Published online Dec 24, 2020. doi: 10.5306/wjco.v11.i12.1018
Peer-review started: April 13, 2020
First decision: August 9, 2020
Revised: August 24, 2020
Accepted: October 27, 2020
Article in press: October 27, 2020
Published online: December 24, 2020
Processing time: 249 Days and 1.4 Hours
Forkhead box P3 (FOXP3) is a specific marker for immunosuppressive regulatory T (T-reg) cells. T-regs and an immunosuppressive enzyme, indoleamine 2,3-dioxygenase (IDO), are associated with advanced disease in cancer.
To evaluate the co-expression of FOXP3 and IDO in triple negative breast cancer (TNBC) with respect to hormone-positive breast cancer patients from Pakistan.
Immunohistochemistry was performed to analyze the expression of FOXP3, IDO, estrogen receptor, progesterone receptor, and human epidermal growth factor receptor on tissues of breast cancer patients (n = 100): Hormone-positive breast cancer (n = 51) and TNBC (n = 49). A total of 100 patients were characterized as FOXP3 negative vs positive and further categorized based on low, medium, and high IDO expression score. Univariate and multivariate logistic regression models were used.
Out of 100 breast tumors, 25% expressed FOXP3 positive T-regs. A significant co-expression of FOXP3 and IDO was observed among patients with TNBC (P = 0.01) compared to those with hormone-positive breast cancer. Two variables were identified as significant independent risk factors for FOXP3 positive: IDO expression high (adjusted odds ratio (AOR) 5.90; 95% confidence interval (CI): 1.22-28.64; P = 0.03) and TNBC (AOR 2.80; 95%CI: 0.96-7.95; P = 0.05).
Our data showed that FOXP3 positive cells might be associated with high expression of IDO in TNBC patients. FOXP3 and IDO co-expression may also suggest its involvement in disease, and evaluation of FOXP3 and IDO expression in TNBC patients may offer a new therapeutic option.
Core Tip: Forkhead box P3 (FOXP3) positive cells might be associated with high expression of indoleamine 2,3-dioxygenase (IDO) in triple negative breast cancer (TNBC) patients. Evaluation of FOXP3 and IDO expression in TNBC patients may provide a novel effective therapeutic strategy.
- Citation: Asghar K, Loya A, Rana IA, Bakar MA, Farooq A, Tahseen M, Ishaq M, Masood I, Rashid MU. Forkhead box P3 and indoleamine 2,3-dioxygenase co-expression in Pakistani triple negative breast cancer patients. World J Clin Oncol 2020; 11(12): 1018-1028
- URL: https://www.wjgnet.com/2218-4333/full/v11/i12/1018.htm
- DOI: https://dx.doi.org/10.5306/wjco.v11.i12.1018
Forkhead box P3 (FOXP3) is a part of the forkhead/winged-helix family of transcription regulators[1]. FOXP3 is a specific marker for regulatory T cells (T-regs)[2], which are crucial mediators of peripheral tolerance[3]. FOXP3 expression has been reported in breast cancer[4-6], and its quantification in this malignancy can be used as an effective tool to monitor disease progression and predict prognosis[7]. The cell count of FOXP3 expressing T-regs increases steadily in breast cancer with increasing stage of disease[7]. The mechanisms underlying are still not clear. High numbers of FOXP3 expressing T-regs provide poor prognosis for relapse-free survival in patients with invasive carcinoma[7], but Lee et al[8] observed the prognostic significance of FOXP3-positive T-regs compared to FOXP3-negative T-regs in triple negative breast cancer (TNBC). Furthermore, they found that improved survival was linked with FOXP3-postive T-regs in TNBC. This finding was in contrast with other types of cancers[8]. Therefore, further studies are required to link FOXP3- positive T-regs to good or worse prognosis.
An immunosuppressive enzyme, indoleamine 2,3-dioxygenase (IDO), catabolizes tryptophan into kynurenines[9,10]. IDO has the ability to inhibit the immune responses and produce immunosuppression through the differentiation and maturation of T-regs[11]. On the other hand, tryptophan depletion by IDO affects the cytotoxicity of T cells[12]. It has been reported that tryptophan downstream metabolites induce apoptosis of T cells in vitro[13]. IDO plays a role in the cancer immune-escape mechanism[14,15]. Evidence has suggested that overexpression of IDO has been observed in both antigen-presenting cells and tumor cells in tumor draining lymph nodes[16]. IDO overexpression may lead to recruitment of T-regs in breast tumor microenvironment and promote metastasis[17].
TNBC is characterized by lack of expression of estrogen receptor (ER), progesterone receptor (PR), and human epidermal growth factor receptor (HER2)[18]. TNBC is a more aggressive tumor than other breast cancers types[19]. Our goal was to quantify FOXP3 expression in relation with IDO expression in patients diagnosed with breast cancer from Pakistan. Pakistan has the highest incidence of breast cancer cases in its region. We further investigated the numbers of FOXP3-positive T-regs in TNBC patients compared to hormone-positive breast cancer patients.
For this retrospective analysis, archived formalin-fixed paraffin-embedded (FFPE) blocks of 100 breast cancer patients were retrieved from the pathology department. The study was conducted at Shaukat Khanum Memorial Cancer Hospital and Research Center (SKMCH&RC) Lahore, Pakistan. All the patients were diagnosed with breast cancer between 2007 and 2009, and all patients selected were treatment naïve. Tumor grade was allocated using the Nottingham Histologic Score. Immunohistochemistry was performed to identify the expression of ER, PR, and HER2 by using standard methods[20]. Clinico-pathological data were obtained from medical reports of the patients. The current study was approved by the Institutional Review Board (IRB) of the SKMCH&RC (#IRB-16-08) and was exempted from informed consent in agreement with the Declaration of Helsinki Guidelines. We used the specimens of hospital registered patients. The data were recorded in such a manner that the individual identity could not be recognized. This study does not include any procedures that would normally require informed consent outside the context of the study.
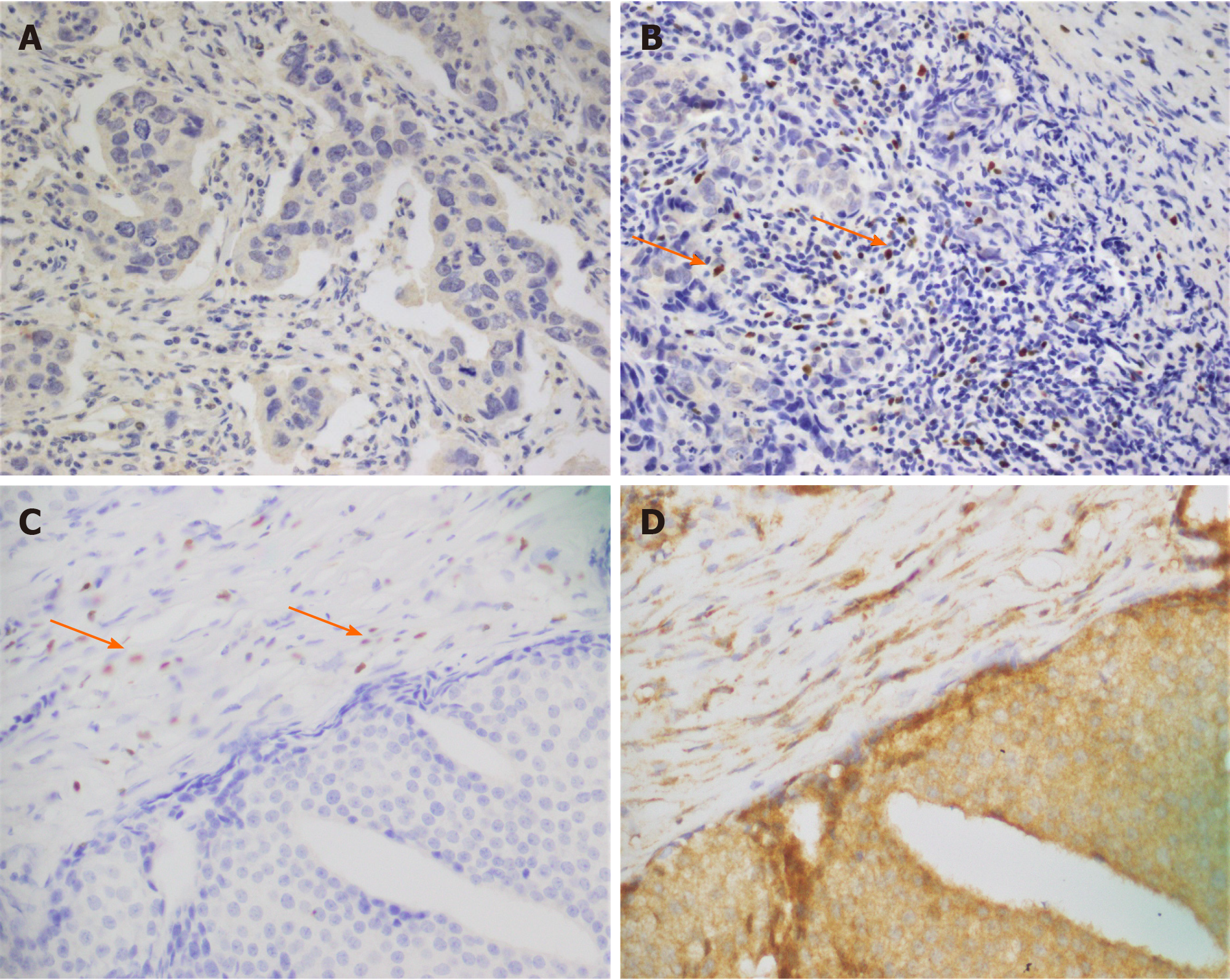
Bond III Leica automated system (Leica Biosystems Melbourne, Australia) was used to perform the immunohistochemistry. Briefly, two sections of FFPE blocks of the same patients were obtained. Bond Dewax solution (#AR922, Leica) was used to deparaffinize the slides. Bond ER-2 (#AR9640, Leica) was used to perform heat induced epitope retrieval on the automated system for 20 min. The primary antibodies FOXP3 (Abcam, #ab22510, Cambridge, United Kingdom) or IDO1 (Abcam, #ab55305) were used at a 1:50 and 1:200 dilution, respectively, in primary antibody diluent and incubated for 5 min. BondTM polymer refine detection kit was used to visualize FOXP3 and IDO labeling. Peroxidase block was applied for 5min. The slides were then incubated with post primary rabbit anti mouse immunoglobulin G for 8 min, followed by incubation with polymer anti-rabbit poly-horseradish peroxidase-immunoglobulin G for 8 min. Three prime-diaminobenzidine tetrahydrochloride hydrate was applied for 10 min. Counterstaining was performed with hematoxylin for 5 min. Two pathologists were involved in the study, and they conducted a blind histopathologic assessment. The discrepancies between the two pathologists were reviewed mutually to reach the consensus. The mean score of both pathologists was considered as the final score. Staining of at least 25% of cells was considered positive for FOXP3. FOXP3 expression had nuclear localization[6]. IDO staining evaluation was based on two factors: (1) Intensity of cytoplasmic staining (0 to 3); and (2) Percentage of cells staining positive (0 to 3). They were categorized as low (1-3), medium (4-6), and high (7-9).
Statistical analysis was carried out using SPSS software (version 20.0; SPSS, Armonk, NY, United States). For continuous variables, mean and standard deviation were used. For categorical variables, percentages (proportions) were used. Chi-square or Fisher exact test was performed for bivariate analysis. Independent t-test was performed for continuous explanatory variables such as age. Risk factors were identified by using the univariable and multivariable logistic regression model.
A total of 100 breast cancer patients were included in this study with an average age of 48 years. Majority of patients belonged to the Punjab region (88%). Fifty-seven percent of tumors were T2/T3, and 7% tumors were T1 (Tumor Node Metastasis classification). According to the grade distribution, 56% presented grade III. Fifty percent of patients were positive for node, and 49% were positive for metastasis. PR (26%), HER2-neu (26%), and ER (31%) were expressed in the tumor tissue (Table 1). We have further categorized baseline characteristics based on TNBC and hormone positive breast cancer in Table 2.
| Variables | Levels | Total, n % |
| Age | mean ± SD | 48.28 ± 11.83 |
| Region | Punjab | 88 (88.0%) |
| Khyber Pakhtunkhwa | 7.0 (7.0%) | |
| Kashmir | 3.0 (3.0%) | |
| Sindh | 2.0 (2.0%) | |
| Histology | Ductal | 91 (91.0%) |
| Others | 9.0 (9.0%) | |
| Grade | II | 35 (35.0%) |
| III | 56 (56.0%) | |
| UNK | 09 (9.0%) | |
| Tumor size | T1 | 7.0 (7.0%) |
| T2/T3 | 57 (57.0%) | |
| UNK | 36 (36.0%) | |
| Nodes | Negative | 37 (37.0%) |
| Positive | 50 (50.0%) | |
| UNK | 13 (13.0%) | |
| Metastasis | Negative | 38 (38.0%) |
| Positive | 49 (49.0%) | |
| UNK | 13 (13.0%) | |
| Estrogen receptor | Negative | 69 (69.0%) |
| Positive | 31 (31.0%) | |
| Progesterone receptor | Negative | 74 (74.0%) |
| Positive | 26 (26.0%) | |
| HER2 status | Negative | 74 (74.0%) |
| Positive | 26 (26.0%) | |
| TNBC | No | 51 (51.0%) |
| Yes | 49 (49.0%) | |
| Status | Alive | 50 (50.0%) |
| Death | 35 (35.0%) | |
| Lost to follow-up | 15 (15.0%) | |
| FOXP3 | Negative | 75 (75.0%) |
| Positive | 25 (25.0%) | |
| IDO score | Low | 24 (24.0%) |
| Medium | 27 (27.0%) | |
| High | 49 (49.0%) |
| Variables | Levels | Triple negative breast cancer, n (%) | Hormone positive breast cancer, n (%) |
| Age | mean ± SD | 47.24 ± 11.5 | 49.27 ± 12.0 |
| Histology | Ductal | 46 (50.5) | 45 (49.5) |
| Others | 3 (33.3) | 6 (66.7) | |
| Total | 49 (49.0) | 51 (51.0) | |
| Grade | II | 9 (25.7) | 26 (74.3) |
| III | 37 (66.1) | 19 (33.9) | |
| UNK | 3 (33.3) | 6 (66.7) | |
| Total | 49 (49.0) | 51 (51.0) | |
| Tumor size | T1 | 4 (57.1) | 3 (42.9) |
| T2/T3 | 26 (45.6) | 31 (54.4) | |
| UNK | 19 (52.8) | 17 (47.2) | |
| Total | 49 (49.0) | 51 (51.0) | |
| Nodes | Negative | 23 (62.1) | 14 (37.8) |
| Positive | 21 (42.0) | 29 (58.0) | |
| UNK | 5 (38.4) | 8 (61.6) | |
| Total | 49 (49.0) | 51 (51.0) | |
| Metastasis | Negative | 23 (60.5) | 15 (39.5) |
| Positive | 21 (42.8) | 28 (57.2) | |
| UNK | 5 (38.4) | 8 (61.6) | |
| Total | 49 (49.0) | 51 (51.0) |
There were 25 out of 100 FOXP3 positive cases (Table 3). Based on immunohistochemistry analysis, FOXP3 expression had nuclear localization. All the cases were invasive ductal carcinoma. Furthermore, 18 out of 25 were TNBC patients. The data of 75 out of 100 FOXP3 negative cases are provided in supplementary data (Supplementary Table 1).
| Case | Histology | Age in yr | Grade | Nodes | Metastasis | ER | PR | HER2 | TNBC |
| 1 | Ductal | 28 | 3 | 0 | - | - | - | - | + |
| 2 | Ductal | 54 | 3 | 14 | + | - | - | - | + |
| 3 | Ductal | 67 | 3 | 1 | + | - | - | - | + |
| 4 | Ductal | 65 | 2 | UNK | UNK | - | - | - | + |
| 5 | Ductal | 45 | 3 | 13 | + | - | - | - | + |
| 6 | Ductal | 45 | 3 | 0 | - | - | - | - | + |
| 7 | Ductal | 55 | 3 | 0 | - | - | - | - | + |
| 8 | Ductal | 23 | 3 | 0 | - | - | - | - | + |
| 9 | Ductal | 47 | 2 | 0 | - | - | - | - | + |
| 10 | Ductal | 73 | 3 | 2 | + | - | - | - | + |
| 11 | Ductal | 35 | 3 | 0 | - | - | - | - | + |
| 12 | Ductal | 35 | 3 | 0 | - | - | - | - | + |
| 13 | Ductal | 52 | 3 | UNK | UNK | - | - | - | + |
| 14 | Ductal | 39 | 3 | 0 | - | - | - | - | + |
| 15 | Ductal | 48 | 3 | 0 | - | - | - | - | + |
| 16 | Ductal | 70 | 3 | 2 | + | - | - | - | + |
| 17 | Ductal | 40 | 3 | 13 | + | - | - | - | + |
| 18 | Ductal | 35 | 3 | 13 | + | - | - | - | + |
| 19 | Ductal | 43 | 3 | 0 | UNK | - | - | + | - |
| 20 | Ductal | 36 | 2 | 1 | + | - | - | + | - |
| 21 | Ductal | 45 | 3 | UNK | UNK | + | + | - | - |
| 22 | Ductal | 71 | 2 | 6 | + | + | - | - | - |
| 23 | Ductal | 30 | 2 | 17 | + | + | + | - | - |
| 24 | Ductal | 40 | 2 | UNK | UNK | - | - | + | - |
| 25 | Ductal | 62 | 2 | 0 | - | + | + | + | - |
In order to validate the immunosuppressive effect of FOXP3 and IDO co-expression, we categorized the patients into TNBC and hormone-positive breast cancer groups. The mean age at diagnosis of FOXP3 positive vs negative breast cancer cases was 47.32 ± 14.19 years and 48.60 ± 11.02 years, respectively (P = 0.64). The majority of patients had grade III tumor (n = 18) and grade II tumor (n = 07). There was a statistically significant association between FOXP3 and high expression of IDO (P = 0.01) and TNBC (P = 0.01), respectively. Remaining explanatory variables are presented in Table 4.
| Variables | Characteristics | FOXP3 negative 75 (75.0%) | FOXP3 positive 25 (25.0%) | P value |
| Age (yr) | mean ± SD | 48.60 ± 11.02 | 47.32 ± 14.20 | 0.64 |
| IDO score | Low | 22 (91.7%) | 2 (8.3%) | 0.01a |
| Medium | 23 (85.2%) | 4 (14.8%) | ||
| High | 30 (61.2%) | 19 (38.8%) | ||
| Grade | II | 28 (80.0%) | 7 (20.0%) | 0.21 |
| III | 38 (67.9%) | 18 (32.1%) | ||
| Metastasis | Negative | 27 (71.1%) | 11 (28.9%) | 0.45 |
| Positive | 39 (79.6%) | 10 (20.4%) | ||
| Tumor size | T1 | 4 (57.1%) | 3 (42.9%) | 0.15 |
| T2/T3 | 46 (80.7%) | 11 (19.3%) | ||
| Lymph nodes involvement | Negative | 26 (70.3%) | 11 (29.7%) | 0.29 |
| Positive | 40 (80.0%) | 10 (20.0%) | ||
| Estrogen receptor | Negative | 48 (69.6%) | 21 (30.4%) | 0.06 |
| Positive | 27 (87.1%) | 4 (12.9%) | ||
| Progesterone receptor | Negative | 52 (70.3%) | 22 (29.7%) | 0.06 |
| Positive | 23 (88.5%) | 3 (11.5%) | ||
| HER2–neu receptor | Negative | 53 (71.6%) | 21 (28.4%) | 0.19 |
| Positive | 22 (84.6%) | 4 (15.4%) | ||
| Triple negative breast cancer | No | 44 (86.3%) | 7 (13.7%) | 0.01a |
| Yes | 31 (63.3%) | 18 (36.7%) |
To evaluate the expression of FOXP3 and IDO, we selected FFPE tumor specimens of the same patients (n = 100). Out of 100 patients, 25 expressed FOXP3-positive T-regs, and 75 expressed FOXP3-negative T-regs (Figure 1). IDO positivity was found in all breast tumor specimens. Synchronal expression of FOXP3 and IDO is shown in Figure 1. Immunostaining of low, medium, and high IDO expression is provided in supplementary data (Figure 1).
Table 5 summarizes the several clinicopathological features that were included in unadjusted and adjusted logistic regression model to identify the FOXP3 correlation with IDO expression and TNBC. Two variables were identified as significant independent risk factors for FOXP3 positive: IDO expression high [adjusted odds ratio (AOR) 5.90; 95% confidence interval (CI): 1.22-28.64; P = 0.03) and TNBC (AOR 2.80; 95%CI: 0.96-7.95, P = 0.05) in multivariable analysis.
| Variables | Characteristics | Univariable analysis odds ratio (95%CI), P value | Multivariable analysis odds ratio (95%CI), P value |
| IDO score | Low | Ref. | Ref. |
| Medium | 1.91 (0.32-11.52), 0.50 | 2.32 (0.37-14.50), 0.37 | |
| High | 6.97 (1.50-33.10), 0.01a | 5.90 (1.22-28.64), 0.03a | |
| Triple negative breast cancer | No | Ref. | Ref. |
| Yes | 3.65 (1.36-9.80), 0.01a | 2.80 (0.96-7.95), 0.05a |
The role of immunosuppression in cancer progression is currently evaluated in various cancers[21-23]. It has been established that immunological factors such as T-regs are involved in the progression of tumor through induction of immune tolerance in the tumor microenvironment[7,22]. T-regs are effective inhibitors of the immune system[22]. T-regs create immunosuppressive environment by suppressing effector immune cells[22]. They are also associated with poor clinical outcomes in various tumors[4,7]. FOXP3 is a specified marker for T-regs[2]. Several studies identified that FOXP3+ T-regs infiltration in tumor microenvironment may affect breast cancer progression[7,24]. Bates et al[7] demonstrated that a high ratio of FOXP3 cells predict worse relapse-free survival and shorten overall survival in patients with invasive breast carcinoma[7]. In another study the researchers observed no difference in overall survival among patients expressing high or low FOXP3[25]. There is contradictory data regarding the involvement of FOXP3+ T-regs in breast cancer patients. Nevertheless, we investigated FOXP3 positive vs negative expression in the current study. FOXP3 expression was identified in 25 breast cancer patients, and a majority of these patients displayed TNBC phenotype. Overall, 36.73% of TNBC patients expressed FOXP3 positive cells, while 13.72% of hormone positive breast cancer patients expressed FOXP3 positive cells. On the other hand, FOXP3 expression was not detected in 63.26% of TNBC patients and 86.27% of hormone positive breast cancer patients. Our findings of FOXP3 T-regs infiltration in TNBC patients is similar to several studies published before that identified the involvement of FOXP3 positive cells in breast cancer progression[7,24].
FOXP3+ T-regs can restrain effector T cells by an IDO dependent mechanism[9]. IDO plays a critical role in the pathogenesis of breast cancer[26]. IDO overexpression is linked with shorter overall survival and poor prognosis[27-34]. FOXP3+ T-regs have prognostic implications in TNBC[8]. IDO expression is also associated with TNBC[26]. Previously we showed high IDO expression in TNBC patients from Pakistan[35]. The aim of our current study was to identify the substantial association between FOXP3-positive T-regs and IDO in TNBC patients. There was a statistically significant association of FOXP3 with high IDO expression (P = 0.01) and TNBC (P = 0.01) respectively. Two variables were recognized as significant independent risk factors for FOXP3 positive: IDO expression high (AOR 5.90; 95%CI: 1.22-28.64; P = 0.03) and TNBC (AOR 2.80; 95%CI: 0.96-7.95; P = 0.05) in multivariable analysis. Although several studies focus on the role of immunosuppression in TNBC, our data provide some insight regarding immunosuppression in association with simultaneous expression of FOXP3 and IDO in TNBC patients.
Our study has some limitations, which have to be mentioned. The study population (n = 100) did not permit us to draw any strong conclusion. Forthcoming projects on breast cancer patients from Pakistan with inclusive cohort studies are required to authenticate conclusive associations.
Identification of an appropriate immunotherapeutic target for TNBC is currently a hot-topic. FOXP3 and IDO co-expression has the ability to inhibit anti-tumor immune responses and may be considered one of the hurdles in the development of successful immunotherapy for cancer. The role of FOXP3 and IDO co-expression is still a subject of rigorous research in breast cancer.
In conclusion, the current data revealed that FOXP3 positive cells might be associated with high IDO expression in TNBC patients. FOXP3 and IDO expression monitoring in TNBC patients may provide an effective therapeutic strategy.
Forkhead box P3 (FOXP3) and indoleamine 2,3-dioxygenase (IDO) are associated with advanced disease in cancer (e.g., breast cancer).
To quantify FOXP3 expression in relation with IDO expression in patients diagnosed with breast cancer from Pakistan.
Our objective was to identify the co-expression of FOXP3 and IDO in triple negative breast cancer (TNBC) patients.
Immunohistochemistry was performed to analyze the expression of FOXP3, IDO, estrogen receptor, progesterone receptor, and human epidermal growth factor receptor in human breast cancer tissues.
A significant association of FOXP3 and IDO co-expression was observed among patients with TNBC (P = 0.01).
FOXP3 positive cells might be associated with high expression of IDO in TNBC patients.
Evaluation of FOXP3 and IDO expression in TNBC patients may be implemented in the future as a therapeutic strategy.
Manuscript source: Unsolicited manuscript
Specialty type: Oncology
Country/Territory of origin: Pakistan
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): 0
Grade C (Good): C, C
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Scaggiante B S-Editor: Fan JR L-Editor: Filipodia P-Editor: Wang LL
| 1. | Coffer PJ, Burgering BM. Forkhead-box transcription factors and their role in the immune system. Nat Rev Immunol. 2004;4:889-899. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 274] [Cited by in RCA: 304] [Article Influence: 14.5] [Reference Citation Analysis (0)] |
| 2. | Curiel TJ. Regulatory T cells and treatment of cancer. Curr Opin Immunol. 2008;20:241-246. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 203] [Cited by in RCA: 200] [Article Influence: 11.8] [Reference Citation Analysis (0)] |
| 3. | Watanabe MA, Oda JM, Amarante MK, Cesar Voltarelli J. Regulatory T cells and breast cancer: implications for immunopathogenesis. Cancer Metastasis Rev. 2010;29:569-579. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 83] [Cited by in RCA: 102] [Article Influence: 7.3] [Reference Citation Analysis (0)] |
| 4. | Ladoire S, Arnould L, Mignot G, Coudert B, Rébé C, Chalmin F, Vincent J, Bruchard M, Chauffert B, Martin F, Fumoleau P, Ghiringhelli F. Presence of Foxp3 expression in tumor cells predicts better survival in HER2-overexpressing breast cancer patients treated with neoadjuvant chemotherapy. Breast Cancer Res Treat. 2011;125:65-72. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 95] [Cited by in RCA: 105] [Article Influence: 7.0] [Reference Citation Analysis (0)] |
| 5. | Zuo T, Wang L, Morrison C, Chang X, Zhang H, Li W, Liu Y, Wang Y, Liu X, Chan MW, Liu JQ, Love R, Liu CG, Godfrey V, Shen R, Huang TH, Yang T, Park BK, Wang CY, Zheng P, Liu Y. FOXP3 is an X-linked breast cancer suppressor gene and an important repressor of the HER-2/ErbB2 oncogene. Cell. 2007;129:1275-1286. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 315] [Cited by in RCA: 322] [Article Influence: 17.9] [Reference Citation Analysis (0)] |
| 6. | Merlo A, Casalini P, Carcangiu ML, Malventano C, Triulzi T, Mènard S, Tagliabue E, Balsari A. FOXP3 expression and overall survival in breast cancer. J Clin Oncol. 2009;27:1746-1752. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 207] [Cited by in RCA: 232] [Article Influence: 14.5] [Reference Citation Analysis (0)] |
| 7. | Bates GJ, Fox SB, Han C, Leek RD, Garcia JF, Harris AL, Banham AH. Quantification of regulatory T cells enables the identification of high-risk breast cancer patients and those at risk of late relapse. J Clin Oncol. 2006;24:5373-5380. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 968] [Cited by in RCA: 923] [Article Influence: 48.6] [Reference Citation Analysis (0)] |
| 8. | Lee S, Cho EY, Park YH, Ahn JS, Im YH. Prognostic impact of FOXP3 expression in triple-negative breast cancer. Acta Oncol. 2013;52:73-81. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 58] [Cited by in RCA: 70] [Article Influence: 5.8] [Reference Citation Analysis (0)] |
| 9. | Mansfield AS, Heikkila PS, Vaara AT, von Smitten KA, Vakkila JM, Leidenius MH. Simultaneous Foxp3 and IDO expression is associated with sentinel lymph node metastases in breast cancer. BMC Cancer. 2009;9:231. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 80] [Cited by in RCA: 87] [Article Influence: 5.4] [Reference Citation Analysis (0)] |
| 10. | Muller AJ, DuHadaway JB, Donover PS, Sutanto-Ward E, Prendergast GC. Inhibition of indoleamine 2,3-dioxygenase, an immunoregulatory target of the cancer suppression gene Bin1, potentiates cancer chemotherapy. Nat Med. 2005;11:312-319. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 808] [Cited by in RCA: 854] [Article Influence: 42.7] [Reference Citation Analysis (0)] |
| 11. | Mellor AL, Munn DH. Tryptophan catabolism and T-cell tolerance: immunosuppression by starvation? Immunol Today. 1999;20:469-473. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 485] [Cited by in RCA: 468] [Article Influence: 18.0] [Reference Citation Analysis (0)] |
| 12. | Lee GK, Park HJ, Macleod M, Chandler P, Munn DH, Mellor AL. Tryptophan deprivation sensitizes activated T cells to apoptosis prior to cell division. Immunology. 2002;107:452-460. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 292] [Cited by in RCA: 314] [Article Influence: 13.7] [Reference Citation Analysis (0)] |
| 13. | Munn DH, Shafizadeh E, Attwood JT, Bondarev I, Pashine A, Mellor AL. Inhibition of T cell proliferation by macrophage tryptophan catabolism. J Exp Med. 1999;189:1363-1372. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1172] [Cited by in RCA: 1249] [Article Influence: 48.0] [Reference Citation Analysis (0)] |
| 14. | Mellor AL, Munn DH. IDO expression by dendritic cells: tolerance and tryptophan catabolism. Nat Rev Immunol. 2004;4:762-774. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1666] [Cited by in RCA: 1770] [Article Influence: 84.3] [Reference Citation Analysis (0)] |
| 15. | Grohmann U, Fallarino F, Puccetti P. Tolerance, DCs and tryptophan: much ado about IDO. Trends Immunol. 2003;24:242-248. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 570] [Cited by in RCA: 562] [Article Influence: 25.5] [Reference Citation Analysis (0)] |
| 16. | Katz JB, Muller AJ, Prendergast GC. Indoleamine 2,3-dioxygenase in T-cell tolerance and tumoral immune escape. Immunol Rev. 2008;222:206-221. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 300] [Cited by in RCA: 316] [Article Influence: 19.8] [Reference Citation Analysis (0)] |
| 17. | Yu J, Sun J, Wang SE, Li H, Cao S, Cong Y, Liu J, Ren X. Upregulated expression of indoleamine 2, 3-dioxygenase in primary breast cancer correlates with increase of infiltrated regulatory T cells in situ and lymph node metastasis. Clin Dev Immunol. 2011;2011:469135. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 47] [Cited by in RCA: 62] [Article Influence: 4.4] [Reference Citation Analysis (0)] |
| 18. | Curigliano G, Goldhirsch A. The triple-negative subtype: new ideas for the poorest prognosis breast cancer. J Natl Cancer Inst Monogr. 2011;2011:108-110. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 41] [Cited by in RCA: 42] [Article Influence: 3.2] [Reference Citation Analysis (0)] |
| 19. | Penault-Llorca F, Viale G. Pathological and molecular diagnosis of triple-negative breast cancer: a clinical perspective. Ann Oncol. 2012;23 Suppl 6:vi19-vi22. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 77] [Cited by in RCA: 110] [Article Influence: 9.2] [Reference Citation Analysis (0)] |
| 20. | Chen X, Cho DB, Yang PC. Double staining immunohistochemistry. N Am J Med Sci. 2010;2:241-245. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 29] [Reference Citation Analysis (0)] |
| 21. | Disis ML, Lyerly HK. Global role of the immune system in identifying cancer initiation and limiting disease progression. J Clin Oncol. 2005;23:8923-8925. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 15] [Cited by in RCA: 13] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 22. | Kalathil SG, Thanavala Y. High immunosuppressive burden in cancer patients: a major hurdle for cancer immunotherapy. Cancer Immunol Immunother. 2016;65:813-819. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 43] [Cited by in RCA: 59] [Article Influence: 6.6] [Reference Citation Analysis (0)] |
| 23. | Penn I, Starzl TE. Immunosuppression and cancer. Transplant Proc. 1973;5:943-947. [PubMed] |
| 24. | Ladoire S, Arnould L, Apetoh L, Coudert B, Martin F, Chauffert B, Fumoleau P, Ghiringhelli F. Pathologic complete response to neoadjuvant chemotherapy of breast carcinoma is associated with the disappearance of tumor-infiltrating foxp3+ regulatory T cells. Clin Cancer Res. 2008;14:2413-2420. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 233] [Cited by in RCA: 244] [Article Influence: 14.4] [Reference Citation Analysis (0)] |
| 25. | Wolf AM, Rumpold H, Wolf D, Gastl G, Reimer D, Jenewein N, Marth C, Zeimet AG. Role of forkhead box protein 3 expression in invasive breast cancer. J Clin Oncol. 2007;25:4499-500; author reply 4500. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 19] [Cited by in RCA: 19] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 26. | Kim S, Park S, Cho MS, Lim W, Moon BI, Sung SH. Strong Correlation of Indoleamine 2,3-Dioxygenase 1 Expression with Basal-Like Phenotype and Increased Lymphocytic Infiltration in Triple-Negative Breast Cancer. J Cancer. 2017;8:124-130. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 26] [Cited by in RCA: 27] [Article Influence: 3.4] [Reference Citation Analysis (0)] |
| 27. | Brandacher G, Perathoner A, Ladurner R, Schneeberger S, Obrist P, Winkler C, Werner ER, Werner-Felmayer G, Weiss HG, Göbel G, Margreiter R, Königsrainer A, Fuchs D, Amberger A. Prognostic value of indoleamine 2,3-dioxygenase expression in colorectal cancer: effect on tumor-infiltrating T cells. Clin Cancer Res. 2006;12:1144-1151. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 438] [Cited by in RCA: 481] [Article Influence: 25.3] [Reference Citation Analysis (0)] |
| 28. | Okamoto A, Nikaido T, Ochiai K, Takakura S, Saito M, Aoki Y, Ishii N, Yanaihara N, Yamada K, Takikawa O, Kawaguchi R, Isonishi S, Tanaka T, Urashima M. Indoleamine 2,3-dioxygenase serves as a marker of poor prognosis in gene expression profiles of serous ovarian cancer cells. Clin Cancer Res. 2005;11:6030-6039. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 284] [Cited by in RCA: 312] [Article Influence: 15.6] [Reference Citation Analysis (0)] |
| 29. | Ino K, Yoshida N, Kajiyama H, Shibata K, Yamamoto E, Kidokoro K, Takahashi N, Terauchi M, Nawa A, Nomura S, Nagasaka T, Takikawa O, Kikkawa F. Indoleamine 2,3-dioxygenase is a novel prognostic indicator for endometrial cancer. Br J Cancer. 2006;95:1555-1561. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 176] [Cited by in RCA: 203] [Article Influence: 10.7] [Reference Citation Analysis (0)] |
| 30. | Huang A, Fuchs D, Widner B, Glover C, Henderson DC, Allen-Mersh TG. Serum tryptophan decrease correlates with immune activation and impaired quality of life in colorectal cancer. Br J Cancer. 2002;86:1691-1696. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 171] [Cited by in RCA: 176] [Article Influence: 7.7] [Reference Citation Analysis (0)] |
| 31. | Ino K, Yamamoto E, Shibata K, Kajiyama H, Yoshida N, Terauchi M, Nawa A, Nagasaka T, Takikawa O, Kikkawa F. Inverse correlation between tumoral indoleamine 2,3-dioxygenase expression and tumor-infiltrating lymphocytes in endometrial cancer: its association with disease progression and survival. Clin Cancer Res. 2008;14:2310-2317. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 153] [Cited by in RCA: 175] [Article Influence: 10.3] [Reference Citation Analysis (0)] |
| 32. | Astigiano S, Morandi B, Costa R, Mastracci L, D'Agostino A, Ratto GB, Melioli G, Frumento G. Eosinophil granulocytes account for indoleamine 2,3-dioxygenase-mediated immune escape in human non-small cell lung cancer. Neoplasia. 2005;7:390-396. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 120] [Cited by in RCA: 121] [Article Influence: 6.1] [Reference Citation Analysis (0)] |
| 33. | Weinlich G, Murr C, Richardsen L, Winkler C, Fuchs D. Decreased serum tryptophan concentration predicts poor prognosis in malignant melanoma patients. Dermatology. 2007;214:8-14. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 130] [Cited by in RCA: 141] [Article Influence: 7.8] [Reference Citation Analysis (0)] |
| 34. | Takao M, Okamoto A, Nikaido T, Urashima M, Takakura S, Saito M, Saito M, Okamoto S, Takikawa O, Sasaki H, Yasuda M, Ochiai K, Tanaka T. Increased synthesis of indoleamine-2,3-dioxygenase protein is positively associated with impaired survival in patients with serous-type, but not with other types of, ovarian cancer. Oncol Rep. 2007;17:1333-1339. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 11] [Cited by in RCA: 12] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 35. | Asghar K, Loya A, Rana IA, Tahseen M, Ishaq M, Farooq A, Bakar MA, Masood I. Indoleamine 2,3-dioxygenase expression and overall survival in patients diagnosed with breast cancer in Pakistan. Cancer Manag Res. 2019;11:475-481. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 17] [Cited by in RCA: 29] [Article Influence: 4.8] [Reference Citation Analysis (0)] |