Published online May 24, 2020. doi: 10.5306/wjco.v11.i5.294
Peer-review started: December 31, 2019
First decision: March 15, 2020
Revised: May 9, 2020
Accepted: May 14, 2020
Article in press: May 14, 2020
Published online: May 24, 2020
Processing time: 144 Days and 20.1 Hours
Colorectal cancer (CRC) is the third most common cause of cancer-related death worldwide. Despite significant advances in screening, surgical management and adjuvant therapies, average 5-year survival seldom exceeds 60% in most developed nations. Metastatic disease represents the primary cause of mortality in patients with CRC, and the liver is the most common location for distant tumour spread. Up to 25% of patients are found to have synchronous liver metastases at the time of diagnosis and a further 30%-40% will develop metachronous disease in the course of follow-up. It has been suggested that primary tumour location [right side versus left side, primary tumour location (PTL)] can influence oncological outcomes in this patient group and that this should be considered in prognostic models and therapeutic decision-making algorithms. This suggestion is not universally accepted and there have been conflicting reports in the literature to date.
To provide a comprehensive summary of the available evidence regarding the impact of PTL on oncological outcomes in patients with colorectal cancer liver metastases (CRCLM).
MEDLINE, EMBASE and COCHRANE were searched for relevant publications using the Preferred Reporting Items for Systematic Reviews and Meta-Analyses methodology. Data on oncological outcomes was then extracted from full text articles that met the predefined inclusion criteria.
A total of 41 studies were identified that met predefined inclusion criteria for this review. In 21 out of 38 studies that provided data on overall survival, a statistically significant improvement in overall survival was reported in patients with left sided primary tumours. These studies included a total of 13897 patients compared with 4306 patients in the studies that did not show a significant difference. Eight studies noted a similar trend towards improved disease-free or progression-free survival. Several authors observed distinct patterns of relapse after treatment of hepatic metastases according to PTL; for example hepatic recurrence after treatment of CRCLM appears to occur more aggressively with right-sided CRC.
Taken together, the findings of the present review indicate that PTL may have a role as an independent prognostic factor when determining treatment and disease surveillance strategies in CRC. The mechanisms responsible for this variation remain poorly understood, but are likely to relate to molecular, histological and embryological differences, as well as inherent differences in therapeutic sensitivity.
Core tip: Primary tumour location is associated with differing oncological outcomes and patterns of hepatic metastatic behaviour in patients with colorectal cancer liver metastases. Specifically, this systematic review indicates that there is improved overall survival in patients undergoing treatment for colorectal cancer liver metastases with left-sided colorectal cancer (CRC), compared with right-sided CRC. These findings suggest that primary tumour location may have a role in developing more individually-tailored staging, treatment and surveillance strategies for patients with CRC in the future. Current chemotherapeutic regimens may require additional modification(s) to take into account the fundamental molecular and embryological differences that underpin primary tumour sidedness.
- Citation: Bingham G, Shetye A, Suresh R, Mirnezami R. Impact of primary tumour location on colorectal liver metastases: A systematic review. World J Clin Oncol 2020; 11(5): 294-307
- URL: https://www.wjgnet.com/2218-4333/full/v11/i5/294.htm
- DOI: https://dx.doi.org/10.5306/wjco.v11.i5.294
Colorectal cancer (CRC) is the third most common cancer subtype world-wide with over 1 million new cases diagnosed in 2018[1]. Metastatic disease represents the primary cause of mortality in CRC, and up to 25% of patients are found to have synchronous metastases at the time of diagnosis. A further 40% will develop metachronous disease and approximately 25%-30% of patients will develop liver metastases in the course of follow-up[2]. Indications for curative intent treatment of CRC liver metastases (CRCLM) have expanded rapidly over the last three decades, and several key factors have led to improvements in outcome, notably enhanced radiological detection, improved chemotherapeutic efficacy and more aggressive surgical treatment[3]. With modern combined-modality treatment approaches, 5-year survival in excess of 50% has been reported in selected patients with CRCLM[4]. Clinico-pathological factors believed to be associated with worse oncological outcome in CRCLM include the presence of synchronous metastases, bi-lobar liver involvement, metastases > 5 cm in size, and the presence of extra-hepatic disease[4,5]. It has also been suggested by a number of authors that primary tumour location (PTL) - right side versus left side, can influence patterns of hepatic metastatic dissemination and survival[6,7]. For example, a number of studies have demonstrated inferior oncological outcome in patients undergoing surgical resection of CRCLM with right-sided versus left-sided colonic primary tumours[8-10]. This has not been a consistent observation, and others have shown no clear association[11,12]. The aim of the present systematic review is to provide a summary of the available evidence on the impact of PTL on oncological outcomes in patients with CRCLM.
An electronic literature search was carried out using MEDLINE (1965 to March 2020), EMBASE (1980 to March 2020) and the Cochrane Library databases. The medical subject heading terms and key words used are as follows: “Colon” or “rectal cancer”, “liver metastasis” or “liver metastases” or “hepatic metastasis” or “hepatic metastases” and “left” and “Right”. Studies, abstracts and citations were scanned for relevance. The latest date of this search was 27 March 2020. The publications deemed relevant were read in full and assessed for inclusion and their references scanned to identify papers not identified in the initial search.
The methodology was designed around the “Preferred Reporting Items for Systematic Reviews and Meta-Analyses” recommendations for improving the standard of systematic reviews[13].
Studies meeting the following criteria were included for review: (1) Language: Full article accessible in English language only; Conference abstracts only were excluded. (2) Patient population: Studies reporting outcomes in ≥ 10 male/female patients aged ≥ 18 years with colorectal cancer and liver metastases. Where multiple publications were identified covering overlapping periods of time from the same institution/research group, the most recent and/or relevant data were selected for inclusion, and (3) Outcome measures: Studies were included if they reported oncological outcome data such as overall survival (OS), progression-free survival (PFS), recurrence-free survival (RFS) or disease-free survival (DFS). Studies reporting oncological outcomes for metastatic colorectal cancer were excluded if results were not reported for liver metastases specifically. Patients with metastases at multiple sites were included if one site was liver.
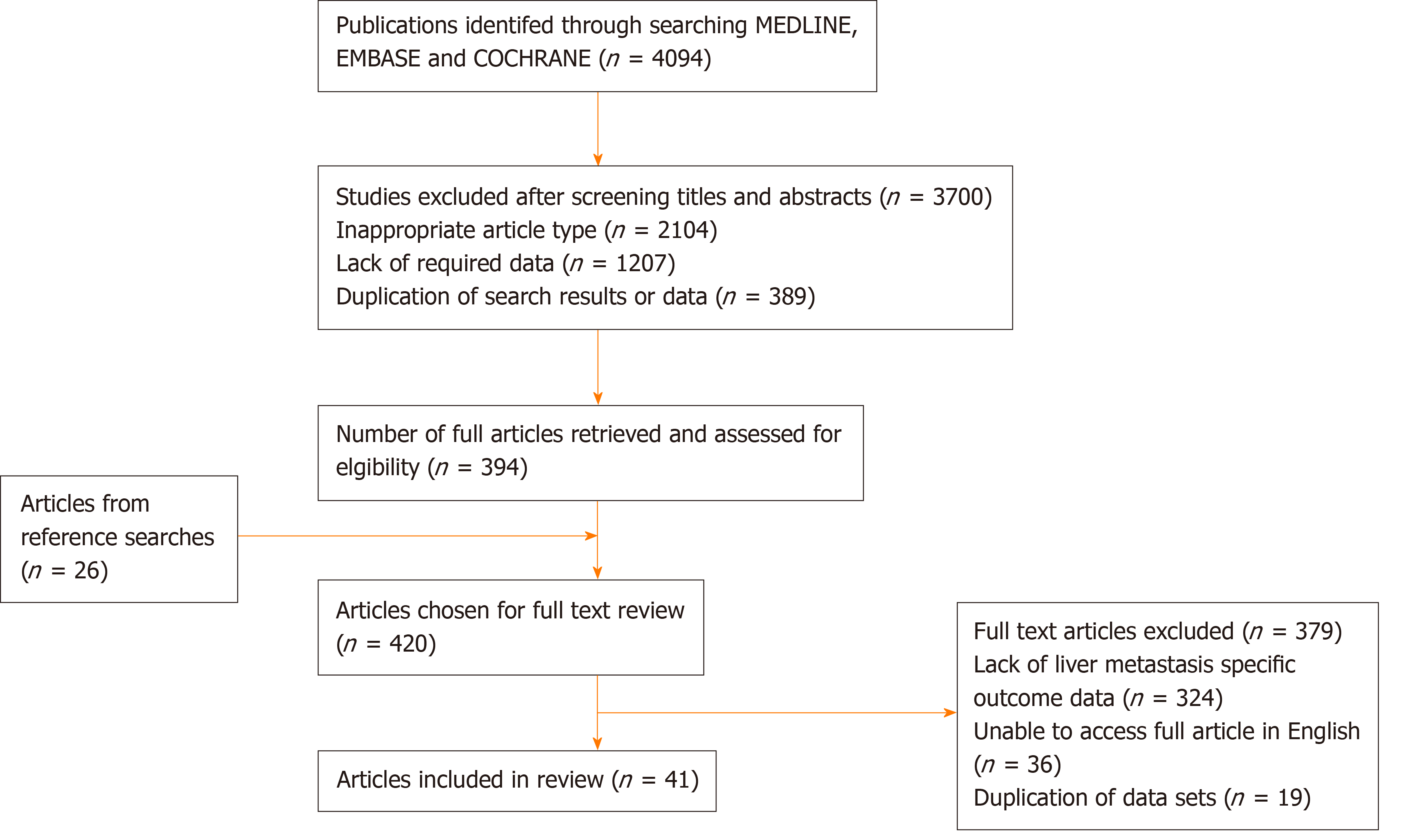
Three authors (Bingham G, Shetye A, Suresh R) independently extracted the following data from eligible studies: First author, year of publication, country of origin, study type, number of patients by gender, site of primary and age, primary study endpoint(s), secondary endpoint(s), extent and distribution of liver metastases, follow-up duration, adjuvant/neoadjuvant management, overall survival, progression-free survival, recurrence-free survival. Where there was uncertainty regarding inclusion a second author was consulted for consensus. All papers included were graded according to level of evidence using the system proposed by the Scottish Intercollegiate Guidelines Network[14]. A Preferred Reporting Items for Systematic Reviews and Meta-Analyses flow-diagram summarising the above search strategy is provided in Figure 1.
A total of 4094 potentially relevant publications were initially identified through the search strategy summarised in Figure 1. After screening of titles and abstracts, 3700 publications were withdrawn, leaving 394 articles for full text review. A reference search from these articles identified a further 26 studies of potential relevance. Of the 420 full text publications that were evaluated, 41 studies, including a total of 18426 patients, were found to meet our predefined inclusion criteria and were included in the review process. Study characteristics from these 41 studies are summarised in Table 1. Study population in these studies ranged from 24 to 3125 patients. Studies comprised two cohort prospective studies[15,16] (evidence level 2+) and 39 retrospective studies (evidence level 2+ - 2++)[8-12,17-50], this included 6 papers with pooled analysis (evidence level 2+-2++)[10,12,15,16,18,49]. There were no randomised controlled trials.
| Ref. | Year | Country of study | Number of patients included | Median follow up (mo) | Primary tumour location (n) | Median age (yr) | ||||
| L | R | Rectum | L | R | Rectum | |||||
| Zhou et al[27] | 2017 | China | 295 | 24 | 89 | 94 | 112 | 57 | 59 | 61 |
| Zhang et al[30] | 2017 | China | 194 | 12 | 144 | 50 | 60 | 55.5 | ||
| Makowiec et al[37] | 2018 | Germany | 221 | 158 | 63 | 64 | 65 | |||
| Chafai et al[29] | 2005 | Australia | 398 | 26.8 | 277 | 71 | ||||
| Rougier et al[15] | 1995 | France | 537 | 197 | 117 | 223 | ||||
| Wang et al[38] | 2018 | China | 420 | 26 | 334 | 86 | 57 | 58.5 | ||
| Gu et al[28] | 2018 | China | 102 | 51 | 51 | 63 | 61.5 | |||
| Gasser et al[12] | 2019 | Austria | 259 | 38.1 | 200 | 59 | 64 | 66 | ||
| de Haas et al[24] | 2010 | France | 750 | 39 | 413 | 154 | 200 | |||
| Marques et al[11] | 2018 | Brazil | 151 | 42 | 124 | 27 | 57 | 61 | ||
| Russolillo et al[21] | 2019 | Italy | 686 | 81 | 364 | 322 | 63 | 66 | ||
| Umeda et al[34] | 2013 | Japan | 100 | 36 | 40 | 23 | 37 | |||
| Zheng et al[31] | 2018 | China | 318 | 233 | 85 | |||||
| Mavros et al[39] | 2013 | United States | 97 | 26.4 | 44 | 24 | 27 | |||
| Viganò et al[20] | 2014 | Italy | 749 | 51.4 | 63 | 87 | 48 | |||
| Connor et al[40] | 2016 | Canada | 63 | 31.5 | 27 | 10 | 21 | |||
| Eefsen et al[41] | 2015 | Denmark | 254 | 44.6 | 125 | 51 | 78 | |||
| Schirripa et al[33] | 2015 | Italy | 309 | 45.6 | 138 | 87 | 82 | |||
| Loosen et al[42] | 2018 | Germany | 125 | 102 | 23 | |||||
| Amikura et al[32] | 2018 | Japan | 342 | 52.7 | 236 | 106 | ||||
| Yamashita et al[26] | 2018 | United States | 725 | 27/41 | 487 | 238 | 56 | 58 | ||
| Dulundu et al[19] | 2017 | Turkey | 108 | 40 | 24 | 44 | 58.5 | 63.2 | 63.8 | |
| Creasy et al[9] | 2016 | United States | 907 | 132 | 578 | 329 | 62.4 | 65.4 | ||
| Sasaki et al[23] | 2016 | United States | 426 | 28.9 | 297 | 129 | ||||
| Palkovics et al[43] | 2018 | Hungary | 319 | 114 | 72 | 133 | ||||
| Dupré et al[8] | 2018 | United Kingdom | 364 | 41.8 | 290 | 74 | 65.1 | 68.6 | ||
| Heise et al[22] | 2017 | Germany | 160 | 21 | 113 | 47 | ||||
| Shigematsu et al[44] | 2018 | Japan | 396 | 36.4 | 155 | 93 | 148 | |||
| Rhu et al[45] | 2017 | South Korea | 410 | 30.5 | 289 | 121 | 58.41 | 59.56 | ||
| Lionti et al[25] | 2018 | Italy | 63 | 40 | 22 | 23 | 18 | |||
| Wang et al[46] | 2017 | China | 159 | 130 | 29 | |||||
| Norén et al[18] | 2015 | Sweden | 3125 | 1109 | 1092 | 924 | ||||
| Berardi et al[47] | 2018 | Belgium | 62 | 24 | 11 | 4 | 47 | |||
| Cremolini et al[16] | 2018 | Italy | 159 | 42.1 | 40 | 64 | 52 | |||
| McCracken et al[48] | 2019 | United States | 612 | 388 | 226 | 55 | 62 | |||
| McVey et al[49] | 2019 | United States | 732 | 26.8 | 397 | 336 | 59 | 62 | ||
| Imai et al[35] | 2019 | Japan | 163 | 38.8 | 127 | 36 | ||||
| Koch et al[36] | 2018 | Germany | 30 | 24 | 5 | 1 | ||||
| Liao et al[10] | 2018 | Taiwan | 1442 | 58. | 888 | 554 | 62.4 | 64.6 | ||
| Adam et al[50] | 2011 | France | 186 | 37 | 106 | 35 | 41 | |||
| Wang et al[17] | 2019 | China | 1508 | 557 | 593 | 358 | ||||
Data on the influence of PTL on OS in CRCLM was provided by 38 of the studies included for review, including a total of 18203 patients. In 21 of these studies (13897 patients -76.3% of the total patient population captured) a statistically significant trend was observed with improved OS in patients with left sided primary tumours undergoing treatment for CRCLM (l-CRCLM). For example, Wang et al[17] in their study of 1508 patients receiving surgical treatment for synchronous CRCLM, of which 593 had right sided primary colorectal tumours (r-CRCLM), found a significant difference in 5-year OS between left and right sided primaries (l-CRCLM 40.1%, r-CRCLM 24.6%, P < 0.001). They also found that patients with r-CRCLM were more likely to be T4 (31.3% vs 20.1%, P < 0.001) N2 (42.5% vs 31.8%, P < 0.001), and poorly differentiated (30.5% vs 15.1%, P < 0.001). Creasy et al[9] in a similar cohort of 907 patients (36% with right sided primaries) undergoing hepatic resection found a found a median OS of 5.2 years in l-CRCLM compared with 3.6 years with in r-CRCLM (P = 0.004), with a hazard ratio (HR) of 1.22 (P = 0.028) on multivariate analysis. In their population database study of 3125 patients in Sweden Norén et al[18] found that l-CRCLM extended median OS by 4 mo (P = 0.02) compared with r-CRCLM. In addition, the authors reported enhanced 5-year OS (45.8% vs 44.5% P = 0.02), with a HR of 0.75 for l-CRCLM (P < 0.001).
A further 17 studies with 4306 patients found no statistically significant difference in OS between the two groups, but there was a trend towards longer OS in patients with l-CRCLM on the whole. For example, Gasser et al[12] found patients with a l-CRCLM had 22 mo longer median overall survival compared to r-CRCLM (P = 0.051). This contrasts with only 2 studies that showed lower OS in patients with l-CRCLM study, Dulundu et al[19] and Viganò et al[20], but neither with statistical significance (P = 0.072 and P < 0.05 respectively). These results are summarised in Table 2.
| Ref. | Year | Median OS (mo) | 5 Year OS (%) | OS Hazard ratio | |||||||||
| L | R | Rectum | P value | L | R | Rectum | P value | L | R | Rectum | P value | ||
| Zhou et al[27] | 2017 | 35 | 33 | 32 | 27.5 | 23.1 | 23 | 0.85 | 1 | 1.02 | |||
| Zhang et al[30] | 2017 | 22 | 12 | 0.012a | |||||||||
| 14 | 10 | ||||||||||||
| Makowiec et al[37] | 2018 | 41 | 46 | ||||||||||
| Chafai et al[29] | 2005 | 22.5 | 9.9 | < 0.001a | 1 | 1.68 | < 0.001a | ||||||
| Rougier et al[15] | 1995 | 8.2 | 4.5 | 7.6 | < 0.001a | ||||||||
| 6.8 | 3.8 | 6.6 | < 0.001a | ||||||||||
| Wang et al[38] | 2018 | 38.3 | 46.5 | 1 | 1.08 | ||||||||
| Gu et al[28] | 2018 | 40.3 | 29.4 | 0.042a | 1 | 6.2 | < 0.001a | ||||||
| Gasser et al[12] | 2019 | 55.5 | 33.5 | 58.2 | 1 | 1.53 | 0.029a | ||||||
| de Haas et al[24] | 2010 | 54 | 36 | 48 | 0.001a | 1 | 1.5 | ||||||
| Marques et al[11] | 2018 | 1 | 2.1 | ||||||||||
| Russolillo et al[21] | 2019 | 63.3 | 35.7 | 0.002a | 0.82 | 1 | < 0.001a | ||||||
| Umeda et al[34] | 2013 | 1.24 | 1 | 1.62 | |||||||||
| Zheng et al[31] | 2018 | 29.5 | 21.9 | < 0.001a | 0.5 | 1 | < 0.001a | ||||||
| Mavros et al[39] | 2013 | 1 | 1.09 | ||||||||||
| Viganò et al[20] | 2014 | 41.3 | 58.3 | 47.4 | 43.1 | 44.7 | 41.3 | ||||||
| Connor et al[40] | 2016 | 1 | 2.4 | 0.0321a | |||||||||
| Eefsen et al[41] | 2015 | 0.63 | 1 | 0.57 | 0.045a | ||||||||
| Schirripa et al[33] | 2015 | 57.3 | 35.5 | 61.1 | 0.017a | 1 | 1.59 | 0.95 | 0.017a | ||||
| Loosen et al[42] | 2018 | 1 | 2.32 | ||||||||||
| Amikura et al[32] | 2018 | 56.1 | 48.4 | 1 | 1.287 | ||||||||
| Yamashita et al[26] | 2018 | 52 | 32 | < 0.0001a | 1 | 2.04 | < 0.0001a | ||||||
| 78 | 55 | 0.003a | 1 | 1.9 | 0.0009a | ||||||||
| Dulundu et al[19] | 2017 | 30.43 | 46.38 | 40.86 | 52.5 | 54.1 | 59 | ||||||
| Creasy et al[9] | 2016 | 62.4 | 43.2 | 50.4 | 38.5 | 0.028a | 1 | 1.22 | 0.028a | ||||
| Sasaki et al[23] | 2016 | 55.3 | 44.1 | 0.033a | 53.7 | 41.5 | 0.76 | 1 | 0.033a | ||||
| Palkovics et al[43] | 2018 | 39 | 36 | 40 | |||||||||
| Dupré et al[8] | 2018 | 45.3 | 34.6 | 0.035a | 37.5 | 25.4 | 0.010a | 1 | 1.429 | 0.036a | |||
| Shigematsu et al[44] | 2018 | 0.67 | 1 | 0.63 | |||||||||
| Rhu et al[45] | 2017 | 0.862 | 1 | ||||||||||
| Wang et al[46] | 2017 | 0.75 | 1 | ||||||||||
| Norén et al[18] | 2015 | 61 | 57 | 48 | 0.02a | 45.8 | 44.5 | 42.6 | 0.02a | 0.75 | 1 | 0.73 | < 0.001a |
| Cremolini et al[16] | 2018 | 0.96 | 1 | ||||||||||
| McCracken et al[48] | 2019 | 75.6 | 54 | < 0.001a | 1 | 1.6 | 0.001a | ||||||
| McVey et al[49] | 2019 | 43 | 44.2 | 1.108 | 1 | ||||||||
| 79.4 | 64.6 | 0.037a | 0.629 | 0.788 | 0.024a | ||||||||
| Imai et al[35] | 2019 | 55.5 | 52.3 | 1 | 3.44 | 0.021a | |||||||
| Koch et al[36] | 2018 | 0.66 | 1 | ||||||||||
| Liao et al[10] | 2018 | 75.2 | 61.7 | 0.005a | |||||||||
| Adam et al[50] | 2011 | 31 | 0 | 36 | 0.003a | 1 | 2.2 | 0.003a | |||||
| Wang et al[17] | 2019 | 40.1 | 24.6 | < 0.001a | 1 | 1.75 | < 0.001a | ||||||
Benefit in DFS was also suggested, but not as convincingly as OS. This data was more sparsely provided, as some authors opted to alternatively provide PFS. Four studies including 3013 patients showed improved DFS in l-CRCLM. Russolillo et al[21] found improved median DFS by almost 1 year (32.7 mo vs 20.8 mo, P = 0.002) in their 364 patients with l-CRCLM (vs 322 patients with r-CRCLM) when assessing patterns of recurrence and survival following resection of liver metastases. In 2017 Heise et al[22] reported a DFS benefit in patients with l-CRCLM undergoing repeat hepatectomy after recurrence of colorectal cancer (HR: 0.19, P = 0.001). Liao et al[10] studied 1442 patients with stage III CRC who went on to develop CRCLM, and found that patients with left-sided colon cancer had better 3-year DFS (70.9% vs 66.5%, P = 0.033) compared to those with r-CRCLM.
In contrast to these observations, only one study by Sasaki et al[23] found significantly improved 3-year DFS in patients with r-CRCLM (28% vs 20.2%, P = 0.001) in their study of 426 patients who were undergoing curative intent hepatectomy.
Thirteen studies with 3423 patients showed no significant difference in DFS between l-CRCLM and r-CRCLM. These results are summarised in Table 3.
| Ref. | Year | Median DFS (mo) | 3 yr DFS (%) | DFS HR | |||||||||
| L | R | Rectum | P value | L | R | Rectum | P value | L | R | Rectum | P value | ||
| Wang et al[38] | 2018 | 22.4 | 29.1 | ||||||||||
| Gasser et al[12] | 2019 | 12.6 | 9.1 | 9.6 | |||||||||
| Marques et al[11] | 2018 | 1 | 1.1 | ||||||||||
| Russolillo et al[21] | 2019 | 32.7 | 20.8 | 0.002a | |||||||||
| Connor et al[40] | 1 | 1.6 | |||||||||||
| Eefsen et al[41] | 0.60 | 1 | 0.92 | ||||||||||
| Schirripa et al[33] | 2015 | 12.0 | 10.7 | 12.6 | 1 | 1.23 | 1.04 | ||||||
| Amikura et al[32] | 2018 | 35.4 | 32.3 | 1.09 | 1 | ||||||||
| Yamashita et al[26] | 2018 | 27 | 15 | 0.001a | 1 | 1.71 | < 0.0001a | ||||||
| 41 | 21 | 0.001a | 1 | 1.48 | < 0.0001a | ||||||||
| Creasy et al[9] | 2016 | 37 | 29 | 1.14 | |||||||||
| Sasaki et al[23] | 2016 | 20.2 | 28 | 0.001a | |||||||||
| Heise et al[22] | 2017 | 0.19 | 1 | 0.001a | |||||||||
| Shigematsu et al[44] | 2018 | 0.85 | 1 | 0.97 | |||||||||
| Wang et al[46] | 2017 | 1.36 | 1 | ||||||||||
| Berardi et al[47] | 2018 | 1.06 | 1 | 1.63 | |||||||||
| Cremolini et al[16] | 2018 | 0.81 | 1 | ||||||||||
| Imai et al[35] | 2019 | 22.9 | 21.3 | ||||||||||
| Liao et al[10] | 2018 | 70.9 | 66.5 | 0.033a | |||||||||
Only five publications provided data on PFS, and these data are summarised in Table 4. These studies including 2805 patients showed significantly improved PFS in l-CRCLM versus r-CRCLM. For example, de Haas et al[24] showed in 726 patients undergoing hepatic resection for CRCLM, that patients with l-CRCLM had a higher 5-year PFS (18% vs 16%, P = 0.009). No papers give significant evidence to the contrary and only one study with 63 patients showing no significant difference[25].
The data summarised in this systematic review appear to support the suggestion that CRCLM arising from right-sided colorectal primary tumours are associated with inferior OS compared with those arising from the left-sided CRC. Specifically, 21 of the 38 studies that provided data on OS reported statistically significant inferior OS in patients with r-CRCLM. Liao et al[10] for example demonstrated in their large study of 1442 patients that patients with l-CRCLM had better 5-year OS, 5-year cancer-specific survival, and 5-year RFS, all with statistical significance. In 2018 Yamashita et al[26] similarly concluded in their cohort of 725 patients undergoing upfront hepatic resection, that there was a significant survival benefit to having l-CRCLM, but that this benefit was no longer evident after neoadjuvant chemotherapy. The relationship between primary site and DFS and PFS with CRCLM is less clear, though again there appears to be a trend towards improved oncological outcome in l-CRCLM. Explaining these variations in oncological outcome is likely to require a deeper understanding of the underlying molecular and embryological differences associated with primary tumour sidedness.
There are subtleties in regards to variable oncological outcome identified in this review that merit further discussion. For example, in terms of DFS, Sasaki et al[23] reported interesting findings in terms of patterns of relapse with l-CRCLM compared with r-CRCLM. Specifically, in their study patients with l-CRCLM exhibited a shorter disease-free interval compared with patients who had undergone treatment for r-CRCLM (P = 0.01). However, irrespective of timing of relapse, and in spite of a longer disease-free interval, when patients with r-CRCLM did succumb to hepatic recurrence, it was consistently found to be with more advanced disease (> 4 recurrent lesions, P < 0.01). As a result, the authors found significantly reduced OS and significantly reduced survival after recurrence in r-CRCLM, compared with l-CRCLM. Thus it is conceivable, for reasons as yet unclear, that the liver is able to “hold off” recurrence of hepatic metastases arising from right-sided primaries for longer, but also that when this does finally occur, it is a more aggressive pattern of progression, leading to the paradoxical observation in some studies of seemingly favourable disease free interval, but ultimately inferior OS with r-CRCLM.
Previously the suggestion has been made that the typically more indolent course of presentation of right CRC, might in part be responsible for inferior outcome with resulting liver metastases[10]. The notion here is that delayed diagnosis of primary tumour results in an increased risk of developing synchronous metastases which are then incurable[51]. This would mean potentially fewer curative-intent resections offered to these patients, resulting in observed abbreviated survival. However, in this study we have also shown that there is evidence that PTL also has prognostic impact in patients with unresectable disease from the outset. For example, Zhou et al[27] reported on outcomes in 295 patients with unresectable CRCLM undergoing palliative radio-frequency ablation, and found similar rates of OS, but that the PFS was significantly better in patients with l-CRCLM (HR: 0.67, P = 0.012). Gu et al[28] also reported outcomes following palliative-intent radio-frequency ablation in patients with CRCLM, finding that patients with l-CRCLM had a significantly lower risk of recurrence outside of the ablation zone, with increased OS of 40.3 mo compared with 29.4 mo in r-CRCLM(P = 0.042). Multivariate analysis confirmed a HR of 6.2 (P = 0.001) for r-CRCLM predicting OS. This data is further supported by findings reported by Chafai et al[29] in 2005, who studied patients with unresected synchronous liver metastases after resection of the primary tumour. They found a significantly shorter survival in palliative patients who had r-CRCLM compared with l-CRCLM (2 years survival 9.9% vs 22.2%, HR: 1.5 P < 0.001). In circumstances where palliative/debulking surgery is offered, differences continue to persist for l-CRCLM versus r-CRCLM. For example, in 2017 Zhang et al[30] found that hepatic palliative resection prolonged median OS by 8 mo in patients with l-CRCLM (palliative resection vs no resection: 22 mo vs 14 mo, P = 0.009); however, by comparison no such improvement in OS was observed for patients with r-CRCLM undergoing palliative resection (12 mo vs 10 mo, P = 0.910).
With regards to defining putative mechanistic explanations for these differences, a number of factors should be considered. Firstly, there is considerable evidence that right sided CRCs are significantly more likely to harbor negative prognostic features; they tend to present at a more advanced stage, often in older patients, with a greater chance of synchronous metastatic disease, are more likely to carry unfavourable genetic mutation(s), and show poor differentiation[18,21,27,31,52]. It could therefore follow that patients with right sided CRC simply present with more advanced and aggressive disease from the outset. This however was not a uniform finding across the studies included in this review. For example, Creasy et al[9] found no such differences between right sided versus left sided CRC in terms of proportion of patients with the largest metastasis > 5 cm, proportion of patients with multiple metastases, or the proportion of patients with extra-hepatic disease. In spite of this relative equipoise, the authors reported significantly improved OS in patients with l-CRCLM and suggest that unique differences based on sidedness are likely to exist that extend beyond the aforementioned conventionally accepted differences.
From this perspective a number of mechanisms have emerged that could play a role in contributing to the inferior oncological outcome observed in patients with r-CRCLM. These broadly can be considered as: (1) Molecular differences; (2) Histopathological differences; (3) Therapeutic sensitivity differences; and (4) Embryological differences.
There are well-established molecular differences between right- and left-sided CRC with the former more often exhibiting KRAS and/or BRAF mutation[12,33,34,53]. RAS mutations have consistently been found to be associated with more aggressive tumour biology and are identified in up to 45% of patients with metastatic CRC. For example, the studies published by Amikura et al[32] and Shindoh et al[54] both demonstrate that RAS mutational status is associated with significantly worse survival in CRCLM (Amikura et al[32]: 5-year OS: 42.4% vs 65.3%, P = 0.0006; Shindoh et al[54]: 3-year DFS 59.9 vs 83.6% P = 0.016). Of note, it has also been reported that among patients put forward for curative intent resection of CRCLMs, the incidence of RAS mutation is only around 10%-15%, indicating that underlying tumour biology, seemingly inseparably linked to PTL, exerts additional prognostic relevance as it appears to indirectly influence surgical candidacy[55]. Goffredo et al[56] evaluated outcomes in 2655 patients undergoing CRCLM resection. They observed a significant increase in likelihood of mutant KRAS with right-sided PTL, compared to left and correspondingly found reduced OS in patients with r-CRCLM. It is likely that additional molecular drivers are responsible for the variations seen according to PTL, and RAS/BRAF likely account for only part of the molecular landscape especially since only a limited proportion of these cases are put forward for resection[54]. This notion is supported by Huang et al[57] who found no significant association between KRAS/BRAF mutational status and prognosis in patients presenting with metachronous CRCLM.
The role of mismatch repair (MMR) status and microsatellite instability (MSI) in the context of PTL seems less certain. Right sided CRC is more frequently associated with deficient MMR and MSI[7,56,58]. These tumours tend to be typified by poor differentiation, mucinous features and lymphocytic invasion. Evidence supports the suggestion that MSI is associated with improved oncological outcome[59,60]. However, this is at odds with the findings of the present review, where r-CRCLM appears to have shortened survival. This may reflect fundamental differences in MMR status according to tumour stage. For example, Jernvall et al[61] found MSI to be a more common finding in right sided Stage II CRC, but this was less frequently observed in stage IV disease. In this review molecular data were only available from a limited number of publications and subdivision of molecular phenotype according to PTL has not been provided in most cases. Hence, we are not able to draw any more definitive conclusions on the precise interplay between molecular factors and PTL. Considering these limitations, The Cancer Genome Atlas Network sought to evaluate a broader panel of genetic mutations and defined cases as “hypermutated” where a mutation rate of > 12/106 bases was found. Out of 276 samples analysed, the majority of hypermutated cases were right sided primary tumours. The group suggest that hypermutated phenotype is a significant negative prognostic feature, and this may in part account for inferior survival with r-CRCLM, as noted in the present review[62].
Several investigators have evaluated tumour histopathological features in order to determine if the difference in sidedness outcomes and tumour aggressiveness can be explained by one or more of these. Desmoplastic growth behaviour, presence of poorly differentiated clusters and tumour budding have all been considered[25,41,63]. Strong evidence, however, relates to the prevalence of mucinous elements. Viganò et al[20] and Russolillo et al[21] have both demonstrated mucinous adenocarcinoma to be more prevalent in right-sided CRC (P = 0.002 and P = 0.001, respectively). Viganò et al[20] reported significantly shortened OS with r-CRCLM versus l-CRCLM. They observed that, when compared with non-mucinous carcinoma, mucinous carcinoma has a higher KRAS mutation rate 61.8% vs 36.4%; P = 0.037) and lower chemotherapy response rate (63.9% vs 85.2%; P = 0.006). Specifically, Viganò et al[20] reported lower 5-year OS (33.2% vs 55.2%; P = 0.010) and DFS (32.5% vs 49.3%; P = 0.037) for mucinous tumours undergoing hepatic resection. One can extrapolate from these observations that inferior survival and right sided PTL are linked by an increased tendency for mucinous histology.
There are also suggestions in the literature that chemosensitivity is important in predicting survival, and that there may be a differing chemosensitivity profile according to PTL. In their meta-analysis of 16 first-line trials evaluating the efficacy of chemotherapy alone vs chemotherapy with targeted biologics in patients with unresectable metastatic CRC, You et al[64] found survival of patients with right sided CRC was inferior to those with left in patients receiving chemotherapy alone, implying that right-sided tumours overall are less chemosensitive. This finding is supported by Yamashita et al[26] found that r-CRCLM were independently associated with “minor pathological response” (defined as cancer cells accounting for ≥ 50% of residual cells), and were thus less sensitive to chemotherapy with worse RFS and OS. Interestingly, Marques et al[11] found that when selecting patients for CRCLM resection based on chemosensitivity, the survival disadvantage seen with r-CRCLM was eliminated. This suggests fundamental differences in tumour biology with the less chemosensitive phenotype more frequently seen with right-sided PTL and in turn associated with poorer survival.
This difference is maintained after the addition of well-established antiangiogenic biologics. You et al[64] found inferior survival in patients with r-CRCLM receiving chemotherapy and bevacizumab compared with l-CRCLM. Zheng et al[31] studied the effect of cetuximab as an addition to chemotherapy in KRAS wild-type patients with initially unresectable hepatic metastases. They found a survival benefit to cetuximab in patients with both r-CRCLM and l-CRCLM, but importantly noted that this effect was more substantial in the latter, with higher rates of effective tumour downstaging and extended OS.
In terms of embryology, Yamashita et al[26] suggest that the mid-gut embryological origin of the right colon may be responsible for the variable responsiveness of r-CRCLM to chemotherapy and differing oncological outcomes. Specifically, in their study of outcomes in 725 patients, they found reduced responsiveness to chemotherapy, reduced RFS and reduced OS in patients with r-CRCLM. The authors reported that this difference was maintained irrespective of RAS mutational status, which is considered to be one of the key oncogenic differences between right- and left-sided colon cancers. It is possible however, that other factors centered around the distinct development of these regions of gut, including unique lymphatic and venous drainage basins, and exposures to unique types of bacterial flora, could be contributing to oncological variability. This is an area that requires further research.
As a systematic review this paper has some inherent limitations, it is restricted by the quality of the literature available. However, all included papers were graded using the SIGN criteria with small studies excluded to mitigate this. Care was taken to perform a complete literature search, but studies and some work in progress may have been missed. Larger studies and well as future meta-analysis will be necessary to more clearly establish this trend and may provide a deeper understanding of the mechanisms at play.
In conclusion, the present review provides compelling data to support the notion that PTL significantly influences oncological outcome in patients with CRCLM. Overall, the data presented indicate that patients with r-CRCLM appear to have truncated overall, disease-free and progression-free survival. Some of these differences are likely to be accounted for by molecular heterogeneity, but other factors such as embryological origin and colonic microbiotal composition are areas that have received comparatively less attention in terms of research, and these may represent promising avenues to explore in the future. With the understanding that PTL could have prognostic relevance, comes the need to adjust treatment pipelines for patients accordingly. For example, patients with right-sided CRC may require abbreviated intervals between surveillance scans and tumour-marker assessment after primary tumour resection. In addition, given their more aggressive pattern of recurrence after hepatic resection/treatment, patients with r-CRCLM may benefit from a more radical first-line treatment of hepatic metastases. For example, the role of non-anatomical resection and the use of locally-ablative techniques in r-CRCLM may need more careful consideration.
Colorectal cancer (CRC) is the third most common cause of cancer related death with liver being the most common metastatic site. It has been long suggested that left and right sided primary tumours exhibit different behaviour but relatively little has been written about how this relates specifically to outcomes in colorectal cancer with liver metastases (CRCLM).
To improve current understanding regarding the impact of PTL on CRCLM given the relative paucity of information in this area. This in turn could have a significant impact on patient morbidity and mortality.
To ascertain whether there is a significant difference in oncological outcome in patients with CRCLM depending on PTL and to present some hypotheses that may explain any differences found. This systematic review demonstrates a significant difference in outcomes based on PTL with inferior oncological outcome for patients with right-sided CRC. . Further work is needed to better characterise the mechanisms responsible for this variation in order to inform clinical decision making.
A systematic review of Medline, Cochrane and Embase using the Terms “The medical subject heading terms and key words used are as follows: “Colon” or “rectal cancer”, “liver metastasis” or “liver metastases” or “hepatic metastasis” or “hepatic metastases” and “left” and “Right”. This search was combined with a bibliographic search to find the relevant publications and extract data from these papers. The methodology was based around the Preferred Reporting Items for Systematic Reviews and Meta-Analyses’ recommendations for systematic reviews
Twenty-one studies with a total of 18203 patients showed a statistically significant trend of improved overall survival in patient with left sided primary tumours undergoing treatment for colorectal cancer liver metastases (l-CRCLM). Four studies including 3013 patients showed improved disease free survival (DFS) in l-CRCLM. Only five publications provided data on progression free survival (PFS). These studies including 2805 patients showed significantly improved PFS in l-CRCLM vs r-CRCLM. The findings of this review are congruent with the accepted premise of superior survival in left sided colorectal cancer, and uniquely show that this remains true in the context of metastatic liver disease. We highlight a number of factors that may contribute to this, including KRAS/BRAF mutational status, presence of mucinous elements, and impaired chemosensitivy –all which are shown to be associated with right-sided PTL. The exact interplay between these known factors, PTL, and the emerging new mutations and molecular markers is yet to be determined and work needs to be done to determine the importance of PTL within the conglomeration.
The findings of this review indicate that PTL may have a role as an independent prognostic factor when determining treatment and disease surveillance strategies specifically in colorectal cancer that has metastasised to the liver. We find improved survival for both resected and unresectable l-CRCLM as well as a maintained trend after addition of biologics to established chemotherapy regimens. Hepatic recurrence after treatment of CRCLM appears to occur more aggressively with right-sided CRC, conferring significantly reduced survival. Explaining these variations in oncological outcome requires a deeper understanding of the underlying molecular and embryological differences associated with primary tumour sidedness. Microsatellite instability, interestingly, whilst more common in right-sided tumours, has been shown to be independently associated with improved survival – a finding somewhat incongruent with the overall picture of inferior survival in r-CRCLM. This suggests alternative mechanisms beyond MMR and microsatellite instability are likely to be involved. KRAS and BRAF mutational status, mucinous adenocarcinoma, and impaired chemosensitivity are all known to be significantly associated with right-sided CRC, and we show here that this association and the accompanying inferior survival persists in r-CRCLM. A better understanding of the role of PTL in the oncological outcomes of metastatic CRC may allow for improved risk stratification and redesigned patient pathways.
There is a considerable amount of data available on the oncological outcomes of patients undergoing liver resection for CRCLM, as related to PTL. This shows with convincing evidence that outcomes are superior for patients with l-CRCLM. Future research should be focused on gathering associated molecular and genetic data as related to PTL to better understand the tumour biology of right-sided CRC. This may allow the determination of ideal molecular markers, both for risk stratification/prognostication, and that may be used as potential therapeutic targets.
Manuscript source: Invited Manuscript
Specialty type: Oncology
Country/Territory of origin: United Kingdom
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): B, B
Grade C (Good): C, C
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Gurzu S, Kucherlapati MH, Osawa S, Sun XT S-Editor: Wang J L-Editor: A E-Editor: Liu MY
| 1. | Bray F, Ferlay J, Soerjomataram I, Siegel RL, Torre LA, Jemal A. Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin. 2018;68:394-424. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 53206] [Cited by in RCA: 55842] [Article Influence: 7977.4] [Reference Citation Analysis (132)] |
| 2. | Vatandoust S, Price TJ, Karapetis CS. Colorectal cancer: Metastases to a single organ. World J Gastroenterol. 2015;21:11767-11776. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 232] [Cited by in RCA: 232] [Article Influence: 23.2] [Reference Citation Analysis (2)] |
| 3. | Tejpar S, Stintzing S, Ciardiello F, Tabernero J, Van Cutsem E, Beier F, Esser R, Lenz HJ, Heinemann V. Prognostic and Predictive Relevance of Primary Tumor Location in Patients With RAS Wild-Type Metastatic Colorectal Cancer: Retrospective Analyses of the CRYSTAL and FIRE-3 Trials. JAMA Oncol. 2017;3:194-201. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 441] [Cited by in RCA: 528] [Article Influence: 66.0] [Reference Citation Analysis (0)] |
| 4. | House MG, Ito H, Gönen M, Fong Y, Allen PJ, DeMatteo RP, Brennan MF, Blumgart LH, Jarnagin WR, D'Angelica MI. Survival after hepatic resection for metastatic colorectal cancer: trends in outcomes for 1,600 patients during two decades at a single institution. J Am Coll Surg. 2010;210:744-752, 752-755. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 327] [Cited by in RCA: 357] [Article Influence: 23.8] [Reference Citation Analysis (1)] |
| 5. | Arru M, Aldrighetti L, Castoldi R, Di Palo S, Orsenigo E, Stella M, Pulitanò C, Gavazzi F, Ferla G, Di Carlo V, Staudacher C. Analysis of prognostic factors influencing long-term survival after hepatic resection for metastatic colorectal cancer. World J Surg. 2008;32:93-103. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 83] [Cited by in RCA: 89] [Article Influence: 4.9] [Reference Citation Analysis (0)] |
| 6. | Jess P, Hansen IO, Gamborg M, Jess T; Danish Colorectal Cancer Group. A nationwide Danish cohort study challenging the categorisation into right-sided and left-sided colon cancer. BMJ Open. 2013;3:e002608. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 58] [Cited by in RCA: 78] [Article Influence: 6.5] [Reference Citation Analysis (0)] |
| 7. | Nitsche U, Stögbauer F, Späth C, Haller B, Wilhelm D, Friess H, Bader FG. Right Sided Colon Cancer as a Distinct Histopathological Subtype with Reduced Prognosis. Dig Surg. 2016;33:157-163. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 60] [Cited by in RCA: 71] [Article Influence: 7.9] [Reference Citation Analysis (0)] |
| 8. | Dupré A, Malik HZ, Jones RP, Diaz-Nieto R, Fenwick SW, Poston GJ. Influence of the primary tumour location in patients undergoing surgery for colorectal liver metastases. Eur J Surg Oncol. 2018;44:80-86. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 32] [Cited by in RCA: 40] [Article Influence: 5.0] [Reference Citation Analysis (0)] |
| 9. | Creasy JM, Sadot E, Koerkamp BG, Chou JF, Gonen M, Kemeny NE, Saltz LB, Balachandran VP, Peter Kingham T, DeMatteo RP, Allen PJ, Jarnagin WR, D'Angelica MI. The Impact of Primary Tumor Location on Long-Term Survival in Patients Undergoing Hepatic Resection for Metastatic Colon Cancer. Ann Surg Oncol. 2018;25:431-438. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 53] [Cited by in RCA: 72] [Article Influence: 9.0] [Reference Citation Analysis (0)] |
| 10. | Liao CK, Chiang JM, Tsai WS, You JF, Hsieh PS, Hung HY, Chen HH, Tang RP, Chen JS, Yeh CY. Primary tumor location in stage III colon cancer has prognostic impact on subsequent liver metastasis. J Surg Oncol. 2018;118:1301-1310. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 8] [Cited by in RCA: 10] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 11. | Marques MC, C Ribeiro HS, Costa WL, de Jesus VHF, de Macedo MP, Diniz AL, Godoy AL, Farias IC, Aguiar S, Riechelmann RSP, S Begnami MDF, Coimbra FJF. Is primary sidedness a prognostic factor in patients with resected colon cancer liver metastases (CLM)? J Surg Oncol. 2018;117:858-863. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 18] [Cited by in RCA: 22] [Article Influence: 3.1] [Reference Citation Analysis (0)] |
| 12. | Gasser E, Braunwarth E, Riedmann M, Cardini B, Fadinger N, Presl J, Klieser E, Ellmerer P, Dupré A, Imai K, Malik H, Baba H, Ulmer H, Schneeberger S, Öfner D, Dinnewitzer A, Stättner S, Primavesi F. Primary tumour location affects survival after resection of colorectal liver metastases: A two-institutional cohort study with international validation, systematic meta-analysis and a clinical risk score. PLoS One. 2019;14:e0217411. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 17] [Cited by in RCA: 36] [Article Influence: 6.0] [Reference Citation Analysis (0)] |
| 13. | Moher D, Liberati A, Tetzlaff J, Altman DG; PRISMA Group. Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. PLoS Med. 2009;6:e1000097. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 52948] [Cited by in RCA: 47198] [Article Influence: 2949.9] [Reference Citation Analysis (0)] |
| 14. | Harbour R, Miller J. A new system for grading recommendations in evidence based guidelines. Br J Med. 2001;323:334–336. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1088] [Cited by in RCA: 1075] [Article Influence: 44.8] [Reference Citation Analysis (0)] |
| 15. | Rougier P, Milan C, Lazorthes F, Fourtanier G, Partensky C, Baumel H, Faivre J. Prospective study of prognostic factors in patients with unresected hepatic metastases from colorectal cancer. Fondation Française de Cancérologie Digestive. Br J Surg. 1995;82:1397-1400. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 140] [Cited by in RCA: 134] [Article Influence: 4.5] [Reference Citation Analysis (0)] |
| 16. | Cremolini C, Milione M, Marmorino F, Morano F, Zucchelli G, Mennitto A, Prisciandaro M, Lonardi S, Pellegrinelli A, Rossini D, Bergamo F, Aprile G, Urbani L, Morelli L, Schirripa M, Cardellino GG, Fassan M, Fontanini G, de Braud F, Mazzaferro V, Falcone A, Pietrantonio F. Differential histopathologic parameters in colorectal cancer liver metastases resected after triplets plus bevacizumab or cetuximab: a pooled analysis of five prospective trials. Br J Cancer. 2018;118:955-965. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 18] [Cited by in RCA: 17] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 17. | Wang Z, Wang X, Zhang Z, Wang X, Chen M, Lu L, Zhu W, Zhang J, Jia H, Chen J. Association between Primary Tumor Location and Prognostic Survival in Synchronous Colorectal Liver Metastases after Surgical Treatment: A Retrospective Analysis of SEER Data. J Cancer. 2019;10:1593-1600. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 18] [Cited by in RCA: 10] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 18. | Norén A, Eriksson HG, Olsson LI. Selection for surgery and survival of synchronous colorectal liver metastases; a nationwide study. Eur J Cancer. 2016;53:105-114. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 58] [Cited by in RCA: 66] [Article Influence: 6.6] [Reference Citation Analysis (0)] |
| 19. | Dulundu E, Attaallah W, Tilki M, Yegen C, Coskun S, Coskun M, Erdim A, Tanrikulu E, Yardimci S, Gunal O. Simultaneous resection for colorectal cancer with synchronous liver metastases is a safe procedure: Outcomes at a single center in Turkey. Biosci Trends. 2017;11:235-242. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 7] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 20. | Viganò L, Russolillo N, Ferrero A, De Rosa G, Ferreri E, Forchino F, Sperti E, Capussotti L. Resection of liver metastases from colorectal mucinous adenocarcinoma: is this a different disease? Results of a case-control study. Ann Surg. 2014;260:878-884; discussion 884-885. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 26] [Cited by in RCA: 31] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 21. | Russolillo N, Sperti E, Langella S, Menonna F, Allieta A, Di Maio M, Ferrero A. Impact of primary tumor location on patterns of recurrence and survival of patients undergoing resection of liver metastases from colon cancer. HPB (Oxford). 2020;22:116-123. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 9] [Cited by in RCA: 18] [Article Influence: 3.6] [Reference Citation Analysis (0)] |
| 22. | Heise D, Bayings W, Tuinhof A, Eickhoff R, Kroh A, Ulmer F, Dejong CHC, Neumann U, Binnebösel M. Long-term outcome and quality of life after initial and repeat resection of colorectal liver metastasis: A retrospective analysis. Int J Surg. 2017;48:281-285. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 13] [Cited by in RCA: 18] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 23. | Sasaki K, Andreatos N, Margonis GA, He J, Weiss M, Johnston F, Wolfgang C, Antoniou E, Pikoulis E, Pawlik TM. The prognostic implications of primary colorectal tumor location on recurrence and overall survival in patients undergoing resection for colorectal liver metastasis. J Surg Oncol. 2016;114:803-809. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 53] [Cited by in RCA: 67] [Article Influence: 8.4] [Reference Citation Analysis (0)] |
| 24. | de Haas RJ, Wicherts DA, Salloum C, Andreani P, Sotirov D, Adam R, Castaing D, Azoulay D. Long-term outcomes after hepatic resection for colorectal metastases in young patients. Cancer. 2010;116:647-658. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 38] [Cited by in RCA: 41] [Article Influence: 2.7] [Reference Citation Analysis (0)] |
| 25. | Lionti S, Reggiani Bonetti L, Bettelli S, Spallanzani A, Gelsomino F, Barresi V. Histopathological variables in liver metastases of patients with stage IV colorectal cancer: potential prognostic relevance of poorly differentiated clusters. Hum Pathol. 2018;78:115-124. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 12] [Cited by in RCA: 13] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 26. | Yamashita S, Brudvik KW, Kopetz SE, Maru D, Clarke CN, Passot G, Conrad C, Chun YS, Aloia TA, Vauthey JN. Embryonic Origin of Primary Colon Cancer Predicts Pathologic Response and Survival in Patients Undergoing Resection for Colon Cancer Liver Metastases. Ann Surg. 2018;267:514-520. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 59] [Cited by in RCA: 64] [Article Influence: 9.1] [Reference Citation Analysis (0)] |
| 27. | Zhou F, Yu X, Liang P, Han Z, Cheng Z, Yu J, Liu F, Hu Y. Does primary tumor location impact the prognosis of colorectal liver metastases patients after microwave ablation? - Lessons from 10 years' experience. Oncotarget. 2017;8:100791-100800. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 14] [Cited by in RCA: 15] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 28. | Gu Y, Huang Z, Gu H, Gao F, Zhang T, Huang S, Huang J. Does the Site of the Primary Affect Outcomes When Ablating Colorectal Liver Metastases with Radiofrequency Ablation? Cardiovasc Intervent Radiol. 2018;41:912-919. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 12] [Cited by in RCA: 16] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 29. | Chafai N, Chan CL, Bokey EL, Dent OF, Sinclair G, Chapuis PH. What factors influence survival in patients with unresected synchronous liver metastases after resection of colorectal cancer? Colorectal Dis. 2005;7:176-181. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 31] [Cited by in RCA: 29] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 30. | Zhang RX, Ma WJ, Gu YT, Zhang TQ, Huang ZM, Lu ZH, Gu YK. Primary tumor location as a predictor of the benefit of palliative resection for colorectal cancer with unresectable metastasis. World J Surg Oncol. 2017;15:138. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 14] [Cited by in RCA: 24] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 31. | Zheng P, Ren L, Feng Q, Zhu D, Chang W, He G, Ji M, Jian M, Lin Q, Yi T, Wei Y, Xu J. Patients with RAS wild-type right-sided unresectable liver-confined mCRC also benefit from cetuximab plus chemotherapy in first-line treatment. Am J Cancer Res. 2018;8:2337-2345. [PubMed] |
| 32. | Amikura K, Akagi K, Ogura T, Takahashi A, Sakamoto H. The RAS mutation status predicts survival in patients undergoing hepatic resection for colorectal liver metastases: The results from a genetic analysis of all-RAS. J Surg Oncol. 2018;117:745-755. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 17] [Cited by in RCA: 25] [Article Influence: 3.1] [Reference Citation Analysis (0)] |
| 33. | Schirripa M, Bergamo F, Cremolini C, Casagrande M, Lonardi S, Aprile G, Yang D, Marmorino F, Pasquini G, Sensi E, Lupi C, De Maglio G, Borrelli N, Pizzolitto S, Fasola G, Bertorelle R, Rugge M, Fontanini G, Zagonel V, Loupakis F, Falcone A. BRAF and RAS mutations as prognostic factors in metastatic colorectal cancer patients undergoing liver resection. Br J Cancer. 2015;112:1921-1928. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 114] [Cited by in RCA: 142] [Article Influence: 14.2] [Reference Citation Analysis (0)] |
| 34. | Umeda Y, Nagasaka T, Mori Y, Sadamori H, Sun DS, Shinoura S, Yoshida R, Satoh D, Nobuoka D, Utsumi M, Yoshida K, Yagi T, Fujiwara T. Poor prognosis of KRAS or BRAF mutant colorectal liver metastasis without microsatellite instability. J Hepatobiliary Pancreat Sci. 2013;20:223-233. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 42] [Cited by in RCA: 51] [Article Influence: 4.3] [Reference Citation Analysis (0)] |
| 35. | Imai K, Yamashita YI, Miyamoto Y, Nakao Y, Yusa T, Itoyama R, Nakagawa S, Okabe H, Hiyoshi Y, Nitta H, Chikamoto A, Baba H. Implication of primary tumor location for the indication of preoperative chemotherapy in patients with colorectal liver metastases. HPB (Oxford). 2019;21:405-412. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 7] [Cited by in RCA: 14] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 36. | Koch C, Schmidt N, Winkelmann R, Eichler K, Pession U, Bechstein WO, Zeuzem S, Waidmann O, Trojan J. Anti-EGF Receptor-Based Conversion Chemotherapy in RAS Wild-Type Colorectal Cancer Patients: Impact on Survival and Resection Rates. Digestion. 2018;98:263-269. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 3] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 37. | Makowiec F, Menzel M, Bronsert P, Holzner PA, Klock A, Lang SA, Fichtner-Feigl S, Neeff HP. Does the site of primary colorectal cancer influence the outcome after resection of isolated liver metastases? Dig Liver Dis. 2018;50:1088-1092. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 8] [Cited by in RCA: 11] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 38. | Wang K, Xu D, Yan XL, Poston G, Xing BC. The impact of primary tumour location in patients undergoing hepatic resection for colorectal liver metastasis. Eur J Surg Oncol. 2018;44:771-777. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 24] [Cited by in RCA: 31] [Article Influence: 4.4] [Reference Citation Analysis (0)] |
| 39. | Mavros MN, Hyder O, Pulitano C, Aldrighetti L, Pawlik TM. Survival of patients operated for colorectal liver metastases and concomitant extra-hepatic disease: external validation of a prognostic model. J Surg Oncol. 2013;107:481-485. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 11] [Cited by in RCA: 13] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 40. | Connor AA, McNamara K, Al-Sukhni E, Diskin J, Chan D, Ash C, Lowes LE, Allan AL, Zogopoulos G, Moulton CA, Gallinger S. Central, But Not Peripheral, Circulating Tumor Cells are Prognostic in Patients Undergoing Resection of Colorectal Cancer Liver Metastases. Ann Surg Oncol. 2016;23:2168-2175. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 21] [Cited by in RCA: 26] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 41. | Eefsen RL, Vermeulen PB, Christensen IJ, Laerum OD, Mogensen MB, Rolff HC, Van den Eynden GG, Høyer-Hansen G, Osterlind K, Vainer B, Illemann M. Growth pattern of colorectal liver metastasis as a marker of recurrence risk. Clin Exp Metastasis. 2015;32:369-381. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 67] [Cited by in RCA: 64] [Article Influence: 6.4] [Reference Citation Analysis (0)] |
| 42. | Loosen SH, Heise D, Dejong CH, Roy S, Tacke F, Trautwein C, Roderburg C, Luedde T, Neumann UP, Binnebösel M. Circulating Levels of Osteopontin Predict Patients' Outcome after Resection of Colorectal Liver Metastases. J Clin Med. 2018;7:390. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 8] [Cited by in RCA: 11] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 43. | Palkovics A, Vereczkei A, Kalmár KN, Fincsur A, Kiss I, Németh B, Papp A. The Issue of Survival After Colorectal Liver Metastasis Surgery: Parenchyma Sparing vs. Radicality. Anticancer Res. 2018;38:6431-6438. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3] [Cited by in RCA: 3] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 44. | Shigematsu Y, Inamura K, Yamamoto N, Mise Y, Saiura A, Ishikawa Y, Takahashi S, Kanda H. Impact of CDX2 expression status on the survival of patients after curative resection for colorectal cancer liver metastasis. BMC Cancer. 2018;18:980. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 7] [Cited by in RCA: 18] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 45. | Rhu J, Heo JS, Choi SH, Choi DW, Kim JM, Joh JW, Kwon CHD. Streamline flow of the portal vein affects the lobar distribution of colorectal liver metastases and has a clinical impact on survival. Ann Surg Treat Res. 2017;92:348-354. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 6] [Cited by in RCA: 14] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 46. | Wang Y, Yuan YF, Lin HC, Li BK, Wang FH, Wang ZQ, Ding PR, Chen G, Wu XJ, Lu ZH, Pan ZZ, Wan DS, Sun P, Yan SM, Xu RH, Li YH. Pathologic response after preoperative therapy predicts prognosis of Chinese colorectal cancer patients with liver metastases. Chin J Cancer. 2017;36:78. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 6] [Cited by in RCA: 13] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 47. | Berardi G, De Man M, Laurent S, Smeets P, Tomassini F, Ariotti R, Hoorens A, van Dorpe J, Varin O, Geboes K, Troisi RI. Radiologic and pathologic response to neoadjuvant chemotherapy predicts survival in patients undergoing the liver-first approach for synchronous colorectal liver metastases. Eur J Surg Oncol. 2018;44:1069-1077. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 15] [Cited by in RCA: 10] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 48. | Elizabeth McCracken EK, Samsa GP, Fisher DA, Farrow NE, Landa K, Shah KN, Blazer DG, Zani S. Prognostic significance of primary tumor sidedness in patients undergoing liver resection for metastatic colorectal cancer. HPB (Oxford). 2019;21:1667-1675. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 6] [Cited by in RCA: 7] [Article Influence: 1.2] [Reference Citation Analysis (1)] |
| 49. | McVey JC, Sasaki K, Margonis GA, Nowacki AS, Firl DJ, He J, Berber E, Wolfgang C, Miller CC, Weiss M, Aucejo FN. The impact of resection margin on overall survival for patients with colon cancer liver metastasis varied according to the primary cancer location. HPB (Oxford). 2019;21:702-710. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5] [Cited by in RCA: 11] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 50. | Adam R, de Haas RJ, Wicherts DA, Vibert E, Salloum C, Azoulay D, Castaing D. Concomitant extrahepatic disease in patients with colorectal liver metastases: when is there a place for surgery? Ann Surg. 2011;253:349-359. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 102] [Cited by in RCA: 113] [Article Influence: 8.1] [Reference Citation Analysis (0)] |
| 51. | Suthananthan AE, Bhandari M, Platell C. Influence of primary site on metastatic distribution and survival in stage IV colorectal cancer. ANZ J Surg. 2018;88:445–449. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 10] [Cited by in RCA: 18] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 52. | Margonis GA, Buettner S, Andreatos N, Kim Y, Wagner D, Sasaki K, Beer A, Schwarz C, Løes IM, Smolle M, Kamphues C, He J, Pawlik TM, Kaczirek K, Poultsides G, Lønning PE, Cameron JL, Burkhart RA, Gerger A, Aucejo FN, Kreis ME, Wolfgang CL, Weiss MJ. Association of BRAF Mutations With Survival and Recurrence in Surgically Treated Patients With Metastatic Colorectal Liver Cancer. JAMA Surg. 2018;153:e180996. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 111] [Cited by in RCA: 161] [Article Influence: 23.0] [Reference Citation Analysis (0)] |
| 53. | Sasaki K, Margonis GA, Wilson A, Kim Y, Buettner S, Andreatos N, Gani F, Amini N, Spolverato G, Pawlik TM. Prognostic Implication of KRAS Status after Hepatectomy for Colorectal Liver Metastases Varies According to Primary Colorectal Tumor Location. Ann Surg Oncol. 2016;23:3736-3743. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 44] [Cited by in RCA: 49] [Article Influence: 5.4] [Reference Citation Analysis (0)] |
| 54. | Shindoh J, Nishioka Y, Yoshioka R, Sugawara T, Sakamoto Y, Hasegawa K, Hashimoto M, Kokudo N. KRAS Mutation Status Predicts Site-Specific Recurrence and Survival After Resection of Colorectal Liver Metastases Irrespective of Location of the Primary Lesion. Ann Surg Oncol. 2016;23:1890-1896. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 41] [Cited by in RCA: 41] [Article Influence: 4.6] [Reference Citation Analysis (0)] |
| 55. | Brudvik KW, Kopetz SE, Li L, Conrad C, Aloia TA, Vauthey JN. Meta-analysis of KRAS mutations and survival after resection of colorectal liver metastases. Br J Surg. 2015;102:1175-1183. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 131] [Cited by in RCA: 155] [Article Influence: 15.5] [Reference Citation Analysis (0)] |
| 56. | Goffredo P, Utria AF, Beck AC, Chun YS, Howe JR, Weigel RJ, Vauthey JN, Hassan I. The Prognostic Impact of KRAS Mutation in Patients Having Curative Resection of Synchronous Colorectal Liver Metastases. J Gastrointest Surg. 2019;23:1957-1963. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 17] [Cited by in RCA: 23] [Article Influence: 3.8] [Reference Citation Analysis (0)] |
| 57. | Huang CJ, Teng HW, Chien CC, Lin JK, Yang SH. Prognostic significance of C-reactive protein polymorphism and KRAS/BRAF in synchronous liver metastasis from colorectal cancer. PLoS One. 2014;8:e65117. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 10] [Cited by in RCA: 13] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 58. | Iacopetta B. Are there two sides to colorectal cancer? Int J Cancer. 2002;101:403-408. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 551] [Cited by in RCA: 597] [Article Influence: 26.0] [Reference Citation Analysis (0)] |
| 59. | Kawakami H, Zaanan A, Sinicrope FA. Microsatellite instability testing and its role in the management of colorectal cancer. Curr Treat Options Oncol. 2015;16:30. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 226] [Cited by in RCA: 294] [Article Influence: 32.7] [Reference Citation Analysis (0)] |
| 60. | Taieb J, Shi Q, Pederson L, Alberts S, Wolmark N, Van Cutsem E, de Gramont A, Kerr R, Grothey A, Lonardi S, Yoshino T, Yothers G, Sinicrope FA, Zaanan A, André T. Prognosis of microsatellite instability and/or mismatch repair deficiency stage III colon cancer patients after disease recurrence following adjuvant treatment: results of an ACCENT pooled analysis of seven studies. Ann Oncol. 2019;30:1466-1471. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 103] [Cited by in RCA: 109] [Article Influence: 18.2] [Reference Citation Analysis (0)] |
| 61. | Jernvall P, Mäkinen MJ, Karttunen TJ, Mäkelä J, Vihko P. Microsatellite instability: impact on cancer progression in proximal and distal colorectal cancers. Eur J Cancer. 1999;35:197-201. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 71] [Cited by in RCA: 78] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 62. | Cancer Genome Atlas Network. Comprehensive molecular characterization of human colon and rectal cancer. Nature. 2012;487:330-337. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 6773] [Cited by in RCA: 6673] [Article Influence: 513.3] [Reference Citation Analysis (0)] |
| 63. | Banias L, Gurzu S, Kovacs Z, Bara T, Bara T, Jung I. Nuclear maspin expression: A biomarker for budding assessment in colorectal cancer specimens. Pathol Res Pract. 2017;213:1227-1230. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 31] [Cited by in RCA: 27] [Article Influence: 3.4] [Reference Citation Analysis (0)] |
| 64. | You XH, Jiang YH, Fang Z, Sun F, Li Y, Wang W, Xia ZJ, Wang XZ, Ying HQ. Chemotherapy plus bevacizumab as an optimal first-line therapeutic treatment for patients with right-sided metastatic colon cancer: a meta-analysis of first-line clinical trials. ESMO Open. 2020;4:e000605. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 19] [Cited by in RCA: 21] [Article Influence: 4.2] [Reference Citation Analysis (0)] |