Published online Jun 24, 2020. doi: 10.5306/wjco.v11.i6.397
Peer-review started: December 24, 2019
First decision: February 20, 2020
Revised: April 13, 2020
Accepted: May 12, 2020
Article in press: May 12, 2020
Published online: June 24, 2020
Processing time: 181 Days and 14.3 Hours
Hyperthermic intraperitoneal chemotherapy (HIPEC) for peritoneal metastases (PM) is considered to be feasible, safe and to improve survival.
To investigate whether an immune response is activated following HIPEC for PM.
Six patients were enrolled in this study. Peripheral blood samples were obtained from each patient prior to (day 0) and post-procedure (day 30), and used to evaluate the number of CD3+ total, CD3+/CD4+ T-Helper, CD3+/CD8+ cytotoxic T, CD3+/CD56+ natural killer and CD19+ B lymphocyte numbers, and CD4+: CD8+ T lymphocyte ratios.
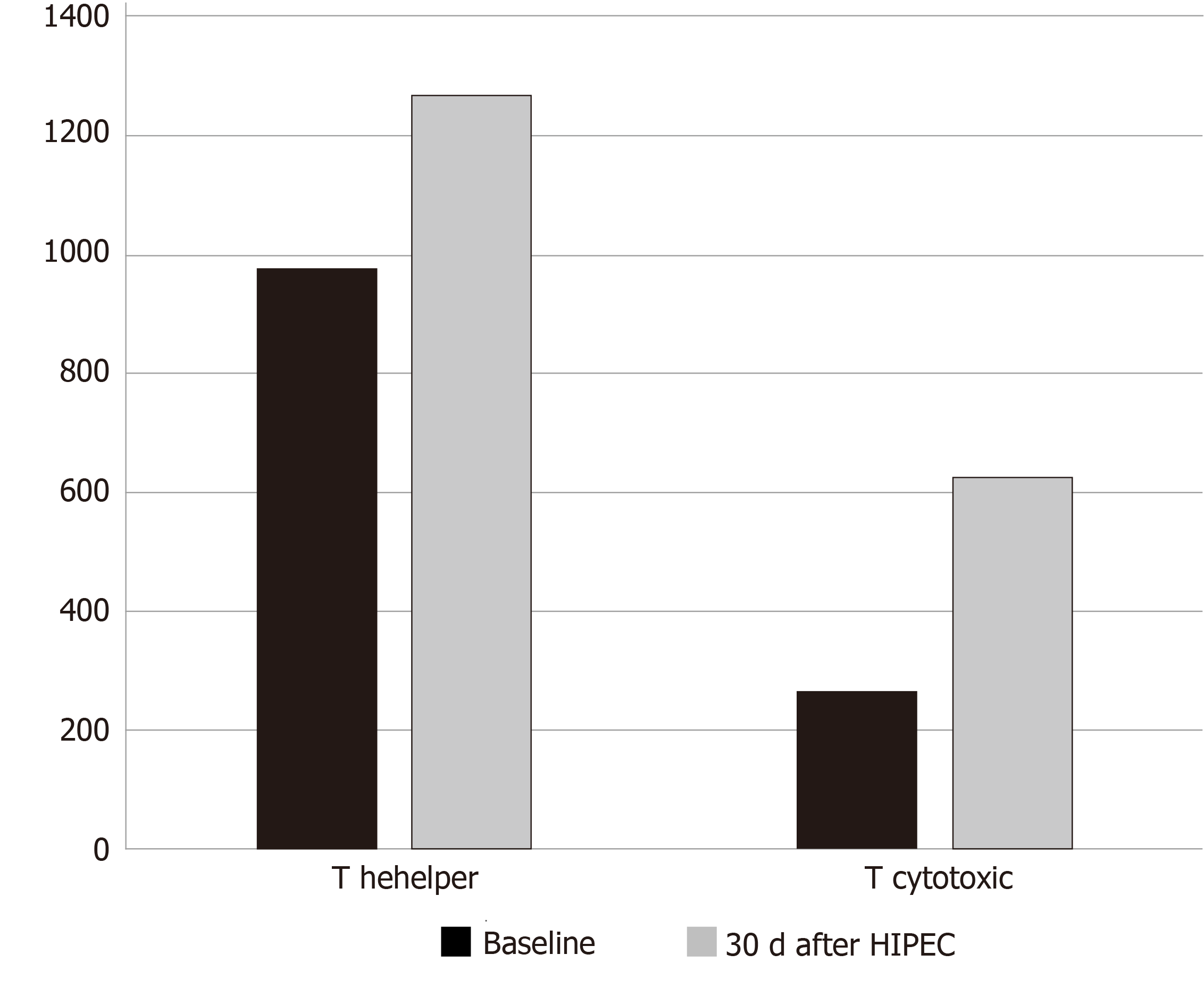
The total numbers of CD3+, CD3+/CD4+ T-Helper, CD3+/CD8+ cytotoxic T, CD3+/CD56+ natural killer and CD19+ B lymphocytes, and CD4+: CD8+ lymphocyte ratios were increased in all but one patient 30 d following the cytoreductive surgery-HIPEC procedure, and these increases were significant (P ≤ 0.05) for CD3+/CD4+ T Helper and CD3+/CD8+ cytotoxic T lymphocyte numbers.
This report provides the first evidence that HIPEC exhibits immunomodulating activity in PM patients, resulting in generalized activation of the adaptive immune response. Moreover, the majority of lymphocyte populations increased following HIPEC and continued to be elevated several weeks following the procedure, consistent with a potential authentic immunomodulating effect rather than a normal inflammatory response, to be fully characterised in future studies.
Core tip: Reports indicate that thermal tumor ablation has potential immunomodulatory activity, illustrated by the intense inflammatory cell response that is associated with this procedure, and characterized by thermally ablated tissue transitional zone infiltration by immunological and inflammatory cell populations, including: Dendritic cells, neutrophils, macrophages, B and T lymphocytes and natural killer cells. In the present study, we evaluated and confirm that hyperthermic intraperitoneal chemotherapy for peritoneal metastases also exhibits immunomodulatory activity, inducing the general activation of an adaptive immune response.
- Citation: Fiorentini G, Sarti D, Patriti A, Eugeni E, Guerra F, Masedu F, Mackay AR, Guadagni S. Immune response activation following hyperthermic intraperitoneal chemotherapy for peritoneal metastases: A pilot study. World J Clin Oncol 2020; 11(6): 397-404
- URL: https://www.wjgnet.com/2218-4333/full/v11/i6/397.htm
- DOI: https://dx.doi.org/10.5306/wjco.v11.i6.397
Cytoreductive surgery (CRS) was originally introduced for debulking peritoneal metastases (PM) in ovarian cancer patients and improved survival[1]. Despite some difficulty in being accepted by the medical oncology community, CRS was subsequently adopted for the treatment of pseudomyxoma peritonei (PMP), with beneficial results, confirming a role for CRS in the treatment of peritoneal disease[1]. CRS methodology was subsequently improved by Sugarbaker et al[2], who introduced six distinct peritonectomy procedures for complete cytoreduction. Intraperitoneal (IP) chemotherapy was subsequently used as an adjuvant therapy to CRS. In several clinical trials, hyperthermic intraperitoneal chemotherapy (HIPEC), in particular, resulted in survival improvements[3]. Today, CRS combined with HIPEC is considered the standard treatment for PMP and peritoneal mesothelioma[4] and, according to current Network NCCN Guidelines[5,6], is also considered for the management of colorectal PM.
HIPEC, pressurized intraperitoneal aerosol chemotherapy, hepatic artery infusion, trans-arterial chemo-embolization and thermal ablation are all under investigation as approaches for locoregional treatment[7,8]. However, only thermal tumor ablation has been reported to induce a potential immunomodulatory effect, illustrated by the intense inflammatory cell response that is associated with thermally ablated tumors[9,10], and characterized by ablated tissue transitional zone infiltration by immune and inflammatory cell populations, including: Dendritic cells, neutrophils, macrophages, B and T lymphocytes and natural killer (NK) cells[10], with a systemic increase in immune cells also detected[8].
The effect of HIPEC is similar to that of radiofrequency ablation, with both procedures inducing hyperemic stress-induced tumor necrosis, suggesting that HIPEC may also exhibit immunomodulatory activity. Therefore, the aim of this study was to investigate whether HIPEC for PM induces an immune response.
In this observational study, data were collected from patients with PM prospectively submitted for CRS and HIPEC, from October 2018 to August 2019, in accordance with Research Ethics Committee regulations. The inclusion criteria were: Age > 18 and < 75 years, diagnosis of PC with T4 stage (including cases with bowel perforation), mucinous subtype, or positive cytology of peritoneal lavage and written informed consent. Exclusion criteria were: Pre-operative chemotherapy or oncology medical treatment, concomitant tumor and autoimmune or rheumatologic disorders.
CRS and HIPEC were performed according to learning curve and training program recommendations[11]. HIPEC, performed immediately after R0 primary tumor debulking (intraoperative administration), involved 60-min infusion/lavage with Oxaliplatin 400 mg/m2 and Mitomycin C 15 mg/m2, with drugs added to perfusion solutions at temperatures ≥ 42°C.
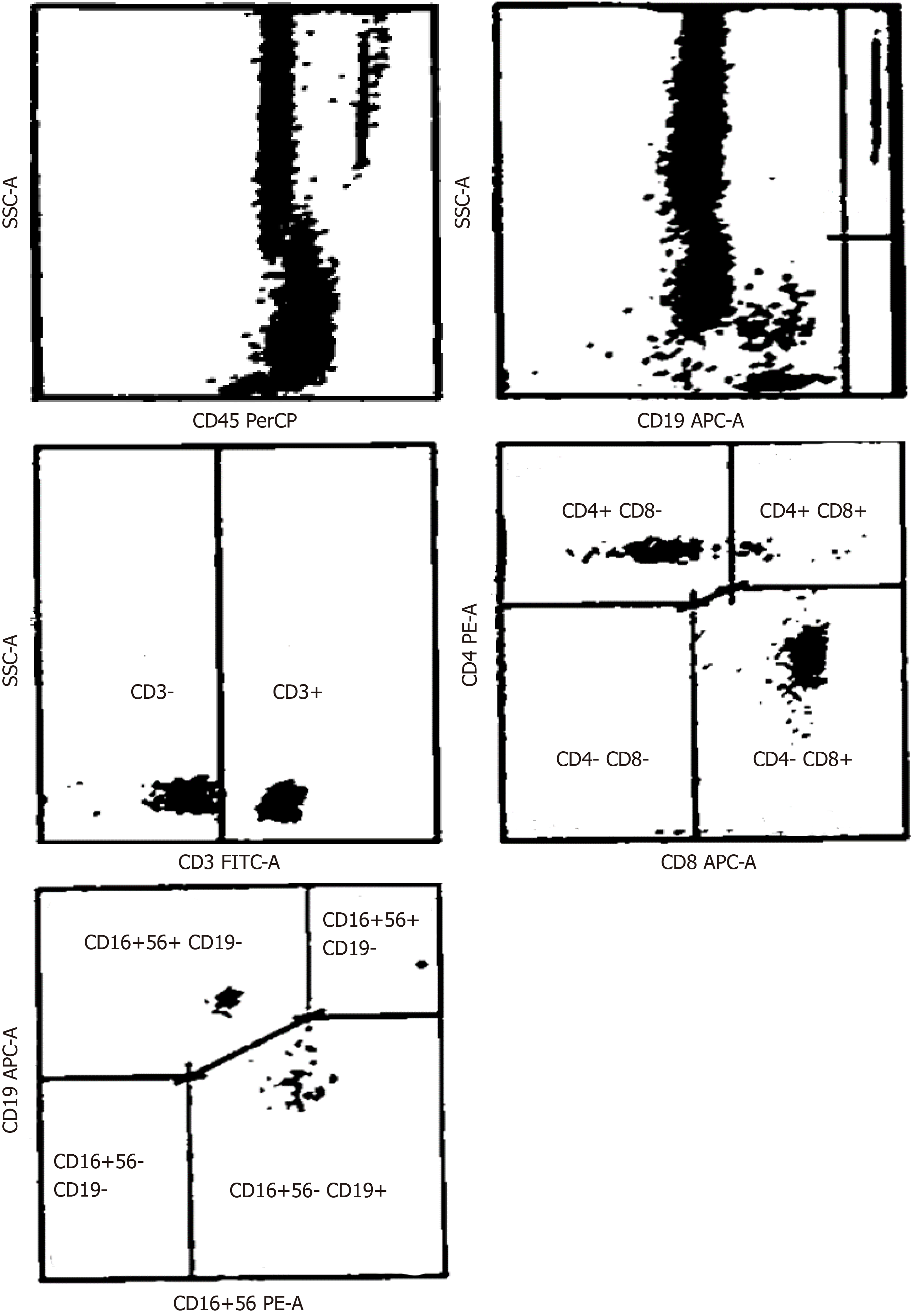
Peripheral blood (PB) samples were collected in ethylenediaminetetraacetic acid, prior to (day 0) and following (day 30) CRS-HIPEC procedures, and immunophenotypic analysis was performed within 24 h (Figure 1). Single-cell suspensions were labelled with fluorochrome-conjugated anti-human CD3, CD4, CD8, CD19 and CD56 monoclonal antibodies or matched isotypes, and CD3+ total, CD3+/CD4+ T-Helper, CD3+/CD8+ cytotoxic T, CD3+/CD56+ NK and CD19+ B lymphocytes, were quantified by fluorescence-activated cell sorting, an antibody-based cell sorting method for heterogeneous mixtures based upon the specific light scattering and fluorescent characteristics of each antibody-labelled cell-type[12]. Additional immunological parameters included the CD4+: CD8+ T lymphocyte ratio. Immunological populations were also analyzed in stained samples, after exclusion of debris and doublets, as previously described[13].
The primary outcome was to assess adaptive immune response activation and decreased immunosuppression, and the secondary outcome was to monitor recurrence-free survival, by computed tomography scan or magnetic resonance imaging.
The characteristics of recruited immune/inflammatory cell populations are described qualitatively and by quantitative variables. Unilateral paired Student’s t-tests were used to demonstrate intra-patient and intra-cohort treatment response variability, trend, and statistical significance associated with a P value of ≤ 0.05.
The 6 patients in this study comprised 3 males (50%) and 3 females (50%), with a median age of 55 years (range 48-71 years). Two patients (33%) presented with PM from colorectal cancer and the other 4 (77%) with PMP (Table 1).
| n | % | |
| Males | 3 | 50 |
| Females | 3 | 50 |
| Appendiceal mucinous neoplasms - pseudomyxoma peritonei | 4 | 67 |
| Colon cancers | 2 | 33 |
| Age (yr) | 55 (median) | 48-71 (range) |
Computed tomography scans, performed 3, 6 and 12 mo following CRS-HIPEC, demonstrated complete responses in all 6 patients, associated with a median progression-free survival of 12 mo. No complications were observed and the mean hospitalization-time, following CRS-HIPEC, was 10 ± 2 d. One patient however, was hospitalized for 58 d with severe oxaliplatin toxicity, characterized by grade 4 hematopoietic toxicity, according to National Cancer Institute, Common Terminology Criteria for Adverse Events (CTCAE v4.03), and grade 3 hematomas in the abdominal wall and pelvis. The other patients did not exhibit any HIPEC drug-associated adverse events.
Evaluation of system lymphocyte populations in patient PB samples (Tables 2 and 3), revealed increases in systemic CD3+ total, CD3+/CD4+ T Helper, CD3+/CD8+ cytotoxic T, CD3-/CD56+ NK, CD19+ B lymphocyte numbers and increased CD4+: CD8+ T lymphocyte ratios, statistically significant for CD3+/CD4+ T Helper and CD3+/CD8+ Cytotoxic T lymphocyte populations (unilateral paired Student’s t test, P < 0.05) (Figure 2). These increases were observed in all but one patient, who exhibited reduced systemic lymphocyte counts post-HIPEC, consistent with severe oxaliplatin toxicity.
| Baseline | 30 d after HIPEC | ||||||||||||
| ID | Tumor type | CD3+ | CD3+/CD4+ T helpe r | CD3+/CD8+ T cytotoxic | CD3-/CD56+ NK | CD19+ B lymphocytes | CD4/CD8 ratio | CD3+ | CD3+/CD4+ T helper | CD3+/CD8+ T cytotoxic | CD3-/CD56+ NK | CD19+ B lymphocytes | CD4/CD8ratio |
| 1 | Colon | 842 | 602 | 188 | 234 | 61 | 3 | 1335 | 835 | 409 | 501 | 78 | 2 |
| 2 | PMP | 970 | 505 | 473 | 238 | 129 | 1 | 487 | 300 | 187 | 57 | 68 | 2 |
| 3 | PMP | 1044 | 1500 | 1700 | 475 | 150 | 1 | 4477 | 2248 | 2179 | 576 | 488 | 1 |
| 4 | Colon | 1244 | 1075 | 158 | 44 | 230 | 7 | 1409 | 1095 | 290 | 116 | 148 | 4 |
| 5 | PMP | 1228 | 874 | 340 | 141 | 134 | 3 | 2304 | 1443 | 846 | 421 | 141 | 2 |
| 6 | PMP | 912 | 776 | 147 | 173 | 152 | 5 | 2002 | 1184 | 782 | 334 | 184 | 2 |
| Mean (standard deviation) baseline | Median (interquartile range) baseline | Mean (standard deviation) 30 d after HIPEC | Median (interquartile range) 30 d after HIPEC | Unilateral paired Student t test | P value | |
| CD3+ | 1040.0 (± 165.8) | 1007.0 (912.0–1228.0) | 2002.3 (± 1364.4) | 1705.5 (1335.0–2304.0) | -1.75 | 0.07 |
| CD3+/CD4+ T helper | 888.7 (± 360.8) | 825.0 (602.0–1075.0) | 1184.2 (± 649.7) | 1139.5 (835.0–1443.0) | -2.05 | 0.048 |
| CD3+/CD8+ T cytotoxic | 501.0 (± 600.9) | 264.0 (158.0–473.0) | 782.2 (± 733.6) | 595.5 (290.0–846.0) | -2.05 | 0.048 |
| CD3-/CD56+ NK | 217.5 (± 144.9) | 203.5 (141.0–238.0) | 334.2 (± 208.9) | 377.5 (116.0–501.0) | -1.69 | 0.07 |
| CD19+ B lymphocytes | 142.7 (± 54.2) | 142.0 (129.0–152.0) | 184.5 (± 155.1) | 144.5 (78.0–184.0) | -0.67 | 0.26 |
| CD4/CD8 ratio | 3.3 (± 2.3) | 3.0 (1.0–5.0) | 2.2 (± 1.0) | 2.0 (2.0–2.0) | 1.78 | 0.07 |
The peritoneum is the second most common site of colon cancer recurrence[14]. However, early imaging detection of peritoneal metastases is difficult and adjuvant systemic treatment does not reduce peritoneal dissemination. Therefore, current international clinical practice guidelines suggest HIPEC for treatment of patients at high risk of PM, as this procedure has been shown to reduce peritoneal recurrences in the long-term and to prolong overall survival[6,14-18].
Hyperthermia induces tissue necrosis immediately adjacent to the thermal source and induces apoptosis in more peripheral or transitional zone tissues, surrounded by tissues unaffected by thermal ablation[10]. Tumor cell necrosis and apoptosis result in the release of tumor-debris, tumor antigens and damage-associated molecular patterns, all of which could theoretically activate the immune system. For this reason, we evaluated systemic immune cell profiles in PM patients treated with CRS-HIPEC, as a potential index of immune response activation by this procedure. The results obtained provide the first evidence that HIPEC induces immune system activation in PM patients, characterized by induction of a generalized adaptive immune response and decrease in immunosuppression. Furthermore, this CRS-HIPEC-induced immunological effect was long-lived and lasted for several weeks, consistent with authentic immunomodulation, rather than a normal inflammatory response.
In our opinion, therefore, HIPEC is not only a useful chemotherapeutic procedure but also stimulates the immune system. In fact, 60-min of HIPEC appears to promote not only efficacious cytotoxicity but also sufficient release of tumor debris, antigens and damage-associated molecules to activate the immune system and promote cytotoxic T lymphocyte maturation.
The limitation of this pilot study, however, is that it only provides preliminary evidence that this methodological approach is appropriate for evaluating immunological changes following CRS and HIPEC, and is, therefore, a forerunner to a future confirmatory large cohort study. Furthermore, this study was not designed to evaluate treatment safety, efficacy or effectiveness[19], justifying the small sample size. The data should also be interpreted with some care, since HIPEC is performed immediately following tumor debulking, implicating surgical trauma as an alternative source of physiological and immunological alteration. Indeed, the normal physiological response to tissue injury involves a complex integration of inflammatory, immunological, neuroendocrine and metabolic mechanisms, and incision, dissection, organ manipulation and vascular alterations all induce acute inflammation for the purpose of host defense and tissue repair[10], with negative immunosuppressive feedback activated to avert an exaggerated immunological/inflammatory response[20]. Notwithstanding this, the difference in the characteristics of acute inflammation due to surgical stress-induced and HIPEC-induced long-lived increases in systemic CD3+, CD3+/CD4+ T Helper, CD3+/CD8+ cytotoxic T, CD3-/CD56+ NK, CD19+ B lymphocytes, and CD4+ : CD8+ T lymphocyte ratio, statistically significant for CD3+/CD4+ T Helper and CD3+/CD8+ cytotoxic T lymphocyte populations, supports the hypothesis that HIPEC activates the immune system, with differences consistent with generalized activation of an adaptive immune response.
In conclusion, this pilot study provides the first evidence that HIPEC activates the immune response in PM patients, supporting an additional immunomodulatory function for this procedure. Of course, results from this small patient cohort pilot study must await confirmation in a larger patient cohort, which could also benefit from comparing the CRS-HIPEC-induced immune response activation to that induced by HIPEC combined with minimally-invasive surgical procedures.
According to current Network NCCN Guidelines, cytoreductive surgery (CRS) combined with hyperthermic intraperitoneal chemotherapy (HIPEC) is considered the standard treatment for pseudomyxoma peritonei and peritoneal mesothelioma. In several clinical trials, HIPEC, in particular, resulted in survival improvements.
Thermal tumor ablation has been reported to induce a potential immunomodulatory effect, illustrated by the intense inflammatory cell response, including: Dendritic cells, neutrophils, macrophages, B and T lymphocytes and natural killer cells. The induction of this immune response might be important in increasing tumor response and improving survival.
The aim of this study was to investigate whether HIPEC for peritoneal metastases induces an immune response, which was determined by analyzing the immune population before and 30 d after HIPEC.
Peripheral blood samples were collected prior to (day 0) and following (day 30) CRS-HIPEC procedures, and immunophenotypic analysis was performed within 24 h. The levels of immunological populations were quantified by fluorescence-activated cell sorting, an antibody-based cell sorting method for heterogeneous mixtures based upon the specific light scattering and fluorescent characteristics of each antibody-labelled cell-type as reported in the literature. Immunological populations were also analyzed in stained samples, after exclusion of debris and doublets, as previously described.
The evaluation of system lymphocyte populations revealed an increase in systemic CD3+ total, CD3+/CD4+ T Helper, CD3+/CD8+ cytotoxic T, CD3-/CD56+ natural killer, CD19+ B lymphocyte levels and CD4+/CD8+ T lymphocyte CD3+/CD8+ Cytotoxic T lymphocyte ratios. Statistical significance was observed for CD3+/CD4+ T Helper and CD3+/CD8+ Cytotoxic T lymphocyte populations (unilateral paired Student’s t test, P < 0.05). These increases were observed in all but one patient, who exhibited reduced systemic lymphocyte counts post-HIPEC, consistent with severe oxaliplatin toxicity.
The results obtained provide new evidence that HIPEC induces immune system activation in pseudomyxoma peritonei patients, characterized by induction of a generalized adaptive immune response and decrease in immunosuppression. Another new finding was that the CRS-HIPEC-induced immunological effect was long-lived and lasted for several weeks, consistently with authentic immunomodulation, rather than a normal inflammatory response.
This pilot study provides the first evidence that HIPEC activates the immune response, supporting an additional immunomodulatory function for this procedure. Further studies are required to confirm these results in a large cohort study and to evaluate treatment safety, efficacy or effectiveness.
Manuscript source: Invited manuscript
Specialty type: Oncology
Country/Territory of origin: Italy
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): B
Grade C (Good): C, C
Grade D (Fair): D
Grade E (Poor): 0
P-Reviewer: Cashin PH, de Melo FF, Morris DL, Zhao XP S-Editor: Zhang L L-Editor: Webster JR E-Editor: Liu MY
| 1. | Neuwirth MG, Alexander HR, Karakousis GC. Then and now: cytoreductive surgery with hyperthermic intraperitoneal chemotherapy (HIPEC), a historical perspective. J Gastrointest Oncol. 2016;7:18-28. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 39] [Reference Citation Analysis (0)] |
| 2. | Sugarbaker PH. Peritonectomy procedures. Ann Surg. 1995;221:29-42. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1124] [Cited by in RCA: 1190] [Article Influence: 39.7] [Reference Citation Analysis (33)] |
| 3. | Morano WF, Khalili M, Chi DS, Bowne WB, Esquivel J. Clinical studies in CRS and HIPEC: Trials, tribulations, and future directions-A systematic review. J Surg Oncol. 2018;117:245-259. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 26] [Cited by in RCA: 38] [Article Influence: 4.8] [Reference Citation Analysis (0)] |
| 4. | Morano WF, Aggarwal A, Love P, Richard SD, Esquivel J, Bowne WB. Intraperitoneal immunotherapy: historical perspectives and modern therapy. Cancer Gene Ther. 2016;23:373-381. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 19] [Cited by in RCA: 32] [Article Influence: 3.6] [Reference Citation Analysis (0)] |
| 5. | Verwaal VJ, van Ruth S, de Bree E, van Sloothen GW, van Tinteren H, Boot H, Zoetmulder FA. Randomized trial of cytoreduction and hyperthermic intraperitoneal chemotherapy versus systemic chemotherapy and palliative surgery in patients with peritoneal carcinomatosis of colorectal cancer. J Clin Oncol. 2003;21:3737-3743. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1396] [Cited by in RCA: 1512] [Article Influence: 68.7] [Reference Citation Analysis (0)] |
| 6. | Network NCCN. 2016 Colon Cancer. NCCN Guidelines Version 2. Available from: https://www.nccn.org/professionals/physician_gls/pdf/colon.pdf. |
| 7. | Guadagni S, Clementi M, Mackay AR, Ricevuto E, Fiorentini G, Sarti D, Palumbo P, Apostolou P, Papasotiriou I, Masedu F, Valenti M, Giordano AV, Bruera G. Real-life multidisciplinary treatment for unresectable colorectal cancer liver metastases including hepatic artery infusion with chemo-filtration and liquid biopsy precision oncotherapy: observational cohort study. J Cancer Res Clin Oncol. 2020;146:1273-1290. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 18] [Cited by in RCA: 13] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 8. | Fiorentini G, Sarti D, Nardella M, Inchingolo R, Nestola M, Rebonato A, Guadagni S. Chemoembolization Alone or Associated With Bevacizumab for Therapy of Colorectal Cancer Metastases: Preliminary Results of a Randomized Study. In Vivo. 2020;34:683-686. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 15] [Cited by in RCA: 15] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 9. | Chu KF, Dupuy DE. Thermal ablation of tumours: biological mechanisms and advances in therapy. Nat Rev Cancer. 2014;14:199-208. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1145] [Cited by in RCA: 1317] [Article Influence: 119.7] [Reference Citation Analysis (0)] |
| 10. | Giardino A, Innamorati G, Ugel S, Perbellini O, Girelli R, Frigerio I, Regi P, Scopelliti F, Butturini G, Paiella S, Bacchion M, Bassi C. Immunomodulation after radiofrequency ablation of locally advanced pancreatic cancer by monitoring the immune response in 10 patients. Pancreatology. 2017;17:962-966. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 73] [Cited by in RCA: 65] [Article Influence: 8.1] [Reference Citation Analysis (0)] |
| 11. | Kusamura S, González-Moreno S, Nizri E, Baratti D, Guadagni S, Guaglio M, Battaglia L, Deraco M. Learning Curve, Training Program, and Monitorization of Surgical Performance of Peritoneal Surface Malignancies Centers. Surg Oncol Clin N Am. 2018;27:507-517. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 22] [Cited by in RCA: 28] [Article Influence: 4.0] [Reference Citation Analysis (0)] |
| 12. | He M, Huang H, Wang M, Chen A, Ning X, Yu K, Li Q, Li W, Ma L, Chen Z, Wang X, Sun Q. Fluorescence-Activated Cell Sorting Analysis of Heterotypic Cell-in-Cell Structures. Sci Rep. 2015;5:9588. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 13] [Cited by in RCA: 15] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 13. | Ito F, Ku AW, Bucsek MJ, Muhitch JB, Vardam-Kaur T, Kim M, Fisher DT, Camoriano M, Khoury T, Skitzki JJ, Gollnick SO, Evans SS. Immune Adjuvant Activity of Pre-Resectional Radiofrequency Ablation Protects against Local and Systemic Recurrence in Aggressive Murine Colorectal Cancer. PLoS One. 2015;10:e0143370. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 26] [Cited by in RCA: 34] [Article Influence: 3.4] [Reference Citation Analysis (0)] |
| 14. | Klaver CE, Musters GD, Bemelman WA, Punt CJ, Verwaal VJ, Dijkgraaf MG, Aalbers AG, van der Bilt JD, Boerma D, Bremers AJ, Burger JW, Buskens CJ, Evers P, van Ginkel RJ, van Grevenstein WM, Hemmer PH, de Hingh IH, Lammers LA, van Leeuwen BL, Meijerink WJ, Nienhuijs SW, Pon J, Radema SA, van Ramshorst B, Snaebjornsson P, Tuynman JB, Te Velde EA, Wiezer MJ, de Wilt JH, Tanis PJ. Adjuvant hyperthermic intraperitoneal chemotherapy (HIPEC) in patients with colon cancer at high risk of peritoneal carcinomatosis; the COLOPEC randomized multicentre trial. BMC Cancer. 2015;15:428. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 103] [Cited by in RCA: 105] [Article Influence: 10.5] [Reference Citation Analysis (0)] |
| 15. | Klaver CEL, Stam R, Sloothaak DAM, Crezee J, Bemelman WA, Punt CJA, Tanis PJ. Colorectal cancer at high risk of peritoneal metastases: long term outcomes of a pilot study on adjuvant laparoscopic HIPEC and future perspectives. Oncotarget. 2017;8:51200-51209. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 17] [Cited by in RCA: 17] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 16. | Pelz JO, Chua TC, Esquivel J, Stojadinovic A, Doerfer J, Morris DL, Maeder U, Germer CT, Kerscher AG. Evaluation of best supportive care and systemic chemotherapy as treatment stratified according to the retrospective peritoneal surface disease severity score (PSDSS) for peritoneal carcinomatosis of colorectal origin. BMC Cancer. 2010;10:689. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 80] [Cited by in RCA: 74] [Article Influence: 4.9] [Reference Citation Analysis (0)] |
| 17. | Klaver YL, Lemmens VE, Nienhuijs SW, Luyer MD, de Hingh IH. Peritoneal carcinomatosis of colorectal origin: Incidence, prognosis and treatment options. World J Gastroenterol. 2012;18:5489-5494. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 103] [Cited by in RCA: 97] [Article Influence: 7.5] [Reference Citation Analysis (0)] |
| 18. | Guaglio M, Sinukumar S, Kusamura S, Milione M, Pietrantonio F, Battaglia L, Guadagni S, Baratti D, Deraco M. Correction to: Clinical Surveillance After Macroscopically Complete Surgery for Low-Grade Appendiceal Mucinous Neoplasms (LAMN) with or Without Limited Peritoneal Spread: Long-Term Results in a Prospective Series. Ann Surg Oncol. 2018;25:987. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3] [Cited by in RCA: 19] [Article Influence: 2.7] [Reference Citation Analysis (0)] |
| 19. | Leon AC, Davis LL, Kraemer HC. The role and interpretation of pilot studies in clinical research. J Psychiatr Res. 2011;45:626-629. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1276] [Cited by in RCA: 1125] [Article Influence: 80.4] [Reference Citation Analysis (0)] |
| 20. | Novitsky YW, Litwin DE, Callery MP. The net immunologic advantage of laparoscopic surgery. Surg Endosc. 2004;18:1411-1419. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 154] [Cited by in RCA: 175] [Article Influence: 8.3] [Reference Citation Analysis (0)] |