Published online Nov 24, 2021. doi: 10.5306/wjco.v12.i11.1009
Peer-review started: March 23, 2021
First decision: July 27, 2021
Revised: August 9, 2021
Accepted: October 18, 2021
Article in press: October 18, 2021
Published online: November 24, 2021
Processing time: 241 Days and 1.1 Hours
The majority of patients with newly diagnosed metastatic prostate cancer (PC) initially respond to androgen deprivation therapy (ADT) and are classified as metastatic castration-sensitive PC (mCSPC). Following months to years of ADT, the disease tends to become resistant to ADT. Recent randomized phase-III trials demonstrated a survival benefit with the addition of upfront docetaxel to ADT in mCSPC. Following its implementation in routine care, this combined treatment strategy requires more detailed evaluation in a real-world setting.
To assess the real-world outcome and safety of upfront docetaxel treatment in mCSPC.
A multicenter retrospective cohort study in the Southeast Health Care Region of Sweden was performed. This region includes approximately 1.1 million citizens and the oncology departments of Linköping, Jönköping, and Kalmar. All patients given upfront docetaxel for mCSPC from July 2015 until December 2017 were included. The primary endpoint was progression-free survival (PFS) at 12 mo, and the secondary endpoints were PFS at 24 mo, overall survival (OS), treatment intensity, adverse events, and unplanned hospitalizations. Exploratory analyses on potential prognostic parameters were performed.
Ninety-four patients were eligible and formed the study cohort. PFS at 12 and 24 mo was 75% (95%CI: 66–84) and 58% (46–70), respectively. OS at 12 and 24 mo was 93% (87–99) and 86% (76–96). A total of 91% of patients (n = 86) were given docetaxel according to the standard protocol of 75 mg/m2 every 3 wk (6 cycles), while 9% (n = 8) received a modified protocol of 50 mg/m2 every 2 wk (9 cycles). The average overall dose intensity for those commencing standard treatment was 91%. Univariate Cox regression analyses show that baseline PSA > 180 vs < 180 and the presence of distant metastases vs locoregional lymph node metastases were only negative prognostic factors (HR 2.86, 95%CI: 1.39–5.87, P = 0.0041 and 3.36, 95%CI: 1.03–10.96, P = 0.045). Following multivariate analysis, statistical significance remained for PSA (2.51, 95%CI: 1.21–5.19, P = 0.013) but not for metastatic status (2.60, 95%CI: 0.78–8.65, P = 0.12). Febrile neutropenia was recorded in 21% (n = 20) of patients, and 26% (n = 24) had at least one episode of unplanned hospitalization under and up to 30 d after the treatment course.
Results from this study support the implementation of upfront docetaxel plus ADT as part of the standard of care treatment strategy in mCSPC.
Core Tip: Two recent trials reported impressive outcomes when upfront docetaxel is added to androgen deprivation therapy in metastatic castration-sensitive prostate cancer (mCSPC). This study presents the outcome and safety of this treatment strategy in a real-world context of all eligible patients in the southeast region of Sweden. While the treatment is toxic in terms of febrile neutropenia and unplanned hospitalizations, the outcome and long-term prognosis appear similar in real life and randomized controlled trial contexts. Further implementation of upfront docetaxel in mCSPC in routine care is encouraged.
- Citation: Isaksson J, Green H, Papantoniou D, Pettersson L, Anden M, Rosell J, Åvall-Lundqvist E, Elander NO. Real-world evaluation of upfront docetaxel in metastatic castration-sensitive prostate cancer. World J Clin Oncol 2021; 12(11): 1009-1022
- URL: https://www.wjgnet.com/2218-4333/full/v12/i11/1009.htm
- DOI: https://dx.doi.org/10.5306/wjco.v12.i11.1009
Prostate cancer (PC) is the second most common malignancy in men. In 2018, more than 1.2 million new cases were reported worldwide, which corresponds to approximately 7% of all cancers[1]. In Sweden, the annual incidence is approximately 10500, with a median age of onset of 68 years[2]. While the 5-year overall survival (OS) (for all stages combined) is continuously improving and now exceeds 90%, the prognosis for patients presenting with upfront metastases remains less optimistic, with expected OS in the range of 30–36 mo and 5-year survival of approximately 30%[3-9].
The majority of patients with newly diagnosed metastatic PC will initially respond to androgen deprivation therapy (ADT) and are classified as castration sensitive (mCSPC). Following months to years of ADT, the disease will tend to become resistant to ADT and thus be defined as metastatic castration refractory prostate cancer (mCRPC). In mCRPC, palliative chemotherapy with docetaxel may offer temporary relief, but survival benefits are usually limited to a few months[4]. However, two recent multicenter trials demonstrated a considerable benefit for docetaxel when this drug was introduced early (i.e., in the initial castration-sensitive phase of the disease)[10,11].
In the CHAARTED study, 790 men were randomized into six cycles of docetaxel plus ADT vs ADT alone. Patients were stratified according to high (visceral metastases or ≥ 4 bone lesions with ≥ 1 beyond the vertebral bodies and pelvis) or low-volume disease. The median OS was 58 mo for men treated with ADT plus docetaxel and 44 mo for men treated with ADT alone. In men with high-volume disease, the additive effect of docetaxel was even better, with a median OS benefit of 17 mo (49 mo vs 32 mo)[11].
STAMPEDE was a multiarmed, multistage trial that included 2962 men with both metastatic and nonmetastatic PC. Stratified randomization (2:1:1:1) allocated men to a standard of care (SOC): ADT with or without radiotherapy, SOC plus docetaxel, SOC plus zoledronic acid, and SOC plus docetaxel plus zoledronic acid. Sixty-one percent had distant metastasis, and 15% had node-positive disease. The remaining 24% presented with nonmetastatic high-risk locally advanced disease [T3/4, PSA ≥ 40 ng/mL, and/or Gleason score (GS) 8–10]. In the STAMPEDE trial, median OS was improved by 10 mo for SOC plus docetaxel compared with SOC alone (81 mo vs 71 mo). For the group with metastatic disease, the OS benefit was 15 mo for SOC plus docetaxel vs SOC alone (60 mo vs 45 mo)[10,12,13].
A smaller French study, GETUG-AFU 15, including approximately 400 patients who were randomized to receive ADT alone or ADT plus docetaxel[14], could not confirm the findings of CHAARTED and STAMPEDE. The French study did not reveal any statistically significant survival benefit with upfront docetaxel treatment (median OS 59 mo in the ADT plus docetaxel group vs 54 mo in the ADT alone group). The different outcomes of the various trials may depend on discrepancies in study populations. In the STAMPEDE and CHAARTED trials, the median PSA levels were nearly twice as high as the median PSA reported in the GETUG-15 population, indicating that the disease stage was generally more advanced in the former cohorts than in the latter. While 66% of CHAARTED patients were reported to have a high volume of metastases, only 48% were classified as such in GETUG-15. Differences were also observed in the GS, with a GS ≥ 8 reported for nearly 61% of the population in CHAARTED and 74% in STAMPEDE compared to 55% in GETUG-15[10,11,14]. Together, these findings suggest that the CHAARTED and STAMPEDE trials included patients with worse prognosis than the subjects enrolled in the GETUG-15 study.
Based on the promising results of STAMPEDE and CHAARTED, the addition of docetaxel to ADT in early mCSPC was introduced in international and national (including Swedish) guidelines, particularly for patients with high-volume disease, in 2015-2016. To be eligible for this therapy, patients should be in good general condition and without significant comorbidities[15]. The eligibility conditions in the Swedish national guidelines were used from the reported characteristics of the STAMPEDE and CHAARTED populations.
Since its introduction in routine care, it remains largely unknown to what extent the outcomes observed in the STAMPEDE and CHAARTED trials are evident in patients treated outside the frame of a randomized controlled trial. This study was therefore designed to assess the real-world outcome and safety of early docetaxel treatment for patients with mCSPC. To completely describe the real-world situation with patients of all ages and with or without concomitant comorbidities, all consecutive patients who received this treatment in the Southeast Health Care Region of Sweden since 2015 were included.
A retrospective multicenter cohort study of all men diagnosed with primary mCSPC in the Southeast Health Care Region of Sweden was designed. This region covers approximately 1.1 million citizens and includes the oncology departments of Linköping, Jönköping, and Kalmar. These three centers provide all oncological treatments in the region. The study was conducted in accordance with the Declaration of Helsinki and was approved by the Regional Ethics Review board in Linköping, Region Östergötland, Sweden (diary number 2018/139–31). Based on the retrospective and noninterventional nature of the study and the absence of publication of individual data, the ethics board did not consider it possible or necessary to obtain written informed consent.
Inclusion criteria were as follows: Male sex, age 18 years or older, evidence of newly diagnosed mCSPC between July 2015 and December 2017 (ICD-10 code C61.x), defined as either node positive (N+) and/or distant metastatic (M+) disease, and administration of at least one cycle of upfront docetaxel chemotherapy in addition to ADT. ADT was initiated before the start of docetaxel, either in conjunction with the diagnosis of mCSPC or earlier (i.e., for patients already receiving ADT in an earlier nonmetastatic disease setting). The exclusion criteria were recurrent disease, castration refractory disease, and patient refusal to undergo ADT. Otherwise, to completely describe the real world situation, no exclusion criteria were applied.
The Swedish Cancer Registry (SCR) was used to identify eligible patients (https://www.socialstyrelsen.se/statistik-och-data/register/alla-register/cancerregistret/). Reporting to the SCR is mandatory, and the registry achieves over 95% coverage for all malignant tumors. The CSAM Cytodos software system (CSAM Health AS, Oslo, Norway), a software program being used for the prescription and administration of chemotherapy at all participating centers, was used to identify those treated with docetaxel.
Medical records were reviewed, and data were registered in a standardized case report form where patient and tumor characteristics, baseline biochemistry, Eastern Cooperative Oncology Group (ECOG) performance status, treatment regimens, toxicity parameters, PSA levels, relapse, and vital status were recorded. Patient and tumor characteristics were recorded according to the TMN classification (8th edition by Union for International Cancer Control 2017), GS according to International Society of Urological Pathology 2014, and histology according to WHO 2004.
The patients included had received docetaxel according to any of two different regimens, either by intravenous infusion every three weeks at doses of 75 mg/m² for a total of six cycles according to the CHAARTED and STAMPEDE treatment protocols or at a dose of 50 mg/m² in two-week cycles for a total of nine cycles according to local guidelines. The latter was used by one site for patients who, for any reason and at the treating oncologist’s discretion, were deemed unfit to receive the 75 mg/m² regimen.
Bone marrow toxicity was evaluated by blood cell counts in addition to other standard biochemical parameters prior to each dose. In general, treatment response was evaluated with PSA levels at days 18–20 in every cycle and, for most patients, with X-ray computed tomography at the end of the sixth cycle (or at the ninth cycle of the 50 mg/m2 two-week cycles).
The primary endpoint was progression-free survival (PFS) at 12 mo. PFS was defined as the time to biochemical progression in accordance with the CHAARTED protocol, where a serologic increase of the PSA level of more than 50% above nadir, reached after initiation of ADT and with two consecutive increases at least two weeks apart, clinical progression due to increasing symptoms, or deterioration or disease progression according to RECIST 1.0 were considered progression. If the PSA nadir was less than 2 μg/L, a minimum increase of more than 2 μg/L was required[10,11]. In addition, an alternative definition of progression according to the Swedish national guidelines, which stipulate serologic increase ≥ 25% from lowest PSA value after start of latest given treatment (and a minimum absolute increase of ≥ 2 μg/L), worsening of clinical symptoms, or radiological disease progression according to RECIST 1.0, was similarly applied[15].
Secondary endpoints were PFS at 24 mo, OS and treatment intensity included dose reductions, premature termination and protocol modifications, and safety of docetaxel treatment in terms of registered bone marrow toxicity and unplanned hospitalizations under and within 30 d after the last docetaxel treatment cycle. Patients were followed until death or May 18, 2018, whichever occurred first.
All statistical analyses were performed in the per-protocol population, defined as all patients who received at least one dose of docetaxel. Patient characteristics and tumor and treatment data were reported as numbers and percentages for categorical variables and medians and ranges for continuous variables. PFS at 12 and 24 mo was estimated according to the CHAARTED[11] and Swedish national guidelines[15] definitions of progressive disease (PD), respectively. Median PFS and OS for the entire cohort and subgroups defined by age over and under median (68); PSA over and under median (180); comorbidities; and presence of distant metastases or locoregional lymph node metastases only were estimated using Kaplan–Meier survival analyses, and the significance of the differences in estimates of median survival was calculated using log rank test. Cox regression analysis with a 95% confidence interval was used to evaluate hazard ratios for the same subgroups. P values below 0.05 were considered statistically significant. Analyses were performed using IBM SPSS statistics software (IBM, version 25). Any missing data are reported in the respective Table.
A total of 94 eligible patients with primary mCSPC treated with docetaxel and ADT were identified and included. Baseline characteristics are shown in Table 1. For comparison, published data from CHAARTED[11] and STAMPEDE[10] are also shown in Table 1. Median age was 68 years (range 49–79). Comorbidities were present in 53% (n = 50) of patients, of which hypertension was the most prevalent. Regarding ECOG PS, data were only available in 64 (70%) of the cases, of which an overwhelming majority were ECOG PS 0–1 (n = 61, 95%). Median PSA at diagnosis was 180 (range 2–7367). Clinical TNM staging was recorded in 84 (90%) of the subjects, with T3N1M1 being the most common staging. Of those with distant metastases, bone metastases were most prevalent (n = 74, 79%), followed by lymph node metastases (n = 54, 57%). Most tumors (n = 60, 64%) were classified as GS 8–10.
| Total, n = 94 (%) | CHAARTED, ADT + Docetaxel | STAMPEDE, Standard of care + Docetaxel | |
| Age, yr | |||
| Median | 68.0 | 64 | 65 |
| Range | 49-79 | 36-88 | 40-81 |
| Prostate-specific antigen (μg/L), at diagnosis | |||
| Median | 180 | 50.9 | 70 |
| Range | 2-7367 | 0.2-8540.1 | 1-9999 |
| Comorbidities, n (%) | 50 (53) | ||
| Diabetes mellitus I and II | 16 (17) | 56 (9) | |
| Hyperlipidemia | 23 (24) | 208 (35) | |
| Hypertension | 38 (40) | ||
| Previous malignant disease1 | 11 (11) | ||
| Performance status (ECOG)2, n (%) | |||
| 0 | 46 (72) | 277 (69.8) | |
| 1 | 15 (23) | 114 (28.7) | |
| 2 | 3 (5) | 6 (1.5) | |
| T category at diagnosis3, n (%) | |||
| T1 | 6 (6) | 0 | |
| T2 | 17 (18) | 60 (10) | |
| T3 | 46 (49) | 390 (66) | |
| T4 | 11 (12) | 105 (18) | |
| TX | 4 (4) | 35 (6) | |
| Not assessed | 10 (11) | ||
| N category at diagnosis3, n (%) | |||
| N0 | 29 (31) | 260 (44) | |
| N1 | 42 (45) | 298 (50) | |
| NX | 23 (24) | 34 (6) | |
| Metastases3, n (%) | |||
| Non-distant metastasis4 | 19 (20) | ||
| Distant metastases | 75 (80) | 362 (61) | |
| Location, n (%) | |||
| Bone metastases | 74 (79) | 307 (52) | |
| Liver metastases | 2 (2) | 6 (1) | |
| Lung metastases | 12 (13) | 13 (2) | |
| Lymph node metastases | 54 (57) | 102 (17) | |
| Gleason sum score, n (%) | |||
| ≤ 6 | 2 (2) | 21 (5.3) | ≤ 7 |
| 7 | 27 (29) | 96 (24.2) | 110 (19%) |
| 8-10 | 60 (64) | 241 (60.7) | 436 (74%) |
| Unknown | 5 (5) | 39 (9.8) | 46 (8%) |
| Histology (WHO 2004), n (%) | |||
| Acinar adenocarcinoma | 86 (92) | ||
| Ductal carcinoma | 1 (1) | ||
| Mixed type | 2 (2) | ||
| Unknown | 5 (5) | ||
| Follow-up, months | 20 | ||
| Median (IQR) | 13-28 | ||
| Status last follow-up, n (%) | |||
| Alive, no disease progression | 15 (16) | ||
| Alive, disease progression | 65 (69) | ||
| Dead of disease | 14 (15) |
Eighty-two (87%) of the patients received a combination of gonadotropin releasing hormone (GnRH) and a nonsteroidal antiandrogen as the castration method, nine (10%) were treated with GnRH alone, and three (3%) were surgically orchidectomized. The median time from the start of ADT until the start of docetaxel was 63 d (range 8–400). The median duration of ADT was 331 d (range 5–1038) (Table 2). Seventy-seven patients (82%) received docetaxel according to the 75 mg/m2 every six weeks schedule, and eight (8%) received docetaxel according to the modified schedule of 50 mg/m2 every two weeks.
| Total, n = 94 (%) | |
| ADT | |
| GnRH and nonsteroidal antiandrogen | 82 (87) |
| GnRH alone | 9 (10) |
| Orchidectomy | 3 (3) |
| ADT | |
| Time from ADT start to Docetaxel start, days | |
| Median (range) | 63 (8-400) |
| ADT duration, days | |
| Median (range) | 331 (5-1038) |
| Docetaxel | |
| 751 mg/m² | 77 |
| Adm mean dose % of full dose | 91 |
| Mean adm dose, mg | 139 |
| Mean acc dose, mg | 758 |
| Completed all cycles | 63 (67) |
| 502 mg/m² | 8 |
| Adm mean dose % of full dose | 83 |
| Mean adm dose, mg | 86 |
| Mean acc dose, mg | 610 |
| Completed all cycles | 4 (50) |
| Switch | 9 |
| Adm mean dose % of full dose | 87 |
| Mean adm dose, mg | 107 |
| Mean acc dose, mg | 641 |
| Completed all cycles | 9 (100) |
| Dose reduction | 33 (35) |
| Dose escalation | 13 (14) |
| Unchanged | 47 (50) |
| Missing | 1 (1) |
| Best response at end of Docetaxel3 | |
| CR | 6 (6) |
| PR | 50 (53) |
| SD | 15 (16) |
| PD | 11 (12) |
| NE | 12 (13) |
| Est. PFS | Mean (95%CI) |
| 12 mo | |
| CHAARTED/STAMPEDE | 75% (66-84) |
| Swedish national guidelines | 71% (61-81) |
| 24 mo | |
| CHAARTED/STAMPEDE | 58% (46-70) |
| Swedish national guidelines | 55% (43-67) |
| OS | |
| 12 mo | 93% (87-99) |
| 24 mo | 86% (76-96) |
Of those 77 patients commencing the 75 mg/m2 regimen, 63 (81%) completed all 6 cycles. The mean administered dose (of 75 mg/m2 full dose) was 91%, corresponding to 139 mg docetaxel (Table 2).
Of those eight patients commencing the 50 mg/m2 schedule, four (50%) completed all 9 planned cycles. For this regimen, the mean administered dose was 83% of the full dose, corresponding to 86 mg of docetaxel.
Nine patients started with the 75 mg/m2 schedule but switched to the 50 mg/m2 regimen during treatment. In this subgroup of patients, all nine fulfilled the expected 6 cycles.
In total, 33 (35%) underwent at least one dose reduction, 13 (14%) had a dose escalation, and 47 (50%) received the initially prescribed dose throughout the treatment period (Table 2).
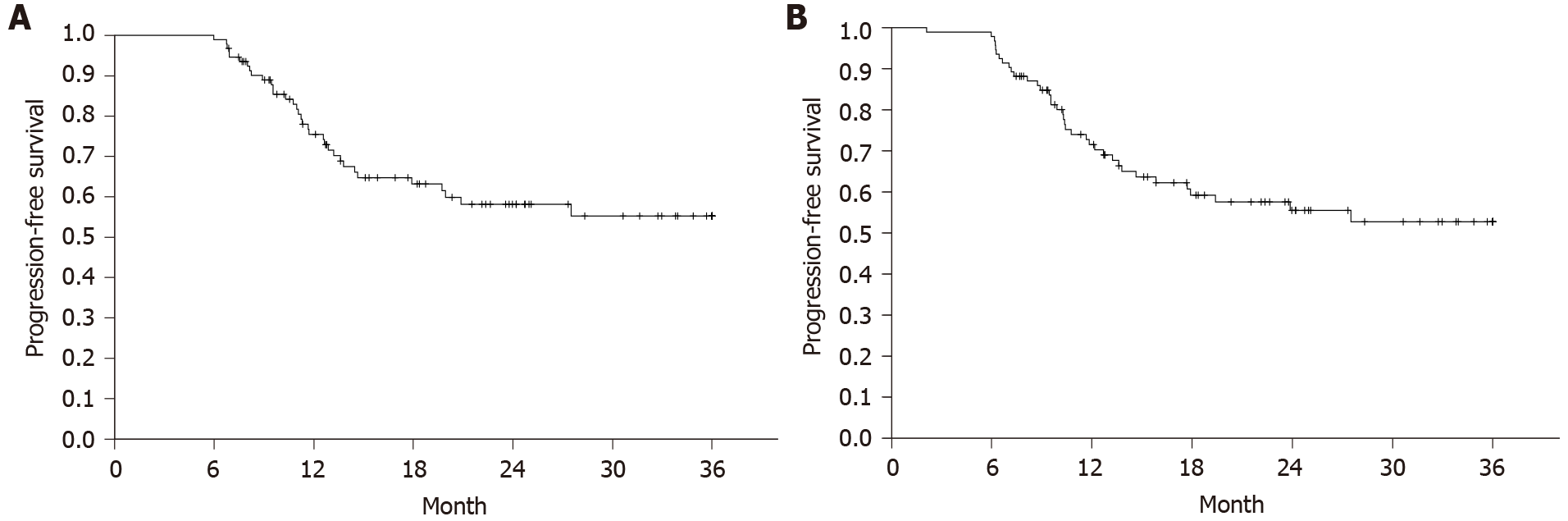
PFS and OS in the entire cohort are shown in Figures 1 and 2. PFS at 12 mo in the total cohort of 94 patients was 75% (95%CI: 66%-84%) or 71% (95%CI: 61%-81%), depending on whether the definition of CHAARTED/STAMPEDE or the Swedish national guidelines was used. The corresponding proportions at 24 mo were 58% (95%CI: 46%-70%) and 55% (95%CI: 43%-67%) (Table 2). The OS rates at 12 and 24 mo were 93% (95%CI: 87%-99%) and 86% (95%CI: 76%-96%), respectively. Median PFS and median OS were not reached by the data cutoff date. At the time of analysis, 65 patients had evidence of disease (69%), and 14 had died (15%) (Table 1). The best response at the end of docetaxel treatment was complete response for six subjects (6%), partial response (n = 50, 53%), stable disease (n = 15, 16%), and PD (n = 11, 12%). Twelve (13%) of the patients were non-evaluable (NE) for PFS (Table 2). Median follow-up was 20 mo.
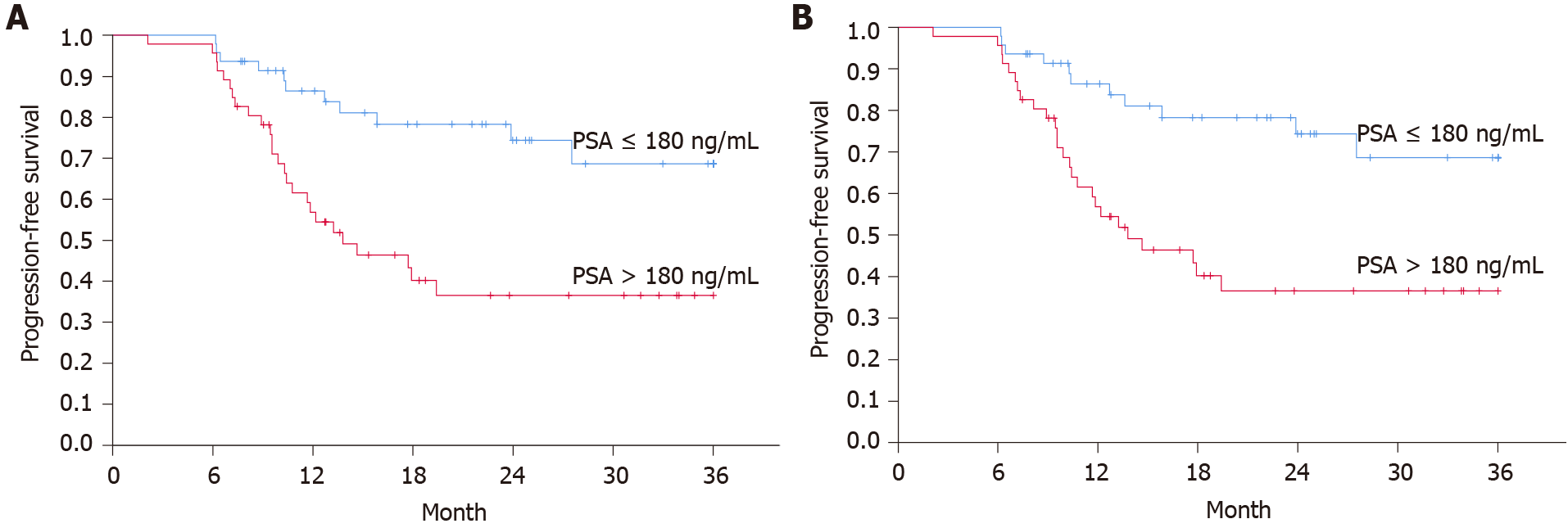
Cox regression analyses were performed to compare PFS and OS in the following subgroups: Age older than 68 years vs 68 years or younger, PSA higher than 180 μg/L vs less than 180 μg/L, comorbidities (yes/no) and absence of distant metastases vs. presence of any distant metastases. For continuous variables (age and PSA), patients were dichotomized according to their below or above the median value of the respective parameter. Univariate Cox regression analyses showed that baseline PSA higher than 180 and the presence of distant metastases were negative prognostic factors (HR 2.86, 95%CI: 1.39–5.87, P = 0.0041 and 3.36, 95%CI: 1.03–10.96, P = 0.045). Following multivariate analysis, statistical significance remained for PSA (2.51, 95%CI: 1.21–5.19, P = 0.013) but not for metastatic status (2.60, 95%CI: 0.78–8.65, P = 0.12) (Table 3). Similar and statistically significant findings on baseline PSA and PFS were evident when the Swedish national guidelines criteria for progressive disease were used (Table 4).
| Number of patients | Number of events | HR, 95%CI, P value (univariate) | HR, 95%CI, P value (multivariate) | |
| Age, yr (median) | ||||
| ≤ 68 | 48 | 21 | 1.00 | 1.00 |
| > 68 | 46 | 15 | 0.78, 0.40-1.51, 0.45 | 0.83, 0.42-1.67, 0.61 |
| PSA (median) | ||||
| ≤ 180 | 48 | 12 | 1.00 | 1.00 |
| > 180 | 46 | 24 | 2.86, 1.39-5.87, 0.0041 | 2.51, 1.21-5.19, 0.013 |
| Comorbidities | ||||
| No | 44 | 15 | 1.00 | 1.00 |
| Yes | 50 | 21 | 1.15, 0.59-2.23, 0.68 | 1.19, 0.60-2.36, 0.63 |
| Distant metastases | ||||
| No | 19 | 3 | 1.00 | 1.00 |
| Yes | 75 | 33 | 3.36, 1.03-10.96, 0.045 | 2.60, 0.78-8.65, 0.12 |
| Number of patients | Number of events | HR, 95%CI, P value (univariate) | HR, 95%CI, P value (multivariate) | |
| Age, yr (median) | ||||
| ≤ 68 | 48 | 22 | 1.00 | 1.00 |
| > 68 | 46 | 17 | 0.83, 0.44-1.56, 0.55 | 0.88, 0.46-1.71, 0.71 |
| PSA (median) | ||||
| ≤ 180 | 48 | 13 | 1.00 | 1.00 |
| > 180 | 46 | 26 | 2.86, 1.44-5.69, 0.0028 | 2.57, 1.28-5.16, 0.0081 |
| Comorbidities | ||||
| No | 44 | 17 | 1.00 | 1.00 |
| Yes | 50 | 22 | 1.06, 0.56-2.00, 0.85 | 1.08, 0.56-2.07, 0.83 |
| Distant metastases | ||||
| No | 19 | 4 | 1.00 | 1.00 |
| Yes | 75 | 35 | 2.69, 0.96-7.59, 0.061 | 2.11, 0.73-6.06, 0.17 |
Table 5 shows registered side effects and bone marrow toxicity according to the National Cancer Institute Common Terminology Criteria for Adverse Events (CTCAE version 4). Most strikingly, 20 (21%) of the patients experienced febrile neutropenia, and 24 (26%) had at least one episode of unplanned hospitalization under and up to 30 d after the docetaxel treatment course. Other reported adverse events, as well as reasons for early termination of the treatment, are shown in detail in Table 5.
| Total, n = 94 (%) | |
| Reason for termination of treatment | |
| Completed as planned | 74 (79) |
| Adverse event | 11 (12) |
| Fatigue | 5 (5) |
| Tumor progression | 2 (2) |
| Patient preference | 1 (1) |
| Other | 1 (1) |
| Bone marrow toxicity | |
| Hemoglobin | |
| Any grade | 16 (17) |
| ≥ grade 3-4 | 0 |
| White blood cell count | |
| Any grade | 20 (21) |
| ≥ grade 3-4 | 15 (16) |
| Neutrophil count | |
| Any grade | 19 (20) |
| ≥ grade 3-4 | 19 (20) |
| Grade missing | 1 |
| Platelet count | |
| Any grade | 2 (2) |
| ≥ grade 3-4 | 0 |
| Unplanned hospitalization under and within 30 d after chemotherapy | 24 (26) |
| Febrile neutropenia | 20 (21) |
This population-based study explored the outcome and safety of combined upfront docetaxel and ADT in mCSPC in a real-world cohort including the first 94 patients undergoing this treatment strategy in the Southeast Health Care Region of Sweden, reporting outcome and safety measures that were similar to previous findings in pivotal randomized controlled trials on the topic[10,11].
In the last few years, treatment of mCSPC with upfront docetaxel in addition to ADT has been widely implemented in routine care in Sweden and elsewhere. This significant change in practice was primarily based on the results of two major randomized controlled trials, CHAARTED and STAMPEDE[10,11]. While the results of these trials were promising, less is known about the outcome and safety of this treatment in a real-world context (i.e., among patients who are treated outside the frame of a randomized controlled trial).
Based on the CHAARTED/STAMPEDE definition of progressive disease, the cohort investigated in this study exhibited PFS rates of 75% and 58% at 12 and 24 mo, respectively, closely corresponding to the outcomes displayed in the CHAARTED and STAMPEDE publications[10,11]. Similarly, OS estimates at 12 and 24 mo of 93% and 86% mirror the Kaplan–Meier curves of the two trial populations. Taken together, these results indicate that the value of upfront docetaxel added to ADT in mCSPC appears similar in randomized controlled populations and this Swedish real-world cohort.
There are some similarities and differences between the real-world population investigated in this study and the patient populations of CHAARTED and STAMPEDE. In this cohort, patients were marginally older with a median of 68 years compared to a median of 64 (CHAARTED) and 65 (STAMPEDE) in the randomized controlled trials. The vast majority of patients (95%–99%) in the real-world cohort as well as the phase-III trial populations exhibited ECOG PS 0–1. Conversely, baseline PSA levels were considerably higher in the real-world cohort (median 180) than in the controlled trial populations, which exhibited median PSA levels of 51 (CHAARTED) and 70 (STAMPEDE), potentially indicating a higher disease burden in the real-world cohort at the start of the treatment. Patients with PSA above the median also had a significantly higher risk of progressive disease, which was reflected in both univariate and multivariable regression analyses (Figure 2).
Also, the extent of metastatic disease was different in the two phase-III trials and this real-world cohort. The STAMPEDE trial reported that 61% of the total study population had metastatic disease, and the CHAARTED trial, which only included patients with evidence of metastatic disease, reported 65% with high volume disease; these latter numbers were not available from the STAMPEDE publication. In this real-world cohort, 80% had distant metastases, while 20% had non-distant metastases only. While metastatic burden was a negative prognostic factor in univariate regression analysis of this cohort, the statistical significance did not remain in multivariable analysis.
Taken together, these findings indicate that the outcome of docetaxel in mCSPC is comparable in real life, where patients are generally older and often present with more advanced disease in terms of baseline PSA levels, and phase-III trial populations with more beneficial baseline characteristics.
Completion of all planned cycles was reported in 86% of the CHAARTED and 76% of the STAMPEDE trial populations. Similar figures were found in this cohort: 81% completed the entire treatment course, and 35% (n = 33) underwent dose reductions. Eight (8%) also received a modified 50 mg/m2 every-two-weeks schedule from the start, and nine (10%) converted from the standard 75 mg/m2 every-three-weeks to this modified 50 mg/m2 protocol (switch). There is currently little evidence for this 50 mg/m2 protocol in mCSPC, and the deviation from SOC probably reflects an eagerness to provide the treatment to frail and/or comorbid patients who otherwise would be considered not eligible for docetaxel. The low number of patients in this subgroup together with the finding that only 50% of the patients who began with 50 mg/m2 were able to fulfil all planned cycles mean that the efficacy and safety for this adapted treatment schedule remain unproven.
Safety data of this study reveal that 21% of the patients experienced febrile neutropenia and, in total, 26% had at least one episode of unplanned hospitalization under or shortly after the docetaxel treatment course. While only 4% had their treatments prematurely terminated due to febrile neutropenia, these findings still emphasize that the docetaxel 75 mg/m2 every-three-weeks regimen is a particularly toxic treatment. This result might be particularly important when upfront docetaxel is considered for older and/or frail patients who would not be eligible for the STAMPEDE and CHAARTED trials.
To our knowledge, this is the first study that systematically reports the real-world outcome of upfront docetaxel in a Scandinavian context. Other real-world studies conducted in other countries and/or ethnic groups corroborate the results of this study. Lavoie et al[16] assessed the clinical effectiveness of upfront docetaxel in a Canadian setting, showing a similar outcome and safety data to those of this study, with 12-mo OS of 91% and 26% experiencing grade 3–4 febrile neutropenia[16], and comparable outcomes were also reported in a German study[17] and in Northern American non-white populations[18].
The primary strengths of this study include the truly real-world setup, covering all eligible patients in a reasonably large geographical region. Because there are no nongovernmental health care providers that offer cancer chemotherapy in the Southeast Region in Sweden, every patient who was given the therapy and met the inclusion criteria was included. Another additional value is that Swedish health care is generally available and publicly funded, meaning that all individuals, regardless of socioeconomic status, are offered similar treatment and follow-up programs. In the current era of novel therapeutic options in the early and late stages of PC, including targeted treatments such as radium 223[19] and lutetium-177 [177Lu]-PSMA-617[20], it becomes increasingly important to assess the efficacy and tolerability of treatments already established in standard practice.
The study’s primary weakness mirrors its primary strength: The retrospective inclusion and, to some extent, different treatment regimens prescribed make it more difficult to define the efficacy and toxicity of the treatment schedule evaluated in the STAMPEDE and CHAARTED trials. The limited sample size means that subgroup analyses should be considered exploratory, and that their results should be interpreted with care.
This study provides additional evidence on the efficacy and safety of upfront docetaxel in a real-world context of mCSPC. Progression-free and OS appear similar in real-world and randomized controlled trial settings. Febrile neutropenia remains a frequent and severe adverse event, and unplanned hospitalizations are common in patients undergoing this treatment. High baseline PSA indicates worse prognosis. In conclusion, results support the implementation of upfront docetaxel plus ADT as part of the SOC treatment strategy in mCSPC.
Randomized phase-III trials indicate that upfront treatment with docetaxel, in addition to androgen deprivation therapy (ADT), improves survival in metastatic castration-sensitive prostate cancer (mCSPC). Less is known about the outcome of such treatment in real-world patients treated outside the frames of a clinical trial.
It is important to assess the outcome and safety of upfront docetaxel and ADT combination therapy following its implementation in real-world patients with mCSPC.
To evaluate the outcome of docetaxel and ADT combination therapy in real-world patients with mCSPC in terms of progression-free survival (PFS), overall survival (OS), and safety.
A multicenter retrospective noninterventional study was performed and included 94 first consecutive real-world patients with mCSPC receiving upfront docetaxel and ADT in the Southeast Health Care Region of Sweden. Univariate and multivariate regression analyses were performed to identify prognostic parameters. Adverse events and unplanned hospitalizations were thoroughly reviewed.
PFS at 12 and 24 mo was 75% and 58%, while OS was 93% and 86% concurrently points, respectively. High baseline PSA levels were associated with worse prognosis in multivariate regression analysis. Twenty-one percent of the patients experienced febrile neutropenia, and 26% had at least one episode of unplanned hospitalization.
The outcome and safety of docetaxel and ADT combination therapy in mCSPC appear similar in real-world and randomized controlled trial populations. This study supports further implementation of this treatment strategy in standard of care.
Future studies must identify clinically useful biomarkers and tools for tailored treatment strategies in patients with mCSPC.
Provenance and peer review: Unsolicited article; Externally peer reviewed.
Specialty type: Oncology
Country/Territory of origin: Sweden
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): 0
Grade C (Good): C
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Dadgar H S-Editor: Fan JR L-Editor: A P-Editor: Fan JR
| 1. | Bray F, Ferlay J, Soerjomataram I, Siegel RL, Torre LA, Jemal A. Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin. 2018;68:394-424. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 53206] [Cited by in RCA: 55814] [Article Influence: 7973.4] [Reference Citation Analysis (132)] |
| 2. | Ayoubi S. Cancer i siffror 2018. Socialstyrelsens artikelnummer, 2018. |
| 3. | Oudard S, Banu E, Beuzeboc P, Voog E, Dourthe LM, Hardy-Bessard AC, Linassier C, Scotté F, Banu A, Coscas Y, Guinet F, Poupon MF, Andrieu JM. Multicenter randomized phase II study of two schedules of docetaxel, estramustine, and prednisone versus mitoxantrone plus prednisone in patients with metastatic hormone-refractory prostate cancer. J Clin Oncol. 2005;23:3343-3351. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 110] [Cited by in RCA: 105] [Article Influence: 5.3] [Reference Citation Analysis (0)] |
| 4. | Tannock IF, de Wit R, Berry WR, Horti J, Pluzanska A, Chi KN, Oudard S, Théodore C, James ND, Turesson I, Rosenthal MA, Eisenberger MA; TAX 327 Investigators. Docetaxel plus prednisone or mitoxantrone plus prednisone for advanced prostate cancer. N Engl J Med. 2004;351:1502-1512. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4284] [Cited by in RCA: 4355] [Article Influence: 207.4] [Reference Citation Analysis (0)] |
| 5. | Maximum androgen blockade in advanced prostate cancer: an overview of 22 randomised trials with 3283 deaths in 5710 patients. Prostate Cancer Trialists' Collaborative Group. Lancet. 1995;346:265-269. [PubMed] |
| 6. | Dearnaley DP, Sydes MR, Mason MD, Stott M, Powell CS, Robinson AC, Thompson PM, Moffat LE, Naylor SL, Parmar MK; Mrc Pr05 Collaborators. A double-blind, placebo-controlled, randomized trial of oral sodium clodronate for metastatic prostate cancer (MRC PR05 Trial). J Natl Cancer Inst. 2003;95:1300-1311. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 181] [Cited by in RCA: 165] [Article Influence: 7.5] [Reference Citation Analysis (0)] |
| 7. | Tangen CM, Hussain MH, Higano CS, Eisenberger MA, Small EJ, Wilding G, Donnelly BJ, Schelhammer PF, Crawford ED, Vogelzang NJ, Powell IJ, Thompson IM Jr. Improved overall survival trends of men with newly diagnosed M1 prostate cancer: a SWOG phase III trial experience (S8494, S8894 and S9346). J Urol. 2012;188:1164-1169. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 80] [Cited by in RCA: 85] [Article Influence: 6.5] [Reference Citation Analysis (0)] |
| 8. | Aus G, Robinson D, Rosell J, Sandblom G, Varenhorst E; South-East Region Prostate Cancer Group. Survival in prostate carcinoma--outcomes from a prospective, population-based cohort of 8887 men with up to 15 years of follow-up: results from three countries in the population-based National Prostate Cancer Registry of Sweden. Cancer. 2005;103:943-951. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 40] [Cited by in RCA: 38] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 9. | James ND, Spears MR, Clarke NW, Dearnaley DP, De Bono JS, Gale J, Hetherington J, Hoskin PJ, Jones RJ, Laing R, Lester JF, McLaren D, Parker CC, Parmar MKB, Ritchie AWS, Russell JM, Strebel RT, Thalmann GN, Mason MD, Sydes MR. Survival with Newly Diagnosed Metastatic Prostate Cancer in the "Docetaxel Era": Data from 917 Patients in the Control Arm of the STAMPEDE Trial (MRC PR08, CRUK/06/019). Eur Urol. 2015;67:1028-1038. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 271] [Cited by in RCA: 309] [Article Influence: 28.1] [Reference Citation Analysis (0)] |
| 10. | James ND, Sydes MR, Clarke NW, Mason MD, Dearnaley DP, Spears MR, Ritchie AW, Parker CC, Russell JM, Attard G, de Bono J, Cross W, Jones RJ, Thalmann G, Amos C, Matheson D, Millman R, Alzouebi M, Beesley S, Birtle AJ, Brock S, Cathomas R, Chakraborti P, Chowdhury S, Cook A, Elliott T, Gale J, Gibbs S, Graham JD, Hetherington J, Hughes R, Laing R, McKinna F, McLaren DB, O'Sullivan JM, Parikh O, Peedell C, Protheroe A, Robinson AJ, Srihari N, Srinivasan R, Staffurth J, Sundar S, Tolan S, Tsang D, Wagstaff J, Parmar MK; STAMPEDE investigators. Addition of docetaxel, zoledronic acid, or both to first-line long-term hormone therapy in prostate cancer (STAMPEDE): survival results from an adaptive, multiarm, multistage, platform randomised controlled trial. Lancet. 2016;387:1163-1177. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1374] [Cited by in RCA: 1557] [Article Influence: 173.0] [Reference Citation Analysis (0)] |
| 11. | Sweeney CJ, Chen YH, Carducci M, Liu G, Jarrard DF, Eisenberger M, Wong YN, Hahn N, Kohli M, Cooney MM, Dreicer R, Vogelzang NJ, Picus J, Shevrin D, Hussain M, Garcia JA, DiPaola RS. Chemohormonal Therapy in Metastatic Hormone-Sensitive Prostate Cancer. N Engl J Med. 2015;373:737-746. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1943] [Cited by in RCA: 2088] [Article Influence: 208.8] [Reference Citation Analysis (0)] |
| 12. | van Soest RJ, de Wit R. Irrefutable evidence for the use of docetaxel in newly diagnosed metastatic prostate cancer: results from the STAMPEDE and CHAARTED trials. BMC Med. 2015;13:304. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 18] [Cited by in RCA: 23] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 13. | Puente J, Grande E, Medina A, Maroto P, Lainez N, Arranz JA. Docetaxel in prostate cancer: a familiar face as the new standard in a hormone-sensitive setting. Ther Adv Med Oncol. 2017;9:307-318. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 44] [Cited by in RCA: 44] [Article Influence: 5.5] [Reference Citation Analysis (0)] |
| 14. | Gravis G, Fizazi K, Joly F, Oudard S, Priou F, Esterni B, Latorzeff I, Delva R, Krakowski I, Laguerre B, Rolland F, Théodore C, Deplanque G, Ferrero JM, Pouessel D, Mourey L, Beuzeboc P, Zanetta S, Habibian M, Berdah JF, Dauba J, Baciuchka M, Platini C, Linassier C, Labourey JL, Machiels JP, El Kouri C, Ravaud A, Suc E, Eymard JC, Hasbini A, Bousquet G, Soulie M. Androgen-deprivation therapy alone or with docetaxel in non-castrate metastatic prostate cancer (GETUG-AFU 15): a randomised, open-label, phase 3 trial. Lancet Oncol. 2013;14:149-158. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 451] [Cited by in RCA: 537] [Article Influence: 44.8] [Reference Citation Analysis (0)] |
| 15. | Zhulina Y, Cao Y, Amcoff K, Carlson M, Tysk C, Halfvarson J. The prognostic significance of faecal calprotectin in patients with inactive inflammatory bowel disease. Aliment Pharmacol Ther. 2016;44:495-504. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 51] [Cited by in RCA: 60] [Article Influence: 6.7] [Reference Citation Analysis (0)] |
| 16. | Lavoie JM, Zou K, Khalaf D, Eigl BJ, Kollmannsberger CK, Vergidis J, Noonan K, Zulfiqar M, Finch D, Chi KN. Clinical effectiveness of docetaxel for castration-sensitive prostate cancer in a real-world population-based analysis. Prostate. 2019;79:281-287. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 12] [Cited by in RCA: 12] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 17. | Mager R, Savko O, Böhm K, Thomas A, Dotzauer R, Borgmann H, Jäger W, Thomas C, Haferkamp A, Höfner T, Tsaur I. Comparative assessment of docetaxel for safety and efficacy between hormone-sensitive and castration-resistant metastatic prostate cancer. Urol Oncol. 2019;37:999-1005. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5] [Cited by in RCA: 7] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 18. | Pathak S, Thekkekara R, Yadav U, Ahmed AT, Yim B, Lad TE, Mullane M, Batra KK, Aronow WS, Psutka SP. Efficacy of Upfront Docetaxel With Androgen Deprivation Therapy for Castration-Sensitive Metastatic Prostate Cancer Among Minority Patients. Am J Ther. 2020;28:e380-e387. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 2] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 19. | Parker C, Nilsson S, Heinrich D, Helle SI, O'Sullivan JM, Fosså SD, Chodacki A, Wiechno P, Logue J, Seke M, Widmark A, Johannessen DC, Hoskin P, Bottomley D, James ND, Solberg A, Syndikus I, Kliment J, Wedel S, Boehmer S, Dall'Oglio M, Franzén L, Coleman R, Vogelzang NJ, O'Bryan-Tear CG, Staudacher K, Garcia-Vargas J, Shan M, Bruland ØS, Sartor O; ALSYMPCA Investigators. Alpha emitter radium-223 and survival in metastatic prostate cancer. N Engl J Med. 2013;369:213-223. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2211] [Cited by in RCA: 2354] [Article Influence: 196.2] [Reference Citation Analysis (0)] |
| 20. | Hofman MS, Violet J, Hicks RJ, Ferdinandus J, Thang SP, Akhurst T, Iravani A, Kong G, Ravi Kumar A, Murphy DG, Eu P, Jackson P, Scalzo M, Williams SG, Sandhu S. [177Lu]-PSMA-617 radionuclide treatment in patients with metastatic castration-resistant prostate cancer (LuPSMA trial): a single-centre, single-arm, phase 2 study. Lancet Oncol. 2018;19:825-833. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 593] [Cited by in RCA: 837] [Article Influence: 119.6] [Reference Citation Analysis (0)] |