Published online May 24, 2019. doi: 10.5306/wjco.v10.i5.201
Peer-review started: January 4, 2019
First decision: January 26, 2019
Revised: March 12, 2019
Accepted: March 26, 2019
Article in press: March 27, 2019
Published online: May 24, 2019
Processing time: 141 Days and 8.5 Hours
Adjuvant chemotherapy using intraperitoneal (IP) treatment has demonstrated survival benefit over intravenous (IV) therapy alone in patients treated with upfront debulking surgery for advanced stage ovarian cancer. Neoadjuvant chemotherapy followed by interim surgery and adjuvant chemotherapy has similar outcome in survival as compared to upfront surgery followed by adjuvant IV chemotherapy. IP chemotherapy has not been widely adopted in clinical practice for a number of reasons. Whether IP chemotherapy delivered in the patients who received neoadjuvant chemotherapy can be well tolerated or confers any clinical benefit has not been well studied.
To evaluate the experience of adjuvant IP chemotherapy in the community cancer clinic setting, and the clinical benefit and tolerability of incorporating IP chemotherapy in patients who received neoadjuvant treatment.
We retrospectively evaluated toxicities and outcomes of patients with stage III and IV ovarian cancer diagnosed at our institution between 07/2007 and 07/2015 who received intraperitoneal chemotherapy after cytoreductive surgery (group 1) or after neoadjuvant chemotherapy followed by interim surgery (group 2).
Thirty eight patients were treated with IP chemotherapy, median age was 54 years old (range 38.6 to 71 years). In group 1 (n = 25), 12 (48%) of the patients completed 4 or more cycle of IP treatment after upfront debulking surgery; while in group 2 (n = 13), 8 (61.5%) of the patients completed all 3 cycles of the assigned IP chemotherapy after receiving neoadjuvant IV chemotherapy followed by surgery, and 2 (15.4%) more patients tolerated more than 3 cycles. In those patients who did not get planned IP chemotherapy, most of them were treated with substitutional IV chemotherapy, and the completion rate for 6 cycles of IV + IP was 92%. Abdominal pain, (64% in group 1 and 38% in group 2), vomiting, (36% in group 1 and 30.8% in group 2), dehydration (16% in group 1 and 15.4% in group 2), and hypomagnesemia (12% in group 1 and 15.4% in group 2) were the most common adverse effects in all patients, while patients who have received neoadjuvant chemotherapy were more likely to get hypokalemia, fatigue and renal insufficiency. Progression free survival (PFS) was 26.5 mo (95% CI 14.9, 38.0) in group 1 and 27.6 mo (95% CI 13.1, 42.1) in group 2. The overall survival was 100.2 mo (95% CI 67.9, 132.5) for group 1 and 68.2 mo (95% CI 32.2, 104.0) for group 2. For the entire cohort, PFS was 26.5 mo (95% CI 15.9, 37.0) and OS was 78.8 mo (95% CI 52.3, 105.4).
The use of IP/IV chemotherapy can be safely administrated in the community cancer clinic setting. The use of IP/IV chemotherapy in patients who have received neoadjuvant chemotherapy followed by surgery is feasible and tolerable. Despite various modification of the IP regimen, incorporation of IP chemotherapy in the adjuvant setting appears to be associated with improved PFS and overall survival.
Core tip: Intraperitoneal chemotherapy has shown survival benefits in the adjuvant setting among the patients with advanced stage ovarian carcinoma undergoing debunking surgery. However, this intraperitoneal route could not be widely adopted due to a number of issues including patients choice and its cumbersome nature. The present study explores its feasibility in a community cancer setting. We have retrospectively analyzed the rates of toxicities and outcome of the patients who received this therapy in our cancer center. We conclude that intraperitoneal chemotherapy can be safely administered in the community cancer setting and improves the overall and progression free survival.
- Citation: Meghal T, Dave V, Tang H, Kumar V, Xu Y. Clinical benefit and tolerability of adjuvant intraperitoneal chemotherapy in patients who have or have not received neoadjuvant chemotherapy for advanced ovarian cancer. World J Clin Oncol 2019; 10(5): 201-212
- URL: https://www.wjgnet.com/2218-4333/full/v10/i5/201.htm
- DOI: https://dx.doi.org/10.5306/wjco.v10.i5.201
Epithelial ovarian cancer is the most common cause of death among women with gynecologic malignancies and the 5th leading cause of cancer death in the United States[1]. Approximately 75% of women have stage III or IV disease at diagnosis[2]. Several randomized studies have demonstrated survival benefit when intraperitoneal (IP) chemotherapy is utilized in the adjuvant treatment after maximal debulking surgery vs only intravenous (IV) chemotherapy[3-5]. Cochrane review of 8 IP studies showed a hazard ratio (HR) of 0.81 to be less likely to die from ovarian cancer after receiving IP vs IV alone[6]. Another long term follow up study using combined data from Gynecologic Oncology Group (GOG) 114 and GOG 172 demonstrated median survival difference of about 10 mo in favor of IP therapy[7]. However, IP chemotherapy has not been widely used in the academic or community cancer centers alike, due to concerns of toxicity, such as abdominal pain, severe nausea and vomiting, catheter associated infection, as well as unfamiliarity of the treatment or unavailability in the facilities[8]. In a retrospective examination of six medical centers in the National Comprehensive Cancer Network, the use of IP was found in up to 50% of the eligible patients which peaked in year 2007-2008, but the usage rate plateaued afterwards[8]. More recently, alternative IV regimens incorporating dose dense delivery of paclitaxel or angiogenesis inhibitor bevacizumab have been reported and have been applied in the clinical practice[9-11].
European Organization for Research on Treatment of Cancer (EORTC) conducted a randomized study comparing neoadjuvant IV chemotherapy followed by interim debulking surgery followed by adjuvant chemotherapy vs upfront debulking surgery followed by adjuvant IV chemotherapy, and showed that the neoadjuvant approach is not inferior to the adjuvant IV treatment[12]. The question then emerges whether patients who have received neoadjuvant IV chemotherapy followed by optimal debulking surgery can still tolerate and benefit from adjuvant IP chemotherapy. An OV21/PETROC study tried to address this question. The first report of the phase II portion did show a lower progression rate at 9 mo as compared to IV chemotherapy suggesting benefit of IP chemotherapy after neoadjuvant treatment[13].
Our community cancer center has started offering IP chemotherapy to eligible ovarian cancer patients since 2005. Since 2010, after the publication of the EORTC study using the neoadjuvant chemotherapy approach, we continued to offer adjuvant IP chemotherapy in patients who received neoadjuvant chemotherapy. In this study, we aimed to examine the experience of conducting IP chemotherapy in a community cancer center setting. We will compare the toxicity profile of IP when used after upfront surgery versus after neoadjuvant chemotherapy and interim debulking surgery, and evaluate the outcomes of patients who received IP treatment either after upfront surgery or after neoadjuvant treatment.
This study was reviewed and approved by the Institutional Review Board. The electronic medical records and hospital tumor registry was queried for all patients who were diagnosed with ovarian, fallopian tube, or primary peritoneal cancer based on the International Classification of Diseases (ICD) 9 and ICD 10 codes. Patients who were diagnosed of stage II, III or IV cancers between July 2005 and July 2015 and received at least 1 treatment of IP chemotherapy were eligible and included in the analysis. Medical records were reviewed for collection of data on demographics, pathology, chemotherapy agents, regimens, dose modifications and side effects. The progression free survival (PFS) and overall survival (OS) were calculated using the day of surgery as the start day, and March 30, 2017 as the last day of censor.
PFS was considered to have ended at the time of cancer progression as shown on radiography, or death from any cause. If progression was first detected on the basis of increased CA125 level, and a computed tomography (CT) scan was performed within 4 wk, then the date of progression would be the date of the scan. If no CT scan was done within 4 wk, then the date of CA125 increase, with levels defined by the Gynecologic Cancer Intergroup criteria[14], would be the date of progression. If a patient was lost for follow up, then the last day of follow up will be the end date for calculation of PFS or OS. In a small number of patients who were lost for follow up and had Medicare insurance, the Medicare data base was checked to estimate the date of death.
Patients treated with IP chemotherapy following surgery for recurrence disease were included. In PFS and OS calculation, the start day was the day of the second surgery.
We hypothesized that IP chemotherapy would be associated with improved survival compared with IV chemotherapy, and our pre-study statistical sample size calculation indicated that at 31 patients will be required to have 80% power to compare to the historical data, assuming a median OS of 30 mo in the primary surgery group[12], and 60 mo for the IP group[7], with SD of 60 and the effect size of 0.5. Kaplan Meier estimation curves were used for estimation of survival and log-rank test was applied. Stata (StataCorp. 2017. Stata Statistical Software: Release 15. College Station, TX: StataCorp LLC) was used for all the calculations.
Between July 2005 and July 2015, 63 patients were diagnosed of stage III ovarian cancer and 38 (60.3%) of those patients were treated with IP chemotherapy. Of the 38 patients included in the analysis, the median age was 55.5 years and range was 38.6 to 73.8 years. Twenty five patients were treated with upfront debulking surgery followed by adjuvant IP and IV chemotherapy (group 1) and 13 patients were treated with neoadjuvant chemotherapy followed by interim debulking surgery followed by adjuvant IP and IV chemotherapy (group 2). The demographics and clinical characteristics of those patients are included in Table 1. Three patients had stage II disease, and the majority had stage III disease. Two patients had stage IV disease at diagnosis, including one with cytology positive pleural effusion which was drained and did not recur after neoadjuvant chemotherapy, and another patient with a malignant umbilical nodule which was resected during surgery. Three patients were treated with IP therapy after surgery for the first recurrence and they were all in group 1 and received adjuvant treatment. Before starting adjuvant treatment, the baseline CA 125 value was abnormal in 15 (39.5%) patients, more in group 1 (12, 48%) than in group 2 (3, 23%).
| Demographics | Total (n = 38) | Adjuvant group (Group 1, n = 25) | Neoadjuvant group (Group 2, n = 13) |
| Median age (yr) | 55.5 | 54.3 | 50.1 |
| Range | 38.6-73.8 | 42.1-68.8 | 38.6-73.8 |
| Age groups | |||
| 30-50 | 16 | 10 | 6 |
| 51-60 | 10 | 8 | 2 |
| 61-75 | 12 | 7 | 5 |
| Primary site | |||
| Ovarian | 37 (97) | 24 (96) | 13 (100) |
| Fallopian tube | 1 (3) | 1 (4) | 0 (0) |
| Tumor stage | |||
| IIB | 1 (2.6) | 0 (0) | 1 (7.7) |
| IIC | 2 (5.3) | 2 (8) | 0 (0) |
| IIIA | 3 (7.9) | 2 (8) | 1 (7.7) |
| IIIB | 5 (13.2) | 4 (16) | 1 (7.7) |
| IIIC | 22 (57.9) | 14 (56) | 8 (61.5) |
| IV | 2 (5.3) | 0 (0) | 2 (15.4) |
| Recurrent (n = 3) | 3 (7.9) | ||
| IIB/C | 2 (8) | 2 (8) | 0 (0) |
| IIIB | 1 (2.6) | 1 (2.6) | 0 (0) |
| Histology | |||
| High grade serous | 37 (97.3) | 25 (100) | 12 (93.3) |
| Poorly differentiated | 1 (2.7) | 0 (0) | 1 (6.7) |
| Surgery optimal debulking (< 1 cm) | 23 (60.5) | 17 (68) | 6 (46.1) |
| No optimal debulking | 3 (7.9) | 1 (4) | 2 (15.4) |
| Data unavailable | 12 (31.6) | 7 (28) | 5 (38.5) |
| Baseline CA 125 value after surgery1 | |||
| Normal | 9 (23.7) | 2 (8) | 7 (53.8) |
| Abnormal | 15 (39.5) | 12 (48) | 3 (23) |
| Not available | 14 (36.8) | 11 (44) | 3 (23) |
| BRCA 1/2 mutations (all germline) | |||
| Positive | 7 (18.5) | 4 (16) | 3 (23.1) |
| Negative | 16 (42.1) | 12 (48) | 4 (30.8) |
| Unknown | 15 (39.4) | 8 (32) | 7 (53.8) |
A modified treatment protocol with Paclitaxel 135 mg/m2 over 3 h on day 1, cisplatin 75 mg/m2 IP on day 2 and paclitaxel 80 mg/m2 IP on day 8 was the standard protocol in this hospital[15]. All patients were treated in the out-patient setting. The first patient who received adjuvant IP and IV treatment in group 1 was in January 2007, and the first patient who received adjuvant IP after neoadjuvant IV treatment was in February 2011.
In group 1, 12 (48%) of the patients completed 4 or more cycle of IP treatment, while the other 52% patients only had 1-3 cycles of IP chemo. In group 2, 8 (61.5%) of the patients completed all 3 cycles of the prescribed IP chemotherapy after surgery, and 2 (15.4%) more patients tolerated more than 3 cycles (Table 2). Twenty three percent of the patients received 1 or 2 IP treatments.
| Treatment characteristics | Total (n = 38) | Adjuvant group (Group 1, n = 25) | Neoadjuvant group (Group 2, n = 13) |
| Median | 3 | 3 | 3 |
| Range | (1-6) | (1-6) | (1-4) |
| Completion of IP cycles | |||
| 1 | 5 (13.2) | 4 (16) | 1 (7.7) |
| 2 | 3 (7.9) | 1 (4) | 2 (15.3) |
| 3 | 16 (42.1) | 8 (32) | 8 (61.5) |
| 4 | 9 (23.7) | 8 (32) | 1 (7.7) |
| 5 | 1 (2.6) | 1 (4) | 0 (0) |
| 6 | 4 (10.5) | 3 (12) | 1 (7.7) |
| IP cisplatin | |||
| Starting dose 75 mg/m2 | 35 (92.1) | 24 (96) | 11 (84.6) |
| Dose reduction to 60 mg/m2 | 11 (28.9) | 6 (24) | 5 (38.5) |
| IP paclitaxel | |||
| Dose reduction | 2 (5.3) | 2 (8) | 0/13 (0) |
| Dose omission | 20 (52.6) | 11 (44) | 9 (69.2) |
| Changed to IV abraxane | 3 (7.9) | 1 (4) | 2 (15.4) |
| Treatment delay | |||
| Delay in starting a new cycle | 8 (21.1) | 5 (20) | 3 (23.1) |
| Delay in day 8 treatment | 8 (21.1) | 5 (20) | 3 (23.1) |
| Prophylactic hydration planned | 10 (26.3) | 7 (18.4) | 3 (21.1) |
A majority of patients were started the treatment with cisplatin IP at 75 mg/m2 dose, with 96% and 84.6% in group 1 and group 2 respectively. Dose reduction of cisplatin to 60 mg/m2 was seen in 24% in group 1 and 38.5% in group 2. In addition, the dose omission of day 8 IP paclitaxel was common, which occurred in 44% in group 1 and 69.2% in group 2. The delay in starting day 8 treatment due to toxicities was about 20% in both groups. The delay in starting a new cycle of treatment occurred in about 20% of the patients in both groups (Table 2). Three patients did not get IP paclitaxel treatment because they developed allergic reactions to IV paclitaxel, and their treatment was switched to IV albumin-bound paclitaxel on day 1 and 8, without day 8 of IP treatment.
The schedule and dosage of IV chemotherapy regimens showed more variations (Table 3). In group 1, those patients who did not complete 6 cycles of IP treatment were more likely to be treated with every 3 wk paclitaxel and carboplatin (14 patients, 56%), and this regimen was used for 7 (54%) patients in group 2 in the neoadjuvant setting. A minority of others used dose dense weekly paclitaxel and carboplatin treatment, or carboplatin backbone in combination with docetaxel, albumin-bound paclitaxel or gemcitabine. A total of 22 (88%) patients in group 1 completed 6 cycles of chemotherapy, including those who received less than 6 cycles of IP containing chemotherapy. Twelve out of 13 (92%) patients in group 2 completed 6 cycles of neoadjuvant IV and adjuvant IV and/or IP treatment.
| Treatment regimens | Total, n = 38 | Adjuvant group, n = 25 | Neoadjuvant group, n = 13 |
| Taxol 175 mg/m2, Carboplatin AUC 5 or 6 every 3 wk | 21 (55.3) | 14 (56) | 7 (54) |
| Carboplatin AUC 5 or 6, Taxol 80 mg/m2 day 1, 8, 15 every 3 wk (dose dense) | 3 (7.9) | 2 (8) | 1 (8)1 |
| Taxol 80 mg/m2 day 1, 8, Carboplatin AUC 2 day 1, 8 every 3 wk (weekly regimen) | 1 (2.6) | 0 (0) | 1 (8) |
| Carboplatin AUC 5 and Docetaxel 75 mg/m2 every 3 wk | 2 (5.2) | 1 (4) | 1 (8) |
| Carboplatin AUC 5 Gemcitabine 800 mg/m2 day 1, 8 every 3 wk (due to peripheral neuropathy) | 1 (2.6) | 1 (4) | 0 (0) |
| Carboplatin AUC 5 or 6, Abraxane D1, 8 every 3 wk (due to allergic reaction to Taxel) | 3 (7.9) | 1 (4) | 2 (15) |
| Carboplatin AUC with Taxol x1, with Docetaxel x1 and with Gemcitabine x1 (due to allergic reaction) | 1 (2.6) | 1 (4) | 0 (0) |
| No IV treatment, IP treatment only | 6 (15.8) | 5 (20) | 1 (8) |
| Completion of ≥ 6 cycles of IV + IP treatment | 34 (89.5) | 22 (88) | 12 (92) |
| Completion of 7-10 cycles of IV + IP | 5 (13.1) | 2 (8) | 3 (23) |
| Subsequent treatment with Bevacizumab at progression | 19 (50) | 13 (52) | 6 (46) |
The occurrence of grade 3 or 4 adverse events is summarized in Table 4. Abdominal pain (64% in group 1 and 38.5% in group 2), vomiting (36% in group 1 and 30.8% in group 2), dehydration (16% in group 1 and 15.4% in group 2), and hypomagnesemia (12% in group 1 and 15.4% in group 2) were the most common adverse effects in all patients, while patients who have received neoadjuvant chemotherapy are more likely to get hypokalemia, fatigue and renal insufficiency. Catheter malfunction was only found in 1 patient and there was no treatment related death. Mild hematological toxicities were seen mainly with neutropenia and anemia, and there was no difference in the 2 groups. Prophylactic hydration was scheduled in 28% of the patient in group 1 and 23% of the patients in group 2. Prophylactic hydration was the routine practice with one physician, and was scheduled for every patient on day 4 or 5 and day 11 or 12. Two of the 3 patients who were found to have renal insufficiency were found on the day of planned hydration, and improved after hydration.
| Toxicities | Adjuvant group, n = 25 (100) | Neoadjuvant group, n = 13 (100) |
| Abdominal pain/pelvic pain | 16 (64) | 5 (38.5) |
| Nausea/vomiting | 9 (36) | 4 (30.8) |
| Fatigue | 2 (8) | 2 (15.4) |
| Renal insufficiency | 0 (0) | 3 (23.1) |
| Dehydration/hypotension | 4 (16) | 2 (15.4) |
| Catheter malfunction/infection | 1 (4) | 0 (0) |
| Anemia | 3 (12) | 1 (7.7) |
| Thrombocytopenia | 1 (4) | 0 (0) |
| Neutropenia | 5 (20) | 1 (7.7) |
| Hypokalemia | 1 (4) | 4 (30.8) |
| Hypomagnesemia | 3 (12) | 2 (15.4) |
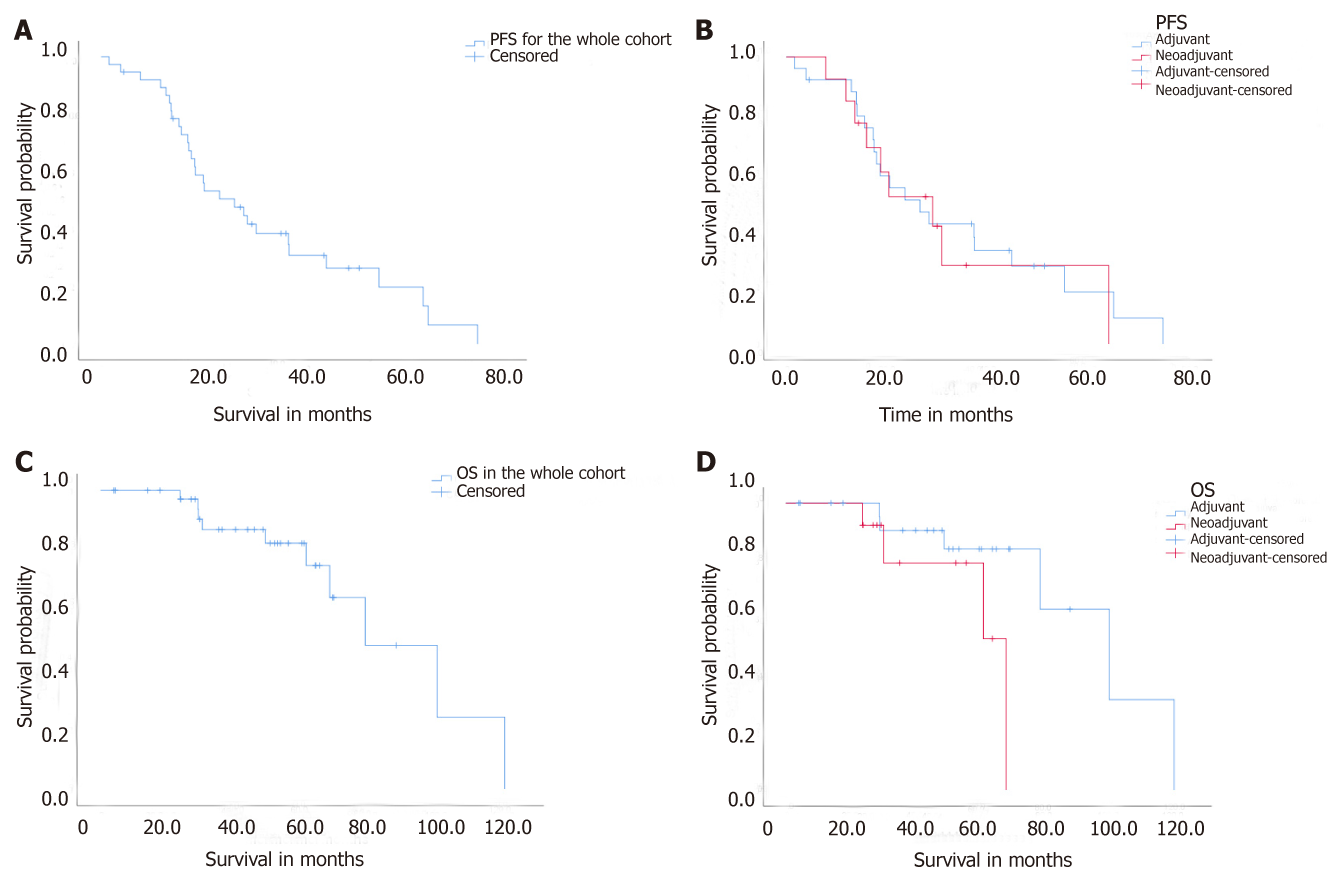
The median follow up of all patients were 48.7 mo (range 4 to 120.3). Ten patients lost to follow up for overall survival (8 in group 1 and 2 in group 2). Four patients lost to follow up for PFS (3 in group 1 and 1 in group 2). For the entire cohort, PFS was 26.5 mo (95% CI 14.9, 38.0). PFS was 26.5 mo (95% CI 14.9, 38.0) in group 1 (adjuvant) and 27.6 mo (95% CI 13.1, 42.1) in group 2 (neoadjuvant) (P > 0.05) (Figure 1A and B). The OS was 78.8 mo (95% CI 52.3, 105.4) for the entire cohort, and 100.2 mo (95% CI 67.9, 132.5) for group 1 and 68.2 mo (95% CI 32.2, 104) for group 2 (Figure 1C and D). Three patients were treated with adjuvant IP at the time of first recurrence and the start day for calculation of PFS and OS was the day of second surgery, instead of the initial diagnosis. Nineteen patients received subsequent Bevacizumab treatment when they had further recurrence, 13 (52%) in group 1 and 6 (46%) in group 2 (Table 3).
Seven patients had detectable germline BRCA 1 mutations (Table 5). Five patients were diagnosed at an age older or equal than 50 years old. Three patients demonstrated PFS longer than 50 mo, and 2 of them have not recurred yet. One patient received PARP inhibitor treatment at recurrence.
| Serial Number | Age at diagnosis (yr) | BRCA mutation | Significance | Group assignment | PFS per study (mo) | PFS1 | Recurrence |
| 3 | 50 | BRCA 1, Q1395X (4302C>T) | Deleterious | 1 | 51.2+ | 73.2+ | No |
| 4 | 52 | BRCA 1, 187del AG | Deleterious | 1 | 15.5 | Yes | |
| 7 | 58 | BRCA 1, Q310X | Deleterious | 1 | 14+ | 36+ | No |
| 22 | 56 | BRCA 1, G1706E | Suspected deleterious | 1 | 23.5 | Yes, deceased | |
| 23 | 54 | BRCA 1, unknown | Deleterious | 1 | 12.9 | Yes | |
| 28 | 41 | BRCA 1, C64G (309T>G) | Deleterious | 2 | 35.7+ | 53 | Splenic recurrence, at 53 mo |
| 35 | 40 | BRCA 1, 187delAG | Deleterious | 2 | 29.9+ | 51.9+ | No |
Five patients (3 in group 1 and 2 in group 2) had no recurrence at the time of censor, the median follow up of these 5 patients were 36.2 mo (range 29.5 to 50.5 mo).
One patient developed a new peritoneal mass which was biopsy proven to be endometroid carcinoma 74 mo after initial surgery while the initial pathology was papillary serous carcinoma. This second diagnosis was treated as a recurrent event in PFS calculation, based on a presumed possibility of an occult mixed histology in the primary occurrence, although a though examination by the pathologist did not show endometroid component.
The 9 mo progression free rate was 88.6% in the entire cohort.
A landmark study (GOG 172) reported median PFS of 23.8 mo and OS of 65.6 mo in patients with advanced ovarian cancer who received IP chemotherapy in the adjuvant setting[4]. However, only 42% patients completed all 6 cycles of IP + IV treatment, and 52% received 4 or more cycles of IP containing therapy in that study. In this retrospective study, we reviewed the outcomes and toxicities of patients who received outpatient IP chemotherapy in a community hospital setting. We found that 48% of the patients tolerated 4 or more cycles of IP chemotherapy after upfront debulking surgery, while 65.5% of the patients could tolerate all 3 cycles of the assigned IP chemotherapy after receiving neoadjuvant IV treatment followed by surgery, and an additional 15.4% patients tolerated 4-6 cycles. Despite a marked variation in the dose and schedule of IV and IP chemotherapy, the entire cohort had a median PFS of 26.5 (95% CI, 15.9, 37.0) mo and OS of 78.8 mo (95% CI 52.3, 105.4). These outcome measures are numerically comparable to those reported in randomized clinical trials[3-5] as well as in the combination analysis[7].
One of the major aims of this study is to study the toxicity profile of IP treatment in patients who have already received 3 cycles of neoadjuvant IV chemotherapy. In this study, we observed abdominal pain (38%-64%), nausea and vomiting (30.8%-36%) and electrolyte abnormalities (4%-30%) to be the most common adverse effects in all patients, while patients who have received neoadjuvant chemotherapy are more likely to get hypokalemia, renal insufficiency and fatigue while receiving IP chemotherapy after surgery. Overall, the magnitude of side effects in this study appeared to be similar to that reported in the GOG 172 study, where the gastrointestinal side effects were 46% and renal side effects were 7%[4]. Importantly, there is no increase in the rate of anemia, neutropenia or thrombocytopenia in the group who have already received neoadjuvant chemotherapy.
Catheter problem only occurred in 1 patient in our study, while it was reported to be about 20% and led to treatment discontinuation in the phase III trial[4], which became one of the major concerns of adopting this treatment in the community. We did not encounter infection or catheter occlusion; and other than proper training our nursing staff received, there was no particular extra care to the IP catheters.
Prophyalctic hydration was a routine practice with one physician and 2 cases of renal insufficiency were found on day 4 or 5 which were planned hydration days. In those patients who did not have planned hydrations, this transient change of renal function could be missed thus underdiagnosed.
Comparing to the most relevant bench marker study, which is the randomized phase II/III OV21/PETROC study presented in American Society of Clinical Oncology 2016[13], the rate of adverse effects in our cohort is much higher. In the above 3 arm study, patients received neoadjuvant chemotherapy followed by surgery and were then randomized to receive IV Paclitaxel day 1, day 8 and carboplatin IV day 1 (arm 1), or the same IV-IP protocol we followed in our cohort, which is cisplatin IP day 1, paclitaxel IV day 1 and paclitaxel IP day 8 (arm 2). The patients in arm 3 received carboplatin AUC 5 or 6 IP substituting cisplatin IP on day 1 with the rest same as in arm 2. The IP cisplatin containing arm (arm 2) was considered to be inferior and was discontinued. In their report, side effects equal or more than grade 3 occurred in only less than 10% of the patients, which is much less than in our patients. One of the reasons for this difference could be due to the elimination of IP cisplatin in early stage of the OV21/PETROC trial. In terms of outcome measure, the progression rate at 9 mo was 42% in arm 1, and 24.5% in arm 3 showing favorable result in the IP arm. In our study, the 9 mo progression free rate of the entire cohort was 88.6%. Due to the small sample size in our study, this large difference may not be statistically significant. However, it did show an excellent treatment response produced in our patients.
Overall, our analysis showed that administrating IP chemotherapy after neoadjuvant chemotherapy and surgery is doable. Although it appeared to be associated with more GI and renal side effects, about half of the patients can endure all three cycles.
Incorporating IP treatment in the adjuvant treatment of stage III and IV ovarian cancer patients in our institution, whether or not they have received neoadjuvant chemotherapy, was inspired by the large difference in PFS (23.8 mo vs 18.3 mo) and OS (65.6 mo vs 49.7 mo) demonstrated in the GOG 172 study[4] and supported by others[3,5]. This approach has been challenged, and it is now a subject of debate regarding the definitive benefit with IP therapy in the era of applying inhibition of vascular endothelial growth factor (VEGF) pathway. Adding VEGF targeting agent Bevacizumab to the chemotherapy backbone and extending its use for a prolonged period has been evaluated in GOG 218[9] and ICON-7 study[10], and both studies showed improvement in PFS and OS in high risk patients. In June 2018, Genentech[16] reported an updated analysis of GOG 218 showing improvement in PFS from 12 mo to 18.2 mo and a hazard ratio of 0.62 by adding Bevacizumab to chemotherapy. Bevacizumab has received approval by Food and Drug Administration for upfront adjuvant treatment in stage III or IV ovarian cancer after initial debulking surgery[16].
Delivery of chemotherapy in a dose dense (weekly) fashion may offer therapeutic advantage, as shown in the Japanese study (median PFS of 28 mo), longer than the conventional every 3 wk chemotherapy (median PFS 17.2 mo)[17]. Data from the GOG 252 study showed a less impressive difference with dose dense treatment chemotherapy (14.2 mo vs 10.3 mo) only among those patients who did not receive bevacizumab as part of the adjuvant treatment[11]. A more direct comparison was carried out by the NRG/GOG 256 and was presented in 2016 SGO meeting[18]. This study randomized patients to IV dose dense chemotherapy, IP carboplatin with IV weekly paclitaxel, and IP cisplatin, IP paclitaxel and IV paclitaxel, and bevacizumab was added in all 3 arms[18]. There was no difference in PFS among the three arms, albeit the PFS was much better in all the arms than that in the previous studies. As all patients received treatment with IV bevacizumab, it is possible that the additional therapeutic effect of bevacizumab has overshadowed the benefit gained from IP therapy. In addition, the dose of IP cisplatin was 100 mg/m2 in the original GOG 172 study, while it was 75 mg/m2 in the NRG study, suggesting the importance of the treatment effect with high dose cisplatin. Adding to the controversy of the benefit of IP chemotherapy is the new report from the phase III study applying hyperthermic IP chemotherapy with cisplatin 100 mg/m2 or not during interim surgery in patients already received neoadjuvant IV chemotherapy[19]. The addition of hyperthermic IP versus surgery alone leads to improvement in both PFS and OS with HR of 0.6. The median recurrence free survival was 10.7 mo in the surgery group and 14.2 mo in the surgery plus hyperthermia group. The median OS was 33.9 mo in the surgery group and 45.7 mo in the surgery plus hyperthermia group. The result supports the intraperitoneal approach of treatment. Whether the therapeutic effect is a result of hyperthermia or the high effective dose of cisplatin IP at 100 mg/m2 is still unclear, and further confirmatory trials are needed[20].
Our observation of median PFS of 26.5 mo and OS of 78.8 mo in the entire cohort of 38 patients who received IP chemotherapy is significant. Despite the variations in dose, schedule, and chemotherapy agent choice, these measures are numerically longer than reported studies in the literature, such as the EORTC neoadjuvant study[12], the IV therapy only arms in GOG 172[4], and the arm with Bevacizumab in the GOG 218 study[9]. Our observation should add useful information to the medical literature regarding the clinical experience and benefit of incorporating IP chemotherapy in ovarian cancer treatment in the community setting.
The limitation of the study is its retrospective nature and its small sample size. There was sometimes limitation and deficiencies in the documentation of adverse events particularly in patients in group 1. When a patient was not scheduled to come back to the clinic for an interim lab test, a nadir in the counts of white blood cell, hemoglobin or platelet counts may be missed. The pattern of management among physicians varied among treatment physicians, and routine schedules of hydrations on day 4 and day 10 were applied by one physician which possibly lead to better capture of adverse events. Our data set is also extremely small in the evaluation of PFS or OS.
In conclusion, our findings suggest that the administration of IP chemotherapy is feasible in both settings of after upfront surgery and after neoadjuvant IV therapy followed by interim surgery. It can be safely administrated in the community cancer clinic setting. The use of IP/IV chemotherapy in patients who have received neoadjuvant chemotherapy is tolerable. Despite various schedule modifications, dose reductions and shortening of treatment courses, incorporation of IP chemotherapy in the adjuvant treatment of ovarian cancer appears to improve disease free survival and OS.
Adjuvant chemotherapy using intraperitoneal (IP) treatment has demonstrated survival benefit over intravenous (IV) therapy alone in patients treated with upfront debulking surgery for advanced stage ovarian cancer based on the Gynecologic Oncology Group (GOG) 172 trial. Neoadjuvant chemotherapy followed by interim surgery and adjuvant chemotherapy has similar outcome in survival as compared to upfront surgery followed by adjuvant IV chemotherapy based on the European Organization for Research on Treatment of Cancer study. IP chemotherapy has not been widely adopted in clinical practice for a number of reasons, mainly due to the concern of side effects. With the wide spread use of neoadjuvant chemotherapy, it is unclear whether IP chemotherapy in the adjuvant setting in those patients is safe and beneficial. There is an ongoing phase III study (OV21/PETROC) addressing this questions, and its preliminary result showed increase in progression free survival (PFS) in the IP arm compared to IV arm (42% vs 24.5%) using 9 mo progression rate as the outcome measure.
There are multiple problems to be addressed regarding IP chemotherapy. (1) What are the side effects of IP treatments, especially off clinical trials in a community cancer center? (2) Would patients experience more side effects after they have received neoadjuvant IV chemotherapy and then receive IP chemotherapy in the adjuvant setting? And (3) Is there benefit or improved outcome in those patients who receive IP chemotherapy? As our cancer center recommended IP chemotherapy to all fit patients as a general practice, we decided to analyze our data to answer those questions. We hope to share our community experience and to show the safety and efficacy data, to decrease the concerns regarding the side effects of IP, and to support the use of IP in the right clinical setting.
We wished to evaluate the experience of adjuvant IP chemotherapy in the community cancer clinic setting, and the clinical benefit and tolerability of incorporating IP chemotherapy in patients who have received neoadjuvant treatment.
We retrospectively evaluated toxicities and outcomes of patients with stage III and IV ovarian cancer diagnosed at our institution between 07/2007 and 07/2015 who received intraperitoneal chemotherapy after cytoreductive surgery (group 1) or after neoadjuvant chemotherapy followed by interim surgery (group 2). We reviewed the electronic records, and documented the regimens used, dose reduction, dose delay, drug variations. We also documented toxicities, patient characteristics.
We performed a sample size calculation to determine the least number of patients to be included in the study to have an 80% power to compare with the historical data (60 mo for the IP group reported in the GOG 172 study), and came up with 31 patients. We actually had 38 patients, which should have the above power to have a comparison.
We specified that PFS will be calculated starting from the date of diagnosis to the date of progression on computed tomography scan or death or last known follow up. Three patients were treated at the first recurrence with IP after surgery, and we defined the diagnosis date to be the date of the second debulking surgery, which was used as the start date for PFS and overall survival (OS) calculations. For some patients who lost for follow up and had Medicare insurance, we checked Medicare data base to extract date of death.
Thirty eight patients were treated with IP chemotherapy, median age was 54 years old (range 38.6 to 71 years). In group 1 (n = 25), 12 (48%) of the patients completed 4 or more cycle of IP treatment after upfront debulking surgery; while in group 2 (n = 13), 8 (61.5%) of the patients completed all 3 cycles of the assigned IP chemotherapy after receiving neoadjuvant IV chemotherapy followed by surgery, and 2 (15.4%) more patients tolerated more than 3 cycles. In those patients who did not get planned IP chemotherapy, most of them were treated with substitutional IV chemotherapy, and the completion rate for 6 cycles of IV + IP was 92%.
Abdominal pain, (64% in group 1 and 38% in group 2), vomiting (36% in group 1 and 30.8% in group 2), dehydration (16% in group 1 and 15.4% in group 2), and hypomagnesemia (12% in group 1 and 15.4% in group 2) were the most common adverse effects in all patients, while patients who have received neoadjuvant chemotherapy were more likely to get hypokalemia, fatigue and renal insufficiency.
PFS was 26.5 mo (95% CI 14.9, 38.0) in group 1 and 27.6 mo (95% CI 13.1, 42.1) in group 2. OS was 100.2 mo (95% CI 67.9, 132.5) for group 1 and 68.2 mo (95% CI 32.2, 104.0) for group 2. For the entire cohort, PFS was 26.5 mo (95% CI 15.9, 37.0) and OS was 78.8 mo (95% CI 52.3, 105.4). The 9-mo PFS rate was 88.6% in the entire cohort.
Our result reflected the real world experience of IP administration, in that most of the patients did not get 6 cycles of IP for adjuvant treatment as in GOG 172 study. About half of the patients can get 3 cycles of IP treatment, which was also true in those patients who have received neoadjuvant treatment. There appears to be benefits in PFS and OS even with the above limitations.
The use of IP/IV chemotherapy can be safely administrated in the community cancer clinic setting. The use of IP/IV chemotherapy in patients who have received neoadjuvant chemotherapy followed by surgery is feasible and tolerable. Despite various modification of the IP regimen, incorporation of IP chemotherapy in the adjuvant setting appears to be associated with improved progression free survival and overall survival.
Our data provides community practice experience and supports the data reported in GOG 172 and Cochran review from clinical trials about the benefits and toxicities of IP therapy. The benefit of IP treatment remains sizable even with reduced cycles of IP and dose variations.
Our study provides new information on the benefits and toxicities of administration of adjuvant IP in patients who have received neoadjuvant IV chemotherapy. A phase III OV21/PETROC study has been designed to address this question, and our 9-mo PFS rate was higher than reported in the study.
In our community practices, administration of IP chemotherapy in the adjuvant treatment for ovarian cancer, and in patients who have received IV chemotherapy in the neoadjuvant setting, is feasible, safe and associated with apparent benefit in PFS and OS. This approach should be further studied in randomized phase III clinical trials.
Manuscript source: Unsolicited manuscript
Specialty type: Oncology
Country of origin: United States
Peer-review report classification
Grade A (Excellent): 0
Grade B (Very good): 0
Grade C (Good): C
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Voutsadakis IA S-Editor: Wang JL L-Editor: A E-Editor: Xing YX
| 1. | Siegel RL, Miller KD, Jemal A. Cancer statistics, 2015. CA Cancer J Clin. 2015;65:5-29. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 9172] [Cited by in RCA: 9957] [Article Influence: 995.7] [Reference Citation Analysis (0)] |
| 2. | Surveillance, Epidemiology, and End Results Program. Cancer Stat Facts: Ovarian Cancer, 2016. Available from: URL: http://seer.cancer.gov/statfacts/html/ovary.html. |
| 3. | Alberts DS, Liu PY, Hannigan EV, O'Toole R, Williams SD, Young JA, Franklin EW, Clarke-Pearson DL, Malviya VK, DuBeshter B. Intraperitoneal cisplatin plus intravenous cyclophosphamide versus intravenous cisplatin plus intravenous cyclophosphamide for stage III ovarian cancer. N Engl J Med. 1996;335:1950-1955. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 977] [Cited by in RCA: 870] [Article Influence: 30.0] [Reference Citation Analysis (0)] |
| 4. | Armstrong DK, Bundy B, Wenzel L, Huang HQ, Baergen R, Lele S, Copeland LJ, Walker JL, Burger RA; Gynecologic Oncology Group. Intraperitoneal cisplatin and paclitaxel in ovarian cancer. N Engl J Med. 2006;354:34-43. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2030] [Cited by in RCA: 1949] [Article Influence: 102.6] [Reference Citation Analysis (0)] |
| 5. | Markman M. Hyperthermic intraperitoneal chemotherapy in the management of ovarian cancer: A critical need for an evidence-based evaluation. Gynecol Oncol. 2009;113:4-5. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 16] [Cited by in RCA: 17] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 6. | Jaaback K, Johnson N. Intraperitoneal chemotherapy for the initial management of primary epithelial ovarian cancer. Cochrane Database Syst Rev. 2006;CD005340. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 62] [Cited by in RCA: 60] [Article Influence: 3.2] [Reference Citation Analysis (0)] |
| 7. | Tewari D, Java JJ, Salani R, Armstrong DK, Markman M, Herzog T, Monk BJ, Chan JK. Long-term survival advantage and prognostic factors associated with intraperitoneal chemotherapy treatment in advanced ovarian cancer: a gynecologic oncology group study. J Clin Oncol. 2015;33:1460-1466. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 198] [Cited by in RCA: 214] [Article Influence: 21.4] [Reference Citation Analysis (0)] |
| 8. | Wright AA, Cronin A, Milne DE, Bookman MA, Burger RA, Cohn DE, Cristea MC, Griggs JJ, Keating NL, Levenback CF, Mantia-Smaldone G, Matulonis UA, Meyer LA, Niland JC, Weeks JC, O'Malley DM. Use and Effectiveness of Intraperitoneal Chemotherapy for Treatment of Ovarian Cancer. J Clin Oncol. 2015;33:2841-2847. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 117] [Cited by in RCA: 143] [Article Influence: 14.3] [Reference Citation Analysis (0)] |
| 9. | Burger RA, Brady MF, Bookman MA, Fleming GF, Monk BJ, Huang H, Mannel RS, Homesley HD, Fowler J, Greer BE, Boente M, Birrer MJ, Liang SX; Gynecologic Oncology Group. Incorporation of bevacizumab in the primary treatment of ovarian cancer. N Engl J Med. 2011;365:2473-2483. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1658] [Cited by in RCA: 1786] [Article Influence: 127.6] [Reference Citation Analysis (0)] |
| 10. | Perren TJ, Swart AM, Pfisterer J, Ledermann JA, Pujade-Lauraine E, Kristensen G, Carey MS, Beale P, Cervantes A, Kurzeder C, du Bois A, Sehouli J, Kimmig R, Stähle A, Collinson F, Essapen S, Gourley C, Lortholary A, Selle F, Mirza MR, Leminen A, Plante M, Stark D, Qian W, Parmar MK, Oza AM; ICON7 Investigators. A phase 3 trial of bevacizumab in ovarian cancer. N Engl J Med. 2011;365:2484-2496. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1501] [Cited by in RCA: 1619] [Article Influence: 115.6] [Reference Citation Analysis (0)] |
| 11. | Pimenta L, Dornelas M, Cezana L. Weekly vs. Every-3-Week Paclitaxel for Ovarian Cancer. N Engl J Med. 2016;374:2602-2603. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 6] [Cited by in RCA: 9] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 12. | Vergote I, Tropé CG, Amant F, Kristensen GB, Ehlen T, Johnson N, Verheijen RH, van der Burg ME, Lacave AJ, Panici PB, Kenter GG, Casado A, Mendiola C, Coens C, Verleye L, Stuart GC, Pecorelli S, Reed NS; European Organization for Research and Treatment of Cancer-Gynaecological Cancer Group; NCIC Clinical Trials Group. Neoadjuvant chemotherapy or primary surgery in stage IIIC or IV ovarian cancer. N Engl J Med. 2010;363:943-953. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1666] [Cited by in RCA: 1770] [Article Influence: 118.0] [Reference Citation Analysis (0)] |
| 13. | Mackay H, Gallagher CJ, Parulekar WR, Ledermann JA, Armstrong DK, Gourley C, Romero I, Feeney A, Bessette P, Hall M, Weberpals JI, Hall G, Lau SK, Gauthier P, Fung-Kee-Fung M, Eisenhauer EA, Winch C, Tu D, Provencher DM. OV21/PETROC: A randomized Gynecologic Cancer Intergroup (GCIG) phase II study of intraperitoneal (IP) versus intravenous (IV) chemotherapy following neoadjuvant chemotherapy and optimal debulking surgery in epithelial ovarian cancer (EOC). J Clin Oncol. 2016;34:LBA5503. |
| 14. | Vergote I, Rustin GJ, Eisenhauer EA, Kristensen GB, Pujade-Lauraine E, Parmar MK, Friedlander M, Jakobsen A, Vermorken JB. Re: new guidelines to evaluate the response to treatment in solid tumors [ovarian cancer]. Gynecologic Cancer Intergroup. J Natl Cancer Inst. 2000;92:1534-1535. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 192] [Cited by in RCA: 200] [Article Influence: 8.0] [Reference Citation Analysis (0)] |
| 15. | Konner JA, Grabon DM, Gerst SR, Iasonos A, Thaler H, Pezzulli SD, Sabbatini PJ, Bell-McGuinn KM, Tew WP, Hensley ML, Spriggs DR, Aghajanian CA. Phase II study of intraperitoneal paclitaxel plus cisplatin and intravenous paclitaxel plus bevacizumab as adjuvant treatment of optimal stage II/III epithelial ovarian cancer. J Clin Oncol. 2011;29:4662-4668. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 37] [Cited by in RCA: 39] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 16. | Genentech Inc. Avastin prescribing information, 2018. Available from: URL: https://www.accessdata.fda.gov/drugsatfda_docs/label/2009/125085s0169lbl.pdf. |
| 17. | Katsumata N, Yasuda M, Takahashi F, Isonishi S, Jobo T, Aoki D, Tsuda H, Sugiyama T, Kodama S, Kimura E, Ochiai K, Noda K; Japanese Gynecologic Oncology Group. Dose-dense paclitaxel once a week in combination with carboplatin every 3 weeks for advanced ovarian cancer: a phase 3, open-label, randomised controlled trial. Lancet. 2009;374:1331-1338. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 571] [Cited by in RCA: 540] [Article Influence: 33.8] [Reference Citation Analysis (0)] |
| 18. | Walker J, Brady MF, DiSilvestro PA, Fujiwara K, Alberts D, Zheng W, Tewari K, Cohn DE, Powell M, Van Le L, Rubin S, Davidson SA, Gray HJ, Waggoner S, Myers T, Aghajanian C, Secord AA, Mannel RS. A phase III trial of bevacizumab with IV versus IP chemotherapy for ovarian, fallopian tube, and peritoneal carcinoma: An NRG Oncology Study. Gynecologic Oncology. 2016;141:208. [RCA] [DOI] [Full Text] [Cited by in Crossref: 13] [Cited by in RCA: 12] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 19. | van Driel WJ, Koole SN, Sikorska K, Schagen van Leeuwen JH, Schreuder HWR, Hermans RHM, de Hingh IHJT, van der Velden J, Arts HJ, Massuger LFAG, Aalbers AGJ, Verwaal VJ, Kieffer JM, Van de Vijver KK, van Tinteren H, Aaronson NK, Sonke GS. Hyperthermic Intraperitoneal Chemotherapy in Ovarian Cancer. N Engl J Med. 2018;378:230-240. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 844] [Cited by in RCA: 987] [Article Influence: 141.0] [Reference Citation Analysis (0)] |
| 20. | Spriggs DR, Zivanovic O. Ovarian Cancer Treatment - Are We Getting Warmer? N Engl J Med. 2018;378:293-294. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 25] [Cited by in RCA: 29] [Article Influence: 4.1] [Reference Citation Analysis (0)] |