Published online Jul 24, 2020. doi: 10.5306/wjco.v11.i7.412
Peer-review started: December 31, 2019
First decision: April 29, 2020
Revised: May 31, 2020
Accepted: June 14, 2020
Article in press: June 14, 2020
Published online: July 24, 2020
Processing time: 201 Days and 2.8 Hours
Lung cancer remains the leading cause of cancer-related deaths worldwide. The treatment of non-small cell lung cancer (NSCLC), which accounts for a vast majority of lung cancers, has shifted to personalized, targeted therapy following discoveries of several targetable oncogenic mutations. Targeting of specific mutations has improved outcomes in many patients. This success has led to several target-specific agents replacing chemotherapy as first-line treatment in certain mutated NSCLC. Several researchers have reported that there may be imaging biomarkers that may be predictive of the presence of these mutations. These features, when present, have the potential in triaging patients into the most appropriate diagnostic and treatment algorithms. Distinct imaging features and patterns of metastases that have been associated with NSCLC with various targetable oncogenic mutations are presented in this review.
Core tip: Non-small cell lung cancer (NSCLC) is a leading cause of cancer-related deaths worldwide. Targeted therapy has improved outcomes in subsets of patients with certain targetable mutations. Several researchers have reported imaging biomarkers, which may predict the presence of these mutations. In this review, we present the primary tumor imaging features and patterns of metastases in NSCLC with oncogenic mutations.
- Citation: Mendoza DP, Piotrowska Z, Lennerz JK, Digumarthy SR. Role of imaging biomarkers in mutation-driven non-small cell lung cancer. World J Clin Oncol 2020; 11(7): 412-427
- URL: https://www.wjgnet.com/2218-4333/full/v11/i7/412.htm
- DOI: https://dx.doi.org/10.5306/wjco.v11.i7.412
Lung cancer results in millions of deaths annually and is the leading cause of cancer-related deaths worldwide[1]. Non-small cell lung cancer (NSCLC) accounts for approximately 85% of lung cancers, and more than half of NSCLC are metastatic at the time of diagnosis[2]. The prognosis in cases of metastatic NSCLC remains dismal despite advances in treatment, with five-year survival rates of approximately 5%[2].
Genotyping studies have revealed genetic heterogeneity in NSCLC and identified several key driver mutations, many of which have been found to be targetable or potentially targetable[3]. Mutations with currently approved targeted therapies (Table 1) include EGFR mutations, ALK rearrangements, ROS1 rearrangements, BRAF mutations, and NTRK gene fusions[4]. There are other driver mutations in NSCLC for which targeted therapies are under investigation in clinical trials or available as off-label use of agents approved for other indications. These include mutations involving rearranged during transfection proto-oncogene (RET), MET, human epidermal growth factor receptor 2 (HER2), and KRAS genes.
| Molecular alteration | Approved targeted therapies |
| EGFR | Afatinib |
| Dacomitinib | |
| Erlotinib | |
| Gefitinib | |
| Osimertinib | |
| ALK | Alectinib |
| Brigatinib | |
| Ceritinib | |
| Crizotinib | |
| Lorlatinib | |
| ROS1 | Crizotinib |
| Entrectinib | |
| BRAF | Dabrafenib + trametinib |
| NTRK | Larotrectinib |
| Entrectinib |
In certain patient subgroups, targeted therapy can improve outcomes, making the detection of these mutations an important step in developing personalized treatment strategies. Several (phenotypic) biomarkers have been reported to suggest the presence of specific mutations in NSCLC and to predict responsiveness to certain targeted therapies. The fundamental principle of these biomarkers is that their presence may be indicative of a specific underlying driver mutation in NSCLC and these biomarkers include clinical, pathologic, as well as imaging features.
Given the success of targeted therapy in certain molecular subsets of patients, screening for driver mutations has become an essential step in the evaluation of patients with newly diagnosed NSCLCs. Current guidelines, including those from the College of American Pathologists, International Association for the Study of Lung Cancer, and Association of Molecular Pathologists, now recommend assessment for the presence of driver mutations in patients with advanced NSCLC, specifically in those with adenocarcinoma[4,5].
While screening for driver mutations has been widely adopted in clinical practice, no standard screening platform has been established. The optimal testing platform would be accurate, cost-effective, and with a fast turnaround time. The methods currently available offer these features to varying degrees. Their sensitivities may also depend on the mutation being assessed. As such, no single platform has emerged as the optimal testing method for all (Table 2).
| Testing technique | High sensitivityin detecting | Lower sensitivityin detecting |
| Direct gene sequencing | BRAF | |
| Requires high tumor cellularity | EGFR | |
| Largely replaced by newer techniques | HER2 | |
| KRAS | ||
| Allele specific sequencing | BRAF | |
| Detects predefined abnormalities | EGFR | |
| Allows for multiplex testing | HER2 | |
| KRAS | ||
| METex14 skipping | ||
| Next Generation sequencing | BRAF | ALK |
| Can detect novel mutations | EGFR | RET |
| Allows for multiplex testing | HER2 | ROS1 |
| Can be costly and time consuming | KRAS | NTRK |
| METex14 skipping | MET amplification | |
| Fluorescent in situ hybridization | ALK | |
| RET | ||
| ROS1 | ||
| NTRK | ||
| MET amplification | ||
| Immunohistochemistry Also used to detect PD-L1 protein expression | ALKROS1 | NTRK fusion |
The techniques most commonly employed in molecular analysis of tumor tissue include direct gene sequencing, allele-specific sequencing by polymerase chain reaction (PCR), next generation sequencing (NGS), fluorescence in situ hybridization (FISH) and evaluation of protein expression by immunohistochemistry (IHC). All of these techniques require tissue samples. Direct gene sequencing (i.e., Sanger sequencing) was one of the first methods used to perform genotyping but has largely been replaced since by the other methods as it requires a higher tumor cellularity in tissue samples and is more prone to false negative results.
In allele-specific tissue testing, raw DNA is amplified using PCR and is then analyzed for specific abnormalities. Amplification with PCR allows for greater sensitivity and allows for testing of more than one abnormality at a time (i.e., multiplex testing). Its main drawback is that is can only test for predefined abnormalities and is unable to detect new mutations.
FISH testing can be used to detect gene rearrangements, amplifications, or deletions. It is highly sensitive in detecting rearrangements in the ALK, ROS1, and RET genes as well as MET amplification and NTRK fusion[6,7]. Tissue IHC has also been found to be highly sensitive and specific in detecting ALK and ROS1 rearrangements by detecting expression of abnormal proteins, but it has not been as helpful in the detection of other mutations[8,9]. It is also routinely used in determining PD-L1 expressivity in tumor cells[10].
Finally, NGS is an automated platform that, like allele-specific sequencing, can simultaneously test for multiple genetic abnormalities. It is highly sensitive in the detection of EGFR, HER2, METex14, BRAF, and KRAS mutations[6,11]. It can also detect ALK, ROS1, and RET rearrangements, but with lower sensitivity[6,11], and identify novel mutations. The main drawback is cost, as NGS demands advanced bioinformatics systems, fast and complex data processing, and large data storage requirements. Another potential challenge of NGS is the detection of novel variants and mutations of indeterminate significance.
Although testing for several driver mutations is now standard of care in the management of advanced NSCLC, significant disparities in compliance with recommended exist around the world, and even within the United States[12,13]. The identification of clinical, pathologic, and imaging biomarkers has the potential to improve compliance and mitigate these disparities. Moreover, identification of these biomarkers has the potential to lower cost by helping to identify the patients who may benefit the most from molecular testing and by assisting in in the selection of the most appropriate testing algorithm.
Several clinicopathologic features have been associated with the presence of certain mutations (Table 3). EGFR mutations are the first molecular alterations in lung cancer shown to confer sensitivity to specific targeted therapies. EGFR mutations are identified in approximately 15% of lung adenocarcinomas in the United States but have been reported in up to approximately 60% of Asian cases[14]. Affected patients tend to be younger with minimal or absent history of smoking[15]. Several generations of tyrosine kinase inhibitors (TKI) have been approved as first-line treatment in advanced EGFR-mutant NSCLC[16-22].
| Clinicopathologic features | EGFR-mutant | ALK-rearranged | ROS1-rearranged | BRAF V600E-mutant | MET exon 14 skipping |
| Age | Younger | Younger | Younger | No specific age predilection | Older compared to another mutated NSCLC |
| Race | More common in Asian populations | More common in Caucasian populations | No specific racial predilection | No specific racial predilection | No specific racial predilection |
| Smoking history | Minimal to no smoking history | Minimal to no smoking history | Minimal to no smoking history | Minimal to no smoking history; positive smoking history in non-V600E mutation | Both smokers and non-smokers |
| Tumor histology | Adenocarcinoma | Adenocarcinoma | Adenocarcinoma | Adenocarcinoma | Increased incidence of METex14 skipping with sarcomatoid histology |
ALK gene rearrangements, most commonly resulting in fusion of ALK to echinoderm microtubule-associated protein-like 4 (EML4), are reported in approximately 5% of NSCLC[23,24]. Similar to EGFR-mutant NSCLC, ALK-positive NSCLCs are more common in younger patients with minimal or no smoking history[25,26]. Several ALK-targeted TKIs have been shown to be highly effective in treating ALK-positive NSCLC and are now Food and Drug Administration-approved[27-32].
ROS1 rearrangements, most commonly genetic translocations between ROS1 and CD74, represent another targetable driver alteration identified in 1%-2% of NSCLC[33,34]. Similar to EGFR mutations and ALK rearrangements, ROS1 rearrangements are also associated with younger age, little to no smoking history, and adenocarcinoma cell type[33,34]. ALK and ROS tyrosine kinase domains share a high degree of homology, making ROS1-positive NSCLC highly sensitive to crizotinib[33]. Entrectinib, a tropomyosin receptor kinase (TRK)/ROS1 inhibitor, has also been found to be effective and has been approved for the treatment of advanced ROS1-positive NSCLC[4].
Mutations in the BRAF gene, which are present in 2%-4% of NSCLC, have emerged as another possible target in the treatment of NSCLC[35,36]. BRAF is a protein kinase, which, when constitutively activated by a mutation, can lead to increased cell proliferation and survival, decreased cell death, and oncogenesis through the RAS/MAPK pathway[36,37]. Several subtypes of BRAF mutations exist and are typically classified as either V600E or non-V600E[36-38]. Unlike mutations involving EGFR, ALK, and ROS1, those with activating non-V600E BRAF mutations are typically current or previous smokers, although those with V600E mutations are typically also less likely to have a history of smoking[36,39,40]. Combination treatment with BRAF and MEK inhibitors, dabrafenib and trametinib, has been approved for advanced NSCLC with BRAF V600E mutations[41].
Fusions involving one of three TRK (NTRK fusions) are seen in less than 1% of NSCLC and has not been shown to have a predilection based on gender, age, smoking history, or histology[42]. Two TRK inhibitors, larotrectinib and entrectinib, have shown efficacy against NSCLC harboring NTRK fusions and have been approved in advanced cases[43,44].
RET fusions are detected in 1%-2% of NSCLC and are more commonly seen in patients with no significant smoking history[45,46]. Multi-targeted TKIs such as cabozantinib and vandetanib have been found to have anti-RET activity[47,48]. Subsequently, highly potent, RET-selective TKIs, pralsetinib (BLU-667) and selpercatinib (LOXO-292), have shown promising preliminary safety and efficacy profiles in patients with advanced solid tumors harboring RET alterations and are under investigation in the treatment of RET-positive NSCLC[49,50].
The MET proto-oncogene encodes a receptor tyrosine kinase, which plays a role in the RAS/MAPK, Rac/Rho, and PI3K/Akt signaling pathways, which mediate cellular growth, anti-apoptosis, and metastasis[51]. MET amplification and overexpression have been found in a wide variety of malignancies including lung cancer, both as a primary driving mutation and as an acquired resistance mechanism in EGFR-mutated NSCLC[52,53]. MET exon 14 (METex14) skipping represents a distinct subset of MET mutations seen in up to 4% of NSCLC and is mutually exclusive of other driver mutations, including EGFR, ALK, and ROS1[3,53]. METex14 skipping mutations tend to affect older patients compared to EGFR and ALK[53-55]. Although most tumors with METex14 skipping mutations are adenocarcinomas, there is increased incidence of the mutation in those with sarcomatoid histology[54]. Crizotinib and cabozantinib have shown promise in treating the treatment of NSCLC harboring METex14 skipping mutations, and several clinical trials are currently underway investigating novel MET-targeted TKIs, including tepotinib and capmatinib[56,57].
HER2 encodes an EGFR family receptor tyrosine kinase, with mutations in HER2 gene detected in approximately 1%-3% of NSCLC[58,59]. These mutations are more commonly seen in lung adenocarcinoma and are more common in nonsmokers and women[59]. There is evidence showing that HER2-mutated NSCLC may respond to trastuzumab-based regimens and ado-trastuzumab emtansine[58-60], and several clinical trials of novel TKIs targeting HER2 are currently underway including poziotinib, TAK-788, pyrotinib and others.
Finally, activating KRAS mutations are the most commonly identified alterations in NSCLC, seen in up to 25% of lung adenocarcinomas[61]. Unlike EGFR and ALK alterations, KRAS mutations are generally seen in smokers. Several previous efforts to identify RAS-specific inhibitors have been unsuccessful. Currently, several agents are under investigation in the treatment of NSCLC with KRAS-G12C mutations, which accounts for approximately 12% of KRAS mutations in NSCLC[62].
There has been increasing awareness of the clinical features (e.g., minimal to no history of smoking, Asian descent, etc.) that are associated with certain mutations in NSCLC, but the association of the imaging features and underlying driver mutations in NSCLC remains under-recognized. Emerging data suggest that there are differences among NSCLC harboring different targetable oncogenic driver mutations with respect to the imaging features of the primary tumor and patterns of metastases (Table 4, Figure 1). These features, when present, can potentially point to certain mutations.
| Imaging feature | EGFR mutation | ALK rearrangement | ROS1 rearrangement | METex14 skipping mutation |
| Primary tumor | Increased ground-glass components | Purely solid lesion | Purely solid lesion | Multifocal primary lung cancers |
| Presence of air bronchograms (pneumonic appearance) | ||||
| Peripheral predilection | Peripheral predilection | Peripheral predilection | ||
| Metastatic patterns | Diffuse lung metastases | Lymphangitic carcinomatosis | Lymphangitic carcinomatosis | Oligometastatic disease |
| Pleural and pericardial metastasis | Pleural metastases | |||
| Intrathoracic and distant lymphadenopathy | Intrathoracic and distant lymphadenopathy | |||
| Lytic bone metastases | Sclerotic bone metastases | Sclerotic bone metastases | Lytic bone metastases | |
| High rates of brain metastases | High rates of brain metastases | High rates of brain metastases, but lower compared to EGFR and ALK | High rates of brain metastases, but lower compared to EGFR and ALK |
Several researchers have investigated the imaging features of the primary tumors in those with mutation-driven NSCLC. The most commonly investigated features are the tumor density, morphology, and location.
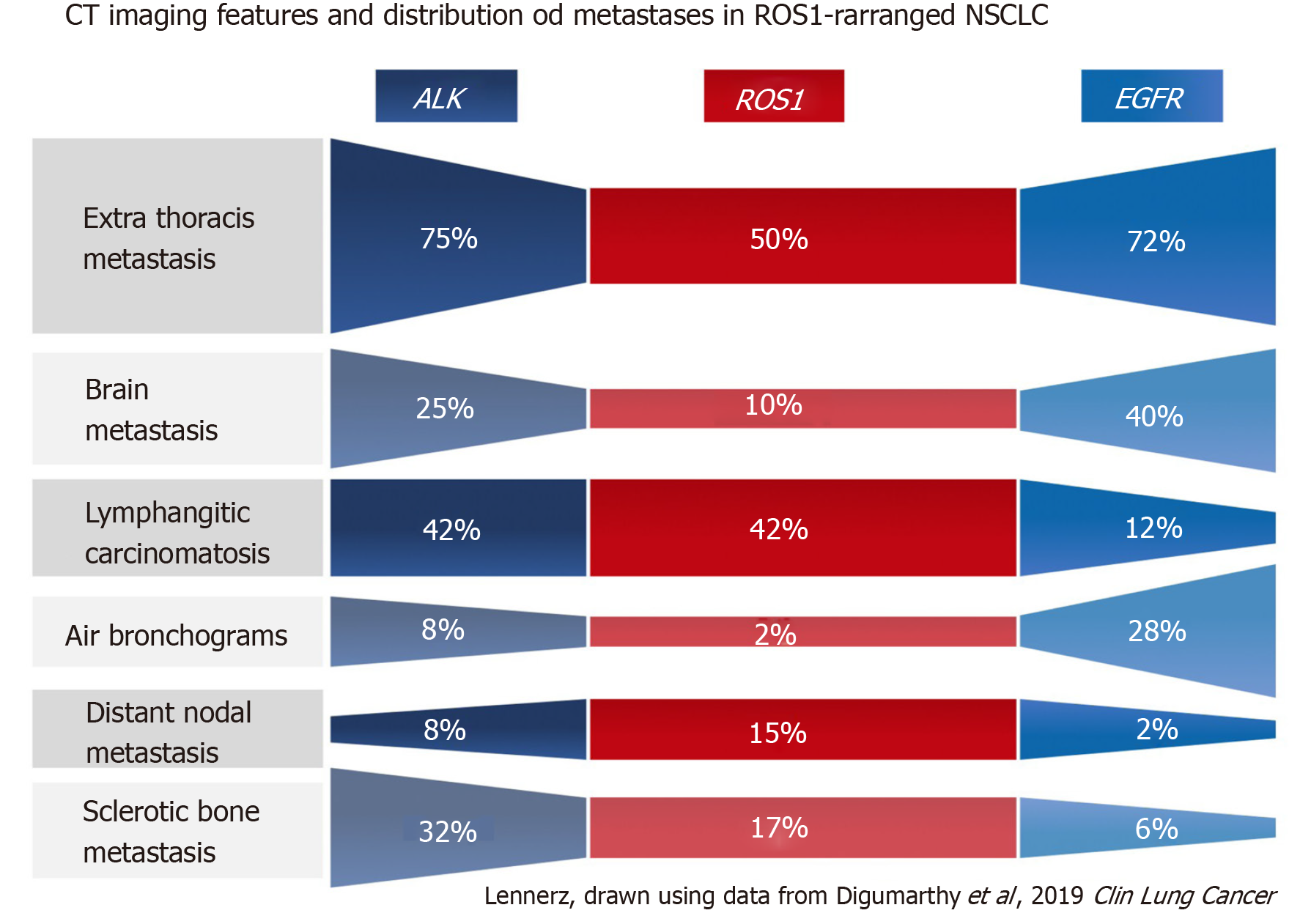
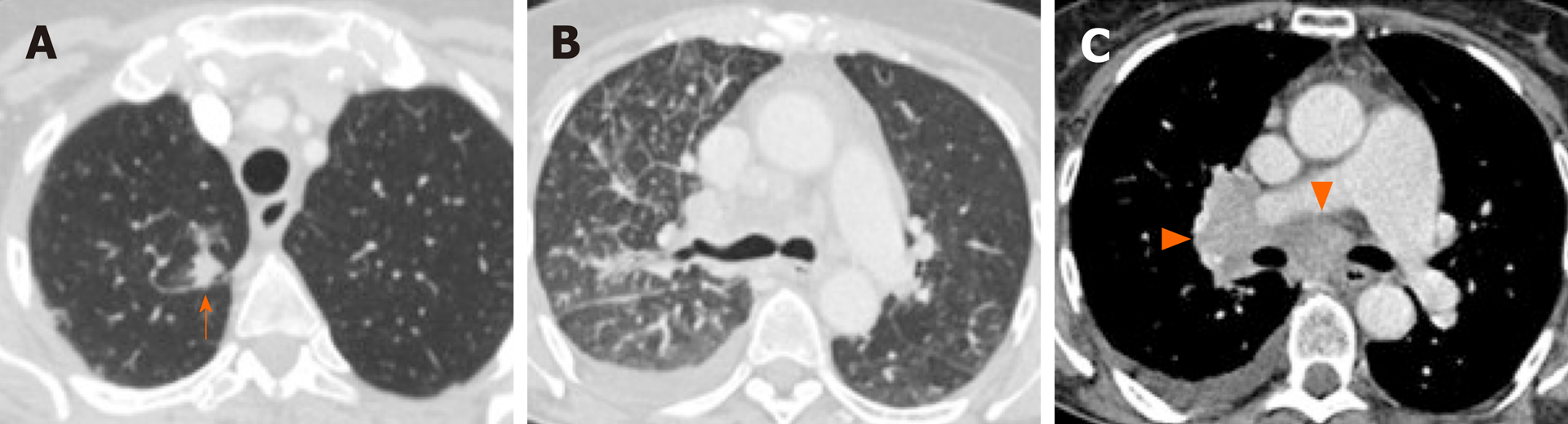
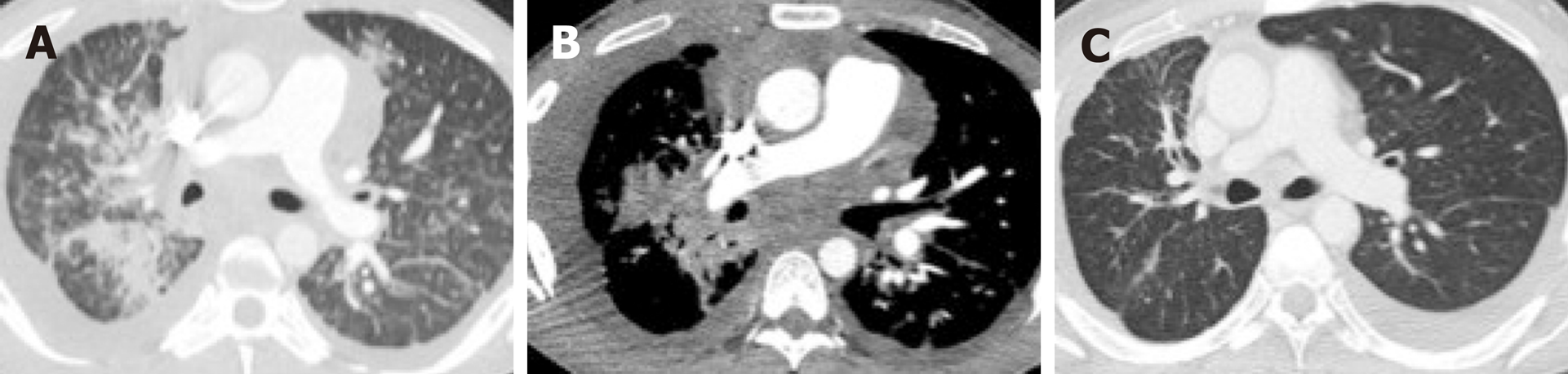
Overwhelmingly, most primary tumors in both mutated and non-mutated NSCLC are solid in density, including those with mutations involving KRAS, EGFR, ALK, ROS1, RET, MET, BRAF, HER2, and KRAS[40,54,63-71]. To date, the imaging features of NSCLC with NTRK fusions have not been studied, likely owing to their rarity. While most lung tumors are typically solid, several studies[63,64,66] have reported increased propensity of primary tumors in EGFR-mutant NSCLC to have a consolidative “pneumonic” appearance with ground-glass components, cavitations, and air-bronchograms (Figure 2A and 4A). This highlights the need for vigilance in the setting of non-resolving consolidations to prevent missed or delayed diagnosis in these patients.
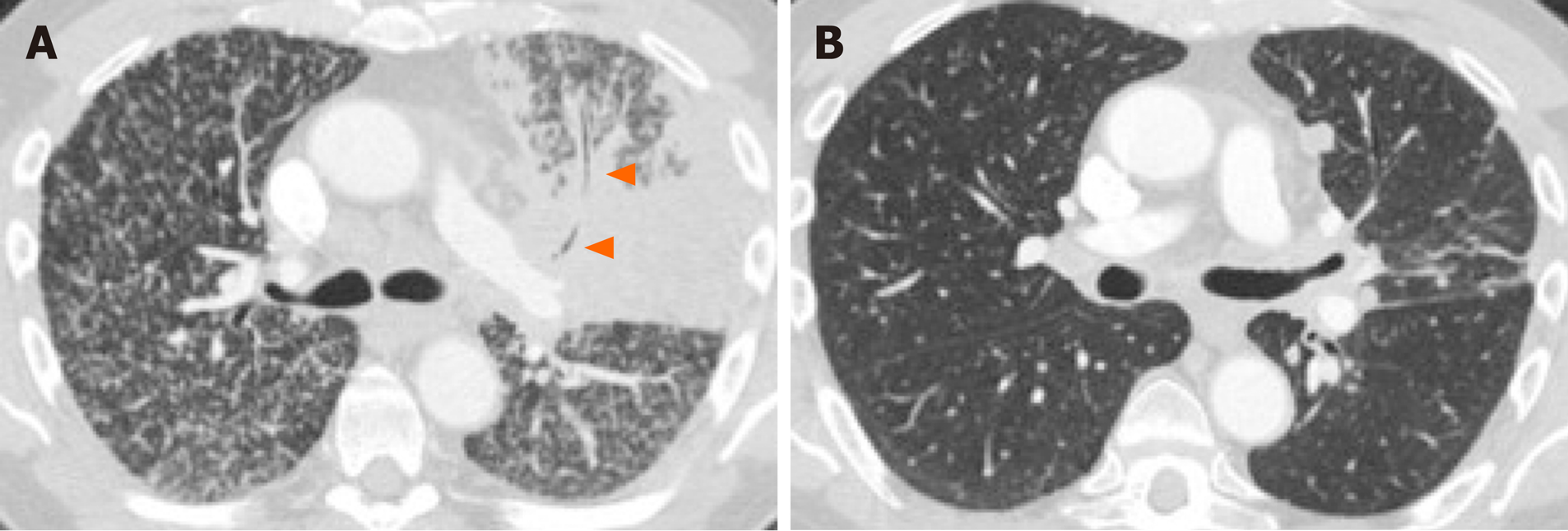
Primary tumor location, particularly the tumor’s axial location (i.e., central versus peripheral location), is another commonly investigated imaging feature. Increased tendency for peripheral rather than central locations has been reported in NSCLC with ALK rearrangements (Figure 3A)[66], RET rearrangements[70,72] and METex14 skipping mutations[54]. Two small studies have also suggested that the primary tumors in ROS1-positive NSCLC tend to be peripheral[70,73], although a subsequent larger study failed to support these findings[71].
More recently, it has been reported that the primary tumors in ALK-positive NSCLC are more likely to occur in the lower lobes, compared to EGFR-wild type and ALK/EGFR-negative tumors[66]. Most lung cancers develop in the upper lobes. Propensity for lung cancer development in the lower lobes has been reported in lung cancers developing in nonsmokers, although the presence or absence of an underlying driver mutation was not included in the study[74]. It has also been suggested that lower lobe tumors may be associated with a worse prognosis, but the studies did not include NSCLC with targetable mutations[75,76]. Tumor location may have implications with respect to accessibility for biopsy, surgery, or radiation therapy.
Nodal status is an important prognostic factor and determinant of treatment offered to patients with lung cancer. A number of studies have suggested that certain driver mutations may have increased predisposition for both intrathoracic and distant nodal metastases. In particular, several studies have reported increased frequency for extensive lymphadenopathy in ALK-positive NSCLC (Figure 3C)[65,66,71,77]. More recently, a similar predilection for intrathoracic and distant nodal metastases have been associated with ROS1-positive NSCLC[71]. The extensive lymphadenopathy seen in ALK-positive and ROS1-positive NSCLC can potentially be misinterpreted initially on imaging as either lymphoma or small cell lung cancer[65,71].
Several studies have reported that there is increased frequency of diffuse “miliary” (i.e., widespread disseminated) lung metastases in EGFR-mutant NSCLC (Figure 2A and 4A). Our group has previously reported up to a six-fold increased incidence of diffuse lung metastases in EGFR-mutant NSCLC compared to EGFR-wild type NSCLC[63]. While diffuse lung metastases are typically associated with worse prognosis, the presence of an EGFR mutation and increased responsiveness to targeted therapy (Figure 2B) can potentially improve outcomes in these patients. In the setting of a dominant lung mass and diffuse “miliary” lung metastases, EGFR-mutant NSCLC should be suspected[63].
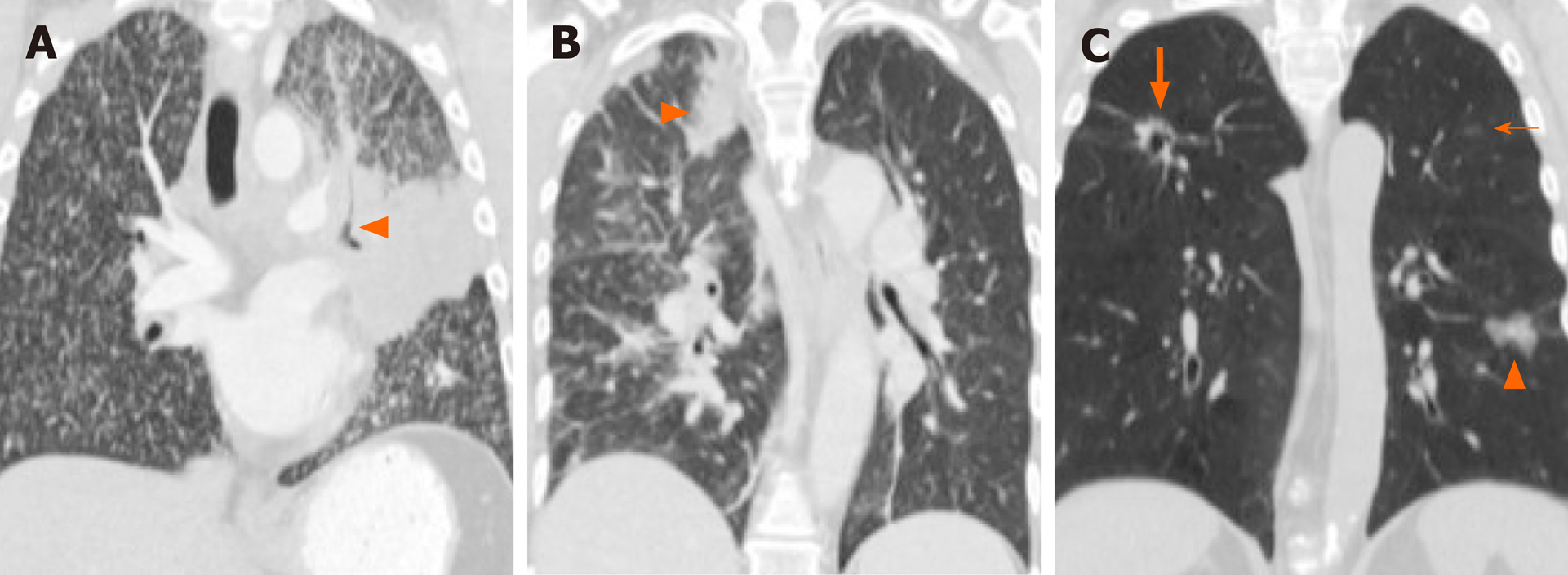
ALK-positive NSCLC, on the other hand, has been associated with lymphangitic carcinomatosis (Figure 3B and 4B) in comparison to EGFR-mutant NSCLC[65-67,78]. More recently, ROS1-positive NSCLC has also been associated with predilection for lymphangitic carcinomatosis (Figure 5A)[71]. On imaging, lymphangitic carcinomatosis is characterized by nodular thickening of the axial and peripheral, subpleural interstitium, with relative sparing of the intralobular interstitium[79]. Lymphangitic carcinomatosis is associated with worse prognosis in various extrapulmonary malignancies, but its prognostic impact in the setting of primary lung malignancies remains unclear du to paucity of data[80]. While it may appear intuitive to that lymphangitic carcinomatosis is suggestive of more advanced disease, a concurrent targetable mutation with either ALK or ROS1 may improve outcomes in these patients (Figure 5C).
More recently, it has been suggested that NSCLC with METex14 skipping mutations may have increased frequency of multifocal, synchronous primary lung cancer at presentation (Figure 4C), which was observed in approximately 1 in 5 patients[54]. The authors suggested that this multifocality may be secondary to synchronous adenocarcinomas with distinct splice site mutations, which has been previously described for METex14-mutated primary lung adenocarcinomas[81].
In addition to increased frequency of lymphangitic carcinomatosis, ALK-positive NSCLC has also been associated with increased frequencies of both pleural (Figure 3C) and pericardial metastases[65], and ROS1-positive NSCLC has also been associated with pleural metastases (Figure 5B)[71]. The mechanism behind these potential differences in metastatic tropisms among the different genotypes remains to be determined.
The brain is a common site of metastasis in NSCLC, with over 20% of patients with advanced NSCLC having brain metastases at the time of diagnosis, and up to approximately 50% developing them within three years[82-84]. Brain metastases present a unique challenge, as their treatment requires agents that can cross and can remain active beyond the blood-brain barrier.
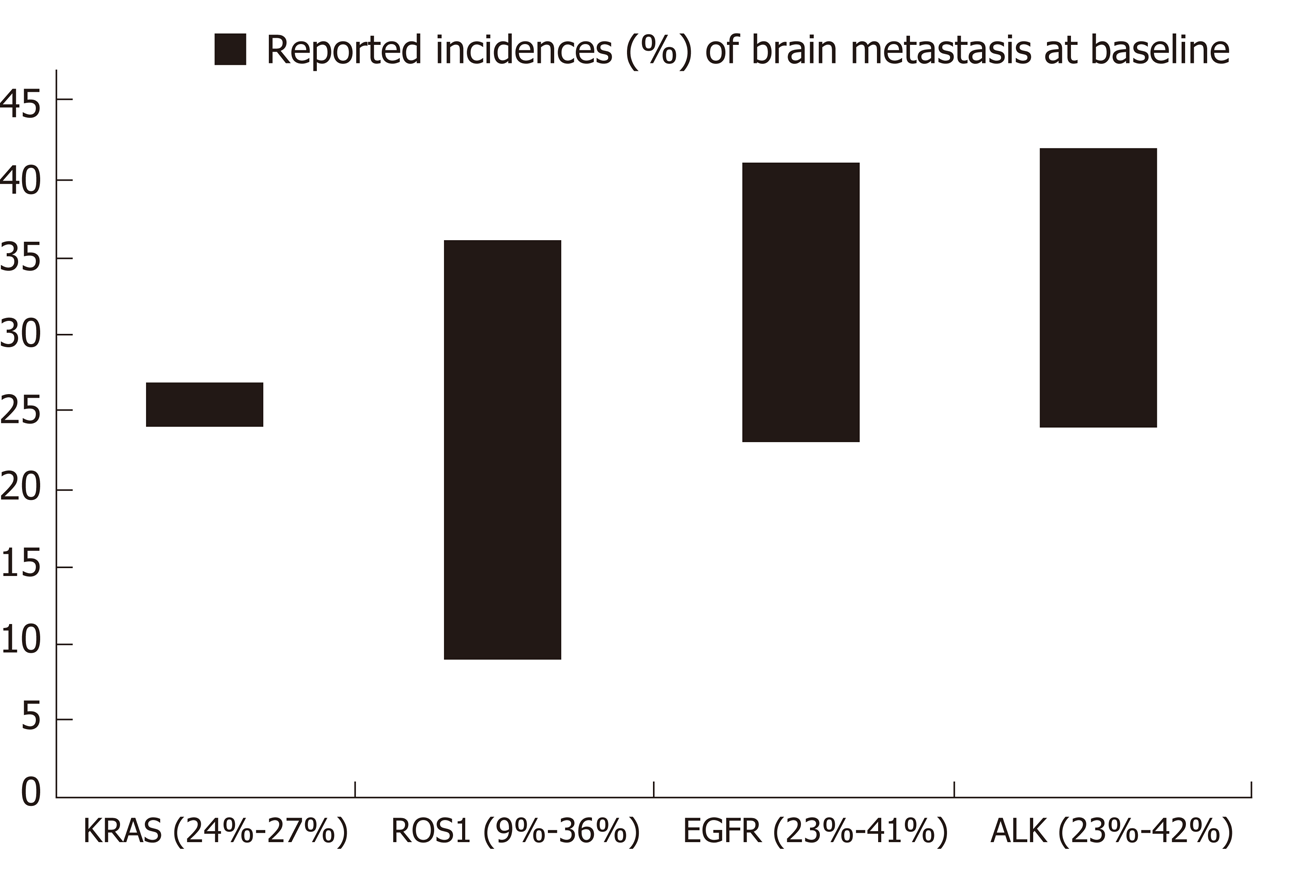
Several studies have suggested potential differences in the frequencies of brain metastases across the different oncogenic drivers in NSCLC[85]. NSCLC harboring alterations in EGFR, ALK, or ROS1 have been associated with increased frequencies of brain metastases[86-89]. Some reports, however, show that there is significant overlap in the frequencies of brain metastases among the different mutation groups[90]. Reported frequencies of brain metastasis at time of diagnosis of advanced disease range from 23%-41% in EGFR-mutant NSCLC[66,86,87], 23%-42% in ALK-positive NSCLC[66,86], and 9%-36% in ROS1-positive NSCLC[71,88,90]. Less data is available with respect to the frequencies of brain metastases in the other mutational subgroups. Incidence of 25% have been reported for both RET-positive[91] and HER2-mutant NSCLC[92], 21% for NSCLC with METex14 skipping mutations[54], and 10% for BRAF-mutant NSCLC. Ranges of reported incidences of brain metastases in the more common molecular subtypes are presented on Figure 6. Further investigation is necessary to determine if differences in tropism to the brain truly exist across the different oncogenic subsets in NSCLC and to determine the underlying mechanism resulting in differences. Nevertheless, the high incidences of brain metastases across several of these mutated tumors underscore the need for targeted agents that have robust CNS activity.
The bones are a common site of metastasis in NSCLC and osseous metastases are seen in up to 40% of patients with advanced lung cancer[93]. Bone metastases are a significant cause of morbidity in cancer patients as they can predispose to pathologic fractures, cause debilitating pain and severely reduce quality of life.
Osseous metastases from a variety of malignancies can be either predominantly lytic or predominantly sclerotic or osteoblastic in appearance. Many lytic lesions may also become sclerotic with treatment. Malignancies classically associated with sclerotic bone metastases are prostate cancer and small cell lung cancer. In general, bone metastases in NSCLC usually present as lytic lesions, with sclerotic metastases rarely seen prior to treatment[94,95].
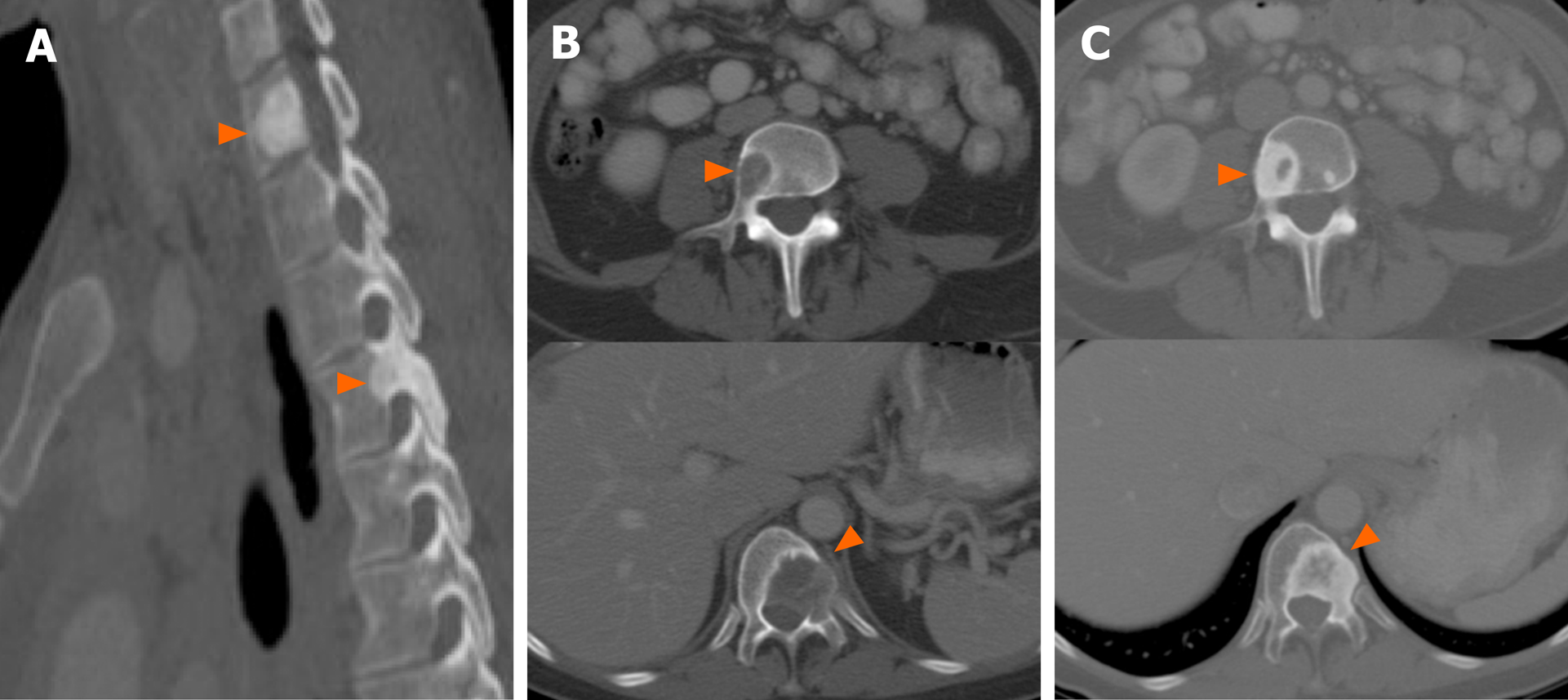
A number of studies, however, have reported that there may be a predisposition to either lytic or sclerotic bone metastasis based on the presence of an underlying driver mutation in NSCLC. ALK-positive NSCLC, for instance, has been associated with sclerotic metastases (Figure 7). In a study comparing the imaging findings of ALK-positive NSCLC to those of EGFR-mutant NSCLC, more than half of the patients with bone metastases in the setting of ALK-positive NSCLC had sclerotic bone metastases prior to any treatment. In contrast, sclerotic bone metastases were seen in only 1 of 6 patients with EGFR-mutant NSCLC[66]. More recently, a different study comparing the imaging features of ROS1-positive NSCLC to those with ALK or EGFR alterations, showed similar frequencies of bone metastases among the three mutational subgroups, but an increased frequency of sclerotic bone metastases in bot ROS1-positive NSCLC and ALK-positive NSCLC compared to EGFR-mutant NSCLC[71]. In contrast, a series presenting the clinicopathologic and imaging features of NSCLC with METex14 skipping mutations reported the bone metastases to be predominantly lytic in these patients[76]. The morphology of bone metastases in the other molecular subgroups has yet to be reported.
No specific imaging biomarker has yet to be identified to suggest the presence of an underlying BRAF mutation in NSCLC[40,96]. It has been suggested, however, that at the time of presentation, patients with lung cancer harboring the V600E BRAF mutation may be more likely to have intrathoracic metastases, particularly pleural metastases, while those with non-V600E BRAF mutations may be more likely to have intra-abdominal metastases[40].
Finally, METex14 skipping mutations in NSCLC have recently been associated with increased incidence of oligometastatic disease[54]. In the case series, the authors reported 4 patients that had only a single site of metastases (3 with adrenal metastasis and 1 one with soft tissue metastasis), although the findings have yet to be validated[54]. Several studies, however, have reported better outcomes in patients with limited metastatic burden when managed with radical treatment with curative intent[97].
The mechanism behind the morphological differences of the primary tumor and the differences in metastatic tropisms among the molecular subgroups of NSCLC remain unclear. While none of the imaging features and metastatic tropisms we discussed can reliably predict the presence of specific genetic alterations in isolation and they are unlikely to replace molecular genotyping in directing the need for targeted therapy, these imaging biomarkers can indicate the presence of specific targetable mutations and can play an adjunctive role. These features can assist in the selection patients who may benefit from expedited pathways for molecular testing or repeat testing when the initial genotyping results are equivocal or discordant with the clinical and imaging presentation. Given the importance of initiating targeted therapy in patients with targetable mutations, it is imperative to use all biomarkers available – clinical, histopathologic, and radiologic – in detecting these mutations.
Manuscript source: Invited manuscript
Specialty type: Oncology
Country/Territory of origin: United States
Peer-review report’s scientific quality classification
Grade A (Excellent): A, A
Grade B (Very good): 0
Grade C (Good): 0
Grade D (Fair): 0
Grade E (Poor): E
P-Reviewer: Deng B, Ju SQ, Lin Q S-Editor: Dou Y L-Editor: A E-Editor: Zhang YL
| 1. | Torre LA, Bray F, Siegel RL, Ferlay J, Lortet-Tieulent J, Jemal A. Global cancer statistics, 2012. CA Cancer J Clin. 2015;65:87-108. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 18694] [Cited by in RCA: 21324] [Article Influence: 2132.4] [Reference Citation Analysis (3)] |
| 2. | National Cancer Institute. SEER Cancer Statistics Review (CSR) 1975-2016. [updated 9 April 2020]. Available from: https://seer.cancer.gov/csr/1975_2016/. |
| 3. | Cancer Genome Atlas Research Network. Comprehensive molecular profiling of lung adenocarcinoma. Nature. 2014;511:543-550. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 3395] [Cited by in RCA: 4262] [Article Influence: 387.5] [Reference Citation Analysis (0)] |
| 4. | Ettinger DS, Aisner DL, Wood DE, Akerley W, Bauman J, Chang JY, Chirieac LR, D'Amico TA, Dilling TJ, Dobelbower M, Govindan R, Gubens MA, Hennon M, Horn L, Lackner RP, Lanuti M, Leal TA, Lilenbaum R, Lin J, Loo BW, Martins R, Otterson GA, Patel SP, Reckamp K, Riely GJ, Schild SE, Shapiro TA, Stevenson J, Swanson SJ, Tauer K, Yang SC, Gregory K, Hughes M. NCCN Guidelines Insights: Non-Small Cell Lung Cancer, Version 5.2018. J Natl Compr Canc Netw. 2018;16:807-821. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 268] [Cited by in RCA: 361] [Article Influence: 60.2] [Reference Citation Analysis (0)] |
| 5. | Lindeman NI, Cagle PT, Aisner DL, Arcila ME, Beasley MB, Bernicker EH, Colasacco C, Dacic S, Hirsch FR, Kerr K, Kwiatkowski DJ, Ladanyi M, Nowak JA, Sholl L, Temple-Smolkin R, Solomon B, Souter LH, Thunnissen E, Tsao MS, Ventura CB, Wynes MW, Yatabe Y. Updated Molecular Testing Guideline for the Selection of Lung Cancer Patients for Treatment With Targeted Tyrosine Kinase Inhibitors: Guideline From the College of American Pathologists, the International Association for the Study of Lung Cancer, and the Association for Molecular Pathology. Arch Pathol Lab Med. 2018;142:321-346. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 509] [Cited by in RCA: 573] [Article Influence: 81.9] [Reference Citation Analysis (0)] |
| 6. | Garinet S, Laurent-Puig P, Blons H, Oudart JB. Current and Future Molecular Testing in NSCLC, What Can We Expect from New Sequencing Technologies? J Clin Med. 2018;7. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 46] [Cited by in RCA: 40] [Article Influence: 5.7] [Reference Citation Analysis (0)] |
| 7. | Dietel M. Molecular Pathology: A Requirement for Precision Medicine in Cancer. Oncol Res Treat. 2016;39:804-810. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 18] [Cited by in RCA: 21] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 8. | Mino-Kenudson M. Immunohistochemistry for predictive biomarkers in non-small cell lung cancer. Transl Lung Cancer Res. 2017;6:570-587. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 37] [Cited by in RCA: 37] [Article Influence: 4.6] [Reference Citation Analysis (0)] |
| 9. | Yoshida A, Tsuta K, Wakai S, Arai Y, Asamura H, Shibata T, Furuta K, Kohno T, Kushima R. Immunohistochemical detection of ROS1 is useful for identifying ROS1 rearrangements in lung cancers. Mod Pathol. 2014;27:711-720. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 107] [Cited by in RCA: 119] [Article Influence: 10.8] [Reference Citation Analysis (0)] |
| 10. | Hirsch FR, McElhinny A, Stanforth D, Ranger-Moore J, Jansson M, Kulangara K, Richardson W, Towne P, Hanks D, Vennapusa B, Mistry A, Kalamegham R, Averbuch S, Novotny J, Rubin E, Emancipator K, McCaffery I, Williams JA, Walker J, Longshore J, Tsao MS, Kerr KM. PD-L1 Immunohistochemistry Assays for Lung Cancer: Results from Phase 1 of the Blueprint PD-L1 IHC Assay Comparison Project. J Thorac Oncol. 2017;12:208-222. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 808] [Cited by in RCA: 981] [Article Influence: 109.0] [Reference Citation Analysis (0)] |
| 11. | Kruglyak KM, Lin E, Ong FS. Next-Generation Sequencing and Applications to the Diagnosis and Treatment of Lung Cancer. Adv Exp Med Biol. 2016;890:123-136. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 35] [Cited by in RCA: 39] [Article Influence: 4.3] [Reference Citation Analysis (0)] |
| 12. | Lee DH, Tsao MS, Kambartel KO, Isobe H, Huang MS, Barrios CH, Khattak A, de Marinis F, Kothari S, Arunachalam A, Cao X, Burke T, Valladares A, de Castro J. Molecular testing and treatment patterns for patients with advanced non-small cell lung cancer: PIvOTAL observational study. PLoS One. 2018;13:e0202865. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 34] [Cited by in RCA: 50] [Article Influence: 7.1] [Reference Citation Analysis (0)] |
| 13. | Illei PB, Wong W, Wu N, Chu L, Gupta R, Schulze K, Gubens MA. ALK testing trends and patterns among community practices in the United States. JCO Precision Oncol. 2018;1-11. [RCA] [DOI] [Full Text] [Cited by in Crossref: 12] [Cited by in RCA: 13] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 14. | Shi Y, Au JS, Thongprasert S, Srinivasan S, Tsai CM, Khoa MT, Heeroma K, Itoh Y, Cornelio G, Yang PC. A prospective, molecular epidemiology study of EGFR mutations in Asian patients with advanced non-small-cell lung cancer of adenocarcinoma histology (PIONEER). J Thorac Oncol. 2014;9:154-162. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 917] [Cited by in RCA: 1119] [Article Influence: 101.7] [Reference Citation Analysis (0)] |
| 15. | Kawaguchi T, Koh Y, Ando M, Ito N, Takeo S, Adachi H, Tagawa T, Kakegawa S, Yamashita M, Kataoka K, Ichinose Y, Takeuchi Y, Serizawa M, Tamiya A, Shimizu S, Yoshimoto N, Kubo A, Isa S, Saka H, Matsumura A. Prospective Analysis of Oncogenic Driver Mutations and Environmental Factors: Japan Molecular Epidemiology for Lung Cancer Study. J Clin Oncol. 2016;34:2247-2257. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 62] [Cited by in RCA: 92] [Article Influence: 10.2] [Reference Citation Analysis (0)] |
| 16. | Park K, Tan EH, O'Byrne K, Zhang L, Boyer M, Mok T, Hirsh V, Yang JC, Lee KH, Lu S, Shi Y, Kim SW, Laskin J, Kim DW, Arvis CD, Kölbeck K, Laurie SA, Tsai CM, Shahidi M, Kim M, Massey D, Zazulina V, Paz-Ares L. Afatinib versus gefitinib as first-line treatment of patients with EGFR mutation-positive non-small-cell lung cancer (LUX-Lung 7): a phase 2B, open-label, randomised controlled trial. Lancet Oncol. 2016;17:577-589. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 663] [Cited by in RCA: 886] [Article Influence: 98.4] [Reference Citation Analysis (0)] |
| 17. | Wu YL, Cheng Y, Zhou X, Lee KH, Nakagawa K, Niho S, Tsuji F, Linke R, Rosell R, Corral J, Migliorino MR, Pluzanski A, Sbar EI, Wang T, White JL, Nadanaciva S, Sandin R, Mok TS. Dacomitinib versus gefitinib as first-line treatment for patients with EGFR-mutation-positive non-small-cell lung cancer (ARCHER 1050): a randomised, open-label, phase 3 trial. Lancet Oncol. 2017;18:1454-1466. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 568] [Cited by in RCA: 852] [Article Influence: 106.5] [Reference Citation Analysis (0)] |
| 18. | Rosell R, Carcereny E, Gervais R, Vergnenegre A, Massuti B, Felip E, Palmero R, Garcia-Gomez R, Pallares C, Sanchez JM, Porta R, Cobo M, Garrido P, Longo F, Moran T, Insa A, De Marinis F, Corre R, Bover I, Illiano A, Dansin E, de Castro J, Milella M, Reguart N, Altavilla G, Jimenez U, Provencio M, Moreno MA, Terrasa J, Muñoz-Langa J, Valdivia J, Isla D, Domine M, Molinier O, Mazieres J, Baize N, Garcia-Campelo R, Robinet G, Rodriguez-Abreu D, Lopez-Vivanco G, Gebbia V, Ferrera-Delgado L, Bombaron P, Bernabe R, Bearz A, Artal A, Cortesi E, Rolfo C, Sanchez-Ronco M, Drozdowskyj A, Queralt C, de Aguirre I, Ramirez JL, Sanchez JJ, Molina MA, Taron M, Paz-Ares L; Spanish Lung Cancer Group in collaboration with Groupe Français de Pneumo-Cancérologie and Associazione Italiana Oncologia Toracica. Erlotinib versus standard chemotherapy as first-line treatment for European patients with advanced EGFR mutation-positive non-small-cell lung cancer (EURTAC): a multicentre, open-label, randomised phase 3 trial. Lancet Oncol. 2012;13:239-246. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4228] [Cited by in RCA: 4334] [Article Influence: 333.4] [Reference Citation Analysis (0)] |
| 19. | Maemondo M, Inoue A, Kobayashi K, Sugawara S, Oizumi S, Isobe H, Gemma A, Harada M, Yoshizawa H, Kinoshita I, Fujita Y, Okinaga S, Hirano H, Yoshimori K, Harada T, Ogura T, Ando M, Miyazawa H, Tanaka T, Saijo Y, Hagiwara K, Morita S, Nukiwa T; North-East Japan Study Group. Gefitinib or chemotherapy for non-small-cell lung cancer with mutated EGFR. N Engl J Med. 2010;362:2380-2388. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3961] [Cited by in RCA: 4366] [Article Influence: 291.1] [Reference Citation Analysis (0)] |
| 20. | Mok TS, Wu Y-L, Ahn M-J, Garassino MC, Kim HR, Ramalingam SS, Shepherd FA, He Y, Akamatsu H, Theelen WS, Lee CK, Sebastian M, Templeton A, Mann H, Marotti M, Ghiorghiu S, Papadimitrakopoulou VA; AURA3 Investigators. Osimertinib or Platinum-Pemetrexed in EGFR T790M-Positive Lung Cancer. N Engl J Med. 2017;376:629-640. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2334] [Cited by in RCA: 2557] [Article Influence: 319.6] [Reference Citation Analysis (0)] |
| 21. | Soria JC, Ohe Y, Vansteenkiste J, Reungwetwattana T, Chewaskulyong B, Lee KH, Dechaphunkul A, Imamura F, Nogami N, Kurata T, Okamoto I, Zhou C, Cho BC, Cheng Y, Cho EK, Voon PJ, Planchard D, Su WC, Gray JE, Lee SM, Hodge R, Marotti M, Rukazenkov Y, Ramalingam SS; FLAURA Investigators. Osimertinib in Untreated EGFR-Mutated Advanced Non-Small-Cell Lung Cancer. N Engl J Med. 2018;378:113-125. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2556] [Cited by in RCA: 3580] [Article Influence: 511.4] [Reference Citation Analysis (0)] |
| 22. | Sequist LV, Yang JC, Yamamoto N, O'Byrne K, Hirsh V, Mok T, Geater SL, Orlov S, Tsai CM, Boyer M, Su WC, Bennouna J, Kato T, Gorbunova V, Lee KH, Shah R, Massey D, Zazulina V, Shahidi M, Schuler M. Phase III study of afatinib or cisplatin plus pemetrexed in patients with metastatic lung adenocarcinoma with EGFR mutations. J Clin Oncol. 2013;31:3327-3334. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2416] [Cited by in RCA: 2556] [Article Influence: 213.0] [Reference Citation Analysis (0)] |
| 23. | Soda M, Choi YL, Enomoto M, Takada S, Yamashita Y, Ishikawa S, Fujiwara S, Watanabe H, Kurashina K, Hatanaka H, Bando M, Ohno S, Ishikawa Y, Aburatani H, Niki T, Sohara Y, Sugiyama Y, Mano H. Identification of the transforming EML4-ALK fusion gene in non-small-cell lung cancer. Nature. 2007;448:561-566. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3816] [Cited by in RCA: 4084] [Article Influence: 226.9] [Reference Citation Analysis (0)] |
| 24. | Shaw AT, Hsu PP, Awad MM, Engelman JA. Tyrosine kinase gene rearrangements in epithelial malignancies. Nat Rev Cancer. 2013;13:772-787. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 231] [Cited by in RCA: 236] [Article Influence: 19.7] [Reference Citation Analysis (0)] |
| 25. | Wong DW, Leung EL, So KK, Tam IY, Sihoe AD, Cheng LC, Ho KK, Au JS, Chung LP, Pik Wong M; University of Hong Kong Lung Cancer Study Group. The EML4-ALK fusion gene is involved in various histologic types of lung cancers from nonsmokers with wild-type EGFR and KRAS. Cancer. 2009;115:1723-1733. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 525] [Cited by in RCA: 568] [Article Influence: 35.5] [Reference Citation Analysis (0)] |
| 26. | Fukui T, Yatabe Y, Kobayashi Y, Tomizawa K, Ito S, Hatooka S, Matsuo K, Mitsudomi T. Clinicoradiologic characteristics of patients with lung adenocarcinoma harboring EML4-ALK fusion oncogene. Lung Cancer. 2012;77:319-325. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 76] [Cited by in RCA: 91] [Article Influence: 7.0] [Reference Citation Analysis (0)] |
| 27. | Peters S, Camidge DR, Shaw AT, Gadgeel S, Ahn JS, Kim DW, Ou SI, Pérol M, Dziadziuszko R, Rosell R, Zeaiter A, Mitry E, Golding S, Balas B, Noe J, Morcos PN, Mok T; ALEX Trial Investigators. Alectinib versus Crizotinib in Untreated ALK-Positive Non-Small-Cell Lung Cancer. N Engl J Med. 2017;377:829-838. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1385] [Cited by in RCA: 1720] [Article Influence: 215.0] [Reference Citation Analysis (0)] |
| 28. | Soria JC, Tan DSW, Chiari R, Wu YL, Paz-Ares L, Wolf J, Geater SL, Orlov S, Cortinovis D, Yu CJ, Hochmair M, Cortot AB, Tsai CM, Moro-Sibilot D, Campelo RG, McCulloch T, Sen P, Dugan M, Pantano S, Branle F, Massacesi C, de Castro G. First-line ceritinib versus platinum-based chemotherapy in advanced ALK-rearranged non-small-cell lung cancer (ASCEND-4): a randomised, open-label, phase 3 study. Lancet. 2017;389:917-929. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 692] [Cited by in RCA: 842] [Article Influence: 105.3] [Reference Citation Analysis (0)] |
| 29. | Shaw AT, Gandhi L, Gadgeel S, Riely GJ, Cetnar J, West H, Camidge DR, Socinski MA, Chiappori A, Mekhail T, Chao BH, Borghaei H, Gold KA, Zeaiter A, Bordogna W, Balas B, Puig O, Henschel V, Ou SI; study investigators. Alectinib in ALK-positive, crizotinib-resistant, non-small-cell lung cancer: a single-group, multicentre, phase 2 trial. Lancet Oncol. 2016;17:234-242. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 413] [Cited by in RCA: 510] [Article Influence: 51.0] [Reference Citation Analysis (0)] |
| 30. | Kim DW, Tiseo M, Ahn MJ, Reckamp KL, Hansen KH, Kim SW, Huber RM, West HL, Groen HJM, Hochmair MJ, Leighl NB, Gettinger SN, Langer CJ, Paz-Ares Rodríguez LG, Smit EF, Kim ES, Reichmann W, Haluska FG, Kerstein D, Camidge DR. Brigatinib in Patients With Crizotinib-Refractory Anaplastic Lymphoma Kinase-Positive Non-Small-Cell Lung Cancer: A Randomized, Multicenter Phase II Trial. J Clin Oncol. 2017;35:2490-2498. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 367] [Cited by in RCA: 448] [Article Influence: 56.0] [Reference Citation Analysis (0)] |
| 31. | Camidge DR, Kim HR, Ahn MJ, Yang JC, Han JY, Lee JS, Hochmair MJ, Li JY, Chang GC, Lee KH, Gridelli C, Delmonte A, Garcia Campelo R, Kim DW, Bearz A, Griesinger F, Morabito A, Felip E, Califano R, Ghosh S, Spira A, Gettinger SN, Tiseo M, Gupta N, Haney J, Kerstein D, Popat S. Brigatinib versus Crizotinib in ALK-Positive Non-Small-Cell Lung Cancer. N Engl J Med. 2018;379:2027-2039. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 506] [Cited by in RCA: 681] [Article Influence: 97.3] [Reference Citation Analysis (0)] |
| 32. | Solomon BJ, Besse B, Bauer TM, Felip E, Soo RA, Camidge DR, Chiari R, Bearz A, Lin CC, Gadgeel SM, Riely GJ, Tan EH, Seto T, James LP, Clancy JS, Abbattista A, Martini JF, Chen J, Peltz G, Thurm H, Ou SI, Shaw AT. Lorlatinib in patients with ALK-positive non-small-cell lung cancer: results from a global phase 2 study. Lancet Oncol. 2018;19:1654-1667. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 386] [Cited by in RCA: 546] [Article Influence: 78.0] [Reference Citation Analysis (0)] |
| 33. | Bergethon K, Shaw AT, Ou SH, Katayama R, Lovly CM, McDonald NT, Massion PP, Siwak-Tapp C, Gonzalez A, Fang R, Mark EJ, Batten JM, Chen H, Wilner KD, Kwak EL, Clark JW, Carbone DP, Ji H, Engelman JA, Mino-Kenudson M, Pao W, Iafrate AJ. ROS1 rearrangements define a unique molecular class of lung cancers. J Clin Oncol. 2012;30:863-870. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1085] [Cited by in RCA: 1214] [Article Influence: 93.4] [Reference Citation Analysis (0)] |
| 34. | Gainor JF, Shaw AT. Novel targets in non-small cell lung cancer: ROS1 and RET fusions. Oncologist. 2013;18:865-875. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 228] [Cited by in RCA: 271] [Article Influence: 22.6] [Reference Citation Analysis (0)] |
| 35. | Baik CS, Myall NJ, Wakelee HA. Targeting BRAF-Mutant Non-Small Cell Lung Cancer: From Molecular Profiling to Rationally Designed Therapy. Oncologist. 2017;22:786-796. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 82] [Cited by in RCA: 87] [Article Influence: 10.9] [Reference Citation Analysis (0)] |
| 36. | Paik PK, Arcila ME, Fara M, Sima CS, Miller VA, Kris MG, Ladanyi M, Riely GJ. Clinical characteristics of patients with lung adenocarcinomas harboring BRAF mutations. J Clin Oncol. 2011;29:2046-2051. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 467] [Cited by in RCA: 529] [Article Influence: 37.8] [Reference Citation Analysis (0)] |
| 37. | Marchetti A, Felicioni L, Malatesta S, Grazia Sciarrotta M, Guetti L, Chella A, Viola P, Pullara C, Mucilli F, Buttitta F. Clinical features and outcome of patients with non-small-cell lung cancer harboring BRAF mutations. J Clin Oncol. 2011;29:3574-3579. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 365] [Cited by in RCA: 425] [Article Influence: 30.4] [Reference Citation Analysis (0)] |
| 38. | Cardarella S, Ogino A, Nishino M, Butaney M, Shen J, Lydon C, Yeap BY, Sholl LM, Johnson BE, Jänne PA. Clinical, pathologic, and biologic features associated with BRAF mutations in non-small cell lung cancer. Clin Cancer Res. 2013;19:4532-4540. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 233] [Cited by in RCA: 283] [Article Influence: 23.6] [Reference Citation Analysis (0)] |
| 39. | Litvak AM, Paik PK, Woo KM, Sima CS, Hellmann MD, Arcila ME, Ladanyi M, Rudin CM, Kris MG, Riely GJ. Clinical characteristics and course of 63 patients with BRAF mutant lung cancers. J Thorac Oncol. 2014;9:1669-1674. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 83] [Cited by in RCA: 102] [Article Influence: 10.2] [Reference Citation Analysis (0)] |
| 40. | Mendoza DP, Dagogo-Jack I, Chen T, Padole A, Shepard JO, Shaw AT, Digumarthy SR. Imaging characteristics of BRAF-mutant non-small cell lung cancer by functional class. Lung Cancer. 2019;129:80-84. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 14] [Cited by in RCA: 16] [Article Influence: 2.7] [Reference Citation Analysis (0)] |
| 41. | Planchard D, Smit EF, Groen HJM, Mazieres J, Besse B, Helland Å, Giannone V, D'Amelio AM Jr, Zhang P, Mookerjee B, Johnson BE. Dabrafenib plus trametinib in patients with previously untreated BRAFV600E-mutant metastatic non-small-cell lung cancer: an open-label, phase 2 trial. Lancet Oncol. 2017;18:1307-1316. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 485] [Cited by in RCA: 677] [Article Influence: 84.6] [Reference Citation Analysis (0)] |
| 42. | Farago AF, Taylor MS, Doebele RC, Zhu VW, Kummar S, Spira AI, Boyle TA, Haura EB, Arcila ME, Benayed R, Aisner DL, Horick NK, Lennerz JK, Le LP, Iafrate AJ, Ou SI, Shaw AT, Mino-Kenudson M, Drilon A. Clinicopathologic Features of Non-Small-Cell Lung Cancer Harboring an NTRK Gene Fusion. JCO Precis Oncol. 2018;2018. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 55] [Cited by in RCA: 101] [Article Influence: 14.4] [Reference Citation Analysis (0)] |
| 43. | Drilon A, Laetsch TW, Kummar S, DuBois SG, Lassen UN, Demetri GD, Nathenson M, Doebele RC, Farago AF, Pappo AS, Turpin B, Dowlati A, Brose MS, Mascarenhas L, Federman N, Berlin J, El-Deiry WS, Baik C, Deeken J, Boni V, Nagasubramanian R, Taylor M, Rudzinski ER, Meric-Bernstam F, Sohal DPS, Ma PC, Raez LE, Hechtman JF, Benayed R, Ladanyi M, Tuch BB, Ebata K, Cruickshank S, Ku NC, Cox MC, Hawkins DS, Hong DS, Hyman DM. Efficacy of Larotrectinib in TRK Fusion-Positive Cancers in Adults and Children. N Engl J Med. 2018;378:731-739. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1577] [Cited by in RCA: 1897] [Article Influence: 271.0] [Reference Citation Analysis (0)] |
| 44. | Siena S, Doebele RC, Shaw AT, Karapetis CS, Tan DS-W, Cho BC, Kim D-W, Ahn M-J, Krebs M, Goto K, Garrido Lopez P, Farago AF, Loong HHF, Tosi D, Barve MA, Simmons BP, Ye C, Eng S, Drilon AE. Efficacy of entrectinib in patients (pts) with solid tumors and central nervous system (CNS) metastases: Integrated analysis from three clinical trials. J Clin Oncol. 2019;37:3017–3017. [RCA] [DOI] [Full Text] [Cited by in Crossref: 18] [Cited by in RCA: 18] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 45. | Wang R, Hu H, Pan Y, Li Y, Ye T, Li C, Luo X, Wang L, Li H, Zhang Y, Li F, Lu Y, Lu Q, Xu J, Garfield D, Shen L, Ji H, Pao W, Sun Y, Chen H. RET fusions define a unique molecular and clinicopathologic subtype of non-small-cell lung cancer. J Clin Oncol. 2012;30:4352-4359. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 352] [Cited by in RCA: 428] [Article Influence: 32.9] [Reference Citation Analysis (0)] |
| 46. | Kohno T, Ichikawa H, Totoki Y, Yasuda K, Hiramoto M, Nammo T, Sakamoto H, Tsuta K, Furuta K, Shimada Y, Iwakawa R, Ogiwara H, Oike T, Enari M, Schetter AJ, Okayama H, Haugen A, Skaug V, Chiku S, Yamanaka I, Arai Y, Watanabe S, Sekine I, Ogawa S, Harris CC, Tsuda H, Yoshida T, Yokota J, Shibata T. KIF5B-RET fusions in lung adenocarcinoma. Nat Med. 2012;18:375-377. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 590] [Cited by in RCA: 667] [Article Influence: 51.3] [Reference Citation Analysis (0)] |
| 47. | Drilon A, Rekhtman N, Arcila M, Wang L, Ni A, Albano M, Van Voorthuysen M, Somwar R, Smith RS, Montecalvo J, Plodkowski A, Ginsberg MS, Riely GJ, Rudin CM, Ladanyi M, Kris MG. Cabozantinib in patients with advanced RET-rearranged non-small-cell lung cancer: an open-label, single-centre, phase 2, single-arm trial. Lancet Oncol. 2016;17:1653-1660. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 323] [Cited by in RCA: 364] [Article Influence: 40.4] [Reference Citation Analysis (0)] |
| 48. | Yoh K, Seto T, Satouchi M, Nishio M, Yamamoto N, Murakami H, Nogami N, Matsumoto S, Kohno T, Tsuta K, Tsuchihara K, Ishii G, Nomura S, Sato A, Ohtsu A, Ohe Y, Goto K. Vandetanib in patients with previously treated RET-rearranged advanced non-small-cell lung cancer (LURET): an open-label, multicentre phase 2 trial. Lancet Respir Med. 2017;5:42-50. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 190] [Cited by in RCA: 241] [Article Influence: 26.8] [Reference Citation Analysis (0)] |
| 49. | Drilon AE, Subbiah V, Oxnard GR, Bauer TM, Velcheti V, Lakhani N, Besse B, Park K, Patel JD, Cabanillas ME, Johnson ML. A phase 1 study of LOXO-292, a potent and highly selective RET inhibitor, in patients with RET-altered cancers. J Clin Oncol. 2018;36:102. [RCA] [DOI] [Full Text] [Cited by in Crossref: 79] [Cited by in RCA: 79] [Article Influence: 11.3] [Reference Citation Analysis (0)] |
| 50. | Subbiah V, Taylor M, Lin J, Hu M, Ou S-HI, Brose MS, Garralda E, Clifford C, Palmer M, Evans E, Shi H, Wolf B, Gainor JF. Abstract CT043: Highly potent and selective RET inhibitor, BLU-667, achieves proof of concept in a phase I study of advanced, RET-altered solid tumors. Cancer Res. 2018;78:CT043–CT043. [RCA] [DOI] [Full Text] [Cited by in Crossref: 15] [Cited by in RCA: 15] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 51. | Organ SL, Tsao MS. An overview of the c-MET signaling pathway. Ther Adv Med Oncol. 2011;3:S7-S19. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 474] [Cited by in RCA: 632] [Article Influence: 48.6] [Reference Citation Analysis (0)] |
| 52. | Engelman JA, Zejnullahu K, Mitsudomi T, Song Y, Hyland C, Park JO, Lindeman N, Gale CM, Zhao X, Christensen J, Kosaka T, Holmes AJ, Rogers AM, Cappuzzo F, Mok T, Lee C, Johnson BE, Cantley LC, Jänne PA. MET amplification leads to gefitinib resistance in lung cancer by activating ERBB3 signaling. Science. 2007;316:1039-1043. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3442] [Cited by in RCA: 3655] [Article Influence: 203.1] [Reference Citation Analysis (0)] |
| 53. | Awad MM, Oxnard GR, Jackman DM, Savukoski DO, Hall D, Shivdasani P, Heng JC, Dahlberg SE, Jänne PA, Verma S, Christensen J, Hammerman PS, Sholl LM. MET Exon 14 Mutations in Non-Small-Cell Lung Cancer Are Associated With Advanced Age and Stage-Dependent MET Genomic Amplification and c-Met Overexpression. J Clin Oncol. 2016;34:721-730. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 380] [Cited by in RCA: 512] [Article Influence: 56.9] [Reference Citation Analysis (0)] |
| 54. | Digumarthy SR, Mendoza DP, Zhang EW, Lennerz JK, Heist RS. Clinicopathologic and Imaging Features of Non-Small-Cell Lung Cancer with MET Exon 14 Skipping Mutations. Cancers (Basel). 2019;11. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 18] [Cited by in RCA: 29] [Article Influence: 4.8] [Reference Citation Analysis (0)] |
| 55. | Heist RS, Shim HS, Gingipally S, Mino-Kenudson M, Le L, Gainor JF, Zheng Z, Aryee M, Xia J, Jia P, Jin H, Zhao Z, Pao W, Engelman JA, Iafrate AJ. MET Exon 14 Skipping in Non-Small Cell Lung Cancer. Oncologist. 2016;21:481-486. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 68] [Cited by in RCA: 83] [Article Influence: 9.2] [Reference Citation Analysis (0)] |
| 56. | Felip E, Horn L, Patel JD, Sakai H, Scheele J, Bruns R, Paik PK. Tepotinib in patients with advanced non-small cell lung cancer (NSCLC) harboring MET exon 14-skipping mutations: Phase II trial. J Clin Oncol. 2018;36:9016–9016. [RCA] [DOI] [Full Text] [Cited by in Crossref: 17] [Cited by in RCA: 17] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 57. | Wolf J, Seto T, Han J-Y, Reguart N, Garon EB, Groen HJM, Tan DS-W, Hida T, De Jonge MJ, Orlov SV, Smit EF, Souquet PJ, Vansteenkiste JF, Giovannini M, Le Mouhaer S, Robeva A, Waldron-Lynch M, Heist RS. Capmatinib (INC280) in METΔex14-mutated advanced non-small cell lung cancer (NSCLC): Efficacy data from the phase II GEOMETRY mono-1 study. J Clin Oncol. 2019;37:9004–9004. [RCA] [DOI] [Full Text] [Cited by in Crossref: 74] [Cited by in RCA: 76] [Article Influence: 12.7] [Reference Citation Analysis (0)] |
| 58. | Cappuzzo F, Ciuleanu T, Stelmakh L, Cicenas S, Szczésna A, Juhász E, Esteban E, Molinier O, Brugger W, Melezínek I, Klingelschmitt G, Klughammer B, Giaccone G; SATURN investigators. Erlotinib as maintenance treatment in advanced non-small-cell lung cancer: a multicentre, randomised, placebo-controlled phase 3 study. Lancet Oncol. 2010;11:521-529. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 856] [Cited by in RCA: 904] [Article Influence: 60.3] [Reference Citation Analysis (0)] |
| 59. | Mazières J, Peters S, Lepage B, Cortot AB, Barlesi F, Beau-Faller M, Besse B, Blons H, Mansuet-Lupo A, Urban T, Moro-Sibilot D, Dansin E, Chouaid C, Wislez M, Diebold J, Felip E, Rouquette I, Milia JD, Gautschi O. Lung cancer that harbors an HER2 mutation: epidemiologic characteristics and therapeutic perspectives. J Clin Oncol. 2013;31:1997-2003. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 430] [Cited by in RCA: 512] [Article Influence: 42.7] [Reference Citation Analysis (0)] |
| 60. | Li BT, Shen R, Buonocore D, Olah ZT, Ni A, Ginsberg MS, Ulaner GA, Offin M, Feldman D, Hembrough T, Cecchi F, Schwartz S, Pavlakis N, Clarke S, Won HH, Brzostowski EB, Riely GJ, Solit DB, Hyman DM, Drilon A, Rudin CM, Berger MF, Baselga J, Scaltriti M, Arcila ME, Kris MG. Ado-Trastuzumab Emtansine for Patients With HER2-Mutant Lung Cancers: Results From a Phase II Basket Trial. J Clin Oncol. 2018;36:2532-2537. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 302] [Cited by in RCA: 394] [Article Influence: 56.3] [Reference Citation Analysis (0)] |
| 61. | O'Byrne KJ, Gatzemeier U, Bondarenko I, Barrios C, Eschbach C, Martens UM, Hotko Y, Kortsik C, Paz-Ares L, Pereira JR, von Pawel J, Ramlau R, Roh JK, Yu CT, Stroh C, Celik I, Schueler A, Pirker R. Molecular biomarkers in non-small-cell lung cancer: a retrospective analysis of data from the phase 3 FLEX study. Lancet Oncol. 2011;12:795-805. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 164] [Cited by in RCA: 148] [Article Influence: 10.6] [Reference Citation Analysis (0)] |
| 62. | Seton-Rogers S. KRAS-G12C in the crosshairs. Nat Rev Cancer. 2020;20:3. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 24] [Cited by in RCA: 25] [Article Influence: 5.0] [Reference Citation Analysis (0)] |
| 63. | Digumarthy SR, Mendoza DP, Padole A, Chen T, Peterson PG, Piotrowska Z, Sequist LV. Diffuse Lung Metastases in EGFR-Mutant Non-Small Cell Lung Cancer. Cancers (Basel). 2019;11. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 11] [Cited by in RCA: 11] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 64. | Zhang H, Cai W, Wang Y, Liao M, Tian S. CT and clinical characteristics that predict risk of EGFR mutation in non-small cell lung cancer: a systematic review and meta-analysis. Int J Clin Oncol. 2019;24:649-659. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 20] [Cited by in RCA: 27] [Article Influence: 4.5] [Reference Citation Analysis (0)] |
| 65. | Mendoza DP, Stowell J, Muzikansky A, Shepard JO, Shaw AT, Digumarthy SR. Computed Tomography Imaging Characteristics of Non-Small-Cell Lung Cancer With Anaplastic Lymphoma Kinase Rearrangements: A Systematic Review and Meta-Analysis. Clin Lung Cancer. 2019;20:339-349. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 13] [Cited by in RCA: 21] [Article Influence: 3.5] [Reference Citation Analysis (0)] |
| 66. | Mendoza DP, Lin JJ, Rooney MM, Chen T, Sequist LV, Shaw AT, Digumarthy SR. Imaging Features and Metastatic Patterns of Advanced ALK-Rearranged Non-Small Cell Lung Cancer. AJR Am J Roentgenol. 2020;214:766-774. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 13] [Cited by in RCA: 12] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 67. | Rizzo S, Petrella F, Buscarino V, De Maria F, Raimondi S, Barberis M, Fumagalli C, Spitaleri G, Rampinelli C, De Marinis F, Spaggiari L, Bellomi M. CT Radiogenomic Characterization of EGFR, K-RAS, and ALK Mutations in Non-Small Cell Lung Cancer. Eur Radiol. 2016;26:32-42. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 156] [Cited by in RCA: 203] [Article Influence: 20.3] [Reference Citation Analysis (0)] |
| 68. | Rizzo S, Raimondi S, de Jong EEC, van Elmpt W, De Piano F, Petrella F, Bagnardi V, Jochems A, Bellomi M, Dingemans AM, Lambin P. Genomics of non-small cell lung cancer (NSCLC): Association between CT-based imaging features and EGFR and K-RAS mutations in 122 patients-An external validation. Eur J Radiol. 2019;110:148-155. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 15] [Cited by in RCA: 19] [Article Influence: 2.7] [Reference Citation Analysis (0)] |
| 69. | Sawan P, Plodkowski AJ, Li AE, Li BT, Drilon A, Capanu M, Ginsberg MS. CT features of HER2-mutant lung adenocarcinomas. Clin Imaging. 2018;51:279-283. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 8] [Cited by in RCA: 8] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 70. | Plodkowski AJ, Drilon A, Halpenny DF, O'Driscoll D, Blair D, Litvak AM, Zheng J, Moskowitz CS, Ginsberg MS. From genotype to phenotype: Are there imaging characteristics associated with lung adenocarcinomas harboring RET and ROS1 rearrangements? Lung Cancer. 2015;90:321-325. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 17] [Cited by in RCA: 24] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 71. | Digumarthy SR, Mendoza DP, Lin JJ, Chen T, Rooney MM, Chin E, Sequist LV, Lennerz JK, Gainor JF, Shaw AT. Computed Tomography Imaging Features and Distribution of Metastases in ROS1-rearranged Non-Small-cell Lung Cancer. Clin Lung Cancer. 2020;21:153-159.e3. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 16] [Cited by in RCA: 16] [Article Influence: 2.7] [Reference Citation Analysis (0)] |
| 72. | Saiki M, Kitazono S, Yoshizawa T, Dotsu Y, Ariyasu R, Koyama J, Sonoda T, Uchibori K, Nishikawa S, Yanagitani N, Horiike A, Ohyanagi F, Oikado K, Ninomiya H, Takeuchi K, Ishikawa Y, Nishio M. Characterization of Computed Tomography Imaging of Rearranged During Transfection-rearranged Lung Cancer. Clin Lung Cancer. 2018;19:435-440.e1. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5] [Cited by in RCA: 5] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 73. | Yoon HJ, Sohn I, Cho JH, Lee HY, Kim JH, Choi YL, Kim H, Lee G, Lee KS, Kim J. Decoding Tumor Phenotypes for ALK, ROS1, and RET Fusions in Lung Adenocarcinoma Using a Radiomics Approach. Medicine (Baltimore). 2015;94:e1753. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 87] [Cited by in RCA: 95] [Article Influence: 9.5] [Reference Citation Analysis (0)] |
| 74. | Jamnik S, Uehara C, da Silva VV. Location of lung carcinoma in relation to the smoking habit and gender. J Bras Pneumol. 2006;32:510-514. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 10] [Cited by in RCA: 10] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 75. | Lee HW, Lee CH, Park YS. Location of stage I-III non-small cell lung cancer and survival rate: Systematic review and meta-analysis. Thorac Cancer. 2018;9:1614-1622. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 23] [Cited by in RCA: 41] [Article Influence: 5.9] [Reference Citation Analysis (0)] |
| 76. | Kudo Y, Saji H, Shimada Y, Nomura M, Usuda J, Kajiwara N, Ohira T, Ikeda N. Do tumours located in the left lower lobe have worse outcomes in lymph node-positive non-small cell lung cancer than tumours in other lobes? Eur J Cardiothorac Surg. 2012;42:414-419. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 20] [Cited by in RCA: 25] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 77. | Choi CM, Kim MY, Hwang HJ, Lee JB, Kim WS. Advanced adenocarcinoma of the lung: comparison of CT characteristics of patients with anaplastic lymphoma kinase gene rearrangement and those with epidermal growth factor receptor mutation. Radiology. 2015;275:272-279. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 50] [Cited by in RCA: 60] [Article Influence: 6.0] [Reference Citation Analysis (0)] |
| 78. | Halpenny DF, Riely GJ, Hayes S, Yu H, Zheng J, Moskowitz CS, Ginsberg MS. Are there imaging characteristics associated with lung adenocarcinomas harboring ALK rearrangements? Lung Cancer. 2014;86:190-194. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 46] [Cited by in RCA: 49] [Article Influence: 4.5] [Reference Citation Analysis (0)] |
| 79. | Mendoza DP, Digumarthy SR. The added value of quantitative 18F-FDG-PET/CT parameters in the assessment of pulmonary lymphangitic carcinomatosis in lung cancer. J Thorac Dis. 2019;11:E239-E242. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4] [Cited by in RCA: 4] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 80. | Rami-Porta R, Bolejack V. Reply to "Inclusion of Lymphangitis as a Descriptor in the New TNM Staging of Lung Cancer: Filling Up the Blank Spaces". J Thorac Oncol. 2015;10:e119-e120. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 5] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 81. | Wang SXY, Lei L, Guo HH, Shrager J, Kunder CA, Neal JW. Synchronous primary lung adenocarcinomas harboring distinct MET Exon 14 splice site mutations. Lung Cancer. 2018;122:187-191. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4] [Cited by in RCA: 4] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 82. | Rangachari D, Yamaguchi N, VanderLaan PA, Folch E, Mahadevan A, Floyd SR, Uhlmann EJ, Wong ET, Dahlberg SE, Huberman MS, Costa DB. Brain metastases in patients with EGFR-mutated or ALK-rearranged non-small-cell lung cancers. Lung Cancer. 2015;88:108-111. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 333] [Cited by in RCA: 386] [Article Influence: 38.6] [Reference Citation Analysis (0)] |
| 83. | Schouten LJ, Rutten J, Huveneers HA, Twijnstra A. Incidence of brain metastases in a cohort of patients with carcinoma of the breast, colon, kidney, and lung and melanoma. Cancer. 2002;94:2698-2705. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 715] [Cited by in RCA: 733] [Article Influence: 31.9] [Reference Citation Analysis (0)] |
| 84. | Shi AA, Digumarthy SR, Temel JS, Halpern EF, Kuester LB, Aquino SL. Does initial staging or tumor histology better identify asymptomatic brain metastases in patients with non-small cell lung cancer? J Thorac Oncol. 2006;1:205-210. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 66] [Cited by in RCA: 81] [Article Influence: 4.5] [Reference Citation Analysis (0)] |
| 85. | Lin JJ, Gainor JF. Time to tackle the blood-brain barrier in HER2-mutant lung cancer. Cancer. 2019;125:4363-4366. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3] [Cited by in RCA: 3] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 86. | Remon J, Besse B. Brain Metastases in Oncogene-Addicted Non-Small Cell Lung Cancer Patients: Incidence and Treatment. Front Oncol. 2018;8:88. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 47] [Cited by in RCA: 58] [Article Influence: 8.3] [Reference Citation Analysis (0)] |
| 87. | Chooback N, Lefresne S, Lau SC, Ho C. CNS Metastases in Epidermal Growth Factor Receptor Mutation-Positive Non-Small-Cell Lung Cancer: Impact on Health Resource Utilization. J Oncol Pract. 2018;14:e612-e620. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5] [Cited by in RCA: 5] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 88. | Gainor JF, Tseng D, Yoda S, Dagogo-Jack I, Friboulet L, Lin JJ, Hubbeling HG, Dardaei L, Farago AF, Schultz KR, Ferris LA, Piotrowska Z, Hardwick J, Huang D, Mino-Kenudson M, Iafrate AJ, Hata AN, Yeap BY, Shaw AT. Patterns of Metastatic Spread and Mechanisms of Resistance to Crizotinib in ROS1-Positive Non-Small-Cell Lung Cancer. JCO Precis Oncol. 2017;2017. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 97] [Cited by in RCA: 150] [Article Influence: 18.8] [Reference Citation Analysis (0)] |
| 89. | Zhao N, Wilkerson MD, Shah U, Yin X, Wang A, Hayward MC, Roberts P, Lee CB, Parsons AM, Thorne LB, Haithcock BE, Grilley-Olson JE, Stinchcombe TE, Funkhouser WK, Wong KK, Sharpless NE, Hayes DN. Alterations of LKB1 and KRAS and risk of brain metastasis: comprehensive characterization by mutation analysis, copy number, and gene expression in non-small-cell lung carcinoma. Lung Cancer. 2014;86:255-261. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 40] [Cited by in RCA: 57] [Article Influence: 5.2] [Reference Citation Analysis (0)] |
| 90. | Patil T, Smith DE, Bunn PA, Aisner DL, Le AT, Hancock M, Purcell WT, Bowles DW, Camidge DR, Doebele RC. The Incidence of Brain Metastases in Stage IV ROS1-Rearranged Non-Small Cell Lung Cancer and Rate of Central Nervous System Progression on Crizotinib. J Thorac Oncol. 2018;13:1717-1726. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 84] [Cited by in RCA: 140] [Article Influence: 20.0] [Reference Citation Analysis (0)] |
| 91. | Drilon A, Lin JJ, Filleron T, Ni A, Milia J, Bergagnini I, Hatzoglou V, Velcheti V, Offin M, Li B, Carbone DP, Besse B, Mok T, Awad MM, Wolf J, Owen D, Camidge DR, Riely GJ, Peled N, Kris MG, Mazieres J, Gainor JF, Gautschi O. Frequency of Brain Metastases and Multikinase Inhibitor Outcomes in Patients With RET-Rearranged Lung Cancers. J Thorac Oncol. 2018;13:1595-1601. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 109] [Cited by in RCA: 153] [Article Influence: 21.9] [Reference Citation Analysis (0)] |
| 92. | Offin M, Feldman D, Ni A, Myers ML, Lai WV, Pentsova E, Boire A, Daras M, Jordan EJ, Solit DB, Arcila ME, Jones DR, Isbell JM, Beal K, Young RJ, Rudin CM, Riely GJ, Drilon A, Tabar V, DeAngelis LM, Yu HA, Kris MG, Li BT. Frequency and outcomes of brain metastases in patients with HER2-mutant lung cancers. Cancer. 2019;125:4380-4387. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 33] [Cited by in RCA: 55] [Article Influence: 9.2] [Reference Citation Analysis (0)] |
| 93. | Popper HH. Progression and metastasis of lung cancer. Cancer Metastasis Rev. 2016;35:75-91. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 228] [Cited by in RCA: 348] [Article Influence: 38.7] [Reference Citation Analysis (0)] |
| 94. | Haghighatkhah HR, Sanei Taheri M, Kharrazi SM, Ghazanfari Amlashi D, Haddadi M, Pourabdollah M. An unusual case of pulmonary adenocarcinoma with multiple and extraordinary metastases. Iran J Radiol. 2012;9:93-98. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 4] [Cited by in RCA: 4] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 95. | Ali Mohammed Hammamy R, Farooqui K, Ghadban W. Sclerotic Bone Metastasis in Pulmonary Adenocarcinoma. Case Rep Med. 2018;2018:1903757. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 7] [Cited by in RCA: 8] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 96. | Halpenny DF, Plodkowski A, Riely G, Zheng J, Litvak A, Moscowitz C, Ginsberg MS. Radiogenomic evaluation of lung cancer - Are there imaging characteristics associated with lung adenocarcinomas harboring BRAF mutations? Clin Imaging. 2017;42:147-151. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 12] [Cited by in RCA: 13] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 97. | Schanne DH, Heitmann J, Guckenberger M, Andratschke NHJ. Evolution of treatment strategies for oligometastatic NSCLC patients - A systematic review of the literature. Cancer Treat Rev. 2019;80:101892. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 30] [Cited by in RCA: 41] [Article Influence: 6.8] [Reference Citation Analysis (0)] |