Published online Jan 10, 2019. doi: 10.5306/wjco.v10.i1.1
Peer-review started: September 29, 2018
First decision: October 26, 2018
Revised: December 7, 2018
Accepted: December 17, 2018
Article in press: December 17, 2018
Published online: January 10, 2019
Processing time: 89 Days and 13.7 Hours
Xerostomia, or dry mouth, is a significant problem affecting quality of life in patients treated with radiation therapy for head and neck cancer. Strategies for reduction of xerostomia burden vary widely, with options including: sialagogue medications, saliva substitutes, acupuncture, vitamins, hyperbaric oxygen, submandibular gland transfer, and acupuncture or associated treatments. In this review, we sought to evaluate long-term outcomes of patients treated with various interventions for radiation-induced xerostomia. A literature search was performed using the terms “xerostomia” and “radiation” or “radiotherapy”; all prospective clinical trials were evaluated, and only studies that reported 1 year follow up were included. The search results yielded 2193 studies, 1977 of which were in English. Of those, 304 were clinical trials or clinical studies. After abstract review, 23 trials were included in the review evaluating the following treatment modalities: pilocarpine (three); cevimeline (one); amifostine (eleven); submandibular gland transfer (five); acupuncture like transcutaneous electrical nerve stimulation (ALTENS) (one); hyperbaric oxygen (one); and acupuncture (one). Pilocarpine, cevimeline, and amifostine have been shown in some studies to improve xerostomia outcomes, at the cost of toxicity. ALTENS has similar efficacy with fewer side effects. Submandibular gland transfer is effective but requires an elective surgery, and thus may not always be appropriate or practical. The use of intensity-modulated radiation therapy, in addition to dose de-escalation in select patients, may result in fewer patients with late xerostomia, reducing the need for additional interventions.
Core tip: Xerostomia is a common side effect of radiation for head and neck cancer, and can impact patient quality of life even years after treatment. In this review, we sought to evaluate the current literature regarding long-term outcomes of interventions for radiation-induced xerostomia, including medical management, submandibular gland transfer, acupuncture, acupuncture like transcutaneous electrical nerve stimulation, and hyperbaric oxygen.
- Citation: Ma SJ, Rivers CI, Serra LM, Singh AK. Long-term outcomes of interventions for radiation-induced xerostomia: A review. World J Clin Oncol 2019; 10(1): 1-13
- URL: https://www.wjgnet.com/2218-4333/full/v10/i1/1.htm
- DOI: https://dx.doi.org/10.5306/wjco.v10.i1.1
Head and neck cancer patients treated with radiation experience changes in their quality of life due to radiation-induced dry mouth or xerostomia[1]. Complications include trouble eating, speaking, and swallowing, which can lead to depression and limited social activities[2,3]. Xerostomia can be avoided by reducing the mean radiation dose delivered to parotid and submandibular glands[4-9]. Intensity-modulated radiation therapy (IMRT) to spare parotid glands has been shown to reduce the incidence of xerostomia and improve quality of life[10-16]. However, despite the improvements seen in quality of life, select patients report persistent xerostomia after IMRT[17,18].
Standard care for radiation-induced xerostomia in head and neck cancer patients remains sialogogue medications, such as pilocarpine and cevimeline[19]. However, these medications have been shown to cause complications such as nausea and sweating[20], in some cases leading to patients withdrawing from the study[21,22] Numerous alternatives have been studied for xerostomia treatment, including amifostine[23], bethanechol[24], saliva substitutes[25], palifermin[26], alpha-tocopherol[27], vitamin C/E[28], thyme honey[29], herbal products, acupuncture[30], transcutaneous electrical nerve stimulations, submandibular gland transfer surgery[31], gene therapy[32], hyperbaric oxygen[33], and hyperthermic, supersaturated humidification—many of which have been reviewed previously in a meta-analysis by Mercadante et al[19]. However, few studies have long-term follow up data for interventions. We performed this review to evaluate various interventions for the long-term management of radiation-induced xerostomia.
We performed a review of journal articles in English in July 2018. Our inclusion criterion was any prospective clinical trial reporting clinical outcomes of interventions for radiation-induced xerostomia, with evaluation for late xerostomia at least 1 year after the radiation or intervention. The exclusion criteria were: (1) review articles, retrospective studies, letters, or case reports; (2) studies that did not show the most updated results when multiple journal articles published from the same patient cohort; (3) xerostomia unrelated to prior radiation therapy; and (4) the use of radioiodine or radionucleotide as a treatment.
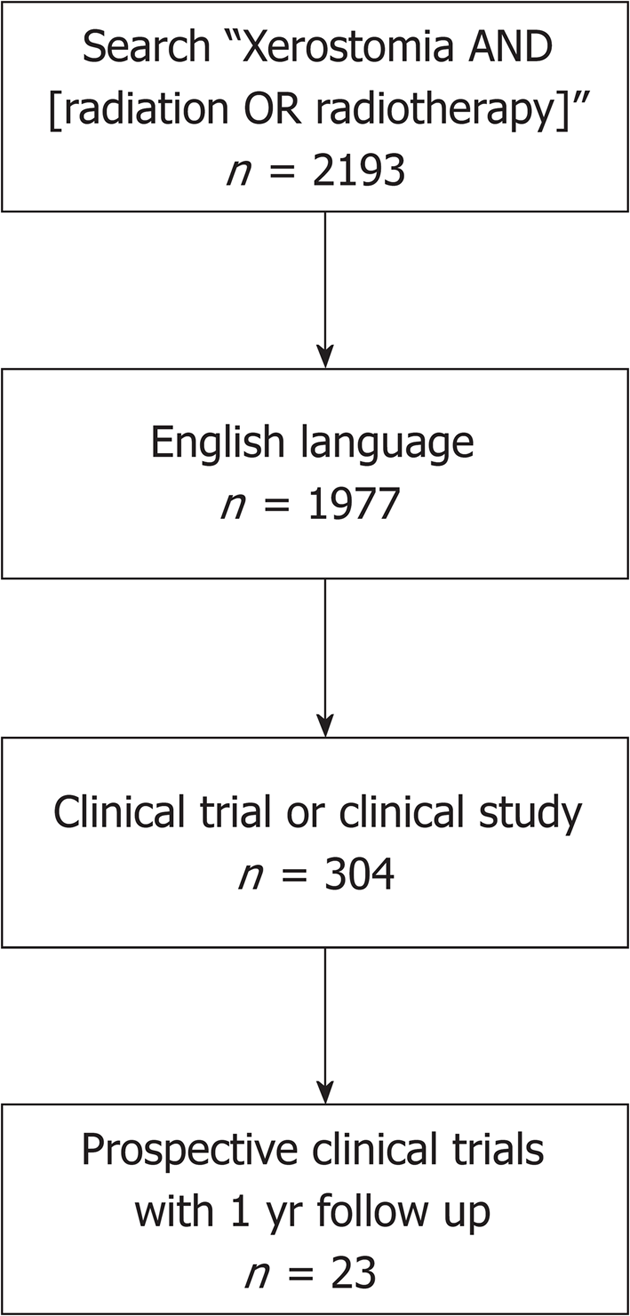
PubMed electronic databases were queried in July 2018 for search terms such as “xerostomia”, “radiotherapy”, and “radiation”. This database query initially resulted in 2193 studies. Of these, 304 studies were prospective trials written in English. With our exclusion criteria, these studies and their reference lists were reviewed to be considered for inclusion (Figure 1).
Twenty-three studies are selected for analysis (Tables 1-6). Of these, three studies evaluated pilocarpine; one evaluated cevimeline; eleven studies evaluated amifostine; five evaluated submandibular transfer; one evaluated ALTENS; one evaluated hyperbaric oxygen; and one evaluated acupuncture. Below we review the results of the studies.
| Author | Type of study | n | Intervention | Xerostomia symptoms | Salivary Function | Toxicity |
| Burlage et al[34], 2008 | Double-blind, randomized, placebo-controlled trial | 170 | PC during RT vs placebo | LENT SOMA: no difference at 1 yr; Patient-reported xero: significantly lower scores in pilocarpine group at 12 mo only if mean parotid dose > 40 Gy | Parotid flow rate complication probability (PFCP): at 1 yr, no diff between arms (except in subset of pts with > 40 Gy mean parotid dose-reduced loss of flow in pilocarpine group) | 2 patients didn't complete treatment, excessive sweating for PC and suspected AE for placebo pt; 1 G2 excessive sweating |
| Mateos et al[35], 2001 | Prospective non-randomized study | 49 | PC 5 mg TID during RT and for 1 yr vs no PC | No significant difference in visual analogue scale between groups | Dynamic salivary scintigraphy: no SS differences between groups | NA |
| Valdez et al[36], 1993 | Double-blind, randomized, placebo-controlled trial | 9 | PC 5 mg four times daily for 3 mo during RT vs placebo | Significantly fewer subjective oral symptoms in pilocarpine group on survey during treatment; no difference at 1 yr (25% in both arms) | Salivary flow rate (resting and stimulated): smaller losses in stim function in PC group at 3 mo (SS) | none reported |
| Chambers et al[37], 2007 | Open-label prospective single-arm study | 255 | Cevimeline for 1 yr 45 mg TID orally | Used mean global eval. score (0-3), at final eval. 59.2% improved, 37.3% no change, 3.5% worse compared with first visit (P < 0.0001 change from baseline to visit 8) | NA | 20.4% G3 AE, most common was sweating; 7.1% severe AE, one possibly attributed to study drug (miscarriage) |
| Author | Type of study | n | Intervention | Xerostomia symptoms | Effect Size |
| Bardet et al[46], 2011 | Phase III randomized trial | 291 | Amifostine IV vs SC | RTOG grading: G2+ xero significantly higher in SC at 1 yr, but not at 2-3 yr | 37% IV vs 62% SC |
| Haddad et al[45], 2009 | Phase II randomized trial | 58 | SC amifostine 500 mg daily (for median 28 doses) no amifostine | CTCAE: no significant difference in Gr2+ xero (minimum follow up 26 mo) | 41% both arms |
| Law et al[44], 2007 | Phase II prospective nonrandomized trial | 20 | SC 500 mg amifostine 30-60 min before RT | G2 xero 42% at 12 mo, 29% at 18 mo; no G3+ xero. G3+ mucositis in 30% of pts. | |
| Anné et al[43], 2007 | Phase II single arm multicenter trial | 54 | SC amifostine | RTOG scoring: G2+ xero = 56%; late G2+ in 45% ; G3+ acute 33% | |
| Jellema et al[23], 2006 | Phase II randomized trial | 91 | No amifostine vs 200 mg/m2 IV daily (3 wk) vs 5 wk | RTOG scoring: significant difference in late G2+ xero at 6 mo between arms; no difference in xero at 12 mo or 24 mo; no dif in acute xero | Late G2+ xero 74% vs 67% vs 52% |
| EORTC QLQ-H and N35: significantly higher mean xerostomia score in no amifostine group | |||||
| Buentzel et al[41], 2006 | Phase III randomized placebo-controlled trial | 132 | IV amifostine 300 mg/m2 days 1-5 and 21-25, 200 mg/m2 on other days vs placebo | RTOG criteria: no significant difference in G2+ acute or late xero | 39% amifostine vs 34% placebo (acute); 39% amifostine vs 24% placebo (late) |
| Wasserman et al[42], 2005 | Phase III randomized trial | 303 | IV amifostine 200 mg/m2 15-30 prior to each RT fraction vs no amifostine | RTOG scoring: significantly lower G2+ xero in amifostine group on longitudinal analysis | 20% vs 36% at 24 mo |
| Thorstad et al[47], 2004 | Pilot clinical trial | 27 | Amifostine concurrent with RT (500 mg SC daily) | not reported | NA |
| Antonadou et al[40], 2002 | Randomized controlled trial | 50 | Amifostine 300 mg/m2 15-30 min prior to RT (daily) vs no amifostine | RTOG/EORTC scoring: significantly lower xero in amifostine group at 18 mo (G1+) | 30.4% vs 4.5% |
| Brizel et al[39], 2000 | Phase III multiinstitutional randomized trial | 303 | Amifostine 200 mg/m2 15-30 min prior to each RT tx vs no amifostine | RTOG scoring: significantly higher G2+ xero (acute and late) in control vs amifostine; higher dose required to cause G2 xero in amifostine pts (60 Gy vs 42 Gy); | 78% vs 51% (acute); 57% 43% (1 yr) |
| Büntzel et al[38], 1998 | Phase II randomized trial | 39 | Amifostine IV 500mg prior to carboplatin (days 1-5 and 21-25) vs no amifostine | Acute G2 xero, G3 mucositis, and G3 thrombocytopenia all significantly decreased with amifostine; at 12 mo, trend toward xero improvement with amifostine | Xero: G2 100% vs 12% (acute); 55% vs 17% (late; P = 0.05) |
| Author | Salivary Production | QOL | Toxicity |
| Bardet et al[46], 2011 | No difference in unstimulated and stimulated salivary flow rate = | No difference in patient-reported salivary function or Gr 2+ xero | No difference in compliance between arms (69% IV vs 71% SC). Acute toxicity 25% IV vs 27% SC (NS). SS higher rate of hypotension in IV arm; significantly higher skin rash and local pain in SC arm. |
| Haddad et al[45], 2009 | No difference in unstimulated or stimulated saliva at all endpoints (up to 1 yr) | No difference in penetration, aspiration, and pharyngeal residue on swallow eval. | G3 mucositis in 75% (amifostine) and 70% (no amifostine); Gr3 skin toxicity in 12 patients in amifostine group (main reason for withholding amifostine) |
| Law et al[44], 2007 | NA | NA | G2 weight loss for all pts, Gr2 or less N/V in 7 pts (35%). No grade 3+ amifostine-related AEs. |
| Anné et al[43], 2007 | NA | PBQ: mean score 8.5 baseline, 6.1 at 4 wk, 7.5 at 1 yr | Nausea, emesis, injection site reaction most common G1-2; G3 dehydration 11%, rash 6%, weight decrease, mucositis, dyspnea, allergic reaction 4% each; one G4 anaphylaxis |
| Jellema et al[23], 2006 | NA | QLQ-C30, QLQ-H and N35: no differences in sticky saliva or other QOL data | Significantly higher N/V in amifostine groups; 28% of patients discontinued amifostine early |
| Buentzel et al[41], 2006 | not assessed: fewer than one-third in each arm had salivary assessment at 1 yr | NA | 42% G3+ toxicity (amifostine) vs 20% (placebo) (SS) |
| Wasserman et al[42], 2005 | no dif. in stimulated; unstimulated higher in amifosine group at 12 mo (SS) | PBQ: amifostine group had SS better mouth dryness at 12, 18, and 24 mo; better score for "use of oral comfort aids" with amifostine at 24 mo | not enough to analyze |
| Thorstad et al[47], 2004 | not reported | not reported | reasons for discontinuing amifostine: nausea (33%), rash (15%), fever (7%), other (11%) |
| Antonadou et al[40], 2002 | NA | NA | SS lower acute mucositis and acute dysphagia in amifostine group; in amifostine group, 1 pt had N/V, 3 pts had transient hypotension |
| Brizel et al[39], 2000 | Whole saliva production higher in amifostine pts at 1 yr (SS) | PBQ: overall score favored amifostine at 1 yr (SS) | 53% nausea and vomiting (5% of total administrations; 3% G3 N, 5% G3 V)); G3 N/V in 7% of pts; median weight loss higher in control group (SS); hypotension 15% (3% G3; < 1% of all doses); venous catheter complications 5%; infections 14%; clotting/vascular 3% (1 pt G4); allergic reaction 5% |
| Büntzel et al[38], 1998 | NA | NA | No significant difference in N/V between groups; hypotension 40% amifostine arm (max drop 20 mmHg) |
| Author | Study design | n | Intervention | Xerostomia symptoms | Effect size |
| Zhang et al[31], 2014 | Randomized controlled trial | 65 | Submandibular transfer vs control | Significantly lower incidence of xerostomia (RTOG/EORTC staging criteria) at 1 yr and 5 yr in transfer group vs control. Significantly lower VAS at 5 yr for transfer group | Xerostomia 18.7% vs 81.8% at 1 yr; 15.4% for transfer vs 76.9% at 5 yr; VAS 3.7 for transfer vs 5.8 for control |
| Rieger et al[51], 2012 | Phase III randomized controlled trial | 69 | Submandibular transfer vs oral PC | EORTC QLQ H and N35: significantly worse dry mouth and sticky saliva at 1 yr in PC group vs submandibular transfer at 1 yr | Dry mouth score 42.6 vs 85.8; sticky saliva score 37.2 vs 66.7 |
| Liu et al[50], 2011 | Prospective non-randomized controlled trial | 70 | Submandibular transfer vs control | At 5 yr, significantly higher mod-to-severe xerostomia in control group; significantly better VAS in transfer group vs control | Mod-to-severe xerostomia 78.6% vs 12.9% |
| Seikaly et al[49], 2004 | Phase II prospective non-randomized | 38 | Submandibular gland transfer vs control | UW-QOL: significantly better xerostomia symptoms (amount and consistency) at 2 yr | 83% vs 0% reporting normal amount of saliva |
| Jha et al[48], 2003 | Phase II prospective single arm | 76 | submandibular gland transfer | UW-QOL: 81% minimal or no xero at end of RT; 65% at 2 mo; 71% at 6 mo (in unshielded pts, 71% had severe xero at 6 mo) | - |
| Author | Salivary function | Quality of life | Toxicity |
| Zhang et al[31], 2004 | Transfer 1.39 g and 1.6 g saliva vs 0.66 and 0.68 g control at 1 yr and 5 yr, respectively. Significantly higher submandibular gland secretion in transfer group at 5 yr (radionuclide scintigraphy). | Significantly improved speech, chewing, swallowing, changes in eating habits, nighttime xero, need to wake up to drink frequently, sleep quality in transfer group | No surgical death or complications occurred in transfer group |
| Rieger et al[51], 2012 | NA | NA | Not reported |
| Liu et al[50], 2011 | Significantly better trapping and excretion (scintigraphy) in transfer group at 5 yr; Significantly higher mean weight of unstimulated saliva in transfer group at 5 yr | Transfer group improved significantly vs control in dry mouth, night rest, drink to speech, drink to eat, water intake, change in feeding pattern, tooth decay, and visual analogue scale | No major complications of surgery (one pt taken back 2 yr later for removal of wire used to mark borders of transferred gland due to pain) |
| Seikaly et al[49], 2004 | Significantly higher stimulated and unstimulated saliva in transfer group at 16 mo | NA | No surgical complications from submandibular transfer |
| Jha et al[48], 2003 | stimulated and unstimulated saliva decrease gradually, then increase at 16 mo (graphical) | NA | No surgical complications |
| Author | Type of study | Sample size | Intervention | Xerostomia symptoms | Salivary function | Quality of life | Toxicity |
| Wong et al[54], 2015 | Phase III randomized controlled trial | 148 | ALTENS vs oral PC (5 mg TID for 12 wk) | NA | Basal WSP and stimulated WSP: no sig difference | XeQOLs: no difference at 15 mo. 83% ALTENS positive responders vs 62.8% PC, SS at 15 mo. | 2 G3 events in PC (dry mouth, blurry vision) vs 1 G3 event in ALTENS (headache). 61.6% of PC had Grade 3 or less non-hematologic AEs vs 20.9% of ALTENS |
| Teguh et al[52], 2009 | randomized controlled trial | 19 | Hyperbaric O2 (30 sessions at 2.5 ATA with O2 breathing for 90 min daily, 5 d a week) vs control | Visual analogue scale dry mo better on O2 (SS) | NA | EORTC QLQ-C30 and H and N35; Sticky saliva better on O2 (SS) and less dry mouth on O2 (SS) | NA |
| Blom et al[53], 1996 | randomized placebo-controlled trial | 38 | acupuncture vs placebo (superficial acupuncture) | NA | salivary flow rate: no dif. between groups; both groups showed increased flow rates after treatment | No specific endpoints | Tiredness, small haematomas at acupuncture sites |
Pilocarpine, a cholinergic agonist, has shown mixed results in the treatment of radiation related xerostomia. Burlage et al[34] evaluated 170 patients randomized to either oral pilocarpine or placebo during radiation therapy. Based on LENT SOMA score, there was no statistical difference between the two arms at one year. However, based on patient reported xerostomia, pilocarpine significantly reduced symptoms in patients who received > 40 Gy mean dose to the parotid. Toxicity was relatively low in this, with 2 patients withdrawing from the study–one in the pilocarpine group (due to sweating) and one in the placebo group due to a suspected adverse event. The only grade 2 reported toxicity was excessive sweating in one patient. In study by Mateos et al[35], 49 patients were divided into two groups. One group received pilocarpine during RT and throughout the year that followed, while the other received radiation alone. Visual analogue scale (VAS) revealed no subjective difference between the two groups. Dynamic salivary scintigraphy also showed no statistically significant advantage to pilocarpine. In contrast, Valdez et al[36] reported a series of 9 patients receiving either pilocarpine for 3 mo or placebo. Based on patient reports surveys, there were significantly fewer symptoms of xerostomia in the pilocarpine group. Interestingly, in this small number of patients, there was a statistically significant difference between the groups in stimulated parotid salivary function at 3 mo. No pilocarpine related toxicities were reported.
Cevimeline has also been studied as an oral agent for treatment of xerostomia. Chambers et al[37] reported a single arm trial of 255 patients taking cevimeline for 1 year. At final evaluation, 59.2% of patients had improved symptoms based on mean global evaluation score, with 37.3% showing no change, and 3.5% with worsening symptoms compared to initial visit. The rate of grade3 toxicity was 20.4% and consisted mostly of sweating; 7.1% of patients experienced a severe adverse event, one of which (miscarriage) was possibly attributed to the study drug. Overall the authors conclude that cevimeline was well tolerated and may provide relief of xerostomia in head and neck cancer patients.
Eleven papers studying amifostine met inclusion criteria. Büntzel et al[38] reported on 39 patients randomized to either IV amifostine 500 mg with carboplatin (days 1-5 and days 21-25) during concurrent chemoradiation, or to chemoradiation alone. The authors report that grade 3 mucositis, grade 2 xerostomia, and grade 3 thrombocytopenia were all significantly decreased in the amifostine group. Brizel et al[39] also found an advantage with amifostine, reporting on 303 patients treated with either radiation along or amifostine 200 mg/m2 15-30 min prior to each RT dose. Grade 2 xerostomia was significantly improved in the amifostine group compared with control (51% vs 78%, P < 0.0001); in addition, a higher threshold dose of radiation was required to cause grade 2 xerostomia in the amifostine group. This study also quantified whole saliva production (WSP) during RT and at follow up; there was significantly higher saliva production in the amifostine patients at 1 year. In terms of amifostine toxicities, 53% of patients experienced nausea and vomiting (5% of total amifostine administrations, with 3% grade3 nausea and 5% grade3 vomiting). Other complications included hypotension, venous catheter complications, infections, and clotting/vascular disorders. Many of these were related to the IV method of administration. The authors concluded that amifostine reduces xerostomia, although alternative delivery methods should be evaluated. In contrast, a separate study did not report significant administration-related toxicities with the use of IV amifostine 300 mg/m2 prior to RT[40]. This randomized trial assigned patients to either chemoradiation alone or chemoradiation with prophylactic amifostine. Toxicities reported included nausea/vomiting (1 patient) and transient hypotension (13.6%). By week 3 of radiation treatment, 100% of the control group and 9.1% of the study group had grade 2 mucositis; by week 5, 97.5% of control group was reported to have moderate to severe mucositis, and 63.6% of the study group. Additionally, treatment duration was significantly shorter in the amifostine group, due to more treatment interruptions from grade 4 mucositis.
Two additional studies evaluated the efficacy and safety of IV amifostine. A randomized trial by Buentzel et al[41] included 132 patients randomized to either IV amifostine 300 mg/m2 on days 1-5 and 200 mg/m2 on other days of RT, or placebo. In contrast to prior studies mentioned, there was no difference in acute or late xerostomia based on RTOG criteria. There was, however, a difference in toxicity, with a 43% vs 20% grade 3 toxicity rate (amifostine and placebo groups, respectively). Of note, less than 1/3 of patients were evaluated at the 1 year time point. Wasserman et al[42] included 303 patients randomized to either amifostine 200 mg/m2 prior to each radiation fraction, or radiation alone. With 2 years of follow up, the amifostine group had: lower grade 2-4 chronic xerostomia, increased unstimulated saliva scores, and better patient reported mouth dryness.
As an alternative delivery method, subcutaneous administration of amifostine has been evaluated in four trials with mixed results. Two phase II single arm trials, with 54 and 20 patients each, treated patients with subcutaneous amifostine and compared results to prior studies using IV, and showed similar results in terms of efficacy[43,44]. One phase II randomized study of 58 patients compared subcutaneous amifostine with no amifostine, and found no significant difference between the two arms[45]. A phase III randomized controlled trial assigned 291 patients to either IV or subcutaneous amifostine[46]. The authors found no significant benefit in terms of patient compliance or efficacy with subcutaneous administration, suggesting that IV should remain the standard treatment. Thorstad et al[47] report toxicity results of a pilot study of 27 patients assessing subcutaneous amifostine delivered concurrently with IMRT. Although compliance rate was not reported, the authors report that not all patients tolerated the treatment, with nausea, rash, and fever being the main complaints that caused discontinuation of amifostine.
Five studies evaluating salivary gland transfer met criteria for inclusion. In a phase II single arm study of 57 patients, Jha el al[48] reported that submandibular salivary gland transfer to the submental space is feasible and safe, with 81% of patients reporting none or minimal xerostomia with median follow up of 14 mo. One phase II prospective non-randomized trial of 38 patients showed improved stimulated and unstimulated saliva as well as improved patient reported xerostomia in the transfer group compared to patients who did not receive transfer[49]. Similarly, in another study including 70 patients, those treated with salivary gland transfer had an incidence of 12.9% of moderate-to-severe xerostomia compared with 78.6% in the control group[50]. Two randomized controlled trials, of 65 and 69 patients each, evaluated submandibular transfer, one comparing to a control group, and the other to oral pilocarpine[31,51]. The first showed a significant reduction in the incidence of xerostomia with salivary gland transfer; the second also showed a significant advantage for salivary gland transfer over pilocarpine with respect to dry mouth and sticky saliva. In all of the above studies, surgery was well tolerated, with no reported complications.
Other studies include treatment with hyperbaric oxygen, which was shown in a randomized trial to improve patient reported dry mouth and sticky saliva[52]. In a sham-controlled study of acupuncture for xerostomia, Blom et al[53] treated patients either with acupuncture or superficial acupuncture, and found no difference in xerostomia outcomes or salivary flow rate between the two groups. Finally, Wong et al[54], in a 148 patient phase III study, reported on the use of acupuncture-like transcutaneous electrical nerve stimulation (ALTENS) compared to oral pilocarpine. While there was no difference in whole salivary production, there was a significantly higher proportion of patients in the ALTENS group that responded positively to treatment. In addition, the rate of adverse events was 61.6% in the pilocarpine group compared with 20.9% in the ALTENS group, although the difference in adverse events was not significant at 9 mo (P = 0.67). There were three grade 3 toxicities overall (dry mouth and blurred vision in the pilocarpine group, headache in the ALTENS group).
Although there have been prior reviews on the management of radiotherapy-induced xerostomia, to our knowledge there has been no review of the current literature with a focus on late xerostomia following radiotherapy for head and neck cancer[19,55,56]. We found a very heterogeneous group of studies in this review, with many different modalities, doses, routes of administration, timing with respect to treatment, and differing quality of life (QOL) endpoints as well as different objective saliva measurements.
In most of the studies reviewed above, amifostine appears to be beneficial in reducing the risk of long term xerostomia, although it likely requires IV administration. Severely limiting clinical utilization, however, toxicity was noted in close to half of the patients treated[39,41]. Similarly toxicity limits the clinical utilization of pilocarpine and cevimeline, which have been shown to improve xerostomia, with treatment related adverse events exceeding 91.4% (20.4% grade 3) with cevimeline[37].
In contrast, ALTENS treatment was shown to be as effective as pilocarpine, with fewer adverse events (20.8% in ALTENS group vs 61.6% in pilocarpine group)[54]. At 15 mo, the treatment response rate was significantly higher in the ALTENS group. ALTENS represents a non-invasive, well tolerated option for treatment of late xerostomia. However, ALTENS devices are not widely available and when offered in a clinical setting, require patients to travel to the clinic twice weekly for 12 wk. Both of these issues limit availability. To address this issue, Iovoli et al[57] have described a case report of excellent improvement in dry mouth with home use of a new, cheap, commercially available device.
Submandibular gland transfer has shown promise in several studies as mentioned above. The use of salivary gland transfer in select patients appears to be effective with regard to xerostomia prevention. Additionally, none of the studies evaluated here reported complications from surgery. However, the use of this procedure is somewhat limited based on several factors including patient selection criteria (for example, it would not be feasible in patients with bilateral positive neck nodes), experience of each surgeon and willingness to perform the procedure, as well as time constraints and potential delay of definitive treatment for an elective procedure.
With the advancement of radiation delivery techniques, the use of IMRT has been shown to reduce dose to selected salivary glands, therefore sparing salivary function. It is generally thought that damage to major salivary glands (submandibular and parotid) is the major cause of xerostomia following radiation therapy, as evaluated with MRI, CT, and ultrasound[58-62]. Pacholke et al[63] retrospectively reviewed 210 patients with xerostomia at least one year following completion of radiation therapy, as measured by the University of Michigan xerostomia QOL score. Higher xerostomia scores were associated with higher salivary gland dose. On multivariate analysis, radiation technique was an independent predictor of xerostomia, favoring IMRT. The PARSPORT trial was a randomized phase III randomized controlled trial that assigned patients with pharyngeal squamous cell carcinoma to either conventional radiotherapy or parotid-sparing IMRT, and found a significant reduction in xerostomia in the IMRT group[10]. In addition to IMRT, the use of intensity modulated proton beam therapy (IMPT) has also been studied in a 150 patient case-matched analysis comparing IMPT to IMRT[64]. With respect to xerostomia, the authors found improved patient-reported symptoms at 3 mo, but no difference at 1 year.
In many cases, however, complete sparing of the parotid or submandibular glands is not possible due to proximity of primary tumor or grossly involved lymph nodes. Recently, there is new evidence that sparing even a portion of the parotid gland may be helpful in preventing xerostomia. Parotid stem cells are thought to be capable of regenerating salivary function, and are located in a concentrated area in the parotid gland around the main salivary ducts, as demonstrated in a study in rats[65]. In this same study, the authors identified a volume in the human parotid gland posterior to the mandible that was most associated with saliva production one year following radiation therapy, and demonstrated that it is possible to spare this area in some patients where sparing the entire parotid is not feasible[65].
Because of the increasing incidence of HPV positive head and neck cancer, there has been interest in de-escalating therapy for this subset of patients[66-68]. By reducing the total radiation dose, xerostomia may become less prevalent in this population, thus reducing the need for alternative treatment of salivary dysfunction.
While pilocarpine, cevimeline and amifostine have been shown to improve late xerostomia outcomes, these treatments often cause side effects that are not tolerable for patients. ALTENS represents a less toxic alternative therapy for prevention of late xerostomia, but has not been widely available until recently[57]. Similarly, submandibular gland transfer is effective, but may not be appropriate for all patients. Salivary gland sparing with improved radiation techniques (IMRT)—in particular sparing of parotid stem cells—is a practical way to reduce late salivary dysfunction. As IMRT becomes more widely available, in conjunction with potential dose de-escalation, the need for alternative xerostomia treatments may become less relevant.
Manuscript source: Invited manuscript
Specialty type: Oncology
Country of origin: United States
Peer-review report classification
Grade A (Excellent): 0
Grade B (Very good): B
Grade C (Good): C, C
Grade D (Fair): 0
Grade E (Poor): 0
P- Reviewer: Chen YK, Kupeli S, Su CC S- Editor: Ma YJ L- Editor: A E- Editor: Song H
| 1. | Langendijk JA, Doornaert P, Verdonck-de Leeuw IM, Leemans CR, Aaronson NK, Slotman BJ. Impact of late treatment-related toxicity on quality of life among patients with head and neck cancer treated with radiotherapy. J Clin Oncol. 2008;26:3770-3776. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 458] [Cited by in RCA: 528] [Article Influence: 31.1] [Reference Citation Analysis (0)] |
| 2. | Greenspan D. Xerostomia: diagnosis and management. Oncology (Williston Park). 1996;10:7-11. [PubMed] |
| 3. | Dirix P, Nuyts S, Vander Poorten V, Delaere P, Van den Bogaert W. The influence of xerostomia after radiotherapy on quality of life: results of a questionnaire in head and neck cancer. Support Care Cancer. 2008;16:171-179. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 118] [Cited by in RCA: 131] [Article Influence: 7.3] [Reference Citation Analysis (0)] |
| 4. | Deasy JO, Moiseenko V, Marks L, Chao KS, Nam J, Eisbruch A. Radiotherapy dose-volume effects on salivary gland function. Int J Radiat Oncol Biol Phys. 2010;76:S58-S63. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 376] [Cited by in RCA: 421] [Article Influence: 28.1] [Reference Citation Analysis (0)] |
| 5. | Malouf JG, Aragon C, Henson BS, Eisbruch A, Ship JA. Influence of parotid-sparing radiotherapy on xerostomia in head and neck cancer patients. Cancer Detect Prev. 2003;27:305-310. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 52] [Cited by in RCA: 43] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 6. | Robin TP, Gan GN, Tam M, Westerly D, Riaz N, Karam SD, Lee N, Raben D. Safety of contralateral submandibular gland sparing in locally advanced oropharyngeal cancers: A multicenter review. Head Neck. 2016;38:506-511. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 12] [Cited by in RCA: 12] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 7. | Miah AB, Schick U, Bhide SA, Guerrero-Urbano MT, Clark CH, Bidmead AM, Bodla S, Del Rosario L, Thway K, Wilson P, Newbold KL, Harrington KJ, Nutting CM. A phase II trial of induction chemotherapy and chemo-IMRT for head and neck squamous cell cancers at risk of bilateral nodal spread: the application of a bilateral superficial lobe parotid-sparing IMRT technique and treatment outcomes. Br J Cancer. 2015;112:32-38. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 27] [Cited by in RCA: 30] [Article Influence: 2.7] [Reference Citation Analysis (0)] |
| 8. | Jen YM, Shih R, Lin YS, Su WF, Ku CH, Chang CS, Shueng PW, Hwang JM, Liu DW, Chao HL, Lin HY, Chang LP, Shum WY, Lin CS. Parotid gland-sparing 3-dimensional conformal radiotherapy results in less severe dry mouth in nasopharyngeal cancer patients: a dosimetric and clinical comparison with conventional radiotherapy. Radiother Oncol. 2005;75:204-209. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 25] [Cited by in RCA: 24] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 9. | Saarilahti K, Kouri M, Collan J, Kangasmäki A, Atula T, Joensuu H, Tenhunen M. Sparing of the submandibular glands by intensity modulated radiotherapy in the treatment of head and neck cancer. Radiother Oncol. 2006;78:270-275. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 123] [Cited by in RCA: 110] [Article Influence: 5.8] [Reference Citation Analysis (0)] |
| 10. | Nutting CM, Morden JP, Harrington KJ, Urbano TG, Bhide SA, Clark C, Miles EA, Miah AB, Newbold K, Tanay M, Adab F, Jefferies SJ, Scrase C, Yap BK, A’Hern RP, Sydenham MA, Emson M, Hall E. Parotid-sparing intensity modulated versus conventional radiotherapy in head and neck cancer (PARSPORT): a phase 3 multicentre randomised controlled trial. Lancet Oncol. 2011;12:127-136. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1395] [Cited by in RCA: 1242] [Article Influence: 88.7] [Reference Citation Analysis (0)] |
| 11. | Lin A, Kim HM, Terrell JE, Dawson LA, Ship JA, Eisbruch A. Quality of life after parotid-sparing IMRT for head-and-neck cancer: a prospective longitudinal study. Int J Radiat Oncol Biol Phys. 2003;57:61-70. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 277] [Cited by in RCA: 252] [Article Influence: 11.5] [Reference Citation Analysis (0)] |
| 12. | Braam PM, Terhaard CH, Roesink JM, Raaijmakers CP. Intensity-modulated radiotherapy significantly reduces xerostomia compared with conventional radiotherapy. Int J Radiat Oncol Biol Phys. 2006;66:975-980. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 106] [Cited by in RCA: 102] [Article Influence: 5.4] [Reference Citation Analysis (0)] |
| 13. | Anand AK, Jain J, Negi PS, Chaudhoory AR, Sinha SN, Choudhury PS, Kumar R, Munjal RK. Can dose reduction to one parotid gland prevent xerostomia?--A feasibility study for locally advanced head and neck cancer patients treated with intensity-modulated radiotherapy. Clin Oncol (R Coll Radiol). 2006;18:497-504. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 25] [Cited by in RCA: 28] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 14. | Kam MK, Leung SF, Zee B, Chau RM, Suen JJ, Mo F, Lai M, Ho R, Cheung KY, Yu BK, Chiu SK, Choi PH, Teo PM, Kwan WH, Chan AT. Prospective randomized study of intensity-modulated radiotherapy on salivary gland function in early-stage nasopharyngeal carcinoma patients. J Clin Oncol. 2007;25:4873-4879. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 556] [Cited by in RCA: 529] [Article Influence: 29.4] [Reference Citation Analysis (0)] |
| 15. | Pow EH, Kwong DL, McMillan AS, Wong MC, Sham JS, Leung LH, Leung WK. Xerostomia and quality of life after intensity-modulated radiotherapy vs. conventional radiotherapy for early-stage nasopharyngeal carcinoma: initial report on a randomized controlled clinical trial. Int J Radiat Oncol Biol Phys. 2006;66:981-991. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 542] [Cited by in RCA: 518] [Article Influence: 28.8] [Reference Citation Analysis (0)] |
| 16. | Saarilahti K, Kouri M, Collan J, Hämäläinen T, Atula T, Joensuu H, Tenhunen M. Intensity modulated radiotherapy for head and neck cancer: evidence for preserved salivary gland function. Radiother Oncol. 2005;74:251-258. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 126] [Cited by in RCA: 120] [Article Influence: 5.7] [Reference Citation Analysis (0)] |
| 17. | Tribius S, Raguse M, Voigt C, Münscher A, Gröbe A, Petersen C, Krüll A, Bergelt C, Singer S. Residual deficits in quality of life one year after intensity-modulated radiotherapy for patients with locally advanced head and neck cancer: Results of a prospective study. Strahlenther Onkol. 2015;191:501-510. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 34] [Cited by in RCA: 38] [Article Influence: 3.8] [Reference Citation Analysis (0)] |
| 18. | Scrimger R, Kanji A, Parliament M, Warkentin H, Field C, Jha N, Hanson J. Correlation between saliva production and quality of life measurements in head and neck cancer patients treated with intensity-modulated radiotherapy. Am J Clin Oncol. 2007;30:271-277. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 48] [Cited by in RCA: 45] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 19. | Mercadante V, Al Hamad A, Lodi G, Porter S, Fedele S. Interventions for the management of radiotherapy-induced xerostomia and hyposalivation: A systematic review and meta-analysis. Oral Oncol. 2017;66:64-74. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 67] [Cited by in RCA: 104] [Article Influence: 13.0] [Reference Citation Analysis (0)] |
| 20. | Scarantino C, LeVeque F, Swann RS, White R, Schulsinger A, Hodson DI, Meredith R, Foote R, Brachman D, Lee N. Effect of pilocarpine during radiation therapy: results of RTOG 97-09, a phase III randomized study in head and neck cancer patients. J Support Oncol. 2006;4:252-258. [PubMed] |
| 21. | Johnson JT, Ferretti GA, Nethery WJ, Valdez IH, Fox PC, Ng D, Muscoplat CC, Gallagher SC. Oral pilocarpine for post-irradiation xerostomia in patients with head and neck cancer. N Engl J Med. 1993;329:390-395. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 350] [Cited by in RCA: 308] [Article Influence: 9.6] [Reference Citation Analysis (0)] |
| 22. | LeVeque FG, Montgomery M, Potter D, Zimmer MB, Rieke JW, Steiger BW, Gallagher SC, Muscoplat CC. A multicenter, randomized, double-blind, placebo-controlled, dose-titration study of oral pilocarpine for treatment of radiation-induced xerostomia in head and neck cancer patients. J Clin Oncol. 1993;11:1124-1131. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 209] [Cited by in RCA: 192] [Article Influence: 6.0] [Reference Citation Analysis (0)] |
| 23. | Jellema AP, Slotman BJ, Muller MJ, Leemans CR, Smeele LE, Hoekman K, Aaronson NK, Langendijk JA. Radiotherapy alone, versus radiotherapy with amifostine 3 times weekly, versus radiotherapy with amifostine 5 times weekly: A prospective randomized study in squamous cell head and neck cancer. Cancer. 2006;107:544-553. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 34] [Cited by in RCA: 33] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 24. | Jaguar GC, Lima EN, Kowalski LP, Pellizzon AC, Carvalho AL, Boccaletti KW, Alves FA. Double blind randomized prospective trial of bethanechol in the prevention of radiation-induced salivary gland dysfunction in head and neck cancer patients. Radiother Oncol. 2015;115:253-256. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 22] [Cited by in RCA: 27] [Article Influence: 2.7] [Reference Citation Analysis (0)] |
| 25. | Nagy K, Urban E, Fazekas O, Thurzo L, Nagy E. Controlled study of lactoperoxidase gel on oral flora and saliva in irradiated patients with oral cancer. J Craniofac Surg. 2007;18:1157-1164. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 38] [Cited by in RCA: 34] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 26. | Brizel DM, Murphy BA, Rosenthal DI, Pandya KJ, Glück S, Brizel HE, Meredith RF, Berger D, Chen MG, Mendenhall W. Phase II study of palifermin and concurrent chemoradiation in head and neck squamous cell carcinoma. J Clin Oncol. 2008;26:2489-2496. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 79] [Cited by in RCA: 70] [Article Influence: 4.1] [Reference Citation Analysis (0)] |
| 27. | Chitra S, Shyamala Devi CS. Effects of radiation and alpha-tocopherol on saliva flow rate, amylase activity, total protein and electrolyte levels in oral cavity cancer. Indian J Dent Res. 2008;19:213-218. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 31] [Cited by in RCA: 35] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 28. | Chung MK, Kim do H, Ahn YC, Choi JY, Kim EH, Son YI. Randomized Trial of Vitamin C/E Complex for Prevention of Radiation-Induced Xerostomia in Patients with Head and Neck Cancer. Otolaryngol Head Neck Surg. 2016;155:423-430. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 14] [Cited by in RCA: 26] [Article Influence: 2.9] [Reference Citation Analysis (0)] |
| 29. | Charalambous A, Lambrinou E, Katodritis N, Vomvas D, Raftopoulos V, Georgiou M, Paikousis L, Charalambous M. The effectiveness of thyme honey for the management of treatment-induced xerostomia in head and neck cancer patients: A feasibility randomized control trial. Eur J Oncol Nurs. 2017;27:1-8. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 33] [Cited by in RCA: 44] [Article Influence: 5.5] [Reference Citation Analysis (0)] |
| 30. | Li LX, Tian G, He J. The standardization of acupuncture treatment for radiation-induced xerostomia: A literature review. Chin J Integr Med. 2016;22:549-554. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 8] [Cited by in RCA: 9] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 31. | Zhang X, Liu F, Lan X, Yu L, Wu W, Wu X, Xiao F, Li S. Clinical observation of submandibular gland transfer for the prevention of xerostomia after radiotherapy for nasopharyngeal carcinoma: a prospective randomized controlled study of 32 cases. Radiat Oncol. 2014;9:62. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 11] [Cited by in RCA: 15] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 32. | Quock RL. Xerostomia: current streams of investigation. Oral Surg Oral Med Oral Pathol Oral Radiol. 2016;122:53-60. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 9] [Cited by in RCA: 11] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 33. | Sherlock S, Way M, Tabah A. Hyperbaric oxygen treatment for the management of radiation-induced xerostomia. J Med Imaging Radiat Oncol. 2018;62:841-846. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 6] [Cited by in RCA: 6] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 34. | Burlage FR, Roesink JM, Kampinga HH, Coppes RP, Terhaard C, Langendijk JA, van Luijk P, Stokman MA, Vissink A. Protection of salivary function by concomitant pilocarpine during radiotherapy: a double-blind, randomized, placebo-controlled study. Int J Radiat Oncol Biol Phys. 2008;70:14-22. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 69] [Cited by in RCA: 62] [Article Influence: 3.4] [Reference Citation Analysis (0)] |
| 35. | Mateos JJ, Setoain X, Ferre J, Rovirosa A, Navalpotro B, Martin F, Ortega M, Lomeña F, Fuster D, Pavia J, Pons F. Salivary scintigraphy for assessing the protective effect of pilocarpine in head and neck irradiated tumours. Nucl Med Commun. 2001;22:651-656. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 25] [Cited by in RCA: 24] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 36. | Valdez IH, Wolff A, Atkinson JC, Macynski AA, Fox PC. Use of pilocarpine during head and neck radiation therapy to reduce xerostomia and salivary dysfunction. Cancer. 1993;71:1848-1851. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 1] [Reference Citation Analysis (0)] |
| 37. | Chambers MS, Jones CU, Biel MA, Weber RS, Hodge KM, Chen Y, Holland JM, Ship JA, Vitti R, Armstrong I, Garden AS, Haddad R. Open-label, long-term safety study of cevimeline in the treatment of postirradiation xerostomia. Int J Radiat Oncol Biol Phys. 2007;69:1369-1376. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 34] [Cited by in RCA: 30] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 38. | Büntzel J, Schuth J, Küttner K, Glatzel M. Radiochemotherapy with amifostine cytoprotection for head and neck cancer. Support Care Cancer. 1998;6:155-160. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 53] [Cited by in RCA: 50] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 39. | Brizel DM, Wasserman TH, Henke M, Strnad V, Rudat V, Monnier A, Eschwege F, Zhang J, Russell L, Oster W, Sauer R. Phase III randomized trial of amifostine as a radioprotector in head and neck cancer. J Clin Oncol. 2000;18:3339-3345. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 624] [Cited by in RCA: 539] [Article Influence: 21.6] [Reference Citation Analysis (0)] |
| 40. | Antonadou D, Pepelassi M, Synodinou M, Puglisi M, Throuvalas N. Prophylactic use of amifostine to prevent radiochemotherapy-induced mucositis and xerostomia in head-and-neck cancer. Int J Radiat Oncol Biol Phys. 2002;52:739-747. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 167] [Cited by in RCA: 155] [Article Influence: 6.7] [Reference Citation Analysis (0)] |
| 41. | Buentzel J, Micke O, Adamietz IA, Monnier A, Glatzel M, de Vries A. Intravenous amifostine during chemoradiotherapy for head-and-neck cancer: a randomized placebo-controlled phase III study. Int J Radiat Oncol Biol Phys. 2006;64:684-691. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 113] [Cited by in RCA: 92] [Article Influence: 4.6] [Reference Citation Analysis (0)] |
| 42. | Wasserman TH, Brizel DM, Henke M, Monnier A, Eschwege F, Sauer R, Strnad V. Influence of intravenous amifostine on xerostomia, tumor control, and survival after radiotherapy for head-and- neck cancer: 2-year follow-up of a prospective, randomized, phase III trial. Int J Radiat Oncol Biol Phys. 2005;63:985-990. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 130] [Cited by in RCA: 101] [Article Influence: 5.1] [Reference Citation Analysis (0)] |
| 43. | Anné PR, Machtay M, Rosenthal DI, Brizel DM, Morrison WH, Irwin DH, Chougule PB, Estopinal NC, Berson A, Curran WJ Jr. A Phase II trial of subcutaneous amifostine and radiation therapy in patients with head-and-neck cancer. Int J Radiat Oncol Biol Phys. 2007;67:445-452. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 45] [Cited by in RCA: 39] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 44. | Law A, Kennedy T, Pellitteri P, Wood C, Christie D, Yumen O. Efficacy and safety of subcutaneous amifostine in minimizing radiation-induced toxicities in patients receiving combined-modality treatment for squamous cell carcinoma of the head and neck. Int J Radiat Oncol Biol Phys. 2007;69:1361-1368. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 24] [Cited by in RCA: 20] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 45. | Haddad R, Sonis S, Posner M, Wirth L, Costello R, Braschayko P, Allen A, Mahadevan A, Flynn J, Burke E, Li Y, Tishler RB. Randomized phase 2 study of concomitant chemoradiotherapy using weekly carboplatin/paclitaxel with or without daily subcutaneous amifostine in patients with locally advanced head and neck cancer. Cancer. 2009;115:4514-4523. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 69] [Cited by in RCA: 67] [Article Influence: 4.2] [Reference Citation Analysis (0)] |
| 46. | Bardet E, Martin L, Calais G, Alfonsi M, Feham NE, Tuchais C, Boisselier P, Dessard-Diana B, Seng SH, Garaud P, Aupérin A, Bourhis J. Subcutaneous compared with intravenous administration of amifostine in patients with head and neck cancer receiving radiotherapy: final results of the GORTEC2000-02 phase III randomized trial. J Clin Oncol. 2011;29:127-133. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 37] [Cited by in RCA: 35] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 47. | Thorstad WL, Chao KS, Haughey B. Toxicity and compliance of subcutaneous amifostine in patients undergoing postoperative intensity-modulated radiation therapy for head and neck cancer. Semin Oncol. 2004;31:8-12. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 19] [Cited by in RCA: 17] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 48. | Jha N, Seikaly H, Harris J, Williams D, Liu R, McGaw T, Hofmann H, Robinson D, Hanson J, Barnaby P. Prevention of radiation induced xerostomia by surgical transfer of submandibular salivary gland into the submental space. Radiother Oncol. 2003;66:283-289. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 91] [Cited by in RCA: 75] [Article Influence: 3.4] [Reference Citation Analysis (0)] |
| 49. | Seikaly H, Jha N, Harris JR, Barnaby P, Liu R, Williams D, McGaw T, Rieger J, Wolfaardt J, Hanson J. Long-term outcomes of submandibular gland transfer for prevention of postradiation xerostomia. Arch Otolaryngol Head Neck Surg. 2004;130:956-961. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 75] [Cited by in RCA: 57] [Article Influence: 2.7] [Reference Citation Analysis (0)] |
| 50. | Liu XK, Su Y, Jha N, Hong MH, Mai HQ, Fan W, Zeng ZY, Guo ZM. Submandibular salivary gland transfer for the prevention of radiation-induced xerostomia in patients with nasopharyngeal carcinoma: 5-Year outcomes. Head Neck. 2011;33:389-395. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5] [Cited by in RCA: 9] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 51. | Rieger JM, Jha N, Lam Tang JA, Harris J, Seikaly H. Functional outcomes related to the prevention of radiation-induced xerostomia: oral pilocarpine versus submandibular salivary gland transfer. Head Neck. 2012;34:168-174. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 26] [Cited by in RCA: 30] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 52. | Teguh DN, Levendag PC, Noever I, Voet P, van der Est H, van Rooij P, Dumans AG, de Boer MF, van der Huls MP, Sterk W, Schmitz PI. Early hyperbaric oxygen therapy for reducing radiotherapy side effects: early results of a randomized trial in oropharyngeal and nasopharyngeal cancer. Int J Radiat Oncol Biol Phys. 2009;75:711-716. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 57] [Cited by in RCA: 60] [Article Influence: 3.8] [Reference Citation Analysis (0)] |
| 53. | Blom M, Dawidson I, Fernberg JO, Johnson G, Angmar-Månsson B. Acupuncture treatment of patients with radiation-induced xerostomia. Eur J Cancer B Oral Oncol. 1996;32B:182-190. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 113] [Cited by in RCA: 103] [Article Influence: 3.6] [Reference Citation Analysis (0)] |
| 54. | Wong RK, Deshmukh S, Wyatt G, Sagar S, Singh AK, Sultanem K, Nguyen-Tân PF, Yom SS, Cardinale J, Yao M, Hodson I, Matthiesen CL, Suh J, Thakrar H, Pugh SL, Berk L. Acupuncture-Like Transcutaneous Electrical Nerve Stimulation Versus Pilocarpine in Treating Radiation-Induced Xerostomia: Results of RTOG 0537 Phase 3 Study. Int J Radiat Oncol Biol Phys. 2015;92:220-227. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 43] [Cited by in RCA: 41] [Article Influence: 4.1] [Reference Citation Analysis (0)] |
| 55. | Lovelace TL, Fox NF, Sood AJ, Nguyen SA, Day TA. Management of radiotherapy-induced salivary hypofunction and consequent xerostomia in patients with oral or head and neck cancer: meta-analysis and literature review. Oral Surg Oral Med Oral Pathol Oral Radiol. 2014;117:595-607. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 43] [Cited by in RCA: 46] [Article Influence: 4.2] [Reference Citation Analysis (0)] |
| 56. | Jensen SB, Pedersen AM, Vissink A, Andersen E, Brown CG, Davies AN, Dutilh J, Fulton JS, Jankovic L, Lopes NN, Mello AL, Muniz LV, Murdoch-Kinch CA, Nair RG, Napeñas JJ, Nogueira-Rodrigues A, Saunders D, Stirling B, von Bültzingslöwen I, Weikel DS, Elting LS, Spijkervet FK, Brennan MT; Salivary Gland Hypofunction/Xerostomia Section; Oral Care Study Group; Multinational Association of Supportive Care in Cancer (MASCC)/International Society of Oral Oncology (ISOO). A systematic review of salivary gland hypofunction and xerostomia induced by cancer therapies: management strategies and economic impact. Support Care Cancer. 2010;18:1061-1079. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 205] [Cited by in RCA: 179] [Article Influence: 11.9] [Reference Citation Analysis (0)] |
| 57. | Iovoli AJ, Singh AK. Accupuncture-like transcutaneous electrical nerve stimulation therapy success using a commercially available unit 8 years post-radiation for xerostomia: a case report. J Radiother Pract. 2017;16:217-220. |
| 58. | Beetz I, Schilstra C, Burlage FR, Koken PW, Doornaert P, Bijl HP, Chouvalova O, Leemans CR, de Bock GH, Christianen ME, van der Laan BF, Vissink A, Steenbakkers RJ, Langendijk JA. Development of NTCP models for head and neck cancer patients treated with three-dimensional conformal radiotherapy for xerostomia and sticky saliva: the role of dosimetric and clinical factors. Radiother Oncol. 2012;105:86-93. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 70] [Cited by in RCA: 83] [Article Influence: 5.9] [Reference Citation Analysis (0)] |
| 59. | Beetz I, Schilstra C, van der Schaaf A, van den Heuvel ER, Doornaert P, van Luijk P, Vissink A, van der Laan BF, Leemans CR, Bijl HP, Christianen ME, Steenbakkers RJ, Langendijk JA. NTCP models for patient-rated xerostomia and sticky saliva after treatment with intensity modulated radiotherapy for head and neck cancer: the role of dosimetric and clinical factors. Radiother Oncol. 2012;105:101-106. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 115] [Cited by in RCA: 153] [Article Influence: 11.8] [Reference Citation Analysis (0)] |
| 60. | Wada A, Uchida N, Yokokawa M, Yoshizako T, Kitagaki H. Radiation-induced xerostomia: objective evaluation of salivary gland injury using MR sialography. AJNR Am J Neuroradiol. 2009;30:53-58. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 30] [Cited by in RCA: 35] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 61. | Belli ML, Scalco E, Sanguineti G, Fiorino C, Broggi S, Dinapoli N, Ricchetti F, Valentini V, Rizzo G, Cattaneo GM. Early changes of parotid density and volume predict modifications at the end of therapy and intensity of acute xerostomia. Strahlenther Onkol. 2014;190:1001-1007. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 21] [Cited by in RCA: 25] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 62. | Yang X, Tridandapani S, Beitler JJ, Yu DS, Chen Z, Kim S, Bruner DW, Curran WJ, Liu T. Diagnostic accuracy of ultrasonic histogram features to evaluate radiation toxicity of the parotid glands: a clinical study of xerostomia following head-and-neck cancer radiotherapy. Acad Radiol. 2014;21:1304-1313. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 8] [Cited by in RCA: 13] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 63. | Pacholke HD, Amdur RJ, Morris CG, Li JG, Dempsey JF, Hinerman RW, Mendenhall WM. Late xerostomia after intensity-modulated radiation therapy versus conventional radiotherapy. Am J Clin Oncol. 2005;28:351-358. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 63] [Cited by in RCA: 65] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 64. | Blanchard P, Garden AS, Gunn GB, Rosenthal DI, Morrison WH, Hernandez M, Crutison J, Lee JJ, Ye R, Fuller CD, Mohamed AS, Hutcheson KA, Holliday EB, Thaker NG, Sturgis EM, Kies MS, Zhu XR, Mohan R, Frank SJ. Intensity-modulated proton beam therapy (IMPT) versus intensity-modulated photon therapy (IMRT) for patients with oropharynx cancer - A case matched analysis. Radiother Oncol. 2016;120:48-55. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 174] [Cited by in RCA: 174] [Article Influence: 19.3] [Reference Citation Analysis (0)] |
| 65. | van Luijk P, Pringle S, Deasy JO, Moiseenko VV, Faber H, Hovan A, Baanstra M, van der Laan HP, Kierkels RG, van der Schaaf A, Witjes MJ, Schippers JM, Brandenburg S, Langendijk JA, Wu J, Coppes RP. Sparing the region of the salivary gland containing stem cells preserves saliva production after radiotherapy for head and neck cancer. Sci Transl Med. 2015;7:305ra147. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 134] [Cited by in RCA: 166] [Article Influence: 18.4] [Reference Citation Analysis (0)] |
| 66. | Chen AM, Zahra T, Daly ME, Farwell DG, Luu Q, Gandour-Edwards R, Vaughan AT. Definitive radiation therapy without chemotherapy for human papillomavirus-positive head and neck cancer. Head Neck. 2013;35:1652-1656. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 27] [Cited by in RCA: 37] [Article Influence: 3.1] [Reference Citation Analysis (0)] |
| 67. | Ang KK, Harris J, Wheeler R, Weber R, Rosenthal DI, Nguyen-Tân PF, Westra WH, Chung CH, Jordan RC, Lu C, Kim H, Axelrod R, Silverman CC, Redmond KP, Gillison ML. Human papillomavirus and survival of patients with oropharyngeal cancer. N Engl J Med. 2010;363:24-35. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 5387] [Cited by in RCA: 4979] [Article Influence: 331.9] [Reference Citation Analysis (0)] |
| 68. | Marur S, Li S, Cmelak AJ, Gillison ML, Zhao WJ, Ferris RL, Westra WH, Gilbert J, Bauman JE, Wagner LI, Trevarthen DR, Balkrishna J, Murphy BA, Agrawal N, Colevas AD, Chung CH, Burtness B. E1308: Phase II Trial of Induction Chemotherapy Followed by Reduced-Dose Radiation and Weekly Cetuximab in Patients With HPV-Associated Resectable Squamous Cell Carcinoma of the Oropharynx- ECOG-ACRIN Cancer Research Group. J Clin Oncol. 2017;35:490-497. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 284] [Cited by in RCA: 361] [Article Influence: 40.1] [Reference Citation Analysis (0)] |