Published online Nov 24, 2021. doi: 10.5306/wjco.v12.i11.1023
Peer-review started: April 13, 2021
First decision: July 3, 2021
Revised: July 26, 2021
Accepted: October 14, 2021
Article in press: October 14, 2021
Published online: November 24, 2021
Processing time: 219 Days and 19.1 Hours
Lynch syndrome (LS) is a hereditary cancer predisposition syndrome associated with increased risk of multiple cancers. While colorectal cancer surveillance decreases mortality in LS and is recommended by guidelines, there is lack of evidence for the efficacy of surveillance for extra-colonic cancers associated with LS, including small intestinal cancer (SIC) and urinary tract cancer (UTC). Given the limited evidence, guidelines do not consistently recommend surveillance for SIC and UTC, and it remains unclear how often individuals will choose to undergo and follow through with extra-colonic surveillance recommendations.
To study factors associated with SIC and UTC surveillance uptake and outcomes in LS.
This is an IRB-approved retrospective analysis of individuals with LS seen at a tertiary care referral center. Included individuals had a pathogenic or likely pathogenic variant in MLH1, MSH2, MSH6, PMS2, or EPCAM, or were a confirmed obligate carrier, and had at least one documented visit to our center. Information regarding SIC and UTC surveillance was captured for each individual, and detailed personal and family history was obtained for individuals who had an initial LS management visit in our center’s dedicated high-risk LS clinic between January 1, 2017 and October 29, 2020. During these initial management visits, all patients had in-depth discussions of SIC and UTC surveillance with 1 of 3 providers experienced in LS management to promote informed decision-making about whether to pursue SIC and/or UTC surveillance. Statistical analysis using Pearson’s chi-squared test and Wilcoxon rank-sum test was completed to understand the factors associated with pursuit and completion of SIC and UTC surveillance, and a P value below 0.05 was deemed statistically significant.
Of 317 individuals with LS, 86 (27%) underwent a total of 105 SIC surveillance examinations, with 5 leading to additional work-up and no SICs diagnosed. Additionally, 99 (31%) patients underwent a total of 303 UTC surveillance examinations, with 19 requiring further evaluation and 1 UTC identified. Of 155 individuals who had an initial LS management visit between January 1, 2017 and October 29, 2020, 63 (41%) chose to undergo SIC surveillance and 58 (37%) chose to undergo UTC surveillance. However, only 26 (41%) and 32 (55%) of those who initially chose to undergo SIC or UTC surveillance, respectively, successfully completed their surveillance examinations. Individuals with a pathogenic variant in MSH2 or EPCAM were more likely to initially choose to undergo SIC surveillance (P = 0.034), and older individuals were more likely to complete SIC surveillance (P = 0.007). Choosing to pursue UTC surveillance was more frequent among older individuals (P = 0.018), and females more frequently completed UTC surveillance (P = 0.002). Personal history of cancer and family history of SIC or UTC were not significantly associated with electing nor completing surveillance. Lastly, the provider discussing SIC/UTC surveillance was significantly associated with subsequent surveillance choices.
Pursuing and completing SIC/UTC surveillance in LS is influenced by several factors, however broad incorporation in LS management is likely unhelpful due to low yield and frequent false positive results.
Core Tip: This retrospective study of a Lynch syndrome (LS) cohort measured the uptake and outcome of small intestinal cancer (SIC) and urinary tract cancer (UTC) surveillance. When given the option of surveillance, a minority of patients elected surveillance, and patient completion of surveillance exams was suboptimal. Completed surveillance exams rarely detected SIC/UTC and resulted in multiple false positives that led to additional follow-up procedures. Pursuing and completing SIC/UTC surveillance in LS was influenced by several factors, however given the low yield and positive predictive value, broad incorporation of SIC/UTC surveillance in LS management is unlikely to be helpful.
- Citation: DeJesse J, Vajravelu RK, Dudzik C, Constantino G, Long JM, Wangensteen KJ, Valverde KD, Katona BW. Uptake and outcomes of small intestinal and urinary tract cancer surveillance in Lynch syndrome. World J Clin Oncol 2021; 12(11): 1023-1036
- URL: https://www.wjgnet.com/2218-4333/full/v12/i11/1023.htm
- DOI: https://dx.doi.org/10.5306/wjco.v12.i11.1023
Lynch syndrome (LS) is an autosomal dominant cancer predisposition syndrome resulting from a disease-causing variant in the MLH1, MSH2, MSH6, PMS2, or EPCAM gene. LS is primarily associated with increased colorectal and endometrial cancer risk but is also associated with increased risk of gastric, small intestinal, hepatobiliary, ovarian, urinary tract, brain, and skin cancers[1-3]. Colorectal cancer surveillance has proven effective in LS, with colonoscopy decreasing colorectal cancer mortality in LS by approximately 65%, which has led to inclusion of frequent colonoscopy in all LS management guidelines[4-8]. There is less agreement about the utility of surveillance for extra-colonic cancers in LS. Although recent reports show upper gastrointestinal cancer surveillance could detect gastric and duodenal cancers in LS[9,10], differences remain in recommended upper gastrointestinal cancer surveillance[5,11]. There is a paucity of evidence supporting efficacy of other types of extra-colonic cancer surveillance in LS, including small intestinal cancer (SIC) and urinary tract cancer (UTC) surveillance, as well as a lack of data addressing provider recommendation and patient uptake of SIC and UTC surveillance.
The cumulative risk of small intestinal adenocarcinoma in LS is up to 11%, markedly higher than the general population risk of 0.3%[5,7,12-14]. Options for SIC surveillance include small bowel follow through (SBFT), video capsule endoscopy (VCE), or CT/MRI enterography, with VCE being considered the most sensitive for small intestinal pathology[15,16]. However, there are limitations to the use of VCE, including cost and possible capsule retention in those with a history of prior abdominal surgery[17]. A study of SIC surveillance with VCE in 35 asymptomatic LS patients identified 2 adenomas and 1 adenocarcinoma, all distal to the duodenum[18]. A separate study of VCE performed on 200 asymptomatic LS patients led to the discovery of a small intestinal adenoma and a small intestinal adenocarcinoma[19]; this same group performed follow-up VCE on 155 patients of the same cohort a mean interval of 2 years after the first VCE which led to no additional small intestinal neoplasms being detected[20]. Other prospective and retrospective studies of LS cohorts concluded that the low frequency of SIC in LS prevented surveillance from being cost effective[20,21]. Given the limited data on SIC surveillance, there is currently no consensus recommendation regarding dedicated SIC surveillance for individuals with LS.
Individuals with LS are also at increased risk of UTC, including cancer of the renal pelvis, bladder, and ureters[21-23]. Cumulative risk of UTC in LS is up to 28%, a 20-fold increase in risk compared to the general population, with the highest risk seen in males with pathogenic MSH2 variants[5,13,21,24,25]. Options for UTC surveillance include urinalysis, urine cytology, CT urogram, or cystoscopy. A study of the Danish HNPCC Registry found that only 2 (0.1%) of 1,868 urine cytology screens in 977 patients led to a diagnosis of an asymptomatic tumor, and 22 screens (1.2%) led to a false positive result, leading the authors to conclude that urine cytology is not an ideal surveillance method in LS[23]. Another study of the same registry found that 78% of UTCs in the cohort occurred in patients without a family history of UTC, and 73% of UTCs were in individuals with a pathogenic MSH2 variant or a first degree relative of a MSH2 carrier, leading the authors to suggest that UTC surveillance should not be limited to patients with a family history of UTC and should be focused on patients with pathogenic MSH2 variants[25]. There has been disagreement amongst LS guidelines regarding the utility of UTC surveillance as some groups, like the U.S. Multi-Society Task Force, have recommended considering routine surveillance[6,26-29], while others, including the Mallorca group, have deemed there is not sufficient evidence to recommend regular surveillance[5,7,23,30].
Apart from whether SIC or UTC surveillance is recommended, it is equally important to understand whether individuals with LS will undergo surveillance if recommended, especially given the already intensive surveillance recommendations often mandated as part of a comprehensive LS surveillance program. Data characterizing extra-colonic cancer surveillance compliance in LS are limited, however a large study of annual UTC surveillance in LS found a compliance rate of only 29%[23]. This compliance is substantially lower than colonoscopy compliance (68%-85%)[31,32]. Furthermore, it remains uncertain what factors influence a patient’s decision to pursue SIC or UTC surveillance in the presence of variable guidelines and recommendations.Herein, we aim to characterize the uptake and outcomes of SIC and UTC surveillance in LS, including patients’ decisions about whether to pursue surveillance despite the limited evidence on efficacy and varying guideline recommendations, whether these individuals successfully complete surveillance, and the yield of the surveillance examinations.
This is a retrospective study of individuals with LS seen at Penn Medicine, approved by the University of Pennsylvania Institutional Review Board. Individuals with LS had a confirmed pathogenic or likely pathogenic variant in MLH1, MSH2, MSH6, PMS2, or EPCAM, or were an obligate carrier of a familial pathogenic or likely pathogenic variant in one of these genes, and had at least one visit to the health system.
The electronic medical records of all LS patients were reviewed for pertinent details regarding demographics, medical history, family history, and surveillance examination results. Data were captured and entered into a REDCap database hosted at the University of Pennsylvania to facilitate statistical analysis. SIC surveillance was defined as a VCE or SBFT ordered in the absence of symptoms concerning for small intestinal pathology. Abnormal SIC surveillance results included any finding that was suspicious for a polyp or neoplastic process. UTC surveillance was defined as a urinalysis or urine cytology ordered in the absence of symptoms concerning for urinary tract pathology. Abnormal UTC surveillance results included a urine dipstick positive for blood, microscopic detection of red blood cells above the upper limit of normal, or atypical/abnormal urothelial cells found on urine cytology.
In an effort to further characterize recent SIC and UTC surveillance decisions, this study reviewed data from individuals who had their initial office visit for LS management in the Penn Medicine Gastrointestinal Cancer Genetics Program between January 1, 2017 and October 29, 2020 and were seen by 1 of 3 providers experienced in LS management. During the initial office visit to formulate the LS surveillance plan, each patient was engaged in detailed discussion about SIC and UTC surveillance covering the risks of surveillance (including false positive results generating additional evaluations), potential benefits, lack of robust data showing surveillance prevents cancer and/or reduces mortality, and lack of consistent guideline recommendations. If after this in-depth discussion a patient decides to pursue SIC and/or UTC surveillance, the provider orders appropriate testing and notes the patient’s decision in their chart. If no evidence of a completed surveillance examination was in the patient’s electronic medical record, the surveillance examination was noted as incomplete.
Statistical analysis using Pearson’s chi-squared test for categorical variables and Wilcoxon rank-sum test for continuous variables was completed with a Type I error rate of 0.05 using Stata software version 16.1.
Three hundred seventeen individuals with LS had a visit to the health system and were included as part to the cohort; the cohort was mostly white (86%), non-Hispanic (98%), and female (59%), with a median age of 49 years (IQR: 38-61 years) (Supplementary Table 1). Distribution of LS genes in the cohort was relatively uniform as the percentage of pathogenic/likely pathogenic variants in MLH1, MSH2/EPCAM, MSH6, and PMS2 was 23%, 35%, 22%, and 20% respectively. Of the 317 individuals with LS, 86 (27%) underwent a total of 105 SIC surveillance examinations with the majority (55%) being VCEs. There were no SICs diagnosed. However, 5 of these surveillance VCEs were suspicious for a small bowel polyp, which led to further work-up with invasive procedures (Table 1). None of the follow-up procedures led to the identification of any neoplastic small intestinal lesions. The positive predictive value (PPV) for VCE was 0% with a one-sided 97.5% confidence interval of 0%-52%. Of this same cohort of 317 individuals with LS, 99 (31%) underwent a total of 303 UTC surveillance examinations, the majority (65%) of which were urinalysis, with 19 of these surveillance tests showing abnormal findings that prompted further evaluation (Table 1). Of the 19 abnormal surveillance results leading to further work up, 10 (53%) were urine cytologies, and 1 surveillance urine cytology led to a single UTC diagnosis of a non-invasive high grade urothelial papillary carcinoma in a 64 year-old male individual with a pathogenic variant in MSH2. This patient had localized disease that was treated with nephroureterectomy and retroperitoneal/pelvic lymph node dissection and is subsequently followed by regular cystoscopy and MRI without reoccurrence. Urinalysis had a PPV of 0% with a one-sided 97.5% confidence interval of 0%-34%, and urine cytology had a PPV of 10% with a 95% confidence interval of 0.25%-45%.
| n = 317 | |
| Individuals who underwent SIC surveillance | 86 (27%) |
| SIC surveillance exams completed per individual, median (IQR) | 1 (1-1) |
| Total completed SIC surveillance exams | 105 |
| VCE | 58 (55%) |
| SBFT | 47 (45%) |
| Abnormal SIC surveillance exams leading to further work-up | 5 (5%) |
| VCE | 5 (100%) |
| SBFT | 0 (0%) |
| Abnormal SIC surveillance exams leading to a SIC diagnosis | 0 (0%) |
| Individuals who underwent UTC surveillance | 99 (31%) |
| UTC surveillance exams completed per individual, median (IQR) | 2 (1-3) |
| Total completed UTC surveillance exams | 303 |
| Urinalysis | 197 (65%) |
| Urine cytology | 106 (35%) |
| Abnormal UTC surveillance exams leading to further work-up | 19 (6%) |
| Urinalysis | 9 (47%) |
| Urine Cytology | 10 (53%) |
| Abnormal UTC surveillance exams leading to a UTC diagnosis | 1 (5%) |
To understand the factors influencing uptake of SIC and UTC surveillance in LS, we further analyzed those individuals with LS who had an initial LS management visit with our program between January 1, 2017 and October 29, 2020. This cohort was comprised of 155 individuals who were primarily white (90%) and female (65%), and the majority had private insurance (86%) (Table 2). There was a near equal distribution of patients across all LS genes, with 70 (45%) of these individuals having a personal history of cancer, including 2 (1%) with SIC and 6 (4%) with UTC. Almost all (97%) of these individuals had a family history of cancer, with 8 (5%) having a family history of SIC, and 35 (23%) having a family history of UTC. A majority of the cohort (78%) was treated by a single provider.
| n = 155 | |
| Age (yr), median (IQR) | 46 (33-58) |
| Female sex | 100 (65%) |
| Race | |
| White | 139 (90%) |
| Black | 3 (2%) |
| Asian | 7 (5%) |
| Other | 2 (1%) |
| Unknown | 4 (3%) |
| Hispanic ethnicity | 2 (1%) |
| Ashkenazi Jewish ancestry | 16 (10%) |
| Lynch syndrome gene | |
| MLH1 | 29 (19%) |
| MSH2 or EPCAM | 40 (26%) |
| MSH6 | 45 (29%) |
| PMS2 | 41 (26%) |
| Personal history of cancer | 70 (45%) |
| Small intestinal | 2 (1%) |
| Urinary tract | 6 (4%) |
| Colorectal | 30 (19%) |
| Family history of cancer | 151 (97%) |
| Small intestinal | 8 (5%) |
| Urinary tract | 35 (23%) |
| Colorectal | 113 (73%) |
| Type of insurance | |
| Private insurance | 134 (86%) |
| Medicare insurance | 16 (10%) |
| Medicaid insurance | 5 (3%) |
| Provider | |
| Provider 1 | 121 (78%) |
| Provider 2 | 17 (11%) |
| Provider 3 | 17 (11%) |
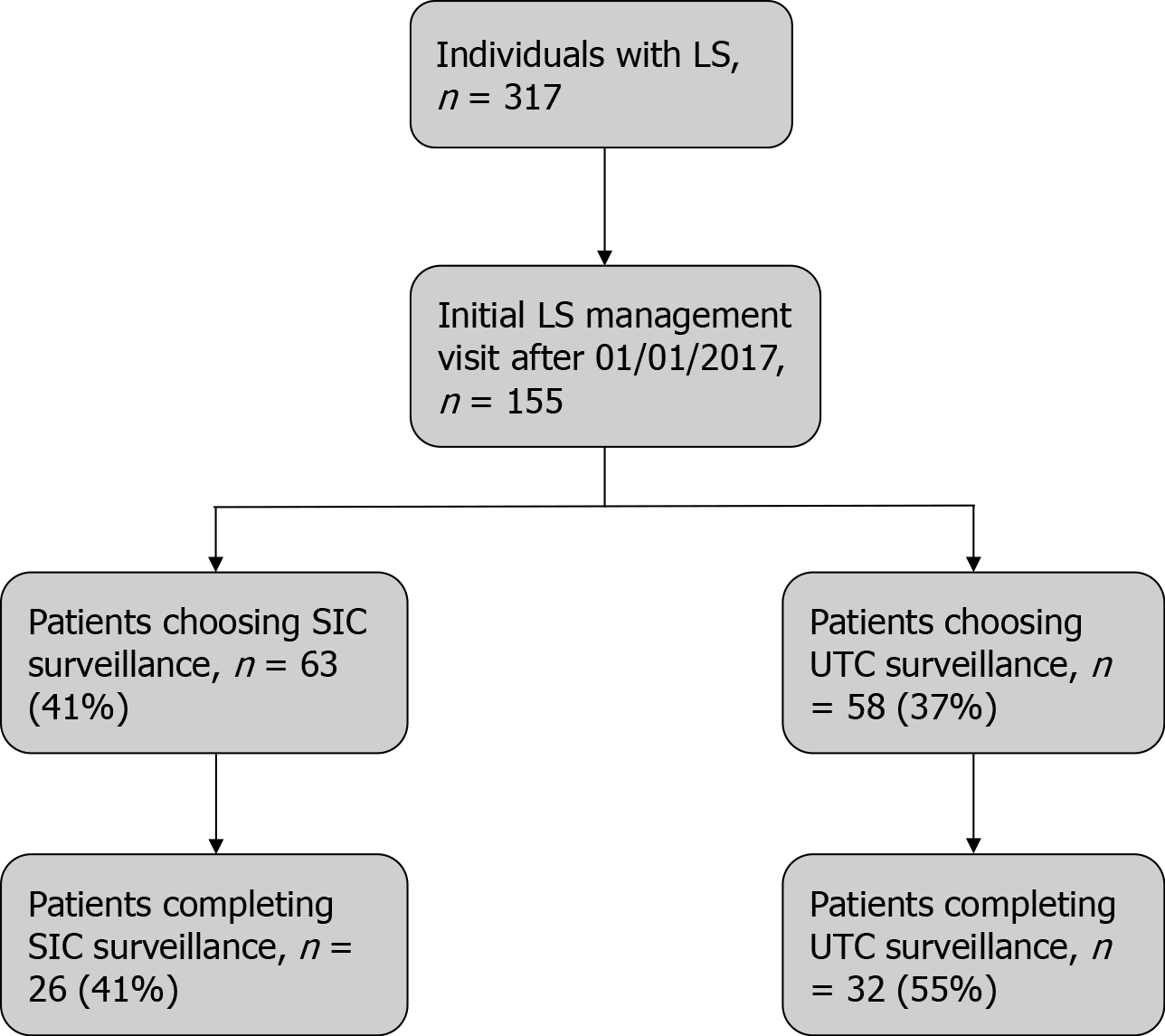
At their initial LS management visit, during which the risks and benefits of SIC and UTC surveillance were reviewed to allow patients to make an informed decision, 63 (41%) patients chose to undergo SIC surveillance and 58 (37%) chose to undergo UTC surveillance (Figure 1). However, of those who chose to undergo SIC and UTC surveillance at their initial management visit, only 26 (41%) and 32 (55%) completed their SIC or UTC surveillance examinations, respectively.
We next assessed for factors associated with choosing to undergo SIC and/or UTC surveillance as well as successfully completing surveillance tests. Individuals with a pathogenic variant in MSH2 or EPCAM were more likely to initially choose to undergo SIC surveillance compared to those with other mutations as this group accounted for 24 (38%) of the individuals who chose SIC surveillance (Table 3, P = 0.034). Older age was associated with completion of SIC surveillance (P = 0.007), as the median age of those who completed SIC surveillance was 56 years—14 years higher than the median age of those who did not complete surveillance. Additionally, there were statistically significant differences in choosing and completing SIC surveillance in a provider-dependent manner.
For UTC surveillance, older age was associated with choosing to undergo surveillance; the median age of those who chose to undergo surveillance was 48 years, 8 years higher than those who chose no surveillance (Table 4, P = 0.018). Female sex was associated with UTC surveillance completion (P = 0.002) as 26 (81%) individuals who completed UTC surveillance were female. Ashkenazi Jewish ancestry was also associated with completion of UTC surveillance (P = 0.006). The individuals who were treated by Provider 1 chose UTC surveillance less frequently (P = 0.000) but were more likely to complete surveillance exams if they chose to pursue them (P = 0.000). Personal history of cancer and family history of SIC or UTC were not associated with initially choosing to undergo surveillance or surveillance completion (Tables 3-4). Race, Hispanic ethnicity, and insurance status were also not associated with choosing nor completing surveillance (Tables 3-4).
| Surveillance chosen (n = 63) | Surveillance not chosen (n = 92) | P value | Surveillance completed (n = 26) | Surveillance not completed (n = 37) | P value | ||
| Age (yr), median (IQR) | 48 (37-59) | 42 (32-57) | 0.114 | 56 (46-62) | 42 (33-54) | 0.007a | |
| Female | 44 (70%) | 56 (61%) | 0.252 | 21 (81%) | 23 (62%) | 0.113 | |
| Race | 0.750 | 0.531 | |||||
| White | 58 (92%) | 81 (88%) | 24 (92%) | 34 (92%) | |||
| Black | 1 (2%) | 2 (2%) | 0 (0%) | 1 (3%) | |||
| Asian | 3 (5%) | 4 (4%) | 2 (8%) | 1 (3%) | |||
| Other | 0 (0%) | 2 (2%) | - | - | |||
| Unknown | 1 (2%) | 3 (3%) | 0 (0%) | 1 (3%) | |||
| Hispanic ethnicity | 1 (2%) | 1 (1%) | 0.912 | 0 (0%) | 1 (3%) | 0.211 | |
| AJ ancestry | 5 (8%) | 11 (12%) | 0.687 | 1 (4%) | 4 (11%) | 0.489 | |
| Lynch syndrome gene | 0.034a | 0.357 | |||||
| MLH1 | 10 (16%) | 19 (21%) | 5 (19%) | 5 (14%) | |||
| MSH2 or EPCAM | 24 (38%) | 16 (17%) | 7 (27%) | 17 (46%) | |||
| MSH6 | 14 (22%) | 31 (34%) | 8 (31%) | 6 (16%) | |||
| PMS2 | 15 (24%) | 26 (28%) | 6 (23%) | 9 (24%) | |||
| Personal history of cancer | 33 (52%) | 37 (40%) | 0.135 | 17 (65%) | 16 (43%) | 0.083 | |
| Family history of SIC | 5 (8%) | 3 (3%) | 0.182 | 1 (4%) | 4 (11%) | 0.340 | |
| Insurance | 0.111 | 0.314 | |||||
| Private | 58 (92%) | 76 (83%) | 25 (96%) | 33 (89%) | |||
| Medicare | 5 (8%) | 11 (12%) | 1 (4%) | 4 (11%) | |||
| Medicaid | 0 (0%) | 5 (5%) | - | - | |||
| Provider | 0.000a | 0.030a | |||||
| Provider 1 | 39 (62%) | 82 (89%) | 17 (65%) | 22 (59%) | |||
| Provider 2 | 13 (21%) | 4 (4%) | 8 (31%) | 5 (14%) | |||
| Provider 3 | 11 (17%) | 6 (7%) | 1 (4%) | 10 (27%) | |||
| Surveillance chosen (n = 58) | Surveillance not chosen(n = 97) | P value | Surveillance completed (n = 32) | Surveillance not completed (n = 26) | P value | |
| Age (yr), median (IQR) | 48 (39-60) | 40 (32-55) | 0.018a | 45 (40-61) | 50 (37-60) | 0.772 |
| Female | 37 (64%) | 63 (65%) | 0.884 | 26 (81%) | 11 (42%) | 0.002a |
| Race | 0.431 | 0.498 | ||||
| White | 54 (93%) | 85 (88%) | 30 (94%) | 24 (92%) | ||
| Black | 1 (2%) | 2 (2%) | 0 (0%) | 1 (4%) | ||
| Asian | 3 (5%) | 4 (4%) | 2 (6%) | 1 (4%) | ||
| Other | 0 (0%) | 2 (2%) | - | - | ||
| Unknown | 0 (0%) | 4 (4%) | - | - | ||
| Hispanic ethnicity | 0 (0%) | 2 (2%) | 0.336 | - | - | - |
| AJ ancestry | 3 (5%) | 13 (13%) | 0.229 | 3 (9%) | 0 (0%) | 0.006a |
| Lynch syndrome gene | 0.640 | 0.171 | ||||
| MLH1 | 8 (14%) | 21 (22%) | 3 (9%) | 5 (19%) | ||
| MSH2 or EPCAM | 17 (29%) | 23 (24%) | 9 (28%) | 8 (31%) | ||
| MSH6 | 17 (29%) | 28 (29%) | 13 (41%) | 4 (15%) | ||
| PMS2 | 16 (28%) | 25 (26%) | 7 (22%) | 9 (35%) | ||
| Personal history of cancer | 29 (50%) | 41 (42%) | 0.349 | 17 (53%) | 12 (46 %) | 0.597 |
| Family history of UTC | 15 (26%) | 20 (21%) | 0.477 | 11 (34%) | 4 (15%) | 0.118 |
| Insurance | 0.631 | 0.402 | ||||
| Private | 50 (86%) | 84 (87%) | 29 (91%) | 21 (81%) | ||
| Medicare | 7 (12%) | 9 (9%) | 3 (9%) | 4 (15%) | ||
| Medicaid | 1 (2%) | 4 (4%) | 0 (0%) | 1 (4%) | ||
| Provider | ||||||
| Provider 1 | 36 (62%) | 85 (88%) | 0.001a | 28 (88%) | 8 (31%) | 0.000 a |
| Provider 2 | 12 (21%) | 5 (5%) | 2 (6%) | 10 (38%) | ||
| Provider 3 | 10 (17%) | 7 (7%) | 2 (6%) | 8 (31%) |
Lynch syndrome is a high-risk cancer predisposition syndrome, with affected individuals requiring lifelong cancer risk management. Whereas some surveillance, such as colorectal cancer surveillance, is strongly recommended in LS, there is a lack of consistent recommendations for SIC and UTC surveillance due to the limited data showing this extra-colonic surveillance is effective. In this study, we investigated uptake of SIC and UTC surveillance in LS and the outcomes of the associated surveillance examinations. Our data shows that after engagement in an in-depth discussion on the potential benefits, risks, and limitations of SIC and UTC surveillance, a majority of patients decided to forgo surveillance, and those that initially chose to pursue surveillance had low completion rates. Additionally, we show a low PPV with frequent false positive results for both SIC and UTC surveillance, and the overall yield of cancer diagnoses was low for all surveillance methods. Taken together, our results do not support regular incorporation of SIC and UTC surveillance into standard LS cancer risk management care.
Effective cancer surveillance in LS should ideally utilize testing that is cost-effective and low risk, and surveillance should ultimately increase survival[7]. An ideal test must also have a high level of sensitivity and specificity, as false positive cancer surveillance results not only lead to further work-up but also lead to emotional distress for the patient as well as decreased compliance rates and follow-up with subsequent surveillance exams[33,34]. Additionally, false positive results can expose patients to possible harms resulting from superfluous follow-up procedures as well as additional medical costs related to these procedures. Our data showed that both SIC and UTC surveillance had a low PPV, with 24 positive surveillance studies leading to the diagnosis of only 1 neoplastic lesion. These data are consistent with a previous study of SIC screening in asymptomatic LS patients that observed 13 VCE results suspicious for SIC, none of which led to the confirmation of a SIC through follow-up testing[20]. While VCE is the most sensitive test for picking up small intestinal pathology[15,16], it may have a downside of being less specific, leading to high rates of false positive test results that require patients to undergo unnecessary invasive procedures. In addition to this observed low specificity, the sensitivity of urine cytology in asymptomatic LS patients has also been previously reported to be poor (29%)[23]. Effective cancer surveillance should also result in improved survival. With only one individual in our study having a surveillance-detected UTC or SIC, we are unable to meaningfully comment on the impact of surveillance on cancer survival, however at this time it remains unclear if early diagnosis of UTC or SIC leads to higher survival rates[7,35]. Together, the low yield and low PPV of SIC and UTC surveillance described in this study do not provide support for broad inclusion of SIC and/or UTC surveillance in LS management; however, whether this surveillance should be considered for certain sub-groups of patients with LS will require future larger studies.
The majority of the patients in our cohort chose not to pursue SIC or UTC surveillance after having an in-depth discussion with their provider about the risks, benefits, and limitations of surveillance. It is likely that this discussion and the provider involved influenced the patients’ decision making; accurate risk perception has previously been observed to impact LS patients’ behaviors towards cancer surveillance[36]. The difference in cancer surveillance behaviors between patients of different providers could result from differing manners in which the providers discussed surveillance options in an individual’s initial LS management visit. In this study, individuals with pathogenic variants in MSH2 or EPCAM chose to pursue SIC surveillance more frequently, while older individuals chose to pursue UTC surveillance more often. In a study of colorectal cancer survivors with Lynch-like syndrome, increased cancer worry was associated with a stronger belief that extra-colonic cancer surveillance was necessary[37]; perhaps learning of the increased cancer risk that comes with age and pathogenic MSH2 or EPCAM variants compelled patients in this study to opt for additional surveillance. The observation that individuals with MSH2 or EPCAM pathogenic variants were more likely to choose to undergo SIC surveillance, but not UTC surveillance, compared to individuals with pathogenic variants in other genes may be due to a Type I error or may also be influenced by other factors that were not captured in this study. The choice to initially pursue surveillance was likely also influenced by other factors not examined in this study. Some individuals may have decided to forgo surveillance due to the emotional distress that comes with the increased surveillance burden and the requirement to navigate potentially challenging health care system infrastructures, factors that have been shown to influence other cancer surveillance in LS[36]. Other variables such as associated costs and familial obligations may also have affected patient decision making. A future prospective study surveying the attitudes and perceptions of individuals with LS about low-evidence surveillance tests would be important to help answer this question.
The completion rate of SIC and UTC surveillance in this cohort was 41% and 55%, respectively, which is higher than a previous study of compliance with UTC surveillance in LS finding a rate of 29%[23]. Our increased completion rate may have resulted from the in-depth discussion on this surveillance between the patient and provider. However, our observed completion rate was lower than the reported compliance rate for colonoscopy within the LS population (68-85%)[31,32]. Colorectal cancer risk is well-recognized as one of the highest cancer risks in LS, and therefore, the lower completion rate compared to colorectal cancer surveillance may be due to the individuals’ perception of the decreased risk of extra-colonic cancers[36]. The discrepancy could also be due to provider emphasis on the effectiveness of colonoscopy to decrease mortality and morbidity. In this study, we observed that providers may influence an individual’s choices towards SIC and UTC surveillance, both in terms of choosing to undergo surveillance and completing surveillance. Additionally, we found completion of SIC surveillance was more frequent among older individuals, and completion of UTC surveillance was more frequent among those of female sex and Ashkenazi Jewish ancestry. These findings present a contrast to another study of a LS cohort which observed that cancer surveillance completion was associated with younger age[38]. This other study also ascertained that surveillance completion was influenced by occupation status, a factor not captured by this study. Other unexamined factors could have played a role in surveillance completion, as well; for instance, the completion of surveillance may have been put on hold due to the management of other ongoing health issues.
Considering the limited information available on the effectiveness of SIC and UTC surveillance in LS, which was further obfuscated by the findings of this study, we do not believe that SIC and UTC surveillance should be broadly performed in all individuals with LS. Instead, we advocate for the individualized incorporation of these surveillance methods in a patient-dependent manner after a detailed discussion of the risks, limitations, benefits, and uncertainties. A larger prospective study would be better equipped to assess the true benefits and risks of SIC and UTC surveillance as well as to understand patients’ interest in and concerns with extra-colonic surveillance. Additionally, the low PPV of the surveillance methods observed in this study emphasize the need for further research on the cost of this surveillance and the effect of early detection of SIC and UTC on patient morbidity and mortality. Qualitative studies could also elucidate patient perspectives as individuals with LS may have negative psychological effects if multiple extra-colonic cancer surveillance studies are incorporated into their management.
Limitations of this study include that the LS cohort is from a single tertiary care center and lacks racial diversity; therefore, the results observed may not be representative of more geographically and racially diverse cohorts. Another limitation is that individuals may have completed SIC or UTC surveillance outside of our medical center, with these completed surveillance tests neither appearing in the individual’s electronic medical record nor being captured by this study. Finally, this study has a relatively small sample size, which may prevent recognition of other significant associations.
This cohort study describes outcomes of SIC and UTC surveillance in LS and identifies factors influencing the SIC and UTC surveillance practices of individuals with LS. This study highlights problems with incorporation of SIC and UTC surveillance into LS care, as illustrated by the low PPV and low overall yields of these tests. The study also shows that the pursuit and completion of these surveillance examinations may depend on the affected individual's age, sex, genotype, and provider; however at this time, there is insufficient evidence to support widespread use of SIC/UTC surveillance in all individuals with LS. Further large-scale studies on SIC and UTC surveillance are needed to better understand the utility of available surveillance tests as well as their cost effectiveness and impact on patient survival.
Lynch syndrome (LS) is an autosomal dominant cancer predisposition syndrome resulting from a disease-causing variant in the MLH1, MSH2, MSH6, PMS2, or EPCAM gene. LS is primarily associated with increased colorectal and endometrial cancer risk, but it is also associated with increased risk of small intestinal cancer (SIC) and urinary tract cancer (UTC). Cancer surveillance management for SIC and UTC has yet to be standardized for LS patients due to a lack of proven efficacy for current surveillance methods, and data regarding provider and patient interest in the current SIC and UTC surveillance methods are also lacking.
This study was interested in describing the efficacy and impact of completed SIC and UTC surveillance exams in a cohort of 317 LS patients. In addition, we were interested in patients’ decisions about whether to pursue surveillance despite the limited evidence on efficacy and varying guideline recommendations and whether these individuals successfully completed surveillance.
To characterize the uptake and outcomes of SIC and UTC surveillance among LS patients at a tertiary care referral center. We intended to analyze the factors influencing individuals' surveillance behaviors and to calculate the yield of completed surveillance exams.
This was a retrospective study of individuals with LS seen at a tertiary care referral center. Information regarding SIC and UTC surveillance was captured for each individual. Additional demographic information and medical history was collected for individuals who had an initial LS management visit in our center’s dedicated high-risk LS clinic between January 1, 2017 and October 29, 2020 to allow for analysis of individuals' behaviors after engaging in an in-depth conversation regarding surveillance with a provider in the clinic. Statistical analysis using Pearson’s chi-squared test and Wilcoxon rank-sum test was completed, and a P value below 0.05 was deemed statistically significant.
Of the 317 individuals with LS in our cohort, 27% underwent a total of 105 SIC surveillance exams, and 31% underwent a total of 303 UTC surveillance exams. Each surveillance method was found to have a low positive predictive value and yield. A single UTC was diagnosed, and 0 SICs were diagnosed. Of 155 individuals who had an initial LS management visit between January 1, 2017 and October 29, 2020, a minority of individuals chose to undergo either SIC (41%) or UTC (37%) surveillance. Only 41% of individuals completed SIC surveillance, and 55% completed UTC surveillance when ordered. Several factors were found to be significantly associated with surveillance pursuit and completion, including age, sex, genotype, and provider.
This study observed a low positive predictive value and yield for completed SIC and UTC surveillance exams, and after an in-depth conversation on the limitations and benefits of SIC and UTC surveillance, there was limited interest for this surveillance among individuals with LS. At this time, there continues to be insufficient evidence to support widespread SIC and UTC surveillance in LS.
This study highlights the need for further research in SIC and UTC surveillance in LS. More data is needed on the cost of SIC and UTC surveillance and the effect of early detection of SIC and UTC on patient morbidity and mortality. Qualitative studies are also needed to elucidate patient perspectives regarding the addition of low-evidence surveillance exams to their cancer surveillance management.
Provenance and peer review: Invited article; Externally peer reviewed.
Specialty type: Oncology
Country/Territory of origin: United States
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): 0
Grade C (Good): 0
Grade D (Fair): D
Grade E (Poor): 0
P-Reviewer: Lamba M S-Editor: Wang LL L-Editor: A P-Editor: Wang LL
| 1. | Aarnio M, Mecklin JP, Aaltonen LA, Nyström-Lahti M, Järvinen HJ. Life-time risk of different cancers in hereditary non-polyposis colorectal cancer (HNPCC) syndrome. Int J Cancer. 1995;64:430-433. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 416] [Cited by in RCA: 378] [Article Influence: 12.6] [Reference Citation Analysis (0)] |
| 2. | Lynch HT, Watson P, Shaw TG, Lynch JF, Harty AE, Franklin BA, Kapler CR, Tinley ST, Liu B, Lerman C. Clinical impact of molecular genetic diagnosis, genetic counseling, and management of hereditary cancer. Part II: Hereditary nonpolyposis colorectal carcinoma as a model. Cancer. 1999;86:2457-2463. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 1] [Reference Citation Analysis (0)] |
| 3. | Kovacs ME, Papp J, Szentirmay Z, Otto S, Olah E. Deletions removing the last exon of TACSTD1 constitute a distinct class of mutations predisposing to Lynch syndrome. Hum Mutat. 2009;30:197-203. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 119] [Cited by in RCA: 118] [Article Influence: 7.4] [Reference Citation Analysis (0)] |
| 4. | Syngal S, Brand RE, Church JM, Giardiello FM, Hampel HL, Burt RW; American College of Gastroenterology. ACG clinical guideline: Genetic testing and management of hereditary gastrointestinal cancer syndromes. Am J Gastroenterol. 2015;110:223-62; quiz 263. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 957] [Cited by in RCA: 1090] [Article Influence: 109.0] [Reference Citation Analysis (0)] |
| 5. | National Comprehensive Cancer Network. Genetic / Familial High-Risk Assessment : Colorectal (Version 1.2020) [Internet]. [cited 20 February 2021]. Available from: https://www.nccn.org/professionals/physician_gls/pdf/genetics_colon.pdf. |
| 6. | Giardiello FM, Allen JI, Axilbund JE, Boland CR, Burke CA, Burt RW, Church JM, Dominitz JA, Johnson DA, Kaltenbach T, Levin TR, Lieberman DA, Robertson DJ, Syngal S, Rex DK; US Multi-Society Task Force on Colorectal Cancer. Guidelines on genetic evaluation and management of Lynch syndrome: a consensus statement by the US Multi-Society Task Force on colorectal cancer. Gastroenterology. 2014;147:502-526. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 332] [Cited by in RCA: 363] [Article Influence: 33.0] [Reference Citation Analysis (0)] |
| 7. | Vasen HF, Blanco I, Aktan-Collan K, Gopie JP, Alonso A, Aretz S, Bernstein I, Bertario L, Burn J, Capella G, Colas C, Engel C, Frayling IM, Genuardi M, Heinimann K, Hes FJ, Hodgson SV, Karagiannis JA, Lalloo F, Lindblom A, Mecklin JP, Møller P, Myrhoj T, Nagengast FM, Parc Y, Ponz de Leon M, Renkonen-Sinisalo L, Sampson JR, Stormorken A, Sijmons RH, Tejpar S, Thomas HJ, Rahner N, Wijnen JT, Järvinen HJ, Möslein G; Mallorca group. Revised guidelines for the clinical management of Lynch syndrome (HNPCC): recommendations by a group of European experts. Gut. 2013;62:812-823. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 556] [Cited by in RCA: 535] [Article Influence: 44.6] [Reference Citation Analysis (1)] |
| 8. | Järvinen HJ, Aarnio M, Mustonen H, Aktan-Collan K, Aaltonen LA, Peltomäki P, De La Chapelle A, Mecklin JP. Controlled 15-year trial on screening for colorectal cancer in families with hereditary nonpolyposis colorectal cancer. Gastroenterology. 2000;118:829-834. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 922] [Cited by in RCA: 906] [Article Influence: 36.2] [Reference Citation Analysis (0)] |
| 9. | Kumar S, Dudzik CM, Reed M, Long JM, Wangensteen KJ, Katona BW. Upper Endoscopic Surveillance in Lynch Syndrome Detects Gastric and Duodenal Adenocarcinomas. Cancer Prev Res (Phila). 2020;13:1047-1054. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 15] [Cited by in RCA: 23] [Article Influence: 4.6] [Reference Citation Analysis (0)] |
| 10. | Ladigan-Badura S, Vangala DB, Engel C, Bucksch K, Hueneburg R, Perne C, Nattermann J, Steinke-Lange V, Rahner N, Schackert HK, Weitz J, Kloor M, Kuhlkamp J, Nguyen HP, Moeslein G, Strassburg C, Morak M, Holinski-Feder E, Buettner R, Aretz S, Loeffler M, Schmiegel W, Pox C, Schulmann K; German Consortium for Familial Intestinal Cancer. Value of upper gastrointestinal endoscopy for gastric cancer surveillance in patients with Lynch syndrome. Int J Cancer. 2021;148:106-114. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 17] [Cited by in RCA: 29] [Article Influence: 5.8] [Reference Citation Analysis (0)] |
| 11. | Vangala DB, Cauchin E, Balmaña J, Wyrwicz L, van Cutsem E, Güller U, Castells A, Carneiro F, Hammel P, Ducreux M, van Laethem JL, Matysiak-Budnik T, Schmiegel W. Screening and surveillance in hereditary gastrointestinal cancers: Recommendations from the European Society of Digestive Oncology (ESDO) expert discussion at the 20th European Society for Medical Oncology (ESMO)/World Congress on Gastrointestinal Cancer, Barcelona, June 2018. Eur J Cancer. 2018;104:91-103. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 38] [Cited by in RCA: 57] [Article Influence: 8.1] [Reference Citation Analysis (0)] |
| 12. | Bonadona V, Bonaïti B, Olschwang S, Grandjouan S, Huiart L, Longy M, Guimbaud R, Buecher B, Bignon YJ, Caron O, Colas C, Noguès C, Lejeune-Dumoulin S, Olivier-Faivre L, Polycarpe-Osaer F, Nguyen TD, Desseigne F, Saurin JC, Berthet P, Leroux D, Duffour J, Manouvrier S, Frébourg T, Sobol H, Lasset C, Bonaïti-Pellié C; French Cancer Genetics Network. Cancer risks associated with germline mutations in MLH1, MSH2, and MSH6 genes in Lynch syndrome. JAMA. 2011;305:2304-2310. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 730] [Cited by in RCA: 794] [Article Influence: 56.7] [Reference Citation Analysis (0)] |
| 13. | Engel C, Loeffler M, Steinke V, Rahner N, Holinski-Feder E, Dietmaier W, Schackert HK, Goergens H, von Knebel Doeberitz M, Goecke TO, Schmiegel W, Buettner R, Moeslein G, Letteboer TG, Gómez García E, Hes FJ, Hoogerbrugge N, Menko FH, van Os TA, Sijmons RH, Wagner A, Kluijt I, Propping P, Vasen HF. Risks of less common cancers in proven mutation carriers with lynch syndrome. J Clin Oncol. 2012;30:4409-4415. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 198] [Cited by in RCA: 220] [Article Influence: 16.9] [Reference Citation Analysis (0)] |
| 14. | Møller P, Seppälä TT, Bernstein I, Holinski-Feder E, Sala P, Gareth Evans D, Lindblom A, Macrae F, Blanco I, Sijmons RH, Jeffries J, Vasen HFA, Burn J, Nakken S, Hovig E, Rødland EA, Tharmaratnam K, de Vos Tot Nederveen Cappel WH, Hill J, Wijnen JT, Jenkins MA, Green K, Lalloo F, Sunde L, Mints M, Bertario L, Pineda M, Navarro M, Morak M, Renkonen-Sinisalo L, Valentin MD, Frayling IM, Plazzer JP, Pylvanainen K, Genuardi M, Mecklin JP, Moeslein G, Sampson JR, Capella G; Mallorca Group. Cancer risk and survival in path_MMR carriers by gene and gender up to 75 years of age: a report from the Prospective Lynch Syndrome Database. Gut. 2018;67:1306-1316. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 303] [Cited by in RCA: 399] [Article Influence: 57.0] [Reference Citation Analysis (0)] |
| 15. | Akin E, Demirezer Bolat A, Buyukasik S, Algin O, Selvi E, Ersoy O. Comparison between Capsule Endoscopy and Magnetic Resonance Enterography for the Detection of Polyps of the Small Intestine in Patients with Familial Adenomatous Polyposis. Gastroenterol Res Pract. 2012;2012:215028. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 7] [Cited by in RCA: 10] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 16. | Tescher P, Macrae FA, Speer T, Stella D, Gibson R, Tye-Din JA, Srivatsa G, Jones IT, Marion K. Surveillance of FAP: a prospective blinded comparison of capsule endoscopy and other GI imaging to detect small bowel polyps. Hered Cancer Clin Pract. 2010;8:3. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 27] [Cited by in RCA: 29] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 17. | Costamagna G, Shah SK, Riccioni ME, Foschia F, Mutignani M, Perri V, Vecchioli A, Brizi MG, Picciocchi A, Marano P. A prospective trial comparing small bowel radiographs and video capsule endoscopy for suspected small bowel disease. Gastroenterology. 2002;123:999-1005. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 565] [Cited by in RCA: 517] [Article Influence: 22.5] [Reference Citation Analysis (0)] |
| 18. | Saurin JC, Pilleul F, Soussan EB, Manière T, D'Halluin PN, Gaudric M, Cellier C, Heresbach D, Gaudin JL; Capsule Commission of the French Society of Digestive Endoscopy (SFED). Small-bowel capsule endoscopy diagnoses early and advanced neoplasms in asymptomatic patients with Lynch syndrome. Endoscopy. 2010;42:1057-1062. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 48] [Cited by in RCA: 46] [Article Influence: 3.1] [Reference Citation Analysis (0)] |
| 19. | Haanstra JF, Al-Toma A, Dekker E, Vanhoutvin SA, Nagengast FM, Mathus-Vliegen EM, van Leerdam ME, de Vos tot Nederveen Cappel WH, Sanduleanu S, Veenendaal RA, Cats A, Vasen HF, Kleibeuker JH, Koornstra JJ. Prevalence of small-bowel neoplasia in Lynch syndrome assessed by video capsule endoscopy. Gut. 2015;64:1578-1583. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 34] [Cited by in RCA: 35] [Article Influence: 3.5] [Reference Citation Analysis (0)] |
| 20. | Haanstra JF, Al-Toma A, Dekker E, Vanhoutvin SALW, Nagengast FM, Mathus-Vliegen EM, van Leerdam ME, de Vos Tot Nederveen Cappel WH, Veenendaal RA, Cats A, Sanduleanu S, Vasen HFA, Kleibeuker JH, Koornstra JJ. Incidence of small bowel neoplasia in Lynch syndrome assessed by video capsule endoscopy. Endosc Int Open. 2017;5:E622-E626. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 12] [Cited by in RCA: 13] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 21. | Watson P, Vasen HFA, Mecklin JP, Bernstein I, Aarnio M, Järvinen HJ, Myrhøj T, Sunde L, Wijnen JT, Lynch HT. The risk of extra-colonic, extra-endometrial cancer in the Lynch syndrome. Int J Cancer. 2008;123:444-449. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 454] [Cited by in RCA: 425] [Article Influence: 25.0] [Reference Citation Analysis (0)] |
| 22. | Sijmons RH, Kiemeney LA, Witjes JA, Vasen HF. Urinary tract cancer and hereditary nonpolyposis colorectal cancer: risks and screening options. J Urol. 1998;160:466-470. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 81] [Cited by in RCA: 79] [Article Influence: 2.9] [Reference Citation Analysis (0)] |
| 23. | Myrhøj T, Andersen MB, Bernstein I. Screening for urinary tract cancer with urine cytology in Lynch syndrome and familial colorectal cancer. Fam Cancer. 2008;7:303-307. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 49] [Cited by in RCA: 54] [Article Influence: 3.2] [Reference Citation Analysis (0)] |
| 24. | Dominguez-Valentin M, Sampson JR, Seppälä TT, Ten Broeke SW, Plazzer JP, Nakken S, Engel C, Aretz S, Jenkins MA, Sunde L, Bernstein I, Capella G, Balaguer F, Thomas H, Evans DG, Burn J, Greenblatt M, Hovig E, de Vos Tot Nederveen Cappel WH, Sijmons RH, Bertario L, Tibiletti MG, Cavestro GM, Lindblom A, Della Valle A, Lopez-Köstner F, Gluck N, Katz LH, Heinimann K, Vaccaro CA, Büttner R, Görgens H, Holinski-Feder E, Morak M, Holzapfel S, Hüneburg R, Knebel Doeberitz MV, Loeffler M, Rahner N, Schackert HK, Steinke-Lange V, Schmiegel W, Vangala D, Pylvänäinen K, Renkonen-Sinisalo L, Hopper JL, Win AK, Haile RW, Lindor NM, Gallinger S, Le Marchand L, Newcomb PA, Figueiredo JC, Thibodeau SN, Wadt K, Therkildsen C, Okkels H, Ketabi Z, Moreira L, Sánchez A, Serra-Burriel M, Pineda M, Navarro M, Blanco I, Green K, Lalloo F, Crosbie EJ, Hill J, Denton OG, Frayling IM, Rødland EA, Vasen H, Mints M, Neffa F, Esperon P, Alvarez K, Kariv R, Rosner G, Pinero TA, Gonzalez ML, Kalfayan P, Tjandra D, Winship IM, Macrae F, Möslein G, Mecklin JP, Nielsen M, Møller P. Cancer risks by gene, age, and gender in 6350 carriers of pathogenic mismatch repair variants: findings from the Prospective Lynch Syndrome Database. Genet Med. 2020;22:15-25. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 196] [Cited by in RCA: 409] [Article Influence: 68.2] [Reference Citation Analysis (0)] |
| 25. | Joost P, Therkildsen C, Dominguez-Valentin M, Jönsson M, Nilbert M. Urinary Tract Cancer in Lynch Syndrome; Increased Risk in Carriers of MSH2 Mutations. Urology. 2015;86:1212-1217. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 53] [Cited by in RCA: 62] [Article Influence: 6.2] [Reference Citation Analysis (0)] |
| 26. | Pradere B, Lotan Y, Roupret M. Lynch syndrome in upper tract urothelial carcinoma: significance, screening, and surveillance. Curr Opin Urol. 2017;27:48-55. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 22] [Cited by in RCA: 30] [Article Influence: 3.8] [Reference Citation Analysis (0)] |
| 27. | Koornstra JJ, Mourits MJ, Sijmons RH, Leliveld AM, Hollema H, Kleibeuker JH. Management of extracolonic tumours in patients with Lynch syndrome. Lancet Oncol. 2009;10:400-408. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 139] [Cited by in RCA: 140] [Article Influence: 8.8] [Reference Citation Analysis (0)] |
| 28. | Mork M, Hubosky SG, Rouprêt M, Margulis V, Raman J, Lotan Y, O'Brien T, You N, Shariat SF, Matin SF. Lynch Syndrome: A Primer for Urologists and Panel Recommendations. J Urol. 2015;194:21-29. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 55] [Cited by in RCA: 46] [Article Influence: 4.6] [Reference Citation Analysis (0)] |
| 29. | van der Post RS, Kiemeney LA, Ligtenberg MJ, Witjes JA, Hulsbergen-van de Kaa CA, Bodmer D, Schaap L, Kets CM, van Krieken JH, Hoogerbrugge N. Risk of urothelial bladder cancer in Lynch syndrome is increased, in particular among MSH2 mutation carriers. J Med Genet. 2010;47:464-470. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 122] [Cited by in RCA: 139] [Article Influence: 9.3] [Reference Citation Analysis (0)] |
| 30. | Huang D, Matin SF, Lawrentschuk N, Roupret M. Systematic Review: An Update on the Spectrum of Urological Malignancies in Lynch Syndrome. Bladder Cancer. 2018;4:261-268. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 17] [Cited by in RCA: 20] [Article Influence: 2.9] [Reference Citation Analysis (0)] |
| 31. | Newton K, Green K, Lalloo F, Evans DG, Hill J. Colonoscopy screening compliance and outcomes in patients with Lynch syndrome. Colorectal Dis. 2015;17:38-46. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 51] [Cited by in RCA: 62] [Article Influence: 6.2] [Reference Citation Analysis (0)] |
| 32. | Stoffel EM, Mercado RC, Kohlmann W, Ford B, Grover S, Conrad P, Blanco A, Shannon KM, Powell M, Chung DC, Terdiman J, Gruber SB, Syngal S. Prevalence and predictors of appropriate colorectal cancer surveillance in Lynch syndrome. Am J Gastroenterol. 2010;105:1851-1860. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 60] [Cited by in RCA: 57] [Article Influence: 3.8] [Reference Citation Analysis (0)] |
| 33. | Tosteson AN, Fryback DG, Hammond CS, Hanna LG, Grove MR, Brown M, Wang Q, Lindfors K, Pisano ED. Consequences of false-positive screening mammograms. JAMA Intern Med. 2014;174:954-961. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 136] [Cited by in RCA: 150] [Article Influence: 13.6] [Reference Citation Analysis (0)] |
| 34. | Alamo-Junquera D, Murta-Nascimento C, Macià F, Baré M, Galcerán J, Ascunce N, Zubizarreta R, Salas D, Román R, Castells X, Sala M; Cumulative False-Positive Risk Group. Effect of false-positive results on reattendance at breast cancer screening programmes in Spain. Eur J Public Health. 2012;22:404-408. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 23] [Cited by in RCA: 27] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 35. | David KA, Mallin K, Milowsky MI, Ritchey J, Carroll PR, Nanus DM. Surveillance of urothelial carcinoma: stage and grade migration, 1993-2005 and survival trends, 1993-2000. Cancer. 2009;115:1435-1447. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 55] [Cited by in RCA: 57] [Article Influence: 3.6] [Reference Citation Analysis (0)] |
| 36. | Watkins KE, Way CY, Fiander JJ, Meadus RJ, Esplen MJ, Green JS, Ludlow VC, Etchegary HA, Parfrey PS. Lynch syndrome: barriers to and facilitators of screening and disease management. Hered Cancer Clin Pract. 2011;9:8. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 23] [Cited by in RCA: 33] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 37. | Katz LH, Burton-Chase AM, Advani S, Fellman B, Polivka KM, Yuan Y, Lynch PM, Peterson SK. Screening adherence and cancer risk perceptions in colorectal cancer survivors with Lynch-like syndrome. Clin Genet. 2016;89:392-398. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 13] [Cited by in RCA: 14] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 38. | Jia S, Wu X, Zhang Y, Zhang M. Chinese Lynch syndrome-associated colorectal cancer patients' self-concept and adherence to surveillance. Eur J Cancer Care (Engl). 2021;30:e13379. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 1] [Article Influence: 0.2] [Reference Citation Analysis (0)] |