Published online Aug 24, 2020. doi: 10.5306/wjco.v11.i8.528
Peer-review started: January 3, 2020
First decision: April 3, 2020
Revised: July 1, 2020
Accepted: July 19, 2020
Article in press: July 19, 2020
Published online: August 24, 2020
Processing time: 230 Days and 4.6 Hours
Spurred by better understanding of disease biology, improvements in molecular diagnostics, and the development of targeted therapies, the treatment of acute myeloid leukemia (AML) has undergone significant evolution in recent years. Arguably, the most exciting shift has come from the success of treatment with the B-cell lymphoma-2 inhibitor venetoclax. When given in combination with a hypomethylating agent or low dose cytarabine, venetoclax demonstrates high response rates, some of which are durable. In spite of this, relapses after venetoclax treatment are common, and much interest exists in elucidating the mechanisms of resistance to the drug. Alterations in leukemic stem cell metabolism have been identified as a possible escape route, and clinical trials focusing on targeting metabolism in AML are ongoing. This review article highlights current research regarding venetoclax treatment and resistance in AML with a focus on cellular metabolism.
Core tip: The B-cell lymphoma-2 inhibitor venetoclax has drastically changed the treatment paradigm for acute myeloid leukemia; however, much is unknown about mechanisms of relapse after treatment with this agent. Alterations in cellular metabolism have been identified as a potential resistance mechanism and may be able to be targeted with novel treatments.
- Citation: Wilde L, Ramanathan S, Kasner M. B-cell lymphoma-2 inhibition and resistance in acute myeloid leukemia. World J Clin Oncol 2020; 11(8): 528-540
- URL: https://www.wjgnet.com/2218-4333/full/v11/i8/528.htm
- DOI: https://dx.doi.org/10.5306/wjco.v11.i8.528
Acute myeloid leukemia (AML) is a heterogeneous group of aggressive hematologic malignancies characterized by the uncontrolled proliferation of genetically altered immature myeloid cells. Accumulated clonal leukemic stem cells (LSC) are inherently nonfunctional and arrested in differentiation causing rapid bone marrow failure and, if untreated, eventual death[1].
An estimated 21500 patients are diagnosed with AML yearly in the United States[2]. Despite advances in molecular prognostication and therapeutic targeting, AML remains a significant cause of morbidity and mortality. The current 5-year survival rate remains < 30%[3]. Patients above age 65 and those with poor performance status, pre-existing comorbidities, or biologically aggressive disease have especially poor prognoses, as do patients who relapse after hematopoietic stem cell transplant[4].
Since the first publication by Yates et al[5] in 1973, the standard therapeutic approach for treating AML has relied upon intensive induction chemotherapy with the 7 + 3 protocol, a cytarabine and anthracycline based regimen. Individuals who were unable to tolerate intensive chemotherapy had few options[5]. It has only been in the last decade that a myriad of new drugs have changed this paradigm and gained approval for the treatment of AML.
Despite improvements in the success of up-front AML therapy, treatment for relapsed disease remains a significant challenge. Relapse occurs due to the emergence of chemotherapy resistant leukemic stem cells[6]. Over the past decade, much has been learned about the complexity of the metabolic and molecular transformations that LSCs undergo. Interestingly, some of the same metabolic dysregulations are seen in other malignancies including colon, breast, and prostate cancer[7]. Whole-genome mapping and targeted sequencing of serial samples of leukemia cells from individual patients has led to the discovery of distinct metabolic aberrations that play a role in relapse and, in some cases, are targets for drug development[8].
Novel therapies such as venetoclax, a specific B-cell lymphoma-2 (Bcl-2) inhibitor, have triggered a paradigm shift in the approach to AML and reinvigorated discussions about the link between metabolism and cancer. Though the majority of patients respond to venetoclax-based treatment, the depth and duration of response remain inadequate[9]. Thus, understanding the metabolic rewiring that allows treatment resistance to develop is crucial. This review summarizes Bcl-2 inhibition in AML with a focus on mechanisms of resistance to venetoclax, in particular those related to leukemic cell metabolism.
During evolution from normal hematopoietic progenitors to LSCs, cells undergo significant alterations in metabolic pathways including glycolysis, amino acid metabolism, and fatty acid metabolism. Similar to normal progenitors, primitive LSCs retain the ability to self-renew and remain in the G0 phase, allowing them to escape eradication by cytotoxic chemotherapy, which targets actively dividing blasts[10].
Leukemogenic cells exist in a stressful hypoxic microenvironment and, in response, upregulate certain energy producing conduits to meet proliferative demand. Enhanced glycolysis plays a prime role in LSC proliferation. Increased glucose flux is directed by activated oncogenes, particularly expression of BCR-ABL and MLL-AF9, along with overexpression of hypoxia inducible factor 1[11]. These genes upregulate glucose transporter 1 receptor expression, thereby promoting glucose entry and subsequent phosphorylation by hexokinase. Increased levels of hypoxia inducible factor 1, hexokinase, and genes upregulate glucose transporter 1 are described in patients with relapsed AML with poor response to chemotherapy[12]. In vivo studies of aggressive leukemia cells have demonstrated a correlation between high glycolysis flux and decreased levels of autophagy, an evolutionary intracellular degradation process that is bypassed by LSCs[13].
Historically, it has been thought that malignant cells preferentially use cytoplasmic anaerobic glycolysis as a major carbon source (the so-called Warburg effect) over mitochondrial oxidative phosphorylation (OX-PHOS)[14]. However, metabolomic studies have shown that mitochondrial OX-PHOS may be upregulated in LSCs as an adaptive mechanism[15]. Excess oxidative stress has been described in various hematologic malignancies as a critical factor in initiation and progression of disease. There is growing evidence showing that AML LSCs generate increased levels of reactive oxygen species (ROS) primarily driven by mitochondrial NADPH oxidase and other pro-oxidant mechanisms. Sallmyr et al[16] suggest that acquired genetic changes in myeloid malignancies lead to DNA damage and defective repair by directly increasing ROS production. Certain genetic abnormalities in AML such as RAS, IDH1/IDH2 and fms-like tyrosine kinase 3 (FLT3)/ITD mutations can directly disturb ROS metabolism causing an eventual shift to amplified ROS production[16].
Interestingly, the majority of LSCs preferentially maintain a low ROS state due to their quiescent nature. These low ROS LSCs were isolated ex vivo and subject to gene expression studies using RNA sequencing methods. Remarkably, they displayed a uniform overexpression of the Bcl-2 protein without upregulation of other anti-apoptotic members[17,18].
The non-essential acid glutamine can be metabolized by glutaminases to glutamate and then α-ketoglutarate, which can go on to fuel the tricarboxylic acid cycle in the mitochondria[19]. To sustain high proliferative advantage, LSCs may adapt a metabolic preference for glutamine to drive biomass. This so-called glutamine addiction has been demonstrated in multiple studies and represents a potential target for anti-leukemic therapy[20-23].
A number of oncogenes and pathways work to potentiate glutamine addiction in AML, including FLT3. In fact, metabolomic studies reveal that FLT3 inhibited LSCs are impaired in their glycolytic function and fittingly switch to utilize glutamine as primary fuel. Therefore, this metabolic dependency on glutamine metabolism poses a potential therapeutic vulnerability when targeted with FLT3 inhibition[24]. Concurrent reduction of glutamine and Bcl-2 inhibition are being studied to compromise mitochondrial energy production and induce apoptosis, respectively[23].
The mammalian target of rapamycin 1 (mTORC1) signaling pathway is involved in numerous cellular processes including metabolism, cell growth, and apoptosis. Moreover, it has been shown to play an integral role in LSC development and proliferation[25-27]. Glutamine availability is a rate-limiting step for mTORC1; therefore, removal of glutamine accordingly inhibits mTORC1 signaling and may be another metabolic mechanism for the treatment of AML[28].
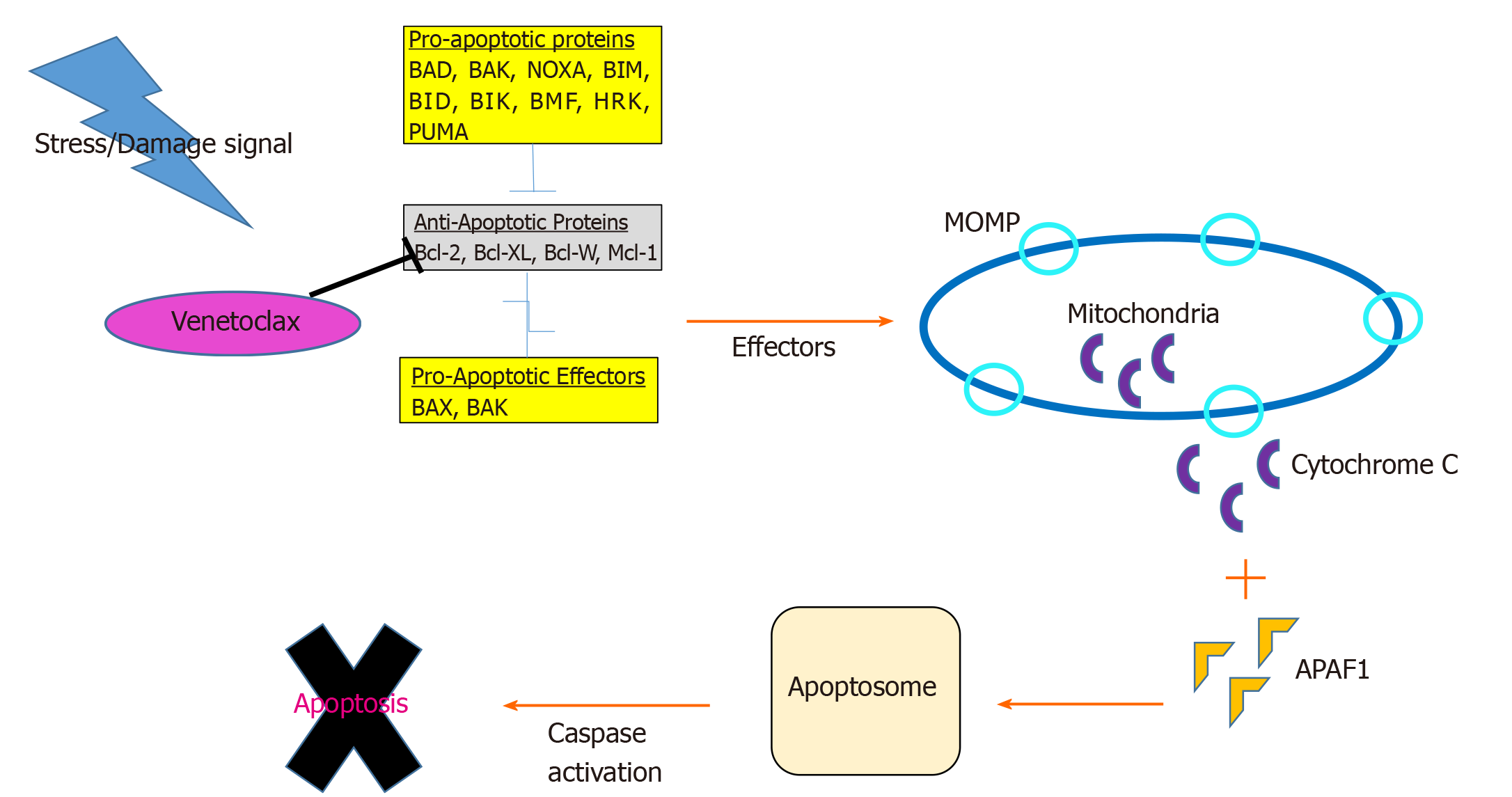
Control of cellular proliferation and apoptosis is deregulated in cancer cells. Mitochondria play an intrinsic role in programmed cell death through release of soluble proteins from the intermembrane space, a process called mitochondrial outer membrane permeabilization (MOMP). A group of over 20 specialized proteins, known as the Bcl-2 family, are the prime mediators of this process[29] (Figure 1).
Apoptosis is tightly regulated by an intricate balance between pro-apoptotic Bax-like proteins (e.g., BAX, BAK and BAD) and anti-apoptotic Bcl-2 like proteins (e.g., Bcl-2, Bcl-XL, Bcl-W and MCL-1) which are predominantly localized in the mitochondria. Bcl-2 prevents apoptosis by inactivating BAX and BAK. Bcl-XL blocks apoptosis by rendering mitochondrial pores impermeable thus inhibiting cytochrome C release. BAX and BAK proteins promote apoptosis by simply opposing Bcl-2 and forming oligomeric pores essential in MOMP[30].
Each of these apoptotic proteins are structurally distinguished by four groups of Bcl-2 homology (BH) domain (1-4). Functionally these BH domains, specifically the “BH3-only proteins” (e.g., BID, BIM, BAD, PUMA, NOXA and BIK/NBK), sense cellular stress, activate pro-death signals, and coordinate the activity of other Bcl-2 proteins[31]. The binding of apoptotic proteins is highly selective: BAD binds exclusively to Bcl-2, Bcl-XL, and Bcl-W, NOXA to MCL-1 and A1, and BIM can bind to all anti-apoptotic members[32]. Upstream of the intrinsic Bcl-2 pathway, PUMA serves as a critical mediator of cell death via p53-dependent and independent activation of BAX, BAK and dismissing inhibition of Bcl-2 family proteins. Most BH3-only proteins exist in an ambiguous conformation and at relatively low levels. Chemotherapeutic agents induce activation of BH3 only proteins to overcome the anti-apoptotic threshold resulting in cell death[33].
In response to cellular derangement, BH3-only proteins concurrently inhibit anti-apoptotic members and activate pro-apoptotic members, BAX and BAK. Intracytoplasmic signaling leads to transformation of BAX into homo-oligomers and translocation of the proteins into the mitochondrial membrane forming pores to induce MOMP. As a result, voltage dependent anion channels are unlocked facilitating release of cytochrome C into cytosol, binding to apoptotic protease-activating factor 1, apoptosome formation, caspase activation, DNA fragmentation, and ultimately cell death[30].
Mitochondrial response to pro-apoptotic members, a process known as “priming”, has been studied as a measure of sensitivity to chemotherapy. Artificial priming of myeloblast mitochondria with BH3-only proteins (BIM or BAD BH3-peptide) supported the hypothesis that Bcl-2 inhibition may be a powerful strategy in targeting AML cells. Analysis of poorly primed, chemo-refractory AML cells showed increased sensitivity to BAD BH3-peptide mediated killing with potential for BH3 mimetic benefit even in low-primed AML[34]. Knowing the level and specificity of priming prior to treatment may help in predicting the synergistic action of chemotherapeutic agents and Bcl-2 inhibitors. This functional approach to predicting mitochondrial response to BH3 peptides, termed BH3 profiling, could distinguish alterations between AML myeloblasts and HSCs. Certain BH3 peptides used for profiling inhibit selective Bcl-2 family proteins (e.g., BAD BH3 peptide indicates dependence on Bcl-2, Bcl-XL, or Bcl-w)[35]. MOMP induced by targeting such peptides hints at specific dependence on certain anti-apoptotic proteins through which they inhibit cell death[36].
Human LSCs were first discovered to modify expression of death receptors (e.g., FAS and TRAIL receptors) to evade apoptosis. LSCs with very immature phenotype of CD34+/CD38- were able to confer both chemotherapy resistance and decreased capacity to induce Fas-induced apoptosis[37]. Prominently, alteration of the Bcl-2 mediated pro-survival pathway and variant expression of effector proteins (BAX and BAK) are potent methods employed by LSCs to inactivate death signals. Bcl-2 is normally expressed in early myeloid progenitors but downregulated during myeloid differentiation. However, transgenic studies have shown that overexpression of Bcl-2 protects LSCs from various apoptosis-inducing stimuli[38]. Bcl-2 overexpression leads to increased LSC numbers in the bone marrow and enhanced colony formation in vitro and in vivo[39]. Remarkably, the bone marrow stromal microenvironment may facilitate this mechanism. Leukemic blasts thrive by exhibiting a higher degree of Bcl-2 when co-cultured with stromal cells. It is therefore possible that eliminating Bcl-2 protein function can eradicate early LSCs[40].
In 2005, ABT-737, a high-affinity small molecule Bcl-2/Bcl-XL/Bcl-W inhibitor, demonstrated single agent mechanistic killing of lymphoma and various solid tumor cell lines. Later studies demonstrated effective killing of primitive CD34+/CD38- populations with independent and synergistic action of conventional chemotherapeutics. Remarkably, this disruption was specific to LSCs without apparent damage to normal HSCs[41]. Certain LSCs with increased MCL-1 and phosphorylated Bcl-2 were unaltered by ABT-737, proposing a potential co-target to bypass resistance in AML[42].
Progenitor blasts and chemo-resistant LSCs are heterogenous and possess a certain degree of metabolic plasticity. As discussed, LSCs adapt to rely on OX-PHOS as their predominant source of carbon as suggested by high mitochondrial mass and increased oxygen consumption[43]. Chemically blocking Bcl-2 causes prompt and severe impairment of OX-PHOS with the potential to cut off a major power source for LSCs[17].
Bcl-2 dependence has been described as a hallmark of multiple hematologic malignancies including AML. This led to the study of venetoclax (ABT-199), an oral Bcl-2 inhibitor, as a single agent and in combination with hypomethylating agents for the treatment of AML. Venetoclax is highly specific for Bcl-2 but also inhibits several other members of the Bcl family, including Bcl-W[17,44]. Strong preclinical data was evidenced by a median IC50 of approximately 10 nmol/L, and mitochondrial apoptosis occurring within 2 h of exposure[45].
Venetoclax monotherapy was first studied in high-risk relapsed/refractory AML patients and was found to have an underwhelming overall response rate of 19%[46]. Given these results, the success of the combination of venetoclax with a hypomethylating agent (HMA) (either 7 d of azacitidine or 5 d of decitabine) or low-dose cytarabine in newly diagnosed, elderly AML patients was somewhat unexpected. Studies have demonstrated 50%-70% response rates for combination therapy in this high-risk population[47,48]. In addition, in the HMA + venetoclax study, median overall survival was increased by 17.5 mo (double that of an HMA alone)[47]. These pivotal results led, in November 2018, to the FDA approval of the combination of venetoclax plus an HMA or low dose cytarabine combo for adults > 75 years who are not candidates for intensive induction chemotherapy[44]. Patients with mutations in FLT3, IDH1/2, or mutations in the nucleophosmin gene were noted to have the most favorable responses[47].
Interim results from a Phase II study of ten-day decitabine plus venetoclax were recently presented and build upon the results of these initial studies. In this heterogeneous cohort of patients, those with newly diagnosed de novo AML had a CR/CRi rate of 95%. Furthermore, 80% of these patients became MRD negative and 90% were alive at 6 mo[49].
Unfortunately, retrospective results for venetoclax combination therapy in the relapsed/refractory setting have not been as promising, however, prospective studies are ongoing. Overall response rates in these patients, some of whom have been heavily pre-treated, range from 21%-64%[50,51]. Patients with secondary AML and those whose AML harbors a TP53 mutation have the poorest responses[52]. Identifying the reasons for the disparity between response rates in newly diagnosed and relapsed disease has been the focus of much investigation, and has centered on a discussion of leukemic cell metabolism.
There is increasing interest in understanding the mechanisms underlying venetoclax resistance. Genomic and protein analyses of expanding clones of LSCs after venetoclax treatment have identified a variety of potential adaptive mechanisms, including alterations in leukemic cell metabolism.
Initial hypotheses about resistance mechanisms focused on alterations in BH3-family protein expression. Reductions in Bcl-2 expression have been shown to promote primary and acquired resistance to venetoclax by alternate pathway activation and upregulated expression of other anti-apoptotic proteins such as MCL-1 and Bcl-xL[53]. A study of AML cell lines in vitro showed a definite and inverse correlation with the ratios of Bcl-2/MCL-1 transcripts and venetoclax sensitivity suggesting the importance of MCL-1 effect on sensitivity[54]. Similarly, Niu et al[55] demonstrated that Bcl-2/MCL-1 transcript ratio may represent a potential biomarker in predicting response[55]. As such, methodical targeting of MCL-1 during venetoclax therapy may delay the acquisition of venetoclax resistance[56]. However, MCL-1 upregulation is only part of the venetoclax resistance story.
To try and better understand the basis of resistance, Chen et al[57] performed a genome-wide CRISPR/Cas9 loss of function screen in venetoclax-sensitive and venetoclax resistant clones (VRCs). The analysis demonstrated that specific genes involved in mitochondrial physiology, namely CLPB with HAX1, contribute to development of VRCs. CLPB, also known as chaperonine, is a protein-coding gene thought to maintain mitochondrial integrity by preventing the release of cytochrome C following death stimulus. Loss of CLPB impairs mitochondrial structure thereby triggering defective OXPHOS and glycolysis. CLPB was notably upregulated in VRCs suggesting a potential dependency and an amenable target. Correspondingly, analysis of CLPB-deficient AML cells showed that they were more sensitive to venetoclax treatment[57].
Similarly, Sharon et al[58], performed a genome-wide CRISPR knockout screen to look for potential genes that could be inactivated to reestablish venetoclax sensitivity[58]. Interestingly, a glycine-to-valine mutation at amino acid position 101 was not identified in the Bcl-2 gene of VRCs. This mutation was previously proposed as an acquired venetoclax resistance mechanism in chronic lymphocytic leukemia[59]. Instead, multiple genes-DAP3, MRPL54, MRPL17, and RBFA- encoding key parts of the mitochondrial translation apparatus were identified. LSCs exposed to the bacterial mitochondrial ribosome inhibitors tedizolid and doxycycline, both alone and in combination with venetoclax, showed a depleted CD34+ fraction with combination therapy, but not with venetoclax alone, suggesting that pharmacologic inhibition of mitochondrial translation may overcome resistance[58].
Findings of a study by Pollyea et al[60] demonstrated that deeper and more durable responses to treatment with venetoclax and azacitadine were due to effective eradication of OXPHOS dependence. Direct in vitro measurement of ETC complex II activity and SDHA glutathionylation in primary AML cells upon venetoclax and azacitidine exposure confirmed decreased glutathione levels and correlating reduction in ETC activity[60]. However, Jones et al[61] showed that OXPHOS levels in the LSCs of patients with relapsed AML are not reduced after HMA and venetoclax exposure suggesting that altered metabolism is an escape route for LSCs[61]. Further evaluation of these LSCs identified an increased reliance on fatty acid metabolism, which may be targetable.
Clinically, combining venetoclax with one or more other agents may be the key to overcoming resistance; many studies of this kind are underway[62]. A comprehensive list of is found in Table 1.
| ClinicalTrials.gov identifier | Treatment combination | Phase | Population |
| NCT03709758 | Venetoclax + daunorubicin + cytarabine | Ib | Untreated |
| NCT03214562 | Venetoclax + fludarabine, cytarabine, filgrastim, idarubicin | Ib/II | Untreated Relapsed/refractory |
| NCT03471260 | Venetoclax + ivosidenib ± azacitidine | I/II | Relapsed/refractory |
| NCT02993523 | Venetoclax + placebo or azacitidine | III | Untreated |
| NCT03069352 | Venetoclax + placebo or low dose cytarabine | III | Untreated |
| NCT03466294 | Venetoclax + azacitidine | II | Untreated-elderly |
| NCT03404193 | Venetoclax + decitabine 10 d | II | Untreated |
| NCT03586609 | Venetoclax + low dose cytarabine+ cladribine + azacitidine | II | Untreated |
| NCT03236857 | Venetoclax ± chemotherapy (various) | I | Relapsed/refractory malignancies (including AML) |
| NCT03455504 | Venetoclax + fludarabine + cytarabine + idarubicin | II | Untreated |
| NCT03629171 | Venetoclax + liposomal daunorubicin -cytarabine | II | Untreated Relapsed/refractory |
| NCT03862157 | Venetoclax + azacitidine + pevonedistat | I/II | Untreated |
| NCT03390296 | Venetoclax + azacitidine + avelumab | I/II | Untreated |
| NCT03390296 | Venetoclax + azacitidine + gemtuzumab ozogamicin + anti-OX40 antibody | I/II | Relapsed/refractory |
| NCT03867682 | Venetoclax + lintuzumab-Ac225 | I/II | Relapsed/refractory |
| NCT03932318 | Venetoclax + azacitidine + lintuzumab-Ac225 | I/II | Relapsed/refractory |
| NCT03672695 | Venetoclax + S64315 | I | Relapsed/refractory |
| NCT03797261 | Venetoclax + AMG-176 | Ib | Relapsed/refractory |
| NCT03063944 | Venetoclax + decitabine + OPB-111077 | Ib/II | Relapsed/refractory |
| NCT03484520 | Venetoclax + dinaciclib | Ib | Relapsed/refractory |
| NCT03441555 | Venetoclax + alvocidib | Ib | Relapsed/refractory |
| NCT02670044 | Venetoclax + cobimetinib; Venetoclax + idasanutlin | I/II | Relapsed/refractory |
| NCT03940352 | Venetoclax + HDM201 | I | Relapsed/refractory |
| NCT03874052 | Venetoclax + ruxolitinib | I | Relapsed/refractory |
| NCT03471260 | Venetoclax + ivosidenib | Ib/II | Relapsed/refractory |
| NCT04092179 | Venetoclax + enasidenib | Ib/II | Relapsed/refractory |
| NCT03735875 | Venetoclax + quizartinib | Ib/II | Relapsed/refractory |
| NCT03625505 | Venetoclax + gilteritinib | I | Relapsed/refractory |
Exploiting dependency on OXPHOS concurrently with Bcl-2 inhibition is a potential therapeutic strategy. Preclinical combination of OPB-111077, an OXPHOS inhibitor, with decitabine synergistically hindered the proliferation LSCs with a tolerable side effect profile. Triplet therapy with OPB-111077 + HMA and venetoclax in AML cells increased apoptosis rates to a greater degree than exposure to single agent OPB-111077 or venetoclax[63]. A Phase I study of the triplet is ongoing.
The OXPHOS inhibitor IACS-010759 is another small molecule with promising in vivo and in vitro activity in LSCs in AML cell lines. This agent binds and inhibits complex I of the electron transport chain (NADH ubiquinone oxidoreductase) and is being studied in a phase I study of patients with relapsed/refractory AML. Safety is yet to be established with dose escalation, but mechanistically this is a sensible combination strategy with venetoclax[64].
Metformin, a biguanide used in diabetes management, has shown potential for anti-leukemic activity by directly targeting electron transport chain complex I activity and inhibition of constitutive mTOR activation. This in turn induces AMPK-independent apoptosis. Promising combination strategies with chemotherapy or other targeted therapies have been described with all-trans retinoic acid, ABT-737 (Bcl-2 inhibitor) and sorafenib in acute promyelocytic leukemia, T-cell acute lymphoblastic leukemia, and FLT3-ITD positive AML[65]. Given its mechanism of action, the combination of metformin with venetoclax may be effective.
Finally, as discussed earlier, CLPB targeting can compromise mitochondrial matrix adding to Bcl-2 inhibition. Interestingly, a bacterial CLPB inhibitor has been developed and proposed as an antimicrobial agent with possible use in this setting[57].
In vitro studies conducted in AML cell lines and patient-derived AML samples have shown that venetoclax in combination with daunorubicin or cytarabine reduced MCL-1 protein levels resulting in increased DNA damage[66]. Preclinical synergy translated to the clinical setting in an open label, multicenter trial study with 82 patients in which CR rate was 54% with a median OS of 10.1 mo. Lower response rates were observed for patients with prior hypomethylating agents[67]. Investigations for Venetoclax with daunorubicin/cytarabine (7 + 3) and consolidation therapy are currently underway.
MCL-1 inhibitors are under development to target VRCs. Direct MCL-1 inhibition with S63845 and A-1210477 plus venetoclax leads to synergistic cell killing of VRCs in vivo and in vitro. Additionally, several studies demonstrate preclinical synergy of A-1210477 and venetoclax where successful neutralization of MCL-1-dependent AML cells have been demonstrated[68]. Dual inhibition of Bcl-2 and MCL-1 (with S55746 and S63845, respectively) has also shown strong activity against LSCs with relative sparing of normal progenitors. Researchers observed prolonged survival of xenograft models of AML with this combination[69].
More recent studies suggest synergy between venetoclax and inhibitors of Cyclin-dependent kinase 9 (CDK9), a transcriptional regulator of MCL-1, via indirect targeting of MCL-1. Drivers of LSC survival like MCL-1 and MYC have very short half-lives making them expeditious targets to CDK9 inhibition. Alvocidib, aka flavopiridol, was the first of the CDK9 agents tested in combination with conventional chemotherapy[70]. A newer agent voruciclib that inhibits CDK9, 4, and 6 kinase diminishes transcription of MCL-1 downstream with better toxicity profile in comparison[71].
Based on preclinical data, mitogen activated protein kinase pathway inhibitors such as cobimetinib (also a MEK1/2 inhibitor) have been studied with concomitant targeting of Bcl-2 in relapsed or refractory AML. Padua et al[72] demonstrated disruption of the RAS/Bcl-2 complex in AML patient derived samples suggesting potential efficacy of the combination[72]. Likewise, Han et al[73] studied co-targeting of Bcl-2 and mitogen activated protein kinase in Bcl-2 protein enriched leukemic cells and synergistic killing was appreciated with over 60% growth inhibition in AML samples, including VRCs[73]. Preliminary phase 1B clinical trial results, however, revealed increased gastrointestinal toxicity, mainly diarrhea, associated with cobimetinib[74]. Newer MAP kinase inhibitors with better safety profiles are currently under development.
Dual Bcl-2 and phosphatidylinositol-3-kinase (PI3K/AKT) inhibition may help overcome both acquired and intrinsic venetoclax resistance requiring and is being evaluated in AML[75]. Co-administration of venetoclax and apitolisib (GDC-0980:PI3K/mTOR inhibitor) or taselisib (GDC-0032: p110β-sparing PI3K inhibitor) induced profound cytochrome C release and apoptosis in various AML cell lines. AKT/mTOR inactivation and MCL-1 downregulation were also noted, with BAX and BAK mediated apoptosis of a CD34+/38-/123+ population while sparing the normal HSCs.
Small molecule mouse double minute 2 homolog (MDM2) antagonists reactivate the tumor suppressor function of wildtype-p53 leading to downstream stimulation of pro-apoptotic BAX and NOXA. Further apoptotic pathways are promoted, like PUMA and BAD, to stabilize and degrade MCL-1. Studies with a combination of Nutlin-3a, a first-generation MDM2 inhibitor, and ABT-737, a Bcl-2 inhibitor, published a decade ago displayed durable induction of mitochondrial apoptosis of AML cells by the combination[76]. Given preclinical rationale, researchers tested the combination of Bcl-2 and MDM2 inhibition (by idasanutlin) in wildtype-AML to boost activity of venetoclax and prevent upfront resistance[77]. Safety and efficacy of venetoclax and idasanutlin has been studied in 39 patients with relapsed refractory elderly AML patients. Overall response rate was 46% with superior responses in IDH1/2, RUNX1, JAK2, MPL, and CALR mutations. TP53 and FLT3 mutations were associated with primary or secondary refractoriness[78]. Additionally, updated data in both safety and efficacy appears to show reasonable tolerance to MDM2 and Bcl-2 inhibition.
JAK inhibitors may combine with venetoclax to counteract bone marrow stroma-mediated resistance in AML. Cytokines activated by JAK/STAT signaling like GM-CSF support AML cell proliferation and switch dependency of Bcl-2 to Bcl-XL[79]. Correspondingly, ex vivo studies of isolated AML blasts expressed sensitivity to venetoclax + ruxolinitib combination as an effective method of killing[80].
The small molecule IDH inhibitors enasidenib (IDH2) and ivosidenib (IDH1) are FDA approved for the treatment of AML. Inhibition of altered IDH1 and IDH2 enzymes along with hypomethylated genes can allow differentiation of LSCs[81]. Studies investigating safety and tolerability of IDH1 and Bcl-2 inhibition are currently ongoing with ivosidenib and venetoclax, respectively[82].
Sequencing studies were performed to assess the combination of venetoclax and the small molecule FLT3 inhibitor quizartinib in specific FLT3 ITD mutated xenograft models. The combination induced durable tumor regression for up to 3 mo after cessation of treatment[83]. However, Chyla et al[84] noted that FLT3-ITD or PTPN11 mutations may confer intrinsic and acquired resistance to venetoclax[84]. Clinical trials evaluating venetoclax and FLT3 inhibitor combination therapy are ongoing[9].
Up-front AML treatment with venetoclax in combination with a hypomethylating agent has shown impressive responses in multiple trials. Unfortunately, response durations are variable and patients still inevitably relapse. Attempts at identifying the cellular and molecular changes that occur after exposure to venetoclax have provided insight into mechanisms of resistance, namely alterations in LSC metabolism. Improved techniques to understand mitochondrial adaptations and the stromal microenvironment may aid in designing new therapeutic strategies. With more potent BH3 mimetics in development and rational combination therapies under investigation, the right strategy for building on the success of venetoclax treatment in AML is within reach.
Manuscript source: Invited manuscript
Specialty type: Oncology
Country/Territory of origin: United States
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): B
Grade C (Good): 0
Grade D (Fair): D
Grade E (Poor): 0
P-Reviewer: Eleftheriadis T, Ju SQ S-Editor: Zhang L L-Editor: A P-Editor: Li JH
| 1. | Grove CS, Vassiliou GS. Acute myeloid leukaemia: a paradigm for the clonal evolution of cancer? Dis Model Mech. 2014;7:941-951. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 147] [Cited by in RCA: 136] [Article Influence: 12.4] [Reference Citation Analysis (0)] |
| 2. | Thein MS, Ershler WB, Jemal A, Yates JW, Baer MR. Outcome of older patients with acute myeloid leukemia: an analysis of SEER data over 3 decades. Cancer. 2013;119:2720-2727. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 165] [Cited by in RCA: 165] [Article Influence: 13.8] [Reference Citation Analysis (0)] |
| 3. | Medeiros BC, Satram-Hoang S, Hurst D, Hoang KQ, Momin F, Reyes C. Big data analysis of treatment patterns and outcomes among elderly acute myeloid leukemia patients in the United States. Ann Hematol. 2015;94:1127-1138. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 179] [Cited by in RCA: 213] [Article Influence: 21.3] [Reference Citation Analysis (0)] |
| 4. | Rashidi A, Weisdorf DJ, Bejanyan N. Treatment of relapsed/refractory acute myeloid leukaemia in adults. Br J Haematol. 2018;181:27-37. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 34] [Cited by in RCA: 49] [Article Influence: 7.0] [Reference Citation Analysis (0)] |
| 5. | Yates J, Glidewell O, Wiernik P, Cooper MR, Steinberg D, Dosik H, Levy R, Hoagland C, Henry P, Gottlieb A, Cornell C, Berenberg J, Hutchison JL, Raich P, Nissen N, Ellison RR, Frelick R, James GW, Falkson G, Silver RT, Haurani F, Green M, Henderson E, Leone L, Holland JF. Cytosine arabinoside with daunorubicin or adriamycin for therapy of acute myelocytic leukemia: a CALGB study. Blood. 1982;60:454-462. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 18] [Cited by in RCA: 18] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 6. | De Kouchkovsky I, Abdul-Hay M. 'Acute myeloid leukemia: a comprehensive review and 2016 update'. Blood Cancer J. 2016;6:e441. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 579] [Cited by in RCA: 899] [Article Influence: 99.9] [Reference Citation Analysis (0)] |
| 7. | Pavlova NN, Thompson CB. The Emerging Hallmarks of Cancer Metabolism. Cell Metab. 2016;23:27-47. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3099] [Cited by in RCA: 3964] [Article Influence: 440.4] [Reference Citation Analysis (0)] |
| 8. | Hackl H, Astanina K, Wieser R. Molecular and genetic alterations associated with therapy resistance and relapse of acute myeloid leukemia. J Hematol Oncol. 2017;10:51. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 88] [Cited by in RCA: 89] [Article Influence: 11.1] [Reference Citation Analysis (0)] |
| 9. | Bohl SR, Bullinger L, Rücker FG. New Targeted Agents in Acute Myeloid Leukemia: New Hope on the Rise. Int J Mol Sci. 2019;20. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 60] [Cited by in RCA: 64] [Article Influence: 10.7] [Reference Citation Analysis (0)] |
| 10. | Stahl M, Kim TK, Zeidan AM. Update on acute myeloid leukemia stem cells: New discoveries and therapeutic opportunities. World J Stem Cells. 2016;8:316-331. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 14] [Cited by in RCA: 16] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 11. | Cheloni G, Poteti M, Bono S, Masala E, Mazure NM, Rovida E, Lulli M, Dello Sbarba P. The Leukemic Stem Cell Niche: Adaptation to "Hypoxia" versus Oncogene Addiction. Stem Cells Int. 2017;2017:4979474. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 11] [Cited by in RCA: 17] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 12. | Song K, Li M, Xu X, Xuan LI, Huang G, Liu Q. Resistance to chemotherapy is associated with altered glucose metabolism in acute myeloid leukemia. Oncol Lett. 2016;12:334-342. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 52] [Cited by in RCA: 80] [Article Influence: 8.9] [Reference Citation Analysis (0)] |
| 13. | Watson AS, Riffelmacher T, Stranks A, Williams O, De Boer J, Cain K, MacFarlane M, McGouran J, Kessler B, Khandwala S, Chowdhury O, Puleston D, Phadwal K, Mortensen M, Ferguson D, Soilleux E, Woll P, Jacobsen SE, Simon AK. Autophagy limits proliferation and glycolytic metabolism in acute myeloid leukemia. Cell Death Discov. 2015;1. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 93] [Cited by in RCA: 113] [Article Influence: 11.3] [Reference Citation Analysis (0)] |
| 14. | Sullivan LB, Gui DY, Vander Heiden MG. Altered metabolite levels in cancer: implications for tumour biology and cancer therapy. Nat Rev Cancer. 2016;16:680-693. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 251] [Cited by in RCA: 298] [Article Influence: 33.1] [Reference Citation Analysis (0)] |
| 15. | Farge T, Saland E, de Toni F, Aroua N, Hosseini M, Perry R, Bosc C, Sugita M, Stuani L, Fraisse M, Scotland S, Larrue C, Boutzen H, Féliu V, Nicolau-Travers ML, Cassant-Sourdy S, Broin N, David M, Serhan N, Sarry A, Tavitian S, Kaoma T, Vallar L, Iacovoni J, Linares LK, Montersino C, Castellano R, Griessinger E, Collette Y, Duchamp O, Barreira Y, Hirsch P, Palama T, Gales L, Delhommeau F, Garmy-Susini BH, Portais JC, Vergez F, Selak M, Danet-Desnoyers G, Carroll M, Récher C, Sarry JE. Chemotherapy-Resistant Human Acute Myeloid Leukemia Cells Are Not Enriched for Leukemic Stem Cells but Require Oxidative Metabolism. Cancer Discov. 2017;7:716-735. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 506] [Cited by in RCA: 622] [Article Influence: 77.8] [Reference Citation Analysis (0)] |
| 16. | Sallmyr A, Fan J, Datta K, Kim KT, Grosu D, Shapiro P, Small D, Rassool F. Internal tandem duplication of FLT3 (FLT3/ITD) induces increased ROS production, DNA damage, and misrepair: implications for poor prognosis in AML. Blood. 2008;111:3173-3182. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 206] [Cited by in RCA: 239] [Article Influence: 14.1] [Reference Citation Analysis (0)] |
| 17. | Lagadinou ED, Sach A, Callahan K, Rossi RM, Neering SJ, Minhajuddin M, Ashton JM, Pei S, Grose V, O'Dwyer KM, Liesveld JL, Brookes PS, Becker MW, Jordan CT. BCL-2 inhibition targets oxidative phosphorylation and selectively eradicates quiescent human leukemia stem cells. Cell Stem Cell. 2013;12:329-341. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 766] [Cited by in RCA: 1015] [Article Influence: 84.6] [Reference Citation Analysis (0)] |
| 18. | Hole PS, Pearn L, Tonks AJ, James PE, Burnett AK, Darley RL, Tonks A. Ras-induced reactive oxygen species promote growth factor-independent proliferation in human CD34+ hematopoietic progenitor cells. Blood. 2010;115:1238-1246. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 93] [Cited by in RCA: 96] [Article Influence: 6.0] [Reference Citation Analysis (0)] |
| 19. | Mazat JP, Ransac S. The Fate of Glutamine in Human Metabolism. The Interplay with Glucose in Proliferating Cells. Metabolites. 2019;9. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 1] [Article Influence: 0.2] [Reference Citation Analysis (0)] |
| 20. | Gregory MA, Nemkov T, Park HJ, Zaberezhnyy V, Gehrke S, Adane B, Jordan CT, Hansen KC, D'Alessandro A, DeGregori J. Targeting Glutamine Metabolism and Redox State for Leukemia Therapy. Clin Cancer Res. 2019;25:4079-4090. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 74] [Cited by in RCA: 122] [Article Influence: 20.3] [Reference Citation Analysis (0)] |
| 21. | Altman BJ, Stine ZE, Dang CV. From Krebs to clinic: glutamine metabolism to cancer therapy. Nat Rev Cancer. 2016;16:619-634. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1308] [Cited by in RCA: 1311] [Article Influence: 145.7] [Reference Citation Analysis (0)] |
| 22. | Jacque N, Ronchetti AM, Larrue C, Meunier G, Birsen R, Willems L, Saland E, Decroocq J, Maciel TT, Lambert M, Poulain L, Hospital MA, Sujobert P, Joseph L, Chapuis N, Lacombe C, Moura IC, Demo S, Sarry JE, Recher C, Mayeux P, Tamburini J, Bouscary D. Targeting glutaminolysis has antileukemic activity in acute myeloid leukemia and synergizes with BCL-2 inhibition. Blood. 2015;126:1346-1356. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 233] [Cited by in RCA: 296] [Article Influence: 29.6] [Reference Citation Analysis (0)] |
| 23. | Matre P, Velez J, Jacamo R, Qi Y, Su X, Cai T, Chan SM, Lodi A, Sweeney SR, Ma H, Davis RE, Baran N, Haferlach T, Su X, Flores ER, Gonzalez D, Konoplev S, Samudio I, DiNardo C, Majeti R, Schimmer AD, Li W, Wang T, Tiziani S, Konopleva M. Inhibiting glutaminase in acute myeloid leukemia: metabolic dependency of selected AML subtypes. Oncotarget. 2016;7:79722-79735. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 103] [Cited by in RCA: 137] [Article Influence: 19.6] [Reference Citation Analysis (0)] |
| 24. | Gallipoli P, Giotopoulos G, Tzelepis K, Costa ASH, Vohra S, Medina-Perez P, Basheer F, Marando L, Di Lisio L, Dias JML, Yun H, Sasca D, Horton SJ, Vassiliou G, Frezza C, Huntly BJP. Glutaminolysis is a metabolic dependency in FLT3ITD acute myeloid leukemia unmasked by FLT3 tyrosine kinase inhibition. Blood. 2018;131:1639-1653. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 107] [Cited by in RCA: 111] [Article Influence: 15.9] [Reference Citation Analysis (0)] |
| 25. | Ghosh J, Kapur R. Role of mTORC1-S6K1 signaling pathway in regulation of hematopoietic stem cell and acute myeloid leukemia. Exp Hematol. 2017;50:13-21. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 26] [Cited by in RCA: 34] [Article Influence: 4.3] [Reference Citation Analysis (0)] |
| 26. | Kharas MG, Okabe R, Ganis JJ, Gozo M, Khandan T, Paktinat M, Gilliland DG, Gritsman K. Constitutively active AKT depletes hematopoietic stem cells and induces leukemia in mice. Blood. 2010;115:1406-1415. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 210] [Cited by in RCA: 221] [Article Influence: 13.8] [Reference Citation Analysis (0)] |
| 27. | Hoshii T, Tadokoro Y, Naka K, Ooshio T, Muraguchi T, Sugiyama N, Soga T, Araki K, Yamamura K, Hirao A. mTORC1 is essential for leukemia propagation but not stem cell self-renewal. J Clin Invest. 2012;122:2114-2129. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 96] [Cited by in RCA: 102] [Article Influence: 7.8] [Reference Citation Analysis (0)] |
| 28. | Willems L, Jacque N, Jacquel A, Neveux N, Maciel TT, Lambert M, Schmitt A, Poulain L, Green AS, Uzunov M, Kosmider O, Radford-Weiss I, Moura IC, Auberger P, Ifrah N, Bardet V, Chapuis N, Lacombe C, Mayeux P, Tamburini J, Bouscary D. Inhibiting glutamine uptake represents an attractive new strategy for treating acute myeloid leukemia. Blood. 2013;122:3521-3532. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 187] [Cited by in RCA: 245] [Article Influence: 20.4] [Reference Citation Analysis (0)] |
| 29. | Suhaili SH, Karimian H, Stellato M, Lee TH, Aguilar MI. Mitochondrial outer membrane permeabilization: a focus on the role of mitochondrial membrane structural organization. Biophys Rev. 2017;9:443-457. [PubMed] |
| 30. | Zheng C, Liu T, Liu H, Wang J. BCL-2 family proteins in apoptosis and its regulation. Curr Protein Pept Sci. 2019;. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 9] [Cited by in RCA: 19] [Article Influence: 4.8] [Reference Citation Analysis (0)] |
| 31. | Lomonosova E, Chinnadurai G. BH3-only proteins in apoptosis and beyond: an overview. Oncogene. 2008;27 Suppl 1:S2-19. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 348] [Cited by in RCA: 331] [Article Influence: 19.5] [Reference Citation Analysis (0)] |
| 32. | Kale J, Osterlund EJ, Andrews DW. BCL-2 family proteins: changing partners in the dance towards death. Cell Death Differ. 2018;25:65-80. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 647] [Cited by in RCA: 1049] [Article Influence: 131.1] [Reference Citation Analysis (0)] |
| 33. | Billard C. Apoptosis inducers in chronic lymphocytic leukemia. Oncotarget. 2014;5:309-325. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 41] [Cited by in RCA: 43] [Article Influence: 4.3] [Reference Citation Analysis (0)] |
| 34. | Vo TT, Ryan J, Carrasco R, Neuberg D, Rossi DJ, Stone RM, Deangelo DJ, Frattini MG, Letai A. Relative mitochondrial priming of myeloblasts and normal HSCs determines chemotherapeutic success in AML. Cell. 2012;151:344-355. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 253] [Cited by in RCA: 280] [Article Influence: 21.5] [Reference Citation Analysis (0)] |
| 35. | Perini GF, Ribeiro GN, Pinto Neto JV, Campos LT, Hamerschlak N. BCL-2 as therapeutic target for hematological malignancies. J Hematol Oncol. 2018;11:65. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 88] [Cited by in RCA: 149] [Article Influence: 21.3] [Reference Citation Analysis (0)] |
| 36. | Deng J, Carlson N, Takeyama K, Dal Cin P, Shipp M, Letai A. BH3 profiling identifies three distinct classes of apoptotic blocks to predict response to ABT-737 and conventional chemotherapeutic agents. Cancer Cell. 2007;12:171-185. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 402] [Cited by in RCA: 410] [Article Influence: 22.8] [Reference Citation Analysis (0)] |
| 37. | Costello RT, Mallet F, Gaugler B, Sainty D, Arnoulet C, Gastaut JA, Olive D. Human acute myeloid leukemia CD34+/CD38- progenitor cells have decreased sensitivity to chemotherapy and Fas-induced apoptosis, reduced immunogenicity, and impaired dendritic cell transformation capacities. Cancer Res. 2000;60:4403-4411. [PubMed] |
| 38. | Andreeff M, Jiang S, Zhang X, Konopleva M, Estrov Z, Snell VE, Xie Z, Okcu MF, Sanchez-Williams G, Dong J, Estey EH, Champlin RC, Kornblau SM, Reed JC, Zhao S. Expression of Bcl-2-related genes in normal and AML progenitors: changes induced by chemotherapy and retinoic acid. Leukemia. 1999;13:1881-1892. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 103] [Cited by in RCA: 110] [Article Influence: 4.2] [Reference Citation Analysis (0)] |
| 39. | Domen J, Cheshier SH, Weissman IL. The role of apoptosis in the regulation of hematopoietic stem cells: Overexpression of Bcl-2 increases both their number and repopulation potential. J Exp Med. 2000;191:253-264. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 258] [Cited by in RCA: 237] [Article Influence: 9.5] [Reference Citation Analysis (0)] |
| 40. | Ciciarello M, Corradi G, Loscocco F, Visani G, Monaco F, Cavo M, Curti A, Isidori A. The Yin and Yang of the Bone Marrow Microenvironment: Pros and Cons of Mesenchymal Stromal Cells in Acute Myeloid Leukemia. Front Oncol. 2019;9:1135. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 22] [Cited by in RCA: 28] [Article Influence: 4.7] [Reference Citation Analysis (0)] |
| 41. | Baev DV, Krawczyk J, O׳Dwyer M, Szegezdi E. The BH3-mimetic ABT-737 effectively kills acute myeloid leukemia initiating cells. Leuk Res Rep. 2014;3:79-82. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 6] [Cited by in RCA: 11] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 42. | Konopleva M, Contractor R, Tsao T, Samudio I, Ruvolo PP, Kitada S, Deng X, Zhai D, Shi YX, Sneed T, Verhaegen M, Soengas M, Ruvolo VR, McQueen T, Schober WD, Watt JC, Jiffar T, Ling X, Marini FC, Harris D, Dietrich M, Estrov Z, McCubrey J, May WS, Reed JC, Andreeff M. Mechanisms of apoptosis sensitivity and resistance to the BH3 mimetic ABT-737 in acute myeloid leukemia. Cancer Cell. 2006;10:375-388. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 818] [Cited by in RCA: 851] [Article Influence: 44.8] [Reference Citation Analysis (0)] |
| 43. | Järås M, Ebert BL. Power cut: inhibiting mitochondrial translation to target leukemia. Cancer Cell. 2011;20:555-556. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 30] [Cited by in RCA: 29] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 44. | Pollyea DA, Amaya M, Strati P, Konopleva MY. Venetoclax for AML: changing the treatment paradigm. Blood Adv. 2019;3:4326-4335. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 108] [Cited by in RCA: 123] [Article Influence: 20.5] [Reference Citation Analysis (0)] |
| 45. | Pan R, Hogdal LJ, Benito JM, Bucci D, Han L, Borthakur G, Cortes J, DeAngelo DJ, Debose L, Mu H, Döhner H, Gaidzik VI, Galinsky I, Golfman LS, Haferlach T, Harutyunyan KG, Hu J, Leverson JD, Marcucci G, Müschen M, Newman R, Park E, Ruvolo PP, Ruvolo V, Ryan J, Schindela S, Zweidler-McKay P, Stone RM, Kantarjian H, Andreeff M, Konopleva M, Letai AG. Selective BCL-2 inhibition by ABT-199 causes on-target cell death in acute myeloid leukemia. Cancer Discov. 2014;4:362-375. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 430] [Cited by in RCA: 566] [Article Influence: 47.2] [Reference Citation Analysis (0)] |
| 46. | Konopleva M, Pollyea DA, Potluri J, Chyla B, Hogdal L, Busman T, McKeegan E, Salem AH, Zhu M, Ricker JL, Blum W, DiNardo CD, Kadia T, Dunbar M, Kirby R, Falotico N, Leverson J, Humerickhouse R, Mabry M, Stone R, Kantarjian H, Letai A. Efficacy and Biological Correlates of Response in a Phase II Study of Venetoclax Monotherapy in Patients with Acute Myelogenous Leukemia. Cancer Discov. 2016;6:1106-1117. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 564] [Cited by in RCA: 831] [Article Influence: 92.3] [Reference Citation Analysis (0)] |
| 47. | DiNardo CD, Pratz K, Pullarkat V, Jonas BA, Arellano M, Becker PS, Frankfurt O, Konopleva M, Wei AH, Kantarjian HM, Xu T, Hong WJ, Chyla B, Potluri J, Pollyea DA, Letai A. Venetoclax combined with decitabine or azacitidine in treatment-naive, elderly patients with acute myeloid leukemia. Blood. 2019;133:7-17. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 861] [Cited by in RCA: 1306] [Article Influence: 186.6] [Reference Citation Analysis (0)] |
| 48. | Jonas BA, Pollyea DA. How we use venetoclax with hypomethylating agents for the treatment of newly diagnosed patients with acute myeloid leukemia. Leukemia. 2019;33:2795-2804. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 76] [Cited by in RCA: 131] [Article Influence: 21.8] [Reference Citation Analysis (0)] |
| 49. | Maiti A, DiNardo CD, Cortes JE, Borthakur G, Pemmaraju N, Benton CB, Kadia TM, Takahashi K, Naqvi K, Ravandi F, Alvarado Y, Short NJ, Daver NG, Sasaki K, Ohanian MN, Garcia-Manero G, Thompson PA, Kornblau SM, Masarova L, Jain N, Jabbour EJ, Andreeff M, Maduike R, Guerrero JA, Zhang Q, Cavazos A, Ma H, Rausch CR, Bivins CA, Vaughan K, Pierce SA, Ning J, Qiao W, Welch JS, Kantarjian HM, Konopleva MY. Interim Analysis of Phase II Study of Venetoclax with 10-Day Decitabine (DEC10-VEN) in Acute Myeloid Leukemia and Myelodysplastic Syndrome. Blood. 2018;132:286-286. [RCA] [DOI] [Full Text] [Cited by in Crossref: 17] [Cited by in RCA: 17] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 50. | Aldoss I, Yang D, Aribi A, Ali H, Sandhu K, Al Malki MM, Mei M, Salhotra A, Khaled S, Nakamura R, Snyder D, O'Donnell M, Stein AS, Forman SJ, Marcucci G, Pullarkat V. Efficacy of the combination of venetoclax and hypomethylating agents in relapsed/refractory acute myeloid leukemia. Haematologica. 2018;103:e404-e407. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 199] [Cited by in RCA: 210] [Article Influence: 30.0] [Reference Citation Analysis (0)] |
| 51. | Gaut D, Burkenroad A, Duong T, Feammelli J, Sasine J, Schiller G. Venetoclax combination therapy in relapsed/refractory acute myeloid leukemia: A single institution experience. Leuk Res. 2020;90:106314. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 35] [Cited by in RCA: 43] [Article Influence: 8.6] [Reference Citation Analysis (0)] |
| 52. | DiNardo CD, Cortes JE. Mutations in AML: prognostic and therapeutic implications. Hematology Am Soc Hematol Educ Program. 2016;2016:348-355. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 106] [Cited by in RCA: 163] [Article Influence: 18.1] [Reference Citation Analysis (0)] |
| 53. | Ramsey HE, Fischer MA, Lee T, Gorska AE, Arrate MP, Fuller L, Boyd KL, Strickland SA, Sensintaffar J, Hogdal LJ, Ayers GD, Olejniczak ET, Fesik SW, Savona MR. A Novel MCL1 Inhibitor Combined with Venetoclax Rescues Venetoclax-Resistant Acute Myelogenous Leukemia. Cancer Discov. 2018;8:1566-1581. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 244] [Cited by in RCA: 262] [Article Influence: 37.4] [Reference Citation Analysis (0)] |
| 54. | Campos EDV, Pinto R. Targeted therapy with a selective BCL-2 inhibitor in older patients with acute myeloid leukemia. Hematol Transfus Cell Ther. 2019;41:169-177. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 13] [Cited by in RCA: 22] [Article Influence: 3.1] [Reference Citation Analysis (0)] |
| 55. | Niu X, Wang G, Wang Y, Caldwell JT, Edwards H, Xie C, Taub JW, Li C, Lin H, Ge Y. Acute myeloid leukemia cells harboring MLL fusion genes or with the acute promyelocytic leukemia phenotype are sensitive to the Bcl-2-selective inhibitor ABT-199. Leukemia. 2014;28:1557-1560. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 69] [Cited by in RCA: 90] [Article Influence: 8.2] [Reference Citation Analysis (0)] |
| 56. | Lin KH, Winter PS, Xie A, Roth C, Martz CA, Stein EM, Anderson GR, Tingley JP, Wood KC. Targeting MCL-1/BCL-XL Forestalls the Acquisition of Resistance to ABT-199 in Acute Myeloid Leukemia. Sci Rep. 2016;6:27696. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 92] [Cited by in RCA: 129] [Article Influence: 14.3] [Reference Citation Analysis (0)] |
| 57. | Chen X, Glytsou C, Zhou H, Narang S, Reyna DE, Lopez A, Sakellaropoulos T, Gong Y, Kloetgen A, Yap YS, Wang E, Gavathiotis E, Tsirigos A, Tibes R, Aifantis I. Targeting Mitochondrial Structure Sensitizes Acute Myeloid Leukemia to Venetoclax Treatment. Cancer Discov. 2019;9:890-909. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 107] [Cited by in RCA: 224] [Article Influence: 37.3] [Reference Citation Analysis (0)] |
| 58. | Sharon D, Cathelin S, Mirali S, Di Trani JM, Yanofsky DJ, Keon KA, Rubinstein JL, Schimmer AD, Ketela T, Chan SM. Inhibition of mitochondrial translation overcomes venetoclax resistance in AML through activation of the integrated stress response. Sci Transl Med. 2019;11. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 86] [Cited by in RCA: 137] [Article Influence: 27.4] [Reference Citation Analysis (0)] |
| 59. | Blombery P, Anderson MA, Gong JN, Thijssen R, Birkinshaw RW, Thompson ER, Teh CE, Nguyen T, Xu Z, Flensburg C, Lew TE, Majewski IJ, Gray DHD, Westerman DA, Tam CS, Seymour JF, Czabotar PE, Huang DCS, Roberts AW. Acquisition of the Recurrent Gly101Val Mutation in BCL2 Confers Resistance to Venetoclax in Patients with Progressive Chronic Lymphocytic Leukemia. Cancer Discov. 2019;9:342-353. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 209] [Cited by in RCA: 316] [Article Influence: 45.1] [Reference Citation Analysis (0)] |
| 60. | Pollyea DA, Stevens BM, Jones CL, Winters A, Pei S, Minhajuddin M, D'Alessandro A, Culp-Hill R, Riemondy KA, Gillen AE, Hesselberth JR, Abbott D, Schatz D, Gutman JA, Purev E, Smith C, Jordan CT. Venetoclax with azacitidine disrupts energy metabolism and targets leukemia stem cells in patients with acute myeloid leukemia. Nat Med. 2018;24:1859-1866. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 328] [Cited by in RCA: 527] [Article Influence: 75.3] [Reference Citation Analysis (0)] |
| 61. | Jones CL, Stevens BM, D'Alessandro A, Reisz JA, Culp-Hill R, Nemkov T, Pei S, Khan N, Adane B, Ye H, Krug A, Reinhold D, Smith C, DeGregori J, Pollyea DA, Jordan CT. Inhibition of Amino Acid Metabolism Selectively Targets Human Leukemia Stem Cells. Cancer Cell. 2018;34:724-740.e4. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 277] [Cited by in RCA: 405] [Article Influence: 57.9] [Reference Citation Analysis (0)] |
| 62. | Grant S. Rational combination strategies to enhance venetoclax activity and overcome resistance in hematologic malignancies. Leuk Lymphoma. 2018;59:1292-1299. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 8] [Cited by in RCA: 10] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 63. | Wilde L, Martinez-Outschoorn U, Palmisiano N, Kasner M. OPB-111077 in Combination with Decitabine and Venetoclax for the Treatment of Acute Myeloid Leukemia. Blood. 2019;134:2597-2597. [RCA] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 2] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 64. | Francesco MED, Marszalek JR, McAfoos T, Carroll CL, Kang Z, Liu G, Theroff JP, Bardenhager JP, Bandi ML, Molina JR, Gera S, Protopopova M, Sun Y, Do MKG, Feng N, Gay JP, Muller F, Konopleva M, Meric-Bernstam F, Toniatti C, Heffernan TP, Draetta GF, Jones P. Abstract 1655: Discovery and development of IACS-010759, a novel inhibitor of Complex I currently in phase I studies to exploit oxidative phosphorylation dependency in acute myeloid leukemia and solid tumors. Cancer Res. 2018;78:1655-1655. [DOI] [Full Text] |
| 65. | Biondani G, Peyron JF. Metformin, an Anti-diabetic Drug to Target Leukemia. Front Endocrinol (Lausanne). 2018;9:446. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 26] [Cited by in RCA: 35] [Article Influence: 5.0] [Reference Citation Analysis (0)] |
| 66. | Niu X, Zhao J, Ma J, Xie C, Edwards H, Wang G, Caldwell JT, Xiang S, Zhang X, Chu R, Wang ZJ, Lin H, Taub JW, Ge Y. Binding of Released Bim to Mcl-1 is a Mechanism of Intrinsic Resistance to ABT-199 which can be Overcome by Combination with Daunorubicin or Cytarabine in AML Cells. Clin Cancer Res. 2016;22:4440-4451. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 125] [Cited by in RCA: 178] [Article Influence: 19.8] [Reference Citation Analysis (0)] |
| 67. | Wei AH, Strickland SA, Hou JZ, Fiedler W, Lin TL, Walter RB, Enjeti A, Tiong IS, Savona M, Lee S, Chyla B, Popovic R, Salem AH, Agarwal S, Xu T, Fakouhi KM, Humerickhouse R, Hong WJ, Hayslip J, Roboz GJ. Venetoclax Combined With Low-Dose Cytarabine for Previously Untreated Patients With Acute Myeloid Leukemia: Results From a Phase Ib/II Study. J Clin Oncol. 2019;37:1277-1284. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 349] [Cited by in RCA: 508] [Article Influence: 84.7] [Reference Citation Analysis (0)] |
| 68. | Wang Q, Wan J, Zhang W, Hao S. MCL-1 or BCL-xL-dependent resistance to the BCL-2 antagonist (ABT-199) can be overcome by specific inhibitor as single agents and in combination with ABT-199 in acute myeloid leukemia cells. Leuk Lymphoma. 2019;60:2170-2180. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 16] [Cited by in RCA: 28] [Article Influence: 4.7] [Reference Citation Analysis (0)] |
| 69. | Moujalled DM, Pomilio G, Ghiurau C, Ivey A, Salmon J, Rijal S, Macraild S, Zhang L, Teh TC, Tiong IS, Lan P, Chanrion M, Claperon A, Rocchetti F, Zichi A, Kraus-Berthier L, Wang Y, Halilovic E, Morris E, Colland F, Segal D, Huang D, Roberts AW, Maragno AL, Lessene G, Geneste O, Wei AH. Combining BH3-mimetics to target both BCL-2 and MCL1 has potent activity in pre-clinical models of acute myeloid leukemia. Leukemia. 2019;33:905-917. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 114] [Cited by in RCA: 131] [Article Influence: 21.8] [Reference Citation Analysis (0)] |
| 70. | Boffo S, Damato A, Alfano L, Giordano A. CDK9 inhibitors in acute myeloid leukemia. J Exp Clin Cancer Res. 2018;37:36. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 100] [Cited by in RCA: 118] [Article Influence: 16.9] [Reference Citation Analysis (0)] |
| 71. | Tibes R, Bogenberger JM. Transcriptional Silencing of MCL-1 Through Cyclin-Dependent Kinase Inhibition in Acute Myeloid Leukemia. Front Oncol. 2019;9:1205. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 24] [Cited by in RCA: 28] [Article Influence: 4.7] [Reference Citation Analysis (0)] |
| 72. | Padua RA, Sarda-Mantel L, Chiquet M, Kappel C, Krief P, Setterblad N, Hontonnou F, Hosten B, Vignal N, Zassadowski F, Cassinat B, Gou P, Giraudier S, Konopleva MY, Andreeff M, PLA M, Ades L, Fenaux P, Chomienne C. BCL-2 Inhibitor Venetoclax (ABT-199) and MEK Inhibitor GDC-0973 Synergise to Target AML Progenitors and Overcome Drug Resistance with the Use of PET Scanning in a Mouse Model of HR-MDS to Monitor Response to Treatment. Blood. 2018;132:5497-5497. [RCA] [DOI] [Full Text] [Cited by in Crossref: 3] [Cited by in RCA: 3] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 73. | Han L, Zhang Q, Dail M, Shi C, Cavazos A, Ruvolo VR, Zhao Y, Kim E, Rahmani M, Mak DH, Jin SS, Chen J, Phillips DC, Koller PB, Jacamo R, Burks JK, DiNardo C, Daver N, Jabbour E, Wang J, Kantarjian HM, Andreeff M, Grant S, Leverson JD, Sampath D, Konopleva M. Concomitant targeting of BCL2 with venetoclax and MAPK signaling with cobimetinib in acute myeloid leukemia models. Haematologica. 2020;105:697-707. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 48] [Cited by in RCA: 85] [Article Influence: 14.2] [Reference Citation Analysis (0)] |
| 74. | Daver N, Pollyea DA, Yee KWL, Fenaux P, Brandwein JM, Vey N, Martinelli G, Kelly KR, Roboz GJ, Garcia JS, Pigneux A, Kshirsagar S, Dail M, Wang J, Mobasher M, Chen LC, Hong WJ, Konopleva M, Andreeff M. Preliminary Results from a Phase Ib Study Evaluating BCL-2 Inhibitor Venetoclax in Combination with MEK Inhibitor Cobimetinib or MDM2 Inhibitor Idasanutlin in Patients with Relapsed or Refractory (R/R) AML. Blood. 2017;130:813-813. [RCA] [DOI] [Full Text] [Cited by in Crossref: 21] [Cited by in RCA: 23] [Article Influence: 2.9] [Reference Citation Analysis (0)] |
| 75. | Rahmani M, Nkwocha J, Hawkins E, Pei X, Parker RE, Kmieciak M, Leverson JD, Sampath D, Ferreira-Gonzalez A, Grant S. Cotargeting BCL-2 and PI3K Induces BAX-Dependent Mitochondrial Apoptosis in AML Cells. Cancer Res. 2018;78:3075-3086. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 56] [Cited by in RCA: 105] [Article Influence: 15.0] [Reference Citation Analysis (0)] |
| 76. | Kojima K, Konopleva M, Samudio IJ, Schober WD, Bornmann WG, Andreeff M. Concomitant inhibition of MDM2 and Bcl-2 protein function synergistically induce mitochondrial apoptosis in AML. Cell Cycle. 2006;5:2778-2786. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 70] [Cited by in RCA: 83] [Article Influence: 4.4] [Reference Citation Analysis (0)] |
| 77. | Pan R, Ruvolo V, Mu H, Leverson JD, Nichols G, Reed JC, Konopleva M, Andreeff M. Synthetic Lethality of Combined Bcl-2 Inhibition and p53 Activation in AML: Mechanisms and Superior Antileukemic Efficacy. Cancer Cell. 2017;32:748-760.e6. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 151] [Cited by in RCA: 218] [Article Influence: 27.3] [Reference Citation Analysis (0)] |
| 78. | Lehmann C, Friess T, Birzele F, Kiialainen A, Dangl M. Superior anti-tumor activity of the MDM2 antagonist idasanutlin and the Bcl-2 inhibitor venetoclax in p53 wild-type acute myeloid leukemia models. J Hematol Oncol. 2016;9:50. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 51] [Cited by in RCA: 78] [Article Influence: 8.7] [Reference Citation Analysis (0)] |
| 79. | Karjalainen R, Pemovska T, Popa M, Liu M, Javarappa KK, Majumder MM, Yadav B, Tamborero D, Tang J, Bychkov D, Kontro M, Parsons A, Suvela M, Mayoral Safont M, Porkka K, Aittokallio T, Kallioniemi O, McCormack E, Gjertsen BT, Wennerberg K, Knowles J, Heckman CA. JAK1/2 and BCL2 inhibitors synergize to counteract bone marrow stromal cell-induced protection of AML. Blood. 2017;130:789-802. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 69] [Cited by in RCA: 85] [Article Influence: 10.6] [Reference Citation Analysis (0)] |
| 80. | Leverson JD, Cojocari D. Hematologic Tumor Cell Resistance to the BCL-2 Inhibitor Venetoclax: A Product of Its Microenvironment? Front Oncol. 2018;8:458. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 31] [Cited by in RCA: 29] [Article Influence: 4.1] [Reference Citation Analysis (0)] |
| 81. | Brunner AM, Neuberg DS, Wander SA, Sadrzadeh H, Ballen KK, Amrein PC, Attar E, Hobbs GS, Chen YB, Perry A, Connolly C, Joseph C, Burke M, Ramos A, Galinsky I, Yen K, Yang H, Straley K, Agresta S, Adamia S, Borger DR, Iafrate A, Graubert TA, Stone RM, Fathi AT. Isocitrate dehydrogenase 1 and 2 mutations, 2-hydroxyglutarate levels, and response to standard chemotherapy for patients with newly diagnosed acute myeloid leukemia. Cancer. 2019;125:541-549. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 20] [Cited by in RCA: 21] [Article Influence: 3.5] [Reference Citation Analysis (0)] |
| 82. | Nassereddine S, Lap CJ, Tabbara IA. Evaluating ivosidenib for the treatment of relapsed/refractory AML: design, development, and place in therapy. Onco Targets Ther. 2019;12:303-308. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 11] [Cited by in RCA: 11] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 83. | Mali RS, Lasater EA, Doyle K, Malla R, Boghaert E, Souers A, Leverson JD, Sampath D. FLT3-ITD Activation Mediates Resistance to the BCL-2 Selective Antagonist, Venetoclax, in FLT3-ITD Mutant AML Models. Blood. 2017;130:1348-1348. [DOI] [Full Text] |
| 84. | Chyla B, Daver N, Doyle K, McKeegan E, Huang X, Ruvolo V, Wang Z, Chen K, Souers A, Leverson J, Potluri J, Boghaert E, Bhathena A, Konopleva M, Popovic R. Genetic Biomarkers Of Sensitivity and Resistance to Venetoclax Monotherapy in Patients With Relapsed Acute Myeloid Leukemia. Am J Hematol. 2018;. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 110] [Cited by in RCA: 121] [Article Influence: 17.3] [Reference Citation Analysis (0)] |