Published online Nov 24, 2020. doi: 10.5306/wjco.v11.i11.898
Peer-review started: June 23, 2020
First decision: July 25, 2020
Revised: July 30, 2020
Accepted: October 12, 2020
Article in press: October 12, 2020
Published online: November 24, 2020
Processing time: 148 Days and 1 Hours
After publication of the PACIFIC trial results, immune checkpoint inhibitor-based immunotherapy was included in the treatment algorithm of locally advanced non-small cell lung cancer (NSCLC). The PACIFIC trial demonstrated that 12 mo of durvalumab consolidation therapy after radical-intent platinum doublet chemotherapy with concomitant radiotherapy improved both progression-free survival and overall survival in patients with unresectable stage III NSCLC. This is the first treatment in decades to successfully improve survival in this clinical setting, with manageable toxicity and without deterioration in quality of life. The integration of durvalumab in the management of locally advanced NSCLC accentuates the need for multidisciplinary, coordinated decision-making among lung cancer specialists, bringing new challenges and controversies as well as important changes in clinical work routines. The aim of the present article is to review—from a practical, multidisciplinary perspective—the findings and implications of the PACIFIC trial. We evaluate the immunobiological basis of durvalumab as well as practical aspects related to programmed cell death ligand 1 determination. In addition, we comprehensively assess the efficacy and toxicity data from the PACIFIC trial and discuss the controversies and practical aspects of incorporating durvalumab into routine clinical practice. Finally, we discuss unresolved questions and future challenges. In short, the present document aims to provide clinicians with a practical guide for the application of the PACIFIC regimen in routine clinical practice.
Core Tip: Durvalumab consolidation therapy is the new standard of care in patients with unresectable stage III non-small cell lung cancer treated with concomitant chemoradiotherapy. In the PACIFIC trial, durvalumab significantly improved both progression-free survival and overall survival compared to placebo, with a manageable toxicity profile. However, several questions surrounding the clinical implementation of this therapy remain including optimal patient selection and timing of treatment initiation, programmed cell death ligand 1 determination, and the impact on clinical routines. These questions make interdisciplinary decision-making more necessary than ever. This review assesses these issues and future challenges from a multidisciplinary approach.
- Citation: Mielgo-Rubio X, Rojo F, Mezquita-Pérez L, Casas F, Wals A, Juan M, Aguado C, Garde-Noguera J, Vicente D, Couñago F. Deep diving in the PACIFIC: Practical issues in stage III non-small cell lung cancer to avoid shipwreck. World J Clin Oncol 2020; 11(11): 898-917
- URL: https://www.wjgnet.com/2218-4333/full/v11/i11/898.htm
- DOI: https://dx.doi.org/10.5306/wjco.v11.i11.898
Lung cancer is the most common type of cancer worldwide, with 2.1 million new cases diagnosed annually; it is also the leading cause of cancer-related mortality, accounting for nearly one in five cancer deaths (1.8 million in 2018). Approximately 30% of patients with non-small cell lung cancer (NSCLC) are diagnosed at stage III, representing about 500000 new cases per year[1]. While NSCLC is potentially curable in patients with locally advanced disease, 5-year survival rates are only 12%-41%, even in patients who receive curative intent treatment[2].
Stage III NSCLC is the most clinically complex stage of lung cancer due to its heterogeneity. Although stage III disease is potentially curable, treatment outcomes depend on both treatment selection and timing (i.e. sequencing), which should be individualized according to the patient’s unique clinical characteristics. Stage III NSCLC comprises a highly heterogeneous group of patients with varying degrees of local and nodal involvement. This heterogeneity is reflected in the 8th edition of the Tumor-Node-Metastasis lung cancer staging system, in which stage III disease is subdivided into three subgroups (stages IIIA, IIIB and IIIC)[2]. Thus, within stage III, some patients could be eligible for upfront surgery (T3N1), while other patients could have potentially resectable disease after neoadjuvant therapy (up to stage T3 with non-bulky N2 involvement), and others might have unresectable tumors (most T4 tumors, bulky N2 disease, and N3).
For decades, the standard of care for patients with unresectable stage III NSCLC has consisted of a combination of platinum doublet-based chemotherapy and concurrent radiotherapy (RT), with a total dose of 66 Gy delivered in 33 daily fractions of 2 Gy/fraction (5 d/wk) for all histological variants, regardless of the molecular characteristics. Median progression-free survival (PFS) in these patients is 8-10 mo, and only 15% of patients survive for 5 or more years[3]. Several unsuccessful attempts have been made to improve long-term survival in these patients, including RT dose escalation and treatment intensification through intensity-modulated radiotherapy (IMRT)[4,5], chemotherapy (CT) consolidation strategies involving 2-3 additional cycles of CT after concomitant treatment[6], and targeted therapies such as cetuximab[7]. Given that the majority of recurrences after concomitant chemoradiotherapy (CRT) present with distant metastases, most research activity in recent years has centered on improving systemic control to improve cure rates[8].
Immune checkpoint inhibitors (ICIs) have proven effective in NSCLC. Initially, ICIs were positioned as second-line treatment and later as upfront palliative treatment for advanced disease. However, as the results of the PACIFIC trial show, ICIs also play an important role in locally advanced disease. Durvalumab is an anti-programmed cell death ligand 1 (PD-L1) human immunoglobulin (Ig) G1 monoclonal antibody that binds with high affinity and specificity to PD-L1 to block the interaction of PD-L1 with programmed cell death protein 1 (PD-1) and B7-1, avoiding inhibition of T-cell function, resulting in enhanced recognition and elimination of tumor cells by T cells. The PACIFIC trial is a double-blind, placebo-controlled, multicenter, phase III study conducted to evaluate the efficacy of durvalumab consolidation therapy after completion of concomitant CRT in patients with unresectable, stage III NSCLC without evidence of disease progression following concurrent CRT. This study demonstrated that 12 mo of treatment with consolidation durvalumab (10 mg/kg intravenous injection [iv] every 2 wk) after concomitant CRT in patients with unresectable stage III NSCLC significantly improved PFS and overall survival (OS), thus meeting an important unmet need after several years without any progress in treating this patient profile[9].
In this article, we describe the immunobiological basis for the benefits obtained by combining immunotherapy with RT in patients with NSCLC. We also review the updated data from the PACIFIC trial and related studies that have administered durvalumab in real-world clinical settings. This review takes a multidisciplinary approach to assessing the role of durvalumab, considering the perspectives of immunologists, pathologists, medical oncologists, and radiation oncologists to better understand the practical issues associated with the application of durvalumab in these patients. We also discuss how patient selection implies a critical need to modify work routines in order to successfully implement durvalumab therapy in routine clinical practice. Finally, we discuss the management of durvalumab-related toxicity and future challenges.
Anti-cancer immunotherapy, especially with ICIs, requires a different approach to conventional cancer treatment. While the main focus of surgery, CT, and RT is the direct elimination of tumor cells, the aim of immunotherapy is to help the body to restore an existing function to act against the tumor. Immunotherapy can help to "revive" the immune system’s response capacity, which—theoretically—should have been sufficient to prevent tumor growth[10]. While several approaches to restoring immune function are available, ICIs are the most widely used.
Although immunotherapy alone has shown efficacy after failure of other treatments, the combination of agents that act on different pathways (i.e. on the tumor or the immune system) clearly increases the overall effectiveness of treatment[11]. Although resistance to treatments that directly target cancer cells (such as RT) should not, in theory, affect the impact of immunotherapy (due to differences in the mechanisms of action), in reality the "collateral effects" of tumor-focused therapies on the immune system can alter the effects of immunotherapy, both positively and negatively.
Conceptually, although different ICIs (cytotoxic T-lymphocyte-associated protein 4 [CTLA4], PD-1, PD-L1, and in the future, T-cell immunoglobulin and mucin domain-3 [TIM-3] or V-domain Ig suppressor of T cell activation) will be prescribed depending on the specific target, all ICIs have the same goal: To restore the immune system’s innate capacity to prevent tumor growth by blocking the function of inhibitory molecules[12]. In this regard, it is essential to avoid weakening the patient’s existing immune function. Moreover, anything that strengthens the immune response (such as immunogenic cell death) is beneficial, as it helps to increase the anti-tumor effect of immunotherapy[13].
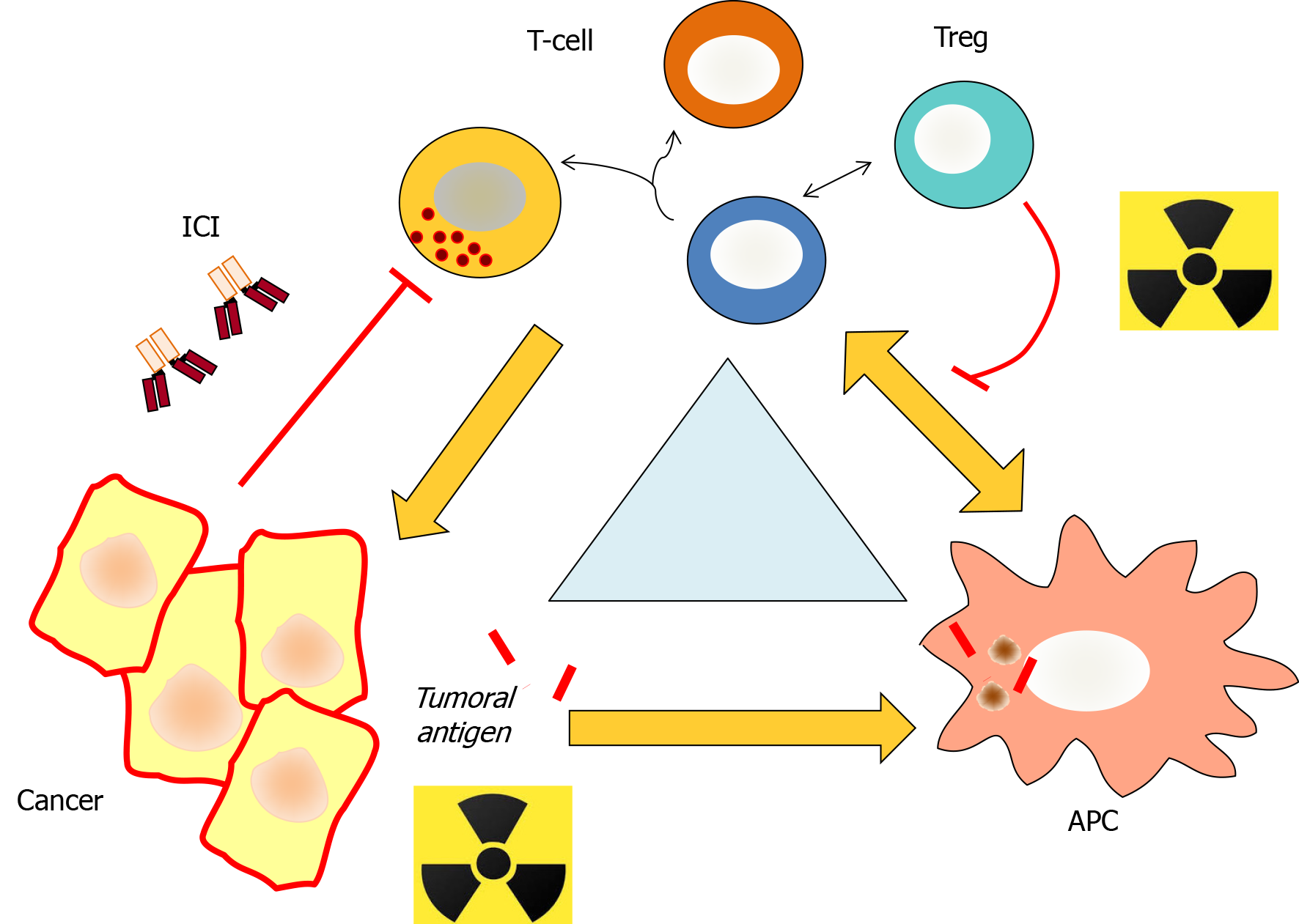
RT can influence the anti-tumor response in several ways, through three main mechanisms, which can produce either positive or negative effects. The first mechanism involves radiation-induced cell death, which can stimulate the release of tumor antigens or induce dysregulation in transcription, leading to increased expression of certain tumor neoantigens, which would not be expressed in unirradiated tumors; both of these effects strengthen the function of anti-tumor lymphocytes by eliminating tumor cells (immunogenic tumor death)[13]. In turn, these tumor antigens activate dendritic cells and cytokines, which recruit and generate an immune response that may have an anti-tumor effect. A second mechanism is the effect that RT has on suppressor cells (regulatory T cells, regulatory B cells, or myeloid-derived suppressor cells). In some cases, RT can eliminate these cells, although in most cases, these cells are highly resistant, and thus RT enhances local anti-tumor immunosuppression. The abscopal effect of RT confirms the distant effects of tumor irradiation (increased suppressor activity distant from the site of irradiation, which would not occur otherwise), and with it the increased immune response due to antigen release in the irradiated tissue, which could migrate—without "the brake"—to other non-irradiated tumors. A third effect of RT on immune response is to induce the production of cytokines and other membrane molecules, including checkpoint molecules such as PD-L1 or TIM-3. Considered together, these effects, which have been demonstrated experimentally[14], help to explain and support the growing interest in combining RT with ICIs to improve post-RT treatment response (Figure 1).
At the physiopathogenic level, it is important to keep in mind that the mechanisms of tumor evasion go far beyond PD1-PD-L1/2 or CTLA4 pathways. The administration of a single ICI assumes that the targeted pathway plays a predominant role in tumor evasion, thus ignoring many other well-established mechanisms that also contribute to reducing the effectiveness of the immune system. If these mechanisms are not targeted therapeutically, they would reduce the effectiveness of immunotherapy. Fortunately, these different mechanisms can be targeted by administering various combinations of different ICIs (including molecules not clearly active in monotherapy such as TIM-3), an approach that is further supported by the optimal timing and form of RT, which also boosts immunotherapy[15,16].
The question of when and how to combine RT with ICIs needs to be defined to further consolidate this combination therapeutic strategy. However, if we take into account the mechanism of action, the optimal treatment sequence should be RT followed by administration of ICIs. In advanced cancers, it is important to avoid irradiating all tumor tissue in order to allow for a complete antigenic stimulation effect, which permits the lymphocytes to migrate to other tumor sites to seek out cancerous tissues, where they can act and amplify. In principle, it should take from 5 to 15 d for the lymphocyte response against the dendritic cells that have captured tumor antigens, causing the proliferation of anti-tumor T lymphocytes, which then locate the tumor while the ICI releases the natural blockade induced by the inhibitory molecules. Furthermore, RT also induces the release of free radicals and cortisol, which can have an immunosuppressive effect that typically reverts 7-15 d after RT (for this reason, the optimal approach—at least theoretically—is to postpone ICI administration for 1–2 wk)[16].
The clinical results of the PACIFIC trial further support the hypothesis regarding the synergistic effects of concomitant CRT and immunotherapy, which we describe in the following paragraphs.
The PACIFIC trial (NCT02125461) was a double-blind, placebo-controlled, multicenter, phase III clinical trial conducted in 253 centers in 26 countries on five continents. The trial was performed to evaluate the efficacy of 12 mo of durvalumab consolidation therapy administered intravenously at doses of 10 mg/kg every 14 d after completion of concomitant CRT in patients with unresectable, stage III NSCLC without evidence of disease progression following concurrent CRT. The primary endpoints were PFS (assessed by blinded independent central review) and OS in the intention-to-treat (ITT) population. The trial included 713 patients, who were randomized (2:1 ratio) to receive durvalumab or placebo starting 1 to 42 d after completing concomitant CRT. The patients were stratified according to age, sex, and smoking status. The initial analysis was performed in 2017 (median follow-up, 14.5 mo). Compared to placebo, durvalumab consolidation therapy significantly improved the following outcome measures: PFS (hazard ratio [HR] = 0.52, 95% confidence interval (CI), 0.42-0.65; P < 0.0001), response rate (28.4% vs 16%; P < 0.001), median duration of response (72.8% vs 46.8% at 18 mo), and median time to death or distant metastasis (23.2 mo vs 14.6 mo; P < 0.001)[17]. These findings were confirmed in an updated analysis in September 2018. At a median follow-up of 25.2 mo, durvalumab therapy significantly improved OS (HR: 0.68, 95%CI: 0.47-0.99; P = 0.0025), with a 24-mo survival rate of 66.3% in the durvalumab group (95%CI: 61.7-70.4) vs 55.6% in the placebo group (95%CI: 48.9-61.8). Median PFS in the active treatment group was 17.2 mo vs 5.6 mo in the placebo group (HR: 0.51, 95%CI: 0.41-0.63), and median survival until death or distant metastasis was 28.3 mo for durvalumab vs 16.2 mo for placebo (HR: 0.53, 95%CI: 0.41-0.68)[9].
Three-year survival outcomes were reported in February 2020 (median follow-up, 33.3 mo [range, 0.2-51.3]). At 3 years, the mortality rate was 44.1% in the durvalumab group vs 56.5% in the placebo group. OS rates for durvalumab vs placebo at 12, 24 and 36 mo were 83.1% vs 74.6%, 66.3% vs 55.3%, and 57.0% vs 43.5%, respectively. Treatment with durvalumab was associated with a 31% reduction in mortality risk (HR: 0.69 [95%CI: 0.55-0.86])[18]. These results strongly support durvalumab consolidation therapy as the standard of care in patients with unresectable, stage III NSCLC who do not progress after concomitant CRT. This indication has been approved by both the Food and Drug Administration (FDA) (February 2018) and the European Medicines Agency (EMA) (September 2018), although the latter limits its use to patients with PD-L1 expression in ≥ 1% of tumor cells, based on data from an unplanned post-hoc study. Scientific societies involved in the management of lung cancer recommend durvalumab consolidation treatment in these patients[19]. However, at present, the question of whether the benefits of durvalumab are limited to patients with positive PD-L1 expression remains controversial, and it is not clear whether durvalumab treatment should be extended to patients with PD-L1-positive tumors < 1% and/or patients with unknown PD-L1 values.
Other clinical trials have been performed to assess the utility and safety of ICI-based consolidation immunotherapy after CRT in stage III NSCLC. In 2018, Durm et al[20] reported the results of a phase II study evaluating the use of pembrolizumab following CRT in 93 patients with unresectable stage III NSCLC. At a median follow-up of 18.6 mo, median time to metastatic disease or death (the primary endpoint) was 22.4 mo, with a median PFS of 17 and an estimated 2-year OS of 61.9%. In terms of adverse effects (AEs), 17.2% of patients developed grade ≥ 2 pneumonitis and 5.4% grade 3 or 4 pneumonitis. Another study, RTOG 3505 — a randomized phase III study of CRT followed by consolidation nivolumab (240 mg iv) or placebo every 2 wk for up to 1 year — was designed to assess survival efficacy and safety of nivolumab consolidation therapy after CRT with cisplatin and etoposide, but the trial was terminated early due to low enrollment (n = 20), due to publication of the PACIFIC findings[21]. Another multicenter, randomized, phase II study of consolidation immunotherapy with nivolumab and ipilimumab or nivolumab alone following CRT in this setting is currently enrolling patients (NCT 03285321). Yan et al[REF] reported safety results from the interim analysis of this study with first 20 patients accrued. Most toxicities were grade 1 or 2 and the most common grade 2 AEs included fatigue (25%), pneumonia (25%), and extremity pain (20%). The incidence of grade 3 or higher immune-related AEs was higher in the nivolumab/ipilimumab arm, but manageable according to the authors. No other studies have evaluated immunotherapy-based consolidation strategy after CRT; consequently, the PACIFIC trial is the first and only randomized phase III, placebo-controlled trial showing a benefit for both PFS and OS in this population to date (Table 1).
| Endpoint | Study and treatment | |||||
| PACIFIC durvalumab | PACIFIC placebo | LUN 14-179 pembrolizumab | PROCLAIM pemetrexed | RTOG 0617 (60 Gy RT) | RTOG 0617 cetuximab | |
| Median follow-up | 33.3 mo | 33.3 mo | 18.6 mo | 22.2 mo | 22.9 mo | 21.3 mo |
| OS | ||||||
| Median | NR | 29.1 mo | 22.4 mo | 26.8 mo | 28.7 mo | 25 mo |
| 12-mo | 83.1% | 74.6% | 74.7% | 76% | 80% | 76.2% |
| 24-mo | 66.3% | 55.3% | 52% | 57.6% | 52.3% | |
| 36-mo | 57% | 43.5% | 40% | |||
| PFS | ||||||
| Median | 17.2 mo | 5.6 mo | 17 mo | 11.4 mo | 11.8 mo | 10.8 mo |
| 12-mo | 55.9% | 35.3% | 60.2% | 49.2% | 44.3% | |
| 24-mo | 44.6% | 29.1% | 24.2% | |||
Obtaining biological samples with optimal (or at least sufficient) quality to assess biomarkers should be a shared, multidisciplinary responsibility. For this reason, it is essential that all clinicians who treat these patients understand the advantages and disadvantages of each sample type. In addition, clear procedures should be established for ordering biomarker analyses and obtaining additional samples (if necessary). In this regard, it should be possible to automatically order new samples based on the findings of the pathologic report.
Accurate biomarker analysis requires a sufficiently large sample of tumor cells (≥ 50-100 viable cells for PD-L1)[22]. The sample must also meet the basic quality requirements for analysis[23], including guarantees to ensure that the time elapsed between extraction and fixation is as short as possible and that fixation is done in a 10% formalin-buffered solution for 6-12 h (small biopsies) or 24-48 h (surgical resections)[24]. In lung cancer, the most common samples used for diagnosis are small biopsies and/or cytological samples (cell blocks, smears, and liquid cytology). All of these are suitable for PD-L1 analysis, and the specific choice will depend on the experience and capacity of the individual laboratory[25]. The use of cytology samples to determine PD-L1 has not yet been validated, although a good correlation has been observed between cytology cell blocks and aspirate smears with biopsy[26].
PD-L1 is a type 1 transmembrane protein (B7-H1) belonging to the B7 family of ligands, which can be expressed in lymphocytes and tumor cells. In advanced NSCLC, PD-L1 overexpression is a predictor of clinical benefit with PD-1/PD-L1 inhibitors[27]. The EMA limits the use of consolidation durvalumab to patients with positive PD-L1 expression (≥ 1% of tumor cells) based on the results of the PACIFIC trial[9].
Various immunohistochemical methods involving antibody clones against PD-L1 are available, the most common being 22C3 and 28-8 (Agilent/Dako), which share the Autostainer Link48 platform, and SP263 (MedImmune/Ventana) and SP142 (Spring/Bioscience/Ventana), which share the Ventana BenchMark platform. Using these four clones, PD-L1 is evaluated as the percentage expression in tumor cells (partial or complete membrane expression) at any intensity; SP142 assess the proportion of the stromal area occupied by immune cells with PD-L1 expression. Three methods—22C3, 28-8, and SP263—have all demonstrated technical equivalency for the determination of PD-L1 expression in tumor cells in patients with lung cancer[27].
Several studies have examined differences in PD-L1 expression between the primary tumor and metastatic lesions[27]. The general consensus is that PD-L1 expression is heterogeneous in 25% of cases, both within the tumor itself and in the different tumor sites, including regional lymph nodes. Studies have shown that treatment with CT or RT increases PD-L1 expression[28]. In this context, large, representative biological samples of the tumor are needed to accurately perform the analysis, and the general recommendation is to analyze pre-treatment samples from the primary tumor and to ensure that additional samples are available as an alternative if this proves inadequate.
When determining the presence of any biomarker (including PD-L1), it is essential to ensure the quality of the results by strictly following laboratory policies. In Europe, the testing laboratory should have ISO 9001 certification and all tests should be accredited according to the UNE-EN ISO15189 standard. This quality policy assumes that trained personnel (technicians, biologists, pathologists) are available and that they adhere to standardized work procedures, using well-maintained instrumentation with CE certification, as well as validated reagents[29]. The use of internal controls for each assay is important, and the laboratory itself should also undergo external quality controls (e.g., SEAP, EMQN, UK-NEQAS). It is also advisable to check the results to verify that the percentage of positive findings is consistent with published reports.
The laboratory report should also meet certain quality parameters, including the following: (1) Recommended response time: 7-10 business days; (2) Compliance with the quality policies described above; and (3) Inclusion of the following data points: Identification of the patient and the person requesting the test; pathological diagnosis; sample type; sample collection and processing date; anatomic origin of sample; date of request, reception, and issuance of the result; type of assay used and description of the limitations; for commercial assays, the following information must be included: Commercial name; batch number; confirmation that the assay is approved for in vitro diagnosis; description of the sample quality; adequacy of the sample; test result given as the percentage of tumor cells with PD-L1 expression; identification of the professional(s) responsible for carrying out the test and (optionally) the name of the laboratory supervisor; any additional information or comment of interest; and, accreditation, certification, or participation in quality assurance programs.
To date, only two studies (both retrospective) have been carried out to evaluate consolidation durvalumab in a real-world clinical setting. Preliminary data from these studies were reported at the 2019 World Conference on Lung Cancer. Grivet et al[30] reported results from a retrospective cohort of 83 patients in Brazil with locally advanced NSCLC who were treated with durvalumab. The cohort was comprised mainly of females (55%) and most patients were smokers (59%), with a median age of 73.5 years. The most common histological finding was adenocarcinoma (67%). The most common chemotherapy regimen was carboplatin-paclitaxel, and the main RT scheme was 60 Gy in 30 fractions (39%). After a median of 1.2 years on durvalumab, only 8 patients had died (9.6%), and none of these deaths was considered treatment-related. The main toxicities were mucositis (63%), cough (52%), and cutaneous rash (48.8%). No cases of grade 5 toxicity were reported. At the same congress, Barbaro et al[31] reported results from a retrospective cohort of 146 patients who had completed concomitant CRT for locally-advanced NSCLC at the Montefiore Medical Center from 2007-2018. Of these patients, 27% did not meet PACIFIC eligibility criteria for durvalumab therapy, mainly due to the presence of concurrent diseases (32%) or additional malignancies (15%); other reasons were disease progression (7%) and pneumonitis (7%). Thus, in the full cohort, only 17 patients received durvalumab, including four who did not meet criteria for durvalumab (due to co-morbidities and/or additional malignancy). The median time to durvalumab initiation following CRT was highly variable, ranging from 56 d prior to durvalumab approval in July 2018 to 30 d afterwards. Although the follow-up was short, there was a trend toward improved OS at 15-mo in patients who received durvalumab vs those who did not (100% vs 87.5%).
The PACIFIC-Real World (PACIFIC-R) trial is currently in progress. The aim of this study is to assess the efficacy and safety of durvalumab treatment after concurrent CRT in a large, real-world population. The study plans to enroll approximately 1200 NSCLC patients through early access programs between 2017 and 2018, with a 5-year follow-up. Interestingly, in this study patients will be enrolled regardless of tumor PD-L1 expression level. Primary endpoints are PFS (investigator-assessed) and OS.
In the PACIFIC trial, the main eligibility criteria were locally advanced, stage III unresectable NSCLC patients treated with definitive concurrent CRT (54 to 66 Gy; V20 < 35%). Additional inclusion criteria were: (1) No progression after CRT; (2) Eastern Cooperative Oncology Group (ECOG) performance status (PS) ≤ 1; and (3) Last radiation dose administered from 1 to 14 d before randomization (after a protocol amendment, this criterion was modified to 1 to 42 d before randomization). Unresolved toxicity ≥ grade 2 and pneumonitis ≥ grade 2 from previous treatment with CRT were the main exclusion criteria. These criteria suggest that some patients will not be eligible for durvalumab therapy after CRT. Currently, published data regarding eligibility criteria in real-life clinical practice area limited; however, a few retrospective, single-center studies have specifically assessed these criteria. Sakaguchi et al[32] evaluated the clinical criteria for durvalumab after CRT in an historical cohort of 73 patients with unresectable stage III NSCLC based on the eligibility criteria established in the PACIFIC trial. This patient cohort was treated between 2011 and 2018 and approximately 30% did not meet eligibility criteria for durvalumab therapy. The most common reasons for ineligibility were radiation pneumonitis (16% of cases), poor PS (10%), and disease progression after CRT (4%). Unfortunately, no data on the V20 or other factors related to radiation quality and risk of pneumonitis were reported.
In another cohort involving 97 patients with stage III unresectable NSCLC patients treated between 2017 and 2019, Shaverdian et al[33]explored the clinical limitations for durvalumab therapy. Durvalumab was not administered in approximately 27% of patients, similar to the results described by Sakaguchi and colleagues. The main exclusion criteria were disease progression and/or CRT-related toxicity. All patients underwent pre-treatment staging with brain magnetic resonance imaging (MRI) and positron emission tomography-computed tomography (PET-CT) to ensure that only stage III patients were included in the study. In contrast to the study by Sakaguchi et al[32], Shaverdian and colleagues reported PTV values and cardiac doses. Consistent with these two retrospective studies, Barbaro et al[31] (see section 4) found that around 27% of patients were not eligible for durvalumab according to PACIFIC criteria. Although these data were obtained in retrospective studies and small cohorts, they consistently show that approximately one out of every 3 or 4 patients (25%-33%) are not eligible for durvalumab therapy after CRT.
Lung cancer is most commonly diagnosed in older people, which may be a limiting factor for the prescription of durvalumab, as some published reports suggest that durvalumab may be more toxic in older patients[34]. In this regard, Socinski et al[35] analyzed data from patients ≥ age 70 included in the PACIFIC trial, who accounted for 22% of the full cohort. Most of these older patients were males with ECOG PS 1 treated with carboplatin-based CT. However, this subgroup analysis did not find any age-related difference in median survival outcomes. In the < 70 years subgroup, median survival in the treatment arm was 16.9 mo vs 5.6 in the placebo arm (HR: 0.53, 95%CI: 0.42-0.67); similarly, in patients ≥ age 70, survival in the treatment arm was 12.3 vs 6.1 mo (HR: 0.62, 95%CI: 0.41-0.95). However, in the patients who received durvalumab, older patients had higher rate of serious AEs (42.6% vs 24.9%) and grade 3/4 AEs (41.6% vs 29.4%), but fewer adverse events of special interest (56.4% vs 67.9%) than younger patients.
In terms of OS, subgroup analyses in the PACIFIC trial showed a greater benefit for durvalumab in certain groups: Females benefitted more (HR: 0.46; 95%CI: 0.3-0.73) than males (HR: 0.78; 95%CI: 0.59-1.03), as did Caucasians (HR: 0.71; 95%CI: 0.54-0.92) vs Asians (HR: 0.62; 95%CI: 0.38-1.01), and American patients (HR: 0.46; 95%CI: 0.3-0.69) vs Asians (HR: 0.67; 95%CI: 0.41-1.11) and Europeans (HR: 0.66; 95%CI: 0.61-1.21)[9].
The PACIFIC trial enrolled patients regardless of tumor PD-L1 expression level. However, a post hoc exploratory analysis performed according to PD-L1 expression (available in 63% of the overall cohort; n = 451)[36] found that 35% had ≥ 25% PD-L1 expression, 67% had ≥1% PD-L1, and 33% were negative (< 1%). Median PFS was higher in the durvalumab arm vs placebo across all these subgroups, as follows: In the subgroup with ≥ 25% PD-L1 expression: 17.8 vs 3.7 mo (HR: 41, 95%CI: 0.26-0.65); in the ≥ 1% PD-L1 group: 17.8 vs 5.6 mo (HR: 0.46, 95%CI: 0.33-0.64); in the negative (< 1% PD-L1 expression) subgroup: 10.7 vs 5.6 mo (HR: 0.73, 95%CI: 0.48-1.11). Finally, in patients with unknown PD-L1 expression, median PFS was 14 mo vs 6.4 mo (HR= 0.59, 95%CI: 0.42-0.83). Based on this subanalysis, in July 2018 the EMA approved durvalumab for patients diagnosed with locally-advanced, unresectable stage III NSCLC who have not progressed following CRT, only for patients whose tumor PD-L1 expression is ≥ 1%. This restricted approval, which differed from the FDA approval (which did not require any minimum PD-L1 expression level) remains controversial in the European oncology community because it means that patients with low and unknown PD-L1 expression are unable to benefit from the improved survival outcomes that consolidation durvalumab provides. In this regard, it is worth noting that the PACIFIC trial found a survival benefit in patients with unknown PD-L1, who comprised 36.7% of the study population[9]. Nevertheless, several ongoing clinical trials will establish the true value of PD-L1 status in these patients[37].
Durvalumab consolidation therapy improved both PFS and OS, irrespective of the type of chemotherapy prescribed, the radiation dose, or time from RT to durvalumab initiation. However, to our knowledge, no large, clinical studies have yet examined all of these variables in a real-world setting.
Chemotherapy-based considerations: The PACIFIC trial showed that several different chemotherapy strategies can be concurrently administered with RT. In that trial, patients received ≥ 2 cycles of platinum-based chemotherapy (etoposide, vinblastine, vinorelbine, a taxane, or pemetrexed) concurrently with definitive RT. Induction chemotherapy (ICT) followed by concurrent CRT was compared to two cycles of chemotherapy delivered concurrently with RT[17]. Spigel et al[38] evaluated the impact of ICT in the PACIFIC trial. Overall, 26% and 29% of patients in the durvalumab and placebo groups, respectively, received ICT. Durvalumab demonstrated a clinical benefit in both groups (with and without ICT) and this was not influenced by the specific ICT regimen. Although safety outcomes were similar (with only minimal differences) across subgroups, the toxicity rate (including pneumonitis) was lower in patients who received ICT, regardless of the treatment arm.
Another important factor to consider is the CRT scheme. Even though concomitant CRT improved outcomes when compared to sequential CRT[39], some patients receive sequential CRT, generally due to the presence of frailty, certain comorbidities, or poor performance status. This subset of patients was not enrolled in the PACIFIC trial, so there is still a lack of evidence for the benefit of durvalumab in this setting. Interestingly, the currently ongoing phase 2 PACIFIC-6 trial (NCT03693300) was designed to assess safety, efficacy, and quality of life (QoL) in 150 patients treated with sequential CRT followed by durvalumab. The primary objective of that study is safety, with secondary endpoints that include PFS, OS, duration of response, response rate, among others. Interestingly, patients with ECOG PS 2 are eligible and there are no biomarker-based enrolment limitations (e.g., PD-L1 expression).
Radiation therapy-based considerations: In the last few years, technological and methodological innovations have improved the accuracy and efficacy of RT. However, RT delivery requires an experienced team and high-quality control measures to ensure that patients receive the most accurate and effective treatment possible to improve clinical outcomes[40].
PET-CT staging is recommended for patients with NSCLC considered candidates for radical RT, as this imaging modality permits accurate staging of stage III NSCLC to better define intrathoracic nodal disease for RT planning, especially compared to conventional staging techniques[41]. Moreover, PET-CT can also detect occult metastatic disease in up to 24% of cases[42]. However, in the PACIFIC trial, PET-CT staging was not mandatory, so some patients with stage IV disease could have been enrolled inadvertently.
Radiation therapy quality assurance (RTQA) is also important, as this ensures the use of high-quality methodologies and procedures to efficiently achieve quality RT. The absence of RTQA has been associated with major violations in clinical trials, with a negative impact on patient outcomes, as reported in the PROCLAIM study[43]. Unfortunately, in the PACIFIC trial, no real-time RTQA quality control was centrally performed, and not all relevant data could be collected after the trial ended.
At present, there are only limited data on durvalumab consolidation in real-world settings, and there are important questions regarding the benefit of the PACIFIC strategy in routine clinical practice. We are certain that large, real-world studies will provide more information about the efficacy of consolidation durvalumab in different subgroups to better determine the patient population most likely to benefit from this therapy. Additionally, such studies could provide an extraordinary source of data to delve deeply into the methodological limitations and quality control issues related to RT that we have emphasized in this review, to fully elucidate the real magnitude of the benefits and risks (e.g., pneumonitis) of durvalumab. In this regard, several ongoing phase 2 and 3 clinical trials (Pacific-R, PACIFIC -2, Pacific-5, Pacific-6) are expected to resolve these open questions.
The toxicity profile was a secondary endpoint in the PACIFIC trial. In general, tolerance to consolidation durvalumab after concomitant CRT was good, with no increase in general toxicity in the active treatment group vs placebo. Adverse event rates of any grade were 96.8% and 94.9% in the durvalumab and placebo groups, respectively; the corresponding rates of grade 3/4 toxicity were 30.5% and 26.1%. However, a higher proportion of patients in the durvalumab group had to interrupt treatment due to toxicity (15.4% vs 9.8%). The most common treatment related toxicities are listed in Table 2. The most frequent AEs leading to treatment interruption were pneumonitis, radiation pneumonitis, and pneumonia. The incidence of radiation pneumonitis or pneumonitis was significantly higher in the durvalumab group (33.9% vs 24.8%), but no significant between-group differences were observed in terms of grade 3/4 pneumonitis (3.4% vs 2.6%). Serious AEs were more common in the durvalumab group (29.1% vs 23.1%), but the mortality rate was significant higher in the placebo group (6.4% vs 4.4%)[44].
| Event | Treatment arm and study | |||||
| Durvalumab in PACIFIC, n = 475 | Placebo in PACIFIC, n = 234 | Pembrolizumab in LUN 14-179 | ||||
| Any grade | Grade 3/4 | Any grade | Grade 3/4 | Any grade | Grade 3/4 | |
| Any event | 96.8% | 30.5% | 94.9% | 26.1% | ||
| Cough | 35.2% | 0.4% | 25.2% | 0.4% | 25.8% | 1.1% |
| Fatigue | 24% | 0.2% | 20.5% | 1.3% | 46.2% | 4.3% |
| Dyspnea | 22.3% | 1.5% | 23.9% | 2.6% | 21.5% | 5.4% |
| Radiation pneumonitis | 20.2% | 1.5% | 15.8% | 0.4% | ||
| Diarrhea | 18.5% | 0.6% | 19.7% | 1.3% | 15.1% | 4.3% |
| Pyrexia | 15.2% | 0.2% | 9.4% | 0% | ||
| Nausea | 14.3% | 0% | 13.2% | 0% | 15.1% | 1.1% |
| Anorexia | 14.3% | 0.2% | 12.8% | 0.9% | 17.2% | 1.1% |
| Pneumonia | 13.3% | 4.4% | 7.7% | 3.8% | ||
| Pneumonitis | 12.6% | 1.9% | 7.7% | 1.7% | 17.2%1 | 5.4% |
| Arthralgia | 12.4% | 0% | 5.1% | 0% | 15.1% | 1.1% |
| Pruritus | 12.4% | 0% | 5.1% | 0% | 10.8% | 0% |
| Rash | 12.2% | 0.2% | 7.7% | 0% | 12.9% | 1.1% |
| Constipation | 11.8% | 0.2% | 8.5% | 0% | ||
| Hypothyroidism | 11.6% | 0.2% | 1.7% | 0% | 7.5% | 0% |
One of the main concerns of administering ICIs in patients treated with radical thoracic RT is the potential for an increased risk of pneumonitis, as studies show that a high percentage of patients receiving radical intent, concomitant thoracic RT develop some degree of pneumonitis[32]. In the phase I KEYNOTE-001 trial carried out to evaluate pembrolizumab in patients with advanced NSCLC, a higher rate of ICI-related thoracic toxicity was observed in patients previously treated with thoracic RT vs those who had not received prior RT (13% vs 1%, P = 0.046)[45]; however, some data suggest that anti-PD-L1 treatments (such as durvalumab) may have a lower risk (compared to anti-PD-1 treatments) of lung toxicity[46]. In general, the addition of consolidation durvalumab to concomitant CRT was accompanied by low rates of severe lung toxicity, a finding that suggests the benefit of durvalumab outweigh the risks. Moreover, this implies that there is no need to restrict the use of durvalumab due to pulmonary toxicity, although it should be noted that the thoracic RT doses in the PACIFIC trial were lower than usual (up to 54 Gy)[47].
In a subgroup analysis performed to identify predisposing factors for pneumonitis in the PACIFIC trial, the pneumonitis rate was higher in Asians (47.9% vs 17.6%) and in patients with EGFR mutations (11% vs 3.8%). No significant associations were found between pneumonitis and other respiratory disorders, the previous RT dose, or the type of CT received (cisplatin vs carboplatin)[48]. However, we lack data from large studies to confirm the absence of predisposing factors for pneumonitis, which could potentially limit the use of consolidation durvalumab therapy. In the PD-L1 subgroup analysis in the PACIFIC trial, any grade pneumonitis was more common in patients treated with durvalumab in all subgroups (ranging from 30.8% to 35.7%) vs placebo (17.4% to 29.9%). However, there were no significant differences in the incidence of grade 3 or 5 pneumonitis (no grade 4 events were reported)[36].
As mentioned above, very few real-world studies of durvalumab have been conducted. However, one multicenter study (n = 36) found that 29% of patients developed symptomatic pneumonitis (grade ≥ 2), with only two cases (5.6%) of grade 3 pneumonitis. In that study, the median time to pneumonitis was 71 d (range, 29-270). The mean duration of pneumonitis was 6 wk (range, 2-19), and the median time to durvalumab interruption was 4.5 wk (range, 2-8). However, 70% of these patients restarted durvalumab after the pneumonitis resolved, with a recurrence rate of 14%. The presence of pneumonitis did not affect survival outcomes[49].
Based on the consensus guidelines and recommendations of the European Respiratory Society, Society for Immunotherapy of Cancer, American Society of Clinical Oncology, and the European Society of Medical Oncology, immune-mediated pneumonitis derived from durvalumab should be graded based on CTCAE Version 5.0 criteria[50]. We recommend the following management approach: Grade 1: Continue treatment with durvalumab but with close follow-up; Grade 2: Interrupt durvalumab therapy and maintain close follow-up. Initiate corticosteroid therapy (oral methylprednisolone 1mg/kg per day or equivalent). Consider restarting durvalumab after resolution or downgrade to grade 1; Grade ≥ 3: Discontinue durvalumab permanently and initiate very high dose corticosteroid therapy (methylprednisolone 2-4 mg/kg per day IV or equivalent) +/- bolus intravenous steroid. If symptoms do not improve within 48 h or clinical worsening is observed, consider infliximab 5 mg/kg, cyclophosphamide 600 mg/m2, or mycophenolate mofetil 1000 mg/12 h.
In cases with symptomatic pneumonitis, steroid treatment, once started, should be maintained for ≥ 6 wk to avoid recurrences. Steroid treatment should be gradually tapered over time[51,52].
In the PACIFIC trial, patient-reported outcomes (PROs) were pre-specified secondary outcomes[14]. In the ITT population, the investigators evaluated PRO symptoms, functioning, global health status, and QoL using the scales most commonly administered in cancer patients (EORTC Quality of Life Questionnaire-Core 30 [QLQ-C30] and the lung cancer version, the QoL-Lung Cancer 13 [QLQ-LC13]). These scales were administered at various time points, including at baseline (randomization), every 8 wk until week 48, and then every 12 wk until progression. In addition, the investigators also assessed changes in key symptoms from baseline to 12 mo (changes ≥ 10 points were defined as clinically relevant), with mixed model for repeated measures (MMRM) and time-to-event analyses.
Hui et al[53] recently reported the results of the PROs analysis. No significant changes were observed in the two treatment arms in the pre-specified longitudinal PROs of interest: Cough (MMRM-adjusted mean of 1.8 with durvalumab vs 0.7 with placebo), dyspnea (3.1 vs 1.4), chest pain (-3.1 vs -3.5), fatigue (-3.0 vs -5.2), appetite loss (-5.8 vs -7.0), and physical functioning (0.1 vs 2.0). In both treatment arms, the global health status/QoL (2.6 with durvalumab vs 1.8 with placebo) remained stable, with no clinically significant variations from baseline. Additionally, no clinically relevant between-group differences were observed in changes from baseline to 12 mo and in time to deterioration in these key PROs. Thus, the durvalumab benefit did not affect overall health and QoL, with no detrimental impact observed in the main indicators of PROs.
Patients with NSCLC classified as stage IIIA due to the presence of mediastinal lymph node involvement (N2) may present differences in the number of nodal stations involved, their size, and/or the existence of capsular rupture. These factors can all influence the prognosis and thus the treatment strategy[54]. Therefore, it is evident that this group of patients is comprised of different subgroups with important differences that can require different treatment strategies. The specific treatment approach should ideally be based on a consensus agreement reached by a multidisciplinary tumor board.
Based on the findings of the PACIFIC trial, 12 mo of durvalumab consolidation therapy is now considered the standard treatment for patients with unresectable stage IIIA NSCLC, provided that no contraindications for immunotherapy are present. For patients with resectable disease, the optimal treatment strategy remains controversial. Upfront surgical resection is indicated in patients without mediastinal lymph node involvement (N0-N1) in whom complete resection is technically feasible (provided the patient's general condition and lung function allow it). In these patients, the treatment indication is adjuvant platinum doublet chemotherapy, as this has been shown to significantly prolong OS[55]. Adjuvant RT should be reserved for patients with positive surgical margins and postoperative mediastinal node involvement[56]. RT should, preferably, be administered sequentially after CT, except in cases with macroscopic residual disease[39,57]. The role of surgery in patients with stage IIIA disease (nodal involvement) is more controversial; in most cases, concomitant CRT is considered the standard option. However, in patients with a single involved mediastinal node < 3 cm with evidence of radiological response after CT or induction RT, surgical resection would be a valid option, although prospective randomized trials have yet to find any difference between these two treatment strategies[39,58]. The data from the PACIFIC trial add to this controversy: Since durvalumab consolidation therapy has been shown to significantly improve OS vs concomitant CRT alone, durvalumab should also be considered the standard of care in stage IIIA disease. That said, this approach could change in coming years based on the highly promising initial data from clinical trials evaluating the role of neoadjuvant immunotherapy combined with CT in patients with resectable (or potentially-resectable) stage III NSCLC. Forde et al[59], in a study involving 23 patients with stage I-IIIA disease who received nivolumab monotherapy, reported a high pathological response rate (45%) with no correlation between PD-L1 expression and efficacy. More recently, Provencio et al[60] reported the results of the NADIM trial, a multicenter phase II clinical trial evaluating nivolumab combined with carboplatin-based CT and neoadjuvant paclitaxel, reporting an even higher pathological response rate (84.6%). If these data are confirmed in large prospective studies, we will need to reopen the debate regarding induction therapy followed by surgery vs concomitant CRT followed by durvalumab consolidation therapy. In any case, it is evident that immunotherapy with PD-1/PD-L1 inhibitors will, one way or another, be incorporated into the treatment regimen for patients with stage IIIA NSCLC.
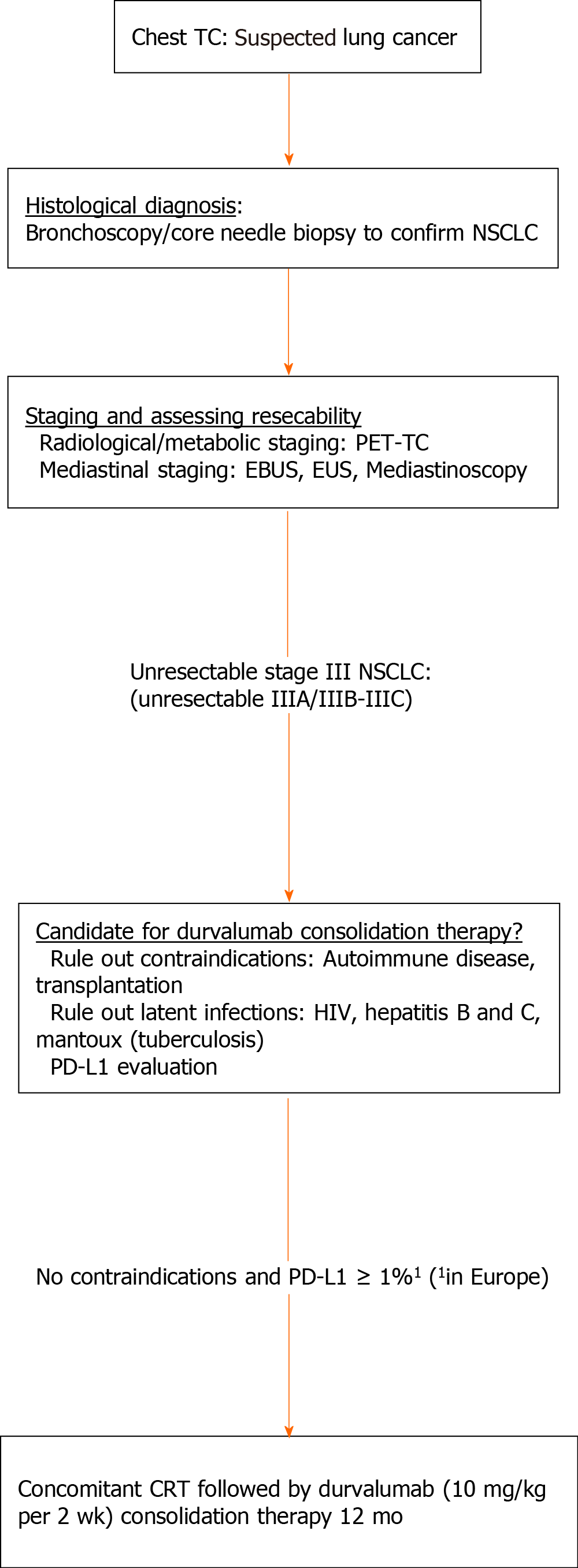
The emergence of durvalumab consolidation therapy in locally advanced disease represents a new challenge for routine clinical practice, requiring clinicians to implement a series of changes in the diagnostic, treatment, and follow-up processes of these patients (Figure 2).
The radiological protocols to rule out distant metastasis remain unchanged, and include PET-CT, brain MRI/CT, and mediastinal staging (endobronchial ultrasonography (EBUS)/esophageal ultrasonography (EUS) or the more invasive technique, mediastinoscopy). By contrast, pathological evaluation has become more important. In the histological analysis, a diagnosis of NSCLC would be sufficient, regardless of the tumor histology (squamous or non-squamous), as the PACIFIC trial demonstrated the efficacy of durvalumab in both histological groups[14].
The EMA authorization limited consolidation durvalumab therapy to patients with PD-L1 expression ≥ 1%, obliging the European cancer care community to intensify efforts to evaluate the role of this biomarker in all patients with unresectable stage III NSCLC. This limitation also increases the need to ensure that adequate tumor sample are obtained and to make use biopsy rather than cytology for the diagnosis, particularly in centers that do not have the capability for PD-L1 determination in cytological samples. Consequently, in some cases the tests (bronchoscopy, EBUS, CT-guided biopsy) will need to be repeated or, alternatively, surgical procedures (the least invasive methods available) will be necessary for the diagnosis.
One near-term objective is to validate cytological samples (e.g., cell block) for the analysis of PD-L1. This would increase the proportion of patients with PD-L1 determination and, in turn, limit the number of diagnostic tests, thus reducing costs, saving time, and also decreasing the risks that these procedures pose for the patient.
Although the PACIFIC trial included patients with and without molecular alterations in EGFR or ALK, immunotherapy is of limited benefit in these patients[40]. However, if the studies currently underway in this population (LAURA, NCT NCT03521154) show a benefit for the use of targeted therapies, we will need to determine the patient’s mutational status to decide whether or not the patient is a candidate for durvalumab therapy.
Goal—patient safety: Pneumonitis is one of the most limiting and well-known toxicities of concomitant CRT. The reported incidence of pneumonitis in the PACIFIC trial was approximately 15% (severe pneumonitis: < 5%); however, this could be higher in real-world clinical settings due to the presence of respiratory comorbidities[43,44]. Consequently, baseline respiratory function should be assessed at least once between completion of RT and initiation of durvalumab therapy. In addition, respiratory function should be periodically monitored by a pulmonologist during treatment and follow-up.
Immunotherapy trials conducted to date have systematically excluded patients with chronic infections (hepatitis B and C, human immunodeficiency virus [HIV], and tuberculosis [TB]) and risk of reactivation. The risk vs benefits of durvalumab in patients with localized disease is unknown, so these infections should be ruled out before initiating treatment. The most common and widely available techniques for this purpose are viral serology (hepatitis and HIV) and the Mantoux test for TB.
Thyroid alterations (thyroid stimulating hormone [TSH], T3, and T4) are common in patients treated with ICIs. For this reason, a baseline determination of these hormones should be performed and their levels measured every 4-6 wk during treatment. Autoimmune diseases should be ruled out to ensure patient safety.
Objective—radiological evaluation: In the PACIFIC trial, early initiation of durvalumab after concomitant CRT (≤ 14 d) was associated with a trend towards higher OS. Chest CT should be performed as soon as possible after completion of RT (preferably < 4 wk): Importantly, the role of chest CT is not to assess response, but rather to rule out local progression and/or signs of severe pneumonitis, which would contraindicate consolidation durvalumab therapy.
The emergence of nodal involvement or enlarged nodes during follow-up without other signs of progression should be confirmed histologically. Granulomatous/ sarcoid-like lesions in the lymph nodes have been described in patients treated with immunotherapy[45], potentially requiring specific management or treatment.
After publication of the PACIFIC trial results, numerous questions have been raised regarding how to optimize immunotherapy in stage III NSCLC in terms of treatment duration, combinations, and RT schemes. This has even prompted a search for new biomarkers or additional treatments.
Based on the data from the PACIFIC trial, the recommended duration of durvalumab therapy is 1 year. There may be a subset of patients (e.g., with low tumor burden, better radiological or metabolic response) who could obtain a similar benefit with a shorter duration of treatment, although data to support this approach are lacking.
RT and PD-L1 inhibitors have a synergistic effect that creates a more immunogenic tumor microenvironment by promoting CD8 T lymphocyte infiltration and suppression of suppressive myeloid cells[61]. Two prospective studies are currently evaluating the combined administration of anti-PD-1/PD-L1 agents during concomitant CRT: PACIFIC-2 (NCT03519971) with durvalumab and KEYNOTE-799 (NCT03631784) with pembrolizumab. However, the potential toxicity of these combinations will need to be observed carefully given findings from previous studies with nivolumab[62] and pembrolizumab[63], both of which showed highly promising results but with an increased risk of pneumonitis. Atezolizumab combined with concomitant CRT is being evaluated in the DETERRED study[64], which will also determine the efficacy and safety of this anti-PD-L1 agent combined with chemotherapy as a consolidation strategy after concomitant CRT.
Although concomitant CRT is the standard treatment in stage III NSCLC, there are some cases—due to the presence of comorbidities, the patient’s functional situation (poor PS and high risk of toxicity)—in which sequential treatment (CT followed by RT) is preferred. As consolidation durvalumab therapy becomes more widely used, the evidence base for sequential treatment in these patients has increased, but prospective data remain unavailable. The prospective, randomized PACIFIC-5 trial (NCT03706690) is currently underway to compare concomitant to sequential therapy. Moreover, that study includes monthly administration of durvalumab (1500 mg iv), which will provide novel efficacy and safety data for that dose level.
Other studies are investigating combined treatment with RT and immunotherapy in which chemotherapy is excluded. The SPRINT study is currently evaluating the efficacy of induction pembrolizumab monotherapy and maintenance RT in patients with PD-L1 > 50%[65]. The DUART study will evaluate the efficacy and safety of durvalumab after RT in patients not eligible for chemotherapy (NCT04249362). Other alternative strategies include combinations with drugs with different mechanisms of action (e.g., anti-CTLA-4) (NCT03663166).
At present, due to its proven efficacy and safety, the standard RT scheme is a total dose of 60 Gy at 2 Gy/d[7].Alternative approaches such as hypofractionated RT combined with immunotherapy are being evaluated in the NRG LUN 004 trial (NCT03801902). The combination of immunotherapy with proton therapy could reduce the cardiopulmonary toxicity associated with conventional RT, thus increasing the safety of hypofractionated schemes. This approach is being prospectively evaluated in another trial (NCT03818776).
At present, access to durvalumab consolidation in Europe is limited to patients with positive PD-L1 expression (> 1%). In addition to the well-known limitations of PD-L1 as a biomarker (interobserver variability, sample heterogeneity, and benefit in tumors without PD-L1 expression, etc.), we must also include temporal variability. RT and CT both alter the tumor microenvironment and PD-L1 expression[66,67]. Therefore, perhaps the decision to administer durvalumab should be based on PD-L1 determination in the tumor sample after completion of concomitant CRT; however, this may be challenging in routine clinical practice (due to patient risks, lack of resources, etc.), and thus more reliable, stable biomarkers are needed.
Some ongoing studies, such as the aforementioned PACIFC-5 trial, will provide prospective data on the drug's efficacy in patients with varying expression level of PD-L1.
Despite the good results achieved with the PACIFIC regimen, a significant proportion of patients still develop disease progression. In fact, 40% of patients required additional treatment after completion of durvalumab therapy, but only 8% received immunotherapy. The efficacy and safety of immunotherapy retreatment in these patients is not known, nor do we know which patient subgroup would most benefit from such an approach (e.g., based on PD-L1 expression, longer progression-free interval, etc.). However, several studies are underway in an effort to answer these questions (NCT03526887), and the results of these trials could potentially be extrapolated to patients treated with durvalumab.
Another area of interest involves cases which progression is exclusively oligometastatic. A recent communication from the ASTRO 2019 congress reported findings from an exploratory analysis of data from the PACIFIC trial, demonstrating that most patients presented evidence of progression in only one or two sites after durvalumab therapy[67]. A retrospective, real-world study that analyzed data from patients treated with durvalumab after concomitant CRT found that nearly half had oligometastatic disease at the first progression[68]. These findings suggest that local ablative therapies could be applied in patients with oligometastatic progression.
Treatment with 12 mo of consolidation durvalumab therapy is the new standard of care in patients with unresectable stage III NSCLC who have received radical-intent concomitant CRT without subsequent progression. The addition of immunotherapy to the treatment of locally-advanced NSCLC represents a paradigm shift based on the findings from the PACIFIC trial showing that consolidation durvalumab (an anti-PD-L1 ICI) significantly improved both PFS and OS compared to placebo. Moreover, the toxicity profile was manageable, with no significant deterioration in quality of life. Even so, implementation of this strategy in routine clinical practice presents numerous challenges, requiring a multidisciplinary approach to decision-making to resolve important controversies such as patient selection, PD-L1 determination, the optimal timing of durvalumab initiation, and changes in clinical work flow practices.
Many questions remain unresolved. However, we will have greater clarity when results are published from the many trials currently underway to evaluate diverse strategies that combine immunotherapy with radical treatment in patients with locally-advanced NSCLC. It seems probable that the management of these patients will continue to evolve in the near future, especially those with stage IIIA disease. Nevertheless, it is evident that immunotherapy for the treatment of locally-advanced NSCLC is here to stay.
Manuscript source: Invited manuscript
Specialty type: Oncology
Country/Territory of origin: Spain
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): B
Grade C (Good): 0
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Zhang K S-Editor: Gao CC L-Editor: Filipodia P-Editor: Wang LL
| 1. | Bray F, Ferlay J, Soerjomataram I, Siegel RL, Torre LA, Jemal A. Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin. 2018;68:394-424. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 53206] [Cited by in RCA: 55773] [Article Influence: 7967.6] [Reference Citation Analysis (132)] |
| 2. | Rami-Porta R, Bolejack V, Crowley J, Ball D, Kim J, Lyons G, Rice T, Suzuki K, Thomas CF Jr, Travis WD, Wu YL; IASLC Staging and Prognostic Factors Committee; Advisory Boards and Participating Institutions. The IASLC Lung Cancer Staging Project: Proposals for the Revisions of the T Descriptors in the Forthcoming Eighth Edition of the TNM Classification for Lung Cancer. J Thorac Oncol. 2015;10:990-1003. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 410] [Cited by in RCA: 561] [Article Influence: 62.3] [Reference Citation Analysis (0)] |
| 3. | Yoon SM, Shaikh T, Hallman M. Therapeutic management options for stage III non-small cell lung cancer. World J Clin Oncol. 2017;8:1-20. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 158] [Cited by in RCA: 186] [Article Influence: 23.3] [Reference Citation Analysis (1)] |
| 4. | Chun SG, Hu C, Choy H, Komaki RU, Timmerman RD, Schild SE, Bogart JA, Dobelbower MC, Bosch W, Galvin JM, Kavadi VS, Narayan S, Iyengar P, Robinson CG, Wynn RB, Raben A, Augspurger ME, MacRae RM, Paulus R, Bradley JD. Impact of Intensity-Modulated Radiation Therapy Technique for Locally Advanced Non-Small-Cell Lung Cancer: A Secondary Analysis of the NRG Oncology RTOG 0617 Randomized Clinical Trial. J Clin Oncol. 2017;35:56-62. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 383] [Cited by in RCA: 541] [Article Influence: 60.1] [Reference Citation Analysis (0)] |
| 5. | Bradley JD, Paulus R, Komaki R, Masters G, Blumenschein G, Schild S, Bogart J, Hu C, Forster K, Magliocco A, Kavadi V, Garces YI, Narayan S, Iyengar P, Robinson C, Wynn RB, Koprowski C, Meng J, Beitler J, Gaur R, Curran W Jr, Choy H. Standard-dose versus high-dose conformal radiotherapy with concurrent and consolidation carboplatin plus paclitaxel with or without cetuximab for patients with stage IIIA or IIIB non-small-cell lung cancer (RTOG 0617): a randomised, two-by-two factorial phase 3 study. Lancet Oncol. 2015;16:187-199. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1197] [Cited by in RCA: 1525] [Article Influence: 152.5] [Reference Citation Analysis (0)] |
| 6. | Ahn JS, Ahn YC, Kim JH, Lee CG, Cho EK, Lee KC, Chen M, Kim DW, Kim HK, Min YJ, Kang JH, Choi JH, Kim SW, Zhu G, Wu YL, Kim SR, Lee KH, Song HS, Choi YL, Sun JM, Jung SH, Ahn MJ, Park K. Multinational Randomized Phase III Trial With or Without Consolidation Chemotherapy Using Docetaxel and Cisplatin After Concurrent Chemoradiation in Inoperable Stage III Non-Small-Cell Lung Cancer: KCSG-LU05-04. J Clin Oncol. 2015;33:2660-2666. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 153] [Cited by in RCA: 195] [Article Influence: 19.5] [Reference Citation Analysis (0)] |
| 7. | Belderbos J, Walraven I, van Diessen J, Verheij M, de Ruysscher D. Radiotherapy dose and fractionation for stage III NSCLC. Lancet Oncol. 2015;156. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 12] [Cited by in RCA: 14] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 8. | Curran WJ Jr, Paulus R, Langer CJ, Komaki R, Lee JS, Hauser S, Movsas B, Wasserman T, Rosenthal SA, Gore E, Machtay M, Sause W, Cox JD. Sequential vs. concurrent chemoradiation for stage III non-small cell lung cancer: randomized phase III trial RTOG 9410. J Natl Cancer Inst. 2011;103:1452-1460. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 760] [Cited by in RCA: 917] [Article Influence: 65.5] [Reference Citation Analysis (1)] |
| 9. | Antonia SJ, Villegas A, Daniel D, Vicente D, Murakami S, Hui R, Kurata T, Chiappori A, Lee KH, de Wit M, Cho BC, Bourhaba M, Quantin X, Tokito T, Mekhail T, Planchard D, Kim YC, Karapetis CS, Hiret S, Ostoros G, Kubota K, Gray JE, Paz-Ares L, de Castro Carpeño J, Faivre-Finn C, Reck M, Vansteenkiste J, Spigel DR, Wadsworth C, Melillo G, Taboada M, Dennis PA, Özgüroğlu M; PACIFIC Investigators. Overall Survival with Durvalumab after Chemoradiotherapy in Stage III NSCLC. N Engl J Med. 2018;379:2342-2350. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1573] [Cited by in RCA: 2057] [Article Influence: 293.9] [Reference Citation Analysis (0)] |
| 10. | Pardoll DM. Immunology beats cancer: a blueprint for successful translation. Nat Immunol. 2012;13:1129-1132. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 139] [Cited by in RCA: 142] [Article Influence: 10.9] [Reference Citation Analysis (0)] |
| 11. | Perez-Gracia JL, Labiano S, Rodriguez-Ruiz ME, Sanmamed MF, Melero I. Orchestrating immune check-point blockade for cancer immunotherapy in combinations. Curr Opin Immunol. 2014;27:89-97. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 81] [Cited by in RCA: 87] [Article Influence: 7.9] [Reference Citation Analysis (0)] |
| 12. | Qin S, Xu L, Yi M, Yu S, Wu K, Luo S. Novel immune checkpoint targets: moving beyond PD-1 and CTLA-4. Mol Cancer. 2019;18:155. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 429] [Cited by in RCA: 829] [Article Influence: 138.2] [Reference Citation Analysis (0)] |
| 13. | Kroemer G, Galluzzi L, Kepp O, Zitvogel L. Immunogenic cell death in cancer therapy. Annu Rev Immunol. 2013;31:51-72. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1808] [Cited by in RCA: 1896] [Article Influence: 158.0] [Reference Citation Analysis (0)] |
| 14. | Kim JE, Patel MA, Mangraviti A, Kim ES, Theodros D, Velarde E, Liu A, Sankey EW, Tam A, Xu H, Mathios D, Jackson CM, Harris-Bookman S, Garzon-Muvdi T, Sheu M, Martin AM, Tyler BM, Tran PT, Ye X, Olivi A, Taube JM, Burger PC, Drake CG, Brem H, Pardoll DM, Lim M. Combination Therapy with Anti-PD-1, Anti-TIM-3, and Focal Radiation Results in Regression of Murine Gliomas. Clin Cancer Res. 2017;23:124-136. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 267] [Cited by in RCA: 354] [Article Influence: 39.3] [Reference Citation Analysis (0)] |
| 15. | Ko EC, Raben D, Formenti SC. The Integration of Radiotherapy with Immunotherapy for the Treatment of Non-Small Cell Lung Cancer. Clin Cancer Res. 2018;24:5792-5806. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 127] [Cited by in RCA: 199] [Article Influence: 28.4] [Reference Citation Analysis (0)] |
| 16. | Torok JA, Salama JK. Combining immunotherapy and radiotherapy for the STAR treatment. Nat Rev Clin Oncol. 2019;16:666-667. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 15] [Cited by in RCA: 25] [Article Influence: 4.2] [Reference Citation Analysis (0)] |
| 17. | Antonia SJ, Villegas A, Daniel D, Vicente D, Murakami S, Hui R, Yokoi T, Chiappori A, Lee KH, de Wit M, Cho BC, Bourhaba M, Quantin X, Tokito T, Mekhail T, Planchard D, Kim YC, Karapetis CS, Hiret S, Ostoros G, Kubota K, Gray JE, Paz-Ares L, de Castro Carpeño J, Wadsworth C, Melillo G, Jiang H, Huang Y, Dennis PA, Özgüroğlu M; PACIFIC Investigators. Durvalumab after Chemoradiotherapy in Stage III Non-Small-Cell Lung Cancer. N Engl J Med. 2017;377:1919-1929. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2750] [Cited by in RCA: 3232] [Article Influence: 404.0] [Reference Citation Analysis (0)] |
| 18. | Gray JE, Villegas A, Daniel D, Vicente D, Murakami S, Hui R, Kurata T, Chiappori A, Lee KH, Cho BC, Planchard D, Paz-Ares L, Faivre-Finn C, Vansteenkiste JF, Spigel DR, Wadsworth C, Taboada M, Dennis PA, Özgüroğlu M, Antonia SJ. Three-Year Overall Survival with Durvalumab after Chemoradiotherapy in Stage III NSCLC-Update from PACIFIC. J Thorac Oncol. 2020;15:288-293. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 218] [Cited by in RCA: 311] [Article Influence: 51.8] [Reference Citation Analysis (0)] |
| 19. | Majem M, Hernández-Hernández J, Hernando-Trancho F, Rodríguez de Dios N, Sotoca A, Trujillo-Reyes JC, Vollmer I, Delgado-Bolton R, Provencio M. Multidisciplinary consensus statement on the clinical management of patients with stage III non-small cell lung cancer. Clin Transl Oncol. 2020;22:21-36. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 20] [Cited by in RCA: 32] [Article Influence: 5.3] [Reference Citation Analysis (0)] |
| 20. | Durm GA, Jabbour SK, Althouse SK, Liu Z, Sadiq AA, Zon RT, Jalal SI, Kloecker GH, Williamson MJ, Reckamp KL, Langdon RM, Kio EA, Gentzler RD, Adesunloye BA, Harb WA, Walling RV, Titzer ML, Hanna NH. A phase 2 trial of consolidation pembrolizumab following concurrent chemoradiation for patients with unresectable stage III non-small cell lung cancer: Hoosier cancer research network LUN 14-179. Cancer. 2020. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 80] [Cited by in RCA: 99] [Article Influence: 19.8] [Reference Citation Analysis (0)] |
| 21. | Gerber DE, Urbanic JJ, Langer C, Hu C, Chang IF, Lu B, Movsas B, Jeraj R, Curran WJ, Bradley JD. Treatment Design and Rationale for a Randomized Trial of Cisplatin and Etoposide Plus Thoracic Radiotherapy Followed by Nivolumab or Placebo for Locally Advanced Non-Small-Cell Lung Cancer (RTOG 3505). Clin Lung Cancer. 2017;18:333-339. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 33] [Cited by in RCA: 45] [Article Influence: 5.0] [Reference Citation Analysis (0)] |
| 22. | Garrido P, Conde E, de Castro J, Gómez-Román JJ, Felip E, Pijuan L, Isla D, Sanz J, Paz-Ares L, López-Ríos F. Updated guidelines for predictive biomarker testing in advanced non-small-cell lung cancer: a National Consensus of the Spanish Society of Pathology and the Spanish Society of Medical Oncology. Clin Transl Oncol. 2020;22:989-1003. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 38] [Cited by in RCA: 59] [Article Influence: 9.8] [Reference Citation Analysis (0)] |
| 23. | Eberhard DA, Giaccone G, Johnson BE; Non-Small-Cell Lung Cancer Working Group. Biomarkers of response to epidermal growth factor receptor inhibitors in Non-Small-Cell Lung Cancer Working Group: standardization for use in the clinical trial setting. J Clin Oncol. 2008;26:983-994. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 229] [Cited by in RCA: 234] [Article Influence: 13.8] [Reference Citation Analysis (0)] |
| 24. | Dietel M, Bubendorf L, Dingemans AM, Dooms C, Elmberger G, García RC, Kerr KM, Lim E, López-Ríos F, Thunnissen E, Van Schil PE, von Laffert M. Diagnostic procedures for non-small-cell lung cancer (NSCLC): recommendations of the European Expert Group. Thorax. 2016;71:177-184. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 114] [Cited by in RCA: 138] [Article Influence: 13.8] [Reference Citation Analysis (0)] |
| 25. | Jennings LJ, Arcila ME, Corless C, Kamel-Reid S, Lubin IM, Pfeifer J, Temple-Smolkin RL, Voelkerding KV, Nikiforova MN. Guidelines for Validation of Next-Generation Sequencing-Based Oncology Panels: A Joint Consensus Recommendation of the Association for Molecular Pathology and College of American Pathologists. J Mol Diagn. 2017;19:341-365. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 349] [Cited by in RCA: 490] [Article Influence: 61.3] [Reference Citation Analysis (0)] |
| 26. | Noll B, Wang WL, Gong Y, Zhao J, Kalhor N, Prieto V, Staerkel G, Roy-Chowdhuri S. Programmed death ligand 1 testing in non-small cell lung carcinoma cytology cell block and aspirate smear preparations. Cancer Cytopathol. 2018;126:342-352. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 79] [Cited by in RCA: 95] [Article Influence: 13.6] [Reference Citation Analysis (0)] |
| 27. | Büttner R, Gosney JR, Skov BG, Adam J, Motoi N, Bloom KJ, Dietel M, Longshore JW, López-Ríos F, Penault-Llorca F, Viale G, Wotherspoon AC, Kerr KM, Tsao MS. Programmed Death-Ligand 1 Immunohistochemistry Testing: A Review of Analytical Assays and Clinical Implementation in Non-Small-Cell Lung Cancer. J Clin Oncol. 2017;35:3867-3876. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 261] [Cited by in RCA: 321] [Article Influence: 40.1] [Reference Citation Analysis (0)] |
| 28. | Boothman AM, Scott M, Ratcliffe M, Whiteley J, Dennis PA, Wadsworth C, Sharpe A, Rizvi NA, Garassino MC, Walker J. Impact of Patient Characteristics, Prior Therapy, and Sample Type on Tumor Cell Programmed Cell Death Ligand 1 Expression in Patients with Advanced NSCLC Screened for the ATLANTIC Study. J Thorac Oncol. 2019;14:1390-1399. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 28] [Cited by in RCA: 42] [Article Influence: 7.0] [Reference Citation Analysis (0)] |
| 29. | Nakhleh RE, Nosé V, Colasacco C, Fatheree LA, Lillemoe TJ, McCrory DC, Meier FA, Otis CN, Owens SR, Raab SS, Turner RR, Ventura CB, Renshaw AA. Interpretive Diagnostic Error Reduction in Surgical Pathology and Cytology: Guideline From the College of American Pathologists Pathology and Laboratory Quality Center and the Association of Directors of Anatomic and Surgical Pathology. Arch Pathol Lab Med. 2016;140:29-40. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 58] [Cited by in RCA: 48] [Article Influence: 5.3] [Reference Citation Analysis (0)] |
| 30. | Grivet F, Montella T, Rufino R, Mafort T, Ferreira CG, Przybysz D. P1.11 Post-Pacific Trial ERA: Patterns of Outcomes and Toxicities From a Single Private Institution in a Developing Country. J Thorac Oncol. 2019;14:S1144-S1145. [RCA] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 1] [Article Influence: 0.2] [Reference Citation Analysis (0)] |
| 31. | Barbaro A, Mienko F, Deng L, Ohri N, Halmos B, Perez-Soler R, Gucalp R, Bodner W, Cheng H. P2.18-11 Real-World Experience of Consolidation Durvalumab for Locally Advanced Non-Small Cell Lung Cancer (NSCLC). J Thorac Oncol. 2019;14:S906-S907. [RCA] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 2] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 32. | Sakaguchi T, Ito K, Furuhashi K, Nakamura Y, Suzuki Y, Nishii Y, Taguchi O, Hataji O. Patients with unresectable stage III non-small cell lung cancer eligible to receive consolidation therapy with durvalumab in clinical practice based on PACIFIC study criteria. Respir Investig. 2019;57:466-471. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 22] [Cited by in RCA: 33] [Article Influence: 5.5] [Reference Citation Analysis (0)] |
| 33. | Shaverdian N, Offin MD, Rimner A, Shepherd AF, Wu AJ, Rudin CM, Hellmann MD, Chaft JE, Gomez DR. Utilization and factors precluding the initiation of consolidative durvalumab in unresectable stage III non-small cell lung cancer. Radiother Oncol. 2020;144:101-104. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 13] [Cited by in RCA: 20] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 34. | Baldini C, Martin Romano P, Voisin AL, Danlos FX, Champiat S, Laghouati S, Kfoury M, Vincent H, Postel-Vinay S, Varga A, Vuagnat P, Ribrag V, Mezquita L, Besse B, Hollebecque A, Lambotte O, Michot JM, Soria JC, Massard C, Marabelle A. Impact of aging on immune-related adverse events generated by anti-programmed death (ligand)PD-(L)1 therapies. Eur J Cancer. 2020;129:71-79. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 28] [Cited by in RCA: 63] [Article Influence: 12.6] [Reference Citation Analysis (0)] |
| 35. | Socinski M, Özgüroğlu M, Villegas A, Daniel D, Vicente D, Murakami S, Hui R, Gray J, Park K, Vincent M, Perrone F, Poole L, Wadsworth C, Dennis P, Antonia S. P1.16-04 Outcomes of Patients <70 or ≥70 Years of Age in PACIFIC. J Thorac Oncol. 2018;13:S628-S629. [RCA] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 2] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 36. | Paz-Ares L, Spira A, Raben D, Planchard D, Cho BC, Özgüroğlu M, Daniel D, Villegas A, Vicente D, Hui R, Murakami S, Spigel D, Senan S, Langer CJ, Perez BA, Boothman AM, Broadhurst H, Wadsworth C, Dennis PA, Antonia SJ, Faivre-Finn C. Outcomes with durvalumab by tumour PD-L1 expression in unresectable, stage III non-small-cell lung cancer in the PACIFIC trial. Ann Oncol. 2020. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 68] [Cited by in RCA: 163] [Article Influence: 32.6] [Reference Citation Analysis (0)] |
| 37. | Garassino M, Faivre-Finn C, Mazieres J, Reck M, Emeribe U, Franks A, Trunova N. P1.01-108 PACIFIC-6: A Phase II Study of Durvalumab Following Sequential Chemoradiotherapy in Patients with Stage III, Unresectable NSCLC. J Thorac Oncol. 2019;14:S403-S404. [RCA] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 2] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 38. | Spigel D, Vansteenkiste J, Reck M, Wakelee H, Özgüroğlu M, Daniel D, Villegas A, Vicente D, Hui R, Murakami S, Paz-Ares L, Poole L, Wadsworth C, Dennis P, Antonia S. P1.16-05 Effect of Induction Chemotherapy in the PACIFIC Study. J Thorac Oncol. 2018;13:S629. [RCA] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 1] [Article Influence: 0.1] [Reference Citation Analysis (0)] |
| 39. | Aupérin A, Le Péchoux C, Rolland E, Curran WJ, Furuse K, Fournel P, Belderbos J, Clamon G, Ulutin HC, Paulus R, Yamanaka T, Bozonnat MC, Uitterhoeve A, Wang X, Stewart L, Arriagada R, Burdett S, Pignon JP. Meta-analysis of concomitant versus sequential radiochemotherapy in locally advanced non-small-cell lung cancer. J Clin Oncol. 2010;28:2181-2190. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1439] [Cited by in RCA: 1368] [Article Influence: 91.2] [Reference Citation Analysis (0)] |
| 40. | Rieber J, Abbassi-Senger N, Adebahr S, Andratschke N, Blanck O, Duma M, Eble MJ, Ernst I, Flentje M, Gerum S, Hass P, Henkenberens C, Hildebrandt G, Imhoff D, Kahl H, Klass ND, Krempien R, Lohaus F, Lohr F, Petersen C, Schrade E, Streblow J, Uhlmann L, Wittig A, Sterzing F, Guckenberger M. Influence of Institutional Experience and Technological Advances on Outcome of Stereotactic Body Radiation Therapy for Oligometastatic Lung Disease. Int J Radiat Oncol Biol Phys. 2017;98:511-520. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 31] [Cited by in RCA: 38] [Article Influence: 4.2] [Reference Citation Analysis (0)] |
| 41. | Vokes EE, Govindan R, Iscoe N, Hossain AM, San Antonio B, Chouaki N, Koczywas M, Senan S. The Impact of Staging by Positron-Emission Tomography on Overall Survival and Progression-Free Survival in Patients With Locally Advanced NSCLC. J Thorac Oncol. 2018;13:1183-1188. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 7] [Cited by in RCA: 10] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 42. | MacManus MP, Hicks RJ, Matthews JP, Hogg A, McKenzie AF, Wirth A, Ware RE, Ball DL. High rate of detection of unsuspected distant metastases by pet in apparent stage III non-small-cell lung cancer: implications for radical radiation therapy. Int J Radiat Oncol Biol Phys. 2001;50:287-293. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 225] [Cited by in RCA: 207] [Article Influence: 8.6] [Reference Citation Analysis (0)] |
| 43. | Brade AM, Wenz F, Koppe F, Lievens Y, San Antonio B, Iscoe NA, Hossain A, Chouaki N, Senan S. Radiation Therapy Quality Assurance (RTQA) of Concurrent Chemoradiation Therapy for Locally Advanced Non-Small Cell Lung Cancer in the PROCLAIM Phase 3 Trial. Int J Radiat Oncol Biol Phys. 2018;101:927-934. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 16] [Cited by in RCA: 23] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 44. |
Kim YH.
Durvalumab after Chemoradiotherapy in Stage III Non-Small-Cell Lung Cancer |
| 45. | Shaverdian N, Lisberg AE, Bornazyan K, Veruttipong D, Goldman JW, Formenti SC, Garon EB, Lee P. Previous radiotherapy and the clinical activity and toxicity of pembrolizumab in the treatment of non-small-cell lung cancer: a secondary analysis of the KEYNOTE-001 phase 1 trial. Lancet Oncol. 2017;18:895-903. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 588] [Cited by in RCA: 831] [Article Influence: 103.9] [Reference Citation Analysis (0)] |
| 46. | Pillai RN, Behera M, Owonikoko TK, Kamphorst AO, Pakkala S, Belani CP, Khuri FR, Ahmed R, Ramalingam SS. Comparison of the toxicity profile of PD-1 versus PD-L1 inhibitors in non-small cell lung cancer: A systematic analysis of the literature. Cancer. 2018;124:271-277. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 169] [Cited by in RCA: 243] [Article Influence: 30.4] [Reference Citation Analysis (0)] |
| 47. | D’Angelillo RM, Ramella S. Pacific trial: a new ocean or an abnormal wave? J Thorac Dis. 2018;10:1225-1226. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 1] [Article Influence: 0.1] [Reference Citation Analysis (0)] |
| 48. | Vansteenkiste J, Naidoo J, Faivre-Finn C, Özgüroğlu M, Villegas A, Daniel D, Murakami S, Hui R, Lee K, Cho BC, Kubota K, Poole L, Wadsworth C, Dennis P, Antonia S. MA05.02 PACIFIC Subgroup Analysis: Pneumonitis in Stage III, Unresectable NSCLC Patients Treated with Durvalumab vs. Placebo After CRT. J Thorac Oncol. 2018;13:S370-S371. [RCA] [DOI] [Full Text] [Cited by in Crossref: 23] [Cited by in RCA: 31] [Article Influence: 4.4] [Reference Citation Analysis (0)] |
| 49. | Sita T, Hassanzadeh C, Savoor R, Samson P, Bradley J, Gentile M, Roach M, Mohindra N, Waqar S, Robinson C, Kruser T. OA03.03 Multi-Institutional Study of Pneumonitis After Treatment with Durvalumab and Chemoradiotherapy for Non-Small Cell Lung Cancer. J Thorac Oncol. 2019;14:S1131-S1132. [RCA] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 2] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 50. | U.S. Department of Health and Human Services. Common Terminology Criteria for Adverse Events (CTCAE) Version 5.0 2017; 2020. Available from: https://ctep.cancer.gov/protocolDevelopment/electronic_applications/docs/CTCAE_v5_Quick_Reference_8.5x11.pdf. |
| 51. | Delaunay M, Prévot G, Collot S, Guilleminault L, Didier A, Mazières J. Management of pulmonary toxicity associated with immune checkpoint inhibitors. Eur Respir Rev. 2019;28. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 35] [Cited by in RCA: 54] [Article Influence: 9.0] [Reference Citation Analysis (0)] |
| 52. | Arbea L, Castanon E, Gil-Bazo I. Guía INMUNO-TOXICIDAD. Diagnóstico y manejo de los efectos secundarios asociados a inmunoterapia en oncología. Primera edición ed. Navarra: EUNSA, 2018. |
| 53. | Hui R, Özgüroğlu M, Villegas A, Daniel D, Vicente D, Murakami S, Yokoi T, Chiappori A, Lee KH, de Wit M, Cho BC, Gray JE, Rydén A, Viviers L, Poole L, Zhang Y, Dennis PA, Antonia SJ. Patient-reported outcomes with durvalumab after chemoradiotherapy in stage III, unresectable non-small-cell lung cancer (PACIFIC): a randomised, controlled, phase 3 study. Lancet Oncol. 2019;20:1670-1680. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 77] [Cited by in RCA: 129] [Article Influence: 21.5] [Reference Citation Analysis (0)] |
| 54. | Biswas T, Sharma N, Machtay M. Controversies in the management of stage III non-small-cell lung cancer. Expert Rev Anticancer Ther. 2014;14:333-347. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 13] [Cited by in RCA: 14] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 55. | Pisters KM, Evans WK, Azzoli CG, Kris MG, Smith CA, Desch CE, Somerfield MR, Brouwers MC, Darling G, Ellis PM, Gaspar LE, Pass HI, Spigel DR, Strawn JR, Ung YC, Shepherd FA; Cancer Care Ontario; American Society of Clinical Oncology. Cancer Care Ontario and American Society of Clinical Oncology adjuvant chemotherapy and adjuvant radiation therapy for stages I-IIIA resectable non small-cell lung cancer guideline. J Clin Oncol. 2007;25:5506-5518. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 251] [Cited by in RCA: 276] [Article Influence: 15.3] [Reference Citation Analysis (0)] |
| 56. | Wang EH, Corso CD, Rutter CE, Park HS, Chen AB, Kim AW, Wilson LD, Decker RH, Yu JB. Postoperative Radiation Therapy Is Associated With Improved Overall Survival in Incompletely Resected Stage II and III Non-Small-Cell Lung Cancer. J Clin Oncol. 2015;33:2727-2734. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 62] [Cited by in RCA: 72] [Article Influence: 7.2] [Reference Citation Analysis (0)] |
| 57. | Francis S, Orton A, Stoddard G, Tao R, Hitchcock YJ, Akerley W, Kokeny KE. Sequencing of Postoperative Radiotherapy and Chemotherapy for Locally Advanced or Incompletely Resected Non-Small-Cell Lung Cancer. J Clin Oncol. 2018;36:333-341. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 27] [Cited by in RCA: 39] [Article Influence: 4.9] [Reference Citation Analysis (0)] |
| 58. | Eberhardt WE, Pöttgen C, Gauler TC, Friedel G, Veit S, Heinrich V, Welter S, Budach W, Spengler W, Kimmich M, Fischer B, Schmidberger H, De Ruysscher D, Belka C, Cordes S, Hepp R, Lütke-Brintrup D, Lehmann N, Schuler M, Jöckel KH, Stamatis G, Stuschke M. Phase III Study of Surgery Versus Definitive Concurrent Chemoradiotherapy Boost in Patients With Resectable Stage IIIA(N2) and Selected IIIB Non-Small-Cell Lung Cancer After Induction Chemotherapy and Concurrent Chemoradiotherapy (ESPATUE). J Clin Oncol. 2015;33:4194-4201. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 197] [Cited by in RCA: 258] [Article Influence: 25.8] [Reference Citation Analysis (0)] |
| 59. | Forde PM, Chaft JE, Smith KN, Anagnostou V, Cottrell TR, Hellmann MD, Zahurak M, Yang SC, Jones DR, Broderick S, Battafarano RJ, Velez MJ, Rekhtman N, Olah Z, Naidoo J, Marrone KA, Verde F, Guo H, Zhang J, Caushi JX, Chan HY, Sidhom JW, Scharpf RB, White J, Gabrielson E, Wang H, Rosner GL, Rusch V, Wolchok JD, Merghoub T, Taube JM, Velculescu VE, Topalian SL, Brahmer JR, Pardoll DM. Neoadjuvant PD-1 Blockade in Resectable Lung Cancer. N Engl J Med. 2018;378:1976-1986. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1051] [Cited by in RCA: 1475] [Article Influence: 210.7] [Reference Citation Analysis (0)] |
| 60. | Provencio-Pulla M, Nadal-Alforja E, Cobo M, Insa A, Costa Rivas M, Majem M, Rodriguez-Abreu D, Lopez-Vivanco G, Domine M, Del Barco Morillo E, Massuti B, Campelo RG, Martinez Marti A, Bernabé R, Franco F, Jove M, Arrabal R, Martin P, Casal J, Calvo V. Neoadjuvant chemo/immunotherapy for the treatment of stages IIIA resectable non-small cell lung cancer (NSCLC): A phase II multicenter exploratory study—NADIM study-SLCG. J Clin Oncol. 2018;36:8521. [RCA] [DOI] [Full Text] [Cited by in Crossref: 32] [Cited by in RCA: 32] [Article Influence: 4.6] [Reference Citation Analysis (0)] |
| 61. | Gong X, Li X, Jiang T, Xie H, Zhu Z, Zhou F, Zhou C. Combined Radiotherapy and Anti-PD-L1 Antibody Synergistically Enhances Antitumor Effect in Non-Small Cell Lung Cancer. J Thorac Oncol. 2017;12:1085-1097. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 130] [Cited by in RCA: 219] [Article Influence: 27.4] [Reference Citation Analysis (0)] |
| 62. | Peters S, Felip E, Dafni U, Belka C, Guckenberger M, Irigoyen A, Nadal E, Becker A, Vees H, Pless M, Martinez-Marti A, Tufman A, Lambrecht M, Andratschke N, Piguet AC, Kassapian M, Roschitzki-Voser H, Rabaglio-Poretti M, Stahel RA, Vansteenkiste J, De Ruysscher D. Safety evaluation of nivolumab added concurrently to radiotherapy in a standard first line chemo-radiotherapy regimen in stage III non-small cell lung cancer-The ETOP NICOLAS trial. Lung Cancer. 2019;133:83-87. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 78] [Cited by in RCA: 117] [Article Influence: 19.5] [Reference Citation Analysis (0)] |
| 63. | Jabbour SK, Berman AT, Decker RH, Lin Y, Feigenberg SJ, Gettinger SN, Aggarwal C, Langer CJ, Simone CB 2nd, Bradley JD, Aisner J, Malhotra J. Phase 1 Trial of Pembrolizumab Administered Concurrently With Chemoradiotherapy for Locally Advanced Non-Small Cell Lung Cancer: A Nonrandomized Controlled Trial. JAMA Oncol. 2020;6:848-855. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 52] [Cited by in RCA: 104] [Article Influence: 26.0] [Reference Citation Analysis (0)] |
| 64. | Lin SH, Lin Y, Yao L, Kalhor N, Carter BW, Altan M, Blumenschein G, Byers LA, Fossella F, Gibbons DL, Kurie JM, Lu C, Simon G, Skoulidis F, Chang JY, Jeter MD, Liao Z, Gomez DR, O'Reilly M, Papadimitrakopoulou V, Thall P, Heymach JV, Tsao AS. Phase II Trial of Concurrent Atezolizumab With Chemoradiation for Unresectable NSCLC. J Thorac Oncol. 2020;15:248-257. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 45] [Cited by in RCA: 101] [Article Influence: 16.8] [Reference Citation Analysis (0)] |
| 65. | Ohri N, Cheng H, Jolly S, Gadgeel SM, Cooper BT, Shum E, Halmos B. The selective personalized radioimmunotherapy for locally advanced NSCLC trial (SPRINT). J Clin Oncol. 2019;37:TPS8571. [RCA] [DOI] [Full Text] [Cited by in Crossref: 5] [Cited by in RCA: 5] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 66. | Dovedi SJ, Cheadle EJ, Popple AL, Poon E, Morrow M, Stewart R, Yusko EC, Sanders CM, Vignali M, Emerson RO, Robins HS, Wilkinson RW, Honeychurch J, Illidge TM. Fractionated Radiation Therapy Stimulates Antitumor Immunity Mediated by Both Resident and Infiltrating Polyclonal T-cell Populations when Combined with PD-1 Blockade. Clin Cancer Res. 2017;23:5514-5526. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 252] [Cited by in RCA: 277] [Article Influence: 34.6] [Reference Citation Analysis (0)] |
| 67. | Raben D, Rimner A, Senan S, Broadhurst H, Pellas T, Dennis PA, Faivre-Finn C. Patterns of Disease Progression with Durvalumab in Stage III Non-small Cell Lung Cancer (PACIFIC). Int J Radiat Oncol Biol Phys. 2019;105:683. [RCA] [DOI] [Full Text] [Cited by in Crossref: 26] [Cited by in RCA: 52] [Article Influence: 8.7] [Reference Citation Analysis (0)] |
| 68. | Offin M, Shaverdian N, Rimner A, Lobaugh S, Shepherd AF, Simone CB 2nd, Gelblum DY, Wu AJ, Lee N, Kris MG, Rudin CM, Zhang Z, Hellmann MD, Chaft JE, Gomez DR. Clinical outcomes, local-regional control and the role for metastasis-directed therapies in stage III non-small cell lung cancers treated with chemoradiation and durvalumab. Radiother Oncol. 2020;149:205-211. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 19] [Cited by in RCA: 46] [Article Influence: 9.2] [Reference Citation Analysis (0)] |