Published online Aug 24, 2020. doi: 10.5306/wjco.v11.i8.644
Peer-review started: January 17, 2020
First decision: March 5, 2020
Revised: May 21, 2020
Accepted: June 27, 2020
Article in press: June 27, 2020
Published online: August 24, 2020
Processing time: 212 Days and 3.9 Hours
The presence of renal cell carcinoma (RCC) and hematologic malignancies (HM) in the same patient is rarely observed. Three primary findings have been described in these patients, including male gender and lymphoid malignancy predominance, and the HM are usually diagnosed before or simultaneously with the RCC. There is a lack of evidence about clinical outcomes in this setting. We report the common characteristics of 9 patients diagnosed with concurrent RCC and HM and their clinical course and response to treatment.
Four (44%) patients were diagnosed with RCC prior to the HM, the diagnosis was simultaneous in 4 (44%) patients, and 1 (11%) patient was diagnosed with the HM prior to the RCC. No patients were treated with cytotoxic chemotherapy or radiation between the diagnosis of RCC and HM. Several unique features were seen in our case series, such as 3 simultaneous cancers in 1 (11%) patient, a splenectomy leading to remission of diffuse large B cell lymphoma without the use of chemotherapy in 1 (11%) patient, chemotherapy and rituximab for lymphoma resulting in a complete response in primary RCC in 1 (11%) patient, and immunotherapy providing an excellent response for primary renal leiomyosarcoma in 1 (11%) patient.
These findings highlight the potential role of immune system dysregulation in patients with the diagnosis of RCC and HM whereby the first malignancy predisposes to the second through an immunomodulatory effect. HM have the potential of being confused with lymph node metastasis from kidney cancer. Lymph node biopsy may be necessary at the time of initial diagnosis or in cases of mixed response to therapy. Long-term medical surveillance is warranted when a patient is diagnosed with RCC or HM. Clinicians should be aware of the higher prevalence of male gender and lymphoid malignancy with concurrent RCC and HM and that either of these conditions may be diagnosed first or they may be diagnosed simultaneously.
Core tip: Renal cell carcinoma (RCC) and hematologic malignancies (HM) in the same patient is rare. We report the common characteristics of 9 patients diagnosed with concurrent RCC and HM and their clinical course and treatment response. None of the patients was treated with cytotoxic chemotherapy or radiation between the diagnosis of RCC and HM. Several features in our series strengthen the immune dysregulation theory as the likely mechanism. Long-term medical surveillance is warranted when a patient is diagnosed with RCC or HM. Clinicians should be aware that either of these conditions may be diagnosed first or they may be diagnosed simultaneously.
- Citation: Shields LB, Kalebasty AR. Concurrent renal cell carcinoma and hematologic malignancies: Nine case reports. World J Clin Oncol 2020; 11(8): 644-654
- URL: https://www.wjgnet.com/2218-4333/full/v11/i8/644.htm
- DOI: https://dx.doi.org/10.5306/wjco.v11.i8.644
Renal cell carcinoma (RCC) and hematologic malignancies (HM) coexisting in a single patient at the same time is a rare phenomenon. RCC is observed in the general population in 12.5 persons per 100000 and HM in 31.8 per 100000[1]. The incidence of RCC and HM occurring in same patient is greater than that expected in the general population[1,2]. A higher than expected incidence of RCC concurrent with non-Hodgkin’s lymphoma (NHL) and multiple myeloma (MM) has also been reported[2-5]. Epidemiological studies have shown that the observed-to-expected ratio for occurrence of RCC in NHL patients were 1.86 to 2.67[2,3]. Furthermore, it has been reported that patients with the diagnosis of NHL have an increased incidence of RCC, bladder carcinoma, lung carcinoma, brain tumors, and melanoma as well as other HM such as acute myeloid leukemia and HL[6,7]. Three predominant features have been described in these patients: (1) Greater number of males; (2) The majority of HM are lymphoid; and (3) The HM are usually diagnosed prior to or simultaneously with the RCC[8].
Several mechanisms have been proposed to account for concurrent RCC and HM, including genetic mutations common to both RCC and HM, hormonal or environmental factors, viral infections, prior cytotoxic chemotherapy or radiation for malignancy, and immune dysregulation[2,3,5,8-12]. It has been suggested that the first malignancy predisposes to the second through an immunomodulatory effect[1-4]. In this respect, immune dysregulation generates the lymphoma which subsequently leads to the development of solid tumors such as RCC. The surveillance for malignancy and immune dysregulation resulting from immune checkpoint inhibitors in these cases are only theories, and the exact mechanisms are unknown.
In this study, we reviewed the medical records and imaging studies of 9 consecutive individuals who were diagnosed with both RCC and HM and evaluated by the same medical oncologist between June 1, 2013 and December 31, 2019 at our Institution (Table 1). Common characteristics of patients with these 2 conditions are presented, and the potential etiology of simultaneous RCC and HM are discussed. We highlight several distinct features of our cases that strengthen the immune dysregulation theory as the most likely mechanism.
| Case | Age at RCC | Grade/Stage/Histology RCC | RCC treatment | Hematologic malignancy | Duration between RCC and HM diagnoses | Treatment for HM |
| 1 | 68 | Grade 2 PRCC with oncocytic features; clinical Stage 1 | None | Grade 2 Stage 4 follicular lymphoma | RCC 10 wk before LM | (1) Bendamustine/rituximab; (2) Follow up 49 mo after kidney biopsy; and (3) Still alive with both malignancies in remission |
| 2 | 70 | Stage 3 Grade 4 CCRCC | Radical nephrectomy | Stage 4 mantle cell lymphoma | Simultaneous | (1) Bendamustine/rituximab; imbruvica; bortezomib; rituximab/lenalidomide; and (2) Acute kidney injury, died 54 mo after nephrectomy as result of mantle cell lymphoma; CCRCC in remission |
| 3 | 55 | Stage 1 Grade 2 CCRCC | Radical nephrectomy | Multiple myeloma | RCC 7 wk before LM | (1) Bortezomib/cyclophosphamide/dexamethasone; (2) Autotransplant; (3) Lanelidomide; pazopanib; cabozantinib; nivolumab/ipilimumab; axitinib; lenvatinib/everolimus; atezolizumab/bevacizumab; and (4) Died 70 mo after nephrectomy due to metastatic CCRCC |
| 4 | 62 | Stage 1 Grade 3 CCRCC | Radical nephrectomy | Stage 3 Grade 3 follicular lymphoma | Simultaneous | (1) Cyclophosphamide/doxorubicin hydrochloride/ palonosetron/rituximab/vincristine (CHOP); rituximab; pazopanib, cabozantinib; and (2) Died 64 mo after nephrectomy and lymph node dissection of progressive metastatic CCRCC |
| 5 | 58 | Stage 1 Unclassified RCC | Partial nephrectomy | Stage 4 non-sclerosing Hodgkin’s lymphoma | RCC 109 mo before LM | (1) Brentuximab vedotin/doxorubicin/vinblastine/dacarbazine (AAVD); and (2) Follow up 23 mo after lymph node biopsy; (3) Still alive with no evidence of recurrent lymphoma or RCC |
| 6 | 55 | Stage 1 CCRCC | Partial nephrectomy | Stage 1E diffuse large B-cell lymphoma | CCRCC 75 mo before LM | (1) No cytotoxic chemotherapy; (2) Splenectomy; (3) Follow up 112 mo after nephrectomy and 37 mo after splenectomy; and (4) Still alive with no evidence of recurrent lymphoma or CCRCC |
| 7 | 59 | Clinical Stage 1 RCC | None | Low-grade follicular center cell lymphoma of skin | LM 9 wk before RCC | (1) No cytotoxic chemotherapy; (2) Excision of skin lymphoma; (3) Follow up 17 mo after kidney biopsy confirming lymphoma; and (4) Kidney CA on surveillance and no recurrent lymphoma |
| 8 | 71 | Stage 4 Grade 2 leiomyosarcoma of the kidney | Kidney biopsy; radical nephrectomy 9 mo later | B cell lymphoma Stage 1E of the kidney | Simultaneous | (1) Gemcitabine/docetaxel anhydrous; ipilimumab/nivolumab; (2) Follow up 11 mo after biopsy confirming leiomyosarcoma of the kidney; and (3) Still alive with continued response of metastatic kidney cancer on check point inhibitor; no treatment for lymphoma |
| 9 | 77 | Clinical Stage 1 RCC | None | Diffuse large B-cell lymphoma subtype activated B-cell, Stage 2E | Simultaneous | (1) Rituximab/cyclophosphamide/etoposide/vincristine/prednisone; (2) Follow up 12 mo after kidney biopsy confirming lymphoma; and (3) Still alive with no evidence of recurrent RCC or lymphoma |
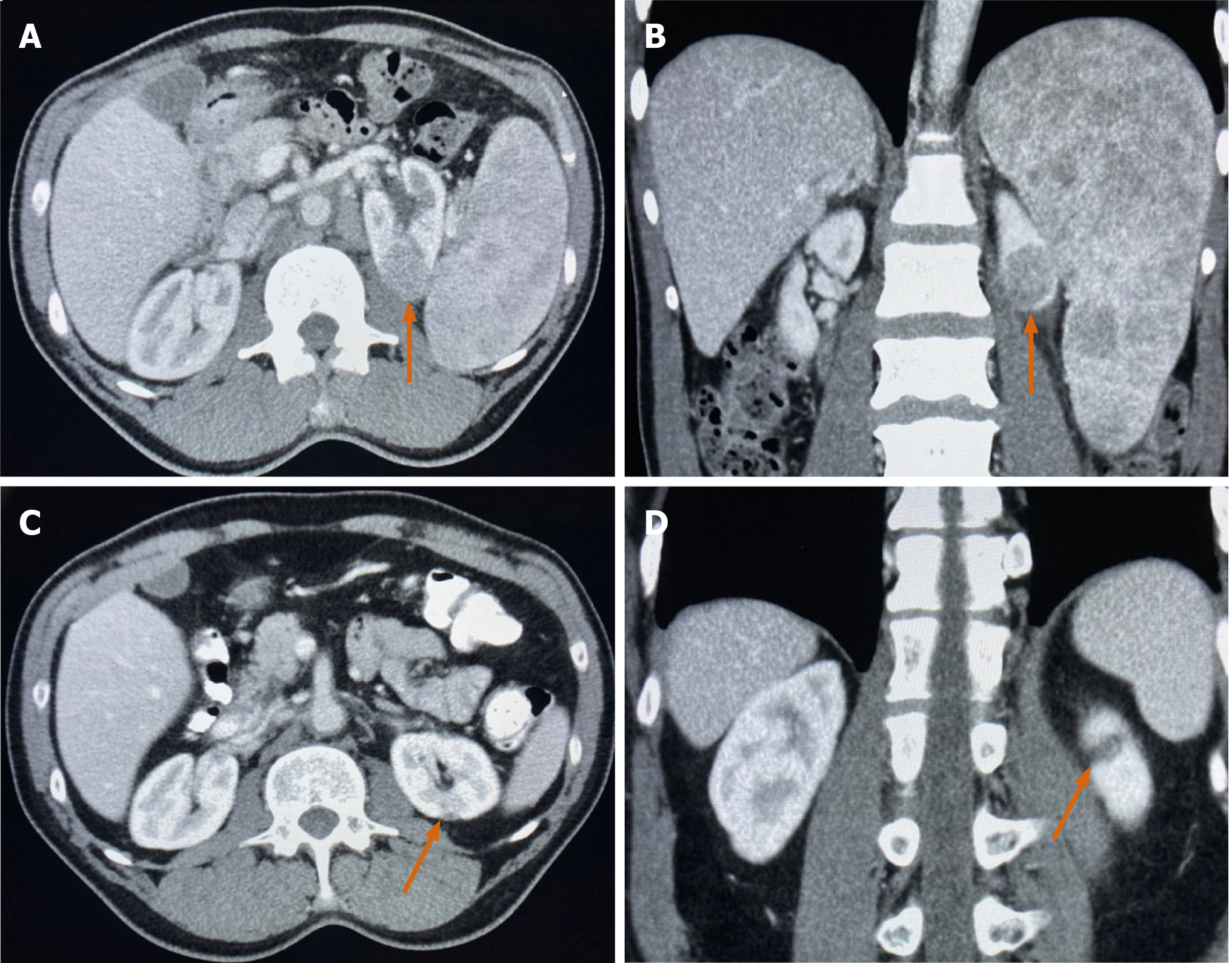
A 68-year-old man underwent a computed tomography (CT) of the chest, abdomen, and pelvis with Gadolinium contrast which revealed a solid and enhancing hypovascular renal mass, axillary adenopathy, splenomegaly with multiple splenic lesions, and spinal bone involvement (Figure 1A and B).
A 70-year-old man underwent a CT of the abdomen and pelvis with Gadolinium contrast which demonstrated a large mass protruding from the lower pole of the left kidney measuring 10.0 cm × 8.2 cm with enlarged lymph nodes in the left common and external iliac groups and left inguinal area.
A 55-year-old man underwent a CT of the abdomen and pelvis with Gadolinium contrast which revealed a 7.0 cm × 5.0 cm left renal mass with bony metastasis of the left acetabulum and superior pubic ramus.
A 62-year-old man underwent a CT of the abdomen and pelvis with Gadolinium contrast that demonstrated a 4.2 cm × 3.9 cm mass arising from the posterior right renal cortex and a 1.9 cm retroperitoneal/paraaortic lymph node.
A 58-year-old man was diagnosed with Stage 1 unclassified RCC after undergoing a partial nephrectomy. The patient was monitored by CT scan surveillance. A CT of the chest, abdomen, and pelvis performed 105 mo after the partial nephrectomy revealed anterior mediastinal axillary lymphadenopathy (LAD).
A 55-year-old man was diagnosed with Stage 1 clear cell RCC (CCRCC) following a partial nephrectomy. The patient was observed by surveillance CT scans. A CT scan performed 75 mo following the partial nephrectomy demonstrated a 5.0 cm splenic mass originally suspected of being metastatic kidney cancer.
A 59-year-old man underwent a CT of the chest, abdomen, and pelvis with Gadolinium contrast which revealed a 15.0 mm solid-appearing enhancing lesion at the superior right kidney.
A 71-year-old man, with a family history of leukemia in his father, underwent a CT of the chest, abdomen, and pelvis with Gadolinium contrast demonstrated a 5.9 cm × 9.2 cm renal mass with lytic bone lesions. A lumbar magnetic resonance imaging (MRI) with Gadolinium contrast showed multiple T1 hypointense T2 hyperintense enhancing lesions of the lower thoracic and lumbar spine, upper sacrum, and superior medial iliac bones with the largest in the L3 lumbar vertebrae.
A 77-year-old man underwent a CT of the abdomen and pelvis with Gadolinium contrast which revealed evidence of a 12.0 cm left upper pole mass with a separate 5.0 cm left lower pole mass with invasion of the spleen and surrounding lymphadenopathy.
A biopsy of a right inguinal lymph node confirmed Grade 2 Stage 4 follicular lymphoma.
The patient was diagnosed with simultaneous Stage 3 Grade 4 CCRCC and Stage 4 mantle cell lymphoma following a radical nephrectomy and retroperitoneal lymph node dissection (LND). Immunohistochemistry confirmed the mantle cell lymphoma in the retroperitoneal lymph node.
The patient was diagnosed with Stage 1 Grade 2 CCRCC following a nephrectomy. A gastric resection was also performed during the nephrectomy which revealed a gastrointestinal stromal tumor (GIST). Biopsy of a lytic bone lesion suspected of metastatic kidney cancer revealed a diagnosis of MM.
The patient was diagnosed with simultaneous Stage 1 Grade 3 CCRCC and Stage 3 Grade 3 follicular lymphoma following a radical nephrectomy and dissection of interaortocaval lymph nodes.
A lymph node biopsy confirmed Stage 4 non-sclerosing HL.
The patient underwent a splenectomy that revealed a Stage 1E diffuse large B-cell lymphoma in the spleen.
The patient underwent an excisional biopsy of the skin of the right arm that revealed a low-grade follicular center cell lymphoma. Nine weeks later an abdominal MRI demonstrated a solid enhancing mass suggestive of RCC (clinical Stage 1).
The patient was diagnosed with simultaneous Stage 4 Grade 2 leiomyosarcoma of the kidney and Stage 1E B cell lymphoma of the kidney by renal biopsy.
The patient was diagnosed with simultaneous clinical Stage 1 RCC and Stage 2E diffuse large B-cell lymphoma subtype activated B-cell with involvement of the retroperitoneal lymph nodes. A kidney biopsy confirmed the diagnosis of lymphoma.
The patient was treated with 6 cycles of bendamustine/rituximab which resulted in a complete response of his lymphoma. Interestingly, his primary kidney mass resolved without any further intervention after treating his lymphoma (Figure 1C and D).
The patient underwent 13 cycles of bendamustine/rituximab following which a pulmonary metastatic nodule was detected on chest CT, reflecting a progression-free survival (PFS) of 37 mo. He was subsequently treated with 2 cycles of imbruvica and 6 cycles of bortezomib after which progressive disease with cervical and LAD was observed on CT (7 mo PFS). He then had 2 cycles of rituximab/lenalidomide.
The patient underwent 4 cycles of bortezomib/cyclophosphamide/dexamethasone followed by an autotransplant. He subsequently underwent 27 cycles of maintenance lanelidomide after which a rib lesion was confirmed as RCC by chest CT (PFS 26 mo). He then had 11 cycles of pazopanib following which there was evidence of another metastatic rib lesion and LAD (PFS 11 mo). The patient underwent 5 cycles cabozantinib and 3 cycles nivolumab/ipilimumab with LAD detected 3 mo later. He was then treated with 4 cycles axitinib with subsequent pulmonary metastatic nodules and LAD by CT (PFS 4 mo). Following 2 cycles of lenvatinib/everolimus, a hepatic metastatic lesion was noted (PFS 3 mo). The patient then underwent 1 cycle of atezolizumab/bevacizumab.
The patient underwent 6 cycles of cyclophosphamide, doxorubicin hydrochloride, vincristine, prednisone, and rituximab followed by 3 cycles of maintenance rituximab. A lytic rib lesion was subsequently confirmed as RCC (PFS 14 mo). He was treated with 18 cycles of pazopanib. A T9 vertebral body lesion was subsequently detected (PFS 23 mo), and pulmonary metastatic nodules and LAD were observed 9 mo later on CT. He was treated with cabozantinib with poor tolerance. Immunotherapy with check point inhibitors was not offered due to active seronegative rheumatoid arthritis.
The patient underwent 6 cycles of brentuximab vedotin, doxorubicin, vinblastine, and dacarbazine.
The patient did not undergo any further systemic treatment for his lymphoma.
The decision was made to continue monitoring the kidney mass rather than performing a partial nephrectomy. The patient had radiation to the skin lesion after the excisional biopsy without receiving any systemic therapy.
The patient underwent 3 cycles of gemcitabine/docetaxel after which a CT detected a spinal lesion confirmed as progression of osseous metastasis (PFS 3 mo). The patient was then treated with 4 cycles of ipilimumab/nivolumab. Progression was noted in the kidney 7 mo afterwards. He subsequently underwent a radical nephrectomy.
The patient underwent 6 cycles of rituximab/cyclophosphamide/etoposide/ vincristine/prednisone for his lymphoma which led to a complete response.
After 49 mo follow-up since the diagnosis of kidney cancer, the patient is alive with both malignancies in remission.
The patient died of acute kidney injury 54 mo after the nephrectomy and LND as a result of mantle cell lymphoma. The RCC remained in remission.
The patient died due to metastatic CCRCC, 70 mo after the nephrectomy. His MM remained in remission.
The patient died of progressive metastatic CCRCC 64 mo after the nephrectomy and LND.
The patient is still alive with no evidence of recurrent lymphoma or RCC, 23 mo after the lymphoma diagnosis.
The patient is still alive with no evidence of recurrent lymphoma or CCRCC (follow up 112 mo since CCRCC).
The patient is still alive with no recurrent lymphoma 17 mo after the skin biopsy, and the RCC remains under surveillance without any significant radiographic progression.
The patient is still alive 4 mo after the nephrectomy with continued response in all metastatic sites of leiomyosarcoma of the kidney on maintenance nivolumab. He did not undergo systemic treatment for the lymphoma.
The patient continued to show evidence of primary RCC by imaging with no significant growth over time. The patient is still alive 12 mo later with no evidence of progressive lymphoma.
The phenomenon of concurrent RCC and HM has been reported in the literature, with a focus on gender, timing of the diagnosis of RCC and HM, and most common HM (Table 2)[1-5,8,10,11]. All except one of the studies in Table 2 documented a higher number of men with both conditions. Interestingly, in Tihan’s and Filippa’s study of 15 patients with coexisting RCC and malignant lymphoma, 11 (73%) of their patients were female[5]. Four (50%) studies in the literature in Table 2 reported a higher number of patients diagnosed with HM before RCC. NHL was the most common HM in the majority of these studies. In Dutcher and colleagues’ review of 199 cases of RCC and HM in the same patient identified in the literature (n = 173) and in their registry (n = 26) between 1991 and 2016, an association between RCC and HM within families was observed, exemplified by 74 patients with RCC who had 95 family members with HM[8]. These authors suggested a genetic correlation between RCC and B-cell malignancies.
| Study | Gender (M/F) | Number of patients with HM diagnosed first | Number of patients with simultaneous diagnosis of RCC and HM | Number of patients with RCC diagnosed first | Most common HM |
| Anderson et al[3], 1988, (n = 41) | 32 (78)/9 (22) | 17 (41) | 8 (20) | 16 (39) | NHL: 41 (100) |
| Nishikubo et al[1], 1996, (n = 8) | 7 (88)/1 (12) | 4 (50) | 0 (0) | 4 (50) | NHL: 2 (25); CLL: 2 (25); MM: 2 (25) |
| Tihan et al[5], 1996, (n = 15) | 4 (27)/ 11 (73) | 0 (0) | 14 (93) | 1 (7) | NHL: 15 (100) |
| Ohsawa et al[11], 1998, (n = 42) | 27 (64)/15 (36) | 38 (90) | 4 (10) | 0 (0) | NHL: 42 (100) |
| Kunthur et al[10], 2006, (n = 9) | 8 (89)/1 (11) | 6 (67) | 1 (11) | 2 (22) | NHL: 6 (67) |
| Choueiri et al[4], 2008, (n = 8) | 6 (75)/2 (25) | 4 (50) | 0 (0) | 4 (50) | MM: 8 (100) |
| Serefhanoglu et al[2], 2010, (n = 5) | 5 (100)/ 0 (0) | 2 (40) | 3 (60) | 0 (0) | CLL: 3 (60) |
| Dutcher et al[8], 2016, (n = 26) | 18 (69)/8 (31) | 16 (62) | 2 (5) | 8 (31) | NHL: 13 (50) |
| Current study, 2020, (n = 9) | 9 (100)/0 (0) | 1 (11) | 4 (44) | 4 (44) | NHL: 7 (78) |
Our case series of 9 patients with concurrent RCC and HM confirms particular aspects in the literature in Table 2, such as the predominance of male gender (all 9 patients in our series) and NHL (7 cases in our series) as the most frequent HM. However, only 1 patient in our series was diagnosed with the HM before the RCC, as either simultaneous diagnosis of the RCC and HM (4 patients) or RCC diagnosis before the HM (4 patients) was more common. We hypothesize that the predilection of male gender in concurrent RCC and HM may be due to the predominant gender in each cancer. In RCC, the male-to-female ratio is almost equal (1.2:1) in patients older than 70 years compared to a 2:1 ratio for patients ages 41 to 60 years old[13]. NHL is significantly more common in males[14]. The lower rate of NHL among females may be explained by direct effects of estrogens on lymphoma cell proliferation or by its effect on anti-tumor immune response[14]. The higher prevalence of males in concurrent RCC and HM may be attributed to more males compared to females affected in both RCC and HM.
A host of mechanisms has been reported as playing a role in developing concurrent RCC and HM. As the greatest number of patients with these combined conditions involves diagnosis of HM first[8,11], it has been proposed that immune dysregulation or breakdown of tumor surveillance associated with the lymphoma may lead to RCC[3,10]. An abnormal immune response may either precipitate lymphoma in patients whose RCC was diagnosed first or predispose a patient to developing both malignancies simultaneously[1,9,10]. As both lymphoma is a neoplasm of the immune system and RCC is a solid tumor that possesses an immune responsive behavior, the simultaneous occurrence of these diseases may be due to failure in tumor surveillance caused by the lymphoma that permits the RCC to develop[3]. In other cases, stimulation of the immune system by the RCC may result in lymphocytic proliferation and clonal proliferation which may spur the development of the HM.
We propose that immune dysregulation may be a potential explanation for both RCC and HM occurring in one host, however, this is solely a theory and the exact mechanisms are not known. It may be partly explained by the fact that both RCC and HM are well-known to respond to immunotherapy although they may have different clinical aggressiveness based on histology and grade. Both malignancies may be monitored without treatment at times based on histology, grade, and clinical behavior. Indolent disease may start to progress quickly due to transformation of the tumor to a more agrressive type. It appears in some cases that the host’s immune system may partially control the tumor growth resulting in “stable disease” without a need for treatment. In such cases, intrinsic or extrinsic immunosuppression may lead to tumor progression.
There is also an increased risk of RCC in patients with NHL that may be attributed to chemotherapy and radiation used in NHL treatment[2,3,11,12]. Additional etiologies for concurrent RCC and HM include viruses such as Epstein-Barr virus, Helicobacter pylori, and human T-lymphotropic virus-1 that have been implicated in lymphomas and carcinomas[2,8]. Interleukin-6 produced by RCC has been shown to stimulate the progression of MM[15]. A common genetic factor may also be involved in concurrent RCC and HM as common chromosomal abnormalities such as 3p and 17p deletions have been observed in both conditions[2,10,16,17]. In addition, PTEN germline mutations have been reported in hereditary RCC, and studies have described abnormalities of PTEN in T-cell and B-cell HM[8]. PTEN abnormalties as a common pathway for the development of RCC and HM in individuals or families remains to be elucidated[8]. The potential similar genetic components in these conditions necessitate a thorough investigation into family histories.
As none of our patients in our series underwent cytotoxic chemotherapy or radiation between the diagnosis of RCC and HM, these treatments did not contribute to the development of either malignancy. Furthermore, as only 1 patient had a family history of leukemia and there were no other definitive environmental, hormonal, or genetic factors, we hypothesize that immune system dysregulation plays the most significant role in the coexistence of RCC and HM. As none of our patients underwent cytotoxic chemotherapy or radiation between the diagnosis of RCC and HM, these treatments did not contribute to the development of either malignancy. Furthermore, as only 1 patient had a family history of leukemia and there were no other definitive environmental, hormonal, or genetic factors, we hypothesize that immune system dysregulation plays the most significant role in the coexistence of RCC and HM.
Several unique features in our patients shed light on the immunological interactions between these 2 conditions. Case #1 did not undergo a nephrectomy or any other treatment for the PRCC which was diagnosed 10 wk before the follicular lymphoma. He underwent cytotoxic chemotherapy (generally not effective in papillary RCC) to treat the latter condition after which both malignancies were in remission. Immune modulation resulting from the lymphoma treatment most likely spurred activation of the patient’s immune response leading to resolution of the PRCC. Interestingly, Case #3 was diagnosed with 3 simultaneous malignancies, including CCRCC, MM, and GIST. These conditions are unrelated and arise from different embryonic origins, specifically, carcinoma, myeloma, and sarcoma. Following only a splenectomy and without any systemic therapy in Case #6, his aggressive diffuse large B-cell lymphoma remains in remission.
Case #8 was diagnosed with the exceedingly rare and aggressive leiomyosarcoma of the kidney which accounts for only 0.12% of renal malignancies and is usually detected in women[18-20]. The cause of female predominance is not fully understood, however, it has been suggested that some malignancies are associated with genes located on X chromosomes that avoid X-inactivation[18,21]. Interestingly, a male predominance has been reported with concurrent RCC and HM, while renal leiomyosarcoma is more common in females. The diagnosis of renal leiomyosarcoma in a male with a simultaneous lymphoma makes our case more unique. A nephrectomy is the treatment of choice for renal leiomyosarcoma; chemotherapy has been reported to be of limited success[18,20]. Case #8 had an excellent response of 7 mo to immunotherapy with check point inhibitors.
Our case series also highlights the important role of biopsy in confirming the diagnosis. Although it may not be possible to biopsy each and every metastatic site, clinicians should consider biopsy when in doubt given the possibility of a second malignancy. Kidney cancer does not follow a certain pattern for lymph node metastasis, and it may be difficult to know whether adenopathy is of benign etiology or metastasis from kidney cancer or another malignancy. Clinicians should consider biopsy in cases of “mixed response” to systemic treatment for kidney cancer.
Strides have been made in cancer immunotherapy with the discovery of checkpoint inhibitors which effectively inhibit the immune system[22]. Programmed cell death 1 receptor (PD-1) signaling plays a role in encouraging cancer development and progression by boosting tumor cell survival[23]. It has been reported that blocking PD-1 signaling significantly promotes antitumor immunity, produces favorable clinical responses, and prolongs survival[23]. Developing antibodies that block PD-1 and programmed cell death receptor ligand 1 have been investigated. The checkpoint inhibitors ipilimumab and nivolumab proved invaluable in the treatment of Case #8 as exemplified by the 7-mo excellent response in this rare kidney cancer histology.
Both RCC and HM may usually be monitored based on clinical and histological factors. RCC has risk stratification criteria (International Metastatic RCC Database Consortium criteria and Memorial Sloan-Kettering Cancer Center criteria) which has been used extensively for offering treatment[24,25]. Watchful waiting may be offered in certain circumstances to patients with RCC. These patients generally have a favorable risk, low disease burden, and a single site of metastasis. Other factors such as metastatic site should be considered in cases of surveillance. Lung, adrenal, and pancreatic metastasis may potentially have a slower clinical course compared to liver and bone metastasis. Diligent monitoring may be performed for some asymptomatic patients with low-grade HM who do not have significant cytopenia.
Long-term medical surveillance is warranted when a patient is diagnosed with RCC or HM. Clinicians should be aware that either of these conditions may be diagnosed first or they may be diagnosed simultaneously. A thorough evaluation into the patient’s history of hormonal or environmental factors, viral infections, and prior cytotoxic chemotherapy or radiation as well as family history of RCC or HM is imperative to delve into the mechanisms that may contribute to these conditions. Further investigation into the immunological dynamics and common genetic abnormalities of RCC and HM may elucidate the relationship between these malignancies.
We acknowledge Norton Healthcare for their continued support.
Manuscript source: Unsolicited manuscript
Specialty type: Oncology
Country/Territory of origin: United States
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): 0
Grade C (Good): C
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: González-González R S-Editor: Dou Y L-Editor: A P-Editor: Wang LL
| 1. | Nishikubo CY, Kunkel LA, Figlin R, Belldegrun A, Rosen P, Elashoff R, Wang H, Territo MC. An association between renal cell carcinoma and lymphoid malignancies. A case series of eight patients. Cancer. 1996;78:2421-2426. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 1] [Reference Citation Analysis (0)] |
| 2. | Serefhanoglu S, Buyukasik Y, Goker H, Akin SC, Akin S, Sayinalp N, Haznedaroglu IC, Ozcebe OI. Concomitant renal cell carcinoma and lymphoid malignancies: a case series of five patients and review of the literature. Med Oncol. 2010;27:55-58. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 7] [Cited by in RCA: 6] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 3. | Anderson CM, Pusztai L, Palmer JL, Cabanillas F, Ellerhorst JA. Coincident renal cell carcinoma and nonHodgkin's lymphoma: the M. D. Anderson experience and review of the literature. J Urol. 1998;159:714-717. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 44] [Cited by in RCA: 27] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 4. | Choueiri TK, Baz RC, McFadden CM, Khasawneh M, Karam MA, Kelly M, Hussein MA. An association between renal cell carcinoma and multiple myeloma: a case series and clinical implications. BJU Int. 2008;101:712-715. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 23] [Cited by in RCA: 24] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 5. | Tihan T, Filippa DA. Coexistence of renal cell carcinoma and malignant lymphoma. A causal relationship or coincidental occurrence? Cancer. 1996;77:2325-2331. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 1] [Reference Citation Analysis (0)] |
| 6. | Travis LB, Curtis RE, Boice JD, Hankey BF, Fraumeni JF. Second cancers following non-Hodgkin's lymphoma. Cancer. 1991;67:2002-2009. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 2] [Reference Citation Analysis (0)] |
| 7. | Travis LB, Curtis RE, Glimelius B, Holowaty E, Van Leeuwen FE, Lynch CF, Adami J, Gospodarowicz M, Wacholder S, Inskip P. Second cancers among long-term survivors of non-Hodgkin's lymphoma. J Natl Cancer Inst. 1993;85:1932-1937. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 150] [Cited by in RCA: 152] [Article Influence: 4.8] [Reference Citation Analysis (0)] |
| 8. | Dutcher JP, Wiernik PH, Varella L, Chintapatla R. Occurrence of renal cell carcinoma and hematologic malignancies (predominantly lymphoid) in individuals and in families. Fam Cancer. 2016;15:677-687. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3] [Cited by in RCA: 2] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 9. | Barişta I. An association between renal cell carcinoma and lymphoid malignancies: a case series of eight patients. Cancer. 1997;80:1004-1005; author reply 1006-1007. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 1] [Reference Citation Analysis (0)] |
| 10. | Kunthur A, Wiernik PH, Dutcher JP. Renal parenchymal tumors and lymphoma in the same patient: case series and review of the literature. Am J Hematol. 2006;81:271-280. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 26] [Cited by in RCA: 26] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 11. | Ohsawa M, Hashimoto M, Yasunaga Y, Shingu N, Aozasa K. Characteristics of non-Hodgkin's lymphoma complicated by renal cell malignancies. Oncology. 1998;55:482-486. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 12] [Cited by in RCA: 12] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 12. | Sargın G, Yavasoglu I, Doger FK, Kadikoylu G, Bolaman Z. A coincidence of renal cell carcinoma and hematological malignancies. Med Oncol. 2012;29:3335-3338. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 7] [Cited by in RCA: 5] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 13. | Hew MN, Zonneveld R, Kümmerlin IP, Opondo D, de la Rosette JJ, Laguna MP. Age and gender related differences in renal cell carcinoma in a European cohort. J Urol. 2012;188:33-38. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 21] [Cited by in RCA: 23] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 14. | Horesh N, Horowitz NA. Does gender matter in non-hodgkin lymphoma? Differences in epidemiology, clinical behavior, and therapy. Rambam Maimonides Med J. 2014;5:e0038. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 38] [Cited by in RCA: 52] [Article Influence: 4.7] [Reference Citation Analysis (0)] |
| 15. | Sakai A, Kawano M, Kuramoto A. Interleukin-6 produced by renal-cell carcinoma cells and progression of multiple myeloma. N Engl J Med. 1991;324:1893-1894. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 30] [Cited by in RCA: 34] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 16. | Anglard P, Tory K, Brauch H, Weiss GH, Latif F, Merino MJ, Lerman MI, Zbar B, Linehan WM. Molecular analysis of genetic changes in the origin and development of renal cell carcinoma. Cancer Res. 1991;51:1071-1077. [PubMed] |
| 17. | Reiter RE, Anglard P, Liu S, Gnarra JR, Linehan WM. Chromosome 17p deletions and p53 mutations in renal cell carcinoma. Cancer Res. 1993;53:3092-3097. [PubMed] |
| 18. | Kwon YS, Salmasi A, Han CS, Hon JD, Singer EA. Renal Leiomyosarcoma: Case Report and Review of the Literature. World J Nephrol Urol. 2015;4:213-217. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 7] [Cited by in RCA: 8] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 19. | Miller JS, Zhou M, Brimo F, Guo CC, Epstein JI. Primary leiomyosarcoma of the kidney: a clinicopathologic study of 27 cases. Am J Surg Pathol. 2010;34:238-242. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 34] [Cited by in RCA: 39] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 20. | Valery JR, Tan W, Cortese C. Renal leiomyosarcoma: a diagnostic challenge. Case Rep Oncol Med. 2013;2013:459282. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 3] [Cited by in RCA: 7] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 21. | Brown CJ, Greally JM. A stain upon the silence: genes escaping X inactivation. Trends Genet. 2003;19:432-438. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 109] [Cited by in RCA: 117] [Article Influence: 5.3] [Reference Citation Analysis (0)] |
| 22. | Azoury SC, Straughan DM, Shukla V. Immune Checkpoint Inhibitors for Cancer Therapy: Clinical Efficacy and Safety. Curr Cancer Drug Targets. 2015;15:452-462. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 73] [Cited by in RCA: 77] [Article Influence: 8.6] [Reference Citation Analysis (0)] |
| 23. | Akinleye A, Rasool Z. Immune checkpoint inhibitors of PD-L1 as cancer therapeutics. J Hematol Oncol. 2019;12:92. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 412] [Cited by in RCA: 503] [Article Influence: 83.8] [Reference Citation Analysis (0)] |
| 24. | MD CALC. IMDC (International Metastatic RCC Database Consortium) Risk Score for RCC. Available from: https://www.mdcalc.com/imdc-international-metastatic-rcc-database-consortium-risk-score-rcc. |
| 25. | MD CALC. Memorial Sloan-Kettering Cancer Center (MSKCC/Motzer) Score for metastatic renal cell carcinoma (RCC). Available from: https://www.mdcalc.com/memorial-sloan-kettering-cancer-center-mskcc-motzer-score-metastatic-renal-cell-carcinoma-rcc. |