Published online Jan 24, 2020. doi: 10.5306/wjco.v11.i1.1
Peer-review started: July 17, 2019
First decision: October 14, 2019
Revised: November 13, 2019
Accepted: November 18, 2019
Article in press: November 18, 2019
Published online: January 24, 2020
Processing time: 174 Days and 3 Hours
The indication for salvage radiotherapy (RT) (SRT) in patients with biochemically-recurrent prostate cancer after surgery is based on prostate-specific antigen (PSA) levels at the time of biochemical recurrence. Although there are clear criteria (pT3-pT4 disease and/or positive margins) for the use of adjuvant radiotherapy, no specific clinical or tumour-related criteria have yet been defined for SRT. In retrospective series, 5-year biochemical progression-free survival (PFS) ranges from 35%-85%, depending on the PSA level at the start of RT. Two phase 3 trials have compared SRT with and without androgen deprivation therapy (ADT), finding that combined treatment (SRT+ADT) improves both PFS and overall survival. Similar to adjuvant RT, the indication for ADT is based on tumour-related factors such as PSA levels, tumour stage, and surgical margins. The number of patients referred to radiation oncology departments for SRT continues to rise. In the present article, we define the clinical, therapeutic, and tumour-related factors that we believe should be evaluated before prescribing SRT. In addition, we propose a decision algorithm to determine whether the patient is fit for SRT. This algorithm will help to identify patients in whom radiotherapy is likely to improve survival without significantly worsening quality of life.
Core tip: Salvage radiotherapy (SRT) is an effective treatment for biochemically-recurrent prostate cancer after prostatectomy. Proper patient selection is crucial. While tumour-related factors are important, the indication for SRT should also be based on clinical factors and dosimetric variables. Patients with non-aggressive tumours who have a life expectancy of less than 10 years are unlikely to benefit from radiotherapy and should thus be considered "unfit" for SRT. The development of advanced imaging techniques such Ga-PSMA positron emission tomography/computed tomography, which are capable of localizing the recurrent lesion when prostate-specific antigen ≤ 0.5 ng/mL, has forced clinicians to reconsider whether patients should undergo radiotherapy without locate first the recurrence.
- Citation: González-San Segundo C, Gómez-Iturriaga A, Couñago F. Are all prostate cancer patients "fit" for salvage radiotherapy? World J Clin Oncol 2020; 11(1): 1-10
- URL: https://www.wjgnet.com/2218-4333/full/v11/i1/1.htm
- DOI: https://dx.doi.org/10.5306/wjco.v11.i1.1
Salvage radiotherapy (RT) (SRT) is the standard treatment for patients with biochemically-recurrent prostate cancer (PCa) following radical prostatectomy[1,2]. Findings from several phase 3 clinical trials demonstrating the value of adjuvant RT in these patients[3-5], together with the growing interest among urologists in the surgical treatment of high-risk PCa, have led to an increase in the number of patients who receive RT postoperatively.
After the findings of those clinical trials confirmed the benefits and efficacy of SRT - especially for early recurrences [defined as prostate-specific antigen (PSA) < 0.5 ng/mL][6-8] - most subsequent studies have focused on the role of tumour-related variables (e.g., PSA levels, PSA kinetics, Gleason score, and surgical margins) in establishing the treatment indication. However, those studies have largely ignored the clinical characteristics that could potentially contraindicate this treatment.
A significant proportion of patients who develop biochemical recurrence (BCR) undergo SRT. However, the use of high-dose, hypofractionated RT in tissues previously subjected to surgery, together with the poor anatomical condition of these tissues (often associated with urinary incontinence), are important factors to consider when deciding whether SRT is indicated given the increased risk of radiation-induced toxicity and the potential to worsen quality of life (QoL).
In the present article, we propose a decision algorithm for SRT. This algorithm was developed after a careful analysis of the literature involving an assessment of a wide range of factors - apart from the well-known tumour characteristics associated with progression-free survival (PFS) - including comorbidities, life expectancy, expected toxicity, and dosimetric variables.
Compared to other malignant tumours, PCa has a long clinical course, which explains why survival outcomes are usually reported at a median follow-up of 10 years. In the United States, data from population registries show that 5-year survival rates in patients with PCa are greater than 90%[9]. In most clinical guidelines, life expectancy ≥ 10 years is an important criterion for treatment selection, especially in patients with low-grade tumours[1,2]. However, in patients with biochemically-recurrent PCa, life expectancy is not usually considered in the treatment selection process, as evidenced in phase 3 trials of postoperative adjuvant RT in which age (< 75 years) is an inclusion criterion but life expectancy is not[3-5]. However, the two randomized clinical trials (RCT) that compared SRT with or without androgen deprivation therapy (ADT)[10,11] did include life expectancy (< 10 years) as an exclusion criterion. Patients who develop BCR after prostatectomy are, on average, 3-5 years older than when the surgery was performed. For this reason, it is important to statistically determine life expectancy, especially in patients with late onset, non-aggressive BCR (based on PSA kinetics and Gleason score). Importantly, patients whose life expectancy is < 10 years at detection of BCR are unlikely to benefit from SRT, except for those with symptomatic, locally-recurrent disease with elevated PSA levels[12], in which case SRT plus ADT can be considered on an individual basis.
Many studies have found that the presence of significant comorbidity is associated with worse survival in PCa patients who undergo radiotherapy[13,14]. Most clinical guidelines recommend the use of validated scales to assess comorbidity in order to facilitate decision-making[1,2]. Specific scales are available to assess comorbidity in patients with PCa[15] and these scales can be used both to predict QoL in the six month period following diagnosis and to estimate the probability of survival in the next 3.5 years. Patients with greater comorbidity, as determined by the total illness burden index for PCa (TIBI-PCa), have a 13-fold greater risk of dying from causes other than PCa in the 3.5 years after diagnosis[15]. Crawford et al[13] showed that survival outcomes in patients with significant comorbidities who underwent RT were significantly worse than in patients who did not receive oncological treatment. At 10-years of follow-up, those patients had a higher risk of PCa-specific mortality (PCSM; 62 deaths in the treatment group vs 42 in the supportive care group, P = 0.08). Moreover, patients with significant comorbidities had a greater risk of mortality of non-PCSM than patients with no or minimal comorbidity (16.1% vs 8.2%)[13].
The RCTs published to date that have evaluated SRT plus ADT have only included patients with performance status ranging from 0-2[10,11]. The TROG 03.06 trial[16] excluded patients with a life expectancy < 5 years (due to the presence of comorbidities). Based on these data, we recommend the use of comorbidity scales at the time of BCR; in addition, patients with a TIBI-PCa > 11 or a Charlson index > 3 should not be offered active treatment because the presence of these risk factors implies a high probability (> 50%) of non-PCSM mortality in the following 3 years.
The use of validated scales such as the International Prostate Symptom Score (IPSS) or the Expanded Prostate Cancer Index to obtain an accurate assessment of urinary symptoms is crucial before deciding whether SRT is indicated. Most studies of postoperative RT have found a direct association between baseline urinary status and the risk of radiation-induced toxicity[17-19]. Patients with poor postoperative urinary function, a previous history of transurethral radical prostatectomy (TURP), or who require repeated bladder catheterization present an increased risk of developing stenosis of the bladder neck and urethra, which can cause a significant deterioration in urinary function. Although the studies that have reported toxicity outcomes associated with postoperative RT have reported similar findings with regard to the impact on urinary function[17-19], this variable was not included in the selection criteria of the prospective trials conducted to date. Neither of the two phase 3 trials that evaluated SRT with or without ADT[10,11], and none of the three phase 3 trials that assessed adjuvant RT[3-5], have reported data on urinary function, nor have they described whether RT negatively impacted urinary function. The SWOG trial only excluded patients who developed total urinary incontinence after surgery[5].
The recently published study by Pollack et al[20] on hypofractionation in patients undergoing primary RT found that late urinary toxicity was significantly higher in patients with high IPSS scores and a history of TURP. The poor urinary status prior to RT in patients who had previously undergone prostatectomy (versus surgery-naive patients) may explain why hypofractionation is not considered standard in this group of patients. In the study by Cozzarini et al[21], the 5-year rate of urinary toxicity rate ≥ grade 3 was 18.1% in the hypofractionated group (2.3-2.9 Gy) versus only 6.9% in the conventional fractionation group.
Given the lack of validated data from prospective studies on the role of urinary function, we cannot recommend a definition of “unfit” based on urinary parameters, nor can we recommend the routine use of hypofractionated schemes. Patients who present poor urinary function prior to RT should be informed of the increased risk of urinary complications (stenosis, hematuria, stranguria, etc.). In addition, it is essential to analyse the risks and benefits of performing RT in patients with poor urinary function. In these patients, dosimetric parameters and clinical variables must be considered together. If the rectal and bladder constraints cannot be met (Table 1), then RT is contraindicated and the recommended treatment approach should be either observation or, in high-risk patients, hormonotherapy.
| Organ at risk | Constraints |
| Bladder | V70 < 30% |
| V55 < 50% | |
| Rectum | V70 < 20% |
| V65 < 25% | |
| V60 < 35% | |
| V50 < 50% | |
| Femoral heads | V50 ≤ 10% |
| Dmax < 45 Gy | |
| Small bowel | V55 < 5 mL |
| V15 < 120 mL |
Although no specific drugs are contraindicated in patients scheduled to undergo SRT, the use of anticoagulant and antiplatelet medications increases the risk of rectal and/or urinary bleeding[17,19,22]. Takeda et al[23] found that anticoagulant use was significantly correlated (P = 0.027) with higher rates of chronic rectal toxicity ≥ grade 2. Even if the use of such medications does not contraindicate RT per se, patients should be informed about the increased risk of bleeding. By contrast, the available evidence indicates that hormonotherapy - sometimes administered concomitantly with SRT - does not increase urinary or radiation-induced rectal toxicity[17,24]. However, in patients with cardiovascular risk factors, the prolonged use of hormonotherapy with SRT should be limited to patients with a poor prognosis, defined as the presence of local and/or regional recurrence, a PSA doubling time (PSADT) < 6 mo, and/or Gleason score > 7.
Recently, our group proposed a risk classification system - similar to the risk stratification used in patients at the initial diagnosis of PCa - to classify patients with biochemically-recurrent PCa into three risk groups[25]. That framework was designed to facilitate decision-making for the use of ADT based on several key prognostic variables (Table 2) assessed at the time of BCR. Low-risk patients, in whom ADT is not indicated, fulfil all of the conditions for good prognosis: PSA ≤ 0.5 ng/mL; PSADT> 12 mo; interval from surgery to recurrence > 18 mo; Gleason score 6 or 7 (3 + 4); free margins; and stage pT2pN0. This subgroup of low-risk patients has the best survival outcomes (PFS) after SRT, which is expected given that they have the least aggressive disease. However, the benefits of RT in this subgroup must be carefully weighed against the risk of radiation-induced toxicity. Two other variables - age and (especially) comorbidities - play a key role in deciding whether to prescribe active treatment or not. We believe that low-risk patients, patients over age 75, and/or those with comorbidities that reduce their life expectancy to < 5-10 years (based on validated scales) should be considered “unfit” for SRT because the treatment is likely to worsen QoL without providing a clear survival benefit.
| Risk group | Factors |
| Low-risk | PSA < 0.6 ng |
| PSA-DT > 12 mo | |
| Gleason score ≤ 7 (ISUP 1,2) | |
| pT2 pN0 | |
| IBR > 18 mo | |
| Negative margins | |
| Intermediate risk | PSA = 0.6 to < 1 ng |
| PSA-DT 6-12 mo | |
| Gleason score 7 (ISUP 3) | |
| pT2-T3a pN0 or pNx | |
| IBR > 18 mo | |
| Positive margins | |
| High-risk | PSA ≥ 1 ng |
| PSA-DT < 6 mo | |
| Gleason score 8-10 (ISUP 4,5) | |
| pT3b pN0 or pNx | |
| IBR < 18 mo | |
| Positive margins |
As early as 2002, Choo et al[26] described the lack of efficacy of SRT - with 5-year biochemical control rates < 35% - in patients with PSA levels > 2 ng/mL or with local macroscopic recurrence. In the meta-analysis by King and colleagues[27], the PSA level prior to SRT was directly related with the probability of disease response and control: for each 0.1 ng/mL increase in the PSA level at the time of BCR, the biochemical relapse-free survival (BRFS) rate decreased by 2.6%. Numerous authors consider PSA ≤ 0.5 ng/mL as the optimal level at which to initiate “early" SRT[6-9]. In their study, Fossati et al[7] found that biochemical control in patients who underwent SRT with PSA levels ≤ 0.5 ng/mL was comparable to that obtained in patients who received adjuvant RT; however, patients with persistently elevated postoperative PSA levels were excluded from the comparison.
The available evidence indicates that the lower the PSA level at the time of BCR, the better the outcomes of SRT. To date, however, no PSA cut-off levels have been established to contraindicate SRT. Choline positron emission tomography/computed tomography (PET/CT) should be performed in patients with PSA values > 1 ng/mL or a PSADT < 6 mo[28]. According to current European Association of Urology Guidelines, prostate-specific membrane antigen (PSMA) PET/CT should be performed prior to SRT in patients with PSA > 0.2 ng/mL at the time of BCR[29]. It is important to keep in mind that administering SRT in patients with PSA levels > 1 ng/mL without first localizing the lesion via imaging tests increases the risk that the affected area (particularly lymph node regions) will not be adequately irradiated.
We recommend performing SRT in patients with PSA values < 0.5 ng/mL provided that the patient has a life expectancy > 10 years and no medical contraindications. Choline or PSMA PET/CT (based on availability) should be performed when PSA values exceed 0.2 ng/mL and/or in cases with PSADT < 6 mo. If there is a visible locoregional recurrence without evidence of distant metastasis, then the radiation target volume can be adjusted to the findings of the imaging tests; in these cases, concomitant ADT is indicated, even in patients with PSA values > 2 ng/mL. Local SRT is not indicated in cases with extrapelvic involvement; instead, systemic therapy should be prescribed after a multidisciplinary tumour board has reviewed and approved the treatment. Finally, in patients with normal imaging tests and PSA values ranging from 0.5-2 ng/mL, the recommendations of the phase 3 GETUG and RTOG trials should be followed[10,11].
Many authors consider the PSADT to be the most important prognostic factor at the time of BCR, even though this variable was not an inclusion criterion in any of the RCTs published to date, nor was it used for risk stratification[3-5,10,11]. However, most clinical guidelines recommend the application of systemic therapy in patients with a PSADT < 6-10 mo at BCR[1]. The PSADT plays no role in determining whether SRT is contraindicated or not, nor should it be used to determine radiation volumes. However, when the PSADT is < 6 mo, ADT should be prescribed, in addition to SRT.
The GETUG study evaluated the influence of the time interval between radical prostatectomy and BCR on treatment outcomes in patients undergoing SRT plus androgen suppression therapy (goserelin)[10]. Patients were grouped into early (< 30 mo) or late BCR. However, no significant differences in biochemical control were observed. By contrast, other authors have found that biochemical control rates are worse in patients with a disease-free interval (DFI) < 18 mo and in patients with persistently-elevated PSA levels after prostatectomy[30], which suggest the presence of high-risk tumours and/or involved surgical margins. Nevertheless, the DFI does not condition the use of SRT, although ADT should be started in patients with a DFI < 18 mo, especially in cases with a short PSADT (< 6 mo). In patients with late onset BCR (> 10 years), the indication for SRT should be evaluated in the context of the patient’s age and comorbidities.
In patients who develop BCR after primary external beam RT, eligibility for salvage should include the patient’s risk group classification at the initial diagnosis of PCa. Local salvage treatment is not advised in high-risk patients and/or those with Gleason 8-10[31]. The phase 3 trials that evaluated adjuvant RT did not include the Gleason score as an inclusion criterion[3-5]. However, the RCTs that have evaluated SRT with and without ADT found no significant between-group differences in survival [PFS or overall survival (OS)] based on the Gleason score, although the course of disease was worse in patients in the placebo group with Gleason scores ≥ 7[10,11].
In recent years, a growing proportion of high-risk patients undergo radical prostatectomy, mainly as part of the multimodal treatment approach supported by urologists. However, the risk of BCR in these patients is high, ranging from 50%-70% in most series[32]. Gandaglia et al[33] found that, together with nodal involvement and stage pT3-T4 disease, the presence of GS 8-10 was the third least favourable factor in patients treated with adjuvant RT. Indeed, patients who presented all three of these unfavourable factors had the worst prognosis, with 10-year OS rates of 62% when no adjuvant RT was performed.
There is no evidence to suggest that the Gleason score or the initial risk group are contraindications for SRT in patients who develop BCR after surgery. However, from a radiation oncology perspective, the presence of these factors creates uncertainties regarding: (1) The optimal target volume (especially in patients who did not undergo initial lymphadenectomy); (2) The indication and duration of concomitant ADT; and especially (3) Whether SRT should be performed in the absence of data from imaging tests ruling out distant disease.
Table 1 shows the recommended dose constraints for the organs at risk used in most studies of SRT. The difficulty of bladder filling in previously-operated patients increases the risk of both acute and chronic urinary toxicity. Numerous publications have recommended limiting the radiation dose and/or treatment volume to avoid an exponential increase in treatment-related complications and long-term sequelae[34-36]. Although the use of rectal spacers has been proven to reduce rectal toxicity in brachytherapy, their efficacy has not been validated in SRT. In patients with unfavourable dose-volume histograms (DVH), no other local measures are available to reduce the dose to the rectum and bladder. Consequently, image-guided RT is imperative in these cases to ensure accuracy and to optimize the dosimetric parameters. In addition, the treating radiation oncologist should discuss with the patient the risks of radiation-induced toxicity (based on the DVH values) and the expected benefits of the radiotherapy treatment. If the patient’s comorbidities are likely to increase the risk of developing toxicity > grade 3 in patients with unfavourable DVH values, then it is reasonable to rule out SRT, just as surgery is often ruled out in high-risk (ASA III-IV) patients.
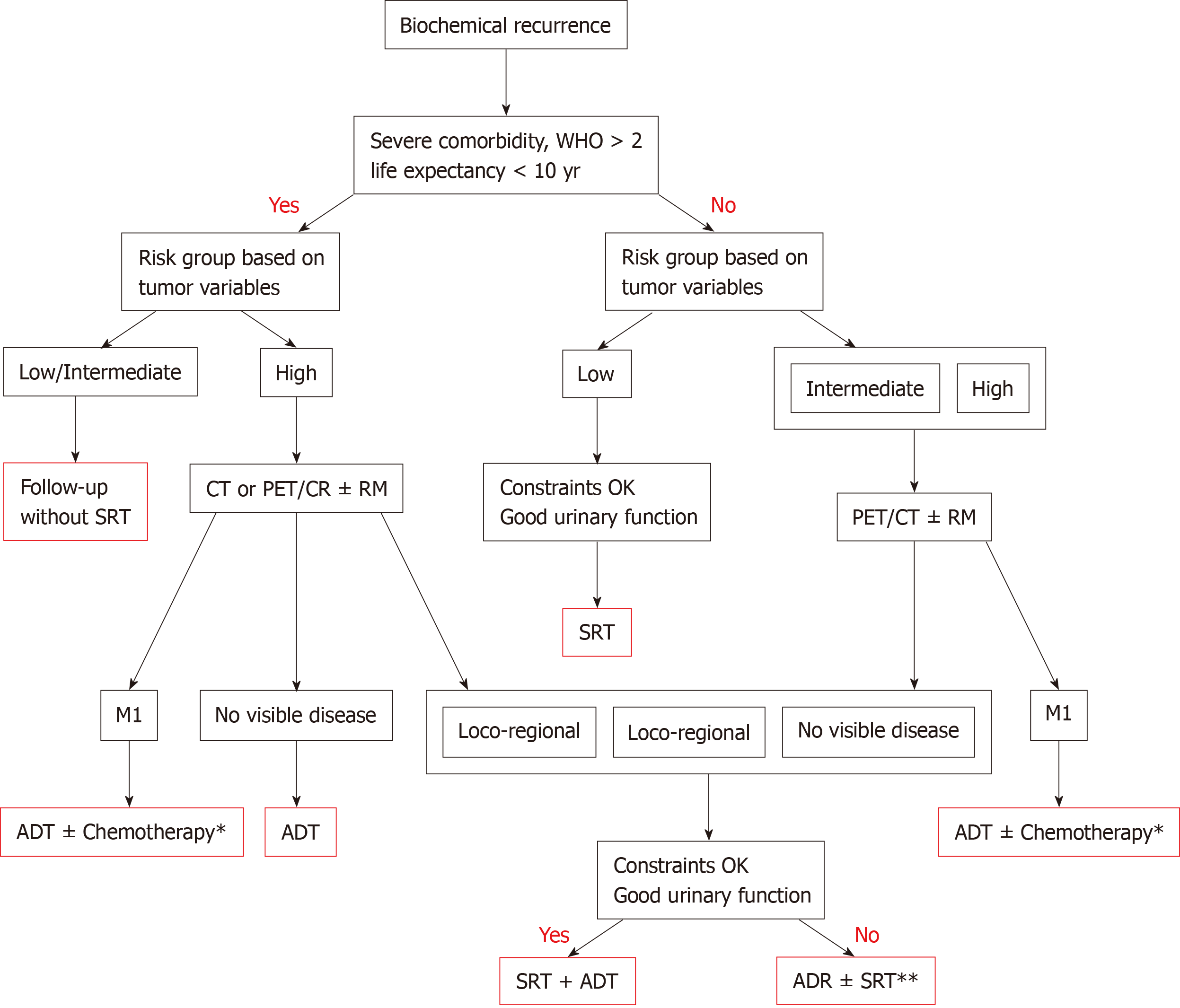
Given the lack of universally-accepted criteria regarding the contraindications of SRT, in Table 3 we provide a summary of the exclusion criteria used in the phase 3 RCTs that have evaluated SRT with and without ADT. That table also includes the exclusion criteria in currently ongoing studies comparing adjuvant RT to SRT. Based on those data, we have developed a decision algorithm to identify patients considered "unfit" for SRT (Figure 1). As with most therapeutic indications, it is important not only to define the patients who are likely to benefit from a given treatment, but also to identify those patients in whom treatment could reduce life expectancy and/or lead to complications without providing a clear clinical benefit. Patients considered “unfit” for SRT would therefore include those who meet several of the following criteria: (1) > 75 years of age; (2) Significant comorbidities; (3) Poor baseline urinary function; (4) Low risk of developing BCR; and (5) Unfavourable DVH values. These patients should be offered alternative approaches, which may include surveillance or hormonal therapy depending on the patient’s individual characteristics, life expectancy, and the "aggressiveness" of the recurrent disease. Finally, in patients with PSA > 1 ng/mL and/or PSADT < 6 mo, SRT should not be performed until the recurrence has been localized on imaging tests or at least until distant metastasis has been ruled out.
| Postoperative radiotherapy | Trial | Exclusion criteria |
| Adjuvant RT | EORTC 22911 | > 75 yr old |
| WHO PS > 1 | ||
| PSA > 0.3 ng/mL | ||
| ARO 96-02/AUOAP 09/95 | > 75 yr old | |
| WHO PS > 1 | ||
| Detectable PSA | ||
| SWOG 8794 | WHO PS > 2 | |
| Total urinary incontinence | ||
| Pelvic infection or urinary extravasation | ||
| Intraoperative rectal injury | ||
| Salvage RT ± ADT | RTOG 9601 | Life expectancy < 10 yr |
| I. Karnofsky < 80% | ||
| Evidence of hepatic disease | ||
| PSA > 4 ng/mL | ||
| GETUG-AFU-16 | WHO PS > 1 | |
| Life expectancy < 10 yr | ||
| Inadequate cardiac function | ||
| Another invasive cancer | ||
| PSA > 2 ng/mL | ||
| Salvage vs adjuvant RT ± ADT | RAVES 08.03 | WHO PS > 1 |
| Concurrent cytotoxic medication | ||
| Hip prosthesis | ||
| Co-morbidities that would interfere with treatment or 5-yr follow-up | ||
| PSA > 0.10 ng/mL | ||
| RADICALS | Other active malignancy | |
| PSA > 0.20 ng/mL | ||
| PAC GETUG | WHO PS > 1 | |
| Other active malignancy | ||
| Life expectancy < 10 yr | ||
| PSA > 0.10 ng/mL | ||
| Severe and uncontrolled arterial hypertension |
Manuscript source: Invited manuscript
Specialty type: Oncology
Country of origin: Spain
Peer-review report classification
Grade A (Excellent): 0
Grade B (Very good): B
Grade C (Good): 0
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Bugaj AM S-Editor: Yan JP L-Editor: A E-Editor: Qi LL
| 1. | Lieng H, Hayden AJ, Christie DRH, Davis BJ, Eade TN, Emmett L, Holt T, Hruby G, Pryor D, Shakespeare TP, Sidhom M, Skala M, Wiltshire K, Yaxley J, Kneebone A. Radiotherapy for recurrent prostate cancer: 2018 Recommendations of the Australian and New Zealand Radiation Oncology Genito-Urinary group. Radiother Oncol. 2018;129:377-386. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 26] [Cited by in RCA: 38] [Article Influence: 5.4] [Reference Citation Analysis (0)] |
| 2. | Mohler JL, Antonarakis ES, Armstrong AJ, D’Amico AV, Davis BJ, Dorff T, Eastham JA, Enke CA, Farrington TA, Higano CS, Horwitz EM, Hurwitz M, Ippolito JE, Kane CJ, Kuettel MR, Lang JM, McKenney J, Netto G, Penson DF, Plimack ER, Pow-Sang JM, Pugh TJ, Richey S, Roach III M, Rosenfeld S, Schaeffer E, Shabsigh A, Small EJ, Spratt DE, Srinivas S, Tward J, Shead DA, Freedman-Cass DA. Prostate Cancer, Version 2.2019, NCCN Clinical Practice Guidelines in Oncology. JNCCN. 2019;17. [RCA] [DOI] [Full Text] [Cited by in Crossref: 628] [Cited by in RCA: 914] [Article Influence: 182.8] [Reference Citation Analysis (0)] |
| 3. | Bolla M, van Poppel H, Tombal B, Vekemans K, Da Pozzo L, de Reijke TM, Verbaeys A, Bosset JF, van Velthoven R, Colombel M, van de Beek C, Verhagen P, van den Bergh A, Sternberg C, Gasser T, van Tienhoven G, Scalliet P, Haustermans K, Collette L; European Organisation for Research and Treatment of Cancer, Radiation Oncology and Genito-Urinary Groups. Postoperative radiotherapy after radical prostatectomy for high-risk prostate cancer: long-term results of a randomised controlled trial (EORTC trial 22911). Lancet. 2012;380:2018-2027. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 654] [Cited by in RCA: 672] [Article Influence: 51.7] [Reference Citation Analysis (0)] |
| 4. | Wiegel T, Bartkowiak D, Bottke D, Bronner C, Steiner U, Siegmann A, Golz R, Störkel S, Willich N, Semjonow A, Stöckle M, Rübe C, Rebmann U, Kälble T, Feldmann HJ, Wirth M, Hofmann R, Engenhart-Cabillic R, Hinke A, Hinkelbein W, Miller K. Adjuvant radiotherapy versus wait-and-see after radical prostatectomy: 10-year follow-up of the ARO 96-02/AUO AP 09/95 trial. Eur Urol. 2014;66:243-250. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 298] [Cited by in RCA: 331] [Article Influence: 30.1] [Reference Citation Analysis (0)] |
| 5. | Thompson IM, Tangen CM, Paradelo J, Lucia MS, Miller G, Troyer D, Messing E, Forman J, Chin J, Swanson G, Canby-Hagino E, Crawford ED. Adjuvant radiotherapy for pathological T3N0M0 prostate cancer significantly reduces risk of metastases and improves survival: long-term followup of a randomized clinical trial. J Urol. 2009;181:956-962. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 910] [Cited by in RCA: 933] [Article Influence: 58.3] [Reference Citation Analysis (0)] |
| 6. | Stish BJ, Pisansky TM, Harmsen WS, Davis BJ, Tzou KS, Choo R, Buskirk SJ. Improved Metastasis-Free and Survival Outcomes With Early Salvage Radiotherapy in Men With Detectable Prostate-Specific Antigen After Prostatectomy for Prostate Cancer. J Clin Oncol. 2016;34:3864-3871. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 133] [Cited by in RCA: 171] [Article Influence: 21.4] [Reference Citation Analysis (0)] |
| 7. | Fossati N, Karnes RJ, Boorjian SA, Moschini M, Morlacco A, Bossi A, Seisen T, Cozzarini C, Fiorino C, Noris Chiorda B, Gandaglia G, Dell'Oglio P, Joniau S, Tosco L, Shariat S, Goldner G, Hinkelbein W, Bartkowiak D, Haustermans K, Tombal B, Montorsi F, Van Poppel H, Wiegel T, Briganti A. Long-term Impact of Adjuvant Versus Early Salvage Radiation Therapy in pT3N0 Prostate Cancer Patients Treated with Radical Prostatectomy: Results from a Multi-institutional Series. Eur Urol. 2017;71:886-893. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 67] [Cited by in RCA: 65] [Article Influence: 7.2] [Reference Citation Analysis (0)] |
| 8. | Valicenti RK, Thompson I, Albertsen P, Davis BJ, Goldenberg SL, Wolf JS, Sartor O, Klein E, Hahn C, Michalski J, Roach M, Faraday MM; American Society for Radiation Oncology/American Urological Association. Adjuvant and salvage radiation therapy after prostatectomy: American Society for Radiation Oncology/American Urological Association guidelines. Int J Radiat Oncol Biol Phys. 2013;86:822-828. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 139] [Cited by in RCA: 139] [Article Influence: 11.6] [Reference Citation Analysis (0)] |
| 9. | Siegel RL, Miller KD, Jemal A. Cancer statistics, 2019. CA Cancer J Clin. 2019;69:7-34. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 13300] [Cited by in RCA: 15456] [Article Influence: 2576.0] [Reference Citation Analysis (2)] |
| 10. | Shipley WU, Seiferheld W, Lukka HR, Major PP, Heney NM, Grignon DJ, Sartor O, Patel MP, Bahary JP, Zietman AL, Pisansky TM, Zeitzer KL, Lawton CA, Feng FY, Lovett RD, Balogh AG, Souhami L, Rosenthal SA, Kerlin KJ, Dignam JJ, Pugh SL, Sandler HM, NRG Oncology RTOG. Radiation with or without Antiandrogen Therapy in Recurrent Prostate Cancer. N Engl J Med. 2017;376:417-428. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 421] [Cited by in RCA: 488] [Article Influence: 61.0] [Reference Citation Analysis (0)] |
| 11. | Carrie C, Hasbini A, de Laroche G, Richaud P, Guerif S, Latorzeff I, Supiot S, Bosset M, Lagrange JL, Beckendorf V, Lesaunier F, Dubray B, Wagner JP, N'Guyen TD, Suchaud JP, Créhange G, Barbier N, Habibian M, Ferlay C, Fourneret P, Ruffion A, Dussart S. Salvage radiotherapy with or without short-term hormone therapy for rising prostate-specific antigen concentration after radical prostatectomy (GETUG-AFU 16): a randomised, multicentre, open-label phase 3 trial. Lancet Oncol. 2016;17:747-756. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 264] [Cited by in RCA: 287] [Article Influence: 31.9] [Reference Citation Analysis (0)] |
| 12. | Shelan M, Odermatt S, Bojaxhiu B, Nguyen DP, Thalmann GN, Aebersold DM, Dal Pra A. Disease Control With Delayed Salvage Radiotherapy for Macroscopic Local Recurrence Following Radical Prostatectomy. Front Oncol. 2019;9:12. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 14] [Cited by in RCA: 15] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 13. | Crawford ED, Grubb R, Black A, Andriole GL, Chen MH, Izmirlian G, Berg CD, D'Amico AV. Comorbidity and mortality results from a randomized prostate cancer screening trial. J Clin Oncol. 2011;29:355-361. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 107] [Cited by in RCA: 107] [Article Influence: 7.6] [Reference Citation Analysis (0)] |
| 14. | Boyle HJ, Alibhai S, Decoster L, Efstathiou E, Fizazi K, Mottet N, Oudard S, Payne H, Prentice M, Puts M, Aapro M, Droz JP. Updated recommendations of the International Society of Geriatric Oncology on prostate cancer management in older patients. Eur J Cancer. 2019;116:116-36. [RCA] [DOI] [Full Text] [Cited by in Crossref: 75] [Cited by in RCA: 150] [Article Influence: 25.0] [Reference Citation Analysis (0)] |
| 15. | Litwin MS, Greenfield S, Elkin EP, Lubeck DP, Broering JM, Kaplan SH. Assessment of prognosis with the total illness burden index for prostate cancer: aiding clinicians in treatment choice. Cancer. 2007;109:1777-1783. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 72] [Cited by in RCA: 68] [Article Influence: 3.8] [Reference Citation Analysis (0)] |
| 16. | Duchesne GM, Woo HH, Bassett JK, Bowe SJ, D'Este C, Frydenberg M, King M, Ledwich L, Loblaw A, Malone S, Millar J, Milne R, Smith RG, Spry N, Stockler M, Syme RA, Tai KH, Turner S. Timing of androgen-deprivation therapy in patients with prostate cancer with a rising PSA (TROG 03.06 and VCOG PR 01-03 [TOAD]): a randomised, multicentre, non-blinded, phase 3 trial. Lancet Oncol. 2016;17:727-737. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 146] [Cited by in RCA: 179] [Article Influence: 19.9] [Reference Citation Analysis (0)] |
| 17. | Shelan M, Abo-Madyan Y, Welzel G, Bolenz C, Kosakowski J, Behnam N, Wenz F, Lohr F. Dose-escalated salvage radiotherapy after radical prostatectomy in high risk prostate cancer patients without hormone therapy: outcome, prognostic factors and late toxicity. Radiat Oncol. 2013;8:276. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 25] [Cited by in RCA: 29] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 18. | Ghadjar P, Jackson A, Spratt DE, Oh JH, Munck af Rosenschöld P, Kollmeier M, Yorke E, Hunt M, Deasy JO, Zelefsky MJ. Patterns and predictors of amelioration of genitourinary toxicity after high-dose intensity-modulated radiation therapy for localized prostate cancer: implications for defining postradiotherapy urinary toxicity. Eur Urol. 2013;64:931-938. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 35] [Cited by in RCA: 36] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 19. | Martínez-Arribas CM, González-San Segundo C, Cuesta-Álvaro P, Calvo-Manuel FA. Predictors of urinary and rectal toxicity after external conformed radiation therapy in prostate cancer: Correlation between clinical, tumour and dosimetric parameters and radical and postoperative radiation therapy. Actas Urol Esp. 2017;41:615-623. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4] [Cited by in RCA: 6] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 20. | Pollack A, Walker G, Horwitz EM, Price R, Feigenberg S, Konski AA, Stoyanova R, Movsas B, Greenberg RE, Uzzo RG, Ma C, Buyyounouski MK. Randomized trial of hypofractionated external-beam radiotherapy for prostate cancer. J Clin Oncol. 2013;31:3860-3868. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 339] [Cited by in RCA: 371] [Article Influence: 30.9] [Reference Citation Analysis (0)] |
| 21. | Cozzarini C, Fiorino C, Deantoni C, Briganti A, Fodor A, La Macchia M, Noris Chiorda B, Rancoita PM, Suardi N, Zerbetto F, Calandrino R, Montorsi F, Di Muzio N. Higher-than-expected severe (Grade 3-4) late urinary toxicity after postprostatectomy hypofractionated radiotherapy: a single-institution analysis of 1176 patients. Eur Urol. 2014;66:1024-1030. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 71] [Cited by in RCA: 89] [Article Influence: 8.1] [Reference Citation Analysis (0)] |
| 22. | Fuentes-Raspall R, Inoriza JM, Rosello-Serrano A, Auñón-Sanz C, Garcia-Martin P, Oliu-Isern G. Late rectal and bladder toxicity following radiation therapy for prostate cancer: Predictive factors and treatment results. Rep Pract Oncol Radiother. 2013;18:298-303. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 20] [Cited by in RCA: 15] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 23. | Takeda K, Ogawa Y, Ariga H, Koto M, Sakayauchi T, Fujimoto K, Narazaki K, Mitsuya M, Takai Y, Yamada S. Clinical correlations between treatment with anticoagulants/antiaggregants and late rectal toxicity after radiotherapy for prostate cancer. Anticancer Res. 2009;29:1831-1834. [PubMed] |
| 24. | Zapatero A, García-Vicente F, Sevillano D, Martín de Vidales C, Ferrer C, Torres JJ, Minguez R, Rabadán M. Is hormone therapy a protective factor for late hematuria after high-dose radiotherapy in prostate cancer? Urology. 2008;72:1130-1134. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 9] [Cited by in RCA: 14] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 25. | Gonzalez-San Segundo C, Couñago F, Gomez-Iturriaga A. Androgen Deprivation Therapy and Salvage Radiotherapy: Are We Missing Something? Eur Urol. 2019;76:260-261. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5] [Cited by in RCA: 4] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 26. | Choo R, Hruby G, Hong J, Bahk E, Hong E, Danjoux C, Morton G, DeBoer G. (IN)-efficacy of salvage radiotherapy for rising PSA or clinically isolated local recurrence after radical prostatectomy. Int J Radiat Oncol Biol Phys. 2002;53:269-276. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 46] [Cited by in RCA: 47] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 27. | King CR. The dose-response of salvage radiotherapy following radical prostatectomy: A systematic review and meta-analysis. Radiother Oncol. 2016;121:199-203. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 74] [Cited by in RCA: 71] [Article Influence: 7.9] [Reference Citation Analysis (0)] |
| 28. | Picchio M, Castellucci P. Clinical Indications of C-Choline PET/CT in Prostate Cancer Patients with Biochemical Relapse. Theranostics. 2012;2:313-317. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 21] [Cited by in RCA: 24] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 29. | Mottet N. European Association of Urology. Guidelines prostate cancer. Available from: https://uroweb.org/guideline/prostate-cancer/. |
| 30. | Ploussard G, Staerman F, Pierrevelcin J, Saad R, Beauval JB, Roupret M, Audenet F, Peyromaure M, Delongchamps NB, Vincendeau S, Fardoun T, Rigaud J, Villers A, Bastide C, Soulie M, Salomon L; Committee of Cancerology of the Association of French Urology. Predictive factors of oncologic outcomes in patients who do not achieve undetectable prostate specific antigen after radical prostatectomy. J Urol. 2013;190:1750-1756. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 39] [Cited by in RCA: 43] [Article Influence: 3.6] [Reference Citation Analysis (0)] |
| 31. | López Torrecilla J, Hervás A, Zapatero A, Gómez Caamaño A, Macías V, Herruzo I, Maldonado X, Gómez Iturriaga A, Casas F, González San Segundo C. Uroncor consensus statement: Management of biochemical recurrence after radical radiotherapy for prostate cancer: From biochemical failure to castration resistance. Rep Pract Oncol Radiother. 2015;20:259-272. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 14] [Cited by in RCA: 15] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 32. | Briganti A, Karnes RJ, Gandaglia G, Spahn M, Gontero P, Tosco L, Kneitz B, Chun FK, Zaffuto E, Sun M, Graefen M, Marchioro G, Frohneberg D, Giona S, Karakiewicz PI, Van Poppel H, Montorsi F, Joniau S; European Multicenter Prostate Cancer Clinical and Translational Research Group (EMPaCT). Natural history of surgically treated high-risk prostate cancer. Urol Oncol. 2015;33:163.e7-163.13. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 84] [Cited by in RCA: 94] [Article Influence: 9.4] [Reference Citation Analysis (0)] |
| 33. | Gandaglia G, Karakiewicz PI, Briganti A, Trudeau V, Trinh QD, Kim SP, Montorsi F, Nguyen PL, Abdollah F, Sun M. Early radiotherapy after radical prostatectomy improves cancer-specific survival only in patients with highly aggressive prostate cancer: validation of recently released criteria. Int J Urol. 2015;22:89-95. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 20] [Cited by in RCA: 21] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 34. | Marks LB, Yorke ED, Jackson A, Ten Haken RK, Constine LS, Eisbruch A, Bentzen SM, Nam J, Deasy JO. Use of normal tissue complication probability models in the clinic. Int J Radiat Oncol Biol Phys. 2010;76:S10-S19. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1352] [Cited by in RCA: 1171] [Article Influence: 78.1] [Reference Citation Analysis (1)] |
| 35. | Sassowsky M, Gut P, Hölscher T, Hildebrandt G, Müller AC, Najafi Y, Kohler G, Kranzbühler H, Guckenberger M, Zwahlen DR, Azinwi NC, Plasswilm L, Takacs I, Reuter C, Sumila M, Manser P, Ost P, Böhmer D, Pilop C, Aebersold DM, Ghadjar P. Use of EORTC target definition guidelines for dose-intensified salvage radiation therapy for recurrent prostate cancer: results of the quality assurance program of the randomized trial SAKK 09/10. Int J Radiat Oncol Biol Phys. 2013;87:534-541. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 17] [Cited by in RCA: 22] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 36. | Croke J, Maclean J, Nyiri B, Li Y, Malone K, Avruch L, Kayser C, Malone S. Proposal of a post-prostatectomy clinical target volume based on pre-operative MRI: volumetric and dosimetric comparison to the RTOG guidelines. Radiat Oncol. 2014;9:303. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 13] [Cited by in RCA: 14] [Article Influence: 1.3] [Reference Citation Analysis (0)] |