Published online Sep 24, 2020. doi: 10.5306/wjco.v11.i9.732
Peer-review started: May 16, 2020
First decision: June 4, 2020
Revised: June 6, 2020
Accepted: August 25, 2020
Article in press: August 25, 2020
Published online: September 24, 2020
Processing time: 125 Days and 8.2 Hours
Inflammation is a well-established enabling factor for cancer development and provides a framework for the high prevalence of colon cancer in inflammatory bowel disease. In accordance, chronic inflammation has recently been implicated in the development of cancer stem cells (CSCs). However, the mechanism whereby anti-inflammatory drugs act in the prevention of colitis-associated cancer (CAC) is only partially understood.
To evaluate the role of diacerein (DAR), an anti-inflammatory drug that mainly acts through the inhibition of interleukin (IL)-1β expression in the development of CSCs and CAC.
The effects of DAR on colon inflammation in mice with CAC were evaluated by inflammatory index, reverse real-time transcription polymerase chain reaction and western blot. Cytokine levels were measured by enzyme-linked immunosorbent assay. Cells assays evaluated the effects of DAR on CSCs. Immunohistochemistry and apoptosis assays were also used to evaluate the effects of DAR on tumorigenesis associated with inflammation.
DAR treatment reduced colon inflammation as well as the number and size of tumors in azoxymethane plus dextran sulphate sodium-treated animals. Accordingly, DAR treatment was associated with reduced intracellular signals of inflammation (inhibitor of nuclear factor kappa B kinase and c-Jun N-terminal kinase phosphorylation) in the colon. In addition, DAR treatment was associated with a decrease in colon CSC formation, suggesting that besides reducing colonic inflammation, DAR has a direct effect on the inhibition of colon carcinogenesis.
Together, these data indicate that DAR-mediated IL-1β suppression attenuates inflammation-induced colon cancer and CSC formation, highlighting DAR as a potential candidate for the chemoprevention of CAC.
Core Tip: Inflammation is a well-established factor for colitis-associated cancer development. In accordance, chronic inflammation has been implicated in cancer stem cell (CSC) development. The present study evaluates the role of diacerein (DAR), an anti-inflammatory drug, in the development of an experimental model of CSCs and colitis-associated cancer. DAR treatment reduced colon inflammation and number and size of tumors. DAR treatment was associated with reduced intracellular signals of inflammation (inhibitor of nuclear factor kappa B kinase and c-Jun N-terminal kinase phosphorylation) in colon. DAR treatment was associated with a decrease in colon CSC formation, suggesting that besides reducing colonic inflammation, DAR has a direct effect on the inhibition of colon carcinogenesis.
- Citation: Paulino DSM, Mendes MCS, Camargo JA, Brambilla SR, Wood Dos Santos T, Ribeiro ML, Carvalheira JBC. Diacerein treatment prevents colitis-associated cancer in mice. World J Clin Oncol 2020; 11(9): 732-746
- URL: https://www.wjgnet.com/2218-4333/full/v11/i9/732.htm
- DOI: https://dx.doi.org/10.5306/wjco.v11.i9.732
Over the past few decades, inflammation has emerged as an important enabling factor for cancer development[1-3]. Although the mechanisms that link inflammation to carcinogenesis remain poorly understood, inflammation favors all stages of tumorigenesis by exerting direct effects on tumor cell proliferation, survival, metastasis and angiogenesis[4,5]. Accordingly, inflammatory bowel diseases (IBDs), the most prevalent of which are ulcerative colitis and Crohn’s disease, significantly increase the risk for future development of colorectal cancer (CRC)[6-8]. Additionally, since the discovery of cancer stem cells (CSCs), growing evidence supports the idea that human cancers, in general, are considered a stem cell disease. In this scenario, it has been demonstrated that apart from its increased ability to self-renew, CSCs are related to tumor progression, metastasis, resistance to therapy and subsequent tumor recurrence[9]. Interestingly, recent studies have linked chronic inflammation to the development of CSCs[10], including colonic CSCs[11], giving support to the hypothesis that colitis-associated cancer (CAC) might be associated with inflammation-mediated disrupted function of intestinal stem cells.
Chemically induced colitis studies have led to the identification of pro-inflammatory cytokines as key elements in the pathogenesis of both colitis and CAC[12]. These studies disclosed a positive correlation between increased serum levels of interleukin (IL)-6 and the incidence of CRC[13]. CRC incidence is reduced in mice lacking the tumor necrosis factor receptor p55 (TNF-Rp55 knockout) treated with azoxymethane (AOM) and dextran sulphate sodium (DSS)[14]. In consonance with these results, IL-1β is increased in an AOM + DSS CAC model, and the administration of an IL-1 receptor antagonist prevented cancer development[15-17]. Moreover, genetic ablation of the inhibitor of nuclear factor kappa B kinase (IKK)β, an intracellular molecule that activates factor nuclear kappa B (NFκB) by pro-inflammatory cytokines, attenuates the development of CAC[18]. Despite the progress in this field, the effect of anti-inflammatory drugs on CAC chemoprevention is not entirely clear[19].
Although some studies have reported that the canonical anti-inflammatory treatment of IBD with 5-aminosalicylate reduces the risk of developing CRC[20], other results are conflicting[21,22]. Interestingly, compounds of a diverse nature with anti-inflammatory properties were described to have chemopreventive effects in animal models of CAC carcinogenesis[23-26]. Furthermore, antibody-mediated TNF neutralization resulted in the reduction of tumor incidence and multiplicity[27]. However, the potential role of pharmacological IL-1β inhibitors in the development of CAC was not fully explored.
Diacerein (DAR; 1,8-diacetoxy-9,10-dioxo-dihydroanthracene-3-carboxylic acid) is a nonsteroidal anti-inflammatory drug (commonly known as NSAID) that was first described in 1980[28]. Mechanistically, DAR and its derived metabolite rhein act mainly by suppressing IL-1β expression[29]. However, other effects have been described, including reducing serum levels of IL-12 and TNF-α, inhibition of chemotaxis and neutrophil phagocytosis, as well as macrophage migration and phagocytic activity[29,30].
Herein, we report that DAR treatment not only suppressed colitis and CAC in animals treated with AOM + DSS but also reduced colon CSC formation. These results reveal DAR as a potential candidate for the treatment of IBD as well as for the chemoprevention of CRC associated with IBD.
Male Swiss mice (ages 8– to 10-wk-old) were maintained at the Animal Breeding Center of the University of Campinas (UNICAMP) (Campinas, São Paulo, Brazil) under pathogen-free conditions in a 12 h/12 h light/dark cycle and a temperature-controlled environment. Mice were fed with a standard diet (AIN-93) and received water ad libitum. All procedures involving animals were approved by the local animal ethics committee of UNICAMP (Protocol No. 3350-1). Mice were immobilized for intragastric gavage; the practice was done carefully using gavage needles specific for mice. All procedures were performed in a manner to minimize the pain and discomfort of the animals.
DAR was provided by TRB-Pharma (Campinas, SP, Brazil), and it was diluted in 0.01 M phosphate-buffered saline (PBS). The animals that were treated with DAR received three different doses of 10, 20 and 50 mg/kg per day by daily gavage, while the control (CTL) group received vehicle (PBS).
AOM (12.5 mg/kg; Sigma-Aldrich, St Louis, MO, United States) was injected intraperitoneally into 10-wk-old Swiss mice. After 1 wk, the animals received 2.5% DSS (molecular weight 36–50 kDa; MP Biomedical, Inc, Santa Ana, CA, United States) in water for 5 d. DSS treatment was repeated once a month for a total of three cycles. Mice were sacrificed 10 d after the last cycle, with the extraction of colon tissue and tumors afterwards[12].
The weights of the animals were measured weekly, and they were inspected daily for the presence of anal bleeding and diarrhea.
Colon tissue and tumor extracts were homogenized in extraction buffer. Then, Laemmli sample buffer containing dithiothreitol (100 mM) was added, and the solution was heated at 100 ºC for 5 min[31]. Equivalent amounts of total extracts were subjected to 8%–10% sodium dodecyl sulfate-polyacrylamide gel electrophoresis. Using a wet transfer apparatus (Bio-Rad, Hercules, CA, United States), the resolved proteins were transferred to nitrocellulose membranes. Subsequently, the membranes were blocked and incubated overnight at 4 °C with specific antibodies. The antibodies used were anti-phospho-Jun N-terminal kinase (JNK) Thr183/Tyr185 (SC-12882-R; Santa Cruz Biotechnology, Dallas, TX, United States), anti-phospho-IKK Ser180/Ser181 (SC-23470-R; Santa Cruz Biotechnology), anti-phospho-protein kinase B (AKT) Ser473 (Cell-9271; Cell Signaling Technology, Danvers, MA, United States), anti-phospho-ribosomal protein S6 kinase (p70S6K) Thr389 (Cell-9205; Cell Signaling Technology), and anti-β-tubulin (Cell-2146; Cell Signaling Technology).
The membranes were washed three times in Tris-buffered saline for 10 min, incubated with peroxidase-labelled anti-mouse or anti-rabbit immunoglobulin (Ig) G antibodies diluted in blocking buffer for 2 h, and then washed again. These membranes were exposed to a chemiluminescence solution (SuperSignal West Pico Chemiluminescent Substrate (Pierce Biotechnology, Rockford, IL, United States)) under constant agitation for approximately 3 min, and then they were subsequently revealed in ChemiDoc MP (Bio-Rad).
The serum cytokine profile (TNF, IL-1β and IL-6) of animals subjected to colon cancer induction was measured using a commercial enzyme-linked immunosorbent assay kit (Millipore, St Charles, MO, United States).
The expression levels of TNF-α, IL-1β and IL-6 genes in the colonic mucosa of Swiss mice were determined by quantitative real-time reverse transcription polymerase chain reaction (RT-PCR) analysis. The total RNA was isolated according to manufacturer recommendations, and then reverse transcribed into cDNA by using the Maxima First Strand cDNA Synthesis Kit (Thermo Scientific, Waltham, MA, United States). The TaqManTM system (Applied Biosystems, Foster City, CA, United States) was used for RT-PCR reactions. The specific primers used for gene amplification were TNF-α (Mn00443260), IL-1β (Mn00434228) and IL-6 (Mn00446190). The β-actin (BAC) gene (Mm00607939_s1) was used for normalization of gene expression. The programs QuantStudio™ 7 software v1.0 (Applied Biosystems) and DataAssist™ software v3.01 (Life Technologies, Carlsbad, CA, United States) were used for data analyses.
DAR dilution: DAR was dissolved in isopropyl alcohol and diluted in Dulbecco’s modified Eagle’s medium (DMEM) (25% final alcohol concentration). For cell assays, concentrations ranging from 0–300 mmol/L (0, 50, 100, 200 and 300 mmol/L) were used.
Cell culture: The human colon carcinoma cell lines HT-29 and CACO-2 were purchased from the American Type Culture Collection (ATCC, Philadelphia, PA, United States). All cell lines were mycoplasma-free and authenticated by GenePrint10System Kit (Promega, Madison, WA, United States). Cells were cultured in DMEM (Invitrogen, Carlsbad, CA, United States) supplemented with 10% fetal calf serum, streptomycin, and penicillin. After expansion, the cells were seeded and divided in two groups: Control (incubated with DMEM) and DAR 100 mmol/L (incubated with DMEM + DAR at 100 mmol/L for 24 h and 48 h). After that, the cells were collected and stored in a -80 ºC freezer. Cells were also treated with 10 ng/mL of IL-1β (Sigma-Aldrich) for 24 h.
Cytotoxicity assay: Cellular toxicity was assessed by the tetrazolium microculture (MTT) assay. Briefly, the cells were cultured in 96-well microplates at 3 × 104 cells per well and treated with DAR (0, 50, 100, 200 and 300 mmol/L) or diluent (25% isopropyl alcohol) and incubated at 37 °C and 5% of CO2 for 24 h and 48 h. At end, the supernatant was discarded, and the MTT solution (tetrazolium 5 mg/mL solution diluted in PBS) was added and incubated for 3 h. Then, 100 μL of 10% sodium dodecyl sulphate solution (10%; 0.01 mol/L HCL) was added to each well, followed by further incubation for 18 h. Absorbance was measured at 540 nm in a microplate reader (Multiskan MS Labsystems, Joensu, Finland).
Spheroids formation assay: Spheroid cultures were performed according to the protocol described by Razian et al[32] (2013) , with some modifications. Briefly, after treatment with DAR 100 mmol/L (previously described), the cells were trypsinized and transferred to 6-well plates (1 × 105 cells per well) pre-treated with 1.5% agarose. Oncospheres were grown in DMEM/F12 medium (Invitrogen) supplemented with 20 ng/mL of epidermal growth factor, basic fibroblast growth factor, N2, and B27. The medium was replaced periodically until the 10th d of culture. At end, the number of spheres obtained in each well was calculated in order to evaluate the influence of the compound on cellular development. The material was also collected and stored in a -80 ºC freezer.
RNA extraction and quantitative RT-PCR: The expression levels of the CSCs’ markers CD33 and CD144 genes[33,34], as well as IL-1β in colon cancer parental and CSCs, were determined by RT-PCR analysis, as previously described.
For assessment of the inflammatory index, animals were sacrificed, and the colon tissue was extracted 10 d after the first DSS dose. The colons extracted from the Swiss mice were fixed in 4% paraformaldehyde for 24 h. Histopathological analysis was performed on paraffin-embedded sections after hematoxylin and eosin staining. Assessment of the inflammatory index was performed checking the severity of areas of epithelial degeneration, focal or multifocal areas, erosions of the epithelium, presence of ulcers, tissue hyperplasia and size of the affected area, as previously described[35].
The slides were deparaffinized, and the antigen retrieval was carried out by immersing the slides in 0.01 mol/L citrate buffer (pH 6) and heating in a microwave oven for 1 min at maximum power level, followed by 9 min at medium power, stopping at each minute to avoid overheating. Endogenous peroxidase blockade was performed with 5% hydrogen peroxide solution in methanol for 10 min at room temperature. Nonspecific antibody reactivity was blocked by incubating the tissue for 1 h in blocking buffer [3% bovine serum albumin (BSA) in PBS] at room temperature. Tissues were incubated overnight at 4 °C with the primary antibody anti-human/mouse CD44 (14-0441-82; eBioscience, San Diego, CA, United States) (1:200) diluted in 1% BSA, followed by secondary antibody goat pAb to rat IgG (ab7097; Abcam, Cambridge, United Kingdom) (1:200) also diluted in 1% BSA for 90 min at room temperature. For colorimetric detection, the chromogen of the diaminobenzidine reaction was added (ACB030 DAB chromogen concentrate; ScyTek Laboratories, Logan, UT, United States) + DAB substrate (ACU250 high contrast; ScyTek Laboratories). The counterstaining was done with Mayer's hematoxylin for 10 seconds and in sequence was assembled with Entellan (1079610100; Merck, Kenilworth, NJ, United States). Photos were documented with the Zeiss-Axiophot 2/CellSens program microscope, and the count of positive cells was performed using ImageJ 4.47t (National Institutes of Health, Bethesda, MD, United States). A total of 20 crypts for each sample, with five repetitions were performed. The sum of positive counts was divided by the number of crypts to obtain the mean per group.
Immunohistochemical analysis for Ki-67 was performed similarly. The primary antibody used was anti-human Ki-67 (AB9260; Millipore) (1:50), the secondary antibody was goat anti-rabbit IgG (31460; Thermo Scientific), and the chromogen was diaminobenzidine tetrahydrochloride hydrate (D5637 DAB; Sigma-Aldrich).
Terminal deoxynucleotidyl transferase-mediated dUTP nick end labelling (11684817910 TUNEL In Situ Cell Death Detection Kit, POD; Roche, Basel, Switzerland) staining was performed according to the manufacturer’s recommendations.
The results were presented as the mean ± standard error. Unless otherwise specified, the differences between groups were analyzed by two-tailed Student’s t-tests. ANOVA was used to evaluate the differences in the inflammatory index assays. The statistical methodology employed for the analysis of Kaplan-Meier curves was an unstratified log-rank test. Multiple measures ANOVA was used to compare treatment effectiveness for rectal bleeding and diarrhea. The significance level adopted was a P value less than 0.05. Data analyses were conducted using Stata version 12 (Statacorp, College Station, TX, United States) and GraphPad Prism, version 5 for Windows (GraphPad Software, San Diego, CA, United States).
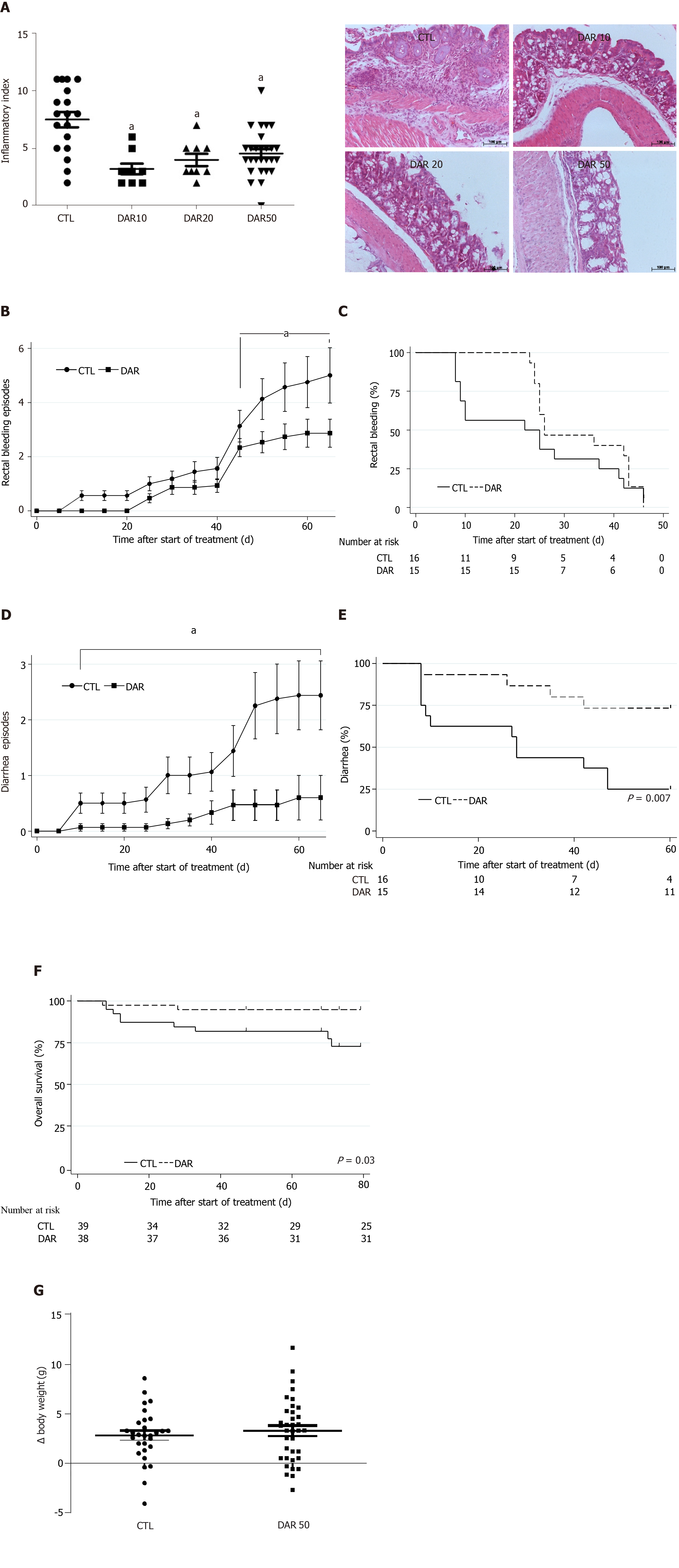
We hypothesized that DAR treatment was able to reduce colon inflammation in animals exposed to AOM + DSS induced-colitis. In order to confirm our hypothesis, we tested three different doses of DAR (10, 20 and 50 mg/kg/d) and observed that all tested doses were able to reduce chemically induced colitis when compared to the control group (P < 0.001, n = 9–27) (Figure 1A). Consistently, treatment with DAR 50 mg/kg/d (DAR50) was associated with a reduced intensity of rectal bleeding, as measured by the number of bleeding episodes over time (Figure 1B); however, the median time to first rectal bleeding episode analysis showed no difference between the two arms (Figure 1C). A previous meta-analysis showed that the use of DAR for osteoarthritis treatment was associated with a relative risk increase of 3.5 in diarrhea[36]. However, consistent with a preponderant effect of DAR in the inflamed colon mucosa, DAR50 treatment reduced the number of episodes of diarrhea in this study, as shown in Figure 1D. In accordance, time to first episode of diarrhea was prolonged with DAR50 vs control [median, not reached vs 28 d, respectively; hazard ratio (HR): 0.25, 95% confidence interval (CI): 0.08-0.78, P < 0.01, n = 15–16 animals/group] (Figure 1E). Accordingly, the analysis of overall survival showed that DAR50 treatment resulted in a 78% reduction in the risk of death compared with no treatment (HR: 0.22; 95%CI: 0.04-1.00; P = 0.05, n = 38–39 animals/group) (Figure 1F). Changes in body weight were not detected after treatment (CTL = 2.81 ± 0.49 g, n = 29; DAR50 = 3.27 ± 0.53 g, n = 36; P = 0.53) (Figure 1G).
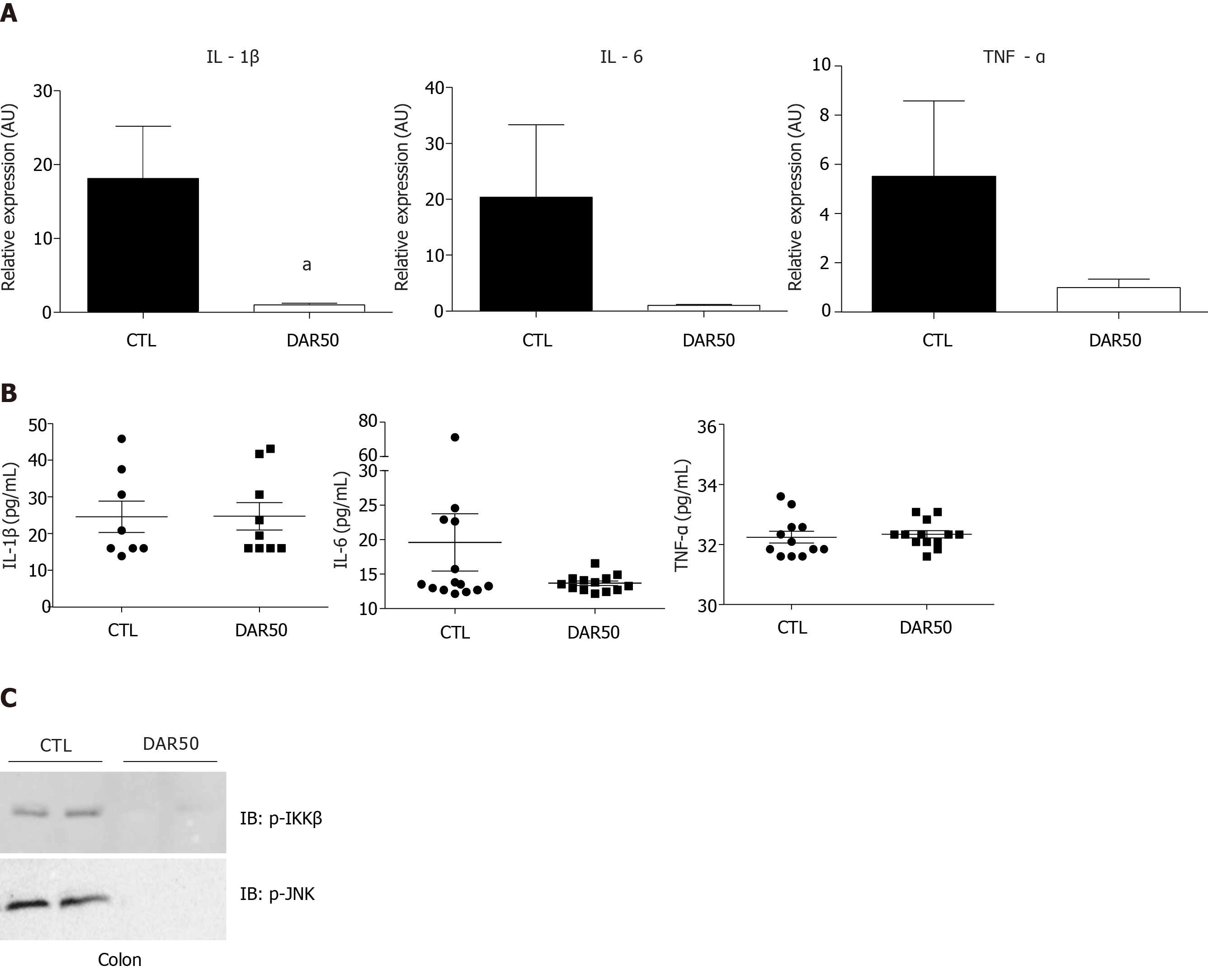
We also measured the expression levels of the pro-inflammatory cytokines IL-1β, TNF-α and IL-6 in colonic mucosa. There was a reduction in IL-1β expression in mice treated with DAR50 and a trend toward a reduction in TNF-α and IL-6 levels (Figure 2A). We did not observe a reduction in serum cytokine levels of IL-1β, TNF-α and IL-6 in animals treated with DAR50 compared to vehicle (Figure 2B). Concordant with these results, DAR50 treatment inhibited the activation of intracellular mediators of inflammation in the colon of Swiss mice, as shown by a dramatic reduction of IKK and JNK phosphorylation in peritumoral colonic tissues (Figure 2C).
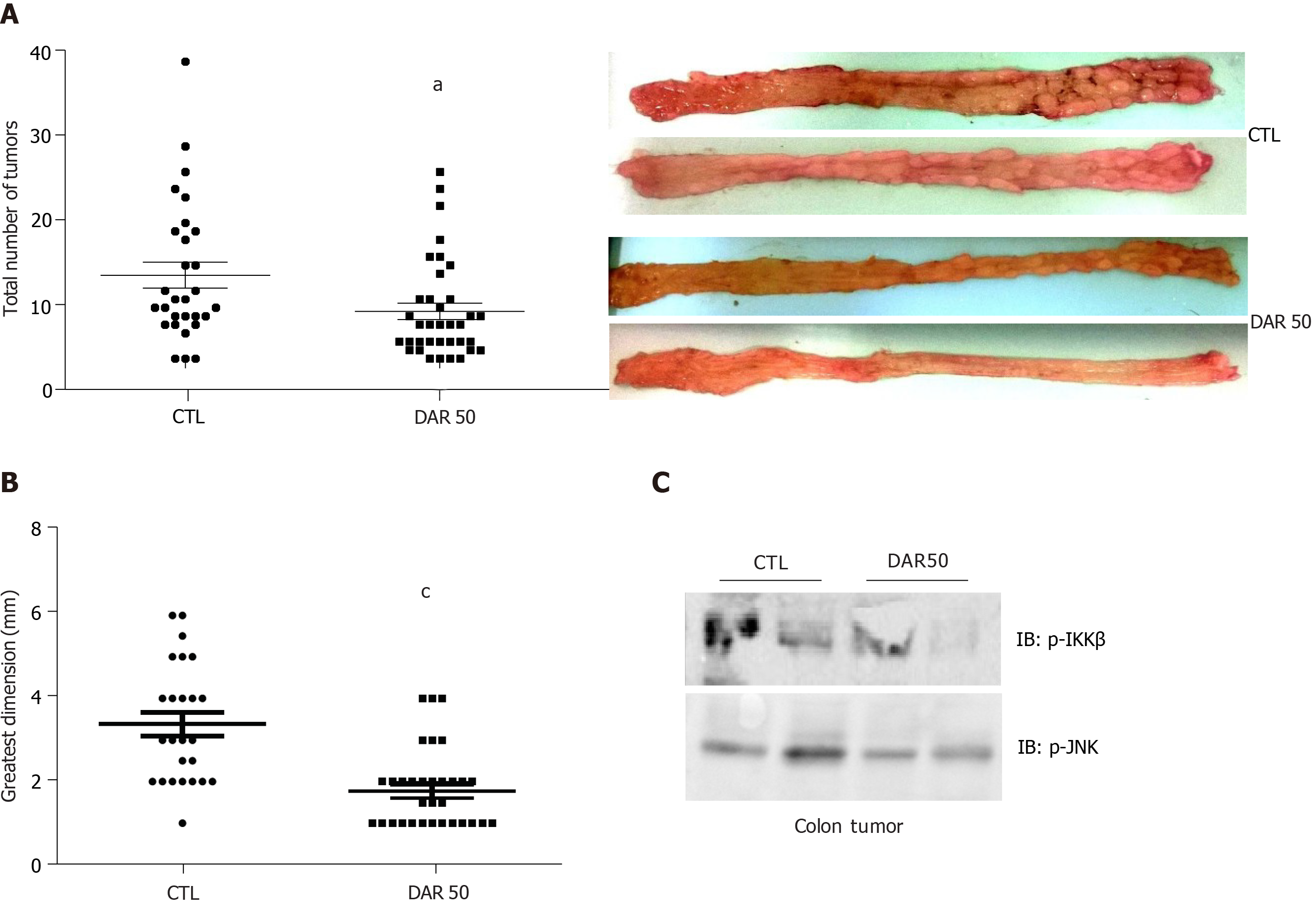
To determine whether pharmacological reduction of IL-1β prevented CAC, we evaluated the effect of DAR administration using the AOM + DSS model. There were fewer (CTL = 9.86 ± 1.53, n = 29; DAR50 = 5.58 ± 0.96, n = 36; P = 0.016) and smaller (CTL = 3.38 ± 0.28 mm, n= 25; DAR = 1.77 ± 0.17 mm, n = 33; P < 0.001) tumors in the DAR50-treated mice than in the control mice (Figure 3). In accordance, mice treated with DAR50 had a reduction of IKK and JNK phosphorylation in colon tumors. In contrast, DAR treatment did not change tumor incidence (CTL = 80%, n = 29; DAR = 80.9%, n = 36; P = 0.94).
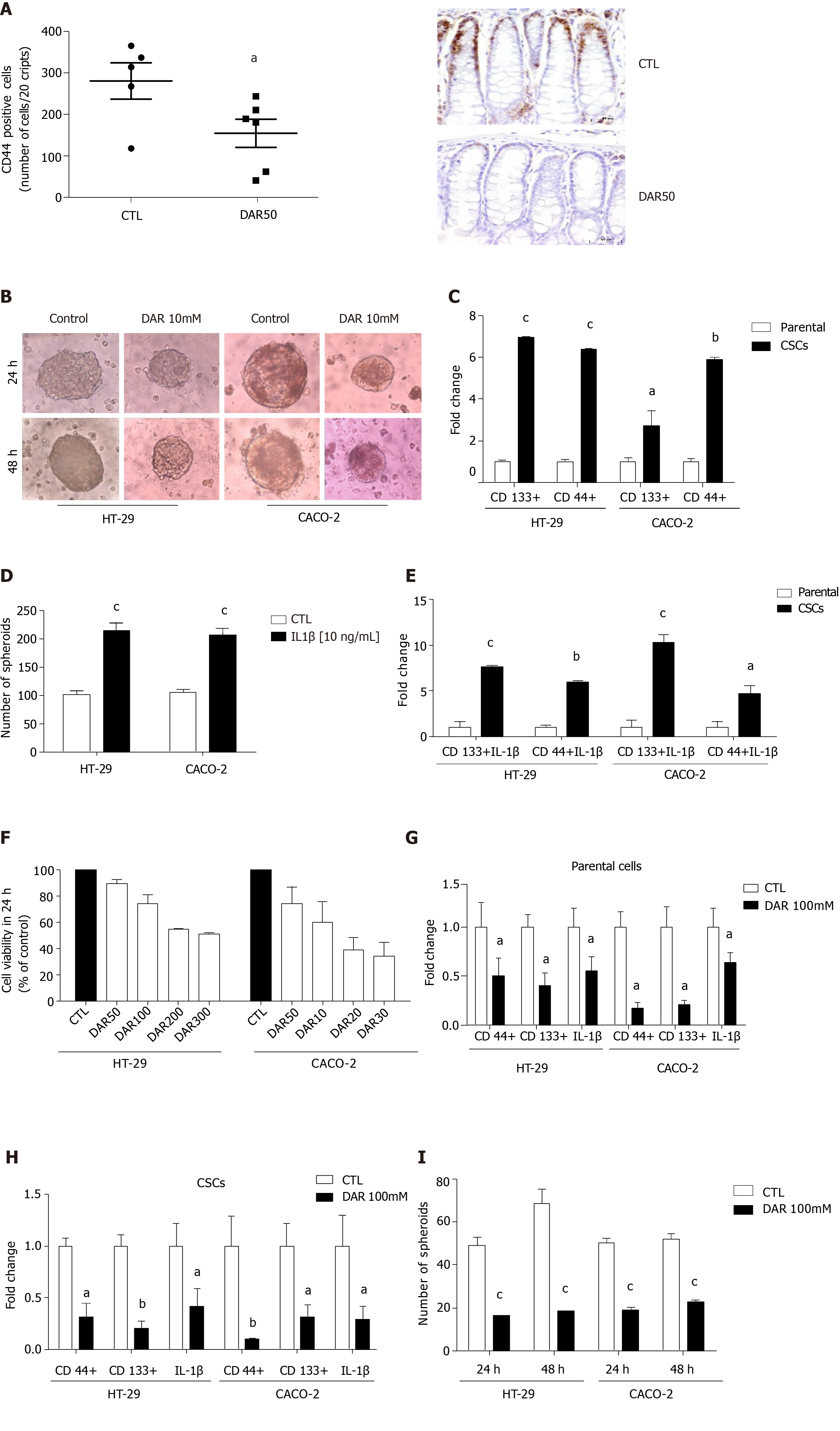
Considering that DAR anti-inflammatory effects might affect CSC formation, we evaluated the levels of the CSC marker CD44 in colonic mucosa. Our results clearly showed that DAR treatment reduced the expression of CD44 in inflamed colonic mucosa (P < 0.05; n = 5–6; five fields per colon section) (Figure 4A).
To better access the mechanisms by which DAR affects the expression of CSCs, we used HT-29 and CACO-2 cell lines. Initially, we demonstrated that these cell lines could form spheroids (Figure 4B), which was confirmed by the increased expression of the CSC markers CD44 and CD133 (Figure 4C). Further, we addressed the hypothesis that DAR-mediated IL-1β reduction modulates the formation of CSCs. Therefore, we treated HT-29 and CACO-2 cells with IL-1β (10 ng/mL) and observed an increase in the number of spheroids formed in both cell lines (Figure 4D). Additionally, we observed that the treatment with this cytokine also leads to an upregulation of CD133 and CD44 expression in CSCs (Figure 4E).
Next, we demonstrated that DAR at 100 mM showed no significant toxicity to the cells (Figure 4F), which was, therefore, the concentration used in subsequent experiments. Consistent with the effects observed in vivo, DAR 100 mM not only attenuated the expression of CD44 but also significantly decreased CD133 and IL-1β levels in both HT-29 and CACO-2 cell lines (parental) (Figure 4G) and in the derived CSCs (Figure 4H). Additionally, our data show that treatment with DAR 100 mM was effective in reducing the formation of CSCs in the HT-29 cell line after 24 h (CTL = 49.13 ± 3.56, n = 8; DAR = 16.44 ± 1.46, n = 9; P < 0.0001) and 48 h (CTL = 68.25 ± 6.93, n = 8; DAR = 18.75 ± 1.03, n = 8; P < 0.0001), and in the CACO-2 cell line after 24 h (CTL = 50.16 ± 2.27, n = 8; DAR = 19.22 ± 1.06, n = 8; P < 0.0001) and 48 h (CTL = 52.13 ± 2.21, n = 8; DAR = 22.70 ± 0.95, n = 8; P < 0.0001) (Figure 4I).
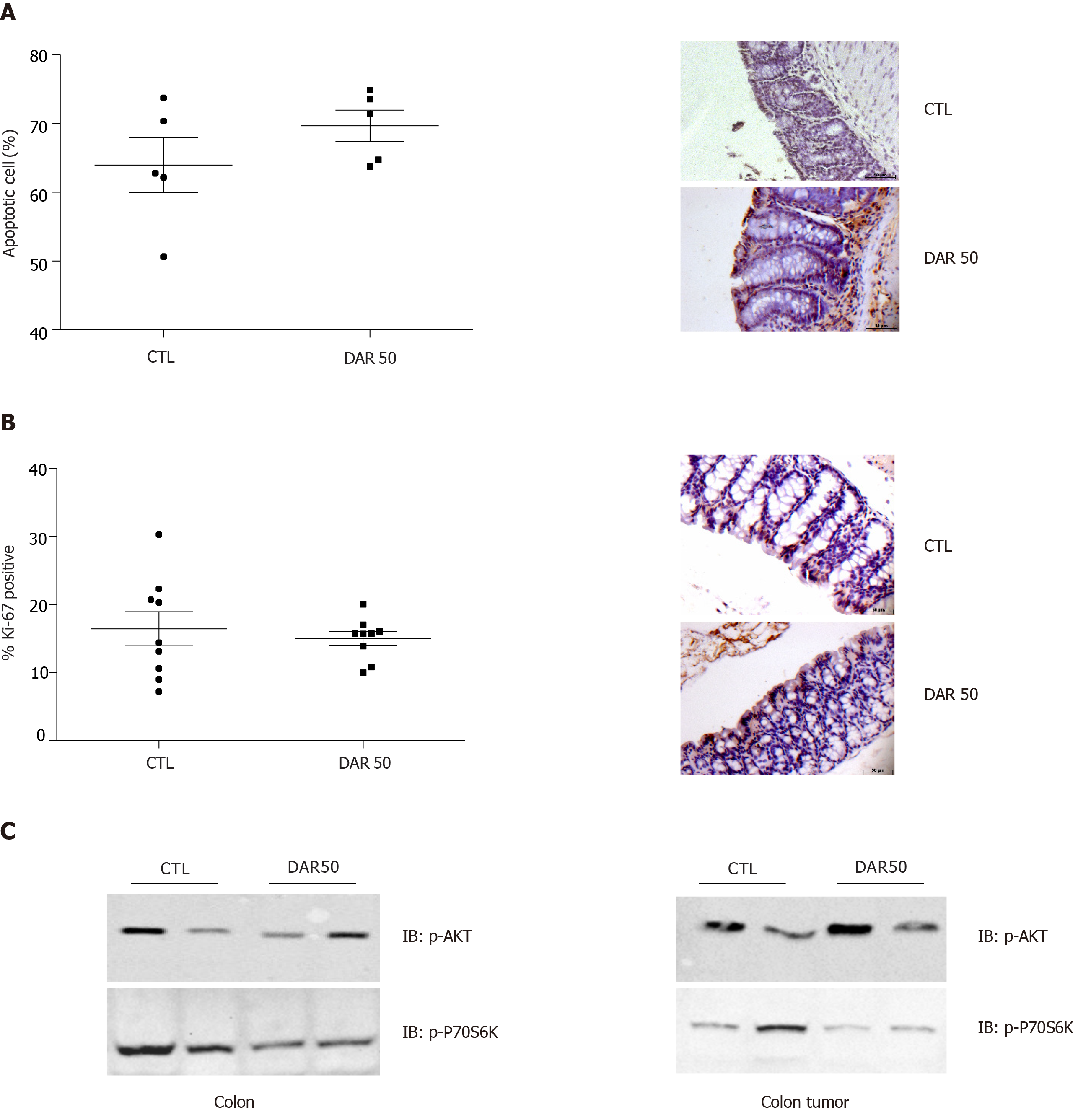
Next, we evaluated the anti-cancer effects of DAR on proliferation and apoptosis. Our results showed that DAR50 treatment did not play a significant role in the apoptosis of tumor cells, as observed by TUNEL staining (CTL = 63.94% ± 3.98%; DAR50 = 69.68% ± 2.28%; P = 0.24; n = 5; five fields per colon section) (Figure 5A). Likewise, treatment with DAR did not affect cell proliferation, measured here by the percentage of cells stained with Ki-67 (CTL = 16.92% ± 1.05%; DAR = 17.16% ± 1.97%; n = 5; five fields per colon section) (Figure 5B). Accordingly, DAR treatment inhibited the activation of proteins involved in proliferation in the colon but not in colon cancer, as demonstrated by a reduction of Akt and p70S6K phosphorylation in peritumoral colonic tissues, whereas no change in the activation of these proteins in colon cancer was observed (Figure 5C).
Colon cancer is a daunting and disastrous consequence of chronic colon inflammation. Despite a recent meta-analysis showing that ulcerative colitis is associated with a 2.4-fold increased risk of CAC, the role of anti-inflammatory drugs as chemopreventive agents remains unclear[19,37]. IL-1β, a dominant mediator of colitis in animal models, occurs in abundant levels in patients with UC[38,39]. DAR is an old drug, commonly used for the treatment of osteoarthritis[36]; however, its effect on control of CAC is unknown. In the present study, we determined that DAR is sufficient to prevent colon cancer in the AOM + DSS model. Our data showed that DAR treatment reduced colonic IL-1β expression that coincided with a reduction in colitis and in the inhibition of the pro-inflammatory intracellular signal mediators JNK and IKK. Furthermore, DAR treatment reduced the formation of CSCs, which may contribute to the reduction of colon cancer development.
DAR treatment reduced IL-1β expression in the colon of AOM + DSS-treated mice, with a trend toward a reduction in the expression of TNF and IL-6. Indeed, previous work reported that the specific blockage of the IL-1β signaling pathway attenuated DSS-induced bowel inflammation[15-17]. Given that the activation of the IKK/NFκB signaling pathway is strongly correlated with CAC carcinogenesis[18,40], it is possible that the DAR-mediated decrease in inflammation is due to its effect on this signaling pathway. Altogether, these data suggest that the DAR-mediated inhibition of IL-1β and resultant suppression of IKK in CAC attenuate the development of colitis.
Aberrant activation of mammalian target of rapamycin (mTOR) signaling has long been associated with colon cancer development[41,42]; however, it was not until recently that it was recognized as a common event during the course of IBD that is key for CAC carcinogenesis[43,44]. Consistent with previous studies showing that the AKT/mTOR signaling pathway is activated by IL-1β[45], our data shows that DAR inhibits AKT and p70S6K phosphorylation in peritumoral colonic tissues, suggesting that IL-1β is a mediator of AKT/mTOR activation in peritumoral colonic tissues and may be involved in the initiation of CAC carcinogenesis.
An important distinction between the pathophysiological contexts of CAC and sporadic CRC is the role of inflammation in the initial steps of carcinogenesis. It is not biologically plausible that inflammation promotes tumor initiation in sporadic CRC, while inflammation precedes CAC by triggering DNA damage and suppressing mismatch of repair enzymes, therefore supporting tumor initiation[1]. It is interesting to note that besides the possible anti-inflammatory effects in colon cancer initiation, DAR not only reduced CSC markers in inflamed colonic mucosa but also inhibited the formation of CSCs in human colon adenocarcinoma cell lines, suggesting, therefore, that its anti-cancer effects may not only be limited to colitis attenuation but also a direct effect inhibiting initial phases of colon carcinogenesis. In accordance, DAR treatment did not change the phenotype of established colon tumors, as shown by similar activation of AKT/mTOR signaling, cancer proliferation and apoptosis rates, suggesting that the effects of DAR are mainly on tumor initiation.
It is well-described that NFκB-mediated inflammatory responses are directly related to proliferation, angiogenesis and cancer cell survival. Additionally, it has recently been shown that the activation of the NFκB pathway induces the expression of stemness regulators of epithelial-mesenchymal transition, generating a CSC phenotype[46]. Thus, the results presented here indicate that the anti-inflammatory effects of DAR might be, at least in part, responsible for inhibition of the formation of CSCs. Consistent with this hypothesis, our data show that DAR treatment significantly reduced IL-1β expression in CSCs as well IL-1β treatment increased the number of CSCs. Therefore, considering the relevance of CSCs on chemotherapy resistance, CSC-targeted therapy may become a very prominent strategy to increase the success of cancer treatments, and DAR would be a novel candidate.
Although neutralizing TNF is an effective approach and changed the history of IBD, a large proportion of patients still relapse or present with persistent disease, which can lead to irreversible colon damage as well as an increased risk for the development of CAC[47,48]. Recent studies have shown that the risk of CRC in IBD patients was not reduced by aspirin or by NSAID use[49,50]. The results presented here indicate that DAR may prevent not only colitis but also the development of CRC by reducing the formation of CSCs in an IL-1β dependent manner. Thus, DAR, a drug with a known safety profile that has the potential to short-cut and reduce the costs of translational research, is an attractive candidate for the chemoprevention of colon cancer in IBD patients.
Inflammation is a well-established enabling factor for cancer development and provides a framework for the high prevalence of colon cancer in inflammatory bowel disease.
To detect the mechanism whereby diacerein (DAR), an anti-inflammatory drug, acts in the prevention of colitis-associated cancer (CAC).
This study aimed to investigate the effects of DAR on colon inflammation in mice with CAC and evaluate the action of DAR in the development of cancer stem cells (CSCs).
The effects of DAR on colon inflammation in mice with CAC were evaluated by inflammatory index, reverse transcription polymerase chain reaction and western blot. Cytokine levels were measured by enzyme-linked immunosorbent assay. Cells assays evaluated the effects of DAR on CSCs. Immunohistochemistry and apoptosis assays were also used to evaluate the effects of DAR on tumorigenesis associated with inflammation.
DAR treatment reduced colon inflammation as well as the number and size of tumors. Accordingly, DAR treatment was associated with reduced intracellular signals of inflammation in the colon. In addition, DAR treatment was associated with a decrease in colon CSC formation.
DAR-mediated interleukin-1β suppression attenuates inflammation-induced colon cancer and CSC formation, suggesting that besides reducing colonic inflammation, DAR has a direct effect on the inhibition of colon carcinogenesis.
The reduction of inflammation, CSC formation and tumorigenesis highlights DAR as a potential candidate for the chemoprevention of CAC.
We thank Mr. Luiz Janeri, Josimo Pinheiro, Dioze Guadagnini, and Andrey Santos of the State University of Campinas, Brazil for technical assistance.
Manuscript source: Unsolicited manuscript
Specialty type: Gastroenterology and hepatology
Country/Territory of origin: Brazil
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): 0
Grade C (Good): C
Grade D (Fair): D
Grade E (Poor): 0
P-Reviewer: Gao F, Xiao M S-Editor: Zhang H L-Editor: Filipodia P-Editor: Wang LL
| 1. | Terzić J, Grivennikov S, Karin E, Karin M. Inflammation and colon cancer. Gastroenterology. 2010;138:2101-2114.e5. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1333] [Cited by in RCA: 1507] [Article Influence: 100.5] [Reference Citation Analysis (0)] |
| 2. | Ullman TA, Itzkowitz SH. Intestinal inflammation and cancer. Gastroenterology. 2011;140:1807-1816. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 738] [Cited by in RCA: 853] [Article Influence: 60.9] [Reference Citation Analysis (0)] |
| 3. | Hanahan D, Weinberg RA. Hallmarks of cancer: the next generation. Cell. 2011;144:646-674. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 51728] [Cited by in RCA: 47059] [Article Influence: 3361.4] [Reference Citation Analysis (5)] |
| 4. | Kraus S, Arber N. Inflammation and colorectal cancer. Curr Opin Pharmacol. 2009;9:405-410. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 193] [Cited by in RCA: 216] [Article Influence: 13.5] [Reference Citation Analysis (0)] |
| 5. | Allavena P, Germano G, Marchesi F, Mantovani A. Chemokines in cancer related inflammation. Exp Cell Res. 2011;317:664-673. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 160] [Cited by in RCA: 158] [Article Influence: 10.5] [Reference Citation Analysis (0)] |
| 6. | Kulaylat MN, Dayton MT. Ulcerative colitis and cancer. J Surg Oncol. 2010;101:706-712. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 57] [Cited by in RCA: 57] [Article Influence: 3.8] [Reference Citation Analysis (0)] |
| 7. | Eaden JA, Abrams KR, Mayberry JF. The risk of colorectal cancer in ulcerative colitis: a meta-analysis. Gut. 2001;48:526-535. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1985] [Cited by in RCA: 2075] [Article Influence: 86.5] [Reference Citation Analysis (1)] |
| 8. | Gillen CD, Walmsley RS, Prior P, Andrews HA, Allan RN. Ulcerative colitis and Crohn's disease: a comparison of the colorectal cancer risk in extensive colitis. Gut. 1994;35:1590-1592. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 375] [Cited by in RCA: 340] [Article Influence: 11.0] [Reference Citation Analysis (0)] |
| 9. | Zeuner A, Todaro M, Stassi G, De Maria R. Colorectal cancer stem cells: from the crypt to the clinic. Cell Stem Cell. 2014;15:692-705. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 252] [Cited by in RCA: 287] [Article Influence: 28.7] [Reference Citation Analysis (0)] |
| 10. | Rinkenbaugh AL, Baldwin AS. The NF-κB Pathway and Cancer Stem Cells. Cells. 2016;5:16. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 148] [Cited by in RCA: 203] [Article Influence: 22.6] [Reference Citation Analysis (0)] |
| 11. | Wang D, Fu L, Sun H, Guo L, DuBois RN. Prostaglandin E2 Promotes Colorectal Cancer Stem Cell Expansion and Metastasis in Mice. Gastroenterology. 2015;149:1884-1895.e4. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 175] [Cited by in RCA: 227] [Article Influence: 22.7] [Reference Citation Analysis (0)] |
| 12. | West NR, McCuaig S, Franchini F, Powrie F. Emerging cytokine networks in colorectal cancer. Nat Rev Immunol. 2015;15:615-629. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 222] [Cited by in RCA: 280] [Article Influence: 28.0] [Reference Citation Analysis (0)] |
| 13. | Bromberg J, Wang TC. Inflammation and cancer: IL-6 and STAT3 complete the link. Cancer Cell. 2009;15:79-80. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 400] [Cited by in RCA: 444] [Article Influence: 27.8] [Reference Citation Analysis (0)] |
| 14. | Popivanova BK, Kitamura K, Wu Y, Kondo T, Kagaya T, Kaneko S, Oshima M, Fujii C, Mukaida N. Blocking TNF-alpha in mice reduces colorectal carcinogenesis associated with chronic colitis. J Clin Invest. 2008;118:560-570. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 103] [Cited by in RCA: 456] [Article Influence: 26.8] [Reference Citation Analysis (0)] |
| 15. | Cominelli F, Nast CC, Duchini A, Lee M. Recombinant interleukin-1 receptor antagonist blocks the proinflammatory activity of endogenous interleukin-1 in rabbit immune colitis. Gastroenterology. 1992;103:65-71. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 138] [Cited by in RCA: 132] [Article Influence: 4.0] [Reference Citation Analysis (0)] |
| 16. | Wang Y, Wang K, Han GC, Wang RX, Xiao H, Hou CM, Guo RF, Dou Y, Shen BF, Li Y, Chen GJ. Neutrophil infiltration favors colitis-associated tumorigenesis by activating the interleukin-1 (IL-1)/IL-6 axis. Mucosal Immunol. 2014;7:1106-1115. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 88] [Cited by in RCA: 117] [Article Influence: 10.6] [Reference Citation Analysis (0)] |
| 17. | Xiao H, Gulen MF, Qin J, Yao J, Bulek K, Kish D, Altuntas CZ, Wald D, Ma C, Zhou H, Tuohy VK, Fairchild RL, de la Motte C, Cua D, Vallance BA, Li X. The Toll-interleukin-1 receptor member SIGIRR regulates colonic epithelial homeostasis, inflammation, and tumorigenesis. Immunity. 2007;26:461-475. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 234] [Cited by in RCA: 251] [Article Influence: 13.9] [Reference Citation Analysis (0)] |
| 18. | Greten FR, Eckmann L, Greten TF, Park JM, Li ZW, Egan LJ, Kagnoff MF, Karin M. IKKbeta links inflammation and tumorigenesis in a mouse model of colitis-associated cancer. Cell. 2004;118:285-296. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1859] [Cited by in RCA: 1961] [Article Influence: 93.4] [Reference Citation Analysis (0)] |
| 19. | Arber N, Levin B. Chemoprevention of colorectal neoplasia: the potential for personalized medicine. Gastroenterology. 2008;134:1224-1237. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 84] [Cited by in RCA: 78] [Article Influence: 4.6] [Reference Citation Analysis (0)] |
| 20. | Velayos FS, Terdiman JP, Walsh JM. Effect of 5-aminosalicylate use on colorectal cancer and dysplasia risk: a systematic review and metaanalysis of observational studies. Am J Gastroenterol. 2005;100:1345-1353. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 426] [Cited by in RCA: 379] [Article Influence: 19.0] [Reference Citation Analysis (0)] |
| 21. | Rutter M, Saunders B, Wilkinson K, Rumbles S, Schofield G, Kamm M, Williams C, Price A, Talbot I, Forbes A. Severity of inflammation is a risk factor for colorectal neoplasia in ulcerative colitis. Gastroenterology. 2004;126:451-459. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 895] [Cited by in RCA: 882] [Article Influence: 42.0] [Reference Citation Analysis (0)] |
| 22. | Ullman T, Croog V, Harpaz N, Hossain S, Kornbluth A, Bodian C, Itzkowitz S. Progression to colorectal neoplasia in ulcerative colitis: effect of mesalamine. Clin Gastroenterol Hepatol. 2008;6:1225-30; quiz 1177. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 60] [Cited by in RCA: 59] [Article Influence: 3.5] [Reference Citation Analysis (0)] |
| 23. | Shirakami Y, Shimizu M, Tsurumi H, Hara Y, Tanaka T, Moriwaki H. EGCG and Polyphenon E attenuate inflammation-related mouse colon carcinogenesis induced by AOM plus DDS. Mol Med Rep. 2008;1:355-361. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 9] [Cited by in RCA: 10] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 24. | Shimizu M, Shirakami Y, Sakai H, Adachi S, Hata K, Hirose Y, Tsurumi H, Tanaka T, Moriwaki H. (-)-Epigallocatechin gallate suppresses azoxymethane-induced colonic premalignant lesions in male C57BL/KsJ-db/db mice. Cancer Prev Res (Phila). 2008;1:298-304. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 78] [Cited by in RCA: 81] [Article Influence: 5.1] [Reference Citation Analysis (0)] |
| 25. | Shimizu M, Shirakami Y, Iwasa J, Shiraki M, Yasuda Y, Hata K, Hirose Y, Tsurumi H, Tanaka T, Moriwaki H. Supplementation with branched-chain amino acids inhibits azoxymethane-induced colonic preneoplastic lesions in male C57BL/KsJ-db/db mice. Clin Cancer Res. 2009;15:3068-3075. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 49] [Cited by in RCA: 58] [Article Influence: 3.6] [Reference Citation Analysis (0)] |
| 26. | Shirakami Y, Shimizu M, Kubota M, Araki H, Tanaka T, Moriwaki H, Seishima M. Chemoprevention of colorectal cancer by targeting obesity-related metabolic abnormalities. World J Gastroenterol. 2014;20:8939-8946. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 15] [Reference Citation Analysis (0)] |
| 27. | Kim YJ, Hong KS, Chung JW, Kim JH, Hahm KB. Prevention of colitis-associated carcinogenesis with infliximab. Cancer Prev Res (Phila). 2010;3:1314-1333. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 61] [Cited by in RCA: 78] [Article Influence: 5.2] [Reference Citation Analysis (0)] |
| 28. | Pomarelli P, Berti M, Gatti MT, Mosconi P. A non steroidal anti-inflammatory drug that stimulates prostaglandin release. Farmaco Sci. 1980;35:836-842. [PubMed] |
| 29. | Spencer CM, Wilde MI. Diacerein. Drugs. 1997;53:98-106; discussion 107-8. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 75] [Cited by in RCA: 73] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 30. | Malaguti C, Vilella CA, Vieira KP, Souza GH, Hyslop S, Zollner Rde L. Diacerhein downregulate proinflammatory cytokines expression and decrease the autoimmune diabetes frequency in nonobese diabetic (NOD) mice. Int Immunopharmacol. 2008;8:782-791. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 32] [Cited by in RCA: 39] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 31. | Laemmli UK. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970;227:680-685. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 185380] [Cited by in RCA: 188842] [Article Influence: 3433.5] [Reference Citation Analysis (0)] |
| 32. | Razian G, Yu Y, Ungrin M. Production of large numbers of size-controlled tumor spheroids using microwell plates. J Vis Exp. 2013;e50665. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 9] [Cited by in RCA: 22] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 33. | Cherciu I, Bărbălan A, Pirici D, Mărgăritescu C, Săftoiu A. Stem cells, colorectal cancer and cancer stem cell markers correlations. Curr Health Sci J. 2014;40:153-161. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 36] [Reference Citation Analysis (0)] |
| 34. | Li L, Duan T, Wang X, Zhang RH, Zhang M, Wang S, Wang F, Wu Y, Huang H, Kang T. KCTD12 Regulates Colorectal Cancer Cell Stemness through the ERK Pathway. Sci Rep. 2016;6:20460. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 24] [Cited by in RCA: 25] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 35. | Cooper HS, Murthy SN, Shah RS, Sedergran DJ. Clinicopathologic study of dextran sulfate sodium experimental murine colitis. Lab Invest. 1993;69:238-249. [PubMed] |
| 36. | Fidelix TS, Macedo CR, Maxwell LJ, Fernandes Moça Trevisani V. Diacerein for osteoarthritis. Cochrane Database Syst Rev. 2014;CD005117. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 53] [Cited by in RCA: 47] [Article Influence: 4.3] [Reference Citation Analysis (0)] |
| 37. | Jess T, Rungoe C, Peyrin-Biroulet L. Risk of colorectal cancer in patients with ulcerative colitis: a meta-analysis of population-based cohort studies. Clin Gastroenterol Hepatol. 2012;10:639-645. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 581] [Cited by in RCA: 659] [Article Influence: 50.7] [Reference Citation Analysis (0)] |
| 38. | Reinecker HC, Steffen M, Witthoeft T, Pflueger I, Schreiber S, MacDermott RP, Raedler A. Enhanced secretion of tumor necrosis factor-alpha, IL-6, and IL-1 beta by isolated lamina propria mononuclear cells from patients with ulcerative colitis and Crohn's disease. Clin Exp Immunol. 1993;94:174-181. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 603] [Cited by in RCA: 648] [Article Influence: 20.3] [Reference Citation Analysis (0)] |
| 39. | McAlindon ME, Hawkey CJ, Mahida YR. Expression of interleukin 1 beta and interleukin 1 beta converting enzyme by intestinal macrophages in health and inflammatory bowel disease. Gut. 1998;42:214-219. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 140] [Cited by in RCA: 148] [Article Influence: 5.5] [Reference Citation Analysis (0)] |
| 40. | Pikarsky E, Porat RM, Stein I, Abramovitch R, Amit S, Kasem S, Gutkovich-Pyest E, Urieli-Shoval S, Galun E, Ben-Neriah Y. NF-kappaB functions as a tumor promoter in inflammation-associated cancer. Nature. 2004;431:461-466. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1879] [Cited by in RCA: 2015] [Article Influence: 96.0] [Reference Citation Analysis (0)] |
| 41. | Aoki K, Tamai Y, Horiike S, Oshima M, Taketo MM. Colonic polyposis caused by mTOR-mediated chromosomal instability in Apc+/Delta716 Cdx2+/- compound mutant mice. Nat Genet. 2003;35:323-330. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 177] [Cited by in RCA: 188] [Article Influence: 8.5] [Reference Citation Analysis (0)] |
| 42. | Shao J, Evers BM, Sheng H. Roles of phosphatidylinositol 3'-kinase and mammalian target of rapamycin/p70 ribosomal protein S6 kinase in K-Ras-mediated transformation of intestinal epithelial cells. Cancer Res. 2004;64:229-235. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 52] [Cited by in RCA: 47] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 43. | Deng L, Zhou JF, Sellers RS, Li JF, Nguyen AV, Wang Y, Orlofsky A, Liu Q, Hume DA, Pollard JW, Augenlicht L, Lin EY. A novel mouse model of inflammatory bowel disease links mammalian target of rapamycin-dependent hyperproliferation of colonic epithelium to inflammation-associated tumorigenesis. Am J Pathol. 2010;176:952-967. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 165] [Cited by in RCA: 183] [Article Influence: 11.4] [Reference Citation Analysis (0)] |
| 44. | Thiem S, Pierce TP, Palmieri M, Putoczki TL, Buchert M, Preaudet A, Farid RO, Love C, Catimel B, Lei Z, Rozen S, Gopalakrishnan V, Schaper F, Hallek M, Boussioutas A, Tan P, Jarnicki A, Ernst M. mTORC1 inhibition restricts inflammation-associated gastrointestinal tumorigenesis in mice. J Clin Invest. 2013;123:767-781. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 25] [Cited by in RCA: 53] [Article Influence: 4.4] [Reference Citation Analysis (0)] |
| 45. | Reddy SA, Huang JH, Liao WS. Phosphatidylinositol 3-kinase in interleukin 1 signaling. Physical interaction with the interleukin 1 receptor and requirement in NFkappaB and AP-1 activation. J Biol Chem. 1997;272:29167-29173. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 190] [Cited by in RCA: 198] [Article Influence: 7.1] [Reference Citation Analysis (0)] |
| 46. | Korkaya H, Liu S, Wicha MS. Regulation of cancer stem cells by cytokine networks: attacking cancer's inflammatory roots. Clin Cancer Res. 2011;17:6125-6129. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 268] [Cited by in RCA: 260] [Article Influence: 18.6] [Reference Citation Analysis (0)] |
| 47. | Ordás I, Feagan BG, Sandborn WJ. Early use of immunosuppressives or TNF antagonists for the treatment of Crohn's disease: time for a change. Gut. 2011;60:1754-1763. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 91] [Cited by in RCA: 96] [Article Influence: 6.9] [Reference Citation Analysis (0)] |
| 48. | Melmed GY, Targan SR. Future biologic targets for IBD: potentials and pitfalls. Nat Rev Gastroenterol Hepatol. 2010;7:110-117. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 60] [Cited by in RCA: 58] [Article Influence: 3.9] [Reference Citation Analysis (0)] |
| 49. | Burr NE, Hull MA, Subramanian V. Does aspirin or non-aspirin non-steroidal anti-inflammatory drug use prevent colorectal cancer in inflammatory bowel disease? World J Gastroenterol. 2016;22:3679-3686. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 25] [Cited by in RCA: 29] [Article Influence: 3.2] [Reference Citation Analysis (0)] |
| 50. | Samadder NJ, Mukherjee B, Huang SC, Ahn J, Rennert HS, Greenson JK, Rennert G, Gruber SB. Risk of colorectal cancer in self-reported inflammatory bowel disease and modification of risk by statin and NSAID use. Cancer. 2011;117:1640-1648. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 45] [Cited by in RCA: 46] [Article Influence: 3.3] [Reference Citation Analysis (0)] |