Published online Nov 24, 2019. doi: 10.5306/wjco.v10.i11.358
Peer-review started: March 4, 2019
First decision: April 11, 2019
Revised: August 13, 2019
Accepted: November 4, 2019
Article in press: November 4, 2019
Published online: November 24, 2019
Processing time: 270 Days and 21.9 Hours
Genetic testing is widely recommended for all epithelial ovarian cancer (EOC) patients. However, an increased probability of identifying germline mutations has been reported in selected patients with risk factors such as a family history or personal history of cancer and high-grade serous carcinoma (HGSC) subtype. HGSC has been reported to be the most common subtype of EOC worldwide (approximately 70%). However, this subtype is less prevalent in Thai patients (reported as only 20%). The difference in the distribution of various subtypes of EOC may reflect the incidence of germline mutations in Thai EOC patients.
To evaluate the frequencies of germline mutations in EOC patients and to compare the frequencies in those with and without clinical risk factors for hereditary ovarian cancer.
This cross-sectional study included 112 nonmucinous EOC patients who underwent primary surgery at our tertiary care hospital. Clinical risk factors for hereditary ovarian cancer were defined as follows: Age below 40 years, a significant family history of cancer, synchronous ovarian and endometrial cancer, and HGSC. Comprehensive germline mutations were detected by next-generation sequencing.
Of a total of 112 patients, 82 (73.2%) patients had ≥ 1 risk factor and 30 (26.8%) patients had no risk factors. Germline mutations were detected in 26 patients: 20 (17.8%) patients had BRCA1/2 mutations, but 6 (5.4%) patients had mutations in other genes, including 1 in MLH1, 1 in MSH2, 1 in RAD51C, 2 in ATM and 1 in CDH1. Germline mutations were only detected in patients with risk factors (26 of 82, 31.7%), not in patients without risk factors (P < 0.001). A significant family history of cancer and HGSC were the only two significant risk factors associated with a higher proportion of germline mutations (56.3% vs 10% for those with and without a history of cancer, respectively, 40.8% vs 9.5% for those with and without HGSC). Germline BRCA mutations were detected in 38.8% of patients with HGSC but in only 1.6% of those with non-HGSC. An age below 40 years, personal history of breast cancer, and synchronous ovarian and endometrial cancer were not significant factors (14.3% vs 23.5%, 33.3% vs 21%, 22.2% vs 22.3%).
Approximately one-third of EOC patients with risk factors had germline mutations. Almost all germline BRCA mutations were found in patients with the HGSC subtype. Selected patients with HGSC and a family history of cancer should be initially considered for genetic analysis in Thailand.
Core tip: Germline mutations could not be detected in any epithelial ovarian cancer patients without risk factors such as age below 40 years, significant family or personal history of cancer, and high-grade serous subtype. Mutations were detected in approximately one-third of patients with these risk factors: 25% had BRCA1/2 mutations, 5% had mutations in other homologous recombination genes, and 2.5% had MMR mutations. Significantly more germline mutations were found in patients with a family history of cancer, especially ovarian cancer. Germline BRCA mutations were detected in 38.8% of patients with the high-grade serous subtype but in only 1.6% of those with a non-high-grade serous subtype. Selected patients with the high-grade serous subtype or a significant family history of cancer should initially be considered for genetic analysis in limited resource settings.
- Citation: Manchana T, Phowthongkum P, Teerapakpinyo C. Germline mutations in Thai patients with nonmucinous epithelial ovarian cancer. World J Clin Oncol 2019; 10(11): 358-368
- URL: https://www.wjgnet.com/2218-4333/full/v10/i11/358.htm
- DOI: https://dx.doi.org/10.5306/wjco.v10.i11.358
Although most epithelial ovarian cancers (EOCs) are sporadic, at least 10% of EOC patients have a genetic predisposition[1]. Risk assessment has been previously used to identify patients at risk for hereditary ovarian cancers and to offer genetic counseling and testing. These risk factors are as follows: Early age of onset, personal history of breast cancer, family history of breast, ovarian cancer, endometrial and colon cancer, and specific histologic subtype such as high-grade serous carcinoma (HGSC)[2]. HGSC has been reported to increase the risk of germline mutations. It has also been reported that approximately 20% of HGSCs harbor BRCA mutations[3]. Recently, the recommendation for genetic testing in EOC patients has changed. Various national professional societies such as the American College of Obstetricians and Gynecologists, the Society of Gynecologic Oncologists, and the National Comprehensive Cancer Network have recommended offering genetic counseling and testing for all women with EOC including fallopian tube cancers and primary peritoneal cancers irrespective of risk factors[4,5]. Firstly, some patients with germline mutations will be missed if the testing is based on these current risk factors. Secondly, genetic results can predict treatment outcomes. Patients with BRCA-mutated EOC have increased platinum sensitivity response rates and significant improvement in survival rate compared with those with non-BRCA- mutated EOC[6]. Thirdly, novel targeted therapy with Poly (ADP-ribose) polymerase inhibitors has been reported to be a promising targeted therapy for BRCA-mutated cancers including homologous recombination (HR) gene-deficient ovarian cancers[7].
The frequency of germline mutations varies across different countries and ethnicities. The incidence of BRCA mutations has been reported to range from 5.8%-24.8%[8]. Our previous study reported that 11.4% of patients with nonmucinous EOC had BRCA mutations[9]. However, that study included a small number of patients, and the mutations were reported only in selected patients with risk factors for hereditary ovarian cancers such as a family history or personal history of cancer and HGSC. Therefore, the incidence of BRCA mutations in unselected EOC patients could be even lower than this number. The incidence of HGSC is a major predictive variable. Although it has been reported as the most common subtype worldwide at approximately 70%[3], only 20% of Thai patients have serous carcinoma[9,10]. The difference in the distribution of various subtypes of EOC between Thai patients and others worldwide may reflect the lower incidence of germline mutations in Thai EOC patients. The objective of this study is to evaluate the frequency of germline mutations in EOC patients and to compare the frequency in those with and without clinical risk factors for hereditary ovarian cancer.
This study was approved by the Institutional Review Board, Faculty of Medicine, Chulalongkorn University. This was a cross-sectional study conducted between November 2015 and February 2018.
Patients with EOC, fallopian tube or primary peritoneal cancer who underwent primary surgery and had a pathologically confirmed nonmucinous subtype were enrolled. Clinical risk factors for hereditary ovarian cancer are defined as follows: (1) Age at diagnosis below 40 years; (2) Significant family history (≥ 1 relative with ovarian cancer at any age, ≥ 1 relative with breast cancer or hereditary nonpolyposis colorectal cancer (HNPCC)-associated cancers such as colon cancer or endometrial cancer diagnosed before 50 years of age, ≥ 2 relatives with breast cancer or HNPCC-associated cancers diagnosed after 50 years of age; (3) Previous diagnosis of breast cancer; (4) Synchronous ovarian cancer and endometrial cancer; and (5) HGSC[9]. All patients who agreed to participate in this study received genetic counseling by a geneticist and provided informed consent.
Comprehensive germline mutation analysis was performed using peripheral blood DNA samples analyzed by a GeneRead DNAseq Mix-n-Match Panel v2 (27-gene panel including APC, ATM, AXIN2, BARD1, BMPR1A, BRCA1, BRCA2, BRIP1, CDH1, CHEK2, EPCAM, MLH1, MLH3, MRE11, MSH2, MSH6, MUTYH, NBN, PALB2, PMS2, PTEN, RAD50, RAD51C, RAD51D, SMAD4, STK11, and TP53) (Qiagen) and next-generation sequencing (NGS) (The Illumina MiSeq System; Illumina, San Diego, CA, United States). DNA was extracted from the patients’ peripheral blood using a QIAamp DNA Blood Mini Kit. DNA quality was assessed using GeneRead DNA QuantiMIZE Kits according to the manufacturer’s instructions (Qiagen, Germany). DNA library preparation and NGS were performed as previously described[9]. Variant pathogenicity was classified based on the American College of Medical Genetics and Genomics and the American Society of Molecular Pathology standard and guidelines for the interpretation of sequence variants[11]. Variant classification was listed as "Pathogenic", "Likely pathogenic", "Variant of Uncertain Clinical Significance", "Likely benign" or "Benign" in decreasing order of clinical importance. The pathogenic and likely pathogenic variants found in this study were confirmed using bi-directional Sanger sequencing.
SPSS version 22 (SPSS Inc., Chicago, IL, United States) was used for the statistical analysis. Comparisons of the proportions of germline mutations between two groups were analyzed by Chi-square or Fisher’s Exact test. P values less than 0.05 were considered statistically significant.
Of the 112 patients, 97 were diagnosed with EOC, 4 with fallopian tube cancer, 2 with primary peritoneal cancer, and 9 with synchronous endometrial and ovarian cancer. The mean age was 52.8 ± 11.0 years. Patient characteristics are shown in Table 1. Eighty-two patients (73.2%) had at least one clinical risk factor, and 27 patients (24.1%) had more than one risk factor. Of the 82 patients, EOC was diagnosed in 67 patients (81.7%), fallopian tube cancer in 4 patients (4.9%), primary peritoneal cancer in 2 patients (2.4%), and synchronous endometrial and ovarian cancer in 9 patients (11.0%). The histologic subtypes in these patients were HGSC in 49 patients (59.8%), low-grade serous carcinoma in 4 patients (4.9%), endometrioid carcinoma in 15 patients (18.3%), clear cell carcinoma in 12 patients (14.6%), mixed endometrioid and clear cell carcinoma in 1 patient (1.2%), and adenocarcinoma in 1 patient (1.2%). Thirty patients (26.8%) had no clinical risk factors of hereditary ovarian cancer, all of whom were diagnosed with EOC. The histologic subtypes in these patients were endometrioid carcinoma in 13 patients (43.3%), clear cell carcinoma in 12 patients (40%), mixed endometrioid and clear cell carcinoma in 3 patients (10%) and low-grade serous carcinoma in 2 patients (6.7%).
| Characteristics | Patients with risk factors (n = 82) | Patients without risk factor (n = 30) | P value |
| Mean age (yr) | 51.8 ± 11.7 | 55.6 ± 8.3 | 0.06 |
| Menopause, n (%) | 39 (47.6) | 17 (56.7) | 0.52 |
| Diagnosis, n (%) | |||
| Epithelial ovarian cancer | 67 (81.7) | 30 (100) | 0.10 |
| Fallopian tube cancer | 4 (4.9 | 0 | |
| Peritoneal cancer | 2 (2.4) | 0 | |
| Synchronous ovarian and endometrial cancer | 9 (11.0) | 0 | |
| Histologic subtype, n (%) | |||
| High-grade serous | 49 (59.8) | 0 | < 0.001 |
| Low-grade serous | 4 (4.9) | 2 (6.7) | |
| Endometrioid | 15 (18.3) | 13 (43.3) | |
| Clear cell | 12 (14.6) | 12 (40.0) | |
| Mixed endometrioid and clear cell | 1 (1.2) | 3 (10.0) | |
| Adenocarcinoma | 1 (1.2) | 0 | |
| Stage, n (%) | |||
| 1 | 27 (32.9) | 13 (43.3) | 0.06 |
| 2 | 10 (12.2) | 8 (26.7) | |
| 3 | 38 (46.3) | 9 (30.0) | |
| 4 | 7 (8.5) | 0 | |
| Platinum sensitivity, n (%) | 66 (80.5) | 23 (76.7) | 0.73 |
| Germline mutation, n (%) | 26 (31.7) | 0 | < 0.001 |
Germline mutations were detected in 26 patients (23.2%), whereas 20 patients (17.8%) had BRCA1 or BRCA2 mutations, and 6 patients (5.4%) had mutations in other genes (Table 2). Germline mutations could not be detected in any of the 30 patients without risk factors. In contrast, all mutations were detected in 26 of 82 patients with ≥ 1 risk factor (31.7%, P < 0.001). The frequency of germline mutations according to various risk factors is shown in Table 3. Significantly more germline mutations were observed in patients with a significant family history of cancer compared with patients without a significant family history (56.3% and 10% for with and without a family history of cancer, respectively, P < 0.001). Fourteen of 32 patients who had a family history of cancer had BRCA mutations (43.7%) and 3 patients (9.4%) had other gene mutations. In contrast, 6/80 (7.5%) patients without a family history of cancers had BRCA mutations and 2/80 (2.5%) had other gene mutations (1 ATM and 1 CDH1). Significantly higher frequencies of germline mutations were observed in patients with a family history of ovarian cancer (70% vs 17.6% for those with and without a family history of ovarian cancer, respectively, P = 0.001) and in patients with a family history of breast cancer (40.9% vs 17.8% for patients with and without a family history of breast cancer, respectively, P = 0.04). Patients with a family history of ovarian cancer had a higher frequency of BRCA mutations than those with a family history of breast cancer (60% vs 40.9%). If other genes were included, the frequency of germline mutations was 70% and 40.9%, in those with family history of ovarian and breast cancers, respectively. HGSC was also observed as another factor associated with a higher frequency of germline mutations (40.8% and 9.5%, P < 0.001). Nineteen patients with HGSC (38.8%) had BRCA mutations. Seven of 63 patients (11.1%) who had types other than HGSC had germline mutations; 1.6% had BRCA mutations and 7.9% had other gene mutations. A sub-analysis of 30 patients with HGSC without the other clinical risk factors revealed that the frequency of BRCA mutations was 16.7% (13.3% with BRCA1 mutations and 3.3% with BRCA2 mutations). The frequency of germline mutations in patients with other risk factors such as age below 40 years, personal history of breast cancer, and synchronous cancer was not significantly different (14.3% vs 23.5%, 33.3% vs 21%, 22.2% vs 22.3%; P > 0.05)
| Age (yr) | Gene | Mutation | Variant classification | Cancer | Family history of cancer | Synchro-nous cancers | Histology | ||
| Nucleotide change | Protein change | Type | |||||||
| 63 | BRCA1 | c.1889delA | p.Asn630IlefsTer2 | Frameshift | Pathogenic | Ovarian cancer IIIC | Ovarian cancer (sister) | - | High-grade serous |
| 64 | BRCA1 | c.981_982delAT | p.Cys328Ter | Frameshift | Pathogenic | Ovarian cancer IIIB | Ovarian cancer (sister) | - | High-grade serous |
| 56 | BRCA1 | c.5072C>A | p.Thr1691Lys | Missense | Likely pathogenic | Fallopian tube cancer IIA | - | - | High-grade serous |
| 52 | BRCA1 | c.3748G>T | p.Glu1250Ter | Nonsense | Pathogenic | Peritoneal cancer IIIC | Breast cancer (mother) ovarian cancer (sister) | - | High-grade serous |
| 46 | BRCA1 | c.2059C>T | p.Gln687Ter | Nonsense | Pathogenic | Ovarian cancer IIIC | Ovarian cancer (grandmother) | - | High-grade serous |
| 72 | BRCA1 | c.3049G>T | p.Glu1017Ter | Nonsense | Pathogenic | Ovarian cancer IIIA | Breast cancer (2 sisters) endometrial cancer (mother) | - | High-grade serous |
| 57 | BRCA1 | c.3770_3771delAG | p.Glu1257GlyfsTer9 | Frameshift | Pathogenic | Ovarian cancer IIIC | Breast cancer (aunt) | - | High-grade serous |
| 51 | BRCA1 | c.1426delC | p.His476MetfsTer2 | Frameshift | Pathogenic | Ovarian cancer IVB | Breast cancer (niece) | - | High-grade serous |
| 35 | BRCA1 | c.3020C>A | p.Ser1007Ter | Nonsense | Pathogenic | Ovarian cancer IIIC | Breast cancer (mother) | - | High-grade serous |
| 63 | BRCA1 | c.3181delA | p.Ile1061Ter | Frameshift | Pathogenic | Tubal cancer IVB | - | - | High-grade serous |
| 69 | BRCA1 | c.1155G>A | p.Trp385Ter | Nonsense | Pathogenic | Tubal cancer IC | - | - | High-grade serous |
| 45 | BRCA1 | c.3049G>T | p.Glu1017Ter | Nonsense | Pathogenic | Ovarian cancer IVB | - | - | High-grade serous |
| 62 | BRCA1 | c.4327C>T | p.Arg1443 | Frameshift | Pathogenic | Peritoneal cancer IIB | Breast cancer (daughter) Breast and ovarian cancer (sister) endometrial cancer (sister) | - | High-grade serous |
| 59 | BRCA1 | c.981_982delAT | p.Cys328Ter | Frameshift | Pathogenic | Ovarian cancer IIIC | Breast cancer (sister) | - | High-grade serous |
| 49 | BRCA2 | c.4126G > T | p.Gly1376Ter | Nonsense | Pathogenic | Ovarian cancer IIIC | Breast cancer (sister) Prostate cancer (uncle) Colon cancer (uncle) | - | High-grade serous |
| 56 | BRCA2 | c.7558C > T | p.Arg2520Ter | Nonsense | Pathogenic | Ovarian cancer IIIC | - | - | High-grade serous |
| 60 | BRCA2 | c.3109C > T | p.Gln1037Ter | Nonsense | Pathogenic | Ovarian cancer IVB | Breast cancer (2 sisters) | Breast cancer | High-grade serous |
| 49 | BRCA2 | c.1367_1368delAG | p.Lys457GlufsTer4 | Frameshift | Pathogenic | Ovarian cancer IIIC | Ovarian cancer (mother) | Breast cancer | High-grade serous |
| 40 | BRCA2 | c.22_23delAG | p.Arg8AlafsTer4 | Frameshift | Pathogenic | Ovarian cancer IIIC | Breast cancer (sister) | - | Clear cell carcinoma |
| 63 | BRCA2 | c.1405_1406delGA | p.Asp469 | Nonsense | Pathogenic | Ovarian cancer IIB | - | Breast cancer | High-grade serous |
| 46 | MLH1 | c.109G > A | p.Glu37Lys | Nonsense | Likely pathogenic | Ovarian cancer IIC and endometrial cancer IAG1 | Colon cancer (grandfather, father and uncle) | - | Well differentiated serous and well differentiated endometrioid |
| 48 | MSH2 | c.1183C > T | p.Gln395Ter | Nonsense | Pathogenic | Ovarian cancer IA and endometrial cancer IAG1 | Endometrial cancer (aunt) | Endometrial cancer | Endometrioid |
| 48 | CDH1 | c.1118C > T | p.Pro373Leu | Missense | Likely pathogenic | Ovarian cancer IC and endometrial cancer IAG3 | - | Endometrial cancer | Endometrioid with clear cell |
| 54 | RAD51C | c.905-2A > C | Not applicable | Splice site loss | Likely pathogenic | Ovarian cancer IIIC | Ovarian cancer (mother) | - | High-grade serous |
| 67 | ATM | c.1402_1403delAA | p.Lys468GlufsTer18 | Frameshift | Likely pathogenic | Ovarian cancer IA | - | Breast cancer (Triple- negative) | Clear cell |
| 52 | ATM | c.8431_8432delAA | p.Lys2811ValfsTer3 | Frameshift | Likely pathogenic | Ovarian cancer IIIC | Colon cancer (mother, aunt) liver cancer (aunt) | - | Clear cell |
| Risk factors | Number of patients | BRCA1, n (%) | BRCA2, n (%) | Other genes, n (%) |
| None | 30 | 0 | 0 | 0 |
| Family history of cancers (breast/ovary/endometrium/colon) | 32 | 10 (31.2) | 4 (12.5) | 4 (12.5) (1ATM, 1RAD51C,1 MLH1, 1MSH2) |
| Family history of breast cancer | 22 | 6 (27.3) | 3 (13.6) | 0 |
| Family history of ovarian cancer | 10 | 5 (50.0) | 1 (10.0) | 1 (10.0) (RAD51C) |
| Personal history of breast cancer | 12 | 0 | 3 (25.0) | 1 (8.3) (ATM) |
| Two primary ovarian and endometrial cancer | 9 | 0 | 0 | 3 (33.3) (1 MLH1, 1MSH2, 1CDH1) |
| High-grade serous carcinoma | 49 | 14 (28.6) | 5 (10.4) | 1 (2.1) (RAD51C) |
| Young age (< 40 yr) | 14 | 1 (7.1) | 1 (7.1) | 0 |
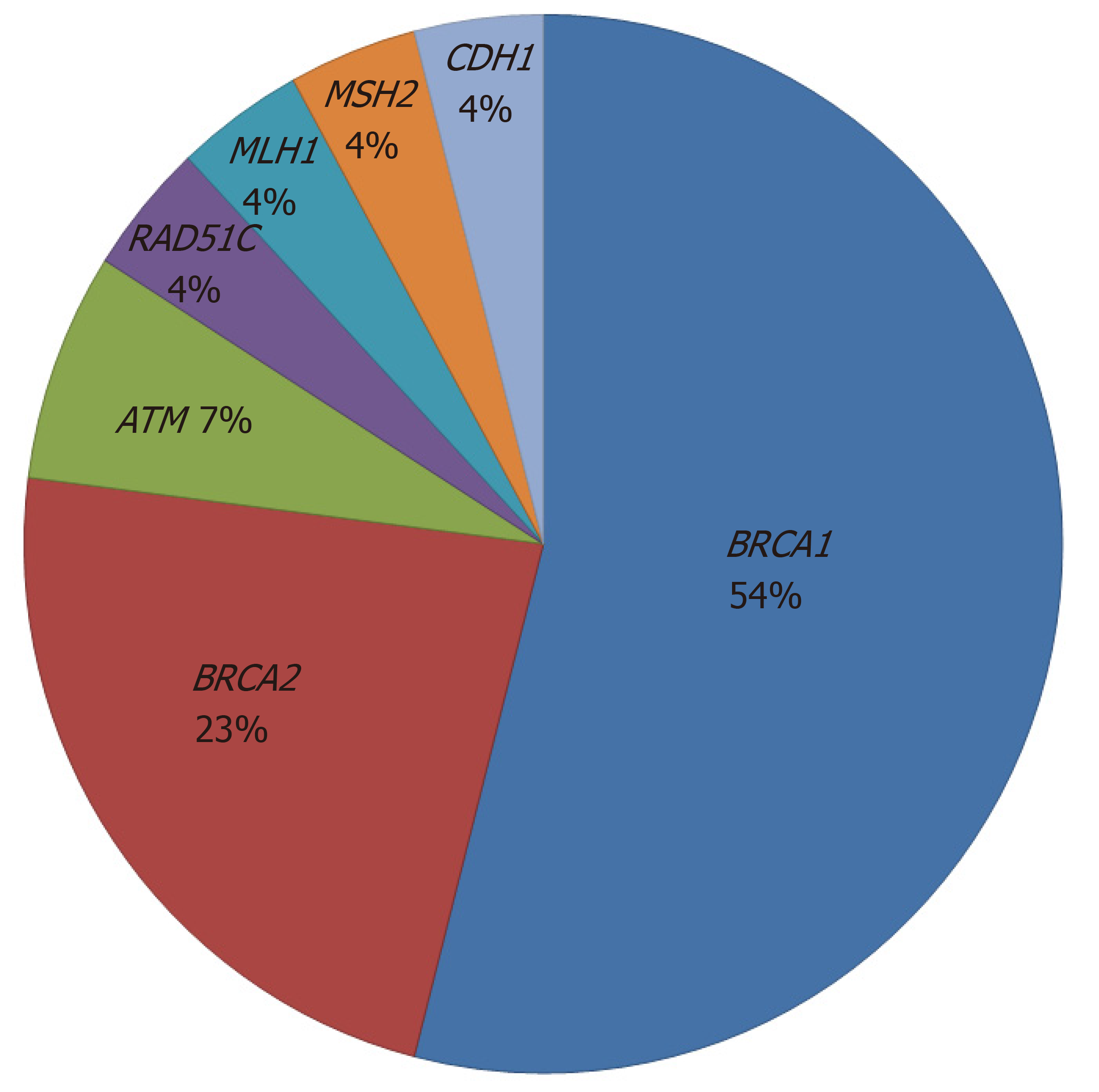
Germline BRCA mutations were found in 20/82 (24.4%) patients with risk factors (14 BRCA1 mutations and 6 BRCA2 mutations). MMR mutations (MLH1 and MSH2) were found in 2 (2.4%) patients who were diagnosed with synchronous endometrial and ovarian cancer. Other gene mutations such as RAD51C, ATM and CDH1 were detected in 4 (4.9%) patients. The proportion of germline mutations is shown in Figure 1. Among 20 patients with BRCA mutations, 14 patients (70%) had a family history of breast and/or ovarian cancer, 19 patients (95%) had HGSC, 3 patients (15%) had a personal history of breast cancer, and 7 patients (35%) were older than 60 years at diagnosis.
At least 10% of EOCs are hereditary, and most germline mutations are associated with BRCA1 and BRCA2[1]. A 10%-15% frequency of germline BRCA mutations has been reported in unselected EOC patients[12-14]. However, mutations in MMR genes (MLH1, MSH2, MSH6, PMS2) associated with Lynch or HNPCC syndrome and other genes in HR pathways have been implicated in inherited susceptibility to ovarian cancer. Approximately a quarter of EOC patients carry germline mutations in HR genes, including BRCA1, BRCA2, and other HR genes such as ATM, BARD1, BRIP1, CHEK1, CHEK2, FAM175A, MRE11A, NBN, PALB2, RAD51C, and RAD51D. Moreover, about 6% of patients carry germline mutations in other HR genes[15,16]. The frequency of germline mutations in this study has been reported to be 31.7% in selected EOC patients with risk factors. Of these patients, 24.4% carried BRCA1 or BRCA2 mutations and 7.3% had mutations in other genes. Other mutations found in this study include those in the RAD51C, ATM, CDH1, MLH1, and MSH2 genes. The RAD51C and ATM genes are involved in DNA repair mechanisms similar to the BRCA1 and BRCA2 genes. These genes have been reported to increase the risk of developing breast and ovarian cancer[17]. Germline CDH1 mutations are known to cause hereditary diffuse gastric cancer syndrome, which increases the lifetime risk of gastric, breast, and colon cancer. However, down-regulation of CDH1 gene expression is involved in cancer invasion and metastasis in many cancers, including ovarian cancer[18]. Mutations in MMR genes are associated with Lynch syndrome, which increase the lifetime risk of colorectal, endometrial and ovarian cancer. The frequency of MMR mutations in this study was 1.8% (2/112 patients), which was slightly higher than that reported in previous studies (0.5%)[14]. A family history or personal history of Lynch syndrome-associated cancers was considered a risk factor in this study. Furthermore, our previous study reported that endometrioid carcinoma was the most common histological subtype among Thai EOC patients[10]. EOC patients with germline MMR mutations usually have the endometrioid subtype. These different subtypes might affect the different frequency of germline mutations in MMR genes in this study.
The selection criteria for germline testing in EOC patients included young age, a family or personal history of cancer, HGSC, and specific ethnicity. Using these criteria, 27.5% of patients with germline BRCA mutations might not have been selected for genetic testing[8]. Although germline BRCA mutations are rarely found in patients aged over 60 years, one-third to half of all BRCA mutations are found in this group of women[1]. Furthermore, a significant family history of cancer is absent in 27%-56% of patients with germline BRCA mutations[1]. Our study showed that 35% of patients with germline BRCA mutations were older than 60 years at diagnosis and 30% had no significant family history of cancer. In addition, each patient might have overlapping risk factors. Therefore, other risk factors such as HGSC or a personal history of cancer in these patients might explain the germline BRCA mutations.
The frequency of germline BRCA mutations in EOC patients with a significant family history of breast/ovarian cancer was 43.7% in this study, which was comparable with that found in previous studies (33%-55%)[12,19-21]. A family history of ovarian cancer increases the chance of identifying germline BRCA mutations compared with a family history of breast cancer (60% vs 40.9%). This finding is consistent with previous studies in Korea and Australia, which reported incidences of BRCA mutations of 55%-63% and 15%-35% for a family history of ovarian and breast cancers, respectively[13,21].
Germline BRCA mutations are particularly frequent in HGSC and have been reported as 11%-23%[12-14]. Our study reported a higher frequency of 38.8%. As mentioned previously, it is likely that each patient has overlapping risk factors. The frequency of germline BRCA mutations was 16.7% in patients with HGSC without other risk factors. Most patients (95%) with germline BRCA mutations had HGSC, which is somewhat higher than the 73.2% reported in a previous study[8]. In contrast, only 1.6% of patients with non-HGSC had BRCA mutations, and 7.9% of these patients had germline mutations in other genes. This finding is in agreement with that of a previous study, which reported that germline BRCA mutations were detected in less than 10% of endometrioid carcinomas and had a very low frequency in clear cell carcinoma[1].
The incidence rates of EOC differ according to various geographic locations. Rates are highest in Western countries and lowest in Asian countries[22]. The age-standardized incidence rate in Thailand has been reported at approximately 6.2 per 100000 people in 2018[22]. Thus, the incidence of germline mutations in EOC may vary by ethnicity and country. Germline BRCA mutations were identified in 13% of Malaysian, 13% of Japanese and 17% of Taiwanese[23-25]. A higher frequency has been reported in 23% of Indian patients, and even higher frequencies have been reported in Korean and Chinese patients (up to 26% and 28.5%, respectively)[25-27]. Different proportions of histologic subtypes are also a major variable. HGSC is the most common subtype worldwide with an incidence rate of 70%[3], but it is much less prevalent in Thai patients (20%-22%). The endometrioid and clear cell carcinoma subtypes are observed more frequently (up to 60%)[9,10]. The reason for the high prevalence of endometrioid and clear cell carcinoma in Thai patients is unknown. Geographic differences may be a possible explanation, as clear cell carcinoma is more prevalent in Asia than in Western countries[27].
In this study, the frequency of germline mutations in patients with risk factors was 31.7% overall and 24.4% for BRCA mutations but was 0% in patients without risk factors. According to our previous study, 30% of EOC patients were classified as high-risk for hereditary ovarian cancer[9]. Most of our patients had non-HGSC and did not have other clinical risk factors. Therefore, the weighted estimated overall frequency of germline mutations in unselected patients should be only 9.5% overall and only 7.3% for BRCA mutations. This number is lower than reported in previous studies from Western countries and some countries in Asia. A much higher proportion of EOC patients who had no clinical risk factors, and HGSC, might be a plausible explanation for the lower incidence of germline BRCA mutations in this study. In general, if the estimated probability of harboring germline mutations is more than 10%, genetic testing is considered cost-effective[28,29]. Nonetheless, genetic testing is rather uncommon in many countries including Thailand because of cost considerations, reimbursement and availability. Since the estimated frequency of germline mutations in unselected patients is below 10%, genetic testing in all EOC patients may not be cost-effective.
The strength of this study was that reliable and comprehensive NGS techniques were used to analyze BRCA genes and other genes associated with HR pathways. The limitation of this study was that not all EOC patients during the study period were included and not all were offered genetic testing. Selection bias might affect the reversed proportion between patients with and without risk factors in this study (73% vs 27%). The overall frequency of germline mutations in this study was presumed and estimated based on the 30% of patients with risk factors. Moreover, BRCA dysfunction by epigenetic silencing was not analyzed. Further studies with larger numbers of patients and multicenter trials should be conducted to better represent the incidence of germline mutations in the Thai population.
In conclusion, approximately one-third of EOC patients with risk factors for hereditary ovarian cancer had germline mutations (24.4% had BRCA mutations and 7.3% had mutations in other genes). However, patients without risk factors did not have germline mutations. The weighted estimated overall frequency of germline mutations in unselected EOC patients was less than 10% and only 7.3% for BRCA mutations. Selected patients with the high-grade serous subtype or a significant family history of ovarian/breast cancers should be initially considered for further genetic analysis and intervention in countries with limited resources.
Genetic testing is widely recommended for all patients with epithelial ovarian cancer (EOC) including fallopian tube and primary peritoneal cancers. A 10%-15% incidence of germline BRCA mutations has been reported in unselected EOC patients. However, universal genetic testing in all patients may not be cost-effective if the estimated probability of harboring germline mutations is less than 10%. The incidence of germline mutations may vary across different countries and ethnicities. Different common histologic subtypes in various countries may be significant variables. The high-grade serous subtype is the most common subtype worldwide (approximately 70%), whereas it is less common in Thai EOC patients (rate of only 20%). Per this result, the incidence of germline mutations might be lower in unselected Thai EOC patients.
Different incidences of germline mutations in EOC patients among various countries may guide different recommendations for genetic testing. Universal genetic testing in all EOC patients may not be cost-effective in Thailand. The risk factors associated with the increased likelihood of having germline mutations should be evaluated.
The objective of this study was to evaluate the frequency of germline mutations in EOC patients and to compare the frequency in those with and without clinical risk factors for hereditary ovarian cancer.
This cross-sectional study was conducted on 112 nonmucinous EOC patients including those with fallopian tube and primary peritoneal cancers who underwent primary surgery at our tertiary care hospital between November 2015 and February 2018. Patients were divided into two groups based on clinical risk factors for hereditary ovarian cancer as follows: Age below 40 years, significant family history of cancer, synchronous ovarian and endometrial cancer, and high-grade serous carcinoma (HGSC). All patients who agreed to participate in this study received genetic counseling by a geneticist and provided informed consent. Comprehensive germline mutations were detected using next-generation sequencing.
Germline mutations were detected in 26 of 112 patients (23.2%); 20 patients (17.8%) had BRCA1 or BRCA2 mutations, but 6 patients (5.4%) had mutations in other genes including 1 with an MLH1, 1 with an MSH2, 1 with a RAD51C, 2 with an ATM and 1 with a CDH1 mutation. All mutations were detected in 26 of 82 patients with ≥ 1 risk factor (31.7%), but none were detected in the 30 patients without risk factors (P < 0.001). A significantly higher frequency of germline mutations was found in patients with a significant family history of cancer (56.3% and 10%, P < 0.001). Patients with a significant family history of ovarian cancer had a higher frequency of BRCA mutations than those with a family history of breast cancer (60% and 40.9%, respectively). HGSC was also associated with a higher frequency of germline mutations (40.8% and 9.5%, P < 0.001). Germline BRCA mutations were detected in 38.8% of patients with HGSC but in only 1.6% of those with non-HGSC. Other risk factors such as age below 40 years, personal history of breast cancer, and synchronous ovarian and endometrial cancer were not significantly different in terms of germline mutations (14.3% vs 23.5%, 33.3% vs 21%, 22.2% vs 22.3%, P > 0.05).
A significant family history of cancer and HGSC were the only two significant risk factors associated with a higher frequency of germline mutations. Germline BRCA mutations were detected in 38.8% of patients with HGSC but in only 1.6% of those with non-HGSC. According to the reverse proportion of histologic subtypes in Thai patients, the estimated overall frequency of germline mutations in unselected EOC patients should be only 9.5% overall and only 7.3% for BRCA mutations. These findings suggest the consideration of genetic testing in selected EOC patients in Thailand.
Although universal genetic testing in all EOC patients is recommended by various national professional societies, it may not apply in every country. The narrow availability of genetic testing, the high cost when not reimbursed, and the limited number of geneticists are major obstacles in Thailand. Selected EOC patients should initially be considered for genetic analysis. As the number of patients in this study is still small and since the study was conducted in only one tertiary hospital, it may not fully represent the Thai population. Further prospective studies with multicenter trials should be conducted. The incidence of germline mutations should be studied in unselected EOC Thai patients to identify significant risk factors. Furthermore, BRCA genes should not be the only focus of germline mutation studies, but these studies should also be expanded to include other homologous recombination and MMR genes.
Manuscript source: Invited manuscript
Specialty type: Oncology
Country of origin: Thailand
Peer-review report classification
Grade A (Excellent): 0
Grade B (Very good): 0
Grade C (Good): C
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Zhang XQ S-Editor: Yan JP L-Editor: A E-Editor: Liu MY
| 1. | Arts-de Jong M, de Bock GH, van Asperen CJ, Mourits MJ, de Hullu JA, Kets CM. Germline BRCA1/2 mutation testing is indicated in every patient with epithelial ovarian cancer: A systematic review. Eur J Cancer. 2016;61:137-145. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 66] [Cited by in RCA: 59] [Article Influence: 6.6] [Reference Citation Analysis (0)] |
| 2. | Committee opinion no.634: Hereditary cancer syndromes and risk assessment. Obstet Gynecol. 2015;125:1538-1543. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 30] [Cited by in RCA: 22] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 3. | Ledermann JA, Drew Y, Kristeleit RS. Homologous recombination deficiency and ovarian cancer. Eur J Cancer. 2016;60:49-58. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 123] [Cited by in RCA: 196] [Article Influence: 21.8] [Reference Citation Analysis (0)] |
| 4. | Committee on Practice Bulletins-Gynecology, Committee on Genetics, Society of Gynecologic Oncology. Practice Bulletin No 182: Hereditary Breast and Ovarian Cancer Syndrome. Obstet Gynecol. 2017;130:e110-e126. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 115] [Cited by in RCA: 137] [Article Influence: 17.1] [Reference Citation Analysis (0)] |
| 5. | National Comprehensive Cancer Network. Genetic/familial high-risk assessment: breast and ovarian. Version 1.2018, October 3, 2017 [cited 10 July 2017]. Available from: https://www.nccn.org/professionals/physician_gls/pdf/genetics_screening.pdf. |
| 6. | Sun C, Li N, Ding D, Weng D, Meng L, Chen G, Ma D. The role of BRCA status on the prognosis of patients with epithelial ovarian cancer: a systematic review of the literature with a meta-analysis. PLoS One. 2014;9:e95285. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 56] [Cited by in RCA: 75] [Article Influence: 6.8] [Reference Citation Analysis (0)] |
| 7. | Gadducci A, Guerrieri ME. PARP inhibitors alone and in combination with other biological agents in homologous recombination deficient epithelial ovarian cancer: From the basic research to the clinic. Crit Rev Oncol Hematol. 2017;114:153-165. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 24] [Cited by in RCA: 21] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 8. | Eccles DM, Balmaña J, Clune J, Ehlken B, Gohlke A, Hirst C, Potter D, Schroeder C, Tyczynski JE, Gomez Garcia EB. Selecting Patients with Ovarian Cancer for Germline BRCA Mutation Testing: Findings from Guidelines and a Systematic Literature Review. Adv Ther. 2016;33:129-150. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 46] [Cited by in RCA: 40] [Article Influence: 4.4] [Reference Citation Analysis (0)] |
| 9. | Chirasophon S, Manchana T, Teerapakpinyo C. High-risk epithelial ovarian cancer patients for hereditary ovarian cancer. J Obstet Gynaecol Res. 2017;43:929-934. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5] [Cited by in RCA: 6] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 10. | Manchana T, Kobwitaya K. Survival outcomes for different subtypes of epithelial ovarian cancer. Clin Res Obstet Gynecol. 2018;1:1-7. |
| 11. | Richards S, Aziz N, Bale S, Bick D, Das S, Gastier-Foster J, Grody WW, Hegde M, Lyon E, Spector E, Voelkerding K, Rehm HL; ACMG Laboratory Quality Assurance Committee. Standards and guidelines for the interpretation of sequence variants: a joint consensus recommendation of the American College of Medical Genetics and Genomics and the Association for Molecular Pathology. Genet Med. 2015;17:405-424. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 19696] [Cited by in RCA: 22206] [Article Influence: 2220.6] [Reference Citation Analysis (0)] |
| 12. | Zhang S, Royer R, Li S, McLaughlin JR, Rosen B, Risch HA, Fan I, Bradley L, Shaw PA, Narod SA. Frequencies of BRCA1 and BRCA2 mutations among 1,342 unselected patients with invasive ovarian cancer. Gynecol Oncol. 2011;121:353-357. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 271] [Cited by in RCA: 317] [Article Influence: 22.6] [Reference Citation Analysis (0)] |
| 13. | Alsop K, Fereday S, Meldrum C, deFazio A, Emmanuel C, George J, Dobrovic A, Birrer MJ, Webb PM, Stewart C, Friedlander M, Fox S, Bowtell D, Mitchell G. BRCA mutation frequency and patterns of treatment response in BRCA mutation-positive women with ovarian cancer: a report from the Australian Ovarian Cancer Study Group. J Clin Oncol. 2012;30:2654-2663. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 795] [Cited by in RCA: 951] [Article Influence: 73.2] [Reference Citation Analysis (0)] |
| 14. | Song H, Cicek MS, Dicks E, Harrington P, Ramus SJ, Cunningham JM, Fridley BL, Tyrer JP, Alsop J, Jimenez-Linan M, Gayther SA, Goode EL, Pharoah PD. The contribution of deleterious germline mutations in BRCA1, BRCA2 and the mismatch repair genes to ovarian cancer in the population. Hum Mol Genet. 2014;23:4703-4709. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 99] [Cited by in RCA: 116] [Article Influence: 10.5] [Reference Citation Analysis (0)] |
| 15. | Walsh T, Casadei S, Lee MK, Pennil CC, Nord AS, Thornton AM, Roeb W, Agnew KJ, Stray SM, Wickramanayake A, Norquist B, Pennington KP, Garcia RL, King MC, Swisher EM. Mutations in 12 genes for inherited ovarian, fallopian tube, and peritoneal carcinoma identified by massively parallel sequencing. Proc Natl Acad Sci USA. 2011;108:18032-18037. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 665] [Cited by in RCA: 728] [Article Influence: 52.0] [Reference Citation Analysis (0)] |
| 16. | Pennington KP, Walsh T, Harrell MI, Lee MK, Pennil CC, Rendi MH, Thornton A, Norquist BM, Casadei S, Nord AS, Agnew KJ, Pritchard CC, Scroggins S, Garcia RL, King MC, Swisher EM. Germline and somatic mutations in homologous recombination genes predict platinum response and survival in ovarian, fallopian tube, and peritoneal carcinomas. Clin Cancer Res. 2014;20:764-775. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 595] [Cited by in RCA: 780] [Article Influence: 65.0] [Reference Citation Analysis (0)] |
| 17. | Castéra L, Harter V, Muller E, Krieger S, Goardon N, Ricou A, Rousselin A, Paimparay G, Legros A, Bruet O, Quesnelle C, Domin F, San C, Brault B, Fouillet R, Abadie C, Béra O, Berthet P; French Exome Project Consortium, Frébourg T, Vaur D. Landscape of pathogenic variations in a panel of 34 genes and cancer risk estimation from 5131 HBOC families. Genet Med. 2018;20:1677-1686. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 45] [Cited by in RCA: 52] [Article Influence: 7.4] [Reference Citation Analysis (0)] |
| 18. | Wang Q, Wang B, Zhang YM, Wang W. The association between CDH1 promoter methylation and patients with ovarian cancer: a systematic meta-analysis. J Ovarian Res. 2016;9:23. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 17] [Cited by in RCA: 19] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 19. | Sekine M, Nagata H, Tsuji S, Hirai Y, Fujimoto S, Hatae M, Kobayashi I, Fujii T, Nagata I, Ushijima K, Obata K, Suzuki M, Yoshinaga M, Umesaki N, Satoh S, Enomoto T, Motoyama S, Tanaka K; Japanese Familial Ovarian Cancer Study Group. Mutational analysis of BRCA1 and BRCA2 and clinicopathologic analysis of ovarian cancer in 82 ovarian cancer families: two common founder mutations of BRCA1 in Japanese population. Clin Cancer Res. 2001;7:3144-3150. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 58] [Cited by in RCA: 58] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 20. | Kang HC, Kim IJ, Park JH, Kwon HJ, Won YJ, Heo SC, Lee SY, Kim KH, Shin Y, Noh DY, Yang DH, Choe KJ, Lee BH, King SB, Park JG. Germline mutations of BRCA1 and BRCA2 in Korean breast and/or ovarian cancer families. Hum Mutat. 2002;20:235. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 38] [Cited by in RCA: 40] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 21. | Lim MC, Kang S, Seo SS, Kong SY, Lee BY, Lee SK, Park SY. BRCA1 and BRCA2 germline mutations in Korean ovarian cancer patients. J Cancer Res Clin Oncol. 2009;135:1593-1599. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 46] [Cited by in RCA: 45] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 22. | GLOBOCAN 2018: IARC Estimated age-standardized incidence rates (World) in 2018, ovary, all ages. 2018 [cited 01 Jan 2019]. Available from: http://gco.iarc.fr/today. |
| 23. | Sakamoto I, Hirotsu Y, Nakagomi H, Ouchi H, Ikegami A, Teramoto K, Amemiya K, Mochizuki H, Omata M. BRCA1 and BRCA2 mutations in Japanese patients with ovarian, fallopian tube, and primary peritoneal cancer. Cancer. 2016;122:84-90. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 46] [Cited by in RCA: 48] [Article Influence: 4.8] [Reference Citation Analysis (0)] |
| 24. | Chao A, Chang TC, Lapke N, Jung SM, Chi P, Chen CH, Yang LY, Lin CT, Huang HJ, Chou HH, Liou JD, Chen SJ, Wang TH, Lai CH. Prevalence and clinical significance of BRCA1/2 germline and somatic mutations in Taiwanese patients with ovarian cancer. Oncotarget. 2016;7:85529-85541. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 33] [Cited by in RCA: 41] [Article Influence: 5.9] [Reference Citation Analysis (0)] |
| 25. | Hasmad HN, Lai KN, Wen WX, Park DJ, Nguyen-Dumont T, Kang PCE, Thirthagiri E, Ma'som M, Lim BK, Southey M, Woo YL, Teo SH. Evaluation of germline BRCA1 and BRCA2 mutations in a multi-ethnic Asian cohort of ovarian cancer patients. Gynecol Oncol. 2016;141:318-322. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 13] [Cited by in RCA: 17] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 26. | Choi MC, Heo JH, Jang JH, Jung SG, Park H, Joo WD, Lee C, Lee JH, Lee JM, Hwang YY, Kim SJ. Germline Mutations of BRCA1 and BRCA2 in Korean Ovarian Cancer Patients: Finding Founder Mutations. Int J Gynecol Cancer. 2015;25:1386-1391. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 23] [Cited by in RCA: 22] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 27. | Wu X, Wu L, Kong B, Liu J, Yin R, Wen H, Li N, Bu H, Feng Y, Li Q, Lu X, Wei J, Zhu X, Mills J, Ellison G, Gutjahr T, Liu Y. The First Nationwide Multicenter Prevalence Study of Germline BRCA1 and BRCA2 Mutations in Chinese Ovarian Cancer Patients. Int J Gynecol Cancer. 2017;27:1650-1657. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 64] [Cited by in RCA: 66] [Article Influence: 8.3] [Reference Citation Analysis (0)] |
| 28. | Mabuchi S, Sugiyama T, Kimura T. Clear cell carcinoma of the ovary: molecular insights and future therapeutic perspectives. J Gynecol Oncol. 2016;27:e31. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 90] [Cited by in RCA: 90] [Article Influence: 10.0] [Reference Citation Analysis (0)] |
| 29. | Nelson HD, Pappas M, Zakher B, Mitchell JP, Okinaka-Hu L, Fu R. Risk assessment, genetic counseling, and genetic testing for BRCA-related cancer in women: a systematic review to update the U.S. Preventive Services Task Force recommendation. Ann Intern Med. 2014;160:255-266. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 166] [Cited by in RCA: 188] [Article Influence: 17.1] [Reference Citation Analysis (0)] |