Published online Aug 24, 2020. doi: 10.5306/wjco.v11.i8.673
Peer-review started: January 20, 2020
First decision: April 3, 2020
Revised: May 7, 2020
Accepted: June 27, 2020
Article in press: June 27, 2020
Published online: August 24, 2020
Processing time: 213 Days and 19.5 Hours
Intravascular lymphoma (IVL) is a rare subtype of lymphoma involving the growth of lymphoma cells within the vessel lumina without lymphadenopathy. Because of various modes of presentation and its rarity, IVL is often diagnosed postmortem. Herein, we report a case of intravascular B-cell lymphoma with hypopituitarism, an extremely rare complication, that was successfully treated with chemotherapy.
An 80-year-old Japanese woman presented with a 7-mo history of a tingling sensation in the lower limbs. She also presented with various other symptoms such as pancytopenia, high fever daily, and unconsciousness with hypoglycemia. Although the doctor who previously treated her diagnosed hypoglycemia as being due to hypopituitarism, the cause of the other symptoms remained uncertain despite a 7-mo evaluation period. We performed bone marrow aspiration to evaluate pancytopenia and found that she had hemophagocytic lymphohistiocytosis (HLH). On the basis of a random skin biopsy for assessing the cause of HLH, she was diagnosed with intravascular B-cell lymphoma. HLH and hypopituitarism were considered secondary to IVL. All her clinical findings matched the presentations of IVL. She was immediately treated with chemotherapy and achieved complete response. She was relapse free two years after treatment.
IVL should be included in the differential diagnosis of hypopituitarism, which although life-threatening, is treatable through prompt diagnosis and appropriate chemotherapy.
Core tip: When encountering cases showing a variety of symptoms that cannot be reasonably explained, general physicians should consider intravascular lymphoma (IVL) and its useful diagnostic tool, i.e., random skin biopsy. Additionally, the mechanisms underlying lymphoma-associated hypopituitarism associated with IVL have not yet been elucidated, thereby necessitating further case studies and laboratory-based research.
- Citation: Kawahigashi T, Teshima S, Tanaka E. Intravascular lymphoma with hypopituitarism: A case report. World J Clin Oncol 2020; 11(8): 673-678
- URL: https://www.wjgnet.com/2218-4333/full/v11/i8/673.htm
- DOI: https://dx.doi.org/10.5306/wjco.v11.i8.673
Intravascular lymphoma (IVL) is a rare subtype of lymphoma, characterized by the selective proliferation of lymphoma cells within the lumina of small-to-medium-sized blood vessels[1]. A B-cell immunophenotype is most commonly observed, although cases with T-cell receptor rearrangements have also been reported[2]. Although various organs can be affected by lymphoma cells, hypopituitarism is an extremely rare complication, and limited cases have been reported[3]. We herein report a case of hypopituitarism due to IVL, which was successfully treated with chemotherapy.
An 80-year-old Japanese woman with no significant comorbidities noticed a bilateral tingling sensation on the anterior surface of her lower limbs.
The sensation was localized below her thighs; however, she felt neither numbness nor weakness, and could walk and ride a bicycle without any problems. Her symptoms persisted for about one month, which resulted in a visit to a local outpatient clinic. Laboratory studies performed in the clinic showed pancytopenia. Although the patient was examined at another general hospital, the cause of her condition was unknown. Subsequently, she experienced a high fever (a temperature of 38°C - 39°C) daily. However, she did not have any other accompanying symptoms, such as chills, pain, and appetite loss, and her general state was relatively normal. Despite a more detailed evaluation, the patient’s diagnosis was inconclusive, and she was observed as an outpatient. Five months after her first visit, she was taken to the emergency department of the hospital at which she was previously treated, because of sudden loss of consciousness. Laboratory studies showed that she had lost consciousness because of hypoglycemia, and further evaluation revealed that hypoglycemia was one of the signs of hypopituitarism. She received glucocorticoid and replacement therapy. Whereas treatment resulted in blood glucose level control, the tingling sensation gradually progressed. Although seven months had elapsed since her first visit, the causes of her symptoms remained unknown. Therefore, she was admitted to the Department of Hematology of our hospital for further assessment.
Her past medical history was noncontributory.
Her family history was noncontributory.
On physical examination, we observed the presence of a tingling sensation on the anterior regions of the lower limbs, under the thighs, and on both palms of the patient. Decreased vibratory sensation on both sides of the lower extremities was also noticed. Hepatosplenomegaly, lymph node enlargement, and skin eruption were not observed.
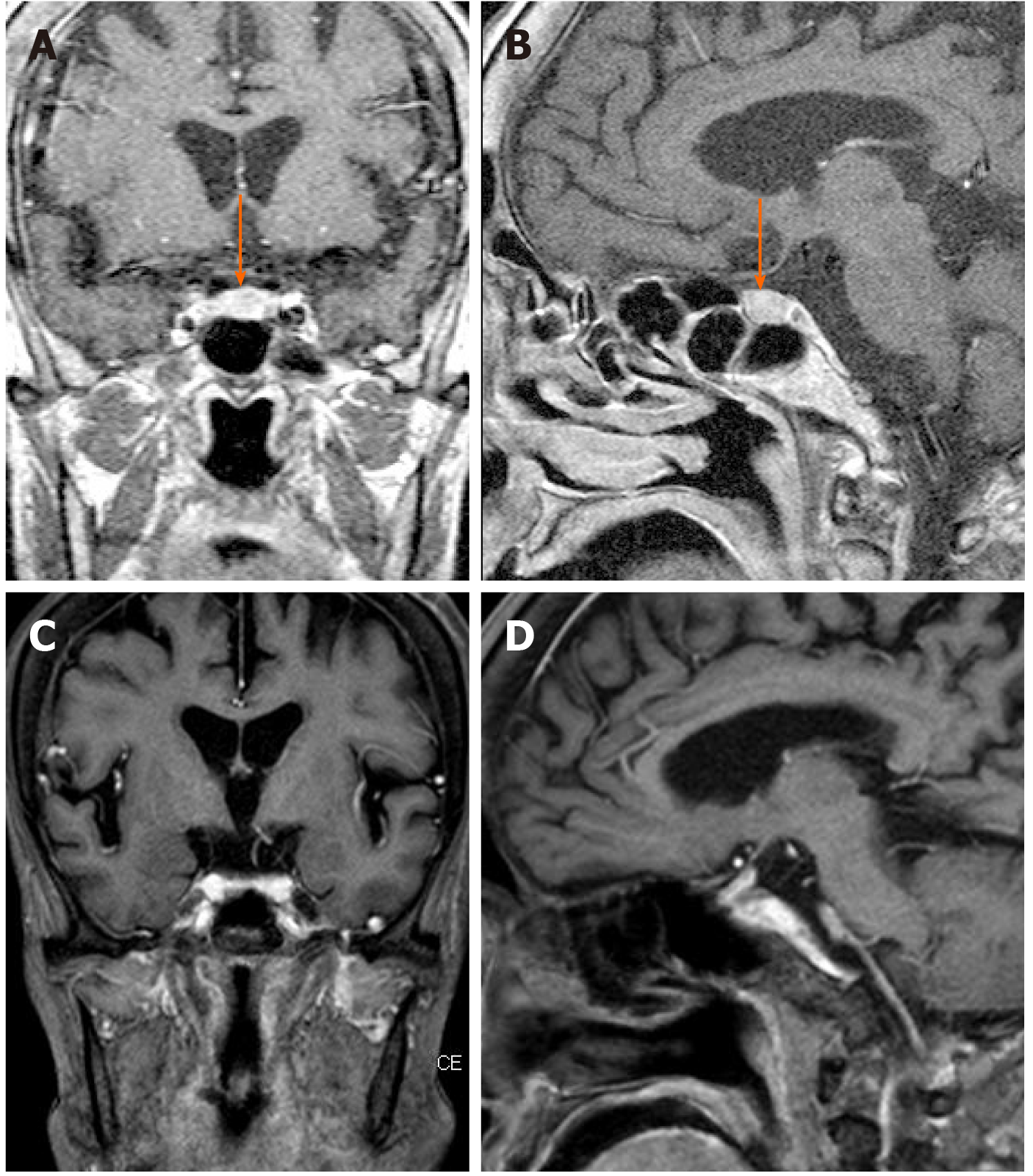
Blood test results were as follows: White blood cell count, 300 cells/μL; Hemoglobin level, 9.0 g/dL; and Platelet count, 113000 platelets/μL, lactate dehydrogenase, 971 IU/L; Ferritin, 710 ng/mL; Soluble interleulin-2 receptor alpha, 3412 U/mL. No evidence of infection or solid tumor was observed on serum examination, culture, and imaging. Brain magnetic resonance imaging (MRI), however, showed enlargement of the pituitary gland (Figure 1A and B). It did not appear to be a tumor as the entire pituitary gland was almost equally contrasted; no other abnormalities were present. We performed stimulating hormone tests, as the patient had been diagnosed with hypopituitarism in the previous hospital. The patient received an injection of four kinds of hormones [growth hormone (GH)-releasing hormone, corticotropin-releasing hormone, luteinizing hormone (LH)-releasing hormone, and thyrotropin-releasing hormone], and we monitored the levels of seven kinds of hormones [GH, LH, follicle-stimulating hormone (FSH), thyroid-stimulating hormone (TSH), cortisol, adrenocorticotropic hormone (ACTH), and prolactin] produced in response. Low levels of six of the seven hormones were observed, with the exception of prolactin, which confirmed the diagnosis of hypopituitarism.
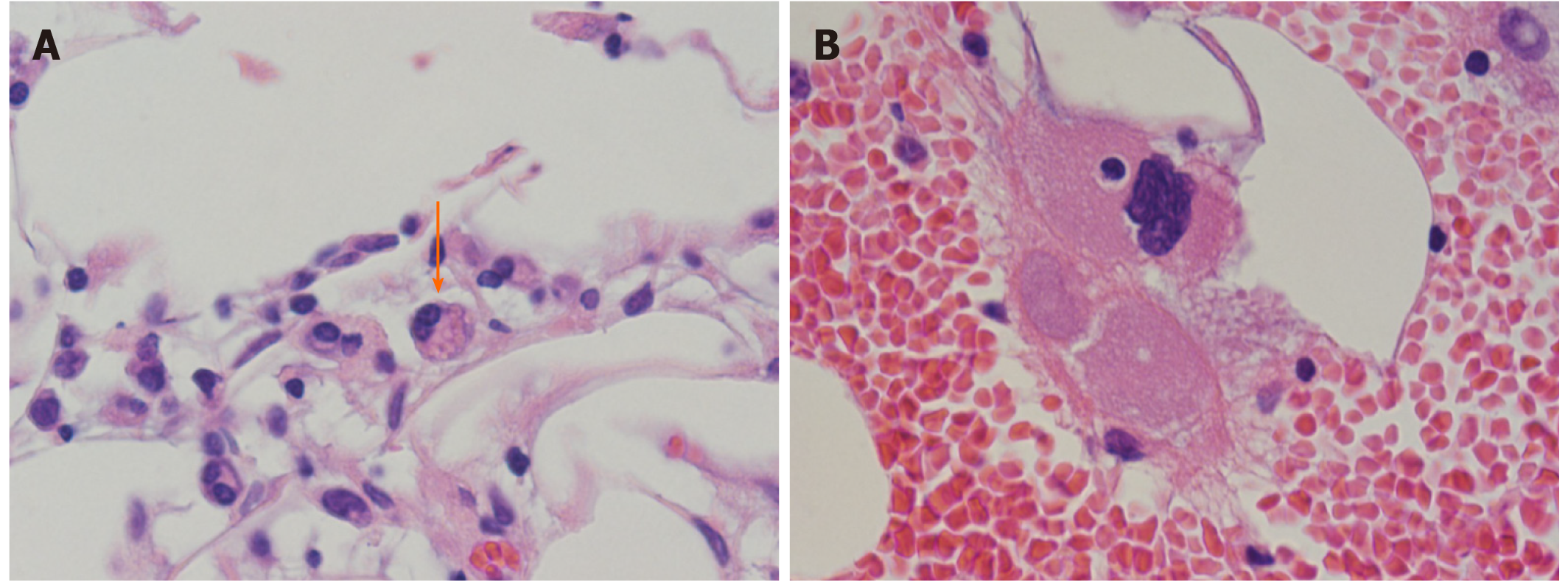
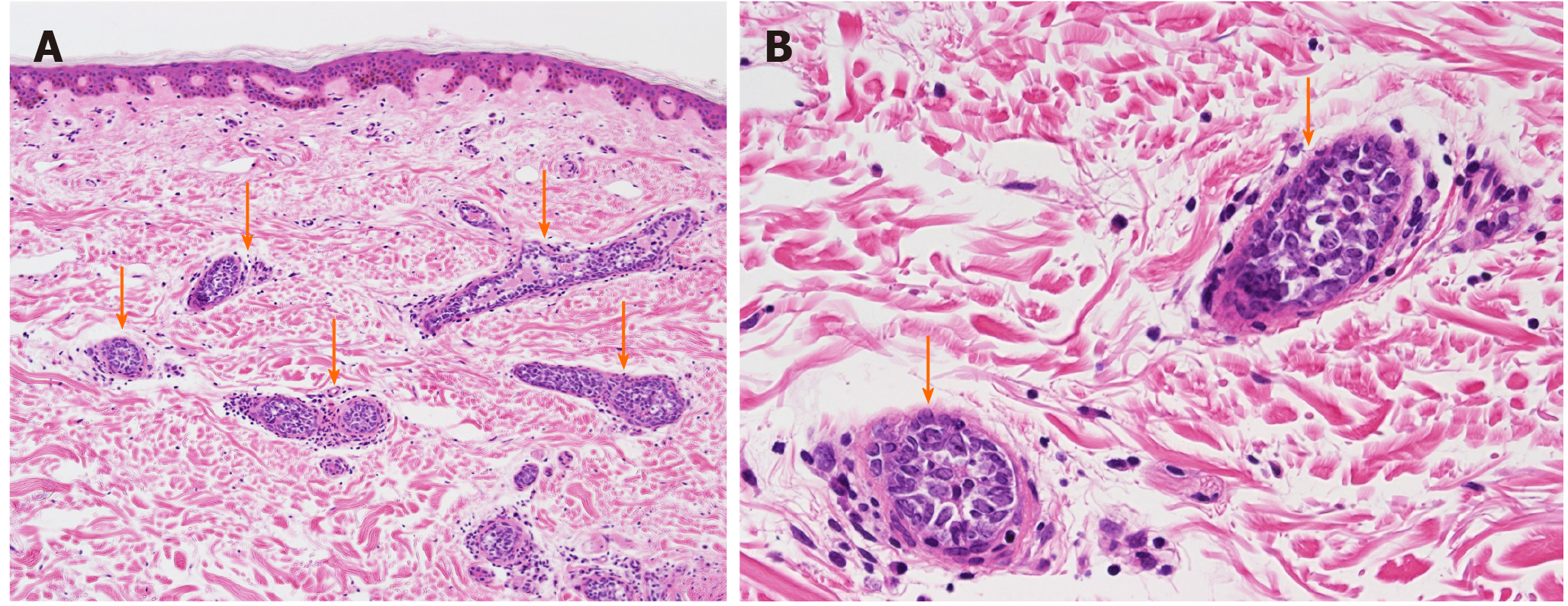
Contrast-enhanced computed tomography scans of the abdomen and pelvis showed a hepatic cyst and mild splenomegaly. There was no evidence of tumor and lymphadenopathy could not be seen. Bone marrow aspiration and biopsy, performed to evaluate pancytopenia, revealed the presence of hemophagocytic lymphohistiocytosis (HLH) (Figure 2A and B). There was no evidence of tumor involvement. She met five of eight criteria of HLH (fever ≥ 38.5°C, splenomegaly, peripheral blood cytopenia, hemophagocytosis in bone marrow, ferritin > 500 ng/mL, elevated soluble IL-2 receptor alpha two standard deviations above age-adjusted laboratory-specific norms)[4]. We suspected lymphoma as a cause of HLH, as no evidence of infection or solid tumors were present. One of the most common causes of adult HLH is lymphoma. However, as the patient did not show lymphadenopathy, a random skin biopsy was performed, which showed that the tumor cells had proliferated predominantly within the small vessels of the dermis with no infiltration outside the vessels (Figure 3). Immunohistochemical staining revealed positivity for the B-cell markers CD20 and CD79a in the absence of staining for T-cell markers. These characteristics were consistent with those of intravascular large B-cell lymphoma.
The patient was diagnosed with IVL, as well as HLH and hypopituitarism secondary to IVL.
The patient was immediately treated with rituximab, cyclophosphamide, doxorubicin, vincristine, and prednisolone. After the first chemotherapy cycle, the tingling sensation partially improved and the symptoms in the palms disappeared. Bone marrow aspiration performed after the first chemotherapy cycle demonstrated the absence of hemophagocytosis, and the bone marrow image was normal, suggesting that HLH had improved with chemotherapy. She completed six cycles of chemotherapy. After the third cycle, head MRI showed that the pituitary gland had returned to its normal size (Figure 1C and D), and it remained at that size after the six cycles. Although the patient required hormone replacement therapy after chemotherapy, her LH, FSH, TSH, free T4, ACTH, and cortisol levels, which were all lower than normal, and prolactin levels, which were higher than normal, returned to normal levels and remained so after completion of the final chemotherapy cycle (Table 1). She did not develop any signs and symptoms associated with hypopituitarism, such as hypoglycemia, after the second cycle of chemotherapy, and her peripheral blood cell count remained normal.
| Hormone | Normal range | Before chemotherapy | During/after chemotherapy | ||
| After the first cycle of chemotherapy | After six cycles of chemotherapy | After 1 yr of chemotherapy | |||
| Thyroid-stimulating hormone | 0.38-4.31 µIU/mL | 0.04 | 0.28 | 0.52 | 0.50 |
| Free T3 | 2.17-3.34 pg/mL | 0.71 | 1.17 | 2.39 | 2.00 |
| Free T4 | 0.82-1.63 ng/dL | 0.63 | 1.22 | 1.22 | 1.74 |
| Growth hormone | 0.13-9.88 ng/mL | 0.68 | 0.33 | 0.35 | 0.24 |
| Prolactin | 3.1-15.4 ng/mL | 24.20 | 25.60 | 16.80 | |
| Adrenocorticotropic hormone | 7.2-63.3 pg/mL | 5.00 | 10.70 | 1.90 | < 1.5 |
| Luteinizing hormone | 5.72-64.31 mIU/mL | < 0.1 | < 0.1 | 7.70 | 8.90 |
| Follicle-stimulating hormone | 0-157.79 mIU/mL | 0.50 | 0.70 | 19.60 | 25.10 |
| Cortisol 8 a.m. | 4.5-21.1 µg/dL | 3.40 | 5.60 | 38.70 | 20.10 |
A complete response was achieved, and the patient was relapse free two years after treatment. Although she still required minimal hormone replacement therapy, 5 mg/d of hydrocortisone, she does not show any signs of hormone deficiency.
Antemortem diagnosis of IVL is often challenging because it affects various organs, resulting in highly variable and unpredictable presentations, although the lymph nodes are typically spared[1]. For example, the presence of a tingling sensation, which was the first complaint in this case, is a common symptom of IVL. The proliferation of tumor cells leads to multiple ischemic necrosis of the central nervous system, nerve roots, cranial nerves, and peripheral nerves[5,6]. This case highlights the importance of a random skin biopsy in diagnosing IVL. IVL is diagnosed by the identification of large lymphoma cells within small-to-medium blood vessels[7]. IVL has been reported following biopsies of various organs, such as the bone marrow, liver, and/or spleen[7]. However, with a random skin biopsy, sufficient specimens can be obtained easily with minimal invasion, and like the bone marrow trephine biopsy, it is highly sensitive for IVL diagnosis[7,8]. Sitthinamsuwan et al[8] demonstrate that random skin biopsy is reliable method for diagnosis of IVL especially in patients with unusual neurological symptoms with co-existing hematologic abnormalities and without lymphadenopathy, such as our case.
In this case, we observed hypopituitarism, a rare complication of IVL. Endocrinopathy is a rare presentation of IVL. Hypopituitarism associated with IVL B-cell lymphoma has been described in fewer than 20 reports[3]. The pituitary gland is a hypervascular organ, and hypopituitarism may be caused by vascular occlusion by the lymphoid tumor cells in the hypothalamus or pituitary gland[3,9]. It is unclear why selective growth of tumor cells occurs[3,9]. Although we did not perform a pituitary biopsy, we diagnosed lymphoma infiltration of the pituitary gland based on poor evidence of other causes of hypopituitarism and pituitary gland enlargement; both conditions improved after chemotherapy. Although most patients of IVL with central nerves system involvement need intrathecal chemotherapy, most patients with pituitary involvement have responded to chemotherapy alone, as this case showed[10].
Although the common clinical or hormonal course after treatment for pituitary involvement with IVL remains uncertain because of the limited number of reported cases, early chemotherapy may be effective. Sawada et al[3] described the case of a patient with hypopituitarism associated with IVL and the endocrinological course that followed. In this case, although the patient showed symptoms of panhypopituitarism before treatment, the levels of LH, FSH, TSH, ACTH, and prolactin returned to normal. She did not receive hormonal supplementation before and after chemotherapy. Sawada et al[3] stated that hematological therapy at an earlier disease stage may contribute to better endocrinological prognoses, and that the amelioration of pituitary infarction by chemotherapy improves pituitary function when the damage is not irreversible, enabling avoidance of hormone replacement therapy. Our case supports their theory, as the function of the pituitary gland in our patient also partially improved after chemotherapy. The infarction-related damage was not irreversible.
In conclusion, we observed a case of IVL with hypopituitarism as a rare complication, which was diagnosed antemortem and successfully treated. Although early chemotherapy is effective, the clinical diagnosis of IVL is challenging. On encountering cases with a variety of symptoms that cannot be explained reasonably, general physicians should consider IVL. Additionally, the mechanisms of lymphoma-associated hypopituitarism associated with IVL have not yet been elucidated, necessitating further case studies and laboratory-based research.
Manuscript source: Unsolicited manuscript
Specialty type: Oncology
Country/Territory of origin: Japan
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): B
Grade C (Good): C
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Gorodetskiy V, Hu B S-Editor: Dou Y L-Editor: A P-Editor: Li JH
| 1. | Shimada K, Kinoshita T, Naoe T, Nakamura S. Presentation and management of intravascular large B-cell lymphoma. Lancet Oncol. 2009;10:895-902. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 185] [Cited by in RCA: 199] [Article Influence: 12.4] [Reference Citation Analysis (0)] |
| 2. | Zuckerman D, Seliem R, Hochberg E. Intravascular lymphoma: the oncologist's "great imitator". Oncologist. 2006;11:496-502. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 139] [Cited by in RCA: 134] [Article Influence: 7.1] [Reference Citation Analysis (0)] |
| 3. | Sawada Y, Ishii S, Koga Y, Tomizawa T, Matsui A, Tomaru T, Ozawa A, Shibusawa N, Satoh T, Shimizu H, Hirato J, Yamada M. Reversible Hypopituitarism Associated with Intravascular Large B-Cell Lymphoma: Case Report of Successful Immunochemotherapy. Tohoku J Exp Med. 2016;238:197-203. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 7] [Cited by in RCA: 8] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 4. | McClain KL, Eckstein O. Clinical features and diagnosis of hemophagocytic lymphohistiocytosis. Waltham, MA: UpToDate Inc. Available from: https://www.uptodate.com/contents/clinical-features-and-diagnosis-of-hemophagocytic-lymphohistiocytosis/. |
| 5. | Kelly JJ, Karcher DS. Lymphoma and peripheral neuropathy: a clinical review. Muscle Nerve. 2005;31:301-313. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 152] [Cited by in RCA: 143] [Article Influence: 7.2] [Reference Citation Analysis (0)] |
| 6. | Nakahara T, Saito T, Muroi A, Sugiura Y, Ogata M, Sugiyama Y, Yamamoto T. Intravascular lymphomatosis presenting as an ascending cauda equina: conus medullaris syndrome: remission after biweekly CHOP therapy. J Neurol Neurosurg Psychiatry. 1999;67:403-406. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 52] [Cited by in RCA: 56] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 7. | Matsue K, Asada N, Odawara J, Aoki T, Kimura S, Iwama K, Fujiwara H, Yamakura M, Takeuchi M. Random skin biopsy and bone marrow biopsy for diagnosis of intravascular large B cell lymphoma. Ann Hematol. 2011;90:417-421. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 56] [Cited by in RCA: 65] [Article Influence: 4.3] [Reference Citation Analysis (0)] |
| 8. | Sitthinamsuwan P, Chinthammitr Y, Pattanaprichakul P, Sukpanichnant S. Random skin biopsy in the diagnosis of intravascular lymphoma. J Cutan Pathol. 2017;44:729-733. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 15] [Cited by in RCA: 16] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 9. | Pekic S, Milicevic S, Colovic N, Colovic M, Popovic V. Intravascular large B-cell lymphoma as a cause of hypopituitarism: gradual and late reversal of hypopituitarism after long-term remission of lymphoma with immunochemotherapy. Endocrine. 2008;34:11-16. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 21] [Cited by in RCA: 18] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 10. | Nizamutdinov D, Patel NP, Huang JH, Fonkem E. Intravascular Lymphoma in the CNS: Options for Treatment. Curr Treat Options Neurol. 2017;19:35. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 19] [Cited by in RCA: 14] [Article Influence: 1.8] [Reference Citation Analysis (0)] |