Published online Jan 10, 2019. doi: 10.5306/wjco.v10.i1.14
Peer-review started: September 25, 2018
First decision: October 16, 2018
Revised: November 29, 2018
Accepted: December 17, 2018
Article in press: December 17, 2018
Published online: January 10, 2019
Processing time: 93 Days and 5.6 Hours
Stereotactic body radiation therapy (SBRT) is the treatment of choice for medically inoperable patients with early stage non-small cell lung cancer (NSCLC). A literature search primarily based on PubMed electronic databases was completed in July 2018. Inclusion and exclusion criteria were determined prior to the search, and only prospective clinical trials were included. Nineteen trials from 2005 to 2018 met the inclusion criteria, reporting the outcomes of 1434 patients with central and peripheral early stage NSCLC. Patient eligibility, prescription dose and delivery, and follow up duration varied widely. Three-years overall survival ranged from 43% to 95% with loco-regional control of up to 98% at 3 years. Up to 33% of patients failed distantly after SBRT at 3 years. SBRT was generally well tolerated with 10%-30% grade 3-4 toxicities and a few treatment-related deaths. No differences in outcomes were observed between conventionally fractionated radiation therapy and SBRT, central and peripheral lung tumors, or inoperable and operable patients. SBRT remains a reasonable treatment option for medically inoperable and select operable patients with early stage NSCLC. SBRT has shown excellent local and regional control with toxicity rates equivalent to surgery. Decreasing fractionation schedules have been consistently shown to be both safe and effective. Distant failure is common, and chemotherapy may be considered for select patients. However, the survival benefit of additional interventions, such as chemotherapy, for early stage NSCLC treated with SBRT remains unclear.
Core tip: Stereotactic body radiation therapy (SBRT) offers excellent local and regional control for early stage non-small cell lung cancer (NSCLC), and is often the treatment of choice for medically inoperable patients. This literature review provides an updated analysis of prospective clinical trials evaluating clinical outcomes following SBRT for early stage NSCLC.
- Citation: Prezzano KM, Ma SJ, Hermann GM, Rivers CI, Gomez-Suescun JA, Singh AK. Stereotactic body radiation therapy for non-small cell lung cancer: A review. World J Clin Oncol 2019; 10(1): 14-27
- URL: https://www.wjgnet.com/2218-4333/full/v10/i1/14.htm
- DOI: https://dx.doi.org/10.5306/wjco.v10.i1.14
Early studies have demonstrated the efficacy of conventionally fractionated radiotherapy for the treatment of stage I non-small cell lung cancer (NSCLC). Haffty et al[1] reported on 43 patients with stage I NSCLC from 1970-1983 who had been deemed medically inoperable or who had refused surgical resection. When treated with a median of 59 Gy in 2 Gy per fraction, 5 year overall survival was reported at 21%. Subsequent studies have demonstrated efficacy for radiation doses exceeding 60 Gy[2,3]. In particular, T1 tumors treated with > 65 Gy had significantly reduced risk of recurrence compared to T2 and T3 tumors or doses ≤ 65 Gy[3]. A more modern analysis of stage I, node negative patients staged with computed tomography (CT) and treated with a median dose of 63.2 Gy showed increased cause-specific survival in the subset of patients who received ≥ 65 Gy[4]. While conventionally fractionated radiotherapy can provide a reasonable alternative to surgical resection in medically inoperable patients, the 5-year overall survival rates reported in these early studies were suboptimal at 10%-30%[1-4]. As the delivery of radiation has improved over time, SBRT has emerged as an alternative to very precisely deliver a high dose of radiation in a small number of fractions[5].
Surgery remains the standard of care for medically operable early stage NSCLC. Stereotactic body radiation therapy (SBRT) also referred to as stereotactic ablative radiotherapy (SABR), has become the preferred treatment option for medically inoperable patients with significant comorbidities or for patients who decline surgery. This article will review major concepts in the use of SBRT for primary early-stage NSCLC, including technical considerations and reported outcomes and toxicities from major clinical trials, with a specific emphasis on fractionation and future directions.
We conducted a comprehensive literature search for journal articles written in English and published between January 2000 and July 2018. The inclusion criterion was any prospective clinical trial reporting clinical outcomes of primary early stage NSCLC definitively treated with SBRT. The exclusion criteria were the following: (1) review articles, case reports, or letters to editors; (2) studies that did not report the most updated outcomes when multiple publications resulted from the same patient cohort; (3) duration of follow-up shorter than one year; (4) sample size fewer than 30; (5) multiple primary lung tumors; and (6) lung oligometastasis or advanced stage NSCLC.
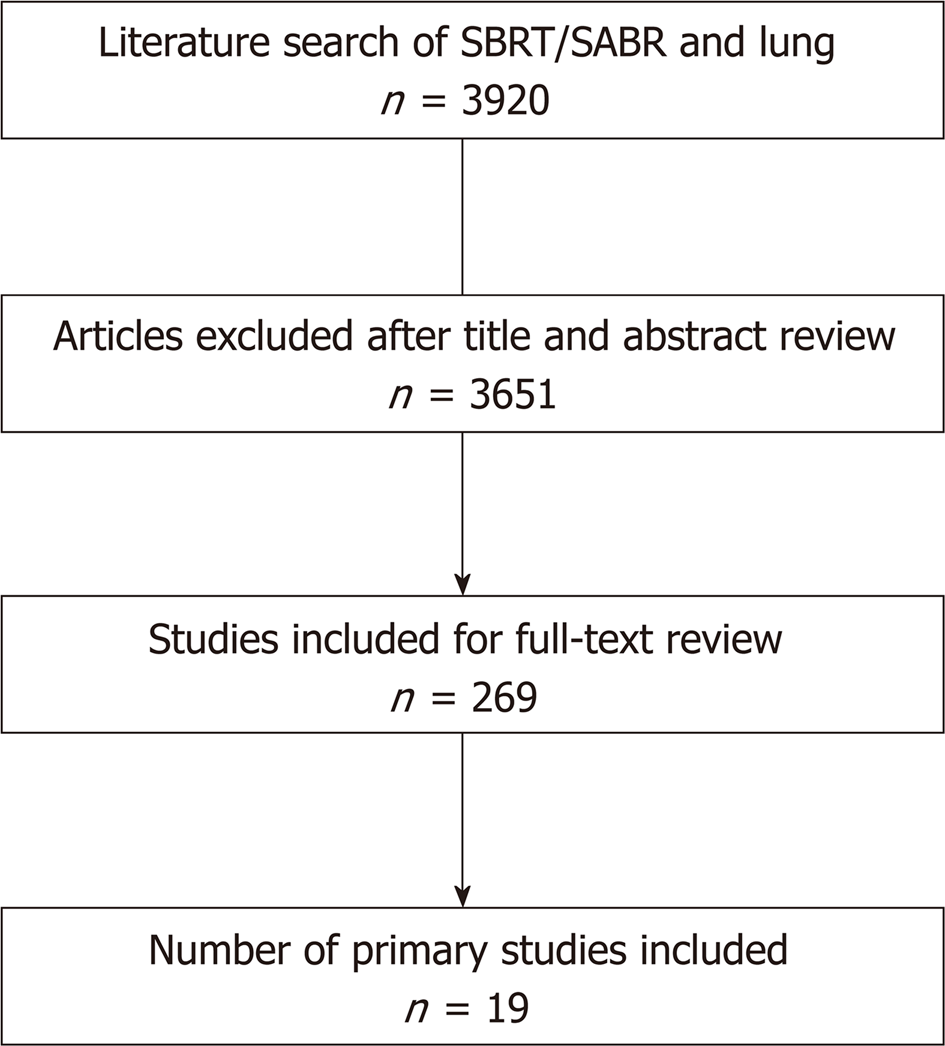
The search was completed in July 2018. Studies included were identified by performing a search of literature existing in the PubMed database. The PubMed electronic database was queried for search terms including “SBRT”, “stereotactic body radiotherapy”, and “SABR”, along with their respective acronyms, and “lung” or “NSCLC”. This database query initially produced 3920 results. Of these, 3631 studies were in the English language. Limiting this selection to prospective clinical trials reduced the results to 269 entries. After a thorough review of the literature, any study meeting the above criteria but not listed in PubMed was additionally included. By applying our inclusion and exclusion criteria, these studies and their reference lists were evaluated by two reviewers to determine their suitability for inclusion (Figure 1).
Nineteen studies meeting our criteria were selected for inclusion in this review (Table 1). Publication years ranged from 2005 to 2017. The mean number of patients included in the trials was 75 (range 31 to 180), with a median follow-up between 16 to 86 mo. Dose fractionation schedules varied widely. Using a α/β ratio of 10, the total biologically effective doses (BED) included were > 100 Gy10 in almost all studies, except for two. Shibamoto et al[6] treated four patients, whose tumors were less than 1.5 cm in diameter, with 44 Gy in 4 fractions. Similarly, a small number of patients were treated with BED < 100 Gy in the dose escalation study authored by McGarry et al[7].
| Study | No. | F/u (median) | Age (median) | Loc | Stage | Dose/fx | OS | LC | RC | DC |
| Miyakawa et al[20], 2017 | 71 | 44 | 77 | C + P | T1-2N0M0 | 48-52 Gy/ 4 fx | 5-yr 65% | 5-yr 85% | NA | NA |
| Sun et al[80], 2017 | 65 | 86 | 71 | C + P | T1-2N0M0 | 50 Gy/4 fx | 7-yr 48% | 7-yr 92% | 7-yr 86% | 7-yr 86% |
| Singh et al[22], 2017, I-124407 | 98 | 27 | NA | P | T1-2N0M0 | 30 Gy/1 fx and 60 Gy/3fx | 2-yr 71% (30 Gy) | NA | NA | NA |
| 2-yr 61% (60 Gy) | ||||||||||
| Bezjak et al[24], 2016, RTOG 0813 | 71 | 33 (57.5 Gy) | NA | C | T1-2N0M0 | 57.5-60 Gy/5 fx | 2-yr 70% (57.5 Gy) | 2-yr 90% (57.5 Gy) | 2-yr 95% (57.5 Gy) | 2-yr 84% (57.5 Gy) |
| 30 (60 Gy) | 2-yr 88% (60 Gy) | 2-yr 88% (60 Gy) | 2-yr 88% (60 Gy) | 2-yr 85% (60 Gy) | ||||||
| Navarro-Martin et al[11], 2016 | 38 | 42 | 74 | P | T1-3N0M0 | 54 Gy/3 fx | 3-yr 66% | 3-yr 94% | 3-yr 79% | 3-yr 87% |
| Nyman et al[14], 2016, SPACE | 102 | 37 | 74 (mean) | P | T1-2N0M0 | 66 Gy/3 fx | 3-yr 54% | 3-yr 86% | 3-yr 93% | 3-yr 76% |
| Chang et al[15], 2015, STARS and ROSEL | 31 | 40 | 67 | C + P | T1-2N0M0 | 54 Gy/3 fx, 50 Gy/4 fx, 60 Gy/5 fx | 3-yr 95% | 3-yr 96% | 3-yr 90% | 3-yr 97% |
| Lindberg et al[19], 2015 | 57 | 42 | 75 (mean) | P | T1-2N0M0 | 45 Gy/3 fx | 5-yr 30% | 5-yr 79% | 3-yr 81% for regional/distant control | NA |
| Nagata et al[12], 2015, JCOG 0403 | 169 | 47 (inop) | 78 | NA | T1N0M0 | 48 Gy/4 fx | 3-yr 60% | 3-yr 87% (inop) | 3-yr 92% (inop) | 3-yr 78% (inop) |
| 67 (op) | 5-yr 43% (inop) | 3-yr 85% (op) | 3-yr 75% (op) | 3-yr 67% (op) | ||||||
| 3-yr 77% | ||||||||||
| 5-yr 54% (op) | ||||||||||
| Shibamoto et al[6], 2015 | 180 | 53 | 77 | C + P | T1-2N0M0 | 44-52 Gy /4 fx | 5-yr 52% | 5-y 83% | 5-yr 84% | 5-yr 76% |
| Videtic et al[13], 2015, RTOG 0915 | 94 | 30 | 75 | P | T1-2N0M0 | 34 Gy/1 fx and 48 Gy/4 fx | 3-yr 56% | 3-yr 98% | NA | NA |
| Timmerman et al[18], 2014, RTOG 0236 | 55 | 48 | 72 | P | T1-2N0M0 | 54 Gy/3 fx | 5-yr 40% | 5-yr 80% | 5-yr 62% (local-regional control) | 5-yr 79% |
| Taremi et al[26], 2012 | 108 | 19 | 73 (mean) | C + P | T1-2N0M0 | 48 Gy/4 fx or 54-60 Gy/3 fx (P) | 4-yr 30% | 4-yr 89% | 4-yr 87% | 4-yr 83% |
| 50-60 Gy /8-10 fx (C) | ||||||||||
| Bral et al[46], 2011 | 40 | 16 | 73 (mean) | C + P | T1-3N0M0 | 60 Gy/3-4 fx | 2-yr 52% | 2-yr 84% | 2 nodal recurrences | 6 distant recurrences |
| Ricardi et al[16], 2010 | 62 | 28 | 74 | P | Stage I | 45 Gy/3 fx | 3-yr 57% | 3-yr 88% | 3-yr 94% | 3-yr 76% |
| Fakiris et al[17], 2009 | 70 | 50 | 70 | C + P | T1-2N0M0 | 60-66 Gy/ 3 fx | 3-yr 43% | 3-yr 88% | 3-yr 91% | 3-yr 87% |
| Koto et al[10], 2007 | 31 | 32 | 77 | C + P | T1-2N0M0 | 45 Gy/3 fx or 60 Gy/8 fx | 3-yr 72% | 3-yr 78% (T1) | 3-yr 94% | 3-yr 81% |
| 3-yr 40% (T2) | ||||||||||
| McGarry et al[7], 2005 | 47 | 27 (Stage IA) | 71 (Stage IA) | C + P | T1-2N0M0 | 24-72 Gy/ 3 fx | NA | 2-yr 81% | 2-yr 81% | 2-yr 79% |
| 19 (Stage IB) | 74 (Stage IB) | |||||||||
| Nagata et al[8], 2005 | 45 | 30 (Stage IA) | 77 (Stage IA) | C + P | T1-2N0M0 | 48 Gy/4 fx | 2-yr 90% (Stage IA) | 1-yr 100% | 2-yr 91% | 2-yr 88% (Stage IA) |
| 22 (Stage IB) | 73 (Stage IB) | 2-yr 72% (Stage IB) | 2-yr 77% (Stage IB) |
Two of the earliest studies for early stage NSCLC treated with SBRT were reported by McGarry et al[7] and Nagata et al[8], who both showed promising local and regional control rates, and distant failure only recorded in patients with T2 disease. In the United States, Timmerman et al[9] reported initial results of a phase I study, demonstrating that SBRT was well tolerated, with updated results finding that the majority of local failure was seen in patients receiving ≤ 48 Gy[7]. Of the included studies that estimated 3-year results, reported overall survival percentages ranging from 43% to 95% and local control rates as high as 98%[10-17]. In the four studies with 5-year outcomes, local control was reported between 79%-85%[6,18-20]. Distant control at three years ranged from 76%-97%[10-17]. Reported outcomes from the included studies are tabulated in Table 1.
Radiation Therapy Oncology Group (RTOG) 0236 was a phase II North American multicenter study of 55 medically inoperable patients with peripheral NSCLC treated with 54 Gy in 3 fractions. The study initially reported 3-year local control rate of 91% and distant failure in 22%[21]. Updated 5-year results showed 5-year local control of 80% and distant failure of 31%[18]. With promising results from the RTOG 0236 3-fraction regimen for peripheral NSCLC, a multicenter, phase II study, I-124407, was undertaken to compare 30 Gy in 1 fraction and 60 Gy in 3 fractions. This study evaluated 98 patients with a median follow up of 27 mo and showed 2-year overall survival of 71% for single fraction and 61% for 3 fraction regimens. There was no difference in survival or toxicity between the regimens[22].
Similarly, building on the results of the 4 fraction regimen by Nagata et al[8], the comparison of 34 Gy in 1 fraction and 48 Gy in 4 fractions was investigated in a multicenter phase II study, RTOG 0915, by Videtic et al[13]. The study assessed 94 patients with a median follow up of 30 mo, showing 2-year overall survival of 61% for single fraction and 78% for 4 fraction regimens. No difference in overall survival, primary tumor control, and toxicity was seen between these regimens.
As conventionally fractionated radiation therapy has also improved over time, the multicenter Scandinavian phase II SPACE trial is the only publication that has reported results comparing SBRT (66 Gy in 3 fractions) to conventionally fractionated radiotherapy (70 Gy in 35 fractions). Despite an imbalance in the number of patients with T2 tumors and of male gender (both of these negative prognostic factors were increased in the SBRT arm), there was no statistically significant difference in 1-, 2-, or 3-year overall survival (81% vs 89%, 68% vs 72%, 54% vs 59%, respectively, for SBRT vs conventionally fractionated arms). Favorable results were also reported for local control (86.4% in the SBRT arm vs 85.7% in the conventional fractionation arm)[14].
Timmerman et al[23] reported a phase II study of 70 medically inoperable patients with both peripheral and central tumors treated with 60-66 Gy in 3 fractions. With a median follow up of 17.5 mo, the study initially reported 2-year local control of 95% with grade 3-4 toxicity seen in 8 patients (11%) and treatment-related death in 6 patients (9%). Central location was initially shown to be an adverse prognostic factor for toxicity, but this did not remain significant in the updated report by Fakiris et al[17].
The NRG/RTOG 0813 phase I/II trial evaluated NSCLC patients with centrally located tumors, defined as within 2 cm of the proximal bronchial tree or adjacent to the mediastinal or pericardial pleura. Successively accruing patients into a dose-escalating 5-fraction SBRT schedule, ranging from 10-12 Gy/fraction, the study was designed to determine the maximal tolerated dose. The highest dose level allowed by the protocol, 12 Gy/fraction, was achieved, with only 7.2% dose-limiting toxicities reported in the preliminary phase I analysis. Two-years overall survival rates were reported at 70%[24].
While many of these trials included medically inoperable patients only, a multicenter Japanese phase II Japan Clinical Oncology Group (JCOG) 0403 study stratified patients who received SBRT for T1N0M0 non-small cell lung tumors into medically operable and inoperable categories. All patients received 48 Gy in 4 fractions. Overall survival at 3 years was reported as 59.9% in the inoperable group vs 76.5% in the operable group[12]. Despite being comprised of a relatively older population (median age of 79 years), their results were similar to other studies with younger median age populations[15,25].
Among operable patients only, lobectomy was compared with SBRT in two phase III trials, STARS (NCT00840749) and ROSEL (NCT00687986), both of which were closed early due to slow accrual. Nonetheless, Chang et al[15] reported a pooled analysis of 58 patients who were enrolled, with a median follow up of 40 mo for SBRT and 35 mo for surgery. In the STARS trial, peripheral and central lung tumors received 54 Gy in 3 fractions and 50 Gy in 4 fractions, respectively. In the ROSEL study, only peripheral lung tumors were included and received either 54 Gy in 3 fractions or 60 Gy in 5 fractions. Overall survival at 3-years was 95% for SBRT and 79% for surgery. Local control at 3 years was 96% for SBRT and 100% for surgery. Distant failure at 3 years was 3% for SBRT and 7% for surgery.
In the collected studies, several toxicity measures were analyzed, with all papers citing National Cancer Institute Common Criteria grading of lung toxicity. The reported toxicities from included studies can be referred to in Table 2.
| Study | Grade 3 + toxicity | Reported adverse events |
| Miyakawa et al[20], 2017 | Grade 3-5, 5.6% | Radiation pneumonitis |
| Sun et al[80], 2017 | Grade 3, 5% | Dermatitis, radiation pneumonitis, chest wall pain |
| Singh et al[22], 2017, I-124407 | Grade 3, 30% | NA |
| Bezjak et al[24], 2016, RTOG 0813 | Grade 3-5, 16%-21% | Respiratory and cardiac toxicities, esophageal perforation, pulmonary hemorrhage |
| Navarro-Martin et al[11], 2016 | Grade 3, 10% | Cough, dyspnea, dermatitis |
| Nyman et al[14], 2016, SPACE | Grade 3, 14% | Dyspnea, cough, skin reactions |
| Chang et al[15], 2015, STARS and ROSEL | Grade 3, 10% | Chest wall pain, cough, fatigue, rib fracture |
| Lindberg et al[19], 2015 | Grade 3-4, 30% | Rib fracture, dyspnea, ventricle tachycardia, cough, fatigue, fibrosis, lung infection, pain, pericardial effusion |
| Inop: Dyspnea, hypoxia, pneumonitis, chest pain, cough | ||
| Nagata et al[12], 2015, JCOG 0403 | Grade 3-4, 13% (inop) Grade 3, 6% (op) | Op: Dyspnea, hypoxia, pneumonitis, chest pain |
| Shibamoto et al[6], 2015 | Grade 3, < 10% | Radiation pneumonitis, pleural effusion, esophagitis, rib fracture, dermatitis |
| Videtic et al[13], 2015, RTOG 0915 | Grade 3-5, 12% | DLCO changes, pneumonitis, PFT changes, 2 treatment-related deaths |
| Timmerman et al[18], 2014, RTOG 0236 | Grade 3-4, 31% | Hypocalcemia, hypoxia, pneumonitis, PFT decreased |
| Taremi et al[26], 2012 | Grade 3, 11% | Fatigue, cough, chest wall pain, rib fracture |
| Bral et al[46], 2011 | Grade 3, 20% | Pneumonitis, cough |
| Ricardi et al[16], 2010 | Grade 3-4, 3% | Radiation pneumonitis |
| Fakiris et al[17], 2009 | Grade 3-5, 16% | Apnea, pneumonia, pleural effusion, hemoptysis, respiratory failure, skin erythema |
| Koto et al[10], 2007 | Grade 3, 3% | Pneumonitis |
| McGarry et al[7], 2005 | Grade 3-4, 15% | Pneumonitis, hypoxia, dermatitis, pericardial effusion, tracheal necrosis |
| Nagata et al[8], 2005 | None | None |
Grade 3 toxicity ranged from 3%-20%, with grade 5 (or fatal) toxicities only detailed by three studies. Fakiris et al[13] noted 12 grade 3-5 toxicities, with the potential treatment-related grade 5 toxicities reported as pneumonia (n = 3), hemoptysis (n = 1), and respiratory failure (n = 1). RTOG 0915 reported one patient death in the single-fraction arm approximately 2 wk after treatment, with the death thought to be unconnected to SBRT. The four-fraction arm had a patient fatality 319 d after treatment due to respiratory failure thought to be related to SBRT. No difference in toxicity was reported between the single fraction vs multi-fraction arms in either RTOG 0915 or I-124407[22].
Rates of toxicities did appear to increase with greater follow-up. For example, 9 patients (16%) with a median follow up of 34 mo were initially reported to have grade 3-4 toxicities in RTOG 0236, but updated results at 4 years found 17 patients (31%) treated with 54 Gy in 3 fractions reporting grade 3-4 toxicities[18,21]. Rib fractures were recorded in 0-18% of patients in the included studies[26]. Late toxicities such as esophageal perforation and fatal pulmonary hemorrhage were documented in the 5 fraction arm of the NRG/RTOG 0813 dose escalation trial for centrally located lung tumors[24].
In the pooled analysis of the STARS and ROSEL studies, Chang et al[15] recorded treatment-related grade 3 toxicities in 10% of patients who underwent SBRT, contrasted with 44% of patients treated surgically who suffered grade 3-4 toxicities, including bleeding, fistula, hernia, anemia, weight loss, and cardiac arrhythmias. One patient died of surgical complications.
When compared to conventionally fractionated radiotherapy, toxicity was shown to be less prevalent in the SBRT arm of the SPACE trial, including rates of esophagitis (8% vs 30%), borderline significant pneumonitis (19% vs 34%) and dyspnea (67% vs 81%)[14]. Additionally, patient-reported quality of life data showed significantly worse dyspnea and chest pain in the three dimensional conformal radiation therapy arm compared to SBRT[14].
Despite the widely varying dose fractionation regimens, patient populations, and primary outcomes included in these prospective trials, results were similarly favorable. High rates of local control and overall survival have been reported, along with favorable toxicity outcomes. These included studies comparing fractionation schemes, operable vs non-operable candidates, and tumor location have paved the way for additional questions to be addressed in future studies.
We acknowledge the limitations of this review. The included studies treated patients over a large time frame with multiple inclusion criteria, differing tumor location, dose fractionation regimens, and prescription methods. Techniques of SBRT delivery were also inconsistent. Different versions of Common terminology criteria for adverse events were used to assess toxicities due to various publication years. Notably, a validity assessment of included studies to evaluate the risk of bias and confidence of results was not undertaken. Unpublished studies are unable to be adequately assessed, and this, too, may lead to an important bias leaning toward the effectiveness of treatment or the under-estimation of toxicities. Despite these limitations, published outcomes with SBRT are consistently promising. Because of this promise, increased attention should be paid to delivering regimens that can improve patients’ quality of life.
Survival and tumor control results were excellent in the included prospective studies, compared to historic controls in this patient population. As radiation techniques have evolved, the delivery of high dose radiation in fewer fractions has also become more precise. The use of intra-fraction volumetric imaging with cone beam CT can reduce target error compared to use of patient setup or bony anatomy alone[27,28]. Intra-fraction imaging is recommended as best practice per ESTRO ACROP guidelines[29]. Because a faster treatment delivery time is likely associated with less patient movement and therefore more accurate treatment delivery, the use of a flattening-filter free setting can help to optimize treatment delivery as well[30-32]. The use of heterogeneity corrections has also been shown in RTOG 0236 to have a significant effect on prescription dose and tumor coverage, and should be considered standard in SBRT treatments[33]. Taken together, these technological advances may also be contributing to improved outcomes in this patient population.
Using an α/β ratio of 10, the vast majority of patients were treated with total BED > 100 Gy10, which has been shown to improve outcomes in NSCLC patients treated with SBRT[34]. Others have argued that biologically effective dose calculations, and the linear quadratic model on which they are constructed, may not be applicable for high fractional doses of radiation[35]. The radiobiological principles upon which the linear quadratic model is based, however, do not account for differences in re-oxygenation, the effects on tumor vasculature and the enhanced host immunity that hypofractionation can produce. Nevertheless, the use of BED > 100 Gy10 has been adopted as a recommendation for SBRT delivery by the National Comprehensive Cancer Network guidelines and American College of Radiology appropriateness criteria[36,37].
Better staging and delivery techniques have helped improve outcomes compared to historical data with conventionally fractionated radiation therapy. The SPACE trial recently demonstrated equivalent survival outcomes compared with SBRT[14]. Although patients treated with SBRT reported better quality of life and decreased toxicity profiles, the improvement of survival and local control seen in conventionally fractionated radiation therapy during the past several decades is still notable[14]. Other trials, such as CHISEL study (NCT01014130) and LUSTRE trial (NCT01968941) are currently ongoing and will further investigate the role of conventionally fractionated radiation therapy.
Given the decreased number of visits and favorable toxicity profiles, SBRT offers increased patient convenience and improved quality of life outcomes compared to conventionally fractionated radiation therapy. It would seem that this advantage would be even greater with a decreasing number of SBRT fractions. Amongst the prospective studies included in this review, widely varying dose fractionations have been studied, with only a few comparisons evaluated. Of note, the 5-fraction regimen, which is a commonly used fractionation schema nationwide[38], has very limited prospective data, and no prospective, comparative data showing superiority. On the other hand, single-fraction dosing, which has been tested in both RTOG 0915 and I-124407, did not show a difference in toxicity or survival outcomes compared to multi-fraction regimens[13,22].
A follow-up study to RTOG 0915 was not funded because the issue of fractionation was not deemed to be of high enough priority by the National Cancer Institute. In the absence of federal funding for further prospective trials of fractionation, retrospective reviews will have to suffice. Our retrospective review of all patients treated with single- vs three-fraction regimens for peripheral early-stage NSCLC at our institution was concordant with the results from our prospective trial[22] and did not show any significant difference in overall survival, progression-free survival, local failure, nodal failure, or distant failure at 24 mo, despite including patients with lower performance status in the single-fraction cohort[39]. A propensity matched cohort analysis of the 3-fraction SBRT regimen used at our institution and a 5-fraction regimen used at another academic institution showed comparable overall survival, progression-free survival, local control and distant control rates[40]. This is consistent with other retrospective analyses[41]. Most recently, we expanded the two-institution analysis to include 163 patients comparing single-fraction vs five-fraction SBRT and again found no difference in survival outcomes or local control[42].
Overall, with robust prospective and retrospective evidence showing high rates of local control and comparable safety outcomes to multi-fraction regimens, our institution has adopted the single-fraction radiation schedule for peripheral, early-stage NSCLCs.
Since the definition of a “No Fly Zone” in the 2006 publication by Timmerman et al[23] the spatial proximity of organs at risk, such as main airways, large blood vessels, the heart and esophagus has been the basis of the distinction between centrally and peripherally located NSCLC. Although updated results 3 years later by Fakiris et al[17] showed there was no difference in survival and toxicity between central and peripheral tumors, several subsequent trials have investigated central or peripheral tumors separately. Overall survival outcomes reported from NRG/RTOG 0813 were noted to be comparable to elderly, medically inoperable patients with peripheral early stage tumors. Despite the safety concerns for the treatment of central tumors, this trial also demonstrated reasonable toxicities, though we await the published manuscript.
A literature review of 20 publications reporting outcomes for 563 central lung tumors treated with SBRT included a majority of single-institution retrospective analyses, with only four prospective studies including 68 patients. Tumor location did not appear to impact overall survival, with overall treatment-related mortality reported as 2.7%. As might be expected, Grade 3 and 4 toxicities were more prevalent for central tumors, but occurred in < 9% of patients[43].
We have previously reported a case of single-fraction SBRT for a solitary metastasis of squamous cell carcinoma in the right hilum which resulted in complete response of the tumor, but sudden grade 4 bronchopulmonary hemorrhage 13 mo after treatment[44]. Given their location near critical organs, treatment of central tumors is inherently risky, with any fractionation schema predisposing to increased toxicity rates compared to tumors located peripherally.
The recently reported Nordic HILUS-Trial was a prospective, multi-center, non-randomized phase II trial of SBRT for central lung tumors (either primary NSCLC or metastasis), which treated patients with 8 fractions of 7 Gy/fraction, and stratified patients based on tumor location near a mainstem bronchus vs a lobar bronchus. Initial results have been published in abstract form. Twenty-one of the 74 included patients developed grade 3 or higher toxicities, with seven patients suffering fatal effects of hemoptysis (n = 6) or pneumonitis (n = 1)[45]. The LungTech trial (EORTC 22113-08113), which aims to evaluate efficiency and toxicity of SBRT in patients with centrally located tumors, is ongoing.
In our review, despite widely varying inclusion criteria, dose fractionation schemas, and institutional protocols, most trials demonstrated excellent local and regional control for early stage NSCLC[6-8,10-19,22,24,26,46]. Among operable patients treated with SBRT, 3-year overall survival was 77%-95%. Grade 3-4 toxicity rates were 10%-30% with a few treatment-related deaths, most notably observed in treatment of central lung tumors[6-8,10-19,22,24,26,46]. These findings are comparable to perioperative complication rates of 15%-25% and the 30-d postoperative mortality rate of 1.7% seen in video-assisted thoracic surgery and open lobectomy in recent trials[47,48].
In the JCOG 0403 trial, the lower median overall survival reported for the patients deemed medically inoperable was likely complicated by the increased number of comorbidities and decreased performance status of that group, making any direct comparison problematic[12]. It would be similarly challenging to draw conclusions about SBRT as a viable alternative to lobectomy from the results of the STARS and ROSELS pooled analysis due to the small sample size and short follow up time[15]. More recently, a brief report was issued regarding results from the single-arm, phase 2 NRG Oncology RTOG 0618 trial, which evaluated SBRT for operable, peripheral T1-2 NSCLC. Of the 26 patients evaluated, only 1 patient had a primary tumor recurrence, and there were no lobular failures at a median follow-up of 48.1 mo. Four-year overall survival was reported as 56%, and median overall survival 55.2 mo[49].
Regardless, distant failure rates of up to 34% are common for both SBRT and surgery[6-8,10-19,22,24,26,46,47]. This is likely due to the fact that despite negative findings in initial nodal sampling, nearly 20% of patients are upstaged pathologically from clinical Stage I[47]. Additional studies have reported up to 30%-35% pathologic upstaging at the time of surgery[50,51]. The incidence of occult mediastinal lymph node metastasis in patients with negative uptake on positron emission tomographic/computed tomographic (PET/CT) imaging was as high as 22%, especially in centrally located NSCLC tumors[52,53]. These findings are unsurprising since PET/CT, mediastinoscopy, and minimally invasive biopsy techniques such as endobronchial ultrasound transbronchial needle aspiration are less sensitive for nodal metastasis compared to nodal dissection[54-57].
A randomized trial of lung resection combined with nodal dissection published results showing improved survival among early stage NSCLC[58]. Despite including three-quarters of patients with stage II-III disease, the distant failure rate for patients undergoing systematic nodal dissection was a promising 22.5% without adjuvant chemotherapy vs 30.7% of patients who had mediastinal lymph node sampling[58]. However, if lymph nodes are sampled extensively prior to surgery to rule out nodal metastasis, systematic nodal dissection does not improve survival or reduce distant failure[59]. At this time, there is no evidence that clinically early stage NSCLC will benefit from intensive lymph node staging prior to SBRT[60], and several trials are currently investigating the potential role of invasive lymph node staging (NCT01786590, NCT02719847).
Our institution has undertaken a pilot study to evaluate the role of trans-cervical extended mediastinal lymphadenectomy (TEMLA) in combination with SBRT for Stage III NSCLC. The methodology of this study has been previously described[61]. TEMLA was completed and then followed by either surgical resection or single-fraction SBRT to the primary site, followed by 10 Gy SBRT directed to the mediastinum and/or positive surgical margin. Ten patients completed the study with preliminary results suggesting that the regimen is both well tolerated and provides good regional control[62]. These findings further suggest that SBRT may be potentially expanded for use in regionally advanced disease.
The SBRT technique allows for a high radiation dose to be delivered to a tumor target while maintaining a rapid drop-off gradient. Since it is assumed that an ablative dose delivered to the target alone should be safe, the toxicity associated with treatment must be related to dose inadvertently deposited in surrounding tissues[63]. These include toxicities such as chest wall pain and rib fractures in treatment of peripheral tumors, and decline in pulmonary function tests, pneumonia, and pleural or pericardial effusions in treatment of tumors in the central chest region[23,46,64,65]. These studies collectively show that toxicity is similar between varied fractionation schema. As mentioned above, toxicity may be increased in central tumors despite the use of prolonged fractionation courses.
The use of chemotherapy has been retrospectively assessed in patients with T1-3N0M0 NSCLC who underwent SBRT, and was found to reduce distant failure and improve overall survival. However, only 26% of the patients (n = 17) received adjuvant chemotherapy[66]. Subsequently, the STEREO trial was opened to investigate the use of adjuvant chemotherapy in medically inoperable patients with early stage NSCLC treated with SBRT (NCT01300299), but given the difficulty in accruing participants (likely due to significant underlying comorbidities of this population), the study was discontinued. Improved overall survival after surgery and adjuvant chemotherapy for stage IB T2N0M0 has been demonstrated in several studies[67-69], but this finding has not been reproduced in larger prospective trials[70-74]. Even when patients were staged clinically and had potential occult nodal metastasis[47,50,51], neoadjuvant or adjuvant chemotherapy with surgery for early stage NSCLC did not improve survival[75]. However, adjuvant chemotherapy may be beneficial in select patients with resected early stage NSCLC, such as the tumor size > 4 cm and solid or micropapillary subtypes of adenocarcinoma[70,76]. Chemotherapy may reduce the risk of distant failure observed in patients treated with either surgery or SBRT alone, but its survival benefits for early stage NSCLC remain unclear.
In addition, other ongoing studies for early stage NSCLC evaluating other treatment regimens and modalities include: immunotherapy with SBRT (NCT02581787, NCT03050554), neoadjuvant SBRT and surgery[77,78], SBRT dose escalation specifically for T2N0M0 large tumors[79], radiofrequency ablation[37], and proton therapy (NCT00875901).
In conclusion, this review shows that SBRT remains the standard of care for medically inoperable patients with early stage NSCLC. While survival and local control outcomes of conventionally fractionated radiation therapy have been shown to be comparable, SBRT still offers better toxicity and quality of life outcomes. Prospective trials evaluating fractionation schema have not shown a clear benefit to multi-fraction regimens for peripheral, early stage NSCLC, and as such, our institution has adopted a single-fraction SBRT scheme. Additionally, further work is being done to evaluate the role of SBRT for regional nodal disease in stage III NSCLC patients. Additional studies are underway to evaluate various modalities and therapy schedules in this challenging patient population.
Manuscript source: Invited manuscript
Specialty type: Oncology
Country of origin: United States
Peer-review report classification
Grade A (Excellent): 0
Grade B (Very good): 0
Grade C (Good): C, C, C
Grade D (Fair): 0
Grade E (Poor): 0
P- Reviewer: Houvenaeghel G, Huo Q, Kanat O S- Editor: Ji FF L- Editor: A E- Editor: Song H
| 1. | Haffty BG, Goldberg NB, Gerstley J, Fischer DB, Peschel RE. Results of radical radiation therapy in clinical stage I, technically operable non-small cell lung cancer. Int J Radiat Oncol Biol Phys. 1988;15:69-73. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 159] [Cited by in RCA: 148] [Article Influence: 4.0] [Reference Citation Analysis (0)] |
| 2. | Armstrong JG, Minsky BD. Radiation therapy for medically inoperable stage I and II non-small cell lung cancer. Cancer Treat Rev. 1989;16:247-255. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 66] [Cited by in RCA: 64] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 3. | Dosoretz DE, Katin MJ, Blitzer PH, Rubenstein JH, Salenius S, Rashid M, Dosani RA, Mestas G, Siegel AD, Chadha TT. Radiation therapy in the management of medically inoperable carcinoma of the lung: results and implications for future treatment strategies. Int J Radiat Oncol Biol Phys. 1992;24:3-9. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 200] [Cited by in RCA: 179] [Article Influence: 5.4] [Reference Citation Analysis (0)] |
| 4. | Kaskowitz L, Graham MV, Emami B, Halverson KJ, Rush C. Radiation therapy alone for stage I non-small cell lung cancer. Int J Radiat Oncol Biol Phys. 1993;27:517-523. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 229] [Cited by in RCA: 199] [Article Influence: 6.2] [Reference Citation Analysis (0)] |
| 5. | Potters L, Kavanagh B, Galvin JM, Hevezi JM, Janjan NA, Larson DA, Mehta MP, Ryu S, Steinberg M, Timmerman R, Welsh JS, Rosenthal SA; American Society for Therapeutic Radiology and Oncology; American College of Radiology. American Society for Therapeutic Radiology and Oncology (ASTRO) and American College of Radiology (ACR) practice guideline for the performance of stereotactic body radiation therapy. Int J Radiat Oncol Biol Phys. 2010;76:326-332. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 293] [Cited by in RCA: 334] [Article Influence: 22.3] [Reference Citation Analysis (0)] |
| 6. | Shibamoto Y, Hashizume C, Baba F, Ayakawa S, Miyakawa A, Murai T, Takaoka T, Hattori Y, Asai R. Stereotactic body radiotherapy using a radiobiology-based regimen for stage I non-small-cell lung cancer: five-year mature results. J Thorac Oncol. 2015;10:960-964. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 57] [Cited by in RCA: 68] [Article Influence: 7.6] [Reference Citation Analysis (0)] |
| 7. | McGarry RC, Papiez L, Williams M, Whitford T, Timmerman RD. Stereotactic body radiation therapy of early-stage non-small-cell lung carcinoma: phase I study. Int J Radiat Oncol Biol Phys. 2005;63:1010-1015. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 381] [Cited by in RCA: 354] [Article Influence: 17.7] [Reference Citation Analysis (0)] |
| 8. | Nagata Y, Takayama K, Matsuo Y, Norihisa Y, Mizowaki T, Sakamoto T, Sakamoto M, Mitsumori M, Shibuya K, Araki N, Yano S, Hiraoka M. Clinical outcomes of a phase I/II study of 48 Gy of stereotactic body radiotherapy in 4 fractions for primary lung cancer using a stereotactic body frame. Int J Radiat Oncol Biol Phys. 2005;63:1427-1431. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 530] [Cited by in RCA: 486] [Article Influence: 24.3] [Reference Citation Analysis (0)] |
| 9. | Timmerman R, Papiez L, McGarry R, Likes L, DesRosiers C, Frost S, Williams M. Extracranial stereotactic radioablation: results of a phase I study in medically inoperable stage I non-small cell lung cancer. Chest. 2003;124:1946-1955. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 15] [Cited by in RCA: 10] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 10. | Koto M, Takai Y, Ogawa Y, Matsushita H, Takeda K, Takahashi C, Britton KR, Jingu K, Takai K, Mitsuya M, Nemoto K, Yamada S. A phase II study on stereotactic body radiotherapy for stage I non-small cell lung cancer. Radiother Oncol. 2007;85:429-434. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 142] [Cited by in RCA: 136] [Article Influence: 7.6] [Reference Citation Analysis (0)] |
| 11. | Navarro-Martin A, Aso S, Cacicedo J, Arnaiz M, Navarro V, Rosales S, de Blas R, Ramos R, Guedea F. Phase II Trial of SBRT for Stage I NSCLC: Survival, Local Control, and Lung Function at 36 Months. J Thorac Oncol. 2016;11:1101-1111. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 34] [Cited by in RCA: 53] [Article Influence: 5.9] [Reference Citation Analysis (0)] |
| 12. | Nagata Y, Hiraoka M, Shibata T, Onishi H, Kokubo M, Karasawa K, Shioyama Y, Onimaru R, Kozuka T, Kunieda E, Saito T, Nakagawa K, Hareyama M, Takai Y, Hayakawa K, Mitsuhashi N, Ishikura S. Prospective Trial of Stereotactic Body Radiation Therapy for Both Operable and Inoperable T1N0M0 Non-Small Cell Lung Cancer: Japan Clinical Oncology Group Study JCOG0403. Int J Radiat Oncol Biol Phys. 2015;93:989-996. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 241] [Cited by in RCA: 307] [Article Influence: 30.7] [Reference Citation Analysis (0)] |
| 13. | Videtic GM, Hu C, Singh AK, Chang JY, Parker W, Olivier KR, Schild SE, Komaki R, Urbanic JJ, Timmerman RD, Choy H. A Randomized Phase 2 Study Comparing 2 Stereotactic Body Radiation Therapy Schedules for Medically Inoperable Patients With Stage I Peripheral Non-Small Cell Lung Cancer: NRG Oncology RTOG 0915 (NCCTG N0927). Int J Radiat Oncol Biol Phys. 2015;93:757-764. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 302] [Cited by in RCA: 289] [Article Influence: 28.9] [Reference Citation Analysis (0)] |
| 14. | Nyman J, Hallqvist A, Lund JÅ, Brustugun OT, Bergman B, Bergström P, Friesland S, Lewensohn R, Holmberg E, Lax I. SPACE-A randomized study of SBRT vs conventional fractionated radiotherapy in medically inoperable stage I NSCLC. Radiother Oncol. 2016;121:1-8. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 187] [Cited by in RCA: 245] [Article Influence: 27.2] [Reference Citation Analysis (0)] |
| 15. | Chang JY, Senan S, Paul MA, Mehran RJ, Louie AV, Balter P, Groen HJ, McRae SE, Widder J, Feng L, van den Borne BE, Munsell MF, Hurkmans C, Berry DA, van Werkhoven E, Kresl JJ, Dingemans AM, Dawood O, Haasbeek CJ, Carpenter LS, De Jaeger K, Komaki R, Slotman BJ, Smit EF, Roth JA. Stereotactic ablative radiotherapy versus lobectomy for operable stage I non-small-cell lung cancer: a pooled analysis of two randomised trials. Lancet Oncol. 2015;16:630-637. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1013] [Cited by in RCA: 1106] [Article Influence: 110.6] [Reference Citation Analysis (0)] |
| 16. | Ricardi U, Filippi AR, Guarneri A, Giglioli FR, Ciammella P, Franco P, Mantovani C, Borasio P, Scagliotti GV, Ragona R. Stereotactic body radiation therapy for early stage non-small cell lung cancer: results of a prospective trial. Lung Cancer. 2010;68:72-77. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 206] [Cited by in RCA: 208] [Article Influence: 13.0] [Reference Citation Analysis (0)] |
| 17. | Fakiris AJ, McGarry RC, Yiannoutsos CT, Papiez L, Williams M, Henderson MA, Timmerman R. Stereotactic body radiation therapy for early-stage non-small-cell lung carcinoma: four-year results of a prospective phase II study. Int J Radiat Oncol Biol Phys. 2009;75:677-682. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 603] [Cited by in RCA: 619] [Article Influence: 38.7] [Reference Citation Analysis (0)] |
| 18. | Timmerman RD, Hu C, Michalski J, Straube W, Galvin J, Johnstone D, Bradley J, Barriger R, Bezjak A, Videtic GM, Nedzi L, Werner-Wasik M, Chen Y, Komaki RU, Choy H. Long-term results for RTOG 0236: A phase II trial of stereotactic body radiation therapy (SBRT) in the treatment of patients with medically inoperable stage I non-small cell lung cancer. Int J Radiat Oncol Biol Phys. 2014;90:S30. [RCA] [DOI] [Full Text] [Cited by in Crossref: 114] [Cited by in RCA: 117] [Article Influence: 10.6] [Reference Citation Analysis (0)] |
| 19. | Lindberg K, Nyman J, Riesenfeld Källskog V, Hoyer M, Lund JÅ, Lax I, Wersäll P, Karlsson K, Friesland S, Lewensohn R. Long-term results of a prospective phase II trial of medically inoperable stage I NSCLC treated with SBRT - the Nordic experience. Acta Oncol. 2015;54:1096-1104. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 49] [Cited by in RCA: 61] [Article Influence: 6.1] [Reference Citation Analysis (0)] |
| 20. | Miyakawa A, Shibamoto Y, Baba F, Manabe Y, Murai T, Sugie C, Yanagi T, Takaoka T. Stereotactic body radiotherapy for stage I non-small-cell lung cancer using higher doses for larger tumors: results of the second study. Radiat Oncol. 2017;12:152. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 7] [Cited by in RCA: 21] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 21. | Timmerman R, Paulus R, Galvin J, Michalski J, Straube W, Bradley J, Fakiris A, Bezjak A, Videtic G, Johnstone D, Fowler J, Gore E, Choy H. Stereotactic body radiation therapy for inoperable early stage lung cancer. JAMA. 2010;303:1070-1076. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 2018] [Cited by in RCA: 1952] [Article Influence: 130.1] [Reference Citation Analysis (0)] |
| 22. | Singh AK, Gomez J, Stephans KL, Bogart JA, Lili T, Malhotra H, Videtic GM, Groman A. A phase 2 randomized study of 2 stereotactic body radiation therapy regimens for medically inoperable patients with node-negative, peripheral non-small cell lung cancer. Int J Radiat Oncol Biol Phys. 2017;98:221-222. [RCA] [DOI] [Full Text] [Cited by in Crossref: 15] [Cited by in RCA: 15] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 23. | Timmerman R, McGarry R, Yiannoutsos C, Papiez L, Tudor K, DeLuca J, Ewing M, Abdulrahman R, DesRosiers C, Williams M, Fletcher J. Excessive toxicity when treating central tumors in a phase II study of stereotactic body radiation therapy for medically inoperable early-stage lung cancer. J Clin Oncol. 2006;24:4833-4839. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1074] [Cited by in RCA: 1133] [Article Influence: 59.6] [Reference Citation Analysis (0)] |
| 24. | Bezjak A, Paulus R, Gaspar L, Timmerman R, Straube W, Ryan WF, Garces Y, Pu AT, Singh AK, Videtic GM, McGarry RC, Iyengar P, Pantarotto JR, Urbanic J, Sun A, Daly ME, Grills IS, Normolle DP, Bradley JD, Choy H. Efficacy and toxicity analysis of NRG Oncology/RTOG 0813 trial of stereotactic body radiation therapy (SBRT) for centrally located non-small cell lung cancer (NSCLC). Int J Radiat Oncol Biol Phys. 2016;96:S8. [RCA] [DOI] [Full Text] [Cited by in Crossref: 57] [Cited by in RCA: 61] [Article Influence: 6.8] [Reference Citation Analysis (0)] |
| 25. | Asamura H, Goya T, Koshiishi Y, Sohara Y, Eguchi K, Mori M, Nakanishi Y, Tsuchiya R, Shimokata K, Inoue H, Nukiwa T, Miyaoka E; Japanese Joint Committee of Lung Cancer Registry. A Japanese Lung Cancer Registry study: prognosis of 13,010 resected lung cancers. J Thorac Oncol. 2008;3:46-52. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 305] [Cited by in RCA: 331] [Article Influence: 19.5] [Reference Citation Analysis (0)] |
| 26. | Taremi M, Hope A, Dahele M, Pearson S, Fung S, Purdie T, Brade A, Cho J, Sun A, Bissonnette JP, Bezjak A. Stereotactic body radiotherapy for medically inoperable lung cancer: prospective, single-center study of 108 consecutive patients. Int J Radiat Oncol Biol Phys. 2012;82:967-973. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 123] [Cited by in RCA: 136] [Article Influence: 9.7] [Reference Citation Analysis (0)] |
| 27. | Purdie TG, Bissonnette JP, Franks K, Bezjak A, Payne D, Sie F, Sharpe MB, Jaffray DA. Cone-beam computed tomography for on-line image guidance of lung stereotactic radiotherapy: localization, verification, and intrafraction tumor position. Int J Radiat Oncol Biol Phys. 2007;68:243-252. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 254] [Cited by in RCA: 265] [Article Influence: 14.7] [Reference Citation Analysis (0)] |
| 28. | Guckenberger M, Meyer J, Wilbert J, Baier K, Mueller G, Wulf J, Flentje M. Cone-beam CT based image-guidance for extracranial stereotactic radiotherapy of intrapulmonary tumors. Acta Oncol. 2006;45:897-906. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 101] [Cited by in RCA: 100] [Article Influence: 5.6] [Reference Citation Analysis (0)] |
| 29. | Guckenberger M, Andratschke N, Dieckmann K, Hoogeman MS, Hoyer M, Hurkmans C, Tanadini-Lang S, Lartigau E, Méndez Romero A, Senan S, Verellen D. ESTRO ACROP consensus guideline on implementation and practice of stereotactic body radiotherapy for peripherally located early stage non-small cell lung cancer. Radiother Oncol. 2017;124:11-17. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 161] [Cited by in RCA: 246] [Article Influence: 30.8] [Reference Citation Analysis (0)] |
| 30. | Prendergast BM, Fiveash JB, Popple RA, Clark GM, Thomas EM, Minnich DJ, Jacob R, Spencer SA, Bonner JA, Dobelbower MC. Flattening filter-free linac improves treatment delivery efficiency in stereotactic body radiation therapy. J Appl Clin Med Phys. 2013;14:4126. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 53] [Cited by in RCA: 59] [Article Influence: 4.9] [Reference Citation Analysis (0)] |
| 31. | Navarria P, Ascolese AM, Mancosu P, Alongi F, Clerici E, Tozzi A, Iftode C, Reggiori G, Tomatis S, Infante M, Alloisio M, Testori A, Fogliata A, Cozzi L, Morenghi E, Scorsetti M. Volumetric modulated arc therapy with flattening filter free (FFF) beams for stereotactic body radiation therapy (SBRT) in patients with medically inoperable early stage non small cell lung cancer (NSCLC). Radiother Oncol. 2013;107:414-418. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 116] [Cited by in RCA: 126] [Article Influence: 10.5] [Reference Citation Analysis (0)] |
| 32. | Annede P, Darreon J, Benkemouche A, Valdenaire S, Tyran M, Kaeppelin B, Macagno A, Barrou J, Cagetti LV, Favrel V, Moureau-Zabotto L, Gonzague L, Fau P, Chargari C, Tallet A, Salem N. Flattening Filter Free vs Flattened Beams for Lung Stereotactic Body Radiation Therapy. Anticancer Res. 2017;37:5133-5139. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 3] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 33. | Xiao Y, Papiez L, Paulus R, Timmerman R, Straube WL, Bosch WR, Michalski J, Galvin JM. Dosimetric evaluation of heterogeneity corrections for RTOG 0236: stereotactic body radiotherapy of inoperable stage I-II non-small-cell lung cancer. Int J Radiat Oncol Biol Phys. 2009;73:1235-1242. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 140] [Cited by in RCA: 131] [Article Influence: 8.2] [Reference Citation Analysis (0)] |
| 34. | Onishi H, Shirato H, Nagata Y, Hiraoka M, Fujino M, Gomi K, Niibe Y, Karasawa K, Hayakawa K, Takai Y, Kimura T, Takeda A, Ouchi A, Hareyama M, Kokubo M, Hara R, Itami J, Yamada K, Araki T. Hypofractionated stereotactic radiotherapy (HypoFXSRT) for stage I non-small cell lung cancer: updated results of 257 patients in a Japanese multi-institutional study. J Thorac Oncol. 2007;2:S94-S100. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 675] [Cited by in RCA: 739] [Article Influence: 41.1] [Reference Citation Analysis (0)] |
| 35. | Kirkpatrick JP, Meyer JJ, Marks LB. The linear-quadratic model is inappropriate to model high dose per fraction effects in radiosurgery. Semin Radiat Oncol. 2008;18:240-243. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 361] [Cited by in RCA: 347] [Article Influence: 20.4] [Reference Citation Analysis (0)] |
| 36. | Videtic GM, Chang JY, Chetty IJ, Ginsburg ME, Kestin LL, Kong FM, Lally BE, Loo BW Jr, Movsas B, Stinchcombe TE, Willers H, Rosenzweig KE; Expert Panel on Radiation OncologyLung. ACR appropriateness Criteria® early-stage non-small-cell lung cancer. Am J Clin Oncol. 2014;37:201-207. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 13] [Cited by in RCA: 14] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 37. | Dupuy DE, Fernando HC, Hillman S, Ng T, Tan AD, Sharma A, Rilling WS, Hong K, Putnam JB. Radiofrequency ablation of stage IA non-small cell lung cancer in medically inoperable patients: Results from the American College of Surgeons Oncology Group Z4033 (Alliance) trial. Cancer. 2015;121:3491-3498. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 117] [Cited by in RCA: 156] [Article Influence: 15.6] [Reference Citation Analysis (0)] |
| 38. | Daly ME, Perks JR, Chen AM. Patterns-of-care for thoracic stereotactic body radiotherapy among practicing radiation oncologists in the United States. J Thorac Oncol. 2013;8:202-207. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 50] [Cited by in RCA: 54] [Article Influence: 4.5] [Reference Citation Analysis (0)] |
| 39. | Ma SJ, Serra LM, Syed YA, Hermann GM, Gomez-Suescun JA, Singh AK. Comparison of Single- and Three-fraction Schedules of Stereotactic Body Radiation Therapy for Peripheral Early-stage Non-Small-cell Lung Cancer. Clin Lung Cancer. 2018;19:e235-e240. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 23] [Cited by in RCA: 21] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 40. | Ma SJ, Cummings M, Serra LM, Syed YA, Hermann GM, Chen Y, Milano MT, Singh AK, Gomez-Suescun JA, Singh DP. Three- Versus Five-Fraction Regimens of Stereotactic Body Radiotherapy for Peripheral Early-Stage Non-Small-Cell Lung Cancer: A Two-Institution Propensity Score-Matched Analysis. Clin Lung Cancer. 2018;19:e297-e302. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 7] [Cited by in RCA: 4] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 41. | Stephans KL, Djemil T, Reddy CA, Gajdos SM, Kolar M, Mason D, Murthy S, Rice TW, Mazzone P, Machuzak M, Mekhail T, Videtic GM. A comparison of two stereotactic body radiation fractionation schedules for medically inoperable stage I non-small cell lung cancer: the Cleveland Clinic experience. J Thorac Oncol. 2009;4:976-982. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 100] [Cited by in RCA: 102] [Article Influence: 6.4] [Reference Citation Analysis (0)] |
| 42. | Cummings MA, Ma SJ, Hermann G, Serra L, Syed Y, Malhotra HK, Chen Y, Milano MT, Gomez-Suescun JA, Singh DP, Singh AK. Comparison of Single- and Five-fraction Regimens of Stereotactic Body Radiation Therapy for Peripheral Early-stage Non-small-cell Lung Cancer: A Two-institution Propensity-matched Analysis. Clin Lung Cancer. 2018;19:511-517. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 16] [Cited by in RCA: 15] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 43. | Senthi S, Haasbeek CJ, Slotman BJ, Senan S. Outcomes of stereotactic ablative radiotherapy for central lung tumours: a systematic review. Radiother Oncol. 2013;106:276-282. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 179] [Cited by in RCA: 200] [Article Influence: 16.7] [Reference Citation Analysis (0)] |
| 44. | Ma SJ, Mix M, Rivers C, Hennon M, Gomez J, Singh AK. Mortality following single-fraction stereotactic body radiation therapy for central pulmonary oligometastasis. J Radiosurg SBRT. 2017;4:325-330. [PubMed] |
| 45. | Lindberg K, Bergstrom P, Brustugun OT, Engelholm S, Grozman V, Hoyer M, Karlsson K, Khalil A, Kristiansen C, Lax I, Loden B, Nyman J, Persson G, Rogg L, Wersall P, Lewensohn R. The Nordic HILUS-Trial - First Report of a Phase II Trial of SBRT of Centrally Located Lung Tumors. J. Thorac Oncol. 2017;S340. [RCA] [DOI] [Full Text] [Cited by in Crossref: 36] [Cited by in RCA: 36] [Article Influence: 4.5] [Reference Citation Analysis (0)] |
| 46. | Bral S, Gevaert T, Linthout N, Versmessen H, Collen C, Engels B, Verdries D, Everaert H, Christian N, De Ridder M, Storme G. Prospective, risk-adapted strategy of stereotactic body radiotherapy for early-stage non-small-cell lung cancer: results of a Phase II trial. Int J Radiat Oncol Biol Phys. 2011;80:1343-1349. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 134] [Cited by in RCA: 137] [Article Influence: 9.1] [Reference Citation Analysis (0)] |
| 47. | Su S, Scott WJ, Allen MS, Darling GE, Decker PA, McKenna RJ, Meyers BF. Patterns of survival and recurrence after surgical treatment of early stage non-small cell lung carcinoma in the ACOSOG Z0030 (ALLIANCE) trial. J Thorac Cardiovasc Surg. 2014;147:747-752: Discussion 752-753. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 50] [Cited by in RCA: 65] [Article Influence: 5.4] [Reference Citation Analysis (0)] |
| 48. | Nwogu CE, D’Cunha J, Pang H, Gu L, Wang X, Richards WG, Veit LJ, Demmy TL, Sugarbaker DJ, Kohman LJ, Swanson SJ; Alliance for Clinical Trials in Oncology. VATS lobectomy has better perioperative outcomes than open lobectomy: CALGB 31001, an ancillary analysis of CALGB 140202 (Alliance). Ann Thorac Surg. 2015;99:399-405. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 110] [Cited by in RCA: 148] [Article Influence: 13.5] [Reference Citation Analysis (0)] |
| 49. | Timmerman RD, Paulus R, Pass HI, Gore EM, Edelman MJ, Galvin J, Straube WL, Nedzi LA, McGarry RC, Robinson CG, Schiff PB, Chang G, Loo BW Jr, Bradley JD, Choy H. Stereotactic Body Radiation Therapy for Operable Early-Stage Lung Cancer: Findings From the NRG Oncology RTOG 0618 Trial. JAMA Oncol. 2018;4:1263-1266. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 158] [Cited by in RCA: 271] [Article Influence: 45.2] [Reference Citation Analysis (0)] |
| 50. | Crabtree TD, Denlinger CE, Meyers BF, El Naqa I, Zoole J, Krupnick AS, Kreisel D, Patterson GA, Bradley JD. Stereotactic body radiation therapy versus surgical resection for stage I non-small cell lung cancer. J Thorac Cardiovasc Surg. 2010;140:377-386. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 169] [Cited by in RCA: 195] [Article Influence: 13.0] [Reference Citation Analysis (0)] |
| 51. | Crabtree T, Puri V, Timmerman R, Fernando H, Bradley J, Decker PA, Paulus R, Putnum JB Jr, Dupuy DE, Meyers B. Treatment of stage I lung cancer in high-risk and inoperable patients: comparison of prospective clinical trials using stereotactic body radiotherapy (RTOG 0236), sublobar resection (ACOSOG Z4032), and radiofrequency ablation (ACOSOG Z4033). J Thorac Cardiovasc Surg. 2013;145:692-699. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 110] [Cited by in RCA: 126] [Article Influence: 9.7] [Reference Citation Analysis (0)] |
| 52. | Al-Sarraf N, Aziz R, Gately K, Lucey J, Wilson L, McGovern E, Young V. Pattern and predictors of occult mediastinal lymph node involvement in non-small cell lung cancer patients with negative mediastinal uptake on positron emission tomography. Eur J Cardiothorac Surg. 2008;33:104-109. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 115] [Cited by in RCA: 124] [Article Influence: 6.9] [Reference Citation Analysis (0)] |
| 53. | Lee PC, Port JL, Korst RJ, Liss Y, Meherally DN, Altorki NK. Risk factors for occult mediastinal metastases in clinical stage I non-small cell lung cancer. Ann Thorac Surg. 2007;84:177-181. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 133] [Cited by in RCA: 172] [Article Influence: 9.6] [Reference Citation Analysis (0)] |
| 54. | Zhao L, He ZY, Zhong XN, Cui ML. (18)FDG-PET/CT for detection of mediastinal nodal metastasis in non-small cell lung cancer: a meta-analysis. Surg Oncol. 2012;21:230-236. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 30] [Cited by in RCA: 36] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 55. | Shingyoji M, Nakajima T, Yoshino M, Yoshida Y, Ashinuma H, Itakura M, Tatsumi K, Iizasa T. Endobronchial ultrasonography for positron emission tomography and computed tomography-negative lymph node staging in non-small cell lung cancer. Ann Thorac Surg. 2014;98:1762-1767. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 44] [Cited by in RCA: 57] [Article Influence: 5.2] [Reference Citation Analysis (0)] |
| 56. | Ong P, Grosu H, Eapen GA, Rodriguez M, Lazarus D, Ost D, Jimenez CA, Morice R, Bandi V, Tamara L, Cornwell L, Green L, Zhu A, Casal RF. Endobronchial ultrasound-guided transbronchial needle aspiration for systematic nodal staging of lung cancer in patients with N0 disease by computed tomography and integrated positron emission tomography-computed tomography. Ann Am Thorac Soc. 2015;12:415-419. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 47] [Cited by in RCA: 55] [Article Influence: 5.5] [Reference Citation Analysis (0)] |
| 57. | Fernandez FG, Kozower BD, Crabtree TD, Force SD, Lau C, Pickens A, Krupnick AS, Veeramachaneni N, Patterson GA, Jones DR, Meyers BF. Utility of mediastinoscopy in clinical stage I lung cancers at risk for occult mediastinal nodal metastases. J Thorac Cardiovasc Surg. 2015;149:35-41, 42.e1. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 19] [Cited by in RCA: 24] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 58. | Wu Yl, Huang ZF, Wang SY, Yang XN, Ou W. A randomized trial of systematic nodal dissection in resectable non-small cell lung cancer. Lung Cancer. 2002;36:1-6. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 214] [Cited by in RCA: 232] [Article Influence: 10.1] [Reference Citation Analysis (0)] |
| 59. | Darling GE, Allen MS, Decker PA, Ballman K, Malthaner RA, Inculet RI, Jones DR, McKenna RJ, Landreneau RJ, Rusch VW, Putnam JB Jr. Randomized trial of mediastinal lymph node sampling versus complete lymphadenectomy during pulmonary resection in the patient with N0 or N1 (less than hilar) non-small cell carcinoma: results of the American College of Surgery Oncology Group Z0030 Trial. J Thorac Cardiovasc Surg. 2011;141:662-670. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 484] [Cited by in RCA: 580] [Article Influence: 41.4] [Reference Citation Analysis (0)] |
| 60. | Wink KCJ, van Baardwijk A, Troost EGC, De Ruysscher D. Nodal recurrence after stereotactic body radiotherapy for early stage non-small cell lung cancer: Incidence and proposed risk factors. Cancer Treat Rev. 2017;56:8-15. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 21] [Cited by in RCA: 25] [Article Influence: 3.1] [Reference Citation Analysis (0)] |
| 61. | Yendamuri S, Battoo A, Dy G, Chen H, Gomez J, Singh AK, Hennon M, Nwogu CE, Dexter EU, Huang M, Picone A, Demmy TL. Transcervical Extended Mediastinal Lymphadenectomy: Experience From a North American Cancer Center. Ann Thorac Surg. 2017;104:1644-1649. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 7] [Cited by in RCA: 6] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 62. | Singh AK, Hennon M, Ma SJ, Demmy TL, Picone A, Dexter EU, Nowgu C, Attwood K, Tan W, Fung-Kee-Fung S, Malhotra HK, Yendamuri S, Gomez-Suescun JA. A Pilot Study of Stereotactic Body Radiation Therapy (SBRT) After Surgery for Stage III Non-Small Cell Lung Cancer. BMC Cancer. 2018;18:1183. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 3] [Cited by in RCA: 5] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 63. | Timmerman RD, Park C, Kavanagh BD. The North American experience with stereotactic body radiation therapy in non-small cell lung cancer. J Thorac Oncol. 2007;2:S101-S112. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 104] [Cited by in RCA: 100] [Article Influence: 5.6] [Reference Citation Analysis (0)] |
| 64. | Voroney JP, Hope A, Dahele MR, Purdie TG, Franks KN, Pearson S, Cho JB, Sun A, Payne DG, Bissonnette JP, Bezjak A, Brade AM. Chest wall pain and rib fracture after stereotactic radiotherapy for peripheral non-small cell lung cancer. J Thorac Oncol. 2009;4:1035-1037. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 69] [Cited by in RCA: 70] [Article Influence: 4.4] [Reference Citation Analysis (0)] |
| 65. | Song SY, Choi W, Shin SS, Lee SW, Ahn SD, Kim JH, Je HU, Park CI, Lee JS, Choi EK. Fractionated stereotactic body radiation therapy for medically inoperable stage I lung cancer adjacent to central large bronchus. Lung Cancer. 2009;66:89-93. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 137] [Cited by in RCA: 143] [Article Influence: 8.9] [Reference Citation Analysis (0)] |
| 66. | Chen Y, Guo W, Lu Y, Zou B. Dose-individualized stereotactic body radiotherapy for T1-3N0 non-small cell lung cancer: long-term results and efficacy of adjuvant chemotherapy. Radiother Oncol. 2008;88:351-358. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 38] [Cited by in RCA: 44] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 67. | Morgensztern D, Du L, Waqar SN, Patel A, Samson P, Devarakonda S, Gao F, Robinson CG, Bradley J, Baggstrom M, Masood A, Govindan R, Puri V. Adjuvant Chemotherapy for Patients with T2N0M0 NSCLC. J Thorac Oncol. 2016;11:1729-1735. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 43] [Cited by in RCA: 72] [Article Influence: 8.0] [Reference Citation Analysis (0)] |
| 68. | Roselli M, Mariotti S, Ferroni P, Laudisi A, Mineo D, Pompeo E, Ambrogi V, Mineo TC. Postsurgical chemotherapy in stage IB nonsmall cell lung cancer: Long-term survival in a randomized study. Int J Cancer. 2006;119:955-960. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 40] [Cited by in RCA: 43] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 69. | Kato H, Ichinose Y, Ohta M, Hata E, Tsubota N, Tada H, Watanabe Y, Wada H, Tsuboi M, Hamajima N, Ohta M; Japan Lung Cancer Research Group on Postsurgical Adjuvant Chemotherapy. A randomized trial of adjuvant chemotherapy with uracil-tegafur for adenocarcinoma of the lung. N Engl J Med. 2004;350:1713-1721. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 615] [Cited by in RCA: 592] [Article Influence: 28.2] [Reference Citation Analysis (0)] |
| 70. | Strauss GM, Herndon JE 2nd, Maddaus MA, Johnstone DW, Johnson EA, Harpole DH, Gillenwater HH, Watson DM, Sugarbaker DJ, Schilsky RL, Vokes EE, Green MR. Adjuvant paclitaxel plus carboplatin compared with observation in stage IB non-small-cell lung cancer: CALGB 9633 with the Cancer and Leukemia Group B, Radiation Therapy Oncology Group, and North Central Cancer Treatment Group Study Groups. J Clin Oncol. 2008;26:5043-5051. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 640] [Cited by in RCA: 727] [Article Influence: 42.8] [Reference Citation Analysis (0)] |
| 71. | Douillard JY, Rosell R, De Lena M, Carpagnano F, Ramlau R, Gonzáles-Larriba JL, Grodzki T, Pereira JR, Le Groumellec A, Lorusso V, Clary C, Torres AJ, Dahabreh J, Souquet PJ, Astudillo J, Fournel P, Artal-Cortes A, Jassem J, Koubkova L, His P, Riggi M, Hurteloup P. Adjuvant vinorelbine plus cisplatin versus observation in patients with completely resected stage IB-IIIA non-small-cell lung cancer (Adjuvant Navelbine International Trialist Association [ANITA]): a randomised controlled trial. Lancet Oncol. 2006;7:719-727. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1087] [Cited by in RCA: 1172] [Article Influence: 61.7] [Reference Citation Analysis (0)] |
| 72. | Arriagada R, Dunant A, Pignon JP, Bergman B, Chabowski M, Grunenwald D, Kozlowski M, Le Péchoux C, Pirker R, Pinel MI, Tarayre M, Le Chevalier T. Long-term results of the international adjuvant lung cancer trial evaluating adjuvant Cisplatin-based chemotherapy in resected lung cancer. J Clin Oncol. 2010;28:35-42. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 264] [Cited by in RCA: 302] [Article Influence: 18.9] [Reference Citation Analysis (0)] |
| 73. | Pignon JP, Tribodet H, Scagliotti GV, Douillard JY, Shepherd FA, Stephens RJ, Dunant A, Torri V, Rosell R, Seymour L, Spiro SG, Rolland E, Fossati R, Aubert D, Ding K, Waller D, Le Chevalier T; LACE Collaborative Group. Lung adjuvant cisplatin evaluation: a pooled analysis by the LACE Collaborative Group. J Clin Oncol. 2008;26:3552-3559. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1426] [Cited by in RCA: 1857] [Article Influence: 109.2] [Reference Citation Analysis (0)] |
| 74. | Butts CA, Ding K, Seymour L, Twumasi-Ankrah P, Graham B, Gandara D, Johnson DH, Kesler KA, Green M, Vincent M, Cormier Y, Goss G, Findlay B, Johnston M, Tsao MS, Shepherd FA. Randomized phase III trial of vinorelbine plus cisplatin compared with observation in completely resected stage IB and II non-small-cell lung cancer: updated survival analysis of JBR-10. J Clin Oncol. 2010;28:29-34. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 304] [Cited by in RCA: 302] [Article Influence: 20.1] [Reference Citation Analysis (0)] |
| 75. | Felip E, Rosell R, Maestre JA, Rodríguez-Paniagua JM, Morán T, Astudillo J, Alonso G, Borro JM, González-Larriba JL, Torres A, Camps C, Guijarro R, Isla D, Aguiló R, Alberola V, Padilla J, Sánchez-Palencia A, Sánchez JJ, Hermosilla E, Massuti B; Spanish Lung Cancer Group. Preoperative chemotherapy plus surgery versus surgery plus adjuvant chemotherapy versus surgery alone in early-stage non-small-cell lung cancer. J Clin Oncol. 2010;28:3138-3145. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 210] [Cited by in RCA: 292] [Article Influence: 19.5] [Reference Citation Analysis (0)] |
| 76. | Tsao MS, Marguet S, Le Teuff G, Lantuejoul S, Shepherd FA, Seymour L, Kratzke R, Graziano SL, Popper HH, Rosell R, Douillard JY, Le-Chevalier T, Pignon JP, Soria JC, Brambilla EM. Subtype Classification of Lung Adenocarcinoma Predicts Benefit From Adjuvant Chemotherapy in Patients Undergoing Complete Resection. J Clin Oncol. 2015;33:3439-3446. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 186] [Cited by in RCA: 218] [Article Influence: 21.8] [Reference Citation Analysis (0)] |
| 77. | Nguyen NA, Isfahanian N, Pond G, Hanna W, Cutz JC, Wright J, Swaminath A, Shargall Y, Chow T, Wierzbicki M, Okawara G, Quan K, Finley C, Juergens R, Tsakiridis T. A Novel Neoadjuvant Therapy for Operable Locally Invasive Non-Small-Cell Lung Cancer. Phase I Study of Neoadjuvant Stereotactic Body Radiotherapy. LINNEARRE I (NCT02433574). Clin Lung Cancer. 2017;18:436-440.e1. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 3] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 78. | Palma DA, Nguyen TK, Kwan K, Gaede S, Landis M, Malthaner R, Fortin D, Louie AV, Frechette E, Rodrigues GB, Yaremko B, Yu E, Dar AR, Lee TY, Gratton A, Warner A, Ward A, Inculet R. Short report: interim safety results for a phase II trial measuring the integration of stereotactic ablative radiotherapy (SABR) plus surgery for early stage non-small cell lung cancer (MISSILE-NSCLC). Radiat Oncol. 2017;12:30. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 8] [Cited by in RCA: 10] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 79. | Onimaru R, Onishi H, Shibata T, Hiraoka M, Ishikura S, Karasawa K, Matsuo Y, Kokubo M, Shioyama Y, Matsushita H, Ito Y, Shirato H. Phase I study of stereotactic body radiation therapy for peripheral T2N0M0 non-small cell lung cancer (JCOG0702): Results for the group with PTV ≥ 100cc. Radiother Oncol. 2017;122:281-285. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 15] [Cited by in RCA: 16] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 80. | Sun B, Brooks ED, Komaki RU, Liao Z, Jeter MD, McAleer MF, Allen PK, Balter PA, Welsh JD, O’Reilly MS, Gomez D, Hahn SM, Roth JA, Mehran RJ, Heymach JV, Chang JY. 7-year follow-up after stereotactic ablative radiotherapy for patients with stage I non-small cell lung cancer: Results of a phase 2 clinical trial. Cancer. 2017;123:3031-3039. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 78] [Cited by in RCA: 121] [Article Influence: 15.1] [Reference Citation Analysis (0)] |