INTRODUCTION
The presumed lack of a graft-versus-tumor effect in autologous stem cell transplantation (ASCT) has been a long-standing argument against a higher curative potential of ASCT that relies mainly on high dose chemotherapy to eradicate tumor cells, compared with allogeneic stem cell transplantation (Allo-SCT)[1]. The clinical evidence of graft-versus-tumor (GVT) effect in Allo-SCT has been attributed to several indirect observations: (1) anecdotal reports demonstrating that abrupt withdrawal of immunosuppression in patients demonstrating tumor relapse post-Allo-SCT can re-establish complete remission; (2) higher risk of relapse in patients receiving syngeneic marrow grafts compared with recipients of allogeneic grafts; (3) patients developing graft-versus-host-disease (GVHD) experienced less relapse after Allo-SCT compared with those who did not; (4) T-cell depletion of an allogeneic graft increases the risk of relapse; and (5) the infusion of donor lymphocytes can induce complete remission after relapse following Allo-SCT[2]. The recognition of the adoptive GVT effect through the infusion of allo-reactive donor lymphocytes has changed our thinking of Allo-SCT from a therapeutic modality limited to high-dose chemotherapy to include immune-mediated GVT as an additional therapeutic intervention[3].
Despite the potential clinical benefit of the adoptive GVT in Allo-SCT, a fundamental problem is observed when using Allo-SCT as an immunotherapeutic modality. The infused allo-reactive donor immunocompetent cells that produce GVT can also target the host, producing GVHD. The transplant-related mortality (TRM) documented in myeloablative Allo-SCT has been reported to be between 40%-50%[3] compared with 3% TRM in ASCT. The rationale for non-myeloablative or reduced-intensity conditioning (RIC) is to decrease the high TRM observed after myeloablative Allo-SCT, though preserving the GVT effect. In lymphoid malignancies, the TRM after RIC has been reported from 5%-8% at day 100 to 19%-25% at 3 years[4]. In myeloid malignancies, the TRM after RIC has been reported around 20%[5]. Thus, the current research efforts in Allo-SCT center on ways of minimizing GVHD without compromising GVT.
The efficacy of ASCT as a treatment modality of cancer has relied mainly on the hope that high-dose chemotherapy will eradicate the resistant tumor clones that survived standard chemotherapy. Recently, we have reported the possibility of an autologous graft-versus-tumor effect without the detrimental effects of GVHD, based on the superior clinical outcomes observed in patients that achieved rapid post-ASCT recovery of their absolute lymphocyte count (ALC)[6]. Early ALC recovery, as a surrogate marker of host immunity in ASCT, is directly dependent on the absolute amount of infused lymphocytes (immune effector cells) harvested during CD34+ stem cell collection[7,8]. Herein, we review the different immune effector cells collected and infused from the stem cell autograft and their impact on immune recovery and survival post-ASCT.
RECOVERY OF ALC POST-ASCT
We reported superior overall survival (OS) and progression-free survival (PFS) in patients with multiple myeloma (MM) and non-Hodgkin lymphoma (NHL) that recovered higher ALC on day 15 (ALC-15) after ASCT[9]. We made similar observations in patients with other hematological malignancies[10-13] and metastatic breast cancer[14]. Several independent groups have confirmed our findings[13-20]. The superior survival observed for the first time post-ASCT based on ALC-15 in different malignancies suggests that patients’ own (autologous) immunity has anti-tumor activity that is not disease specific[1,6]. More importantly, none these patients developed GVHD, favoring a more-specific immune response against the tumor and not the host in the ASCT setting[1,6]. We confirmed the prognostic ability of ALC-15 for survival post-ASCT prospectively[21].
ALC SOURCES POST-ASCT
The ALC-15 discovery as a prognostic factor for survival post-ASCT led to study of the sources affecting ALC-15 recovery post-ASCT. ALC-15 sources post-ASCT can be divided into two categories: (1) host lymphocytes; and (2) infused lymphocytes from the stem cell autograft[1]. From the host, lymphocyte sources include host stem cells and host lymphocytes surviving high dose chemotherapy. The host stem cells surviving high-dose chemotherapy most likely do not contribute to ALC-15 recovery post-ASCT because without stem cell rescue these patients remained myelosuppressed for a prolonged period of time. To identify host lymphocytes is more difficult in comparison with Allo-SCT where the development of mixed chimerism in Allo-SCT allows discrimination of host versus donor lymphocytes. Such discrimination is currently not possible in ASCT in the absence of marking studies for host lymphocytes.
The second possible source for ALC-15 recovery post-ASCT is the lymphocytes collected and infused from the stem cell autograft[1]. From the stem cell autograft ALC-15 recovery could come from: (1) infused stem cells (CD34+); or (2) infused autograft lymphocytes collected during the apheresis process. In order to understand the impact of infused autografts on post-ASCT ALC-15 recovery, we evaluated the association of infused CD34 and/or lymphocytes from the autograft on the kinetics of post-ASCT lymphocyte recovery. No association was identified between infused CD34 stem cells and ALC-15. However, a strong correlation was found between the infused autograft lymphocyte numbers [autograft absolute lymphocyte count (A-ALC)] and ALC-15 recovery. Patients infused with an autograft containing higher A-ALC recovered greater numbers of ALC-15, resulting in better survival in NHL and MM post-ASCT[22,23]. An infused A-ALC ≥ 0.5 × 109 lymphocytes/kg was associated with a superior survival post-ASCT. This finding has been supported by other investigators[24]. These data suggest for the first time that the ASCT stem cell autograft should not be viewed only for the bone marrow rescue procedure to harvest CD34 stem cells necessary for hematologic engraftment, but also as an adoptive immunotherapeutic maneuver in which autograft lymphocyte content directly affects tumor-related clinical outcomes in multiple clinical settings.
The association between A-ALC and ALC-15 provides the first clinical evidence of an autologous GVT effect as the infusion of autograft lymphocytes has a direct impact on immune reconstitution and survival post-ASCT, similar to the GVT effect observed in Allo-SCT from the infused donor immune effector cells[6]. Therefore the identification of specific immune effector cell(s) infused from the autograft could be used as an immunotherapeutic strategy to improve immune recovery and survival post-ASCT.
Autograft CD4+ T cells
Schmidmaier et al[25] reported better event-free survival in MM patients infused with higher numbers of CD4+ helper T lymphocytes (HTL). Patients with a high percentage of HTL infused experienced a prolonged event-free survival (EFS) (2179 ± 170 d vs 1670 ± 212 d, P < 0.003). CD4 + T-cells with low HLA-DR expression produced a better EFS and overall survival compared with those that were HLA-DR+. Infusion of MM cells from the autograft did not affect survival, suggesting that the relapse post-ASCT is due to the number of malignant cells surviving high-dose chemotherapy in the host and not due to the malignant cells infused from the autograft[26].
Autograft CD8+ T cells
Infused autograft CD8+ T cells have been associated with early lymphocyte recovery (ELR) post-ASCT. Defining ELR as an ALC ≥ 500 cells/μL at day 12 post-ASCT, Atta et al[27] reported a faster ELR in patients infused with a CD8+ autograft lymphocyte dose of 0.1 × 109/kg. The authors stated that the autograft CD8+ lymphocyte dose can be used as a marker of a faster ELR, thus translating to better clinical outcomes post-ASCT.
Autograft natural killer cells
Natural killer (NK) cells have shown to be the earliest lymphocyte subset that recovered early post-Allo-SCT and post-ASCT[1]. We reported that the dose of infused autograft NK cells directly correlated with day 15 absolute NK cells counts (NK-15) post-ASCT[28]. Patients with an NK-15 ≥ 80 cells/μL experienced superior OS and PFS compared with those who did not (median OS: not reached vs 5.4 mo, P < 0.0001; and median PFS: not reached vs 3.3 mo, P < 0.0001, respectively)[21].
Autograft dendritic cells
Dendritic cells (DC) are the antigen-presenting cells required for priming of naïve T-cells. DCs that express CD11c are classified as DC1 and they have a myeloid morphology and, when stimulated with tumor necrosis factor, produce high levels of interleukin-12 causing antigen naïve CD4+CD45RA+ T-cell differentiation to Th1 cells[29]. DC2, known as plasmacytoid DCs, have a CD11c-/CD123+ phenotype; they are the precursors of lymphoid DCs and serve to stimulate antigen naïve CD4+CD45RA+ T cells to differentiate into Th2 cells[29]. Dean et al[29] reported that the total number of collected and infused DCs affect survival post-ASCT. In patients infused with a DC dose ≥ 9.10 × 106/kg, the median OS was not reached compared with a median OS of 11.5 mo for patients infused with a DC dose < 9.10 × 106/kg (P < 0.022)[29]. More interesting, in patients infused with DC1 ≥ 4.00 × 106/kg, the median OS was also not reached vs 11.3 mo for patients infused with a DC1 dose < 4.00 × 106/kg. No association with survival was observed with infused DC2[29]. This finding suggests that the polarization of the host immunity towards an anti-tumor Th1 response (DC1) conveyed a superior survival than a Th2 anti-tumor down regulating immune response (DC2).
HIGH-DOSE CHEMOTHERAPY AND INFUSED AUTOGRAFT IMMUNE EFFECTOR CELLS ANTI-TUMOR ACTIVITY
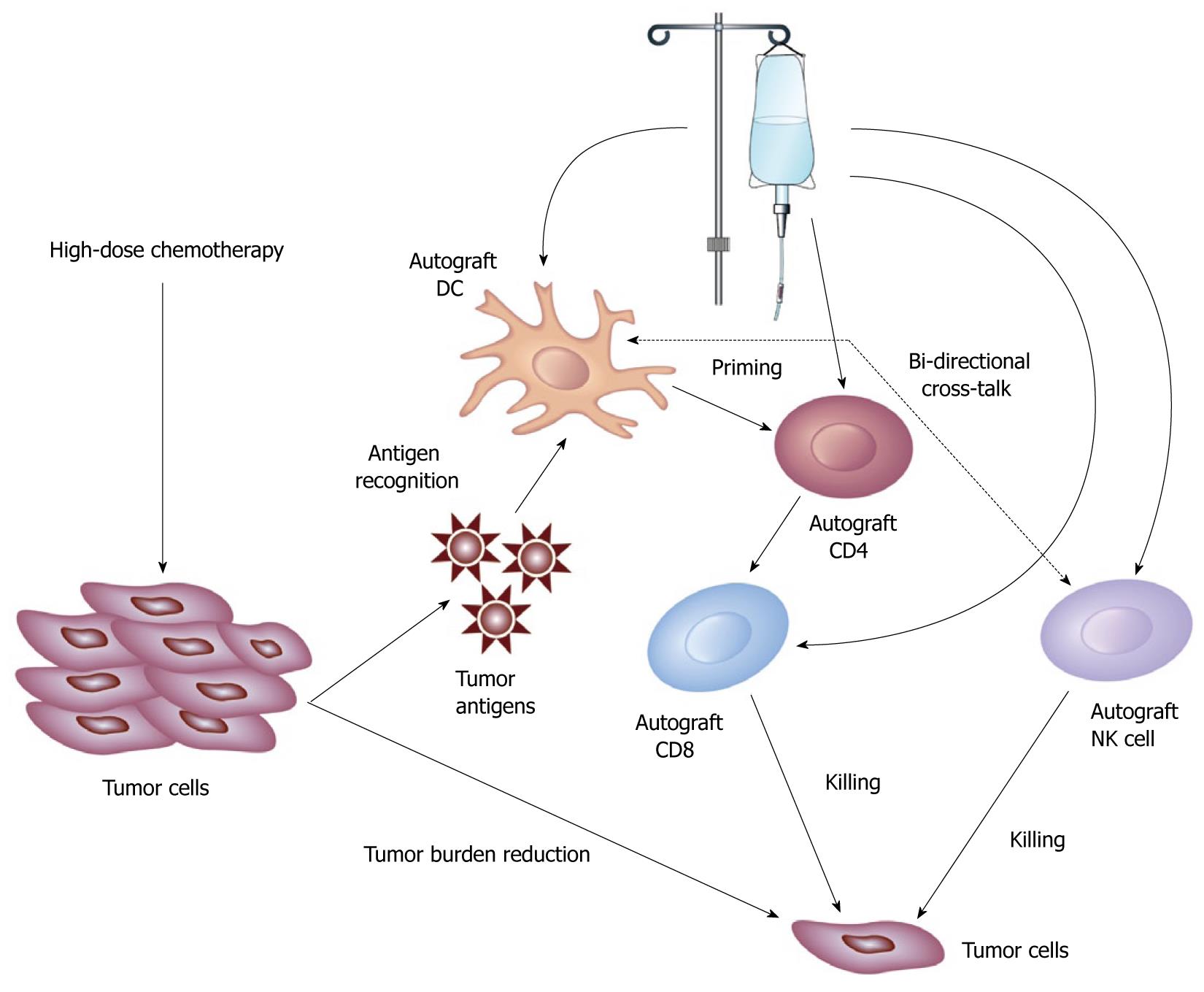
In Allo-SCT, the role of the conditioning regimen (high-dose chemotherapy) has changed from mainly a direct anti-cancer therapy to prevent host versus graft rejection to a therapy which allows the donor immune effector cells to eradicate the tumor cells[3]. In addition, Allo-SCT conditioning regimens can be viewed as a therapeutic strategy to suppress tumor growth to allow the allogeneic graft-versus-tumor effect to develop, as resistance to allogeneic GVT has been observed in acute lymphoblastic lymphoma and high-grade lymphomas whereas the rapid tumor proliferation outgrows the establishment of GVT[30]. In ASCT, high-dose chemotherapy (HDC) has several important roles to allow the host graft (infused autograft immune effector cells) to create an autologous GVT effect. First the HDC in ASCT will reduce the tumor burden to help the host immunity eradicate minimal residual disease. The concept of tumor burden has been proven in animal models where animals inoculated with a tumor containing ≥ 106 cells the immune system is unable to eradicate the tumor compared with animals inoculated with 106 cells[31]. In ASCT, delayed ALC recovery by day 30 post-ASCT is associated with worse OS and PFS compared with ALC recovery by day 15 post-ASCT, arguing that delayed host immunity recovery will allow rapid tumor growth post-ASCT counteracting the anti-tumor activity of the autologous GVT effect[32]. Second, by reducing the tumor burden, it is reasonable to assume that HDT will lower the immunosuppressive effect the tumor uses to evade immune recognition in the microenvironment. Third, by destroying the tumor, HDC might release tumor antigens that the infused DC1 can recognize and thus primed infused naïve CD4+ T-cells that in turn will prime infused cytotoxic CD8+ T cells from the autograft to eradicate minimal residual disease (the adaptive immune response from the autologous GVT effect). The innate immune response from the autologous GVT effect lies in the infused autograft NK cells that can recognize malignant cells without the need for antigen-presenting cells or with the help of antigen-presenting cells as recent reports have shown a bidirectional cross-talk between DCs and NK cells[33]. Thus, the combinations of the effects of HDC of reducing the tumor burden and re-setting the tumor microenvironment allow the infused autograft immune effector cells to create an autologous GVT effect, leading to the eradication of minimal residual disease and improved clinical outcomes post-ASCT (Figure 1).
Figure 1 Schematic representation of the role of high-dose chemotherapy and infused autograft immune effector cells in the eradication of tumor cells post-autologous stem cell transplantation.
The effects of high-dose chemotherapy (HDC) include tumor burden reduction and the release of tumor antigens. The infused autograft immune effectors cells include dendritic cells (DCs) that can recognize tumor antigens to priming infused autograft CD4+ T-cells, which in turn, activate infused autograft cytotoxic CD8+ T-cells to target tumor cells. The innate immune response component from the autograft is performed by the infused natural killer (NK) cells that can recognize tumor cells and NK cells anti-tumor function can be enhance with the help of DCs.
STRATEGIES TO ENHANCE AUTOGRAFT IMMUNE EFFECTOR CELL COLLECTION
The same concept of using a stem cell mobilization regimen to mobilize enough CD34 stem cells from the bone marrow into the peripheral blood for stem cell collection for ASCT applies to lymphocyte harvesting. As the ALC-15 directly depends on the amount of infused A-ALC, it is logical to assume that the collection of A-ALC will depend on the peripheral blood ALC at the time of collection (PC-ALC). We identified a positive correlation between PC-ALC and A-ALC[23,24]. Thus, any interventions that might result in pre-collection lymphopenia may negatively impact on A-ALC collection and ultimately lead to poor clinical outcomes in ASCT. This has been shown in MM patients. MM patients mobilized with granulocyte-colony stimulating-factor (G-CSF) and cyclophosphamide collected fewer A-ALC compared with MM patients that were mobilized with G-CSF alone[34].
However, the development of strategies to mobilize more peripheral blood lymphocytes and maximize lymphocyte harvesting should translate into higher A-ALC numbers, leading to faster immune recovery and improved clinical outcomes post-ASCT.
Interleukin-2 (IL-2) has been used in combination with G-CSF to mobilize NK cells for collection in the autograft. Sosman et al[35] found higher NK cell recovery by day 14 post-ASCT in patients in the IL-2 + G-CSF group. Other combinations of NK cells specific cytokines such as IL-15[36] and IL-21[37] could be studied to assess their ability to mobilize NK cells for harvesting in the autograft. Plerixafor is a CXCR4 inhibitor that has been approved for stem cell mobilization. We reported that plerixafor can also enhance lymphocyte mobilization for harvesting with the hope to improve immune recovery post-ASCT[38]. The number of apheresis collections is determined by the target dose of collected CD34 stem cells. Similarly, patients that had ≥ 4 apheresis collections harvested more lymphocytes compared with those who had < 4 apheresis collections[34]. Thus, the number of collections could be used as a strategy to achieve a target dose of A-ALC to maximize immune recovery and survival post-ASCT.
Another maneuver to enhance lymphocyte collection during apheresis is to reset the apheresis machine to not only collect enough CD34+ stem cells but also high numbers of A-ALC. Three apheresis machines have been used for stem cell collection in the ASCT setting, including the COBE Spectra, the Fenwal CS 3000, and the Baxter Amicus. We identified that the COBE Spectra collected more A-ALC than the other two machines and better survival post-ASCT was observed in patients whose cells were collected by the Spectra machine compared to the others[39]. The survival benefit observed by the Spectra machine was not due to the machine itself; instead it was due to the fact that the Spectra machine collected more A-ALC. Because of this finding, we are currently doing a double blind randomized study where stem cells will be collected from patients the standard way versus a modified version of stem cell collection to maximize lymphocyte collection to assess if the new modified apheresis machine settings not only collect enough CD34 stem cells, but also more A-ALC, to affect survival post-ASCT.
CONCLUSION
The discovery of A-ALC affecting ALC-15, which in turn improved clinical outcome post-ASCT, created a new concept of viewing the stem cell autograft as an adoptive immunotherapeutic strategy with direct impact on survival post-ASCT. Further studies are warranted to understand how host immunity improves survival post-ASCT.
Peer reviewers: Maurizio Bendandi, MD, PhD, Associate Proessor, Laboratoire of Immunotherapy, Division of Oncology, Center for Applied Medical Research, University of Navarre, Cima Avda. Pio XII 55, 31008 Pamplona, Spain; Peter Hersey, FRACP, DPhil, Senior Staff Specialist in Immunology and Oncology, Conjoint Professor in Oncology, Room 443, David Maddison Building, Cnr King and Watt Streets, Newcastle, NSW 2300, Australia
S- Editor Cheng JX L- Editor O’Neill M E- Editor Ma WH