Published online Aug 24, 2020. doi: 10.5306/wjco.v11.i8.629
Peer-review started: April 28, 2020
First decision: June 20, 2020
Revised: June 23, 2020
Accepted: July 26, 2020
Article in press: July 26, 2020
Published online: August 24, 2020
Processing time: 114 Days and 10.7 Hours
Neuroblastoma (NB) is a heterogeneous disease with variable outcomes among countries. Little is known about NB in low- and middle-income countries (LMICs).
The aim of this review was to evaluate regional management protocols and challenges in treating NB in paediatric oncology units in LMICs compared to high-income countries (HICs).
PubMed, Global Health, Embase, SciELO, African Index Medicus and Google Scholar were searched for publications with keywords pertaining to NB, LMICs and outcomes. Only English language manuscripts and abstracts were included. A descriptive review was done, and tables illustrating the findings were constructed.
Limited information beyond single-institution experiences regarding NB outcomes in LMICs was available. The disease characteristics varied among countries for the following variables: sex, age at presentation, MYCN amplification, stage and outcome. LMICs were found to be burdened with a higher percentage of stage 4 and high-risk NB compared to HICs. Implementation of evidence-based treatment protocols was still a barrier to care. Many socioeconomic variables also influenced the diagnosis, management and follow-up of patients with NB.
Patients presented at a later age with more advanced disease in LMICs. Management was limited by the lack of resources and genetic studies for improved NB classification. Further research is needed to develop modified diagnostic and treatment protocols for LMICs in the face of limited resources.
Core tip: Neuroblastoma (NB) is a childhood malignancy of the sympathetic system that accounts for a large percentage of the childhood malignancy mortality. The heterogenous presentation contributes to various treatment challenges especially in low- and middle-income countries (LMICs). NB in LMICs has not been investigated beyond single institutions, but the limited reports differ from those in high-income countries (HICs). The incidence of NB in LMICs has been reported to be lower than HICs, but the disease presents with a higher incidence of high-risk and advanced disease. Furthermore, the limited resources in these countries contribute to the challenges in the management of NB that leads to a high mortality rate. The genetic profile of NB in LMICs is also not known due to limited capacity to perform genetic investigations. This article aims to comprehensively describe NB in LMICs.
- Citation: van Heerden J, Kruger M. Management of neuroblastoma in limited-resource settings. World J Clin Oncol 2020; 11(8): 629-643
- URL: https://www.wjgnet.com/2218-4333/full/v11/i8/629.htm
- DOI: https://dx.doi.org/10.5306/wjco.v11.i8.629
The burden of disease in low- and middle-income countries (LMICs) is predominantly infectious in origin[1,2]. Yet, it is shifting towards non-communicable diseases such as congenital diseases, malignancies and road traffic incidents[2,3]. To date, the focus in research has been on communicable paediatric diseases with the World Health Organization’s initial integrated management of childhood illness programme being one example[2]. Building the capacity of health care professionals to identify childhood malignancies has not been optimal[4]. This possibly explains the 28%-49% childhood malignancy gap reported between LMICs and high-income countries (HICs)[5].
Neuroblastoma (NB) data from HICs are well documented, whereas data from LMICs are limited. NB, predominantly a childhood malignancy, remains a major contributor to childhood cancer mortality and accounts for up to 15% of paediatric malignancy-related deaths[6]. Even with increased-intensity treatment in HICs, the five-year overall survival (OS) remains approximately 60%[7]. However, there is a major divide between HICs and LMICs due to the advances in diagnostics, treatment options and outcomes of NB in HICs[8].
Because of the variability of NB symptoms, they can easily be misdiagnosed as infections, bone marrow failure, neuropathology and obstructive enteropathies in LMICs by primary health care workers. Nurse-led primary care clinics or general practitioners may not have the expertise to recognise rare diseases in children and are often the first contact versus HICs where the first contact is usually more experienced health care workers[9].
Early diagnosis is crucial and necessitates a high index of suspicion with appropriate risk stratification and treatment[5]. The prognosis of NB is determined by a set of well-described prognostic factors that include patient factors (age at diagnosis), biochemical factors (lactate dehydrogenase and ferritin), tumour-related factors (primary site, tumour histology and stage), biological factors (MYCN amplification, ploidy and loss of chromosome 1p) and management factors (post-induction metastatic remission and degree of resection)[10,11]. NB pathophysiology and biological features, predominantly MYCN status, loss of chromosome 1p and ploidy, determine the spontaneous regression or aggressive growth and spread of metastases but do not explain the international difference in characteristics completely[6]. Similarly, notable differences in outcomes have been reported for risk classifications between LMICs and HICs with similar therapies[12-16]. The aim of this narrative review was to evaluate regional variations in the diagnosis and management of NB in LMICs versus HICs.
A comprehensive literature review of publications on PubMed, Global Health, Embase, SciELO, African Index Medicus and Google Scholar with medical subject headings pertaining to NB and outcomes relating to LMICs was done. Search terms included (but were not limited to) ”neuroblastoma”, ”limited resources”, ”low-income”, ”middle-income” and names of LMICs. The search was conducted from April 2019 to January 2020 with terms adapted according to search engines without limitations on the date or language, provided that English summaries or abstracts were included. Conference proceedings were included. No authors were contacted regarding publications.
Due to the variability in reporting, nonstandard application of definitions in the reported clinical results, heterogeneous data and paucity of information, the authors constructed limited tables to evaluate clinical and/or biological characteristics to report in the descriptive review.
The systemic literature search retrieved 127 articles, abstracts and documents on NB in LMICs. After removing 11 documents for possible duplicated reporting, the 116 remaining documents consisted of 13 cancer registry-based reports and 103 non-registry-based documents. Twenty-three non-registry-based, nonrandomised studies (two prospective studies and 21 retrospective studies) were selected. All 116 articles, including the remaining 83 articles that were not specific to NB but contained epidemiological and non-interventional data on NB, were utilised to draw descriptive conclusions regarding epidemiological elements and outcomes for NB in the respective countries. Despite significant population numbers, certain LMIC regions were underrepresented in this review due to possible publication bias of reports.
Data from Asia (China, India, Pakistan, Thailand and Vietnam)[13,17-22], the Middle East and North Africa (Egypt, Iran, Iraq and Morocco)[23-28] and the Americas (Argentina, Brazil, Chile, Cuba, Mexico and Uruguay)[12,16,29-35] were accessible, but reports from sub-Saharan Africa and the Pacific Ocean were limited to single reports from the French-African Paediatric Oncology Group (GFAOP) and reunion[15,36]. The differences between HICs and LMICs could be evaluated from these reports, but complete management and outcome data for interregional variations among LMIC regions were less robust.
In sub-Saharan Africa, the incidence of NB was low, ranging from 0.4 cases per million in Niger to 5.9 cases per million in Kenya[37], compared to HICs such as North America and Europe where the respective incidences were reported as 10.5 and 11.6 cases per million per year in children younger than 15 years [11,38,39]. South Africa reported an incidence of 2.68 cases per million in children under 15 years of age between 1985 and 2007[40]. In Argentina, intraregional variations in incidence were demonstrated with a higher incidence being associated with areas of high socioeconomic status[29]. Yet, the international incidences have remained stable regardless of economic status[41]. As perinatal and low-risk (LR) NB can be asymptomatic and/or spontaneously regress, underdiagnosis of cases is a possible reason[5,37] but the degree of discrepancy is not known.
Difference in age at presentation: In LMICs, the majority of patients were under the age of 5 years, but the percentages of infants reported for China (16.3%) and India (5.9%) (Table 1) were low. The mean or median age of presentation was delayed in some LMICs. In Thailand, the median was 34.8 mo of age and in India as high as 48 mo of age. The median age of presentation in the 16 paediatric oncology units (POUs) of the GFAOP study was 48 mo as well[15]. The age-standardised rates varied among countries, but the ratio of patients under 12 to 60 mo could be as low as 2.3:1 in Argentina and 1.2: 1 in Brazil compared to an HIC like Germany with a 4:1 ratio (Table 2). However, other LMICs such as Cuba (4.8:1), with a good reputation for health care, and Reunion (2.7:1), a French territory in Africa, compared favourably with the United States of America (2.4:1) in this regard (Table 1). The median age of presentation in HICs was reported to be between 17 and 18 mo of age, of whom approximately 40% were diagnosed under 1 year of age[41]. Many studies have reproduced the 18-mo watershed dividing good prognosis (under the age of 18 mo) and poorer prognosis (over the age of 18 mo). Stage 4 patients were per definition below 12 mo of age with a good prognosis. In HICs, 90% of NB patients were younger than 5 years at diagnosis, with a median age at diagnosis of 19 mo, and 37% of patients had been diagnosed as infants[11]. The ATRX-gene is associated with advanced-age presentations, especially over 9 years of age, conferring a poorer prognosis in adolescents and adults[42]. The paucity of genetic studies in LMICs limited the interpretation of gene mutations related to age at diagnosis.
| Country | n | < 12 mo | < 18 mo | < 60 mo | < 120 mo | < 180 mo | Mean | Median |
| Asia | ||||||||
| China (2008-2013)[17] | 59 | 44% | 56% | 24 | ||||
| China (2000-2006)[18] | 98 | 16.3% | 4.1% | 53% | 21.5% | 4% | 48 | |
| India (1990-2004)[19] | 103 | 0%-5.9% | 77%-98.1% | 1.9% | 41 | - | ||
| Pakistan (2015-2016)[20] | 70 | 30% | 63% | 7% | 36 | |||
| South America | ||||||||
| Argentina (2000-2012)[29] | 753 | 30% | 52.2% | 12.9% | 45.3% | 26.4 | ||
| Brazil (1991-2012)[30] | 258 | 29% | 49% | 17% | 5% | 40.5 | 28.9 | |
| Brazil (1990-2000)[16] | 125 | 26% | 13% | 41% | 20% | 38.2 | 33 | |
| Middle East and North Africa | ||||||||
| Egypt (2005-2010)[23] | 142 | 24.2% | 75.8% | 30 | ||||
| Egypt (2001-2010)[24] | 53 | 22.6% | 77.4% | |||||
| Iran (1974-2005)[25] | 219 | 21.5% | 78.5% | 40.5 | ||||
| Iraq (2008-2014)[26] | 62 | 30.6% | 50% | 16.1% | 3.2% | 37 | ||
| Sub-Saharan Africa | ||||||||
| Ethiopia (2010-2013)[79] | 5 | 0 | 40% | 40% | 20% | |||
| Kenya (1997-2005)[44] | 22 | 31.8% | 50% | 18.2% | 60 | |||
| Country | n | < 12 mo | < 60 mo | Ratio < 12: < 60 | < 120 mo | < 180 mo | Total incidence |
| South America | |||||||
| Argentina (2000-2012)[29] | 753 | 32.9 | 14.6 | 2.3: 1 | 2.8 | 1.0 | 8.3 |
| Uruguay (2001-2010)[35] | 69 | 63.1 | 18.1 | 3.4: 1 | 2.3 | 0 | 9.1 |
| Chile (2007-2012)[31] | 88 | 21.9 | 6.7 | 3.2: 1 | 2.1 | 0.3 | 4.7 |
| Brazil (1998-2002)[33] | 372 | 15.3 | 12.4 | 1.2: 1 | 3.8 | 1.3 | 5.9 |
| Central America and the Caribbean | |||||||
| Mexico (1996-2005)[32] | 72 | 18.5 | 5.4 | 3.4: 1 | 1.1 | 0.2 | 3.8 |
| Cuba (2001-2003)[34] | 46 | 3.9 | 0.8 | 4.8: 1 | 0.5 | 0.2 | 0.1 |
| Sub-Saharan Africa | |||||||
| Reunion (2005-2011)[36] | 12 | 44.1 | 15.8 | 2.7: 1 | 4.1 | 0 | 9.6 |
Gender distribution at diagnosis: The GFAOP reported that the male to female ratio for 16 African POUs was 2: 1[15]. In other LMICs, the male predominance as well as the greater male to female ratio was reproducible (Table 3). The ratios varied from 1.06: 1 to 2: 1. Previous studies from Southern Africa reported a ratio of 1.7:1[43] in keeping with the male predominance, while a Mexican study reported a lower NB incidence of 2.5-4.1 cases per million per year, in keeping with the situation in other LMICs, yet the male to female ratio of 1.1:1 was similar to HICs[32]. Kenya also reported a 1: 1 ratio in an LMIC setting[44]. The incidences based on gender have not been explained by other biological features. These findings were in contrast to the reported surveillance, epidemiology, and end results programme data from North America and European data, according to which a slight male predominance with a ratio of 1.1:1 was noted[38,45].
| Country | Total | Male | Female | Ratio M: F |
| Asia | ||||
| Pakistan (2015-2016)[20] | 70 | 1.8: 1 | ||
| India (2000-2017)[64] | 85 | 57 (67%) | 28 (33%) | 2: 1 |
| India (1990-2004)[19] | 103 | 76 (74%) | 27 (26%) | 2.8: 1 |
| Thailand (2000-2007)[21] | 67 | 39 (58.2%) | 23(34.3%) | 1.7: 1 |
| Vietnam (2010-2012)[22] | 130 | 76(58.5%) | 54 (41.6%) | 1.4: 1 |
| China (2008-2013)[17] | 59 | 35 (59%) | 24 (40.1%) | 1.5: 1 |
| China (2000-2006)[18] | 98 | 1.3: 1 | ||
| South America | ||||
| Brazil (1991-2012)[30] | 258 | 148 (57%) | 110 (43%) | 1.3: 1 |
| Brazil (1990-2000)[16] | 125 | 68 (54.4%) | 57 (45.6%) | 1.2: 1 |
| Argentina (1999-2015)[12] | 39 | 21 (54%) | 18 (46%) | 1.2: 1 |
| Argentina (2000-2012)[29] | 971 | 509 (52%) | 462 (48%) | 1.1: 1 |
| Middle East and North Africa | ||||
| Iran (1974-2005)[25] | 219 | 1.9: 1 | ||
| Iraq (2008-2014)[26] | 62 | 37 (59.7%) | 25 (40.3%) | 1.5: 1 |
| Morocco (2012-2015)[27] | 40 | 26 (65%) | 14 (35%) | 1.8: 1 |
| Egypt (2005-2010)[23] | 142 | 68 (51.5%) | 64 (48.5%) | 1.06: 1 |
| Egypt (2001-2010)[24] | 53 | 35 (66%) | 18 (35%) | 1.9: 1 |
| Egypt (2007-2011)[28] | 271 | 169 (62.4%) | 102 (37.6%) | 1.65: 1 |
| Sub-Saharan Africa | ||||
| Northern Nigeria (2003-2009)[79] | 14 | 10 (71.4%) | 4 (28.6%) | 2.5: 1 |
| Southern Africa (South Africa and Namibia) (1983-1997)[43] | 1.7: 1 | |||
| Ethiopia (2010-2013)[78] | 5 | 3 (60%) | 2 (40%) | 1.5: 1 |
| Kenya (1997-2005)[44] | 22 | 11 (50%) | 11 (50%) | 1: 1 |
Population variations: Population variations related to epidemiology and pathophysiology contributed to a difference in the presentation of high-risk (HR) disease but not non-HR disease[46]. Independent from social circumstances, certain ethnicities were diagnosed at an older median age (> 20 mo) and had a higher prevalence of stage 4 disease and unfavourable histology tumours (undifferentiated cells)[46]. Studies amongst Alaskan indigenous ethnicities (a heterogeneous group of Eskimos, Native Indians and Aleuts) reported an incidence of 0.7 cases per million[47]. In Australia, Aboriginal and Torres Strait Island children were 1.83 times more likely to die from neuroblastoma than nonindigenous children while only contributing 3.7% of diagnoses[48]. The lower incidence of NB among indigenous ethnicities was not reproduced in LMICs of South America or the Pacific Islands[49,50].
Difference in stage during presentation: Many LMICs reported stage 4 rates upward of 50%, with India and Pakistan reporting 71.8% and 79% stage 4 tumours respectively (Table 4). Egypt, Pakistan and Iran did not report any patients with stage 1 tumours, while China and India reported 3% and 1% stage 1 diagnosis respectively[18-20,25]. The GFAOP reported metastatic disease for up to 80% of patients except Burkina Faso and Morocco, where it varied from 20% to 50%[15]. Kenya reported the highest percentage of metastatic disease at 92.3%[44]. The data suggested that presentation in LMIC was usually metastatic.
| Country | n | Stage 1 | Stage 4 | Non-MYCN amplified | MYCN amplified | Non-HR | HR |
| Asia | |||||||
| China (2008-2013)[17] | 59 | 6.8% | 37.3% | 55% | 45% | 53% | 47% |
| China (2000-2006)[18] | 98 | 3% | 50% | ||||
| India (1990-2004)[19] | 103 | 1% | 71.8% | ||||
| Pakistan (2015-2016)[20] | 70 | 0% | 79% | > 61.1% | |||
| South America | |||||||
| Argentina (2000-2012)[29] | 753 | 12% | 55.5% | 80% | 20% | ||
| Brazil (1991-2012)[30] | 258 | 15% | 46% | 75% | 25% | ||
| Brazil (1990-2000)[16] | 125 | 7% | 64% | 53% | 47% | ||
| Middle East and North Africa | |||||||
| Egypt (2005-2010)[23] | 142 | 0% | 64.7% | 24.2% | 75.8% | ||
| Egypt (2001-2010)[24] | 53 | 0% | 67.9% | 79.2% | 20.8% | 32% | 68% |
| Iran (1974-2005)[25] | 219 | 14.5% | 53.8% | ||||
| Iraq (2008-2014)[26] | 62 | 1.6% | 69.4% | 45.2% | 54.8% | ||
| Sub-Saharan Africa | |||||||
| Kenya (1997-2005)[44] | 26 | 0% | 92.3% | ||||
Difference in MYCN amplification: Molecular and genetic diagnostics were not available in the greater number of reports and were recorded as a challenge in the literature[13,15,51]. In the GFOAP study, only North African countries could determine MYCN status[15] with Namibia and South Africa reporting MYCN studies in Southern Africa[44]. MYCN is present in about 20% of tumours[51,52]. Limited data are available on biological studies, especially genetic studies, in LMICs mainly due to resource constraints. In Iran, MYCN amplification was reported in 80% of NB patients, while Vietnam, Argentina and Egypt respectively reported rates of 17.8%, 20% and 20.8% (Table 4)[14,17,19].
Intra-risk group classification variability: Age groups, biological information and treatment protocols were not standardised in the literature, due to the development of classifications and changing treatments during the review period. Of note, risk classification was either not possible or was done retrospectively. Management protocols focus on administering risk-based treatments after identification of the classification of each patient yet many patients were treated on the basis of stage[39]. LMICs concluded that optimal treatment was doubtful due to the suboptimal classification of tumours[9,15,19]. The International Neuroblastoma Risk Group classification and the Children’s Oncology Group classification rely on histological and genetic information (mitosis-karyorrhexis index, MYCN amplification, 11q aberration and DNA ploidy) to determine classification[11], which is not available in many resource-limited settings. Even when available, the lack of consistent cytogenetic evaluation, as was the case in Argentina, relegated patients in need of high-intensity treatment to LR categories and suboptimal treatment[12]. Due to the aggressive nature of especially HR NB, palliative rather than curative options have been pursued in LMICs[11]. Yet, variability in outcomes has been described within each risk class, highlighting that individual assessment is probably suboptimal Therefore, the International Society for Paediatric Oncology (SIOP)-Paediatric Oncology for Developing Countries (PODC) has adapted the approach to risk stratification with therapy based on available resources and utilising available diagnostic techniques[11]. The classification relies on age, stage and the common available nonspecific tumour markers ferritin and lactate dehydrogenase for risk classification[11]. Morocco has implemented this classification system in the prospective NB protocol and has concluded that it allowed for more accurate diagnosis and systematic treatment[27]. For more accurate comparisons across resource-limited settings, classifications such as the SIOP-PODC classification should be standardly applied.
Reports from LMICs were predominantly single-institution reports. A multi-institutional survey by the GFAOP[15] and a review from India including 17 institutions and 11 cities[3] described the epidemiology, heterogeneous management approaches and outcomes of NB in LMICs[5]. Sub-Saharan African countries reported lower incidences of NB (3%-7.5%) among childhood malignancies compared to North-African countries (7%-30%)[15]. The same study identified the limitations of reporting: Plain radiography, ultrasonography, computed tomography and magnetic resonance imaging were available at all centres, but access to imaging studies was variable. None of the sub-Saharan centres had metaiodobenzylguanidine scans. The North African centres had these scans, but only Algeria had consistent access due to government funding[15]. In Honduras and the Philippines, diagnostic resources were available in large cities but were inaccessible to most patients living in rural areas[50]. This is a typical problem in LMICs[53]. An Indian multi-study review concluded that variability in India included treatment protocols, reporting of outcomes and calculation of survival rates[13]. This conclusion could also be applied to other LMICs. Morocco and Argentina were the only LMICs to describe prospective national studies regarding NB[27,29]. This is representative of the diverse, nonstandardised approach to NB in most LMICs. Most studies found a lack of access to biological tests for stratification (based on HIC-validated data), the presentation of advanced disease, poor socioeconomic circumstances and a significant percentage of patients who absconded from treatment[23,24]. Advanced disease and higher than average percentages of HR disease were described (Table 4). The PODC committee of the SIOP has developed adapted guidelines for the management of NB in LMICs[11]. Yet, in the field of paediatric oncology, especially in sub-Saharan Africa, a prioritised, stepwise approach has been advised in limited-resource settings, prioritising pain management, supportive care, comorbid diseases and malignancies with a higher incidence and relatively uncomplicated treatment regimens above rare childhood malignancies[54]. In Africa, only Morocco has published data from standardised prospective NB protocols from four POUs based on the PODC guidelines[27].
Clinical presentation, index of suspicion and misdiagnosis: Because of its heterogeneous clinical presentation, NB can be challenging to diagnose[30]. The presenting signs of NB can be similar to those of non-malignant diseases and can confound recognition of the disease[10,55]. Symptoms of an NB abdominal mass can be misdiagnosed as more common childhood illnesses such as constipation[56]. In LMICs, similar to HICs, the most common presentation reported in 19%-87% of patients was an abdominal mass (Table 5)[18,19,23,30]. Other common presentations were nonspecific abdominal pain (22%-73.5%)[18,30] and fever (25%-65%)[18,19,23,30], metastatic manifestations such as bilateral proptosis (27%-42.4%)[19,23], bone pain (19%)[30] and pancytopaenia, and constitutional symptoms such as loss of weight[56]. The clinical progression of the tumour involves a spectrum of behaviour from aggressive advancement to metastatic disease or spontaneous regression and mature differentiation of cell types such as ganglioneuroma[29,57]. Health care practitioners must have a high index of suspicion for NB with a varied clinical picture[35]. Misdiagnosing NB from other abdominal tumours prevents accurate registration of the diagnosis[29]. In resource-limited settings, the diagnosis of asymptomatic benign clinical types is less common, possibly due to underdiagnosis. Early detection by screening in HICs neither impacted outcomes nor was it cost-effective[57]. While the incidence was increased during active screening of the disease in the European, North American and Japanese context, surgical interventions were increased without improvement of survival[57].
| Asia | ||||
| China (2000-2006)[18] | Abd pain (73.5%) | Abd mass (54.1%) | Fever (45.9%) | Limb pain (25.5%) |
| India (1990-2004)[19] | Fever (65%) | Abd mass (54%) | Bone pain (31%) | Proptosis (27%) |
| South America | ||||
| Brazil (1991-2012)[30] | Fever (25%) | Abd pain (22%) | Abd mass (19%) | Bone pain (19%) |
| Middle East and North Africa | ||||
| Egypt (2005-2010)[23] | Abd mass (87%) | Pallor (57.6%) | Fever (45.5%) | Proptosis (42.4%) |
| Sub-Saharan Africa | ||||
| Kenya (1997-2005)[44] | Abd mass (53.8%) | Bone pain (50%) | Proptosis (38.5%) | Fever (19.8%) |
Access to and assignment of treatment: The number and capacities of POUs varied substantially among LMICs, and capacities also varied among POUs in a single country[50]. Basic paediatric oncology components were not available in the Philippines and Senegal[50], while Venezuela and Egypt had adequate intensive care facilities and even transplant services[50]. This is also true of POUs in South Africa[44]. Furthermore, paediatric services may not even exist in certain countries or often compete with adult services for resources[54].
Current treatment protocols are based on risk stratification[11]. The LMIC reports included treatments over four decades[13,30]. Therefore, outcomes were predominantly reported per stage and, subsequently, as classification systems evolved, research describing the treatment of LR and intermediate-risk (IR) patients but focussing primarily on HR disease as the greatest NB burden was reported.
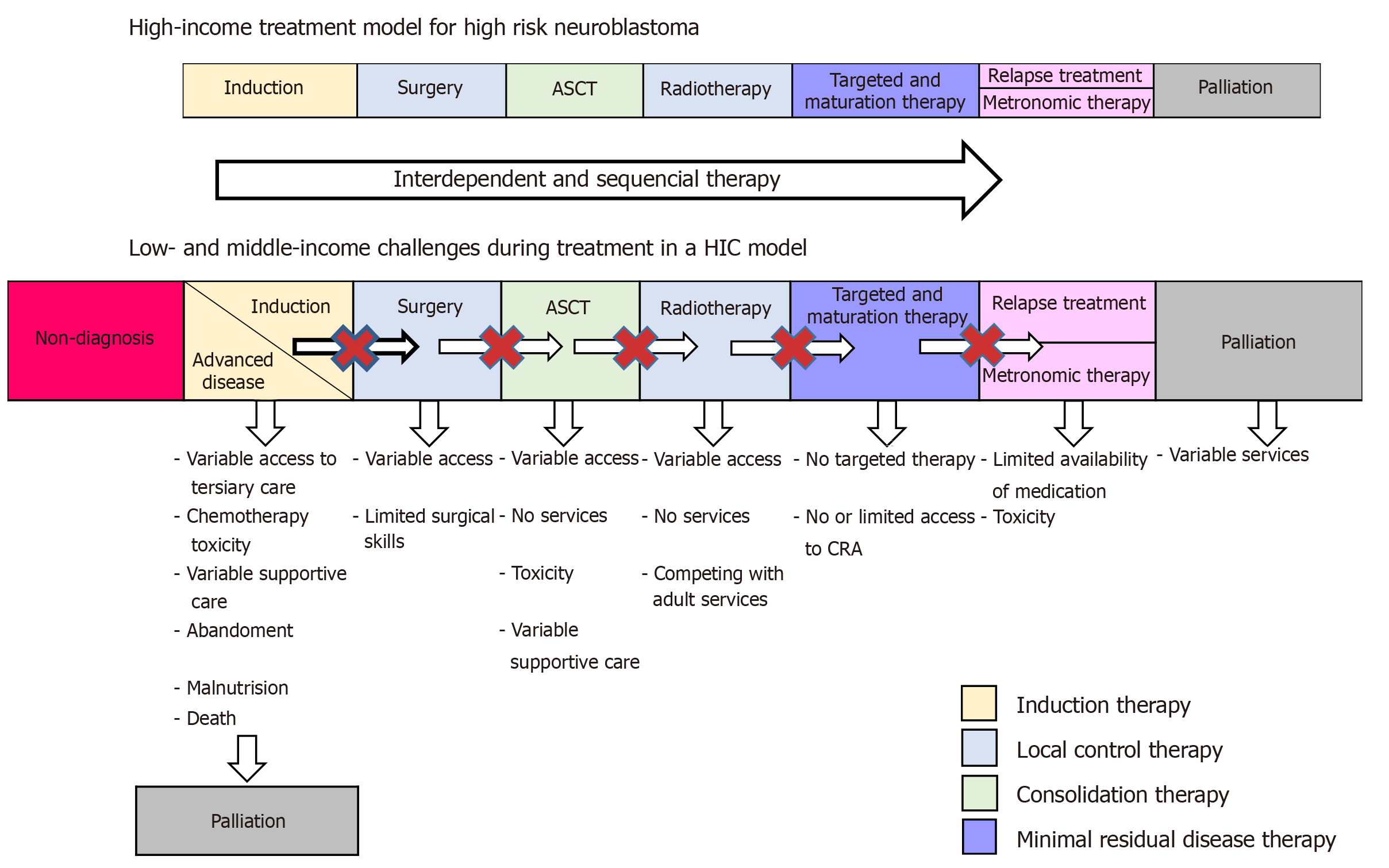
In many LMICs, NB treatment choices are limited to mainly chemotherapy, surgery and radiotherapy[1]. In HR NB, multimodal therapy is of vital importance for cure and five-year OS of up to 60% (Figure 1).
Due to advanced disease at diagnosis, palliative treatment is often the only plausible option (Figure 1). Other challenges for the management of NB include lack of surgical and radiotherapy skills or equipment as well as lack of chemotherapy[1,11]. Poor outcomes have necessitated the development of palliative strategies, yet many LMICs where drug insecurity is high do not have even basic medicines for palliation[58]. Resources, drug security and expertise in institutions influence treatment decisions to a similar extent as treatment adherence and response to treatment. The ability of facilities to provide supportive care, in terms of antibiotics, intensive care and granulocyte-stimulating factors, influences decision making regarding the intensity of treatment that patients receive[10,11].
Treatment protocols utilised in low- and medium-income countries and outcomes: Over the past decades, guidelines for the treatment of NB have changed as a result of an improved understanding of biological prognostic factors and changing classification systems yet chemotherapy remains based on etoposide and platinum (cisplatin and/or carboplatin) backbones plus dose- and time-intensive administration of chemotherapy[11]. Some approaches include doxorubicin in the regimens, while the SIOP-PODC treatment guidelines for NB are based on settings relating to the level of supportive care and resources available in a POU[11]. Indicators for reporting outcomes were not consistent over the same period. Some studies reported according to stage, while others reported according to risk classification.
The GFAOP administered various local and international protocols based on the standard backbone including doxorubicin[15]. Individual POUs reported a long-term OS of less than 10% for metastatic disease. Tunisia reported an OS of 78% for non-metastatic disease, while Senegal reported an OS (metastatic plus non-metastatic) of 38.9%. The report concluded that with all countries having access to surgical options, the outcomes were ”generally poor” and standardised protocols were being developed for multicentre use[15]. In Morocco, a GFAOP member, a national prospective protocol divided into an HR protocol and a non-HR protocol based on the risk-adapted SIOP-PODC treatment guidelines was studied[11,15,27]. Long-term outcomes were not reported, but 60.6% of HR patients experienced a partial or very good partial response, receiving local control with surgery or consolidation therapy[27]. The study concluded that risk stratification and treatment guidelines adapted for LMICs improved the accuracy of diagnosis and access to systematic treatment[27]. The protocol was also suitable for multicentre use[27].
A Chinese study administered OPEC by modifying the Japanese study group protocol[18]. The five-year OS was 80% for stages 1 and 2 and 48.3% and 20% for stages 3 and 4 respectively, which was less than the Japanese outcomes[18].
Egyptian and Indian centres based their HR treatment on the North American CCG-3891 protocols, while other LMIC centres administered chemotherapy according the European protocols from France and the International Society of Paediatric Oncology European Neuroblastoma Research Network (SIOPEN)[59]. Indian institutions followed a non-standardised approach including OPEC/OJEC, doxorubicin-containing and Ifosfamide-containing regimens[13]. Iran and Egypt used OPEC/OJEC regimens[23-25], while Brazil, Thailand and China followed doxorubicin-based regimens[16-18,21,30]. Stage 1 disease had a five-year OS of 100% in Brazil[16], China[17,18] and Thailand[21], while stage 4 OS was under 20%[16,18]. The three-year OS for stage 4 disease in Thailand and China was less than 35%[17,21]. While the outcomes for stage 1 disease were comparable to HICs, the poorer stage 4 outcomes were less optimal than in HICs[10]. The same conclusion was reached in an Indian study with three-year OS and event-free survival for non-metastatic disease of 77% and 54% respectively[60].
Argentina alternated between rapid COJEC and the modified N7 for HR disease according to the SIOPEN HR NBL-1 protocol[12]. The five-year OS was 24%. The study concluded that improved supportive care, optimal treatment and maximising available resources were needed[12]. A second Argentinian study associated lower socioeconomic status with poorer outcomes independent of treatment[29].
In LMICs, no conformity was found in the management of NB amongst regions within countries. Failing to complete one aspect of the sequential treatment protocol relegates the outcome to being suboptimal. This is often the case in LMICs with limited access to health care and limited resources for optimal treatment[61]. It is possible that without genetic factors to distinguish more clearly between IR and HR disease, the IR cohorts in LMICs contain a number of HR patients, thereby affecting outcomes[11].
Main factors affecting outcomes: LMICs have identified treatment-related, tumour-related and social factors that affect the outcomes of children with NB. Delayed diagnosis[30] and inaccurate diagnosis of tumours due to limited radiologic and pathology resources were cited as major obstacles[25,27,60]. The limited ability to perform biological testing impaired accurate risk stratification[25,27,30,62]. Centres with higher levels of supportive care reported the inability to perform bone marrow transplants as a limitation to improving outcomes[24,60]. The variability of tumours and nonspecific presentation contributed to late diagnosis and the incidence of advanced disease[12,25,27,30,62]. Yet, the greatest problems were the abandonment of treatment and patients lost to follow-up of up to 50%[11,70,62], which were linked to social factors and the distance from treatment centres[12].
Social circumstances and outcomes: A Brazilian study reported intraregional variation in the incidence of NB based on socioeconomic status[33]. The study concluded that patients from regions with a lower socioeconomic status had poorer outcomes[33]. In South African populations, socioeconomic and/or cultural factors related to access to or utilisation of health care services are a possible contributing factor to poorer outcomes[1]. A large proportion of rural inhabitants have restricted access to medical facilities and thus experience a delay in treatment[1,63,64]. A Harvard study concluded that in the United States of America, NB diagnosis was influenced by social circumstances[65]. According to the study, the Human Development Index showed a direct relationship between socioeconomic status and the incidence of NB[65].
Factors influencing health-seeking behaviour: The heterogeneous and aggressive pathophysiology of NB demands prompt response and immediate medical intervention for nonspecific symptoms[66,67]. The economic structure of LMICs influences the affordability of healthcare and parental education[68-70]. These factors determine the promptness of the response to and the action taken with regard to nonspecific symptoms associated with the initial phases of childhood malignancies. The steadfast belief in traditional medicine as a first treatment option and cultural systems in which elders or a single authority figure decide about seeking medical intervention may delay action towards directed care[71,72]. Political stability and government policies have a direct impact on the availability, accessibility and quality of health care systems in treating childhood cancer[73,74].
The focus of research for LMICs should be on creating greater awareness in the diagnosis of NB, improving diagnostics and establishing social support strategies for successful, harmonised management protocols and homogenous treatment facilities to improve outcomes[55,75]. The main priority should be accurate tumour registries to document not only the most common or treatable childhood malignancies but also the rarer tumours such as NB[37]. In resource-limited settings, the need for genetic markers to develop more accurate risk classifications exists, especially to distinguish clearly between IR and HR patients. This is important in the case of stage 2 and stage 4 patients with adverse biology tumours who have in a higher risk classification compared to patients with non-adverse biology tumours[11,25,29]. Genome and exome sequencing have improved the understanding of the pathophysiology of NB in HICs[76]. However, knowledge regarding genetics of NB in the diverse ethnicities in LMICs is limited. A further challenge would be to make treatments and advanced diagnostics, such as liquid biopsies and biological tests, more widely available to all countries, whether HICs or LMICs, to improve diagnostic capacities and outcomes[75]. In advanced disease, palliative research could contribute to a greater understanding of the role of metronomic therapies and disease control in the context of NB[77].
Childhood malignancy awareness and advocacy still face great challenges, especially in LMICs, notably countries with large rural populations and great geographical divides, in accurately diagnosing malignancies, especially heterogeneous tumours such as NB. The lack of uniform treatment protocols for this variable disease is still a barrier to care. Epidemiological data are reproducible in different international studies, but data from across the world are not uniform. More research regarding tumour biology, specifically genomics, is needed not only in HICs but also in LMICs to determine underlying differences in molecular biology of the tumours, genetic targets and drug processing of NB patients, especially in heterogeneous populations. This information must then be made available to treatment centres where biological investigation is not possible, ready for clinical application to achieve improved outcomes for NB worldwide.
Neuroblastoma (NB) is a well-documented childhood malignancy with the greatest source of knowledge originating from high-income countries. The management of NB in low- and middle countries (LMIC) is less robust due to various social and resource limitations.
The outcomes of various LMIC during the same period like South America, Francophone/North African countries, Asia and South Pacific Islands was evaluated.
This literature review was to evaluate regional development of management protocols, the challenges in treating NB in paediatric oncology units in LMIC as compared to high-income countries, new laboratory and clinical developments in the treatment of NB.
A literature review of publications searched on PubMed, Medline, Global Health, Embase, SciELO and Google Scholar with keywords in keeping with NB and outcomes. Due to the variability in reporting, nonstandard application of definitions in the reported clinical results, heterogeneous data and paucity of information, the authors constructed limited tables to evaluate clinical and/or biological characteristics to report in the descriptive review.
Childhood malignancy awareness and advocacy still face great challenges, especially in LMICs, in accurately diagnosing malignancies, especially heterogeneous tumours such as NB. The lack of uniform treatment protocols for this variable disease is still a barrier to care. Epidemiological data are reproducible in different international studies, but data from across the world are not uniform.
More research regarding tumour biology, specifically genomics, is needed not only in high-income countries but also in LMICs to determine underlying differences in molecular biology of the tumours, genetic targets and drug processing of NB patients, especially in heterogeneous populations.
The focus of research for LMICs should be on creating greater awareness in the diagnosis of NB, improving diagnostics and establishing social support strategies for successful, harmonised management protocols and homogenous treatment facilities to improve outcomes. In resource-limited settings, the need for genetic markers to develop more accurate risk classifications exists. A further challenge would be to make treatments and advanced diagnostics, such as liquid biopsies and biological tests, more widely available to all countries. With advanced disease, palliative research could contribute to a greater understanding of the role of metronomic therapies and disease control in the context of NB.
Dr. van Heerden, as staff member of the Department of Paediatric Haematology and Oncology, Antwerp University Hospital, University of Antwerp, acknowledges the department for research support. Our gratitude to Annamarie du Preez for language editing of the article.
Manuscript source: Unsolicited manuscript
Specialty type: Oncology
Country/Territory of origin: South Africa
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): B
Grade C (Good): 0
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Cenciarelli C S-Editor: Zhang H L-Editor: A P-Editor: Li JH
| 1. | Magrath I, Steliarova-Foucher E, Epelman S, Ribeiro RC, Harif M, Li CK, Kebudi R, Macfarlane SD, Howard SC. Paediatric cancer in low-income and middle-income countries. Lancet Oncol. 2013;14:e104-e116. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 236] [Cited by in RCA: 275] [Article Influence: 22.9] [Reference Citation Analysis (0)] |
| 2. | Global Burden of Disease Pediatrics Collaboration, Kyu HH, Pinho C, Wagner JA, Brown JC, Bertozzi-Villa A, Charlson FJ, Coffeng LE, Dandona L, Erskine HE, Ferrari AJ, Fitzmaurice C, Fleming TD, Forouzanfar MH, Graetz N, Guinovart C, Haagsma J, Higashi H, Kassebaum NJ, Larson HJ, Lim SS, Mokdad AH, Moradi-Lakeh M, Odell SV, Roth GA, Serina PT, Stanaway JD, Misganaw A, Whiteford HA, Wolock TM, Wulf Hanson S, Abd-Allah F, Abera SF, Abu-Raddad LJ, AlBuhairan FS, Amare AT, Antonio CA, Artaman A, Barker-Collo SL, Barrero LH, Benjet C, Bensenor IM, Bhutta ZA, Bikbov B, Brazinova A, Campos-Nonato I, Castañeda-Orjuela CA, Catalá-López F, Chowdhury R, Cooper C, Crump JA, Dandona R, Degenhardt L, Dellavalle RP, Dharmaratne SD, Faraon EJ, Feigin VL, Fürst T, Geleijnse JM, Gessner BD, Gibney KB, Goto A, Gunnell D, Hankey GJ, Hay RJ, Hornberger JC, Hosgood HD, Hu G, Jacobsen KH, Jayaraman SP, Jeemon P, Jonas JB, Karch A, Kim D, Kim S, Kokubo Y, Kuate Defo B, Kucuk Bicer B, Kumar GA, Larsson A, Leasher JL, Leung R, Li Y, Lipshultz SE, Lopez AD, Lotufo PA, Lunevicius R, Lyons RA, Majdan M, Malekzadeh R, Mashal T, Mason-Jones AJ, Melaku YA, Memish ZA, Mendoza W, Miller TR, Mock CN, Murray J, Nolte S, Oh IH, Olusanya BO, Ortblad KF, Park EK, Paternina Caicedo AJ, Patten SB, Patton GC, Pereira DM, Perico N, Piel FB, Polinder S, Popova S, Pourmalek F, Quistberg DA, Remuzzi G, Rodriguez A, Rojas-Rueda D, Rothenbacher D, Rothstein DH, Sanabria J, Santos IS, Schwebel DC, Sepanlou SG, Shaheen A, Shiri R, Shiue I, Skirbekk V, Sliwa K, Sreeramareddy CT, Stein DJ, Steiner TJ, Stovner LJ, Sykes BL, Tabb KM, Terkawi AS, Thomson AJ, Thorne-Lyman AL, Towbin JA, Ukwaja KN, Vasankari T, Venketasubramanian N, Vlassov VV, Vollset SE, Weiderpass E, Weintraub RG, Werdecker A, Wilkinson JD, Woldeyohannes SM, Wolfe CD, Yano Y, Yip P, Yonemoto N, Yoon SJ, Younis MZ, Yu C, El Sayed Zaki M, Naghavi M, Murray CJ, Vos T. Global and National Burden of Diseases and Injuries Among Children and Adolescents Between 1990 and 2013: Findings From the Global Burden of Disease 2013 Study. JAMA Pediatr. 2016;170:267-287. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 477] [Cited by in RCA: 429] [Article Influence: 47.7] [Reference Citation Analysis (0)] |
| 3. | Bollyky TJ, Templin T, Cohen M, Dieleman JL. Lower-Income Countries That Face The Most Rapid Shift In Noncommunicable Disease Burden Are Also The Least Prepared. Health Aff (Millwood). 2017;36:1866-1875. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 108] [Cited by in RCA: 143] [Article Influence: 20.4] [Reference Citation Analysis (0)] |
| 4. | Geel JA, Stevenson BT, Jennings RB, Krook LE, Winnan SJ, Katz BT, Fox TJ, Nyati L. Enough is not enough: Medical students' knowledge of early warning signs of childhood cancer. S Afr Med J. 2017;107:585-589. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4] [Cited by in RCA: 4] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 5. | Howard SC, Lam CG, Arora RS. Cancer epidemiology and the “incidence gap” from non-diagnosis. Pediatr Hematol Oncol J. 2018;3:75-78. [RCA] [DOI] [Full Text] [Cited by in Crossref: 4] [Cited by in RCA: 3] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 6. | Cao Y, Jin Y, Yu J, Wang J, Yan J, Zhao Q. Research progress of neuroblastoma related gene variations. Oncotarget. 2017;8:18444-18455. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 20] [Cited by in RCA: 26] [Article Influence: 3.3] [Reference Citation Analysis (1)] |
| 7. | Whittle SB, Smith V, Doherty E, Zhao S, McCarty S, Zage PE. Overview and recent advances in the treatment of neuroblastoma. Expert Rev Anticancer Ther. 2017;17:369-386. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 176] [Cited by in RCA: 279] [Article Influence: 34.9] [Reference Citation Analysis (0)] |
| 8. | Knaul FM, Arreola-Ornelas H, Rodriguez NM, Méndez-Carniado O, Kwete XJ, Puentes-Rosas E, Bhadelia A. Avoidable Mortality: The Core of the Global Cancer Divide. J Glob Oncol. 2018;4:1-12. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 9] [Cited by in RCA: 11] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 9. | Erdmann F, Feychting M, Mogensen H, Schmiegelow K, Zeeb H. Social Inequalities Along the Childhood Cancer Continuum: An Overview of Evidence and a Conceptual Framework to Identify Underlying Mechanisms and Pathways. Front Public Health. 2019;7:84. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 32] [Cited by in RCA: 34] [Article Influence: 5.7] [Reference Citation Analysis (0)] |
| 10. | National Cancer Institute. Neuroblastoma. [cited 22 July 2019]. Available from: https://www.cancer.gov/types/neuroblastoma. |
| 11. | Parikh NS, Howard SC, Chantada G, Israels T, Khattab M, Alcasabas P, Lam CG, Faulkner L, Park JR, London WB, Matthay KK; International Society of Pediatric Oncology. SIOP-PODC adapted risk stratification and treatment guidelines: Recommendations for neuroblastoma in low- and middle-income settings. Pediatr Blood Cancer. 2015;62:1305-1316. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 70] [Cited by in RCA: 67] [Article Influence: 6.7] [Reference Citation Analysis (0)] |
| 12. | Easton JC, Gomez S, Asdahl PH, Conner JM, Fynn AB, Ruiz C, Ojha RP. Survival of high-risk pediatric neuroblastoma patients in a developing country. Pediatr Transplant. 2016;20:825-830. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 7] [Cited by in RCA: 8] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 13. | Kulkarni KP, Marwaha RK. Outcome of neuroblastoma in India. Indian J Pediatr. 2013;80:832-837. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 8] [Cited by in RCA: 9] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 14. | Greengard E, Hill-Kayser C, Bagatell R. Treatment of high-risk neuroblastoma in children: recent clinic trial results. Clin Invest. 2013;3:1071–1081. [RCA] [DOI] [Full Text] [Cited by in Crossref: 3] [Cited by in RCA: 3] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 15. | Traoré F, Eshun F, Togo B, Yao JJA, Lukamba MR. Neuroblastoma in Africa: A Survey by the Franco-African Pediatric Oncology Group. J Glob Oncol. 2016;2:169-173. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 9] [Cited by in RCA: 10] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 16. | Parise IZ, Haddad BR, Cavalli LR, Pianovski MA, Maggio EM, Parise GA, Watanabe FM, Ioshii SO, Rone JD, Caleffe LG, Odone Filho V, Figueiredo BC. Neuroblastoma in southern Brazil: an 11-year study. J Pediatr Hematol Oncol. 2006;28:82-87. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 9] [Cited by in RCA: 10] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 17. | Shao JB, Lu ZH, Huang WY, Lv ZB, Jiang H. A single center clinical analysis of children with neuroblastoma. Oncol Lett. 2015;10:2311-2318. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 6] [Cited by in RCA: 10] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 18. | Li K, Dong K, Gao J, Yao W, Xiao X, Zheng S. Neuroblastoma management in Chinese children. J Invest Surg. 2012;25:86-92. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 8] [Cited by in RCA: 10] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 19. | Bansal D, Marwaha RK, Trehan A, Rao KL, Gupta V. Profile and outcome of neuroblastoma with convertional chemotherapy in children older than one year: a 15-years experience. Indian Pediatr. 2008;45:135-139. [PubMed] |
| 20. | Ahmad A, Asghar N, Masood N, Najamuddin, Khan FS, Rathore Z, Rathore AW. Clinical spectrum of Advanced Neuroblastoma. Journal of Rawalpindi Medical College (JRMC). 2017;21:229-232. |
| 21. | Shuangshoti S, Shuangshoti S, Nuchprayoon I, Kanjanapongkul S, Marrano P, Irwin MS, Thorner PS. Natural course of low risk neuroblastoma. Pediatr Blood Cancer. 2012;58:690-694. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 10] [Cited by in RCA: 11] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 22. | Bui C, Nguyen U, Trong D, Vo N. Neuroblastoma in Vietnam: A retrospective analysis of MYCN status and clinical features to inform prognosis and improve outcome. Pediatr Dimensions. 2019;4:1-6. [RCA] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 2] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 23. | Al-Tonbary Y, Badr M, Mansour A, El Safy U, Saeed S, Hassan T, Elashery R, Nofal R, Darwish A. Clinico-epidemiology of neuroblastoma in north east Egypt: A 5-year multicenter study. Oncol Lett. 2015;10:1054-1062. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 7] [Cited by in RCA: 10] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 24. | El-Sayed MI, Ali AM, Sayed HA, Zaky EM. Treatment results and prognostic factors of pediatric neuroblastoma: a retrospective study. Int Arch Med. 2010;3:37. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 15] [Cited by in RCA: 15] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 25. | Mehdiabadi GB, Arab E, Rafsanjani KA, Ansari S, Moinzadeh AM. Neuroblastoma in Iran: an experience of 32 years at a referral childrens hospital. Asian Pac J Cancer Prev. 2013;14:2739-2742. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 11] [Cited by in RCA: 12] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 26. | Abdallah BK, Rashid NG, Tawfiq SA. Neuroblastoma in Iraq-KRG-Sulaimani. J Cancer Sci Clin Ther. 2018;2:001-008. |
| 27. | Salman Z, Kababri M, Hessissen L, Khattab M. An Intensive Induction Protocol for High Risk Neuroblastoma in Morocco. J Glob Oncol. 2016;2 Suppl:80s-81s. [RCA] [DOI] [Full Text] [Cited by in Crossref: 5] [Cited by in RCA: 5] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 28. | Moussa E, Fawzy F, Younis A, Shafei M. Combined Treatment Strategy and Outcome of High-Risk Neuroblastoma: Experience of the Children’s Cancer Hospital-Egypt. J Cancer Ther. 2013;4:1435-1442. [RCA] [DOI] [Full Text] [Cited by in Crossref: 5] [Cited by in RCA: 6] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 29. | Moreno F, Marti JL, Palladino M, Lobos P, Gaultieri A, Cacciavillano W. Childhood Neuroblastoma: Incidence and Survival in Argentina. Report from the National Pediatric Cancer Registry, ROHA Network 2000–2012. Pediatr Blood Cancer. 2016;63:1362-1367. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 19] [Cited by in RCA: 19] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 30. | Lucena JN, Alves MTS, Abib SCV, Souza GO, Neves RPC, Caran EMM. Clinical and epidemiological characteristics and survival outcomes of children with neuroblastoma: 21 years of experience of the Instituto du Oncologia Pediatrica, Sao Paulo, Brazil. Rev Paul Pediatrics. 2018;36:254-260. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 8] [Cited by in RCA: 12] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 31. | Rice HE, Englum BR, Gulack BC, Adibe OO, Tracy ET, Kreissman SG, Routh JC. Use of patient registries and administrative datasets for the study of pediatric cancer. Pediatr Blood Cancer. 2015;62:1495-500. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 30] [Cited by in RCA: 26] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 32. | Juárez-Ocaña S, Palma-Padilla V, González-Miranda G, Siordia-Reyes AG, López-Aguilar E, Aguilar-Martínez M, Mejía-Aranguré JM, Carreón-Cruz R, Rendón-Macías ME, Fajardo-Gutiérrez A. Epidemiological and some clinical characteristics of neuroblastoma in Mexican children (1996-2005). BMC Cancer. 2009;9:266. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 13] [Cited by in RCA: 12] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 33. | de Camargo B, de Oliveira Ferreira JM, de Souza Reis R, Ferman S, de Oliveira Santos M, Pombo-de-Oliveira MS. Socioeconomic status and the incidence of non-central nervous system childhood embryonic tumours in Brazil. BMC Cancer. 2011;11:160. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 25] [Cited by in RCA: 27] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 34. | Torres P, Galán Y, Lence J, García M, Lezcano M, Fernández L. Childhood cancer incidence in Cuba, 2001 to 2003. MEDICC Rev. 2010;12:19-25. [PubMed] |
| 35. | Howard SC, Metzger ML, Wilimas JA, Quintana Y, Pui CH, Robison LL, Ribeiro RC. Childhood cancer epidemiology in low-income countries. Cancer. 2008;112:461-472. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 174] [Cited by in RCA: 174] [Article Influence: 10.2] [Reference Citation Analysis (0)] |
| 36. | Ramiandrisoa J, Jehanne M, Sauvat F, Reguerre Y, Chamouine A, Chirpaz E. Incidence and survival of childhood cancer in the French islands of Reunion and Mayotte (2005-2011). Cancer Epidemiol. 2017;49:61-65. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3] [Cited by in RCA: 3] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 37. | Stefan C, Bray F, Ferlay J, Liu B, Maxwell Parkin D. Cancer of childhood in sub-Saharan Africa. Ecancermedicalscience. 2017;11:755. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 55] [Cited by in RCA: 65] [Article Influence: 8.1] [Reference Citation Analysis (0)] |
| 38. | Spix C, Pastore G, Sankila R, Stiller CA, Steliarova-Foucher E. Neuroblastoma incidence and survival in European children (1978-1997): report from the Automated Childhood Cancer Information System project. Eur J Cancer. 2006;42:2081-2091. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 141] [Cited by in RCA: 137] [Article Influence: 7.2] [Reference Citation Analysis (0)] |
| 39. | Ward E, DeSantis C, Robbins A, Kohler B, Jemal A. Childhood and adolescent cancer statistics, 2014. CA Cancer J Clin. 2014;64:83-103. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1416] [Cited by in RCA: 1579] [Article Influence: 143.5] [Reference Citation Analysis (1)] |
| 40. | Stefan DC, Stones DK, Wainwright RD, Kruger M, Davidson A, Poole J, Hadley GP, Forman D, Colombet M, Steliarova-Foucher E. Childhood cancer incidence in South Africa, 1987-2007. S Afr Med J. 2015;105:939-947. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 27] [Cited by in RCA: 32] [Article Influence: 3.6] [Reference Citation Analysis (0)] |
| 41. | Johnsen JI, Dyberg C, Wickström M. Neuroblastoma-A Neural Crest Derived Embryonal Malignancy. Front Mol Neurosci. 2019;12:9. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 90] [Cited by in RCA: 169] [Article Influence: 28.2] [Reference Citation Analysis (0)] |
| 42. | Cheung NK, Zhang J, Lu C, Parker M, Bahrami A, Tickoo SK, Heguy A, Pappo AS, Federico S, Dalton J, Cheung IY, Ding L, Fulton R, Wang J, Chen X, Becksfort J, Wu J, Billups CA, Ellison D, Mardis ER, Wilson RK, Downing JR, Dyer MA; St Jude Children's Research Hospital–Washington University Pediatric Cancer Genome Project. Association of age at diagnosis and genetic mutations in patients with neuroblastoma. JAMA. 2012;307:1062-1071. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 316] [Cited by in RCA: 329] [Article Influence: 25.3] [Reference Citation Analysis (0)] |
| 43. | Hesseling PB, Ankone K, Wessels G, Schneider JW, Du Plessis L, Moore S. Neuroblastoma in southern Africa: epidemiological features, prognostic factors and outcome. Ann Trop Paediatr. 1999;19:357-363. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 11] [Cited by in RCA: 11] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 44. | Kitonyi GW, Macharia WM, Mwanda OW, Pamnani R. Clinico-pathologic characteristics and treatment outcomes in children with neuroblastoma at the Kenyatta National Hospital, Nairobi. East Afr Med J. 2009;86:S39-S46. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 4] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 45. | Panagopoulou P, Georgakis MK, Baka M, Moschovi M, Papadakis V, Polychronopoulou S, Kourti M, Hatzipantelis E, Stiakaki E, Dana H, Tragiannidis A, Bouka E, Antunes L, Bastos J, Coza D, Demetriou A, Agius D, Eser S, Gheorghiu R, Šekerija M, Trojanowski M, Žagar T, Zborovskaya A, Ryzhov A, Dessypris N, Morgenstern D, Petridou ET. Persisting inequalities in survival patterns of childhood neuroblastoma in Southern and Eastern Europe and the effect of socio-economic development compared with those of the US. Eur J Cancer. 2018;96:44-53. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 13] [Cited by in RCA: 11] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 46. | Henderson TO, Bhatia S, Pinto N, London WB, McGrady P, Crotty C, Sun CL, Cohn SL. Racial and ethnic disparities in risk and survival in children with neuroblastoma: a Children's Oncology Group study. J Clin Oncol. 2011;29:76-82. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 89] [Cited by in RCA: 110] [Article Influence: 7.3] [Reference Citation Analysis (0)] |
| 47. | Lanier AP, Holck P, Ehrsam Day G, Key C. Childhood cancer among Alaska Natives. Pediatrics. 2003;112:e396. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 44] [Cited by in RCA: 35] [Article Influence: 1.6] [Reference Citation Analysis (1)] |
| 48. | Valery PC, Youlden DR, Baade PD, Ward LJ, Green AC, Aitken JF. Cancer survival in Indigenous and non-Indigenous Australian children: what is the difference? Cancer Causes Control. 2013;24:2099-2106. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 15] [Cited by in RCA: 17] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 49. | Chatenoud L, Bertuccio P, Bosetti C, Levi F, Negri E, La Vecchia C. Childhood cancer mortality in America, Asia, and Oceania, 1970 through 2007. Cancer. 2010;116:5063-5074. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 40] [Cited by in RCA: 40] [Article Influence: 2.7] [Reference Citation Analysis (0)] |
| 50. | Ribeiro RC, Steliarova-Foucher E, Magrath I, Lemerle J, Eden T, Forget C, Mortara I, Tabah-Fisch I, Divino JJ, Miklavec T, Howard SC, Cavalli F. Baseline status of paediatric oncology care in ten low-income or mid-income countries receiving My Child Matters support: a descriptive study. Lancet Oncol. 2008;9:721-729. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 177] [Cited by in RCA: 192] [Article Influence: 11.3] [Reference Citation Analysis (0)] |
| 51. | Maris JM. Recent advances in neuroblastoma. N Engl J Med. 2010;362:2202-2211. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1588] [Cited by in RCA: 1479] [Article Influence: 98.6] [Reference Citation Analysis (0)] |
| 52. | Louis CU, Shohet JM. Neuroblastoma: molecular pathogenesis and therapy. Annu Rev Med. 2015;66:49-63. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 220] [Cited by in RCA: 263] [Article Influence: 23.9] [Reference Citation Analysis (0)] |
| 53. | Usmani GN. Pediatric oncology in the third world. Curr Opin Pediatr. 2001;13:1-9. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 25] [Cited by in RCA: 25] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 54. | Israels T, Ribeiro RC, Molyneux EM. Strategies to improve care for children with cancer in Sub-Saharan Africa. Eur J Cancer. 2010;46:1960-1966. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 47] [Cited by in RCA: 46] [Article Influence: 3.1] [Reference Citation Analysis (0)] |
| 55. | Smith MA, Altekruse SF, Adamson PC, Reaman GH, Seibel NL. Declining childhood and adolescent cancer mortality. Cancer. 2014;120:2497-2506. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 313] [Cited by in RCA: 357] [Article Influence: 32.5] [Reference Citation Analysis (0)] |
| 56. | Heck JE, Ritz B, Hung RJ, Hashibe M, Boffetta P. The epidemiology of neuroblastoma: a review. Paediatr Perinat Epidemiol. 2009;23:125-143. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 118] [Cited by in RCA: 110] [Article Influence: 6.9] [Reference Citation Analysis (0)] |
| 57. | Brodeur GM. Spontaneous regression of neuroblastoma. Cell Tissue Res. 2018;372:277-286. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 142] [Cited by in RCA: 138] [Article Influence: 19.7] [Reference Citation Analysis (0)] |
| 58. | Logie DE, Harding R. An evaluation of a morphine public health programme for cancer and AIDS pain relief in Sub-Saharan Africa. BMC Public Health. 2005;5:82. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 54] [Cited by in RCA: 56] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 59. | SIOPEN. European association involved in the research and care of children with neuroblastoma. [cited 28 October 2019]. Available from: https://www.siopen.net. |
| 60. | Radhakrishnan V, Raja A, Dhanushkodi M, Ganesan TS, Selvaluxmy G, Sagar TG. Real World Experience of Treating Neuroblastoma: Experience from a Tertiary Cancer Centre in India. Indian J Pediatr. 2019;86:417-426. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5] [Cited by in RCA: 5] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 61. | Friedman DL. High-risk neuroblastoma: challenges in management in low- and middle-income countries. Pediatr Transplant. 2016;20:742-743. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 1] [Article Influence: 0.1] [Reference Citation Analysis (0)] |
| 62. | Bhatnagar SN, Sarin YK. Neuroblastoma: a review of management and outcome. Indian J Pediatr. 2012;79:787-792. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 19] [Cited by in RCA: 18] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 63. | Statistics South Africa. General household survey 2010. In: stats sa. [cited 22 July 2019]. Available from: http://www.statssa.gov.za. |
| 64. | Swaminathan R, Sankaranarayanan R. Under-diagnosis and under-ascertainment of cases may be the reasons for low childhood cancer incidence in rural India. Cancer Epidemiol. 2010;34:107-108. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 19] [Cited by in RCA: 21] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 65. | Kamihara J, Ma C, Fuentes Alabi SL, Garrido C, Frazier AL, Rodriguez-Galindo C, Orjuela MA. Socioeconomic status and global variations in the incidence of neuroblastoma: call for support of population-based cancer registries in low-middle-income countries. Pediatr Blood Cancer. 2017;64:321-323. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 18] [Cited by in RCA: 18] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 67. | Poyiadjis S, Tuyisenge L, Stefan D. (2014) Early Warning Signs of Cancer in Children/Models for Early Diagnosis. Stefan D. Rodriguez-Galindo C. Pediatric Hematology-Oncology in Countries with Limited Resources. New York: Springer 2014; pp 65. |
| 68. | Viana MB, Fernandes RA, de Oliveira BM, Murao M, de Andrade Paes C, Duarte AA. Nutritional and socio-economic status in the prognosis of childhood acute lymphoblastic leukemia. Haematologica. 2001;86:113-120. [PubMed] |
| 69. | Louie DS, Liang JP, Owyang C. Characterization of a new CCK antagonist, L364, 718: in vitro and in vivo studies. Am J Physiol. 1988;255:G261-G266. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 82] [Cited by in RCA: 95] [Article Influence: 8.6] [Reference Citation Analysis (0)] |
| 70. | Mostert S, Sitaresmi MN, Gundy CM, Janes V, Sutaryo, Veerman AJ. Comparing childhood leukaemia treatment before and after the introduction of a parental education programme in Indonesia. Arch Dis Child. 2010;95:20-25. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 48] [Cited by in RCA: 48] [Article Influence: 3.2] [Reference Citation Analysis (0)] |
| 71. | Arora RS, Pizer B, Eden T. Understanding refusal and abandonment in the treatment of childhood cancer. Indian Pediatr. 2010;47:1005-1010. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 51] [Cited by in RCA: 53] [Article Influence: 3.8] [Reference Citation Analysis (0)] |
| 72. | Peltzer K. Utilization and practice of traditional/complementary/alternative medicine (TM/CAM) in South Africa. Afr J Tradit Complement Altern Med. 2009;6:175-185. [PubMed] |
| 73. | Hiatt RA, Breen N. The social determinants of cancer: a challenge for transdisciplinary science. Am J Prev Med. 2008;35:S141-S150. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 90] [Cited by in RCA: 86] [Article Influence: 5.1] [Reference Citation Analysis (0)] |
| 74. | Coovadia H, Jewkes R, Barron P, Sanders D, McIntyre D. The health and health system of South Africa: historical roots of current public health challenges. Lancet. 2009;374:817-834. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 710] [Cited by in RCA: 660] [Article Influence: 41.3] [Reference Citation Analysis (0)] |
| 75. | Carter NH, Avery AH, Libes J, Lovvorn HN, Hansen EN. Pediatric Solid Tumors in Resource-Constrained Settings: A Review of Available Evidence on Management, Outcomes, and Barriers to Care. Children (Basel). 2018;5. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 6] [Cited by in RCA: 6] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 76. | Liu Z, Thiele CJ. Molecular Genetics of Neuroblastoma. Diagnostic and Therapeutic Nuclear Medicine for Neuroendocrine Tumors. In: Pacak K, Taïeb D. Diagnostic and Therapeutic Nuclear Medicine for Neuroendocrine Tumors, Contemporary Endocrinology. Switzerland: Springer International Publishing, Cham, 2017: 83-125. |
| 77. | Pasquier E, Street J, Pouchy C, Carre M, Gifford AJ, Murray J, Norris MD, Trahair T, Andre N, Kavallaris M. β-blockers increase response to chemotherapy via direct antitumour and anti-angiogenic mechanisms in neuroblastoma. Br J Cancer. 2013;108:2485-2494. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 107] [Cited by in RCA: 128] [Article Influence: 10.7] [Reference Citation Analysis (0)] |
| 78. | Yifru S, Muluye D. Childhood cancer in Gondar University Hospital, Northwest Ethiopia. BMC Res Notes. 2015;8:474. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 14] [Cited by in RCA: 24] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 79. | Ibrahim M, Abdullahi SU, Hassan-Hanga F, Atanda A. Pattern of childhood malignant tumors at a teaching hospital in Kano, Northern Nigeria: A prospective study. Indian J Cancer. 2014;51:259-261. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 1] [Article Influence: 0.1] [Reference Citation Analysis (0)] |