Published online Jun 24, 2020. doi: 10.5306/wjco.v11.i6.378
Peer-review started: December 20, 2019
First decision: April 2, 2020
Revised: May 11, 2020
Accepted: June 2, 2020
Article in press: June 2, 2020
Published online: June 24, 2020
Processing time: 187 Days and 8.9 Hours
Preoperative evaluations aiming to assess high-risk features in clinical stage 1 endometrial cancer patients are crucial to refer these patients to gynecologic oncologists. Cancer antigen 125 (CA125) and human epididymis protein 4 (HE4) have been reported in endometrial cancer patients with poor prognostic factors.
To evaluate the association between preoperative levels of CA125 and HE4 and high-risk features and establish optimal cut-off values in clinical stage 1 endometrial cancer.
A retrospective study was conducted in clinical stage 1 endometrial cancer patients who underwent primary surgery between January 2013 and December 2018. A total of 128 patients had preoperative serum CA125 and HE4 measurements. High-risk features included grade 3 tumors, large tumor sizes (more than 2 cm), deep myometrial invasion (more than 50%), lymphovascular space invasion (LVSI), cervical involvement, extrauterine involvement and node metastasis. Receiver operating characteristic (ROC) curves were generated to analyze the optimal cut-off values.
The mean age of the patients was 57.4 years, and 69.5% of them were postmenopausal. Most patients presented with stage I disease (67.2%) and had the endometrioid subtype (97.7%). The median CA125 and HE4 levels in all patients were 22.1 U/mL and 104.7 pmol/L, respectively. CA125 and HE4 levels were significantly elevated in those with large tumor sizes, deep myometrial invasion, LVSI, extrauterine metastasis, and advanced stage, but node metastasis was associated with elevated CA125 only. According to the ROC curve, both serum markers had statistical significance for the prediction of high-risk features only in postmenopausal patients, with an optimal cut-off value of 20 U/mL for CA125 [area under the concentration-time curve (AUC) = 0.72, P = 0.002] and 113 pmol/L for HE4 (AUC = 0.70, P = 0.006). The combination of both serum markers had 80% sensitivity and 64.4% positive predictive value. Significantly worse 5-year disease-free survival was observed in patients with high levels of CA125 and HE4 (78.4% and 100%, respectively; P = 0.01).
Preoperative CA125 levels greater than 20 U/mL or HE4 levels greater than 113 pmol/L are associated with an increased risk of having high-risk features and present as prognostic factors in clinical stage 1 postmenopausal endometrial cancer patients. This information is helpful for general gynecologists to refer high-risk patients to gynecologic oncologists to perform complete surgical staging.
Core tip: Standard surgical treatment in endometrial cancer consists of hysterectomy with bilateral salpingo-oophorectomy. Node dissection is performed in selected patients with high-risk features such as grade 3 tumors, large tumor sizes (more than 2 cm), deep myometrial invasion (more than 50%), lymphovascular space invasion, cervical involvement, and extrauterine involvement. Preoperative cancer antigen 125 (CA125) and human epididymis protein 4 (HE4) levels were significantly elevated in clinical stage 1 postmenopausal endometrial cancer patients with these high-risk features and an increased risk of upstaging. CA125 levels above 20 U/mL and HE4 levels above 113 pmol/L were used as the optimal cut-off values for the prediction of high-risk features, and they were also shown to be poor prognostic factors. Preoperative CA125 and HE4 may be an acceptable screening test for general gynecologists to consider the referral of these high-risk patients to gynecologic oncologists to perform complete surgical staging.
- Citation: Panyavaranant P, Manchana T. Preoperative markers for the prediction of high-risk features in endometrial cancer. World J Clin Oncol 2020; 11(6): 378-388
- URL: https://www.wjgnet.com/2218-4333/full/v11/i6/378.htm
- DOI: https://dx.doi.org/10.5306/wjco.v11.i6.378
Endometrial cancer is one of the most common female genital tract cancers, and approximately 380000 new cases are diagnosed each year. In developed countries, endometrial cancer is the most common female genital tract cancer[1]. The incidence of endometrial cancer has a rising trend, with the increased incidence of obese women being one of the main reasons. Primary surgical treatment is the main option for all stages of endometrial cancer. Standard treatment consists of hysterectomy with bilateral salpingo-oophorectomy. Additional pelvic and/or para-aortic lymph node dissection is also considered based on various histological factors, such as tumor size, grade, subtype, myometrial invasion, lymphovascular space invasion (LVSI), cervical involvement, and extrauterine metastasis. Lymphadenectomy may not be necessary in endometrial cancer patients with a tumor size less than two centimeters, tumor grade 1-2, the endometrioid subtype, and less than 50% myometrial invasion[2]. The chance of node metastasis in these patients is less than 5%[3]. Women with these low-risk features can be operated on safely by general gynecologists. In contrast, grade 3, nonendometrioid histology, LVSI, and cervical and extrauterine involvement are considered high-risk features. Complete lymphadenectomy should be performed. Preoperative evaluations aiming to assess high-risk features are crucial, and if these features are detected, the patient should be referred to gynecologic oncologists.
Cancer antigen 125 (CA125) and human epididymis protein 4 (HE4) are serum tumor markers that are widely used in epithelial ovarian cancer. Currently, these tumor markers have been reported in endometrial cancer patients, especially those with poor prognostic factors, including grade 3 tumors, deep myometrial invasion, node metastasis, and extrauterine metastasis[4-7]. There is also previous evidence that preoperative serum CA125 combined with HE4 is an ideal marker for the separation of endometrial cancer patients and normal patients[8,9]. However, CA125 still has some disadvantages. CA125 levels can increase in many nonmalignant conditions, especially in premenopausal women. Therefore, the diagnostic ability of CA125 is still unsatisfactory, and the use of CA125 in endometrial cancer patients requires further detailed and intensive study. HE4 is a novel marker, and high levels of HE4 are also associated with high-risk features and extrauterine metastasis[10]. HE4 has been reported to be more sensitive in early-stage endometrial cancer than CA125[7]. However, the cut-off value of HE4 reported in different studies varied[10-16].
This study was conducted to assess the association between preoperative levels of CA125 and HE4 and high-risk features, including grade 3 tumors, large tumor sizes (more than 2 cm), deep myometrial invasion, LVSI, cervical involvement, extrauterine metastasis and node metastasis. Moreover, the cut-off values of each tumor marker were analyzed to predict high-risk features in clinical stage 1 endometrial cancer patients.
This retrospective study was approved by the Institutional Review Board (IRB), Faculty of Medicine, Chulalongkorn University, Bangkok, Thailand. Endometrial cancer patients who underwent primary surgery between January 2010 and December 2018 at King Chulalongkorn Memorial Hospital, Bangkok, Thailand were sorted by the diagnosis according to International Classification of Diseases and Related Health Problems 10th Revision. Endometrial cancer patients with clinical stage 1 and the endometrioid subtype were included. Patients with advanced stages, the nonendometrioid subtype, synchronous ovarian or colorectal cancers and incomplete medical records were excluded. The patients’ demographic data, clinicopathological characteristics, and preoperative serum biomarkers, including CA125 and HE4 levels, were collected. The levels of CA125 and HE4 were investigated using Modular analytics E170, and Cobas® e601 and e602 analyzers with the electrochemiluminescence immunoassay (ECLIA) technique (Roche Diagnostics®, Germany). The normal reference value of CA125 was 0-35 U/mL and that of HE4 was 100-150 pmol/L.
Standard surgical treatment included hysterectomy with bilateral salpingo-oophorectomy with either conventional or minimally invasive approaches. Pelvic and paraaortic lymphadenectomy was performed in the indicated patients. The surgical staging procedures were performed according to National Comprehensive Cancer Network guidelines[17]. Frozen sectioning was not a part of the routine practice in our institute. The surgical specimens were sent for pathological examination. The tumor size, percentage of myometrial invasion, tumor grading, LVSI, cervical involvement, nodal metastasis, and extrauterine disease were systemically examined by gynecologic pathologists. The patients were surgically staged according to the 2009 International Federation of Gynecology and Obstetrics staging system. High-risk features included grade 3 tumors, large tumor sizes (more than 2 cm), deep myometrial invasion (more than 50%), LVSI, cervical metastasis, extrauterine metastasis and nodal metastasis.
Comparisons between serum CA125 and HE4 levels and high-risk features were analyzed using the Wilcoxon rank sum test for two independent factors. Correlations between clinicopathological characteristics and CA125 and HE4 levels were analyzed by Pearson correlation and the Mann-Whitney U test. Receiver operating characteristic (ROC) curves were plotted. The optimal cut-off value was determined by identifying the optimal point of the best sensitivity and specificity. All diagnostic performance metrics were calculated, including sensitivity, specificity, positive predictive value (PPV) and negative predictive value (NPV). Survival analysis was performed using Kaplan–Meier curves and the log rank test to assess the differences between survival curves. Statistical analyses were performed using SPSS version 22.0 (SPSS Inc., Chicago, IL, United States). A P value < 0.05 was considered to indicate statistical significance.
A total of 128 patients were included in the analysis. Clinicopathological characteristics are shown in Table 1. The mean age of the patients was 57.4 years; 47.7% of them were nulliparous, and 69.5% of them were postmenopausal. The mean BMI was 27.7 kg/m2, and 37 patients (28.9%) had a BMI greater than 30 kg/m2. Eighty patients (62.5%) had coexisting medical diseases, and 72 patients (56.3%) had benign gynecologic diseases such as leiomyoma, adenomyosis, or pelvic and ovarian endometriosis. Eighty-six patients (67.2%) were confirmed as surgical stage 1, but 32.8% were upstaged. Most patients (97.7%) had endometrioid adenocarcinoma. Tumor grade 1 was found in 55.5% of patients, and 18% and 24.2% of them had grades 2 and 3, respectively. Three patients with preoperative grade 3 endometrioid carcinoma had a change in the diagnosis to papillary serous carcinoma in 2 patients (1.6%) and carcinosarcoma in 1 patient (0.8%).
| Characteristics | n = 128 |
| Age (yr), mean ± SD | 57.4 ± 10.9 |
| BMI (kg/m2), mean ± SD | 27.7 ± 7.5 |
| Obesity (BMI > 30 kg/m2) | 37 (28.9) |
| Parity | |
| Nulliparous | 61 (47.7) |
| Multiparous | 67 (52.3) |
| Menopausal status | |
| Premenopause | 39 (30.5) |
| Postmenopause | 89 (69.5) |
| Coexisting medical diseases1 | 80 (62.5) |
| Pre-existing breast cancer | 12 (9.4) |
| Coexisting benign gynecologic diseases2 | 72 (56.3) |
| Surgical stage of disease | |
| Stage I | 86 (67.2) |
| Stage II | 15 (11.7) |
| Stage III | 19 (14.8) |
| Stage IV | 8 (6.3) |
| Histologic grade | |
| Grade 1 endometrioid carcinoma | 71 (55.5) |
| Grade 2 endometrioid carcinoma | 23 (18.0) |
| Grade 3 endometrioid carcinoma | 31 (24.2) |
| Uterine papillary serous carcinoma | 2 (1.6) |
| Carcinosarcoma | 1 (0.8) |
The median CA125 and HE4 levels in all patients were 22.1 U/mL (range 5.3-786.8 U/mL) and 104.7 pmol/L (range 30.5-1719.0 pmol/L), respectively. Significantly older age and postmenopausal status were reported in patients with high-risk features. The levels of CA125 and HE4 were significantly higher in patients with high-risk features (Table 2). The associations between CA125 and HE4 and high-risk features are shown in Table 3. Almost all high-risk features were significantly associated with elevated CA125 and HE4 except grade 3 tumors and cervical involvement. The median CA125 levels were significantly higher in patients with large tumor sizes (23.8 U/mL vs 16.1 U/mL, P = 0.03), deep myometrial invasion (27.5 U/mL vs 19.1 U/mL, P = 0.01), LVSI (55.8 U/mL vs 19.4 U/mL, P < 0.001), extrauterine metastasis (54.6 U/mL vs 19.1 U/mL, P < 0.001), and node metastasis (57.0 U/mL vs 19.3 U/mL, P = 0.004) than in patients without those features. Moreover, advanced-stage patients had significantly higher CA125 levels than early-stage patients (57.0 U/mL vs 19.3 U/mL, P < 0.001).
| Characteristics | Patients with high-risk features (n = 74) | Patients without high-risk features (n = 54) | P value |
| Age (yr), mean ± SD | 60.1 ± 10.7 | 53.7 ± 10.2 | 0.001 |
| BMI (kg/m2), mean ± SD | 27.1 ± 7.8 | 28.5 ± 7.1 | 0.32 |
| Obese (BMI > 30 kg/m2) | 16 (21.6) | 21 (38.9) | 0.05 |
| Nulliparous | 41 (47.7) | 26 (48.1) | 0.48 |
| Postmenopause | 59 (79.7) | 30 (55.6) | 0.006 |
| Coexisting medical diseases | 46 (62.2) | 34 (63.0) | 1.00 |
| Coexisting benign gynecologic diseases | 42 (56.8) | 30 (55.56) | 1.00 |
| CA125 level, median (min-max) | 27.0 (7.1-786.8) | 16.3 (5.3-432.1) | 0.002 |
| HE4 level, median (min-max) | 130.6 (39.4-1719.0) | 81.7 (30.5-293.9) | 0.005 |
| Pathological characteristics | No. of cases | CA125 (U/mL) | HE4 (pmol/L) | ||||
| Median | Min-Max | Median | Min-Max | ||||
| Tumor size | ≤ 2 cm | 38 | 16.1 | 6.9-556.4 | 68.9 | 30.5-649.1 | |
| > 2 cm | 90 | 23.8 | 5.3-786.8 | 128.3 | 35.0-1719.0 | ||
| P = 0.03 | P < 0.001 | ||||||
| Tumor grade | 1 + 2 | 93 | 22.1 | 5.3-786.8 | 98.8 | 30.5-1132.0 | |
| 3 | 35 | 20.6 | 7.1-556.4 | 117.7 | 43.6-1719.0 | ||
| P = 0.71 | P = 0.78 | ||||||
| Myometrial invasion | ≤ 50% | 69 | 19.1 | 5.3-786.8 | 83.0 | 30.5-860.2 | |
| > 50% | 59 | 27.5 | 7.1-479.5 | 130.6 | 39.4-1719.0 | ||
| P = 0.01 | P = 0.006 | ||||||
| Lymphovascular space invasion | No | 95 | 19.4 | 5.3-786.8 | 88.3 | 30.5-1132.0 | |
| Yes | 33 | 55.8 | 11.7-479.5 | 165.7 | 39.4-1719.0 | ||
| P < 0.001 | P = 0.003 | ||||||
| Cervical involvement | No | 102 | 21.3 | 5.3-786.8 | 94.3 | 30.5-860.2 | |
| Yes | 26 | 25.0 | 10.0-479.5 | 300.0 | 39.4-1719.0 | ||
| P = 0.15 | P = 0.05 | ||||||
| Extrauterine metastasis | No | 91 | 19.1 | 5.3-556.4 | 87.0 | 30.5-585.3 | |
| Yes | 37 | 54.6 | 10.0-786.8 | 183.5 | 39.4-1719.0 | ||
| P < 0.001 | P < 0.001 | ||||||
| Node metastasis | Negative | 106 | 19.3 | 5.3-786.8 | 93.0 | 30.5-1132.0 | |
| Positive | 13 | 57.0 | 11.7-439.2 | 128.6 | 39.4-1719.0 | ||
| P = 0.004 | P = 0.15 | ||||||
| Any high-risk features | No | 55 | 16.3 | 5.3-432.1 | 81.7 | 30.5-293.9 | |
| Yes | 73 | 27.5 | 7.1-786.8 | 130.5 | 39.4-1719.0 | ||
| P < 0.001 | P < 0.001 | ||||||
| Stage of disease | Early | 101 | 19.3 | 5.3-786.8 | 89.5 | 30.5-1132.0 | |
| Advanced | 27 | 57.0 | 9.5-479.5 | 283.0 | 39.40 - 1719.0 | ||
| P < 0.001 | P < 0.001 | ||||||
The median HE4 levels were significantly higher in patients with large tumor sizes (128.3 pmol/L vs 68.9 pmol/L, P < 0.001), deep myometrial invasion (130.6 pmol/L vs 83.0 pmol/L, P = 0.006), LVSI (165.7 pmol/L vs 88.3 pmol/L, P = 0.003), and extrauterine metastasis (183.5 pmol/L vs 87.0 pmol/L, P < 0.001). Advanced stage patients also had higher HE4 levels than early stage patients (283.0 pmol/L vs 89.5 pmol/L, P < 0.001).
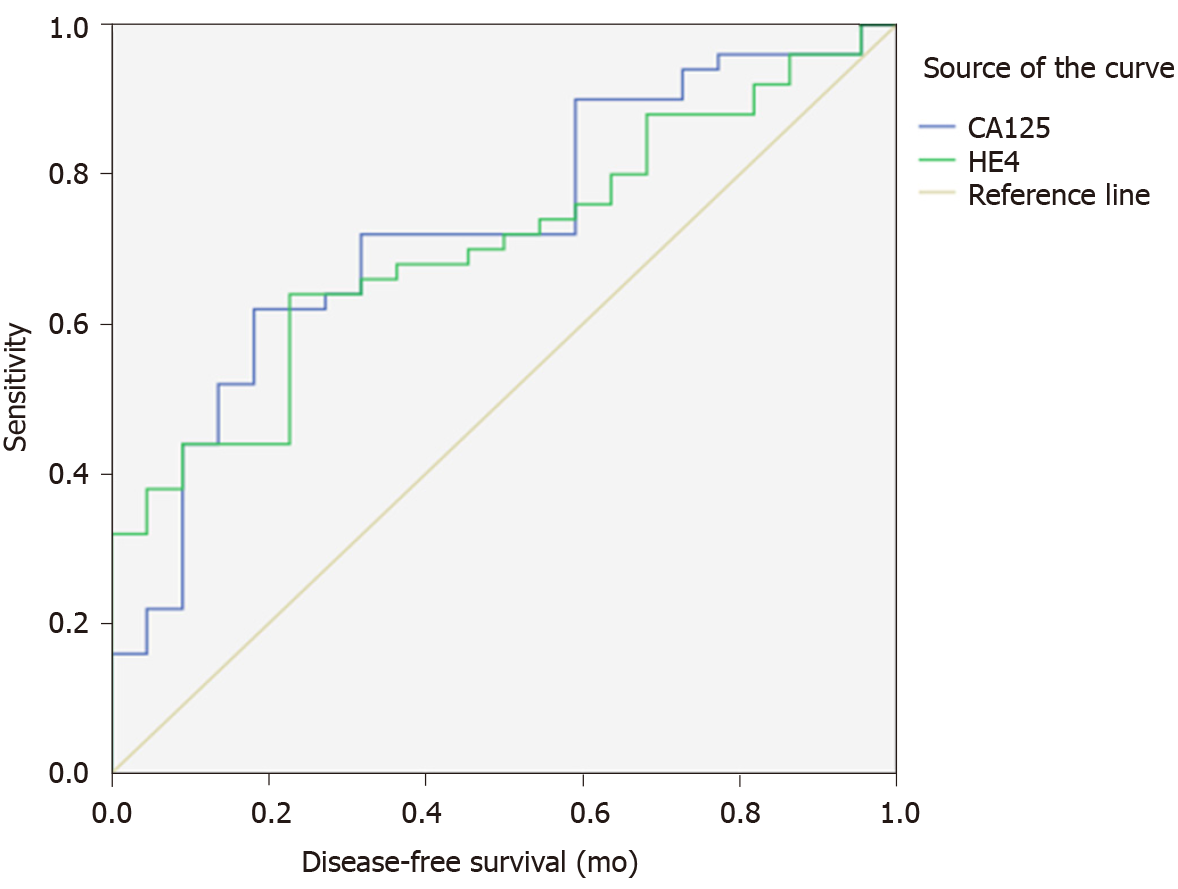
ROC curves were generated in pre- and postmenopausal patients. Both CA125 and HE4 had no statistical significance for the prediction of high-risk features in premenopausal patients (P = 0.14 and 0.08, respectively). The optimal cut-off values of CA125 and HE4 for the prediction of high-risk features in postmenopausal women were 20 U/mL and 113 pmol/L, respectively. At the cut-off CA125 value of 20 U/mL, the area under the concentration-time curve (AUC) was 0.72 (P = 0.002), with a sensitivity of 64.0%, a specificity of 72.3%, a PPV of 69.8% and an NPV of 66.8% (Figure 1 and Table 4). At the cut-off HE4 value of 113 pmol/L, the AUC was 0.70 (P = 0.006), with a sensitivity of 64.0%, a specificity of 77.3%, a PPV of 73.8% and an NPV of 68.2%. The combination of both serum markers with CA125 above 20 U/mL and/or HE4 above 113 pmol/L had a sensitivity of 80%, a specificity of 55.8%, a PPV of 64.4%, and an NPV of 73.6% for the prediction of high-risk features in endometrial cancer.
| Cut-off value | AUC | P value | Sensitivity (%) | Specificity (%) | PPV (%) | NPV (%) | |
| CA125 | 20 U/mL | 0.72 | 0.002 | 64.0 | 72.3 | 69.8 | 66.8 |
| HE4 | 113 pmol/L | 0.70 | 0.006 | 64.0 | 77.3 | 73.8 | 68.2 |
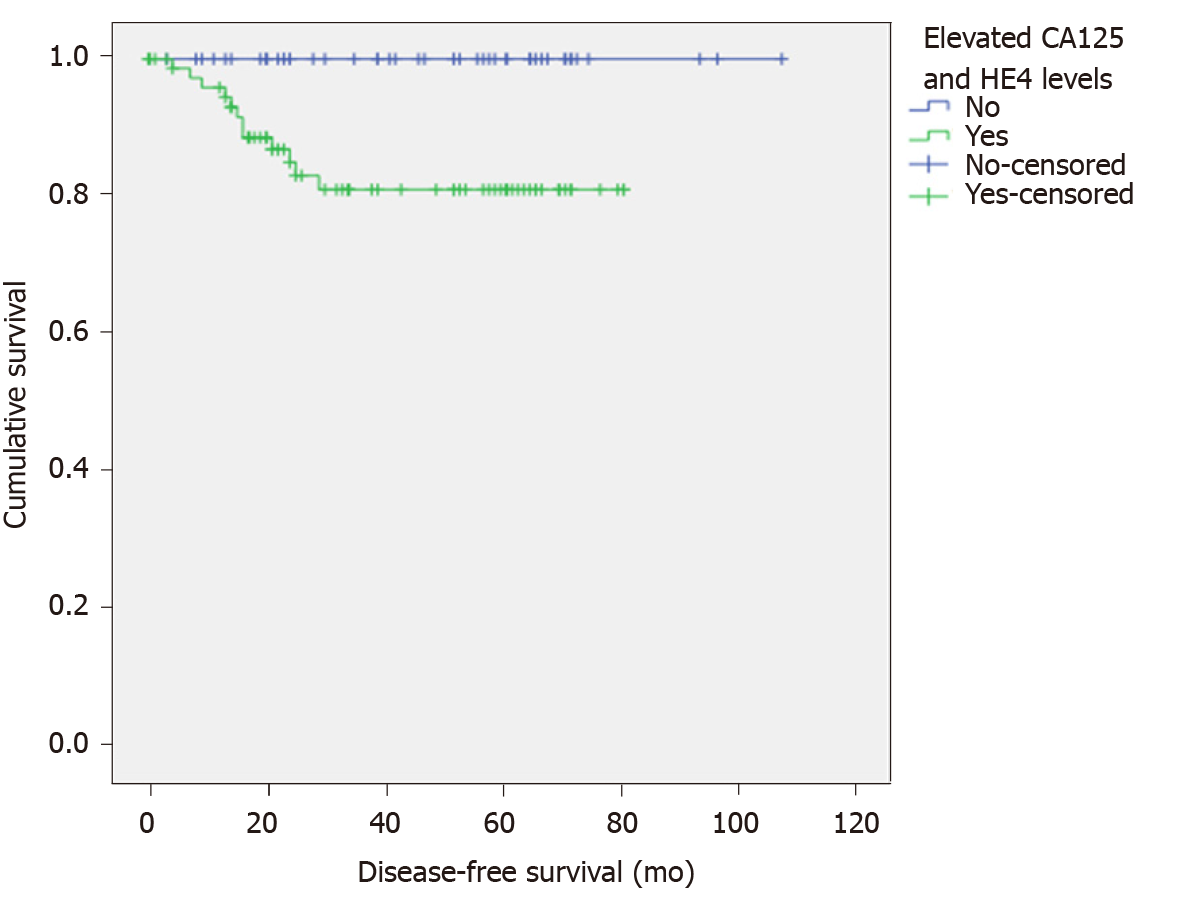
The median follow-up time in this study was 39 mo (0-108 mo). Fifteen patients (11.7%) were lost to follow-up, 12 patients (9.4%) developed recurrent disease, and only 1 patient died from recurrent disease. All recurrent patients had elevated preoperative CA125 and/or HE4 levels. Using the cut-off values of CA125 above 20 U/mL or HE4 above 113 pmol/L, the patients with higher levels than the cut-off values had a significantly shorter median disease-free survival (DFS) of 25.5 mo compared with 53 mo (P = 0.03), and the 5-year DFS was 78.4% and 100%, respectively (P = 0.01) (Figure 2).
Most endometrial cancer patients (85%) were clinically diagnosed with stage I endometrial cancer[18]. Nevertheless, endometrial cancer is accurately staged according to surgical staging because the clinical stage is imprecise. In this study, one-third of clinical stage I endometrial cancer was upstaged. Only 67.25% of the patients were diagnosed correctly as surgical stage 1. These results are consistent with previous data that only 75% of stage I endometrial cancer patients were diagnosed correctly as surgical stage 1[18]. Complete surgical staging, including lymphadenectomy, is crucial especially in patients with high-risk features to provide prognostic information. In developing countries, some patients are managed by general gynecologists. Therefore, stratifying patients preoperatively into high-risk and low-risk groups is necessary to refer high-risk patients to experienced gynecologic oncologists.
Preoperative imaging, such as transvaginal ultrasound, computed tomography, magnetic resonance imaging (MRI), and F-fluorodeoxyglucose PET-CT, can be used to identify various high-risk features, such as large tumor sizes, deep myometrial invasion, cervical involvement, extrauterine involvement and node metastasis. Although transvaginal ultrasound is least costly and has similar accuracy for the assessment of myometrial and cervical invasion, it has limitations in assessing node metastasis[19]. MRI and PET-CT have been accepted to have the highest efficacy. However, a major limitation is the poor detection of microscopic node metastasis. Intraoperative assessment with frozen sections has been accepted as the most reliable method to assess tumor grade and myometrial invasion. The accuracy rate was reported to be as high as 90%[20]. Although frozen sections are reliable, they are not widely available. Moreover, the centers must have gynecologic oncologists who should be available to perform staging if high-risk factors are found from frozen sections. Therefore, preoperative imaging and frozen sections may not be practical in developing countries or countries with limited resources due to the high expense and limited number of gynecologic oncologists.
Previous studies have examined the predictors of high-risk features in endometrial cancer, and serum tumor markers are the most promising. Many tumor markers can be elevated in endometrial cancer, such as CA15-3, CA125, HE4, and YKL-40[21]. Nevertheless, tumor markers that have the most reliable data are CA125 and HE4. CA125 and HE4 can be used as biomarkers to stratify patients preoperatively into high-risk and low-risk groups. Preoperative CA125 levels were significantly correlated with deep myometrial invasion, advanced stage, and extrauterine and node metastasis[4,6,10]. The current study also confirmed similar results with the additional finding that large tumor size and LVSI also correlated with increased levels of CA125. HE4 levels were also significantly higher in patients with all high-risk features except node metastasis. This finding is consistent with a previous study that showed that CA125 is a strong predictive factor for node metastasis and is more precise than HE4[22]. Although not all endometrial cancer patients receive complete node dissection, almost 93% of our patients received pelvic node dissection with or without para-aortic node dissection. The incidence of node metastasis in this study was 10.9%, which was similar to that of a previous report[2]. Therefore, the incidence of node metastasis in this study should be reliable.
Age has been reported as a prognostic factor in endometrial cancer. Older patients are more likely to have high-risk features and present in an advanced stage[23]. The current study also demonstrated that high-risk features were uncommon in premenopausal patients. Only 20% of premenopausal patients had high-risk features compared to 80% in postmenopausal patients. Elevated CA125 levels can occur in various benign gynecologic conditions in premenopausal women, such as leiomyoma and endometriosis. As a result, CA125 may not be a good serum biomarker for the prediction of high-risk features in premenopausal patients. The ROC curves showed that the optimal cut-off value of CA125 for the prediction of high-risk features in postmenopausal patients was 20 U/mL (sensitivity of 64%, specificity of 72.3%, PPV of 69.8%, and NPV of 66.8%). This value was consistent with previous studies that reported values varying between 20 U/mL and 40 U/mL[22,24-26]. Elevated preoperative serum CA125 levels were not only associated with various high-risk features but also appeared to have significantly worse 5-year DFS[24]. Furthermore, CA125 can be used during postoperative surveillance. A level above 20 U/mL may indicate relapse of the disease[25].
The optimal cut-off value of HE4 for the prediction of high-risk features was 113 pmol/L (sensitivity of 64.0%, specificity of 77.3%, PPV of 73.8% and NPV of 68.2%). The cut-off value for HE4 is varied, and a level of 70 pmol/L was used in most studies[7,11,12,16]. The cut-off value in the current study was as high as 113 pmol/L; however, this finding is similar to those of previous studies[10,15]. HE4 levels increase by age[13]. Most patients in the current study were postmenopausal, which might be a plausible explanation. Elevated HE4 levels above 113 pmol/L are associated with high-risk factors such as advanced stage and deep myometrial invasion and have also been reported as a poor prognostic biomarker[10,15,16]. Other studies also confirmed the finding that a high preoperative level of HE4 alone or in combination with a high level of CA125 was an independent prognostic marker in endometrial cancer[14,27], but they used a different cut-off HE4 value of 81 pmol/L and CA125 value of 35 U/mL[14].
The current study showed that the combination of CA125 above 20 U/mL and HE4 above 113 pmol/L increased the sensitivity for the prediction of high-risk features in postmenopausal patients to 80%, and the PPV was 64.4%. Therefore, preoperative CA125 and HE4 may be acceptable screening tests for general gynecologists to consider the referral of these high-risk patients to gynecologic oncologists for complete surgical staging.
The current study has some limitations due to its retrospective design. Although elevated CA125 and HE4 levels were associated with high-risk features and had worse prognosis in clinical stage 1 endometrial cancer patients, the cut-off values could be analyzed only in postmenopausal patients. Because high-risk features were less common in premenopausal patients, a larger sample size should be evaluated. The optimal cut-off values of these two serum markers should be evaluated in premenopausal endometrial cancer patients. Furthermore, a longer follow-up time should be conducted to confirm these markers as independent prognostic factors.
In conclusion, preoperative CA125 levels above 20 U/mL or HE4 levels above 113 pmol/L can predict high-risk features such as large tumor sizes, deep myometrial invasion, LVSI, advanced stage, and extrauterine metastasis in clinical stage 1 postmenopausal endometrial cancer patients. However, only a high level of CA125 was associated with node metastasis. Combining elevated levels of CA125 with HE4 preoperatively enhanced the sensitivity to 80% and PPV to 64.4%. It may be a helpful tool to direct patients to gynecologic oncologists to perform complete surgical staging. This result is clinically useful for individualized treatment and provides information about the prognosis of disease.
Standard surgical treatment in endometrial cancer consists of hysterectomy with bilateral salpingo-oophorectomy. Node dissection is performed in selected patients with high-risk features such as grade 3 tumors, large tumor sizes (more than 2 cm), deep myometrial invasion (more than 50%), lymphovascular space invasion (LVSI), cervical involvement, and extrauterine involvement. Preoperative evaluation aiming to assess high-risk features in clinical stage 1 endometrial cancer patients are crucial. Preoperative imaging still has some limitations in assessing microscopic node metastasis. Intraopeative frozen section has been accepted as the most reliable method but it is not widely available especially in developing countries.
Cancer antigen 125 (CA125) and human epididymis protein 4 (HE4) have been reported in endometrial cancer patients with poor prognostic factors. These serum tumor markers can be used as biomarkers to stratify patients preoperatively into high-risk and low-risk groups aiming to refer high-risk patients to gynecologic oncologists for complete surgical staging.
The objective of this study was to assess the association between preoperative levels of CA125 and HE4 and high-risk features, including grade 3 tumors, large tumor sizes (more than 2 cm), deep myometrial invasion, LVSI, cervical involvement, extrauterine metastasis and node metastasis. The cut-off values of each tumor marker were analyzed to predict high-risk features in clinical stage 1 endometrial cancer patients.
This retrospective study was conducted on 128 clinical stage 1 endometrioid endometrial cancer patients were included. Patients with advanced stages, the nonendometrioid subtype, synchronous ovarian or colorectal cancers and incomplete medical records were excluded. The patients’ demographic data, clinicopathological characteristics, and preoperative serum biomarkers, including CA125 and HE4 levels, were collected. High-risk features included grade 3 tumors, large tumor sizes (more than 2 cm), deep myometrial invasion (more than 50%), LVSI, cervical involvement, extrauterine involvement and node metastasis. Receiver operating characteristic (ROC) curves were generated to analyze the optimal cut-off values.
The mean age of the patients was 57.4 years, and 69.5% of them were postmenopausal. Most patients presented with stage I disease (67.2%) and had the endometrioid subtype (97.7%). The median CA125 and HE4 levels in all patients were 22.1 U/mL and 104.7 pmol/L, respectively. CA125 and HE4 levels were significantly elevated in those with large tumor sizes, deep myometrial invasion, LVSI, extrauterine metastasis, and advanced stage, but node metastasis was associated with elevated CA125 only. According to the ROC curve, both serum markers had statistical significance for the prediction of high-risk features only in postmenopausal patients, with an optimal cut-off value of 20 U/mL for CA125 (AUC = 0.72, P = 0.002) and 113 pmol/L for HE4 (AUC = 0.70, P = 0.006). The combination of both serum markers had 80% sensitivity and 64.4% positive predictive value. Significantly worse 5-year disease-free survival was observed in patients with high levels of CA125 and HE4 (78.4% and 100%, respectively; P = 0.01).
Preoperative CA125 levels above 20 U/mL or HE4 levels above 113 pmol/L can predict high-risk features such as large tumor sizes, deep myometrial invasion, LVSI, advanced stage, and extrauterine metastasis in clinical stage 1 postmenopausal endometrial cancer patients. However, only a high level of CA125 was associated with node metastasis. Combining elevated levels of CA125 with HE4 preoperatively enhanced the sensitivity to 80% and PPV to 64.4%. It may be a helpful tool to direct patients to gynecologic oncologists to perform complete surgical staging. This result is clinically useful for individualized treatment and provides information about the prognosis of disease.
Elevated CA125 and HE4 levels were associated with high-risk features and had worse prognosis in clinical stage 1 endometrial cancer patients, the cut-off values could be analyzed only in postmenopausal patients in this study. Because high-risk features were less common in premenopausal patients, a larger sample size should be further evaluated and the optimal cut-off values should be evaluated in premenopausal endometrial cancer patients. Furthermore, combination with novel biomarkers should be further investigated aiming to increase efficacy.
Manuscript source: Invited manuscript
Specialty type: Oncology
Country/Territory of origin: Thailand
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): 0
Grade C (Good): C, C, C
Grade D (Fair): D
Grade E (Poor): 0
P-Reviewer: de Melo FF, Guo K, Kok V, Viswanath Y S-Editor: Liu M L-Editor: A E-Editor: Ma YJ
| 1. | Bray F, Ferlay J, Soerjomataram I, Siegel RL, Torre LA, Jemal A. Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin. 2018;68:394-424. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 53206] [Cited by in RCA: 55834] [Article Influence: 7976.3] [Reference Citation Analysis (132)] |
| 2. | Kumar S, Podratz KC, Bakkum-Gamez JN, Dowdy SC, Weaver AL, McGree ME, Cliby WA, Keeney GL, Thomas G, Mariani A. Prospective assessment of the prevalence of pelvic, paraaortic and high paraaortic lymph node metastasis in endometrial cancer. Gynecol Oncol. 2014;132:38-43. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 115] [Cited by in RCA: 142] [Article Influence: 11.8] [Reference Citation Analysis (0)] |
| 3. | Amant F, Mirza MR, Koskas M, Creutzberg CL. Cancer of the corpus uteri. Int J Gynaecol Obstet. 2018;143 Suppl 2:37-50. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 181] [Cited by in RCA: 279] [Article Influence: 39.9] [Reference Citation Analysis (0)] |
| 4. | Chen YL, Huang CY, Chien TY, Huang SH, Wu CJ, Ho CM. Value of pre-operative serum CA125 level for prediction of prognosis in patients with endometrial cancer. Aust N Z J Obstet Gynaecol. 2011;51:397-402. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 23] [Cited by in RCA: 34] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 5. | Han SS, Lee SH, Kim DH, Kim JW, Park NH, Kang SB, Song YS. Evaluation of preoperative criteria used to predict lymph node metastasis in endometrial cancer. Acta Obstet Gynecol Scand. 2010;89:168-174. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 35] [Cited by in RCA: 36] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 6. | Kim HS, Park CY, Lee JM, Lee JK, Cho CH, Kim SM, Kim JW. Evaluation of serum CA-125 levels for preoperative counseling in endometrioid endometrial cancer: a multi-center study. Gynecol Oncol. 2010;118:283-288. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 27] [Cited by in RCA: 39] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 7. | Moore RG, Brown AK, Miller MC, Badgwell D, Lu Z, Allard WJ, Granai CO, Bast RC Jr, Lu K. Utility of a novel serum tumor biomarker HE4 in patients with endometrioid adenocarcinoma of the uterus. Gynecol Oncol. 2008;110:196-201. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 148] [Cited by in RCA: 159] [Article Influence: 9.4] [Reference Citation Analysis (0)] |
| 8. | Dong C, Liu P, Li C. Value of HE4 Combined with Cancer Antigen 125 in the Diagnosis of Endometrial Cancer. Pak J Med Sci. 2017;33:1013-1017. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 16] [Cited by in RCA: 19] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 9. | Knific T, Osredkar J, Smrkolj Š, Tonin I, Vouk K, Blejec A, Frković Grazio S, Rižner TL. Novel algorithm including CA-125, HE4 and body mass index in the diagnosis of endometrial cancer. Gynecol Oncol. 2017;147:126-132. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 21] [Cited by in RCA: 36] [Article Influence: 4.5] [Reference Citation Analysis (0)] |
| 10. | Brennan DJ, Hackethal A, Metcalf AM, Coward J, Ferguson K, Oehler MK, Quinn MA, Janda M, Leung Y, Freemantle M; ANECS Group, Webb PM, Spurdle AB, Obermair A. Serum HE4 as a prognostic marker in endometrial cancer--a population based study. Gynecol Oncol. 2014;132:159-165. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 65] [Cited by in RCA: 75] [Article Influence: 6.3] [Reference Citation Analysis (0)] |
| 11. | Angioli R, Plotti F, Capriglione S, Montera R, Damiani P, Ricciardi R, Aloisi A, Luvero D, Cafà EV, Dugo N, Angelucci M, Benedetti-Panici P. The role of novel biomarker HE4 in endometrial cancer: a case control prospective study. Tumour Biol. 2013;34:571-576. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 69] [Cited by in RCA: 82] [Article Influence: 6.3] [Reference Citation Analysis (0)] |
| 12. | Prueksaritanond N, Cheanpracha P, Yanaranop M. Association of Serum HE4 with Primary Tumor Diameter and Depth of Myometrial Invasion in Endometrial Cancer Patients at Rajavithi Hospital. Asian Pac J Cancer Prev. 2016;17:1489-1492. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 14] [Cited by in RCA: 15] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 13. | Bolstad N, Øijordsbakken M, Nustad K, Bjerner J. Human epididymis protein 4 reference limits and natural variation in a Nordic reference population. Tumour Biol. 2012;33:141-148. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 76] [Cited by in RCA: 85] [Article Influence: 6.1] [Reference Citation Analysis (0)] |
| 14. | Mutz-Dehbalaie I, Egle D, Fessler S, Hubalek M, Fiegl H, Marth C, Widschwendter A. HE4 is an independent prognostic marker in endometrial cancer patients. Gynecol Oncol. 2012;126:186-191. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 66] [Cited by in RCA: 67] [Article Influence: 5.2] [Reference Citation Analysis (0)] |
| 15. | Presl J, Ovesna P, Novotny Z, Vlasak P, Bouda J, Kostun J, Topolcan O, Kucera R, Bednarikova M, Weinberger V. Importance of Preoperative Knowledge of the Biomarker HE4 in Early-stage Endometrial Cancer Regarding Surgical Management. Anticancer Res. 2017;37:2697-2702. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5] [Cited by in RCA: 5] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 16. | Stiekema A, Lok C, Korse CM, van Driel WJ, van der Noort V, Kenter GG, Van de Vijver KK. Serum HE4 is correlated to prognostic factors and survival in patients with endometrial cancer. Virchows Arch. 2017;470:655-664. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 20] [Cited by in RCA: 24] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 17. | Koh WJ, Abu-Rustum NR, Bean S, Bradley K, Campos SM, Cho KR, Chon HS, Chu C, Cohn D, Crispens MA, Damast S, Dorigo O, Eifel PJ, Fisher CM, Frederick P, Gaffney DK, George S, Han E, Higgins S, Huh WK, Lurain JR 3r, Mariani A, Mutch D, Nagel C, Nekhlyudov L, Fader AN, Remmenga SW, Reynolds RK, Tillmanns T, Ueda S, Wyse E, Yashar CM, McMillian NR, Scavone JL. Uterine Neoplasms, Version 1.2018, NCCN Clinical Practice Guidelines in Oncology. J Natl Compr Canc Netw. 2018;16:170-199. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 322] [Cited by in RCA: 441] [Article Influence: 73.5] [Reference Citation Analysis (0)] |
| 18. | Sirisabya N, Manchana T, Worasethsin P, Khemapech N, Lertkhachonsuk R, Sittisomwong T, Vasuratna A, Termrungruanglert W, Tresukosol D. Is complete surgical staging necessary in clinically early-stage endometrial carcinoma? Int J Gynecol Cancer. 2009;19:1057-1061. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 14] [Cited by in RCA: 13] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 19. | Morice P, Leary A, Creutzberg C, Abu-Rustum N, Darai E. Endometrial cancer. Lancet. 2016;387:1094-1108. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 934] [Cited by in RCA: 1163] [Article Influence: 129.2] [Reference Citation Analysis (0)] |
| 20. | Ugaki H, Kimura T, Miyatake T, Ueda Y, Yoshino K, Matsuzaki S, Fujita M, Kimura T, Morii E, Enomoto T. Intraoperative frozen section assessment of myometrial invasion and histology of endometrial cancer using the revised FIGO staging system. Int J Gynecol Cancer. 2011;21:1180-1184. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 13] [Cited by in RCA: 5] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 21. | Aggarwal P, Kehoe S. Serum tumour markers in gynaecological cancers. Maturitas. 2010;67:46-53. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 36] [Cited by in RCA: 42] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 22. | Antonsen SL, Høgdall E, Christensen IJ, Lydolph M, Tabor A, Loft Jakobsen A, Fagö-Olsen CL, Andersen ES, Jochumsen K, Høgdall C. HE4 and CA125 levels in the preoperative assessment of endometrial cancer patients: a prospective multicenter study (ENDOMET). Acta Obstet Gynecol Scand. 2013;92:1313-1322. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 44] [Cited by in RCA: 59] [Article Influence: 4.9] [Reference Citation Analysis (0)] |
| 23. | Lee NK, Cheung MK, Shin JY, Husain A, Teng NN, Berek JS, Kapp DS, Osann K, Chan JK. Prognostic factors for uterine cancer in reproductive-aged women. Obstet Gynecol. 2007;109:655-662. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 126] [Cited by in RCA: 126] [Article Influence: 7.0] [Reference Citation Analysis (0)] |
| 24. | Chung HH, Kim JW, Park NH, Song YS, Kang SB, Lee HP. Use of preoperative serum CA-125 levels for prediction of lymph node metastasis and prognosis in endometrial cancer. Acta Obstet Gynecol Scand. 2006;85:1501-1505. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 75] [Cited by in RCA: 83] [Article Influence: 4.6] [Reference Citation Analysis (0)] |
| 25. | Kurihara T, Mizunuma H, Obara M, Andoh K, Ibuki Y, Nishimura T. Determination of a normal level of serum CA125 in postmenopausal women as a tool for preoperative evaluation and postoperative surveillance of endometrial carcinoma. Gynecol Oncol. 1998;69:192-196. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 56] [Cited by in RCA: 57] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 26. | Powell JL, Hill KA, Shiro BC, Diehl SJ, Gajewski WH. Preoperative serum CA-125 levels in treating endometrial cancer. J Reprod Med. 2005;50:585-590. [PubMed] |
| 27. | Wang Y, Han C, Teng F, Bai Z, Tian W, Xue F. Predictive value of serum HE4 and CA125 concentrations for lymphatic metastasis of endometrial cancer. Int J Gynaecol Obstet. 2017;136:58-63. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 23] [Cited by in RCA: 32] [Article Influence: 3.6] [Reference Citation Analysis (0)] |