinib for patients with metastatic non-clear cell renal cell carcinoma (ASPEN): a multicentre, open-label, randomised phase 2 trial. Lancet Oncol. 2016;17:378-388. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 248] [Cited by in RCA: 330] [Article Influence: 36.7] [Reference Citation Analysis (0)] |
| 9. | Motzer RJ, Hutson TE, Cella D, Reeves J, Hawkins R, Guo J, Nathan P, Staehler M, de Souza P, Merchan JR, Boleti E, Fife K, Jin J, Jones R, Uemura H, De Giorgi U, Harmenberg U, Wang J, Sternberg CN, Deen K, McCann L, Hackshaw MD, Crescenzo R, Pandite LN, Choueiri TK. Pazopanib versus sunitinib in metastatic renal-cell carcinoma. N Engl J Med. 2013;369:722-731. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1350] [Cited by in RCA: 1471] [Article Influence: 122.6] [Reference Citation Analysis (0)] |
| 10. | Escudier B, Porta C, Bono P, Powles T, Eisen T, Sternberg CN, Gschwend JE, De Giorgi U, Parikh O, Hawkins R, Sevin E, Négrier S, Khan S, Diaz J, Redhu S, Mehmud F, Cella D. Randomized, controlled, double-blind, cross-over trial assessing treatment preference for pazopanib versus sunitinib in patients with metastatic renal cell carcinoma: PISCES Study. J Clin Oncol. 2014;32:1412-1418. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 306] [Cited by in RCA: 311] [Article Influence: 28.3] [Reference Citation Analysis (0)] |
| 11. | Buti S, Bersanelli M, Maines F, Facchini G, Gelsomino F, Zustovich F, Santoni M, Verri E, De Giorgi U, Masini C, Morelli F, Vitale MG, Sava T, Prati G, Librici C, Fraccon AP, Fornarini G, Maruzzo M, Leonardi F, Caffo O. First-Line PAzopanib in NOn-clear-cell Renal cArcinoMA: The Italian Retrospective Multicenter PANORAMA Study. Clin Genitourin Cancer. 2017;15:e609-e614. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 25] [Cited by in RCA: 43] [Article Influence: 5.4] [Reference Citation Analysis (0)] |
| 12. | Matrana MR, Baiomy A, Campbell M, Alamri S, Shetty A, Teegavarapu P, Kalra S, Xiao L, Atkinson B, Corn P, Jonasch E, Elsayes KM, Tannir NM. Outcomes of Patients With Metastatic Non-Clear-Cell Renal Cell Carcinoma Treated With Pazopanib. Clin Genitourin Cancer. 2017;15:e205-e208. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 20] [Cited by in RCA: 23] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 13. | Eisenhauer EA, Therasse P, Bogaerts J, Schwartz LH, Sargent D, Ford R, Dancey J, Arbuck S, Gwyther S, Mooney M, Rubinstein L, Shankar L, Dodd L, Kaplan R, Lacombe D, Verweij J. New response evaluation criteria in solid tumours: revised RECIST guideline (version 1.1). Eur J Cancer. 2009;45:228-247. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 15860] [Cited by in RCA: 21602] [Article Influence: 1350.1] [Reference Citation Analysis (1)] |
| 14. | U.S. Department of Health and Human Services. Common Terminology Criteria for Adverse Events (CTCAE) Version 4.0 Published: June 14, 2010. Available from: https://www.eortc.be/services/doc/ctc/ctcae_4.03_2010-06-14_quickrefe. |
| 15. | Pal SK, Tangen C, Thompson IM Jr, Balzer-Haas N, George DJ, Heng DYC, Shuch B, Stein M, Tretiakova M, Humphrey P, Adeniran A, Narayan V, Bjarnason GA, Vaishampayan U, Alva A, Zhang T, Cole S, Plets M, Wright J, Lara PN Jr. A comparison of sunitinib with cabozantinib, crizotinib, and savolitinib for treatment of advanced papillary renal cell carcinoma: a randomised, open-label, phase 2 trial. Lancet. 2021;397:695-703. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 178] [Cited by in RCA: 181] [Article Influence: 45.3] [Reference Citation Analysis (0)] |
| 16. | McDermott DF, Lee JL, Ziobro M, Suarez C, Langiewicz P, Matveev VB, Wiechno P, Gafanov RA, Tomczak P, Pouliot F, Donskov F, Alekseev BY, Shin SJ, Bjarnason GA, Castellano D, Silverman RK, Perini RF, Schloss C, Atkins MB. Open-Label, Single-Arm, Phase II Study of Pembrolizumab Monotherapy as First-Line Therapy in Patients With Advanced Non-Clear Cell Renal Cell Carcinoma. J Clin Oncol. 2021;39:1029-1039. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 119] [Cited by in RCA: 157] [Article Influence: 39.3] [Reference Citation Analysis (0)] |
| 17. | Emamekhoo H, Olsen M, Carthon BC, Drakaki A, Tykodi SS. Safety and efficacy of nivolumab plus ipilimumab (NIVO+IPI) in patients with advanced renal cell carcinoma (aRCC) with brain metastases: Interim analysis of CheckMate 920. J Clin Oncol. 2019;37:4517-4517. [RCA] [DOI] [Full Text] [Cited by in Crossref: 24] [Cited by in RCA: 24] [Article Influence: 4.0] [Reference Citation Analysis (0)] |
| 18. | Iacovelli R, Ciccarese C, Bria E, Bracarda S, Porta C, Procopio G, Tortora G. Patients with sarcomatoid renal cell carcinoma - re-defining the first-line of treatment: A meta-analysis of randomised clinical trials with immune checkpoint inhibitors. Eur J Cancer. 2020;136:195-203. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 32] [Cited by in RCA: 54] [Article Influence: 10.8] [Reference Citation Analysis (0)] |