Published online Aug 24, 2020. doi: 10.5306/wjco.v11.i8.655
Peer-review started: March 12, 2020
First decision: June 7, 2020
Revised: June 11, 2020
Accepted: July 18, 2020
Article in press: July 18, 2020
Published online: August 24, 2020
Processing time: 161 Days and 14.4 Hours
Sinonasal malignancies are rare but demanding due to complex anatomy, usually late diagnosis, and inconsistent therapy strategy based on multimodality approaches. Squamous cell carcinoma (SCC) is the most common histology, with poorer prognosis. In the setting of orbital invasion, an orbital exenteration may be required. However, in case of primary rejection of disfiguring surgery or unresectable disease, proton beam therapy (PBT) should be largely considered, allowing for better sparing of neighboring critical structures and improved outcomes by dose escalation.
A 62-year-old male presented with a recurrent SCC in the nasal septum abutting frontal skull base and bilateral orbits at 7 mo after primary partial nasal amputation. Because of refusal of face-deforming surgery and considerable adverse effects of conventional radiotherapy, the patient underwent a PBT by hyperfractionated accelerated scheme, resulting in complete response and moderate toxicities. After 2 years, a nasal reconstruction was implemented with satisfactory appearance and recurrence-freedom to date. Another patient with an initially extended sinonasal SCC, invading right orbit and facial soft tissue, declined an orbital exenteration and was treated with a normofractionated PBT to the gross tumor and elective cervical lymphatics. The follow-up showed a continuous tumor remission with reasonable late toxicities, such as cataract and telangiectasia on the right. Despite T4a stage and disapproval of concurrent chemotherapy owing to individual choice, both patients still achieved outstanding treatment outcomes with PBT alone.
PBT enabled orbit preservation and excellent tumor control without severe adverse effects on both presented patients with locally advanced sinonasal SCC.
Core tip: The treatment of sinonasal squamous cell carcinoma is exceedingly challenging owing to complex anatomy, delayed diagnosis, and lack of randomized clinical studies about multimodality approaches. In particular, locally advanced disease with indication of orbital exenteration or other disfiguring surgeries, as well as unresectable gross tumor require modern non-surgical treatment options like proton beam therapy, as presented in this case report, to achieve a long-term tumor control without severe late toxicities, such as blindness and cerebral radiation necrosis.
- Citation: Lin YL. Proton beam therapy of periorbital sinonasal squamous cell carcinoma: Two case reports and review of literature. World J Clin Oncol 2020; 11(8): 655-672
- URL: https://www.wjgnet.com/2218-4333/full/v11/i8/655.htm
- DOI: https://dx.doi.org/10.5306/wjco.v11.i8.655
Sinonasal malignancies (SNMs) occur very seldom and account for only 3% of all head and neck cancers and 1% of all malignant tumor diseases, with a peak incidence in the 5th to 7th decades and predominance among males. The prodromes, such as nasal congestion and discharge, epistaxis and lacrimation, are often misjudged as rhinosinusitis and consequently neglected by both the patients and physicians. At the presence of late symptoms like facial edema, sensory failures and cranial neuropathy, the patient is first referred to sinonasal endoscopy and imaging[1]. At this time, however, over 50% of the cases are diagnosed in an advanced stage (T3/4), with poor prognostic outcome[2,3]. Female sex, nasal cavity tumor, adenocarcinoma and low clinical stage have been identified as positive predictors[4]. Among the epithelial tumors, the squamous cell carcinoma (SCC) is the most common (80%), followed by adenocarcinoma. Both histological subtypes are etiologically associated with occupational exposure to wood, leather and textile dusts, organic solvents, welding fumes, arsenic, etc.[5,6].
For sinonasal (SN) SCC (SNSCC), higher age and tumor stage are adverse prognostic factors, while surgery has been shown to improve survival significantly[3]. Based on the analysis of the United States National Cancer Database, surgical approach represents the therapeutic mainstay of SNSCC, whereas neoadjuvant chemoradiotherapy (CRT) is associated with improved R0 resectability[7]. Although recent retrospective studies have validated superior outcomes by multimodality, the optimal combination and sequence of surgery, radiotherapy (RT) and chemotherapy remain controversial[7-9]. Furthermore, locally advanced SNM with orbital invasion is actually challenging for clinicians due to the complexity of complete gross resection, that largely requires an orbital exenteration and consecutive aesthetic restoration by means of plastic surgery, prosthesis and rehabilitation. Given the correlated burden to the patient’s psyche and quality of life, the information about prognosis, multimodal therapy approaches and supportive adjuvant measures should be comprehensively discussed between the patient and attending physicians before the therapeutic decision[10].
Chief complaints: A 62-year-old German male presented with a relapsed tumor in the nasal septum, extending to dual-sided ethmoidal sinuses and abutting frontal skull base, as well as a suspicious lymph node metastasis in the left parotid gland, in an interval of 7 mo after the primary surgery to address a nasal SCC.
History of present illness: The tumor recurrence was confirmed by a sampling excision of nasal mucosa in August 2014, which showed moderately differentiated keratinizing SCC. According to the assessment of otorhinolaryngology, a resection was possible in principle but would have been accompanied by enormous physical defect and face distortion due to the requisite removal of bilateral medial canthi and glabella. The patient rejected the surgery and tried to gain information about RT. Consultation with the radiation oncology team of the university hospital close to his home led to recommendation of a combined CRT or proton beam therapy (PBT). The patient preferred the latter, after he became educated about the more vehement toxicities of conventional RT with photons, such as necessity of artificial nutrition owing to pharyngitis, malfunction of sense of smell and taste, deafness of left ear, and blindness in 2-5 years. Consequently, he contacted three particle therapy institutes in Germany but obtained refusal from two for the following reasons: The benefit of particle therapy for SNSCC was not completely clarified and could not be offered out of clinical trials. Besides, the sinonasal airspaces causing uncertainties in the treatment planning was unfavorable for the exact calculation of dose distribution in the target volume. Therefore, a conventional RT via modern technique [(e.g., intensity-modulated RT (IMRT)] with concomitant platinum-based chemotherapy was recommended. On the contrary, Rinecker Proton Therapy Center was the only one of the three consulted institutes which accepted the patient for PBT.
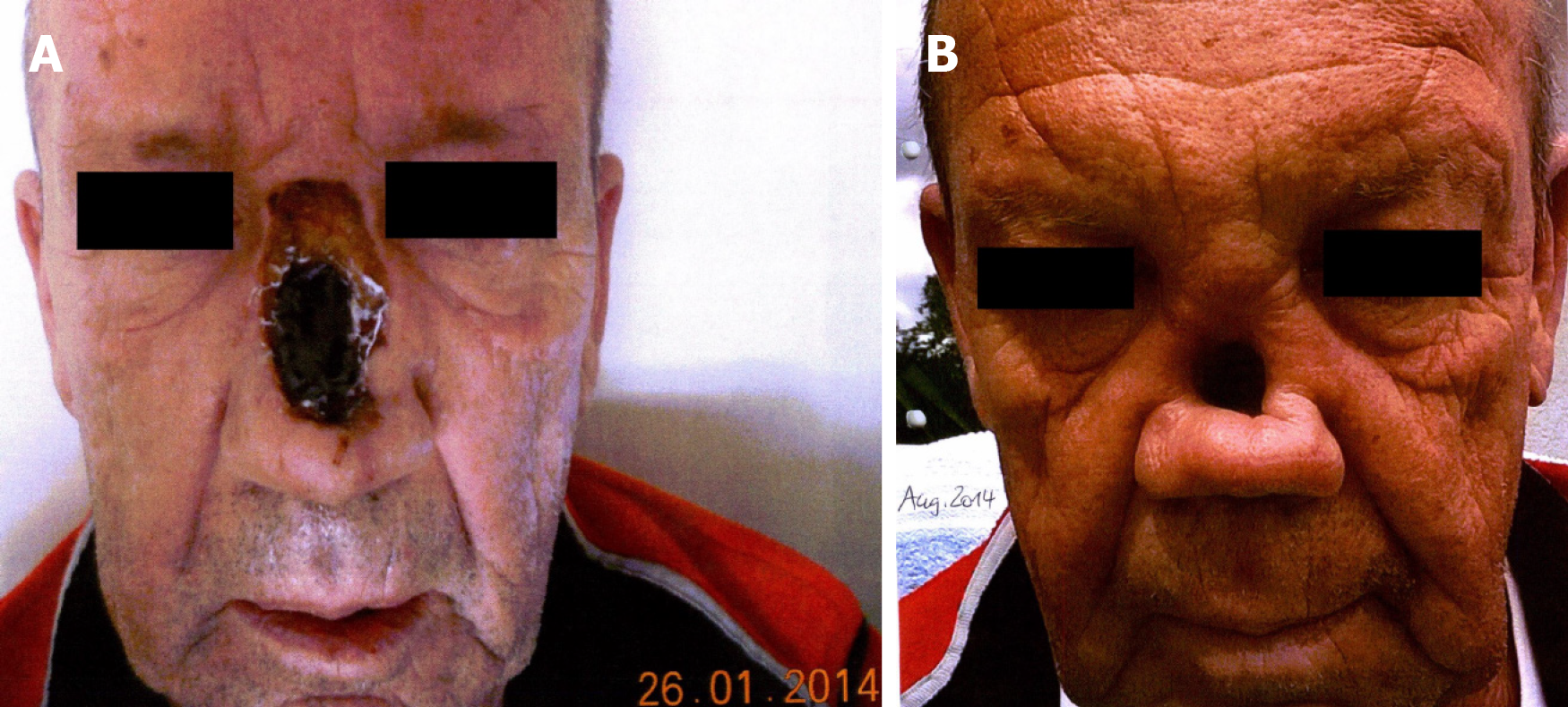
History of past illness: The nasal SCC had been initially noted in October 2013 by recurrent epistaxis with swelling and enlargement of the nose and foreign body feeling. Endoscopy demonstrated an exophytic lesion in the nasal septum, reaching to the nasal floor. Imaging examinations, including computed tomography (CT) scan of head, neck and thorax and ultrasound of neck, showed a tumor perforating the anterior nasal septum with infiltration of nasal bridge and destruction of nasal bone, emphasized on the left side, as well as a suspicious Warthin’s tumor in the left parotid gland. In January 2014, the patient underwent a partial nasal ablation (Figure 1A) and selective neck dissection (level I-III) on both sides, with postoperative tumor stage determined to be pT2 pN0 G3 R0 cM0.
Personal and family history: The patient was in good general condition and worked at his own gym. Apart from chronic nicotine abuse (at least 50 pack-years), there was no relevant comorbidity known.
Physical examination upon admission: There was an obvious substance defect in the middle nasal portion with tumorous skin thickening all-round after the partial amputation (Figure 1B), so that the original nasal epithesis no longer fit within. The common clinical examination yielded normal findings.
Laboratory examinations: No special laboratory test was arranged.
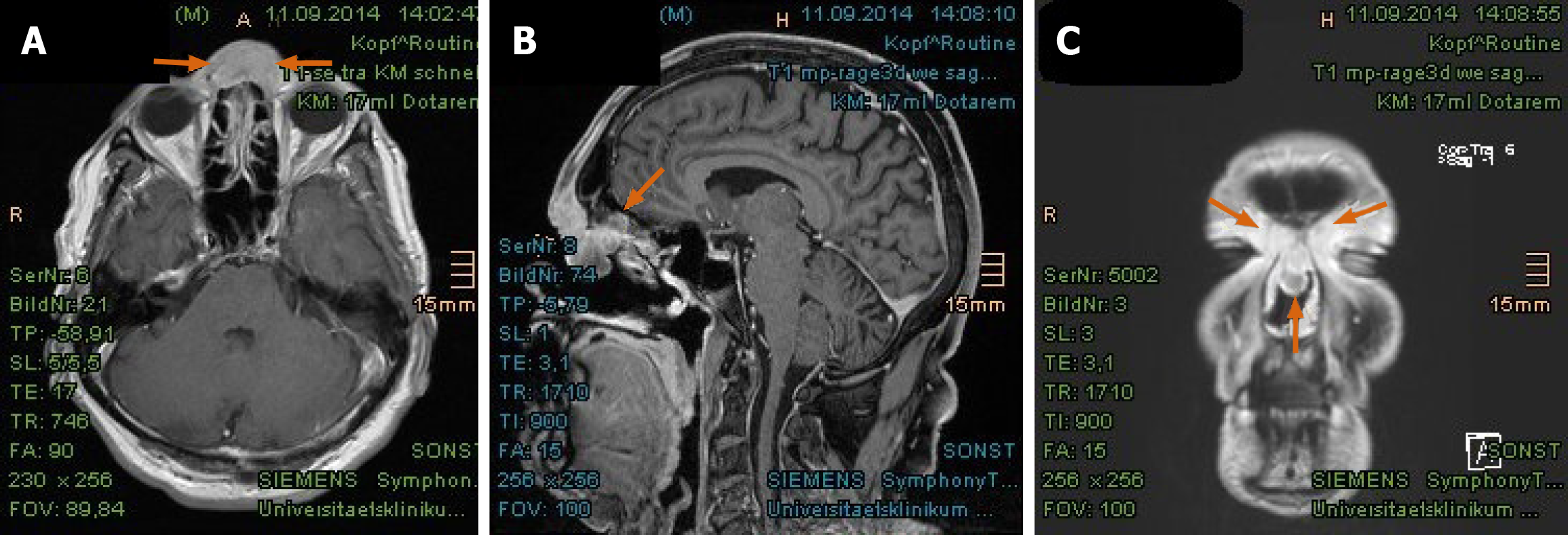
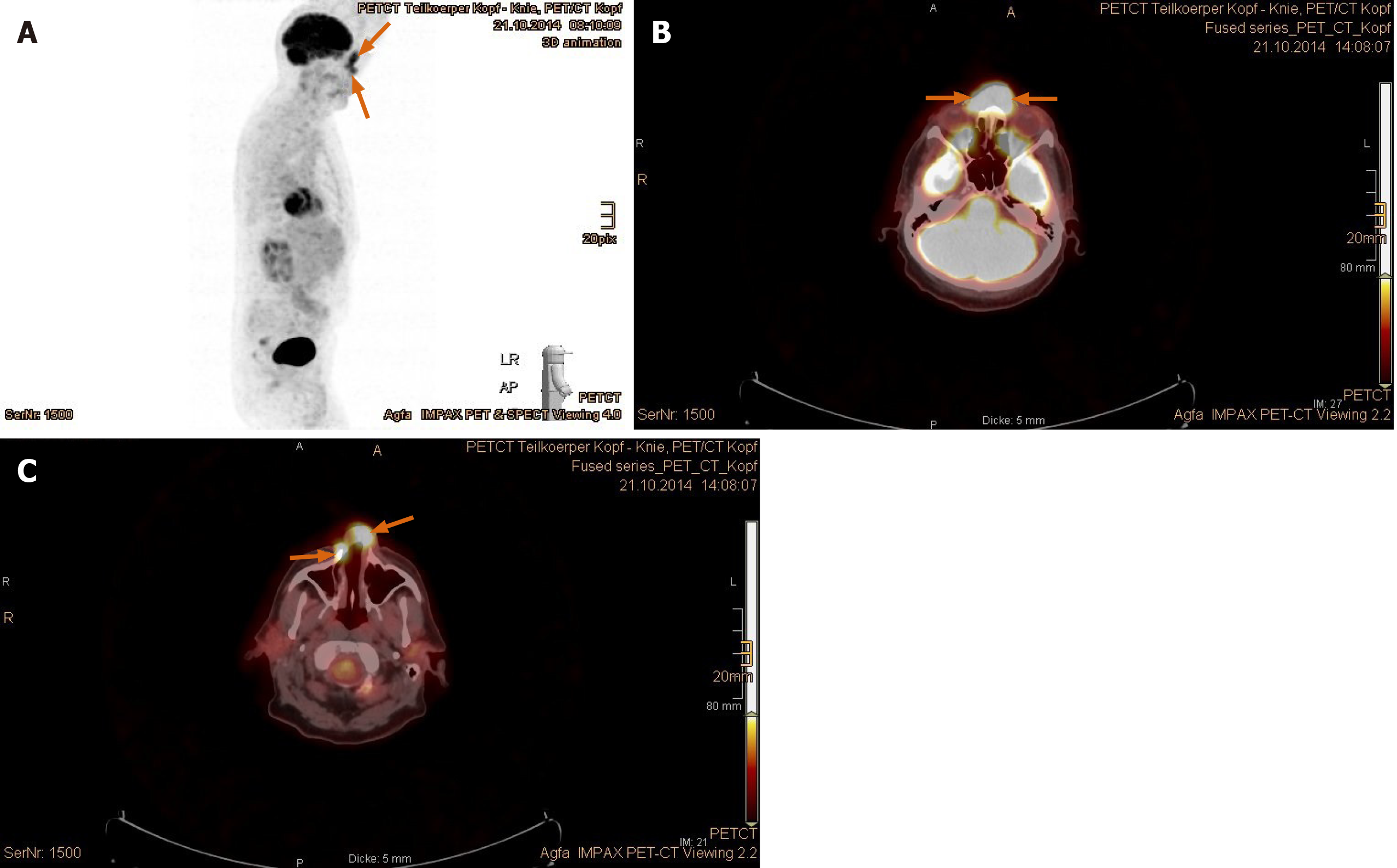
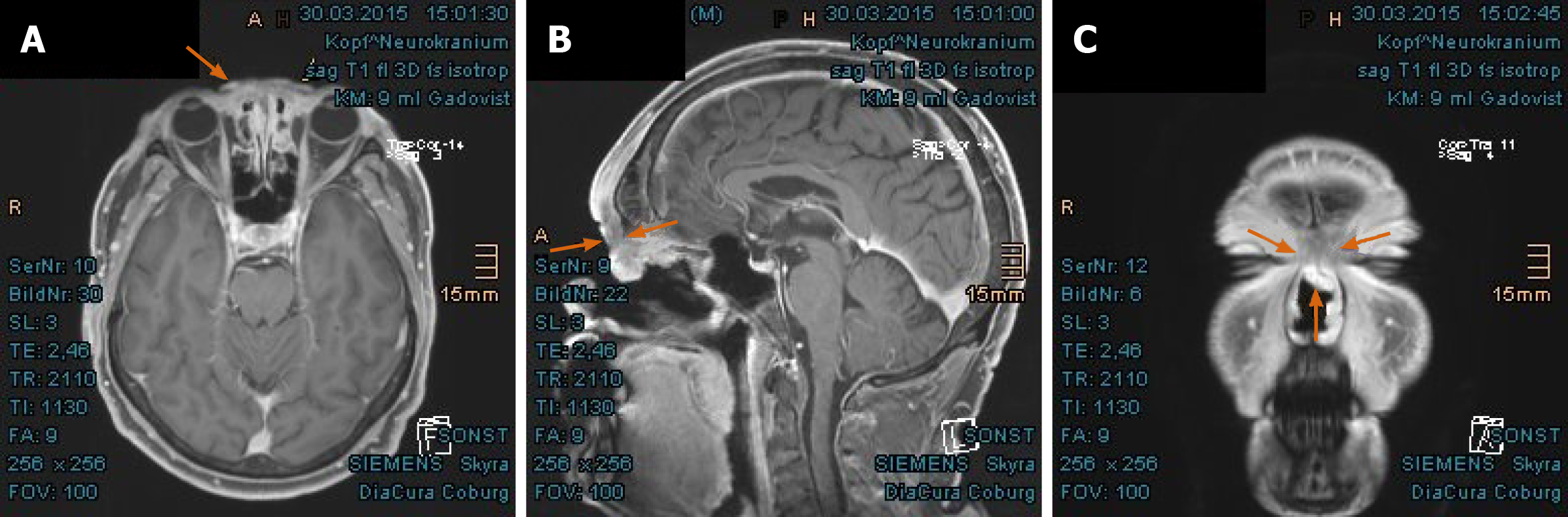
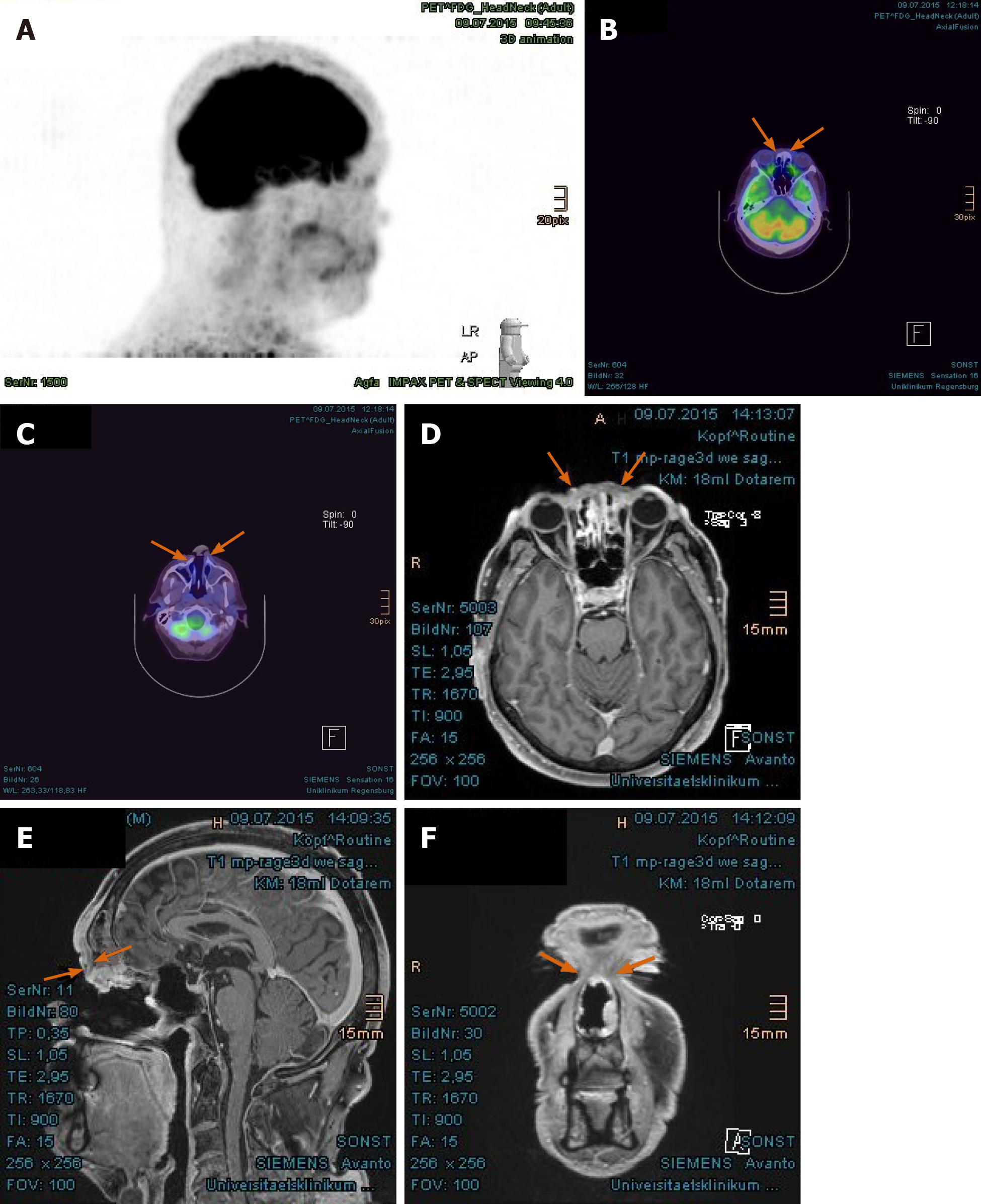
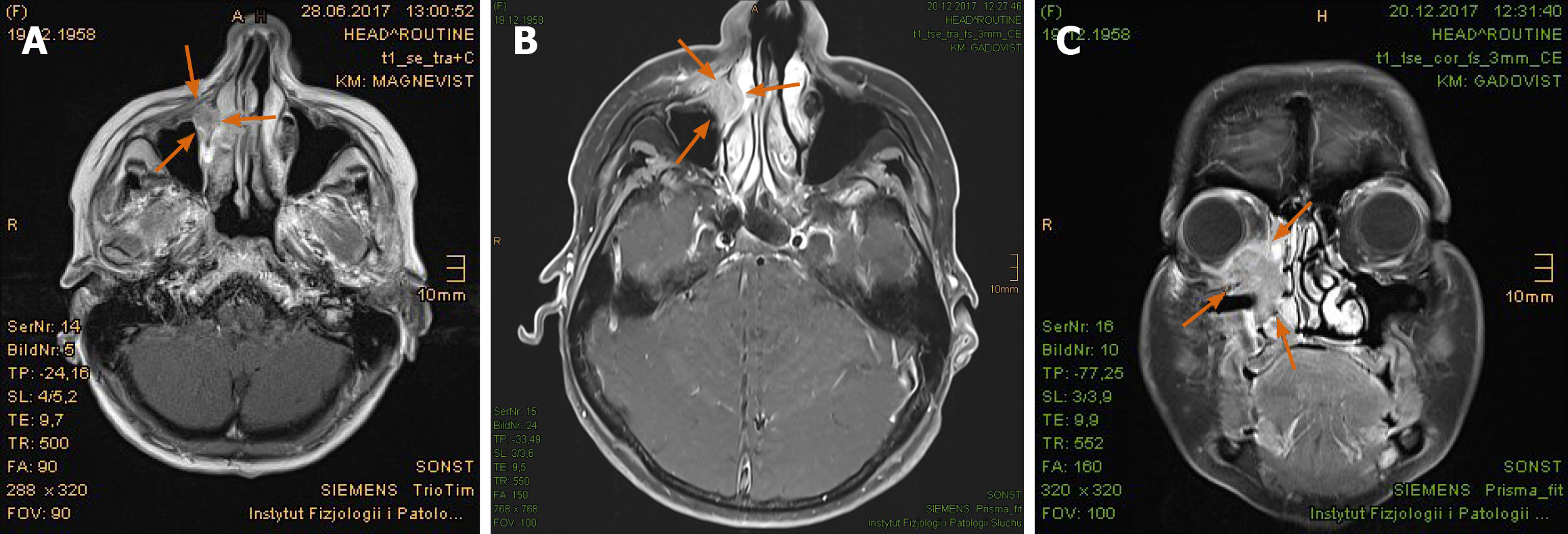
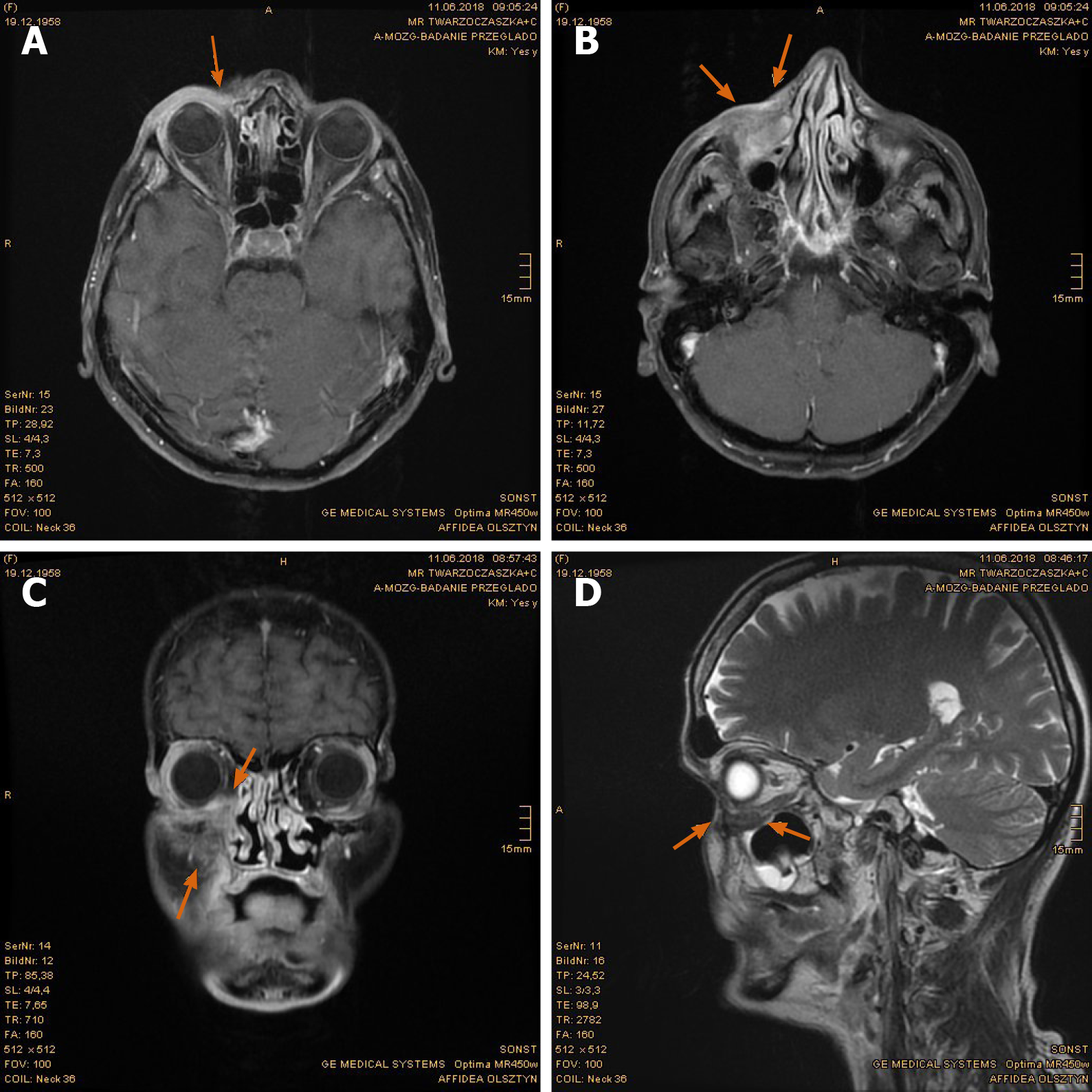
Imaging examinations: The magnetic resonance imaging (MRI) prior to PBT showed intensive contrast enhancement in the central nasal cavity with soft tissue swelling of nasal bridge until nostrils, measuring approximately 29 mm × 15 mm × 22 mm, abutting the frontal sinus and skull base (Figure 2A-C). The ethmoidal air cells were partially involved by tumor infiltration as well as mucosal swelling. Apart from at least one strong enhancing nodule of 8 mm × 11 mm diameter at the lower pole of left parotid gland, no pathological cervical lymph node was detected. To complete the restaging examination, the patient underwent additional positron emission tomography with 2-deoxy-2-fluorine-18-fluoro-D-glucose (18F-FDG-PET/CT). Both suspicious tumor recurrences in the nasal bridge and left parotid gland exhibited a maximum standardized uptake value [SUV(max)] of 5.2 and 2.7 in each (Figure 3A-C). Although the parotid lesion was initially interpreted as Warthin’s tumor, which is FDG-avid on principle, this finding was assessed by our specialist of nuclear medicine and radiology as highly suspicious of intraparotid lymph node metastasis.
Final diagnosis: Recurrent periorbital SNSCC, tumor stage rpT4a rpN1 G2 cM0.
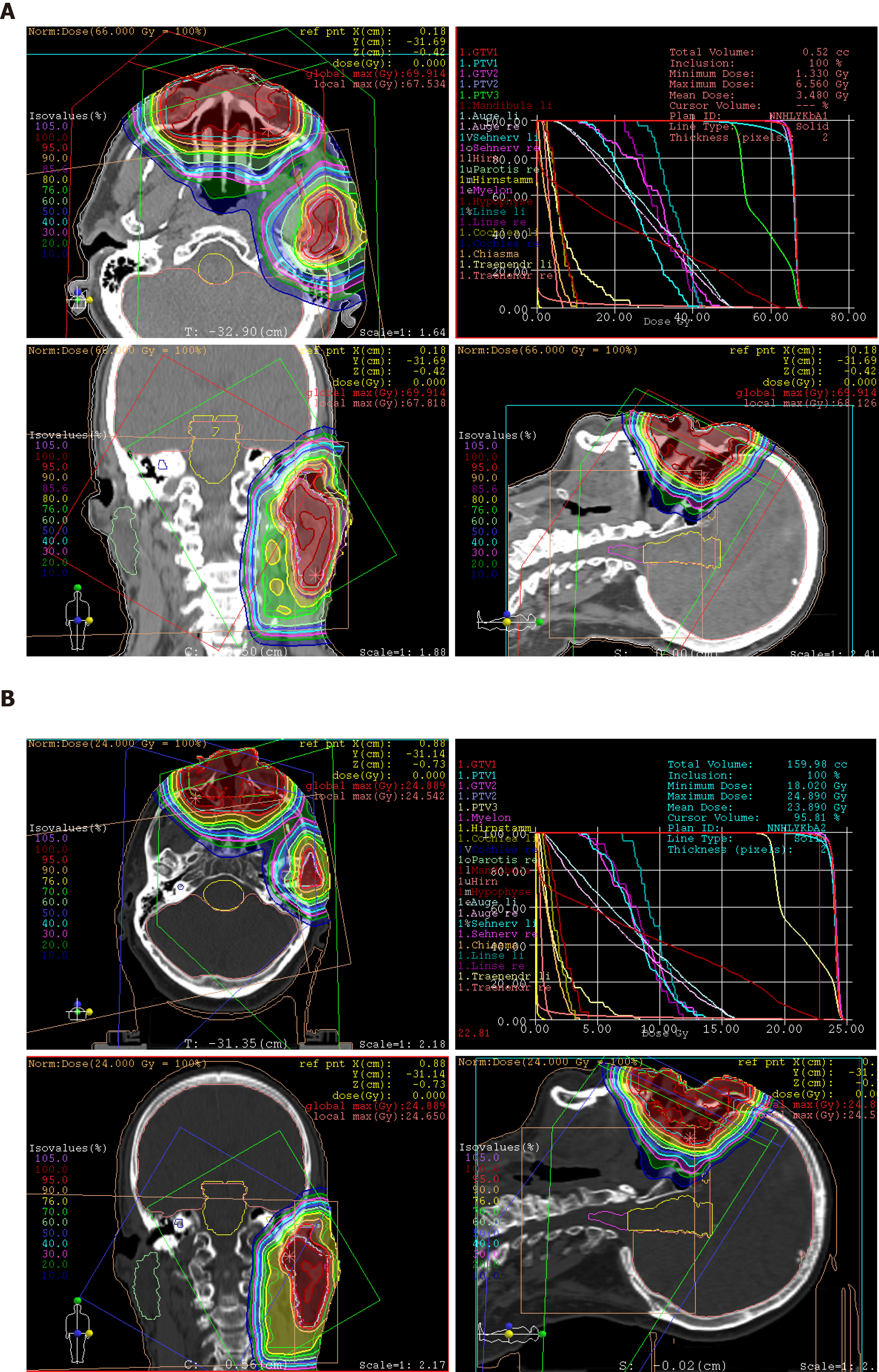
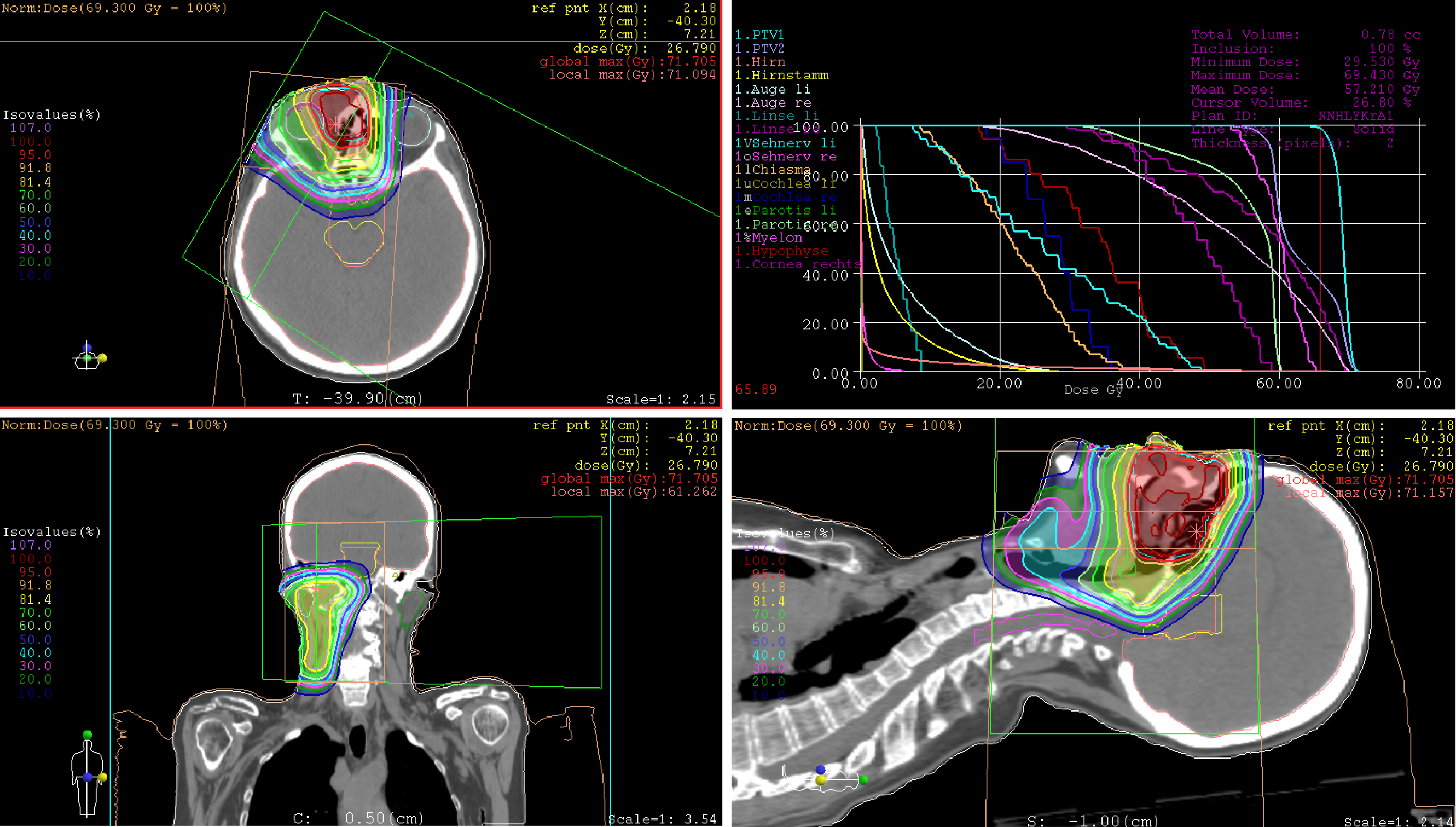
Treatment: At express request due to continuation of working during the treatment, the patient was treated with hyperfractionated accelerated scheme within 37 d, from October to December 2014. The informed consent was obtained prior to the initiation of the treatment. The PBT was delivered in 44 fractions and single dose of 1.50 Gy [relative biological effectiveness (RBE)], twice a day with minimum interim of 4 h, at a total dose of 66.00 Gy (RBE) to the tumor recurrences in the nasal bridge and left parotid gland. Simultaneously, the left cervical lymphatic drainage, including nodi lymphatici parotidei, submandibulares and jugulares superiores, received 52.80 Gy (RBE) in total, with single dose of 1.20 Gy (RBE). The entire target volume was irradiated from three gantry angles of 30°, 330° and 80° using the pencil beam scanning technique (Figure 4A). After 28 fractions, the safety margin to both eyeballs was reduced because of incipient tumor shrinkage, noticed by weekly-performed low-dose CT scans, as well as for the purpose of better eye sparing (Figure 4B). Since the statutory health insurance refused to reimburse the cost of PBT, the patient deliberately declined a concurrent chemotherapy, in order to demonstrate afterwards that he was exclusively cured by PBT alone.
Outcome and follow-up: Generally, the patient tolerated PBT well and drove 300 km daily between our center and his home. At the beginning of the treatment, he complained of intumescence of the nose, with boring pain in the evening, that was mitigated by anti-edematous medication (dexamethasone 8-16 mg per day) and analgesics. In the further course, he developed increasing radiation dermatitis with superinfection in the middle face, especially at the inner corners of both eyes, corresponding to grade 2-3 by Common Terminology Criteria for Adverse Events (commonly referred to as CTCAE). At the final examination, the skin finding was improved by intensified skin care and disinfectant measures taken immediately after daily irradiation. The patient denied visual impairment and dry eyes as well as dysphagia and changes in taste and smell. Xerostomia only occurred temporarily.
In the first follow-up, at 3 mo after the PBT, MRI scan displayed a significant tumor reduction in the nasal bridge (Figure 5A-C). At this stage, it was normally hard to distinguish between residual tumor and inflammation tissue. Nonetheless, the biopsy from the nasal bridge revealed a chronic granulating mucosal ulcer with no evidence of malignancy. As post-radiogenic changes, the mucosa of the nasal cavity and paranasal sinuses was still distinctly swollen, accompanied by fluid accumulation in the left petrous bone. Subjectively, the patient reported, first, deterioration of moist desquamation after finishing the PBT, which was alleviated by use of a special ointment mixture containing cortisone (prescribed by his dermatologist) after 10 d. Second, he complained of excessive lacrimation, lymphedema of the face and hypacusis on account of post-radiogenic tympanic effusion in the left ear. Other late toxicities, such as visual, olfactory and gustatory disturbances and dry mouth, were absent.
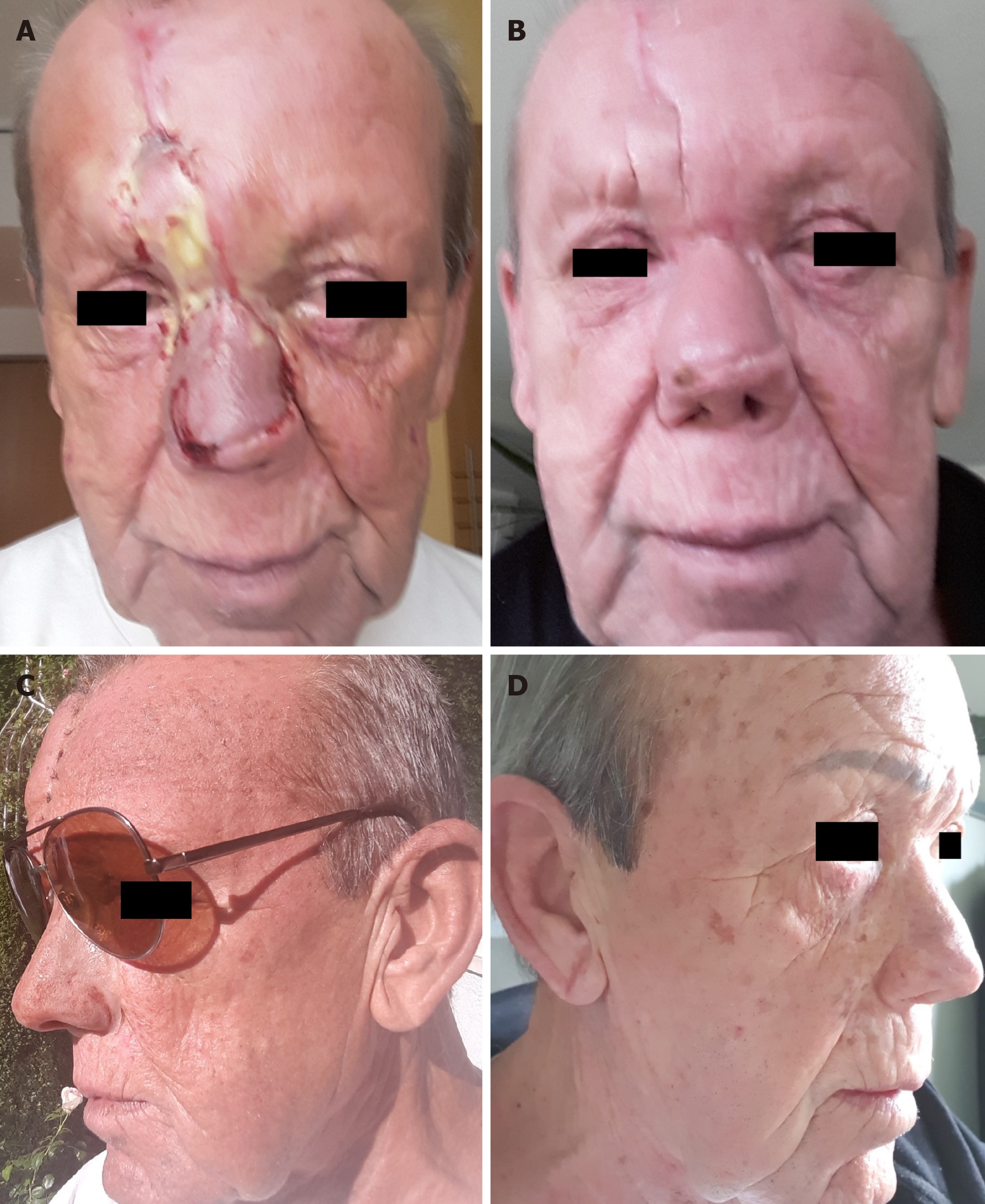
The PET/CT and MRI scans performed at 8 mo after PBT showed a complete tumor remission (Figure 6A-F). The lymphedema in the facial and left retroauricular area regressed by frequent lymphatic drainage massage. Except sustained reinforced shedding of tears, no sensory impairment was present, and the epithesis of nose fit in again. At the 23rd mo after PBT, the patient reported bilateral cataracts, dry eyes, permanent loss of medial eyebrows, eyelashes and nasal hair, and use of lubricating eye drops steadily. Since a tumor recurrence was continuously excluded in the PET/CT and MRI scans (Figure 7A-E), the patient was accepted to undergo a nasal reconstruction in five sessions, carried out between 2016-2017, in cooperation with the otorhinolaryngology and plastic surgery departments[40] (Figure 8A-C). To date, the patient is content with the cosmetic result (Figure 8D) and remains free of tumor recurrence as well as visual and auditory impairment. Despite his objection in view of the successful treatments, the health insurance still declines to refund the expenditure of PBT and reconstruction surgery.
Chief complaints: A 59-year-old Polish female was diagnosed with a space-occupying lesion of the right lacrimal sac adjoining nasal cavity and maxillary sinus in the ophthalmology, initially in summer 2017 (Figure 9A).
History of present illness: The patient was referred to the otorhinolaryngology department for the further examinations. Owing to lack of an apparent tumor in the nasal cavity, presence of ulceration and unfavorable curvature of the nasal septum, instead of an endoscopic approach, the histopathology was obtained in January 2018 by an open biopsy through the lower eyelid, submitting moderately differentiated keratinizing SCC. Because the tumor invaded the medial orbit and adjacent paranasal sinuses (Figure 9B and C), an orbital exenteration on the right was defined as the therapy of choice but was rejected by the patient. She then contacted our center for the purpose of organ preservation via definitive RT with PBT.
History of past illness: Not specified.
Personal and family history: The patient was in reduced general condition, being wheelchair-bound (Karnofsky Performance Score 60) by rheumatoid arthritis and on long-term treatment with methotrexate.
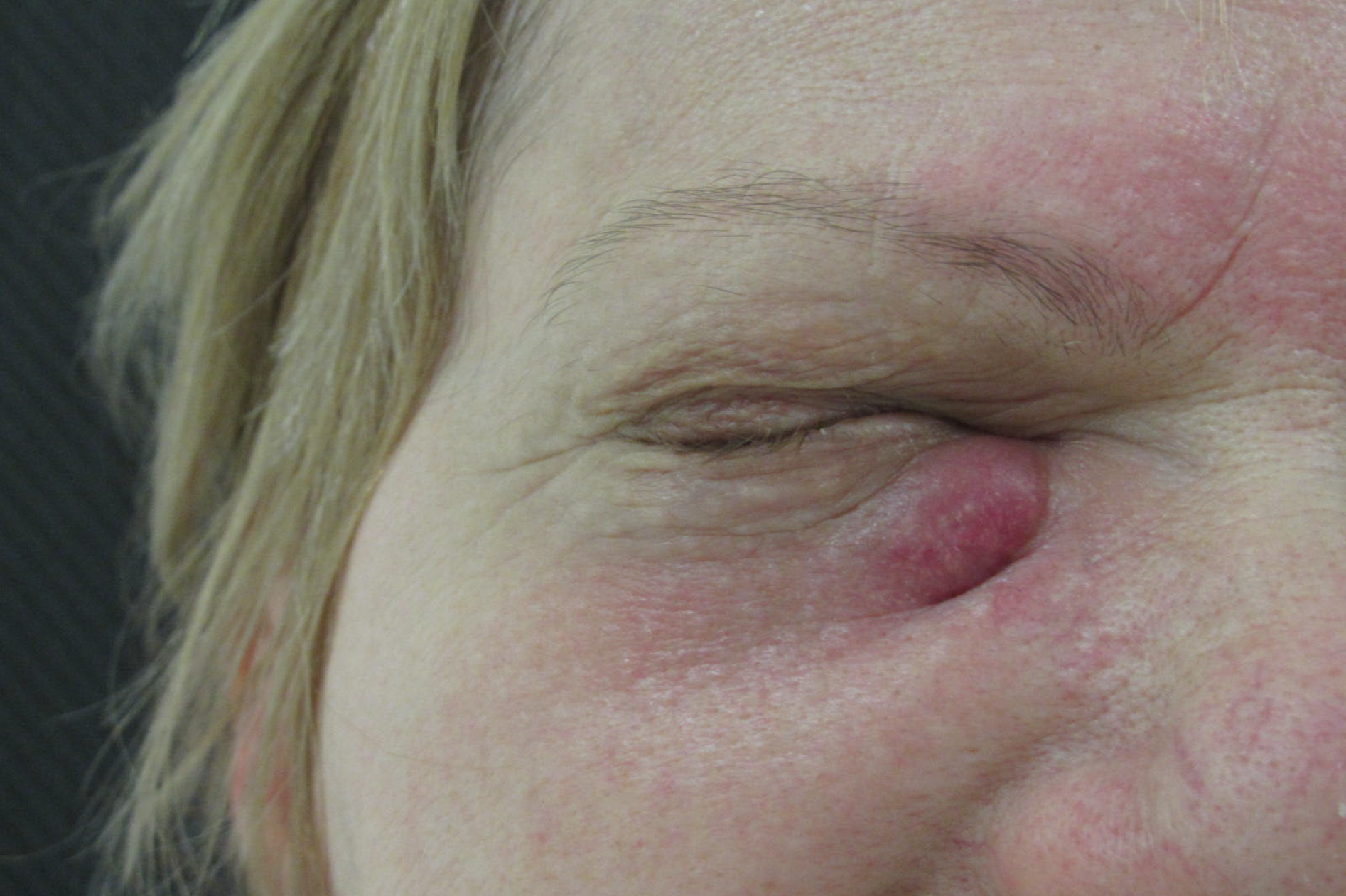
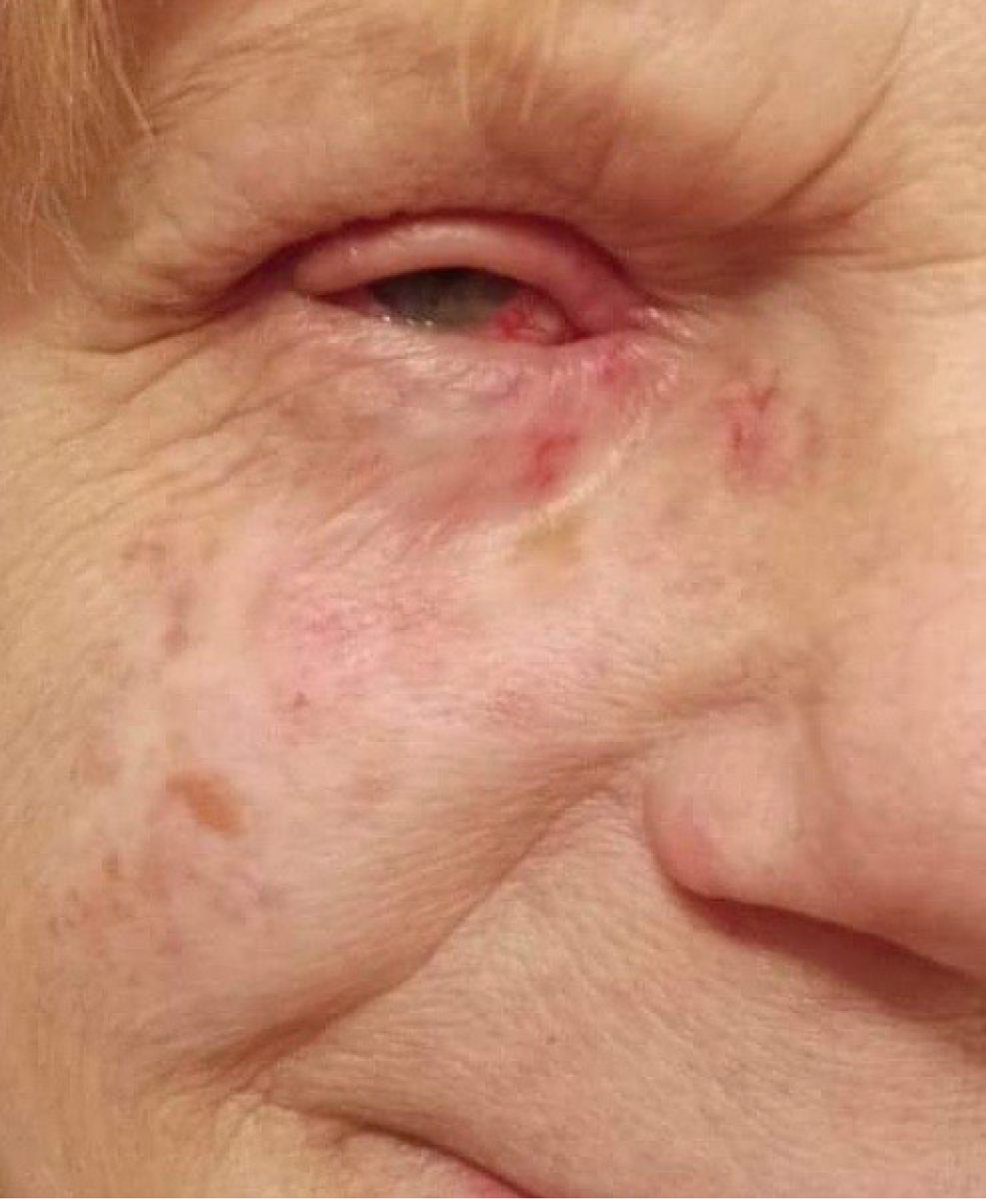
Physical examination upon admission: The patient presented with a bean-like flushed elevation inferiorly to the medial canthus of the right eye (Figure 10). The cervical lymph nodes were not as enlarged as to be palpable. No suspicious findings of tumor spread were apparent in the common clinical examination.
Laboratory examinations: No special laboratory test was arranged.
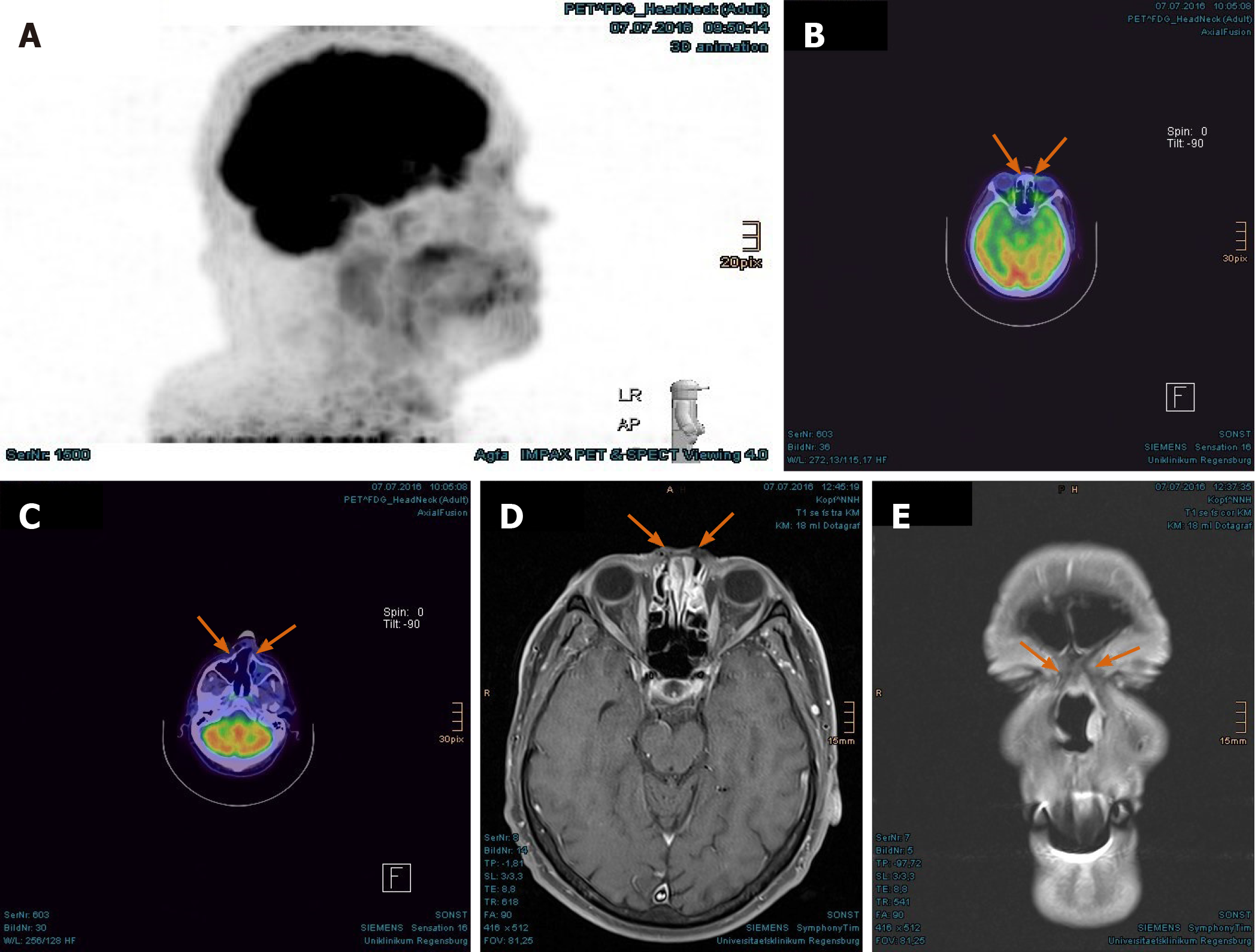
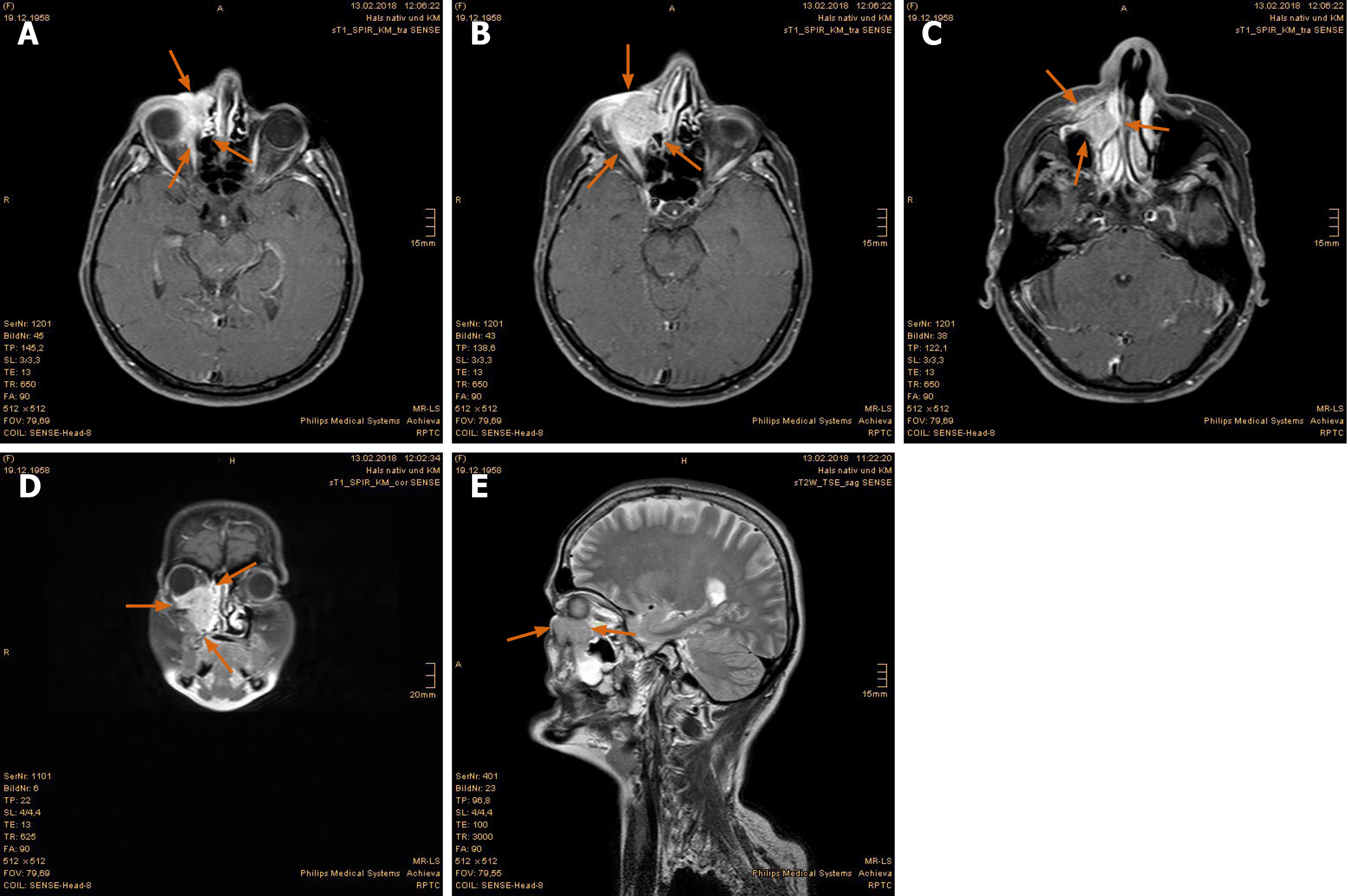
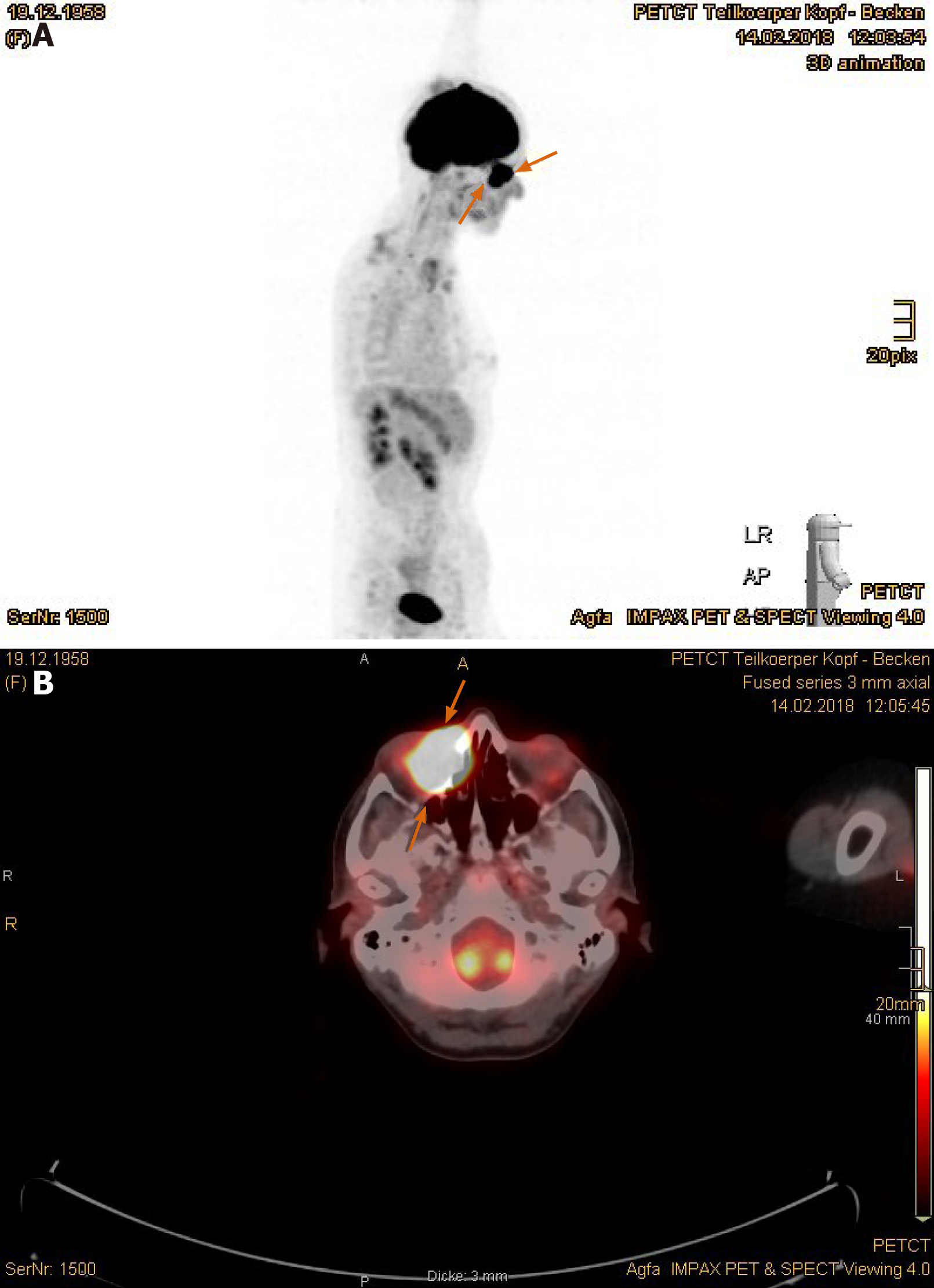
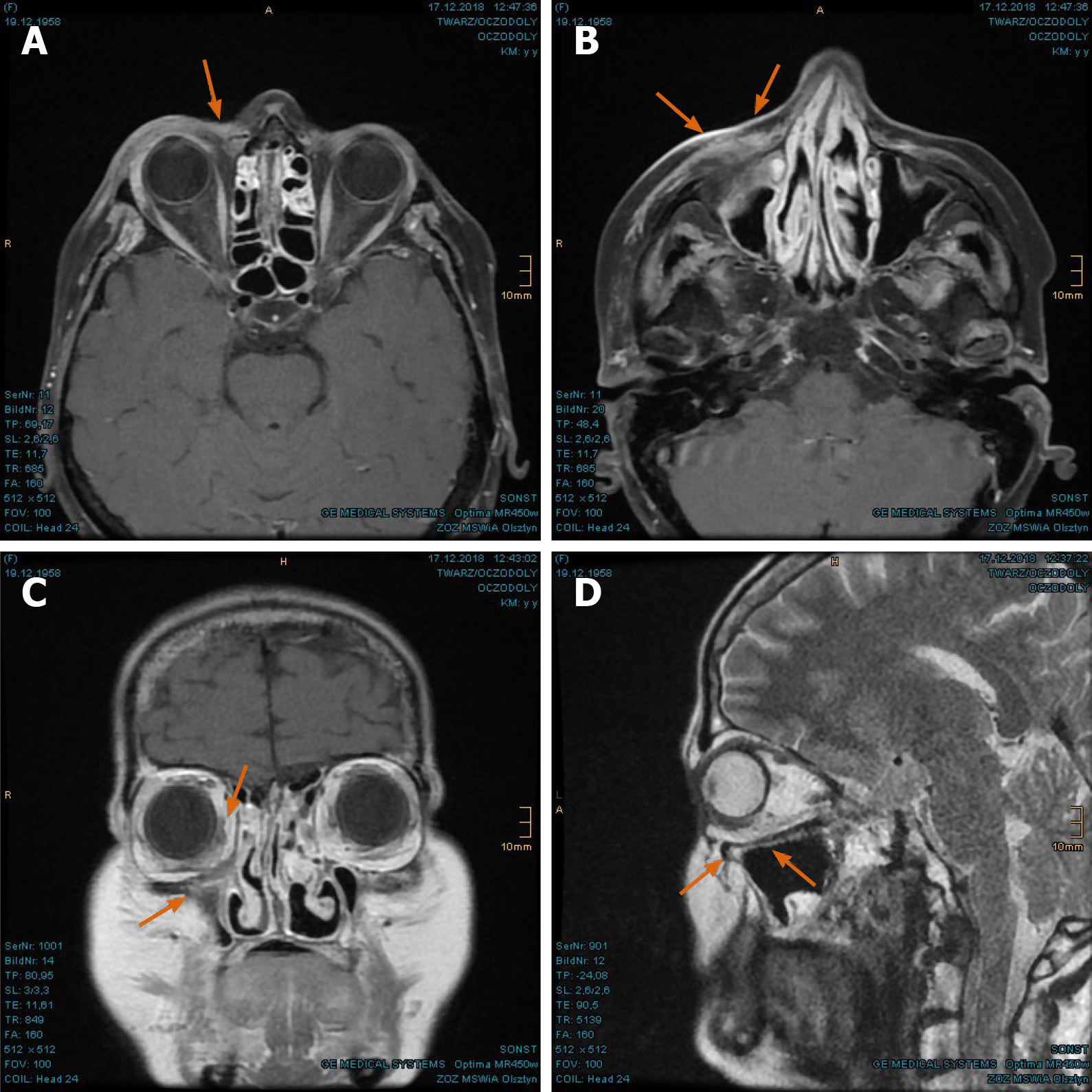
Imaging examinations: The MRI scan in February 2018 demonstrated nodular progress of the naso-orbital tumor up to 3.7 cm × 2.5 cm × 2.5 cm with bony destruction of the right infero-medial eye socket, possible invasion of the muscle cone and shift of the right eye to apico-lateral (Figure 11A and B). Furthermore, the tumor penetrated the neighboring ethmoidal sinus and nasal cavity with involvement of turbinates as well as the antero-medial recess of right maxillary sinus and facial soft tissue in the naso-labial fold and zygomatic area (Figure 11C-E). The 18F-FDG-PET/CT revealed an increased uptake [SUV(max): 12.0] of the multi-compartment SCC in splanchnocranium without definite evidence of metastasis (Figure 12A and B).
Final diagnosis: Periorbital SNSCC, tumor stage cT4a cN0 G2 cM0.
Treatment: The patient has undergone a normofractionated PBT in 33 fractions, from February to April 2018. The informed consent was obtained prior to the initiation of the treatment. The primary tumor manifestation was treated with single dose of 2.10 Gy (RBE) at a total dose of 69.30 Gy (RBE), while the right lymphatic basins including buccofacial, parotideal, retropharyngeal, submandibular and suprajugulary nodal stations received 59.40 Gy (RBE) overall with a single dose of 1.80 Gy (RBE). Under immobilization with head, neck and shoulder mask and vacuum cushion (BlueBAGTM; Medical Intelligence, Schwabmünchen, Germany), the target was irradiated from two gantry angles of 5° and 300° using the pencil beam scanning technique (Figure 13). Because of lack in remarkable change of tumor size (according to weekly-performed low-dose CT scans), adaptation of the treatment plan was not required. Given the aforementioned comorbidity, simultaneous chemotherapy was dismissed by the patient.
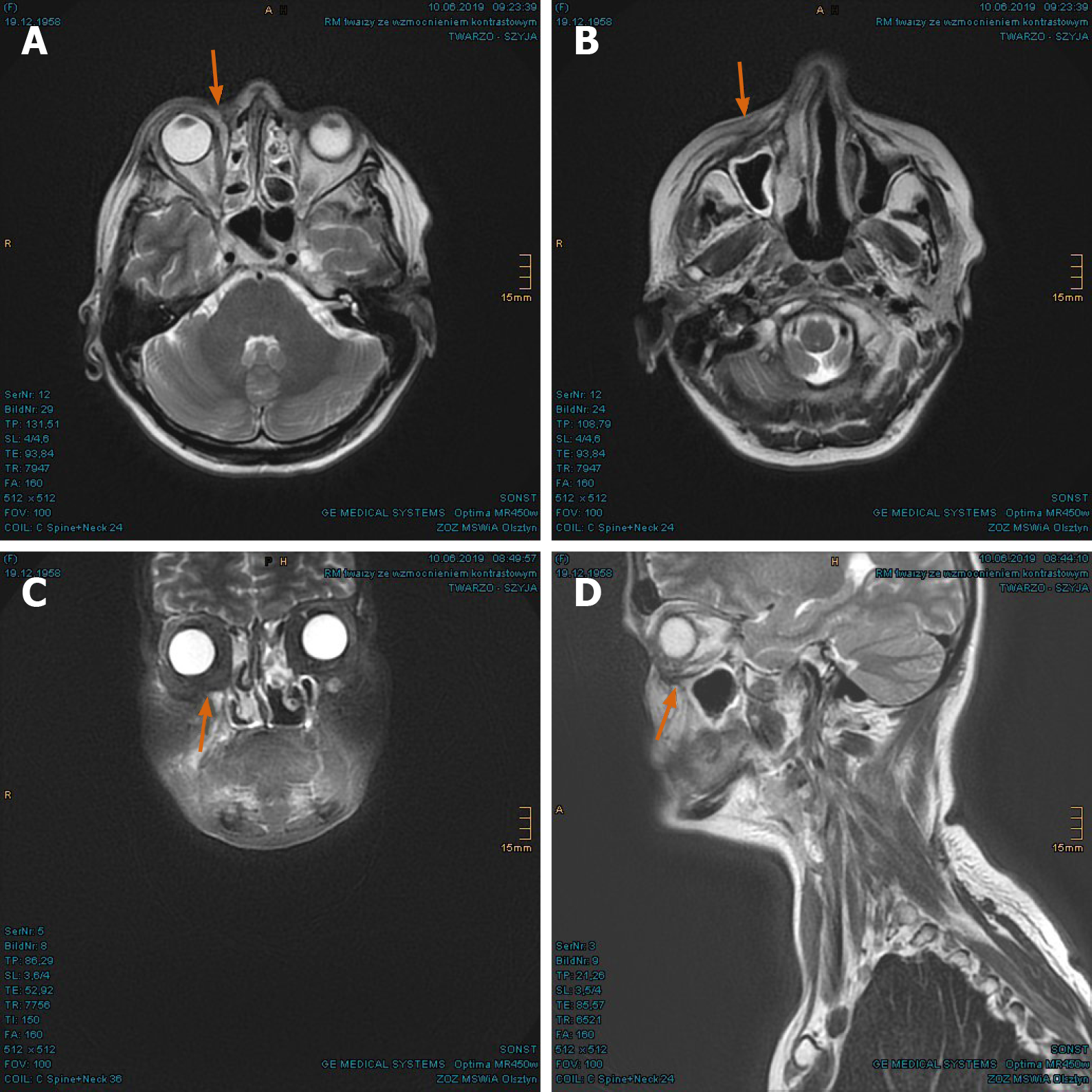
Outcome and follow-up: During the treatment, the patient developed moderate dysphagia, odynophagia, nasal congestions, and conjunctivitis. The greatest effect was painful radiation dermatitis (CTCAE grade 2-3) on the right cheek, extending from the right orbit to the lips. In the first follow-up at 3 mo after PBT, the patient reported significant amelioration of pharyngitis, dermatitis, and swelling of the nasal mucosa. The motility disorders of eye muscle regressed as well. In the first MRI scan in June 2018, the tumor mass was found to have dwindled considerably (Figure 14A-D). The consequent control at 8 mo and 14 mo showed complete tumor remission (Figures 15A-D, and 16A-D). As late toxicities, telangiectasia on the right infraorbital fold and cataract of the right eye were indicated at 18 mo after the PBT (Figure 17). The first was corrected by laser skin treatment, while a cataract surgery is still pending as of the writing of this report. Apart from nasal mucosa dryness, repeated conjunctivitis and right nasolacrimal duct obstruction, the patient is continuously free of tumor recurrence and radiation-related symptoms to date.
SNSCC is not only related to occupational exposures, as mentioned above; in a population-based case-control study, tobacco smoking emerged as a strong risk factor for nasal cancer, with 60% increased risk in ever-smokers and an increment of 6% annually[11]. Smoking also favored malignant transformation and relapse of sinonasal inverted papilloma after surgical resection[12,13]. Similar to pharyngeal and cervical SCC, the impact of human papillomavirus (HPV) on the carcinogenesis and prognosis of SNSCC was investigated progressively. HPV positivity is more common in SCC of nasal cavity and nonkeratinizing SNSCC, yielding improved overall survival[14,15]. With reference to this, both cases presented herein were not attributed to professional exposures, as the HPV status remained unknown because of missing testing at the time of tumor diagnosis. Nevertheless, the patient with recurrent nasal SCC showed long-standing smoking habits, and the other with right periorbital SCC was under long-term immunosuppression with methotrexate, that is significantly associated with various malignancies[16].
SNSCC is typically earmarked by bony destruction of the adjoining sinus walls and oftentimes accompanied by invasion of the orbital wall, infratemporal fossa, skull base and contralateral site, owing to delayed diagnosis. Besides, complex anatomy and diverse normal variants of the sinonasal tract aggravate the identification of tumor origin and extension[17]. In case of tumor invasion of orbit, orbital exenteration - one of the most face-deforming operations - with removal of all the orbital contents, including eyelid and periosteum, is indicated to achieve better survival outcomes[18,19]. Flaps, such as temporoparietal, galeal, free gracilis and free vastus lateralis musculocutaneous flap, are available for the reconstruction of defects; however, they should be employed with special diligence, due to the known comorbidities and postoperative complications[20,21]. In the recent publications, there is a trend of eye-sparing surgery without previously assumed survival disadvantages, especially in combination with adjuvant RT[22-25].
In the past few decades, there have been progressions in endoscopic endonasal surgery, microvascular reconstruction, RT, and systemic therapy. Even though surgery, with or without subsequent RT or CRT, remains the standard regime in most of the cases, Cracchiolo et al[26] pointed out that the choice of therapeutic strategy was influenced by multiple tumor and non-tumor factors, stating apparent deviation from the National Comprehensive Cancer Network guidelines for the treatment of SNSCC. When utilizing a primary surgical approach, constant tumor factors and variable treatment factors, preeminently negative margin resection, were associated with improved survival. Additionally, patients with advanced tumor stage and positive margin resection profited significantly from adjuvant RT or CRT.
Concerning the regional metastases of SNM, levels I, II and III, and retropharyngeal lymphatic basins are frequently involved. Notwithstanding, the accurate assessment of elective nodal treatment in clinically N0 neck is fastidious, with an estimated risk for occult disease of 10%-20% or more. Notably, in tumor stage III-IV of SNSCC, elective neck irradiation should be intended in absence of selective neck dissection[27]. In a retrospective review, Peck et al[28] identified the histologic types of SNM as the most impacting factors in predicting regional metastases, whereas the invasion of adjacent structures like dura, infratemporal fossa, palate and facial soft tissue was associated with increased occurrence of regional metastases. Taking this into account, we had decided to perform an elective nodal irradiation of the ipsilateral neck for both patients, surrendering an effective locoregional control and adequate tolerability.
Because of the rare occurrence and heterogeneous histologic subtypes and primary sites of SNM, there have been no randomized clinical trials to compare the various treatment modalities. In principle, early stage tumor is adequate to be managed with surgery alone, while locally advanced disease requires multimodality approaches. For patients who refuse up-front surgery, a RT-based approach is a legitimate option as well. In view of rapid growth and aggressive local spread of SNSCC to the neighboring organs at risk, such as optic nerves, eye globes, orbitofrontal and temporopolar cortex, as presented in our case report, sufficient local control (LC) is crucial for improved survival. Among SNMs, SCC incidentally seems to submit lower survival rates in comparison to other histologies[29,30]. Novel development of RT technique, above all PBT and carbon ion therapy (CIT), should be generally considered to ameliorate treatment outcomes, to prevent long-term radiation-induced toxicities, and to facilitate organ preservation. Although photon irradiation stays the RT paradigm, more and more particle therapy institutions, mainly in the United States and Japan on account of generous availability, have delivered convincing results in the treatment of SNM[29-34]. In their multi-institutional Proton Collaborative Group registry study, Yu et al[30] reported promising outcomes of 69 patients with SNM treated with PBT predominantly, which was provided as de novo RT or reirradiation in curative intention. Late ≥ grade 3 toxicities, such as vision loss and symptomatic brain necrosis, were not notified.
As recapitulation, the advantages of charged particle therapy are known to be comparatively low entrance dose and minimum exit dose, according to the physical feature of PBT and CIT, the so-called Bragg peak, as well as higher RBE and linear energy transfer than photons, which is utterly relevant for treating radioresistant tumor histologies. Based on the privileged physical and biological characteristics, the sophisticated amendment of dose distribution may provide superior conformality of target coverage with feasible dose escalation. Particularly, locally advanced, unresectable gross tumors may benefit from higher dose regimes. Toyomasu et al[33] reported 3-year/5-year overall survival and LC rates of 56.2%/41.6% and 54.0%/50.4% in the largest retrospective study of SNSCC treated with particle therapy alone. Of the patients, over one-third had unresectable disease, while almost half of the entire cohort obtained 65.0 Gy (RBE) in 26 fractions. Another study dealing with dose-intensified, hyperfractionated PBT to SNM with or without concurrent chemotherapy[29] also showed magnificent 3-year LC rates (of 90% for gross total resection and PBT, 61% for primary PBT, and 59% for patients with gross residual disease). Analogous to our patient in “Case 1”, these patients obtained 1.20 Gy (RBE) twice daily, to a median total dose of 73.80 Gy (RBE). The incidence of ≥ grade 3 late toxicities was 24%, and in another study with CIT of SNM, the high-grade late toxicities occurred in 17% of the cohort[34].
On the other hand, the utility of dose escalation in the former investigations using photons and neutrons was equivocal. Hoppe et al[35] demonstrated improved progression-free survival and overall survival in patients receiving RT dose ≥ 65 Gy, while other studies exhibited poorer survival outcomes as this dose limit was surpassed[36,37]. That might be ascribed to increment of potentially life-threatening dose-related toxicities, like radiation necrosis of temporal lobe and blindness. However, the utilization of pencil beam scanning technique allowing for intensity modulated proton therapy (IMPT, used on our patients presented) may reduce overall toxicities, largely by sparing of adjoining normal tissues, and increase LC, by delivering higher dose to the target[38-39, 41-42]. In the setting of extended ipsilateral orbital invasion as reported in “Case 2”, moderate excess of maximum dose to the right optic nerve [65.30 Gy (RBE)] and right eyeball [70.11 Gy (RBE)] was deliberately permitted due to decreased integral dose of the critical structures by means of IMPT. Herein, the mean doses for the right optic nerve and eyeball were 60.06 Gy (RBE) and 52.36 Gy (RBE) respectively. At a follow-up period of 2 years, severe ocular toxicity was not observed.
Furthermore, unlike CIT, with confined irradiation field size, and aforementioned publications, mostly on the ground of obsolete passive scattering PBT, IMPT using active scanning technique facilitates the implementation of an elective neck irradiation simultaneously at uncertain nodal metastases. Even for manifest nodal disease as our patient in “Case 1”, PBT can be affiliated with an inferior demand of opioids and a reduced rate of gastrostomy tube dependence. In comparison of acute toxicities between PBT and IMRT for nasopharyngeal and sinonasal cancers with comprehensive head and neck irradiation, the mean doses to the oral cavity, esophagus, larynx and parotid glands was significantly lower when utilizing PBT, corresponding to a retrospective study of McDonald et al[43]. To estimate the potential benefit for PBT over IMRT in terms of dose reduction in organs at risk, normal tissue complication probability models may support treatment selection for head and neck cancer patients[39,42].
Both cases with locally advanced periorbital SNSCC treated with PBT alone demonstrate excellent results in view of tumor control and quality of life at a follow-up period of 5 years and 2 years. In general, the therapy regimes of SNM should be managed individually according to histology, tumor stage, prior treatments, personal risk factors, and patient preference. Both multimodality and non-surgical approaches are overdue to be reviewed profoundly in prospective randomized trials. Still, given the dosimetric advantages of PBT, especially in reducing the ocular and brain toxicities for unresectable gross disease, it is somehow unethical to withhold IMPT from the patients on account of random allocation of study design, limited availability of IMPT, lack in clinical experience, and insurance status. A model-based approach on normal tissue complication probability may relieve the selection of suitable patients with clinically significant benefit from PBT.
Manuscript source: Unsolicited manuscript
Specialty type: Oncology
Country/Territory of origin: Germany
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): 0
Grade C (Good): C
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Sridharan G S-Editor: Yan JP L-Editor: A P-Editor: Wang LL
| 1. | Eggesbø HB. Imaging of sinonasal tumours. Cancer Imaging. 2012;12:136-152. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 82] [Cited by in RCA: 84] [Article Influence: 6.5] [Reference Citation Analysis (0)] |
| 2. | Bhattacharyya N. Factors affecting survival in maxillary sinus cancer. J Oral Maxillofac Surg. 2003;61:1016-1021. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 71] [Cited by in RCA: 75] [Article Influence: 3.4] [Reference Citation Analysis (0)] |
| 3. | Jain S, Li Y, Kuan EC, Tajudeen BA, Batra PS. Prognostic Factors in Paranasal Sinus Squamous Cell Carcinoma and Adenocarcinoma: A SEER Database Analysis. J Neurol Surg B Skull Base. 2019;80:258-263. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 21] [Cited by in RCA: 26] [Article Influence: 3.7] [Reference Citation Analysis (0)] |
| 4. | Thorup C, Sebbesen L, Danø H, Leetmaa M, Andersen M, Buchwald C, Kristensen CA, Bentzen J, Godballe C, Johansen J, Grau C. Carcinoma of the nasal cavity and paranasal sinuses in Denmark 1995-2004. Acta Oncol. 2010;49:389-394. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 61] [Cited by in RCA: 66] [Article Influence: 4.4] [Reference Citation Analysis (0)] |
| 5. | d'Errico A, Pasian S, Baratti A, Zanelli R, Alfonzo S, Gilardi L, Beatrice F, Bena A, Costa G. A case-control study on occupational risk factors for sino-nasal cancer. Occup Environ Med. 2009;66:448-455. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 56] [Cited by in RCA: 58] [Article Influence: 3.6] [Reference Citation Analysis (0)] |
| 6. | Binazzi A, Ferrante P, Marinaccio A. Occupational exposure and sinonasal cancer: a systematic review and meta-analysis. BMC Cancer. 2015;15:49. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 69] [Cited by in RCA: 98] [Article Influence: 9.8] [Reference Citation Analysis (0)] |
| 7. | Robin TP, Jones BL, Gordon OM, Phan A, Abbott D, McDermott JD, Goddard JA, Raben D, Lanning RM, Karam SD. A comprehensive comparative analysis of treatment modalities for sinonasal malignancies. Cancer. 2017;123:3040-3049. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 93] [Cited by in RCA: 131] [Article Influence: 16.4] [Reference Citation Analysis (0)] |
| 8. | Danesh-Sani SA, Sarafraz A, Chamani M, Derakhshandeh H. Paranasal sinuses malignancies: A 12-year review of clinical characteristics. Med Oral Patol Oral Cir Bucal. 2016;21:e626-e630. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 5] [Cited by in RCA: 8] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 9. | Chen NX, Chen L, Wang JL, Wang JY, Yan F, Ma L, Zhang XX. A clinical study of multimodal treatment for orbital organ preservation in locally advanced squamous cell carcinoma of the nasal cavity and paranasal sinus. Jpn J Clin Oncol. 2016;46:727-734. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 16] [Cited by in RCA: 16] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 10. | Herzog M. [Tumors of the paranasal sinus invading the orbit]. HNO. 2018;66:730-742. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 6] [Cited by in RCA: 5] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 11. | Greiser EM, Greiser KH, Ahrens W, Hagen R, Lazszig R, Maier H, Schick B, Zenner HP. Risk factors for nasal malignancies in German men: the South-German Nasal cancer study. BMC Cancer. 2012;12:506. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 14] [Cited by in RCA: 16] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 12. | Hong SL, Kim BH, Lee JH, Cho KS, Roh HJ. Smoking and malignancy in sinonasal inverted papilloma. Laryngoscope. 2013;123:1087-1091. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 31] [Cited by in RCA: 32] [Article Influence: 2.7] [Reference Citation Analysis (0)] |
| 13. | Moon IJ, Lee DY, Suh MW, Han DH, Kim ST, Min YG, Lee CH, Rhee CS. Cigarette smoking increases risk of recurrence for sinonasal inverted papilloma. Am J Rhinol Allergy. 2010;24:325-329. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 33] [Cited by in RCA: 30] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 14. | Kılıç S, Kılıç SS, Kim ES, Baredes S, Mahmoud O, Gray ST, Eloy JA. Significance of human papillomavirus positivity in sinonasal squamous cell carcinoma. Int Forum Allergy Rhinol. 2017;7:980-989. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 38] [Cited by in RCA: 39] [Article Influence: 4.9] [Reference Citation Analysis (0)] |
| 15. | Oliver JR, Lieberman SM, Tam MM, Liu CZ, Li Z, Hu KS, Morris LGT, Givi B. Human papillomavirus and survival of patients with sinonasal squamous cell carcinoma. Cancer. 2020;126:1413-1423. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 29] [Cited by in RCA: 47] [Article Influence: 7.8] [Reference Citation Analysis (0)] |
| 16. | Inose R, Hashimoto N, Hosomi K, Yokoyama S, Takada M. Association between malignancy and methotrexate and biological disease-modifying antirheumatic drugs in patients with rheumatoid arthritis. Int J Clin Pharmacol Ther. 2020;58:131-138. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 9] [Cited by in RCA: 16] [Article Influence: 3.2] [Reference Citation Analysis (0)] |
| 17. | Kawaguchi M, Kato H, Tomita H, Mizuta K, Aoki M, Hara A, Matsuo M. Imaging Characteristics of Malignant Sinonasal Tumors. J Clin Med. 2017;6:116. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 38] [Cited by in RCA: 70] [Article Influence: 8.8] [Reference Citation Analysis (0)] |
| 18. | Hoffman GR, Jefferson ND, Reid CB, Eisenberg RL. Orbital Exenteration to Manage Infiltrative Sinonasal, Orbital Adnexal, and Cutaneous Malignancies Provides Acceptable Survival Outcomes: An Institutional Review, Literature Review, and Meta-Analysis. J Oral Maxillofac Surg. 2016;74:631-643. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 27] [Cited by in RCA: 27] [Article Influence: 2.7] [Reference Citation Analysis (0)] |
| 19. | Safi AF, Behn L, Rothamel D, Guntinas-Lichius O, Beutner D, Nickenig HJ, Zöller J, Kreppel M. Therapy of sinonasal malignancies invading the orbit-orbital exenteration versus preservation of the orbit plus radiotherapy. J Craniomaxillofac Surg. 2017;45:258-261. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 21] [Cited by in RCA: 19] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 20. | Nassab RS, Thomas SS, Murray D. Orbital exenteration for advanced periorbital skin cancers: 20 years experience. J Plast Reconstr Aesthet Surg. 2007;60:1103-1109. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 60] [Cited by in RCA: 60] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 21. | Karabekmez FE, Selimoglu MN, Duymaz A, Karamese MS, Keskin M, Savaci N. Management of neglected periorbital squamous cell carcinoma requiring orbital exenteration. J Craniofac Surg. 2014;25:729-734. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 12] [Cited by in RCA: 17] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 22. | Skinner HD, Garden AS, Rosenthal DI, Ang KK, Morrison WH, Esmaeli B, Pinnix CC, Frank SJ. Outcomes of malignant tumors of the lacrimal apparatus: the University of Texas MD Anderson Cancer Center experience. Cancer. 2011;117:2801-2810. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 47] [Cited by in RCA: 57] [Article Influence: 4.1] [Reference Citation Analysis (0)] |
| 23. | Lisan Q, Kolb F, Temam S, Tao Y, Janot F, Moya-Plana A. Management of orbital invasion in sinonasal malignancies. Head Neck. 2016;38:1650-1656. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 33] [Cited by in RCA: 45] [Article Influence: 5.0] [Reference Citation Analysis (0)] |
| 24. | Hu W, Hu J, Gao J, Yang J, Qiu X, Kong L, Lu JJ. Outcomes of orbital malignancies treated with eye-sparing surgery and adjuvant particle radiotherapy: a retrospective study. BMC Cancer. 2019;19:776. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 10] [Cited by in RCA: 13] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 25. | Li R, Tian S, Zhu Y, Zhu W, Wang S. Management of orbital invasion in sinonasal squamous cell carcinoma: 15 years' experience. Int Forum Allergy Rhinol. 2020;10:243-255. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 10] [Cited by in RCA: 13] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 26. | Cracchiolo JR, Patel K, Migliacci JC, Morris LT, Ganly I, Roman BR, McBride SM, Tabar VS, Cohen MA. Factors associated with a primary surgical approach for sinonasal squamous cell carcinoma. J Surg Oncol. 2018;117:756-764. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 24] [Cited by in RCA: 28] [Article Influence: 3.5] [Reference Citation Analysis (0)] |
| 27. | Castelnau-Marchand P, Levy A, Moya-Plana A, Mirghani H, Nguyen F, Del Campo ER, Janot F, Kolb F, Ferrand FR, Temam S, Blanchard P, Tao Y. Sinonasal squamous cell carcinoma without clinical lymph node involvement : Which neck management is best? Strahlenther Onkol. 2016;192:537-544. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 7] [Cited by in RCA: 14] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 28. | Peck BW, Van Abel KM, Moore EJ, Price DL. Rates and Locations of Regional Metastases in Sinonasal Malignancies: The Mayo Clinic Experience. J Neurol Surg B Skull Base. 2018;79:282-288. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 11] [Cited by in RCA: 19] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 29. | Dagan R, Bryant C, Li Z, Yeung D, Justice J, Dzieglewiski P, Werning J, Fernandes R, Pirgousis P, Lanza DC, Morris CG, Mendenhall WM. Outcomes of Sinonasal Cancer Treated With Proton Therapy. Int J Radiat Oncol Biol Phys. 2016;95:377-385. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 49] [Cited by in RCA: 56] [Article Influence: 6.2] [Reference Citation Analysis (0)] |
| 30. | Yu NY, Gamez ME, Hartsell WF, Tsai HK, Laramore GE, Larson GL, Simone CB 2nd, Rossi C, Katz SR, Buras MR, Golafshar MA, Vargas CE, Patel SH. A Multi-Institutional Experience of Proton Beam Therapy for Sinonasal Tumors. Adv Radiat Oncol. 2019;4:689-698. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 24] [Cited by in RCA: 31] [Article Influence: 5.2] [Reference Citation Analysis (0)] |
| 31. | Patel SH, Wang Z, Wong WW, Murad MH, Buckey CR, Mohammed K, Alahdab F, Altayar O, Nabhan M, Schild SE, Foote RL. Charged particle therapy versus photon therapy for paranasal sinus and nasal cavity malignant diseases: a systematic review and meta-analysis. Lancet Oncol. 2014;15:1027-1038. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 150] [Cited by in RCA: 172] [Article Influence: 15.6] [Reference Citation Analysis (0)] |
| 32. | Russo AL, Adams JA, Weyman EA, Busse PM, Goldberg SI, Varvares M, Deschler DD, Lin DT, Delaney TF, Chan AW. Long-Term Outcomes After Proton Beam Therapy for Sinonasal Squamous Cell Carcinoma. Int J Radiat Oncol Biol Phys. 2016;95:368-376. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 47] [Cited by in RCA: 58] [Article Influence: 6.4] [Reference Citation Analysis (0)] |
| 33. | Toyomasu Y, Demizu Y, Matsuo Y, Sulaiman NS, Mima M, Nagano F, Terashima K, Tokumaru S, Hayakawa T, Daimon T, Fuwa N, Sakuma H, Nomoto Y, Okimoto T. Outcomes of Patients With Sinonasal Squamous Cell Carcinoma Treated With Particle Therapy Using Protons or Carbon Ions. Int J Radiat Oncol Biol Phys. 2018;101:1096-1103. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 13] [Cited by in RCA: 18] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 34. | Koto M, Demizu Y, Saitoh JI, Suefuji H, Tsuji H, Okimoto T, Ohno T, Shioyama Y, Ikawa H, Nemoto K, Nakano T, Kamada T; Japan Carbon-Ion Radiation Oncology Study Group. Definitive Carbon-Ion Radiation Therapy for Locally Advanced Sinonasal Malignant Tumors: Subgroup Analysis of a Multicenter Study by the Japan Carbon-Ion Radiation Oncology Study Group (J-CROS). Int J Radiat Oncol Biol Phys. 2018;102:353-361. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 36] [Cited by in RCA: 42] [Article Influence: 6.0] [Reference Citation Analysis (0)] |
| 35. | Hoppe BS, Stegman LD, Zelefsky MJ, Rosenzweig KE, Wolden SL, Patel SG, Shah JP, Kraus DH, Lee NY. Treatment of nasal cavity and paranasal sinus cancer with modern radiotherapy techniques in the postoperative setting--the MSKCC experience. Int J Radiat Oncol Biol Phys. 2007;67:691-702. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 173] [Cited by in RCA: 167] [Article Influence: 8.8] [Reference Citation Analysis (0)] |
| 36. | Jansen EP, Keus RB, Hilgers FJ, Haas RL, Tan IB, Bartelink H. Does the combination of radiotherapy and debulking surgery favor survival in paranasal sinus carcinoma? Int J Radiat Oncol Biol Phys. 2000;48:27-35. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 97] [Cited by in RCA: 82] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 37. | Chopra S, Kamdar DP, Cohen DS, Heilbrun LK, Smith D, Kim H, Lin HS, Jacobs JR, Yoo G. Outcomes of nonsurgical management of locally advanced carcinomas of the sinonasal cavity. Laryngoscope. 2017;127:855-861. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 26] [Cited by in RCA: 26] [Article Influence: 2.9] [Reference Citation Analysis (0)] |
| 38. | McKeever MR, Sio TT, Gunn GB, Holliday EB, Blanchard P, Kies MS, Weber RS, Frank SJ. Reduced acute toxicity and improved efficacy from intensity-modulated proton therapy (IMPT) for the management of head and neck cancer. Chin Clin Oncol. 2016;5:54. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 19] [Cited by in RCA: 25] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 39. | Blanchard P, Wong AJ, Gunn GB, Garden AS, Mohamed ASR, Rosenthal DI, Crutison J, Wu R, Zhang X, Zhu XR, Mohan R, Amin MV, Fuller CD, Frank SJ. Toward a model-based patient selection strategy for proton therapy: External validation of photon-derived normal tissue complication probability models in a head and neck proton therapy cohort. Radiother Oncol. 2016;121:381-386. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 78] [Cited by in RCA: 80] [Article Influence: 8.9] [Reference Citation Analysis (0)] |
| 40. | Gassner H, Sadick H, Haubner F, Artinger V, Kuehnel T. Prelamination to reconstruct internal nasal lining. Facial Plast Surg. 2013;29:411-416. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5] [Cited by in RCA: 7] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 41. | Mimica X, Yu Y, McGill M, Barker CA, McBride S, Ganly I, Cracchiolo JR, Dunn LA, Katabi N, Sine K, Mah D, Lee A, Lee N, Cohen MA. Organ preservation for patients with anterior mucosal squamous cell carcinoma of the nasal cavity: Rhinectomy-free survival in those refusing surgery. Head Neck. 2019;41:2741-2747. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4] [Cited by in RCA: 7] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 42. | Blanchard P, Gunn GB, Lin A, Foote RL, Lee NY, Frank SJ. Proton Therapy for Head and Neck Cancers. Semin Radiat Oncol. 2018;28:53-63. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 64] [Cited by in RCA: 76] [Article Influence: 10.9] [Reference Citation Analysis (0)] |
| 43. | McDonald MW, Liu Y, Moore MG, Johnstone PA. Acute toxicity in comprehensive head and neck radiation for nasopharynx and paranasal sinus cancers: cohort comparison of 3D conformal proton therapy and intensity modulated radiation therapy. Radiat Oncol. 2016;11:32. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 46] [Cited by in RCA: 59] [Article Influence: 6.6] [Reference Citation Analysis (0)] |