Copyright
©The Author(s) 2017.
World J Clin Oncol. Feb 10, 2017; 8(1): 21-36
Published online Feb 10, 2017. doi: 10.5306/wjco.v8.i1.21
Published online Feb 10, 2017. doi: 10.5306/wjco.v8.i1.21
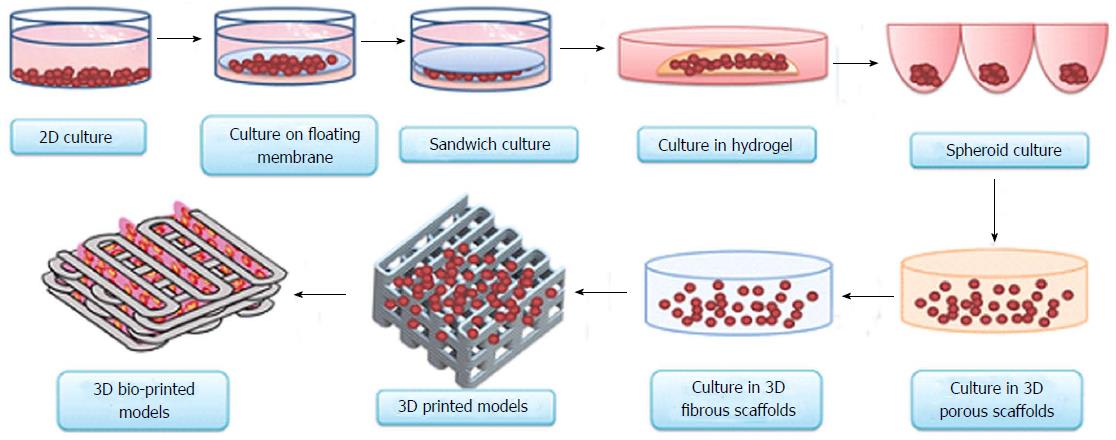
Figure 1 Evolution of cell-culture models from simple two-dimensional to complex three-dimensional bio-printed models.
Currently, 3D bio-printing is the most sophisticated technique used to make tissue/organ constructs[65]. 3D: Three-dimensional.
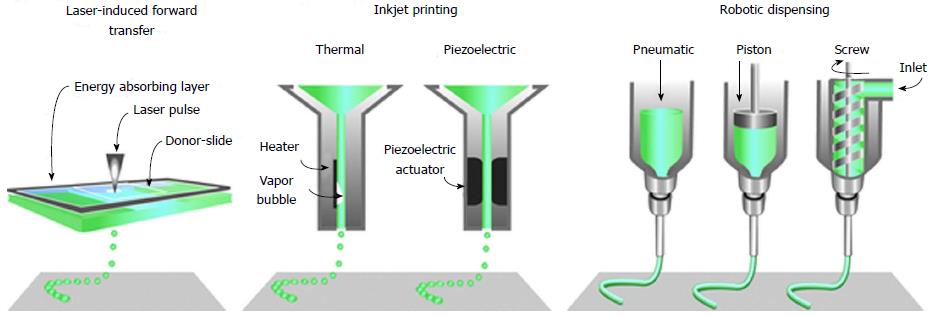
Figure 2 The common approaches currently used to bio-print tissue, are laser-assisted, inkjet-based and extrusion-based robotic dispensing techniques[110].
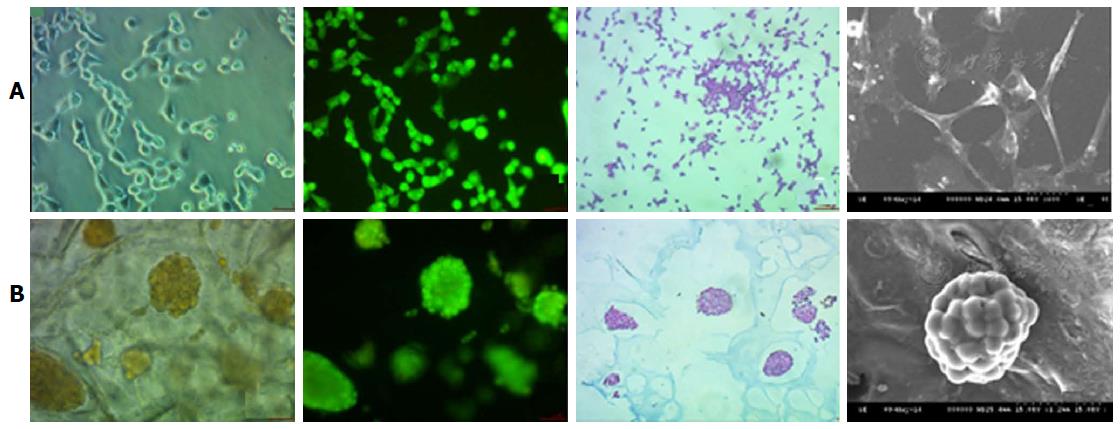
Figure 3 Nonsmall-cell lung cancer 95D cell morphology under two-dimensional and three-dimensional culture conditions.
The 2D cultured cells (A) are tiled, polygonal, of long spindle shape and display more pseudopodia. In contrast, 3D morphology culture groups (B) are a combination of round and oval shapes, display intercellular tight aggregation and adhesion. Furthermore, there is evidence of multiple sizes of cells distributed in different scaffold pores[60]. 2D: Two-dimensional; 3D: Three-dimensional.
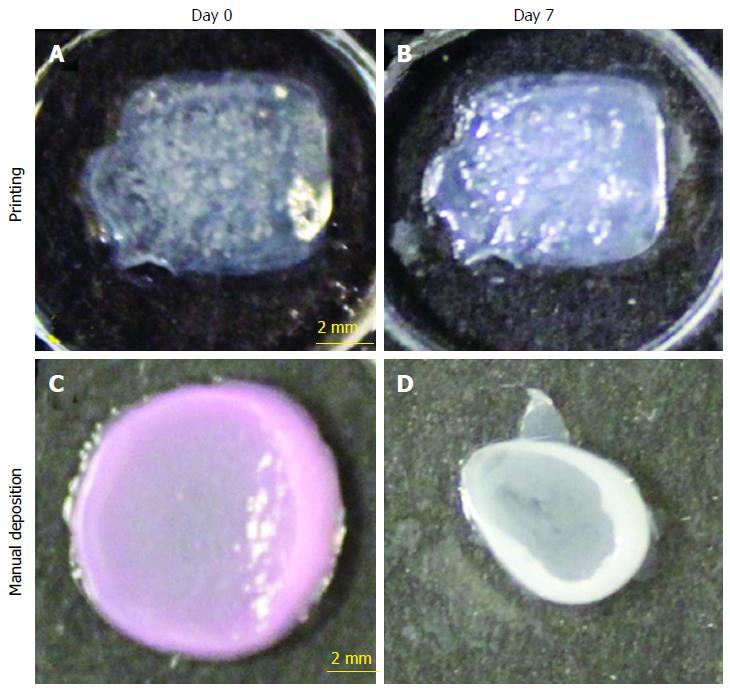
Figure 4 Shape and form of printed skin tissue.
A comparison of skin tissues fabricated via 3D bio-printing and manual deposition indicates that printed skin samples (A, B) retain their form (dimensions) and shape, whereas manually deposited structures (C, D) shrink and form concave shapes (buckle) under submerged culture condition after 7 d.
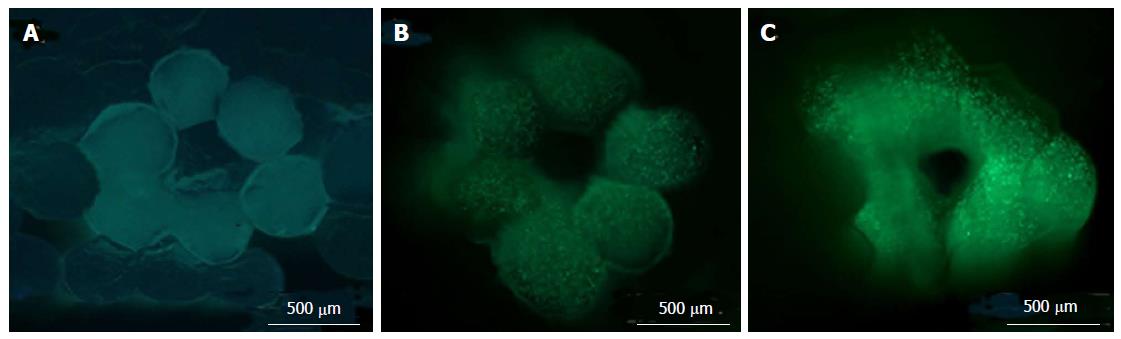
Figure 5 Cross-sectional images of three-dimensional bio-printed tissue (NIH 3T3 cells) containing an encapsulated fluorescent HA-BODIPY tracer for increased visualisation.
Cross-sectional views of the bio-printed vascular constructs were taken (A) immediately after printing; (B) at 14 d; and (C) at 28 d of culture using LIVE/DEAD staining to highlight viable and dead cells. Green fluorescence indicates calcein AM-stained live cells[70].
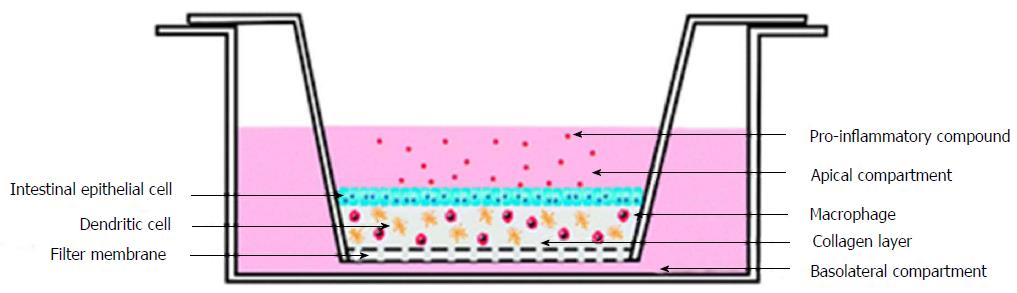
Figure 6 Experimental setup of three-dimensional co-culture comprising of intestinal epithelial cells, macrophages and dendritic cells[74].
- Citation: Charbe N, McCarron PA, Tambuwala MM. Three-dimensional bio-printing: A new frontier in oncology research. World J Clin Oncol 2017; 8(1): 21-36
- URL: https://www.wjgnet.com/2218-4333/full/v8/i1/21.htm
- DOI: https://dx.doi.org/10.5306/wjco.v8.i1.21