Published online Mar 24, 2019. doi: 10.5306/wjco.v10.i3.110
Peer-review started: January 14, 2019
First decision: January 26, 2019
Revised: February 23, 2019
Accepted: March 12, 2019
Article in press: March 12, 2019
Published online: March 24, 2019
Processing time: 68 Days and 13.5 Hours
Malignant vascular tumors of the liver include rare primary hepatic mesenchymal tumors developed in the background of a normal liver parenchyma. Most of them are detected incidentally by the increased use of performing imaging techniques. Their diagnosis is challenging, involving clinical and imaging criteria, with final confirmation by histology and immunohistochemistry. Surgery represents the mainstay of treatment. Liver transplantation (LT) has improved substantially the prognosis of hepatic epithelioid hemangioendothelioma (HEHE), with 5-year patient survival rates of up to 81%, based on the European Liver Intestine Transplantation Association-European Liver Transplant Registry study. Unfortunately, the results of surgery and LT are dismal in cases of hepatic angiosarcoma (HAS). Due to the disappointing results of very short survival periods of approximately 6-7 mo after LT, because of tumor recurrence and rapid progression of the disease, HAS is considered an absolute contraindication to LT. Recurrences after surgical resection are high in cases of HEHE and invariably present in cases of HAS. The discovery of reliable prognostic markers and the elaboration of prognostic scores following LT are needed to provide the best therapeutic choice for each patient. Studies on a few patients have demonstrated the stabilization of the disease in a proportion of patients with hepatic vascular tumors using novel targeted antiangiogenic agents, cytokines or immunotherapy. These new approaches, alone or in combination with other therapeutic modalities, such as surgery and classical chemotherapy, need further investigation to assess their role in prolonging patient survival. Personalized therapeutic algorithms according to the histopathological features, behavior, molecular biology and genetics of the tumors should be elaborated in the near future for the management of patients diagnosed with primary malignant vascular tumors of the liver.
Core tip: Primary malignant vascular tumors of the liver are rare mesenchymal tumors, most commonly detected incidentally using modern imaging techniques. They have variable clinical and imaging features, histology, immunohistochemistry, and molecular findings; therefore, their diagnosis may be difficult. Surgery represents the mainstay of treatment. Hepatic angiosarcoma has a dismal outcome and represents a contraindication for liver transplantation. Development of novel antiangiogenic and other molecular targeted treatments are needed to improve the patient outcome. This paper provides an overview of this group of tumors based on the most recent literature data, encompassing modern information regarding diagnostic challenges, prognostic factors and perspective therapeutic approaches.
- Citation: Lazăr DC, Avram MF, Romoșan I, Văcariu V, Goldiș A, Cornianu M. Malignant hepatic vascular tumors in adults: Characteristics, diagnostic difficulties and current management. World J Clin Oncol 2019; 10(3): 110-135
- URL: https://www.wjgnet.com/2218-4333/full/v10/i3/110.htm
- DOI: https://dx.doi.org/10.5306/wjco.v10.i3.110
Because of their wide spectrum of clinical presentations, degrees of malignancy, histopathological and imaging features, molecular biology and genetics of the tumor, as well as tumor aggressiveness, vascular hepatic tumors in adults may raise diagnostic difficulties. In these cases, a definitive diagnosis implies the corroboration of clinical suspicion data with suggestive imaging and histological features, including typically positive immunohistochemical markers and specific molecular findings. Their pathogenesis is not yet fully understood. Due to their rarity, late diagnosis because of the lack/nonspecific symptoms, and diagnostic difficulties, to date, these primary mesenchymal (endothelial) hepatic tumors in adults represent a group of tumors that does not yet have well-established therapeutic guidelines. Challenges in their management are due to their wide range of tumor behavior, from definite benign tumors such as hepatic hemangiomas, benign/low-grade malignancies such as hepatic small vessel neoplasias (HSVNs), tumors with high malignant potential such as hepatic perivascular epithelioid cell tumors (PEComas), and hepatic hemangiopericytomas (HPCs), other tumors with intermediate degrees of malignancy such as hepatic epithelioid hemangioendotheliomas (HEHEs), up to malignancies developed more often in the context of immunodeficiency syndrome–Kaposi sarcomas (KSs), and high-grade malignancies with a poor outcome such as hepatic angiosarcomas (HASs)[1,2].
This paper provides a comprehensive overview of this rare and particular group of hepatic vascular tumors, focusing on the malignant histological subtypes of neoplasia in adult patients, by searching through the most recent literature data. This review will comprise essential information regarding diagnostic challenges, modern prognostic factors and therapeutic approaches designed to improve patient survival (PS).
Epithelioid hemangioendothelioma (EHE) represents a rare vascular tumor encompassing cords of epithelioid cells surrounded by myxohyaline stroma that may involve soft tissue and visceral organs, classified by World Health Organization (WHO) in 2002 as being a lesion with metastatic potential. This borderline entity was described and termed for the first time by Weiss and Enzinger[3] (1982) as a soft tissue tumor of endothelial origin associated with an intermediate clinical outcome between benign hemangioma and malignant angiosarcoma. It can develop in any site, most frequently in the liver, liver plus lung, lung and bone as unique sites, respectively (HEARD support group) but also in many other organs, including the spleen, brain and meninges, breast, heart, head and neck region, soft tissue, skin, lymph nodes and stomach[3,4].
HEHE was first described by Ishak et al[5] in a series of 32 cases (1984) as a tumor with primary liver involvement, most often presenting as multiple liver nodules mimicking metastases and having a low-to-intermediate grade of malignancy. In fact, this entity may be associated with variable malignant potential. In some of the patients, the lesions are slow-progressive; in others, the lesions are associated with a more rapid progression. The tumors are constituted by epithelioid or spindle cells that are either spreading along preformed vessels or creating new vessels[5].
Primary malignant HEHE is estimated to be a very rare tumor, involving 1/1 million inhabitants, most commonly individuals aged between 30 and 40 years, and women (61% of cases), with a female per male ratio of 3:2[6,7].
In contrast to other primary hepatic tumors, HEHE does not develop in a background of chronic liver disease. Risk factors for its development remain unknown. There have been some risk factors suggested, such as the use of oral contraceptives, prior liver trauma or hepatitis, alcohol consumption, long-term exposure to some substances such as asbestos, vinyl chloride, or Thorotrast; however, to date, no clear evidence has supported these speculations exists[8,9].
Although some etiopathogenic factors for the development of HEHE remain unclear, recent data have demonstrated the presence of the vascular endothelial growth factor (VEGF)-vascular endothelial growth factor receptor (VEGFR) signaling pathway activation[10].
HEHE is most often represented by ill-defined multifocal lesions disseminated in both hepatic lobes that vary in diameter from a few millimeters to several centimeters.
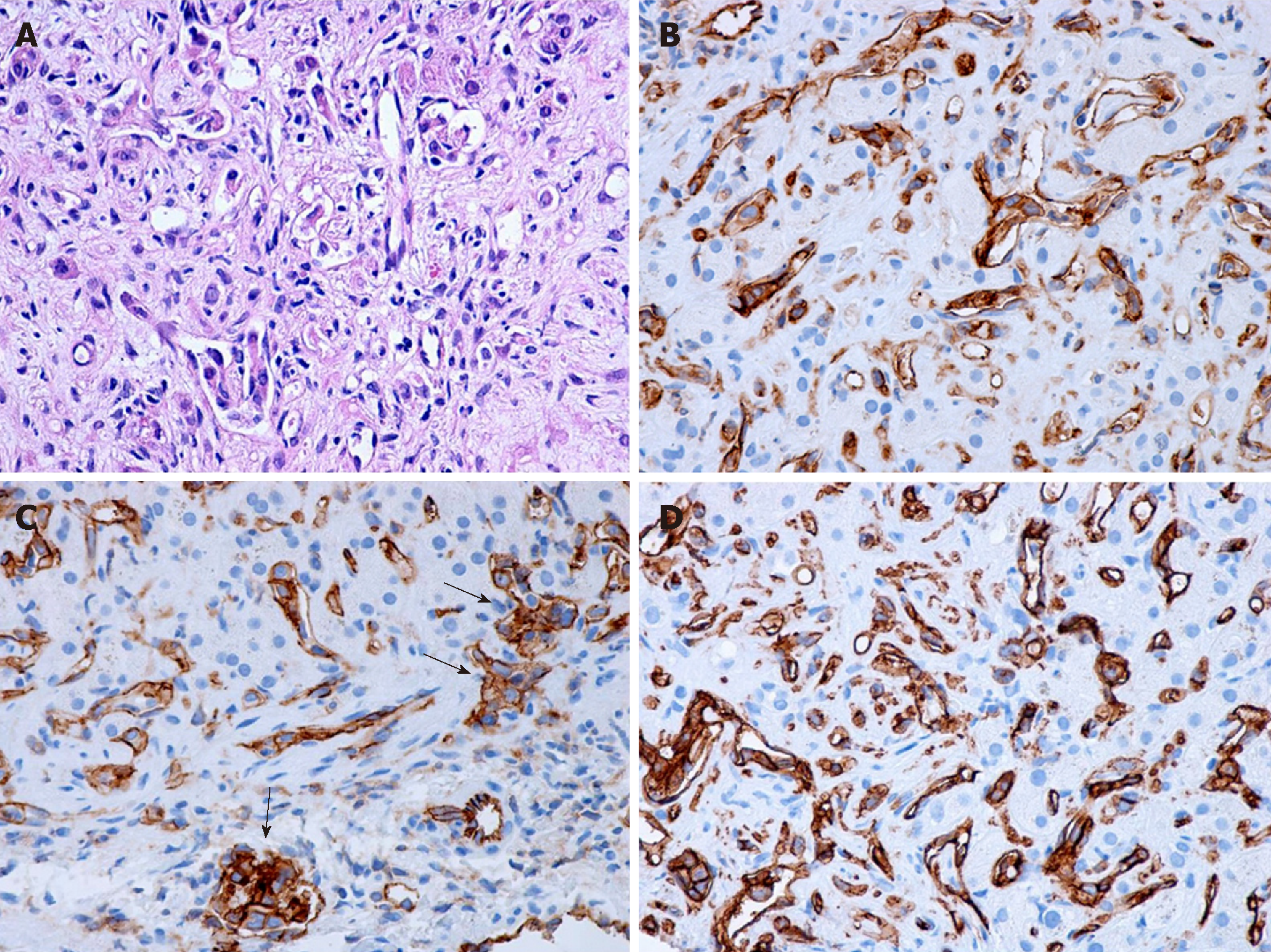
Macroscopically, HEHE appearance shows firm, tan- to white-colored nodules on sectioning, sometimes with the presence of calcifications and usually with a hyperemic periphery. Histologically, the nodules comprise multiple contiguous acini, and the tumor cells spread along preexisting hepatic sinusoids, terminal hepatic venules, and portal vein branches, frequently invading the Glisson capsule (Figure 1A). Intravascular growth of the tumor may take the aspect of a solid plug or a polypoid projection. There are two patterns described, a dendritic and an epithelioid type[11].
The dendritic pattern includes spindle cells or stellate cells with irregular shapes and multiple interdigitating projections dispersed on a dense, fibrous, myxoid stroma, sometimes with a glandular appearance. Frequently, the presence of cytoplasmic vacuoles (corresponding to intracellular vascular lumens), some of them containing erythrocytes, can be observed. The epithelioid pattern contains tumor cells with a more rounded shape, large atypical cells with abundant cytoplasm, disposed in solid areas surrounded by an inflammatory infiltrate. As the lesions evolve, they are accompanied by progressive fibrosis and calcifications. Additionally, the tumor cells and the vascular nature of the tumor may not be visible and recognized inside the tumor center because of the dense stroma often with the presence of calcifications. Therefore, needle biopsy specimens taken from these regions may pose diagnostic challenges. This tumor may resemble veno-occlusive disease, being associated with a high tendency of vascular invasion.
Tumor cells are positive for endothelial markers such as CD31, CD34 and factor VIII (Figure 1B-D). Additionally, podoplanin (D2-40) may be highly expressed in the tumoral cells of hepatic EH. Transcription factors ERG and Friend leukemia integration 1 are two additional markers helpful for defining the vascular nature of HEHE, being transcriptional factors belonging to the ETS family expressed in endothelial cells. In the study of Flucke et al[12] on 39 cases of HEHE, almost one-third of cases expressed pankeratin and the same percentage stained positive for CK 8.18; TFE3 showed a nuclear positive immunoreaction in 21/24 cases, irrespective of TFE3 rearrangement; this protein is a member of the microphthalmia transcription factor family associated with oncogenic properties in several tumors[12]. However, the usefulness of its immunohistochemical detection remains questionable. The presence of nuclear CAMTA1 expression encountered in approximately 85%-90% of cases is currently the main diagnosis of HEHE.
The histopathological differential diagnosis includes other vascular tumors (such as angiosarcoma, which is a more aggressive tumor, KS, bacillary angiomatosis), metastatic adenocarcinoma, and different tumors with a fibrous stroma such as cholangiocarcinoma, scirrhous hepatocellular carcinoma, or sclerosed hemangioma, as well as nonneoplastic conditions such as veno-occlusive disease. The characteristic vascular vacuole contained by the tumor cells of HEHE may be mistaken for steatotic/mucin vacuoles of an adenocarcinoma, but mucin staining are negative. Compared with HEHE, angiosarcoma is much more aggressive and destructive, obliterating the acinar landmarks and leading to the appearance of cavities. In the case of cholangiocarcinoma, tumor cells are disposed in a tubular or glandular pattern, often with mucin production, positive staining for cytokeratin and negative staining for endothelial markers[13-15].
Electron microscopy: Ultrastructurally, tumor cells present with endothelial differentiation, tight junctions, pinocytotic vesicles and Weibel-Palade bodies (approximately 30% of patients)[12].
The t(1;3)(p36;q23-25) or t(11;X)(q13;p11) translocations seem to represent early oncogenic events involved in the development of HEHE, producing a modified transcription program in cells endowed with endothelial properties. One of the mechanisms may rely on the manifestation as chimeric transcription factors by the corresponding fusion proteins, namely, WWTR1-CAMTA1 and YAP1-TFE3, exhibiting their oncogenic properties through a promoter switch. Another possible oncogenic mechanism may be initiated due to the loss of regulatory domains of the C-terminus of either WWTR1 or YAP1 and N-terminus of either CAMTA1 or TFE3.
WWTR1 encodes a transcriptional coactivator responsible for mesenchymal stem cell differentiation and is highly expressed in endothelial cells, while CAMTA1 encodes a calmodulin-binding transcription activator with oncogenic effects under the control of the WWTR1 promoter, possibly due to the occurrence of an in-frame fusion of the C terminus of CAMTA1 to WWTR1[16,17].
Recently, in a small subset of patients with HEHE (mainly young individuals), and distinct morphology, the presence of a YAP1-TFE3 in-frame fusion was detected; YAP1 represents a member of the FAT gene family that encodes another WW-domain-containing transcriptional coactivator. Again, the study of Flucke et al[12] revealed a high accuracy of FISH and RT-PCR methods in detecting the fusion genes to diagnose patients with HEHE. To date, the methods for molecular genetics determination are not routinely available[15].
The clinical manifestations of HEHE are nonspecific, varying largely from the lack of symptoms up to the development of portal hypertension or liver failure. At diagnosis, about one-quarter of patients are asymptomatic, and, among those presenting symptoms and signs, the most common ones are represented by epigastric or right upper quadrant discomfort/pain (60%-70% of cases), followed by hepatomegaly and weight loss, an altered general status and jaundice. Approximately 10% of patients present with pulmonary symptoms[8].
The most common clinical sign, encountered in approximately half of all cases, is hepatosplenomegaly. Portal hypertension may develop due to venous compression/ infiltration by the tumor. The tumor may also manifest as Budd-Chiari or Kasabach-Merritt syndrome (consumption coagulopathy) (exceptionally). Rupture of large tumors may occur, causing hemoperitoneum. Additionally, in about half of all cases, EHE may arise in other sites, such as other visceral organs, lungs, lymph nodes and bones.
Biologically, approximately 15% of cases do not show any changes; the most frequently encountered laboratory findings are cholestasis (60% of cases) and cytolytic syndrome (40%), usually with normal serum values of tumor markers (such as α-fetoprotein, carcinoembryonic antigen, and CA 19-9)[18].
Frequently, HEHEs are incidentally detected at various imaging investigations such as abdominal ultrasound (US), computed tomography (CT scan), magnetic resonance imaging (MRI), or positron emission tomography (PET scan) recommended for other indications. Two distinct patterns of the tumor can be identified using imaging techniques, namely, the early “peripheral pattern”, including the nodular type (most often with bilobar involvement) and the “diffuse pattern”, referring to the confluent type that may be associated with invasion of the great vessels.
On abdominal US, HEHE appears usually as a hypoechoic mass; however, sometimes, it can also show mixed or increased echogenicity. It may take the aspect of hepatic nodules or, in the case of the confluent type, an extensive heterogeneous structure of the liver may be seen in the area of tumor involvement.
CT scan aspects of HEHE include the presence of multiple hypervascularized nodules or a large hypodense lesion showing peripheral contrast enhancement. The characteristic CT scan features of HEHE are represented by the presence of multiple hepatic hypoattenuating lesions with a bilobar location that tend to confluent in larger hypoattenuating tumors distributed in a peripheral or subcapsular manner; in case of larger tumors, it may typically present a halo or target-type pattern of contrast enhancement. Native CT scan is best to assess the extent of the tumor and may reveal the presence of focal atrophy associated with retraction of the liver capsule. In approximately 20% of cases, calcifications are present[8].
MRI usually identifies hypo-intense lesions on T1-weighted images, gaining a hyperintense heterogeneous pattern on T2-weighted images. The heterogeneity of contrast enhancement on T2-weighted images can be explained by the presence of areas of calcifications, hemorrhage or necrosis situated in the tumor center, generating decreased signal intensity, and peripheral areas of viable tumor or edematous tissue responsible for increased signal intensity. Similar to CT scan investigations, larger tumors may show a peripheral halo or a target-type of enhancement of the gado-linium contrast, sometimes with the presence of a peripheral hypo-intense rim of avascular tissue. Diffusion-weighted imaging usually reveals a rim of diffusion restriction in the periphery of the tumor and variable signal in the central core because of T2 shine through effects. The extent of the tumor may be better evidenced using ferumoxide-enhanced T2-weighted images, but the precise limit between the tumor and normal liver parenchyma may be difficult to be identified on all sequences. Frequently, imaging investigations may detect the presence of extrahepatic metastasis, enabling patients to be diagnosed at an advanced stage of the disease. Seldom, MRI may detect the presence of ascites and portal hypertension. Therefore, summarizing all the above-mentioned imaging aspects of HEHE, we may emphasize that the pattern of the signal emitted by the lesions on MRI as well as the characteristic on CT scan is similar to other hypervascularized liver tumors or their metastasis.
PET scan shows increased fluorodeoxyglucose (FDG) uptake by this type of tumor. Because the presence of extrahepatic metastases does not seem to influence the treatment, the role of PET scan in assessing the extent of the disease and management of HEHE is currently not very well defined[19,20].
Extrahepatic tumor extension beyond portal lymph nodes was shown to represent a negative prognostic factor. Because of the high incidence of extrahepatic localization and recurrence development after surgical approaches, there was a need to discover prognostic markers that may guide treatment. Lung or multiorgan involvement, disease progression, the presence of ascites, age ≥ 55 years, and male gender were found to be associated with a worse prognosis[4]. The European Liver Intestine Transplantation Association (ELITA)-European Liver Transplant Registry (ELTR) study elaborated a prognostic score using a specific formula that stratifies patients according to their post-LT recurrence risk: low score (excellent DFS of 94%), intermediate score (DSF of 77%) and high score (worse DSF of 38.5%)[21,22].
Because the prediction of tumor behavior and prognosis is difficult, establishing the best therapeutic algorithm for each patient is a challenging task. The therapeutic modalities for patients with HEHE include liver resection, liver transplantation (LT), systemic or locoregional radiation therapy, percutaneous ablative techniques such as radiofrequency ablation (RFA), systemic chemotherapy (anti-angiogenic or anti-tumor pharmacological treatment), locoregional chemotherapy such as transarterial chemoembolization (TACE), hormonal therapy, immunotherapy or only surveillance.
Comparing these therapeutic approaches, Mehrabi et al[13] reported a 5-year PS rate of 75% in patients with hepatic resection, 20% in those treated with chemo/ radiotherapy and only 4.5% in the followed-up patients without any specific treatment, proving the superiority of surgical vs nonsurgical approaches; another study showed rates of 86% for resection and 73% for LT. In the study, the 3-year PS rate was 74.1% for hepatic resection and 81.6% for TACE[13].
By analyzing these data, we can conclude that liver resection, LT and TACE showed similar results, but their indications differ. Hepatic resection is recommended in cases of resectable intrahepatic lesions, while the other two modalities are recommended in cases of unresectable liver lesions[23]. The role of nonsurgical treatment modalities was investigated only in small studies; therefore, their specific place in the therapeutic scheme needs to be further investigated. Additionally, they may play a role as adjuvant or alternative approaches in the case of tumor recurrence after the application of one therapeutic modality[24,25].
The two studies of the ELITA-ELTR including the analysis of 57 patients transplanted for HEHE during the period 1989-2004 plus another 92 patients transplanted during the period 2008-2015, reported 5-year and 10-year disease-free survival (DFS) rates of 79% and 73%, respectively; the 5-year and 10-year posttransplantation PS rates were 81% and 77%, respectively[21,22]. The United Network for Organ Sharing (UNOS) registry reported a 5-year survival rate of 64% in 110 LT recipients for HEHE. Unfortunately, in countries with a limited number of deceased donors, HEHE may not be a priority for LT; therefore, living-donor LT may be considered (with caution)[26]. A study reported several efficient sequential (lung after liver) or simultaneous liver-lung transplantations, despite invasion of the pleura and diaphragm/presence of bone metastases at the moment of transplantation[27]. Recurrences after transplantation are frequently encountered (approximately 25% of cases) and should be managed aggressively.
Because HEHE was demonstrated to contain VEGF and VEGF receptors, small studies have been undertaken using anti-VEGF treatments such as the anti-VEGF monoclonal antibody bevacizumab, tyrosine kinase inhibitors (like sorafenib, sunitinib, and pazopanib) and paclitaxel, as well as other antiangiogenic agents such as PDGFR, angiopoietin peptibodies and endoglin inhibitors[28]. Treatments using anti-VEGF agents were investigated in phase II trials designated to advanced, nonresectable, metastatic HEHE that were performed by the French sarcoma group (sorafenib)[29] and Eastern Cooperative Oncology Group (bevacizumab)[30], showing stabilization of the disease up to 10 mo in 20% and 40% of cases, respectively. Additionally, other vascular target agents such as thalidomide, lenalidomide, interferon, and beta-blockers (considering the high tumor content of beta adrenergic receptors) have been studied, along with standard chemotherapy using doxorubicin, cyclophosphamide and the carboplatin-etoposide combination[31-34]. Radiotherapy is used only to control local pain.
To improve the patient outcome and efficacy of novel treatments, there is an imperative need for better insight into the pathology, molecular biology and genetics of HEHE, to improve the identification of tumors with aggressive behavior and to personalize treatment.
Primary HAS is a rare high-grade malignant tumor resulting from the proliferation of tumoral endothelial cells of blood or lymphatic vessels, and it is endowed with an aggressive behavior. This mesenchymal malignant tumor comprises 2% of all soft tissue sarcomas. HAS can involve any organ, but it is more frequently encountered in the head and neck region, as well as in the skin. Although only approximately 200 cases are diagnosed globally each year, it represents the most common malignant mesenchymal hepatic tumor, accounting for approximately 2% of all primary hepatic neoplasms[35].
HAS is rarely encountered in children; it develops most commonly in the sixth and seventh decades of life and in men, with a male-to-female ratio of 3-4:1[1].
Tumor angiogenesis is stimulated by growth factors such as basic fibroblast growth factor, VEGF, and transforming growth factor. Growth hormone (GH) determines the proliferation of vascular tissue cells (smooth muscle cells, fibroblasts, and endothelial cells), suggesting its involvement in vascular tumor growth. Some proangiogenic growth factors use the phosphoinositide 3-kinases signaling pathway. The expression of the VEGF gene and angiogenesis may be regulated by phosphatase and tensin homolog (PTEN), with PTEN gene mutations representing a molecular event in HAS development. Although approximately one-quarter of HAS seem to be associated with chemical carcinogens, most of the tumors have an unknown etiology.
Tumor development proved to be associated with numerous environmental carcinogens, such as iatrogenic exposure to the radiocontrast agent Thorotrast (colloidal thorium dioxide), industrial exposure to vinyl chloride monomers, chronic exposure to arsenical compounds and exposure to pesticides, external radiation, and radium. Additionally, cyclophosphamide, urethane, diethylstilbestrol, the use of oral contraceptives, phenylhydrazine, iron, androgenic or anabolic steroids proved to be risk factors associated with HAS development[36,37].
HAS is also associated with diseases such as neurofibromatosis and hemo-chromatosis; a possible association with alcoholic cirrhosis has also been described. Although the role of viral hepatitis in the pathogenesis of HAS was also studied, it was not proven to affect tumor development[38].
HAS due to chemical exposure is associated with a latency period between 10 and 40 years. After approximately two decades of use, an association between Thorotrast and the development of malignancies and organ injuries was demonstrated; therefore, it was no longer used as a radiocontrast medium (early 1950s). Presently, exposure to environmental carcinogens (excepting possibly androgenic steroids) was strongly diminished; therefore, most HAS cases seem to be idiopathic.
Macroscopically, HAS is usually multicentric and affects both lobes, or even the entire liver. Tumor foci have a heterogeneous structure, an infiltrative behavior, and variable sizes, forming a conglomerate of sponge-like hemorrhagic nodules involving the whole liver. On sectioning, solid grayish-white areas intermingle with red-brown hemorrhagic tumor areas, or the tumor presents central necrosis, sometimes with the possible development of irregular large cavities filled with liquid or clotted blood. Ill-defined, highly vascularized smaller satellite nodules may also appear. In cases associated with prior Thorotrast exposure, a reticular pattern of fibrosis may be identified[39].
Histologically, tumor cells spread along preexistent vascular channels, such as sinusoids, terminal hepatic venules and portal vein branches and replace normal endothelial cells. This type of growth is sustained by reticulin fibers and is associated with the atrophy of hepatocytes and alteration of the plates, areas of infarctization and fibrosis, with possible subsequent development of cavities presenting uneven walls delineated by tumor cells and sometimes with papillary projections, filled with clotted blood and debris. Tumor invasion of terminal hepatic venules and portal vein branches with secondary obstruction of the vessels is commonly noticed. Solid tumor areas resembling fibrosarcoma and multinucleated giant cells may also be en-countered.
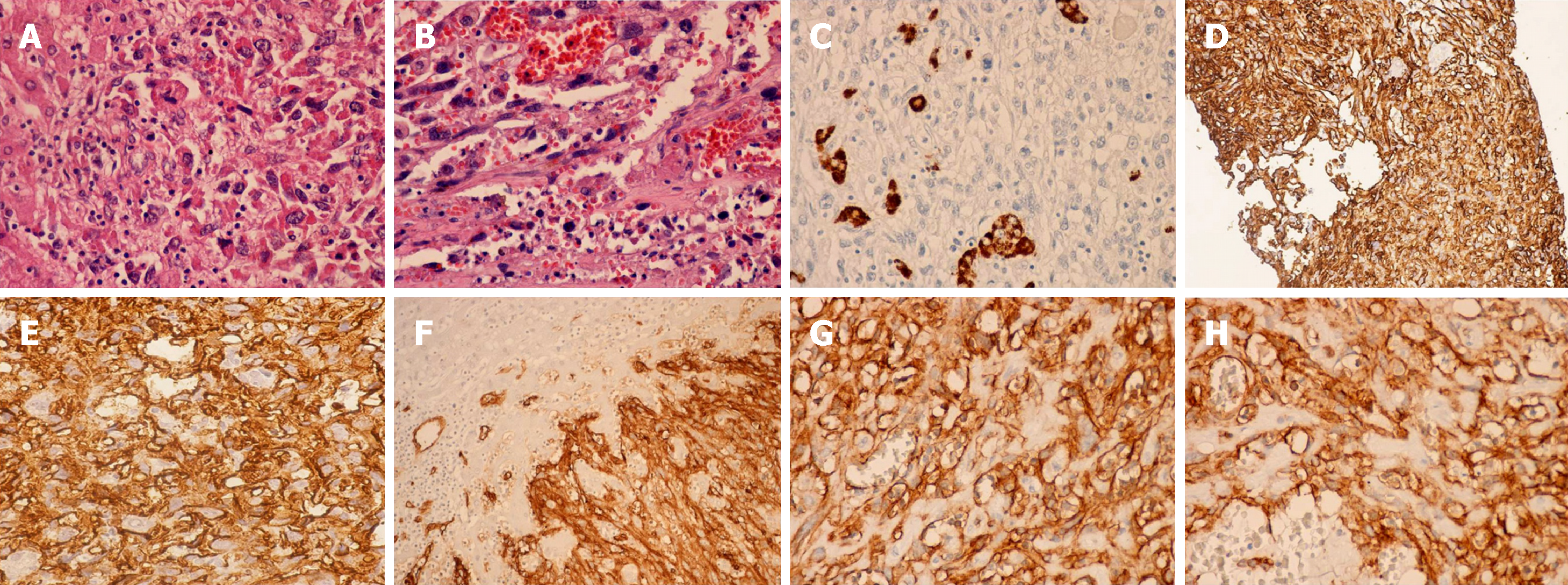
The tumor cells are spindle-shaped, with ill-defined borders, a slightly eosinophilic cytoplasm, hyperchromatic elongated nuclei; mitotic figures are frequently seen [39] (Figure 2A and B).
Macroscopically, Ito et al[40] categorized HAS associated with Thorotrast exposure into four types of growth: Diffuse micronodular, multinodular, massive and mixed (multinodular and massive). Microscopically, HAS is represented by two cell types, spindle-shaped and polyhedral tumor cells, and three growth patterns: sinusoidal, cavernous, and solid. The sinusoidal and cavernous types consist of sinusoid-like or dilated vascular areas delineated by enlarged spindle tumor cells positive for endothelial markers. Tumors of the solid type of growth encompass malignant spindle cells without creating evident vascular spaces, and it is associated with diagnostic difficulties because poorly differentiated tumors usually exhibit weak immunopositivity for endothelial markers.
Thorotrast depositions are identified in reticuloendothelial cells, in connective tissue located in portal areas, inside the walls of terminal hepatic venules, and in the Glisson capsule, and may be visualized using hematoxylin-eosin staining and electron microscopy[40].
A definite diagnosis of HAS requires the presence of specific molecular markers. Tumors are immunoreactive for endothelial factors such as factor VIII, CD31, CD34 and Ulex europaeus agglutinin I, confirming their vascular nature. Moreover, HAS may exhibit positive immunostaining for other markers such as vimentin, desmin, GPC-3, ERG, Ki-67, and pancytokeratin (CK) (approximately 10% of cases). Of all the above mentioned markers, CD31 seems to be the more reliable one. Recently, the study of Wang et al[39] revealed that ERG represents a more accurate marker for diagnosing HAS than classical endothelial markers, with a positivity of 100% of the studied cases. The study of Miettinen et al[41] showed that the immunohistochemical expression of VEGFR2 is strong in most angiosarcomas, irrespective of site, subtype or degree of differentiation but is not detected in most nonendothelial mesenchymal neoplasms. Moreover, VEGFR2 demonstrated a diagnostic usefulness in identifying poorly differentiated angiosarcomas. Additionally, VEGFR2 showed almost equal accuracy for angiosarcoma compared with other endothelial markers such as CD31 and ERG. Their results showed high expression of VEGFR2 in angiosarcomas regardless of the degree of differentiation representing the basis for using targeted therapy with VEGFR2 inhibitors in the treatment of angiosarcomas[41] (Figure 2C-H).
On liver biopsy, the positive diagnosis of HAS is difficult and is made with other sarcomas such as KS and fibrosarcoma; it is almost impossible to be differentiated from HEHE. While the former one has a poor prognosis, the latter can be manageable using LT.
Additionally, HAS must be differentiated from inflammatory and benign vascular conditions of the liver, liver metastasis from primary angiosarcomas arising in other sites or liver metastasis from any other type of primary tumor, as well as he-patocellular carcinoma.
Sometimes, the confirmation of the diagnosis may need to prove the existence of Weibel-Palade bodies.
TP53 mutations were found in HAS associated with vinyl chloride exposure; additionally, a high rate of KRAS-2 mutations has been discovered in both sporadic and Thorotrast-associated HAS[42,43].
Patients present symptoms such as abdominal pain or discomfort, fatigue, anorexia, weight loss, or the detection of an abdominal mass. Other symptoms include nausea, vomiting, malaise, and fever. Symptoms are more expressed than those generated by HEHE. Studies have revealed that up to 40% of cases present extrahepatic disseminations at diagnosis, most often in the lungs, hilar lymph nodes, spleen, adrenal glands, and bones. Patients may present symptoms related to the presence of metastasis such as dyspnea, chest discomfort, or hemoptysis. Sometimes, features of portal hypertension or acute liver failure may be present, the latter due to occlusion of the portal vein by malignant thrombi or replacement of hepatocytes by tumor cells. Severe complications that may develop are represented by tumor rupture leading to acute abdomen and hemoperitoneum, and the development of disseminated intravascular coagulation. Clinical signs include hepatomegaly, jaundice, ascites, peripheral edema, and splenomegaly[44-46].
Because imaging does not reveal specific features of HAS, histological confirmation is mandatory for a definite diagnosis.
Percutaneous Trucut biopsy is not recommended for diagnosing HAS. Instead, several studies recommend the use of fine-needle aspiration cytology (FNAC), but the literature data are conflicting regarding the safety of the procedure. Some studies have reported cases of massive bleeding leading to death following FNA for HAS because the vascular nature of this tumor is prone to hemorrhage complications after needle biopsy, while others claim that FNAC is a safe procedure, not associated with serious complications and capable to bring a definite diagnosis of HAS. Following cytological smear, HAS shows morphologic characteristics that make it potentially recognizable. Because the presence of vasoformative features, although strongly suggestive, are not specific for angiosarcoma, a high clinical suspicion and specific positive immunohistochemical markers are required for a correct diagnosis. Due to the high risk of bleeding, some authors recommend avoidance of percutaneous liver biopsy, and most of the studies plead for open biopsy[35,47].
HAS is sometimes detected incidentally using imaging techniques and exhibits nonspecific features. In the case of HAS imaging, tests show usually the presence of multiple masses or a dominant mass with a heterogeneous aspect.
Abdominal X-rays may detect the presence of the deposition of thorium dioxide at the hepatic periphery in the case of HAS secondary to Thorotrast exposure.
Abdominal US reveals the presence of multiple masses or a solitary lesion showing heterogeneous echogenicity due to the presence of necrotic or hemorrhagic areas inside the tumor and can detect the presence of hemoperitoneum. The tumor behavior at CEUS examination shows central nonenhancement of the contrast and irregular enhancement of the tumor periphery in the arterial and portal phase, with complete wash-out of the contrast in the late phase[48].
Native CT scan images show usually a hypoattenuating pattern of the tumor, more pronounced in cases associated with previous exposure to Thorotrast, due to the displacement of the hyperattenuating linear deposits of Thorotrast. Most of the literature data consider that contrast-enhanced CT scan represent the reference imaging modality for diagnosing HAS, describing the existence of hypodense lesions with various patterns of contrast enhancement; after contrast administration, tumor masses appear mostly isodense compared with the normal liver parenchyma. Large tumors may exhibit a heterogeneous structure with various patterns of early contrast enhancement showing sometimes focal peripheral or intratumoral irregular areas of enhancement or peripheral rim enhancement. CT angiography has diagnostic usefulness, showing the presence of multiple or sometimes solitary hypervascular masses with heterogeneous early and progressive contrast enhancement. Dynamic CT scan may differentiate between HAS with a solid growth pattern and cavernous hemangioma; in the case of a malignant tumor, this investigation reveals early central contrast enhancement and arterioportal shunting[49].
MRI is useful in demonstrating the heterogeneous and hemorrhagic structure, as well as the hypervascular nature of a dominant mass with progressive contrast enhancement. HAS is usually T2 hyperintense with heterogeneous signal towards the center of tumor, while the unenhanced T1 signal is less than that of surrounding liver parenchyma, sometimes with zones of high internal signal demonstrating intra-lesional hemorrhage. Diffusion-weighted MRI detects different values of apparent diffusion coefficients (ADCs) among lesions, the mean ADC value of HAS being slightly higher than that of other hepatic malignancies. Using gadolinium-based contrast enhancement, HAS studies have reported mild enhancement in the early phase, followed by progressive homogeneous enhancement, with complete tumor wash-out in the delayed and parenchymal phase[20,50,51].
There are scarce data regarding the usefulness of PET scan in assessing HAS, some data suggesting that FDG accumulates inside the tumor, and this technique may be a helpful method for tumor staging and the identification of metastasis, which is important to avoid unnecessary surgical interventions[52].
HAS is associated with a very poor prognosis-in the absence of treatment, most patients die in the first 6 mo after the diagnosis; under treatment, survival exceeds 2 years only in approximately 3% of cases, and the mean survival is less than 7 mo after LT (according to ELTR reports)[53].
To date, there are no established treatment guidelines. The mainstay of treatment consists of radical tumor resection or hepatic resection. Surgical radical resection is accompanied by superior survival and less morbidity vs LT, especially when associated with targeted treatments/adjuvant chemotherapies[54]. Due to the disappointing results generated by extremely short survival periods of approximately 6-7 mo after LT because of tumor recurrence and rapid progression of the disease, both ELTR and UNOS consider HAS as an absolute contraindication to LT[55,56].
Tumor resection should be considered in the case of limited disease, as well as when the rest of the liver is relatively normal. Unfortunately, most of the tumors are multinodular and disseminated in both hepatic lobes; even in the presence of a solitary mass, the resection rate does not exceed 20% of cases. The survival was prolonged by combining surgery with adjuvant chemotherapy up to 84 mo in the case of solitary or confined hepatic tumors[57].
In the case of unresectable multifocal hepatic lesions, when the entire liver parenchyma is replaced by the tumor, or in the presence of distant metastases, palliative chemotherapy is the only therapeutic solution. To date, there are no specific chemotherapeutic regimens. Kim et al[58] demonstrated an improved survival in some of the patients by administering 5-FU-carboplatin in combination with doxorubicin or ifosfamide. Another study reported that first-line treatment using adriamycin, ifosfamide, cisplatin and paclitaxel was associated with a small positive effect[59].
Radiotherapy has no benefit in the treatment of HAS because it is a radio-resistant tumor. TACE is used as an emergency procedure to stop active bleeding after tumor rupture and with palliative intent with only little impact on survival[60].
Because its poor prognosis and aggressive behavior, it is of major importance to improve the survival of patients with HAS, by finding the most appropriate treatment in accordance with the expression of antiangiogenic growth factor receptors (such as VEGF and VEGFR) and tumor biology, using specific antiangiogenic or vascular-targeted agents[61], cytokines (e.g., recombinant interleukin 2)[62], and immunotherapy with or without classical chemotherapeutic regimens[63]. These agents have been investigated to show partial responses and need larger studies to certify their role in the management of patients with HAS.
HPC is a rare vascular tumor encompassing less than 2% of soft tissue sarcomas, as well as less than 1% of all vascular tumors; it arises from the pericytes of Zim-mermann, which are small cells surrounding capillaries. The most commonly involved areas are the abdominal and retroperitoneal spaces, lower limbs, head and neck, spine and cranium, while liver involvement is rarely encountered (< 1%). Hepatic HPC can be primary or metastatic, with primary hepatic tumor being seldom reported[1].
It is diagnosed in both male and female adult patients, in the fifth or sixth decades of life.
Some studies have suggested an association of HPC with prolonged steroid intake, trauma or hypertension[1].
Macroscopically, HPC presents as a well-circumscribed solitary lesion delineated by a pseudo capsule, sometimes with a cystic appearance. On sectioning, HPC presents numerous dilated vascular areas with features of hemorrhage and cystic dege-neration[64].
Histologically, HPC is a hypervascular neoplasia, and the tumor cells are mostly spindle shaped. Approximately 50% of the tumors are considered malignant, with parameters suggesting malignant transformation represented by a large tumor size (more than 20 cm), increased mitotic activity (more than 4 mitotic figures per high-power field), cellular pleomorphism with a chromatin pattern, and the presence of central necrosis or intratumoral hemorrhagic areas[65,66].
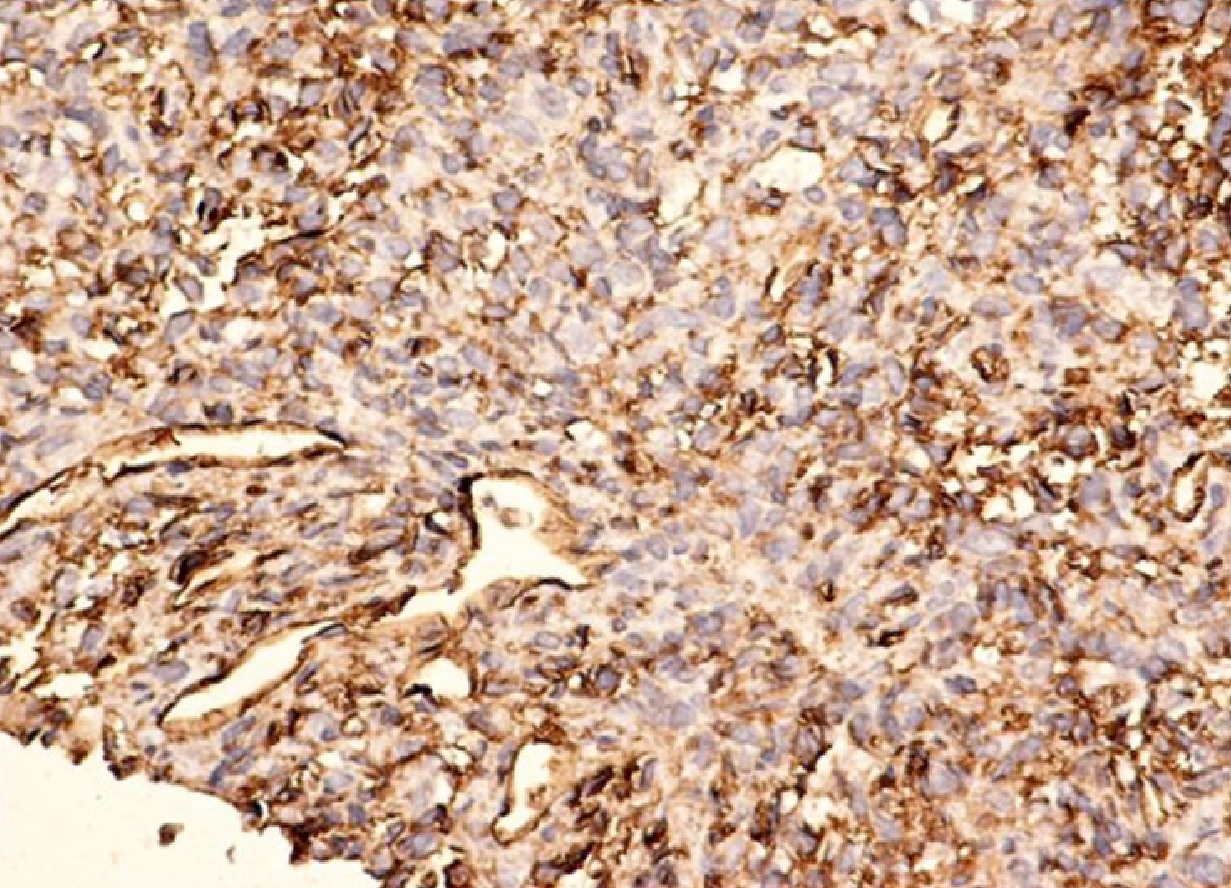
HPC presents positive immunostaining for vimentin, S-100, muscle-specific actin, smooth muscle actin (SMA), CD34, and factor XIIIa[67] (Figure 3).
Histological and immunohistochemical assessment enable its differentiation from other types of sarcomas.
In some cases, 12q13-15 alterations may be present, associated with frequent deletions affecting STAT6, caused by somatic fusions of two genes (NAB2 and STAT6)[68].
Clinical manifestations of hepatic HPC are highly variable and may range from an asymptomatic tumor to the presence of metastases. Sometimes, it may manifest more dramatically as an acute abdomen due to rupture of the tumor. Most frequently, HPC metastasizes in the lung, liver, and bone.
Paraneoplastic syndromes may be present at the time of diagnosis or when metastases develop. In advanced stages, the most frequent paraneoplastic ma-nifestation is hypoglycemia due to the release of insulin-like growth factors[64].
To assess hepatic HPC, investigations such as contrast-enhanced CT scan, MRI, and angiography can be used. The most common enhancement patterns show he-terogeneous early enhancement with persistence into delayed phases. HPC can be hyperechoic and highly vascular on US. On native CT scan, HPC may be hypodense to isodense to surrounding liver. On contrast-enhanced CT, primary hepatic HPC may present as a lobulated tumor with both solid zones showing contrast enhancement, as well as cystic areas, with speckled calcifications. CT angiography shows a highly vascular tumor. On T1-weighted MRI, HPC is hypointense to liver, while on T2-weighted phase it shows a heterogeneously hyperintense signal. MRI shows heterogeneous enhancement of the extracellular contrast. PET scan is a reliable investigation modality to diagnose HPC and for follow-up (if the tumor uptakes the tracer). The imaging features may be suggestive of HPC, but they cannot confirm the diagnosis, which needs histological and immunohistochemical testing[1,20,69].
Half of the patients diagnosed with malignant HPC present 5-year disease free survival after Ro surgery.
The treatment modality of choice is aggressive surgery, both in the case of the primary tumor and distant metastases, but this technique has proven to be associated with a high recurrence rate, even in the case of R0 resections. Unfortunately, detection of these recurrences is quite difficult because there are no specific markers[70].
In the case of localized disease, surgery is the treatment of choice, with 10-year overall survival rates between 54% and 89% after R0 resection. For patients who develop local or distant disease recurrence (approximately 20% of cases), repetitive resections may be needed, but they are sometimes difficult or impossible to perform.
Four patients were included in the ELITA-ELTR study, two of them with primary/metastatic HPC. One metastatic patient had a DFS of 12 years after LT, but it was associated with multiple other surgical interventions. After R0 surgery, the 5-year DFS is approximately 50%, but the recurrence rate remains 10% after 5 years. In the case of paraneoplastic syndrome development, surgery may be needed again, especially in the case of severe hypoglycemia[1,22,71].
Treatment strategies for the successful management of unresectable tumors are scarcely available, including radiotherapy for selected cases or systemic che-motherapy, which has been demonstrated to be rarely associated with tumor response. Therefore, the development of novel therapeutic options is needed for disease control and an improved quality of life[72].
Doxorubicin plus ifosfamide represents the standard combined regimen of systemic chemotherapy for several subtypes of soft tissue sarcomas; another feasible choice consists of combining gemcitabine with docetaxel. Unfortunately, the response of HPC to these combined schemes has been only rarely reported, and, to date, no clinical trial has highlighted an efficient chemotherapeutic regimen for inoperable tumors[73-76].
It has been shown that soft tissue sarcomas (other than GIST), when treated with chemotherapeutic or biologic agents, express patterns of response resembling those of GISTs under imatinib treatment. This behavior indicates that, despite less significant tumor shrinkage, patients may present long time-to-progression periods.
In the study of Park et al[77] on 14 patients with locally advanced, recurrent, or metastatic HPC treated with temozolomide and bevacizumab, partial responses of the tumors were obtained in almost 80% of cases, with some patients showing prolonged disease-free progression (DFP) periods, and 5 of them demonstrating a time-to-progression period exceeding 20 mo. The effects of temozolomide, a chemo-therapeutic alkylating agent whose active metabolite is similar to that of dacarbazine (an active drug against soft tissue sarcomas), seem to be potentiated by an association with bevacizumab, a monoclonal antibody targeting VEGF.
Some anti-VEGF receptor tyrosine kinase inhibitors such as sorafenib and sunitinib have also shown some efficacy in HPC treatment and have been associated with promising early results, with tumor responses exceeding 6 mo in patients treated with sunitinib, as well as partial responses and disease stabilization for up to 22 mo in cases treated with sorafenib[78,79]. Further research will be needed to highlight the most efficient therapeutic targets in HPC and define specific molecular tumor markers that may suggest a good response to certain targeted therapies.
Bonetti et al[80] were the first to describe the existence of a neoplasm family derived from perivascular epithelioid cells (PECs) (1992), reporting in both angiomyolipoma (AML) and lung clear cell “sugar” tumor (CCST) the presence of epithelioid cells with clear-acidophilic cytoplasm, and perivascular disposal, expressing specific positive immunostaining for melanocytic markers[81,82].
The PEC tumor family (PEComas) include, besides AML and CCST, some rare intraabdominal, visceral (most frequently gastrointestinal, gynecological and genitourinary), soft tissue (usually abdominal, pelvic, retroperitoneal and cutaneous) and bone tumors. This latter rare group of tumors was termed non-AML, non lymphangioleiomyomatosis (LAM), non-CCST PEComas, or PEComas-not otherwise specified (NOS)[83,84].
The WHO categorizes PEComas as mesenchymal tumors derived from PECs, with distinct histological and immunohistochemical characteristics. These tumors have been reported in various sites, such as the pancreas, small and large intestines, bladder, uterus, vulva, ovary, breast, broad ligament, prostate, heart, base of skull, liver, and soft tissue[84-88].
HPEComas-NOS may be categorized as tumors with a “high malignant potential” or at high risk of aggressive behavior”.
A study reported 30 cases of hepatic AMLs diagnosed starting 1999 around the world, most of them in women, and the AMLs were considered as a benign tumors requiring conservative treatment[89]. However, studies conducted by University of Verona, differentiate PEComas from classic AMLs, by the absence of adipocytes, presence of thin capillaries, perivascular distribution of tumor cells, and specific immu-nohistochemical markers[90].
Currently, several hypotheses have been suggested regarding the origin of PECs. One considered that PECs are originated from multipotent stem cells of the neural crest, exhibiting phenotypes of both smooth muscle and melanocytic differentiation; a second is that PECs originate from myoblasts with later acquisition of histological and immunohistochemical phenotypes of melanocytic marker expression; a third is that PEComas-NOS are originated from pericytes[91]. Association with risk factors is unkown.
Macroscopically, the tumor is pale tan and friable and has a soft consistency on sectioning.
Histologically, the tumor is composed mostly of medium/large-sized epithelioid cells, intermingled with foci of spindle-shaped cells, disposed around small vessels as cellular nests, exhibiting a trabecular pattern, and including multiple vascular spaces. The tumor cells are mainly epithelioid, occasionally spindle shaped, with abundant cytoplasm, central vesicular nuclei, expressing pleomorphism and hyperchromatism. Mitotic figures are seldom encountered; calcifications may be present[92,93].
Immunohistochemically, the tumor cells are characterized by strong and diffuse coexpression of melanocytic markers such as gp 100 protein (HMB-45 mAb), human melanosome-specific antigen HMSA-1, Melan A, microphthalmia transcription factor (Mitf), and muscle markers such as SMA, vimentin and more rarely desmin, as well as CD34; local immunostaining for Ki-67 is present, while epithelial markers such as EMA and cytokeratin are rarely expressed. PEComas do not exhibit S100 protein, the hepatic carcinoma marker AFP or CD 117[94-97].
The diagnosis of PEComa is based on characteristic morphological features of perivascular tumor cells (epithelioid or spindle-shaped cells), which are im-munohistochemically positive for melanocytic and muscle markers (HMB-45 and SMA).
The differential diagnosis includes hepatocellular carcinoma, hepatic adenoma, gastrointestinal stromal tumors (GISTs), leiomyoma, melanoma and sarcoma. Other possible differential diagnoses include HEHE, paraganglioma, and metastatic chromophobe renal cell carcinoma and adrenocortical carcinoma.
Most PEComas-NOS are sporadic, but a subset has proven to be associated with genetic changes of the tuberous sclerosis complex (TSC) and deletion of 16p (the locus of the TSC2 gene)[98,99]. TSC genes were demonstrated to play a crucial role in the regulation of the mTOR pathway; therefore, inhibition of the mTOR pathway proved to be beneficial in the treatment of malignant PEComa.
Most often patients with HPEComa are asymptomatic or have nonspecific digestive symptoms, and the tumors are found incidentally, by performing abdominal radiological tests for other indications. Large lesions may be associated by epigastric pain, and, rarely, rupture of a large subcapsular tumor may lead to hemoperi-toneum[100].
A preoperative diagnosis of hepatic PEComa is difficult to be made because of nonspecific features on radiologic tests. Abdominal US may show a heterogeneous hypoechoic lesion. Native CT scan may reveal a low-density mass. On contrast-enhanced CT scan, the lesion may show heterogeneous and intense enhancement on the arterial phase, with a slightly hypodense aspect on the portal phase and enhancing rim on the delayed phase, features that may also be confused with hepatocellular carcinoma in the case of underlying diffuse liver disease. Therefore, HPEComas seem to have similar enhanced imaging patterns to those of hepatocellular carcinoma. Some studies have suggested that PEComas should be considered even in the context of an inhomogeneous intratumoral vascular pattern with no signs of hemorrhage inside the lesion, with normal background hepatic parenchyma and negative hepatitis virus markers. The few published data on the role of FDG-PET/CT for the diagnosis and staging of PEComas are controversial[101-104].
Literature data have concluded that the natural history of primary HPEComas may be relatively varied and, currently, not very clearly defined or predictable. Although most of the tumors are considered to have a benign outcome, recently, more cases of malignant PEComas with diverse origins such as the small intestine, prostate, base of the skull and soft tissue have been detected.
Recently, among diagnostic criteria for malignancy, a tumor dimension more than 5 cm with infiltrative borders, nuclear pleomorphism, high mitotic activity (more than 1/50 high power field), necrosis, high nuclear atypia, vascular invasion and aggressive behavior have been included. In the case of the existence of more than 2 high risk features, the tumors are considered as malignant. However, because many PEComas present some atypical features without expressing necessarily aggressive behavior, these criteria should be considered with caution[104].
Malignant transformation of benign PEComas both with a sarcoma-like or a carcinoma-like appearance has been reported. Moreover, some cases may develop late metastasis, many years after the diagnosis of the primary tumor.
Because HPEComas-NOS are considered tumors with malignant potential or at high risk of an aggressive outcome, and because presently there are no firm diagnostic criteria for malignancy, the long-term follow-up of the tumors is recommended.
Although there are no specific treatment protocols and no definitive evidence concerning the benefits of adjuvant therapy, the mainstay of treatment is represented by radical resection of the hepatic primary tumor followed by monitoring. Sometimes, tumor resection may be difficult because of the abundant intratumoral vasculature leading to hemorrhagic complications[105]. Usually, there is no tumor recurrence after surgical resection, and subsequent development of distant metastases is not so often encountered. In the case of inoperable tumors, the use of neoadjuvant stereotactic body radiation seems to be a good strategy for HPEComas showing PET-tracer positivity at initial imaging investigation, capable of converting the tumor to respectability[106]. Another alternative therapeutic modality for patients not suitable for resection resides in performing interventional treatment using transarterial embolization and/or RFA[107].
Some studies have shown the beneficial role of neoadjuvant treatment using the mTOR inhibitor sirolimus in unresectable PEComas to facilitate tumor shrinkage and surgical removal[108]. A review of 234 malignant PEComas assessed the efficacy of neoadjuvant chemoradiotherapy, describing response rates ranging from 0 to 80% with some cases demonstrating disease progression while on treatment; additionally, some of the studied patients received various types of adjuvant treatment using chemo- or radiotherapy, hormonal, or even immunotherapy. Most of the patients treated by adjuvant chemotherapy developed recurrent disease within the next two years, while those presenting with metastatic disease died within an interval ranging from 4 to 30 mo. After initial resection of the tumor, treatment of the metastatic stage included different modalities, such as repetitive surgical resection, chemotherapy, radiotherapy, the tyrosine kinase inhibitor imatinib, and sometimes mTOR inhibitors, showing inconstant responses[109].
Several reports have described cases of metastatic PEComas treated with mTOR inhibitors because the tumors express p70S6K, which is involved in the mTOR pathway. Patients with progression after first-line treatment using resection and imatinib were further treated with mTOR inhibitors, such as everolimus or sirolimus plus etoposide, showing a partial response and survival of more than 3 years[110].
KS represents a low-grade angioproliferative tumor developed in association with human herpesvirus-8 (HHV-8) that can harbor several clinical variants. The first or “classical” variant affects men of Ashkenazi Jewish ethnicity and from the Mediterranean area and shows cutaneous involvement and slow evolution. The second type affects mostly Africans, and the clinical manifestation consists of lymphadenopathy development; it has an aggressive behavior, leading to death in few years. The third variant is “iatrogenic”, due to HHV-8 activation following the administration of immunosuppressive drugs for autoimmune disorders or in transplant recipients. The most frequently encountered type is acquired immune deficiency syndrome (AIDS)-related KS, which has an aggressive behavior and the highest rate of hepatic involvement of all variants[111].
The most frequent manifestation of KS is cutaneous papular lesions located in the lower limbs, oral cavity and genitalia; among visceral locations, the gastrointestinal tract is most often encountered. Moritz Kaposi was the first to describe a hepatic KS (1872) at an autopsy report.
In the United States, HHV-8 accounts for approximately 5% of HIV-uninfected men compared with 25%-60% of HIV-positive men having sex with men. Patients with AIDS present a 20000 higher risk of developing KS than the general population. After the introduction of antiretroviral therapy, an 80% decrease in the AIDS-related KS incidence was observed, presently accounting for less than 1% of AIDS patients[112,113].
Because it is mostly asymptomatic, the real incidence of liver involvement of KS is difficult to assess; autopsy series have reported the prevalence of hepatic involvement ranging between 8.3% and 34% in AIDS-related tumors. In the study, KS was reported in liver biopsies in 18.6% of AIDS cases, representing the most frequent histological diagnosis made in AIDS patients. In the non-HIV infected population, although there is a very well-known burden of posttransplant cases of proliferative disorders, including KS, no case of KS with liver involvement has been reported to date[114,115].
HHV-8 demonstrates high tropism for hematopoietic cells, monocytes, B lymphocytes, hepatocytes and endothelial cells, the latter undergoing oncogenic transformation under the effect of this virus. The active lytic infection implies viral replication and activation of numerous genes and may be induced by many triggers, including HIV infection[116,117].
In the case of hepatocyte infection, positive cellular immunohistochemistry for latency-associated nuclear antigen-1 (LANA-1) can be detected; the virus also demonstrated a direct oncogenic potential because of its ability to bind to the tumor-suppression protein p53; moreover, several kinases, such as hepatocyte growth factor, promote KS development by inducing viral lytic replication[118-120].
The tumor affects portal areas, but it can also infiltrate into the liver parenchyma from the vicinity.
Macroscopically, it is characterized by irregular multiple red-brown spongiform masses of various sizes disseminated throughout the liver.
Histologically, hepatic KS comprises tumor spindle cells, characteristic of angioproliferative HHV-8-infected cells undergoing malignant transformation, separated by slit-like vascular channels, and inflammatory cells including mononuclear and hemosiderin-laden macrophages[121].
Tumor cells characteristically express HHV-8 LANA, endothelial markers such as CD31, CD34 and factor VIII, as well as lymphatic vessel endothelial receptor-1[122].
The differential diagnosis of KS is made with angiosarcoma. Bacillary angiomatosis develops similar to KS, in patients with AIDS due to Bartonella quintana/henselae infection, which can be demonstrated ultrastucturally using Warthin-Starry stain or by polymerase chain reaction.
HHV-8 DNA detection is characteristic for KS.
Usually, hepatic KS is asymptomatic and rarely diagnosed during life. Additionally, liver function tests are generally close to normal in cases of hepatic involvement. The literature data have described few patients with clinically significant liver involvement, associated with a rapid evolution toward acute liver failure, multiorgan failure and death[123,124].
Typical imaging findings may help define clinically significant hepatic KS. Abdominal US may show the presence of a heterogeneous cystic lesion, with the presence of solid areas and hyperechoic strands surrounding peripheral portal branches. CT scan reveals an inhomogeneous liver structure, with multiple hypodense scattered small-sized nodules that are mostly located in periportal regions. On MRI imaging, these nodules appear hypointense on T1-weighted in-phase scanning and hyperintense on T1-weighted out-of-phase scanning, without demonstrating any other specific findings on other phases of examination[125,126].
Biopsy from hepatic nodules in cases of liver involvement suspicion may demonstrate the presence of hyaline globules, hemosiderin depositions, enlarged portal spaces with fibrotic changes, macrovesicular steatosis, the formation of new bile ducts, and typical spindle-shaped tumor cells showing large vesicular nuclei, expressing positive immunostaining for endothelial markers.
If KS is suspected, extensive examination of the teguments, including oral and rectal regions, should be performed. Visceral or cutaneous biopsy reporting typical histological and immunohistochemical features is required to confirm the diagnosis. Visceral involvement needs further investigation in cases of fecal occult bleeding or the presence of adenopathies. If the patients present, besides cutaneous KS, iron deficiency anemia, fecal occult bleeding or digestive manifestations, they require gastrointestinal endoscopy; in cases of adenopathies, the patients should undergo a complete CT scan of the thorax and abdomino-pelvic region.
In cases of KS, the TNM staging system cannot predict accurately the prognosis and guide the management of patients. Therefore, the Aids Clinical Trials Group (ACTG) Oncology Committee has elaborated a staging system that stratifies patients with AIDS-associated KS into low and high risk, respectively, based on three criteria: tumor burden (T) (presence of extensive oral, cutaneous or visceral involvement), immune status (I) (defined by CD4 count), and systemic illness (S) (defined by general symptoms and performance status)[127].
Patients with AIDS-associated KS demonstrate a higher mortality risk than HIV patients. Although the initiation of antiretroviral (ARV) HIV treatment usually leads to progression of KS lesions, long-term treatment determines the decrease in tumor incidence. Because the degree of tumor control seems to be related to the degree of HIV control, current guidelines recommend the treatment of underlying immunodeficiency using ARV therapy. In cases of extensive cutaneous or symptomatic visceral involvement and the presence of immune reconstitution inflammatory syndrome (IRIS), patients need the addition of systemic treatments. Standard radiotherapy, electron beam radiation therapy, and the application of retinoid products represent therapeutic modalities associated with good responses for cutaneous lesions[128,129].
A meta-analysis demonstrated that systemic chemotherapy can reduce the progression of KS, although without demonstrating a statistically significant impact on survival[130]. First-line treatment for advanced disease consists of liposomal anthracyclines, such as pegylated liposomal doxorubicin, which have proven to be associated with significant better response rates vs classical chemotherapy (58.7% vs 23.3%), and slight improvement in survival. Second-line treatment includes the administration of paclitaxel, which is associated with response rates of approximately 59%-71%. Third-line agents include etoposide, vincristine, vinblastine and bleomycin, which are associated with response rates of 23%-36%, median survival periods of 11-13 mo, and significant side effects[131-134].
Interferon-alpha seems to detain some efficiency in the treatment of AIDS-related KS due to its antiviral and antiangiogenic actions, but its use is limited because of hepatotoxicity. Novel approaches have also been explored, such as antiangiogenic agents (e.g., the anti-VEGF monoclonal antibody bevacizumab), cytokines (e.g., IL-12), matrix metalloproteinases or immunotherapy (e.g., cytotoxic T-lymphocyte antigen 4 antibody ipilimumab, and the antibody against programmed cell death 1 nivolumab), and their roles in the treatment of KS are being currently investigated in different phases of clinical trials. In a small-sized phase II clinical study, approximately one-third of cases presented partial responses under imatinib treatment[135].
Treatment using HHV-8 replication inhibitors such as foscarnet and ganciclovir has also been investigated. HHV-8 infection may present long-term remissions under specific treatment, but therapy is indicated only in patients with progressive hepatic disease. On the other hand, ARV treatment in coinfected patients (HHV-8 + HIV) may determine worsening of the disease as shown in the KS AIDS AntiRetroviral Therapy trial, in which approximately 21% of patients presented rapid progression of the disease, by developing KS-IRIS[136,137].
HSVNs are recently characterized, rare, benign or low-grade malignancy vascular neoplasms of the liver, encompassing small vessels with an infiltrative border, without diagnostic features of cavernous hemangioma or HAS. The differentiation between benign and malignant vascular tumors is usually simple. Cavernous hemangioma is a benign vascular tumor with a well-circumscribed macroscopic aspect, diagnosed histologically by its typically large vascular spaces delineated by uniform endothelial cells and fibrous septa found in the background, while malignant angiosarcoma is constituted by scarce stroma and the tendency to infiltrate into hepatic sinusoids and to displace hepatic plates. HSVNs are vascular tumors of uncertain malignant potential that have an infiltrative growth pattern; therefore, they may be confused with HAS but show less pronounced cytologic atypia.
The study of Gill et al[138] reported that the mean age for HSVN patients was 54 years, with a marked male predominance.
If this tumor is considered to have a behavior resembling cavernous hemangioma, then future studies could investigate other possible genetic factors and associations with estrogen and several autoimmune diseases. HAS is associated with exposure to specific toxins and drugs (as we have previously described), but most cases are idiopathic in nature. Studies have found no specific associations with previous toxins or drug exposures in cases of HSVN.
Macroscopically, features of HSVN demonstrate the presence of a poorly circumscribed nonencapsulated hemorrhagic tumor, with a pale to brown color on sectioning, without cystic degeneration or macroscopically visible vessels.
Histologically, HSVN is an infiltrative neoplasia formed by thin-walled small vascular spaces with the detection of intraluminal erythrocytes and rarely thrombosis, delineated by flat-oval endothelial cells with a hobnail-like appearance, without features of multistratification, nuclear pleomorphism, mitotic figures or the presence of nucleoli. Hepatic parenchyma in the vicinity may show aspects of hepatocyte plate expansion and nodular-hyperplasia that may mimic hepatocellular carcinoma. The infiltrative tumor border may be better highlighted using immunohistochemical vascular markers.
Studies have shown positive and strong immunostaining for vascular markers (CD34, CD 31, and FLI-1) in HSVN cases. Immunohistochemical reactions for potential malignant behavior (GLUT-1, p53, Ki-67, c-myc) revealed a higher Ki-67 proliferative index vs. cavernous hemangioma but a significant lower Ki-67 proliferative index for HSVN than for HAS (3.7% vs 42.8%); therefore, this index proved to be a reliable discriminator between these two types of tumors. A cutoff value of 10% for the diagnosis of HAS was associated with a 100% accuracy for differentiating between HAS and HSVN. Moreover, intense positive nuclear p53 immunoreactions and positive GLUT-1 and c-myc immunostainings were detected only in cases of HAS[138].
HSVN may be mistaken for “anastomosing” hemangioma, HAS, or even hepatocellular carcinoma.
Molecular results following the sequencing of 510 genes showed that most HSVN cases present an activating hotspot GNAQ mutation due to a somatic p.Q209H mutation, expressing a clonal or neoplastic proliferation described also in cases of uveal melanoma and blue nevi. Additionally, an activating hotspot mutation in PIK3CA and a stop-gain mutation in AMER1 have been described. These molecular features have not been identified in cases of cavernous hemangioma. This GNAQ mutation (sometimes even associated with PIK3CA mutation) seems to be a crucial first step in HSVN tumorigenesis and malignant potential. A hotspot mutation in PIK3CA also represents an oncogenic mutation encountered in various cancers. HSVN did not show mutations or amplifications in genes associated with HAS[139,140].
Most of the cases are asymptomatic; usually, a unique liver mass is identified incidentally by imaging tests performed for another indication. Rarely, mild elevation of liver function tests, difficult to correlate directly with the presence of the tumor, is encountered.
Imaging methods do not show typical features in cases of HSVN because the aspect may range from an atypical vascular tumor to neuroendocrine neoplasia and even hepatocellular carcinoma; therefore, biopsy with histologic and immunohistochemical confirmation is needed for a definite diagnosis. Abdominal US examination may describe the presence of a hypoechoic and heterogenous tumor. On CEUS, the tumor presented intense early and homogeneous enhancement in the arterial phase that continued in the portal phase but was isoechoic in the delayed phase. MRI findings seem also to be nonspecific. Some cases present a pronounced expansion of hepatocyte plates in the vicinity of the tumor, an aspect that was reported in cases of hemangiomas, or the surrounding hepatic tissue may show focal-nodular hyperplasia-like alterations, resembling a hepatic nodule on imaging tests that must be differentiated from hepatocellular carcinoma[141].
The diagnosis needs a thorough histological examination, immunohistochemical and molecular testing. On small core biopsies, immunohistochemistry for potential malignant behavior, especially Ki-67, p53, and c-Myc, can aid in differentiating HSVN from HAS. Furthermore, molecular biology may be useful in the diagnosis and management of these cases; detection of an activating hotspot GNAQ mutation may reflect a recurrent genetic abnormality[142].
Although, due to their infiltrative behavior, HSVNs can mimic HAS, these tumors are considered benign or low-grade neoplasias because of the absence of cellular atypia or uncontrolled proliferation. Due to their rarity and scarcity of prognostic literature data, the true nature (benign vs low-grade neoplasia) of these tumors remains unclear. Although studies have detected no definite metastasis, there is no extended follow-up study of HSVN that enables definitive exclusion of latent metastasis development or tumor recurrence[138].
Although HSVN usually has a benign behavior and because of the current limited number of follow-up studies, infiltrative growth pattern of the tumor and molecular changes encountered also in several malignancies, resection and long- term follow-up of the tumor are recommended[143]. Main clinical-pathological characteristics of malignant vascular liver tumors are revealed in Table 1.
| Tumor type | HEHE | HAS | HPC | HPEComas-NOS | KS | HSVNs |
| Epidemiology | Very rare, 30-40 yr, F: M ratio 3:2 | 2% of primary hepatic neoplasms, 50-60 yr, M:F ratio 3-4:1 | Very rare, 40-50 yr, M:F ratio 1:1 | Extremely rare, mostly female | 8.3%-34% of AIDS-related tumors | Rare, mean age 54 yr, male predominance |
| Etiology | - | Thorotrast, vinyl chloride monomers and arsenical compounds exposure, radiation | - | - | HHV-8, HIV infection | - |
| Gross pathology | Multiple ill-defined firm, tan- to white-colored nodules | Multicentric infiltrative sponge-like hemorrhagic nodules | Well-circumscribed solitary lesion, with hemorrhage and cystic degeneration on sectioning | Pale tan, friable soft tumor | Multiple irregulat red-brown masses | Poorly circumscribed, single nonencapsulated hemorrhagic tumor |
| Histology | Epithelioid/spindle cells surrounded by myxoid stroma, presence of cytoplasmic vacuoles, intravascular tumor growth | Spindle-shaped/epithelioid tumor cells with ill-defined borders, frequent mitotic figures | Hypervascular tumor, spindle-shaped cells | Large epithelioid tumor cells surrounding small vessels | Spindle tumor cells, separated by slit-like vascular channels | Thin-walled small vascular spaces , delineated by hobnail-like endothelial cells, no mitotic figures |
| Immunohistochemical markers | CAMTA 1 expression, Ki-67 expression > 10% | ERG, VEGFR2 | Vimentin, S-100, muscle-specific actin, smooth muscle actin, CD 34 | gp 100 protein, HMSA-1, SMA, vimentin | HHV8-LANA 1 | CD34, CD31, FLI1, Ki-67 index < 10% |
| Molecular features | WWTR1-CAMTA1 and YAP1-TFE3 fusion genes | TP53, KRAS-2 mutations | 12q13-15 alterations in some cases | Genetic changes of the TSC genes | HHV8- DNA detection | Hotspot GNAQ, PIK3CA mutation |
| Clinical features | Oligosymptomatic → portal hypertension, venooclusive disease | Abdominal pain, weight loss, malaise, portal hypertension, hemoperitoneum | Asymptomatic → hemoperitoneum→ paraneoplastic syndromes (hypoglycemia) | Mostly asymptomatic → hemoperitoneum | Asymtomatic/oligosymptomatic | Mostly asymptomatic |
| Imaging US/CEUS | Hypoechoic heterogeneous mass/nodules | Heterogeneous echogenicity; CEUS: Central nonenhancement, irregular enhancement of the tumor periphery in arterial and portal phase, complete wash-out in the late phase | Hypoechoic, hypervascular tumors | Heterogeneous hypoechoic lesion | Heterogeneous cystic lesion, solid areas and hyperechoic strands surrounding peripheral portal branches | Hypoechoic and heterogenous tumor. ceus: Intense early and homogeneous enhancement in the arterial phase, continuing in the portal phase, isoechoic in delayed phase |
| Native CT scan | Extent of the tumor assessment, focal atrophy, retraction of the liver capsule | Hypoattenuating pattern of the tumor | Hypodense/ isodense tumor | Low-density mass | Inhomogeneous liver structure, multiple hypodense scattered small-sized nodules, mostly located in periportal regions | Nonspecific |
| Contrast-enhanced CT scan | Multiple hepatic bilobar hypoattenuating lesions; larger tumors: halo or target-type pattern of contrast enhancement (typically) | Hypodense lesions, various patterns of contrast enhancement; isodense after contrast administration; large tumors: heterogeneous structure, various patterns of early contrast enhancement ± focal irregular areas/ peripheral rim enhancement; arterioportal shunting | Lobulated tumor: Solid zones with contrast enhancement, cystic areas, with speckled calcifications | Heterogeneous and intense enhancement on arterial phase, slightly hypodense aspect on portal phase and enhancing rim on delayed phase | Nonspecific | Nonspecific |
| CT angiography | Nonspecific | Multiple/solitary hypervascular masses, heterogeneous early and progressive contrast enhancement | Highly vascular tumor | Nonspecific | Nonspecific | Nonspecific |
| MRI T1-weighted | Hypo-intense lesions | Heterogeneous hiperintense pattern | Hypo-intense lesion | Nonspecific | Hypointense on T1-weighted in-phase scanning and hyperintense on T1-weighted out-of-phase scanning | Nonspecific |
| MRI T2-weighted | Hyper-intense heterogeneous pattern | Hyper-intense heterogeneous pattern | Nonspecific | |||
| MRI DW | Variable | Not known | ||||
| Extracellular contrast MRI | Larger tumors: peripheral halo or target-type of enhancement, ± peripheral hypo-intense rim of avascular tissue | Mild enhancement in the early phase, progressive homogeneous enhancement, with complete tumor wash-out in delayed and parenchymal phase | Heterogeneous contrast enhancement | |||
| FDG PET | Variable uptake | Increased uptake | Increased uptake | Controversial results | Nonspecific | Nonspecific |
| Prognosis | 75% 5-yr survival rate following surgery | Very poor, 2-yr survival rate under treatment < 3% | 50% 5-yr disease free survival after Ro surgery | Not very clearly defined | Reserved | Benign/low-grade neoplasia – currently, prognosis not well defined |
| Treatment | Liver resection Liver transplantation; TACE; Chemotherapy, antiangiogenic agents | Tumor/hepatic resection; Liver transplantation contraindicated; Adjuvant/palliative chemotherapy, antiangiogenic agents, immunotherapy | Aggressive surgery; Radiotherapy, chemotherapy, antiangiogenic treatment | Follow-up; Surgical resection Chemo-, radiotherapy, mTOR inhibitors, immunotherapy; SRBT, TAE, RFA | ARV HIV treatment; Systemic chemotherapy; Novel targeted treatments under study; HHV-8 replication inhibitors | Resection and long-term follow-up |
Malignant vascular tumors of the liver frequently raise diagnostic challenges due to their low incidence, lack of clinical awareness and their clinical, imaging and histological variability. Differentiation from other tumors is mainly based on histology and typical immunohistochemical features, if needed in conjunction with molecular studies.
In cases of HEHE, surgery, including liver resection or LT, represents the mainstay of treatment, reaching 5-year PS rates after transplantation of approximately 81%. Posttransplantation recurrences are frequently encountered and should always be managed aggressively. The risk of developing recurrences may be estimated using prognostic scores. In patients with a high risk of recurrence, further addition in the treatment algorithm of novel antiangiogenic or other molecular targeted agents would be beneficial.
For HAS, the mainstay of treatment consists of radical tumor resection or hepatic resection. Due to dismal results after LT, HAS is considered an absolute con-traindication to LT. Because its poor prognosis, there is an urgent need to improve the survival of patients with HAS by finding the most appropriate treatment in accordance with tumor biology, using specific anti-VEGF, or other antiangiogenic or vascular-targeted agents, cytokines, immunotherapy with or without standard chemotherapy.
In cases of localized HPC, surgery is the elective treatment, with 10-year overall survival rates between 54% and 89% after radical resection. Isolated cases of HPC have been reported that have undergone LT to improve the quality of life. The management of unresectable tumors includes radio- or chemotherapy, unfortunately only rarely associated with tumor responses. On the other hand, treatment using anti-VEGF agents has shown promising results.
The mainstay of treatment in hepatic PEComas-NOS is represented by radical resection of the hepatic primary tumor followed by surveillance. Some reports have described positive results in cases of metastatic PEComas treated with mTOR inhibitors.
For patients with AIDS-related KS, current guidelines recommend the treatment of underlying immunodeficiency using antiretroviral therapy. The literature data have shown that systemic chemotherapy can reduce the progression of KS but without reaching a statistically significant impact on PS.
Due to their low incidence and few literature data, the true nature of HSVN is still debated; therefore, a longer follow-up is needed to clarify its potentially malignant behavior.
Tailored treatment algorithms according to the histology and immuno-histochemistry, as well as the molecular features and genetics of the primary malignant vascular tumors of the liver, are needed in the near future to improve PS and the quality of life.
Manuscript source: Invited manuscript
Specialty type: Oncology
Country of origin: Romania
Peer-review report classification
Grade A (Excellent): 0
Grade B (Very good): B
Grade C (Good): C
Grade D (Fair): D
Grade E (Poor): 0
P- Reviewer: Liu G, Patel HRH, Soldera J S- Editor: Ji FF L- Editor: A E- Editor: Wu YXJ
| 1. | Lerut J, Iesari S. Vascular tumours of the liver: a particular story. Transl Gastroenterol Hepatol. 2018;3:62. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 17] [Cited by in RCA: 13] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 2. | Theodosopoulos T, Dellaportas D, Tsangkas A, Tsangkas N, Psychogiou V, Yiallourou A, Polymeneas G, Kondi-Pafiti A. Clinicopathological features and management of hepatic vascular tumors. A 20-year experience in a Greek University Hospital. J BUON. 2013;18:1026-1031. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 1] [Article Influence: 0.1] [Reference Citation Analysis (0)] |
| 3. | Weiss SW, Enzinger FM. Epithelioid hemangioendothelioma: a vascular tumor often mistaken for a carcinoma. Cancer. 1982;50:970-981. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 1] [Reference Citation Analysis (0)] |
| 4. | Lau K, Massad M, Pollak C, Rubin C, Yeh J, Wang J, Edelman G, Yeh J, Prasad S, Weinberg G. Clinical patterns and outcome in epithelioid hemangioendothelioma with or without pulmonary involvement: insights from an internet registry in the study of a rare cancer. Chest. 2011;140:1312-1318. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 140] [Cited by in RCA: 177] [Article Influence: 12.6] [Reference Citation Analysis (1)] |
| 5. | Ishak KG, Sesterhenn IA, Goodman ZD, Rabin L, Stromeyer FW. Epithelioid hemangioendothelioma of the liver: a clinicopathologic and follow-up study of 32 cases. Hum Pathol. 1984;15:839-852. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 341] [Cited by in RCA: 284] [Article Influence: 6.9] [Reference Citation Analysis (0)] |
| 6. | Hsieh MS, Liang PC, Kao YC, Shun CT. Hepatic epithelioid hemangioendothelioma in Taiwan: a clinicopathologic study of six cases in a single institution over a 15-year period. J Formos Med Assoc. 2010;109:219-227. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 15] [Cited by in RCA: 16] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 7. | Sardaro A, Bardoscia L, Petruzzelli MF, Portaluri M. Epithelioid hemangioendothelioma: an overview and update on a rare vascular tumor. Oncol Rev. 2014;8:259. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 100] [Cited by in RCA: 142] [Article Influence: 12.9] [Reference Citation Analysis (0)] |
| 8. | Hu HJ, Jin YW, Jing QY, Shrestha A, Cheng NS, Li FY. Hepatic epithelioid hemangioendothelioma: Dilemma and challenges in the preoperative diagnosis. World J Gastroenterol. 2016;22:9247-9250. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 16] [Cited by in RCA: 15] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 9. | Treska V, Daum O, Svajdler M, Liska V, Ferda J, Baxa J. Hepatic Epithelioid Hemangioendothelioma - a Rare Tumor and Diagnostic Dilemma. In Vivo. 2017;31:763-767. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 18] [Cited by in RCA: 21] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 10. | Miettinen M, Rikala MS, Rys J, Lasota J, Wang ZF. Vascular endothelial growth factor receptor 2 as a marker for malignant vascular tumors and mesothelioma: an immunohistochemical study of 262 vascular endothelial and 1640 nonvascular tumors. Am J Surg Pathol. 2012;36:629-639. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 79] [Cited by in RCA: 82] [Article Influence: 6.3] [Reference Citation Analysis (0)] |
| 11. | Mistry AM, Gorden DL, Busler JF, Coogan AC, Kelly BS. Diagnostic and therapeutic challenges in hepatic epithelioid hemangioendothelioma. J Gastrointest Cancer. 2012;43:521-525. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 28] [Cited by in RCA: 23] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 12. | Flucke U, Vogels RJ, de Saint Aubain Somerhausen N, Creytens DH, Riedl RG, van Gorp JM, Milne AN, Huysentruyt CJ, Verdijk MA, van Asseldonk MM, Suurmeijer AJ, Bras J, Palmedo G, Groenen PJ, Mentzel T. Epithelioid Hemangioendothelioma: clinicopathologic, immunhistochemical, and molecular genetic analysis of 39 cases. Diagn Pathol. 2014;9:131. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 149] [Cited by in RCA: 174] [Article Influence: 15.8] [Reference Citation Analysis (0)] |
| 13. | Mehrabi A, Kashfi A, Fonouni H, Schemmer P, Schmied BM, Hallscheidt P, Schirmacher P, Weitz J, Friess H, Buchler MW, Schmidt J. Primary malignant hepatic epithelioid hemangioendothelioma: a comprehensive review of the literature with emphasis on the surgical therapy. Cancer. 2006;107:2108-2121. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 388] [Cited by in RCA: 308] [Article Influence: 16.2] [Reference Citation Analysis (1)] |
| 14. | Makhlouf HR, Ishak KG, Goodman ZD. Epithelioid hemangioendothelioma of the liver: a clinicopathologic study of 137 cases. Cancer. 1999;85:562-582. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 1] [Reference Citation Analysis (0)] |
| 15. | Demetris AJ, Minervini M, Raikow RB, Lee RG. Hepatic epithelioid hemangioendothelioma: biological questions based on pattern of recurrence in an allograft and tumor immunophenotype. Am J Surg Pathol. 1997;21:263-270. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 55] [Cited by in RCA: 56] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 16. | Doyle LA, Fletcher CD, Hornick JL. Nuclear Expression of CAMTA1 Distinguishes Epithelioid Hemangioendothelioma From Histologic Mimics. Am J Surg Pathol. 2016;40:94-102. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 169] [Cited by in RCA: 173] [Article Influence: 19.2] [Reference Citation Analysis (0)] |
| 17. | Shibuya R, Matsuyama A, Shiba E, Harada H, Yabuki K, Hisaoka M. CAMTA1 is a useful immunohistochemical marker for diagnosing epithelioid haemangioendothelioma. Histopathology. 2015;67:827-835. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 75] [Cited by in RCA: 80] [Article Influence: 8.0] [Reference Citation Analysis (0)] |
| 18. | Bioulac-Sage P, Laumonier H, Laurent C, Blanc JF, Balabaud C. Benign and malignant vascular tumors of the liver in adults. Semin Liver Dis. 2008;28:302-314. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 96] [Cited by in RCA: 98] [Article Influence: 5.8] [Reference Citation Analysis (0)] |
| 19. | Briongos-Figuero LS, Zamora-Martínez T. A rare vascular tumor of the liver. Clin Gastroenterol Hepatol. 2015;13:e46-e47. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 1] [Article Influence: 0.1] [Reference Citation Analysis (0)] |
| 20. | Ehman EC, Torbenson MS, Wells ML, Welch BT, Thompson SM, Garg I, Venkatesh SK. Hepatic tumors of vascular origin: imaging appearances. Abdom Radiol (NY). 2018;43:1978-1990. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 7] [Cited by in RCA: 6] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 21. | Lai Q, Feys E, Karam V, Adam R, Klempnauer J, Oliverius M, Mazzaferro V, Pascher A, Remiszewski P, Isoniemi H, Pirenne J, Foss A, Ericzon BG, Markovic S, Lerut JP; European Liver Intestine Transplant Association (ELITA). Hepatic Epithelioid Hemangioendothelioma and Adult Liver Transplantation: Proposal for a Prognostic Score Based on the Analysis of the ELTR-ELITA Registry. Transplantation. 2017;101:555-564. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 84] [Cited by in RCA: 69] [Article Influence: 8.6] [Reference Citation Analysis (0)] |
| 22. | Lerut JP, Weber M, Orlando G, Dutkowski P. Vascular and rare liver tumors: a good indication for liver transplantation? J Hepatol. 2007;47:466-475. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 23] [Cited by in RCA: 16] [Article Influence: 0.9] [Reference Citation Analysis (1)] |
| 23. | Jung DH, Hwang S, Hong SM, Kim KH, Lee YJ, Ahn CS, Moon DB, Ha TY, Song GW, Park GC, Yu E, Lee SG. Clinicopathological Features and Prognosis of Hepatic Epithelioid Hemangioendothelioma After Liver Resection and Transplantation. Ann Transplant. 2016;21:784-790. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 13] [Cited by in RCA: 16] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 24. | Galvão FH, Bakonyi-Neto A, Machado MA, Farias AQ, Mello ES, Diz ME, Machado MC. Interferon alpha-2B and liver resection to treat multifocal hepatic epithelioid hemangioendothelioma: a relevant approach to avoid liver transplantation. Transplant Proc. 2005;37:4354-4358. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 32] [Cited by in RCA: 24] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 25. | Cardinal J, de Vera ME, Marsh JW, Steel JL, Geller DA, Fontes P, Nalesnik M, Gamblin TC. Treatment of hepatic epithelioid hemangioendothelioma: a single-institution experience with 25 cases. Arch Surg. 2009;144:1035-1039. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 43] [Cited by in RCA: 46] [Article Influence: 3.1] [Reference Citation Analysis (0)] |
| 26. | Rodriguez JA, Becker NS, O'Mahony CA, Goss JA, Aloia TA. Long-term outcomes following liver transplantation for hepatic hemangioendothelioma: the UNOS experience from 1987 to 2005. J Gastrointest Surg. 2008;12:110-116. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 86] [Cited by in RCA: 80] [Article Influence: 4.7] [Reference Citation Analysis (0)] |
| 27. | Desie N, Van Raemdonck DE, Ceulemans LJ, Nevens F, Verslype C, Vansteenbergen W, Pirenne J, Monbaliu D, Roskams T, Verbeken EK, Neyrinck AP, Dupont LJ, Yserbyt J, Verleden GM, Vos R. Combined or Serial Liver and Lung Transplantation for Epithelioid Hemangioendothelioma: A Case Series. Am J Transplant. 2015;15:3247-3254. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 11] [Cited by in RCA: 13] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 28. | Semenisty V, Naroditsky I, Keidar Z, Bar-Sela G. Pazopanib for metastatic pulmonary epithelioid hemangioendothelioma-a suitable treatment option: case report and review of anti-angiogenic treatment options. BMC Cancer. 2015;15:402. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 46] [Cited by in RCA: 50] [Article Influence: 5.0] [Reference Citation Analysis (0)] |
| 29. | Chevreau C, Le Cesne A, Ray-Coquard I, Italiano A, Cioffi A, Isambert N, Robin YM, Fournier C, Clisant S, Chaigneau L, Bay JO, Bompas E, Gauthier E, Blay JY, Penel N. Sorafenib in patients with progressive epithelioid hemangioendothelioma: a phase 2 study by the French Sarcoma Group (GSF/GETO). Cancer. 2013;119:2639-2644. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 77] [Cited by in RCA: 89] [Article Influence: 7.4] [Reference Citation Analysis (0)] |
| 30. | Agulnik M, Yarber JL, Okuno SH, von Mehren M, Jovanovic BD, Brockstein BE, Evens AM, Benjamin RS. An open-label, multicenter, phase II study of bevacizumab for the treatment of angiosarcoma and epithelioid hemangioendotheliomas. Ann Oncol. 2013;24:257-263. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 243] [Cited by in RCA: 290] [Article Influence: 22.3] [Reference Citation Analysis (0)] |
| 31. | Soape MP, Verma R, Payne JD, Wachtel M, Hardwicke F, Cobos E. Treatment of Hepatic Epithelioid Hemangioendothelioma: Finding Uses for Thalidomide in a New Era of Medicine. Case Rep Gastrointest Med. 2015;2015:326795. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 7] [Cited by in RCA: 9] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 32. | Pallotti MC, Nannini M, Agostinelli C, Leoni S, Scioscio VD, Mandrioli A, Lolli C, Saponara M, Pileri S, Bolondi L, Biasco G, Pantaleo MA. Long-term durable response to lenalidomide in a patient with hepatic epithelioid hemangioendothelioma. World J Gastroenterol. 2014;20:7049-7054. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 16] [Cited by in RCA: 22] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 33. | Stiles JM, Amaya C, Rains S, Diaz D, Pham R, Battiste J, Modiano JF, Kokta V, Boucheron LE, Mitchell DC, Bryan BA. Targeting of beta adrenergic receptors results in therapeutic efficacy against models of hemangioendothelioma and angiosarcoma. PLoS One. 2013;8:e60021. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 69] [Cited by in RCA: 74] [Article Influence: 6.2] [Reference Citation Analysis (0)] |
| 34. | Lakkis Z, Kim S, Delabrousse E, Jary M, Nguyen T, Mantion G, Heyd B, Lassabe C, Borg C. Metronomic cyclophosphamide: an alternative treatment for hepatic epithelioid hemangioendothelioma. J Hepatol. 2013;58:1254-1257. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 27] [Cited by in RCA: 24] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 35. | Chaudhary P, Bhadana U, Singh RA, Ahuja A. Primary hepatic angiosarcoma. Eur J Surg Oncol. 2015;41:1137-1143. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 53] [Cited by in RCA: 86] [Article Influence: 8.6] [Reference Citation Analysis (0)] |
| 36. | Coulier B, Pierard F, Gielen I, Maldague P. Hepatic angiosarcoma occurring 65 years after thorium dioxide (Thorotrast) exposure: imaging, surgical and histo- pathologic findings of a historical case. JBR-BTR. 2014;97:254-258. [PubMed] |
| 37. | Collins JJ, Jammer B, Sladeczek FM, Bodnar CM, Salomon SS. Surveillance for angiosarcoma of the liver among vinyl chloride workers. J Occup Environ Med. 2014;56:1207-1209. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 9] [Cited by in RCA: 11] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 38. | Huang NC, Wann SR, Chang HT, Lin SL, Wang JS, Guo HR. Arsenic, vinyl chloride, viral hepatitis, and hepatic angiosarcoma: a hospital-based study and review of literature in Taiwan. BMC Gastroenterol. 2011;11:142. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 57] [Cited by in RCA: 49] [Article Influence: 3.5] [Reference Citation Analysis (0)] |
| 39. | Wang ZB, Wei LX. [Primary hepatic angiosarcoma: a clinical and pathological analysis]. Zhonghua Bing Li Xue Za Zhi. 2013;42:376-380. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 6] [Reference Citation Analysis (0)] |
| 40. | Ito Y, Kojiro M, Nakashima T, Mori T. Pathomorphologic characteristics of 102 cases of thorotrast-related hepatocellular carcinoma, cholangiocarcinoma, and hepatic angiosarcoma. Cancer. 1988;62:1153-1162. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 1] [Reference Citation Analysis (0)] |
| 41. | Miettinen M, Wang ZF, Paetau A, Tan SH, Dobi A, Srivastava S, Sesterhenn I. ERG transcription factor as an immunohistochemical marker for vascular endothelial tumors and prostatic carcinoma. Am J Surg Pathol. 2011;35:432-441. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 251] [Cited by in RCA: 253] [Article Influence: 18.1] [Reference Citation Analysis (0)] |
| 42. | Hollstein M, Marion MJ, Lehman T, Welsh J, Harris CC, Martel-Planche G, Kusters I, Montesano R. p53 mutations at A:T base pairs in angiosarcomas of vinyl chloride-exposed factory workers. Carcinogenesis. 1994;15:1-3. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 139] [Cited by in RCA: 126] [Article Influence: 4.1] [Reference Citation Analysis (0)] |
| 43. | Przygodzki RM, Finkelstein SD, Keohavong P, Zhu D, Bakker A, Swalsky PA, Soini Y, Ishak KG, Bennett WP. Sporadic and Thorotrast-induced angiosarcomas of the liver manifest frequent and multiple point mutations in K-ras-2. Lab Invest. 1997;76:153-159. [PubMed] [DOI] [Full Text] |
| 44. | Zhu YP, Chen YM, Matro E, Chen RB, Jiang ZN, Mou YP, Hu HJ, Huang CJ, Wang GY. Primary hepatic angiosarcoma: A report of two cases and literature review. World J Gastroenterol. 2015;21:6088-6096. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 49] [Cited by in RCA: 51] [Article Influence: 5.1] [Reference Citation Analysis (0)] |
| 45. | Leowardi C, Hormann Y, Hinz U, Wente MN, Hallscheidt P, Flechtenmacher C, Buchler MW, Friess H, Schwarzbach MH. Ruptured angiosarcoma of the liver treated by emergency catheter-directed embolization. World J Gastroenterol. 2006;12:804-808. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 40] [Cited by in RCA: 35] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 46. | Alves VAF, Rimola J. Malignant Vascular Tumors of the Liver in Adults. Semin Liver Dis. 2019;39:1-12. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5] [Cited by in RCA: 6] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 47. | Wong JW, Bedard YC. Fine-needle aspiration biopsy of hepatic angiosarcoma: report of a case with immunocytochemical findings. Diagn Cytopathol. 1992;8:380-383. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 22] [Cited by in RCA: 22] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 48. | Wang L, Lv K, Chang XY, Xia Y, Yang ZY, Jiang YX, Dai Q, Tan L, Li JC. Contrast-enhanced ultrasound study of primary hepatic angiosarcoma: a pitfall of non-enhancement. Eur J Radiol. 2012;81:2054-2059. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 25] [Cited by in RCA: 23] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 49. | Park YS, Kim JH, Kim KW, Lee IS, Yoon HK, Ko GY, Sung KB. Primary hepatic angiosarcoma: imaging findings and palliative treatment with transcatheter arterial chemoembolization or embolization. Clin Radiol. 2009;64:779-785. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 58] [Cited by in RCA: 60] [Article Influence: 3.8] [Reference Citation Analysis (0)] |
| 50. | Kamatani T, Iguchi H, Okada T, Yamazaki H, Tsunoda H, Watanabe M, Oda M, Ohbu M, Yokomori H. Co-registered positron emission tomography/computed tomography and gadolinium-ethoxybenzyl-diethylenetriamine pentaacetic acid magnetic resonance imaging features of multiple angiosarcoma of the liver. Hepatol Res. 2014;44:E297-E303. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 8] [Cited by in RCA: 8] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 51. | Bruegel M, Muenzel D, Waldt S, Specht K, Rummeny EJ. Hepatic angiosarcoma: cross-sectional imaging findings in seven patients with emphasis on dynamic contrast-enhanced and diffusion-weighted MRI. Abdom Imaging. 2013;38:745-754. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 33] [Cited by in RCA: 38] [Article Influence: 3.2] [Reference Citation Analysis (0)] |
| 52. | Maeda T, Tateishi U, Hasegawa T, Ojima H, Arai Y, Sugimura K. Primary hepatic angiosarcoma on coregistered FDG PET and CT images. AJR Am J Roentgenol. 2007;188:1615-1617. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 36] [Cited by in RCA: 34] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 53. | Li DB, Si XY, Wan T, Zhou YM. A pooled analysis of treatment and prognosis of hepatic angiosarcoma in adults. Hepatobiliary Pancreat Dis Int. 2018;17:198-203. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 11] [Cited by in RCA: 16] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 54. | Lin YH, Lin CC, Concejero AM, Yong CC, Kuo FY, Wang CC. Surgical experience of adult primary hepatic sarcomas. World J Surg Oncol. 2015;13:87. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 24] [Cited by in RCA: 28] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 55. | Orlando G, Adam R, Mirza D, Soderdahl G, Porte RJ, Paul A, Burroughs AK, Seiler CA, Colledan M, Graziadei I, Garcia Valdecasas JC, Pruvot FR, Karam V, Lerut J. Hepatic hemangiosarcoma: an absolute contraindication to liver transplantation--the European Liver Transplant Registry experience. Transplantation. 2013;95:872-877. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 80] [Cited by in RCA: 60] [Article Influence: 5.0] [Reference Citation Analysis (0)] |
| 56. | Husted TL, Neff G, Thomas MJ, Gross TG, Woodle ES, Buell JF. Liver transplantation for primary or metastatic sarcoma to the liver. Am J Transplant. 2006;6:392-397. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 55] [Cited by in RCA: 49] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 57. | Huang NC, Kuo YC, Chiang JC, Hung SY, Wang HM, Hung YM, Chang YT, Wann SR, Chang HT, Wang JS, Ho SY, Guo HR. Hepatic angiosarcoma may have fair survival nowadays. Medicine (Baltimore). 2015;94:e816. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 23] [Cited by in RCA: 20] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 58. | Kim HR, Rha SY, Cheon SH, Roh JK, Park YN, Yoo NC. Clinical features and treatment outcomes of advanced stage primary hepatic angiosarcoma. Ann Oncol. 2009;20:780-787. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 71] [Cited by in RCA: 70] [Article Influence: 4.4] [Reference Citation Analysis (1)] |
| 59. | Asmane I, Litique V, Heymann S, Marcellin L, Métivier AC, Duclos B, Bergerat JP, Kurtz JE. Adriamycin, cisplatin, ifosfamide and paclitaxel combination as front-line chemotherapy for locally advanced and metastatic angiosarcoma. Analysis of three case reports and review of the literature. Anticancer Res. 2008;28:3041-3045. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5] [Cited by in RCA: 5] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 60. | Pierce DB, Johnson GE, Monroe E, Loggers ET, Jones RL, Pollack SM, Padia SA. Safety and Efficacy Outcomes of Embolization in Hepatic Sarcomas. AJR Am J Roentgenol. 2018;210:175-182. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 12] [Cited by in RCA: 18] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 61. | Young RJ, Woll PJ. Anti-angiogenic therapies for the treatment of angiosarcoma: a clinical update. Memo. 2017;10:190-193. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 14] [Cited by in RCA: 23] [Article Influence: 2.9] [Reference Citation Analysis (0)] |
| 62. | Mitsui F, Aikata H, Hashimoto Y, Nagaoki Y, Kimura Y, Katamura Y, Kawaoka T, Takaki S, Hiraga N, Tsuge M, Waki K, Hiramatsu A, Imamura M, Kawakami Y, Takahashi S, Arihiro K, Chayama K. A first case of hepatic angiosarcoma treated with recombinant interleukin-2. Hiroshima J Med Sci. 2011;60:91-96. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 3] [Article Influence: 0.2] [Reference Citation Analysis (0)] |
| 63. | Sindhu S, Gimber LH, Cranmer L, McBride A, Kraft AS. Angiosarcoma treated successfully with anti-PD-1 therapy - a case report. J Immunother Cancer. 2017;5:58. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 65] [Cited by in RCA: 90] [Article Influence: 11.3] [Reference Citation Analysis (0)] |
| 64. | Pandiaraja J, Subramanian V. A Case of Primary Hemangiopericytoma of Liver. J Med Sci. 2015;35:169-172. [RCA] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 2] [Article Influence: 0.2] [Reference Citation Analysis (0)] |
| 65. | Guillou L, Fletcher JA, Fletcher CD, Mandahl N, Fletcher CDM, Unni KK, Mertens F. Extrapleural solitary fibrous tumor and hemangiopericytoma. Fletcher CDM, Unni KK, Mertens F. WHO classification of tumours: Pathology and genetics of tumours of soft tissue and bone. Lyon: IARC Press 2002; 86-90. |
| 66. | Lehmann C, Mourra N, Tubiana JM, Arrivé L. [Solitary fibrous tumor of the liver]. J Radiol. 2006;87:139-142. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 14] [Cited by in RCA: 14] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 67. | Liang YM, Li XH, Lü YL, Zhong M. [Morphology and immunohistochemical characteristics of hepatic primary and metastatic malignant spindle cell tumors]. Zhonghua Yi Xue Za Zhi. 2005;85:96-100. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 1] [Reference Citation Analysis (0)] |
| 68. | Zimmermann A. Solitary fibrous tumor/hemangiopericytoma of the liver. In: Tumors and tumor-like lesions of the hepatobiliary tract. Springer, Cham. 2017;1057-1067. [DOI] [Full Text] |
| 69. | Caruso S, Gruttadauria S, Minervini MI, Miraglia R, Milazzo M, Luca A, Gridelli B. Primary hemangiopericytoma of the liver: sonographic findings. J Clin Ultrasound. 2009;37:305-307. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 8] [Cited by in RCA: 5] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 70. | Adams J, Lodge JP, Parker D. Liver transplantation for metastatic hemangiopericytoma associated with hypoglycemia. Transplantation. 1999;67:488-489. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 21] [Cited by in RCA: 17] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 71. | Flores-Stadler EM, Chou P, Walterhouse D, Bourtsos E, Gonzalez-Crussi F. Hemangiopericytoma of the liver: immunohistochemical observations, expression of angiogenic factors, and review of the literature. J Pediatr Hematol Oncol. 1997;19:449-454. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 10] [Cited by in RCA: 7] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 72. | Uemura S, Kuratsu J, Hamada J, Yoshioka S, Kochi M, Ushio Y, Nakahara T, Kishida K. Effect of radiation therapy against intracranial hemangiopericytoma. Neurol Med Chir (Tokyo). 1992;32:328-332. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 30] [Cited by in RCA: 28] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 73. | Beadle GF, Hillcoat BL. Treatment of advanced malignant hemangiopericytoma with combination adriamycin and DTIC: a report of four cases. J Surg Oncol. 1983;22:167-170. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 48] [Cited by in RCA: 45] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 74. | Galanis E, Buckner JC, Scheithauer BW, Kimmel DW, Schomberg PJ, Piepgras DG. Management of recurrent meningeal hemangiopericytoma. Cancer. 1998;82:1915-1920. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 1] [Reference Citation Analysis (0)] |
| 75. | Chamberlain MC, Glantz MJ. Sequential salvage chemotherapy for recurrent intracranial hemangiopericytoma. Neurosurgery. 2008;63:720-6; author reply 726-7. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 51] [Cited by in RCA: 56] [Article Influence: 3.5] [Reference Citation Analysis (0)] |
| 76. | Constantinidou A, Jones RL, Scurr M, Al-Muderis O, Judson I. Systemic therapy in solitary fibrous tumour [abstract #39407]. 15th Connective Tissue Oncologic Society Annual Meeting, Miami, FL. 2009;. |
| 77. | Park MS, Patel SR, Ludwig JA, Trent JC, Conrad CA, Lazar AJ, Wang WL, Boonsirikamchai P, Choi H, Wang X, Benjamin RS, Araujo DM. Activity of temozolomide and bevacizumab in the treatment of locally advanced, recurrent, and metastatic hemangiopericytoma and malignant solitary fibrous tumor. Cancer. 2011;117:4939-4947. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 198] [Cited by in RCA: 175] [Article Influence: 12.5] [Reference Citation Analysis (0)] |
| 78. | Casali PG, Stacchiotti S, Palassini E, Marrari A, Morosi C, Messina A. Evaluation of the antitumor activity of sunitinib malate (SM) in solitary fibrous tumor (SFT). J Clin Oncol. 2009;27 (15S); abstr 10571. |
| 79. | Maki RG, D'Adamo DR, Keohan ML, Saulle M, Schuetze SM, Undevia SD, Livingston MB, Cooney MM, Hensley ML, Mita MM, Takimoto CH, Kraft AS, Elias AD, Brockstein B, Blachère NE, Edgar MA, Schwartz LH, Qin LX, Antonescu CR, Schwartz GK. Phase II study of sorafenib in patients with metastatic or recurrent sarcomas. J Clin Oncol. 2009;27:3133-3140. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 475] [Cited by in RCA: 427] [Article Influence: 26.7] [Reference Citation Analysis (0)] |
| 80. | Bonetti F, Pea M, Martignoni G, Zamboni G. PEC and sugar. Am J Surg Pathol. 1992;16:307-308. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 296] [Cited by in RCA: 307] [Article Influence: 9.3] [Reference Citation Analysis (0)] |
| 81. | Folpe AL, Kwiatkowski DJ. Perivascular epithelioid cell neoplasms: pathology and pathogenesis. Hum Pathol. 2010;41:1-15. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 258] [Cited by in RCA: 264] [Article Influence: 16.5] [Reference Citation Analysis (0)] |
| 82. | Parfitt JR, Bella AJ, Izawa JI, Wehrli BM. Malignant neoplasm of perivascular epithelioid cells of the liver. Arch Pathol Lab Med. 2006;130:1219-1222. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 3] [Reference Citation Analysis (0)] |
| 83. | Bonetti F, Martignoni G, Colato C, Manfrin E, Gambacorta M, Faleri M, Bacchi C, Sin VC, Wong NL, Coady M, Chan JK. Abdominopelvic sarcoma of perivascular epithelioid cells. Report of four cases in young women, one with tuberous sclerosis. Mod Pathol. 2001;14:563-568. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 152] [Cited by in RCA: 149] [Article Influence: 6.2] [Reference Citation Analysis (0)] |
| 84. | Zhao LJ, Yang YJ, Wu H, Huang SM, Liu K. Perivascular epithelioid cell tumor of the liver: a case report and literature review. Eur Rev Med Pharmacol Sci. 2013;17:1665-1668. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 10] [Cited by in RCA: 13] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 85. | Harris GC, McCulloch TA, Perks G, Fisher C. Malignant perivascular epithelioid cell tumour ("PEComa") of soft tissue: a unique case. Am J Surg Pathol. 2004;28:1655-1658. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 49] [Cited by in RCA: 44] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 86. | Lehman NL. Malignant PEComa of the skull base. Am J Surg Pathol. 2004;28:1230-1232. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 39] [Cited by in RCA: 37] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 87. | Sadeghi S, Krigman H, Maluf H. Perivascular epithelioid clear cell tumor of the common bile duct. Am J Surg Pathol. 2004;28:1107-1110. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 26] [Cited by in RCA: 23] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 88. | Silva EG, Deavers MT, Bodurka DC, Malpica A. Uterine epithelioid leiomyosarcomas with clear cells: reactivity with HMB-45 and the concept of PEComa. Am J Surg Pathol. 2004;28:244-249. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 119] [Cited by in RCA: 103] [Article Influence: 4.9] [Reference Citation Analysis (0)] |
| 89. | Tsui WM, Colombari R, Portmann BC, Bonetti F, Thung SN, Ferrell LD, Nakanuma Y, Snover DC, Bioulac-Sage P, Dhillon AP. Hepatic angiomyolipoma: a clinicopathologic study of 30 cases and delineation of unusual morphologic variants. Am J Surg Pathol. 1999;23:34-48. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 283] [Cited by in RCA: 226] [Article Influence: 8.7] [Reference Citation Analysis (0)] |
| 90. | Martignoni G, Pea M, Reghellin D, Zamboni G, Bonetti F. PEComas: the past, the present and the future. Virchows Arch. 2008;452:119-132. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 337] [Cited by in RCA: 372] [Article Influence: 20.7] [Reference Citation Analysis (0)] |
| 91. | Pan CC, Chung MY, Ng KF, Liu CY, Wang JS, Chai CY, Huang SH, Chen PC, Ho DM. Constant allelic alteration on chromosome 16p (TSC2 gene) in perivascular epithelioid cell tumour (PEComa): genetic evidence for the relationship of PEComa with angiomyolipoma. J Pathol. 2008;214:387-393. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 148] [Cited by in RCA: 146] [Article Influence: 8.6] [Reference Citation Analysis (0)] |
| 92. | Zhang X, Wang L, Jiang Y, Wan Z, Li W, Yao C, Geng Z, Lv Y. Hepatic perivascular epithelioid cell tumors-not otherwise specified: a case report. Nan Fang Yi Ke Da Xue Xue Bao. 2014;34:1-4. [PubMed] |
| 93. | Folpe AL, Mentzel T, Lehr HA, Fisher C, Balzer BL, Weiss SW. Perivascular epithelioid cell neoplasms of soft tissue and gynecologic origin: a clinicopathologic study of 26 cases and review of the literature. Am J Surg Pathol. 2005;29:1558-1575. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 640] [Cited by in RCA: 636] [Article Influence: 33.5] [Reference Citation Analysis (0)] |
| 94. | Petrolla AA, Xin W. Hepatic angiomyolipoma. Arch Pathol Lab Med. 2008;132:1679-1682. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 3] [Reference Citation Analysis (0)] |
| 95. | Fadare O, Parkash V, Yilmaz Y, Mariappan MR, Ma L, Hileeto D, Qumsiyeh MB, Hui P. Perivascular epithelioid cell tumor (PEComa) of the uterine cervix associated with intraabdominal "PEComatosis": A clinicopathological study with comparative genomic hybridization analysis. World J Surg Oncol. 2004;2:35. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 84] [Cited by in RCA: 84] [Article Influence: 4.0] [Reference Citation Analysis (0)] |
| 96. | Zavala-Pompa A, Folpe AL, Jimenez RE, Lim SD, Cohen C, Eble JN, Amin MB. Immunohistochemical study of microphthalmia transcription factor and tyrosinase in angiomyolipoma of the kidney, renal cell carcinoma, and renal and retroperitoneal sarcomas: comparative evaluation with traditional diagnostic markers. Am J Surg Pathol. 2001;25:65-70. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 80] [Cited by in RCA: 66] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 97. | Priola AM, Priola SM, Cataldi A, Marci V, Fava C. Acute abdomen as an unusual presentation of hepatic PEComa. A case report. Tumori. 2009;95:123-128. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 16] [Cited by in RCA: 13] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 98. | Son HJ, Kang DW, Kim JH, Han HY, Lee MK. Hepatic perivascular epithelioid cell tumor (PEComa): a case report with a review of literatures. Clin Mol Hepatol. 2017;23:80-86. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 12] [Cited by in RCA: 29] [Article Influence: 3.6] [Reference Citation Analysis (0)] |
| 99. | Stone CH, Lee MW, Amin MB, Yaziji H, Gown AM, Ro JY, Têtu B, Paraf F, Zarbo RJ. Renal angiomyolipoma: further immunophenotypic characterization of an expanding morphologic spectrum. Arch Pathol Lab Med. 2001;125:751-758. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 6] [Cited by in RCA: 6] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 100. | Tan Y, Zhang H, Xiao EH. Perivascular epithelioid cell tumour: dynamic CT, MRI and clinicopathological characteristics--analysis of 32 cases and review of the literature. Clin Radiol. 2013;68:555-561. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 41] [Cited by in RCA: 47] [Article Influence: 3.6] [Reference Citation Analysis (0)] |
| 101. | Maebayashi T, Abe K, Aizawa T, Sakaguchi M, Ishibashi N, Abe O, Takayama T, Nakayama H, Matsuoka S, Nirei K, Nakamura H, Ogawa M, Sugitani M. Improving recognition of hepatic perivascular epithelioid cell tumor: Case report and literature review. World J Gastroenterol. 2015;21:5432-5441. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 30] [Cited by in RCA: 35] [Article Influence: 3.5] [Reference Citation Analysis (0)] |
| 102. | Tirumani SH, Shinagare AB, Hargreaves J, Jagannathan JP, Hornick JL, Wagner AJ, Ramaiya NH. Imaging features of primary and metastatic malignant perivascular epithelioid cell tumors. AJR Am J Roentgenol. 2014;202:252-258. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 47] [Cited by in RCA: 60] [Article Influence: 5.5] [Reference Citation Analysis (0)] |
| 103. | Kumasaka S, Arisaka Y, Tokue A, Higuchi T, Nakajima T, Tsushima Y. A case of multiple hepatic angiomyolipomas with high (18) F-fluorodeoxyglucose uptake. BMC Med Imaging. 2014;14:17. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 13] [Cited by in RCA: 14] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 104. | Deng YF, Lin Q, Zhang SH, Ling YM, He JK, Chen XF. Malignant angiomyolipoma in the liver: a case report with pathological and molecular analysis. Pathol Res Pract. 2008;204:911-918. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 61] [Cited by in RCA: 50] [Article Influence: 2.9] [Reference Citation Analysis (0)] |
| 105. | Khaja F, Carilli A, Baidas S, Sriharan A, Norford S. PEComa: A Perivascular Epithelioid Cell Tumor in the Liver-A Case Report and Review of the Literature. Case Rep Med. 2013;2013:904126. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 17] [Cited by in RCA: 23] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 106. | Kirste S, Kayser G, Zipfel A, Grosu AL, Brunner T. Unresectable hepatic PEComa: a rare malignancy treated with stereotactic body radiation therapy (SBRT) followed by complete resection. Radiat Oncol. 2018;13:28. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 10] [Cited by in RCA: 18] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 107. | Guan H, Zou Y, Lv Y, Wang C. Hepatic perivascular epithelioid cell tumor treated by transarterial embolization plus radiofrequency ablation: A case report and literature review. Medicine (Baltimore). 2017;96:e6969. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 7] [Cited by in RCA: 9] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 108. | Bergamo F, Maruzzo M, Basso U, Montesco MC, Zagonel V, Gringeri E, Cillo U. Neoadjuvant sirolimus for a large hepatic perivascular epithelioid cell tumor (PEComa). World J Surg Oncol. 2014;12:46. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 29] [Cited by in RCA: 30] [Article Influence: 2.7] [Reference Citation Analysis (0)] |
| 109. | Bleeker JS, Quevedo JF, Folpe AL. "Malignant" perivascular epithelioid cell neoplasm: risk stratification and treatment strategies. Sarcoma. 2012;2012:541626. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 99] [Cited by in RCA: 135] [Article Influence: 10.4] [Reference Citation Analysis (0)] |
| 110. | Wagner AJ, Malinowska-Kolodziej I, Morgan JA, Qin W, Fletcher CD, Vena N, Ligon AH, Antonescu CR, Ramaiya NH, Demetri GD, Kwiatkowski DJ, Maki RG. Clinical activity of mTOR inhibition with sirolimus in malignant perivascular epithelioid cell tumors: targeting the pathogenic activation of mTORC1 in tumors. J Clin Oncol. 2010;28:835-840. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 269] [Cited by in RCA: 278] [Article Influence: 18.5] [Reference Citation Analysis (0)] |
| 111. | Tacconi D, Vergori A, Lapini L, Magnolfi A, Carnevali A, Caremani M. Hepatic Kaposi's sarcoma in a patient affected by AIDS: Correlation between histology and imaging. J Ultrasound. 2012;15:215-219. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 12] [Cited by in RCA: 10] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 112. | Giuliani M, Cordiali-Fei P, Castilletti C, Di Carlo A, Palamara G, Boros S, Rezza G. Incidence of human herpesvirus 8 (HHV-8) infection among HIV-uninfected individuals at high risk for sexually transmitted infections. BMC Infect Dis. 2007;7:143. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 21] [Cited by in RCA: 23] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 113. | Lodi S, Guiguet M, Costagliola D, Fisher M, de Luca A, Porter K; CASCADE Collaboration. Kaposi sarcoma incidence and survival among HIV-infected homosexual men after HIV seroconversion. J Natl Cancer Inst. 2010;102:784-792. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 105] [Cited by in RCA: 95] [Article Influence: 6.3] [Reference Citation Analysis (0)] |
| 114. | Bhutani M, Polizzotto MN, Uldrick TS, Yarchoan R. Kaposi sarcoma-associated herpesvirus-associated malignancies: epidemiology, pathogenesis, and advances in treatment. Semin Oncol. 2015;42:223-246. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 92] [Cited by in RCA: 108] [Article Influence: 9.8] [Reference Citation Analysis (0)] |
| 115. | Shiels MS, Pfeiffer RM, Gail MH, Hall HI, Li J, Chaturvedi AK, Bhatia K, Uldrick TS, Yarchoan R, Goedert JJ, Engels EA. Cancer burden in the HIV-infected population in the United States. J Natl Cancer Inst. 2011;103:753-762. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 552] [Cited by in RCA: 563] [Article Influence: 40.2] [Reference Citation Analysis (0)] |
| 116. | Antman K, Chang Y. Kaposi's sarcoma. N Engl J Med. 2000;342:1027-1038. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 672] [Cited by in RCA: 608] [Article Influence: 24.3] [Reference Citation Analysis (0)] |
| 117. | Russo JJ, Bohenzky RA, Chien MC, Chen J, Yan M, Maddalena D, Parry JP, Peruzzi D, Edelman IS, Chang Y, Moore PS. Nucleotide sequence of the Kaposi sarcoma-associated herpesvirus (HHV8). Proc Natl Acad Sci USA. 1996;93:14862-14867. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1209] [Cited by in RCA: 1157] [Article Influence: 39.9] [Reference Citation Analysis (0)] |
| 118. | Mercader M, Taddeo B, Panella JR, Chandran B, Nickoloff BJ, Foreman KE. Induction of HHV-8 lytic cycle replication by inflammatory cytokines produced by HIV-1-infected T cells. Am J Pathol. 2000;156:1961-1971. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 109] [Cited by in RCA: 101] [Article Influence: 4.0] [Reference Citation Analysis (0)] |
| 119. | Friborg J, Kong W, Hottiger MO, Nabel GJ. p53 inhibition by the LANA protein of KSHV protects against cell death. Nature. 1999;402:889-894. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 542] [Cited by in RCA: 542] [Article Influence: 20.8] [Reference Citation Analysis (0)] |
| 120. | Nakamura T, Mizuno S. The discovery of hepatocyte growth factor (HGF) and its significance for cell biology, life sciences and clinical medicine. Proc Jpn Acad Ser B Phys Biol Sci. 2010;86:588-610. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 323] [Cited by in RCA: 382] [Article Influence: 25.5] [Reference Citation Analysis (0)] |
| 121. | Hong YK, Foreman K, Shin JW, Hirakawa S, Curry CL, Sage DR, Libermann T, Dezube BJ, Fingeroth JD, Detmar M. Lymphatic reprogramming of blood vascular endothelium by Kaposi sarcoma-associated herpesvirus. Nat Genet. 2004;36:683-685. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 281] [Cited by in RCA: 281] [Article Influence: 13.4] [Reference Citation Analysis (0)] |
| 122. | Hammock L, Reisenauer A, Wang W, Cohen C, Birdsong G, Folpe AL. Latency-associated nuclear antigen expression and human herpesvirus-8 polymerase chain reaction in the evaluation of Kaposi sarcoma and other vascular tumors in HIV-positive patients. Mod Pathol. 2005;18:463-468. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 47] [Cited by in RCA: 31] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 123. | Schneiderman DJ, Arenson DM, Cello JP, Margaretten W, Weber TE. Hepatic disease in patients with the acquired immune deficiency syndrome (AIDS). Hepatology. 1987;7:925-930. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 113] [Cited by in RCA: 95] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 124. | Read PJ, Lucas S, Morris S, Kulasegaram R. Immune reconstitution inflammatory syndrome Kaposi sarcoma in the liver manifesting as acute obstructive hepatitis: another potential role for montelukast? Int J STD AIDS. 2013;24:156-158. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4] [Cited by in RCA: 4] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 125. | Hammerman AM, Kotner LM, Doyle TB. Periportal contrast enhancement on CT scans of the liver. AJR Am J Roentgenol. 1991;156:313-315. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 14] [Cited by in RCA: 15] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 126. | Valls C, Cañas C, Turell LG, Pruna X. Hepatosplenic AIDS-related Kaposi's sarcoma. Gastrointest Radiol. 1991;16:342-344. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 26] [Cited by in RCA: 16] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 127. | Nasti G, Talamini R, Antinori A, Martellotta F, Jacchetti G, Chiodo F, Ballardini G, Stoppini L, Di Perri G, Mena M, Tavio M, Vaccher E, D'Arminio Monforte A, Tirelli U; AIDS Clinical Trial Group Staging System in the Haart Era--the Italian Cooperative Group on AIDS and Tumors and the Italian Cohort of Patients Naive from Antiretrovirals. AIDS-related Kaposi's Sarcoma: evaluation of potential new prognostic factors and assessment of the AIDS Clinical Trial Group Staging System in the Haart Era--the Italian Cooperative Group on AIDS and Tumors and the Italian Cohort of Patients Naive From Antiretrovirals. J Clin Oncol. 2003;21:2876-2882. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 113] [Cited by in RCA: 110] [Article Influence: 5.0] [Reference Citation Analysis (0)] |
| 128. | Maskew M, Fox MP, van Cutsem G, Chu K, Macphail P, Boulle A, Egger M, Africa FI. Treatment response and mortality among patients starting antiretroviral therapy with and without Kaposi sarcoma: a cohort study. PLoS One. 2013;8:e64392. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 31] [Cited by in RCA: 37] [Article Influence: 3.1] [Reference Citation Analysis (0)] |
| 129. | Donato V, Guarnaccia R, Dognini J, de Pascalis G, Caruso C, Bellagamba R, Morrone A. Radiation therapy in the treatment of HIV-related Kaposi's sarcoma. Anticancer Res. 2013;33:2153-2157. [PubMed] |
| 130. | Gbabe OF, Okwundu CI, Dedicoat M, Freeman EE. Treatment of severe or progressive Kaposi's sarcoma in HIV-infected adults. Cochrane Database Syst Rev. 2014;CD003256. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 26] [Cited by in RCA: 51] [Article Influence: 4.6] [Reference Citation Analysis (0)] |
| 131. | Van Leer-Greenberg B, Kole A, Chawla S. Hepatic Kaposi sarcoma: A case report and review of the literature. World J Hepatol. 2017;9:171-179. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 14] [Cited by in RCA: 11] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 132. | Stewart S, Jablonowski H, Goebel FD, Arasteh K, Spittle M, Rios A, Aboulafia D, Galleshaw J, Dezube BJ. Randomized comparative trial of pegylated liposomal doxorubicin versus bleomycin and vincristine in the treatment of AIDS-related Kaposi's sarcoma. International Pegylated Liposomal Doxorubicin Study Group. J Clin Oncol. 1998;16:683-691. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 251] [Cited by in RCA: 223] [Article Influence: 8.3] [Reference Citation Analysis (0)] |
| 133. | Grünaug M, Bogner JR, Loch O, Goebel FD. Liposomal doxorubicin in pulmonary Kaposi's sarcoma: improved survival as compared to patients without liposomal doxorubicin. Eur J Med Res. 1998;3:13-19. [PubMed] |
| 134. | Gill PS, Wernz J, Scadden DT, Cohen P, Mukwaya GM, von Roenn JH, Jacobs M, Kempin S, Silverberg I, Gonzales G, Rarick MU, Myers AM, Shepherd F, Sawka C, Pike MC, Ross ME. Randomized phase III trial of liposomal daunorubicin versus doxorubicin, bleomycin, and vincristine in AIDS-related Kaposi's sarcoma. J Clin Oncol. 1996;14:2353-2364. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 322] [Cited by in RCA: 271] [Article Influence: 9.3] [Reference Citation Analysis (0)] |
| 135. | Martellotta F, Berretta M, Vaccher E, Schioppa O, Zanet E, Tirelli U. AIDS-related Kaposi's sarcoma: state of the art and therapeutic strategies. Curr HIV Res. 2009;7:634-638. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 54] [Cited by in RCA: 52] [Article Influence: 3.5] [Reference Citation Analysis (0)] |
| 136. | Gantt S, Casper C. Human herpesvirus 8-associated neoplasms: the roles of viral replication and antiviral treatment. Curr Opin Infect Dis. 2011;24:295-301. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 87] [Cited by in RCA: 75] [Article Influence: 5.4] [Reference Citation Analysis (0)] |
| 137. | La Ferla L, Pinzone MR, Nunnari G, Martellotta F, Lleshi A, Tirelli U, De Paoli P, Berretta M, Cacopardo B. Kaposi' s sarcoma in HIV-positive patients: the state of art in the HAART-era. Eur Rev Med Pharmacol Sci. 2013;17:2354-2365. [PubMed] |
| 138. | Gill RM, Buelow B, Mather C, Joseph NM, Alves V, Brunt EM, Liu TC, Makhlouf H, Marginean C, Nalbantoglu I, Sempoux C, Snover DC, Thung SN, Yeh MM, Ferrell LD. Hepatic small vessel neoplasm, a rare infiltrative vascular neoplasm of uncertain malignant potential. Hum Pathol. 2016;54:143-151. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 29] [Cited by in RCA: 37] [Article Influence: 4.1] [Reference Citation Analysis (0)] |
| 139. | Behjati S, Tarpey PS, Sheldon H, Martincorena I, Van Loo P, Gundem G, Wedge DC, Ramakrishna M, Cooke SL, Pillay N, Vollan HKM, Papaemmanuil E, Koss H, Bunney TD, Hardy C, Joseph OR, Martin S, Mudie L, Butler A, Teague JW, Patil M, Steers G, Cao Y, Gumbs C, Ingram D, Lazar AJ, Little L, Mahadeshwar H, Protopopov A, Al Sannaa GA, Seth S, Song X, Tang J, Zhang J, Ravi V, Torres KE, Khatri B, Halai D, Roxanis I, Baumhoer D, Tirabosco R, Amary MF, Boshoff C, McDermott U, Katan M, Stratton MR, Futreal PA, Flanagan AM, Harris A, Campbell PJ. Recurrent PTPRB and PLCG1 mutations in angiosarcoma. Nat Genet. 2014;46:376-379. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 255] [Cited by in RCA: 245] [Article Influence: 22.3] [Reference Citation Analysis (0)] |
| 140. | Antonescu CR, Yoshida A, Guo T, Chang NE, Zhang L, Agaram NP, Qin LX, Brennan MF, Singer S, Maki RG. KDR activating mutations in human angiosarcomas are sensitive to specific kinase inhibitors. Cancer Res. 2009;69:7175-7179. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 230] [Cited by in RCA: 209] [Article Influence: 13.1] [Reference Citation Analysis (0)] |
| 141. | Lewis S, Aljarallah B, Trivedi A, Thung SN. Magnetic resonance imaging of a small vessel hepatic hemangioma in a cirrhotic patient with histopathologic correlation. Clin Imaging. 2015;39:702-706. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 8] [Cited by in RCA: 8] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 142. | Joseph NM, Brunt EM, Marginean C, Nalbantoglu I, Snover DC, Thung SN, Yeh MM, Umetsu SE, Ferrell LD, Gill RM. Frequent GNAQ and GNA14 Mutations in Hepatic Small Vessel Neoplasm. Am J Surg Pathol. 2018;42:1201-1207. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 34] [Cited by in RCA: 53] [Article Influence: 8.8] [Reference Citation Analysis (0)] |
| 143. | Walcott-Sapp S, Tang E, Kakar S, Shen J, Hansen P. Resection of the largest reported hepatic small vessel neoplasm. Hum Pathol. 2018;78:159-162. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 12] [Cited by in RCA: 15] [Article Influence: 2.1] [Reference Citation Analysis (0)] |