Published online Oct 24, 2021. doi: 10.5306/wjco.v12.i10.926
Peer-review started: March 13, 2021
First decision: June 7, 2021
Revised: June 25, 2021
Accepted: September 8, 2021
Article in press: September 8, 2021
Published online: October 24, 2021
Processing time: 222 Days and 17.7 Hours
Breast cancer (BC) frequency in males is extremely low and tumor features vary from its female counterpart. Breast cancer clinical and pathological features differ by race in women. Tumor infiltrating lymphocyte (TIL) levels, mismatch repair (MMR) protein loss, androgen receptor (AR) expression, and PIK3CA gene mutations are predictive biomarkers of response to biological therapy in female BC. There is limited information about clinical and pathological features as well as predictive biomarkers in males of non-Caucasian races with BC.
To investigate clinicopathological features and biomarkers of BC tumors in males and their prognostic value in Peruvian population.
This study looked at a single-institution series of 54 Peruvian males with invasive BC who were diagnosed from Jan 2004 to June 2018. Standard pathological features, TIL levels, MMR proteins, AR immunohistochemistry staining, and PIK3CA gene mutations were prospectively evaluated in cases with available paraffin material. Percentage of AR and estrogen receptor (ER) positive cells was additionally calculated by software after slide scanning. Statistical analyses included association tests, intraclass correlation test and Kaplan Meier overall survival curves.
The median age was 63 years and most cases were ER-positive (85.7%), HER2 negative (87.2%), Luminal-A phenotype (60%) and clinical stage II (41.5%) among our male breast tumors. Median TIL was 10% and higher levels tended to be associated with Luminal-B phenotype and higher grade. AR-positive was found in 85.3% and was correlated with ER (intraclass index of 0.835, P < 0.001). Loss of MMR proteins was found in 15.4% and PIK3CA mutation (H1047R) in 14.3% (belonged to the Luminal-A phenotype). Loss of MMR proteins was associated with AR-negative (P = 0.018) but not with ER (P = 0.43) or TIL (P = 0.84). Early stages (P < 0.001) and lower grade (P = 0.006) were associated with longer overall survival. ER status, phenotype, AR status, TIL level, MMR protein loss nor PIK3CA mutation was not associated with survival (P > 0.05).
Male BC is usually ER and AR positive, and Luminal-A. MMR loss and PIK3CA mutations are infrequent. Stage and grade predicted overall survival in our South American country population.
Core Tip: Most male breast cancers were estrogen receptor-positive, HER2-negative, androgen receptor (AR)-positive, and Luminal A phenotype. Loss of mismatch repair (MMR) and PIK3CA mutations was found in around 15% of the cases. AR was correlated with ER expression and without loss of MMR proteins. Stage and grade are prognostic features in Peruvian male breast cancer.
- Citation: Castaneda CA, Castillo M, Bernabe LA, Sanchez J, Torres E, Suarez N, Tello K, Fuentes H, Dunstan J, De La Cruz M, Cotrina JM, Abugattas J, Guerra H, Gomez HL. A biomarker study in Peruvian males with breast cancer. World J Clin Oncol 2021; 12(10): 926-934
- URL: https://www.wjgnet.com/2218-4333/full/v12/i10/926.htm
- DOI: https://dx.doi.org/10.5306/wjco.v12.i10.926
Male breast cancer (BC) represents less than 1% of mammary carcinomas. Male BC has a higher expression rate of estrogen receptor (ER), higher proliferative activity, and generally more aggressive behavior than in females. The etiological attribution of inheritance for BC is more prevalent among males than females, but only a small fraction is attributed to BRCA2 mutation, and even less to BRCA1[1-5].
Combination of immunohistochemical biomarkers predicts response to therapy and allows us to classify molecular subtypes in female BC[6]. Molecular subtypes distribution and clinical features differ regarding racial populations, for instance, high triple negative breast cancer (TNBC) prevalence and young age at diagnosis are described in Latin-American women[6]. However, only a few small reports have evaluated frequency of molecular BC subtypes in Latin-American males [7,8].
Androgen-receptor (AR) expression[9], tumor-infiltrating-lymphocytes (TIL)[10], loss of mismatch-repair (MMR) proteins (biomarker of the hereditary nonpolyposis colorectal cancer (HNPCC) syndrome)[11] and PI3K mutations[12] have been associated with response to target therapy in female BC. There is a need to develop epidemiological and therapeutic information in the male counterpart. This work aims to evaluate the prevalence of clinical and molecular biomarkers of BC tumors in Peruvian males, as well as their impact on survival.
Fifty-four male BC cases who were histologically diagnosed at the Instituto Nacional de Enfermedades Neoplasicas between 2004 and 2018 were included. Clinical information was obtained from the patients' medical records. Live status of patients with not accurate follow-up was obtained from the Peruvian national registry (https://www.reniec.gob.pe) through the Epidemiology Department of the institution. The institutional review board waived informed consent and approved this retrospective case series.
TIL assessment on Hematoxylin and Eosin-stained slide was possible in 42 available samples. TIL and histological grade was prospectively evaluated by three experienced pathologists (JS, ET and HG), who were blinded to the clinical data, following international recommendations[7].
Tissue samples were fixed in 10% buffered formalin to obtain 4 μm paraffinized histological sections from 35 available tissue paraffin blocks. Sections were transferred onto adhesive slides and were dried at 60 ℃ for 30 min. After incubation with the primary antibodies, immunodetection was performed using biotinylated anti-mouse immunoglobulin, followed by peroxidase-labeled streptavidin. The labeled streptavidin biotin kit was used, and 3,3′-diaminobenzidine chromogen was used as a substrate. Immunohistochemical staining for androgen-receptor protein (AR antibody, Dako), estrogen-receptor protein (ER antibody, Zhongshan Bio), Mut L Protein Homolog protein (MLH1 antibody, Dako), DNA mismatch repair protein Msh2 (MSH2 antibody, Dako), MutS Protein Homolog 6 protein (MSH6, Dako) and Postmeiotic Segregation Increased 2 protein (PMS2 antibody, Dako) were carried out according to the manufacturer’s instructions. Normal prostatic tissue was used as a positive control of AR. Phosphate-buffered saline was used to replace the primary antibody and served as the negative control.
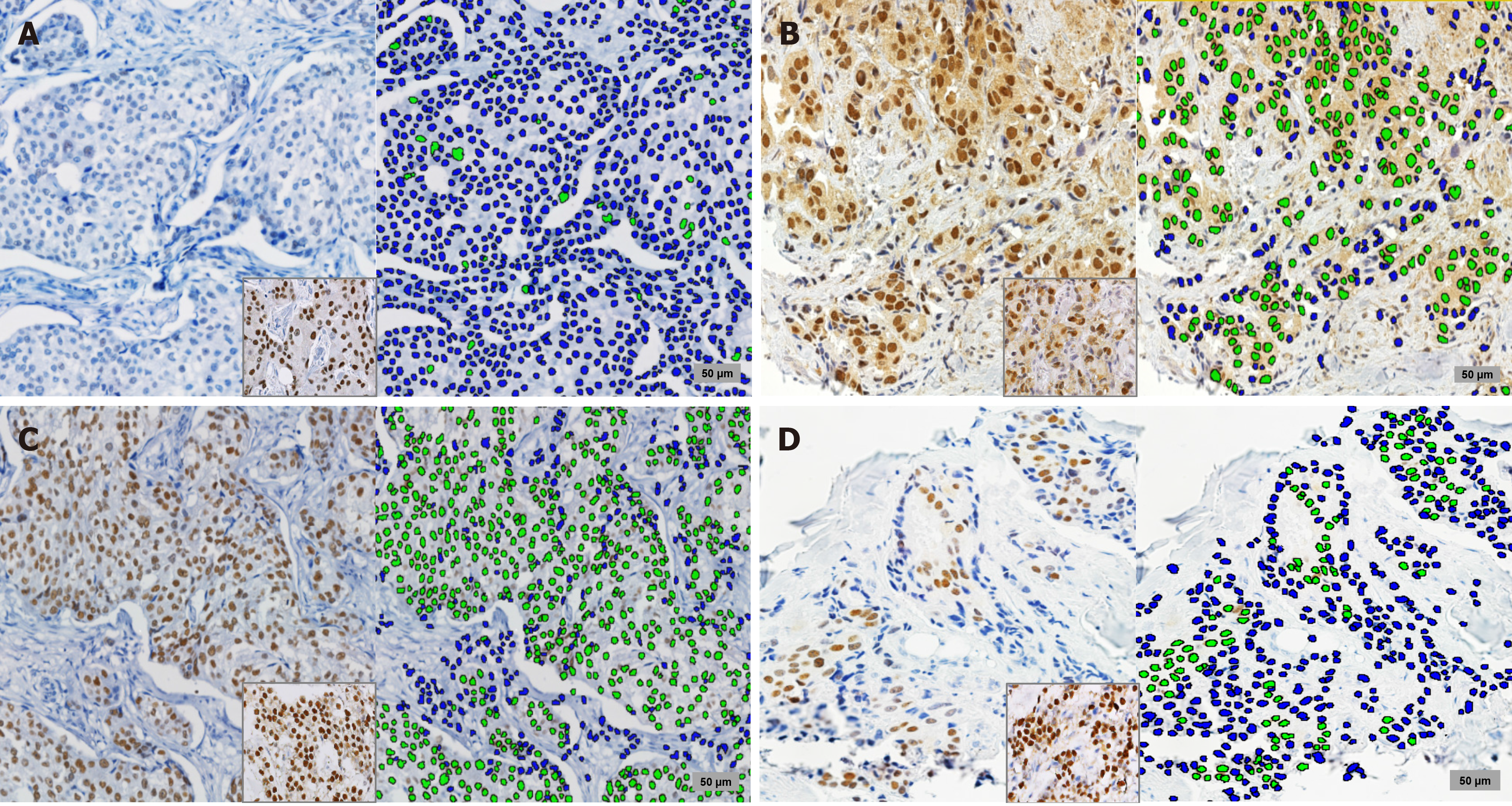
Tumors that had more than 10% of cells exhibiting a moderate or strong intensity of AR expression were considered positive[7]. Additionally, slides of AR and ER staining were scanned in BX63 Olympus (Tokyo, Japan) and the analysis was performed through Visiopharm software in 34 male BC cases. Negative and positive cells were marked in blue and green by TissueMorph Software, (Visopharm, Hoerlson, Denmark), and the proportion of cell count was obtained through the ratio of AR and ER on number of positive overall cells in 5 high power fields (Figure 1) under supervision of a pathologist as previously described (JS)[7]. Non-malignant stromal cells were used as internal positive controls for MLH1, MSH2, MSH6, and PMS2, while the loss of MMR was considered when lacking nuclear staining for at least one was found[13].
Tumor DNA from paraffin-embedded samples was extracted using the QIAamp DNA FFPE Tissue Kit (Qiagen; Hilden, Germany) in the available 14 tumor samples. TaqMan-based real-time PCR analysis was conducted using a LightCycler® 96 Real-Time PCR System (Roche Applied Science, Mannheim, Germany) to detect the three ‘‘hot spot’’ PIK3CA mutations (H1047R, E545K and E542K). Custom TaqMan primers and probes were designed for the PIK3CA mutations (PI3KCA 760: c.1624 G (VIC) >A (FAM), PI3KCA 763: c.1633 G (VIC) >A (FAM), PI3KCA 775: c.3140 A (VIC) >G (FAM), ThermoFisher scientific). The thermal cycler protocol was as follows: 10 min at 96 C, 39 cycles at 60 ℃ for 2 min, 98 ℃ for 30 s, and 60 ℃ for 1 min. All samples were analyzed in a single assay for each mutation. The mutational threshold was determined by measuring the WT HDx FFPE reference standards (Horizon Diagnostics, Cambridge, UK), and was 1%. In contrast, the threshold of reagents inducing false positives was assumed to be 0.5% of the mutation frequency.
Associations of clinical-pathological variables were performed by the Chi-square test or the Fisher’s exact test. Associations of ordinary variables were performed by the Kruskal-Wallis test. Identification of co-expression of AR and ER was performed by the intraclass correlation test. Overall survival (OS) analysis was estimated using the Kaplan–Meier method. Log-rank or Breslow tests were used to find differences between categories, according to the case. A P < 0.05 was considered significant. Analyses were performed using the SPSS statistical package (version 26.0.0, IBM SPSS Statistical).
There were included 54 male BC patients and their median age was 63 years. Twenty-eight (51.9%) tumors were located on the left side, concurrent in-situ component in 41.4% and the most frequent clinical stage was II (41.5%) (Table 1). A family cancer history was found in 12 (22.2%) cases. Regarding treatment, mastectomy, tumorec
| Features | n | % | OS-5yr | P value |
| Age | ||||
| Median (yr) | 63 | 0.105a | ||
| < 63 | 25 | 53.7 | 56.0 | |
| ≥ 63 | 29 | 46.3 | 69.0 | |
| Clinical stage (n = 53) | < 0.001a | |||
| I | 2 | 3.8 | 100.0 | |
| II | 22 | 41.5 | 86.4 | |
| III | 20 | 37.7 | 50.0 | |
| IV | 9 | 17.0 | 22.2 | |
| Histological grade (n = 46) | 0.006a | |||
| 1 | 7 | 15.2 | 85.7 | |
| 2 | 26 | 56.5 | 73.1 | |
| 3 | 13 | 28.3 | 30.8 | |
| sTIL (n = 42) | 0.397b | |||
| < 10% | 12 | 28.6 | 50.0 | |
| ≥ 10% | 30 | 71.4 | 66.7 | |
| ER status (n = 49) | 0.567a | |||
| Positive | 42 | 85.7 | 66.7 | |
| Negative | 7 | 14.3 | 57.1 | |
| PgR status (n = 49) | 0.305a | |||
| Positive | 43 | 87.8 | 67.4 | |
| Negative | 6 | 12.2 | 50.0 | |
| HER2 status (n = 47) | 0.088b | |||
| Positive | 6 | 12.8 | 33.3 | |
| Negative | 41 | 87.2 | 70.7 | |
| Ki67 (n = 40) (median 5%) | 0.232a | |||
| 0%-5% | 22 | 55.0 | 59.1 | |
| > 5% | 18 | 45.0 | 66.7 | |
| Phenotype (n = 45) | 0.356a | |||
| Luminal A | 27 | 60.0 | 63.0 | |
| Luminal B | 13 | 28.9 | 76.9 | |
| TNBC | 5 | 11.1 | 40.0 | |
| AR (n = 35) | 0.294a | |||
| 0%-9% | 5 | 14.3 | 60.0 | |
| ≥ 10% | 30 | 85.7 | 63.3 | |
| Mismatch repair loss (n = 26) | 0.501a | |||
| No | 20 | 76.9 | 65.00 | |
| Yes | 4 | 15.4 | 75.00 | |
| Undetermined | 2 | 7.7 | 100.00 | |
| PIK3CA mutation H1047R (n = 14) | 0.844b | |||
| Positive | 2 | 14.3 | 50.0 | |
| Negative | 12 | 85.7 | 75.0 |
Most cases were ER-positive (85.7%), HER2-negative (87.2%) and belonged to Luminal-A phenotype (Table 1). ER status was not associated with MMR-loss (P = 0.43). No association between ER percentage and MMR-loss was found (P = 0.22).
Positive AR status was found in 85.3% of patients and the two TNBC cases were negative for AR. MMR-loss was associated with AR-negative (75% vs 10%, P = 0.018). A lower median percentage of AR-positive cells was found in cases with MMR-loss (5% vs 70%, P = 0.02).
Evaluation of similar areas for ER and AR found that a median of 3738 cells (52.9%) (range 0%-87%) from 7577 cells (range 2139-11883 cells) were positive for ER, and 3795 cells (55%) (range 0%-87.8%) from 7366 (range 2520-12135 cells) were positive for AR. Intraclass correlation coefficient of the means for AR and ER was 0.835 (P < 0.001) (Figure 2). A lower median of AR-positive tumor cell count proportion was also found in cases with MMR-loss (8% vs 65%, P = 0.018). No association between median of ER-positive tumor cell count proportion and MMR-loss was found (P = 0.163).
Median TIL was 10% and higher levels tended to be associated with Luminal-B (P = 0.058) (Figure 2). TIL ≥ 50% was associated with higher grade (P = 0.039), but not to age (P = 0.44), clinical stage (P = 0.59), MMR-loss (20% vs 15%, P = 0.72) nor PIK3CA mutation status (P = 0.53).
Loss of MMR protein expression was found in 4 (15.4%) cases. It was not associated with age (P = 0.69), clinical-stage (P = 0.68), grade (P = 0.53), molecular subtypes (P = 0.91) or PIK3CA mutation status (P = 0.41).
PIK3CA mutations were found in 2 (14.3%) of the 14 evaluated cases and both were positive for H1047R (1321 copies/microL-15.07% and 1019 copies/microL-18.06%). Both cases belonged to the Luminal-A phenotype.
Longer OS was associated with early stage (P < 0.001) and lower grade (P = 0.006). Survival was not associated with ER status (P = 0.305), phenotype (P = 0.152), AR status (P = 0.613), TIL level (P = 0.397), MMR protein loss (P = 0.501) nor PIK3CA mutation status (P = 0.844).
We found a high frequency of AR expression and low frequency of both MMR-loss and PIK3CA mutations in our BC series in male Peruvian population.
Expression of AR was found in 85% of our male BC cases which is in the previously described range (40%–90%)[1], however we didn’t find its association with prognosis. Androgen-receptor is a key driver of proliferation and cell survival, and although some retrospective male BC series describe its association with better prognosis[3] others like ours not[4]. Additionally, recent basic research and clinical trials describe that AR can predict activity of targeting drugs[9].
Our findings of high rates of ER-positive status and Luminal phenotype have been extensively described in Caucasian series[2,5]. Large sample size studies describe that ER-positive status would have a favorable prognostic effect (similar to the female BC) and predicts response to endocrine modulation[5,14,15]. The fact that we did not find an association with OS could be because of the small size of our series.
There was absence of HER2 enriched phenotype in our series and HER2- positive status was very infrequent. This corroborates previously published information that HER2 overexpression is less frequent than in female series, but it is expected to behave as a predictive feature to anti-HER2 therapies[2,5].
Our findings of 15% of cases with MMR loss have not previously been described in male BC. The HNPCC syndrome is an autosomal dominant genetic disorder characterized by predisposition to extracolonic malignancies at various sites including BC, is detected by loss of MMR proteins and is associated with high response to anti-PDL1 therapy. Studies with small sample size in female BC describe the lack or reduced expression of hMSH2 and hMLH1 in less than 20%[11,16]. Boyd et al[17] described a case of male BC belonging to a large HNPCC kindred that harbors a germline mutation of the MLH1. And recently, Piscuoglio et al[2] developed a pathway and network analysis of 241 genes in a series of 59 male BC and described an enrichment of mutations affecting DNA repair-related genes. We found that MMR-loss was associated with the previously described marker of endocrine response[3,4], the lower AR expression. Furthermore, this association appears to be specific for this steroid marker and not for ER, despite AR and ER expression were co-related. Haricharan et al[18] suggest that alteration in DNA damage repair genes could produce endocrine resistance because of the finding that defects in MMR pathway genes doesn’t allow an accurate CDK4 suppression by endocrine therapy.
PIK3CA mutations were found in only 2 of 14 evaluated male BC cases that is lower than previously reported by our group in women with BC[19,20]. Piscuoglio et al[2] sequenced 241 genes in 59 male BC and found that most recurrent mutations affected the PIK3CA gene (20%). However, these rates would be lower than their female counterparts that have been detected in approximately 30%-40% of female BC[21]. In vitro and translational research in tumor samples from clinical trials found that PIK3CA mutations reduce sensitivity to anti-HER2 drugs[22] and endocrine modula
TIL is a well-described prognostic and predictive biomarker for anti-PDL1-therapy in female BC[10]. Vermeulen et al[22] found that high TIL density evaluated by a method different than the currently recommended in international guidelines was associated with Luminal-B HER2-positive subtype and longer OS in a 1483 male BC series. We also found a trend of higher TILs in Luminal-B subtype but not a relationship with longer OS (P = 0.378). Contrary to the female counterpart or other malignancies, we did not find that higher TIL levels were associated with AR or ER percentage nor with MMR-loss.
Finally, our finding that early stage and lower grade features achieve significant association with better prognosis is consistent with previous reports, and supports the current management of this entity[2,5].
The weakness of our study was that several patients were lost of follow-up; however, obtaining information about their live status from the national registry allowed us to build OS curves. The number of included cases is also small because it is a very unusual entity, representing less than 0.5% of their female counterpart (18552 female new BC cases diagnosed in our center during the same period). Our strongness is the prospective evaluation of biomarkers by pathologists and biologist authors. Our results serve to complement male BC knowledge in the South American population, which has been under-studied.
We conclude that early-stage and low-grade features identify favorable prognoses in male BC. Most Peruvian BC cases are ER-positive, HER2-negative, AR-positive and Luminal-A tumors. MMR loss and PIK3CA mutations are infrequent, and MMR loss was associated with AR negative.
Information about clinicopathological features associated with treatment response and prognosis has been extensively described for female breast cancer, and differences regarding races has been described. Breast cancer in males is much less frequent, has important clinicopathological differences and is less studied than their female breast cancer counterpart.
Discussion and new information about features and biomarkers of Breast cancer (BC) in males have been included in recent cancer-related meetings, and more than 30000 articles have been published in the last two years. However, very few of them have evaluated a South American population.
To describe rates of currently accepted biomarkers for prognosis and for prediction of treatment response in Peruvian males with BC.
Clinical files and tumor slides were reviewed. Tumor-infiltrating lymphocytes, mismatch repair proteins (MMR), PIK3CA gene mutations, estrogen (ER) and, androgen receptors (AR) were prospectively evaluated in available paraffin material.
In our series of 54 Peruvian males with invasive breast cancer, we found that most cases were Luminal-A phenotype (60%), ER-positive (85.7%), AR-positive (85.3%), and the median of tumor-infiltrating lymphocytes was 10%. MMR loss was found in 15.4% and PIK3CA mutation (H1047R) in 14.3% (all in the Luminal-A group). MMR loss was associated with AR-negative (P = 0.018). Longer overall survival was associated with early stages (P < 0.001) and lower grade (P = 0.006).
Most breast cancer tumors in Peruvian males are ER and AR-positive, and MMR loss and PIK3CA mutations are infrequent. MMR loss was associated with hormone receptor-negative.
Biomarkers identified for women with breast cancer need to be validated for the male counterpart. Research in race disparities needs to be extended also for males of non-Caucasian races. Association between MMR loss and activity of AR pathways requires further evaluation.
Manuscript source: Unsolicited manuscript
Corresponding Author's Membership in Professional Societies: European Society of Medical Oncology, 5275.
Specialty type: Oncology
Country/Territory of origin: Peru
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): 0
Grade C (Good): C, C
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Exbrayat JM, Rachmi E S-Editor: Wang LL L-Editor: A P-Editor: Xing YX
| 1. | Kornegoor R, Verschuur-Maes AH, Buerger H, Hogenes MC, de Bruin PC, Oudejans JJ, van der Groep P, Hinrichs B, van Diest PJ. Molecular subtyping of male breast cancer by immunohistochemistry. Mod Pathol. 2012;25:398-404. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 92] [Cited by in RCA: 94] [Article Influence: 7.2] [Reference Citation Analysis (0)] |
| 2. | Piscuoglio S, Ng CK, Murray MP, Guerini-Rocco E, Martelotto LG, Geyer FC, Bidard FC, Berman S, Fusco N, Sakr RA, Eberle CA, De Mattos-Arruda L, Macedo GS, Akram M, Baslan T, Hicks JB, King TA, Brogi E, Norton L, Weigelt B, Hudis CA, Reis-Filho JS. The Genomic Landscape of Male Breast Cancers. Clin Cancer Res. 2016;22:4045-4056. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 88] [Cited by in RCA: 111] [Article Influence: 12.3] [Reference Citation Analysis (0)] |
| 3. | Humphries MP, Sundara Rajan S, Honarpisheh H, Cserni G, Dent J, Fulford L, Jordan LB, Jones JL, Kanthan R, Litwiniuk M, Di Benedetto A, Mottolese M, Provenzano E, Shousha S, Stephens M, Kulka J, Ellis IO, Titloye AN, Hanby AM, Shaaban AM, Speirs V. Characterisation of male breast cancer: a descriptive biomarker study from a large patient series. Sci Rep. 2017;7:45293. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 34] [Cited by in RCA: 51] [Article Influence: 6.4] [Reference Citation Analysis (0)] |
| 4. | Wenhui Z, Shuo L, Dabei T, Ying P, Zhipeng W, Lei Z, Xiaohui H, Jingshu G, Hongtao S, Qingyuan Z. Androgen receptor expression in male breast cancer predicts inferior outcome and poor response to tamoxifen treatment. Eur J Endocrinol. 2014;171:527-533. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 19] [Cited by in RCA: 15] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 5. | Cardoso F, Bartlett JMS, Slaets L, van Deurzen CHM, van Leeuwen-Stok E, Porter P, Linderholm B, Hedenfalk I, Schröder C, Martens J, Bayani J, van Asperen C, Murray M, Hudis C, Middleton L, Vermeij J, Punie K, Fraser J, Nowaczyk M, Rubio IT, Aebi S, Kelly C, Ruddy KJ, Winer E, Nilsson C, Lago LD, Korde L, Benstead K, Bogler O, Goulioti T, Peric A, Litière S, Aalders KC, Poncet C, Tryfonidis K, Giordano SH. Characterization of male breast cancer: results of the EORTC 10085/TBCRC/BIG/NABCG International Male Breast Cancer Program. Ann Oncol. 2018;29:405-417. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 158] [Cited by in RCA: 256] [Article Influence: 42.7] [Reference Citation Analysis (0)] |
| 6. | Carey LA, Perou CM, Livasy CA, Dressler LG, Cowan D, Conway K, Karaca G, Troester MA, Tse CK, Edmiston S, Deming SL, Geradts J, Cheang MC, Nielsen TO, Moorman PG, Earp HS, Millikan RC. Race, breast cancer subtypes, and survival in the Carolina Breast Cancer Study. JAMA. 2006;295:2492-2502. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2859] [Cited by in RCA: 2768] [Article Influence: 145.7] [Reference Citation Analysis (0)] |
| 7. | Rebaza LP, Castaneda CA, Castillo M, Bernabe LA, Sanchez J, Calderon G, Dunstan J, la Cruz M de, Cotrina JM, Abugattas J. Androgen expression and clinicopathological features in male breast cancer. Breast Cancer Manag. 2018;7:BMT07. [RCA] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 1] [Article Influence: 0.1] [Reference Citation Analysis (0)] |
| 8. | Thuler LC, Bergmann A. Male breast cancer: clinical-epidemiological characteristics of 1189 Brazilian patients. Aging Male. 2015;18:118-123. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 8] [Cited by in RCA: 9] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 9. | Traina TA, Miller K, Yardley DA, Eakle J, Schwartzberg LS, O'Shaughnessy J, Gradishar W, Schmid P, Winer E, Kelly C, Nanda R, Gucalp A, Awada A, Garcia-Estevez L, Trudeau ME, Steinberg J, Uppal H, Tudor IC, Peterson A, Cortes J. Enzalutamide for the Treatment of Androgen Receptor-Expressing Triple-Negative Breast Cancer. J Clin Oncol. 2018;36:884-890. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 302] [Cited by in RCA: 354] [Article Influence: 50.6] [Reference Citation Analysis (0)] |
| 10. | Adams S, Loi S, Toppmeyer D, Cescon DW, De Laurentiis M, Nanda R, Winer EP, Mukai H, Tamura K, Armstrong A, Liu MC, Iwata H, Ryvo L, Wimberger P, Rugo HS, Tan AR, Jia L, Ding Y, Karantza V, Schmid P. Pembrolizumab monotherapy for previously untreated, PD-L1-positive, metastatic triple-negative breast cancer: cohort B of the phase II KEYNOTE-086 study. Ann Oncol. 2019;30:405-411. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 404] [Cited by in RCA: 450] [Article Influence: 75.0] [Reference Citation Analysis (0)] |
| 11. | Fusco N, Lopez G, Corti C, Pesenti C, Colapietro P, Ercoli G, Gaudioso G, Faversani A, Gambini D, Michelotti A, Despini L, Blundo C, Vaira V, Miozzo M, Ferrero S, Bosari S. Mismatch Repair Protein Loss as a Prognostic and Predictive Biomarker in Breast Cancers Regardless of Microsatellite Instability. JNCI Cancer Spectr. 2018;2:pky056. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 44] [Cited by in RCA: 62] [Article Influence: 8.9] [Reference Citation Analysis (0)] |
| 12. | André F, Ciruelos E, Rubovszky G, Campone M, Loibl S, Rugo HS, Iwata H, Conte P, Mayer IA, Kaufman B, Yamashita T, Lu YS, Inoue K, Takahashi M, Pápai Z, Longin AS, Mills D, Wilke C, Hirawat S, Juric D; SOLAR-1 Study Group. Alpelisib for PIK3CA-Mutated, Hormone Receptor-Positive Advanced Breast Cancer. N Engl J Med. 2019;380:1929-1940. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1607] [Cited by in RCA: 1708] [Article Influence: 284.7] [Reference Citation Analysis (0)] |
| 13. | Lynch HT, Lynch JF, Lynch PM. Toward a consensus in molecular diagnosis of hereditary nonpolyposis colorectal cancer (Lynch syndrome). J Natl Cancer Inst. 2007;99:261-263. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 40] [Cited by in RCA: 37] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 14. | Zagouri F, Sergentanis TN, Chrysikos D, Dimopoulos MA, Psaltopoulou T. Fulvestrant and male breast cancer: a pooled analysis. Breast Cancer Res Treat. 2015;149:269-275. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 34] [Cited by in RCA: 31] [Article Influence: 3.1] [Reference Citation Analysis (0)] |
| 15. | Wedam S, Fashoyin-Aje L, Bloomquist E, Tang S, Sridhara R, Goldberg KB, Theoret MR, Amiri-Kordestani L, Pazdur R, Beaver JA. FDA Approval Summary: Palbociclib for Male Patients with Metastatic Breast Cancer. Clin Cancer Res. 2020;26:1208-1212. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 47] [Cited by in RCA: 93] [Article Influence: 15.5] [Reference Citation Analysis (0)] |
| 16. | Murata H, Khattar NH, Gu L, Li GM. Roles of mismatch repair proteins hMSH2 and hMLH1 in the development of sporadic breast cancer. Cancer Lett. 2005;223:143-150. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 38] [Cited by in RCA: 42] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 17. | Boyd J, Rhei E, Federici MG, Borgen PI, Watson P, Franklin B, Karr B, Lynch J, Lemon SJ, Lynch HT. Male breast cancer in the hereditary nonpolyposis colorectal cancer syndrome. Breast Cancer Res Treat. 1999;53:87-91. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 59] [Cited by in RCA: 60] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 18. | Haricharan S, Punturi N, Singh P, Holloway KR, Anurag M, Schmelz J, Schmidt C, Lei JT, Suman V, Hunt K, Olson JA Jr, Hoog J, Li S, Huang S, Edwards DP, Kavuri SM, Bainbridge MN, Ma CX, Ellis MJ. Loss of MutL Disrupts CHK2-Dependent Cell-Cycle Control through CDK4/6 to Promote Intrinsic Endocrine Therapy Resistance in Primary Breast Cancer. Cancer Discov. 2017;7:1168-1183. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 43] [Cited by in RCA: 55] [Article Influence: 6.9] [Reference Citation Analysis (0)] |
| 19. | Xiao W, Zhang G, Chen B, Chen X, Wen L, Lai J, Li X, Li M, Liu H, Liu J, Han-Zhang H, Lizaso A, Liao N. Mutational Landscape of PI3K-AKT-mTOR Pathway in Breast Cancer: Implications for Targeted Therapeutics. J Cancer. 2021;12:4408-4417. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 18] [Cited by in RCA: 27] [Article Influence: 6.8] [Reference Citation Analysis (0)] |
| 20. | Miricescu D, Totan A, Stanescu-Spinu II, Badoiu SC, Stefani C, Greabu M. PI3K/AKT/mTOR Signaling Pathway in Breast Cancer: From Molecular Landscape to Clinical Aspects. Int J Mol Sci. 2020;22. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 291] [Cited by in RCA: 478] [Article Influence: 95.6] [Reference Citation Analysis (0)] |
| 21. | Castaneda CA, Lopez-Ilasaca M, Pinto JA, Chirinos-Arias M, Doimi F, Neciosup SP, Rojas KI, Vidaurre T, Balko JM, Arteaga CL, Gomez HL. PIK3CA mutations in Peruvian patients with HER2-amplified and triple negative non-metastatic breast cancers. Hematol Oncol Stem Cell Ther. 2014;7:142-148. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 8] [Cited by in RCA: 18] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 22. | Vermeulen MA, Slaets L, Cardoso F, Giordano SH, Tryfonidis K, van Diest PJ, Dijkstra NH, Schröder CP, van Asperen CJ, Linderholm B, Benstead K, Foekens R, Martens JWM, Bartlett JMS, van Deurzen CHM. Pathological characterisation of male breast cancer: Results of the EORTC 10085/TBCRC/BIG/NABCG International Male Breast Cancer Program. Eur J Cancer. 2017;82:219-227. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 57] [Cited by in RCA: 68] [Article Influence: 8.5] [Reference Citation Analysis (0)] |