Published online Nov 24, 2020. doi: 10.5306/wjco.v11.i11.935
Peer-review started: April 23, 2020
First decision: June 15, 2020
Revised: September 16, 2020
Accepted: October 5, 2020
Article in press: October 5, 2020
Published online: November 24, 2020
Processing time: 208 Days and 20.7 Hours
Female breast cancer is the leading type of cancer worldwide with an incidence of approximately 2.1 million in 2018. Hormone receptor status plays a vital role in its management.
To determine the molecular expression pattern of biomarkers in breast cancer and their correlation with tumor variables.
This prospective study was designed to analyze expression patterns of estrogen receptor(ER), progesterone receptor (PR) and human epidermal growth factor receptor(HER2/neu) in breast cancer patients. The dataset has been taken from the Department of Radiotherapy and Oncology of Usmanu Danfodiyo University Teaching Hospital Sokoto, Nigeria from 1 January 2015 to 2 December 2019. The dataset had 259 records and 7 attributes. SPSS version 23.0 for statistical analysis was used. The data analyzed demographic and other clinicopathological characteristics as categorical variables. The mean and standard deviation were determined for the quantitative variable.
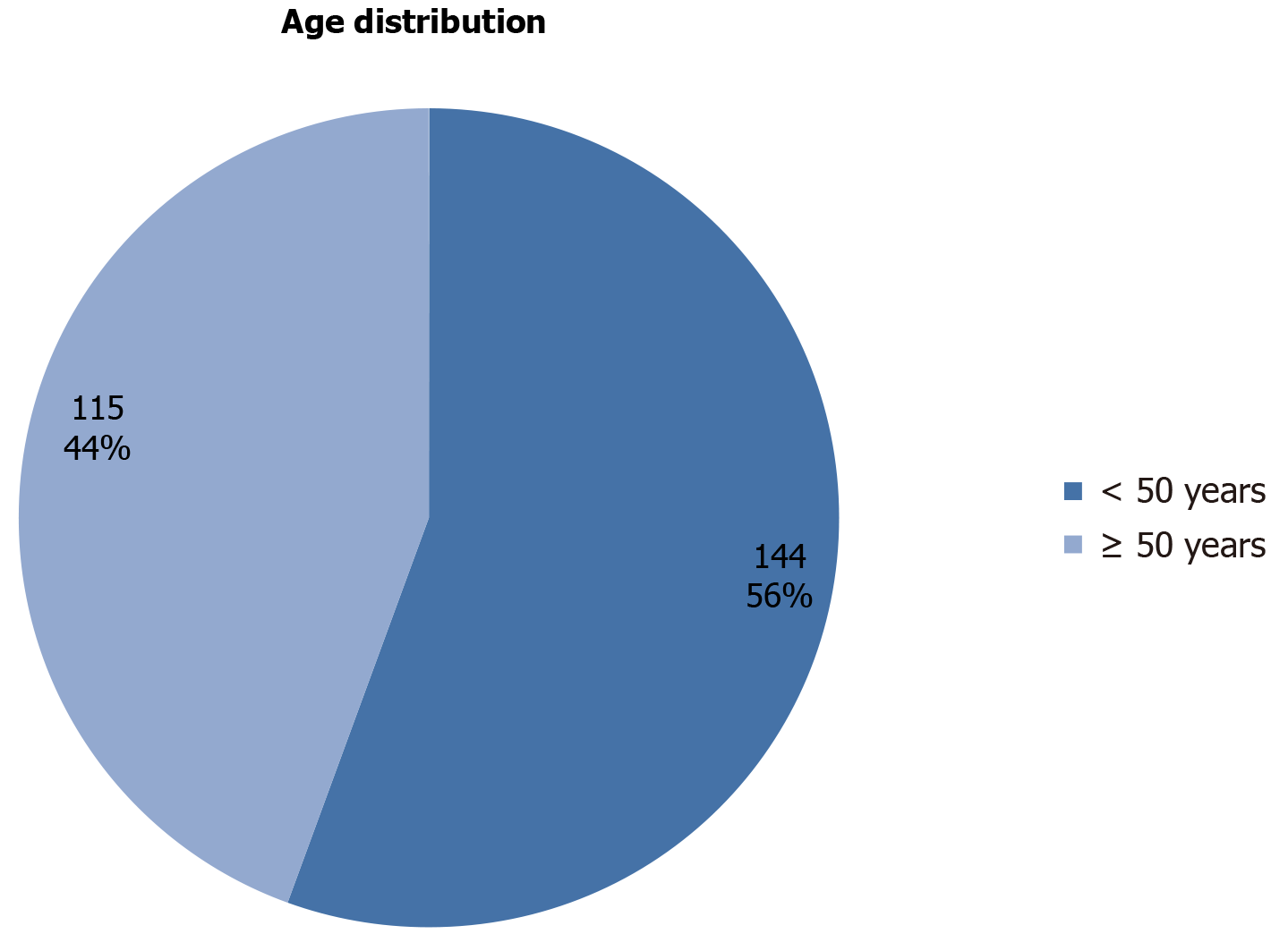
A total of 259 breast cancer cases were included in the study. The mean age was 48.3 ± 11.0, with an age range of 26-80 years and a median age of 46 years. The morphological categories were invasive ductal carcinoma 258 (99.6%) and invasive lobular carcinoma 1 (0.4%). ER, positivity increased in 73 patients (50%) under the age of 50 years, as well as PR positivity increased in 34 patients (23.6%) under the age of 50 years. HER/2neupositivity decreased in 8 patients (5.6%) under the age of 50 years. Hormonal receptors were statistically significant with clinicopathological characteristics (P < 0.05).
Our study showed that ER, PR and HER2/neuexpression had a strong correlation with age, tumor grade, tumor size and lymph node status. Hence, hormone receptor assessment is highly recommended because of its significance in clinical management and prognostication.
Core Tip: Breast cancer is the most common malignancy in Nigeria as it is in the whole world. Assessment of immunohistochemical profile and its tumor characteristic is paramount to the management of this disease entity. The paucity of literature on breast cancer biomarkers in sub-Saharan Africa (e.g., Nigeria) has contributed to the increase in morbidity and mortality recorded in the region.
- Citation: Aliyu UM, Musa AA. Assessment of breast cancer immunohistochemistry and tumor characteristics in Nigeria. World J Clin Oncol 2020; 11(11): 935-944
- URL: https://www.wjgnet.com/2218-4333/full/v11/i11/935.htm
- DOI: https://dx.doi.org/10.5306/wjco.v11.i11.935
Female breast cancer is the most common malignancy worldwide, with over two million cases diagnosed in 2018[1]. Prognostic indicators are features that are unrelated to adjuvant therapy that determines the outcome of disease, while predictive indicators are related to response to therapy[2]. The treatment and outcome of this disease entity vary depending on the biological characteristics. Hence, modalities for treatment employ those features to individualized breast cancer therapy[3]. These characteristics can also determine the prognosis and outcome[2]. In the last three decades, tremendous progress has been made in the biological characterization of breast cancers with the advent of immunohistochemistry and immunophenotyping. Various biomarkers such as hormone receptors, vascular endothelial growth factors, epithermal growth factor, tumor suppressor genes, multidrug resistant genes and adhesion molecules have been identified[2,4].
Currently, determination of estrogen receptor (ER), progesterone receptor (PR) and human epidermal receptor growth factor 2 (HER2/neu) receptor is routine in the diagnosis of breast cancer[5,6]. The International Breast Cancer Study Group classified breast cancer into three[6,7] categories based on the proportion of cells that are receptor positive [responsive (10%), response uncertain (1%-9%) and nonresponsive (0%)]. At least 1% positivity is necessary for commencement of hormone therapy. Molecular assays give an insight into the current status of the tumor, which often differs from the primary when it metastasizes. A comparative study involving 658 and 418 of ER/PR samples showed a dissonant rate of 29% and 27%, respectively, for primary and metastatic tumors[3,8].
ER is a biomarker found in over 65% of invasive breast cancer and contributes significantly to its pathobiology. ER positivity makes it responsive to hormonal therapy, resulting in a more favorable outcome. PR, like ER, is also a transcription factor, which is largely controlled by ER and to a lesser extent by growth factors. About 55% to 65% of invasive breast cancers show PR positivity. PR commonly coexists with ER. Both receptors require at least 1% nuclear staining to be considered positive according to the American Society of Clinical Oncology/College of American Pathologists guidelines[11,12]. Allred scoring and H-scoring are two other commonly used systems for ER and PR evaluations[9-11].
HER2/neu has the potential of enhancing proliferation and survival of tumor cells. Hence its overexpression, which occurs in about 12%-20% of inflammatory breast cancer, results in a more aggressive growth and poor response to treatment. According to the American Society of Clinical Oncology/College of American Pathologists guidelines, membrane staining of 10% or more is considered positive and an indication of a favorable response to anti-HER2/neu therapy[11,13]. HER2/neu can be evaluated either by immunohistochemistry or fluorescence in situ hybridization. Although there are many ways to test for this receptor, the above two are the only approved methods[14-16].
This study aimed to determine the molecular expression of biomarkers in breast cancer in Sokoto, Nigeria and their correlation with tumor variables.
This prospective study was designed to analyze expression patterns of ER, PR and HER2/neu in breast cancer patients. The dataset was taken from the Department of Radiotherapy and Oncology of Usmanu Danfodiyo University Teaching Hospital Sokoto, Nigeria from 1 January 2015 to 2 December 2019. The dataset had 259 records and 7 attributes. The data analyzed demographic and other clinicopathological characteristics as categorical variables. The mean and standard deviation were determined for the quantitative variable. All variables were coded as binary dummy variables. For example tumor site (right breast = 1, left breast = 2). Patients were grouped according to age < 50 years and ≥ 50 years. Histological grading stratification of tumor size was performed in three groups (< 2 cm, 2-5 cm and > 5 cm), and lymph nodes were grouped as 1-3 and ≥ 4 based on tumor node metastasis staging for breast carcinoma.
Data were presented as charts, and frequency distribution was generated for all categorical variables. Descriptive and inferential statistics [chi-squared, Fisher exact test, bi-variate correlation (Kendall’s test) and multivariate logistic regression analysis] were used to explore association, the strength of the association and odds ratio (OR) or relative risk between clinicopathological variables and biomarkers expressed (ER, PR and HER2/neu). P ≤ 0.05 was considered statistically significant. SPSS version 23.0 (Chicago, IL, United States) was used.
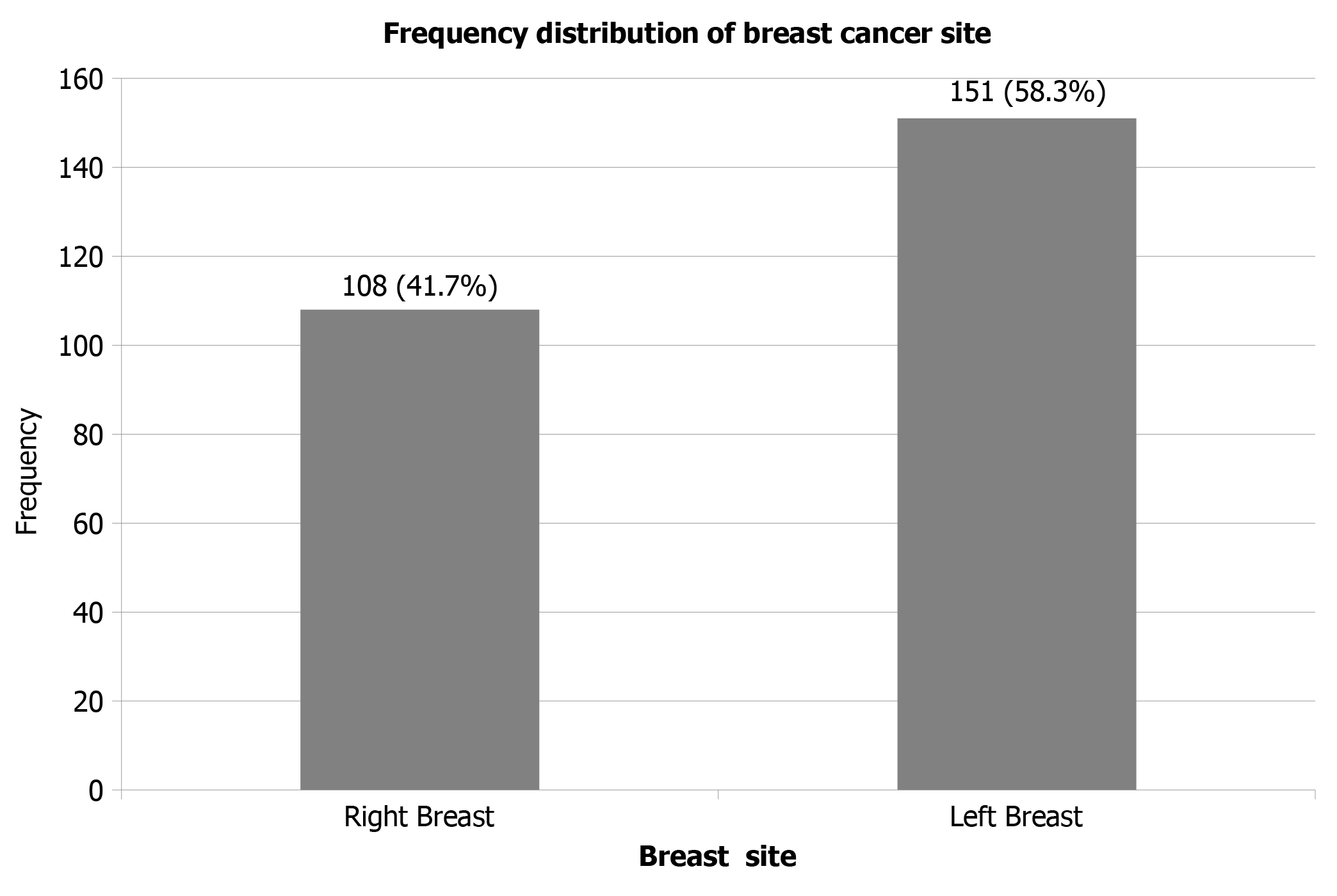
Two hundred and fifty-nine subjects that fulfilled the inclusion criteria were recruited into the study. The mean age was 48.3 ± 11.0. The ages ranged between 26 and 80 years, and the median age was 46 years. Most of the patients 144 (55.6%) were less than 50 years at diagnosis (Figure 1). The left side was the predominant site of breast cancer 151 (58.3%) (Figure 2). The morphological categories were invasive ductal carcinoma (258 patients, 99.6%) and invasive lobular carcinoma (1 patient, 0.4%). Sixty-two (23.9%) cases were grade I, 131 (50.6%) were grade II and 66 (25.5%) were grade III. Tumor sizes were stratified into three categories: 62 patients (23.9%) were < 2.0 cm, 131 patients (50.6%) were 2-5 cm and 66 patients (25.5%) were > 5.0 cm (Table 1).
| Histologic type | Frequency | Percentage |
| Invasive ductal carcinoma | 258 | 99.6 |
| Invasive lobular carcinoma | 1 | 0.4 |
| Grading | ||
| I | 62 | 23.9 |
| II | 131 | 50.6 |
| III | 66 | 25.5 |
| Tumour size | ||
| < 2.0 cm | 62 | 23.9 |
| 2-5 cm | 131 | 50.6 |
| > 5.0 cm | 66 | 25.5 |
| Lymph node involvement | ||
| No | 22 | 8.5 |
| 1-3 | 196 | 75.7 |
| ≥ 4 | 41 | 15.8 |
| Hormonal receptors | ||
| ER + ve | 113 | 43.6 |
| ER - ve | 52 | 20.1 |
| PR + ve | 42 | 16.2 |
| PR - ve | 12 | 4.6 |
| HER/2 neu + ve | 23 | 8.9 |
| HER/2 neu - ve | 17 | 6.6 |
| Total | 259 | 100 |
ER positivity increased in 73 patients (50%) under the age of 50 years, and PR positivity increased in 34 patients (23.6%) under the age of 50 years. HER2/neu positivity decreased in 8 patients (5.6%) under the age of 50 years (P < 0.01). ER positivity was found in 35 patients (56.5%), 55 patients (42.0%) and 23 patients (34.8%) with grade I, II and III carcinomas, respectively. PR positivity was found in 3 patients (3.2%), 29 patients (22.1%) and 10 patients (15.2%) with grade I, II and III carcinomas, respectively. HER2/neu positivity was found 3 patients (4.8%), 13 patients (9.9%) and 7 patients(10.6%) with grade I, II and III carcinomas, respectively. ER and PR positivity were much less in high-grade tumors than in those with intermediate grade (ER 34.8% vs 42%; PR 15.2% vs 22.1%).
The patients were categorized into three groups based on tumor size: Group 1 (tumor size ≤ 2 cm); group 2 (2-5 cm); and group 3 (≥ 5 cm). The total number of group 1 tumors was 62 [35 ER positive (56.5%); 3 PR positive (4.8%); and 3 HER2/neu positive (4.8%)]. The total number of group 2 tumors was 131 [55 ER positive (42.0%); 29 PR positive (22.1%); and 13 HER2/neu positive (9.9%)]. The total number of group 3 tumors was 66 [23 ER positive (34.8%); 10 PR positive (15.2%); and 7 HER2/neu positive (10.6%)]. ER expression in the HER2/neu positive, large-sized tumors was significantly decreased compared with smaller tumors (P < 0.05). Metastasis in axillary lymph nodes was seen in 237 (91.5%) patients. Among the ER positive cases, 86 (43.9%) had axillary lymph node involvement ranging from 1-3. Among the PR positive cases, 37 (17.3%) had positive axillary lymph nodes positive for metastasis. Nineteen (9.7%) participants with HER2/neu positive disease were positive for axillary lymph nodes metastasis. Tumors with axillary lymph node metastasis showed significant association with ER, PR and HER2/neu (P < 0.03) (Table 2).
| Age | Hormonal receptors | Total | P value | |||||
| ER + ve | ER - ve | PR + ve | PR - ve | HER/2 neu + ve | HER/2 neu - ve | |||
| < 50 | 73 (50.70%) | 17 (11.80%) | 34 (23.60%) | 9 (6.30%) | 8 (5.60%) | 3 (2.10%) | 144 | 0.01 |
| ≥ 50 | 40 (34.80%) | 35 (30.40%) | 8 (7.00%) | 3 (2.60%) | 15 (13.00%) | 14 (12.20%) | 115 | |
| Grading | ||||||||
| I | 35 (56.50%) | 18 (29.00%) | 3 (4.80%) | 2 (3.20%) | 3 (4.80%) | 1 (1.60%) | 62 | 0.02 |
| II | 55 (42.00%) | 23 (17.60%) | 29 (22.10%) | 5 (3.80%) | 13 (9.90%) | 6 (4.60%) | 131 | |
| III | 23 (34.80%) | 11 (16.70%) | 10 (15.20%) | 5 (7.60%) | 7 (10.60%) | 10 (15.20%) | 66 | |
| Tumour size | ||||||||
| < 2.0 cm | 35 (56.50%) | 18 (29.00%) | 3 (4.80%) | 2 (3.20%) | 3 (4.80%) | 1 (1.60%) | 62 | 0.02 |
| 2-5 cm | 55 (42.00%) | 23 (17.60%) | 29 (22.10%) | 5 (3.80%) | 13 (9.90%) | 6 (4.60%) | 131 | |
| > 5.0 cm | 23 (34.80%) | 11 (16.70%) | 10 (15.20%) | 5 (7.60%) | 7 (10.60%) | 10 (15.20%) | 66 | |
| Lymph node involvement | ||||||||
| No | 11 (50.00%) | 3 (13.60%) | 3 (13.60%) | 2 (9.10%) | 2 (9.10%) | 1 (4.50%) | 22 | 0.03 |
| 1-3 | 86 (43.90%) | 43 (21.90%) | 34 (17.30%) | 6 (3.10%) | 19 (9.70%) | 8 (4.10%) | 196 | |
| ≥ 4 | 16 (39.00%) | 6 (14.60%) | 5 (12.20%) | 4 (9.80%) | 2 (4.90%) | 8 (19.50%) | 41 | |
Age was significantly correlated with ER, PR and HER2/neu (P < 0.05). However, there was no significant correlation in PR negative disease (P > 0.05). We found that ER and HER2/neu expression compared to tumor grade was statistically significant (P < 0.05) while PR expression was not significant (P > 0.05) (Table 3).
| Clinic pathologic feature | Estimators | Hormonal receptors | |||||
| ER + ve | ER - ve | PR + ve | PR - ve | Her-2 + ve | Her-2 - ve | ||
| Age | R | -0.159a | 0.231b | -0.224b | -0.086 | 0.131a | 0.202b |
| P value | 0.01 | 0.01 | 0.01 | 0.167 | 0.036 | 0.001 | |
| Grading | R | -0.144b | -0.101 | 0.089 | 0.07 | 0.067 | 0.184b |
| P value | 0.015 | 0.087 | 0.133 | 0.236 | 0.26 | 0.002 | |
| Tumour size | R | -0.144a | -0.101 | 0.089 | 0.07 | 0.067 | 0.184b |
| P value | 0.015 | 0.087 | 0.133 | 0.236 | 0.26 | 0.002 | |
| Lymph node | R | -0.051 | -0.018 | -0.024 | 0.045 | -0.047 | 0.184b |
| P value | 0.401 | 0.761 | 0.689 | 0.455 | 0.438 | 0.002 | |
| Total | 259 | 259 | 259 | 259 | 259 | 259 | |
This study also found that women younger than 50 years are likely to present with ER positive disease (P = 0.01, OR = 8.517, 95% confidence interval: 2.309-31.413) and PR negative disease (P = 0.04, OR = 14.00, 95% confidence interval: 2.300-85.218). Similarly, most of the tumors that presented with grade I disease were 15 times more likely to be ER positive than those in grade II or III (P = 0.01, OR 15.2, 95% confidence interval 1.296-12.252) (Table 4 and 5).
| Hormonal receptors | P value | OR | 95% confidence interval | ||
| Lower bound | Upper bound | ||||
| ER + ve | < 50 yr | 0.001 | 8.517 | 2.309 | 31.413 |
| ≤ 50 yr | 1 | ||||
| ER - ve | < 50 yr | 0.243 | 2.267 | 0.573 | 8.965 |
| ≤ 50 yr | 1 | ||||
| PR + ve | < 50 yr | 0 | 19.833 | 4.58 | 85.883 |
| ≤ 50 yr | 1 | ||||
| PR - ve | < 50 yr | 0.004 | 14 | 2.3 | 85.218 |
| ≤ 50 yr | 1 | ||||
| HER/2 neu + ve | < 50 yr | 0.238 | 2.489 | 0.548 | 11.308 |
| ≤ 50 yr | 1 | ||||
| Hormonal receptors | Grading | P value | OR | 95% confidence interval | |
| ER + ve | Grade I | 0.012 | 15.217 | 1.823 | 127.02 |
| Grade II | 0.016 | 3.986 | 1.296 | 12.252 | |
| Grade III | 1 | ||||
| ER - ve | Grade I | 0.012 | 16.364 | 1.835 | 145.95 |
| Grade II | 0.049 | 3.485 | 1.007 | 12.057 | |
| Grade III | 1 | ||||
| PR + ve | Grade I | 0.375 | 3 | 0.265 | 33.974 |
| Grade II | 0.013 | 4.833 | 1.397 | 16.725 | |
| Grade III | 1 | ||||
| PR - ve | Grade I | 0.301 | 4 | 0.288 | 55.471 |
| Grade II | 0.532 | 1.667 | 0.336 | 8.258 | |
| Grade III | 1 | ||||
| HER/2 neu + ve | Grade I | 0.246 | 4.286 | 0.366 | 50.197 |
| Grade II | 0.105 | 3.095 | 0.789 | 12.144 | |
| Grade III | 1 | ||||
This study revealed that most of the patients (56%) were less than 50 years at diagnosis, and the mean age of the study participants was 48 years. This corresponds to what was reported by Sengal et al[17] and Errahhali et al[18] where the mean ages of the participants were 48 years. On the contrary, a similar study by Chand et al[19] reported a mean age of 55 years. In this study, the most predominant histologic type was invasive ductal carcinoma. A similar study in Sudan also reported invasive ductal carcinoma as the most common histological type[17]. The majority of the ER (50%) and PR (23.6%) positive cases were seen in an age less than 50 years. This report agreed with other similar investigations in Sudan and the northern region of India[17,20]. HER2/neu positivity was observed in patients > 50 years. Our report was not in line with Alzaman et al[21] in Bahrain. There was a significant correlation between the age and ER, PR and HER2/neu positivity (P < 0.05). This was consistent with a similar study conducted by Ramic et al[22].
The majority of ER positive cases (57.0%) were observed in grade I carcinomas. Most PR positive cases (22.0%) were seen in grade II, and most HER2/neu positive cases (10.6%) were seen in grade III. This finding did not correspond to Siadati et al[23] where most of the ER positive cases were grade II[23]. A significant association was established between tumor grade, ER positivity and HER2/neu negativity (P < 0.05). A study conducted by Dodiya et al[24] in the western region of India showed that ER and PR correlated with the grade but not HER2/neu.
One hundred and thirty-one cases had a tumor size ranging from 2-5 cm. We observed that 42.0% were ER positive, 22.1% were PR positive and 9.9% were HER2/neu positive. Our finding indicated that there was a negative correlation in ER positivity status and a positive correlation in HER2/neu positivity status with the tumor size (r = -0.144, P = 0.02). This agreed with a study by Almasri et al[25] that reported a significant correlation between tumor size and hormone receptors.
Metastasis in axillary lymph nodes was observed to be higher in ER positive cases (49.3%), while metastasis to axillary lymph nodes was present in 17.3% of the PR positive cases and 9.7% of HER2/neu positive cases. Tumors with axillary lymph node metastasis showed significant association with ER, PR and HER2/neu (P < 0.03). These findings agreed with other similar studies that showed a significant association between axillary lymph node and hormone receptors[19,26,27].
This study also found that women younger than 50 years were more likely to be present with ER positive and PR negative breast cancer. Similarly, most of the tumors that presented with grade I disease were 15 times more likely to be ER positive than those in grade II or III. On the contrary, Sengal et al[17] reported that younger women are 2 times more likely to develop ER negative disease, and grade III tumors are 2 times more likely to be ER negative compared with grade I or II tumors.
Our study showed that ER, PR and HER2/neu expression had a strong correlation with age, tumor grade, tumor size and lymph node status. Hence, hormone receptor assessment is highly recommended because of its significance in clinical management and prognostication.
Female breast cancer is the leading type of cancer worldwide with an incidence of approximately 2.1 million in 2018. Hormone receptor status plays a vital role in its management.
Expression of biomarkers to treat breast cancer play a significant role in its management.
To determine the molecular expression pattern of biomarkers in breast cancer and their correlation with tumor variables.
This prospective study was designed to analyze expression patterns of estrogen receptor(ER), progesterone receptor (PR) and HER2/neu in breast cancer patients. The dataset was taken from the Department of Radiotherapy and Oncology of Usmanu Danfodiyo University Teaching Hospital Sokoto, Nigeria from 1 January 2015 to 2 December 2019. The dataset had 259 records and 7 attributes. SPSS version 23.0 was used for statistical analysis. The data analyzed demographic and other clinicopathological characteristics as categorical variables. The mean and standard deviation were determined for the quantitative variable.
A total of 259 breast cancer cases were included in the study. The mean age was 48.3 ± 11.0, with an age range of 26-80 years and a median age of 46 years. The morphological categories were invasive ductal carcinoma (99.6%) and invasive lobular carcinoma (0.4%). Estrogen receptor positivity increased in 73 patients (50.0%) under the age of 50 years. Progesterone receptor positivity increase in 34 patients (23.6%) under the age of 50 years. Human epidermal receptor growth factor 2 positivity decreased in 8 patients (5.6%) under the age of 50 years. Hormone receptors were statistically significant with clinicopathological characteristics (P < 0.05).
Our study showed that ER, PR and HER2/neu expression had a strong correlation with age, tumor grade, tumor size and lymph node status. Therefore, assessment of hormone receptors for clinical management of a breast cancer patient is strongly recommended to provide prognostic information and therapeutic measurement.
In low-resource countries, there are paucities in the evaluation of the molecular expression of biomarkers in breast cancer. Therefore, there is a need for further studies to improve prevention and early detection of breast carcinoma in developing countries.
Manuscript source: Unsolicited manuscript
Specialty type: Oncology
Country/Territory of origin: Nigeria
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): 0
Grade C (Good): C
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Peng X S-Editor: Wang DM L-Editor: Filipodia P-Editor: Wang LL
| 1. | Musa AA, Aliyu UM. Application of Machine Learning Techniques in Predicting of Breast Cancer Metastases Using Decision Tree Algorithm, in Sokoto Northwestern Nigeria. J Data Mining Genomics Proteomics. 2020;11:220. [DOI] [Full Text] |
| 2. | Leong AS, Zhuang Z. The changing role of pathology in breast cancer diagnosis and treatment. Pathobiology. 2011;78:99-114. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 93] [Cited by in RCA: 79] [Article Influence: 5.6] [Reference Citation Analysis (0)] |
| 3. | Rodenhiser DI, Andrews JD, Vandenberg TA, Chambers AF. Gene signatures of breast cancer progression and metastasis. Breast Cancer Res. 2011;13:201. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 31] [Cited by in RCA: 33] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 4. | Leong AS, Lee AK. Biological indices in the assessment of breast cancer. Clin Mol Pathol. 1995;48:M221-M238. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 12] [Cited by in RCA: 12] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 5. | Zaha DC. Significance of immunohistochemistry in breast cancer. World J Clin Oncol. 2014;5:382-392. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 1] [Reference Citation Analysis (0)] |
| 6. | Barnes DM, Harris WH, Smith P, Millis RR, Rubens RD. Immunohistochemical determination of oestrogen receptor: comparison of different methods of assessment of staining and correlation with clinical outcome of breast cancer patients. Br J Cancer. 1996;74:1445-1451. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 180] [Cited by in RCA: 182] [Article Influence: 6.3] [Reference Citation Analysis (0)] |
| 7. | Fisher ER, Anderson S, Dean S, Dabbs D, Fisher B, Siderits R, Pritchard J, Pereira T, Geyer C, Wolmark N. Solving the dilemma of the immunohistochemical and other methods used for scoring estrogen receptor and progesterone receptor in patients with invasive breast carcinoma. Cancer. 2005;103:164-173. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 66] [Cited by in RCA: 63] [Article Influence: 3.2] [Reference Citation Analysis (0)] |
| 8. | Franco A, Col N, Chlebowski RT. Discordance in estrogen (ER) and progestin receptor (PR) status between primary metastatic breast cancer: a meta-analysis. J Clin Oncol (Meeting Abstracts). 2004;22 (14_suppl):539. [RCA] [DOI] [Full Text] [Cited by in Crossref: 5] [Cited by in RCA: 5] [Article Influence: 0.2] [Reference Citation Analysis (0)] |
| 9. | Harvey JM, Clark GM, Osborne CK, Allred DC. Estrogen receptor status by immunohistochemistry is superior to the ligand-binding assay for predicting response to adjuvant endocrine therapy in breast cancer. J Clin Oncol. 1999;17:1474-1481. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1646] [Cited by in RCA: 1547] [Article Influence: 59.5] [Reference Citation Analysis (0)] |
| 10. | McCarty KS Jr, Miller LS, Cox EB, Konrath J, McCarty KS Sr. Estrogen receptor analyses. Correlation of biochemical and immunohistochemical methods using monoclonal antireceptor antibodies. Arch Pathol Lab Med. 1985;109:716-721. [PubMed] |
| 11. | Tang P, Tse GM. Immunohistochemical Surrogates for Molecular Classification of Breast Carcinoma: A 2015 Update. Arch Pathol Lab Med. 2016;140:806-814. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 79] [Cited by in RCA: 113] [Article Influence: 14.1] [Reference Citation Analysis (0)] |
| 12. | Hammond ME, Hayes DF, Wolff AC, Mangu PB, Temin S. American society of clinical oncology/college of american pathologists guideline recommendations for immunohistochemical testing of estrogen and progesterone receptors in breast cancer. J Oncol Pract. 2010;6:195-197. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 445] [Cited by in RCA: 487] [Article Influence: 32.5] [Reference Citation Analysis (0)] |
| 13. | Wolff AC, Hammond ME, Hicks DG, Dowsett M, McShane LM, Allison KH, Allred DC, Bartlett JM, Bilous M, Fitzgibbons P, Hanna W, Jenkins RB, Mangu PB, Paik S, Perez EA, Press MF, Spears PA, Vance GH, Viale G, Hayes DF; American Society of Clinical Oncology; College of American Pathologists. Recommendations for human epidermal growth factor receptor 2 testing in breast cancer: American Society of Clinical Oncology/College of American Pathologists clinical practice guideline update. J Clin Oncol. 2013;31:3997-4013. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2910] [Cited by in RCA: 3048] [Article Influence: 254.0] [Reference Citation Analysis (0)] |
| 14. | Yeh IT, Mies C. Application of immunohistochemistry to breast lesions. Arch Pathol Lab Med 2008; 132: 349-358 [PMID: 18318578 DOI: 10. 1043/1543-2165(2008)132. |
| 15. | Carlson RW, Moench SJ, Hammond ME, Perez EA, Burstein HJ, Allred DC, Vogel CL, Goldstein LJ, Somlo G, Gradishar WJ, Hudis CA, Jahanzeb M, Stark A, Wolff AC, Press MF, Winer EP, Paik S, Ljung BM. HER2 testing in breast cancer: NCCN Task Force report and recommendations. J Natl Compr Canc Netw. 2006;4 Suppl 3:S1-S22; quiz S23. [RCA] [DOI] [Full Text] [Cited by in Crossref: 11] [Cited by in RCA: 12] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 16. | Wolff AC, Hammond ME, Schwartz JN, Hagerty KL, Allred DC, Cote RJ, Dowsett M, Fitzgibbons PL, Hanna WM, Langer A, McShane LM, Paik S, Pegram MD, Perez EA, Press MF, Rhodes A, Sturgeon C, Taube SE, Tubbs R, Vance GH, van de Vijver M, Wheeler TM, Hayes DF; American Society of Clinical Oncology; College of American Pathologists. American Society of Clinical Oncology/College of American Pathologists guideline recommendations for human epidermal growth factor receptor 2 testing in breast cancer. J Clin Oncol. 2007;25:118-145. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 798] [Cited by in RCA: 851] [Article Influence: 77.4] [Reference Citation Analysis (0)] |
| 17. | Sengal AT, Haj-Mukhtar NS, Elhaj AM, Bedri S, Kantelhardt EJ, Mohamedani AA. Immunohistochemistry defined subtypes of breast cancer in 678 Sudanese and Eritrean women; hospitals based case series. BMC Cancer. 2017;17:804. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 14] [Cited by in RCA: 16] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 18. | Elidrissi Errahhali M, Elidrissi Errahhali M, Ouarzane M, El Harroudi T, Afqir S, Bellaoui M. First report on molecular breast cancer subtypes and their clinico-pathological characteristics in Eastern Morocco: series of 2260 cases. BMC Womens Health. 2017;17:3. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 11] [Cited by in RCA: 26] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 19. | Chand P, Garg A, Singla V, Rani N. Evaluation of Immunohistochemical Profile of Breast Cancer for Prognostics and Therapeutic Use. Niger J Surg. 2018;24:100-106. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 16] [Cited by in RCA: 19] [Article Influence: 2.7] [Reference Citation Analysis (0)] |
| 20. | Kaul R, Sharma J, Minhas SS, Mardi K. Hormone receptor status of breast cancer in the himalayan region of northern India. Indian J Surg. 2011;73:9-12. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 14] [Cited by in RCA: 9] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 21. | AlZaman AS, Mughal SA, AlZaman YS, AlZaman ES. Correlation between hormone receptor status and age, and its prognostic implications in breast cancer patients in Bahrain. Saudi Med J. 2016;37:37-42. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 15] [Cited by in RCA: 16] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 22. | Ramić S, Asić K, Balja MP, Paić F, Benković V, Knežević F. Correlation of phosphorylated HER2 with clinicopathological characteristics and efficacy of trastuzumab treatment for breast cancer. Anticancer Res. 2013;33:2509-2515. [PubMed] |
| 23. | Siadati S, Sharbatdaran M, Nikbakhsh N, Ghaemian N. Correlation of ER, PR and HER-2/Neu with other Prognostic Factors in Infiltrating Ductal Carcinoma of Breast. Iran J Pathol. 2015;10:221-226. [PubMed] |
| 24. | Dodiya H, Patel A, Patel D, Kaushal A, Vijay DG. Study of hormone receptors and epidermal growth factor expression in invasive breast cancers in a cohort of Western India. Indian J Clin Biochem. 2013;28:403-409. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5] [Cited by in RCA: 7] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 25. | Almasri NM, Al Hamad M. Immunohistochemical evaluation of human epidermal growth factor receptor 2 and estrogen and progesterone receptors in breast carcinoma in Jordan. Breast Cancer Res. 2005;7:R598-R604. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 39] [Cited by in RCA: 49] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 26. | Ali EM, Ahmed RH, Ali AM. Correlation of breast cancer subtypes based on ER, PR and HER2/neu expression with axillary lymph node status. Cancer Oncol Res. 2014;2:51-57. |
| 27. | Azizun-Nisa, Bhurgri Y, Raza F, Kayani N. Comparison of ER, PR and HER-2/neu (C-erb B 2) reactivity pattern with histologic grade, tumor size and lymph node status in breast cancer. Asian Pac J Cancer Prev. 2008;9:553-556. [PubMed] |