Published online May 24, 2020. doi: 10.5306/wjco.v11.i5.250
Peer-review started: December 30, 2019
First decision: April 3, 2020
Revised: April 10, 2020
Accepted: April 23, 2020
Article in press: April 23, 2020
Published online: May 24, 2020
Processing time: 147 Days and 8.7 Hours
Lung carcinoma is associated with a high mortality worldwide, being the leading cause of cancer death. It is mainly classified into squamous non-small cell lung cancer (NSCLC), non-squamous NSCLC, and small cell lung cancer. However, such malignancy has been increasingly subdivided into histological and molecular subtypes to guide treatment. Therapies can be used in adjuvant and palliative settings. Regarding immunotherapy, it has been widely tested in both first or subsequent palliative lines. In this sense, drugs such as pembrolizumab, nivolumab, atezolizumab, ipilimumab, avelumab, and durvalumab have been assessed in large studies. Some of these trials have also studied these medicines in adjuvant and in maintenance therapy. In recent years, advances in immunotherapy have raised the hope that the unfavorable prognosis observed in several affected individuals can be changed. Immunotherapy has increased the overall survival in squamous NSCLC, non-squamous NSCLC, and small cell lung cancer. However, it has added to the oncology practice some side effects that are unusual in standard chemotherapy and require special clinical support. In order to show how immunotherapy is being applied in the treatment of lung carcinoma, we reviewed the main studies in adjuvant and palliative scenarios. What is the better scheme? What is the better combination? What is the better dose? When should we use immunotherapy? Does programmed cell death ligand 1 expression significantly interfere in immunotherapy efficiency? Some of these questions have already been answered, while others require more investigations.
Core tip: Immunotherapy has represented a new hope in the treatment of lung cancer. Improvements in global survival curves in metastatic disease and in local advanced disease have been observed with that therapeutic modality. However, some side effects that are unusual in standard chemotherapy have been frequently observed in immunotherapy, and they require special clinical support. This review aims to discuss some aspects regarding immunotherapy in non-small cell lung cancer and the perspectives about the use of this treatment in the adjuvant scenario and in small cell lung cancer.
- Citation: Pinheiro FD, Teixeira AF, de Brito BB, da Silva FAF, Santos MLC, de Melo FF. Immunotherapy – new perspective in lung cancer. World J Clin Oncol 2020; 11(5): 250-259
- URL: https://www.wjgnet.com/2218-4333/full/v11/i5/250.htm
- DOI: https://dx.doi.org/10.5306/wjco.v11.i5.250
The immunotherapy was thought in the 19th century by Coley in the treatment of sarcoma in patients with erysipelas[1]. In the 20th century, the idea of immunologic approaches in cancer treatment was presented and has been evaluated in different tumors. Interestingly, in 1986, interferon-alpha was approved for hairy-cell leukemia[2], and, in the 90’s, BCG was approved for adjuvant setting in bladder cancer. Furthermore, interleukin-2 was a new approach for metastatic melanoma in 1993[3]. However, during 20 years the immunotherapy was forgotten, arising in 2011 with the approval of anti-cytotoxic T-lymphocyte antigen 4 for metastatic melanoma[4].
Lung cancer is the first cause of death in oncology and the past global survival curve used to be poor. The revolution provided by immunotherapy in oncology has represented a new hope in the treatment of this disease. But new different adverse effects have also been observed accompanying these advances, most of them immune-mediated[5]. In addition, improvements in global survival curves in metastatic disease and local advanced disease have been noted with immunotherapy[6]. In this review, we discuss some aspects regarding immunotherapy in non-small cell lung cancer (NSCLC) and some perspectives about the use of this therapeutic modality in the adjuvant scenario and in small cell lung cancer (SCLC).
Immunotherapy with checkpoint inhibitors has become the standard treatment for advanced nondriver-mutated non-squamous NSCLC, mainly with anti-programmed cell death protein 1 and anti-programmed cell death ligand 1 (anti-PD-L1) and, in a few cases, with anti-cytotoxic T-lymphocyte antigen 4. The selection of the adequate therapy for each patient is guided by a proper immunohistochemistry, according IALSC Atlas of PD-L1 immunohistochemistry testing in lung cancer (2017)[5]. It is important to be highlighted that the above-mentioned analysis allows the stratification of patients in 3 groups of PD-L1 expression: < 1%, 1%-49%, and > 50%, and this classification correlates with overall rate response to treatment[6]. Nowadays, various challenges have been faced in the anatomopathological examination scenario. Increasingly, biopsies have been performed using less invasive procedures, generating less anatomopathological material for analysis. That circumstances increase the need of careful handling of the biopsy specimen material. Moreover, an aggravating situation is the demand for the identification of a growing number of molecular targets for treatment[7].
A phase 2 trial, keynote-021, has compared pemetrexed with carboplatin with or without pembrolizumab in metastatic non-squamous NSCLC. The overall rate response was better (56.7%) in the group with pembrolizumab after 23.9 mo when compared with patients that did not receive this drug (30.2%), as well as progression-free survival (PFS), with a hazard ratio (HR) = 0.53. The HR for overall survival (OS) was 0.56 in favor of pembrolizumab[7]. After this trial, there were many trials analyzing the effectiveness of immunotherapy in NSCLC. In patients with the expression of PD-L1 < 50%, some trials tried to have a north in treatment.
The keynote-189, a phase III trial that included unselected patients for PD-L1, also evaluated the above-mentioned treatment. This trial showed that the immunotherapy with chemotherapy vs chemotherapy alone presents a significant difference in OS within 12 mo, 69.2% vs 49.5% respectively. Adverse events (AEs) occurred with grade 3 or higher in 67.2% and 65.8%. It is interesting to notice that acute kidney injury grade 3 or higher was more present in pembrolizumab group (5.2% vs 0.5%). Referring to immune-mediated effects the group with immunotherapy had 22.7% of grade 3 or higher vs 11.9% in the group that lacked pembrolizumab. It is important to note three deaths by pneumonitis (immune-mediated side effect) in that group. Moreover, the correlation between expression of PD-L1 and time of progression or death was also reaffirmed[8].
As an alternative treatment for the patient with non-squamous NSCLC, there are combinations with atezolizumab with bevacizumab, carboplatin and paclitaxel (ABCP). This combination was tested in IMpower 150 trial, in which patients were randomized for the combination above or bevacizumab, carboplatin and paclitaxel (BCP) or atezolizumab, carboplatin and paclitaxel (ACP). After the phase of induction they received bevacizumab or atezolizumab or both in combination until progression or unmanageable toxic effects. From 1202 patients enrolled in three groups, almost half were PD-L1 negative. It is important to be highlighted that patients with drive mutation were included in this study because the specific therapy was not available in their countries. PFS was significantly longer in the group of atezolizumab, 8.3 mo vs 6.8 mo (BCP). The same occurred with OS: 19.2 mo vs 14.7 mo. The median duration of response was also longer in immunotherapy group (ABCP and ACP), 9.0 mo vs 5.7 mo (BCP). Immune-mediated AEs grade 1 and 2 were present in 77.4% of patients with atezolizumab, but no grade 5 side-effect was observed. Deaths by AEs were similar in each group[9].
In 2019, another trial was published with a subgroup of patients from IMpower 150 that were affected by liver metastasis at presentation. Improved median OS was observed in favor of combination of anti-VEGF with immunotherapy group vs bevacizumab group, 13.3 mo vs 9.4 mo respectively. Importantly, the OS didn’t differ when groups ACP vs BCP were evaluated. However, the groups ABCP and ACP were not compared[10].
Another trial that validated atezolizumab was the IMpower 130. This trial compared a combination of chemotherapy with atezolizumab vs chemotherapy alone. The scheme of chemotherapy was carboplatin with nab-paclitaxel. The median OS and PFS was significantly longer for immunotherapy group, 18.6 mo vs 13.9 mo and 7.0 mo vs 5.5 mo[11].
Nivolumab and Ipilimumab are an option to treat in first-line non-squamous and squamous NSCLC. This therapy was evaluated by a study conducted by Hellmann et al[12], 2019. In such a study, almost 70% of included patients had non-squamous NSCLC. Patients with PD-L1 > 1% were separated into three groups: Nivolumab alone, nivolumab with ipilimumab or chemotherapy alone. On the other hand, patients with PD-L1 < 1% were randomized for nivolumab plus ipilimumab, nivolumab with chemotherapy or chemotherapy alone. The median OS in PD-L1 > 1% was 17.1 mo in nivolumab and ipilimumab vs 14.9 mo in the chemotherapy group, while in PD-L1 < 1% patients, that measure was of 17.2 mo in ipilimumab and nivolumab vs 12.2 mo in the chemotherapy group. With regards to AEs we could observe similarity between groups, 32.8% with ipilimumab and nivolumab vs 36% in chemotherapy group. In immunotherapy combination, the most common side effects were skin reactions and endocrine events. Treatment-related deaths were too similar, 8 patients in immunotherapy combination vs 6 in chemotherapy group[12].
A different trial evaluated pembrolizumab vs chemotherapy in patients with more than 50% of PD-L1 expression. Almost 30% of the patients had squamous NSCLC. The median OS was 30 mo in pembrolizumab group vs 14.2 mo in the chemotherapy group. AEs grade 3 to 5, incidences of serious treatment-related effects and treatment discontinuation were similar in both groups. Immune-mediated reactions occurred in 33.8% in pembrolizumab groups and in 5.3% in the chemotherapy group. There were 2 deaths in the immunotherapy group vs 3 in the chemotherapy one[13].
In keynote 042 the included patients had squamous (38%) and non-squamous (62%) NSCLC. The population was divided into stratum of PD-L1 expression, ≥ 50%, ≥ 20% and ≥ 1%. In patients with PD-L1 ≥ 50% the OS of pembrolizumab group was 20 mo vs 12.2 mo in patients that did not receive the therapy. The same was observed in PD-L1 ≥ 20%, with 17.7 mo vs 13 mo, and in PD-L1 ≥ 1%, 16.7 mo vs 12.1 mo. Adverse immune-mediated events and infusion reactions, grade 3 or worse, were higher in pembrolizumab group, 8% vs 1%, and, in all grade side effects, 28% vs 7%. The most common grade 3 or worse in immune-mediated adverse effects in the immunotherapy group were pneumonitis, severe skin reactions and hepatitis[14].
Since we have no trials comparing pembrolizumab vs chemotherapy and pembrolizumab, Zhou et al[15], 2019 published an indirect comparison meta-analysis trying to solve this problem. It was evaluated the efficacy of pembrolizumab plus chemotherapy vs pembrolizumab alone (pem) in first-line treatment of NSCLC and PD-L1 ≥ 50%. Comparisons were made with 2 blocks of two groups. Arm A with pembrolizumab plus chemotherapy vs arm C with chemotherapy and arm B with pembrolizumab vs arm C with chemotherapy. Five trials were selected, keynote 021, 189, 407, 024 and 042. In direct meta-analysis OS was better for the use of pembrolizumab with chemotherapy or alone vs chemotherapy alone, HR = 0.51 and 0.67 respectively. In indirect meta-analysis, however, the result was not observed, HR = 0.76, confidence interval: 0.51–1.14[15].
Nivolumab as first-line therapy was not successful. A trial published in 2017 compared nivolumab alone vs chemotherapy alone. The median PFS was 4.2 mo with nivolumab vs 5.9 mo with chemotherapy and median OS was 14.4 vs 13.2 mo respectively. AEs of grade 3 or 4 were lower in nivolumab vs chemotherapy group, 18% vs 51%. The most immune-mediated AEs were skin-related[16].
Regarding Durvalumab, in an abstract published in 2018, 1118 patients were randomized for durvalumab vs chemotherapy and durvalumab plus Tremelimumab vs chemotherapy in NSCLC. However, no statistical significance in OS and PFS was observed between groups[17]. Two abstracts show better OS when patients had PD-L1 ≥ 25% and used statistical methods to evaluate the effect of subsequent immunotherapy, HR = 0.66 in favor of durvalumab[18,19]. Another abstract was published analyzing patients with high tumor mutational burden. In this group of patients median OS was better in durvalumab alone and associated with tremelimumab vs chemotherapy, HR = 0.77 and 0.49 respectively[20].
Nivolumab was compared with docetaxel as second-line after platinum-based doublet chemotherapy. The median OS was longer in the immunotherapy group, 12.2 mo vs 9.4 mo. AEs grade 3 and 4 were lower in nivolumab group, 10% vs 54%. The most common AEs in nivolumab group were fatigue, nausea, decreased appetite, and asthenia. In immunotherapy treatment, pneumonitis (3% vs < 1%) and hypothyroidism (7% vs 0%) were more frequent. There were 2 deaths, one in each group (by encephalitis in the nivolumab group and by febrile neutropenia in the docetaxel group)[21]. In an update of this study, the median OS in two years was 29% in nivolumab group vs 16% in docetaxel group. The duration of response was longer in the nivolumab group, with 34% of the responders having a response even within two years. In patients in whom immune-modulating medications were administered to manage treatment-related AEs, nearly all AEs resolved[22]. In patients pools of checkmate 017 (squamous NSCLC) and 057 (non-squamous NSCLC), mean treatment duration of patients treated with nivolumab and docetaxel was 8.3 mo and 3.1 mo, respectively[23]. It was suggested that nivolumab provides health-related quality of life advantages compared with docetaxel, in addition to longer OS, in previously treated patients with advanced non-squamous or squamous NSCLC[24].
Atezolizumab is another option to treat patients after at least one line of treatment. A trial with non-squamous and squamous NSCLC evaluated patients to receive atezolizumab or docetaxel. It had excluded patients with previous immunotherapy or docetaxel use. The median OS was better in immunotherapy vs docetaxel group, 13.8 mo vs 9.6 mo. Patients with PD-L1 expression ≥ 50% had the greatest benefit from atezolizumab, median OS 20.5 mo vs 8.9 mo. AEs grade 3 to 4 were 37% in atezolizumab group vs 54% in docetaxel group. The most common AEs were fatigue, nausea, decreased appetite and asthenia in the immunotherapy group. Musculoskeletal pain and pruritus, immune-mediated AEs, were more common in this group. Complementarily, pneumonitis was observed in 6 patients (grade 3), hepatitis was present in 2 patients (grade 3) and colitis also affected 2 patients (grade 2)[25]. The 24-mo landmark OS rate (30.9% vs 21.1%) and HR (0.75) were better for atezolizumab group. In this trial, the most common immune-related AEs were rash (16.9%) and hepatitis (12.6%). Hypothyroidism (4.8%) and pneumonitis (2.3%) were also present[26].
Pembrolizumab after first-line was tested in 2016. In keynote 010, NSCLC patients were randomized in pembrolizumab (high or low dose) and docetaxel groups, of which almost 20% had squamous NSCLC and about 70% had non-squamous NSCLC. Moreover, all of them presented PD-L1 expression ≥ 1%. The median OS was 12.7 mo (high dose) and 10.4 mo (low dose) vs 8.5 mo (docetaxel)[27]. An update with patients that completed 2 years of treatment and patients who received a second course of pembrolizumab was published. The OS remained longer for immunotherapy treatment with HR = 0.53 and 0.69 for PD-L1 expression ≥ 50% and ≥1%, respectively[28]. The health-related quality of life was evaluated in keynote 010 and it was better for patients in immunotherapy group[29]. The most common AEs were hypothyroidism, hyperthyroidism, and pneumonitis. Deaths occurred in five patients of each group, pneumonitis was the cause of 3 deaths in pembrolizumab group[27].
In JAVELIN lung 200 trial, avelumab was evaluated vs docetaxel after at least one line of treatment. All patients had expression of PD-L1. The median OS did not differ significantly between both groups, 11.4 mo vs 10.3 mo[30].
There were fewer trials with only squamous NSCLC patients evaluating immuno-therapy since most studies mainly evaluated patients with non-squamous NSCLC. The checkmate 227, keynote 042 and keynote 024 cited above had 30%, 38%, and 29% of squamous NSCLC patients, respectively[12,13,14].
The keynote 407 evaluated pembrolizumab only in patients with squamous NSCLC with PD-L1 expression ≥ 1%, as first-line treatment. The individuals were randomized for pembrolizumab vs placebo with carboplatin in combination with paclitaxel or nab-paclitaxel for 4 cycles. Pembrolizumab was maintained up for 35 cycles. The median OS was better in immunotherapy group, 15.9 mo vs 11.3 mo. Immune-mediated AEs and infusion reactions occurred in 28.8% in pembrolizumab group vs 8.6% in placebo group. Grade 3 or higher AEs were present in pembrolizumab group (10.8%) and placebo group (3.2%). There was one death in each group, both by pneumonitis. In pembrolizumab group, the most common grade 3 to 5 immune-mediated AEs were pneumonitis (2.5%), colitis (2.2%), and hepatitis (1.8%), while the other ones accounted less than 1%[31].
Another trial that evaluated only squamous NSCLC was IMpower 131. Patients were randomized for atezolizumab vs placebo, both associated with chemotherapy. The median PFS was 6.3 mo vs 5.6 mo, better for immunotherapy[32]. An update of that study published and presented by Jotte et al[33], 2019, at the International Association for the Study of Lung Cancer 2019 World Conference on Lung Cancer, concluded that median OS was 14.2 mo for atezolizumab vs 13.5 mo for placebo. In high expression PD-L1 patients, the median OS was 23.4 mo vs 10.2 mo, better for atezolizumab group. It is important to emphasize that the above-mentioned data were published only in abstracts[33].
There is a trial published in 2015 that compared nivolumab vs chemotherapy after at least one prior therapy in metastatic setting in patients with squamous NSCLC. The median OS was better for the immunotherapy group, 9.2 mo vs 6 mo. All patients had an expression of PD-L1. The major cause of discontinuation of treatment in nivolumab group was pneumonitis (2%), and no death was related in this group[5].
Durvalumab was compared to placebo in stage III NSCLC patients after unsuccessful chemoradiotherapy. The 24-mo OS rate was better in the immunotherapy group (66.3% vs 55.6%). The median time to death or distant metastasis was 28.3 mo vs 16.2 mo also better in the durvalumab group. The most frequent AE leading to the discontinuation of treatment was pneumonitis (4.8% in durvalumab group vs 2.6% in placebo group). Death due to AEs occurred in durvalumab (4.4%) and placebo group (6.4%)[34]. After that trial, the idea of immunotherapy for adjuvant setting was rekindled, and, nowadays, have been evaluated by an amount of current studies[35] (Table 1).
| Trial | Aims | Registry number1 |
| ANVIL | To compare nivolumab vs placebo in stage IB-IIIA squamous NSCLC | NCT02595944 |
| PEARLS | To evaluate pembrolizumab vs placebo in stage IB, II and IIIA NSCLC | NCT02504372 |
| IMpower 010 | To randomize stage IB-IIIA NSCLC patients to receive atezolizumab following adjuvant platinum-based chemotherapy or best supportive care | NCT02486718 |
| BR31 | To assess durvalumab vs placebo in completely resected tumors | NCT02273375 |
SCLC patients present lower survival rates when compared to NSCLC. Immuno-therapy presents various limitations in SCLC, but advances in this field have been achieved.
There is no data on the use of immunotherapy as primary or adjuvant therapy in limited-stage SCLC. However, atezolizumab has been incorporated for extensive-stage disease. A phase III trial randomized patients with extensive-stage SCLC for carboplatin and etoposide with atezolizumab or placebo for four cycles followed by maintenance phase. The median OS was 12.3 mo (atezolizumab) vs 10.3 mo (placebo). It is interesting to note that the patients were not tested for PD-L1 due to the non-standardization of samples obtained and because a low PD-L1 prevalence in tumor cells were expected. Moreover, no evidence of correlation between PD-L1 expression and atezolizumab activity have been described. The HR was 0.7 in benefit for atezolizumab. AEs of any grade were similar in both groups. In the atezolizumab group the most common grade 3 and 4 AEs were neutropenia, anemia and decreased neutrophil count. There were three deaths in each group. In atezolizumab group, they occured due to pneumonia in one patient and neutropenia in another patient. Moreover, one death verified in this group presented unspecified cause. Regarding placebo group, deaths were due to pneumonia, septic shock and cardiopulmonary failure. Immune-mediated AEs, mainly hypothyroidism and rash, occurred in 39.9% vs 24.5% in atezolizumab and placebo group, respectively. Most grade 3 and 4 immune-mediated AEs in atezolizumab group were rash (2%), infusion-related reaction (2%), hepatitis (1.5%), and colitis (1%)[36].
In the treatment of patients suffering relapses of 6 mo or fewer, some immunotherapy options have been tried, but the treatment standardization is being sought by some trials. In this framework, one possibility is the combination of ipilimumab and nivolumab. There is a phase 1/2 trial that tested nivolumab alone or nivolumab with ipilimumab for patients with limited or extensive-stage diseases after progression with at least one platinum-based chemotherapy regimen. Due to the same reasons reported by the above-mentioned trial, PD-L1 expression was not tested, and this parameter was assessed only retrospectively. There were four groups with different doses of nivolumab and ipilimumab. The objective response was 23% in nivolumab 1 mg/kg plus ipilimumab 3 mg/kg group vs 10% in nivolumab 3 mg/kg group vs 19% in nivolumab 3 mg/kg plus ipilimumab 1 mg/kg group vs 33% in nivolumab 1 mg/kg plus ipilimumab 1 mg/kg group (just 3 patients in this group). Grade 3 and 4 AEs occurred in 30%, 13%, 19%, 0% patients, respectively, and the most common of them were increased lipase and diarrhoea[37].
An update of Checkmate 032 was published as abstract in 2017. In which, the randomization was to nivolumab or nivolumab 1 mg/kg plus ipilimumab 3 mg/kg. The median disease control rate was 36% vs 49%, respectively[38].
Pembrolizumab is another possibility in SCLC immunotherapy. That drug was tested in phase 2 trial with advanced SCLC with one or two previous treatment. All patients were tested for PD-L1 expression and it was positive if ≥ 1%. The objective response rate was 35.7% in positive PD-L1 expression and 6% in negative PD-L1 expression. The median OS was 14.6 mo in PD-L1 positive group and 7.7 mo in PD-L1 negative group[39].
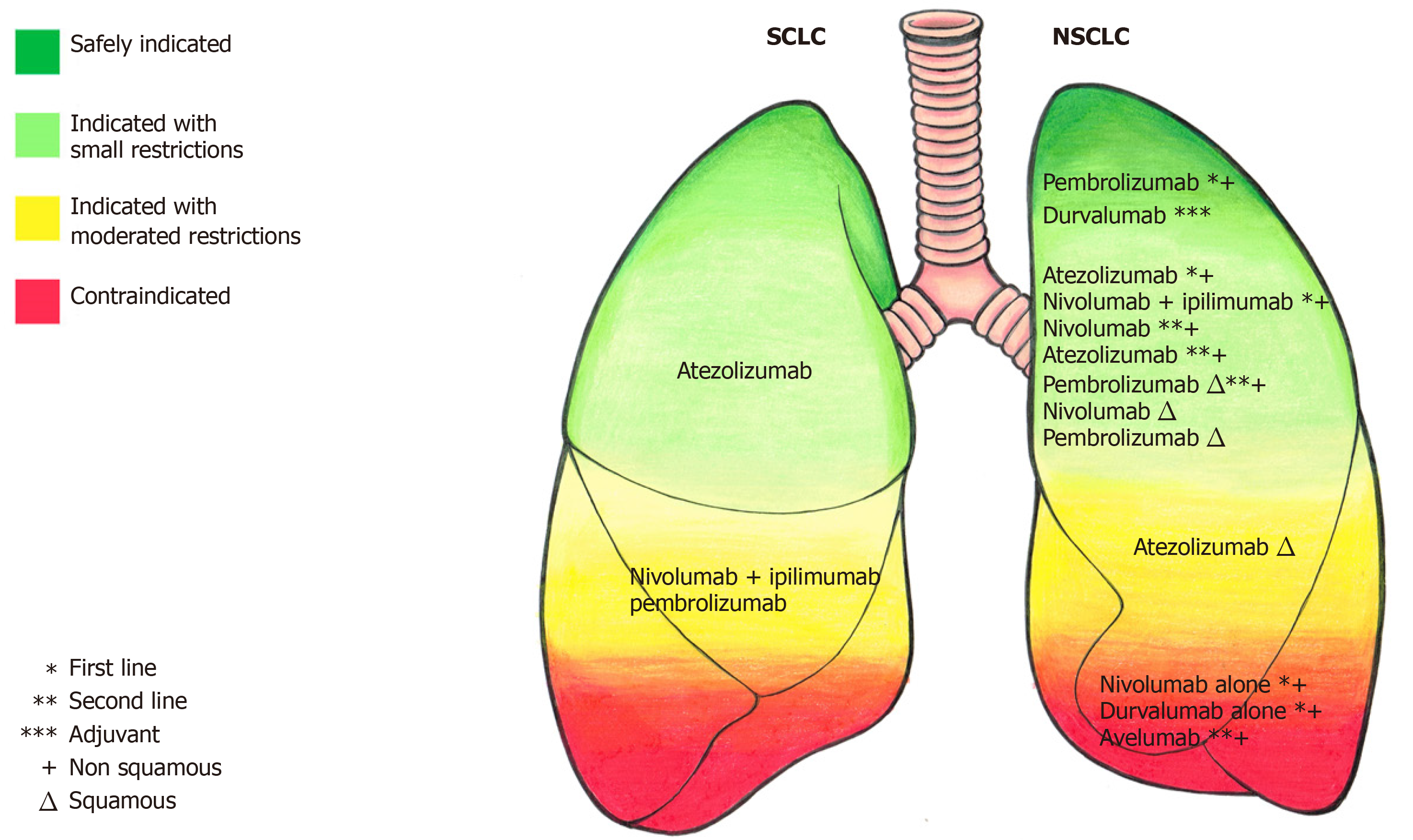
There are few studies published for immunotherapy in SCLC, but there are almost 30 open trials with immunotherapy in different lung cancer settings[40]. Figure 1 summarizes the safety level for an indication of immunotherapeutic drugs in the treatment of lung cancer.
As a conclusion, immunotherapy is considered the new standard in advanced and metastatic NSCLC, with or without chemotherapy. Moreover, it is evident that adequate immunohistochemistry is essential in NSCLC approach since it strongly correlates with treatment response. The benefit of immunotherapy was documented in patients with different sites of metastasis, chemotherapy combination, and expression of PD-L1, although OS in patients with PD-L1 expression ≥ 50% was higher. It is important to be highlighted that promising results have been achieved in both squamous and non-squamous NSCLC, as well as in SCLC. Furthermore, either in first-line or after at least one line, immunotherapy has presented good effects. However, almost all the trials that tested immunotherapy showed immune-mediated AEs and even deaths related to this. In addition, when two immunotherapies are combined, the immune-mediated side effects tend to be worse. The most commonly observed immune-mediated AEs were pneumonitis, hepatitis and skin reactions. In a near future, it is expected that new treatment schemes involving immunotherapy and its combinations will be established. Even now, there are many doubts on what are the optimal doses and the adequate duration for immunotherapies. Finally, the expansion of the knowledge about the use of this therapeutic modality as adjuvant treatment, and new studies on the immune-mediated adverse effects due to these treatments will improve their application in clinical practice.
Manuscript source: Invited manuscript
Specialty type: Oncology
Country/Territory of origin: Brazil
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): B, B
Grade C (Good): 0
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Sugimura H, Yao DF S-Editor: Tang JZ L-Editor: A E-Editor: Xing YX
| 1. | Coley WB. II. Contribution to the Knowledge of Sarcoma. Ann Surg. 1891;14:199-220. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 437] [Cited by in RCA: 481] [Article Influence: 26.7] [Reference Citation Analysis (0)] |
| 2. | Quesada JR, Hersh EM, Manning J, Reuben J, Keating M, Schnipper E, Itri L, Gutterman JU. Treatment of hairy cell leukemia with recombinant alpha-interferon. Blood. 1986;68:493-497. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 165] [Cited by in RCA: 152] [Article Influence: 3.9] [Reference Citation Analysis (0)] |
| 3. | Atkins MB, Lotze MT, Dutcher JP, Fisher RI, Weiss G, Margolin K, Abrams J, Sznol M, Parkinson D, Hawkins M, Paradise C, Kunkel L, Rosenberg SA. High-dose recombinant interleukin 2 therapy for patients with metastatic melanoma: analysis of 270 patients treated between 1985 and 1993. J Clin Oncol. 1999;17:2105-2116. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1498] [Cited by in RCA: 1470] [Article Influence: 56.5] [Reference Citation Analysis (0)] |
| 4. | Hodi FS, O'Day SJ, McDermott DF, Weber RW, Sosman JA, Haanen JB, Gonzalez R, Robert C, Schadendorf D, Hassel JC, Akerley W, van den Eertwegh AJ, Lutzky J, Lorigan P, Vaubel JM, Linette GP, Hogg D, Ottensmeier CH, Lebbé C, Peschel C, Quirt I, Clark JI, Wolchok JD, Weber JS, Tian J, Yellin MJ, Nichol GM, Hoos A, Urba WJ. Improved survival with ipilimumab in patients with metastatic melanoma. N Engl J Med. 2010;363:711-723. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 10799] [Cited by in RCA: 11757] [Article Influence: 783.8] [Reference Citation Analysis (0)] |
| 5. | Brahmer J, Reckamp KL, Baas P, Crinò L, Eberhardt WE, Poddubskaya E, Antonia S, Pluzanski A, Vokes EE, Holgado E, Waterhouse D, Ready N, Gainor J, Arén Frontera O, Havel L, Steins M, Garassino MC, Aerts JG, Domine M, Paz-Ares L, Reck M, Baudelet C, Harbison CT, Lestini B, Spigel DR. Nivolumab versus Docetaxel in Advanced Squamous-Cell Non-Small-Cell Lung Cancer. N Engl J Med. 2015;373:123-135. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5686] [Cited by in RCA: 6728] [Article Influence: 672.8] [Reference Citation Analysis (0)] |
| 6. | Langer CJ, Gadgeel SM, Borghaei H, Papadimitrakopoulou VA, Patnaik A, Powell SF, Gentzler RD, Martins RG, Stevenson JP, Jalal SI, Panwalkar A, Yang JC, Gubens M, Sequist LV, Awad MM, Fiore J, Ge Y, Raftopoulos H, Gandhi L; KEYNOTE-021 investigators. Carboplatin and pemetrexed with or without pembrolizumab for advanced, non-squamous non-small-cell lung cancer: a randomised, phase 2 cohort of the open-label KEYNOTE-021 study. Lancet Oncol. 2016;17:1497-1508. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 877] [Cited by in RCA: 1149] [Article Influence: 127.7] [Reference Citation Analysis (0)] |
| 7. | Ming ST, Keith MK, MB, Sanja D, Yasushi Y, Fred RH. IALSC atlas of PD-L1 immunohistochemistry testing in lung cancer. International Association for the Study of Lung Cancer, 2017; 84. |
| 8. | Gandhi L, Rodríguez-Abreu D, Gadgeel S, Esteban E, Felip E, De Angelis F, Domine M, Clingan P, Hochmair MJ, Powell SF, Cheng SY, Bischoff HG, Peled N, Grossi F, Jennens RR, Reck M, Hui R, Garon EB, Boyer M, Rubio-Viqueira B, Novello S, Kurata T, Gray JE, Vida J, Wei Z, Yang J, Raftopoulos H, Pietanza MC, Garassino MC; KEYNOTE-189 Investigators. Pembrolizumab plus Chemotherapy in Metastatic Non-Small-Cell Lung Cancer. N Engl J Med. 2018;378:2078-2092. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3449] [Cited by in RCA: 4770] [Article Influence: 681.4] [Reference Citation Analysis (0)] |
| 9. | Socinski MA, Jotte RM, Cappuzzo F, Orlandi F, Stroyakovskiy D, Nogami N, Rodríguez-Abreu D, Moro-Sibilot D, Thomas CA, Barlesi F, Finley G, Kelsch C, Lee A, Coleman S, Deng Y, Shen Y, Kowanetz M, Lopez-Chavez A, Sandler A, Reck M; IMpower150 Study Group. Atezolizumab for First-Line Treatment of Metastatic Nonsquamous NSCLC. N Engl J Med. 2018;378:2288-2301. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2076] [Cited by in RCA: 2754] [Article Influence: 393.4] [Reference Citation Analysis (0)] |
| 10. | Reck M, Mok TSK, Nishio M, Jotte RM, Cappuzzo F, Orlandi F, Stroyakovskiy D, Nogami N, Rodríguez-Abreu D, Moro-Sibilot D, Thomas CA, Barlesi F, Finley G, Lee A, Coleman S, Deng Y, Kowanetz M, Shankar G, Lin W, Socinski MA; IMpower150 Study Group. Atezolizumab plus bevacizumab and chemotherapy in non-small-cell lung cancer (IMpower150): key subgroup analyses of patients with EGFR mutations or baseline liver metastases in a randomised, open-label phase 3 trial. Lancet Respir Med. 2019;7:387-401. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 432] [Cited by in RCA: 710] [Article Influence: 118.3] [Reference Citation Analysis (0)] |
| 11. | West H, McCleod M, Hussein M, Morabito A, Rittmeyer A, Conter HJ, Kopp HG, Daniel D, McCune S, Mekhail T, Zer A, Reinmuth N, Sadiq A, Sandler A, Lin W, Ochi Lohmann T, Archer V, Wang L, Kowanetz M, Cappuzzo F. Atezolizumab in combination with carboplatin plus nab-paclitaxel chemotherapy compared with chemotherapy alone as first-line treatment for metastatic non-squamous non-small-cell lung cancer (IMpower130): a multicentre, randomised, open-label, phase 3 trial. Lancet Oncol. 2019;20:924-937. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 649] [Cited by in RCA: 1179] [Article Influence: 196.5] [Reference Citation Analysis (0)] |
| 12. | Hellmann MD, Paz-Ares L, Bernabe Caro R, Zurawski B, Kim SW, Carcereny Costa E, Park K, Alexandru A, Lupinacci L, de la Mora Jimenez E, Sakai H, Albert I, Vergnenegre A, Peters S, Syrigos K, Barlesi F, Reck M, Borghaei H, Brahmer JR, O'Byrne KJ, Geese WJ, Bhagavatheeswaran P, Rabindran SK, Kasinathan RS, Nathan FE, Ramalingam SS. Nivolumab plus Ipilimumab in Advanced Non-Small-Cell Lung Cancer. N Engl J Med. 2019;381:2020-2031. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1360] [Cited by in RCA: 1960] [Article Influence: 326.7] [Reference Citation Analysis (0)] |
| 13. | Reck M, Rodríguez-Abreu D, Robinson AG, Hui R, Csőszi T, Fülöp A, Gottfried M, Peled N, Tafreshi A, Cuffe S, O'Brien M, Rao S, Hotta K, Vandormael K, Riccio A, Yang J, Pietanza MC, Brahmer JR. Updated Analysis of KEYNOTE-024: Pembrolizumab Versus Platinum-Based Chemotherapy for Advanced Non-Small-Cell Lung Cancer With PD-L1 Tumor Proportion Score of 50% or Greater. J Clin Oncol. 2019;37:537-546. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 707] [Cited by in RCA: 1141] [Article Influence: 190.2] [Reference Citation Analysis (0)] |
| 14. | Mok TSK, Wu YL, Kudaba I, Kowalski DM, Cho BC, Turna HZ, Castro G, Srimuninnimit V, Laktionov KK, Bondarenko I, Kubota K, Lubiniecki GM, Zhang J, Kush D, Lopes G; KEYNOTE-042 Investigators. Pembrolizumab versus chemotherapy for previously untreated, PD-L1-expressing, locally advanced or metastatic non-small-cell lung cancer (KEYNOTE-042): a randomised, open-label, controlled, phase 3 trial. Lancet. 2019;393:1819-1830. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1597] [Cited by in RCA: 2397] [Article Influence: 399.5] [Reference Citation Analysis (0)] |
| 15. | Zhou Y, Lin Z, Zhang X, Chen C, Zhao H, Hong S, Zhang L. First-line treatment for patients with advanced non-small cell lung carcinoma and high PD-L1 expression: pembrolizumab or pembrolizumab plus chemotherapy. J Immunother Cancer. 2019;7:120. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 37] [Cited by in RCA: 61] [Article Influence: 10.2] [Reference Citation Analysis (0)] |
| 16. | Carbone DP, Reck M, Paz-Ares L, Creelan B, Horn L, Steins M, Felip E, van den Heuvel MM, Ciuleanu TE, Badin F, Ready N, Hiltermann TJN, Nair S, Juergens R, Peters S, Minenza E, Wrangle JM, Rodriguez-Abreu D, Borghaei H, Blumenschein GR, Villaruz LC, Havel L, Krejci J, Corral Jaime J, Chang H, Geese WJ, Bhagavatheeswaran P, Chen AC, Socinski MA; CheckMate 026 Investigators. First-Line Nivolumab in Stage IV or Recurrent Non-Small-Cell Lung Cancer. N Engl J Med. 2017;376:2415-2426. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1609] [Cited by in RCA: 2012] [Article Influence: 251.5] [Reference Citation Analysis (0)] |
| 17. | Rizvi NA, Cho BC, Reinmuth N, Lee KH, Ahn MJ, Luft A, van den Heuvel M, Cobo M, Smolin A, Vicente D, Moiseyenko V, Antonia SJ, Le Moulec S, Robinet R, Natale R, Nakagawa K, Zhao L, Stockman PK, Chand V, Peters S. LBA6 Durvalumab with or without tremelimumab vs platinum-based chemotherapy as first-line treatment for metastatic non-small cell lung cancer: MYSTIC. Ann Oncol. 2018;29:x41. [RCA] [DOI] [Full Text] [Cited by in Crossref: 17] [Cited by in RCA: 17] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 18. | Reinmuth N, Cho BC, Lee KH, Luft A, Ahn MJ, Schneider J, Shepherd FA, Geater SL, Pápai-Székely Z, Van Ngoc T, Garassino MC, Liu F, Clemett D, Thiyagarajah P, Ouwens M, Scheuring U, Peters S, Rizvi N. Effect of post-study immunotherapy (IO) on overall survival (OS) outcome in patients with metastatic (m) NSCLC treated with first-line durvalumab (D) vs chemotherapy (CT) in the phase III MYSTIC study. Ann Oncol. 2019;30 Suppl 2:ii77. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5] [Cited by in RCA: 5] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 19. | Cho BC, Reinmuth N, Lee KH, Ahn MJ, Luft A, Van den Heuvel M, Dols MC, Smolin A, Vicente D, Moiseyenko V, Antonia SJ, Moulec SL, Robinet G, Natale R, Garon EB, Nakagawa K, Liu F, Thiyagarajah P, Peters S, Rizvi NA. Efficacy and safety of first-line durvalumab (D) ± tremelimumab (T) vs platinum-based chemotherapy (CT) based on clinical characteristics in patients with metastatic (m) NSCLC: Results from MYSTIC. Ann Oncol. 2019;30 Suppl 2:ii79-ii80. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5] [Cited by in RCA: 5] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 20. | Peters S, Cho BC, Reinmuth N, Lee KH, Luft A, Ahn MJ, Baas P, Dols MC, Smolin A, Vicente D, Moiseyenko V, Antonia SJ, Nakagawa K, Goldberg SB, Kim E, Raja R, Brohawn P, Clemett D, Thiyagarajah P, Scheuring U, Liu F, Rizvi N. Tumor mutational burden (TMB) as a biomarker of survival in metastatic non-small cell lung cancer (mNSCLC): Blood and tissue TMB analysis from MYSTIC, a Phase III study of first-line durvalumab ± tremelimumab vs chemotherapy. Proceedings of the AACR Annual Meeting 2019; 2019 Mar 29-Apr 3; Atlanta, GA, United States. Amsterdam: Elsevier, 2019: CT074. |
| 21. | Borghaei H, Paz-Ares L, Horn L, Spigel DR, Steins M, Ready NE, Chow LQ, Vokes EE, Felip E, Holgado E, Barlesi F, Kohlhäufl M, Arrieta O, Burgio MA, Fayette J, Lena H, Poddubskaya E, Gerber DE, Gettinger SN, Rudin CM, Rizvi N, Crinò L, Blumenschein GR, Antonia SJ, Dorange C, Harbison CT, Graf Finckenstein F, Brahmer JR. Nivolumab versus Docetaxel in Advanced Nonsquamous Non-Small-Cell Lung Cancer. N Engl J Med. 2015;373:1627-1639. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 6945] [Cited by in RCA: 7508] [Article Influence: 750.8] [Reference Citation Analysis (0)] |
| 22. | Horn L, Spigel DR, Vokes EE, Holgado E, Ready N, Steins M, Poddubskaya E, Borghaei H, Felip E, Paz-Ares L, Pluzanski A, Reckamp KL, Burgio MA, Kohlhäeufl M, Waterhouse D, Barlesi F, Antonia S, Arrieta O, Fayette J, Crinò L, Rizvi N, Reck M, Hellmann MD, Geese WJ, Li A, Blackwood-Chirchir A, Healey D, Brahmer J, Eberhardt WEE. Nivolumab Versus Docetaxel in Previously Treated Patients With Advanced Non-Small-Cell Lung Cancer: Two-Year Outcomes From Two Randomized, Open-Label, Phase III Trials (CheckMate 017 and CheckMate 057). J Clin Oncol. 2017;35:3924-3933. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 473] [Cited by in RCA: 685] [Article Influence: 85.6] [Reference Citation Analysis (0)] |
| 23. | Vokes EE, Ready N, Felip E, Horn L, Burgio MA, Antonia SJ, Arén Frontera O, Gettinger S, Holgado E, Spigel D, Waterhouse D, Domine M, Garassino M, Chow LQM, Blumenschein G, Barlesi F, Coudert B, Gainor J, Arrieta O, Brahmer J, Butts C, Steins M, Geese WJ, Li A, Healey D, Crinò L. Nivolumab versus docetaxel in previously treated advanced non-small-cell lung cancer (CheckMate 017 and CheckMate 057): 3-year update and outcomes in patients with liver metastases. Ann Oncol. 2018;29:959-965. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 263] [Cited by in RCA: 373] [Article Influence: 62.2] [Reference Citation Analysis (0)] |
| 24. | Reck M, Brahmer J, Bennett B, Taylor F, Penrod JR, DeRosa M, Dastani H, Spigel DR, Gralla RJ. Evaluation of health-related quality of life and symptoms in patients with advanced non-squamous non-small cell lung cancer treated with nivolumab or docetaxel in CheckMate 057. Eur J Cancer. 2018;102:23-30. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 43] [Cited by in RCA: 61] [Article Influence: 8.7] [Reference Citation Analysis (0)] |
| 25. | Rittmeyer A, Barlesi F, Waterkamp D, Park K, Ciardiello F, von Pawel J, Gadgeel SM, Hida T, Kowalski DM, Dols MC, Cortinovis DL, Leach J, Polikoff J, Barrios C, Kabbinavar F, Frontera OA, De Marinis F, Turna H, Lee JS, Ballinger M, Kowanetz M, He P, Chen DS, Sandler A, Gandara DR; OAK Study Group. Atezolizumab versus docetaxel in patients with previously treated non-small-cell lung cancer (OAK): a phase 3, open-label, multicentre randomised controlled trial. Lancet. 2017;389:255-265. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2868] [Cited by in RCA: 3689] [Article Influence: 461.1] [Reference Citation Analysis (0)] |
| 26. | Fehrenbacher L, von Pawel J, Park K, Rittmeyer A, Gandara DR, Ponce Aix S, Han JY, Gadgeel SM, Hida T, Cortinovis DL, Cobo M, Kowalski DM, De Marinis F, Gandhi M, Danner B, Matheny C, Kowanetz M, He P, Felizzi F, Patel H, Sandler A, Ballinger M, Barlesi F. Updated Efficacy Analysis Including Secondary Population Results for OAK: A Randomized Phase III Study of Atezolizumab versus Docetaxel in Patients with Previously Treated Advanced Non-Small Cell Lung Cancer. J Thorac Oncol. 2018;13:1156-1170. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 129] [Cited by in RCA: 194] [Article Influence: 27.7] [Reference Citation Analysis (0)] |
| 27. | Herbst RS, Baas P, Kim DW, Felip E, Pérez-Gracia JL, Han JY, Molina J, Kim JH, Arvis CD, Ahn MJ, Majem M, Fidler MJ, de Castro G, Garrido M, Lubiniecki GM, Shentu Y, Im E, Dolled-Filhart M, Garon EB. Pembrolizumab versus docetaxel for previously treated, PD-L1-positive, advanced non-small-cell lung cancer (KEYNOTE-010): a randomised controlled trial. Lancet. 2016;387:1540-1550. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4116] [Cited by in RCA: 5039] [Article Influence: 559.9] [Reference Citation Analysis (0)] |
| 28. | Herbst RS, Garon EB, Kim D, Cho BC, Pérez Gracia JL, Han J, Arvis CD, Majem M, Forster M, Monnet I, Novello S, Szalai Z, Gubens MA, Su W, Ceresoli GL, Samkari A, Jensen E, Lubiniecki GM, Baas P. Long-term follow-up in the KEYNOTE-010 study of pembrolizumab (pembro) for advanced NSCLC, including in patients (pts) who completed 2 years of pembro and pts who received a second course of pembro. Proceedings of the ESMO Immuno-Oncology Congress 2018; 2018 Dec 15; Geneva, Switzerland. Amsterdam: Elsevier, 2018: x39-x43. |
| 29. | Barlesi F, Garon EB, Kim DW, Felip E, Han JY, Kim JH, Ahn MJ, Fidler MJ, Gubens MA, de Castro G, Surmont V, Li Q, Deitz AC, Lubiniecki GM, Herbst RS. Health-Related Quality of Life in KEYNOTE-010: a Phase II/III Study of Pembrolizumab Versus Docetaxel in Patients With Previously Treated Advanced, Programmed Death Ligand 1-Expressing NSCLC. J Thorac Oncol. 2019;14:793-801. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 32] [Cited by in RCA: 55] [Article Influence: 9.2] [Reference Citation Analysis (0)] |
| 30. | Barlesi F, Vansteenkiste J, Spigel D, Ishii H, Garassino M, de Marinis F, Özgüroğlu M, Szczesna A, Polychronis A, Uslu R, Krzakowski M, Lee JS, Calabrò L, Arén Frontera O, Ellers-Lenz B, Bajars M, Ruisi M, Park K. Avelumab versus docetaxel in patients with platinum-treated advanced non-small-cell lung cancer (JAVELIN Lung 200): an open-label, randomised, phase 3 study. Lancet Oncol. 2018;19:1468-1479. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 239] [Cited by in RCA: 342] [Article Influence: 48.9] [Reference Citation Analysis (0)] |
| 31. | Paz-Ares L, Luft A, Vicente D, Tafreshi A, Gümüş M, Mazières J, Hermes B, Çay Şenler F, Csőszi T, Fülöp A, Rodríguez-Cid J, Wilson J, Sugawara S, Kato T, Lee KH, Cheng Y, Novello S, Halmos B, Li X, Lubiniecki GM, Piperdi B, Kowalski DM; KEYNOTE-407 Investigators. Pembrolizumab plus Chemotherapy for Squamous Non-Small-Cell Lung Cancer. N Engl J Med. 2018;379:2040-2051. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1941] [Cited by in RCA: 2650] [Article Influence: 378.6] [Reference Citation Analysis (0)] |
| 32. | Jotte RM, Cappuzzo F, Vynnychenko I, Stroyakovskiy D, Abreu DR, Hussein M, Soo R, Conter HJ, Kozuki T, da Silva C, Graupner V, Sun SW, Lin R, Jessop H, Kowanetz M, Hoang T, Sandler A, Socinski MA. IMpower131: Primary PFS and safety analysis of a randomized phase III study of atezolizumab + carboplatin + paclitaxel or nab-paclitaxel vs carboplatin + nab-paclitaxel as 1L therapy in advanced squamous NSCLC. J Clin Oncol. 2018;36:LBA9000. [RCA] [DOI] [Full Text] [Cited by in Crossref: 137] [Cited by in RCA: 138] [Article Influence: 19.7] [Reference Citation Analysis (0)] |
| 33. | Jotte R, Cappuzzo F, Vynnychenko I, Stroyakovskiy D, Abreu DR, Hussein M, Soo R, Conter H, Kozuki T, Huang K, Graupner V, Sun S, Hoang T, Jessop H, Mccleland M, Ballinger M, Sandler A, Socinski M. OA14.02 IMpower131: Final OS Results of Carboplatin + Nab-Paclitaxel ± Atezolizumab in Advanced Squamous NSCLC. J Thorac Oncol. 2019;14:S243-S244. [RCA] [DOI] [Full Text] [Cited by in Crossref: 15] [Cited by in RCA: 15] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 34. | Antonia SJ, Villegas A, Daniel D, Vicente D, Murakami S, Hui R, Kurata T, Chiappori A, Lee KH, de Wit M, Cho BC, Bourhaba M, Quantin X, Tokito T, Mekhail T, Planchard D, Kim YC, Karapetis CS, Hiret S, Ostoros G, Kubota K, Gray JE, Paz-Ares L, de Castro Carpeño J, Faivre-Finn C, Reck M, Vansteenkiste J, Spigel DR, Wadsworth C, Melillo G, Taboada M, Dennis PA, Özgüroğlu M; PACIFIC Investigators. Overall Survival with Durvalumab after Chemoradiotherapy in Stage III NSCLC. N Engl J Med. 2018;379:2342-2350. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1573] [Cited by in RCA: 2052] [Article Influence: 293.1] [Reference Citation Analysis (0)] |
| 35. | Moujaess E, Haddad FG, Eid R, Kourie HR. The emerging use of immune checkpoint blockade in the adjuvant setting for solid tumors: a review. Immunotherapy. 2019;11:1409-1422. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 18] [Cited by in RCA: 28] [Article Influence: 4.7] [Reference Citation Analysis (0)] |
| 36. | Horn L, Mansfield AS, Szczęsna A, Havel L, Krzakowski M, Hochmair MJ, Huemer F, Losonczy G, Johnson ML, Nishio M, Reck M, Mok T, Lam S, Shames DS, Liu J, Ding B, Lopez-Chavez A, Kabbinavar F, Lin W, Sandler A, Liu SV; IMpower133 Study Group. First-Line Atezolizumab plus Chemotherapy in Extensive-Stage Small-Cell Lung Cancer. N Engl J Med. 2018;379:2220-2229. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1567] [Cited by in RCA: 2359] [Article Influence: 337.0] [Reference Citation Analysis (0)] |
| 37. | Antonia SJ, López-Martin JA, Bendell J, Ott PA, Taylor M, Eder JP, Jäger D, Pietanza MC, Le DT, de Braud F, Morse MA, Ascierto PA, Horn L, Amin A, Pillai RN, Evans J, Chau I, Bono P, Atmaca A, Sharma P, Harbison CT, Lin CS, Christensen O, Calvo E. Nivolumab alone and nivolumab plus ipilimumab in recurrent small-cell lung cancer (CheckMate 032): a multicentre, open-label, phase 1/2 trial. Lancet Oncol. 2016;17:883-895. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 901] [Cited by in RCA: 997] [Article Influence: 110.8] [Reference Citation Analysis (0)] |
| 38. | Hellmann MD, Ott PA, Zugazagoitia J, Ready NE, Hann CL, De Braud FG, Antonia SJ, Ascierto PA, Moreno V, Atmaca A, Salvagni S, Taylor MH, Amin A, Camidge DR, Horn L, Calvo E, Cai WG, Fairchild JP, Callahan MK, Spigel DR. Nivolumab (nivo) ± ipilimumab (ipi) in advanced small-cell lung cancer (SCLC): First report of a randomized expansion cohort from CheckMate 032. J Clin Oncol. 2017;35:8503. [RCA] [DOI] [Full Text] [Cited by in Crossref: 58] [Cited by in RCA: 58] [Article Influence: 7.3] [Reference Citation Analysis (0)] |
| 39. | Chung CH, Lopez-Martin JA, Kao SCH, Miller WH, Ros W, Gao B, Marabelle A, Gottfried M, Zer A, Delord JP, Penel N, Jalal SI, Xu L, Zeigenfuss S, Pruitt SK, Piha-Paul SA. Phase 2 study of pembrolizumab in advanced small-cell lung cancer (SCLC): KEYNOTE-158. J Clin Oncol. 2018;36:8506. [RCA] [DOI] [Full Text] [Cited by in Crossref: 110] [Cited by in RCA: 111] [Article Influence: 15.9] [Reference Citation Analysis (0)] |
| 40. | USA National Cancer Institute. Treatment Clinical Trials for Small Cell Lung Cancer. 2019 Sep 18. [cited 20 December 2019]. In: Cancer.gov [Internet]. Washington, DC: National Institute of Health 2019-. [about 3 screens]. Available from: URL: https://www.cancer.gov/about-cancer/treatment/clinical-trials/disease/small-cell-lung-cancer/treatment. |