Published online Oct 24, 2020. doi: 10.5306/wjco.v11.i10.809
Peer-review started: February 24, 2020
First decision: July 25, 2020
Revised: August 9, 2020
Accepted: September 14, 2020
Article in press: September 14, 2020
Published online: October 24, 2020
Processing time: 241 Days and 16.7 Hours
Cancer patients account for 15% of all admissions to intensive care unit (ICU) and 5% will experience a critical illness resulting in ICU admission. Mortality rates have decreased during the last decades because of new anticancer therapies and advanced organ support methods. Since early critical care and organ support is associated with improved survival, timely identification of the onset of clinical signs indicating critical illness is crucial to avoid delaying. This article focused on relevant and current information on epidemiology, diagnosis, and treatment of the main clinical disorders experienced by critically ill cancer patients.
Core Tip: Cancer patients are commonly admitted to intensive care unit because of acute respiratory failure due to pulmonary infiltrates or pneumonia, healthcare associated infection by multidrug-resistant pathogens, postoperative care, cardiovascular complications, and neurological disorders. Early critical care and organ support is associated with improved outcomes. Standardized diagnosis strategy and evidence-based therapy are critical in the management of specific clinical disorders.
- Citation: Martos-Benítez FD, Soler-Morejón CD, Lara-Ponce KX, Orama-Requejo V, Burgos-Aragüez D, Larrondo-Muguercia H, Lespoir RW. Critically ill patients with cancer: A clinical perspective. World J Clin Oncol 2020; 11(10): 809-835
- URL: https://www.wjgnet.com/2218-4333/full/v11/i10/809.htm
- DOI: https://dx.doi.org/10.5306/wjco.v11.i10.809
Cancer is one of the main causes of morbidity and mortality worldwide[1]. Overall cancer-death rates have decreased in both men and women due to reduced tobacco use, improved early detection (e.g., colorectal, breast, and cervix) and enhanced treatment options[2-4]. Currently, critical care medicine contributes as a supportive care for patients with cancer.
In the past decades, patients with advanced hematological or oncological disease were not candidates for entry to intensive care unit (ICU) due to low survival rates; however, expectancy in life-span has changed over the last 25 years because of a breakthrough in terms of new anticancer therapies and organ support methods[5]. Cancer patients account for 15% of all admissions to ICU[6] and 5% will experience a critical illness resulting in ICU admission[7]. These frequencies may grow considering the current global burden of cancer and demographic features. Analysis from large databases suggests that a higher proportion of cancer patients survive at ICU discharge[7,8]. Since the five-year survival rate is 41%[9], reluctance to admit cancer patients to the ICU must be avoided from medical practice. In fact, most recent evidences support an increased ICU admission because of improved ICU and hospital outcomes[8-11].
Patients with hematological malignancy or solid tumor are at higher risk for ICU admission as a consequence of acute respiratory failure due to pulmonary infiltrates or pneumonia, healthcare associated infection by multidrug-resistant pathogens related or not with immunosuppression, postoperative care, cardiovascular complications, and neurological disorders[6,12-14].
The aim of this article was to provide critical care clinicians with an overview on relevant and current information on epidemiology, diagnosis, and treatment of the main clinical disorders experienced by cancer patients with a critical illness.
The main reasons for admitting cancer patients to ICU are postoperative care, acute respiratory failure (ARF), and sepsis. Other clinical situations are cardiac complications, neurological disorders, acute kidney injury, bleeding, and oncological emergencies[15]. Mucositis, acute graft-versus-host disease, diffuse alveolar hemorrhage, cardiac dysfunction, hypertension and hepatic venoocclusive disease are other causes in hematological malignancies[16] (Table 1).
| Cause | Comment |
| Postoperative care | Elective or emergency |
| Acute respiratory failure | (1) Infectious: Bacterial, viral, fungal; and (2) Noninfectious: Diffuse alveolar hemorrhage, interstitial lung disease, pulmonary drug toxicity, transfusion-related acute lung injury |
| Cardiovascular disorders | Sepsis and septic shock, pulmonary embolism, drug-induced cardiomyopathy |
| Bleeding disorders | Tumor erosion, coagulopathy, thrombocytopenia |
| Alteration of mental status | (1) Metabolic: Sepsis, drugs, multiorgan system failure, seizure, hyponatremia, hypoxia, hipercapnea; (2) Mass effect: Central nervous system bleeding, tumor effects; and (3) Others: Posterior reversible encephalopathy syndrome |
| Oncologic emergency | Tumor lysis syndrome, leukostasis, superior vena cava syndrome, cardiac tamponade, hipercalcemia |
| Acute decompensated chronic comorbidity | Chronic obstructive pulmonary disease, cardiac disorders (e.g., cardiomyopathy, coronary artery disease), chronic kidney disease, chronic hepatopathy |
| Others | Initiation of chemotherapy for surveillance |
Several studies indicate that early ICU admission is associated with higher survival rates[17-21]; thus, timely identification of patients at onset of clinical signs indicating critical deterioration is crucial to avoid delayed organ support[6]. The ability of physician to identify what patient is expected to benefit from ICU management is limited. As proposed by Ñamendys-Silva et al[22], the following criteria may help for this purpose: Sequential Organ Failure Assessment (SOFA) score between 7 and 10 or < 3 organ failures, recent diagnosis of oncohematological disease, cancer-related medical emergencies (e.g., tumor lysis syndrome, pulmonary infiltrates in patients with leukemia or leukostasis as the initial manifestation of leukemia), likelihood of cure or disease control, Eastern Cooperative Oncology Group scale between 0 and 2, and postoperative intensive care for patients undergoing complex surgical procedures who require hemodynamic monitoring and/or ventilatory support.
As decision-making for ICU admission and management may be challenging, the following strategy is recommended: (1) Full-code ICU management: Full organ support methods (e.g., invasive mechanical ventilation, vasopressors, renal replacement therapy, nutritional support) without limitations of ICU resources for patients with curative therapeutic options, patients in remission, and those with expected life-span ≥ 1 year[5]; (2) Time-limited ICU trial: ICU management with full-code status for a limited period. Although the time of full-code should be judged in accordance with the patient´s clinical course rather than with a fixed time, a reasonable interval could be 2 wk in hematology patients (1 wk if multiple organ failure) and 1 wk in patients with solid tumors (4-5 d if multiple organ failure)[5,6,23]; (3) Patients with poor performance status not eligible for further anticancer therapy, dying patients, and those rejecting critical care treatment should not be admitted to the ICU in general[5]; and (4) Other indications: Exceptional ICU admission (same as time-limited ICU trial) for patients in whom new drugs (approved or not) are available; prophylactic ICU admission with full-code for high-risk patients (e.g., patients at risk of tumor lysis syndrome or acute respiratory failure after chemotherapy); palliative ICU admission for optimizing medical care with noninvasive strategies (e.g., noninvasive ventilation, vasopressors without invasive hemodynamic monitoring, electrical cardioversion, pneumothorax decompression, optimizing pain relieve)[6].
ARF is the leading cause of unplanned ICU admission in cancer patients[24]. The incidence is higher in patients with acute lymphoblastic leukemia, acute myeloid leukemia, hematopoietic stem cell transplant (HSCT), neutropenia, and lung cancer[25-29]. Ventilatory support is used in 35%-50% of critically ill cancer patients[11,30,31]; the associated ICU and hospital mortality rate is as high as 50% and 65%, respectively[31,32] (Table 2). Mortality-related factors in patients with ARF can be grouped into five categories: (1) Respiratory failure associated with organ dysfunction; (2) Factors inherent to delayed ICU admission; (3) Factors associated with chronic underlying comorbidities; (4) Factors involved with the initial treatment of respiratory failure; and (5) Factors related to the etiology of respiratory failure.
| Incidence | Need for ICU admission | Hospital mortality | |
| Hematological malignancy | |||
| Acute myeloid leukemia | 22%-84 % | 66% | 45% |
| Acute lymphoblastic leukemia | 7%-18.5% | 12%-15% | 38.5% |
| Lymphoproliferative diseases | 8% | 8% | 40%-50% |
| Myelodysplastic syndrome | 29.4% | 20% | 17% |
| Autologous hematopoietic stem cell transplant | 3%-28% | 42% | 3%-55% |
| Allogeneic hematopoietic stem cell transplant | 24%-30% | 50% | 51% |
| Prolonged neutropenia | 8%-29.5% | 11%-16% | 5%-12% |
| Solid tumor | |||
| Lung cancer | 26%-50% | 100% | 11.2%-60% |
| Other solid tumors | 0.7%-10.3% | 100% | 6.1%-55% |
| Patients on immunotherapy | 1.3%-3.6% | 1.3% | - |
Five major pathophysiological mechanisms can explain ARF in cancer patients (Table 3). Ventilation/ perfusion mismatch is the most common mechanism, usually caused by pulmonary infiltrates, pneumonia, atelectasis or pulmonary embolism. Increased intrapulmonary shunt occurs in primary or secondary acute respiratory distress syndrome. Drug-associated interstitial lung disease and high-degree metastasized lungs explain disorders of oxygen diffusion[33].
| Mechanism | PaO2 | PaCO2 | DA-aO2 | Comments |
| Disorders in oxygen diffusion | ↓ | ↓ | ↑ | Decreased surface area or short time for hematosis |
| Ventilation/ perfusion mismatch | ↓ | ↑ | ↑ | (1) Decreased ventilation in normally perfused lung regions |
| Increased intrapulmonary shunt | ↓ | ↓ | ↑↑ | Pulmonary venous blood bypasses ventilated alveoli |
| Hypoventilation | ↓ | ↑↑ | N | Hypoventilation |
| Decrease in pressure of inspired oxygen | ↓ | ↓ | N | Decreased pressure of inspired oxygen |
Primary tumor location, clinical stage, admission from the emergency department, medical admission, malignancy-unrelated ICU admission, sepsis, adverse event to chemotherapy, and Acute Physiology And Chronic Health Evaluation (APACHE) II score have been found as risk factor for severe ARF requiring invasive mechanical ventilation[34]. Causes of ARF in patients with cancer are depicted in Table 4[35].
| CNS and neuromuscular disorders | Chest wall and pleural disorders | Vascular disorders | Airway disorders | Parenchymal disorders |
| Drug intoxications: Narcotics; Sedatives; Neuroleptics | Pleural disorder: Malignant pleural effusion; Pleural tumor (primary or metastatic); Tension pneumothorax | Acute pulmonary embolism; Tumor embolism; Pulmonary venooclusive disease | Airway obstruction: Endobronchial metastases; External airway compression; Primary tumor of periglottic area | Pneumonitis: Infection; Chemotherapy; Radiotherapy; Aspiration |
| Encephalopathies: Infection; Metabolic; Seizure | Chest wall disorders: Chest wall tumor (primary or metastatic); Rib fracture | Others: Tracheoesophageal fistula; Bronchiolitis obliterans | Acute respiratory distress syndrome: Infection; Chemotherapy; Radiotherapy; Transfusion | |
| Intracranial tumors: Primary; Metastatic | Complications of HSCT: Peri-engraftment respiratory distress syndrome; Diffuse alveolar hemorrhage; Idiopathic pneumonia syndrome | |||
| Neuropathies/myopathies: Nerve palsy | Others: Lymphangitic carcinomatosis; Pulmonary leukostasis; Bronchiolitis obliterans organizing pneumonia | |||
| Paraneoplastic syndromes: Eaton-Lambert syndrome; Myasthenia gravis; Guillain-Barré syndrome |
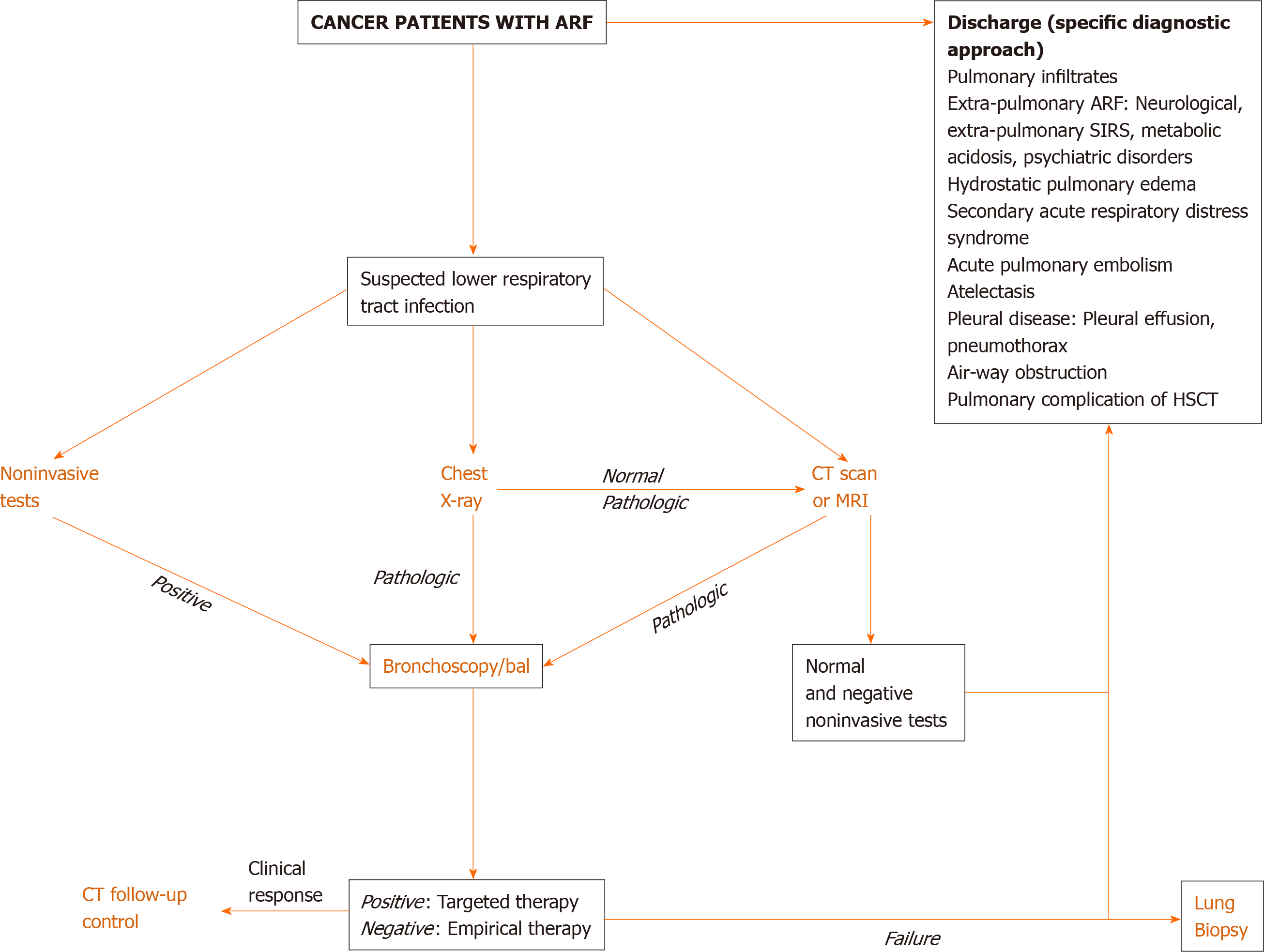
Direct actions focused on the best therapeutic options are required in cancer patients with ARF. Clinical examination is crucial since pulmonary infiltrates and respiratory symptoms (e.g., increased respiratory rate, cough, sputum, rales, thoracic pain, and hemoptysis) are associated with increased ventilatory support and mortality rates[13]. DIRECT approach may suggest the cause of ARF[36]: Identification of the type and duration (D) of respiratory symptoms, assessment of immunosuppressive (I) therapy, interpretation of X-ray (R) pattern, clinician’s experience (E), clinical (C) finding, and high-resolution computed tomography scan (T)[36,37]. Figure 1 depicts a diagnostic algorithm for ARF.
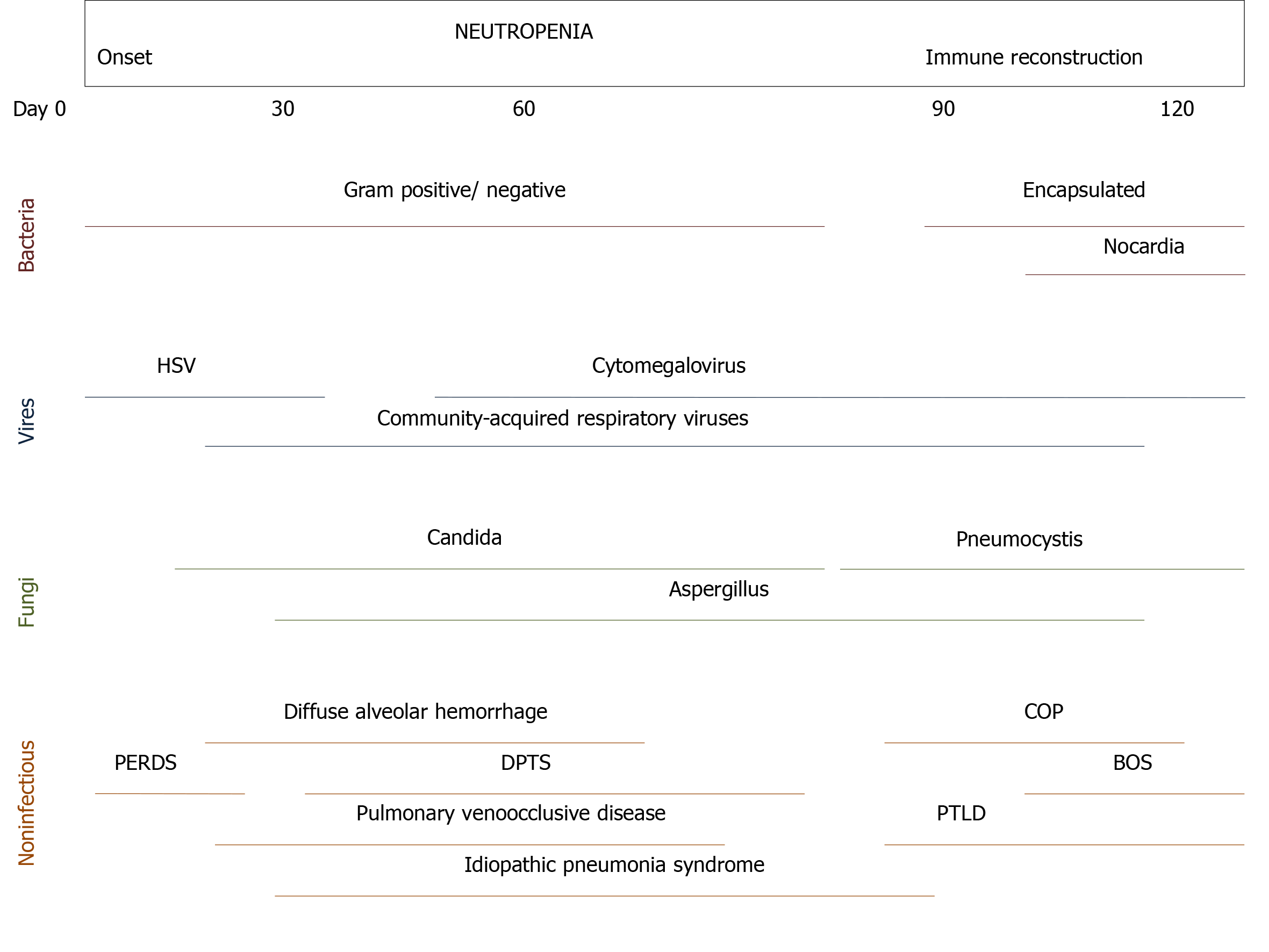
Bacterial infection, usually in immunocompromised patients, is the main cause of ARF[30]. It is common in early stages of lymphoproliferative disorders[38]. Opportunistic infections have been reported prior to initiation of anticancer therapy in patients with T-cell diseases[39]. Noninfectious causes (e.g., lung infiltrates, leukemic infiltrates, diffuse alveolar hemorrhage, drug-related interstitial lung diseases, and noninfectious lung diseases after HSCT) are difficult to identify, therefore more invasive diagnostic studies are needed for reaching diagnosis such as bronchoscopy and bronchoalveolar lavage (BAL)[25,26,40]. Thrombocytopenia, bleeding disorders, and hypoxemia may preclude bronchoscopy and/or lung biopsy. Pattern of computed tomography (CT) scan may help to identify the cause of ARF[37]. Table 5 summarizes invasive and noninvasive diagnostic procedures in cancer patients with ARF. Infectious and noninfectious causes of pulmonary complications following HSCT are depicted in Figure 2[41].
| Diagnostic procedure | Comments |
| Blood cultures | Hospital-acquired bacteria |
| Multislice or high-resolution CT scan | In most cases without contrast media; MRI if a pulmonary CT scan is not feasible |
| Echocardiography | Cardiac evaluation |
| Sputum examination | Bacteria; Fungi; Mycobacteria |
| Induced sputum | Pneumocystis jiroveci |
| Nasopharyngeal aspirates or nasal swabs | Adenovirus, metapneumovirus, coronavirus, parainfluenza virus types 1, 2, 3 and 4; influenza virus types A and B, respiratory syncytial virus A and B; rhinovirus A, B, and C; bocavirus and enterovirus |
| Polymerase chain reaction blood test | Herpesviridae; Cytomegalovirus; Epstein-Barr virus |
| Circulating Aspergillus galactomannan | Aspergillus spp. |
| Serologic tests | Chlamydia pneumoniae; Mycoplasma pneumoniae; Legionella pneumophila |
| Urine antigen | Legionella pneumophila; Streptococcus pneumoniae |
| BAL (mandatory) | (1) Cytospin preparation including Giemsa stain for cytological diagnostics and Gram stain; (2) Quantitative or semi-quantitative bacteriological cultures including culture media to detect Legionella spp., mycobacteria and fungi; (3) Calcofluor white or equivalent stain (assessment of fungi); (4) Quantitative (if possible) PCR for Pneumocystis jirovecii; (5) Direct immunofluorescence test for Pneumocystis jirovecii; (6) Aspergillus antigen (Galactomannan ELISA); and (7) Mycobacterium tuberculosis PCR, atypical mycobacteria |
| BAL (optional) | (1) PCR for cytomegalovirus, respiratory syncytial virus, influenza A/B virus, parainfluenza virus, human metapneumovirus, adenovirus, varicella zoster virus, and Pneumocystis jirovecii (quantitative); and (2) Aspergillus antigen (Galactomannan ELISA); Panfungal or Aspergillus/ mucormycetes PCR |
| Transbronchial biopsies | Not recommended in general in febrile neutropenic and/or thrombocytopenic patients as the first line procedure |
Etiology of ARF may be identified using the information of clinical, laboratory, imagenological, and invasive investigations as following[25,42-44]: (1) Acute or subacute onset; symptoms of upper respiratory tract with fever plus centrilobular nodules or ground-glass opacities on CT scan: Viral infection or atypical pneumonia. Exclude bacterial co-infection; (2) Acute onset; suspect bacterial infection plus alveolar consolidation on X-ray o CT scan: Bacterial infection. Consider bronchoscopy and BAL if sputum cannot be obtained; (3) Subacute onset; T-cell deficiency without prophylaxis for Pneumocystis jirovecii plus diffuse ground-glass opacities on CT scan: Pneumocystis pneumonia; (4) Subacute onset; risk factors for invasive aspergillosis (e.g., prolonged neutropenia, allogeneic HSCT, graft versus host disease, T-cell deficiency) plus consolidation or cavitation on X-ray or CT scan: Invasive pulmonary aspergillosis; and (5) Acute or subacute onset; variable clinical presentation: Disease-related infiltrates, diffuse alveolar hemorrhage, alveolar proteinosis, drug-related pulmonary toxicity.
Hydrostatic pulmonary edema (Biomarkers: Natriuretic peptide or N-terminal pro-B-type natriuretic peptide; echocardiography), pulmonary embolism (Biomarkers: D-dimer, high-sensitive cardiac troponin; echocardiography; CT pulmonary angiogram), pleural effusion/ pneumothorax (X-ray; ultrasound; CT scan), and cardiac tamponade (Echocardiography) should be ruled out.
Treatment of cancer patients with ARF is focused to restore oxygenation, relieve dyspnea and respiratory distress, and improve patient comfort[25]. Mortality rates remain high[17,34]; thus, noninvasive devices are preferred. Although early noninvasive ventilation (NIV) was associated with improved survival rates[45], failure of NIV or high-flow nasal oxygen therapy (HFNO) was associated with increased mortality[24,32]. The most challenging issue is choosing those patients in which a specific respiratory strategy is beneficial over others. Physicians need to consider the following risk factors for NIV failure[5]: (1) Prior to NIV: Vasopressor need, multiple organ failure, airway involvement by malignancy, acute respiratory distress syndrome, unknown etiology of ARF, and delayed-onset ARF; and (2) During NIV: Patient not tolerating NIV, not improvement of arterial blood gases within 6 h, respiratory rate > 30 breath per minute, NIV dependency ≥ 3 d, clinical or respiratory deterioration, and unknown etiology of ARF.
A trial of NIV is recommended for most patients with ARF by reversible underlying cause[32,45,46]; however, HFNO is a promising alternative to NIV[47-50]. In a France-Belgium 28-center-randomized controlled trial of 374 immunocompromised patients with ARF, Lemiale et al[51] found no difference in primary and secondary outcomes between intermittent NIV and standard oxygen therapy. A recent meta-analysis of immunocompromised patients showed that intubation rate was lower in the HFNO group than those in the conventional oxygen therapy group and NIV group; however, HFNO did not improve survival or length of stay[52]. The ongoing FLORALI-IM randomized controlled trials may contribute to clarify these findings[53].
Chemotherapy in patients with cancer has resulted in improved survival, although increased the number of cases with neutropenia. Hematological malignancies and myelodysplastic syndromes are other causes of neutropenia[54,55]. Neutropenia is related to severe invasive infections, septic shock, multiple organ dysfunction, and increased mortality[56,57]. Mokart et al[57] found a hospital mortality rate of 45.3% in patients admitted to ICU. In 7512 critically ill patients with cancer included in a recent systematic review, neutropenia was independently associated with unfavorable outcomes; nevertheless, granulocyte colony-stimulating factor was related to reduced mortality rate[58].
According to the absolute neutrophil count, neutropenia is classified as mild (1000-1500 cells/mm3), moderate (500-999 cells/mm3), severe (100-499 cells/mm3), and deep (< 100 cells/mm3)[54]. Infection usually appear with severe or deep neutropenia[59]. Febrile neutropenia (FN) is defined as a single oral or axillary temperature > 38.3 °C (101 °Fahrenheit) or a temperature > 38.0 °C (100.4 °Fahrenheit) sustained over 60 min in patients with severe neutropenia[56].
Fever may be the earliest and only sign of infection in neutropenic cancer patient. The incidence of FN varies between 10% and 50% in patients with solid tumors receiving antineoplastic therapy and up to 80% in patients with hematological malignancies[54]. The risk of infection is high in severe neutropenia, moderate neutropenia expected to decline to severe within 48 h, and moderate neutropenia lasting more than seven days.
The main independent prognostic factors for mortality in neutropenic patient are age > 60 years, APACHE scores, Simplified Acute Physiology Score scores, SOFA score, need for mechanical ventilation, high serum procalcitonin, need for renal replacement therapy, and allogeneic HSCT[10,55,57].
Risk-stratification scores allow a quick and objective risk assessment. Several risk scores have been validated to evaluate the risk of complications in patients with FN (Table 6[54,60-62]). Because increased complication and mortality rates, high-risk patients are the following: Group 1-3 of the Talcott classification system, < 20 points in the Multinational Association for Supportive Care in Cancer risk index, and ≥ 3 points in the Clinical Index of Stable Febrile Neutropenia score. High-risk patients generally require in-hospital treatment and intravenous administration of broad-spectrum antibiotics[60-62].
| Description/Criteria | Group/ Points |
| Talcott classification system | |
| Patients hospitalized at onset of fever and neutropenia (inpatient at presentation) | 1 |
| Outpatients at presentation but with comorbidities which require hospitalization | 2 |
| Outpatients at presentation with uncontrolled cancer but without comorbidities | 3 |
| Outpatients at presentation without comorbidities and controlled cancer | 4 |
| Multinational association of supportive care of cancer (MASCC) risk-index | |
| Burden of febrile neutropenia | |
| No or mild symptoms: No fever, hemodynamic compromise or clinically significant signs and symptoms of particular site of infection | 5 |
| Moderate symptoms: Any others not included in mild or severe symptoms | 3 |
| Severe symptoms: High grade fever, any hemodynamic compromise or any of the serious complications requiring high dependency unit support | 0 |
| No hypotension (systolic blood pressure > 90 mmHg) | 5 |
| Solid tumor or hematological malignancy with no previous fungal infection | 4 |
| No chronic obstructive pulmonary disease | 4 |
| No dehydration requiring parenteral fluids | 3 |
| Outpatient status | 3 |
| Age < 60 yr | 2 |
| Clinical Index of Stable Febrile Neutropenia (CISNE) score | |
| Eastern Cooperative Oncology Group performance status ≥ 2 | 2 |
| Stress-induced hyperglycemia | 2 |
| Chronic obstructive pulmonary disease (on steroids, supplemental oxygen, or bronchodilators) | 1 |
| Chronic cardiovascular disease (excluding single uncomplicated episode of atrial fibrillation) | 1 |
| Mucositis (at least the presence of patchy ulcerations or pseudomembranes, or moderate pain with modified diet) | 1 |
| Monocytes < 200 cells/mm3 | 1 |
Other risk factors in high-risk patients are the following[55,63-65]: (1) Planned deep neutropenia for more than 7 d; (2) Evidence of liver failure: Abnormal aminotransferases > 5-fold upper limit of normal value or hyperbilirubinemia; (3) Renal impairment: Serum creatinine increase > 50% or > 26.5 μmol/L within 48 h, urine output < 0.5 mL/kg/h for 6 h, or increased concentration in newer biomarkers for sepsis associated-acute kidney injury (e.g., insulin like growth factor binding protein-7, kidney injury molecule-1, neutrophil gelatinase associated lipocalin, tissue inhibitor of metalloproteinase-2); and (4) Pathophysiological imbalance and comorbidities such as, but not limited to: (a) Hemodynamic instability: Hypotension, decreased capillary refill or mottling, hyperlactatemia, central venous oxygen saturation < 70%, and central venous-to-arterial carbon dioxide difference > 6.0 mmHg; (b) Oral or gastrointestinal mucositis interfering with swallowing; (c) Gastrointestinal symptoms: Ileus, severe diarrhea, pain, nausea, and vomiting; (d) Neurological disorders or changes in mental status; (e) Intravascular catheter-related infection; (f) New pulmonary infiltrates or hypoxemia, or decompensated chronic lung disease; and (g) Coagulation abnormalities: International normalized ratio > 1.5, activated partial thromboplastin time > 60 s, or platelet count < 100000 cells/mm3.
Most patients with FN have scarce clinical features. Clinically documented infection is only reported in 20%-35%[54]. Thus, the International Immunocompromised Host Society recommends three categories of patients[66]: (1) Microbiologically documented infection: Clinical site of infection and the associated pathogen is identified; (2) Clinically documented infection: Clinical site of infection is identified, but without isolation of the pathogen; and (3) Unexplained fever: Clinical site of infection and pathogen are not identified. The most patients with FN have unexplained fever.
Since the risk of infection is related to the intensity and duration of neutropenia, the risk for developing FN and its severity must be anticipated for an early diagnosis and treatment of unexplained fever; underlying disease, immune status, co-morbidities, and type of intervention (e.g., chemotherapy scheme, intrinsic hematological toxicity, dose and duration) need to be evaluated. Qualitative disorders of neutrophil function may also increase the risk of infections even with normal neutrophil count[55].
There has been a change in the epidemiological patterns of infections because of a wide spread multidrug-resistant bacteria amongst humans, animals and environmental reservoirs[67]. Microorganisms causing infection mostly come from the normal flora of the skin, oropharyngeal cavity, and gastrointestinal tract. Infection is localized in approximately 30% of cases, mainly in the upper respiratory tract or skin, but only 20%-40% are microbiologically documented[59]. Among gram-negative bacteria, carbapenem-resistant Enterobacteriaceae such as Escherichia coli[67-74] and Klebsiella pneumoniae[67-69,72-75], Pseudomonas aeruginosa[68,69,72-74] and Acinetobacter baumannii[67,68,76] prevail. The most common gram-positive pathogens are methicillin-sensible[68] and methicillin-resistant Staphylococcus aureus[67,68,77], Streptococcus viridians[68,71] and Streptococcus pneumonia[68]; vancomycin-resistant Enteroccocus faecium may be found[67,69]. The main fungi identified are Candida and Aspergillus species[55,78]. Approximately 50% of invasive aspergillosis are found in patients with hematological malignancy or imunocompromissed patients with prolonged severe neutropenia[78]. Mortality rates of invasive fungal infection exceeds 30%[79].
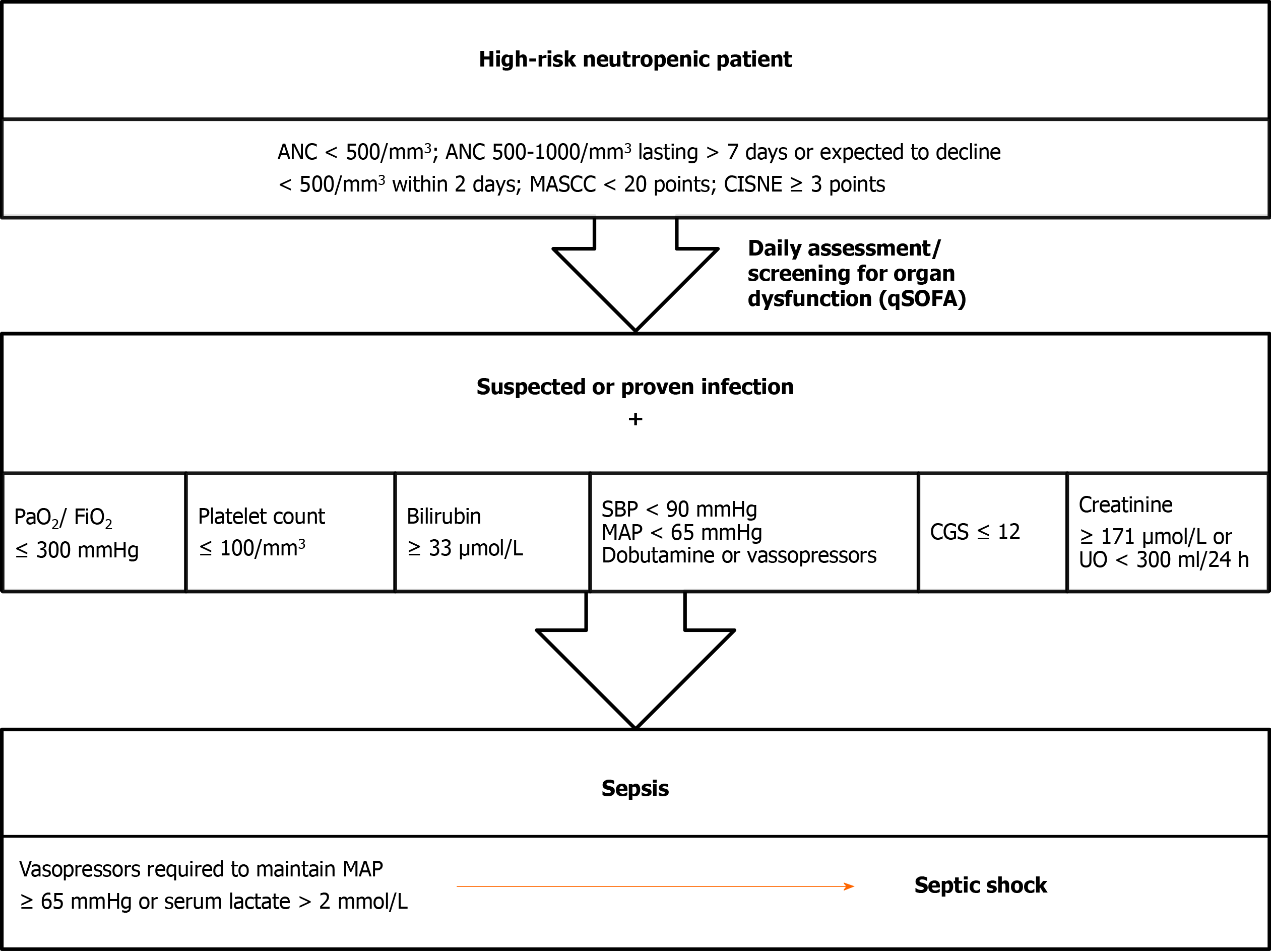
The Third International Consensus Definitions for Sepsis and Septic Shock is recommended to use in FN patients (Figure 3)[80]. In a large meta-analysis, neutropenia was independently associated with poor outcomes[81]; therefore FN should be treated as infectious disease until proven otherwise and must be considered as medical emergency. Therapeutic approach is based on the risk of complications and death, presence of life-threatening infection and magnitude and duration of neutropenia. High-risk patients are vulnerable to develop septic shock; early intravenous administration of broad-spectrum antibiotics against gram-negative and gram-positive bacteria is obligatory. Low-risk patients could be treated in-hospital with intravenous antibiotics or as outpatient with oral antibiotics depending on the clinical picture and comorbidities.
Culture samples must be taken before the onset of antibacterial agents. Information of the general/local epidemiology and resistance profiles is of paramount importance to guide empirical antibiotic therapy[82]. Broad-spectrum antibiotics covering Pseudomonas spp. and methicillin-resistant Staphylococcus aureus are used (Table 7[40,54,55,79,83]). Empirical antimycotic therapy must be promptly started if invasive fungal infection is suspected[79].
| Antibiotherapy | Indication |
| Antipseudomonal β-lactam agent (cefepime, ceftazidime) | All patients with febrile neutropenia |
| OR | |
| Carbapenem (meropenem or mipenem/cilastatin) | Hemodynamic instability |
| OR | |
| Piperacillin/tazobactam | |
| OR | |
| Novel cephalosporin/β-lactamase inhibitor (Ceftolozane/tazobactam or Ceftazidime/avibactam) | |
| PLUS | |
| Aminoglycosides (optional) | |
| PLUS | |
| Vancomycin | |
| Vancomycin, linezolid or daptomycin | Suspected catheter-related infections |
| Skin or soft-tissue infection | |
| Risk of methicillin-resistant Staphylococcus aureus | |
| Linezolid or daptomycin | Risk of vancomycin-resistant Enterococcus spp. |
| Carbapenem | Risk of extended-spectrum β-lactamase-producing gram negative bacteria |
| Polymyxin-colistin or tigecycline | Risk of Klebsiella pneumonia carbapenemase |
| Ciprofloxacin + clindamycin | Penicillin-allergic patients |
| OR | |
| Aztreonam + vancomycin | |
| Trimethoprim/sulfamethoxazole | Suspected Pneumocystis pneumonia |
| Antifungal drugs (echinocandins, amphotericin B lipid-based formulations) | Suspected invasive mycosis |
Treatment needs to be reassessed within 48-72 h; clinical and microbiological data help to modify therapy. In patients with documented infection, duration of therapy is based on the isolated organism and the site of infection. It is usually continued until recovering severe neutropenia; granulocyte colony-stimulating factors may be used[84]. In patients with FN of unidentified etiology, antibiotic therapy should be discontinued after 72 h of apyrexia and clinical recovery irrespective of absolute neutrophil count[56,85].
Several cardiovascular disorders may be developed in cancer patients such as sepsis/septic shock, chemotherapy-associated cardiotoxic disease (CACD), pulmonary embolism, and cardiac tamponade. In this section we refer to CACD since sepsis/septic shock is treated in other section, and pulmonary embolism and cardiac tamponade are nonspecific complication of cancer patients described in other high-quality articles[86,87].
Cardiovascular diseases and cancer are interwoven because of increased cancer survival and cardiotoxic anticancer therapy[88]. Up to 33% of cancer survivors may die due to heart disease[89]. Mortality rates in patients with CACD are 3.5-fold higher than those in patients with idiopathic cardiomyopathies[90]. Cardiovascular effects of chemotherapy may also affect the quality of life and compromise survival expectation.
Left ventricular dysfunction is the most common and serious consequence of CACD, usually secondary to cardiomyopathy or myocarditis (Table 8)[91,92]. Early CACD may be detected in up to 48% while late-onset disorders may be seen in up to 30%[93]. The highest incidence of CACD is reached by anthracyclines such as doxorubicin (3%-26%), alkylating agents such as cyclophosphamide (7%-28%), and monoclonal antibody such as trastuzumab (2%-28%)[94].
| Cardiovascular complications | Types | Oncological therapies |
| Left ventricular dysfunction | Cardiomyopathy or myocarditis | Anthracyclines (e.g., doxorubicin, aunorubicin, epirubicin, idarubicin), antiangiogenic agents (e.g., bevacisumab, sunitinib, sorafenib), alkylating agents (e.g., cyclophosphamide, cisplatin), monoclonal antibodies (e.g., trastuzumab, lapatinib), tyrosine kinase inhibitors (e.g., imatinib, dasatinib, nilotinib, sunitinib, sorafenib, lapatinib) |
| Arrhythmias | QT prolongation, bradycardia, heart block; Atrial arrhythmias; Ventricular arrhythmias or sudden cardiac death | Taxanes, arsenic trioxide, tyrosine kinase inhibitors (e.g., imatinib, dasatinib, nilotinib, sunitinib, sorafenib, lapatinib), anthracyclines(e.g., doxorubicin, aunorubicin, epirubicin, idarubicin) |
| Coronary artery disease | Acute coronary syndromes (included acute myocardial infarction); Chronic ischemic heart disease | Antimetabolites (e.g., gemcitabine, cytarabine), cisplatin, taxanes, thalidomide, bevacisumab, radiotherapy |
| Pericardial disease | Pericarditis (effusive or constrictive form) | Radiotherapy |
| Hypertension | New-onset or worsening | Vascular endothelial growth factor inhibitors, antiangiogenic agents (e.g., bevacisumab, sunitinib, sorafenib), cisplatin, interleukins, interferon |
CACD is classified as type 1 or type 2 depending on the administered therapy[95]. CACD type 1 is typically related to anthracycline drugs, not reversible with cessation of therapy, and dose-dependent; necrosis, vacuoles and disruption of sarcomeres are seen as histopathological findings. CACD type 2, usually associated with monoclonal antibodies such as trastuzumab, is reversible with cessation of therapy, dose-independent, and no ultrastructural disruption in cardiomyocyte cell is found.
There is no consensus to define CACD, but there is convergence regarding clinical or echocardiographic left ventricular dysfunction as the main condition[95]. Diagnosis of CACD can be made if at least one of the following criteria is reached[95,96]: (1) Cardiomyopathy with compromised left ventricular function; (2) Symptoms or signs of heart failure linked to the presence of third noise, tachycardia, or both; (3) Left ventricular ejection fraction (LVEF) less than 55% with a symptomatic decrease of 5%, or an asymptomatic decrease of 10%; and (4) Reduced LVEF > 10% from baseline or LVEF < 53% (the normal reference value for 2D echocardiography), confirmed in two consecutive echocardiography assessments within 2-3 wk apart.
Echocardiography was widely disseminated due to its easy availability, low cost, free of radiation, and information concerning the hemodynamic status and valvular diseases. There is no agreement regarding the time and frequency to achieve echocardiography in cancer patients on chemotherapy, although it is suitable before starting therapy (especially if there are cardiovascular risk factors or history of cardiac disease), during treatment, and 6 to 12 mo after completion[97].
Measurement of LVEF alone may overlook small changes. A variation in myocardial deformation, assessed by myocardial strain image, may precede significant decline in LVEF[98-100]. Magnetic resonance image (MRI) is very useful to determine the size of heart chambers and their function, but cannot be used at bedside for critically ill patients in the ICU. Thus, it should be used when other tests are inconclusive[91,95].
The high-sensitive cardiac troponins and N-terminal pro-B-type natriuretic peptide are cardiac biomarkers without general recommendation for diagnosis of cardiotoxicity; however, these noninvasive diagnostic methods are cheaper than other imaging studies or myocardial biopsy. Cardiac troponins are associated with prognosis in patients on anticancer therapy; thus, higher plasma concentration requires closer monitoring and a possible therapy modification[91,101]. Cut-off points have not been established.
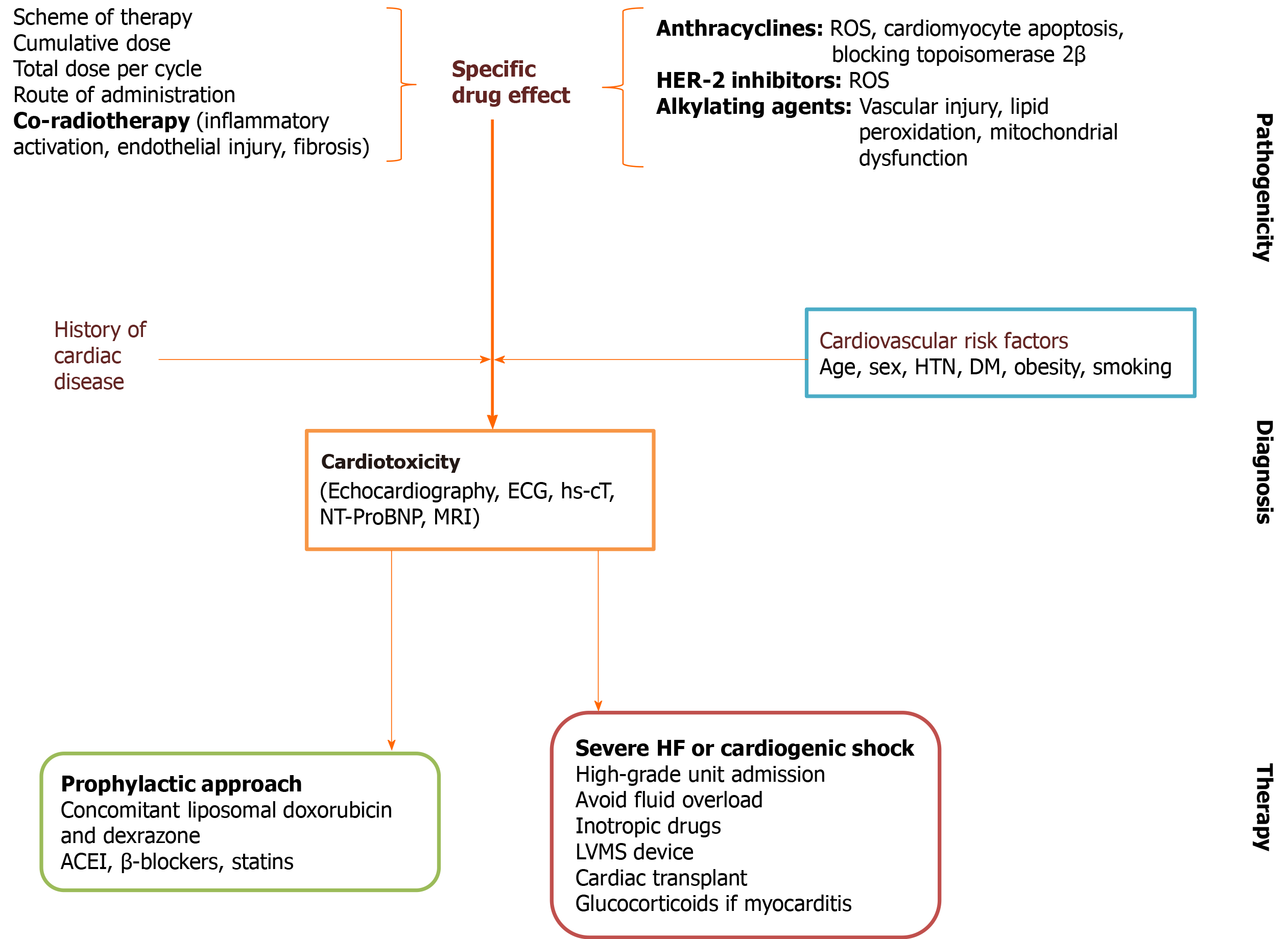
The treatment of adverse-side effects of antineoplastic therapy should be individualized depending on the risk factors for cardiotoxicity, severity, and prognosis (Figure 4[92,102,103]). The International Cardio Oncology Society-One trial found beneficial effects of prophylactic enalapril in patients on anthracycline therapy[104]. A recent study conducted in Brazil showed that carvedilol administered during chemotherapy reduced troponin levels and the risk of systolic dysfunction[105]. A recent meta-analysis showed the usefulness of β-blockers to preserve left ventricular function during anthracycline therapy[106]. Consequently, we would expect an increased cardiac tolerability with higher doses of chemotherapy with little or no interruption.
β-blockers may have further positive effects on malignancy. Since β-adrenergic receptors are overexpressed in malignant breast tissue, propranolol was tested on early-stage breast cancer patients[107]. Molecular analysis showed reduced Ki67 protein expression and decreased phosphorylation of mitogenic signaling regulators; additionally, reduced tumor proliferative indices, metastases rate, and mortality rate were also found[107]. Propranolol also modifies mitogenic and apoptotic signaling in late-stage breast cancer[108]. Long-term β-blockers improved survival outcomes in older ovarian cancer patients with cardiovascular disease[109].
Statins are drugs commonly used in patients with cardiovascular diseases and cancer. Statins regulates cell membrane integrity, cell signaling, protein synthesis, and cell cycle progression; they also modify angiogenesis and tumor growth[110]. Several studies demonstrated that statins are associated with reduced mortality rates in patients with breast cancer, renal cell carcinoma, and colorectal cancer[111,112]. A recent meta-analysis showed that statins was associated with improved outcomes in patients with lung cancer[113], but it was not supported by powered randomized controlled trials. Conversely, other meta-analysis of randomized controlled trials evidenced that statins did not improve overall survival rates or progression-free survival rates in patients with active cancer[114].
For patients with severe heart failure or cardiogenic shock, inotropic drugs and left ventricular mechanical support devices must be considered[115,116]. Glucocorticoids are the first-line therapy, and tumor necrosis factor-α inhibitors as second choice, for myocarditis with lymphocyte infiltration in patients treated with immune checkpoint inhibitors[91]. Cardiac transplant may be an option in selected patients. As expected, the criteria for transplanting these patients differ according to institution and country because active malignancy is generally considered as an absolute contraindication; nevertheless, it is interesting that survival rates after cardiac transplant in cancer patients is similar to those in noncancer patients[102,117].
Recently, cardio-oncology has emerged as a clinical (and scientific) area dedicated to diagnose and treat anticancer therapy-related cardiovascular complications to avoid interruption of treatment. This new discipline combine together cardiologists, oncologists, and hematologists in specialized units[118,119]. In institution without cardio-oncology unit, cancer patients with potentially fatal cardiovascular complications must be admitted to the ICU. Thus, it is necessary to adopt clinical guidelines according to the center resources to provide the best care, especially in cases with acute decompensated heart failure, cardiogenic shock, hypertensive emergency, and arrhythmias.
ICU is commonly required for cancer patients in the postoperative period because of the complexity of surgical procedure and potential complications. Topics regarding anesthetic management and surgical issues were addressed in this review.
Effects of chemotherapy and radiotherapy on respiratory system must be recognized before orotracheal intubation. Severe mucositis lead to pseudomembranous material, edema, and bleeding, which compromises the airway and increases the risk of aspiration during endotracheal intubation. Radiation on the head and neck region may produce permanent tissular fibrosis limiting mouth opening and tongue mobility. Radiotherapy-associated airway fibrosis and tracheal stenosis, usually not recognized on physical examination, may affect intubation and ventilation[120]; thus, monitoring of pulse oximetry and arterial blood gases in perioperative period is mandatory.
Excessive perioperative fluid administration has been correlated with surgical and pulmonary complications; therefore, fluid administration need to be monitored using dynamic indexes to optimize volume status[121,122]. Chemotherapy drugs such as bleomycin and mitomycin may cause lung toxicity[123]. However, in a large cohort of patients from the cancer registry of the Mayo Clinic, only seven patients receiving systemic bleomycin developed acute respiratory distress syndrome after surgery[124].
Transfusion-related immunomodulation is associated with decreased survival rates in cancer patients. This is a secondary phenomenon produced by multiple immunomodulatory mediators derived from white blood cells, red blood cells, and platelets of the donor[125]. Transfusion of red blood cells in the perioperative period affects the survival of cancer patient; thus, reducing blood transfusions could have a positive impact on outcome[126]. On the other hand, Manning-Geist et al[127] observed that perioperative transfusion of red blood cells after debulking surgery in ovarian cancer was not related with wound complication and thrombosis.
Cancer surgery induces neuroendocrine and immune stress response, which may be reduced by regional anesthesia. Surgical manipulation is associated with spreading of tumorigenic cells and releasing cancer-growth factors[128]; thus, immune system modulation may contribute to reduce the incidence of metastases[129].
Changes in immune system has been reported with anesthetic gases[130]. Volatile anesthetics inhibit leukocyte activity and stimulate angiogenesis and metastases[131]; however, evidence is not conclusive because most studies were carried out in vitro. In ovarian cancer cells, isoflurane was related to cell cycle progression and cell proliferation, and increased expression of tumorigenic markers such as insulin-like growth factor 1 within the first 24 h[131]. In breast cancer surgery, preserved natural killer (NK) cells activity was found with propofol-paravertebral anesthesia while reduced NK activity was demonstrated using sevoflurane[132].
Hong et al[133] found that cancer patients treated with volatile inhaled anesthesia had a 5-year overall survival rate similar to those on total intravenous anesthesia. The ENIGMA-II trial did not show negative effects of nitrous oxide on cancer recurrence or mortality[134]. Further randomized controlled trials are required.
Recent studies suggest that opioids inhibit the cellular and humoral immunity, promote proliferation and migration of tumor cells, and facilitate angiogenesis[129,135]. Opioid-induced immunomodulation is manifested in two ways: (1) Direct effects on immune cells via μ receptor and toll-like receptor 4 expressed in the surface of NK cells, macrophages and T-cells (peripheral effects)[136]; and (2) Indirect effects through the sympathetic nervous system and hypothalamic-pituitary-adrenal axis, which suppress lymphocyte proliferation and NK cell cytotoxicity in lymphoid organs (central effects)[137,138]. Nonetheless, the type of drug and the administration period may modify the immunological effects of opioids[139,140] (Table 9[131,140-142]).
Propofol, a sedative drug commonly used in operating room and ICU, has been associated with tumor growth inhibition and reduced risk of metastasis. In patients undergoing hepatectomy for hepatocellular carcinoma and colon cancer surgery, propofol-based total intravenous anesthesia was associated with improved survival rates and reduced postoperative metastases compared with desflurane anesthesia[143,144]. Instead, Huang et al[145] observed no significant difference in locoregional recurrence or overall 5-year survival rates using desflurane or propofol anesthesia in patients undergoing breast cancer surgery.
High-local chemotherapy concentration is reached using the hyperthermic intraperitoneal chemotherapy method. Systemic toxicity, including hematological toxicity, is less common than those with systemic administration of chemotherapy[146]; nonetheless, hematological and pulmonary toxicity may be occasionally produced with potentially fatal outcomes[147].
The Enhanced Recovery After Surgery (ERAS) program reduces surgical stress and improve recovery for an early hospital discharge. This approach includes three components[148,149]: (1) Preoperative: Preadmission counseling, early discharge planning, reduced fasting duration, carbohydrate loading, no/selective bowel preparation, antibiotic prophylaxis, thromboprophylaxis, pre-warming, and no premedication; (2) Intraoperative: Short-acting anesthetic agents, mid-thoracic epidural anesthesia/ analgesia, surgical techniques, no drains, avoidance of fluid overload, and maintenance of normothermia; and (3) Postoperative: Mid-thoracic epidural analgesia, no nasogastric tube, prevention of nausea and vomiting, avoidance of salt and fluid overload, early removal of catheters, early oral nutrition, nonopioid oral analgesia/nonsteroidal anti-inflammatory drugs, early mobilization, stimulation of gut motility (e.g., chewing gum), defined discharge criteria, and audit of compliance and outcomes.
ERAS program has become a widely accepted surgical practice worldwide. Positive outcomes have been found in several surgical locations including elective and emergency surgery[149-151]. ERAS protocols have led to decreased length of hospitalization by 30% to 50%, as well as reduced complications, readmission rates, and health costs[149,142].
Laparoscopic surgery within ERAS protocols in cancer patients has also shown optimistic outcomes[153,154]. ERAS program resulted in improved outcomes, reduced hospitalization cost, and enhanced quality of life as shown by Wang et al[155] in a meta-analysis of elective gastric cancer surgery.
Neurological symptoms and signs are commonly seen in cancer patients. Neurological symptoms may be the initial expression of undiagnosed cancer, emerge during the course of disease, or appear linked to treatment[156]. Cancer patient may also develop nonmalignancy-related neurological disorders, which require a rational approach to exclude cancer-related complications.
Neurological disorders require early diagnosis and treatment to reduce functional loss. Surgical treatment is often required, for which the multidisciplinary approach is mandatory[157]. Neurological disorders in cancer patient is produced by[158]: (1) Direct effects of tumor: Brain metastases, cerebral edema, seizures, spinal cord compression, hydrocephalus, leptomeningeal carcinomatosis; (2) Indirect effects of tumor: Paraneoplastic syndromes, stroke, cerebral venous thrombosis, infection, metabolic and electrolytic disorders; and (3) Treatment effects: Convulsions, cerebrovascular accident (e.g., intracranial hemorrhage due to thrombocytopenia, venous sinus thrombosis), leukoencephalopathy, loss of vision or hearing, peripheral neuropathy, aseptic meningitis, opportunistic infections, acute or late post-radiation necrosis.
The most common neurological emergencies are brain metastases, seizures, and obstructive hydrocephalus[156]. Intracranial hypertension related to cerebral edema, hydrocephalus or mass effect is commonly seen.
Brain metastases complicate up to 20% of cancer patients and are 10-fold more frequent than primary brain tumors[159]. Cancer producing metastases are lung (e.g., nonsmall cells), breast (e.g., HER-2), kidney and melanoma[158,160]. Fifty percent of metastases are solitarylesion. Distribution of brain metastases are cerebral hemispheres (80%), cerebellum (15%) and brainstem (3%). Cerebral edema associated with metastases produces intracranial hypertension. Pathogenesis of edema is complex, including vasogenic edema secondary to capillary leakage, venous stasis, and cerebrospinal fluid obstruction[159,160].
Cerebral metastases may be the initial feature of cancer in 8-10% of cases. It may be characterized by intracranial hypertension with alterations in level of consciousness, headache, and vomiting; focal neurological deficit such as sensory or motor defects, speech disorders, instability, and cognitive impairment; or asymptomatic. Seizures almost always occur when there are multiple metastases, intralesional bleeding, herniation, hydrocephalus, or sudden-onset ischemia of large vessels[158,160]. Diagnosis is made by contrast enhanced MRI. Contrasted CT is useful if MRI is contraindicated or intracranial hemorrhage is suspected, but it is less sensitive for posterior fossa or small tumors[159,160].
Treatment is discouraging. Several factors such as type and location of primary tumor, age, and extracranial disease are involved in prognosis. Therapeutic options include surgical resection, radiotherapy, stereotactic radiosurgery and chemotherapy[159-167]. In patients with good performance status and known primary tumor, surgical resection of unique lesions of noneloquent areas followed by radiation therapy is recommended[159]. In eloquent area lesions, radiation therapy is preferred. Radiation of the entire skull is chosen for multiple and symptomatic metastases, although prognosis is not improved and almost half of patients die due to neurological progression[162,163]. Traditional cytotoxic chemotherapy is not routinely used in the treatment of brain metastases because of low response rates[164]; however, germ-cell tumors and non-Hogdkin lymphoma with nervous system involvement are treated with chemotherapy[166,167]. Metastases secondary to melanoma and renal carcinoma do not respond to chemotherapy[158]. Targeted therapies and immunotherapy are promising oncospecific therapies[168-172].
Despite aggressive treatment, many patients develop malignant cerebral edema and seizures. Cerebral edema occurs through disruption of blood-brain barrier by direct effect of metastases, as well as released several cytokines and growth factors by the tumor cells including the endothelial vascular growth factor with promoting angiogenesis[159]. These factors favor endothelial clefts formation with fragmentation and fenestration of endothelium, and consequently, injury to the basement membrane[159]. Vasogenic edema with fluid leakage and increased interstitial fluid pressure is then developed. Peritumoral edema eventually leads to symptoms and signs of mass effect and increased intracranial pressure.
Glucocorticoids are indicated for all symptomatic patients with metastases-associated cerebral edema. Dexamethasone is the most used due to its long half-life and lower mineralocorticoid activity. Recommended dose is 4-8 mg/d (up to 16 mg/d in very severe symptomatic patients). Higher doses have not additional benefits and side effects may occur. Dexamethasone doses should be progressively decreased in 2 or more weeks to avoid complications of chronic steroid administration (e.g., immunosuppression, hyperglycemia, increased risk of opportunistic infections)[173]. Other therapies include hyperventilation, hypertonic sodium chloride or mannitol 20% in severe intracranial hypertension to prevent herniation in neurocritically ill patients[174].
Seizure complicating brain tumor is commonly found as simple, complex-partial or generalized epilepsy[158]. Status epilepticus may also be developed. Seizures depend on the type and location of brain tumor, as well as cancer-related complication. The reasons of cancer-related seizure are listed in Table 10. Diagnosis is made by clinical feature and electroencephalography showing epileptic changes on brain waves. Epileptiform waves could be present even in absence of clinically visible seizures[175]. Hemiplegia and other focal symptoms may appear up 75% of patients depending on tumor location; infratentorial disease is related toataxia, vomiting, dysarthria and nystagmus[158].
| Causes | Comments |
| Cancer-related seizure | |
| Low-grade tumors | Glioma and oligodendroglioma have intrinsic epileptogenic activity as a result of their long survival and reduced seizure threshold |
| High-grade tumors | Usually secondary to necrosis, hemorrhage or edema |
| Brain metastases | Up to 40% |
| Tumor location | Cortical tumors and those on epileptogenic areas (e.g., mesial temporal lobe and insula) are associated with intractable epilepsy |
| Stroke | Ischemic or hemorrhagic |
| Drug toxicity | Cytarabine, methotrexate, cisplatin, vincristine, cyclophosphamide, anthracyclines |
| Neoplastic meningitis | |
| Paraneoplastic encephalitis | |
| Central nervous system infections | |
| Electrolytic imbalance | Hyponatremia, hypocalcaemia |
| Metabolic disorders | Hypoglycemia |
| Liver or kidney failure | |
| Aggravated preexisting epilepsy | Withdrawal medication |
| Cancer-related acute hydrocephalus | |
| Stopped CSF flow by tumor obstruction of ventricular system | Colloid cysts, ependymoma, intraventricular meningioma, choroid plexus papilloma or posterior fossa tumor; in adults it is often due to leptomeningeal carcinomatosis and intra-ventricular extension of metastasis |
| Increased CSF content due to deficit in reabsorption | Venous sinus thrombosis, infectious meningitis, metastatic seeding or subarachnoid hemorrhage |
Seizure prophylaxis is not recommended for patients with brain tumor; however, this is a controversial recommendation because of improved accurate diagnosis and prognosis using the current continuous electroencephalography, and the introduction of newer and less toxic anticonvulsants (e.g., levetiracetam, lamotrigine, and lacosamide)[176]. Once airway, breathing, circulation, and “dextrose” (the “ABCDs”) have been addressed, acute seizure is treated with parenteral benzodiazepines (e.g., IV lorazepam or diazepam; intramuscular midazolam) as first-line agents. Second-line therapy (e.g., phenytoin/fosphenytoin, valproic acid, phenobarbital, or levetiracetam) should be initiated within 30 min if first-line treatment failed. If second-line agents are ineffective, treatment is escalated to anesthetic agents such as continuous infusion of midazolam, propofol, or pentobarbital[177]. When mass effect or worsening edema is present, dexamethasone can be effective in controlling seizures. For metastasis-related seizures, chemotherapy, radiotherapy and surgery are alternative therapies. Surgical resection is recommended for patients with tumor located in the posterior fossa. Early diagnosis and urgent correction are required for metabolic or electrolytic imbalance-induced seizures.
Maintenance anticonvulsant medication requires more careful evaluation since the old anticonvulsants (e.g., phenytoin, carbamazepine, and phenobarbital) simultaneously induce CYP coenzymes. These coenzymes accelerate the metabolism of steroids and chemotherapeutic agents. Newer antiepileptic drugs such as levetiracetam, without CYP metabolism, are recommended in these circumstances. Other options are gabapentin, pregabalin, lamotrigine, and lacosamide[178].
Acute hydrocephalus is a medical emergency caused by a stopped cerebrospinal fluid (CSF) flow or an increased CSF content. Table 10 lists the reasons of acute hydrocephalus in cancer patients. Since 80% of maximum ventricular dilation is reached in almost 6 h, acute hydrocephalus may be rapidly developed. Clinical diagnosis is suspected in patients presenting headache, blurred vision, transient loss of visual field, ataxia, vomiting, and impaired consciousness. Headache is present in 50% of cases (on occipital region if increased intracranial pressure), exacerbates with the Valsalva maneuver, and is associated with nausea and vomiting[158,160]. Papilledema and focal neurological signs may be present. Tumor interference the CSF flow with a valve-way mechanism at the level of third or fourth ventricle may result in periodic increased intracranial pressure[160]. Noncontrasted CT scan allows identifying the size of ventricles. Obstructive hydrocephalus is classically characterized by ventriculomegaly proximal to the site of obstruction and periventricular edema.
The treatment of acute hydrocephalus should be early and effective. Several procedures have been described such as emergency ventriculostomy, ventricular bypass, endoscopic ventriculostomy, aqueductoplasty (due to aqueduct stenosis), septostomy (in isolated lateral hydrocephalus), and in some cases tumor debunking. Radiotherapy and chemotherapy are options in patients with hydrocephalus secondary to leptomeningeal carcinomatosis or metastatic CSF seeding. Patients with nonsevere obstructive hydrocephalus could be treated with osmotic agents to reduce intracranial pressure (e.g., manitol 20% or hypertonic sodium chloride) and/ or drug interfering with CSF production such as acetazolamide, furosemide, and glucocorticoids[179].
Chemotherapy in ICU may be an option for patients with critical illness driven by the oncological disease, scheduled or ongoing chemotherapy in absence of contraindications, and requirement for monitoring or preventing potentially severe chemotherapy-side effects in high risk patients. Particularly, anticancer chemo or radiotherapy is necessary in cases of acute respiratory failure due to high grade non-Hodgkin lymphoma or hyperleukocytosis[5]. Antineoplastic drugs used by ICU team may be challenging due to little experience; indeed, associated sepsis or organ support methods at the time of chemotherapy onset are erroneously considered as a contraindication[6].
Organ support therapies accompanied by chemotherapy may be beneficial in critically ill patients with cancer-related organ dysfunction[180]. Patient’s consent, comorbidities, performance status, cancer-related life expectancy, and life-span-expanding treatment are necessary to be evaluated to improve outcome. A close collaboration with the attending oncologist or hematologist is mandatory. Organizational issues should be assured for success, including clinical protocols, securing of the medication circuit, consultation with pharmacist and experienced nurses, and daily rounds with the attending oncologist or hematologist[181].
Studies identifying prognostic factors and outcomes of patients receiving chemotherapy in the ICU are scarce and have several limitations such as retrospective design, small sample size, and several nature of cancer[182-184]. Additionally, the following period and subgroup of analyzed patients may have an impact on clinical response (e.g., solid tumor vs. hematological malignancy; traditional chemotherapy vs. targeted immunotherapy; urgent vs. maintenance chemotherapy), which need to be considered to state prognosis.
ICU and hospital mortality rates for patients with solid tumors who received chemotherapy in ICU range from 25% to 54%, and 58% to 77%, respectively[182-184]. One-year survival rates are as low as 7%-12%[182,183]. Lung cancer and acute respiratory failure due to airway compression or pulmonary infiltrates may explain the high mortality rates[182-184]. In patients with hematological malignancy 25%-40% die in the ICU; 30-d, 6-mo and 1-year mortality rates is 40%, 51%-77% and 50%, respectively[10,185]. Risk factors for mortality are degree of organ dysfunction and life-support methods such as ventilatory support, vasopressors, and renal replacement therapy.
Patients with cancer and organ dysfunction need to be early admitted to ICU for improving survival. Clinical and pathophysiological condition, cancer status, and expected life-span must be collectively evaluated to decide full or time-limited organ support methods. Specific disorders require a specialized and well-trained medical staff to optimize diagnosis, enhance treatment, and improve outcomes.
Manuscript source: Invited manuscript
Specialty type: Critical care medicine
Country/Territory of origin: Cuba
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): B
Grade C (Good): C, C
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Guo K, Vega MI S-Editor: Wang JL L-Editor: A P-Editor: Wang LL
| 1. | Heron M. Deaths: Leading Causes for 2016. Natl Vital Stat Rep. 2018;67:1-77. [PubMed] |
| 2. | Jemal A, Ward EM, Johnson CJ, Cronin KA, Ma J, Ryerson B, Mariotto A, Lake AJ, Wilson R, Sherman RL, Anderson RN, Henley SJ, Kohler BA, Penberthy L, Feuer EJ, Weir HK. Annual Report to the Nation on the Status of Cancer, 1975-2014, Featuring Survival. J Natl Cancer Inst. 2017;109:djx030. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 748] [Cited by in RCA: 1109] [Article Influence: 138.6] [Reference Citation Analysis (0)] |
| 3. | Yeh ET, Chang HM. Oncocardiology-Past, Present, and Future: A Review. JAMA Cardiol. 2016;1:1066-1072. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 66] [Cited by in RCA: 84] [Article Influence: 9.3] [Reference Citation Analysis (0)] |
| 4. | American Society of Clinical Oncology. The State of Cancer Care in America, 2017: A Report by the American Society of Clinical Oncology. J Oncol Pract. 2017;13:e353-e394. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 138] [Cited by in RCA: 145] [Article Influence: 18.1] [Reference Citation Analysis (0)] |
| 5. | Kiehl MG, Beutel G, Böll B, Buchheidt D, Forkert R, Fuhrmann V, Knöbl P, Kochanek M, Kroschinsky F, La Rosée P, Liebregts T, Lück C, Olgemoeller U, Schalk E, Shimabukuro-Vornhagen A, Sperr WR, Staudinger T, von Bergwelt Baildon M, Wohlfarth P, Zeremski V, Schellongowski P; Consensus of the German Society of Hematology and Medical Oncology (DGHO), Austrian Society of Hematology and Oncology (OeGHO), German Society for Medical Intensive Care Medicine and Emergency Medicine (DGIIN), and Austrian Society of Medical and General Intensive Care and Emergency Medicine (ÖGIAIN). Consensus statement for cancer patients requiring intensive care support. Ann Hematol. 2018;97:1271-1282. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 45] [Cited by in RCA: 56] [Article Influence: 8.0] [Reference Citation Analysis (0)] |
| 6. | Azoulay E, Schellongowski P, Darmon M, Bauer PR, Benoit D, Depuydt P, Divatia JV, Lemiale V, van Vliet M, Meert AP, Mokart D, Pastores SM, Perner A, Pène F, Pickkers P, Puxty KA, Vincent F, Salluh J, Soubani AO, Antonelli M, Staudinger T, von Bergwelt-Baildon M, Soares M. The Intensive Care Medicine research agenda on critically ill oncology and hematology patients. Intensive Care Med. 2017;43:1366-1382. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 141] [Cited by in RCA: 117] [Article Influence: 14.6] [Reference Citation Analysis (0)] |
| 7. | Puxty K, McLoone P, Quasim T, Sloan B, Kinsella J, Morrison DS. Risk of Critical Illness Among Patients With Solid Cancers: A Population-Based Observational Study. JAMA Oncol. 2015;1:1078-1085. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 92] [Cited by in RCA: 89] [Article Influence: 8.9] [Reference Citation Analysis (0)] |
| 8. | Shimabukuro-Vornhagen A, Böll B, Kochanek M, Azoulay É, von Bergwelt-Baildon MS. Critical care of patients with cancer. CA Cancer J Clin. 2016;66:496-517. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 86] [Cited by in RCA: 74] [Article Influence: 8.2] [Reference Citation Analysis (0)] |
| 9. | Bos MM, Verburg IW, Dumaij I, Stouthard J, Nortier JW, Richel D, van der Zwan EP, de Keizer NF, de Jonge E. Intensive care admission of cancer patients: a comparative analysis. Cancer Med. 2015;4:966-976. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 52] [Cited by in RCA: 62] [Article Influence: 6.2] [Reference Citation Analysis (0)] |
| 10. | Wigmore T, Farquhar-Smith P. Outcomes for Critically Ill Cancer Patients in the ICU: Current Trends and Prediction. Int Anesthesiol Clin. 2016;54:e62-e75. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 10] [Cited by in RCA: 10] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 11. | Torres VB, Vassalo J, Silva UV, Caruso P, Torelly AP, Silva E, Teles JM, Knibel M, Rezende E, Netto JJ, Piras C, Azevedo LC, Bozza FA, Spector N, Salluh JI, Soares M. Outcomes in Critically Ill Patients with Cancer-Related Complications. PLoS One. 2016;11:e0164537. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 21] [Cited by in RCA: 26] [Article Influence: 2.9] [Reference Citation Analysis (0)] |
| 12. | De la Hoz A, Cortés JA. Bacterial and Atypical Infections in Critically Ill Cancer Patients. In: Nates JL, Price KJ, editors. Oncologic Critical Care. Cham: Springer, 2019: 1-22. [DOI] [Full Text] |
| 13. | Schellongowski P, Kiehl M, Kochanek M, Staudinger T, Beutel G; Intensive Care in Hematologic-Oncologic Patients (iCHOP). Intensive care for cancer patients: An interdisciplinary challenge for cancer specialists and intensive care physicians. Memo. 2016;9:39-44. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 14] [Cited by in RCA: 10] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 14. | Martos-Benítez FD, Soto-García A, Gutiérrez-Noyola A. Clinical characteristics and outcomes of cancer patients requiring intensive care unit admission: a prospective study. J Cancer Res Clin Oncol. 2018;144:717-723. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 29] [Cited by in RCA: 18] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 15. | Soubani AO. Critical Care Prognosis and Outcomes in Patients with Cancer. Clin Chest Med. 2017;38:333-353. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 20] [Cited by in RCA: 14] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 16. | Biskup E, Cai F, Vetter M, Marsch S. Oncological patients in the intensive care unit: prognosis, decision-making, therapies and end-of-life care. Swiss Med Wkly. 2017;147:w14481. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 6] [Cited by in RCA: 12] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 17. | Azoulay E, Mokart D, Pène F, Lambert J, Kouatchet A, Mayaux J, Vincent F, Nyunga M, Bruneel F, Laisne LM, Rabbat A, Lebert C, Perez P, Chaize M, Renault A, Meert AP, Benoit D, Hamidfar R, Jourdain M, Darmon M, Schlemmer B, Chevret S, Lemiale V. Outcomes of critically ill patients with hematologic malignancies: prospective multicenter data from France and Belgium--a groupe de recherche respiratoire en réanimation onco-hématologique study. J Clin Oncol. 2013;31:2810-2818. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 389] [Cited by in RCA: 410] [Article Influence: 34.2] [Reference Citation Analysis (0)] |
| 18. | Mokart D, Lambert J, Schnell D, Fouché L, Rabbat A, Kouatchet A, Lemiale V, Vincent F, Lengliné E, Bruneel F, Pene F, Chevret S, Azoulay E. Delayed intensive care unit admission is associated with increased mortality in patients with cancer with acute respiratory failure. Leuk Lymphoma. 2013;54:1724-1729. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 105] [Cited by in RCA: 122] [Article Influence: 9.4] [Reference Citation Analysis (0)] |
| 19. | Lengliné E, Raffoux E, Lemiale V, Darmon M, Canet E, Boissel N, Schlemmer B, Dombret H, Azoulay E. Intensive care unit management of patients with newly diagnosed acute myeloid leukemia with no organ failure. Leuk Lymphoma. 2012;53:1352-1359. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 75] [Cited by in RCA: 88] [Article Influence: 6.8] [Reference Citation Analysis (0)] |
| 20. | Hanzelka KM, Yeung SC, Chisholm G, Merriman KW, Gaeta S, Malik I, Rice TW. Implementation of modified early-goal directed therapy for sepsis in the emergency center of a comprehensive cancer center. Support Care Cancer. 2013;21:727-734. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 21] [Cited by in RCA: 15] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 21. | Song JU, Suh GY, Park HY, Lim SY, Han SG, Kang YR, Kwon OJ, Woo S, Jeon K. Early intervention on the outcomes in critically ill cancer patients admitted to intensive care units. Intensive Care Med. 2012;38:1505-1513. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 84] [Cited by in RCA: 91] [Article Influence: 7.0] [Reference Citation Analysis (0)] |
| 22. | Ñamendys-Silva SA, Plata-Menchaca EP, Rivero-Sigarroa E, Herrera-Gómez A. Opening the doors of the intensive care unit to cancer patients: A current perspective. World J Crit Care Med. 2015;4:159-162. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 15] [Cited by in RCA: 11] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 23. | Shrime MG, Ferket BS, Scott DJ, Lee J, Barragan-Bradford D, Pollard T, Arabi YM, Al-Dorzi HM, Baron RM, Hunink MG, Celi LA, Lai PS. Time-Limited Trials of Intensive Care for Critically Ill Patients With Cancer: How Long Is Long Enough? JAMA Oncol. 2016;2:76-83. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 61] [Cited by in RCA: 69] [Article Influence: 7.7] [Reference Citation Analysis (0)] |
| 24. | Azoulay E, Pickkers P, Soares M, Perner A, Rello J, Bauer PR, van de Louw A, Hemelaar P, Lemiale V, Taccone FS, Martin Loeches I, Meyhoff TS, Salluh J, Schellongowski P, Rusinova K, Terzi N, Mehta S, Antonelli M, Kouatchet A, Barratt-Due A, Valkonen M, Landburg PP, Bruneel F, Bukan RB, Pène F, Metaxa V, Moreau AS, Souppart V, Burghi G, Girault C, Silva UVA, Montini L, Barbier F, Nielsen LB, Gaborit B, Mokart D, Chevret S; Efraim investigators and the Nine-I study group. Acute hypoxemic respiratory failure in immunocompromised patients: the Efraim multinational prospective cohort study. Intensive Care Med. 2017;43:1808-1819. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 124] [Cited by in RCA: 177] [Article Influence: 22.1] [Reference Citation Analysis (0)] |
| 25. | Azoulay E, Mokart D, Kouatchet A, Demoule A, Lemiale V. Acute respiratory failure in immunocompromised adults. Lancet Respir Med. 2019;7:173-186. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 103] [Cited by in RCA: 113] [Article Influence: 18.8] [Reference Citation Analysis (0)] |
| 26. | Bergeron A, Chevret S, Peffault de Latour R, Chagnon K, de Margerie-Mellon C, Rivière F, Robin M, Mani J, Lorillon G, Socié G, Tazi A. Noninfectious lung complications after allogeneic haematopoietic stem cell transplantation. Eur Respir J. 2018;51:1702617. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 42] [Cited by in RCA: 65] [Article Influence: 9.3] [Reference Citation Analysis (0)] |
| 27. | Mayer S, Pastores SM, Riedel E, Maloy M, Jakubowski AA. Short- and long-term outcomes of adult allogeneic hematopoietic stem cell transplant patients admitted to the intensive care unit in the peritransplant period. Leuk Lymphoma. 2017;58:382-390. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 16] [Cited by in RCA: 19] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 28. | Cupp J, Culakova E, Poniewierski MS, Dale DC, Lyman GH, Crawford J. Analysis of Factors Associated With In-hospital Mortality in Lung Cancer Chemotherapy Patients With Neutropenia. Clin Lung Cancer. 2018;19:e163-e169. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 16] [Cited by in RCA: 27] [Article Influence: 3.4] [Reference Citation Analysis (0)] |
| 29. | Gupta M, Sahi MS, Bhargava AK, Talwar V. A Prospective Evaluation of Symptom Prevalence and Overall Symptom Burden Among Cohort of Critically Ill Cancer Patients. Indian J Palliat Care. 2016;22:118-124. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 10] [Cited by in RCA: 13] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 30. | Yoo H, Suh GY, Jeong BH, Lim SY, Chung MP, Kwon OJ, Jeon K. Etiologies, diagnostic strategies, and outcomes of diffuse pulmonary infiltrates causing acute respiratory failure in cancer patients: a retrospective observational study. Crit Care. 2013;17:R150. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 20] [Cited by in RCA: 23] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 31. | Almeida IC, Soares M, Bozza FA, Shinotsuka CR, Bujokas R, Souza-Dantas VC, Ely EW, Salluh JI. The impact of acute brain dysfunction in the outcomes of mechanically ventilated cancer patients. PLoS One. 2014;9:e85332. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 19] [Cited by in RCA: 28] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 32. | Azevedo LCP, Caruso P, Silva UVA, Torelly AP, Silva E, Rezende E, Netto JJ, Piras C, Lobo SMA, Knibel MF, Teles JM, Lima RA, Ferreira BS, Friedman G, Rea-Neto A, Dal-Pizzol F, Bozza FA, Salluh JIF, Soares M; Brazilian Research in Intensive Care Network (BRICNet). Outcomes for patients with cancer admitted to the ICU requiring ventilatory support: results from a prospective multicenter study. Chest. 2014;146:257-266. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 114] [Cited by in RCA: 114] [Article Influence: 10.4] [Reference Citation Analysis (0)] |
| 33. | Lamba TS, Sharara RS, Singh AC, Balaan M. Pathophysiology and Classification of Respiratory Failure. Crit Care Nurs Q. 2016;39:85-93. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 10] [Cited by in RCA: 25] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 34. | Martos-Benítez FD, Gutiérrez-Noyola A, Badal M, Dietrich NA. Risk factors and outcomes of severe acute respiratory failure requiring invasive mechanical ventilation in cancer patients: A retrospective cohort study. Med Intensiva. 2018;42:354-362. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5] [Cited by in RCA: 5] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 35. | Pastores SM, Voigt LP. Acute respiratory failure in the patient with cancer: diagnostic and management strategies. Crit Care Clin. 2010;26:21-40. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 42] [Cited by in RCA: 31] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 36. | Zampieri FG, Bozza FA, Moralez GM, Mazza DD, Scotti AV, Santino MS, Ribeiro RA, Rodrigues Filho EM, Cabral MM, Maia MO, D'Alessandro PS, Oliveira SV, Menezes MA, Caser EB, Lannes RS, Alencar Neto MS, Machado MM, Sousa MF, Salluh JI, Soares M. The effects of performance status one week before hospital admission on the outcomes of critically ill patients. Intensive Care Med. 2017;43:39-47. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 40] [Cited by in RCA: 56] [Article Influence: 6.2] [Reference Citation Analysis (0)] |
| 37. | Elicker BM, Jones KT, Naeger DM, Frank JA. Imaging of Acute Lung Injury. Radiol Clin North Am. 2016;54:1119-1132. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 34] [Cited by in RCA: 34] [Article Influence: 3.8] [Reference Citation Analysis (0)] |
| 38. | Azoulay É, Canet E, Raffoux E, Lengliné E, Lemiale V, Vincent F, de Labarthe A, Seguin A, Boissel N, Dombret H, Schlemmer B. Dexamethasone in patients with acute lung injury from acute monocytic leukaemia. Eur Respir J. 2012;39:648-653. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 57] [Cited by in RCA: 66] [Article Influence: 4.7] [Reference Citation Analysis (0)] |
| 39. | Schellongowski P, Staudinger T, Kundi M, Laczika K, Locker GJ, Bojic A, Robak O, Fuhrmann V, Jäger U, Valent P, Sperr WR. Prognostic factors for intensive care unit admission, intensive care outcome, and post-intensive care survival in patients with de novo acute myeloid leukemia: a single center experience. Haematologica. 2011;96:231-237. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 88] [Cited by in RCA: 102] [Article Influence: 6.8] [Reference Citation Analysis (0)] |
| 40. | Maschmeyer G, Carratalà J, Buchheidt D, Hamprecht A, Heussel CP, Kahl C, Lorenz J, Neumann S, Rieger C, Ruhnke M, Salwender H, Schmidt-Hieber M, Azoulay E. Diagnosis and antimicrobial therapy of lung infiltrates in febrile neutropenic patients (allogeneic SCT excluded): updated guidelines of the Infectious Diseases Working Party (AGIHO) of the German Society of Hematology and Medical Oncology (DGHO). Ann Oncol. 2015;26:21-33. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 111] [Cited by in RCA: 94] [Article Influence: 9.4] [Reference Citation Analysis (0)] |
| 41. | Wieruszewski PM, Herasevich S, Gajic O, Yadav H. Respiratory failure in the hematopoietic stem cell transplant recipient. World J Crit Care Med. 2018;7:62-72. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 16] [Cited by in RCA: 13] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 42. | White PL, Backx M, Barnes RA. Diagnosis and management of Pneumocystis jirovecii infection. Expert Rev Anti Infect Ther. 2017;15:435-447. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 48] [Cited by in RCA: 65] [Article Influence: 8.1] [Reference Citation Analysis (0)] |
| 43. | Blanchard E, Gabriel F, Jeanne-Leroyer C, Servant V, Dumas PY. [Invasive pulmonary aspergillosis]. Rev Mal Respir. 2018;35:171-187. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 9] [Cited by in RCA: 10] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 44. | Koo HJ, Lim S, Choe J, Choi SH, Sung H, Do KH. Radiographic and CT Features of Viral Pneumonia. Radiographics. 2018;38:719-739. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 382] [Cited by in RCA: 411] [Article Influence: 58.7] [Reference Citation Analysis (0)] |
| 45. | Rochwerg B, Brochard L, Elliott MW, Hess D, Hill NS, Nava S, Navalesi P Members Of The Steering Committee, Antonelli M, Brozek J, Conti G, Ferrer M, Guntupalli K, Jaber S, Keenan S, Mancebo J, Mehta S, Raoof S Members Of The Task Force. Official ERS/ATS clinical practice guidelines: noninvasive ventilation for acute respiratory failure. Eur Respir J. 2017;50:1602426. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 617] [Cited by in RCA: 844] [Article Influence: 105.5] [Reference Citation Analysis (0)] |
| 46. | Berbenetz N, Wang Y, Brown J, Godfrey C, Ahmad M, Vital FM, Lambiase P, Banerjee A, Bakhai A, Chong M. Non-invasive positive pressure ventilation (CPAP or bilevel NPPV) for cardiogenic pulmonary oedema. Cochrane Database Syst Rev. 2019;4:CD005351. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 27] [Cited by in RCA: 50] [Article Influence: 8.3] [Reference Citation Analysis (0)] |
| 47. | Frat JP, Thille AW, Mercat A, Girault C, Ragot S, Perbet S, Prat G, Boulain T, Morawiec E, Cottereau A, Devaquet J, Nseir S, Razazi K, Mira JP, Argaud L, Chakarian JC, Ricard JD, Wittebole X, Chevalier S, Herbland A, Fartoukh M, Constantin JM, Tonnelier JM, Pierrot M, Mathonnet A, Béduneau G, Delétage-Métreau C, Richard JC, Brochard L, Robert R; FLORALI Study Group; REVA Network. High-flow oxygen through nasal cannula in acute hypoxemic respiratory failure. N Engl J Med. 2015;372:2185-2196. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1232] [Cited by in RCA: 1374] [Article Influence: 137.4] [Reference Citation Analysis (0)] |
| 48. | Lemiale V, Mokart D, Mayaux J, Lambert J, Rabbat A, Demoule A, Azoulay E. The effects of a 2-h trial of high-flow oxygen by nasal cannula versus Venturi mask in immunocompromised patients with hypoxemic acute respiratory failure: a multicenter randomized trial. Crit Care. 2015;19:380. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 84] [Cited by in RCA: 98] [Article Influence: 9.8] [Reference Citation Analysis (0)] |
| 49. | Mauri T, Alban L, Turrini C, Cambiaghi B, Carlesso E, Taccone P, Bottino N, Lissoni A, Spadaro S, Volta CA, Gattinoni L, Pesenti A, Grasselli G. Optimum support by high-flow nasal cannula in acute hypoxemic respiratory failure: effects of increasing flow rates. Intensive Care Med. 2017;43:1453-1463. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 113] [Cited by in RCA: 168] [Article Influence: 21.0] [Reference Citation Analysis (0)] |
| 50. | Frat JP, Ragot S, Girault C, Perbet S, Prat G, Boulain T, Demoule A, Ricard JD, Coudroy R, Robert R, Mercat A, Brochard L, Thille AW; REVA network. Effect of non-invasive oxygenation strategies in immunocompromised patients with severe acute respiratory failure: a post-hoc analysis of a randomised trial. Lancet Respir Med. 2016;4:646-652. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 137] [Cited by in RCA: 154] [Article Influence: 17.1] [Reference Citation Analysis (0)] |
| 51. | Lemiale V, Mokart D, Resche-Rigon M, Pène F, Mayaux J, Faucher E, Nyunga M, Girault C, Perez P, Guitton C, Ekpe K, Kouatchet A, Théodose I, Benoit D, Canet E, Barbier F, Rabbat A, Bruneel F, Vincent F, Klouche K, Loay K, Mariotte E, Bouadma L, Moreau AS, Seguin A, Meert AP, Reignier J, Papazian L, Mehzari I, Cohen Y, Schenck M, Hamidfar R, Darmon M, Demoule A, Chevret S, Azoulay E; Groupe de Recherche en Réanimation Respiratoire du patient d’Onco-Hématologie (GRRR-OH). Effect of Noninvasive Ventilation vs Oxygen Therapy on Mortality Among Immunocompromised Patients With Acute Respiratory Failure: A Randomized Clinical Trial. JAMA. 2015;314:1711-1719. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 232] [Cited by in RCA: 246] [Article Influence: 24.6] [Reference Citation Analysis (0)] |
| 52. | Cheng LC, Chang SP, Wang JJ, Hsiao SY, Lai CC, Chao CM. The Impact of High-Flow Nasal Cannula on the Outcome of Immunocompromised Patients with Acute Respiratory Failure: A Systematic Review and Meta-Analysis. Medicina (Kaunas). 2019;55:693. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 14] [Cited by in RCA: 9] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 53. | Coudroy R, Frat JP, Ehrmann S, Pène F, Terzi N, Decavèle M, Prat G, Garret C, Contou D, Bourenne J, Gacouin A, Girault C, Dellamonica J, Malacrino D, Labro G, Quenot JP, Herbland A, Jochmans S, Devaquet J, Benzekri D, Vivier E, Nseir S, Colin G, Thévenin D, Grasselli G, Assefi M, Guerin C, Bougon D, Lherm T, Kouatchet A, Ragot S, Thille AW; REVA Network. High-flow nasal oxygen therapy alone or with non-invasive ventilation in immunocompromised patients admitted to ICU for acute hypoxemic respiratory failure: the randomised multicentre controlled FLORALI-IM protocol. BMJ Open. 2019;9:e029798. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 8] [Cited by in RCA: 12] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 54. | Klemencic S, Perkins J. Diagnosis and Management of Oncologic Emergencies. West J Emerg Med. 2019;20:316-322. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 17] [Cited by in RCA: 19] [Article Influence: 3.2] [Reference Citation Analysis (0)] |
| 55. | Hansen BA, Wendelbo Ø, Bruserud Ø, Hemsing AL, Mosevoll KA, Reikvam H. Febrile Neutropenia in Acute Leukemia. Epidemiology, Etiology, Pathophysiology and Treatment. Mediterr J Hematol Infect Dis. 2020;12:e2020009. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 25] [Cited by in RCA: 46] [Article Influence: 9.2] [Reference Citation Analysis (0)] |
| 56. | Stern A, Carrara E, Bitterman R, Yahav D, Leibovici L, Paul M. Early discontinuation of antibiotics for febrile neutropenia versus continuation until neutropenia resolution in people with cancer. Cochrane Database Syst Rev. 2019;1:CD012184. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 7] [Cited by in RCA: 17] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 57. | Mokart D, Darmon M, Resche-Rigon M, Lemiale V, Pène F, Mayaux J, Rabbat A, Kouatchet A, Vincent F, Nyunga M, Bruneel F, Lebert C, Perez P, Renault A, Hamidfar R, Jourdain M, Meert AP, Benoit D, Chevret S, Azoulay E. Prognosis of neutropenic patients admitted to the intensive care unit. Intensive Care Med. 2015;41:296-303. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 61] [Cited by in RCA: 67] [Article Influence: 6.7] [Reference Citation Analysis (0)] |
| 58. | Georges Q, Azoulay E, Mokart D, Soares M, Jeon K, Oeyen S, Rhee CK, Gruber P, Ostermann M, Hill QA, Depuydt P, Ferra C, Toffart AC, Schellongowski P, Müller A, Lemiale V, Tinquaut F, Bourmaud A, Darmon M. Influence of neutropenia on mortality of critically ill cancer patients: results of a meta-analysis on individual data. Crit Care. 2018;22:326. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 29] [Cited by in RCA: 41] [Article Influence: 5.9] [Reference Citation Analysis (0)] |
| 59. | Rivas-Ruiz R, Villasis-Keever M, Miranda-Novales G, Castelán-Martínez OD, Rivas-Contreras S. Outpatient treatment for people with cancer who develop a low-risk febrile neutropaenic event. Cochrane Database Syst Rev. 2019;3:CD009031. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 9] [Cited by in RCA: 9] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 60. | Taj M, Nadeem M, Maqsood S, Shah T, Farzana T, Shamsi TS. Validation of MASCC Score for Risk Stratification in Patients of Hematological Disorders with Febrile Neutropenia. Indian J Hematol Blood Transfus. 2017;33:355-360. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 6] [Cited by in RCA: 12] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 61. | Coyne CJ, Le V, Brennan JJ, Castillo EM, Shatsky RA, Ferran K, Brodine S, Vilke GM. Application of the MASCC and CISNE Risk-Stratification Scores to Identify Low-Risk Febrile Neutropenic Patients in the Emergency Department. Ann Emerg Med. 2017;69:755-764. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 35] [Cited by in RCA: 43] [Article Influence: 4.8] [Reference Citation Analysis (0)] |
| 62. | Ahn S, Rice TW, Yeung SJ, Cooksley T. Comparison of the MASCC and CISNE scores for identifying low-risk neutropenic fever patients: analysis of data from three emergency departments of cancer centers in three continents. Support Care Cancer. 2018;26:1465-1470. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5] [Cited by in RCA: 13] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 63. | Gavelli F, Teboul JL, Monnet X. How can CO2-derived indices guide resuscitation in critically ill patients? J Thorac Dis. 2019;11:S1528-S1537. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 32] [Cited by in RCA: 42] [Article Influence: 7.0] [Reference Citation Analysis (0)] |
| 64. | Poston JT, Koyner JL. Sepsis associated acute kidney injury. BMJ. 2019;364:k4891. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 243] [Cited by in RCA: 474] [Article Influence: 79.0] [Reference Citation Analysis (0)] |
| 65. | Gotts JE, Matthay MA. Sepsis: pathophysiology and clinical management. BMJ. 2016;353:i1585. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 473] [Cited by in RCA: 658] [Article Influence: 73.1] [Reference Citation Analysis (0)] |
| 66. | From the Immunocompromised Host Society. The design, analysis, and reporting of clinical trials on the empirical antibiotic management of the neutropenic patient. Report of a consensus panel. J Infect Dis. 1990;161:397-401. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 207] [Cited by in RCA: 205] [Article Influence: 5.9] [Reference Citation Analysis (0)] |
| 67. | Perdikouri EIA, Arvaniti K, Lathyris D, Apostolidou Kiouti F, Siskou E, Haidich AB, Papandreou C. Infections Due to Multidrug-Resistant Bacteria in Oncological Patients: Insights from a Five-Year Epidemiological and Clinical Analysis. Microorganisms. 2019;7:277. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 21] [Cited by in RCA: 32] [Article Influence: 5.3] [Reference Citation Analysis (0)] |
| 68. | Jung SM, Kim YJ, Ryoo SM, Sohn CH, Seo DW, Lim KS, Kim WY. Cancer patients with neutropenic septic shock: etiology and antimicrobial resistance. Korean J Intern Med. 2020;35:979-987. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 8] [Cited by in RCA: 7] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 69. | Satlin MJ, Walsh TJ. Multidrug-resistant Enterobacteriaceae, Pseudomonas aeruginosa, and vancomycin-resistant Enterococcus: Three major threats to hematopoietic stem cell transplant recipients. Transpl Infect Dis. 2017;19:e12762. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 55] [Cited by in RCA: 71] [Article Influence: 8.9] [Reference Citation Analysis (0)] |
| 70. | Ben-Chetrit E, Eldaim MA, Bar-Meir M, Dodin M, Katz DE. Associated factors and clinical outcomes of bloodstream infection due to extended-spectrum β-lactamase-producing Escherichia coli and Klebsiella pneumoniae during febrile neutropenia. Int J Antimicrob Agents. 2019;53:423-428. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 9] [Cited by in RCA: 11] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 71. | Torfoss D, Fladhagen T, Holte H, Brinch L, Schjesvold FH, Fløisand Y, Nyquist E, Dalgaard J, Meyer P, Lehmann AK, Hammerstrøm J, Skjelbakken T, Høiby EA, Sandvik L, Kvaløy S. Benzylpenicillin plus an aminoglycoside versus meropenem in neutropenic lymphoma and leukaemia patients with a suspected bacterial infection: a randomized, controlled trial. Clin Microbiol Infect. 2017;23:179-187. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 8] [Cited by in RCA: 9] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 72. | Gudiol C, Royo-Cebrecos C, Tebe C, Abdala E, Akova M, Álvarez R, Maestro-de la Calle G, Cano A, Cervera C, Clemente WT, Martín-Dávila P, Freifeld A, Gómez L, Gottlieb T, Gurguí M, Herrera F, Manzur A, Maschmeyer G, Meije Y, Montejo M, Peghin M, Rodríguez-Baño J, Ruiz-Camps I, Sukiennik TC, Carratalà J; BICAR study group. Clinical efficacy of β-lactam/β-lactamase inhibitor combinations for the treatment of bloodstream infection due to extended-spectrum β-lactamase-producing Enterobacteriaceae in haematological patients with neutropaenia: a study protocol for a retrospective observational study (BICAR). BMJ Open. 2017;7:e013268. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 6] [Cited by in RCA: 8] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 73. | Gutiérrez-Gutiérrez B, Salamanca E, de Cueto M, Hsueh PR, Viale P, Paño-Pardo JR, Venditti M, Tumbarello M, Daikos G, Cantón R, Doi Y, Tuon FF, Karaiskos I, Pérez-Nadales E, Schwaber MJ, Azap ÖK, Souli M, Roilides E, Pournaras S, Akova M, Pérez F, Bermejo J, Oliver A, Almela M, Lowman W, Almirante B, Bonomo RA, Carmeli Y, Paterson DL, Pascual A, Rodríguez-Baño J; REIPI/ESGBIS/INCREMENT Investigators. Effect of appropriate combination therapy on mortality of patients with bloodstream infections due to carbapenemase-producing Enterobacteriaceae (INCREMENT): a retrospective cohort study. Lancet Infect Dis. 2017;17:726-734. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 276] [Cited by in RCA: 352] [Article Influence: 44.0] [Reference Citation Analysis (0)] |
| 74. | Gutiérrez-Gutiérrez B, Pérez-Galera S, Salamanca E, de Cueto M, Calbo E, Almirante B, Viale P, Oliver A, Pintado V, Gasch O, Martínez-Martínez L, Pitout J, Akova M, Peña C, Molina J, Hernández A, Venditti M, Prim N, Origüen J, Bou G, Tacconelli E, Tumbarello M, Hamprecht A, Giamarellou H, Almela M, Pérez F, Schwaber MJ, Bermejo J, Lowman W, Hsueh PR, Mora-Rillo M, Natera C, Souli M, Bonomo RA, Carmeli Y, Paterson DL, Pascual A, Rodríguez-Baño J. A Multinational, Preregistered Cohort Study of β-Lactam/β-Lactamase Inhibitor Combinations for Treatment of Bloodstream Infections Due to Extended-Spectrum-β-Lactamase-Producing Enterobacteriaceae. Antimicrob Agents Chemother. 2016;60:4159-4169. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 105] [Cited by in RCA: 129] [Article Influence: 14.3] [Reference Citation Analysis (0)] |
| 75. | Bassetti M, Giacobbe DR, Giamarellou H, Viscoli C, Daikos GL, Dimopoulos G, De Rosa FG, Giamarellos-Bourboulis EJ, Rossolini GM, Righi E, Karaiskos I, Tumbarello M, Nicolau DP, Viale PL, Poulakou G; Critically Ill Patients Study Group of the European Society of Clinical Microbiology and Infectious Disease (ESCMID); Hellenic Society of Chemotherapy (HSC) and Società Italiana di Terapia Antinfettiva (SITA). Management of KPC-producing Klebsiella pneumoniae infections. Clin Microbiol Infect. 2018;24:133-144. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 106] [Cited by in RCA: 129] [Article Influence: 16.1] [Reference Citation Analysis (0)] |
| 76. | Kengkla K, Kongpakwattana K, Saokaew S, Apisarnthanarak A, Chaiyakunapruk N. Comparative efficacy and safety of treatment options for MDR and XDR Acinetobacter baumannii infections: a systematic review and network meta-analysis. J Antimicrob Chemother. 2018;73:22-32. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 128] [Cited by in RCA: 117] [Article Influence: 16.7] [Reference Citation Analysis (0)] |
| 77. | Hassoun A, Linden PK, Friedman B. Incidence, prevalence, and management of MRSA bacteremia across patient populations-a review of recent developments in MRSA management and treatment. Crit Care. 2017;21:211. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 351] [Cited by in RCA: 390] [Article Influence: 48.8] [Reference Citation Analysis (0)] |
| 78. | Schmiedel Y, Zimmerli S. Common invasive fungal diseases: an overview of invasive candidiasis, aspergillosis, cryptococcosis, and Pneumocystis pneumonia. Swiss Med Wkly. 2016;146:w14281. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 39] [Cited by in RCA: 76] [Article Influence: 8.4] [Reference Citation Analysis (0)] |
| 79. | Chen K, Wang Q, Pleasants RA, Ge L, Liu W, Peng K, Zhai S. Empiric treatment against invasive fungal diseases in febrile neutropenic patients: a systematic review and network meta-analysis. BMC Infect Dis. 2017;17:159. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 29] [Cited by in RCA: 26] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 80. | Singer M, Deutschman CS, Seymour CW, Shankar-Hari M, Annane D, Bauer M, Bellomo R, Bernard GR, Chiche JD, Coopersmith CM, Hotchkiss RS, Levy MM, Marshall JC, Martin GS, Opal SM, Rubenfeld GD, van der Poll T, Vincent JL, Angus DC. The Third International Consensus Definitions for Sepsis and Septic Shock (Sepsis-3). JAMA. 2016;315:801-810. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 15803] [Cited by in RCA: 17142] [Article Influence: 1904.7] [Reference Citation Analysis (2)] |
| 81. | Kochanek M, Schalk E, von Bergwelt-Baildon M, Beutel G, Buchheidt D, Hentrich M, Henze L, Kiehl M, Liebregts T, von Lilienfeld-Toal M, Classen A, Mellinghoff S, Penack O, Piepel C, Böll B. Management of sepsis in neutropenic cancer patients: 2018 guidelines from the Infectious Diseases Working Party (AGIHO) and Intensive Care Working Party (iCHOP) of the German Society of Hematology and Medical Oncology (DGHO). Ann Hematol. 2019;98:1051-1069. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 101] [Cited by in RCA: 101] [Article Influence: 16.8] [Reference Citation Analysis (0)] |
| 82. | Averbuch D, Tridello G, Hoek J, Mikulska M, Akan H, Yanez San Segundo L, Pabst T, Özçelik T, Klyasova G, Donnini I, Wu D, Gülbas Z, Zuckerman T, Botelho de Sousa A, Beguin Y, Xhaard A, Bachy E, Ljungman P, de la Camara R, Rascon J, Ruiz Camps I, Vitek A, Patriarca F, Cudillo L, Vrhovac R, Shaw PJ, Wolfs T, O'Brien T, Avni B, Silling G, Al Sabty F, Graphakos S, Sankelo M, Sengeloev H, Pillai S, Matthes S, Melanthiou F, Iacobelli S, Styczynski J, Engelhard D, Cesaro S. Antimicrobial Resistance in Gram-Negative Rods Causing Bacteremia in Hematopoietic Stem Cell Transplant Recipients: Intercontinental Prospective Study of the Infectious Diseases Working Party of the European Bone Marrow Transplantation Group. Clin Infect Dis. 2017;65:1819-1828. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 119] [Cited by in RCA: 122] [Article Influence: 15.3] [Reference Citation Analysis (0)] |
| 83. | Kolonen A, Sinisalo M, Huttunen R, Syrjänen J, Aittoniemi J, Huhtala H, Sankelo M, Rintala H, Räty R, Jantunen E, Nousiainen T, Säily M, Kauppila M, Itälä-Remes M, Ollikainen H, Rauhala A, Koistinen P, Elonen E; Finnish Leukemia Group. Bloodstream infections in acute myeloid leukemia patients treated according to the Finnish Leukemia Group AML-2003 protocol - a prospective nationwide study. Infect Dis (Lond). 2017;49:799-808. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 15] [Cited by in RCA: 23] [Article Influence: 2.9] [Reference Citation Analysis (0)] |
| 84. | Wang Y, Chen L, Liu F, Zhao N, Xu L, Fu B, Li Y. Efficacy and tolerability of granulocyte colony-stimulating factors in cancer patients after chemotherapy: A systematic review and Bayesian network meta-analysis. Sci Rep. 2019;9:15374. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 22] [Cited by in RCA: 13] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 85. | Aguilar-Guisado M, Espigado I, Martín-Peña A, Gudiol C, Royo-Cebrecos C, Falantes J, Vázquez-López L, Montero MI, Rosso-Fernández C, de la Luz Martino M, Parody R, González-Campos J, Garzón-López S, Calderón-Cabrera C, Barba P, Rodríguez N, Rovira M, Montero-Mateos E, Carratalá J, Pérez-Simón JA, Cisneros JM. Optimisation of empirical antimicrobial therapy in patients with haematological malignancies and febrile neutropenia (How Long study): an open-label, randomised, controlled phase 4 trial. Lancet Haematol. 2017;4:e573-e583. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 104] [Cited by in RCA: 151] [Article Influence: 18.9] [Reference Citation Analysis (0)] |
| 86. | Huisman MV, Barco S, Cannegieter SC, Le Gal G, Konstantinides SV, Reitsma PH, Rodger M, Vonk Noordegraaf A, Klok FA. Pulmonary embolism. Nat Rev Dis Primers. 2018;4:18028. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 155] [Cited by in RCA: 227] [Article Influence: 32.4] [Reference Citation Analysis (0)] |
| 87. | Appleton C, Gillam L, Koulogiannis K. Cardiac Tamponade. Cardiol Clin. 2017;35:525-537. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 48] [Cited by in RCA: 58] [Article Influence: 8.3] [Reference Citation Analysis (0)] |
| 88. | Cappetta D, De Angelis A, Sapio L, Prezioso L, Illiano M, Quaini F, Rossi F, Berrino L, Naviglio S, Urbanek K. Oxidative Stress and Cellular Response to Doxorubicin: A Common Factor in the Complex Milieu of Anthracycline Cardiotoxicity. Oxid Med Cell Longev. 2017;2017:1521020. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 279] [Cited by in RCA: 261] [Article Influence: 32.6] [Reference Citation Analysis (0)] |
| 89. | Nebigil CG, Désaubry L. Emergence of cardio-oncology. Ann Pharm Fr. 2018;76:504-506. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 1] [Article Influence: 0.1] [Reference Citation Analysis (0)] |
| 90. | Bloom MW, Hamo CE, Cardinale D, Ky B, Nohria A, Baer L, Skopicki H, Lenihan DJ, Gheorghiade M, Lyon AR, Butler J. Cancer Therapy-Related Cardiac Dysfunction and Heart Failure: Part 1: Definitions, Pathophysiology, Risk Factors, and Imaging. Circ Heart Fail. 2016;9:e002661. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 172] [Cited by in RCA: 233] [Article Influence: 25.9] [Reference Citation Analysis (0)] |
| 91. | Totzeck M, Schuler M, Stuschke M, Heusch G, Rassaf T. Cardio-oncology - strategies for management of cancer-therapy related cardiovascular disease. Int J Cardiol. 2019;280:163-175. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 80] [Cited by in RCA: 135] [Article Influence: 22.5] [Reference Citation Analysis (0)] |
| 92. | Yeh ETH, Ewer MS, Moslehi J, Dlugosz-Danecka M, Banchs J, Chang HM, Minotti G. Mechanisms and clinical course of cardiovascular toxicity of cancer treatment I. Oncology. Semin Oncol. 2019;46:397-402. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 15] [Cited by in RCA: 15] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 93. | Michel L, Rassaf T. Cardio-oncology: need for novel structures. Eur J Med Res. 2019;24:1. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 17] [Cited by in RCA: 20] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 94. | Yeh ET, Bickford CL. Cardiovascular complications of cancer therapy: incidence, pathogenesis, diagnosis, and management. J Am Coll Cardiol. 2009;53:2231-2247. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 853] [Cited by in RCA: 884] [Article Influence: 55.3] [Reference Citation Analysis (0)] |
| 95. | Wickramasinghe CD, Nguyen KL, Watson KE, Vorobiof G, Yang EH. Concepts in cardio-oncology: definitions, mechanisms, diagnosis and treatment strategies of cancer therapy-induced cardiotoxicity. Future Oncol. 2016;12:855-870. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 17] [Cited by in RCA: 17] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 96. | Plana JC, Galderisi M, Barac A, Ewer MS, Ky B, Scherrer-Crosbie M, Ganame J, Sebag IA, Agler DA, Badano LP, Banchs J, Cardinale D, Carver J, Cerqueira M, DeCara JM, Edvardsen T, Flamm SD, Force T, Griffin BP, Jerusalem G, Liu JE, Magalhães A, Marwick T, Sanchez LY, Sicari R, Villarraga HR, Lancellotti P. Expert consensus for multimodality imaging evaluation of adult patients during and after cancer therapy: a report from the American Society of Echocardiography and the European Association of Cardiovascular Imaging. J Am Soc Echocardiogr. 2014;27:911-939. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 827] [Cited by in RCA: 942] [Article Influence: 85.6] [Reference Citation Analysis (0)] |
| 97. | Kang Y, Scherrer-Crosbie M. Echocardiography Imaging of Cardiotoxicity. Cardiol Clin. 2019;37:419-427. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 9] [Cited by in RCA: 8] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 98. | Arciniegas Calle MC, Sandhu NP, Xia H, Cha SS, Pellikka PA, Ye Z, Herrmann J, Villarraga HR. Two-dimensional speckle tracking echocardiography predicts early subclinical cardiotoxicity associated with anthracycline-trastuzumab chemotherapy in patients with breast cancer. BMC Cancer. 2018;18:1037. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 33] [Cited by in RCA: 63] [Article Influence: 9.0] [Reference Citation Analysis (0)] |
| 99. | Alvarez-Cardona J, Lenihan DJ. Anthracycline Cardiotoxicity: It Is Possible to Teach an Old Dog Some New Tricks. Cardiol Clin. 2019;37:355-363. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 6] [Cited by in RCA: 6] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 100. | Ye L, Yang ZG, Selvanayagam JB, Luo H, Yang TZ, Perry R, Diao KY, Huang S, Yang MX, Yang P, Jin Y, Guo YK. Myocardial Strain Imaging by Echocardiography for the Prediction of Cardiotoxicity in Chemotherapy-Treated Patients: A Meta-Analysis. JACC Cardiovasc Imaging. 2020;13:881-882. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 14] [Cited by in RCA: 12] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 101. | Simões R, Silva LM, Cruz ALVM, Fraga VG, de Paula Sabino A, Gomes KB. Troponin as a cardiotoxicity marker in breast cancer patients receiving anthracycline-based chemotherapy: A narrative review. Biomed Pharmacother. 2018;107:989-996. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 28] [Cited by in RCA: 37] [Article Influence: 5.3] [Reference Citation Analysis (0)] |
| 102. | Ryan TD, Nagarajan R, Godown J. Cardiovascular Toxicities in Pediatric Cancer Survivors. Cardiol Clin. 2019;37:533-544. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 14] [Cited by in RCA: 16] [Article Influence: 3.2] [Reference Citation Analysis (0)] |
| 103. | von Minckwitz G, Procter M, de Azambuja E, Zardavas D, Benyunes M, Viale G, Suter T, Arahmani A, Rouchet N, Clark E, Knott A, Lang I, Levy C, Yardley DA, Bines J, Gelber RD, Piccart M, Baselga J; APHINITY Steering Committee and Investigators. Adjuvant Pertuzumab and Trastuzumab in Early HER2-Positive Breast Cancer. N Engl J Med. 2017;377:122-131. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 918] [Cited by in RCA: 981] [Article Influence: 122.6] [Reference Citation Analysis (0)] |
| 104. | Cardinale D, Ciceri F, Latini R, Franzosi MG, Sandri MT, Civelli M, Cucchi G, Menatti E, Mangiavacchi M, Cavina R, Barbieri E, Gori S, Colombo A, Curigliano G, Salvatici M, Rizzo A, Ghisoni F, Bianchi A, Falci C, Aquilina M, Rocca A, Monopoli A, Milandri C, Rossetti G, Bregni M, Sicuro M, Malossi A, Nassiacos D, Verusio C, Giordano M, Staszewsky L, Barlera S, Nicolis EB, Magnoli M, Masson S, Cipolla CM; ICOS-ONE Study Investigators. Anthracycline-induced cardiotoxicity: A multicenter randomised trial comparing two strategies for guiding prevention with enalapril: The International CardioOncology Society-one trial. Eur J Cancer. 2018;94:126-137. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 119] [Cited by in RCA: 163] [Article Influence: 23.3] [Reference Citation Analysis (0)] |
| 105. | Avila MS, Ayub-Ferreira SM, de Barros Wanderley MR, das Dores Cruz F, Gonçalves Brandão SM, Rigaud VOC, Higuchi-Dos-Santos MH, Hajjar LA, Kalil Filho R, Hoff PM, Sahade M, Ferrari MSM, de Paula Costa RL, Mano MS, Bittencourt Viana Cruz CB, Abduch MC, Lofrano Alves MS, Guimaraes GV, Issa VS, Bittencourt MS, Bocchi EA. Carvedilol for Prevention of Chemotherapy-Related Cardiotoxicity: The CECCY Trial. J Am Coll Cardiol. 2018;71:2281-2290. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 250] [Cited by in RCA: 350] [Article Influence: 50.0] [Reference Citation Analysis (0)] |
| 106. | Shah P, Garris R, Abboud R, Vasudev R, Patel H, Doshi R, Shamoon F, Bikkina M. Meta-Analysis Comparing Usefulness of Beta Blockers to Preserve Left Ventricular Function During Anthracycline Therapy. Am J Cardiol. 2019;124:789-794. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 11] [Cited by in RCA: 21] [Article Influence: 3.5] [Reference Citation Analysis (0)] |
| 107. | Montoya A, Amaya CN, Belmont A, Diab N, Trevino R, Villanueva G, Rains S, Sanchez LA, Badri N, Otoukesh S, Khammanivong A, Liss D, Baca ST, Aguilera RJ, Dickerson EB, Torabi A, Dwivedi AK, Abbas A, Chambers K, Bryan BA, Nahleh Z. Use of non-selective β-blockers is associated with decreased tumor proliferative indices in early stage breast cancer. Oncotarget. 2017;8:6446-6460. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 64] [Cited by in RCA: 94] [Article Influence: 13.4] [Reference Citation Analysis (0)] |
| 108. | Montoya A, Varela-Ramirez A, Dickerson E, Pasquier E, Torabi A, Aguilera R, Nahleh Z, Bryan B. The beta adrenergic receptor antagonist propranolol alters mitogenic and apoptotic signaling in late stage breast cancer. Biomed J. 2019;42:155-165. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 30] [Cited by in RCA: 48] [Article Influence: 8.0] [Reference Citation Analysis (0)] |
| 109. | Baek MH, Kim DY, Kim SO, Kim YJ, Park YH. Impact of beta blockers on survival outcomes in ovarian cancer: a nationwide population-based cohort study. J Gynecol Oncol. 2018;29:e82. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 18] [Cited by in RCA: 28] [Article Influence: 4.7] [Reference Citation Analysis (0)] |
| 110. | Mullen PJ, Yu R, Longo J, Archer MC, Penn LZ. The interplay between cell signalling and the mevalonate pathway in cancer. Nat Rev Cancer. 2016;16:718-731. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 336] [Cited by in RCA: 486] [Article Influence: 54.0] [Reference Citation Analysis (2)] |
| 111. | Collins R, Reith C, Emberson J, Armitage J, Baigent C, Blackwell L, Blumenthal R, Danesh J, Smith GD, DeMets D, Evans S, Law M, MacMahon S, Martin S, Neal B, Poulter N, Preiss D, Ridker P, Roberts I, Rodgers A, Sandercock P, Schulz K, Sever P, Simes J, Smeeth L, Wald N, Yusuf S, Peto R. Interpretation of the evidence for the efficacy and safety of statin therapy. Lancet. 2016;388:2532-2561. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1267] [Cited by in RCA: 1271] [Article Influence: 141.2] [Reference Citation Analysis (0)] |
| 112. | El-Hamamsy M, Elwakil H, Saad AS, Shawki MA. A Randomized Controlled Open-Label Pilot Study of Simvastatin Addition to Whole-Brain Radiation Therapy in Patients With Brain Metastases. Oncol Res. 2016;24:521-528. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 14] [Cited by in RCA: 19] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 113. | Xia DK, Hu ZG, Tian YF, Zeng FJ. Statin use and prognosis of lung cancer: a systematic review and meta-analysis of observational studies and randomized controlled trials. Drug Des Devel Ther. 2019;13:405-422. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 24] [Cited by in RCA: 41] [Article Influence: 6.8] [Reference Citation Analysis (0)] |
| 114. | Farooqi MAM, Malhotra N, Mukherjee SD, Sanger S, Dhesy-Thind SK, Ellis P, Leong DP. Statin therapy in the treatment of active cancer: A systematic review and meta-analysis of randomized controlled trials. PLoS One. 2018;13:e0209486. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 40] [Cited by in RCA: 51] [Article Influence: 7.3] [Reference Citation Analysis (0)] |
| 115. | van Diepen S, Katz JN, Albert NM, Henry TD, Jacobs AK, Kapur NK, Kilic A, Menon V, Ohman EM, Sweitzer NK, Thiele H, Washam JB, Cohen MG; American Heart Association Council on Clinical Cardiology; Council on Cardiovascular and Stroke Nursing; Council on Quality of Care and Outcomes Research; and Mission: Lifeline. Contemporary Management of Cardiogenic Shock: A Scientific Statement From the American Heart Association. Circulation. 2017;136:e232-e268. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 693] [Cited by in RCA: 1154] [Article Influence: 144.3] [Reference Citation Analysis (0)] |
| 116. | Chakravarthy M, Tsukashita M, Murali S. A Targeted Management Approach to Cardiogenic Shock. Crit Care Clin. 2018;34:423-437. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 6] [Cited by in RCA: 6] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 117. | Lenneman CG, Sawyer DB. Cardio-Oncology: An Update on Cardiotoxicity of Cancer-Related Treatment. Circ Res. 2016;118:1008-1020. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 218] [Cited by in RCA: 317] [Article Influence: 35.2] [Reference Citation Analysis (0)] |
| 118. | Nhola LF, Villarraga HR. Rationale for Cardio-Oncology Units. Rev Esp Cardiol (Engl Ed). 2017;70:583-589. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 2] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 119. | Mitroi C, Martín-García A, Mazón Ramos P, Virizuela Echaburu JA, Arenas-Prat M, García-Sanz R, Arrarte Esteban V, García-Pinilla JM, Cosín-Sales J, López-Fernández T. Current functioning of cardio-oncology units in Spain. Clin Transl Oncol. 2020;22:1418-1422. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4] [Cited by in RCA: 4] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 120. | Noone J, Barclay C. Head and Neck Cancer Patients – Information for the General Dental Practitioner. Dent Update. 2017;44:209-210, 213-215. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 6] [Cited by in RCA: 6] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 121. | Fantoni D, Shih AC. Perioperative Fluid Therapy. Vet Clin North Am Small Anim Pract. 2017;47:423-434. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 9] [Cited by in RCA: 16] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 122. | Heming N, Moine P, Coscas R, Annane D. Perioperative fluid management for major elective surgery. Br J Surg. 2020;107:e56-e62. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 17] [Cited by in RCA: 31] [Article Influence: 6.2] [Reference Citation Analysis (0)] |
| 123. | Necchi A, Miceli R, Oualla K, Sonpavde G, Giannatempo P, Raggi D, Nicolai N, Boffi R, Busia A, Mariani L, Salvioni R. Effect of Bleomycin Administration on the Development of Pulmonary Toxicity in Patients With Metastatic Germ Cell Tumors Receiving First-Line Chemotherapy: A Meta-Analysis of Randomized Studies. Clin Genitourin Cancer. 2017;15:213-220.e5. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 18] [Cited by in RCA: 20] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 124. | Aakre BM, Efem RI, Wilson GA, Kor DJ, Eisenach JH. Postoperative acute respiratory distress syndrome in patients with previous exposure to bleomycin. Mayo Clin Proc. 2014;89:181-189. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 16] [Cited by in RCA: 17] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 125. | Remy KE, Hall MW, Cholette J, Juffermans NP, Nicol K, Doctor A, Blumberg N, Spinella PC, Norris PJ, Dahmer MK, Muszynski JA; Pediatric Critical Care Blood Research Network (Blood Net). Mechanisms of red blood cell transfusion-related immunomodulation. Transfusion. 2018;58:804-815. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 87] [Cited by in RCA: 147] [Article Influence: 21.0] [Reference Citation Analysis (0)] |
| 126. | Connor JP, O'Shea A, McCool K, Sampene E, Barroilhet LM. Peri-operative allogeneic blood transfusion is associated with poor overall survival in advanced epithelial ovarian Cancer; potential impact of patient blood management on Cancer outcomes. Gynecol Oncol. 2018;151:294-298. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 18] [Cited by in RCA: 26] [Article Influence: 3.7] [Reference Citation Analysis (0)] |
| 127. | Manning-Geist BL, Alimena S, Del Carmen MG, Goodman A, Clark RM, Growdon WB, Horowitz NS, Berkowitz RS, Muto MG, Worley MJ. Infection, thrombosis, and oncologic outcome after interval debulking surgery: Does perioperative blood transfusion matter? Gynecol Oncol. 2019;153:63-67. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 9] [Cited by in RCA: 15] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 128. | Torella MC, Duarte B, Villarroel M, Lasa J, Zubiaurre I. Increased Risk of Synchronous Colorectal Lesions in Patients Referred for Endoscopic Mucosal Resection of Lateral Spreading Tumors. Arq Gastroenterol. 2019;56:276-279. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 2] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 129. | Byrne K, Levins KJ, Buggy DJ. Can anesthetic-analgesic technique during primary cancer surgery affect recurrence or metastasis? Can J Anaesth. 2016;63:184-192. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 89] [Cited by in RCA: 104] [Article Influence: 11.6] [Reference Citation Analysis (0)] |
| 130. | Wall T, Sherwin A, Ma D, Buggy DJ. Influence of perioperative anaesthetic and analgesic interventions on oncological outcomes: a narrative review. Br J Anaesth. 2019;123:135-150. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 111] [Cited by in RCA: 146] [Article Influence: 24.3] [Reference Citation Analysis (0)] |
| 131. | Luo X, Zhao H, Hennah L, Ning J, Liu J, Tu H, Ma D. Impact of isoflurane on malignant capability of ovarian cancer in vitro. Br J Anaesth. 2015;114:831-839. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 75] [Cited by in RCA: 82] [Article Influence: 7.5] [Reference Citation Analysis (0)] |
| 132. | Buckley A, McQuaid S, Johnson P, Buggy DJ. Effect of anaesthetic technique on the natural killer cell anti-tumour activity of serum from women undergoing breast cancer surgery: a pilot study. Br J Anaesth. 2014;113 Suppl 1:i56-i62. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 121] [Cited by in RCA: 152] [Article Influence: 13.8] [Reference Citation Analysis (0)] |
| 133. | Hong B, Lee S, Kim Y, Lee M, Youn AM, Rhim H, Hong SH, Kim YH, Yoon SH, Lim C. Anesthetics and long-term survival after cancer surgery-total intravenous versus volatile anesthesia: a retrospective study. BMC Anesthesiol. 2019;19:233. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 21] [Cited by in RCA: 30] [Article Influence: 5.0] [Reference Citation Analysis (0)] |
| 134. | Myles PS, Leslie K, Chan MT, Forbes A, Peyton PJ, Paech MJ, Beattie WS, Sessler DI, Devereaux PJ, Silbert B, Schricker T, Wallace S; ANZCA Trials Group for the ENIGMA-II investigators. The safety of addition of nitrous oxide to general anaesthesia in at-risk patients having major non-cardiac surgery (ENIGMA-II): a randomised, single-blind trial. Lancet. 2014;384:1446-1454. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 147] [Cited by in RCA: 143] [Article Influence: 13.0] [Reference Citation Analysis (0)] |
| 135. | Kim SY, Kim NK, Baik SH, Min BS, Hur H, Lee J, Noh HY, Lee JH, Koo BN. Effects of Postoperative Pain Management on Immune Function After Laparoscopic Resection of Colorectal Cancer: A Randomized Study. Medicine (Baltimore). 2016;95:e3602. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 21] [Cited by in RCA: 27] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 136. | Shao YJ, Liu WS, Guan BQ, Hao JL, Ji K, Cheng XJ, Wang K. Contribution of Opiate Analgesics to the Development of Infections in Advanced Cancer Patients. Clin J Pain. 2017;33:295-299. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 14] [Cited by in RCA: 20] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 137. | Boland JW, Pockley AG. Influence of opioids on immune function in patients with cancer pain: from bench to bedside. Br J Pharmacol. 2018;175:2726-2736. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 87] [Cited by in RCA: 135] [Article Influence: 16.9] [Reference Citation Analysis (0)] |
| 138. | Xie N, Gomes FP, Deora V, Gregory K, Vithanage T, Nassar ZD, Cabot PJ, Sturgess D, Shaw PN, Parat MO. Activation of μ-opioid receptor and Toll-like receptor 4 by plasma from morphine-treated mice. Brain Behav Immun. 2017;61:244-258. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 36] [Cited by in RCA: 41] [Article Influence: 5.1] [Reference Citation Analysis (0)] |
| 139. | Liang X, Liu R, Chen C, Ji F, Li T. Opioid System Modulates the Immune Function: A Review. Transl Perioper Pain Med. 2016;1:5-13. [PubMed] |
| 140. | Plein LM, Rittner HL. Opioids and the immune system - friend or foe. Br J Pharmacol. 2018;175:2717-2725. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 277] [Cited by in RCA: 322] [Article Influence: 46.0] [Reference Citation Analysis (0)] |
| 141. | Gong L, Qin Q, Zhou L, Ouyang W, Li Y, Wu Y, Li Y. Effects of fentanyl anesthesia and sufentanil anesthesia on regulatory T cells frequencies. Int J Clin Exp Pathol. 2014;7:7708-7716. [PubMed] |
| 142. | Sacerdote P, Bianchi M, Gaspani L, Manfredi B, Maucione A, Terno G, Ammatuna M, Panerai AE. The effects of tramadol and morphine on immune responses and pain after surgery in cancer patients. Anesth Analg. 2000;90:1411-1414. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 239] [Cited by in RCA: 233] [Article Influence: 9.3] [Reference Citation Analysis (1)] |
| 143. | Lai HC, Lee MS, Lin C, Lin KT, Huang YH, Wong CS, Chan SM, Wu ZF. Propofol-based total intravenous anaesthesia is associated with better survival than desflurane anaesthesia in hepatectomy for hepatocellular carcinoma: a retrospective cohort study. Br J Anaesth. 2019;123:151-160. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 47] [Cited by in RCA: 72] [Article Influence: 12.0] [Reference Citation Analysis (0)] |
| 144. | Wu ZF, Lee MS, Wong CS, Lu CH, Huang YS, Lin KT, Lou YS, Lin C, Chang YC, Lai HC. Propofol-based Total Intravenous Anesthesia Is Associated with Better Survival Than Desflurane Anesthesia in Colon Cancer Surgery. Anesthesiology. 2018;129:932-941. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 99] [Cited by in RCA: 142] [Article Influence: 23.7] [Reference Citation Analysis (0)] |
| 145. | Huang YH, Lee MS, Lou YS, Lai HC, Yu JC, Lu CH, Wong CS, Wu ZF. Propofol-based total intravenous anesthesia did not improve survival compared to desflurane anesthesia in breast cancer surgery. PLoS One. 2019;14:e0224728. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 35] [Cited by in RCA: 52] [Article Influence: 8.7] [Reference Citation Analysis (0)] |
| 146. | Hakeam HA, Arab A, Azzam A, Alyahya Z, Eldali AM, Amin T. Incidence of leukopenia and thrombocytopenia with cisplatin plus mitomycin-c versus melphalan in patients undergoing cytoreductive surgery (CRS) and hyperthermic intraperitoneal chemotherapy (HIPEC). Cancer Chemother Pharmacol. 2018;81:697-704. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 11] [Cited by in RCA: 9] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 147. | Abel ML, Kokosis G, Blazer DG. Pulmonary toxicity after intraperitoneal mitomycin C: a case report of a rare complication of HIPEC. World J Surg Oncol. 2017;15:49. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 3] [Cited by in RCA: 5] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 148. | Segelman J, Nygren J. Best practice in major elective rectal/pelvic surgery: enhanced recovery after surgery (ERAS). Updates Surg. 2017;69:435-439. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 9] [Cited by in RCA: 4] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 149. | Pędziwiatr M, Mavrikis J, Witowski J, Adamos A, Major P, Nowakowski M, Budzyński A. Current status of enhanced recovery after surgery (ERAS) protocol in gastrointestinal surgery. Med Oncol. 2018;35:95. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 122] [Cited by in RCA: 202] [Article Influence: 28.9] [Reference Citation Analysis (0)] |
| 150. | Lohsiriwat V, Jitmungngan R. Enhanced recovery after surgery in emergency colorectal surgery: Review of literature and current practices. World J Gastrointest Surg. 2019;11:41-52. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 37] [Cited by in RCA: 40] [Article Influence: 6.7] [Reference Citation Analysis (1)] |
| 151. | Aarts MA, Rotstein OD, Pearsall EA, Victor JC, Okrainec A, McKenzie M, McCluskey SA, Conn LG, McLeod RS; iERAS group. Postoperative ERAS Interventions Have the Greatest Impact on Optimal Recovery: Experience With Implementation of ERAS Across Multiple Hospitals. Ann Surg. 2018;267:992-997. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 95] [Cited by in RCA: 129] [Article Influence: 21.5] [Reference Citation Analysis (0)] |
| 152. | Ljungqvist O, Scott M, Fearon KC. Enhanced Recovery After Surgery: A Review. JAMA Surg. 2017;152:292-298. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1487] [Cited by in RCA: 2226] [Article Influence: 278.3] [Reference Citation Analysis (0)] |
| 153. | Mingjie X, Luyao Z, Ze T, YinQuan Z, Quan W. Laparoscopic Radical Gastrectomy for Resectable Advanced Gastric Cancer Within Enhanced Recovery Programs: A Prospective Randomized Controlled Trial. J Laparoendosc Adv Surg Tech A. 2017;27:959-964. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 21] [Cited by in RCA: 30] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 154. | Liu G, Jian F, Wang X, Chen L. Fast-track surgery protocol in elderly patients undergoing laparoscopic radical gastrectomy for gastric cancer: a randomized controlled trial. Onco Targets Ther. 2016;9:3345-3351. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 8] [Cited by in RCA: 26] [Article Influence: 2.9] [Reference Citation Analysis (0)] |
| 155. | Wang LH, Zhu RF, Gao C, Wang SL, Shen LZ. Application of enhanced recovery after gastric cancer surgery: An updated meta-analysis. World J Gastroenterol. 2018;24:1562-1578. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 54] [Cited by in RCA: 60] [Article Influence: 8.6] [Reference Citation Analysis (0)] |
| 156. | Thandra K, Salah Z, Chawla S. Oncologic Emergencies-The Old, the New, and the Deadly. J Intensive Care Med. 2020;35:3-13. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 7] [Cited by in RCA: 10] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 157. | Todd KH, Thomas CR. An Inflection Point in the Evolution of Oncologic Emergency Medicine. Ann Emerg Med. 2016;68:712-716. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 13] [Cited by in RCA: 12] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 158. | Baldwin KJ, Zivković SA, Lieberman FS. Neurologic emergencies in patients who have cancer: diagnosis and management. Neurol Clin. 2012;30:101-128, viii. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 16] [Cited by in RCA: 16] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 159. | Achrol AS, Rennert RC, Anders C, Soffietti R, Ahluwalia MS, Nayak L, Peters S, Arvold ND, Harsh GR, Steeg PS, Chang SD. Brain metastases. Nat Rev Dis Primers. 2019;5:5. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 393] [Cited by in RCA: 681] [Article Influence: 113.5] [Reference Citation Analysis (0)] |
| 160. | Halfdanarson TR, Hogan WJ, Madsen BE. Emergencies in Hematology and Oncology. Mayo Clin Proc. 2017;92:609-641. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 20] [Cited by in RCA: 20] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 161. | Stea B, Skrepnik T, Hsu CC, Abendroth R. The role of radiation therapy in the treatment of metastatic cancer. Clin Exp Metastasis. 2018;35:535-546. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 2] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 162. | Kemmerer E, Shah S. Update on Radiotherapy for Central Nervous System Malignancies. Surg Oncol Clin N Am. 2017;26:347-355. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3] [Cited by in RCA: 3] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 163. | Xu Y, Ma P, Xu Y, Dai J. Selection of prescription isodose line for brain metastases treated with volumetric modulated arc radiotherapy. J Appl Clin Med Phys. 2019;20:63-69. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 4] [Cited by in RCA: 12] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 164. | Kotecha R, Gondi V, Ahluwalia MS, Brastianos PK, Mehta MP. Recent advances in managing brain metastasis. F1000Res. 2018;7:1772. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 49] [Cited by in RCA: 69] [Article Influence: 9.9] [Reference Citation Analysis (0)] |
| 165. | Niranjan A, Monaco E, Flickinger J, Lunsford LD. Guidelines for Multiple Brain Metastases Radiosurgery. Prog Neurol Surg. 2019;34:100-109. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 30] [Cited by in RCA: 58] [Article Influence: 9.7] [Reference Citation Analysis (0)] |
| 166. | Mayer SA, Solimando DA, Waddell JA. Cancer Chemotherapy Update: Bevacizumab, Etoposide, and Cisplatin Regimen for Refractory Brain Metastases. Hosp Pharm. 2017;52:394-399. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 1] [Article Influence: 0.1] [Reference Citation Analysis (0)] |
| 167. | Kalra M, Adra N, Hanna N, Abonour R, Einhorn LH. High-dose chemotherapy plus peripheral blood stem cell transplantation for patients with relapsed germ cell tumors and active brain metastases. Cancer. 2020;126:1202-1207. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5] [Cited by in RCA: 6] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 168. | Wu YL, Ahn MJ, Garassino MC, Han JY, Katakami N, Kim HR, Hodge R, Kaur P, Brown AP, Ghiorghiu D, Papadimitrakopoulou VA, Mok TSK. CNS Efficacy of Osimertinib in Patients With T790M-Positive Advanced Non-Small-Cell Lung Cancer: Data From a Randomized Phase III Trial (AURA3). J Clin Oncol. 2018;36:2702-2709. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 214] [Cited by in RCA: 370] [Article Influence: 52.9] [Reference Citation Analysis (0)] |
| 169. | Economopoulou P, Mountzios G. Non-small cell lung cancer (NSCLC) and central nervous system (CNS) metastases: role of tyrosine kinase inhibitors (TKIs) and evidence in favor or against their use with concurrent cranial radiotherapy. Transl Lung Cancer Res. 2016;5:588-598. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 17] [Cited by in RCA: 30] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 170. | Venur VA, Leone JP. Targeted Therapies for Brain Metastases from Breast Cancer. Int J Mol Sci. 2016;17:1543. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 57] [Cited by in RCA: 74] [Article Influence: 8.2] [Reference Citation Analysis (0)] |
| 171. | McArthur GA, Maio M, Arance A, Nathan P, Blank C, Avril MF, Garbe C, Hauschild A, Schadendorf D, Hamid O, Fluck M, Thebeau M, Schachter J, Kefford R, Chamberlain M, Makrutzki M, Robson S, Gonzalez R, Margolin K. Vemurafenib in metastatic melanoma patients with brain metastases: an open-label, single-arm, phase 2, multicentre study. Ann Oncol. 2017;28:634-641. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 129] [Cited by in RCA: 159] [Article Influence: 19.9] [Reference Citation Analysis (0)] |
| 172. | Berghoff AS, Venur VA, Preusser M, Ahluwalia MS. Immune Checkpoint Inhibitors in Brain Metastases: From Biology to Treatment. Am Soc Clin Oncol Educ Book. 2016;35:e116-e122. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 34] [Cited by in RCA: 64] [Article Influence: 7.1] [Reference Citation Analysis (0)] |
| 173. | King T, Faiman B. Steroid-Associated Side Effects: A Symptom Management Update on Multiple Myeloma Treatment. Clin J Oncol Nurs. 2017;21:240-249. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 12] [Cited by in RCA: 21] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 174. | Carney N, Totten AM, O'Reilly C, Ullman JS, Hawryluk GW, Bell MJ, Bratton SL, Chesnut R, Harris OA, Kissoon N, Rubiano AM, Shutter L, Tasker RC, Vavilala MS, Wilberger J, Wright DW, Ghajar J. Guidelines for the Management of Severe Traumatic Brain Injury, Fourth Edition. Neurosurgery. 2017;80:6-15. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1786] [Cited by in RCA: 2211] [Article Influence: 276.4] [Reference Citation Analysis (1)] |
| 175. | Claassen J, Taccone FS, Horn P, Holtkamp M, Stocchetti N, Oddo M; Neurointensive Care Section of the European Society of Intensive Care Medicine. Recommendations on the use of EEG monitoring in critically ill patients: consensus statement from the neurointensive care section of the ESICM. Intensive Care Med. 2013;39:1337-1351. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 266] [Cited by in RCA: 276] [Article Influence: 23.0] [Reference Citation Analysis (0)] |
| 176. | Wychowski T, Wang H, Buniak L, Henry JC, Mohile N. Considerations in prophylaxis for tumor-associated epilepsy: prevention of status epilepticus and tolerability of newer generation AEDs. Clin Neurol Neurosurg. 2013;115:2365-2369. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 29] [Cited by in RCA: 30] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 177. | Billington M, Kandalaft OR, Aisiku IP. Adult Status Epilepticus: A Review of the Prehospital and Emergency Department Management. J Clin Med. 2016;5:74. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 11] [Cited by in RCA: 9] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 178. | Farrokh S, Tahsili-Fahadan P, Ritzl EK, Lewin JJ, Mirski MA. Antiepileptic drugs in critically ill patients. Crit Care. 2018;22:153. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 38] [Cited by in RCA: 36] [Article Influence: 5.1] [Reference Citation Analysis (0)] |
| 179. | Del Bigio MR, Di Curzio DL. Nonsurgical therapy for hydrocephalus: a comprehensive and critical review. Fluids Barriers CNS. 2016;13:3. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 63] [Cited by in RCA: 65] [Article Influence: 7.2] [Reference Citation Analysis (0)] |
| 180. | Barth C, Soares M, Toffart AC, Timsit JF, Burghi G, Irrazabal C, Pattison N, Tobar E, Almeida BF, Silva UV, Azevedo LC, Rabbat A, Lamer C, Parrot A, Souza-Dantas VC, Wallet F, Blot F, Bourdin G, Piras C, Delemazure J, Durand M, Salluh J, Azoulay E, Lemiale V; Lung Cancer in Critical Care (LUCCA) Study Investigators. Characteristics and outcome of patients with newly diagnosed advanced or metastatic lung cancer admitted to intensive care units (ICUs). Ann Intensive Care. 2018;8:80. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 14] [Cited by in RCA: 25] [Article Influence: 3.6] [Reference Citation Analysis (0)] |
| 181. | Soares M, Bozza FA, Azevedo LC, Silva UV, Corrêa TD, Colombari F, Torelly AP, Varaschin P, Viana WN, Knibel MF, Damasceno M, Espinoza R, Ferez M, Silveira JG, Lobo SA, Moraes AP, Lima RA, de Carvalho AG, do Brasil PE, Kahn JM, Angus DC, Salluh JI. Effects of Organizational Characteristics on Outcomes and Resource Use in Patients With Cancer Admitted to Intensive Care Units. J Clin Oncol. 2016;34:3315-3324. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 73] [Cited by in RCA: 81] [Article Influence: 9.0] [Reference Citation Analysis (0)] |
| 182. | Zerbib Y, Rabbat A, Fartoukh M, Bigé N, Andréjak C, Mayaux J, De Prost N, Misset B, Lemiale V, Bruneel F, Maizel J, Ricome S, Jacobs F, Bornstain C, Dupont H, Baudin F, Azoulay E, Pène F; Groupe de Recherche sur la Réanimation Respiratoire en Onco-Hématologie (GRRR-OH). Urgent Chemotherapy for Life-Threatening Complications Related to Solid Neoplasms. Crit Care Med. 2017;45:e640-e648. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 21] [Cited by in RCA: 24] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 183. | de Oliveira MCF, Ferreira JC, Nassar Junior AP, Dettino ALA, Caruso P. Impact of Urgent Chemotherapy in Critically Ill Patients. J Intensive Care Med. 2020;35:347-353. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 9] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 184. | Chen YF, Lin JW, Ho CC, Yang CY, Chang CH, Huang TM, Chen CY, Chen KY, Shih JY, Yu CJ. Outcomes of cancer therapy administered to treatment-naïve lung cancer patients in the intensive care unit. J Cancer. 2017;8:1995-2003. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 13] [Cited by in RCA: 16] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 185. | Pastores SM, Goldman DA, Shaz DJ, Kostelecky N, Daley RJ, Peterson TJ, Tan KS, Halpern NA. Characteristics and outcomes of patients with hematologic malignancies receiving chemotherapy in the intensive care unit. Cancer. 2018;124:3025-3036. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 9] [Cited by in RCA: 16] [Article Influence: 2.3] [Reference Citation Analysis (0)] |